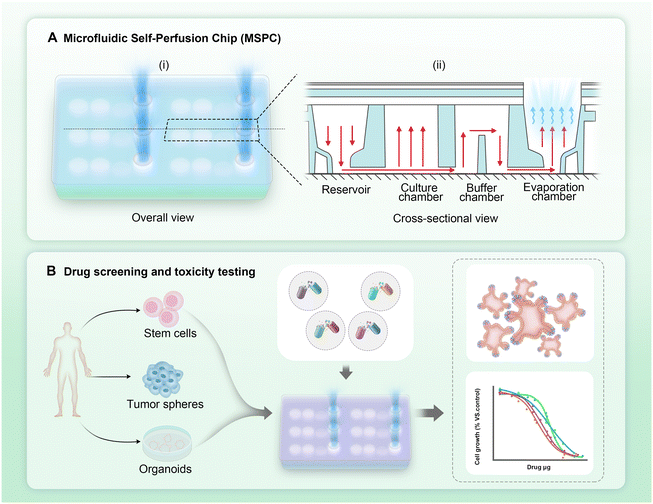
A novel microfluidic self-perfusion chip (MSPC) for pumpless 3D cell, microtissue and organoid culture
Guohua
Wu
abc,
Di
Wu
d,
Wenqi
Hu
bc,
Qinrui
Lu
bc,
Yusen
Zhou
bci,
Jie
Liu
bc,
Qijun
Du
bc,
Zhi
Luo
 e,
Haijie
Hu
f,
Hongwei
Jiang
*a,
Bangchuan
Hu
*g and
Shuqi
Wang
e,
Haijie
Hu
f,
Hongwei
Jiang
*a,
Bangchuan
Hu
*g and
Shuqi
Wang
 *bchi
*bchi
aLuoyang Key Laboratory of Clinical Multiomics and Translational Medicine, Henan Key Laboratory of Rare Diseases, Endocrinology and Metabolism Center, The First Affiliated Hospital, College of Clinical Medicine of Henan University of Science and Technology, Luoyang 471003, China. E-mail: jianghw@haust.edu.cn
bNational Engineering Research Center for Biomaterials, Sichuan University, Chengdu, 610065, China. E-mail: shuqi@scu.edu.cn
cCollege of Biomedical Engineering, Sichuan University, Chengdu, 610065, China
dFrontiers Science Center Disease-related Molecular Network, Laboratory Pulmonary Immunology and Inflammation, Sichuan University, Chengdu, 610213, China
eDepartment of Biomedical Engineering, Southern University of Science and Technology, Shenzhen, 518055, China
fDivision of Biliary Surgery, Department of General Surgery, West China Hospital, Sichuan University, Chengdu, 610041, China
gEmergency and Critical Care Center, ICU, Zhejiang Provincial People's Hospital (Affiliated People's Hospital, Hangzhou Medical College), Hangzhou, 310014, China. E-mail: hubangchuanicu@163.com
hClinical Research Center for Respiratory Disease, West China Hospital, Sichuan University, Chengdu, 610065, China
iTianfu Jincheng Laboratory, City of Future Medicine, Chengdu, 641400, China
First published on 10th April 2025
Abstract
Microfluidic systems have revolutionized biological research by enabling precise control over cellular environments at microscale volumes. However, traditional pump-driven systems face challenges such as complexity, cost, cell-damaging shear stress, and limited portability. This study introduces a novel adjustable microfluidic self-perfusion chip (MSPC) that uses evaporation as a driving force, eliminating the need for external pumps. Our design offers improved metabolic waste management and simplified control over fluid dynamics. The chip features adjustable evaporation pore sizes, demonstrating a robust linear relationship (R2 = 0.95) between the pore size and fluid evaporation rate. This ensures consistent fluid flow and effective waste removal, shown by lower ammonia and lactate levels compared to conventional cultures. Its unidirectional flow system and integrated one-way valve maintain cell viability, even under complete evaporation conditions. This innovative platform facilitates the cultivation of complex tissue-like structures, providing a valuable tool for tissue and organ model development, as well as drug screening and toxicity testing. By addressing key limitations of traditional systems, our adjustable MSPC represents a significant advancement in microfluidic cell culture technology, offering improved accessibility and applicability in biological research.
Introduction
Organ-on-a-chip technology, as a major application of microfluidic systems, has emerged as an indispensable tool in biomedical research.1–3 This method offers researchers unprecedented opportunities to investigate human physiology,4 disease mechanisms,5 and drug responses6 by precisely replicating the microenvironment and functions of human organs. Organ-on-chip platforms utilize the advantages of microfluidic technology, such as precise control over cellular microenvironments, enabling the creation of highly realistic three-dimensional models of human organs.7–9 These “organs-on-chips” not only serve as valuable tools for fundamental biological research but also demonstrate great promise in many fields, including drug screening, toxicity testing, and personalized medicine.Despite the potential of microfluidic technologies, traditional pump-driven systems face critical limitations. The reliance on external pumps increases complexity and cost, limiting accessibility for many laboratories.10–12 Long-term operation often introduces bubble formation and media leakage, which can lead to experimental failure.13,14 Additionally, dependence on external power sources and equipment restricts portability and adaptability across experimental settings.15 These limitations have motivated researchers to explore alternative fluid manipulation strategies that could simplify microfluidic operations while better meeting the needs of biological experiments.
To address the limitations of traditional pump-driven microfluidic systems, researchers have explored various pumpless alternatives. These devices use shaking,16 gravity,17–19 hydrophilic fibers20 or paper-based microfluidics21,22 to induce fluid flow. Shaking-based systems can generate fluid movement but lack precise control and introduce turbulence. Gravity-driven devices provide simplicity; however, their dependence on orientation and variability in flow rates can limit their suitability for long-term or complex biological experiments. Hydrophilic fiber-based chips provide a promising mechanism for passive fluid movement; however, they face challenges such as sample retention, contamination, and inconsistent fluid dynamics. Paper-based microfluidic devices5 provide pumpless fluid control, but are not suitable for long-term cell culture. One study utilized heating-based evaporation to induce fluid flow, but required heating elements and was limited to simple 2D cultures.23 Therefore, there is an urgent need to develop a novel microfluidic chip to address these challenges and provide a more reliable and accurate pump-less fluid control system.
