Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Metal cations recognition by bowl-shaped N-pyrrolic polycyclic aromatic hydrocarbons†
Daria
Szeląg
a,
Jakub S.
Cyniak
 a,
Joachim
Ażgin
a,
Joachim
Ażgin
 a,
Jakub
Wagner
a,
Jakub
Wagner
 b,
Marcin
Lindner
b,
Marcin
Lindner
 *b,
Wojciech
Wróblewski
*b,
Wojciech
Wróblewski
 a and
Artur
Kasprzak
a and
Artur
Kasprzak
 *a
*a
aFaculty of Chemistry, Warsaw University of Technology, Noakowskiego 3, 00-664 Warsaw, Poland. E-mail: artur.kasprzak@pw.edu.pl
bInstitute of Organic Chemistry, Polish Academy of Sciences, Kasprzaka 44/52, 01-224 Warsaw, Poland. E-mail: marcin.lindner@icho.edu.pl
First published on 30th July 2024
Abstract
Bowl-shaped, nitrogen-doped polycyclic aromatic hydrocarbons were examined for the first time as molecular receptors for the recognition of metal cations. Potentiometric and spectrofluorimetric assays, supported with density functional theory computations, revealed that the title compounds recognise metal cations with a special focus on caesium (Cs+) cations.
Bowl-shaped compounds are an intriguing group of molecules, whose synthesis, properties, and functions have been of significant interest in recent years. The curvature in the structure of such compounds influences their unique properties and functions, commonly superior in comparison to their flat congeners.1–3 The curvature not only influences the improved photophysical properties of bowl-shaped molecules, but also enables the creation of interesting molecular arrangements and supramolecular systems. The nonplanar polycyclic aromatic hydrocarbons (PAHs)3 constitute a unique class of buckybowl arrangements4,5 accompanied by pronounced redox and optical properties modulated by heteroatom dopant(s), such as mainly nitrogen (N) but also sulphur,6 phosphorus, selenium,7 and tellurium8,9 atoms, installed at both their central or peripheral parts. Hence, a heteropine analogue of azacorannulene was demonstrated to exhibit unique p-type semiconducting characteristics with high charge carrier mobility for use in single-crystal field-effect transistors.5b While considering the possible utility of a hetera-buckybowl scaffold as an organic light-emitting diode emitter, our group has introduced a new paradigm for bowl-shaped dibenzoazepine-based PAHs attributed to electron-accepting phenazine species, demonstrating distinctive thermally-activated delayed fluorescence (TADF) properties.10 Building on that, subsequent derivatization with twisted electron-rich moieties towards D–A–D emitters was accomplished.11 This led to a significant performance leap, achieving a PLQY as high as 96% and an EQE of 21.9% for orange OLED devices.
On the other hand, electron-rich and π-expanded architectures were anticipated to show remarkable binding features, forming supramolecular complexes between electron-deficient C60 and twizzer-like azacorannulene.12 Recently, the possibility of applying the C3-symmetric sumanene buckybowl in the construction of caesium (Cs+) cation selective sensors was demonstrated.13,14 The importance of the bowl shape of sumanene towards providing Cs+ recognition ability was elucidated. Moreover, tellurium- and phosphorus-doped sumanenes turned out to be potent coordinating partners for silver(I) cations.15 Seeking detection possibilities towards more challenging cations, it could be reasoned that our parent bipolar N-doped PAH could be a perfectly suited system for fluorescent probing of metal cations, not only because of the well-defined emissive properties but even more importantly because of the affinity of the pyramidal centrally positioned N-atom encircled by π-electron units to attract a specific cation. In the light of these considerations, herein we report on the first application of bowl-shaped N-doped PAHs as molecular receptors of metal cations. Their binding abilities were examined using potentiometric methods, spectrofluorimetry, and theoretical calculations (DFT) showing Cs+ selectivity, together with satisfactory apparent binding constant (Kapp) and limit of detection (LOD) values for the formed systems at the level of 105 M−1 and 10−6 M, respectively.
