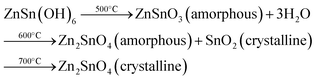Open Access Article
Open Access ArticleComprehensive review of micro/nanostructured ZnSnO3: characteristics, synthesis, and diverse applications
Moksodur Rahman
 ab,
Muhammad Shahriar Bashar
ab,
Muhammad Shahriar Bashar b,
Md. Lutfor Rahman
b,
Md. Lutfor Rahman b and
Faisal Islam Chowdhury
b and
Faisal Islam Chowdhury
 *a
*a
aDepartment of Chemistry, University of Chittagong, Chattogram, Bangladesh. E-mail: faisal@cu.ac.bd
bBangladesh Council of Scientific and Industrial Research (BCSIR), Dhaka, Bangladesh
First published on 23rd October 2023
Abstract
Generally, zinc stannate (ZnSnO3) is a fascinating ternary oxide compound, which has attracted significant attention in the field of materials science due to its unique properties such high sensitivity, large specific area, non-toxic nature, and good compatibility. Furthermore, in terms of both its structure and properties, it is the most appealing category of nanoparticles. The chemical stability of ZnSnO3 under normal conditions contributes to its applicability in various fields. To date, its potential as a luminescent and photovoltaic material and application in supercapacitors, batteries, solar cells, biosensors, gas sensors, and catalysts have been extensively studied. Additionally, the efficient energy storage capacity of ZnSnO3 makes it a promising candidate for the development of energy storage systems. This review focuses on the notable progress in the structural features of ZnSnO3 nanocomposites, including the synthetic processes employed for the fabrication of various ZnSnO3 nanocomposites, their intrinsic characteristics, and their present-day uses. Specifically, we highlight the recent progress in ZnSnO3-based nanomaterials, composites, and doped materials for their utilization in Li-ion batteries, photocatalysis, gas sensors, and energy storage and conversion devices. The further exploration and understanding of the properties of ZnSnO3 will undoubtedly lead to its broader implementation and contribute to the advancement of next-generation materials and devices.
1. Introduction
The creation and fabrication of materials at the nanoscale have witnessed a significant breakthrough in the 21st century, revolutionizing numerous industries, including photocatalysis, energy storage and conversion systems, biosensors, and biological applications. Binary metal-oxides, such as TiO2, ZnO, and SnO2, which are known for their favourable optical and electrical properties, have been extensively investigated and widely applied in various sectors, such as photovoltaic devices,1 thin-film displays,2 electrochromic systems,3 and gas sensing technologies.4 Nevertheless, the practical application of these materials is impeded by their poor thermal and chemical stability when exposed to different environments.Accordingly, to overcome this limitation and improve their characteristics, researchers are actively engaged in the development of ternary oxides, such as ZnSnO3 and In–Zn–O.5–7 Among them, nanostructures of ZnSnO3 (various nano shapes, such as wires, rods, rings, tubes, cubes, and spheres) have attracted considerable interest owing to their advantageous chemical sensitivity, wide energy bandgap, high transmittance percentage, electron mobility, low price, non-toxicity, and earth abundance.5,8–13 The performance of energy storage devices and catalysis is greatly affected by the morphology, structure, and physical characteristics of the active electrode materials. Furthermore, LN-type ZnSnO3, which possesses a high spontaneous polarization (theoretical value Pr ≈ 59 μC cm−2), has been experimentally observed in epitaxial thin films with a value of Pr ≈ 47 μC cm−2.14 These films demonstrate superior photocatalytic activity and exhibit piezoelectric properties.
ZnSnO3 possesses remarkable morphological properties, making it an attractive material with diverse energy and biological applications. The synthesis and fabrication techniques employed greatly influence the morphological characteristics of ZnSnO3. Various factors, such as the capping agent, surfactant, reaction temperature, annealing temperature, concentration of metal precursors, and reaction time, play a crucial role in the development of different synthetic processes.15–17 Researchers have reported the synthesis of well-organized ZnSnO3 nanopowders, composites, and films using a range of methods. These methods include solid-state,18 sol–gel,19,20 ion-exchange,21 high temperature calcination,22,23 thermal evaporation,24,25 magnetron sputtering,26–29 hydrothermal process,8,12 laser ablation,30 and vapor deposition.31 The different crystal structures of ZnSnO3, such as that with the Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m, R
m, R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) , and R3c space groups, have been extensively investigated. The perovskite structure (with the Pm
, and R3c space groups, have been extensively investigated. The perovskite structure (with the Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m space group) of ZnSnO3, together with its face-centred cubic and orthorhombic phases can be achieved through the calcination of various ZnSn(OH)6 precursors. Recent studies have focused on the advancements in ZnSnO3 nanostructures for energy and biological applications. However, considering the limited literature to date on this subject, the present review serves as a necessary contribution to bridge the knowledge gap.
m space group) of ZnSnO3, together with its face-centred cubic and orthorhombic phases can be achieved through the calcination of various ZnSn(OH)6 precursors. Recent studies have focused on the advancements in ZnSnO3 nanostructures for energy and biological applications. However, considering the limited literature to date on this subject, the present review serves as a necessary contribution to bridge the knowledge gap.
This review presents the latest progress in the field of ZnSnO3-based nanomaterials, composites, and doped materials, focusing on their application in the key areas of energy and biology. ZnSnO3-based materials have attracted significant interest due to their potential application in energy storage and conversion technologies, such as lithium/sodium-ion batteries and dye-sensitized solar cells. Additionally, they demonstrate promising prospects as catalysts for the removal of dye/organic pollutants and as gas sensors for various biological uses. This review provides comprehensive insights into the advancements and potential of ZnSnO3-based materials in these specific domains.
2. Crystal structure and physical properties
ZnSnO3 exhibits various types of crystal structures, including perovskite, ilmenite, LiNbO3-type, CdSnO3-type, HgSnO3-type, and post-perovskite with the Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m, R
m, R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) , R3c, Pnma,
, R3c, Pnma, ![[R with combining macron]](https://www.rsc.org/images/entities/i_char_0052_0304.gif) 3c, and Cmcm space groups, respectively, which are all feasible.
3c, and Cmcm space groups, respectively, which are all feasible.
ZnSnO3 with the Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m, R
m, R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) , and R3c space groups has been the subject of numerous study. The perovskite structure (with Pm
, and R3c space groups has been the subject of numerous study. The perovskite structure (with Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m space group) of ZnSnO3 includes face-centred cubic (FCC) and orthorhombic phases, which is typically synthesized by annealing different ZnSn(OH)6 precursors. In terms of surface energy, ZnSnO3 crystals with an FCC structure generally exhibit the order of (111) < (100) < (110). This demonstrates that the normal surfaces of ZnSnO3 crystals tend to exhibit (111) facets, while facets with higher surface energies such as {100} or {110} may not appear during realistic thermodynamic growth processes.32
m space group) of ZnSnO3 includes face-centred cubic (FCC) and orthorhombic phases, which is typically synthesized by annealing different ZnSn(OH)6 precursors. In terms of surface energy, ZnSnO3 crystals with an FCC structure generally exhibit the order of (111) < (100) < (110). This demonstrates that the normal surfaces of ZnSnO3 crystals tend to exhibit (111) facets, while facets with higher surface energies such as {100} or {110} may not appear during realistic thermodynamic growth processes.32
Zinc tin oxide/zinc stannate occur in two individual oxides with distinct crystal structures and varying Zn/Sn ratios. These oxides are known as orthorhombic or perovskite ZnSnO3 and cubic spinel-type Zn2SnO4.33 Based on the available data, Zn2SnO4 demonstrates higher thermal stability compared to ZnSnO3. Zn2SnO4 possesses a cubic spinel arrangement, which has been previously established as the most thermodynamically stable form. Alternatively, ZnSnO3 is formed under non-equilibrium conditions, such as high pressure, suggesting its thermodynamic metastability as a crystal phase. The formation of metastable ZnSnO3 requires high pressure and energy conditions. The phase transition from metastannate to orthostannate begins at approximately 500 °C, with an activation energy of around 0.965 eV. Subsequently, recrystallization occurs, leading to the formation of the orthostannate phase with an inverse spinel structure, which is typically observed at around 750 °C. This investigation provides valuable insights into the behaviour of perovskite ZnSnO3 undergoing a phase change to inverse spinel Zn2SnO4 during calcination.33
Previous studies have indicated that metastable ZnSnO3 structures tend to degrade at temperatures exceeding 500 °C. However, Rovisco et al. demonstrated that breakdown can take place at much lower temperatures (e.g., 220 °C for 24 h) or longer reaction durations (e.g., 200 °C for 36 h) because of the high energy involved in the hydrothermal technique.34 This underscores the benefits of utilizing hydrothermal techniques to acquire metastable nanostructures composed of multiple components, such as ZnSnO3, at reduced temperatures. It also underscores the requirement of carefully managing and comprehending all aspects of fabrication to attain the targeted structures successfully. The growth mechanisms of nanostructures throughout the reaction period pose a significant challenge in the synthesis of nanomaterials. Particularly, in the case of fabricating ZnSnO3 nanowire, the metastable nature of this phase adds complexity to its complete comprehension.34 By increasing the reaction time and overall energy available, the development of nanostructures and their corresponding phases can be observed, which is primarily due to the meticulous optimization of the physio-chemical parameters employed in this study. Their primary objective was to generate ZnSnO3 nanowires, but the formation of Zn2SnO4 nanostructures was also observed, particularly under the conditions of very short synthesis durations, lower temperatures, and smaller reaction volumes. This observation implies that the formation of Zn2SnO4 requires comparatively less energy. The aforementioned procedure is illustrated in Fig. 1.
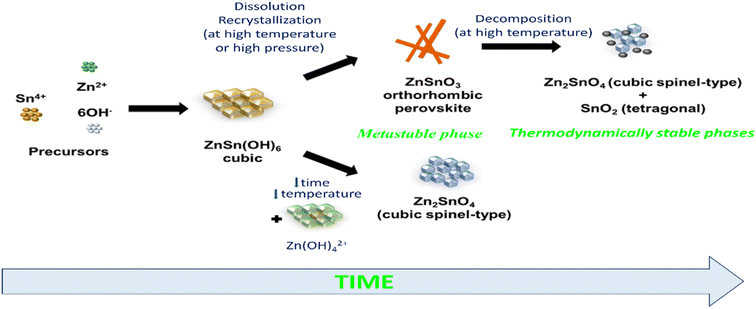 | ||
| Fig. 1 Schematic illustration of phase rearrangements during the hydrothermal process of zinc stannate nanostructures as a function of reaction energy and synthesis time.34 | ||
The LiNbO3-type configuration (R3c) is distinguished by the substantial displacement of Zn atoms, which is caused by the robust covalent bonds formed between Zn and three oxygen atoms. This bonding configuration gives rise to piezoelectric, ferroelectric, pyroelectric, and non-linear optical properties. The crystal lattice of ZnSnO3 in the LN-type arrangement, as depicted in Fig. 2a, is comprised of interconnected octahedral units. Interlocking occurs between the Zn octahedra, where each octahedron shares its corners with another octahedron of the same type. Similarly, Sn octahedra exhibit corner sharing, forming connections with other Sn octahedral structures. The cation arrangement follows a pattern of Sn–Zn-vacancy-Sn-Zn-vacancy-Sn, aligned along the z-axis. In ZnSnO3, the bond valence sums for Zn, Sn, and O were calculated to be 1.79, 4.08, and 1.96, respectively. Notably, the Zn–O bond in ZnSnO3 is found to be under-bonded compared to the ideal values. The Sn–O distances in ZnSnO3 were observed to be 0.2005 nm (×3) and 0.2094 nm (×3), deviating from the distances typically observed in perovskite-type stannates, which feature SnO6 octahedra.35
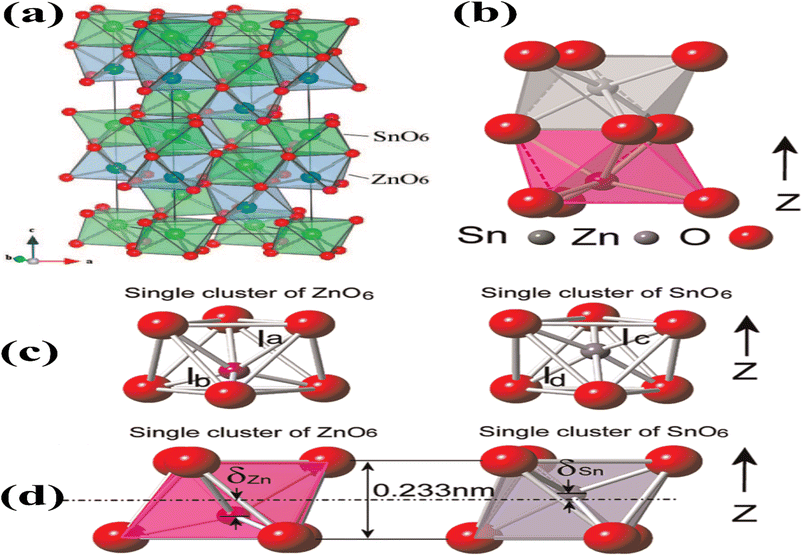 | ||
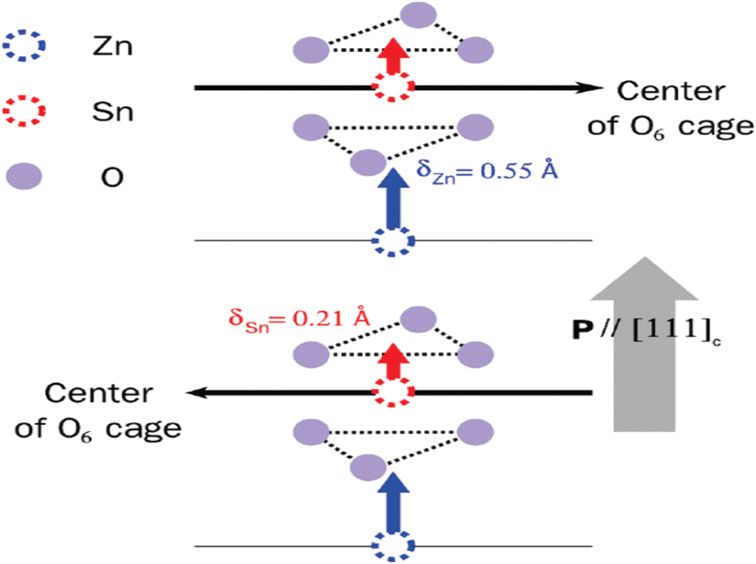
| Fig. 2 (a) Crystal structure of ZnSnO3 is visualized in a three-dimensional image, revealing the arrangement of atoms in an octahedral framework [reprinted with permission from J. Am. Chem. Soc. 2008, 130, 21, 6704–6705. Copyright 2008, the American Chemical Society]. (b) Lower side of the structure consists of ZnO6 octahedra, while the upper side is formed by SnO6 octahedra. (c) Separate and distinct clusters of ZnO6 and SnO6. (d) Displacement of Zn ions (δZn) and Sn ions (δSn) from their equilibrium positions along the z-axis causes a variation in the bonding length between oxygen (O) and Zn (O–Zn–O) or Sn (O–Sn–O) atoms, respectively.48 | ||
Fig. 2b illustrates the presence of two octahedral structures, where one is composed of ZnO6 and the other SnO6. The SnO6 and ZnO6 octahedra are connected to the neighbouring octahedra through shared edges and faces. Fig. 2c illustrates a single ZnO6 and SnO6 cluster. The Zn–O bonding lengths consist of three long bonds (approximately 0.2308 nm) on the upper side and three short bonds (approximately 0.2040 nm) on the bottom side, which are labelled as la and lb in the ZnO6 cluster, respectively. The Sn–O bonding lengths in the SnO6 cluster, labelled as lc and ld, consist of three short bonds (approximately 0.2008 nm) on the upper side and three long bonds (approximately 0.2093 nm) on the lower side. Fig. 2d shows individual clusters of ZnO6 and SnO6, highlighting the variation in bonding length along the z-axis. In the ZnO6 cluster, the Zn ion exhibits a displacement (δZn) of 0.5 Å, while in the SnO6 cluster, the Sn ion has a displacement (δSn) of 0.2 Å. Along the z-axis, the Zn ion experiences a larger displacement than the Sn ion, resulting in the creation of spontaneous polarization, which is the origin of piezoelectricity in this material.36,37
In ref. 38 and 39, density functional theory (DFT) and the extended gradient estimation were employed to investigate the structural, electrical, and optical properties of ZnSnO3. The analysis of the electronic structures revealed that ZnSnO3 is a semiconductor characterized by a direct band gap of 1.0 eV. The examination of the optical spectra revealed that inter-band transitions occur in the ZnSnO3 compound. These transitions occur between the O 2p levels in the valence band (VB) and either the Sn 5s level or the higher CB Zn 3d levels in the lower energy level. Additionally, inter-band transitions were observed between the O 2p levels and either the Sn 5p or Zn 4p conduction bands (CB) in the higher energy level. These transitions contributed to the computed optical spectra.
Dielectric properties are also crucial properties for the application of nanoparticles as dielectric materials.40–42 ZnSnO3 materials display excellent electromagnetic wave attenuation characteristics and a wide frequency range, making them suitable for various applications such as ground-penetrating radar systems, microwave absorbers, communication systems, and energy storage devices.43–45 To obtain the real (ε1) and imaginary (ε2) part of the dielectric constant, the Kramers–Kronig equation was utilized.46 Given that ZnSnO3 possesses a hexagonal shape, the evaluation was focused on incoming light polarized along the [1 0 0] and [0 0 1] crystallographic axes. They observed that there was no significant anisotropy in both the real and imaginary parts of the equation. The peaks in ε2 were associated with electron excitation. Furthermore, the computed static dielectric constant, ε1(0), of ZnSnO3 along the [1 0 0] and [0 0 1] directions was determined to be 4.05 and 3.96 eV, respectively.39 These results are significantly lower than that of BaTiO3 (5.12) and PbZrO3 (5.34), indicating the distinct dielectric behaviour of ZnSnO3 compared to these materials.47
ZnSnO3 also exhibits superior ferroelectric properties. Shin et al. conducted research on the ferroelectric characteristics of ZnSnO3.49 They examined the hysteresis loop of a Pt/ZnSnO3/SrRuO3 capacitor at a measurement frequency of 10 kHz to investigate its ferroelectric properties. This exhibited a coercive electric field of 130 kV cm−1 and improved remnant polarization of 47 C cm−2 (2Pr of 94 C cm−2). The epitaxial ZnSnO3 demonstrated a saturation polarization of 58 C cm−2, which was marginally higher than the residual polarization. This observation suggests the presence of a well-formed crystalline structure in the material. To gain a deeper understanding, they investigated the hysteresis loops across a range of frequencies. This analysis aimed to examine the behaviour of the capacitor at various frequency regimes. As depicted in Fig. 3a, the coercivity increased gradually as the measurement frequency increased. Additionally, a square pulse with a voltage of 5 V was employed to evaluate the switching current. The switching behaviour was found to be rapid, with a fast-switching time of 100 ns, as shown in Fig. 3b. This indicates that the capacitor can transition between states efficiently and quickly.
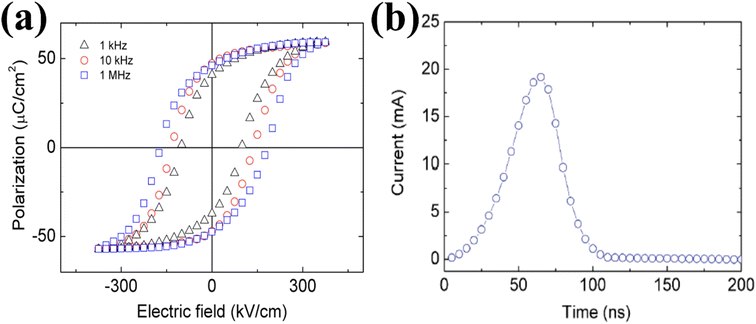 | ||
| Fig. 3 (a) Hysteresis loops of the Pt/ZnSnO3/SrRuO3 capacitor observed at different measurement frequencies. (b) Switching-current as a bias of 5 V. The fast-switching behaviour is within 100 ns.49 | ||
To model the interactions between ions and electrons, projector expanded wave potentials were employed, considering the effects of both ions and electrons.49 The exchange and correlation energies of the electrons were calculated using a local density approximation, providing insights into their behaviour in the system. The estimated lattice constants were a = 5.24 Å and c = 13.88 Å per formula unit cell, with a cell volume of 54.92 Å. Fig. 4 presents the measurements of the deviations of Zn and Sn ions from the oxygen octahedral core. The data revealed that the rearrangement of the A cation (Zn) was 0.55 Å, which exceeds the rearrangement of the B cation (Sn) at 0.21 Å. This disparity can be attributed to the larger available space in the A site, facilitating greater movement for the Zn atom. The difference in rearrangement arises from the fact that Zn, which has a covalent radius of 1.31 Å, is smaller in size compared to Sn, with a covalent radius of 1.41 Å.49 Employing the Berry phase technique, a polarization value of 60 C cm−2 was determined along the pseudo cubic109 direction. This value closely matches both their experimental findings and the results obtained from analytical measurements based on the ionic rearrangements and atomic valences.
 | ||
| Fig. 4 Ionic rearrangements of Zn and Sn in the R3c phase of ZnSnO3.49 | ||
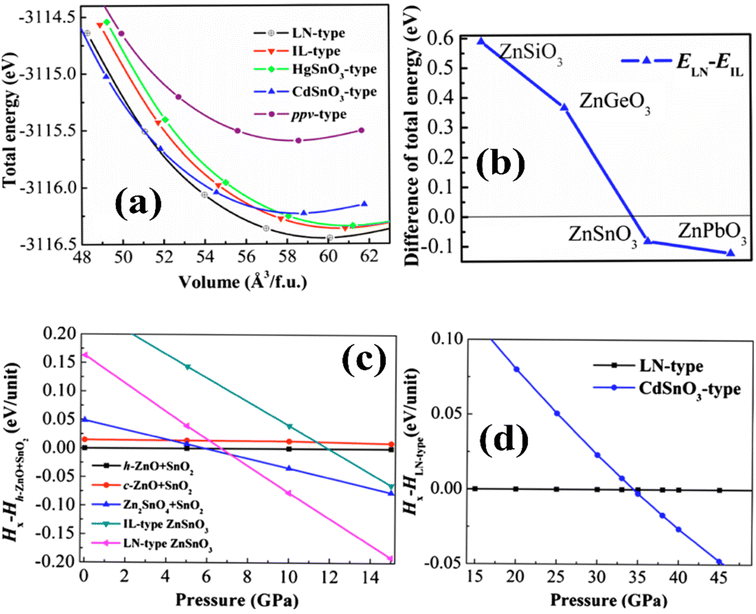
The preferred ground-state structure of ZnSnO3 was found to be the LN-type (LiNbO3-type) phase, given that it exhibits a lower total energy compared to the IL-type (ilmenite-type) structure. The energy difference between the LN and IL phases is merely 0.09 eV per unit of formula, indicating the potential occurrence of a structural shift from the LN to the IL when exposed to high pressure or temperature. However, investigations by Gao et al. revealed that the total energy–volume curves of the LN and IL phases do not cross when subjected to compression, indicating that the structural transition is unlikely to occur under high pressure.39
Fig. 5a illustrates the calculated E–V curves for different structures. When T = 0 K, the LN structure exhibits the lowest total energy among the feasible structures. Alternatively, the cubic perovskite structure has a considerably higher total energy, with a difference of up to 3.76 eV per formula unit compared to the LN-type structure. This substantial energy variation implies that the formation of the cubic perovskite phase of ZnSnO3 is challenging to achieve under normal conditions. Additionally, the combined energy of the CdSnO3-type phase crosses paths with both the IL and LN phases, indicating the possibility of structural changes in severe settings.50–52
 | ||
| Fig. 5 (a) E–V curve for six feasible phases. (b) Energy variation in ZnXO3. (c) Enthalpy variation among heterogenous component oxides. (d) Enthalpy variation in LN-type and CdSnO3-type phases.65 | ||
Moreover, Fig. 5b illustrates the evaluation of the total energy variation in ZnXO3 compounds (where, X = Si, Ge, Sn, and Pb) between the IL and LN structures. The results indicate a monotonic decrease in the total energy difference moving from Si (0.60 eV) to Ge (0.37 eV) to Sn (0.09 eV) phases, proposing that the IL phase is strenuously more favourable than the LN phase for these elements. However, for the Pb-containing phase, the LN phase is more energetically favourable than the IL phase. In the range where the zero-point energy (ZPE) correction becomes significant for assessing the relative structural stability, the overall energy gap between ZnSnO3 in ionic liquid (IL) and layered perovskite (LN) forms is comparatively insignificant. The ZPE calculated from the partial density of states (PDOS) was found to be 0.23 eV per unit for the IL-type structure and 0.31 eV per unit for the LN structure. When accounting for ZPE, the overall energy gap between the IL and LN structures is approximately 0.005 eV per unit. This implies that there is a possibility for these two phases to exist together under normal environmental conditions.50,53–55
The lattice parameters of a semiconductor are typically affected by multiple parameters, as follows:56–59 (i) the concentration of free electrons, which affects the deformation potential of the conduction-band minimum occupied by these electrons, (ii) the concentration of foreign atoms and defects and their difference in ionic radius compared to the host matrix ion, (iii) external strains caused by factors such as substrate-induced stress, and (iv) temperature. To accurately determine the lattice parameters of a crystalline material, high-resolution X-ray diffraction (HRXRD) is commonly employed. The bond method is utilized with a combination of symmetrical and asymmetrical reflections to measure and analyse the lattice parameters.60–63 In the case of ZnSnO3, the calculated lattice parameters are provided in Table 1.