In this study, we develop a novel adjustable microfluidic self-perfusion chip (MSPC) that utilizes evaporation as the primary driving force for fluid flow, eliminating the need for any external equipment. The chip shows a robust linear relationship (R2 = 0.95) between the evaporation surface area and evaporation rate, enabling adjustment of the evaporation rate to match specific culture needs. Our pumpless design offers precise microenvironmental control through adjustable evaporation rates, significantly reducing ammonia and lactate accumulation compared to conventional static cultures, thus facilitating efficient metabolic waste management and nutrient replenishment, particularly beneficial for high-density and long-term cultures. A unique partition design in the buffer chamber ensures stability of the liquid level in the culture chamber and prevents backflow. Through comprehensive assays including evaporation measurements, waste product quantification, and cell viability assessments, we demonstrate that this chip can effectively support the culture of diverse cell lines, tissue clusters, and complex organoids. The results demonstrate that this chip can effectively support the culture of diverse cell lines, tissue clusters, and complex organoids. Furthermore, its utility extends to drug testing, offering a platform for evaluating targeted therapies and immunotherapies.
Materials and methods
Microfluidic device fabrication
The chip was designed using computer-aided design (CAD) software. Microfluidic patterns were directly fabricated onto poly(methyl methacrylate) (PMMA) sheets using a laser cutting machine. PMMA was chosen over PDMS due to its superior optical transparency for real-time imaging, ease of scalable fabrication via laser cutting without requiring complex molding processes, and proven biocompatibility for cell culture applications. Each chip contained four independent culture units. Three different chip designs with varying evaporation surface areas were created, corresponding to the well diameters of traditional 12-well, 24-well, and 48-well plates. In this study, these designs are designated as 12-MSPC, 24-MSPC, and 48-MSPC, respectively. The chip contains 4 major chambers and can hold approximately 3.4 ml of culture medium in total, with the reservoir capable of holding about 1.5 ml of medium. Before cell culture, all MSPC chips were sterilized using ethylene oxide gas. Specific parameters of the chip are presented in Table 1.| Drain channel | Characteristics | Designed (mm) |
|---|---|---|
| Reservoir | Diameter (reservoir layer) | 16.2 mm |
| Height (reservoir layer) | 7 mm | |
| Diameter (inlet) | 1.7 mm | |
| Height (inlet) | 4 mm | |
| Culture chamber | Diameter | 6.8 mm |
| Height | 11 mm | |
| Buffer chamber | Stepped interior, prevents backflow | — |
| Evaporation chamber | Diameter 1 (12-MSC, evaporation layer) | 22.7 mm |
| Diameter 2 (48-MSC, evaporation layer) | 10.7 mm | |
| Diameter 3 (24-MSC, evaporation layer) | 16.2 mm | |
| Height (evaporation layer) | 7 mm | |
| Diameter (outlet) | 1.7 mm | |
| Height (outlet) | 4 mm |
Characterization of the evaporation-driven flow rate
To evaluate the evaporation rate of the microfluidic self-perfusion chip, an organoid culture medium was introduced into the 12-MSPC, 24-MSPC, and 48-MSPC, with three biological replicates set for each pore size. The evaporation rate of the culture medium was quantified using a gravimetric method. At the beginning of the experiment (0 h) and after incubation for 24, 48, and 72 hours, the weight of the chip was measured. The evaporation rate of each chip was determined by calculating the change in chip weight over time. The average daily evaporation rate for each well was obtained.Characterization of AC16, LO2 and BEAS-2B cells under 3D conditions in MSPCs
AC16 (cardiomyocytes), LO2 (hepatic cells), and BEAS-2B (lung epithelial cells) cells (retained in our laboratory) were cultured in Dulbecco's modified Eagle medium (DMEM) supplemented with 1% penicillin–streptomycin and 10% fetal bovine serum (FBS, Gibco, Melbourne, Australia). All cell cultures were maintained at 37 °C with 5% CO2 and 95% humidity. For 3D cell culture, 1 × 106 AC16, LO2, and BEAS-2B cells were uniformly mixed with 30 μL of 8% porous gelatin methacryloyl (GelMA, EFL-GM-PR-002, Suzhou, China), placed into the culture chambers of the chips, and exposed to ultraviolet light for 20 seconds to fix the porous GelMA. The evaporative chips were then incubated under standard culture conditions. The culture medium was replaced every 3 days. On days 2, 4, and 6, the cells were stained using a live/dead cell viability assay kit (calcein-AM/propidium iodide, Dojindo, C542, Japan). The cells were stained with 4 μM calcein-AM and 2 μM propidium iodide in phosphate buffered saline (PBS) and incubated in the dark for 15 minutes. Live cells were stained green by calcein-AM, while dead cells were stained red by propidium iodide. Imaging was performed using confocal microscopy, and ImageJ software was used to quantify the number of live and dead cells. Cell viability was calculated as the ratio of live cells to the total number of cells in each specimen, expressed as a percentage.Characterization of tissue clusters in traditional plates and MSPCs
Adult Sprague–Dawley (SD) rats were euthanized, and liver tissues were excised immediately. The tissues were minced into 1–2 mm3 pieces and then passed through 100 μm and 40 μm cell strainers sequentially to obtain tissue clusters with a size range of 40–100 μm. The isolated tissue clusters were mixed with ice-cold Matrigel and seeded onto either traditional 96-well plates or 96-MSPCs at a density of 1000 clusters per well. The plates were then incubated at 37 °C with 5% CO2 and 95% humidity. The culture medium was replaced every 3 days. On days 3 and 6, the clusters were stained using a live/dead cell viability assay kit. Confocal microscopy was used to image the clusters, and ImageJ software was used to quantify the number of live and dead cells.Measurement of ammonia and lactic acid concentrations
L929 cells (retained in our laboratory) were cultured in 3D hydrogel medium using traditional 96-well plates or 96-MSPCs. Cells were seeded at varying densities of 104, 2 ×104, 5 × 104, and 105 cells per well. The culture medium was replaced every 3 days. Culture medium supernatants were collected at days 2, 4, 6, and 8 post-seeding to evaluate the temporal changes in metabolite concentrations. Before collection, the plates were centrifuged at 300g for 5 minutes to ensure cell-free samples. Supernatants were carefully aspirated and immediately stored at −80 °C until analysis to prevent degradation. The ammonia and lactic acid concentrations in the culture medium supernatants were quantified using a Roche CEDEX BIO analyzer.Generation of organoids