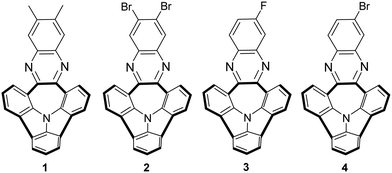
The structures of bowl-shaped and N-doped PAHs 1–4 selected for our receptor studies are presented in Fig. 1. Among our library of such derivatives,10 molecules 1–4 were exclusively chosen due to their satisfactory solubility profiles and different electronic nature of the substituents at the outer benzene ring.
The metal-cation recognition properties of the bowl-shaped N-pyrrolic PAHs were first studied using the potentiometric method. Derivatives 1 and 2, possessing electron-donating (methyl groups) or electron-withdrawing (halogen) substituents in the outer benzene rings, have been selected as model receptors and introduced into polymeric membranes of ion-selective electrodes.16
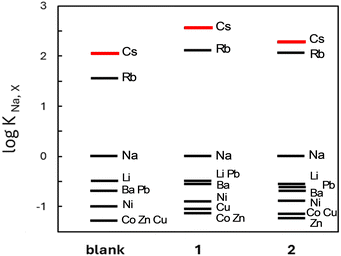
The ion binding ability of these receptors was evaluated on the basis of potentiometric selectivity coefficients17 (see Fig. 2 and values of selectivity coefficients in Table S1, ESI†), expressing quantitatively the influence of a given cation on the signal of a potentiometric sensor. It should be emphasized that the values of the selectivity coefficients were determined in a heterogeneous system (polymeric membrane/water solution), and therefore did not strictly reflect the supramolecular interactions taking place in solution. Moreover, an anion-exchanger – potassium tetrakis[3,5-bis(trifluoromethyl)phenyl]borate (KTFPB) – has been added to PVC membranes to provide proper operation of the sensors containing receptors 1 or 2, assumed to function as neutral carriers.18 The presence of anionic additive KTFPB favours ion-selectivity of the membranes towards more lipophilic cations, i.e., characterized by less negative values of standard molar Gibbs free energy of hydration, like for example caesium (Cs+) or tetramethylammonium cations.19
Despite the presence of KTFPB, the examined membranes exhibited increased affinity towards rubidium (Rb+) and especially Cs+ cations with respect to other metals ions, even in comparison with the so-called blank membranes, containing only the ion-exchanger. For instance, the membranes based on receptor 1 were 350 times more selective for Cs+ against sodium cations (Na+) and at least 1000 times more selective against lithium cations (Li+) as well as divalent metal ions. Although a similar selectivity pattern was observed for membranes based on both receptors, the derivative 1 provided higher Cs+ selectivity (i.e., a higher value of log KNa,Cs was determined). According to our expectations, the membranes exhibited significantly worse selectivity towards transition metal cations (negative values of log KNa,X, comparable with those calculated for the blank membrane), which can be explained by their high hydrophilicity, i.e., very low value of molar Gibbs free energy of hydration (compare for example: −1915 kJ mol−1 and −250 kJ mol−1 for cobalt (Co2+) and Cs+ ions, respectively19).
The tested ion-selective electrodes were selective but also sensitive towards Cs+ cations, enabling their fast and selective quantitative analysis in environmental samples. Potentiometric responses of the sensors based on membranes doped with receptor 1 or 2, determined in 0.01 M NaNO3 solution, exhibited a wide linear range with a detection limit of 25 μM Cs+ (refer to Fig. S1, ESI†). Comparable sensitivity, i.e., slopes of calibration curves close to 50 mV decade−1, as well as short response times in the range of 120–180 s were noticed for both sensors.
