Iron-catalyzed cascade C–C/C–O bond formation of 2,4-dienals with donor–acceptor cyclopropanes: access to functionalized hexahydrocyclopentapyrans†
Manmath
Mishra
,
Kshitiz
Verma
 ,
Sonbidya
Banerjee
and
Tharmalingam
Punniyamurthy
,
Sonbidya
Banerjee
and
Tharmalingam
Punniyamurthy
 *
*
Department of Chemistry, Indian Institute of Technology Guwahati, Guwahati 781039, India. E-mail: tpunni@iitg.ac.in
First published on 9th February 2024
Abstract
Iron-catalyzed cascade C–C and C–O bond formation of 2,4-dienals with donor–acceptor cyclopropanes (DACs) has been developed to furnish hexahydrocyclopentapyrans. Optically active DACs can be coupled stereospecifically (>97% ee). Chirality transfer, use of iron-catalysis and substrate scope are the salient practical features.
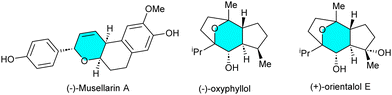
Pyrans are the structural constituents of a broad spectrum of natural products, exhibiting interesting biological and medicinal properties (Fig. 1).1 The development of effective synthetic methods for the construction of these structural scaffolds would thus be valuable.2 Cascade C–C and C-heteroatom bond formation represents a powerful synthetic tool for the conversion of simple substrates into complex molecules with structural diversity.3 In this context, α,β,γ,δ-unsaturated aldehydes allow the construction of C–C and C–O bonds, leaving the unsaturated C
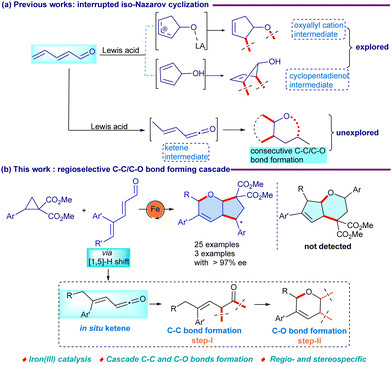
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C for further modifications.4 Precisely, 2,4-dienals in the presence of Lewis acid can convert into an oxyallyl cation, which can be trapped by suitable carbon or heteroatom nucleophiles in an interrupted iso-Nazarov process to construct valuable organic parallels (Scheme 1a).5 In addition, 4π-conrotatory cyclization of 2,4-dienals may give 1,3-dipolar species that can be explored in a cascade fashion as an effective 1,3-synthon. Despite these advances, selective coupling at the C2–C3 backbone is quite challenging due to the competing 1,2- and 1,4-additions in 2,4-dienal.6 Furthermore, DAC has emerged as a versatile building block for the construction of five-membered cyclic scaffolds.7,8
C for further modifications.4 Precisely, 2,4-dienals in the presence of Lewis acid can convert into an oxyallyl cation, which can be trapped by suitable carbon or heteroatom nucleophiles in an interrupted iso-Nazarov process to construct valuable organic parallels (Scheme 1a).5 In addition, 4π-conrotatory cyclization of 2,4-dienals may give 1,3-dipolar species that can be explored in a cascade fashion as an effective 1,3-synthon. Despite these advances, selective coupling at the C2–C3 backbone is quite challenging due to the competing 1,2- and 1,4-additions in 2,4-dienal.6 Furthermore, DAC has emerged as a versatile building block for the construction of five-membered cyclic scaffolds.7,8
The cycloaddition of aldehydes with DAC has been achieved using Sn(OTf)2-catalysis to provide tetrahydrofurans.8c Later, a stoichiometric amount of FeCl3 was used for the reaction of heterocumulenes with DACs to provide 2-pyrrolidines, where the chirality transfer was not consistently observed.8d Recently, the coupling of ketenes with DAC has been shown utilizing InBr3–EtAlCl2 dual catalysis to yield cyclopentanones.8f Herein, we present an iron-catalyzed stereospecific cascade C–C and C–O bond formation of 2,4-dienal with DAC to give functionalized hexahydrocyclopentapyran derivatives (Scheme 1b). Excellent chirality transfer, use of iron-catalysis, cascade C–C and C–O bond formation for the construction of the bicyclic ethers and substrate scope are the important practical features.