In ref. 50, the formation enthalpy [ΔH = Etotal(ZnSnO3) − {Etotal(ZnO) + Etotal(SnO2)}] was estimated to gain insights into the impact of different synthesis pathways in experiments. The calculated formation enthalpies for all the proposed ZnSnO3 phases are positive, as shown in Table 1. This indicates that ZnSnO3 is not energetically favourable and cannot be produced through solid-state fabrication pathways such as combining ZnO and SnO2 under normal environmental conditions. However, experimental evidence suggests that these polymorphs have the potential to remain stable when subjected to extreme conditions, such as elevated pressure and temperature. To comprehend the structural transition of ZnSnO3, the enthalpy variation between heterogeneous component oxides such as (ZnO + SnO2), ((Zn2SnO4 + SnO2)/2), IL- and LN-type phases was also estimated. This analysis is depicted in Fig. 5c and d). Below 5.9 GPa, the heterogeneous component oxides (h-ZnO + SnO2) are more favourable than the various potential phases. In the range of 5.9 to 7.1 GPa, the heterogeneous component oxides of Zn2SnO4 + SnO2 become increasingly favourable, aligning with experimental results at intermediate pressure levels. At low temperatures and >7.1 GPa, the LN-type ZnSnO3 phase is more favourable than its constituent phases, which is consistent with experimental data, suggesting the formation of LN-type ZnSnO3 at 7 GPa. At a pressure of 34.5 GPa, the LN-type phase undergoes a transition to the orthorhombic CdSnO3-type phase, which is significantly higher than the transition pressures observed for ZnGeO3 (15.6 GPa) and MgGeO3 (17.9 GPa).51,57,64
Elastic constants are significant parameters that provide insight into the crystallite structure and bonding strength among atoms. In hexagonal structures such as ZnSnO3, the elastic parameters exhibit positive values and adhere to the stability requirement outlined by Born–Huang, suggesting the elastic stability of both the LN and IL phases. Table 2 presents the elastic constants for various space groups.
| Space group | C11 | C12 | C13 | C14 | C15 | C22 | C23 | C33 | C44 | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| R3c | 310.4 | 137.1 | 100.8 | 1.4 | — | — | — | 175.3 | 77.3 | 66 |
R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) |
268.8 | 132.8 | 78.8 | 14.7 | 14.3 | — | — | 185.9 | 2.6 | |
Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m m |
284.4 | 74.1 | — | — | — | — | — | — | 33.8 | |
| Pnma | 290.8 | 103.8 | 116.7 | — | — | 247.9 | 100.4 | 273.3 | 74.7 | |
| Cmcm | 316.9 | 51.8 | 47.8 | — | — | 210.4 | 77.5 | 223.9 | 239.5 |
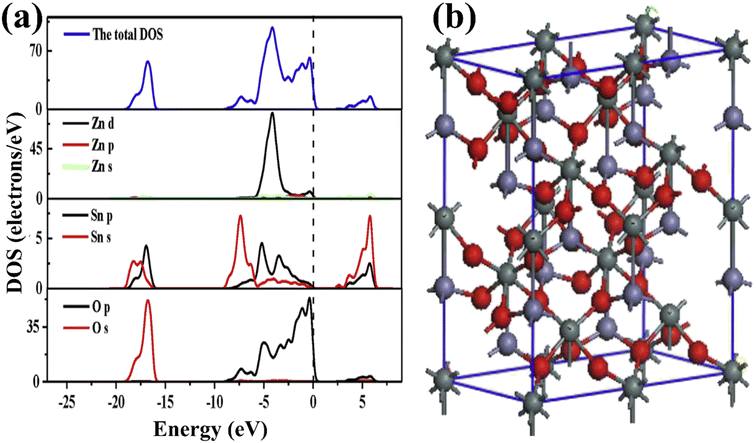
The structural properties of ZnSnO3 can be understood based on density functional theory (DFT) calculations. The ZnSnO3 supercells used in the study consist of 60 atoms (Zn12Sn12O36). The VB of ZnSnO3 primarily consists of Zn 3d104s2 states, Sn 5s25p2 states, and O 2s2p4 states. Fig. 6a and b illustrate the supercells and the determined density of states (DOS) and partial density of states (PDOS) of ZnSnO3.67
 | ||
| Fig. 6 (a) DOS and PDOS for ZnSnO3. (b) Supercells of Zn12Sn12O36.69 | ||
The energy band at [−18.4, −15.3] eV in the VB is largely filled by O 2s states interspersed with few Sn 4d levels. The energy range of approximately [−8.1, −5.5] eV displays bands that can be attributed to the presence of Sn 5s states, together with a combination of O 2p and Zn 3d states. On the other hand, within the range of [−5.5, −3.4] eV, the dominating factor is the Zn 3d states, accompanied by O 2p states and a small contribution from Sn 5p states. At the upper end of the valence band ([3.4, 0] eV), the O 2p state takes precedence and dominates. However, it is accompanied by a mixture of Zn 3d and minor presence of Sn 4d states, suggesting substantial hybridization between these states. The Sn 5s and Zn 4s states dominate the CB area of [1, 5.5] eV, but Sn 5p dominates the Zn 4p state when the energy is more than 5.5 eV. For energies less than 5.5 eV, the Zn PDOS is minimal, and thus negligible. Consequently, the Sn 5s and O 2p states should control the electrical characteristics of ZnSnO3. Both the top of the VB and the bottom of the CB are located at the Γ-point. Consequently, a direct band gap of 1.0 eV is generated, showing that ZnSnO3 is a semiconductor. Due to the absence of an experimental band gap result for comparison, it is important to note that DFT often underemphasizes the energy gap of semiconductor solids.67,68
3. Synthesis technique/routes
Over the past few decades, several synthetic techniques have been employed to synthesize micro/nanostructures of ZnSnO3. However, to achieve the controlled synthesis of these structures and expand their practical applications, it is crucial to comprehensively summarize the emerging growth mechanisms and develop new techniques. The two primary methods used for the synthesis of nanostructures are solution-based and vapor phase approaches. The size, crystal phase, and crystallinity of the synthesized materials significantly impact their band-gap energies and the separation of charge carriers in semiconductor oxides. Therefore, the quality of the synthetic conditions plays a key function in determining the efficiency of semiconductor oxides. Comparing with various synthesis routes, the solution-based procedure emerges as a simple and low energy-consuming technique for the production of ZnSnO3 nanomaterials. By modifying the experimental factors such as solvent, precursor ingredients, and reaction environments, precise control of the nanostructure morphologies can be achieved. This straightforward technique also enables greater control of the particle size of nanostructures. The solution-based methods employed for the synthesis of ZnSnO3 nanostructures include hydrothermal,8,12 sol–gel,19,20 precipitation,70 electrochemical-deposition,36 solvothermal,71 microwave,72 wet chemical, and electrospinning method.73 Among the different methods available, the simple sol–gel process is particularly appealing for the synthesis of ZnSnO3 nanocomposites. This method offers several advantages, including low cost, reliability, good repeatability, and the ability to easily control the physical properties and morphologies.32The activity of ZnSnO3 can be enhanced through the precise control of various factors, including band gap, size, morphology, crystal structure, surface area, stability, reusability, and preparation of composite materials. Among them, the crystal structure and specific surface area play a crucial role in improving the performance of ZnSnO3. A significant parameter for achieving superior results is a large surface area, which is closely interconnected with the structure and mean particle size of the material. In the study by Yu et al., they synthesized homogeneous ZnSnO3 nanocubes using a low-temperature solution route.74 They observed that decreasing the reaction temperature from 80 °C to 0 °C led to a decrease in the particle size of the fabricated ZnSnO3 nanocubes from 600 nm to 40 nm, while the surface area increased from 36.4 to 109.5 m2 g−1. The reduction in particle size resulted in an increase in the overall surface area of the material. A larger surface area provides more reactive sites, leading to higher surface reactivity, greater surface-to-volume ratios, and increased availability of surface-active sites.
Moreover, vapor phase techniques have also been used for the synthesis of nanostructured materials. These techniques include laser ablation,75 vapour–liquid–solid,37 thermal evaporation,76 molecular beam epitaxy (MBE),77 metal–organic chemical vapour deposition78 and magnetron sputtering.79 The choice of the method for the synthesis of ZnSnO3 primarily relies on the desired dimensions of the nanostructures. The impact of various fabrication techniques and starting materials on the morphologies of ZnSnO3 is presented in Table 3. Furthermore, in the application domain, the influence of the morphology of ZnSnO3 on is efficiency has also been investigated.
| Material | Morphologies | Precursors | Method | Ref. |
|---|---|---|---|---|
| ZnSnO3 | Polyhedral | Zn(Ac)2·2H2O, CTAB/SDBS | Solution-based | 32 |
| ZnSnO3 | Nanocubes | SnCl4·5H2O/Zn(Ac)2·2H2O | HNO3 etching | 92 |
| ZnSnO3 | Nanocubes | SnCl4·5H2O/ZnSO4 | Solution-based | 74 |
| ZnSnO3 | Hollow-cubes | Zn(NO3)2/SnCl4 | HCl etching | 131 |
| ZnSnO3 | Hollow-cages | SnCl2/Zn(Ac)2·2H2O | Solution-based | 132 |
| ZnSnO3 | Hollow-cubes | SnCl4·5H2O/ZnCl2 | Solution-based | 125 |
| ZnSnO3 | Hollow-cages | SnCl4·5H2O/Zn(Ac)2·2H2O | Solution-based | 127 |
| ZnSnO3 | Face-centred trigonal | Na2SnO3·3H2O/Zn(NO3)2·6H2O | Sol–gel | 99 |
| ZnSnO3 | Polycrystalline ilmenite-type | Zn(Ac)2·2H2O/SnCl4·5H2O/ethylene glycol | Sol–gel | 100 |
| ZnSnO3 | Nanostructures | ZnCl2/SnCl2/Tepa/trimesic acid | Sol–gel | 103 |
| ZnSnO3 | Hollow cubes | SnCl4/ZnCl2 | NaOH etching | 133 and 134 |
| ZnSnO3 | Nanoplates | Zn(Ac)2·2H2O, SnCl4·5H2O, ethanol, MEA | Sol–gel | 135 |
| ZnSnO3 | Hierarchical nanocages | SnCl4·5H2O/Zn(Ac)2·2H2O/NaOH, (CH2)6N4 | Hydrothermal | 128 |
| ZnSnO3 | Hollow microspheres | SnCl4·5H2O/Zn(Ac)2·2H2O/NaOH, CTAB | Hydrothermal | 126 |
| ZnSnO3 | Nanocages | SnCl4·5H2O/Zn(Ac)2·2H2O/NaOH, (CH2)6N4 | Hydrothermal | 136 |
| ZnSnO3 | Nanocubes | Zn(Ac)2·2H2O/SnCl4·5H2O, NaOH | Hydrothermal | 137 |
| ZnSnO3 | Nanowires | ZnCl2/SnCl4·5H2O/PEG (200) | Hydrothermal | 138 |
| ZnSnO3 | Nanowires | Zn(Ac)2·2H2O/SnCl4·5H2O/NaOH, PEG (4 K) | Single-step hydrothermal | 91 |
| ZnSnO3 | Nanowires | ZnO/SnCl4·5H2O/PEG (10 K) | Hydrothermal | 139 |
| ZnSnO3 | Nanocubes | SnK2O3·3H2O/ZnC4H6O4·2H2O/urea | MA hydrothermal | 72 |
| ZnSnO3 | Nanocubes | ZnSO4·7H2O/Na2SnO3·3H2O | Ionic substitution | 140 |
| ZnSnO3 | Nanobelts/microbelts | Zn and Sn powder/graphite powder | Vapour–liquid–solid | 36 |
| ZnSnO3 | Triangular-belts | Zn and Sn/graphite powder | Vapour–liquid–solid | 37 |
| ZnSnO3 | Nanosheets | Na2SnO3·3H2O/Zn(Ac)2·2H2O/ethylene glycol | Solvothermal | 71 |
| ZnSnO3 | Hollow cubes | ZnCl2, SnCl4·5H2O/sodium citrate/NaOH | Coprecipitation | 116 |
| ZnSnO3 | Solid/hollow microspheres | Zn(NO3)2·6H2O/SnCl4·5H2O/NaOH | Precipitation | 70 |
| ZnSnO3 | Nano-urchins | Zinc plate/ZnOx(OH)y/NH3 | Laser ablation | 75 |
| ZnSnO3 | Amorphous | ZnO/SnO2 | Magnetron sputtering | 79, 141 and 142 |
| Ag–ZnSnO3 | Hollow cubes | ZnCl2/SnCl4·5H2O/sodium citrate/Zn(NO3)2·6H2O | Coprecipitation | 143 |
| CDs-ZnSnO3 | Nanocubes | SnCl4·5H2O/ZnSO4·7H2O/CDs powder | Precipitation–calcination | 144 |
| ZnSnO3/rGO | Nanocubes | ZnSO4·7H2O/SnCl4·5H2O/PDDA/GO | Coprecipitation | 145 and 146 |
| S-ZnSnO3 | Hollow cubes | Zn(NO3)2·6H2O, Na2SnO3·4H2O, C4H6N2, CH3CSNH2 | Ion-exchange | 147 |
| ZnSnO3/rGO | Nanosheets | Zn(NO3)2·6H2O/SnCl2·2H2O/GO | Thermal decomposition | 76 |
| ZnSnO3/C | Nanofibers | SnCl2·2H2O/ZnCl2/DMF/PVP | Electrospinning | 73 |
3.1. Hydrothermal technique
Hydrothermal synthesis is a widely employed technique for the preparation of ZnSnO3, utilizing a solution-based reaction approach. This method involves placing starting material dispersion in a sealed stainless-steel Teflon-lined autoclave. Subsequently, the autoclave is subjected to heating in an oven under precise temperature, duration, and autogenous pressure settings. The temperature typically ranges from 140 °C to 200 °C for the hydrothermal synthesis of zinc stannate.80 The hydrothermal synthesis of ZnSnO3 nanoparticles involves the use of different mineralizers such as ammonium hydroxide, sodium hydroxide, hexadecyl trimethyl ammonium bromide, various amines, and sodium carbonate. This leads to the creation of nanocrystals with different shapes (cubic, spherical, and rod like). Careful control of the chemical properties of the mineralizer is essential given that it influences the surface charges of the resulting metal oxides, which is critical for the formation of nanoparticles in the hydrothermal synthesis process.81–85In hydrothermal fabrication of ZnSnO3, the compositions of synthesized nanomaterials can be precisely controlled through liquid-phase or multiphase chemical reactions. An advantage of the hydrothermal method is its capability to generate crystalline phases that are not stable at higher temperatures.86–89
The hydrothermal method is commonly used for the synthesis of ZnSnO3 with orthorhombic (orth) and face-centred cubic (fcc) phases. In a typical synthesis procedure, both discrete orth and fcc ZnSnO3 phases are simultaneously fabricated in a single solution through hydrothermal treatment. This is achieved by treating a solution having a small amount of ZnO-covered foil, urea, and potassium stannate trihydrate under hydrothermal conditions.90 According to their study, the synthesis process starts by hydrothermally treating a solution of pure zinc-foil and C20H37NaO7S, OT, and 1.50 g L−1 to obtain the ZnO precursor. Subsequently, the obtained ZnO-foil is again hydrothermally treated in an alcohol–water solution containing urea (CO(NH2)2) and potassium stannate trihydrate (K2SnO3·3H2O). Subsequently, the resulting microspheres are dried in air at 60 °C for 6 h. Consequently, pure orth-ZnSnO3 solid microspheres are formed in the solution, as illustrated in Fig. 7. Additionally, pure fcc-ZnSnO3 hollow microspheres are observed on the ZnO-induced template, as illustrated in Fig. 8.90 The following is a description of the process for the formation of the two products:
| CO(NH2)2 + H2O → NH4+ + OH− + CO2 |
| K2SnO3 + ZnO(l) + 2OH− → orth-ZnSnO3 + 2KOH + H2O |
| K2SnO3 + ZnO(s) + 2OH− → fcc-ZnSnO3 + 2KOH + H2O |
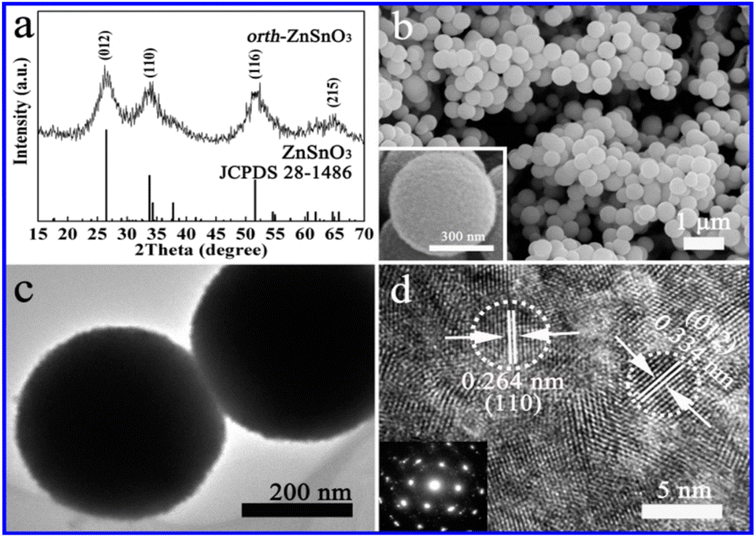 | ||
| Fig. 7 (a) XRD pattern, (b) SEM image, (c) TEM image, and (d) HRTEM image of ZnSnO3 solid microspheres.90 | ||
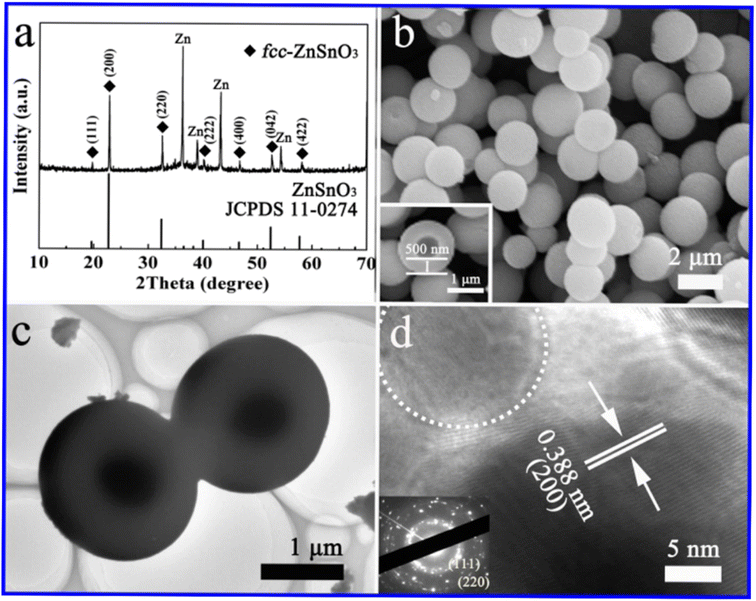 | ||
| Fig. 8 (a) XRD pattern, (b) SEM image, (c) TEM image, and (d) HRTEM image of ZnSnO3 hollow microspheres.90 | ||
To achieve controlled morphologies of the synthesized materials, different surfactants and polymers such as polyethylene glycol (PEG) can be introduced. Numerous studies have been published on the hydrothermal fabrication of nanoparticles, nanorods, nanotubes, nanowires, and hollow spheres. The presence of PEG in the hydrothermal reaction has been observed to facilitate the formation of nanowire morphologies.
In ref. 91, ZnSnO3 nanowire arrays were synthesized using a single-step hydrothermal method. In their study, Zn(Ac)2·2H2O, SnCl4·5H2O, and polyethylene glycol (PEG) were used as the precursors. PEG, acting as a complex agent, reacted with Zn(CH3CO2)2·2H2O and SnCl4·5H2O to form a soluble ZnSn(OH)n(PEG)6−n complex. The presence of PEG was crucial for obtaining the desired morphologies of ZnSnO3 nanowires. The molar ratio of Zn(CH3CO2)2·2H2O to SnCl4·5H2O to PEG (4000) was approximately 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and the reaction was performed at a temperature of 200 °C for a duration of 12 h.
1, and the reaction was performed at a temperature of 200 °C for a duration of 12 h.
Hu and colleagues developed a straightforward chemical solution technique to achieve the large-scale production of well-defined faceted cubic ZnSnO3 and octahedral Zn2SnO4 microcrystals.92 They employed an acid-etching technique, which allowed the originally synthesized zinc stannate faceted microcrystals to undergo a transformation into hollow structures, while preserving their original shape. The synthesis process involved using specific starting materials, namely, tin tetrachloride (SnCl4·5H2O), zinc acetate (ZnAc2·2H2O), and NaOH, which were diluted in distilled water to create a clear solution. In a typical procedure, a 0.02 M ZnAc2 solution was added to a 0.02 M SnCl4 solution at RT and vigorously agitated for 10 min to form a mixed solution for the synthesis of cubic ZnSnO3 microcrystals. Subsequently, this mixture was combined with a 0.2 M NaOH solution (15 mL) and continuously stirred for an additional 10 min. The proportion of Zn2+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sn4+
Sn4+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na+ was kept at a molar ratio of 1
Na+ was kept at a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. Subsequently, a hydrothermal procedure was conducted at 130 °C for 6 h inside a reactor with a 60 mL capacity. After the reaction, the resulting white materials were washed multiple times with ethanol and distilled water.
10. Subsequently, a hydrothermal procedure was conducted at 130 °C for 6 h inside a reactor with a 60 mL capacity. After the reaction, the resulting white materials were washed multiple times with ethanol and distilled water.
The reactions that lead to the creation of ZnSnO3 can be summarized as follows:92
| Zn2+ + Sn4+ + 6OH− → ZnSn(OH)6 |
| ZnSn(OH)6 → ZnSnO3 + 3H2O |
| ZnAc2 + SnCl4 + 6NaOH = ZnSnO3 + 4NaCl + 2NaAc + 3H2O |
Under hydrothermal conditions, the creation, and then breaking of the weak-phase ZnSn(OH)6 results in the nucleation and development of ZnSnO3 nanoparticles, involving the aforementioned chemical processes (step 1). More ZnSnO3 nanocrystallites are generated after adding more reactants to the reaction mixture, which are further combined and bound into comparatively augmented nanocrystallites. Noticeably, the obtained ZnSnO3 undergoes a dissolution–recrystallisation process to reduce the high-energy surfaces. Next, the particles combine to form larger aggregates, adhering to the principles of Ostwald ripening (step 2). By extending the reaction duration, the crystals undergo morphological changes and acquire a cubic shape with dimensions measuring several microns. The cubic crystals exhibit 100 lattice planes on their basal surfaces, achieved by adjusting the growth conditions to promote the desired crystal facet (step 3). Lastly, the nanocrystals present in the reaction system adhere to the surfaces of the ZnSnO3 cubic crystals, resulting in the production of huge cubes.92Fig. 9 schematically depicts the growth process.
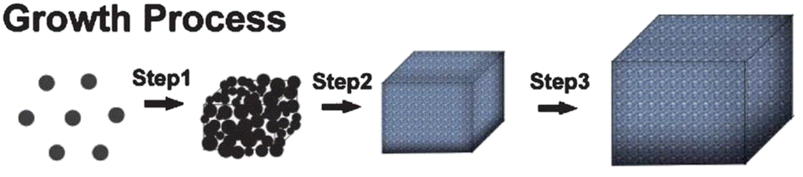 | ||
| Fig. 9 Possible mechanism for the growth of ZnSnO3 cubic microcrystals.92 | ||
Exercising meticulous control over the chemical characteristics of the mineralizer is crucial, given that it determines the specific surface charges present on the resulting metal oxides. These surface charges serve as a critical factor in the overall process, significantly influencing and facilitating the creation of nanoparticles during the hydrothermal synthesis procedure. Generally, the careful management of the properties of the mineralizer directly impacts the outcome of nanoparticle formation, underscoring its pivotal role in this intricate process. However, the reliability and reproducibility of the process are limited, the necessary equipment is expensive, a longer reaction time is required, and it consumes plenty energy.
3.2. Solvothermal
The solvothermal procedure is similar to the hydrothermal method, excepted for the use of organic solvents instead of water. In the case of alcohols and glycerol as the reaction medium, the reactions are referred to as alcohothermal and gyrothermal, respectively. For the synthesis of NCs with good crystalline characteristics, these synthetic techniques are crucial.93Wang and colleagues used the solvothermal route to achieve a phase transformation from 3D fcc ZnSnO3 nanosheets to 2D orth ZnSnO3 nanosheets. In the conventional route, a white suspension of ZnSn(OH)6 products was promptly formed by combining a solution of Na2SnO3·3H2O and Zn(Ac)2·H2O in a mixture of ethylene glycol–deionized water. Subsequently, the ZnSn(OH)6 was moved to a stainless-steel Teflon-lined autoclave and kept at 180 °C for 12 h. Next, the obtain powder was annealed at 500 °C for 4 h at a rate of 2.8 °C min−1. Finally, two-dimensional ZnSnO3 nanosheets were created.71
With the shift from 3D nanocubes to 2D ZnSnO3 nanosheets, a straightforward solvothermal pathway was investigated in ref. 94. To form aqueous solutions, Na2SnO3·3H2O and Zn(Ac)2·3H2O were mixed in ethanol–water solution in the usual manner. Subsequently, the Na2SnO3 solution was added slowly to the Zn(CH3COO)2 solution with stirring, resulting in the formation of a white ZnSn(OH)6 suspension. Then, the suspension was transferred to a Teflon-lined autoclave and kept at 180 °C for 12 h. The resulting ZnSn(OH)6 was washed and annealed at 600 °C for 3 h. Firstly, the reaction happened due to the hydrolysis of the SnO32− ions, resulting in the creation of blend H2SnO3. Later, the Zn2+ ions undergo a reaction with OH ions, resulting in the formation of the Zn(OH)42− phase. This reaction occurs due to the hydrolysis of both CH3COO and Zn2+ ions, as follows:94
| Sn32− + 2H2O ↔ H2SnO3 + 2OH− |
| CH3COO− + H2O ↔ CH3COOH + OH− |
| Zn2+ + 4H2O ↔ Zn(OH)42− + 4H+ |
| H2SnO3 + Zn(OH)42− + 2H+ → ZnSn(OH)6↓ + H2O |
Under hydrothermal conditions, the metastate ZnSn(OH)6 nanocubes will undergo decomposition and subsequent recrystallization as the temperature and pressure increases. This process follows the breaking-recrystallization pathway, resulting in the formation of a stable nanostructure. Fig. 10 depicts the hypothetical growth process graphically.