Human intrahepatic cholangiocarcinoma (ICC) tissues were obtained from patients undergoing surgery at West China Hospital. The study protocol adhered to the ethical guidelines of the Declaration of Helsinki and was approved by the Research Ethics Committee of West China Hospital (2023 Review No. 108). Informed consent was obtained from human participants of this study. Additional approval and guidelines were obtained from the Animal Ethics Committee of West China Hospital of Sichuan University (No. 20230309045) for animal-related experiments. Whether using human or mouse tissue, visible adipose tissue was removed from the sample and washed thoroughly with DPBS. The tissue was placed in a 1.5 mL centrifuge tube and cut into approximately 1 mm3 pieces using sterile scissors. A tissue digestion solution was then added to the system for a 2 hour digestion process. The digestion was terminated by adding twice the volume of DPBS. The digested mixture was passed through a 70 μm cell strainer, and the resulting cell suspension was centrifuged at 300g for 4 min to collect the cells. If visible red blood cells were present in the pellet, 2 mL of red blood cell lysis buffer was used to lyse the red blood cells, followed by another round of centrifugation to obtain a clean cell pellet. The cells were counted and mixed with Matrigel before being seeded into MSPCs at a density of 2 × 104 cells per well. After 30 min, an ICC organoid or mouse normal organoid medium was added. Once the organoids matured, they were digested into single cells using dissociation enzymes and further cultured.Organoid immunostaining
Organoids were harvested using a cell recovery solution (Corning, 354253, USA) and fixed in 4% paraformaldehyde at 4 °C for 30 min, followed by washing three times with phosphate buffered solution (PBST). For histological analysis, the sections were stained using Harris's hematoxylin and eosin (H&E). For immunofluorescence staining, organoids were blocked with a blocking solution (Beyotime) at room temperature for 2 hours. The organoids were then incubated overnight at 4 °C with primary antibodies against SOX9 (Abcam, ab185966, 1 μg ml−1), HNF4α (Abcam, ab201460, 0.5 μg ml−1), and AFP (mouse: Abcam, ab213328,4 μg ml−1; human: Abcam, ab169552, 5 μg ml−1). After washing three times with PBS, secondary antibodies (AlexaFluor®594, ab150080, 2 μg ml−1) were incubated at room temperature for 1 hour. Nuclei were stained with DAPI (Beyotime, C1002, China). The organoids were carefully transferred to glass-bottom confocal dishes and observed using a Zeiss LSM 880 confocal microscope.Drug test
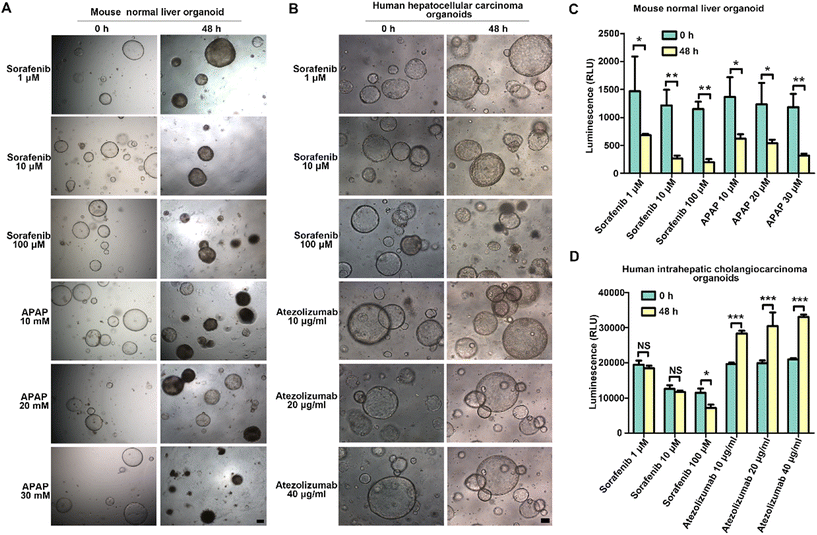
Mouse and human liver organoids were cultured in a 96-MSPC and exposed to various concentrations of sorafenib (MedChemExpress, HY-10201, USA), atezolizumab (MedChemExpress, HY-P9904, USA), and acetaminophen (APAP, MedChemExpress, HY-66005, USA) for cytotoxicity evaluation at 0 and 48 hours. The organoid culture medium was first diluted with the Real-Time Glo reagent (Promega, G9712, USA) according to the manufacturer's instructions, and this mixture was further used to dilute the respective compounds to the desired concentrations. Following treatment, images were captured at 0 and 48 hours using an inverted fluorescence microscope to observe morphological changes. Luminescence, an indicator of cellular viability, was measured at the same time points using a microplate reader to quantify organoid survival and viability.Statistical analysis
The data are presented as means ± standard deviations (SD) from three independent replicates. Statistical significance between groups was evaluated using one-way analysis of variance (ANOVA) in GraphPad Prism, with a threshold of p < 0.05 indicating statistical significance.Results
Characterization of microfluidic self-perfusion chips
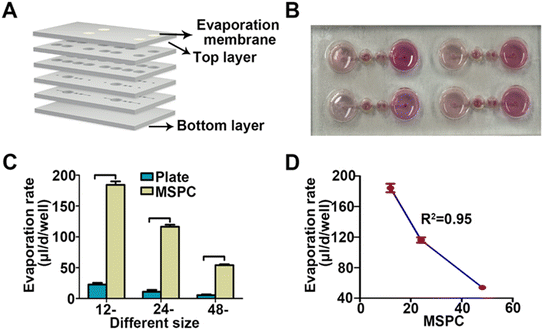
We designed a microfluidic device that drives fluid flow through evaporation, eliminating the need for complex external pumps and providing a user-friendly chip system. The microfluidic chip was fabricated using PMMA, a material with excellent optical transparency, suitable for imaging applications. Additionally, the fabrication process using PMMA was simple and conducive to large-scale production. Each chip contains four independent culture units and microfluidic channels (Fig. 1A-i). Each culture unit was divided into a reservoir, culture chamber, buffer chamber, and evaporation chamber. The buffer chamber has a unique partition design that ensured a stable liquid level in the culture chamber. Even when the liquid in the evaporation chamber was evaporated, the culture chamber maintained a certain amount of liquid for a period of time (Fig. 1A-ii). A 0.22 μm polytetrafluoroethylene (PTFE) microporous membrane covered the evaporation chamber, overlaid with the PMMA material of varying pore sizes. This design allowed for adjustment of the evaporation chamber's size, thereby controlling the evaporation rate as needed.The MSPCs consist of multiple layers, shown in the exploded view schematic (Fig. 2A). We evaluated the system under cell culture conditions by adding a cell culture medium (Fig. 2B). Fig. 2C shows the evaporation rates measured in traditional well plates and MSPCs for each plate format. For traditional 12-well, 24-well, and 48-well culture plates, the average daily evaporation rates per well were 22.59 ± 2.88 μl, 10.88 ± 3.01 μl, and 5.36 ± 1.27 μl, respectively. In contrast, the average daily evaporation rates per well in the corresponding 12-MSPCs, 24-MSPCs, and 48-MSPCs were 184.30 ± 5.60 μl, 116.33 ± 3.44 μl, and 54.00 ± 1.60 μl. The evaporation rates in the MSPCs were significantly higher than those in the traditional well plates (p < 0.05) (Fig. 2C). Furthermore, as the evaporation surface area increased, the evaporation rate increased proportionally, showing a linear relationship between the evaporation surface area and rate (R2 = 0.95) (Fig. 2D). Based on these evaporation rates, we calculated the corresponding flow rates through the culture chamber: 7.68 ± 0.23 μl per hour for the 12-MSPC, 4.85 ± 0.14 μl per hour for the 24-MSPC, and 2.25 ± 0.07 μl per hour for the 48-MSPC. Given the clear linear relationship, we prioritized the 96-MSPCs (evaporation membrane diameter: 6.8 mm) for subsequent experiments due to its high-throughput capacity and compatibility with experimental requirements. For this 96-MSPC format, the flow rate was calculated to be 1.13 ± 0.04 μl per hour.