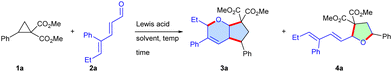
First, we commenced the optimization studies with dimethyl 2-phenylcyclopropane-1,1-dicarboxylate 1a and 4-phenylhepta-2,4-dienal 2a as the model substrates (Table 1 and Table S1, ESI†). To our delight, the coupling occurred to furnish the cyclic scaffolds 3a and 4a in 31% and 27% yields, respectively, when the substrates were stirred with 10 mol% FeCl3 in 1,2-dichloroethane for 4 h at room temperature. Subsequent screening of the solvent, quantity (20 mol%) of Lewis acid and temperature led to the production of 3a in 78% yield along with a trace amount of 4a in toluene at 60 °C, whereas THF, CH3CN and HFIP afforded a mixture of 3a and 4a in moderate yields. Lewis acids such as Sc(OTf)3, Yb(OTf)3, Cu(OTf)2, Zn(OTf)2 and CoCl2 yielded inferior outcomes. A control experiment confirmed that in the absence of the Lewis acid, the formation of the cycloadduct was unsuccessful.
| Entry | Lewis acid | Solvent | Yieldb | |
|---|---|---|---|---|
| 3a | 4a | |||
| a Reaction conditions: 1a (0.1 mmol), 2a (0.12 mmol), Lewis acid (10 mol%), solvent (1.5 mL), 4 h, room temperature. b Isolated yield. c FeCl3 (0.5 equiv). d FeCl3 (20 mol%). e FeCl3 (20 mol%), 60 °C, 2 h. f FeCl3 (20 mol%), 80 °C, 2 h.n.d. = not detected. | ||||
| 1 | FeCl3 | (CH2Cl)2 | 31 | 27 |
| 2c | FeCl3 | Toluene | 45 | 52 |
| 3d | FeCl3 | Toluene | 57 | 22 |
| 4e | FeCl3 | Toluene | 78 | Trace |
| 5f | FeCl3 | Toluene | 72 | Trace |
| 6 | — | Toluene | n.d. | n.d. |
Having optimized the reaction conditions, the scope of the procedure was examined, engaging a series of substituted DACs 1b–t with 2a as the standard substrate (Scheme 2). The 2-tolyl DAC 1b underwent reaction to furnish 3b in 72% yield, whereas 1c with an electron withdrawing 3-CF3 substituent delivered 3c in 76% yield. Furthermore, the 4-substituted DACs viz., methyl 1d, fluoro 1e, bromo 1f, tert-butyl 1g, nitro 1h and cyano 1i groups reacted to furnish the target scaffolds 3d–i in 64–78% yields, which suggested that electron donating and withdrawing groups were well compatible. In addition, 2-pyrenyl 1j and thienyl 1k substrates conveyed the target products 3j and 3k (X-ray, CCDC = 2298985, see ESI†) in 73% and 77% yields, respectively. Under these conditions, the ester functionality of the DACs was varied, and the linear diethyl variant 1l gave 3l in 72% yield, whereas bulkier iso-propyl 1m and benzyl 1n gave no desired cycloadduct due to the steric effect. Similarly, cyclohexyl bearing 1m was an unsuccessful substrate, which suggests that the electrophilicity of the cyclopropyl carbon is crucial for the coupling. Also, no cycloaddition was observed when the phenyl ring of DAC was altered with a heterocyclic 4-pyridyl 1p, indicating the complexation of the catalyst with the active N-atom of pyridyl. However, the natural product-derived DACs such as (±)-α-tocopherol 1q and cholesterol 1r successfully reacted to produce the scaffolds 3q and 3r in 77% and 76% yields, respectively. Moreover, terpenoid derived DACs 1s and 1t underwent coupling to afford the bicyclic ethers 3s (d.r. 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.45) and 3t (d.r. 1
0.45) and 3t (d.r. 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.35) in 80% and 81% yields, respectively.
0.35) in 80% and 81% yields, respectively.