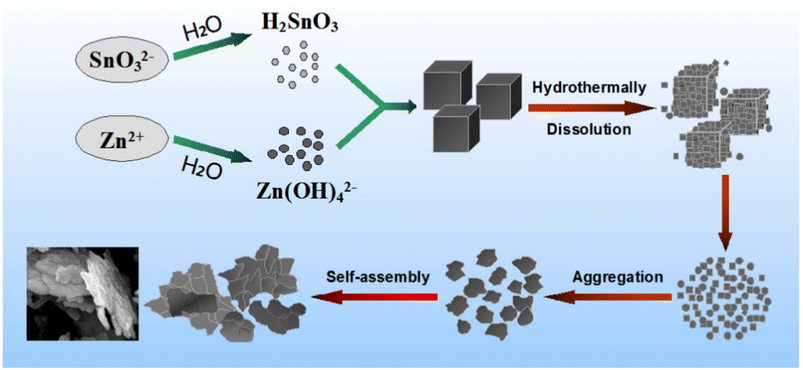 | ||
| Fig. 10 Diagram depicting the probable process for the formation of the precursor ZnSn(OH)6.95 | ||
3.3. Sol–gel technique
The sol–gel method is a chemical process that creates oxide-based materials from hydrolysable precursors through hydrolysis and condensation reactions. These precursors contain weaker ligands than water, such as halides, nitrates, sulfates, alkoxides, and carboxylates. The hydrolysed precursors form small colloidal nanoparticles in a liquid sol, which can undergo further polycondensation to create a network of polymeric oxide-based materials with oxo-bridges. The initial gels formed through this method consist of both a gel network and a significant liquid phase. Drying these gels, whether at room temperature or through heating, removes the solvent phase and produces dense materials.96–98Due to its simplicity, cost-effectiveness, and capability to produce large-area films, the sol–gel method is widely regarded as an excellent technique for the preparation of ZnSnO3. As demonstrated in ref. 99, the face-centred trigonal perovskite structure of ZnSnO3 was synthesized using a sol gel. They used Na2SnO3·3H2O (0.01 mol) and Zn(NO3)2·6H2O (0.01 mol) as the precursors and deionized water (100 mL) as the solvent. Finally, the white ZnSnO3 nanoparticles were dried at 70 °C in an oven for 12 h.
The authors of ref. 100 fabricated polycrystalline IL-type ZnSnO3via the sol–gel process at atmospheric pressure utilizing Zn(CH3COO)2·2H2O, SnCl4·5H2O, and ethylene glycol as the precursors. To determine the impact of sintering on the ZnSnO3 structure, the resulting product was sintered at 450 °C, 550 °C, and 650 °C. Upon sintering below 500 °C, they observed that the phase formation is incomplete with the significant presence of ZnO and SnO2 as secondary phases. In contrast, sintering at 650 °C led to the creation of pure-phase ZnSnO3.
Li et al. synthesized Zn–Sn–O thin-films and investigated the impact of sintering (300–1000 °C) on their microstructure, morphological, and optical characteristics.67 Spinel Zn2SnO4 was formed in the temperature range of 400–700 °C, while perovskite ZnSnO3 emerged at 800 °C. Alternatively, the formation of Zn2SiO4 only occurred at temperatures exceeding 1000 °C. Furthermore, the grain size decreased as the temperature increased from 400 °C to 800 °C. The proposed explanation is that the Zn2SnO4 grain grows via the surface diffusion pathway, and SnO2 is dispersed on the surface of Zn2SnO4, inhibiting grain formation. Furthermore, when the temperature exceeds 800 °C, the lattice properties clearly decrease. The formation of ZnSnO3 is responsible for this phenomenon, as follows:
| Sn2+ + 0.5O2 → Sn4+ + O2− |
| Zn2SnO4 + SnO2 → 2ZnSnO3 |
Authors of ref. 101 synthesized porous ZnSnO3 nanocubes via the conventional solution-based process together with a calcination process. When annealed at 500 °C, ZnSn(OH)6 lost three water molecules to form amorphous ZnSnO3, which decomposed into amorphous Zn2SnO4 and crystalline SnO2 at 600 °C. The amorphous Zn2SnO4 became crystalline Zn2SnO4 when the annealing temperature increased to 700 °C. The proposed conversion of ZnSn(OH)6 into different morphologies is as follows:
As demonstrated in ref. 103, ZnSnO3 nanostructures were prepared via the sol–gel method using tepa as the gelling medium and trimeric acid hydrolysis medium, which increased the hydrolysis rate and led to initially smaller nucleation, controlling the final particle size. Here, 0.8 mmol ZnCl2 and 0.8 mmol SnCl2 were dispersed in 10 mL DI individually, and then mixed with the gelling agent and hydrolysis agent at 80 °C. Subsequently, the fabricated ZnSnO3 was calcined at 700 °C for 2 h. A straightforward and cost-effective method was employed by Geng et al. for the successful synthesis of polyhedral ZnSnO3 with a variety of morphologies without further heat treatment.32 They showed the morphology conversion between octahedra and 14-faceted ZnSnO3 using surfactant. In the conventional synthesis, they used tin tetrachloride and Zn(Ac)2 as the starting precursors. Different surfactants in varying concentrations and NaOH were used to control the polyhedral shape. In a beaker, the combined sol was treated at 85 °C with stirring. Finally, centrifugation was used to collect the white polyhedral ZnSnO3 products. When an anionic surfactant (SDBS) was employed, the synthesis of ZnSnO3 yielded different polyhedral shapes depending on the concentration of SDBS such as (1) at 0.25 M, 14-faceted polyhedra were synthesized, (2) at a moderate range of 0.3 to 0.5 M, truncated octahedra were formed, and (3) at 0.5 to 1.0 M, typical octahedra were synthesized. These observations demonstrate how the SDBS concentration influences the morphology of the resulting ZnSnO3 particles. Fig. 11 depicts this shape-evolution pattern. Again, at varying concentrations of the cationic CTAB surfactant, the synthesis of ZnSnO3 yielded different polyhedral shapes such as (1) at 0.2 M, regular octahedra were created, (2) at an intermediate CTAB range of 0.3 to 0.5 M, truncated octahedra were formed, and (3) at 0.5 to 1.0 M, the resulting products exhibited regular 14-faceted polyhedra. These findings demonstrate the influence of CTAB concentration on the morphology of the synthesized ZnSnO3 particles. This shape-evolution form is depicted in Fig. 12.
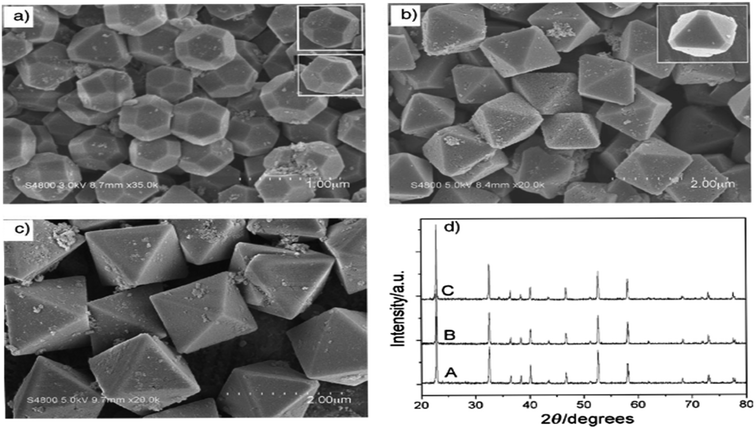 | ||
| Fig. 11 SEM micrograph of ZnSnO3 obtained using: (a) SDBS 0.15 M, (b) SDBS 0.4 M, and (c) SDBS 0.75 M. (d) XRD pattern of (A) 14-faceted polyhedra, (B) truncated octahedra and (C) octahedra.102 | ||
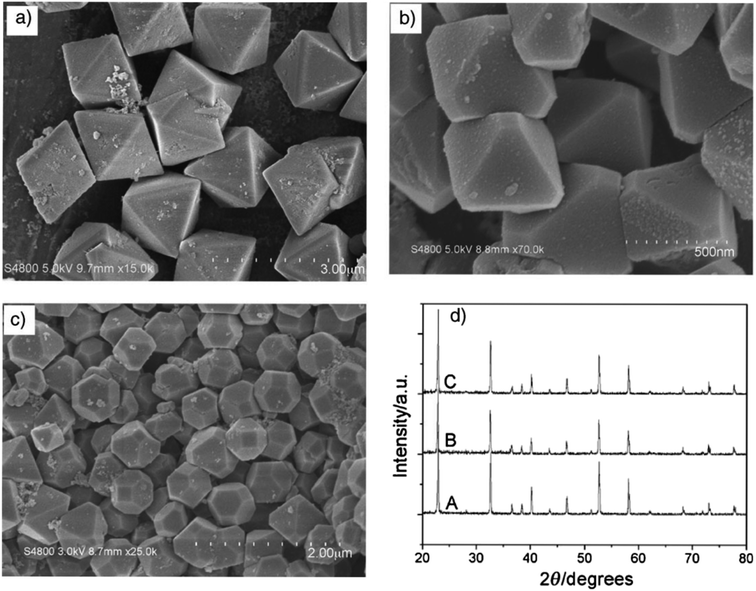 | ||
| Fig. 12 Typical SEM images of the as-prepared ZnSnO3 products: (a) CTAB 0.15 M, (b) CTAB 0.4 M, and (c) CTAB 0.75 M. (d) Corresponding XRD patterns of the as-prepared ZnSnO3 polyhedra, (A) octahedra, (B) truncated octahedra and (C) 14-faceted polyhedra.102 | ||
When the concentration of the anionic SDBS surfactant increased, the R value also increased. Consequently, the shape of the ZnSnO3 crystals changed from 14-faceted polyhedra to truncated octahedra, and eventually to regular octahedra. In contrast, when the concentration of the cationic CTAB surfactant increased, the R value decreased. This resulted in a different trend compared to the anionic SDBS surfactant, where increasing the CTAB concentration led to a transition from regular octahedra to truncated octahedra, and ultimately to 14-faceted polyhedra (Fig. 13).102
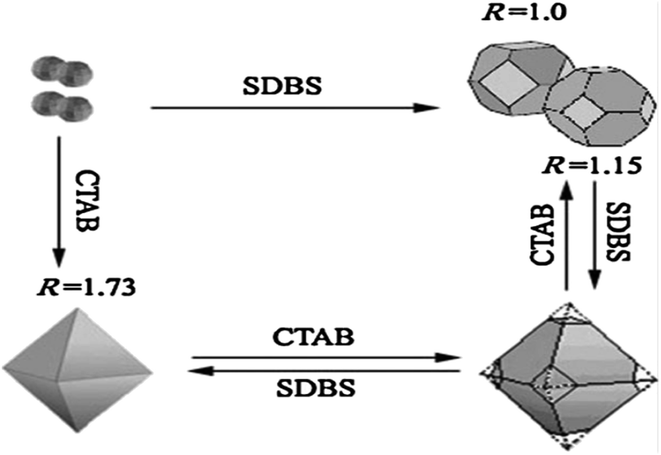 | ||
| Fig. 13 Schematic diagram depicting the relationship between the ratio R and the transformation of the crystal shape.102 | ||
The production of ZnSnO3 using the sol–gel method presents significant technical and chemical challenges. This is primarily due to the fact that the precursors used to introduce Sn4+ ions typically contain highly electronegative ions (such as Cl−) or additional metallic ions (such as Na2+), which reduce the likelihood of effective bonding between Sn4+ and Zn2+. Additionally, ZnSnO3 exhibits lower thermal stability, leading to its decomposition into Zn2SnO4, ZnO, and SnO2 at elevated temperatures. Thus, to address these issues, alternative low-temperature synthesis techniques such as co-precipitation and hydrothermal synthesis have been employed.104
3.4. Vapor–liquid–solid technique
In recent decades, the vapor–liquid–solid (VLS) growth technique has witnessed substantial advancements.105 Originally used for whisker growth, it has evolved into a practical method for producing semiconductor nanowires. These nanowires have applications in various fields such as nanoscale electronics, optoelectronics, sensing, and energy conversion.106–108 However, based on a previous literature survey, few research endeavours have been reported on the synthesis of ZnSnO3 using the VLS method.Wang et al. employed the widely recognized vapor–liquid–solid (VLS) mechanism to establish a thermal reaction method for synthesizing ZnSnO3 nanoparticles, as described in ref. 36. The typical synthesis procedure utilized in their study is as follows. Initially, a Zn–Sn buffer layer was deposited as a seed layer on Si and alumina substrates. Subsequently, separate starting materials consisting of Zn and Sn powders were set in an alumina boat. Moreover, to enhance the carbon-thermal reaction while sintering, a blend of source materials containing 50 wt% graphite powder was placed in the central region of a quartz reactor with a stable temperature of 1173 K and left to react at a pressure of 10–20 torr for a duration of 3 h. Throughout the reaction, the flow rate of argon and oxygen gases was maintained at 200 sccm and 10–20 sccm, respectively. Subsequently, the resulting product comprised of single microbelt nanogenerators was constructed using enlarged ZnSnO3 belts measuring 300–1000 μm in length. Furthermore, the power output and piezoelectric properties of these microbelts were assessed.36
Wang et al. also synthesized lead-free ZnSnO3 triangular-belts using a high-temperature carbon-thermal approach. In this method, the starting materials consisted of Zn and Sn together with graphite powder. These materials were set in the middle point of the reactor to facilitate the process. The system was left to react at a stable temperature of 900 °C and a pressure of 10–20 torr for a duration of 3 h. The flow rate of Ar and O2 gas during the reaction process was set at 200 and 10–20 sccm, respectively.37
Although the vapor–liquid–solid (VLS) technique is a potent method for the synthesis of nanowires, it is associated with certain challenges. For example, it requires specialized, costly equipment for handling the potentially hazardous precursor gases, and achieving uniformity in nanowire properties can be difficult. Furthermore, the incorporation of contaminants during growth process and the energy-intensive high-temperature conditions are additional concerns,109–111 together with its limited scalability and precursor availability. Nevertheless, VLS is valuable due to its precise control of the nanowire properties, and thus ongoing research seeks to address the above-mentioned challenges for its broader utilization.
3.5. Coprecipitation technique
The coprecipitation method offers several advantages for the synthesis of ZnSnO3. Firstly, it is a relatively simple and cost-effective technique, which facilitates precise control of the composition and stoichiometry of the final product. This method enables the simultaneous precipitation of zinc and tin ions in a homogeneous manner, leading to a well-mixed precursor solution.112–114 Additionally, coprecipitation is conducted in aqueous solutions at relatively low temperatures, reducing the energy consumption and environmental impact compared to high-temperature synthesis methods.115 The coprecipitation technique involves the precipitation of metal hydroxides from a salt precursor using a base in a solvent. By carefully controlling the release of anions and cations, the nucleation and growth kinetics of the particles can be regulated, leading to the synthesis of uniformly dispersed nanoparticles. Proper regulation of the experimental parameters such as pH, reactant and ion concentration, and temperature is crucial for controlling the morphological characteristics during the precipitation process.In a study conducted by the authors of ref. 116, double-shelled perovskite ZnSn(OH)6 hollow-cubes were fabricated using a rapid coprecipitation method. This involved mixing Zn2+, Sn4+, and OH− ions with Na3C6H5O7 and NaOH at RT. Subsequently, the obtained ZnSn(OH)6 cubes were transformed into ZnSnO3 double-shelled nanocubes through sintering at 300 °C for 3 h in an N2 atmosphere.
According to ref. 70, ZnSnO3 hollow spheres were fabricated via a straightforward and template-free in situ precipitation technique, followed by a dehydration step. During this process, a solution comprised of a water–alcohol mixture was created and subjected to constant stirring. The solution consisted of Zn(NO3)2·6H2O, SnCl4·5H2O, and NaOH mixed with the above-mentioned solution. The experimental conditions were kept constant throughout, except for the growth temperature, which was varied from 60 °C to 100 °C to investigate its impact on the morphology of ZnSnO3. The results showed a significant alteration in the shape of ZnSnO3 with an increase in the growth temperature, as depicted in Fig. 14. At 60 °C, the sample primarily consisted of uniform and monodisperse polyhedrons with a diameter of approximately 2.4 μm (Fig. 12a). When the growth temperature was increased to 80 °C, the ZnSnO3 nanoparticles assembled into hollow microspheres (Fig. 14b). However, at 100 °C, uneven ZnSnO3 spheres were obtained, with some small nanoparticles attached to their surface (Fig. 14c). Fig. 14d displays the X-ray diffraction peaks of the ZnSn(OH)6 precursor at various growth temperatures.
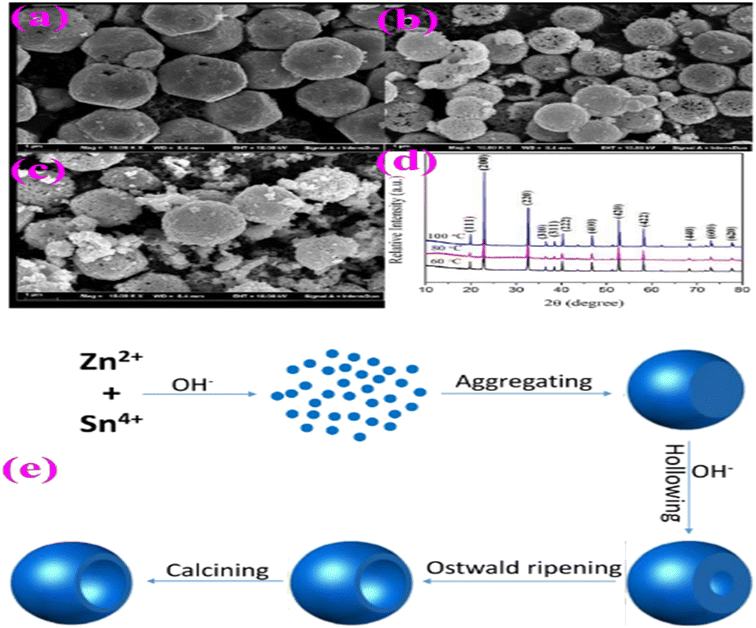 | ||
| Fig. 14 SEM images of ZnSnO3 obtained at different reaction temperatures: (a) 60 °C, (b) 80 °C, and (c) 100 °C. (d) XRD patterns of ZnSn(OH)6 precursor. (e) Schematic illustration of the possible formation mechanism of ZnSnO3 hollow spheres.70 | ||
A schematic representation of the fabrication route of the ZnSnO3 hollow-spheres is shown in Fig. 14e. Initially, a significant amount of ZnSn(OH)6 is generated in the presence of Zn2+ and Sn4+ ions at high concentrations of OH−. Subsequently, these ZnSn(OH)6 particles develop into spherical shapes through rapid aggregation. In the second stage, the surface of the ZnSn(OH)6 microspheres undergo etching due to the dissolution approach in the highly alkaline environment, as the concentration of OH− ions increases. Concurrently, the re-crystallization of ZnSn(OH)6 occurs in the system, maintaining dynamic-equilibrium.70
| ZnSn(OH)6 + 4OH− ↔ Sn(OH)62− + Zn(OH)42− |
The resulting ZnSnO3 nanoparticles typically exhibit high purity, fine particle size, and enhanced crystallinity, making coprecipitation an attractive choice for the production of ZnSnO3 for various applications, including catalysis, sensors, and photovoltaics.112,115 However, one drawback of the coprecipitation method for synthesizing ZnSnO3 is controlling the particle size and morphology of the resulting material. This method often yields nanoparticles with a wide size distribution and irregular shapes, which can negatively impact the properties and performance of the materials in specific applications.117,118 Achieving precise control over the particle size and shape can be difficult, requiring additional steps such as post-synthesis annealing and the use of surfactants.119 Moreover, coprecipitation may also introduce impurities or defects in the final product, which can be undesirable in some applications, such as electronic devices and photocatalysis, where material purity and uniformity are critical for optimal performance.
3.6. Magnetron sputtering technique
Sputtering involves bombarding a target material with high-energy particles, causing the discharge of atoms/molecules from the surface. However, diode sputtering has notable drawbacks, including low deposition rates and high cost. In contrast, magnetron sputtering is a vacuum-coating process known for its high deposition rates, allowing the deposition of metals, alloys, and compounds on various substrates with thicknesses up to the millimeter scale. This technique offers several significant advantages compared to other vacuum coating processes, making it suitable for diverse commercial applications ranging from microelectronic manufacturing to the creation of decorative coatings.120ZnSnO3 films were produced using variable magnetron sputtering settings, employing mixed powder targets of ZnO and SnO2. In the resulting films, ZnSnO3 nanocrystalline phases were preferentially formed in the columnar grain structure when a short deposition time of 2 h was used. To create the target, pure SnO2 and ZnO particles with a size of 1 μm were blended in a rotatable drum, maintaining an atomic ratio of Zn/Sn = 1. Subsequently, the mixture was placed on a copper plate, ensuring uniform thickness and a compacted surface. The deposition process involved evacuating the system to a base pressure below 3 × 10−3 Pa, followed by backfilling with Ar gas to achieve a pressure of 0.1–0.5 Pa, depending on the specific array parameters. The glass made of sodium as the substrate was subjected to an in-place cleaning process through RF sputtering for 15 min at 100 W prior to deposition. Subsequently, the films were deposited via RF sputtering for 4 h, using target powers in the range of 200 to 400 W and substrate spacing in the range of 80 to 180 mm based on the specific line-up run surroundings. The deposition rate of the film thickness increased with a variation in the power and spacing, obtaining improved optical properties. The average transmittance of the ZnSnO3 films was about 80%. Moreover, the ZnSnO3 films exhibited optical band gaps in the range of 2.6–3.4 eV and resistivity in the range of 10−3–10−4 Ω cm.79
A drawback associated with the magnetron sputtering technique for the synthesis of ZnSnO3 is its restricted capacity to accurately control the stoichiometry and composition. This method relies on the sputtering of target materials (in this case, Zn and Sn) on a substrate, and achieving the desired stoichiometry can be challenging due to the differences in the sputter rates between the elements. Consequently, achieving a precise ZnSnO3 composition may require careful process optimization and monitoring, which can be time-consuming and may lead to variations in material quality. Additionally, magnetron sputtering is typically conducted in a vacuum environment, which can be expensive and may limit the scalability of the production process for large-scale applications.
3.7. Electrospinning technique
Standard single-needle electrospinning processes have demonstrated significant performance advantages and wide-ranging applications in various sectors, due to their unique structural characteristics. Electrospinning is influenced by factors such as viscosity, operating voltage, temperature, pressure, and flow velocity.121To prepare the electrospinning precursor solution, tin(II) chloride dihydrate (SnCl2·H2O) and zinc chloride (ZnCl2) were dispersed in a mixture of dimethylformamide and absolute ethanol with vigorous stirring, maintaining a Zn2+ to Sn4+ molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Subsequently, polyvinylpyrrolidone (PVP) was added to the mixture, while continuously stirring. Then, the resulting solution was transferred to a plastic syringe with a volume of 10 mL, which was outfitted with a blunt-tip needle of 22-gauge size, serving as the electrospinning precursor solution. A syringe pump was employed to control the feeding rate. Aluminium foil was positioned 20 cm away from the needle to collect the electrospun fibers. To fabricate ZnSnO3 nanofibers, the collected precursor fibers were subjected to calcination in an air environment at 450 °C for a duration of 24 h.73
1. Subsequently, polyvinylpyrrolidone (PVP) was added to the mixture, while continuously stirring. Then, the resulting solution was transferred to a plastic syringe with a volume of 10 mL, which was outfitted with a blunt-tip needle of 22-gauge size, serving as the electrospinning precursor solution. A syringe pump was employed to control the feeding rate. Aluminium foil was positioned 20 cm away from the needle to collect the electrospun fibers. To fabricate ZnSnO3 nanofibers, the collected precursor fibers were subjected to calcination in an air environment at 450 °C for a duration of 24 h.73
An inherent limitation when employing the electrospinning technique for the synthesis of ZnSnO3 is the potential difficulty in achieving the desired crystalline structure and phase purity. Electrospinning primarily produces materials in the form of nanofibers or nanofibrous mats, which may require additional heat treatment or annealing steps to transform them into the desired ZnSnO3 crystalline structure. This post-treatment can introduce challenges in terms of controlling the grain size and phase purity of the final product, given that the annealing conditions need to be carefully optimized.