Supramolecular interactions between the metal cations and investigated bowl-shaped N-doped PAHs were further examined in solution with fluorescence spectroscopy. Since exclusive Cs+ recognition abilities were observed during potentiometric experiments, the interactions between Cs+ and receptor 1, featuring the best solubility profile among the compounds tested, were first investigated. Due to the insolubility of the tested receptors in water, the addition of organic solvent was necessary to enable running spectroscopic investigations at reasonable receptor concentration. To our delight, receptor 1 was found to be soluble in the THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O 1
H2O 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 vol/vol solvent system, which was selected for spectrofluorimetric assays. Cs+ cations were introduced to the solution in the form of the corresponding hexafluorophosphate (PF6−) salt.
1 vol/vol solvent system, which was selected for spectrofluorimetric assays. Cs+ cations were introduced to the solution in the form of the corresponding hexafluorophosphate (PF6−) salt.
Fig. 3 shows the fluorescence spectra results of titration of receptor 1 with Cs+ (refer to ESI,† Section S3 for all the spectra and data on fluorescence spectra assays). Upon the addition of further portions (molar equivalents) of Cs+, turn-on fluorescence behaviour was observed. This feature was ascribed to the dynamic formation of cation–π systems,20,21 revealing the recognition of Cs+ by the tested bowl-shaped PAH also in solution. The stoichiometry of the formed complexes between 1 and Cs+, analysed with Job's plot method,22 was equal to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (see plots for 1 in Section S3, ESI†). The Kapp value for the system, estimated with the Benesi–Hildebrand method,23 was on the level of 104 M−1, while the LOD value was on the micromolar concentration level (Table 1). Thus, satisfactory binding parameters for the formed systems can be concluded. Respective Cs+ recognition studies were also performed for bowl-shaped receptors 3–4 (receptor 2 was excluded from fluorescence spectroscopy studies due to solubility limitations). As compared with receptor 1, interactions between Cs+ and receptors 3–4 were characterised by ca. 10-fold higher binding constants (see data in Table 1). This result might suggest that the presence of electron-withdrawing groups in the outer phenyl rings of the tested N-doped PAHs should have a beneficial effect on Kapp. The LOD for the system containing 3 was similar to that of 1, whereas recognition of Cs+ by 4 was characterised by ca. 10-fold higher LOD.
1 (see plots for 1 in Section S3, ESI†). The Kapp value for the system, estimated with the Benesi–Hildebrand method,23 was on the level of 104 M−1, while the LOD value was on the micromolar concentration level (Table 1). Thus, satisfactory binding parameters for the formed systems can be concluded. Respective Cs+ recognition studies were also performed for bowl-shaped receptors 3–4 (receptor 2 was excluded from fluorescence spectroscopy studies due to solubility limitations). As compared with receptor 1, interactions between Cs+ and receptors 3–4 were characterised by ca. 10-fold higher binding constants (see data in Table 1). This result might suggest that the presence of electron-withdrawing groups in the outer phenyl rings of the tested N-doped PAHs should have a beneficial effect on Kapp. The LOD for the system containing 3 was similar to that of 1, whereas recognition of Cs+ by 4 was characterised by ca. 10-fold higher LOD.