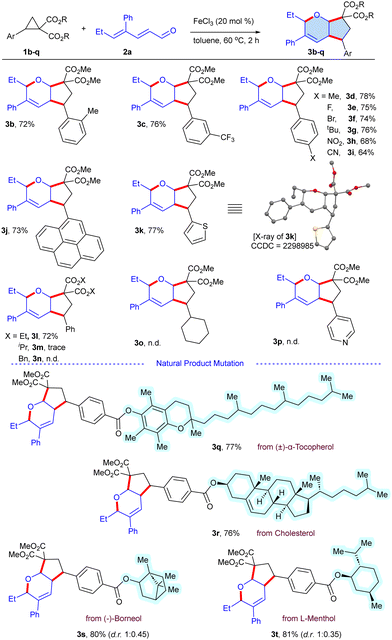 | ||
Scheme 2 Substrate scope of D–A cyclopropanes.a,b a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Reaction conditions: 1b–t (0.1 mmol), 2a (0.12 mmol), FeCl3 (20 mol%), toluene (1.5 mL), 60 °C, 2 h. b Reaction conditions: 1b–t (0.1 mmol), 2a (0.12 mmol), FeCl3 (20 mol%), toluene (1.5 mL), 60 °C, 2 h. b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Isolated yield. n.d. = not detected. Isolated yield. n.d. = not detected. | ||
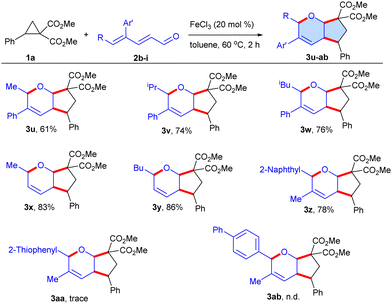
Next, the diversification of 2,4-dienals 2b–i was investigated utilizing dimethyl 2-phenylcyclopropane-1,1-dicarboxylate 1a as the standard substrate (Scheme 3). The presence of aliphatic substituents at the C-5 position of the 2,4-dienals, such as methyl 2b, iso-propyl 2c and iso-butyl 2d, led to the production of the target cyclic ethers 3u–w in 61–76% yields. In addition, aliphatic trans,trans-2,4-hexadienal 2e and trans,trans-2,4-nonadienal 2f were amenable, furnishing 3x and 3y in 83% and 86% yields, respectively. Intriguingly, 2-naphthyl substituted 2g installed at the C-5 position of the 2,4-dienal performed excellently delivering 3z in 78% yield, whereas the thiophenyl 2h yielded 3aa in a trace amount. Furthermore, a biphenyl derivative 2i failed to give the desired cycloadduct 3ab. This might be ascribed to the restriction in H-shift at the C-5 position by the large terminal biphenyl group, which hinders all cis C–C double bond formation that plays a key role in the reaction.
 | ||
Scheme 3 Substrate scope of 2,4-dienals.a,b a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Reaction conditions: 1a (0.1 mmol), 2b–i (0.12 mmol), FeCl3 (20 mol%), toluene (1.5 mL), 60 °C, 2 h. b Reaction conditions: 1a (0.1 mmol), 2b–i (0.12 mmol), FeCl3 (20 mol%), toluene (1.5 mL), 60 °C, 2 h. b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Isolated yield. n.d. = not detected. Isolated yield. n.d. = not detected. | ||
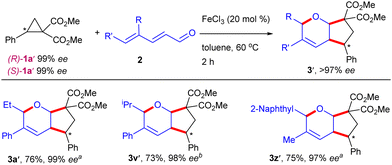
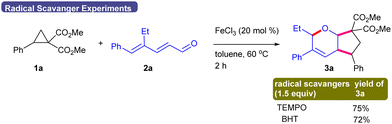
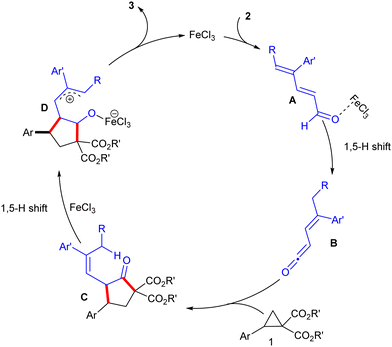
To get an insight into the reaction pathway, the coupling of the optically pure DACs (R)-1a′ and (S)-1a′ was examined as the representative examples (Scheme 4). The coupling of dienal 2a with (R)-1a′ produced 3a′ in >99% ee, whereas 2c and 2g underwent coupling with (S)-1a′ and (R)-1a′ to yield 3v′ and 3z′ in 98% and 97% ee, respectively. These results suggest that the coupling is regio- and stereospecific with excellent chirality transfer. In addition, the coupling of 1a and 2a occurred efficiently in the presence of the radical scavengers, 2,2,6,6-tetra-methylpiperidine-1-oxyl (TEMPO) and 2,6-di-tert-butyl-4-methylphenol (BHT), which suggested that the radical pathway might not be involved (Scheme 5). Thus, FeCl3-catalyzed9 [1,5]-H shift10a–c of 2,4-dienal 2 may deliver the ketene B, which can couple with DAC 1 stereospecifically to furnish the cyclic scaffold C (Scheme 6).8f In another [1,5]-H shift, the allylic intermediate D may undergo a nucleophilic attack on the C-5 center to give the target bicyclic ether 3.10d