3.8. Etching technique
An advantage of the etching technique for the synthesis of ZnSnO3 is its ability to create finely tailored nanostructures with a high degree of precision.122 By selectively removing certain components from the precursor material, etching enables the fabrication of intricate and well-defined nanoarchitectures of ZnSnO3, such as nanofibers.73 Furthermore, the etching technique enables the incorporation of ZnSnO3 into specific device architectures, offering versatility and customization in the design of advanced materials for various technological applications.73,122,123A successful etching approach was employed to produce hollow ZnSnO3 nanocube architectures. Depending on the type of etching agent utilized, two paths can be distinguished, i.e., acid and basic agents. In the case of utilizing NaOH solution as both the reactant and etching agent, hollow ZnSnO3 architectures can be synthesized.124 Initially, solid ZnSn(OH)6 cubic compounds were formed by combining SnCl4/ZnCl2. Subsequently, ZnSn(OH)6 hollow cube structures were obtained by introducing additional NaOH. Finally, the ZnSn(OH)6 hollow cubes were sintered at an elevated temperature to form hollow ZnSnO3. Fig. 15 illustrates the progression of the hollow ZnSnO3 microstructure. In further investigations, Xu et al. discovered that only the combination of SnCl4/ZnCl2/NaOH solution allowed the fabrication of ZnSnO3 hollow-boxes.125 Similarly, by subjecting a mixture of SnCl4/Zn(Ac)2/NaOH to hydrothermal heating based on Ostwald ripening, hierarchically hollow ZnSnO3 cages were synthesized in a one-pot reaction, requiring an excess amount of NaOH.126–128
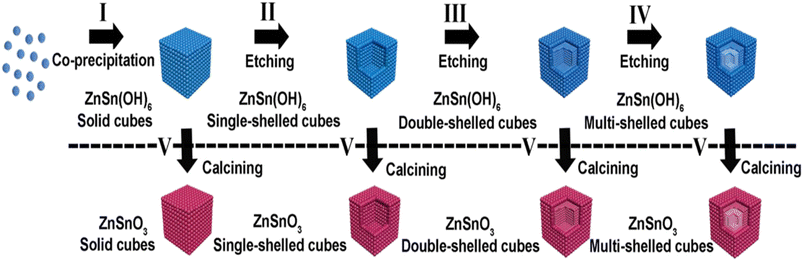 | ||
| Fig. 15 Schematic illustration of the fabrication of ZnSnO3 hollow-boxes under basic-etching and calcination.129 | ||
Cubic microcrystals of ZnSnO3 can be readily transformed into hollow structures by utilizing nitric acid (HNO3) as an alternative etching agent. Fig. 16 presents the proposed mechanism of the acid etching process, together with TEM images depicting the products obtained at different stages of etching the cubic ZnSnO3 templates.92
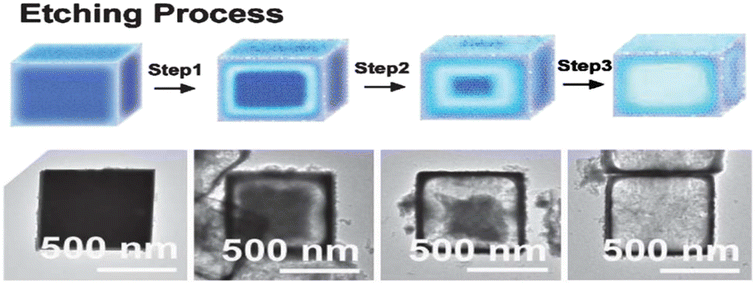 | ||
| Fig. 16 Potential mechanism illustrating the acid etching process and the resulting synthesized products when employing cubic ZnSnO3 templates.92 | ||
The etching technique for the synthesis of ZnSnO3 has drawbacks, including challenges in controlling the composition and morphology precisely. Etching involves selectively removing components from a precursor material, which is difficult for complex oxide systems such as ZnSnO3. This can lead to non-uniformity in the final product, affecting the properties and performance. The process is time-consuming, requiring careful optimization of the etching agents and conditions, making synthesis more intricate compared to other methods.
3.9. Mechanochemical technique
Mechanochemical blending is a solid preparation method involving the coupling of mechanical and chemical phenomena on the molecular scale. It is an efficient strategy for synthesizing nanosized metal lattice composite powders due to its simplicity and the ability to achieve composite powder particles with a uniform distribution of grain sizes. In the study reported in ref. 130, the orthorhombic structure of ZnSnO3 was created through mechanochemical grinding. A stoichiometric amount of ZnO and SnO2 was ground for 20 min to obtain a fine powder, which was annealed at 500 °C for 3 h. Subsequently, the calcination process was repeated for an additional 20 n at 800 °C, with intermittent milling after each 3 h interval. Finally, the mixture was heated to the desired temperature at a ramping speed of 10 °C per minute. However, the mechanochemical technique for synthesizing ZnSnO3 has the drawbacks of extended processing times and high energy consumption. Specifically, this method involves mechanical milling or grinding of precursor materials to induce chemical reactions. Although it can yield high-purity ZnSnO3, the need for prolonged milling can be time-consuming and energy-intensive.Building on the comprehensive review presented earlier, the precise synthesis of diverse ZTO micro-/nanostructures represents a captivating avenue for achieving enhanced performance and investigating the interplay between structure, properties, and performance. Additionally, the utilization of hollow shapes with customizable sizes, morphologies, and compositions has gained significant attention in the fields of sensors and catalysts owing to their exceptional attributes including improved surface area, reduced density, and extensive volume. These remarkable features make them highly desirable for various applications.
4. Application
In this section, we present some of the common uses of morphological ZnSnO3 nanoparticles in the fields of photocatalysts, sensing, storage, and energy conversion devices.The ability to design and synthesize ZnSnO3 micro/nanostructures with precise control of their morphology, phase, and homogeneous size holds great potential for expanding their applications. Particularly, the crystalline phase plays a crucial role in energy harvesting applications. Moreover, by enhancing the structural or electrical properties of ZnSnO3 nanocomposites, they can be utilized in a broad range of applications. Extensive studies exhibited the exceptional potential of ZnSnO3 materials in various fields, including gas-sensing, photocatalysis, piezocatalysts, lithium-ion batteries, transparent conductors, and energy storage. Their remarkable electron mobility and electrical conductivity are the main factors responsible for this characteristic.
In this section, we explore the diverse morphology-based applications of ZnSnO3 nanocomposites in photocatalysis, sensing, energy storage and conversion devices, highlighting their significant contributions to these fields.
4.1. Photodegradation of pollutants
Zinc stannate is a ternary metal oxide that holds great potential for photocatalytic and pyroelectric wastewater treatment. Previous studies have established that ZnSnO3 exhibits ferroelectric properties, and similar to ferroelectrics with a perovskite structure, display piezoelectric behaviour, making it an important candidate for wastewater treatment.148–150 Among the six possible crystal structures, LN-type-ZnSnO3 has been found to possess superior photocatalytic activity due to its piezoelectric property.The enhanced photocatalytic activity of LN-type ZnSnO3 can be attributed to a specific feature of this crystallite orientation. In LN-type ZnSnO3, the displacement of Zn ions next to the z-axis is higher than Sn ions, resulting in spontaneous polarization. The theoretical high spontaneous polarization of LN-type ZnSnO3 thin films is estimated to be approximately Pr ≈ 59 C cm−2. However, experimental studies have revealed slightly lower values, such as Pr ≈ 47 C cm−2 at a coercive field of Ec ≈ 130 kV cm−1 and Pr ≈ 30 C cm−2 at Ec ≈ 25 kV cm−1 in single crystal-oriented LN-type ZnSnO3 thin films. Vertically aligned LN-type ZnSnO3 films exhibit a measured spontaneous polarization of Pr ≈ 26 C cm−2.14
In the study in ref. 99, the authors employed the sol–gel method to synthesize ZnSnO3 catalyst nanoparticles and investigated their behaviour in the process of synergistic pyro- and photo-bi-catalysis. When subjected to UV light and thermal cycles in the range of 20 °C to 65 °C, the ZnSnO3 catalyst exhibited a remarkable bi-catalytic dye degradation efficiency of about 98.1%. This efficiency significantly surpassed the photocatalytic degradation (76.8%) and the pyrocatalytic degradation (20.2%). The enhanced photoactivity of ZnSnO3 can be attributed to its ability to facilitate faster separation of electron–hole (e−–h+) pairs, effectively acting as an electron trap. Consequently, the piezo-photocatalyst demonstrated a higher decomposition/breakdown performance than the ordinary photocatalyst. This improvement can be attributed to the synergistic catalytic effect achieved by combining the activity of piezo-phototronics with piezoelectricity, semiconductors, and photonics. This coupling effectively reduces the recombination of e−–h+ pairs generated by light and improves their mobility by inducing energy band distortion through applied stresses.
In the study in ref. 151, it was demonstrated that the efficiency of the ZnSnO3/ZnO composite was significantly higher than that of ZnSnO3 alone in the degradation of phenol. The ZnSnO3/ZnO nanocomposite exhibited an improved photocatalytic breakdown with a reaction rate of 0.023 min−1 than that of 0.0168 min−1 for ZnSnO3. This improvement in the reaction rate constant was ascribed to the mixed oxides of the composite with increased surface area of ZnSnO3/ZnO nanocomposites. Consequently, the effective charge separation of the e−–h+ pairs occurred. This study also investigated the surface properties of the ZnSnO3/ZnO nanocomposite by studying the isoelectric point (IEP) using the pH drift method. The IEP refers to the pH at which a semiconductor metal oxide carries no net charge, significantly affecting the surface characteristics. The most significant photodegradation of phenol occurred in mildly acidic medium, specifically at a pH of ∼6.4. The efficiency of the photocatalytic reaction is directly influenced by the pH of the solution, given that it impacts the formation of hydroxyl radicals and leads to changes in the surface characteristics of the photocatalyst.
Furthermore, in another study in ref. 91, ZnSnO3 nanowires were found to exhibit excellent piezophotocatalytic activity, with a rate constant of ∼0.0176 min−1. This enhanced performance can be attributed to the large surface area, superb arrangement, piezo-potential build-up, and band deforming characteristics of the ZnSnO3 nanowires.
The optical-absorption activity of a photocatalyst plays a crucial part in photocatalysis. The authors in ref. 147 synthesized a nanocomposite of S-doped ZnSnO3, which exhibited improved adsorption abilities and a narrow energy gap. The S-ZnSnO3 material exhibited a significantly high specific surface area, reaching 80.63 m2 g−1, which facilitated the effective adsorption of reactants. Additionally, the introduction of S-doping in ZnSnO3 led to a rapid reduction in the band gap from 3.7 to 2.4 eV. This significant reduction in the band gap resulted in a highly effective photocatalyst for the decomposition of RhB, achieving an efficiency of almost 90%, which is substantially higher than when using pure ZnSnO3.
Furthermore, the manipulation of the surface morphologies of ZnSnO3 also improves its photocatalytic capabilities by reducing the photon-induced recombination e−–h+ pairs and enhancing their mobility. In the in ref. 116, the authors designed double-shelled ZnSnO3 hollow-cubes to enhance the photocatalytic breakdown of antibiotic wastewater. These double-shelled ZnSnO3 hollow cubes provide a high surface area, offer large reactive sites, and allow multi-scattering of incident light, thereby enhancing the photocatalytic performance. The trapping experiments conducted in the study detected the primary reactive species in the photo breakdown activity as ·OH and h+. These active species successfully encourage the breakdown of organic dyes under exposure to simulated sunlight.
Hollow ZnSnO3 nanospheres/rGO nanocomposites have been proven to be effective photocatalysts for degrading metronidazole in aqueous solutions. These nanocomposites demonstrated remarkable photodegradation capabilities, particularly under visible light irradiation for a duration of 180 min. The hollow ZnSnO3 nanospheres achieved a degradation efficiency of 42.1%, while the ZnSnO3/rGO nanocomposites exhibited an even higher efficiency of 72.5%. In contrast, the pure rGO and ZnSnO3 with rGO physical mixtures displayed a very low performance in terms of photodegradation. The outstanding performance of ZnSnO3/rGO can be related to several factors. Firstly, the hybrid nanocomposite possesses higher adsorption effectiveness for the target dyes. Additionally, it exhibits enhanced absorption of visible light due to the presence of rGO. Finally, the distinctive electronic system of the ZnSnO3/rGO hybrid nanocomposite contributes to its enhanced photocatalytic properties. This finding was reported in ref. 132.
The probable reaction mechanism pathway in the breakdown of dye can be outlined as follows:116,147
| ZnSnO3 + hθ → e− + h+ |
| OH− + h+ → ·OH |
| H2O + h+ → OH + H+ |
| e− + O2 → ·O2− |
| Dye + O2−/·OH/h+ → degradation products |
When light of frequency ν equaling the Eg of metal oxide semiconductors illuminates them, the electrons (e−) in the semiconductors become excited and transition from the VB to the CB. This process generates holes (h+) in the VB, resulting in the formation of e−–h+ pairs. These h+ and e− can engage in reactions with H2O molecules, OH− ions, and dissolved O2, leading to the creation of ·OH radicals and ·O2− radicals, respectively. Conversely, the organic dye can also undergo photosensitization. When a photosensitized organic dye undergoes dissociation in water, it can react with ·OH radicals and ·O2− radicals to produce H2O and CO2.
After band-gap excitation and the generation of free charge carriers (electrons and holes), various mechanisms of de-excitation occur, as shown in Fig. 17a and b.116 Together with the desired redox reactions (pathways 1 and 2), several recombination processes compete with each other, hindering the successful transfer of the carriers to acceptor molecules at the surface.
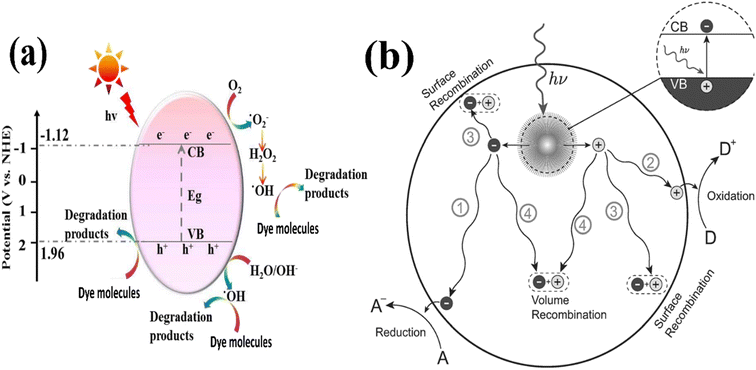 | ||
| Fig. 17 Illustration of (a) photocatalytic mechanism. (b) Photoinduced formation of an electron hole pair in a semiconductor.148,217 | ||
These competing recombination processes include the recombination of carriers with their oppositely charged counterparts trapped on the surface (route 3) and the recombination of two carriers in the bulk of the semiconductor (pathway 4). Both of these mechanisms contribute to a decrease in the efficiency of the photocatalytic reaction. The rate of recombination is influenced by several factors, including the mobility and trapping behaviour of the charge carriers, the density of defects in the semiconductor lattice, and the presence of an interface with a secondary material, which acts as a sink for either electrons or holes. These factors collectively affect the recombination rates, and consequently impact the overall effectiveness of the photocatalytic process (Table 4).152
| Materials | Morphology | Light source | Time | Dye | Efficiency (%) | Ref. |
|---|---|---|---|---|---|---|
| a Pyro-/photo-bi-catalysis. b pH = 6.5. c pH = 7.5. | ||||||
| ZnSnO3/rGO | Nanosheets | Visible-light | 35 | MB | 85 | 76 |
| 90 | Phenol | 72.89 | ||||
| ZnSnO3 | Nanocubes | UV | 80 | RhB | 98.1a | 99 |
| 76.8 | ||||||
| S-ZnSnO3 | Hollow cubes | Visible-light | 100 | RhB | 90 | 147 |
| ZnSnO3/rGO/MoS2 | Nanosheets | UV | 100 | MB | 86 | 153 |
| RhB | 78 | |||||
| ZnSnO3 | Hollow cubes | Xe | 100 | MB | 96.52 | 116 |
| CIP | 85.9 | |||||
| ZnSnO3/rGO | 3D folded | Visible | 120 | CIP | 100 | 154 |
| ZnSnO3 | Nanostructures | UV | 120 | Acid brown 14 | 92 | 103 |
| ZnSnO3/rGO | Hollow nanospheres | Visible light | 180 | Metronidazole | 72.5 | 132 |
| ZnSnO3/ZnO | Nanocubes | UV | 60 | Phenol | 47 | 151 |
| AgI/ZnSn(OH)6 | Nanocubes | Visible light | 40 | RhB | 100 | 155 |
| 60 | TOC | 91 | ||||
| ZnSnO3 | Nanourchins | UV | 90 | MO | 98 | 75 |
| 2,5-DCP | 95 | |||||
| ZnSnO3/g-C3N4 | Nanosheets | Visible | 120 | Tetracycline | 85 | 156 |
| ZnSnO3/g-C3N4 | Nanocubes | Visible | 120 | Tetracycline | 90.8 | 157 |
| Cu3P/ZnSnO3/g-C3N4 | Nanocubes | Xe lamp | 60 | Tetracycline | 98.45 | 158 |
| CoP/ZnSnO3 | Nanocubes | Visible | 60 | Tetracycline | 96.44 | 159 |
| CDs-ZnSnO3 | Nanocubes | Visible | 60 | Tetracycline | 81.76 | 144 |
| NDs/ZnSnO3 | Nanocubes | Visible | 120 | Tetracycline | 90 | 160 |
| Ag–ZnSnO3 | Hollow nanocubes | UV | 240 | Caffeine | 68b | 143 |
| 100c | ||||||
4.2. Gas sensors
Extensive research has been conducted in recent decades on gas-sensing materials due to their crucial role in detecting hazardous gases. This research has significant implications in industry, environmental monitoring, agriculture, medical diagnosis, military, human health, and aerospace.161–164 Gas sensors based on n-type semiconductors have been employed to detect inflammable and hazardous gases such as H2, H2S, CH4, C2H5OH, and CO. However, a major limitation of these sensors is their lack of selectivity.Thus, to address this limitation, the n-type ZnSnO3 semiconductor has emerged as a promising solution for enhancing the selectivity of gas sensors towards various gases. The utilization of the sensitive material has been greatly improved by the substantial increase in the specific surface area of ZnSnO3. This enhanced specific surface area of the semiconductor allows for better capacity to adsorb gas molecules. Additionally, the double-shelled hollow morphology with mesoporous features not only facilitates the adsorption of gas molecules on the surface of the sensitive/reactive material but also allows for deep penetration of gas molecules inside the hollow spheres. This unique structure enables the rapid diffusion of gas molecules and oxygen, thereby improving the overall performance of the gas sensor.
ZnSnO3 is widely recognized as an n-type semiconductor, primarily governed by the behaviour of its conduction electrons. When ZnSnO3-based sensors come into contact with air, oxygen molecules tend to adsorb on the sample surface, effectively capturing free electrons from the CB. This process leads to the formation of ionized oxygen species, including O2−, O− and O2−. Consequently, the consumption of electrons results in the creation of a substantial electron depletion layer on the surface of the sample. This electron depletion layer significantly increases the resistance of the sensor, thereby influencing its overall performance.
ZnSnO3 has been extensively studied as a highly effective material for detecting gases that are reducing and combustible. A gas sensor fabricated using polyhedral ZnSnO3 demonstrated exceptional sensitivity, quick recovery, and reliable results for the detection of HCHO gas. Among the various polyhedral shapes, the 14-faceted ZnSnO3, consisting of 6 {100} facets and 8 {111} facets, exhibited significantly greater sensitivity compared to the octahedral shape and commercially available powder. This improved sensitivity is attributed to the larger exposure of the active {100} facets on the 14-faceted ZnSnO3. The increased number of active {100} facets in the 14-faceted ZnSnO3 led to a higher specific surface area, providing more reactive sites for ZnSnO3 to bind gas molecules. Consequently, this results in higher sensitivity. Considering these findings, it is promising to explore the potential of using cubic ZnSnO3 entirely exposed with {100} facets for gas sensing applications.32
The researchers in ref. 165 have successfully developed hollow ZnSnO3 nanocages, which showed remarkable gas sensing properties, particularly when detecting formaldehyde gas. These nanocages demonstrated a high gas response, together with short response and recovery times, superior selectivity, and good repeatability and stability, even at low concentrations of HCHO. This study confirmed that the highest response, with a value of 57.6, was achieved when exposed to 50 parts per million (ppm) of HCHO gas at 350 °C. The response time for the nanocages was measured to be 3 s, while the recovery time was 5 s. Alternatively, the sensitivity of these nanocages towards other gases such as C2H5OH, (CH3)2CO, CH3OH, and NH3 was significantly lower compared to formaldehyde gas.
To enhance the selectivity of sensors, the researchers in ref. 126 investigated the use of hollow cubic nanoparticles of zinc stannate with a surface area of 22.6319 m2 g−1, which were synthesized through the hydrothermal process. The sensor fabricated using these samples exhibited significant improvements in selectivity. When exposed to 500 ppm of butane gas at an operating temperature of 380 °C, the response of the hollow microspheres was measured to be 5.79, whereas the response of the solid microspheres was 3.92. Moreover, the response time and recovery time of the hollow ZnSnO3 microspheres were recorded to be 0.3 s and 0.65 s, while that of the solid ZnSnO3 microspheres was 1.3 s and 13.7 s, respectively. These superior features of the sensor were ascribed to the larger specific surface area of the hollow particles in comparison to the solid particles. This enabled better access to the surface, leading to an increased ability to adsorb gas molecules.
Jiang et al. employed a hydrothermal technique to prepare perovskite-type ZnSnO3 and conducted gas sensitivity experiments.166 The results obtained from calculations revealed that acetone molecules strongly interacted with pre-adsorbed O2− and O− on the ZnSnO3 (001) surface, leading to charge transfer and resistance changes in the material. These observed phenomena form the basis for utilizing ZnSnO3 as a sensing material for detecting acetone. The experimental findings demonstrated that the perovskite-type ZnSnO3 exhibited a sheet like morphology with micro-holes on its surface, which contributed to enhanced sensitivity towards (CH3)2CO gas. Through gas sensing tests, the ideal performance temperature was determined to be 350 °C, with a response time of 4 s and recovery time of 27 s. The sensitivity of the ZnSnO3 nanosheets produced in this study towards 100 ppm and 10 ppm of (CH3)2CO gas was found to be 125.444 and 8.37, respectively. Furthermore, the sensor based on the sheet-like ZnSnO3 maintained 89.64% and 94.74% of its original sensitivity towards 10/100 ppm acetone gas, respectively, after undergoing a stability and repeatability test for five weeks.
One approach to enhance the selectivity towards specific gases involves doping or modifying the gas-sensitive material through the use of another substance and creating composites. This was demonstrated in ref. 167, where Ag/ZnSnO3 structures exhibited sensitivity towards acetone. In this study, it was found that the response to 100 ppm of acetone was 30, with a response time of 2 s and recovery time of 3 s. The optimal operating temperature was determined to be 280 °C. In another study, ref. 168, focused on increasing the sensitivity towards ethanol vapor by doping with NiO. The sensitivity to 20 ppm of C2H5OH was measured at 23.95, with a response time of 56 s and recovery time of 48 s. The optimal operating temperature for this system was found to be 160 °C. In ref. 169, the authors synthesized zinc stannate nanofibers with a surface area of 88.4 m2 g−1 using the electrospinning method. The sensitivity towards acetone was enhanced by doping with Au/In–ZnSnO3. The response to 50 ppm of ethanol was recorded at 19.3, with a response time of 10 s and recovery time of 13 s at 200 °C.
The gas-sensing properties of conventional ZnSnO3 nanoparticles are often unsatisfactory for sensor applications due to their high operating temperatures. These sensors typically require temperatures exceeding 200 °C, which limits their practical use. At lower temperatures, the thermal energy is insufficient to overcome the activation energy barrier, resulting in inadequate reactions with sensitive materials. Simultaneously, as the operating temperature increases, gas molecules gain higher thermal energy, enabling them to surpass the activation energy barrier. This leads to enhanced reactions among gas molecules and adsorbed oxygen, as well as enhanced ion adsorption on the surface of the semiconductor material.
Thus, to address this issue and achieve high-performance gas sensors with reduced working temperatures, various modifications have been applied, such as preparing nanomaterials with different morphologies and doping. In ref. 170, the authors synthesized 3D flower-like ZnSnO3 using the co-precipitation method and improved the C2H5OH sensing properties at RT by incorporating nano-TiO2. This modification significantly enhanced the ethanol response at room temperature, reaching a maximum value of 36.6. The response and recovery time for a low concentration of 10 ppm C2H5OH were recorded to be 4 s and 17 s, respectively. The authors attributed the formation of the 3D flower-like structure to the presence of the stabilizer and pH balancer triethanolamine (TEOA) during the synthesis of ZnSnO3.