Comparative analysis of ammonia (NH3) and lactic acid concentrations in L929 cells cultured in 3D hydrogel using traditional 96-well plates and 96-MSPCs
In our study, we measured NH3 and lactic acid concentrations to evaluate metabolic waste accumulation in 3D cultures and compared their levels between traditional culture plates and MSPCs at various cell densities and time points (Fig. 3). At evaluated time points and cell densities, NH3 concentrations were consistently and significantly lower in the 96-MSPCs compared to those in the traditional 96 well plates (p < 0.001) (Fig. 3A). The lactic acid concentration demonstrated a clear dependency on cell density (Fig. 3B). At relatively lower cell densities (104, 2 × 104, and 5 × 104 cells per well), there were no significant differences in lactic acid concentrations between the traditional plates and MSPCs at all time points (p > 0.05). In contrast, at the highest cell density (105 cells per well) on days D2, D4, D6, and D8, the lactic acid concentrations in the traditional 96 well plates were 760.72 ± 122.11 mg L−1, 513.12 ± 90.77 mg L−1, 465.67 ± 107.11 mg L−1, and 493.35 ± 162.61 mg L−1, respectively, while in the 96-MSPCs, the concentrations were 268.37 ± 58.57 mg L−1, 191.81 ± 22.21 mg L−1, 205.95 ± 22.59 mg L−1, and 220.29 ± 17.91 mg L−1, respectively. At the highest cell density, the lactic acid concentrations in the traditional 96 well plates were consistently lower than those in the 96-MSPCs (p < 0.001).Cell viability of multiple cell lines and tissue clusters in the 96-MSPC microenvironment
We investigated the growth and viability of three cell lines (AC16, L02, and BEAS-2B) and microtissue in 96-MSPCs (Fig. 4). All three cell lines exhibited an increase in cell density over time, as indicated by the higher number of fluorescent cells observed in the D6 images compared to those in D2 (Fig. 4A).The cell viability analysis was quantitatively assessed for three cell lines: AC16, L02, and BEAS-2B (Fig. 4B–D). The AC16 cells (Fig. 4B) showed viability rates of 97.97 ± 1.38%, 97.76 ± 0.87%, and 98.27 ± 1.45% at days 2, 4, and 6, respectively. Similarly, L02 cells (Fig. 4C) demonstrated 98.41 ± 1.40%, 97.39 ± 0.85%, and 98.15 ± 1.54%, while BEAS-2B cells (Fig. 4D) exhibited 98.56 ± 0.46%, 98.11 ± 1.04%, and 97.66 ± 1.52% viability at the same time points. The results showed that all three cell lines maintained consistently high viability throughout the 6-day culture period in the 96-MSPCs.
Cell viability in SD rat kidney tissue clusters was evaluated using a live/dead fluorescence assay during a 6-day culture period in both traditional plates and 96-MSPCs (Fig. 4E and F). Live cells exhibited green fluorescence, while dead cells displayed red fluorescence (Fig. 4E). Quantitative analysis of cell viability demonstrated significant differences between the traditional 96-well plates and 96-MSPCs at both D3 and D6 of culture. At D3, tissue clusters in the 96-MSPCs exhibited 88.82 ± 7.75% viability, significantly higher than the 61.39 ± 9.18% observed in the traditional 96-well plates (p < 0.001). At D6, the differential effect was more pronounced, with the 96-MSPCs maintaining 72.76 ± 7.13% viability, while viability in the traditional 96-well plates decreased substantially to 0.96 ± 0.72% (p < 0.001) (Fig. 4F).
Morphological and histological evaluation of organoids cultured in 96-MSPCs
Histological and immunofluorescence analyses (Fig. 5B) were performed to assess cellular differentiation. Hematoxylin and eosin (H&E) staining revealed a well-organized cellular architecture. Immunofluorescence analysis revealed successful hepatic organoid development, characterized by the expression of multiple lineage-specific markers: SOX9 (hepatic progenitor cells), HNF4α (hepatocytes), and AFP (alpha-fetoprotein). Nuclear counterstaining was performed with DAPI.
Human intrahepatic cholangiocarcinoma (ICC) organoids
The morphological progression of human ICC organoids is shown in Fig. 5C. At day 0, the organoids were small and densely packed. By day 3, there was a noticeable increase in size and number of organoids. After passage 1, the organoids exhibit further growth and structural complexity. The organoids demonstrated robust phenotypic stability through multiple passages (passage 3).Histological and immunofluorescence staining (Fig. 5D) further characterized these organoids. H&E staining demonstrated the cellular organization and structure. Immunofluorescence staining for hepatic progenitor cells (SOX9), hepatocytes (HNF4α), and alpha-fetoprotein (AFP) confirmed the differentiation and maturation of the ICC organoids.