 | ||
| Fig. 3 Fluorescence Cs+ titration of representative bowl-shaped and N-doped PAH receptor 1 (λex = 320 nm). | ||
| Receptor | K app [M−1] | LOD [μM] |
|---|---|---|
| 1 | 3.8 × 104 | 3.7 |
| 3 | 3.0 × 105 | 4.8 |
| 4 | 2.9 × 105 | 23.6 |
Building on the above-described procedure, receptor 1 was also titrated with Na+, Co2+ or Rb+ cations, as a representative small s-block cation, d-block cation and cation featuring similar van der Waals radius to Cs+, respectively. Receptor 1 featured the property of recognising these metal cations in solution with similar turn-on fluorescence behaviour as in Cs+ titration assay (refer to ESI,† Section S3 for these spectra). The Kapp values for the systems were slightly higher than for Cs+, equalling 7.9 × 104 M−1, 6.6 × 104 M−1 and 5.4 × 104 M−1, for Na+, Co2+ and Rb+, respectively. The LOD values for Rb+ (1.9 μM) and Co2+ (3.4 μM) were slightly lower (slightly higher for Na+ – 9.1 μM) in comparison to the respective value for Cs+ (3.7 μM). While enhanced selectivity of receptor 1 towards recognising Rb+ was also observed in potentiometric experiments, we believe that Na+ and Co2+ binding observed during fluorescence assays resulted from the differences between the used techniques. In potentiometric experiments, the water-immiscible phase was doped with the receptor and formed a heterogeneous system contacting the solution, limiting the system dynamics in the light of the recognition process. On the other hand, analyses in solution (fluorescence titration assays) provided higher system dynamics which enabled the formation of complexes between 1 and such cations as small s-block cation Na+ and d-block cation Co2+.
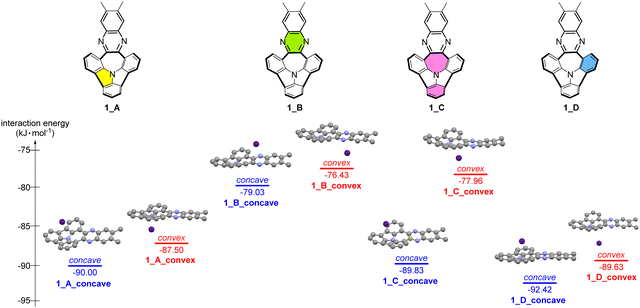
To further study the investigated non-covalent systems, DFT calculations with pseudopotential plane wave (PSPW)24 were conducted (simulation cell was 100 Å). The computational works were performed with molecule 1 as a representative N-doped PAH receptor. Complexes containing one Cs+ cation per receptor molecule (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex stoichiometry) were subjected to calculation experiments, according to the above-discussed spectrofluorimetric results. Eight different complex arrangements were subjected to DFT calculations. These included Cs+ concave or convex bound to different structural parts of the receptor, namely six- or seven-membered rings, mostly N-doped ones, see Fig. 4. One considered arrangement (labelled as 1_B) included Cs+ bound to the non-bowl-shaped part of the receptor, while other arrangements (labelled as 1_A, 1_C, 1_D) assumed binding Cs+ by bowl-shaped parts of the receptor.
1 complex stoichiometry) were subjected to calculation experiments, according to the above-discussed spectrofluorimetric results. Eight different complex arrangements were subjected to DFT calculations. These included Cs+ concave or convex bound to different structural parts of the receptor, namely six- or seven-membered rings, mostly N-doped ones, see Fig. 4. One considered arrangement (labelled as 1_B) included Cs+ bound to the non-bowl-shaped part of the receptor, while other arrangements (labelled as 1_A, 1_C, 1_D) assumed binding Cs+ by bowl-shaped parts of the receptor.
Fig. 4 presents the DFT calculated interaction energies for each complex. The interaction energies for all considered systems featured negative values, confirming the formation of energetically favourable complexes. According to our computational results, binding of Cs+ at the bowl parts of the receptor (arrangements 1_A, 1_C, 1_D) was energetically more favoured than binding at the flat part (arrangement 1_B). This conclusion was supported by further DFT calculations on electrostatic surface potential (ESP), which indicated a non-uniform charge distribution within the receptor molecule (see Fig. S24, ESI†). It emphasized the importance of curvature in the considered N-doped PAHs towards providing metal cation recognition features. Furthermore, the formation of both concave- but also convex-bound 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes was expected, since in most cases no significant differences in the concave- versus convex-oriented interaction energies of Cs+ binding were found, with the exception of one arrangement 1_C (refer also to the calculated geometry of the HOMOs, Fig. S25, ESI†). Interestingly, our DFT analyses suggest that the most energetically favourable Cs+ complex might be the concave-bound system including the six-membered ring located between N-doped rings of the considered PAH (1_D_concave, interaction energy −92.42 kJ mol−1).