ZnSnO3 is a conventional n-type metal-oxide semiconductor and its gas sensing mechanism operates through surface control, relying on changes in conductivity during the gas-sensing reaction. Upon exposure to air, ZnSnO3 nanocubes adsorb oxygen ions on their surface, as follows:171
| O2 + e− → O2(ads)− | (1) |
| O2(ads) + 2e− → 2O(ads)− | (2) |
| O2(ads) + 4e− → 2O(ads)2− | (3) |
When the ZnSnO3 nanocubes are exposed to C2H5OH/(CH3)2CO/HCHO, the C2H5OH/(CH3)2CO/HCHO molecules will interact with absorbed oxygen ions as follows:
| C2H5OH/(CH3)2CO/HCHO + O2(ads)− → H2O + CO2 + e− | (4) |
| C2H5OH/(CH3)2CO/HCHO + 2O(ads)− → H2O + CO2 + 2e− | (5) |
| C2H5OH/(CH3)2CO/HCHO + 2O(ads)2− → H2O + CO2 + 4e− | (6) |
Eqn (1) to (3) describe the process in which electrons are extracted from the CB of ZnSnO3, leading to an increase in resistance. Alternatively, eqn (4) to (6) depict the process where electrons are returned to the CB of ZnSnO3, resulting in a decrease in resistance. This gas-sensing mechanism highlights that the quantity of surface-adsorbed oxygen ions plays a crucial role in the gas-sensing characteristics of ZnSnO3. As electrons are consumed in the surface region of the sensitive material, a depletion layer is formed, leading to an increase in the resistance of the material, as shown in Fig. 18.170
 | ||
| Fig. 18 Schematic band diagrams of ZnSnO3 hollow microspheres exposed to (a) air and (b) CH3COCH3, and ZnSnO3/SnO2 hollow urchin gas sensors exposed to (c) air and (d) CH3COCH3.218 | ||
4.3. Anode for lithium-ion batteries
Lithium-ion batteries (LIBs) have revolutionized the energy storage landscape and become the backbone of portable electronic devices, electric vehicles, and grid storage applications. The remarkable combination of high energy density, long cycle life, and low self-discharge rate has made Li-ion batteries the preferred choice in various industries.186–188 Over the years, extensive research efforts have been directed towards enhancing the performance and safety of Li-ion batteries, driven by the growing demand for sustainable and efficient energy storage solutions. In this case, ZnSnO3 possesses a unique crystal structure and a wide bandgap, making it an intriguing material for Li-ion battery applications. When used as an electrode material, ZnSnO3 exhibits an exceptional theoretical capacity of 1320 mA h g−1, surpassing that of many traditional alternatives.71 This high capacity implies that ZnSnO3 has potential to store more lithium ions, resulting in increased energy storage capabilities (Table 5).| Sensing materials | Morphology | Surface area (m2 g−1) | Target | Concentration (ppm) | Operating temp. (°C) | Response (Ra/Rg) | Response-recovery time (s) | Ref. |
|---|---|---|---|---|---|---|---|---|
| ZnSnO3 | Hollow microspheres | 62.50 | Ethanol | 500 | 320 | 144.3 | 7, 8 | 90 |
| Solid microspheres | 40.43 | 260 | 80 | 4, 7 | ||||
| CeO2/ZnSnO3 | Hollow microspheres | — | Ethanol | 100 | 200 | 219.2 | 12, 22 | 172 |
| CuO/ZnSnO3 | Hollow microspheres | — | Ethanol | 100 | 160 | 131 | 13, 8 | 173 |
| Ag–ZnSnO3 | Nanocubes | — | Ethanol | 100 | 200 | 83.96 | 1, 50 | 174 |
| ZnSnO3 | Hollow microspheres | 30.21 | Ethanol | 100 | 280 | 24 | — | 70 |
| NiO/ZnSnO3 | Microsphere-decorated fibers | 60 | Ethanol | 20 | 160 | 23.95 | 56, 48 | 168 |
| ZnSnO3 | Hollow spheres | — | Ethanol | 50 | 270 | 27.8 | 0.9, 2.2 | 127 |
| ZnSnO3 | 2D nanosheets | 85.1 | HCHO | 10 | 100 | 57 | 36, 42 | 175 |
| Nanocubes | 59.7 | Ethanol | 200 | 20.3 | 41, 105 | |||
| ZnSnO3 | 3D flower-like | — | Ethanol | 1000 | RT(25) | 36.6 | 2, 14 | 170 |
| ZnSnO3 | Hollow nanocages | 76.4 | HCHO | 50 | 350 | 57.6 | 3, 5 | 165 |
| Ethanol | 34.5 | |||||||
| Acetone | 23.6 | |||||||
| ZnSnO3 | Nanosheets | 57.86 | Ethanol | 50 | 320 | 33 | 0.36, 9 | 94 |
| Acetone | 440 | 41 | 0.18, 2.6 | |||||
| ZnSnO3 | Nanocubes | 71 | TEA | 100 | 350 | 57.5 | 41, 040 | 72 |
| ZnSnO3 | Hierarchical | 67.8 | HCHO | 10 | 300 | 34.1 | 132, 15 | 171 |
| ZnSnO3 | Hollow cubes | 98 | HCHO | 100 | 220 | 37.2 | 1, 59 | 124 |
| ZnSnO3 | Hollow cubes | — | HCHO | 200 | 270 | 22.5 | — | 136 |
| ZnSnO3 | Nanocubes | 70.001 | HCHO | 50 | 210 | 21.2 | — | 137 |
| ZnSnO3 | Porous nanostructures | — | HCHO | 100 | 240 | 13.5 | 1, 10 | 176 |
| ZnSnO3 | Porous cubes | 33.7 | Toluene | 100 | 300 | 16.56 | 2, 11 | 177 |
| HCHO | 36.81 | 3, 12 | ||||||
| Zn2SnO4 | Hierarchical | 23.7 | Toluene | 100 | 280 | 25.2 | 1, 3.5 | 178 |
| Spherical | 20.6 | 19.2 | ||||||
| Cubic | 9.1 | 11.7 | ||||||
| ZnSnO3 | Sheet-like | — | Acetone | 100 | 350 | 125.44 | 7, 27 | 166 |
| ZnSnO3/rGO | Hierarchical | 33.14 | Acetone | 50 | 350 | 80.37 | 4, 27 | 179 |
| Ag/ZnSnO3 | Hollow nanocubes | 19.1 | Acetone | 100 | 280 | 30 | 2, 3 | 167 |
| ZnSnO3/SnO2 | Concave microcubes | 69.7 | Acetone | 50 | 260 | 19.1 | 5.6 | 180 |
| ZnSnO3 | Hollow polyhedrons | — | Acetone | 50 | 240 | 12.48 | 17, 10 | 181 |
| Au/In–ZnSnO3 | Nanofibers | 88.4 | Acetone | 50 | 200 | 19.3 | 10, 13 | 169 |
| ZnSnO3 | Nanocages | — | H2S | 100 | 310 | 29.4 | 20, 50 | 128 |
| ZnSnO3–C1 | Hollow cubes | — | H2S | 100 | 335 | 14.18 | 6, 22 | 182 |
| ZnSnO3 | Hollow hexahedron | 152 | CO2 | 400 | RT | 4, 65 | 73.2, 79.5 | 183 |
| PdO–ZnSnO3 | Hollow microspheres | — | n-Propanol | 100 | 140 | 30.8 | 1, 25 | 184 |
| ZnSnO3 | Hollow spheres | 53.91 | n-Butanol | 50 | 200 | 7.1 | 2, 40 | 185 |
| ZnSnO3 | Hollow microspheres | 22.632 | Butane | 500 | 380 | 5.79 | 0.3, 0.65 | 126 |
| Solid microspheres | — | 3.92 | 1.3, 13.7 |
Although the various structures of ZnSnO3 demonstrate appealing characteristics in LIBs, this material still faces notable changes in volume during repeated charging and discharging cycles. Consequently, its rate performance is compromised and it experiences considerable capacity loss, impeding its practical use in real-world applications.188,189 Thus, to address the aforementioned limitations of ZnSnO3, scientists have implemented diverse approaches, including the creation of micro–nano structures, incorporating transition metals through modification, and combining the material with carbon substances.190–193
A reported study demonstrated that a sandwich-like C/ZnSnO3 composite exhibited comparatively higher reversible capacity even after long-term cycling tests.194 Binary ZnSnO3 nanocomposites combine two practical materials, displaying an improved synergistic performance. The integration of these elements results in an enhancement of their individual properties, including improved electrical and ionic conductivity, enhanced electrochemical reactivity, and increased mechanical stability. The sandwich-like C/ZnSnO3 nanocomposite displayed a reversible capacity of 1107 mA h g−1 under a current density of 100 mA g−1, with a coulombic output of ∼98.5% after the 200th cycle. This performance was better than that of sheet-like ZnSnO3, which showed a coulombic output of ∼96.2% after 100 cycles. Fig. 19 illustrates the synthesis procedure of the sandwich-like ZnSnO3@C. The charge–discharge data of the sandwich-like ZnSnO3@C and sheet-like ZnSnO3 at a current density of 100 mA g−1 and the cycling performance of the nanocomposite are shown in Fig. 20a–d. The complete electrochemical reaction pathway can be outlined as follows:71,194
| Zn + xLi+ + xe− ↔ LixZnx ≤ 1 |
| C + yLi+ + ye− ↔ LiyC |
| Sn + zLi+ + ze− ↔ LizSnO ≤ z ≤ 4.4 |
| 4Li+ + ZnSnO3 + 4e− → Sn + 2Li2O + ZnO |
| 6Li+ + ZnSnO3 + 6e− → Sn + 3Li2O + Zn |
 | ||
| Fig. 19 Schematic illustration depicting the synthesis of sandwich-like ZnSnO3@C.194 | ||
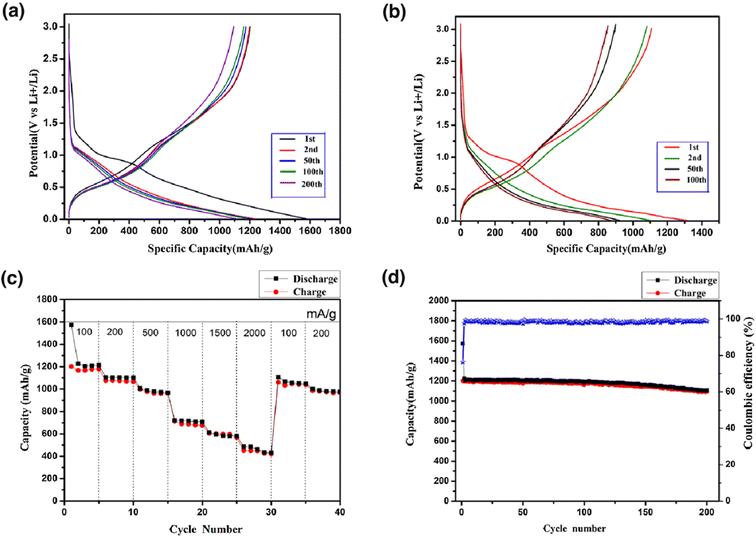 | ||
| Fig. 20 Charge–discharge profiles of (a) sandwich-like ZnSnO3@C and (b) sheet-like ZnSnO3 at 100 mA g−1. (c) Cycling activity at various current densities. (d) Cycling activity at 100 mA g−1 of the sandwich-like ZnSnO3@C.194 | ||
Overall, the unique nanostructure of the sandwich-like C/ZnSnO3 composite, with its interconnected carbon nanoplates, encapsulated ZnSnO3 nanoparticles, and high specific surface area (106 m2 g−1), enables enhanced Li storage activity by facilitating efficient charge transfer, preventing material detachment, and accommodating volume changes during electrochemical reactions.194
Amorphous ZnSnO3 double-shell hollow microcubes (D-ZnSnO3) possessed an average edge length of 1.6 mm and displayed favourable electrochemical characteristics when utilized as anode materials.195 Compared to their ZnSnO3 yolk–shell (Y-ZnSnO3) counterparts, the D-ZnSnO3 anodes exhibited a superior performance. They maintained a high coulombic efficiency of 99% at a current density of 100 mA g−1 and retained a significant reversible capacity of 741 mA h g−1 after 50 cycles. The exceptional electrochemical properties of D-ZnSnO3 originate from its amorphous nature and unique box-in-box hollow architecture. The amorphous structure of D-ZnSnO3 contributed to its isotropy, effectively relieving mechanical strain and preserving the electrode integrity throughout cycling. Furthermore, the hollow box-in-box architecture provided ample space to accommodate the substantial volume changes associated with the insertion and extraction of Li+ ions.195
Lu et al. successfully fabricated amorphous isotropic ZnSnO3 nanocubes with a well-defined mesoporous morphology, which were coated with a uniform thin carbon layer, forming core–shell nanoparticles that were interconnected to create a cohesive framework.196 Comparative electrochemical analysis of the amorphous ZnSnO3@C and crystalline ZnSnO3@C revealed that the former exhibited a superior performance, including a higher specific capacity, superior cyclic stability, and favourable rate capability.
Although the capacity and cyclic stability of the crystalline ZnSnO3@C, with excellent crystallinity, showed a slight increase, reaching a capacity of 791 mA h g−1 after 100 cycles, the amorphous ZnSnO3@C demonstrates an improved reversible specific capacity and a more stable capacity retention. The amorphous material achieved reversible capacities exceeding 1020 mA h g−1 during the initial 20 cycles, with a marginal increase to 1060 mA h g−1 after 100 cycles. This enhancement was attributed to the improved accessibility of lithium ions in the structure. The uninterrupted carbon nanocoating, interconnected porous structure, well-established mesoporosity, and homogeneous amorphous composition all significantly contributed and played essential roles in the accommodation of mechanical strain, buffering of volume changes, and maintenance the structural integrity of ZnSnO3 to achieve a stable cycling performance.
Additionally, this unique structure facilitates rapid electron and Li-ion transfer, thereby enhancing the application of active materials and enabling high-reversible cathode performances.196 The utilization of ZnO@ZnSnO3 quantum dot (QD) core–shell nanorod (NR) arrays as anodes led to significant improvements in their electrochemical performance.197 The incorporation of a highly conductive ZnSnO3 QD shell on the ZnO@ZnSnO3 QD core–shell NRs resulted in larger free spaces, which were retained after extended cycling at high current densities. This indicates that the unique arrangement of the ZnO@ZnSnO3 QD core–shell NRs successfully accommodated the volume changes during cycling.
The use of a binder-free electrode offers several advantages, such as establishing good contact with the electrolyte and maintaining a short Li-ion diffusion path within the ZnO@ZnSnO3 QD electrode. Moreover, after 450 cycles at a current density of 1000 mA g−1, there were still more free spaces observed among the ZnO@ZnSnO3 QDs. This suggests that the barrier posed by significant volume expansion can be overcome when the surface of ZnO nanorods is enhanced with ZnSnO3 QD shells.197 The incorporation of ZnSnO3 hollow cubes in flexible reduced graphene oxide (rGO) sheets, resulting in a porous architecture, was proven to be advantageous in lithium-ion battery applications.146 This design effectively mitigated the issues associated with significant volume enlargement throughout repetitive charge–discharge cycles. Additionally, the 3D networks created by the ZnSnO3 hollow cubes encased in rGO sheets facilitated the rapid transport of lithium ions and electrons.
As a result of these features, the ZnSnO3-graphene assemblies (ZGAs) exhibited an improved rate capacity, achieving a high specific capacity of 552.6 mA h g−1 at a current density of 1200 mA g−1. Furthermore, ZGAs demonstrated excellent cycling stability, maintaining a specific capacity of 745.4 mA h g−1 after 100 cycles at a current density of 100 mA g−1. These impressive electrochemical properties indicate that ZGAs have substantial prospect as superior-performance anode materials for LIBs.146
In the study described in ref. 198, cube-like, interconnected, amorphous ZnSnO3@TiO2 nanocomposites were fabricated using a simple co-precipitation followed by TiO2 coating procedure. The incorporation of an interconnected TiO2 wrapping and numerous mesopores in the composite structure served to alleviate volume expansion stress, resulting in significantly improved electrochemical stability and performance. The hierarchical heterostructure of the ZnSnO3@TiO2 nanocomposite exhibited impressive electrochemical characteristics. It demonstrated a high initial discharge capacity of 1590 mA h g−1 at a current density of 100 mA g−1 and a capacity retention of 780 mA h g−1 after 200 cycles, surpassing that of pure ZnSnO3 nanomaterial anodes. The benefits of the hierarchical nanocomposite morphology, together with the synergistic activity of ZnSnO3 and TiO2 contributed to the improved cycle life of the ZnSnO3@TiO2 anode. Several factors contribute to its improved performance, as follows: (1) the hierarchical porous 3D morphology reduced the transportation length of lithium ions and electrolyte molecules, increased the contact area between the electrolyte and active material, and enhanced the penetration capability of the electrolyte molecules. (2) The hierarchical porous morphology helped mitigate the significant volume enlargement that occurs during repetitive charge–discharge cycles. (3) The TiO2 coating layer effectively alleviated the stress induced by volume changes during the Li+ insertion–extraction conversion reaction. (4) The synergistic effect of the high capacity of ZnSnO3 and superior structural stability of TiO2 contributed to the superior electrochemical performance of the ZnSnO3@TiO2 composite. Overall, the hierarchical ZnSnO3@TiO2 nanocomposite demonstrated promising electrochemical properties, making it a potential candidate for high-performance anode materials in LIBs.198
A sustainable cubic ZnSnO3/carbon composite with a high surface area of 896.39 m2 g−1 was successfully synthesized using a low-cost combustion process.199 The electrochemical performance of supercapacitors using the ZnSnO3/C nanocomposite as electrode materials was demonstrated, revealing a good rate capability and exceptional cycling stability. The presence of ZnSnO3 in the composite facilitated rapid ion transfer, resulting in a capacitance retention of approximately 80% after 3000 charge–discharge cycles at different current densities in 6 M KOH electrolyte. These results indicate that the ZnSnO3/carbon composite shows promise as a lightweight electrode material for supercapacitors due to its quick charge–discharge characteristics and strong endurance.
Furthermore, it is anticipated that unique morphology of anode materials, such as porous hollow nanostructures198 and hybrid carbon-based nanocomposites,199 can be a viable approach for enhancing lithium storage. However, it is necessary to improve the existing system for evaluating anode materials and assessing their charge–discharge capacity (Table 6).
| Materials | Initial capacity (mA h g−1) | Reversible capacity (mA h g−1) | Cycle number (n) | Current density (mA g−1) | Capacity retention (%) | Ref. |
|---|---|---|---|---|---|---|
| Sandwich-like ZnSnO3@C | 1573 | 1107 | 200 | 100 | 70 | 194 |
| Sheet-like ZnSnO3 | 1321 | 872 | 100 | 100 | 66 | 194 |
| ZnO@ZnSnO3 core–shell | 1275 | 1073 | 110 | 200 | 84 | 197 |
| Amorphous ZnSnO3@C | 1633 | 1060 | 100 | 200 | 65 | 196 |
| Crystalline ZnSnO3@C | 1350 | 791 | 100 | 200 | 59 | 196 |
| Cube-like ZnSnO3@TiO2 | 1590 | 780 | 200 | 100 | 49 | 198 |
| ZnSnO3/rGO | 1987.5 | 745 | 100 | 100 | 38 | 146 |
| ZnSnO3/rGO | 2013 | 718 | 100 | 100 | 36 | 145 |
| ZnSnO3/rGO | 1691 | 713 | 100 | 100 | 42 | 200 |
| S@Ni/NiO/ZnSnO3 | 1070 | 493 | 400 | 335 | 46 | 201 |
| S@ZnSnO3 | 781 | 312 | 400 | 335 | 40 | 201 |
| ZnSnO3/C nanofibers | 1412 | 586 | 100 | 100 | 42 | 73 |
| ZnSnO3/C | 1469 | 695 | 200 | 100 | 57 | 189 |
| ZnSnO3@CNF | 1183 | 582.6 | 100 | 100 | 49 | 188 |
| ZnSnO3 nanocubes | 1470 | 590 | 50 | 200 | 40 | 101 |
| Double-shell-ZnSnO3 hollow microcubes | 2134 | 741 | 50 | 100 | 35 | 195 |
| Yolk–shell ZnSnO3 | 1570 | 628 | 50 | 100 | 40 | 195 |
| ZnSnO3–C hollow microcubes | 2255 | 703 | 50 | 100 | 31 | 202 |
| ZnSnO3 amorphous hollow microcubes | 1921 | 400 | 50 | 100 | 21 | 202 |
| Sheet-like ZnSnO3 | 1390 | 625 | 50 | 100 | 45 | 71 |
| ZnSnO3@C/rGO | 1984 | 1040 | 45 | 100 | 52 | 203 |
| Ag/ZnSnO3 | 874.5 | 464.5 | 45 | 300 | 53 | 204 |
4.4. Nanodevices
Materials with piezoelectric properties have the remarkable ability to transform mechanical energy into electrical energy. In 2006, Wang206 pioneered the concept of piezoelectric nanogenerators (PENGs), and since then, there has been an explosion of innovation in the design of nanostructured materials to serve as PENGs for harnessing diverse forms of energy from their surroundings.121,206,207 This surge in research has led to the development of various piezoelectric materials, including inorganic materials, to further advance this field.205,208–210In the study conducted by Wang et al., a lead-free nanogenerator was developed using a ZnSnO3 microbelts, which demonstrated an impressive performance.36 When subjected to compressive and releasing strain ranging from 0.8% to 1.0%, the ZnSnO3 microbelts, anchored at their ends on a flexible polystyrene substrate, produced an output power of approximately 3 nW. The resulting output voltage and current were measured to be 100 mV and 30 nA, respectively. These values correspond to an energy conversion efficiency in the range of 4.2% to 6.6% based on the applied strain of 0.8% to 1%. These findings highlight the significant advancements in lead-free ZnSnO3 nanogenerators, showcasing their potential for high output power generation and efficient energy conversion through piezoelectric effects.
Datta and colleagues made a significant contribution by demonstrating the remarkable ferroelectric properties of ZnSnO3 nanowire arrays.211 They discovered that these arrays exhibited an exceptional remanent polarization value of approximately 30 μC cm−2. This groundbreaking finding provides valuable insights for the future development of ZnSnO3-based ferroelectric devices, enabling a more coordinated and targeted design approach.
Piezoelectric nanogenerators (PENGs) are highly valued for their ability to convert ambient mechanical stimuli into electrical energy, making them ideal for self-powered sensors. In ref. 212, the researchers employed a low-temperature solution-based approach to synthesize aluminium-doped zinc stannate (ZnSnO3) PENGs, which exhibited high electrical outputs in response to external forces. The improved output was ascribed to several factors, including the modified crystalline structure of ZnSnO3, the incorporation of aluminium cations with specific modifications, and the reduced size of the ZnSnO3 nanocube particles. Through their experiments, the researchers determined that the optimal concentration of aluminium dopant was 2 wt%. The resulting Al-doped ZnSnO3 PENG generated impressive electrical outputs of 110 V, 13 μA, and 1200 mW m−2 with just 120 beats per min (2 Hz) finger tapping as the input. Remarkably, the PENG charged a 2.2 μF capacitor to 3 V in a mere 8 s with the simple act of finger tapping. Leveraging the high output-to-input ratio, the researchers integrated the PENG with a helmet, enabling it to function as a motion sensor. By detecting the mechanical movements of the wearer, the device converted each footstep into a wireless signal, showcasing its potential applications in capturing human motion data.
The improved electrical output of nanogenerators is attributed to the enhanced dielectric features, enhanced electric dipole moment, and piezoelectric coefficient exhibited by piezoelectric materials. To harness the synergistic effects of ZnSnO3 and polydimethylsiloxane (PDMS) polymer, Yu and colleagues synthesized a composite polymer matrix (CPM) by embedding ZnSnO3 nanocrystals (NCs) in triboelectric PDMS.213 The resulting hybrid nanogenerator (HNG) demonstrated an impressive performance, with open-circuit voltage (VOC), short-circuit current (ISC), power density, and charge density values of approximately ∼410 V, 13 μA, 2 W m−2, and 95 C m−2, respectively, for the 0.15 g reduced graphene oxide (rGO)-ZnSnO3/PDMS CPM-based HNG. The electrical energy produced by harvesting mechanical energy using the HNG was investigated during various human body actions, including finger tapping, hand punching, walking, and running, as depicted in Fig. 21a–d. The obtained electrical output, as represented by the VOC/ISC curves of the HNG, was approximately ∼40 V/1.5 μA, ∼80 V/2 μA, ∼160 V/4 μA, and ∼190 V/6 μA for tapping with a finger, punching with a hand, walking, and running, respectively. These results demonstrate the remarkable potential of the HNG in converting mechanical energy from human actions into electrical energy.
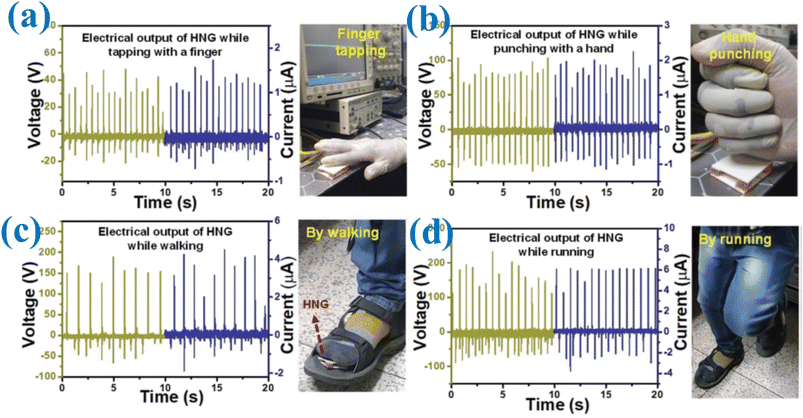 | ||
| Fig. 21 HNG device showing promising potential for real-time and practical applications in harvesting mechanical energy from everyday human activities. Visual illustrations and the associated electrical data showing the performance and output of the HNG device for (a) finger tapping, (b) hand punching, (c) walking, and (d) running.213 | ||
Furthermore, the integration of single-crystalline piezoelectric perovskite ZnSnO3 nanocubes with PDMS for the construction of hybrid piezoelectric nanogenerators (HP-NGs) is a significant development. Lee et al. successfully demonstrated a highly stable and powerful HP-NG without the need for electrical poling treatment.214 The HP-NGs incorporating ZnSnO3 nanocubes showed remarkable mechanical durability, robustness, and power-generation capabilities. To evaluate their piezoelectric power generation and mechanical resilience, a direct impact approach was employed. In one experiment, a large-scale HP-NG was placed on a road and subjected to vertical compressive force exerted by a heavy motor vehicle. Fig. 22 captures a snapshot of this experimental motion.