Evaluation of targeted therapies and immunotherapies in mouse normal liver and human ICC organoids using 96-MSPCs
The effects of sorafenib, APAP, and atezolizumab on mouse normal liver organoids and human ICC organoids were evaluated over 48 hours (Fig. 6). To investigate the effects of sorafenib and acetaminophen (APAP) on mouse normal liver organoids, we treated the organoids with various concentrations of sorafenib (1 μM, 10 μM, 100 μM) and APAP (10 mM, 20 mM, 30 mM) for 48 hours. As shown in Fig. 6A, the morphological changes in the organoids were assessed at 0 hours and 48 hours. At the highest concentration of sorafenib (100 μM), significant shrinkage of the organoid structure was observed, indicating a strong cytotoxic effect. APAP treatment also resulted in notable morphological changes, with higher concentrations (20 mM and 30 mM) causing substantial organoid disintegration and loss of structural integrity.The luminescence assay results depicted in Fig. 6C quantitatively support these observations. Specifically, for sorafenib treatment at 1 μM, 10 μM, and 100 μM, the luminescence values at 0 hours were 1467.67 ± 620.17, 1216.67 ± 275.38, and 1151.00 ± 131.73, respectively. After 48 hours, the luminescence values were 686.33 ± 23.86, 266.00 ± 51.45, and 201.33 ± 52.99, respectively. After 48 hours, a significant decrease in luminescence was observed at all concentrations (p < 0.05), indicating reduced cell viability. Similarly, for APAP treatments at 10 mM, 20 mM, and 30 mM, the luminescence values at 0 hours were 1366.67 ± 351.19, 1233.33 ± 381.88, and 1183.33 ± 236.29, respectively. After 48 hours, the luminescence values were 619.00 ± 81.46, 539.33 ± 63.79, and 317.33 ± 36.64, respectively. All APAP treatment groups exhibited a significant reduction in luminescence after 48 hours (p < 0.05), consistent with the observed morphological changes.
Human ICC organoids exhibited a similar response to sorafenib, with growth inhibition apparent at higher concentrations (Fig. 6B). Atezolizumab treatment (10 μg ml−1, 20 μg ml−1, 40 μg ml−1) appeared to have a minimal impact on the organoid morphology or size over 48 hours. Specifically, treatment with different concentrations of sorafenib (1 μM, 10 μM, and 100 μM) resulted in luminescence values of 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 416.33 ± 1203.17, 12
416.33 ± 1203.17, 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 591.33 ± 1068.00, and 11
591.33 ± 1068.00, and 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 440.81 ± 1281.35 at 0 hours, respectively. At 48 hours, the luminescence values were 18
440.81 ± 1281.35 at 0 hours, respectively. At 48 hours, the luminescence values were 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 418.78 ± 724.33, 11
418.78 ± 724.33, 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 708.78 ± 411.65, and 7152.00 ± 977.01, respectively (Fig. 6D). While there was no significant change in luminescence with 1 μM and 10 μM sorafenib, a significant decrease was noted with 100 μM sorafenib (p < 0.05).
708.78 ± 411.65, and 7152.00 ± 977.01, respectively (Fig. 6D). While there was no significant change in luminescence with 1 μM and 10 μM sorafenib, a significant decrease was noted with 100 μM sorafenib (p < 0.05).
Notably, treatment with different concentrations of atezolizumab (10 μg ml−1, 20 μg ml−1, and 40 μg ml−1) resulted in luminescence values of 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 576.89 ± 465.63, 19
576.89 ± 465.63, 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 888.89 ± 777.41, and 20
888.89 ± 777.41, and 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 962.44 ± 368.42 in human ICC organoids at 0 hours, respectively. After 48 hours, the luminescence values were 28
962.44 ± 368.42 in human ICC organoids at 0 hours, respectively. After 48 hours, the luminescence values were 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 319.00 ± 783.51, 30
319.00 ± 783.51, 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 422.56 ± 3893.08, and 33
422.56 ± 3893.08, and 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 022.33 ± 688.42 (p < 0.001), respectively (Fig. 6D). These findings suggest that this immunotherapeutic agent does not exhibit cytotoxic effects on these ICC organoids.
022.33 ± 688.42 (p < 0.001), respectively (Fig. 6D). These findings suggest that this immunotherapeutic agent does not exhibit cytotoxic effects on these ICC organoids.
Discussion
Traditional microfluidic systems face several issues such as high complexity and cost,24,25 limiting their use for long-term cell culture and drug testing. To address these challenges, we introduce a novel adjustable microfluidic self-perfusion chip (MSPC) that offers a simpler, more efficient solution for biological research. Our pumpless fluid control system uses evaporation as the primary driving force for fluid flow. Fluid flow is driven by evaporation-induced pressure gradients through the PTFE membrane (Fig. 1A-ii), creating a negative pressure that draws fresh medium from the reservoir. The buffer chamber's stepped partition stabilizes liquid levels and prevents backflow, ensuring continuous nutrient delivery under variable evaporation This innovative approach offers several significant advantages over traditional systems. First, it simplifies microfluidic operations by eliminating the need for external pumps and associated equipment, reducing complexity and cost. Second, the chip demonstrates superior metabolic waste management, as evidenced by significantly lower ammonia and lactate levels compared to conventional static cell culture in a 96-well plate. This feature is crucial for maintaining cellular health in long-term cultures, particularly for 3D cell cultures with a high cell density. Additionally, the chip's design allows for precise control over the microenvironment through adjustable evaporation rates, providing researchers with an adjustable system for a wide range of experimental conditions. The versatility of our chip is further demonstrated by its successful application in culturing multiple cell lines, tissue clusters, and complex organoids, providing a new strategy for advanced disease modeling and drug screening applications.Microfluidic chip designs have evolved from traditional systems relying on complex external pumps to pumpless alternatives,26 requiring auxiliary equipment like rockers,27–29 shakers,30 gravity-assisted devices,17 hydrophilic fibers20 or hydrostatic pressure31 for fluid movement. However, our study's novel adjustable chip eliminates the need for any external materials or equipment. The chip is made of polymethyl methacrylate (PMMA), which not only offers excellent optical transparency, a crucial feature for imaging applications, but is also suitable for large-scale production.32,33 The evaporation well is covered with a 0.22 μm polytetrafluoroethylene (PTFE) microporous membrane, combined with an adjustable PMMA cover layer, enabling fine-tuning of the evaporation rate (Fig. 1). Compared to capillary-driven systems (e.g., hydrophilic fibers), our evaporation-driven MSPC reduces environmental dependency through adjustable evaporation pores and minimizes contamination risks by avoiding direct fluid–material interaction. The buffer chamber's partition design (Fig. 1A-ii) ensures unidirectional flow and sustained metabolite management (Fig. 3), demonstrating superior long-term stability over static cultures.