1 complexes was expected, since in most cases no significant differences in the concave- versus convex-oriented interaction energies of Cs+ binding were found, with the exception of one arrangement 1_C (refer also to the calculated geometry of the HOMOs, Fig. S25, ESI†). Interestingly, our DFT analyses suggest that the most energetically favourable Cs+ complex might be the concave-bound system including the six-membered ring located between N-doped rings of the considered PAH (1_D_concave, interaction energy −92.42 kJ mol−1).
In conclusion, this work revealed that bowl-shaped and N-doped PAHs constitute a new class of molecular receptors dedicated to the recognition of metal cations. According to our potentiometric experiments, a preference towards Cs+ binding could be concluded, whereas spectrofluorimetric analyses in solution pointed out satisfactory Kapp and LOD values for the formed systems at the level of 105 M−1 and 10−6 M, respectively. The importance of the bowl shape towards providing Cs+ recognition properties of the studied receptors was supported with DFT calculations. We believe that this work opens up new avenues in yet-unexplored applications of bowl-shaped PAHs.
M. L., W. W. and A. K. conceived the project, whereas W. W. and A. K. supervised the experiments; D. S. performed most potentiometric and spectrofluorimetric analyses; J. S. C. performed part of the spectrofluorimetric analyses; J. A. performed DFT calculations; J. W. synthesised the compounds; A. K. conceptualized the manuscript and corresponded with the Editor and Reviewers; M. L., W. W. and A. K. contributed to writing of the manuscript, including preparation of the revised version.
The financial support from the National Science Centre, Poland, grant no. 2021/43/B/ST4/00114 (A. K.), UMO-2018/31/D/ST5/00426 (J. W., M. L.), and Warsaw University of Technology (statutory support) are acknowledged. The computational studies were carried out with the support of the Interdisciplinary Centre for Mathematical and Computational Modelling University of Warsaw (ICM UW) under computational allocation no G91-1416. M. L. is a recipient of a scholarship awarded by the Polish Ministry of Education and Science to outstanding young scientists (2/DSP/2021). J. W. acknowledges the “START” scholarship awarded by the Foundation for Polish Science (093.2023).
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Notes and references
- M. Saito, H. Shinokubo and H. Sakurai, Mater. Chem. Front., 2018, 2, 635–661 RSC.
- E. Nestoros and M. C. Stuparu, Chem. Commun., 2018, 54, 6503–6519 RSC.
- W.-S. Wong and M. Stępień, Trends Chem., 2022, 4, 573–576 CrossRef CAS.
- A. Borissov, Y. K. Maurya, L. Moshniaha, W.-S. Wong, M. Żyła-Karwowska and M. Stępień, Chem. Rev., 2022, 122, 565–788 CrossRef CAS PubMed.
- (a) W. Wang and X. Shao, Org. Biomol. Chem., 2021, 19, 101–122 RSC; (b) W. Wang, F. Hanindita, Y. Tanaka, K. Ochiai, H. Sato, Y. Li and T. Yasuda, Angew. Chem., Int. Ed., 2023, 62, e202218176 CrossRef CAS PubMed.
- K. Imamura, K. Takimiya, T. Otsubo and Y. Aso, Chem. Commun., 1999, 1859–1860 RSC.
- X. Li, Y. Zhu, J. Shao, B. Wang, S. Zhang, Y. Shao, X. Jin, X. Yao, R. Fang and X. Shao, Angew. Chem., Int. Ed., 2014, 53, 535–538 CrossRef CAS PubMed.
- S. Wang, J. Shang, C. Yan, W. Wang, C. Yuan, H.-L. Zhang and X. Shao, Org. Chem. Front., 2019, 6, 263–272 RSC.
- W. Wang, F. Hanindita, Y. Tanaka, K. Ochiai, H. Sato, Y. Li, T. Yasuda and S. Ito, Angew. Chem., Int. Ed., 2023, 62, e202218176 CrossRef CAS PubMed.
- J. Wagner, P. Zimmermann Crocomo, M. A. Kochman, A. Kubas, P. Data and M. Lindner, Angew. Chem., Int. Ed., 2022, 61, e202202232 CrossRef CAS PubMed.
- J. Wagner, D. Kumar, M. A. Kochman, T. Gryber, M. Grzelak, A. Kubas, P. Data and M. Lindner, ACS Appl. Mater. Interfaces, 2023, 15, 37728–37740 CrossRef CAS PubMed.
- Y. Tokimaru, S. Ito and K. Nozaki, Angew. Chem., Int. Ed., 2018, 130, 9966–9970 CrossRef.
- J. S. Cyniak, Ł. Kocobolska, N. Bojdecka, A. Gajda-Walczak, A. Kowalczyk, B. Wagner, A. M. Nowicka, H. Sakurai and A. Kasprzak, Dalton Trans., 2023, 52, 3137–3147 RSC.
- J. Ażgin, M. Wesoły, K. Durka, H. Sakurai, W. Wróblewski and A. Kasprzak, Dalton Trans., 2024, 53, 2964–2972 RSC.
- S. Wang, C. Yan, J. Shang, W. Wang, C. Yuan, H. Zhang and X. Shao, Angew. Chem., Int. Ed., 2019, 58, 3819–3823 CrossRef CAS PubMed.
- Membranes contained: 1% wt receptor 1–2, 65% wt plasticizer (o-NPOE) and 33% wt PVC. Assuming the 1:1 stoichiometry of the formed complexes, 50% mol vs receptor of KTFPB was added to the membranes to gain their highest selectivity.
- According to the Nikolski-Eisenman equation, the values of the selectivity coefficients (log
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KNa,X) express quantitatively the influence of a given interfering ion X (in our case X = Cs+, Rb+, Li+, Ba2+, Pb2+ and transition metal cations) on the potential of the ion-selective electrode sensitive towards arbitrary chosen primary ion (in our case: Na+); the higher the value of the selectivity coefficient the higher selectivity towards given interfering cation X.
KNa,X) express quantitatively the influence of a given interfering ion X (in our case X = Cs+, Rb+, Li+, Ba2+, Pb2+ and transition metal cations) on the potential of the ion-selective electrode sensitive towards arbitrary chosen primary ion (in our case: Na+); the higher the value of the selectivity coefficient the higher selectivity towards given interfering cation X. - P. C. Meier, W. E. Morf, M. Läubli and W. Simon, Anal. Chim. Acta, 1984, 156, 1–8 CrossRef CAS.
- Y. Marcus, J. Chem. Soc., Faraday Trans., 1991, 87, 2995–2999 RSC.
- A. Kasprzak, Angew. Chem., Int. Ed., 2024, 63, e202318437 CrossRef CAS PubMed.
- K. Sikligar, S. P. Kelley, G. A. Baker and J. L. Atwood, Cryst. Grow. Des., 2022, 22, 2806–2811 CrossRef CAS.
- J. S. Renny, L. L. Tomasevich, E. H. Tallmadge and D. B. Collum, Angew. Chem., Int. Ed., 2013, 52, 11998–12013 CrossRef CAS PubMed.
- H. A. Benesi and J. H. Hildebrand, J. Am. Chem. Soc., 1949, 71, 2703–2707 CrossRef CAS.
- E. J. Bylaska, Annu. Rep. Comput. Chem., 2017, 13, 185–228 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Materials and methods, Experimental section, additional data on potentiometric and spectrofluorimetric experiments, and DFT calculations. See DOI: https://doi.org/10.1039/d4cc02586e |
| This journal is © The Royal Society of Chemistry 2024 |