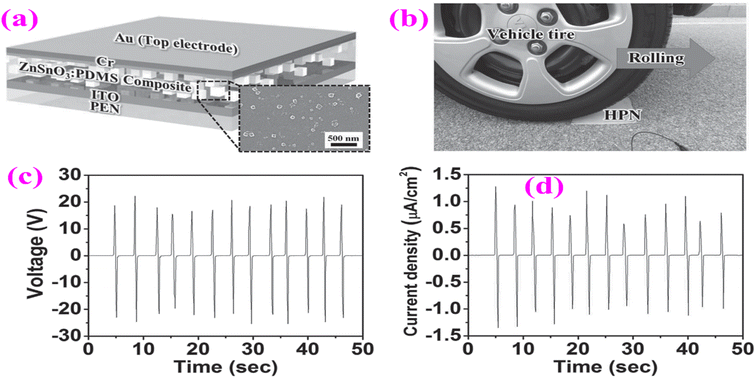 | ||
| Fig. 22 Structure and power generation by HP-NG when a tire is rolled. (a) Illustration of the structure of the ZnSnO3:PDMS nanocomposite-based flexible HP-NG as seen by the cross-sectional FESEM image. (b) Experimental setup for power production under the rolling of a vehicle tire, in which ZnSnO3:PDMS HP-NG was attached to the road. (c) Output voltage and (d) current density from HP-NG induced by the loading and unloading of a vehicle tire.214 | ||
The observed the output voltage and current from the 10 cm × 10 cm HP-NG are depicted in Fig. 22c and d, demonstrating a notable recorded output voltage of approximately ∼20 V and output current density of about 1 μA cm−2.
The utilization of ZnSnO3 triangular belts as lead-free nanogenerators has yielded groundbreaking results [Z]. By applying a mechanical deformation of 0.1%, these nanogenerators could generate a current of 0.13 μA and voltage of 5.3 50 V, marking the first verification of high output power from lead-free ZnSnO3 nanogenerators.37
Fig. 23 presents a schematic diagram illustrating the working mechanism of generating power from a piezoelectric nanogenerator (PENG). Research has confirmed that when subjected to longitudinal compressive stress, the electric dipoles in the crystal align effectively in a single direction due to the phenomenon known as the stress-induced poling effect.215 When a tapping force is applied to the device, this pressure causes the separation of positive and negative charges in the ZnSnO3 nanorods, creating an electric dipole.216 After the cancellation of the interior charges with opposite signs, excess charges remain at both surfaces, leading to a net polarization effect, as depicted in Fig. 7b. This resulting polarization induces opposite charges at the corresponding electrodes, initiating the flow of electrons through the external circuit, thereby generating voltage and current. This mechanism generates a piezoelectric potential across the surface, allowing free electrons to move from the top and bottom electrodes through the external circuit. To balance this generated piezoelectric potential, the electrons accumulate at the interfaces between the nanocubes and electrodes. Subsequently, when the nanocubes decompress after removing the applied load or object, the accumulated electrons return through the external load, and the piezoelectric potential is neutralized.215,216
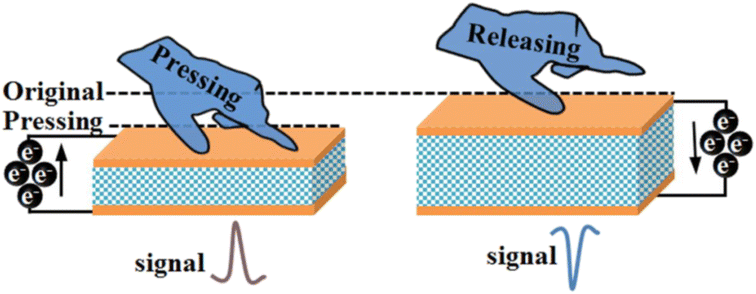 | ||
| Fig. 23 Working mechanism of generating power from a piezoelectric nanogenerator.215 | ||
On pressing it will give a positive peak, whereas on releasing the pressure, it produces a negative peak as the electrons move in the opposite direction. Higher and smaller peaks were also observed when compression occurred more rapidly than decompression and the magnitude of the both output voltage and current strongly depends on the compression frequency and applied pressure.210,215,216
5. Conclusion and perspectives
Nanostructured materials based on ZnSnO3 possess a multitude of features. These include optical properties, a high isoelectric point, large specific area, non-toxic nature, mechanical characteristics, bending strength, and flexibility. Each of these properties is significant and unique, contributing to the functionality and potential applications of ZnSnO3-based nanostructures. Researchers have dedicated their efforts to exploring the fundamental aspects of developing, modifying, and utilizing ZnSnO3-based nanostructures in various energy and biological applications due to their intriguing characteristics. In this study, we focused on ZnSnO3-based nanostructures and provided a comprehensive overview, with particular emphasis on their applications in Li-ion batteries, photocatalysis, biosensors, and the biological domain. Our review primarily addresses the advancements made in ZnSnO3-based materials to achieve higher performances across diverse applications.ZnSnO3-based materials exhibit photocatalytic properties, enabling the decomposition of dye molecules upon exposure to visible light. The efficiency of catalysts in photodegradation and their long-term stability are influenced by factors such as structural morphology, crystal orientation, particle size, and oxygen defects. To enhance the photocatalytic activity of ZnSnO3, several strategies have been employed, including the fabrication of ZnSnO3 nanostructured arrays, incorporating additional components such as noble metals, introducing dopants, and integrating carbon materials. These approaches aim to improve the overall photocatalytic performance of ZnSnO3-based materials. However, there are still challenges and opportunities in the field of ZnSnO3 photocatalysis. Despite extensive research in controlled environments, key factors such as surface excess charges, surface additives, and the states of adsorbates in the photocatalytic process are not well-understood. Additionally, there is no consensus on the nature of photogenerated charge carriers and their relationship with charge/energy transfer and bond breaking/forming due to a lack of detailed knowledge about their interactions with adsorbates. Thus, to address these issues, it is crucial to complement traditional ensemble-averaged experimental techniques with time-resolved methods. These experiments aim to uncover the dynamic aspects of ZnSnO3 photocatalysis, leading to a deeper understanding and the potential development of new dynamic-based photocatalysis models.
Researchers have shown that ZnSnO3-based nanostructured materials possess the capability to bind gaseous molecules. In sensing applications, the utilization of nanostructures aims to increase the surface-to-volume ratio, enhancing their reactivity and facilitating their interaction with analytes by facilitating their translocation across cell membranes. To satisfy to biosensing and environmental requirements, scientists have developed electrochemical sensors utilizing ZnSnO3 nanostructures and composite materials. These advancements open possibilities for improved gas sensing and environmental monitoring applications. Due to the benefits mentioned earlier, we emphasize the synthesis strategy to enhance the efficiency of ZnSnO3 and optimize its operating temperature. It is advisable to investigate techniques that can produce ZnSnO3 structures characterized by significant porosity and substantial specific surface areas. Given that surface resistance plays a crucial role in gas sensing, the creation of highly porous structures is advantageous, offering additional reactive sites for the absorption and interaction of the desired gas molecules, and thereby promoting the formation of an electron depletion region. Despite the extensive use of ZnSnO3 nanomaterials and their composites for gas sensing, their selectivity and high operating temperature remain questionable. Thus, to enhance selectivity for specific gases, it is recommended to conduct dynamic tests at varying temperatures. Different temperatures influence the adsorption and desorption processes of distinct gas molecules on the material surface, resulting in temperature-dependent interactions between the sensing material and specific gas molecules. This dynamic testing approach enables the confirmation of the selectivity of a sensor for a particular gas at a specific temperature. Consequently, in practical applications, a single sensor can detect multiple gases by adjusting the working temperature, enabling the accurate identification of the components in mixed gases and significantly expanding the detection capabilities of the sensor.
ZnSnO3-based nanostructures have been found to exhibit promising energy storage characteristics, indicating their potential use as electrode materials in supercapacitors and lithium-ion batteries. ZnSnO3 is widely recognized as a viable active material for batteries, demonstrating a typical reversible energy of 1107 mA h g−1 after 200 cycles. These findings highlight the suitability of ZnSnO3 for energy storage applications and suggest its potential for enhancing the performance of supercapacitors and LIBs. Although various related structures have been created and considerable progress has been made in energy storage, there are still many challenges that need further exploration. First, cleverly designed structures optimize certain properties of LIBs, but simultaneously these anodes present other issues. For example, porous or hollow structures can accommodate the volume changes but reduce the volumetric capacity of the whole cell. For the prevalent application of portable, light, small electric devices, volumetric capacity and specific capacity are highly valued.
The comprehensive assessment of anode materials and structures is necessary, aiming to produce electrode architecture designs endowed with better overall performances. Although composites of multi-material blending and combination of different structures are beneficial, more theoretical research including modelling and computing should be applied to maximize the features leading to improved performances, while also minimizing the shortcomings among the different materials and structures.
The exceptional properties of nanostructured ZnSnO3-based materials in energy storage, biosensor, biological, and PENG applications highlight their potential for further scientific advancements in interdisciplinary research. There is significant scope for enhancing the performance of ZnSnO3-based materials in the energy, environmental, and biology fields. Accordingly, further investigations into the tunable characteristics of these materials are anticipated, aiming to unlock their full potential and contribute to advancements in these diverse fields.
Author contributions
Moksodur Rahman: conceptualization, formal analysis, investigation, methodology, visualization, writing – original draft; Muhammad Shahriar Bashar: supervision, resources, validation, writing – review & editing; Md. Lutfor Rahman: validation, visualization, writing – review & editing; Faisal Islam Chowdhury: conceptualization, supervision, project administration, writing – review & editing.Conflicts of interest
There are no conflicts to declare.References
- A. Yamada and M. Konagai, Atomic layer deposition of ZnO films and their application to solar cells, Solid State Phenom., 1999, 67, 237–247, DOI:10.4028/www.scientific.net/ssp.67-68.237.
- M. Ashraf, S. M. J. Akhtar, A. F. Khan, Z. Ali and A. Qayyum, Effect of annealing on structural and optoelectronic properties of nanostructured ZnSe thin films, J. Alloys Compd., 2011, 509, 2414–2419, DOI:10.1016/j.jallcom.2010.11.032.
- K. A. Gesheva, T. M. Ivanova and G. Bodurov, Transition metal oxide films: Technology and “smart Windows” electrochromic device performance, in Prog Org Coat, Elsevier, 2012, pp. 635–639. DOI:10.1016/j.porgcoat.2011.07.016.
- R. S. Niranjan, K. R. Patil, S. R. Sainkar and I. S. Mulla, High H2S-sensitive copper-doped tin oxide thin film, Mater. Chem. Phys., 2003, 80, 250–256, DOI:10.1016/S0254-0584(02)00467-4.
- I. M. El Radaf, Promising novel transparent conductive F-doped ZnSnO3 thin films for optoelectronic applications, J. Mater. Sci.: Mater. Electron., 2023, 34, 215, DOI:10.1007/s10854-022-09600-z.
- S. Y. Bang, F. C. Mocanu, T. H. Lee, J. Yang, S. Zhan, S. M. Jung, D. W. Shin, Y. H. Suh, X. B. Fan, S. Lee, H. W. Choi, L. G. Occhipinti, S. D. Han and J. M. Kim, Robust In-Zn-O Thin-Film Transistors with a Bilayer Heterostructure Design and a Low-Temperature Fabrication Process Using Vacuum and Solution Deposited Layers, ACS Omega, 2020, 5, 21593–21601, DOI:10.1021/acsomega.0c02225.
- Z. Wu, B. Fan, L. Zhang, Y. Yao, S. Hong, H. Yu and Y. Jia, Strongly enhanced piezoelectric-catalysis of ZnSnO3/graphite hybrid materials for dye wastewater decomposition, Ceram. Int., 2023, 49(18), 29614–29621, DOI:10.1016/j.ceramint.2023.06.180.
- J. Xu, X. Jia, X. Lou and J. S. Shen, One-step hydrothermal synthesis and gas sensing property of ZnSnO3 microparticles, Solid-State Electronics, 2006, 50(3), 504–507, DOI:10.1016/j.sse.2006.02.001.
- P. Wadkar, D. Bauskar and P. Patil, High performance H2 sensor based on ZnSnO3 cubic crystallites synthesized by a hydrothermal method, Talanta, 2013, 105, 327–332, DOI:10.1016/j.talanta.2012.10.051.
- Y. Zeng, T. Zhang, H. Fan, G. Lu and M. Kang, Synthesis and gas-sensing properties of ZnSnO3 cubic nanocages and nanoskeletons, Sens. Actuators, B, 2009, 143, 449–453, DOI:10.1016/j.snb.2009.07.021.
- Z. Wang, J. Liu, F. Wang, S. Chen, H. Luo and X. Yu, Size-Controlled Synthesis of ZnSnO3 Cubic Crystallites at Low Temperatures and Their HCHO-Sensing Properties, J. Phys. Chem. C, 2010, 114, 13577–13582, DOI:10.1021/jp104733e.
- X. J. Zhu, L. M. Geng, F. Q. Zhang, Y. X. Liu and L. B. Cheng, Synthesis and performance of Zn2SnO4 as anode materials for lithium ion batteries by hydrothermal method, J. Power Sources, 2009, 189, 828–831, DOI:10.1016/j.jpowsour.2008.07.028.
- C. Wang, B. Q. Xu, X. Wang and J. Zhao, Preparation and photocatalytic activity of ZnO/TiO2/SnO2 mixture, J. Solid State Chem., 2005, 178, 3500–3506, DOI:10.1016/j.jssc.2005.09.005.
- D. Mukherjee, A. Datta, C. Kons, M. Hordagoda, S. Witanachchi and P. Mukherjee, Intrinsic anomalous ferroelectricity in vertically aligned LiNbO3-type ZnSnO3 hybrid nanoparticle-nanowire arrays, Appl. Phys. Lett., 2014, 105, 1–6, DOI:10.1063/1.4902557.
- E. Y. Shaba, J. O. Jacob, J. O. Tijani and M. A. T. Suleiman, A critical review of synthesis parameters affecting the properties of zinc oxide nanoparticle and its application in wastewater treatment, Appl. Water Sci., 2021, 11, 48, DOI:10.1007/s13201-021-01370-z.
- T. Udayabhaskararao, M. Kazes, L. Houben, H. Lin and D. Oron, Nucleation, Growth, and Structural Transformations of Perovskite Nanocrystals, Chem. Mater., 2017, 29, 1302–1308, DOI:10.1021/acs.chemmater.6b04841.
- A. Perejón, N. Murafa, P. E. Sánchez-Jiménez, J. M. Criado, J. Subrt, M. J. Diánez and L. A. Pérez-Maqueda, Direct mechanosynthesis of pure BiFeO3 perovskite nanoparticles: Reaction mechanism, J. Mater. Chem. C, 2013, 1, 3551–3562, 10.1039/c3tc30446a.
- Y. Cao, D. Jia, J. Zhou and Y. Sun, Simple solid-state chemical synthesis of ZnSnO3 nanocubes and their application as gas sensors, Eur. J. Inorg. Chem., 2009, 4105–4109, DOI:10.1002/ejic.200900146.
- G. Fu, H. Chen, Z. Chen, J. Zhang and H. Kohler, Humidity sensitive characteristics of Zn 2SnO4-LiZnVO4 thick films prepared by the sol-gel method, Sens. Actuators, B, 2002, 81, 308–312, DOI:10.1016/S0925-4005(01)00971-6.
- P. Song, Q. Wang and Z. Yang, Ammonia gas sensor based on PPy/ZnSnO3 nanocomposites, Mater. Lett., 2011, 65, 430–432, DOI:10.1016/j.matlet.2010.10.087.
- D. Kovacheva and K. Petrov, Preparation of crystalline ZnSnO3 from Li2SnO3 by low-temperature ion exchange, Solid State Ion., 1998, 109(3–4), 327–332, DOI:10.1016/S0167-2738(97)00507-9.
- J. X. Wang, S. S. Xie, H. J. Yuan, X. Q. Yan, D. F. Liu, Y. Gao, Z. P. Zhou, L. Song, L. F. Liu, X. W. Zhao, X. Y. Dou, W. Y. Zhou and G. Wang, Synthesis, structure, and photoluminescence of Zn2SnO4 single-crystal nanobelts and nanorings, Solid State Commun., 2004, 131, 435–440, DOI:10.1016/j.ssc.2004.06.009.
- Z. Lu and Y. Tang, Two-step synthesis and ethanol sensing properties of Zn2SnO4SnO2 nanocomposites, Mater. Chem. Phys., 2005, 92, 5–9, DOI:10.1016/j.matchemphys.2004.11.029.
- J. X. Wang, S. S. Xie, Y. Gao, X. Q. Yan, D. F. Liu, H. J. Yuan, Z. P. Zhou, L. Song, L. F. Liu, W. Y. Zhou and G. Wang, Growth and characterization of axially periodic Zn2SnO4 (ZTO) nanostructures, J. Cryst. Growth, 2004, 267, 177–183, DOI:10.1016/j.jcrysgro.2004.03.052.
- X. Y. Xue, Y. J. Chen, Q. H. Li, C. Wang, Y. G. Wang and T. H. Wang, Electronic transport characteristics through individual nanowires, Appl. Phys. Lett., 2006, 88, 182102, DOI:10.1063/1.2199612.
- Y.-Y. Choi, H.-K. Kim, H.-W. Koo, T.-W. Kim and S.-N. Lee, Flexible ZnSnO3/Ag/ZnSnO3 multilayer electrodes grown by roll-to-roll sputtering on flexible polyethersulfone substrates, J. Vac. Sci. Technol., A, 2011, 29, 061502, DOI:10.1116/1.3632999.
- Y. Y. Choi, S. J. Kang and H. K. Kim, Rapid thermal annealing effect on the characteristics of ZnSnO3 films prepared by RF magnetron sputtering, Curr. Appl. Phys., 2012, 12(4), S104–S107, DOI:10.1016/j.cap.2012.05.014.
- H. Cao, Z. Hu, X. Wei, H. Wang, X. Tian and S. Ding, Conductometric ethanol gas sensor based on a bilayer film consisting of SnO2 film and SnO2/ZnSnO3 porous film prepared by magnetron sputtering, Sens. Actuators, B, 2023, 382 DOI:10.1016/j.snb.2023.133562.
- I. Riahi, B. Khalfallah and F. Chaabouni, Physico-chemical properties of perovskite ZnSnO3 thin films deposited on glass and silicon wafers by RF magnetron sputtering, Opt. Quantum Electron., 2022, 54 DOI:10.1007/s11082-022-03907-1.
- K. Lee, H. S. Han, J. H. Ryu, S. Kang, K. Jung, Y. K. Kim, T. Song, S. Mhin and K. M. Kim, Laser-driven formation of ZnSnO3/CNT heterostructure and its critical role in boosting performance of the triboelectric nanogenerator, Carbon, 2023, 212 DOI:10.1016/j.carbon.2023.118120.
- Q. R. Hu, P. Jiang, H. Xu, Y. Zhang, S. L. Wang, X. Jia and W. H. Tang, Synthesis and photoluminescence of Zn2SnO4 nanowires, J. Alloys Compd., 2009, 484, 25–27, DOI:10.1016/j.jallcom.2009.05.057.
- B. Geng, C. Fang, F. Zhan and N. Yu, Synthesis of polyhedral ZnSnO3 microcrystals with controlled exposed facets and their selective gas-sensing properties, Small, 2008, 4, 1337–1343, DOI:10.1002/smll.200701177.
- T. Bora, M. H. Al-Hinai, A. T. Al-Hinai and J. Dutta, Phase Transformation of Metastable ZnSnO3 Upon Thermal Decomposition by In Situ Temperature-Dependent Raman Spectroscopy, J. Am. Ceram. Soc., 2015, 98, 4044–4049, DOI:10.1111/jace.13791.
- A. Rovisco, R. Branquinho, J. Martins, E. Fortunato, R. Martins and P. Barquinha, Growth Mechanism of Seed-Layer Free ZnSnO3 Nanowires : E ff ect of Physical Parameters, ( 2019) 37–41 Search PubMed.
- Y. Inaguma, M. Yoshida and T. Katsumata, A polar oxide ZnSnO3 with a LiNbO3-type structure, J. Am. Chem. Soc., 2008, 130, 6704–6705, DOI:10.1021/ja801843v.
- J. M. Wu, C. Xu, Y. Zhang and Z. L. Wang, Lead-Free Nanogenerator Made from Single ZnSnO3 Microbelt, ACS Nano, 2012, 6, 4335–4340, DOI:10.1021/nn300951d.
- C. Jyh Ming Wu, C. Xu, Y. Zhang, Y. Yang, Y. Zhou, Z. Lin Wang, J. M. Wu, C. Xu, Y. Zhang, Y. Yang, Y. Zhou and Z. L. Wang, Flexible and Transparent Nanogenerators Based on a Composite of Lead-Free ZnSnO3 Triangular-Belts, Adv. Mater., 2012, 24, 6094–6099, DOI:10.1002/adma.201202445.
- H. Wang, H. Huang and B. Wang, First-principles study of structural, electronic, and optical properties of ZnSnO3, Solid State Commun., 2009, 149, 1849–1852, DOI:10.1016/j.ssc.2009.07.009.
- H. Gou, F. Gao and J. Zhang, Structural identification, electronic and optical properties of ZnSnO3: First principle calculations, Comput. Mater. Sci., 2010, 49, 552–555, DOI:10.1016/j.commatsci.2010.05.049.
- Y. Fan, X. Huang, G. Wang and P. Jiang, Core-Shell Structured Biopolymer@BaTiO3 Nanoparticles for Biopolymer Nanocomposites with Significantly Enhanced Dielectric Properties and Energy Storage Capability, J. Phys. Chem. C, 2015, 119, 27330–27339, DOI:10.1021/acs.jpcc.5b09619.
- W. Routray and V. Orsat, Recent advances in dielectric properties–measurements and importance, Curr. Opin. Food Sci., 2018, 23, 120–126, DOI:10.1016/j.cofs.2018.10.001.
- A. Chaudhary, P. Malik, R. Mehra and K. K. Raina, Influence of ZnO nanoparticle concentration on electro-optic and dielectric properties of ferroelectric liquid crystal mixture, J. Mol. Liq., 2013, 188, 230–236, DOI:10.1016/j.molliq.2013.09.020.
- S. Gao, C. Wu, Y. Zhang and H. Li, Dielectric regulation of high-graphitized fine ash wrapped cube-like ZnSnO3 composites with boosted microwave absorption performance, Ceram. Int., 2021, 47, 4994–5002, DOI:10.1016/j.ceramint.2020.10.074.
- D. Anadkat, C. Badampudi, A. Gor and A. V. Sanchela, Investigation of frequency dependent dielectric properties of La-doped BaSnO3-ZnSnO3 solid-solutions, J. Alloys Compd., 2023, 958 DOI:10.1016/j.jallcom.2023.170350.
- A. Sasmal, A. Patra, P. S. Devi and S. Sen, Space charge induced augmented dielectric permittivity and improved energy harvesting ability of nano-Ag decorated ZnSnO3 filled PVDF based flexible nanogenerator, Compos. Sci. Technol., 2021, 213 DOI:10.1016/j.compscitech.2021.108916.
- R. Love, Application of Kramers-Kronig relations to the interpretation of dielectric data, J. Phys. C: Solid State Phys., 1974, 7 Search PubMed , http://iopscience.iop.org/0022-3719/7/23/024.
- C. Li, H. Wang, B. Wang and R. Wang, First-principles study of the structure, electronic, and optical properties of orthorhombic BiInO3, Appl. Phys. Lett., 2007, 91 DOI:10.1063/1.2770761.
- J. M. Wu, C. Xu, Y. Zhang and Z. L. Wang, Lead-Free Nanogenerator Made from Single ZnSnO3 Microbelt, ACS Nano, 2012, 6, 4335–4340, DOI:10.1021/nn300951d.
- J. Y. Son, G. Lee, M. Jo, H. Kim, H. M. Jang and Y. Shin, Heteroepitaxial Ferroelectric ZnSnO3 Thin Film, J. Am. Chem. Soc., 2009, 8386–8387, DOI:10.1021/ja903133n.
- H. Gou, J. Zhang, Z. Li, G. Wang, F. Gao, R. C. Ewing and J. Lian, Energetic stability, structural transition, and thermodynamic properties of ZnSnO3, Appl. Phys. Lett., 2011, 98, 2011–2014, DOI:10.1063/1.3562013.
- Y. Inaguma, M. Yoshida and T. Katsumata, A polar oxide ZnSnO3 with a LiNbO3-type structure, J. Am. Chem. Soc., 2008, 130, 6704–6705, DOI:10.1021/ja801843v.
- J. Ko and C. T. Prewitt, High-pressure phase transition in MnTiO3 from the ilmenite to the LiNbO3 structure, Phys. Chem. Miner., 1988, 15, 355–362, DOI:10.1007/BF00311040.
- W. Zhong and D. Vanderbilt, Effect of quantum fluctuations on structural phase transitions in SrTiO3 and BaTiO3, Phys. Rev. B, 1996, 53, 5047, DOI:10.1103/PhysRevB.53.5047.
- Y. Wang, J. A. Yan and M. Y. Chou, Electronic and vibrational properties of γ-AlH3, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 77 DOI:10.1103/PhysRevB.77.014101.
- X. Q. Chen, W. Wolf, R. Podloucky and P. Rogl, Ab initio study of structural stability, elastic, vibrational, and electronic properties of TiPd2, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 76 DOI:10.1103/PhysRevB.76.092102.
- M. Leszczyński, E. Litwin-Staszewska, T. Suski, J. Bąk-Misiuk and J. Domagała, Lattice Constant Of Doped Semiconductor, Acta Phys. Pol. A, 1995 Search PubMed.