The results demonstrate that this design ensures evaporation rates in 12-, 24-, and 48-MSPCs significantly higher than in traditional well plates (Fig. 2C). Moreover, a robust linear relationship (R2 = 0.95) exists between the evaporation surface area and the evaporation rate in the chip (Fig. 2D). This linearity allows researchers to precisely adjust fluid flow rates by modifying the size of the evaporation pores, thereby meeting various experimental requirements. Furthermore, the chip's unique partition design in the buffer chamber ensures stability of the liquid level in the culture (Fig. 1A-ii). This design not only prevents backflow of liquid from the evaporation chamber to the culture chamber but also ensures that some culture medium remains in the culture chamber to maintain cell viability for a period of time, even when the evaporation chamber is completely dry. Notably, due to the use of the PMMA material and the absence of any external materials or equipment needs, the MSPC system demonstrates excellent scalability potential. While our current study validated the design with a four-unit device, the system can be easily expanded to a well-plate format, with each unit maintaining consistent evaporation characteristics due to the linear relationship between the evaporation surface area and rate. This scalable design further enhances the high-throughput capabilities of MSPC for applications such as drug screening and toxicity testing, while preserving its advantage of requiring no external pumps or complex equipment.
The efficient management of metabolic waste in cell culture systems is crucial for maintaining cellular health.34–36 Traditional organ-on-a-chip systems utilizing external pumps effectively remove metabolic waste but increase costs and introduce operational challenges like air bubbles, leakage, and contamination.37–39 However, our novel adjustable chip, based on an evaporation mechanism without the use of complex external equipment, reduces the accumulation of ammonia and lactic acid during culture. Across all cell densities and time points, the ammonia concentrations observed in our 96-MSPCs were consistently lower than those in traditional 96-well plates (Fig. 3A). Interestingly, the lactic acid concentrations showed a cell density-dependent response, with significant reductions only observed at higher cell densities (105 cells per well) (Fig. 3B). These results can be attributed to the intrinsic properties of the metabolic by-products ammonia and lactic acid, as well as their differential behavior under varying culture conditions. Ammonia, a primary product of amino acid catabolism, can be eliminated through various pathways in cell culture systems, predominantly via cellular reutilization and media exchange.40 In this study, the innovative MSPC technology significantly reduced ammonia concentrations in the culture environment by continuously supplying fresh media to the cells in the culture pool. This approach not only optimized cell culture conditions but also potentially mitigated the adverse effects of ammonia on cell growth and metabolism. In contrast, lactate is a product of anaerobic glycolysis.41 Under high cell density conditions, traditional plate cultures may lead to localized oxygen depletion, prompting cells to rely more heavily on anaerobic metabolism, thus increasing lactate production. By providing more efficient gas exchange, the adjustable MSPCs likely reduce the cells' dependence on anaerobic metabolism, thereby decreasing lactate accumulation. By maintaining a stable metabolic microenvironment without complex external equipment, it offers a cost-effective and accessible platform for studying cellular processes in high-density and long-term cultures.
The adjustable MSPC's ability to maintain a stable metabolic microenvironment for cells under high-density and long-term conditions makes it particularly advantageous for cultivating both cells and tissue clusters. Three cell lines AC16, L02, and BEAS-2B were cultured in the 96-MSPCs over 6 days (Fig. 4A). Remarkably, all three cell lines maintained consistently high viability throughout the culture period, with rates exceeding 97% at days 2, 4, and 6 (Fig. 4B–D). These results indicate the chip's ability to provide a stable and supportive microenvironment for various cell types. Furthermore, the results obtained from the culture of SD rat kidney tissue clusters are particularly compelling (Fig. 4E and F). The marked superiority of the MSPCs over traditional 96-well plates in maintaining tissue viability was evident, especially by day 6 of culture. This disparity (72.76% viability in MSPCs versus 0.96% in traditional plates) underscores the potential of this system to revolutionize ex vivo tissue culture methodologies.
Based on our findings demonstrating the MSPC system's advantages in maintaining tissue cluster viability and reducing metabolic waste accumulation, we further investigated its potential for culturing organoids, which demand more stringent microenvironmental conditions. The observed morphological development of mouse liver organoids and human hepatobiliary cancer organoids, progressing from small, dense clusters to larger, more complex structures, aligns with the typical developmental trajectory reported in conventional 3D culture systems.42,43 The expression of key markers such as SOX9, HNF4α, and AFP in these organoids indicates that our chip can recapitulate the complex cellular hierarchies present in liver tissue. The application of our adjustable MSPC in drug testing scenarios demonstrates its potential as a powerful tool for preclinical evaluation of targeted therapies and immunotherapies. Mouse organoids treated with sorafenib and acetaminophen exhibited dose-dependent morphological alterations and decreased viability (Fig. 6A–C), consistent with previous research findings using microfluidic systems.6,44 This indicates that our system can effectively recapitulate mechanisms of drug-induced liver injury observed in other dynamic culture platforms. The differential response of human intrahepatic cholangiocarcinoma (ICC) organoids to sorafenib and atezolizumab further underscores the potential of our system in advancing personalized medicine approaches (Fig. 6B–D). The observed growth inhibition at higher sorafenib concentrations is consistent with its known anti-tumor effects on ICC reported in studies using static culture systems, suggesting that our MSPC system can reliably reproduce drug responses across different culture methodologies.45,46 Interestingly, the limited impact of atezolizumab on ICC organoids underscores the complexity of immunotherapy responses in tumors.47,48 This finding aligns with clinical observations of variable immunotherapy efficacy in ICC patients.49,50 The system's capacity to simultaneously evaluate drug effects on both normal and cancerous tissues could provide valuable insights into therapeutic windows and potential side effects, potentially improving the translation of preclinical findings to clinical outcomes.
Despite the significant advantages of our adjustable MSPC, certain limitations remain. One challenge is the potential variability in evaporation rates under differing environmental conditions, such as temperature and humidity, which could affect long-term culture stability. Future iterations of the chip could integrate sensors to monitor and adjust these fluctuations,51 ensuring more precise control of the microenvironment. Future work should focus on addressing these limitations, potentially through the development of modular designs or the integration of smart materials for more dynamic control over evaporation rates. These advancements could further enhance the versatility and impact of our evaporation-based microfluidic system in biological research and drug development.