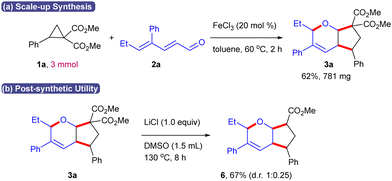
To demonstrate the synthetic utility, a scale-up of the reaction was investigated using 3 mmol of 1a with 2a as the representative substrate to produce 3a in 62% yield (Scheme 7a). In addition, Krapcho decarboxylation of 3a using LiCl afforded the monoester 6 in 67% yield (d.r. 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25) (Scheme 7b).
0.25) (Scheme 7b).
In summary, we have described the iron-catalyzed cascade C–C and C–O bond formation of 2,4-dienals with DACs to furnish functionalized bicyclic cyclopentapyran derivatives. The use of iron-catalysis, excellent chirality transfer and substrate scope are the important practical features.
We thank CSIR (02(0458)/21/EMR-II) for the financial support and CIF and NECBH (BT/CoE/34/SP28408/2018), and DST-FIST (SR/FST/CS-II/2017/23c) for NMR, mass and X-ray analyses. M. M. acknowledges the CSIR for the fellowship and T. P. thanks SERB for the J. C. Bose Fellowship (JCB/2022/000037). We also thank Mr Sandeep Kumar for solving the X-ray crystallography.
Conflicts of interest
There are no conflicts to declare.Notes and references
- For examples, see: (a) D. S. Jang, E. J. Park, M. E. Hawthorne, J. S. Vigo, J. G. Graham, F. Cabieses, B. D. Santarsiero, A. D. Mesecar, H. H. S. Fong, R. G. Mehta, J. M. Pezzuto and A. D. Kinghorn, J. Agric. Food Chem., 2002, 50, 6330 CrossRef CAS PubMed; (b) Y.-Q. Li, X.-S. Huang, K. Ishida, A. Maier, G. Kelter, Y. Jiang, G. Peschel, K.-D. Menzel, M.-G. Li, M.-L. Wen, L.-H. Xu, S. Grabley, H.-H. Fiebig, C.-L. Jiang, C. Hertweck and I. Sattler, Org. Biomol. Chem., 2008, 6, 3601 RSC; (c) L.-B. Dong, J. He, X.-Y. Li, X.-D. Wu, X. Deng, G. Xu, L.-Y. Peng, Y. Zhao, Y. Li, X. Gong and Q.-S. Zhao, Nat. Prod. Bioprospect., 2011, 1, 41 CrossRef CAS; (d) X. Han, G. Peh and P. E. Floreancig, Eur. J. Org. Chem., 2013, 1193 CrossRef CAS; (e) X.-F. He, X.-K. Zhang, C.-A. Geng, J. Hu, X.-M. Zhang, Y.-Q. Guo and J.-J. Chen, Bioorg. Chem., 2020, 96, 103638 CrossRef CAS PubMed.
- For examples, see: (a) D. G. Hall, T. Rybak and T. Verdelet, Acc. Chem. Res., 2016, 49, 2489 CrossRef CAS PubMed; (b) J. Xuan and A. Studer, Chem. Soc. Rev., 2017, 46, 4329 RSC; (c) F. Vetica, P. Chauhan, S. Dochain and D. Enders, Chem. Soc. Rev., 2017, 46, 1661 RSC; (d) V. S. Thirunavukkarasu, M. Donati and L. Ackermann, Org. Lett., 2012, 14, 3416 CrossRef CAS PubMed.
- For examples, see: (a) K. Tanaka, Y. Otake, H. Sagae, K. Noguchi and M. Hirano, Angew. Chem., Int. Ed., 2008, 47, 1312 CrossRef CAS PubMed; (b) T. N. Tekavec and J. Louie, J. Org. Chem., 2008, 73, 2641 CrossRef CAS PubMed; (c) H. Yu, R. Lee, H. Kim and D. Lee, Org. Lett., 2021, 23, 1135 CrossRef CAS PubMed.
- For examples, see: (a) W. S. Jen, J. J. M. Wiener and D. W. C. MacMillan, J. Am. Chem. Soc., 2000, 122, 9874 CrossRef CAS; (b) A. G. Csákÿ, G. de la Herrán and M. C. Murcia, Chem. Soc. Rev., 2010, 39, 4080 RSC; (c) K. L. Jensen, G. Dickmeiss, H. Jiang, Ł. Albrecht and K. A. Jørgensen, Acc. Chem. Res., 2012, 45, 248 CrossRef CAS PubMed; (d) C. Curti, L. Battistini, A. Sartori and F. Zanardi, Chem. Rev., 2020, 120, 2448 CrossRef CAS PubMed; (e) C. Guo, M. Fleige, D. Janssen-Müller, C. G. Daniliuc and F. Glorius, J. Am. Chem. Soc., 2016, 138, 7840 CrossRef CAS PubMed.
- For examples, see: (a) Z.-J. Jia, K. Jiang, Q.-Q. Zhou, L. Dong and Y.-C. Chen, Chem. Commun., 2013, 49, 5892 RSC; (b) A.-S. Marques, V. Coeffard, I. Chataigner, G. Vincent and X. Moreau, Org. Lett., 2016, 18, 5296 CrossRef CAS PubMed; (c) A.-S. Marques, J. Marrot, I. Chataigner, V. Coeffard, G. Vincent and X. Moreau, Org. Lett., 2018, 20, 792 CrossRef CAS PubMed; (d) A.-S. Marques, T. Duhail, J. Marrot, I. Chataigner, V. Coeffard, G. Vincent and X. Moreau, Angew. Chem., Int. Ed., 2019, 58, 9969 CrossRef CAS PubMed; (e) A. La-Venia, L. Passaglia, L. Gurgone, V. Gandon and M. J. Riveira, J. Org. Chem., 2022, 87, 13469 CrossRef CAS PubMed.
- For examples, see: (a) H. Yanai, A. Takahashi and T. Taguchi, Chem. Commun., 2010, 46, 8728 RSC; (b) L. Crouillebois, L. Pantaine, J. Marrot, V. Coeffard, X. Moreau and C. Greck, J. Org. Chem., 2015, 80, 595 CrossRef CAS PubMed; (c) L. Yang, L. Wei and J.-P. Wan, Chem. Commun., 2018, 54, 7475 RSC; (d) C.-Y. Wang, J.-B. Han, L. Wang and X.-Y. Tang, J. Org. Chem., 2019, 84, 14258 CrossRef CAS PubMed; (e) A. Topolska, S. Frankowski and Ł. Albrecht, Org. Lett., 2022, 24, 955 CrossRef CAS PubMed.
- For examples, see: (a) H.-U. Reissig and R. Zimmer, Chem. Rev., 2003, 103, 1151 CrossRef CAS PubMed; (b) T. F. Schneider, J. Kaschel and D. B. Werz, Angew. Chem., Int. Ed., 2014, 53, 5504 CrossRef CAS PubMed; (c) M. A. Cavitt, L. H. Phun and S. France, Chem. Soc. Rev., 2014, 43, 804 RSC; (d) H. K. Grover, M. R. Emmett and M. A. Kerr, Org. Biomol. Chem., 2015, 13, 655 RSC; (e) N. R. O’Connor, J. L. Wood and B. M. Stoltz, Isr. J. Chem., 2016, 56, 431 CrossRef PubMed; (f) E. Budynina, K. Ivanov, I. Sorokin and M. Melnikov, Synthesis, 2017, 3035 CrossRef CAS; (g) O. A. Ivanova and I. V. Trushkov, Chem. Rec., 2019, 19, 2189 CrossRef CAS PubMed.
- For examples, see: (a) E. Wenkert, M. E. Alonso, B. L. Buckwalter and K. J. Chou, J. Am. Chem. Soc., 1977, 99, 4778 CrossRef CAS; (b) E. Piers and H.-U. Reissig, Angew. Chem., Int. Ed. Engl., 1979, 18, 791 CrossRef; (c) P. D. Pohlhaus, S. D. Sanders, A. T. Parsons, W. Li and J. S. Johnson, J. Am. Chem. Soc., 2008, 130, 8642 CrossRef CAS PubMed; (d) A. F. G. Goldberg, N. R. O’Connor, R. A. Craig II and B. M. Stoltz, Org. Lett., 2012, 14, 5314 CrossRef CAS PubMed; (e) M. Mondal, M. Panda, V. McKee and N. J. Kerrigan, J. Org. Chem., 2019, 84, 11983 CrossRef CAS PubMed; (f) S. Das, C. G. Daniliuc and A. Studer, Angew. Chem., Int. Ed., 2017, 56, 11554 CrossRef CAS PubMed; (g) M. Mondal, M. Panda, N. W. Davis, V. McKee and N. J. Kerrigan, Chem. Commun., 2019, 55, 13558 RSC; (h) M.-S. Xie, G.-F. Zhao, T. Qin, Y.-B. Suo, G.-R. Qu and H.-M. Guo, Chem. Commun., 2019, 55, 1580 RSC; (i) W. Luo, Z. Sun, E. H. N. Fernando, V. N. Nesterov, T. R. Cundari and H. Wang, ACS Catal., 2019, 9, 8285 CrossRef CAS; (j) A. A. Suleymanov, E. Le Du, Z. Dong, B. Muriel, R. Scopelliti, F. Fadaei-Tirani, J. Waser and K. Severin, Org. Lett., 2020, 22, 4517 CrossRef CAS PubMed; (k) S. Nicolai and J. Waser, Angew. Chem., Int. Ed., 2022, 61, e202209006 CrossRef CAS PubMed; (l) O. Dalkilic, O. Turbedaroglu, F. Lafzi and H. Kilic, J. Org. Chem., 2023, 88, 11834 CrossRef CAS PubMed; (m) M. Bao, K. Lopez, R. Gurung, H. Arman and M. P. Doyle, ACS Catal., 2023, 13, 1621 CrossRef CAS.
- For examples, see: (a) A. Correa, O. García Mancheño and C. Bolm, Chem. Soc. Rev., 2008, 37, 1108 RSC; (b) C.-L. Sun, B.-J. Li and Z.-J. Shi, Chem. Rev., 2011, 111, 1293 CrossRef CAS PubMed; (c) Y.-Y. Li, S.-L. Yu, W.-Y. Shen and J.-X. Gao, Acc. Chem. Res., 2015, 48, 2587 CrossRef CAS PubMed; (d) R. Shang, L. Ilies and E. Nakamura, Chem. Rev., 2017, 117, 9086 CrossRef CAS PubMed; (e) Y. Liu, T. You, H.-X. Wang, Z. Tang, C.-Y. Zhou and C.-M. Che, Chem. Soc. Rev., 2020, 49, 5310 RSC; (f) X.-F. Duan, Chem. Commun., 2020, 56, 14937 RSC.
- For examples, see: (a) S. J. Pastine and D. Sames, Org. Lett., 2005, 7, 5429 CrossRef CAS PubMed; (b) J. Yu, N. Li, D.-F. Chen and S.-W. Luo, Tetrahedron Lett., 2014, 55, 2859 CrossRef CAS; (c) R. Tamura, E. Kitamura, R. Tsutsumi, M. Yamanaka, T. Akiyama and K. Mori, Org. Lett., 2019, 21, 2383 CrossRef CAS PubMed; (d) T. Sakaguchi, Y. Okuno, Y. Tsutsumi, H. Tsuchikawa and S. Katsumura, Org. Lett., 2011, 13, 4292 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2298985. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3cc06261a |
| This journal is © The Royal Society of Chemistry 2024 |