- E. Ching-Prado, C. A. Samudio, J. Santiago-Aviles and S. Velumani, Electronic structure and optical properties of SnO2:F from PBE0 hybrid functional calculations, J. Mater. Sci.: Mater. Electron., 2018, 29, 15423–15435, DOI:10.1007/s10854-018-9067-3.
- P. G. Sundell, M. E. Björketun and G. Wahnström, Thermodynamics of doping and vacancy formation in BaZrO3 perovskite oxide from density functional calculations, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 73 DOI:10.1103/PhysRevB.73.104112.
- M. Bououdina, S. Azzaza, R. Ghomri, M. N. Shaikh, J. H. Dai, Y. Song, W. Song, W. Cai and M. Ghers, Structural and magnetic properties and DFT analysis of ZnO:(Al,Er) nanoparticles, RSC Adv., 2017, 7, 32931–32941, 10.1039/c7ra01015j.
- L. Q. Jiang, J. K. Guo, H. B. Liu, M. Zhu, X. Zhou, P. Wu and C. H. Li, Prediction of lattice constant in cubic perovskites, J. Phys. Chem. Solids, 2006, 67, 1531–1536, DOI:10.1016/j.jpcs.2006.02.004.
- D. Kucharczyk and Z. Niklewski, Accurate X-ray Determination of the Lattice Parameters and the Thermal Expansion Coefficients of VO2 near the Transition Temperature, J. Appl. Cryst., 1979, 12, 370–373, DOI:10.1107/S0021889879012711.
- Y. Okada and Y. Tokumaru, Precise determination of lattice parameter and thermal expansion coefficient of silicon between 300 and 1500 K, J. Appl. Phys., 1984, 56, 314–320, DOI:10.1063/1.333965.
- S. Shikata, T. Tanno, T. Teraji, H. Kanda, T. Yamada and J. I. Kushibiki, Precise measurements of diamond lattice constant using Bond method, Jpn. J. Appl. Phys., 2018, 57 DOI:10.7567/JJAP.57.111301.
- H. Yusa, M. Akaogi, N. Sata, H. Kojitani, R. Yamamoto and Y. Ohishi, High-pressure transformations of ilmenite to perovskite, and lithium niobate to perovskite in zinc germanate, Phys. Chem. Miner., 2006, 33, 217–226, DOI:10.1007/s00269-006-0070-5.
- H. Gou, J. Zhang, Z. Li, G. Wang, F. Gao, R. C. Ewing and J. Lian, Energetic stability, structural transition, and thermodynamic properties of ZnSnO3, Appl. Phys. Lett., 2011, 98, 2011–2014, DOI:10.1063/1.3562013.
- Q. J. Liu, H. Qin, Z. Jiao, F. S. Liu and Z. T. Liu, First-principles calculations of structural, elastic, and electronic properties of trigonal ZnSnO3 under pressure, Mater. Chem. Phys., 2016, 180, 75–81, DOI:10.1016/j.matchemphys.2016.05.041.
- H. L. Yuan and J. C. Li, Effect of annealing temperature on the growth of Zn-Sn-O nanocomposite thin films, J. Alloys Compd., 2017, 714, 114–119, DOI:10.1016/j.jallcom.2017.04.230.
- H. Wang, H. Huang and B. Wang, First-principles study of structural, electronic, and optical properties of ZnSnO3, Solid State Commun., 2009, 149, 1849–1852, DOI:10.1016/j.ssc.2009.07.009.
- H. L. Yuan and J. C. Li, Effect of annealing temperature on the growth of Zn-Sn-O nanocomposite thin films, J. Alloys Compd., 2017, 714, 114–119, DOI:10.1016/j.jallcom.2017.04.230.
- X. Jia, M. Tian, R. Dai, D. Lian, S. Han, X. Wu and H. Song, One-pot template-free synthesis and highly ethanol sensing properties of ZnSnO3 hollow microspheres, Sens. Actuators, B, 2017, 240, 376–385, DOI:10.1016/j.snb.2016.08.146.
- T. W. Yuejiao Chen, B. Qu, M. Lin, D. Lei, L. Chen and Q. Li, Synthesis of ZnSnO3 Mesocrystals from Regular Cube-like to Sheet-like Structures and Their Comparative Electrochemical Properties in Li-ion Batteries, J. Mater. Chem. C, 2015, 3, 10715–10722, 10.1039/b000000x.
- B. S. Sá, C. A. Zito, T. M. Perfecto and D. P. Volanti, Porous ZnSnO3 nanocubes as a triethylamine sensor, Sens. Actuators, B, 2021, 338 DOI:10.1016/j.snb.2021.129869.
- Y. Zhang, J.-L. Wei, X.-Y. Jin, M.-C. Yu, L. Wang, Yu-H. Guo and S.-T. Dong, Electrospun ZnSnO3-C Nanofiber as an Anode Material for Lithium-Ion Batteries, J. Electron. Mater., 2021, 50, 4945–4953, DOI:10.1007/s11664-021-09036-x.
- Z. Wang, J. Liu, F. Wang, S. Chen, H. Luo and X. Yu, Size-controlled synthesis of ZnSnO3 cubic crystallites at low temperatures and their HCHO-sensing properties, J. Phys. Chem. C, 2010, 114, 13577–13582, DOI:10.1021/jp104733e.
- Z. Tian, C. Liang, J. Liu, H. Zhang and L. Zhang, Zinc stannate nanocubes and nanourchins with high photocatalytic activity for methyl orange and 2,5-DCP degradation, J. Mater. Chem., 2012, 22, 17210–17214, 10.1039/c2jm32406g.
- G. Gnanamoorthy, V. K. Yadav, D. Latha, V. Karthikeyan and V. Narayanan, Enhanced photocatalytic performance of ZnSnO3/rGO nanocomposite, Chem. Phys. Lett., 2020, 739 DOI:10.1016/j.cplett.2019.137050.
- K. Fujiwara, H. Minato, J. Shiogai, A. Kumamoto, N. Shibata and A. Tsukazaki, Thin-film stabilization of LiNbO3-type ZnSnO3 and MgSnO3 by molecular-beam epitaxy, APL Mater., 2018, 7, 022505, DOI:10.1063/1.5054289.
- Y. Xu, L. Y. Hou and X. M. Zhang, Zinc tin oxide thin films prepared by MOCVD with different Sn/Zn ratios, Rare Met., 2017, 36, 753–757, DOI:10.1007/s12598-015-0583-5.
- F. W. J. Li, Y. Qi and W. D. Y. Guo, Characteristics of the Structure and Properties of ZnSnO3 Films by Varying the Magnetron Sputtering Parameters, Acta Metall. Sin. (Engl. Lett.), 2016, 29, 827–833, DOI:10.1007/s40195-016-0458-2.
- G. Yang and S. J. Park, Conventional and microwave hydrothermal synthesis and application of functional materials: A review, Materials, 2019, 12 DOI:10.3390/ma12071177.
- A. Annamalai, D. Carvalho, K. C. Wilson and M. J. Lee, Properties of hydrothermally synthesized Zn2SnO4 nanoparticles using Na2CO3 as a novel mineralizer, Mater. Charact., 2010, 61, 873–881, DOI:10.1016/j.matchar.2010.05.011.
- X. Fu, X. Wang, J. Long, Z. Ding, T. Yan, G. Zhang, Z. Zhang, H. Lin and X. Fu, Hydrothermal synthesis, characterization, and photocatalytic properties of Zn2SnO4, J. Solid State Chem., 2009, 182, 517–524, DOI:10.1016/j.jssc.2008.11.029.
- X. Ji, X. Huang, J. Liu, J. Jiang, X. Li, R. Ding, Y. Hu, F. Wu and Q. Li, Hydrothermal synthesis of novel Zn2SnO4 octahedron microstructures assemblec with hexagon nanoplates, J. Alloys Compd., 2010, 503 DOI:10.1016/j.jallcom.2009.12.038.
- J. Zeng, M. Di Xin, K. W. Li, H. Wang, H. Yan and W. Zhang, Transformation process and photocatalytic activities of hydrothermally synthesized Zn2SnO4 nanocrystals, J. Phys. Chem. C, 2008, 112, 4159–4167, DOI:10.1021/jp7113797.
- M. Ben Ali, F. Barka-Bouaifel, H. Elhouichet, B. Sieber, A. Addad, L. Boussekey, M. Férid and R. Boukherroub, Hydrothermal synthesis, phase structure, optical and photocatalytic properties of Zn2SnO4 nanoparticles, J. Colloid Interface Sci., 2015, 457, 360–369, DOI:10.1016/j.jcis.2015.07.015.
- L. Zhang, X. Zhang, Y. Zou, Y. H. Xu, C. L. Pan, J. S. Hu and C. M. Hou, Hydrothermal synthesis, influencing factors and excellent photocatalytic performance of novel nanoparticle-assembled Bi25FeO40 tetrahedrons, CrystEngComm, 2015, 17, 6527–6537, 10.1039/c5ce00743g.
- Y. Li, Y. L. Lu, K. Di Wu, D. Z. Zhang, M. Debliquy and C. Zhang, Microwave-assisted hydrothermal synthesis of copper oxide-based gas-sensitive nanostructures, Rare Met., 2021, 40, 1477–1493, DOI:10.1007/s12598-020-01557-4.
- F. Majid, J. Rauf, S. Ata, I. Bibi, A. Malik, S. M. Ibrahim, A. Ali and M. Iqbal, Synthesis and characterization of NiFe2O4 ferrite: Sol–gel and hydrothermal synthesis routes effect on magnetic, structural and dielectric characteristics, Mater. Chem. Phys., 2021, 258 DOI:10.1016/j.matchemphys.2020.123888.
- Y. Wang, Y. J. Hu, X. Hao, P. Peng, J. Y. Shi, F. Peng and R. C. Sun, Hydrothermal synthesis and applications of advanced carbonaceous materials from biomass: a review, Adv. Compos. Hybrid Mater., 2020, 3, 267–284, DOI:10.1007/s42114-020-00158-0.
- Y. Wang, P. Gao, D. Bao, L. Wang, Y. Chen, X. Zhou, P. Yang, S. Sun and M. Zhang, One pot, two phases: Individual orthorhombic and face-centered cubic ZnSnO3 obtained synchronously in one solution, Inorg. Chem., 2014, 53, 12289–12296, DOI:10.1021/ic5014126.
- C. H. Chou, S. Y. Lee and K. S. Chang, High-density ZnSnO3 nanowire arrays fabricated using single-step hydrothermal synthesis, J. Am. Ceram. Soc., 2020, 103, 4129–4139, DOI:10.1111/jace.17100.
- G. Ma, R. Zou, L. Jiang, Z. Zhang, Y. Xue, L. Yu, G. Song, W. Li and J. Hu, Phase-controlled synthesis and gas-sensing properties of zinc stannate (ZnSnO3 and Zn2SnO4) faceted solid and hollow microcrystals, CrystEngComm, 2012, 2172–2179, 10.1039/c2ce06272k.
- H. Zhong, T. Mirkovic and G. D. Scholes, Nanocrystal Synthesis, Compr. Nanosci. Nanotechnol., 2011, 1–5, DOI:10.1016/B978-0-12-374396-1.00051-9.
- Y. Chen, L. Yu, Q. Li, Y. Wu, Q. Li and T. Wang, An evolution from 3D face-centered-cubic ZnSnO3 nanocubes to 2D orthorhombic ZnSnO3 nanosheets with excellent gas sensing performance, Nanotechnology, 2012, 23, 415501, DOI:10.1088/0957-4484/23/41/415501.
- Y. Chen, L. Yu, Q. Li, Y. Wu and Q. Li, An evolution from 3D face-centered-cubic ZnSnO3 nanocubes to 2D orthorhombic ZnSnO3 nanosheets with excellent gas sensing performance, Nanotechnology, 2012, 23, 415501 CrossRef PubMed.
- B. C. Dave and S. B. Lockwood, Sol-Gel Method, Encyclopedia of Nanotechnology, 2012, pp. 2459–2470, DOI:10.1007/978-90-481-9751-4_359.
- E. Yilmaz and M. Soylak, Functionalized nanomaterials for sample preparation methods, in Handbook of Nanomaterials in Analytical Chemistry: Modern Trends in Analysis, Elsevier, 2019, pp. 375–413, DOI:10.1016/B978-0-12-816699-4.00015-3.
- S. Sakka, Sol-Gel Process and Applications, in Handbook of Advanced Ceramics: Materials, Applications, Processing, and Properties, 2nd edn, Elsevier Inc., 2013, pp. 883–910, DOI:10.1016/B978-0-12-385469-8.00048-4.
- J. Chen, W. Luo, S. Yu, X. Yang, Z. Wu, H. Zhang, J. Gao, Y. W. Mai, Y. Li and Y. Jia, Synergistic effect of photocatalysis and pyrocatalysis of pyroelectric ZnSnO3 nanoparticles for dye degradation, Ceram. Int., 2020, 46, 9786–9793, DOI:10.1016/j.ceramint.2019.12.251.
- T. A. Para, H. A. Reshi and V. Shelke, Synthesis of ZnSnO3 nanostructure by sol gel method, AIP Conf. Proc., 2016, 1731, 1–4, DOI:10.1063/1.4947656.
- B. Li, X. Li, J. Zai and X. Qian, Facile Synthesis of Porous Zn–Sn–O Nanocubes and Their Electrochemical Performances, Nanomicro Lett., 2016, 8, 174–181, DOI:10.1007/s40820-015-0075-z.
- B. Geng, C. Fang, F. Zhan and N. Yu, Synthesis of polyhedral ZnSnO3 microcrystals with controlled exposed facets and their selective gas-sensing properties, Small, 2008, 4, 1337–1343, DOI:10.1002/smll.200701177.
- F. Beshkar, O. Amiri and Z. Salehi, Synthesis of ZnSnO3 nanostructures by using novel gelling agents and their application in degradation of textile dye, Sep. Purif. Technol., 2017, 184, 66–71, DOI:10.1016/j.seppur.2017.04.024.
- T. A. Para, H. A. Reshi and V. Shelke, Synthesis of ZnSnO3 nanostructure by sol gel method, in AIP Conf Proc, American Institute of Physics Inc., 2016, DOI:10.1063/1.4947656.
- R. S. Wagner and W. C. Ellis, Vapor-liquid-solid mechanism of single crystal growth, Appl. Phys. Lett., 1964, 4, 89–90, DOI:10.1063/1.1753975.
- Z. Zhu, M. Suzuki, K. Nagashima, H. Yoshida, M. Kanai, G. Meng, H. Anzai, F. Zhuge, Y. He, M. Boudot, S. Takeda and T. Yanagida, Rational Concept for Reducing Growth Temperature in Vapor-Liquid-Solid Process of Metal Oxide Nanowires, Nano Lett., 2016, 16, 7495–7502, DOI:10.1021/acs.nanolett.6b03227.
- S. Li, Y. C. Lin, X. Y. Liu, Z. Hu, J. Wu, H. Nakajima, S. Liu, T. Okazaki, W. Chen, T. Minari, Y. Sakuma, K. Tsukagoshi, K. Suenaga, T. Taniguchi and M. Osada, Wafer-scale and deterministic patterned growth of monolayer MoS2 via vapor-liquid-solid method, Nanoscale, 2019, 11, 16122–16129, 10.1039/c9nr04612g.
- T. Haffner, M. Zeghouane, F. Bassani, P. Gentile, A. Gassenq, F. Chouchane, N. Pauc, E. Martinez, E. Robin, S. David, T. Baron and B. Salem, Growth of Ge1−xSnx Nanowires by Chemical Vapor Deposition via Vapor–Liquid–Solid Mechanism Using GeH4 and SnCl4, Phys. Status Solidi A, 2018, 215, DOI:10.1002/pssa.201700743.
- C. W. Pinion, J. D. Christesen and J. F. Cahoon, Understanding the vapor-liquid-solid mechanism of Si nanowire growth and doping to synthetically encode precise nanoscale morphology, J. Mater. Chem. C, 2016, 4, 3890–3897, 10.1039/c5tc03898g.
- H. Anzai, M. Suzuki, K. Nagashima, M. Kanai, Z. Zhu, Y. He, M. Boudot, G. Zhang, T. Takahashi, K. Kanemoto, T. Seki, N. Shibata and T. Yanagida, True Vapor-Liquid-Solid Process Suppresses Unintentional Carrier Doping of Single Crystalline Metal Oxide Nanowires, Nano Lett., 2017, 17, 4698–4705, DOI:10.1021/acs.nanolett.7b01362.
- X. Xue, Z. Zhou, B. Peng, M. M. Zhu, Y. J. Zhang, W. Ren, Z. G. Ye, X. Chen and M. Liu, Review on nanomaterials synthesized by vapor transport method: growth and their related applications, RSC Adv., 2015, 5, 79249–79263, 10.1039/c5ra13349a.
- T. Zhang, T. Zhang, R. Zhang, J. Deng, G. Lu and L. Wang, Highly sensitive sensing platform based on ZnSnO3 hollow cubes for detection of ethanol, Appl. Surf. Sci., 2017, 400, 262–268, DOI:10.1016/j.apsusc.2016.12.183.
- P. T. Lan Huong, N. Tu, H. Lan, L. H. Thang, N. Van Quy, P. A. Tuan, N. X. Dinh, V. N. Phan and A. T. Le, Functional manganese ferrite/graphene oxide nanocomposites: Effects of graphene oxide on the adsorption mechanisms of organic MB dye and inorganic As(v) ions from aqueous solution, RSC Adv., 2018, 8, 12376–12389, 10.1039/c8ra00270c.
- J. Zhang, L. Fan, J. Li, X. Liu, R. Wang, L. Wang and G. Tu, Growth mechanism of CsPbBr3 perovskite nanocrystals by a co-precipitation method in a CSTR system, Nano Res., 2019, 12, 121–127, DOI:10.1007/s12274-018-2190-x.
- R. Jiang, Y. Wang, C. Gao, A. Li, Y. Liu, D. Li and J. Zhang, Hollow ZnSnO3 cubes@carbon/reduced graphene oxide ternary composite as anode of lithium ion batteries with enhanced electrochemical performance, Ceram. Int., 2017, 43, 11556–11562, DOI:10.1016/j.ceramint.2017.05.031.
- S. Dong, L. Cui, W. Zhang, L. Xia, S. Zhou, C. K. Russell, M. Fan, J. Feng and J. Sun, Double-shelled ZnSnO3 hollow cubes for efficient photocatalytic degradation of antibiotic wastewater, Chem. Eng. J., 2020, 384, 123279, DOI:10.1016/j.cej.2019.123279.
- H. Dong and G. M. Koenig, A review on synthesis and engineering of crystal precursors produced: Via coprecipitation for multicomponent lithium-ion battery cathode materials, CrystEngComm, 2020, 22, 1514–1530, 10.1039/c9ce00679f.
- A. V. Rane, K. Kanny, V. K. Abitha, S. Thomas and S. Thomas, Methods for Synthesis of Nanoparticles and Fabrication of Nanocomposites, in Synthesis of Inorganic Nanomaterials: Advances and Key Technologies, Elsevier, 2018, pp. 121–139, DOI:10.1016/B978-0-08-101975-7.00005-1.
- N. S. Bajaj and R. A. Joshi, Energy materials: synthesis and characterization techniques, Energy Mater., 2021, 61–82, DOI:10.1016/B978-0-12-823710-6.00019-4.
- I. V. Tudose, F. Comanescu, P. Pascariu, S. Bucur, L. Rusen, F. Iacomi, E. Koudoumas and M. P. Suchea, Chemical and physical methods for multifunctional nanostructured interface fabrication, Functional Nanostructured Interfaces for Environmental and Biomedical Applications, Micro Nano Technol., 2019, 15–26, DOI:10.1016/B978-0-12-814401-5.00002-5.
- V. S. Kavarthapu, S. A. Graham, P. Manchi, M. V. Paranjape and J. S. Yu, Electrospun ZnSnO3/PVDF-HFP Nanofibrous Triboelectric Films for Efficient Mechanical Energy Harvesting, Adv. Fiber Mater., 2023 DOI:10.1007/s42765-023-00295-3.
- Z. Li, Q. Li, X. Liu, C. Yang and Y. Zhou, Simultaneous photocatalytic removal of heavy metal and organic dye over nitrogen and sulfur co-doped hierarchical ZnSnO3/Zn2SnO4 hollow octahedrons, Mater. Res. Bull., 2022, 156 DOI:10.1016/j.materresbull.2022.111980.
- Z. Li, Y. Xiong, D. Bi, Q. Liu, C. Yang and J. Zhang, Continuously improved gas-sensing performance of Zn2SnO4 porous octahedrons by structure evolution and further ZnSnO3 nanosheets decoration, J. Alloys Compd., 2022, 901 DOI:10.1016/j.jallcom.2022.163744.
- T. Zhou, T. Zhang, R. Zhang, Z. Lou, J. Deng and L. Wang, Hollow ZnSnO3 Cubes with Controllable Shells Enabling Highly Efficient Chemical Sensing Detection of Formaldehyde Vapors, ACS Appl. Mater. Interfaces, 2017, 9, 14525–14533, DOI:10.1021/acsami.7b03112.
- L. P. Wang, Y. Zhao, C. Wei, C. Wong, M. Srinivasan and Z. J. Xu, Polycrystalline zinc stannate as an anode material for sodium-ion batteries, J. Mater. Chem. A, 2015, 3, 14033–14038, 10.1039/c5ta02734a.
- H. Fan, Y. Zeng, X. Xu, N. Lv and T. Zhang, Hydrothermal synthesis of hollow ZnSnO3 microspheres and sensing properties toward butane, Sens. Actuators, B, 2011, 153, 170–175, DOI:10.1016/j.snb.2010.10.026.
- Y. Bing, Y. Zeng, C. Liu, L. Qiao, Y. Sui, B. Zou, W. Zheng and G. Zou, Assembly of hierarchical ZnSnO3 hollow microspheres from ultra-thin nanorods and the enhanced ethanol-sensing performances, Sens. Actuators, B, 2014, 190, 370–377, DOI:10.1016/j.snb.2013.08.015.
- Y. Zeng, K. Zhang, X. Wang, Y. Sui, B. Zou, W. Zheng and G. Zou, Rapid and selective H2S detection of hierarchical ZnSnO 3 nanocages, Sens. Actuators, B, 2011, 159, 245–250, DOI:10.1016/j.snb.2011.06.080.
- T. Zhou, T. Zhang, R. Zhang, Z. Lou, J. Deng and L. Wang, Hollow ZnSnO3 Cubes with Controllable Shells Enabling Highly Efficient Chemical Sensing Detection of Formaldehyde Vapors, ACS Appl. Mater. Interfaces, 2017, 9, 14525–14533, DOI:10.1021/acsami.7b03112.
- A. V. Borhade and Y. R. Baste, Study of photocatalytic asset of the ZnSnO3 synthesized by green chemistry, Arabian J. Chem., 2017, 10, S404–S411, DOI:10.1016/j.arabjc.2012.10.001.
- P. Song, Q. Wang and Z. Yang, Biomorphic synthesis of ZnSnO3 hollow fibers for gas sensing application, Sens. Actuators, B, 2011, 156, 983–989, DOI:10.1016/j.snb.2011.03.017.
- S. Dong, J. Sun, Y. Li, C. Yu, Y. Li and J. Sun, ZnSnO3 hollow nanospheres/reduced graphene oxide nanocomposites as high-performance photocatalysts for degradation of metronidazole, Appl. Catal., B, 2014, 144, 386–393, DOI:10.1016/j.apcatb.2013.07.043.
- T. Zhou, T. Zhang, R. Zhang, Z. Lou, J. Deng and L. Wang, Hollow ZnSnO3 Cubes with Controllable Shells Enabling Highly Efficient Chemical Sensing Detection of Formaldehyde Vapors, ACS Appl. Mater. Interfaces, 2017, 9, 14525–14533, DOI:10.1021/acsami.7b03112.
- J. F. Duan, S. C. Hou, S. G. Chen and H. G. Duan, Synthesis of amorphous ZnSnO3 hollow nanoboxes and their lithium storage properties, Mater. Lett., 2014, 122, 261–264, DOI:10.1016/j.matlet.2014.02.060.
- L. Xu, W. Kuang, M. Lai, J. Miao, L. Zhang and R. Zhang, Preparation of Sn-Zn-O thin film for its potential applications in photodegradation of organic dyes, Mater. Lett., 2018, 233, 42–46, DOI:10.1016/j.matlet.2018.08.142.
- Y. Zeng, T. Zhang, H. Fan, G. Lu and M. Kang, Synthesis and gas-sensing properties of ZnSnO3 cubic nanocages and nanoskeletons, Sens. Actuators, B, 2009, 143, 449–453, DOI:10.1016/j.snb.2009.07.021.
- J. Zheng, H. Hou, H. Fu, L. Gao and H. Liu, Size-controlled synthesis of porous ZnSnO3 nanocubes for improving formaldehyde gas sensitivity, RSC Adv., 2021, 11, 20268–20277, 10.1039/d1ra01852c.
- Y. T. Wang and K. S. Chang, Piezopotential-Induced Schottky Behavior of Zn1−xSnO3 Nanowire Arrays and Piezophotocatalytic Applications, J. Am. Ceram. Soc., 2016, 99, 2593–2600, DOI:10.1111/jace.14264.
- M. K. Lo, S. Y. Lee and K. S. Chang, Study of ZnSnO3-nanowire piezophotocatalyst using two-step hydrothermal synthesis, J. Phys. Chem. C, 2015, 119, 5218–5224, DOI:10.1021/acs.jpcc.5b00282.