Conclusions
In conclusion, our novel microfluidic self-perfusion chip represents a significant advancement in microfluidic cell culture technology. The system demonstrates superior performance in maintaining cell viability, supporting complex organoid cultures, and facilitating drug testing applications. By eliminating the need for external pumps and other equipment, combined with independent culture units, this platform addresses key limitations of traditional microfluidic systems, enabling researchers to conduct high-throughput applications as needed. The improved waste management and long-term culture capabilities offer the potential for constructing more physiologically relevant in vitro models. Therefore, this innovative approach could have profound implications for biomedical research, drug development, and personalized medicine, potentially accelerating the translation of laboratory findings to clinical applications.Data availability
The data supporting this study's findings are available from the corresponding author upon reasonable request.Author contributions
GW generated the concept, designed the experiment, performed the data analyses and was involved in writing the manuscript. DW, WH, and QL designed the experiment and were involved in writing the manuscript. YZ performed the experiment and helped perform the analysis with constructive discussions. JL, QD, ZL, and HH contributed to the conception of the study and helped perform the analysis with constructive discussions. SW, BH, and HJ generated the concept, designed the experiment and were involved in writing the manuscript.Conflicts of interest
The authors declare that they have no conflicts of interest.Acknowledgements
The authors would like to acknowledge the support from the National Key Research and Development Program of China (2022YFA1105200, 2022YFB3804700), the National Natural Science Foundation of China (82272188), the 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (ZYYC21010), the Organ-on-a-Chip Innovation Base, Central Government-Guided Special Project for Local Scientific and Technological Development in Sichuan Province (2023ZYD0166), and the Chengdu City “Unveiling and Commanding” Science and Technology Project (2024-JB00-00018-GX).References
- D. R. Reyes, M. B. Esch, L. Ewart, R. Nasiri, A. Herland, K. Sung, M. Piergiovanni, C. Lucchesi, J. T. Shoemaker, J. Vukasinovic, H. Nakae, J. Hickman, K. Pant, A. Taylor, N. Heinz and N. Ashammakhi, Lab Chip, 2024, 24, 1076–1087 RSC.
- K. Ronaldson-Bouchard and G. Vunjak-Novakovic, Cell Stem Cell, 2018, 22, 310–324 CrossRef CAS.
- L. A. Low, C. Mummery, B. R. Berridge, C. P. Austin and D. A. Tagle, Nat. Rev. Drug Discovery, 2021, 20, 345–361 CrossRef CAS.
- W. Hu, H. P. Bei, H. Jiang, D. Wu, X. Yu, X. Zhou, Q. Sun, Q. Lu, Q. Du, L. Wang, Z. Luo, G. Wu, X. Zhao and S. Wang, Lab Chip, 2024, 24, 3718–3727 RSC.
- D. Wu, J. Wu, H. Liu, S. Shi, L. Wang, Y. Huang, X. Yu, Z. Lei, T. Ouyang, J. Shen, G. Wu and S. Wang, Lab Chip, 2023, 23, 4708–4725 RSC.
- G. Wu, J. Wu, Z. Li, S. Shi, D. Wu, X. Wang, H. Xu, H. Liu, Y. Huang, R. Wang, J. Shen, Z. Dong and S. Wang, Bio-Des. Manuf., 2022, 5, 437–450 CrossRef.
- C. Ma, Y. Peng, H. Li and W. Chen, Trends Pharmacol. Sci., 2021, 42, 119–133 CrossRef PubMed.
- S. H. Lee and J. H. Sung, Adv. Healthcare Mater., 2018, 7, 1700419 CrossRef PubMed.
- M. Mao, Z. Meng, J. He and D. Li, Trends Biotechnol., 2024, 42, 1331–1334 CrossRef PubMed.
- K. Dradrach, M. Zmyslony, Z. Deng, A. Priimagi, J. Biggins and P. Wasylczyk, Nat. Commun., 2023, 14, 1877 CrossRef PubMed.
- S. M. Scott and Z. Ali, Micromachines, 2021, 12, 319 CrossRef PubMed.
- M. A. Alioglu, Y. P. Singh, M. Nagamine, S. H. A. Rizvi, V. Pal, E. M. Gerhard, S. Saini, M. H. Kim and I. T. Ozbolat, Addit. Manuf., 2023, 70, 103566 CAS.
- X. Wang, D. T. Phan, A. Sobrino, S. C. George, C. C. Hughes and A. P. Lee, Lab Chip, 2016, 16, 282–290 RSC.
- J. D. Stucki and O. T. Guenat, Lab Chip, 2015, 15, 4393–4397 Search PubMed.
- G. M. Whitesides, Nature, 2006, 442, 368–373 CrossRef CAS.
- M. Hagiwara, T. Kawahara, Y. Yamanishi, T. Masuda, L. Feng and F. Arai, Lab Chip, 2011, 11, 2049–2054 RSC.
- A. K. Fajrial, A. Vega, G. Shakya and X. Ding, Lab Chip, 2021, 21, 4772–4778 RSC.
- M. B. Esch, H. Ueno, D. R. Applegate and M. L. Shuler, Lab Chip, 2016, 16, 2719–2729 RSC.
- M. Busek, A. Aizenshtadt, T. Koch, A. Frank, L. Delon, M. A. Martinez, A. Golovin, C. Dumas, J. Stokowiec, S. Gruenzner, E. Melum and S. Krauss, Lab Chip, 2023, 23, 591–608 RSC.
- L. C. Delon, A. Nilghaz, E. Cheah, C. Prestidge and B. Thierry, Adv. Healthcare Mater., 2020, 9, e1901784 CrossRef PubMed.
- J. L. Osborn, B. Lutz, E. Fu, P. Kauffman, D. Y. Stevens and P. Yager, Lab Chip, 2010, 10, 2659–2665 RSC.
- C. Nie, A. J. Frijns, R. Mandamparambil and J. M. den Toonder, Biomed. Microdevices, 2015, 17, 47 CrossRef.
- K.-E. C. Kuan-Yu Chen and K. Wang, presented in part at 2012 7th IEEE International Conference on Nano/Micro Engineered and Molecular Systems (NEMS), Kyoto, Japan, March, 2012 Search PubMed.