- C. Liu, R. Röder, L. Zhang, Z. Ren, H. Chen, Z. Zhang, C. Ronning and P. X. Gao, Highly efficient visible-light driven photocatalysts: A case of zinc stannate based nanocrystal assemblies, J. Mater. Chem. A, 2014, 2, 4157–4167, 10.1039/c3ta14611a.
- I. Lee, N. Sung, K. Hwa and R. Conley, Characterization of zinc – tin – oxide films deposited by radio frequency magnetron sputtering at various substrate temperatures, Thin Solid Films, 2013, 548, 385–388, DOI:10.1016/j.tsf.2013.08.067.
- Y. Choi, S. J. Kang and H. Kim, Rapid thermal annealing effect on the characteristics of ZnSnO3 films prepared by RF magnetron sputtering, Curr. Appl. Phys., 2012, 12, 104–107, DOI:10.1016/j.cap.2012.05.014.
- C. B. Anucha, I. Altin, E. Bacaksiz, V. N. Stathopoulos, I. Polat, A. Yasar and Ö. F. Yüksel, Silver doped zinc stannate (Ag-ZnSnO3) for the photocatalytic degradation of caffeine under uv irradiation, Water, 2021, 13, 5–7, DOI:10.3390/w13091290.
- F. Guo, X. Huang, Z. Chen, H. Sun and W. Shi, Investigation of visible-light-driven photocatalytic tetracycline degradation via carbon dots modified porous ZnSnO3 cubes: Mechanism and degradation pathway, Sep. Purif. Technol., 2020, 253, 117518, DOI:10.1016/j.seppur.2020.117518.
- Y. Ma, R. Jiang, Y. Dong, Y. Liu and J. Zhang, Embedding ultrafine ZnSnO3 nanoparticles into reduced graphene oxide composites as high-performance electrodes for lithium ion batteries, Biomed. Mater., 2020, 0–16, DOI:10.1088/1361-6528/aab07e.
- Y. Wang, D. Li, Y. Liu and J. Zhang, Self-assembled 3D ZnSnO3 hollow cubes@reduced graphene oxide aerogels as high capacity anode materials for lithium-ion batteries, Electrochim. Acta, 2016, 203, 84–90, DOI:10.1016/j.electacta.2016.03.195.
- R. Guo, R. Tian, D. Shi, H. Li and H. Liu, S-Doped ZnSnO3 Nanoparticles with Narrow Band Gaps for Photocatalytic Wastewater Treatment, ACS Appl. Nano Mater., 2019, 2, 7755–7765, DOI:10.1021/acsanm.9b01804.
- S. Dong, L. Cui, W. Zhang, L. Xia, S. Zhou, C. K. Russell, M. Fan, J. Feng and J. Sun, Double-shelled ZnSnO3 hollow cubes for efficient photocatalytic degradation of antibiotic wastewater, Chem. Eng. J., 2020, 384, 123279, DOI:10.1016/j.cej.2019.123279.
- J. Chen, W. Luo, S. Yu, X. Yang, Z. Wu, H. Zhang, J. Gao, Y. W. Mai, Y. Li and Y. Jia, Synergistic effect of photocatalysis and pyrocatalysis of pyroelectric ZnSnO3 nanoparticles for dye degradation, Ceram. Int., 2020, 46, 9786–9793, DOI:10.1016/j.ceramint.2019.12.251.
- A. Biswas, S. Saha and N. R. Jana, ZnSnO3 Nanoparticle-Based Piezocatalysts for Ultrasound-Assisted Degradation of Organic Pollutants, ACS Appl. Nano Mater., 2019, 2, 1120–1128, DOI:10.1021/acsanm.9b00107.
- M. Najam Khan and J. Dutta, Comparison of photocatalytic activity of zinc stannate particles and zinc stannate/zinc oxide composites for the removal of phenol from water, and a study on the effect of pH on photocatalytic efficiency, Mater. Sci. Semicond. Process., 2015, 36, 124–133, DOI:10.1016/j.mssp.2015.03.011.
- S. N. Habisreutinger, L. Schmidt-Mende and J. K. Stolarczyk, Photocatalytic reduction of CO2 on TiO2 and other semiconductors, Angew. Chem., Int. Ed., 2013, 52, 7372–7408, DOI:10.1002/anie.201207199.
- G. Venkatesh, N. Elavarasan, M. Srinivasan, G. Palanisamy, R. R. Macadangdang, S. Vignesh, P. Ramasamy, H. E. Ali, M. Shkir and Z. Ahmad, Z-scheme heterojunction ZnSnO3/rGO/MoS2 nanocomposite for excellent photocatalytic activity towards mixed dye degradation, Int. J. Hydrogen Energy, 2022, 47, 11863–11876, DOI:10.1016/j.ijhydene.2022.01.240.
- S. Dong, L. Xia, X. Chen, L. Cui, W. Zhu, Z. Lu, J. Sun and M. Fan, Interfacial and electronic band structure optimization for the adsorption and visible-light photocatalytic activity of macroscopic ZnSnO3/graphene aerogel, Composites, Part B, 2021, 215, 108765, DOI:10.1016/j.compositesb.2021.108765.
- F. Chen, Q. Yang, C. Niu, X. Li, C. Zhang, J. Zhao, Q. Xu, Y. Zhong, Y. Deng and G. Zeng, Enhanced visible light photocatalytic activity and mechanism of ZnSn(OH)6 nanocubes modified with AgI nanoparticles, Catal. Commun., 2016, 73, 1–6, DOI:10.1016/j.catcom.2015.10.003.
- X. Huang, F. Guo, M. Li, H. Ren, Y. Shi and L. Chen, Hydrothermal synthesis of ZnSnO3 nanoparticles decorated on g-C3N4 nanosheets for accelerated photocatalytic degradation of tetracycline under the visible-light irradiation, Sep. Purif. Technol., 2020, 230, 115854, DOI:10.1016/j.seppur.2019.115854.
- X. Zhu, F. Guo, J. Pan, H. Sun, L. Gao, J. Deng, X. Zhu and W. Shi, Fabrication of visible-light-response face-contact ZnSnO3@g-C3N4 core–shell heterojunction for highly efficient photocatalytic degradation of tetracycline contaminant and mechanism insight, J. Mater. Sci., 2021, 56, 4366–4379, DOI:10.1007/s10853-020-05542-1.
- F. Guo, X. Huang, Z. Chen, L. Cao, X. Cheng, L. Chen and W. Shi, Construction of Cu3P-ZnSnO3-g-C3N4 p-n-n heterojunction with multiple built-in electric fields for effectively boosting visible-light photocatalytic degradation of broad-spectrum antibiotics, Sep. Purif. Technol., 2021, 265, 118477, DOI:10.1016/j.seppur.2021.118477.
- Z. Chen, X. Chu, X. Huang, H. Sun, L. Chen and F. Guo, Fabrication of visible-light driven CoP/ZnSnO3 composite photocatalyst for high-efficient photodegradation of antibiotic pollutant, Sep. Purif. Technol., 2021, 257, 117900, DOI:10.1016/j.seppur.2020.117900.
- J. Pan, F. Guo, H. Sun, Y. Shi and W. Shi, Nanodiamonds anchored on porous ZnSnO3 cubes as an efficient composite photocatalyst with improved visible-light photocatalytic degradation of tetracycline, Sep. Purif. Technol., 2021, 263, 118398, DOI:10.1016/j.seppur.2021.118398.
- D. Kohl, Function and applications of gas sensors, 2001. http://iopscience.iop.org/0022-3727/34/19/201 Search PubMed.
- Z. Xiao, L. B. Kong, S. Ruan, X. Li, S. Yu, X. Li, Y. Jiang, Z. Yao, S. Ye, C. Wang, T. Zhang, K. Zhou and S. Li, Recent development in nanocarbon materials for gas sensor applications, Sens. Actuators, B, 2018, 274, 235–267, DOI:10.1016/j.snb.2018.07.040.
- S. Gupta Chatterjee, S. Chatterjee, A. K. Ray and A. K. Chakraborty, Graphene-metal oxide nanohybrids for toxic gas sensor: A review, Sens. Actuators, B, 2015, 221, 1170–1181, DOI:10.1016/j.snb.2015.07.070.
- S. S. Varghese, S. Lonkar, K. K. Singh, S. Swaminathan and A. Abdala, Recent advances in graphene based gas sensors, Sens. Actuators, B, 2015, 218, 160–183, DOI:10.1016/j.snb.2015.04.062.
- W. Guo, T. Liu, W. Yu, L. Huang, Y. Chen and Z. Wang, Rapid selective detection of formaldehyde by hollow ZnSnO3 nanocages, Phys. E, 2013, 48, 46–52, DOI:10.1016/j.physe.2012.11.021.
- L. Jiang, Z. Chen, Q. Cui, S. Xu and F. Tang, Experimental and DFT-D3 study of sensitivity and sensing mechanism of ZnSnO3 nanosheets to C3H6O gas, J. Mater. Sci., 2022, 57, 3231–3251, DOI:10.1007/s10853-021-06855-5.
- Y. Yin, F. Li, N. Zhang, S. Ruan, H. Zhang and Y. Chen, Improved gas sensing properties of silver-functionalized ZnSnO3 hollow nanocubes, Inorg. Chem. Front., 2018, 5, 2123–2131, 10.1039/c8qi00470f.
- M. ul Haq, Z. Zhang, X. Chen, N. Rahman, S. Khan, R. Khatoon, S. S. Hassan, Z. Ye and L. Zhu, A two-step synthesis of microsphere-decorated fibers based on NiO/ZnSnO3 composites towards superior ethanol sensitivity performance, J. Alloys Compd., 2019, 777, 73–83, DOI:10.1016/j.jallcom.2018.10.361.
- Q. Chen, Y. Wang, M. Wang, S. Ma, P. Wang, G. Zhang, W. Chen, H. Jiao, L. Liu and X. Xu, Enhanced acetone sensor based on Au functionalized In-doped ZnSnO3 nanofibers synthesized by electrospinning method, J. Colloid Interface Sci., 2019, 543, 285–299, DOI:10.1016/j.jcis.2019.02.055.
- X. Wang, B. Ding, Y. Liu, X. Zhu, H. Li, M. Xia, H. Fu and M. Li, Synthesis of 3D flower-like ZnSnO3 and improvement of ethanol-sensing properties at room temperature based on nano-TiO2 decoration and UV radiation, Sens. Actuators, B, 2018, 264, 119–127, DOI:10.1016/j.snb.2018.02.178.
- W. Guo, B. Zhao, M. Fu, C. Wang and R. Peng, One pot synthesis of hierarchical and porous ZnSnO3 nanocubes and gas sensing properties to formaldehyde, Results Phys., 2019, 15, 102606, DOI:10.1016/j.rinp.2019.102606.
- S. Yu, X. Jia, J. Yang, S. Wang, Y. Li and H. Song, Highly sensitive and low detection limit of ethanol gas sensor based on CeO2 nanodot-decorated ZnSnO3 hollow microspheres, Ceram. Int., 2022 DOI:10.1016/j.ceramint.2022.02.023.
- S. Yu, X. Jia, J. Yang, S. Wang, Y. Li and H. Song, Highly sensitive ethanol gas sensor based on CuO/ZnSnO3 heterojunction composites, Mater. Lett., 2021, 291, 129531, DOI:10.1016/j.matlet.2021.129531.
- Y. Yin, Y. Shen, S. Zhao, J. Bai, Y. Qi, C. Han and D. Wei, Effect of noble metal elements on ethanol sensing properties of ZnSnO3 nanocubes, J. Alloys Compd., 2021, 887, 161409, DOI:10.1016/j.jallcom.2021.161409.
- D. Wang, X. Pu, X. Yu, L. Bao, Y. Cheng, J. Xu, S. Han, Q. Ma and X. Wang, Controlled preparation and gas sensitive properties of two-dimensional and cubic structure ZnSnO3, J. Colloid Interface Sci., 2022, 608, 1074–1085, DOI:10.1016/j.jcis.2021.09.167.
- L. Du, Y. Li, Y. Tong and M. Zhang, Biotemplates based preparation of hierarchical ZnSnO3 porous nanostructures for fast detection of formaldehyde, Ceram. Int., 2021, 47, 13139–13146, DOI:10.1016/j.ceramint.2021.01.178.
- J. Huang, X. Xu, C. Gu, W. Wang, B. Geng, Y. Sun and J. Liu, Size-controlled synthesis of porous ZnSnO3 cubes and their gas-sensing and photocatalysis properties, Sens. Actuators, B, 2012, 171–172, 572–579, DOI:10.1016/j.snb.2012.05.036.
- L. Wang, T. Zhou, R. Zhang, Z. Lou, J. Deng and T. Zhang, Comparison of toluene sensing performances of zinc stannate with different morphology-based gas sensors, Sens. Actuators, B, 2016, 227, 448–455, DOI:10.1016/j.snb.2015.12.097.
- L. Jiang, K. Xue, Z. Chen, Q. Cui and S. Xu, Hydrothermal synthesis of ZnSnO3/rGO composite material and highly gas sensing performance to acetone, Mater. Sci. Semicond. Process., 2021, 134 DOI:10.1016/j.mssp.2021.106051.
- J. Zhang, X. Jia, D. Lian, J. Yang, S. Wang, Y. Li and H. Song, Enhanced selective acetone gas sensing performance by fabricating ZnSnO3/SnO2 concave microcube, Appl. Surf. Sci., 2021, 542 DOI:10.1016/j.apsusc.2020.148555.
- Q. Chen, S. Y. Ma, H. Y. Jiao, G. H. Zhang, H. Chen, X. L. Xu, H. M. Yang and Z. Qiang, Synthesis of novel ZnSnO3 hollow polyhedrons with open nanoholes: Enhanced acetone-sensing performance, Ceram. Int., 2017, 43, 1617–1621, DOI:10.1016/j.ceramint.2016.10.094.
- Z. Wang, J. Miao, H. Zhang, D. Wang and J. Sun, Hollow cubic ZnSnO3 with abundant oxygen vacancies for H2S gas sensing, J. Hazard. Mater., 2020, 391, 122226, DOI:10.1016/j.jhazmat.2020.122226.
- K. Liu, Z. Zheng, J. Xu and C. Zhang, Enhanced visible light-excited ZnSnO3 for room temperature ppm-level CO2 detection, J. Alloys Compd., 2022, 907, 164440, DOI:10.1016/j.jallcom.2022.164440.
- L. Du, D. Wang, K. Gu and M. Zhang, Construction of PdO-decorated double-shell ZnSnO3 hollow microspheres for: N-propanol detection at low temperature, Inorg. Chem. Front., 2021, 8, 787–795, 10.1039/d0qi01292k.
- G. Feng, Y. Che, C. Song, J. Xiao, X. Fan, S. Sun, G. Huang and Y. Ma, Morphology-controlled synthesis of ZnSnO3 hollow spheres and their n-butanol gas-sensing performance, Ceram. Int., 2021, 47, 2471–2482, DOI:10.1016/j.ceramint.2020.09.090.
- P. Shi, Z.-H. Fu, M.-Y. Zhou, X. Chen, N. Yao, L.-P. Hou, C.-Z. Zhao, B.-Q. Li, J.-Q. Huang, X.-Q. Zhang and Q. Zhang, Inhibiting intercrystalline reactions of anode with electrolytes for long-cycling lithium batteries, 2022. https://www.science.org Search PubMed.
- Z. Y. Zhang, Z. W. Li, Q. Luo, B. Z. Yang, Y. Liu, Y. Y. Hu, X. Bin Liu, Y. H. Yin, Y. S. Li and Z. P. Wu, Spontaneous nanominiaturization of silicon microparticles with structural stability as flexible anodes for lithium ion batteries, Carbon, 2022, 188, 238–245, DOI:10.1016/j.carbon.2021.11.059.
- X. Li, G. Guan, C. Yu, B. Cheng, X. Chen, K. Zhang and J. Xiang, Enhanced electrochemical performances based on ZnSnO3 microcubes functionalized in-doped carbon nanofibers as free-standing anode materials, Dalton Trans., 2023 10.1039/D3DT01642K.
- K. Wang, S. Zhang, Z. Hou, L. Wang, P. An, J. Jia, Y. Li and P. Zhang, Nanofibrous ZnSnO3/C composite derived from natural cellulose substance as an enhanced lithium-ion battery anode, Mater. Lett., 2023, 331 DOI:10.1016/j.matlet.2022.133435.
- Y. Chen, B. Qu, L. Mei, D. Lei, L. Chen, Q. Li and T. Wang, Synthesis of ZnSnO3 mesocrystals from regular cube-like to sheet-like structures and their comparative electrochemical properties in Li-ion batteries, J. Mater. Chem., 2012, 22, 25373–25379, 10.1039/c2jm33123c.
- X. Chen, Y. Huang, H. Huang, M. Wang and K. Wang, Silver-modified hollow ZnSnO3 boxes as high capacity anode materials for Li-ion batteries, Mater. Lett., 2015, 149, 33–36, DOI:10.1016/j.matlet.2015.02.060.
- Q. Xie, Y. Ma, X. Zhang, H. Guo, A. Lu, L. Wang, G. Yue and D. L. Peng, Synthesis of amorphous ZnSnO3-C hollow microcubes as advanced anode materials for lithium ion batteries, Electrochim. Acta, 2014, 141, 374–383, DOI:10.1016/j.electacta.2014.07.095.
- Y. Ma, R. Jiang, D. Li, Y. Dong, Y. Liu and J. Zhang, Embedding ultrafine ZnSnO3 nanoparticles into reduced graphene oxide composites as high-performance electrodes for lithium ion batteries, Nanotechnology, 2018, 29 DOI:10.1088/1361-6528/aab07e.
- P. Luo, H. Zhang, L. Liu, L. Fang and Y. Wang, Sandwich-like nanostructure of amorphous ZnSnO3 encapsulated in carbon nanosheets for enhanced lithium storage, Electrochim. Acta, 2016, 219, 734–741, DOI:10.1016/j.electacta.2016.10.085.
- Y. Ma, Q. Xie, X. Liu, Y. Zhao, D. Zeng, L. Wang, Y. Zheng and D. L. Peng, Synthesis of amorphous ZnSnO3 double-shell hollow microcubes as advanced anode materials for lithium ion batteries, Electrochim. Acta, 2015, 182, 327–333, DOI:10.1016/j.electacta.2015.09.102.
- F. Han, W. Li, C. Lei, B. He, K. Oshida and A. Lu, Selective Formation of Carbon-Coated, Metastable Amorphous ZnSnO3 Nanocubes Containing Mesopores for Use as High-Capacity Lithium-Ion Battery, Small, 2014, 10, 1–8, DOI:10.1002/smll.201400371.
- H. Tan, H. W. Cho and J. J. Wu, Binder-free ZnO@ZnSnO3 quantum dots core-shell nanorod array anodes for lithium-ion batteries, J. Power Sources, 2018, 388, 11–18, DOI:10.1016/j.jpowsour.2018.03.066.
- Y. L. Qin, F. F. Zhang, X. C. Du, G. Huang, Y. C. Liu and L. M. Wang, Controllable synthesis of cube-like ZnSnO3@TiO2 nanostructures as lithium ion battery anodes, J. Mater. Chem. A, 2015, 3, 2985–2990, 10.1039/c4ta06055e.
- C. K. Sim, S. R. Majid and N. Z. Mahmood, ZnSnO3/mesoporous biocarbon composite towards sustainable electrode material for energy storage device, Microchem. J., 2021, 164, 105968, DOI:10.1016/j.microc.2021.105968.
- Y. Wang, D. Li, Y. Liu and J. Zhang, Fabrication of novel rugby-like ZnSnO3/reduced graphene oxide composites as a high-performance anode material for lithium-ion batteries, Mater. Lett., 2016, 167, 222–225, DOI:10.1016/j.matlet.2015.12.107.
- M. Jiang, J. Wu, H. Guo, H. Zhang, M. Zhu and X. Xu, Controlled synthesis of cube-like ZnSnO3 decorated by nickel-based films and electrochemical applications on lithium-sulfur batteries, Compos. Interfaces, 2022, 1–12, DOI:10.1080/09276440.2022.2044108.
- Q. Xie, Y. Ma, X. Zhang, H. Guo, A. Lu, L. Wang, G. Yue and D. L. Peng, Synthesis of amorphous ZnSnO3-C hollow microcubes as advanced anode materials for lithium ion batteries, Electrochim. Acta, 2014, 141, 374–383, DOI:10.1016/j.electacta.2014.07.095.
- R. Jiang, Y. Wang, C. Gao, A. Li, Y. Liu, D. Li and J. Zhang, Hollow ZnSnO3 cubes@carbon/reduced graphene oxide ternary composite as anode of lithium ion batteries with enhanced electrochemical performance, Ceram. Int., 2017, 43, 11556–11562, DOI:10.1016/j.ceramint.2017.05.031.
- X. Chen, Y. Huang, H. Huang, M. Wang and K. Wang, Silver-modified hollow ZnSnO3 boxes as high capacity anode materials for Li-ion batteries, Mater. Lett., 2015, 149, 33–36, DOI:10.1016/j.matlet.2015.02.060.
- A. Rovisco, A. Dos Santos, T. Cramer, J. Martins, R. Branquinho, H. Águas, B. Fraboni, E. Fortunato, R. Martins, R. Igreja and P. Barquinha, Piezoelectricity Enhancement of Nanogenerators Based on PDMS and ZnSnO3 Nanowires through Microstructuration, ACS Appl. Mater. Interfaces, 2020, 12, 18421–18430, DOI:10.1021/acsami.9b21636.
- Z. L. Wang and J. Song, Piezoelectric nanogenerators based on zinc oxide nanowire arrays, Science, 2006, 312, 242–246, DOI:10.1126/science.1124005.
- R. Guo, Y. Guo, H. Duan, H. Li and H. Liu, Synthesis of Orthorhombic Perovskite-Type ZnSnO3 Single-Crystal Nanoplates and Their Application in Energy Harvesting, ACS Appl. Mater. Interfaces, 2017, 9, 8271–8279, DOI:10.1021/acsami.6b16629.
- C. Cheng, U. J. Jung, W. Heo, W. Park and J. Park, Enhanced piezoelectric performance of ZnSnO3@PVDF composite films by control of embedded contents of ZnSnO3 nanoparticles, Surf. Interfaces, 2023, 41 DOI:10.1016/j.surfin.2023.103177.
- Y. Fu, Y. Nie, Y. Zhao, P. Wang, L. Xing, Y. Zhang and X. Xue, Detecting liquefied petroleum gas (LPG) at room temperature using ZnSnO3/ZnO nanowire piezo-nanogenerator as self-powered gas sensor, ACS Appl. Mater. Interfaces, 2015, 7, 10482–10490, DOI:10.1021/acsami.5b01822.
- J. M. Wu, K. H. Chen, Y. Zhang and Z. L. Wang, A self-powered piezotronic strain sensor based on single ZnSnO3 microbelts, RSC Adv., 2013, 3, 25184–25189, 10.1039/c3ra45027a.
- A. Datta, D. Mukherjee, C. Kons, S. Witanachchi and P. Mukherjee, Evidence of superior ferroelectricity in structurally welded ZnSnO3 nanowire arrays, Small, 2014, 10, 4093–4099, DOI:10.1002/smll.201401249.
- S. M. A. Z. Shawon, Z. D. Carballo, V. S. Vega, C. Lin, M. S. Rafaqut, A. X. Sun, J. J. Li and M. J. Uddin, Surface modified hybrid ZnSnO3 nanocubes for enhanced piezoelectric power generation and wireless sensory application, Nano Energy, 2022, 92, 106653, DOI:10.1016/j.nanoen.2021.106653.
- P. Manchi, S. A. Graham, H. Patnam, M. V. Paranjape and J. S. Yu, rGO-ZnSnO3 Nanostructure-Embedded Triboelectric Polymer-Based Hybridized Nanogenerators, Adv. Mater. Technol., 2022, 2101460, DOI:10.1002/admt.202101460.
- K. Y. Lee, D. Kim, J. H. Lee, T. Y. Kim, M. K. Gupta and S. W. Kim, Unidirectional high-power generation via stress-induced dipole alignment from ZnSnO3 nanocubes/polymer hybrid piezoelectric nanogenerator, Adv. Funct. Mater., 2014, 24, 37–43, DOI:10.1002/adfm.201301379.
- S. Paria, S. K. Karan, R. Bera, A. K. Das, A. Maitra and B. B. Khatua, A Facile Approach to Develop a Highly Stretchable PVC/ZnSnO3 Piezoelectric Nanogenerator with High Output Power Generation for Powering Portable Electronic Devices, Ind. Eng. Chem. Res., 2016, 55, 10671–10680, DOI:10.1021/acs.iecr.6b02172.
- S. Paria, S. Ojha, S. K. Karan, S. K. Si, R. Bera, A. K. Das, A. Maitra, L. Halder, A. De and B. B. Khatua, Approach for Enhancement in Output Performance of Randomly Oriented ZnSnO3 Nanorod-Based Piezoelectric Nanogenerator via p–n Heterojunction and Surface Passivation Layer, ACS Appl. Electron. Mater., 2020, 2, 2565–2578, DOI:10.1021/acsaelm.0c00467.
- S. N. Habisreutinger, L. Schmidt-Mende and J. K. Stolarczyk, Photocatalytic reduction of CO2 on TiO2 and other semiconductors, Angew. Chem., Int. Ed., 2013, 52, 7372–7408, DOI:10.1002/anie.201207199.
- D. Lian, B. Shi, R. Dai, X. Jia and X. Wu, Synthesis and enhanced acetone gas-sensing performance of ZnSnO3/SnO2 hollow urchin nanostructures, J. Nanopart. Res., 2017, 19 DOI:10.1007/s11051-017-4094-1.
| This journal is © The Royal Society of Chemistry 2023 |