- E. K. Sackmann, A. L. Fulton and D. J. Beebe, Nature, 2014, 507, 181–189 CrossRef CAS PubMed.
- S. Halldorsson, E. Lucumi, R. Gómez-Sjöberg and R. M. T. Fleming, Biosens. Bioelectron., 2015, 63, 218–231 CrossRef CAS PubMed.
- Y. I. Wang, C. Oleaga, C. J. Long, M. B. Esch, C. W. McAleer, P. G. Miller, J. J. Hickman and M. L. Shuler, Exp. Biol. Med., 2017, 242, 1701–1713 CrossRef CAS PubMed.
- S. J. Trietsch, E. Naumovska, D. Kurek, M. C. Setyawati, M. K. Vormann, K. J. Wilschut, H. L. Lanz, A. Nicolas, C. P. Ng, J. Joore, S. Kustermann, A. Roth, T. Hankemeier, A. Moisan and P. Vulto, Nat. Commun., 2017, 8, 262 CrossRef.
- P. G. Miller and M. L. Shuler, Biotechnol. Bioeng., 2016, 113, 2213–2227 CrossRef CAS PubMed.
- V. van Duinen, A. van den Heuvel, S. J. Trietsch, H. L. Lanz, J. M. van Gils, A. J. van Zonneveld, P. Vulto and T. Hankemeier, Sci. Rep., 2017, 7, 18071 CrossRef CAS.
- Z. Chen, J. Zilberberg and W. Lee, Biomed. Microdevices, 2020, 22, 58 Search PubMed.
- M. Komeya, K. Hayashi, H. Nakamura, H. Yamanaka, H. Sanjo, K. Kojima, T. Sato, M. Yao, H. Kimura, T. Fujii and T. Ogawa, Sci. Rep., 2017, 7, 15459 CrossRef PubMed.
- W. Zhang, S. Lin, C. Wang, J. Hu, C. Li, Z. Zhuang, Y. Zhou, R. A. Mathies and C. J. Yang, Lab Chip, 2009, 9, 3088–3094 RSC.
- Z. Chen, Z. Yu and G. Chen, Talanta, 2010, 81, 1325–1330 CrossRef CAS.
- L. Liste-Calleja, M. Lecina, J. Lopez-Repullo, J. Albiol, C. Sola and J. J. Cairo, Appl. Microbiol. Biotechnol., 2015, 99, 9951–9960 CrossRef CAS.
- K. Chen, Q. Liu, L. Xie, P. A. Sharp and D. I. C. Wang, Biotechnol. Bioeng., 2001, 72, 55–61 CrossRef CAS PubMed.
- M. Yang, Q. Wang, Y. Zhu, K. Sheng, N. Xiang and X. Zhang, Trends Food Sci. Technol., 2023, 138, 564–576 CrossRef CAS.
- S. Schneider, M. Bubeck, J. Rogal, H. J. Weener, C. Rojas, M. Weiss, M. Heymann, A. D. van der Meer and P. Loskill, Lab Chip, 2021, 21, 3963–3978 RSC.
- A. Sontheimer-Phelps, B. A. Hassell and D. E. Ingber, Nat. Rev. Cancer, 2019, 19, 65–81 Search PubMed.
- B. Zhang, A. Korolj, B. F. L. Lai and M. Radisic, Nat. Rev. Mater., 2018, 3, 257–278 Search PubMed.
- I. W. M. Markus Schneider and U. von Stockar, J. Biotechnol., 1996, 46, 161–185 CrossRef.
- E. Ahn, P. Kumar, D. Mukha, A. Tzur and T. Shlomi, Mol. Syst. Biol., 2017, 13, 953 CrossRef PubMed.
- H. Hu, H. Gehart, B. Artegiani, C. Löpez-Iglesias, F. Dekkers, O. Basak, J. van Es, S. M. Chuva de Sousa Lopes, H. Begthel, J. Korving, M. van den Born, C. Zou, C. Quirk, L. Chiriboga, C. M. Rice, S. Ma, A. Rios, P. J. Peters, Y. P. de Jong and H. Clevers, Cell, 2018, 175, 1591–1606.e1519 CrossRef CAS.
- G. Sorrentino, S. Rezakhani, E. Yildiz, S. Nuciforo, M. H. Heim, M. P. Lutolf and K. Schoonjans, Nat. Commun., 2020, 11, 3416 CrossRef CAS PubMed.
- S. Lu, F. Cuzzucoli, J. Jiang, L.-G. Liang, Y. Wang, M. Kong, X. Zhao, W. Cui, J. Li and S. Wang, Lab Chip, 2018, 18, 3379–3392 RSC.
- A. Huether, M. Hopfner, V. Baradari, D. Schuppan and H. Scherubl, Biochem. Pharmacol., 2007, 73, 1308–1317 CrossRef CAS PubMed.
- C. F. Maier, L. Zhu, L. K. Nanduri, D. Kuhn, S. Kochall, M. L. Thepkaysone, D. William, K. Grutzmann, B. Klink, J. Betge, J. Weitz, N. N. Rahbari, C. Reissfelder and S. Scholch, Int. J. Mol. Sci., 2021, 22, 8675 CrossRef CAS.
- C. D. Jakubowski and N. S. Azad, Chin. Clin. Oncol., 2020, 9, 2 CrossRef PubMed.
- D. C. Guven, B. Stephen, T. K. Sahin, I. Y. Cakir, E. Erul and S. Aksoy, Crit. Rev. Oncol. Hematol., 2022, 174, 103700 CrossRef.
- R. S. Finn, S. Qin, M. Ikeda, P. R. Galle, M. Ducreux, T.-Y. Kim, M. Kudo, V. Breder, P. Merle, A. O. Kaseb, D. Li, W. Verret, D.-Z. Xu, S. Hernandez, J. Liu, C. Huang, S. Mulla, Y. Wang, H. Y. Lim, A. X. Zhu and A.-L. Cheng, N. Engl. J. Med., 2020, 382, 1894–1905 CrossRef CAS PubMed.
- J. M. Llovet, R. Montal, D. Sia and R. S. Finn, Nat Rev Clin Oncol., 2018, 15, 599–616 CrossRef PubMed.
- K. Liu, H. Ding, Z. Chong, Y. Zeng, Y. Niu, J. Zhang, Y. Kang, X. Du and Z. Gu, Chem. Eng. J., 2024, 482, 148679 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |