Scale and morphology design of metal-based catalysts for enhanced Li–CO2 battery performance
Jingzhao
Wang
a,
Xiangming
Cui
a,
Mi
Zhou
a,
Xin
Chen
a,
Shiyi
Sun
a,
Kai
Yang
b,
Jianan
Wang
 *a and
Wei
Yan
*a and
Wei
Yan
 a
a
aDepartment of Environmental Science and Engineering, State Key Laboratory of Multiphase Flow in Power Engineering, School of Energy and Power Engineering, Xi'an Jiaotong University, Xi'an 710049, China. E-mail: wangjn116@xjtu.edu.cn
bAdvanced Technology Institute, University of Surrey, Guildford, Surrey GU2 7XH, UK
First published on 20th November 2024
Abstract
Li–CO2 batteries (LCBs) have garnered significant attention due to their impressive high-energy densities and unique carbon storage capability. However, the sluggish transformation kinetics of CO2 induced high overpotential and poor cycle life greatly impeding the practical application of LCBs. The imperative task for the development of advanced LCBs is to design a bidirectional catalyst with remarkable catalytic activity, selectivity and exceptional electrochemical stability. In this review, the charge and discharge reaction mechanisms of LCBs are systematically presented, and various reaction pathways may occur based on specific reaction conditions. Then the scale and morphology regulation strategies of metal-based catalysts are highlighted, which include their effect on electronic states, coordination environments, and adsorption strengths. The recent progress of promising catalysts with different nanostructures is systematically addressed. Finally, the critical challenges and perspectives for scale and morphology design of metal-based catalysts in LCBs are discussed.
1. Introduction
Since the second industrial revolution, greenhouse gases released during energy production from traditional fossil fuels have caused serious environmental problems, hindering global sustainable development.1–3 Carbon dioxide, the most significant greenhouse gas, plays a critical role in climate change mitigation.4,5 Recently, various Carbon Capture, Utilization, and Storage (CCUS) technologies have been employed to process carbon dioxide for the achievement of carbon neutrality.6,7 However, challenges related to energy efficiency and cost have severely restricted the large-scale application of some traditional technologies (e.g. chemical absorption, mineral carbonation etc.).8,9The lithium–carbon dioxide battery (LCB), as an emerging green and sustainable energy technology, can improve CO2 capture and energy conversion efficiency via direct electrochemical CO2 reduction (CO2RR), addressing the economic constraints of traditional CCUS technologies. Based on the electrochemical reaction 3CO2 + 4Li ↔ 2Li2CO3 + C, the LCB provides a theoretical equilibrium potential up to 2.8 V and an exceptionally high energy density of 1876 W h kg−1, along with significant potential in electrochemical carbon fixation.10,11 With its high energy density and carbon cycling advantages, the LCB can potentially overcome the range limitations of current electric vehicles and drive the industry development more efficiently, economically and sustainably.12,13 Additionally, in future space exploration (e.g., Mars, Jupiter) and deep-sea explorations in CO2-rich environments, theses unique advantages of the LCB have garnered extensive attention from researchers.14
However, because the LCB involves complex multiphase transition reactions, its practical applications have not yet lived up to the initial high expectations. Specifically, the slow reaction kinetics of CO2 and the irreversible electrochemical decomposition of the insulating discharge product Li2CO3 result in large overpotentials and impractical energy efficiency.15 Meanwhile, as a wide band gap material, Li2CO3 with high thermodynamic stability easily leads to a decomposition voltage of up to 4.3 V (vs. Li/Li+) to induce electrolyte decomposition and numerous side reactions, severely impacting the battery cycle life.16 Furthermore, insufficient rate performance significantly obstructs the large-scale application of LCBs. Hence, it is crucial to develop and design highly active bifunctional electrocatalysts to expedite the CO2 reduction reaction (CO2RR) and the evolution reaction (CO2ER).
Over the past few years, electrocatalysts used in LCBs have been widely researched, including carbon-based (graphene, carbon nanotubes, etc.),17,18 noble metal-based (Ru, Ir, Pt, etc.),19–21 transition metals and their compounds (W2C, MoS2, etc.)22,23 and macromolecule compound catalysts.24,25 Design strategies involving chemical composition, morphology and size have been applied to improve the electrocatalytic activity of various heterogeneous catalysts. Notably, the size control of the cathode catalyst is crucial for optimizing the practical performance of LCBs and addressing the conflict between high preparation costs and commercial applications (this study focuses on metal-based catalysts). In terms of the scale design of metal-based catalysts, some factors (such as size, atomic coordination, composition, phase state, valence state, surface condition, end structure, defects, and surface area) play key roles and collectively affect the electronic and geometric structures of catalysts thus determining their reactivity and selectivity.26,27 Especially in heterogeneous catalysts, the size of the metal components becomes a significant structural factor. Catalyst nanoparticles with different shapes have different crystal shapes, different surface atom arrangements, densities and even electronic states. These factors can sometimes have a more pronounced impact on specific reaction rates and product selectivity than catalyst size itself, as they are directly related to chemical adsorption properties and reactivity.28,29 Therefore, an in-depth study of the size effects of metal nanocatalysts is of great significance for designing efficient LCB catalysts in the future.
2. The conversion reaction mechanism of Li–CO2 batteries
A comprehensive and in-depth understanding of the reaction mechanism of LCBs is the fundamental cornerstone for constructing and optimizing catalyst design. Despite the relatively short development history of LCBs, substantial advancements have been achieved in understanding the electrochemical reaction mechanisms of this advanced system in recent years (Fig. 1). In this section, we firstly introduce the mechanism of CO2RR and classify it in detail based on different reduction products, thereby revealing the diversity and complexity of reaction pathways. The electrochemical process of CO2ER is further explored mainly focusing on the self-decomposition of Li2CO3 and its electrochemical reaction with carbon. During this process, the self-decomposition of Li2CO3 and its electrochemical reaction with carbon are the main focus of our attention. By thoroughly analyzing the LCB reaction mechanism, we can provide a robust theoretical basis for catalyst design for guiding the future development of LCB frontier technology. | ||
| Fig. 1 The main breakthrough in the working mechanism of the Li–CO2 battery. Hou et al.30 Copyright © 2017 Wiley-VCH. Reproduced by permission of copyright owner. Qiao et al.31 Copyright © 2017 Elsevier. Reproduced by permission of copyright owner. Yang et al.32 Copyright © 2020 American Chemical Society. Reproduced by permission of copyright owner. Zhao et al.33 Copyright © 2022 American Chemical Society. Reproduced by permission of copyright owner. | ||
2.1. Discharge mechanism of Li–CO2 batteries
The Li–CO2 electrochemical reaction is affected by multiple factors, such as temperature, current density, discharge depth, working gas composition, catalyst, and electrolyte composition. While the exact reaction pathways remain contentious, the final reduction products identified under various conditions could be C, Li2C2O4, and CO. The following sections introduce all the products from CO2 in LCBs in detail.| 4Li + 3CO2 → 2Li2CO3 + C | (1) |
The following year, Liu et al. used LiCF3SO3 dissolved in TEGDME (1 M) as the electrolyte, with Ketjen Black (KB) as the catalyst, to assemble an LCB capable of reversible discharge and charge, achieving a reversible capacity of 1032 mA h g−1 (based on KB mass).35 Besides the XRD and FTIR spectra, surface-enhanced Raman spectroscopy and electron energy loss spectroscopy were further carried out to reveal the discharge products and observe the formation of amorphous carbon with porous gold as a reference. Subsequently, numerous studies have employed a range of characterization techniques, including in situ differential electrochemical mass spectroscopy (DEMS), in situ gas chromatography–mass spectrometry (GC-MS), and X-ray photoelectron spectroscopy (XPS), to further confirm the reversible formation of Li2CO3 and C.
Subsequent work further identified carbonates on the cathode using the GC-MS method, discovering that the evolution rate of CO2 gas, based on area integration compared to the theoretical mass of reduced CO2, produced a fixed amount of Li2CO3 (accounting for 62.3%). Therefore, a CO2 fixation pathway strategy shown in reaction (2) was proposed.31
| CO2 + 4/3Li+ + 4/3e− → 2/3Li2Co3 + 1/3C | (2) |
Nonetheless, the detailed discharge reaction pathways of LCBs are still not well-defined. Based on the regularity of the LiO2 disproportionation reaction in lithium–oxygen batteries (LOBs), Chen's group inferred that a similar disproportionation reaction might occur in LCBs. Considering the identified discharge products as Li2CO3 and C, they detailed a possible discharge mechanism (pathway I).30 In this pathway, carbon dioxide undergoes single electron reduction on the surface of carbon materials to form C2O42− (reaction (3)).
Pathway I:
| 2CO2 + 2e− → C2O42− | (3) |
| C2O42− → CO22− + CO2 | (4) |
| CO22− + C2O42− → 2CO32− + C | (5) |
| 2Li+ + CO32− → Li2CO3 | (6) |
Based on this, additional potential reaction mechanisms have been investigated using first-principles calculations.32Reactions (7) to (10) illustrate different electron transfer methods in the formation of C2O42−, ending with the same chemical disproportionation reaction as Pathway I to produce Li2CO3 and C.
Pathway II:
| CO2 + 2e− → CO22− | (7) |
| CO2 + CO22− → C2O42− | (8) |
Pathway III:
| 2CO2 + e− → C2O4− | (9) |
| C2O4− + e− → C2O42− | (10) |
Typically, the formation reaction of Li2CO3 occurs under a voltage condition of 2.5 V. However, Qiao et al. made a significant finding in their research, observing a distinctive discharge plateau at a lower voltage (1.8 V). Through in-depth analysis of in situ Raman spectroscopy, they noticed a new peak gradually appearing at 520 cm−1, which is likely related to the formation of Li2O.31 To deeply explain this new plateau, they integrated first-principles calculation results and proposed reaction (11) to illustrate this discharge process:
| 4Li + CO2 → 2Li2O + C | (11) |
In the subsequent initial charging phase, O2 release was observed. Furthermore, according to the 4e−/O2 mass-to-charge ratio obtained during this phase, the decomposition pathway of the resulting Li2O can be further identified, as shown in reaction (12).
| 2Li2O → O2 + 4Li+ + 4e− | (12) |
| 2Li + 2CO2 → Li2C2O4 | (13) |
| 2Li + 2CO2 → Li2CO3 + CO | (14) |
Meanwhile, Zhao et al. conducted a more detailed study on the reaction pathway by utilizing surface-enhanced Raman spectroscopy to capture clear evidence of CO2, CO, and Li2CO3 on a gold electrode coated with a monolayer of Cu.33 They further elucidated the detailed process of CO2 conversion to CO, which can be described by a series of intermediate steps and reaction eqn (15)–(17) with the aid of DFT calculations. Additionally, these studies confirmed the feasibility of deriving high-value carbon-based products from the LCB system.
| CO2 + e− → CO2− | (15) |
| CO2− + CO2 → CO3− + CO | (16) |
| 2Li+ + CO3− + e− → Li2CO3 | (17) |
The currently identified reduction processes for the three products are listed in Table 1. In the initial stage of discharge, CO2 is firstly reduced to CO2−. Generally, it further converts to C2O42−, which then undergoes disproportionation with Li+ to produce Li2CO3 and C. However, the intermediate product Li2C2O4 can stabilize and serve as the final product in certain circumstances. Furthermore, for certain cathode materials like zinc, the formation of C2O42− is thermodynamically unfavorable, thus CO2− tends to be reduced to CO and combine with Li+ to form Li2CO3. The reduction products of CO2 in aprotic LCBs will exhibit diversity due to the multiple valence states of carbon. Hence, plenty of efforts are still required to thoroughly comprehend the intrinsic mechanisms of this complex reaction system.
| Possible discharge pathways for LCBs | ||
|---|---|---|
| Pathway I: | Pathway II: | Pathway III: |
| 2CO2 + 2e− → C2O42− | 2CO2 + 2e− → C2O42− | CO2 + e− → CO2− |
| C2O42− → CO22− + CO2 | 2Li+ + C2O42− → Li2C2O4 | CO2− + CO2 → CO3− + CO |
| CO22− + C2O42− → CO32− + C | 2Li+ + CO3− + e− → Li2CO3 | |
| 4Li + 3CO2 → 2Li2CO3 + C, E0 = 2.80 V versus Li/Li+ | 2Li + 2CO2 → Li2C2O4, E0 = 3.01 V versus Li/Li+ | 2Li + 2CO2 → Li2CO3 + CO, E0 = 2.50 V versus Li/Li+ |
2.2. Charge mechanism of Li–CO2 batteries
Unlike the single product in LOBs, the two critical products produced in LCBs generally render the charging process not entirely reversible. Upon thorough investigation of the CO2ER mechanism, it was found that Li2CO3 occupies a central position in most CO2RR pathways. However, due to its electrical insulation, wide band gap (5.026 eV) and thermodynamic stability, Li2CO3 has high decomposition energy and slow reaction kinetics, which severely constrain the practical energy efficiency and cycle life of LCBs.39,40Table 2 lists the possible reaction pathways and corresponding reaction potentials during the charging process. This section emphasizes the self-decomposition of Li2CO3 and the co-decomposition process involving Li2CO3 and C.| Possible reactions | ΔrG0 (kJ mol−1) | E 0 (V versus Li/Li+) |
|---|---|---|
| 2Li2CO3 → 4Li+ + O2+ 2CO2 + 4e− | 737.72 | 3.82 |
| 2Li2CO3 → 4Li+ + O2˙− + 2CO2 + 3e− | N/A | N/A |
| 2Li2CO3 → 4Li+ + 1O2 + 2CO2 + 4e− | 737.72 | 3.82 |
| 2Li2CO3 + C → 4Li+ + 3CO2 + 4e− | 540.52 | 2.80 |
| 2Li2CO3 → 4Li+ + O2 + 2CO2 + 4e− | (18) |
Nevertheless, O2 generation is not consistently observed during the charging process. Consequently, Peng et al., based on prior studies, defined an O2˙− mediated pathway. The generated O2˙− is produced by the decomposition of Li2CO3 as a strong super-nucleophilic reagent (reaction (19)).41
| 2Li2CO3 → 4Li+ + O2˙− + 2CO2 + 3e− | (19) |
Qiao et al. further utilized DEMs to quantitatively analyze the reaction process in a pure CO2 battery system. The results show that O2˙− tends to form at low currents, while O2 generation is more significant at high current condition, with the decomposition pathway largely dependent on kinetic factors.31 Subsequently, Mahne et al. employed selective chemical probes and online mass spectrometry to verify the formation of 1O2, elucidating the O2 absence phenomenon (reaction (20)).42 However, the initial reaction potential for this reaction is as high as 3.8 V, resulting in significant energy loss. In conclusion, the active oxygen generated from the self-decomposition of Li2CO3 and the required high charging voltage result in electrolyte decomposition and electrode corrosion, which are unfavorable for the practical application of high-performance LCBs.
| 2Li2CO3 → 4Li+ + 1O2 + 2CO2 + 4e− | (20) |
| Li2CO3 + C → 4Li+ + 3CO2 + 4e− | (21) |
3. Metal-based catalyst scale and morphology design
Typically, a conventional LCB is composed of several key components, including a lithium metal anode, electrolyte, separator, and cathode/catalyst (Fig. 2). Although each component influences the electrochemical performance of the LCB, the key CO2 conversion reactions are mainly governed by the catalyst in the gas electrode.45–47 This heterogeneous catalysis occurs on the surface of the catalyst particles, more precisely at the interface between the solid catalyst, carbon dioxide gas, and electrolyte.48,49 Therefore, the development of nanoscale catalysts is inevitable, as they expose abundant catalytic surfaces to gas reactants, electrolyte, and lithium ions. Moreover, the reduction in catalyst size causes significant changes in the electronic states and coordination environment of surface atoms, thereby markedly altering their catalytic selectivity and activity.The proportion of catalyst surface atoms increases significantly with decreasing catalyst size, resulting in coordination deficiency and the creation of numerous dangling bonds and unsaturated bonds. This endows the catalysts with higher surface activity, making it easy to combine with other atoms to catalyze CO2 transformation quickly.50,51 Additionally, the electronic states of metal nanoparticles vary with size reduction, even transitioning from a metallic state to a molecular state, fundamentally altering their catalytic performance.52,53 Heterogeneous catalysis in LCBs occurs at the solid–liquid–gas interface through the crucial step of surface adsorption at the catalytic sites on the catalyst. In surface science theory, if the catalytic reaction follows the Langmuir–Hinshelwood mechanism, the reactant molecules, dissociated species and intermediates typically must interact with the atoms at the catalytic sites.
The magnitude of this adsorption energy is closely related to the proportion of uncoordinated metal atoms. Generally, catalyst atoms with low coordination numbers have stronger adsorption capacities for a given molecule compared to those with high coordination numbers.54,55 Furthermore, the proportion of low-coordination surface atoms in the catalyst, which is scale-dependent, determines the cohesive energy of the catalyst, thereby affecting the chemisorption activation energy of adsorbates on it.56 In summary, if the dissociation of reaction molecules is the rate-determining step in heterogeneous catalysis, size-dependent catalytic activity may occur. However, when low-coordination metal atoms easily dissociate reactants and strongly bind with the dissociated species (e.g., the O–O bond of O2 molecules), the activity may decrease as the catalyst size decreases. Additionally, different crystal surfaces of metal particles have varying geometric structures and electronic states, leading to different activities/selectivities in the same catalytic reactions.57 It is now accepted that not every surface or crystal structure of a catalyst is active in gas conversion.58 Thus, identifying and further exposing more active catalytic sites to improve reaction kinetics is equally essential.
Recently, various dimensional nanostructures (such as nanoparticles, nanofibers, nanosheets, and single-atom catalysts (SACs)) have been developed to enhance the contact surface between the catalyst and the surrounding reaction environment. In this section, we classify metal-based catalysts into four typical nanostructures (ranging from microscopic two-dimensional (2D)/3D to atom scale), and provide a detailed overview of the intrinsic correlation between catalyst size design and their catalytic activity, including catalyst composition and the electrochemical performance of LCBs, as shown in Table 3.
| Typical morphology | Morphological properties | Cathodes | Overpotential | Discharge capacity | Stability | Ref. |
|---|---|---|---|---|---|---|
| Nanosheet/block | • Open 2D channels for ion transport | RuRh nanosheet | 1.00 V at 200 mA g−1 | 9600 mA h g−1 at 200 mA g−1 | 190 cycles at 200 mA g−1 | 59 |
| • High cost for synthesis | Cu-TCPP | 1.21 V at 100 mA g−1 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 393 mA h g−1 at 200 mA g−1 393 mA h g−1 at 200 mA g−1 |
123 cycles at 200 mA g−1 | 60 | |
| Ti3C2–MnO | 0.89 V at 100 mA g−1 | 5722 mA h g−1 at 200 mA g−1 | 220 cycles at 100 mA g−1 | 61 | ||
| MoS2/Co9S8@CP | 0.68 V at 20 μA cm−2 | 3954 μA h cm−2 at 40 μA cm−2 | 640 h at 20 μA cm−2 | 62 | ||
| SA Ru–Co3O4/CC | 1.05 V at 100 mA g−1 | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 915 mA h g−1 at 100 mA g−1 915 mA h g−1 at 100 mA g−1 |
251 cycles at 200 mA g−1 | 63 | ||
| Ni–Fe–δ-MnO2 | 1.32 V at 100 mA g−1 | 8287 mA h g−1 at 100 mA g−1 | 100 cycles at 100 mA g−1 | 64 | ||
| Nanotube/wire | • Mechanical reliability | N,S-doped CNTs | 1.67 V at 200 mA g−1 | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 560 mA h g−1 at 200 mA g−1 560 mA h g−1 at 200 mA g−1 |
538 cycles at 200 mA g−1 | 65 |
| • Ability to build highly conductive continuous networks | Holey CNTs | 1.18 V at 50 mA g−1 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 mA h g−1 at 100 mA g−1 500 mA h g−1 at 100 mA g−1 |
150 cycles at 100 mA g−1 | 66 | |
| VA-NCNT | 1.33 V at 50 mA g−1 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 652 mA h g−1 at 100 mA g−1 652 mA h g−1 at 100 mA g−1 |
603 cycles at 500 mA g−1 | 67 | ||
| Co–MnO2/CC | 0.73 V at 100 mA g−1 | 8160 mA h g−1 at 100 mA g−1 | 500 cycles at 100 mA g−1 | 68 | ||
| CC@MoN NFs | 0.36 V at 10 μA cm−2 | 6542 μA h cm−2 at 20 μA cm−2 | 86 cycles at 100 μA cm−2 | 69 | ||
| Mo3N2 NFs | 0.64 V at 10 μA cm−2 | 8361 μA h cm−2 at 20 μA cm−2 | 391 cycles at 100 μA cm−2 | 70 | ||
| Nanoparticle | • Highly active area | Ru@super P | 1.29 V at 100 mA g−1 | 8229 mA h g−1 at 100 mA g−1 | 80 cycles at 100 mA g−1 | 43 |
| • Easy preparation | Ir/CNFs | 1.38 V at 100 mA g−1 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 045 mA h g−1 at 50 mA g−1 045 mA h g−1 at 50 mA g−1 |
45 cycles at 50 mA g−1 | 71 | |
| • Low cost | Ni-NG | 1.48 V at 100 mA g−1 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 625 mA h g−1 at 100 mA g−1 625 mA h g−1 at 100 mA g−1 |
101 cycles at 100 mA g−1 | 72 | |
| • High resistance | IrRu/N-CNTs | 1.45 V at 100 mA g−1 | 6228 mA h g−1 at 100 mA g−1 | 600 cycles at 100 mA g−1 | 73 | |
| Ni/Ru HNPs | 0.88 V at 200 mA g−1 | ∼9800 mA h g−1 at 200 mA g−1 | 120 cycles at 200 mA g−1 | 74 | ||
| Single-atom | • High activity | Fe-ISA/N,S-HG | 1.17 V at 100 mA g−1 | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 174 mA h g−1 at 100 mA g−1 174 mA h g−1 at 100 mA g−1 |
210 cycles at 1000 mA g−1 | 75 |
| • High atomic efficiency | TeAC@NCNS | 0.97 V at 25 μA cm−2 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 351 μA h cm−2 at 50 μA cm−2 351 μA h cm−2 at 50 μA cm−2 |
120 cycles at 100 μA cm−2 | 76 | |
| • Hard preparation | Cd SAs/NC | 1.31 V at 200 mA g−1 | 160![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 045 mA h g−1 at 500 mA g−1 045 mA h g−1 at 500 mA g−1 |
1685 cycles at 1 A g−1 | 77 | |
| RuAC+SA@NCB | 1.05 V at 100 mA g−1 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 652 mA h g−1 at 100 mA g−1 652 mA h g−1 at 100 mA g−1 |
230 h at 300 mA g−1 | 78 |
3.1. Nanosheet/block catalysts (2D/3D)
Nanosheet catalysts, due to their ultra-thin 2D structure, have a high specific surface area, allowing the entire 2D surface to be in close contact with the electrolyte, thereby achieving rapid charge transfer.79 Additionally, this 2D structure can significantly enhance in-plane electronic conductivity to improve catalytic efficiency.80,81 The unique electronic structure and surface properties of nanosheet catalysts result in superior catalytic activity in the electrocatalysis of LCBs.79 In a representative study, Xing et al. developed ultra-thin triangular RuRh alloy nanosheets as highly efficient catalysts, greatly improving the electrochemical performance of the LCB.59 Using transmission electron microscopy (TEM) and high-angle annular dark-field scanning TEM (HAADF-STEM) analysis, each nanosheet was determined to be just 1.9 ± 0.5 nm thick (about 6–8 atomic layers), demonstrating its ultra-thin characteristics (Fig. 3a and b). The synthesized RuRh catalyst was evenly deposited on a carbon support to serve as the LCB cathode for catalytic activity assessment. Under harsh conditions with a discharge current of 1000 mA g−1 and a capacity up to 1000 mA h g−1, the RuRh battery could stably cycle 180 times, maintaining a low overpotential below 1.35 V (Fig. 3c). DFT calculations elucidated the superior catalytic performance, indicating that Ru and Rh interact through d–d orbitals, significantly optimizing the electron transfer capability of Ru surface atoms and balancing the CO2 adsorption performance at Ru sites (Fig. 3d).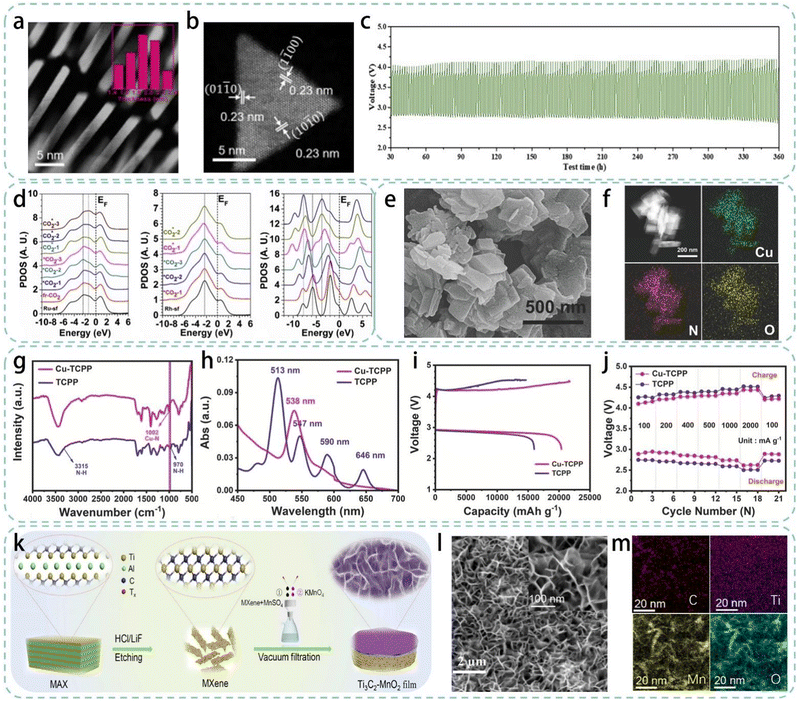 | ||
| Fig. 3 (a) HAADF-STEM images of RuRh nanosheets perpendicular to the TEM grid (the inset is the thickness distribution of RuRh nanosheets), (b) HAADF-STEM images of RuRh nanosheets, (c) cycling performance of the LCBs with the VC72 and RuRh/VC72 as cathodes, (d) 4d-band PDOS evolutions of different CO2-adsorption configurations on the Ru-sf site and the middle is the Rh-sf site, the left is the PDOS of each adsorbing Li2CO3 with relation to adsorption stabilities.59 Copyright © 2020 Elsevier. Reproduced by permission of copyright owner. (e) SEM image of Cu-TCPP, (f) element mappings of C, Cu, N, and O, (g) FT-IR spectra, (h) UV-vis spectra in the range of 450–700 nm of Cu-TCPP and TCPP, (i) the full charge and discharge capacities and (j) rate performance of Cu-TCPP and TCPP.60 Copyright © 2022 Wiley-VCH. Reproduced by permission of copyright owner. (k) Schematic diagram of the synthesis of Ti3C2–MnO2 films, (l) top-view SEM image and (m) element mappings of the Ti3C2–MnO2 film.61 Copyright © 2021 American Chemical Society. Reproduced by permission of copyright owner. | ||
Besides the traditional highly active noble metal-based catalysts, the morphological design of transition metals is another way to enhance catalytic activity. In a recent study, researchers employed a straightforward solvothermal method to integrate Cu catalytic sites with porphyrin organic ligands, creating an efficient LCB cathode catalyst (designated as Cu-TCPP).60 The morphology of Cu-TCPP with nanosheets is characterized by SEM (Fig. 3e). The corresponding elemental mapping results indicate that the elements C, Cu, N, and O are uniformly distributed on Cu-TCPP (Fig. 3f). The formation of Cu–N coordination was verified by Fourier transform infrared spectroscopy (FTIR) and ultraviolet-visible spectroscopy (UV-vis), altering the chemical environment of N (Fig. 3g and h). Benefiting from the electron-gathering ability of Cu–N4 active sites and the excellent CO2 adsorption of porphyrin ligands, the LCB assembled with Cu-TCPP as the catalyst exhibited a high discharge capacity of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 393 mA h g−1 at 100 mA g−1 and an excellent rate performance (Fig. 3i and j).
393 mA h g−1 at 100 mA g−1 and an excellent rate performance (Fig. 3i and j).
MXenes, derived from transition metal carbides, nitrides, or carbonitrides, are a new class of 2D nanomaterials with excellent electronic and ionic conductivity and abundant exposed metal active sites.82 These excellent electrochemical properties have attracted widespread attention in the fields of energy storage and conversion.83 Wang et al. prepared MXene-MnO2 composites using vacuum-assisted filtration and in situ reduction methods, applying them directly as self-supporting cathodes of the LCB (as shown in Fig. 3k).61 Scanning electron microscopy (SEM) observation of the Ti3C2–MnO2 film confirmed that the few-layer MXene film was well dispersed and the in situ grown MnO2 nanosheets were uniformly loaded on it (Fig. 3l). Additionally, the elemental mapping clearly exhibited the uniform distribution of C, Ti, O, and Mn, demonstrating the coexistence of Ti3C2 film and MnO2 nanosheets (Fig. 3m). This work demonstrates a significant performance enhancement by using MXene as a self-supporting cathode in the LCB (low overpotential: 0.89 V, long cycle life: 220 cycles), providing a new perspective for the design of rechargeable LCB catalysts.
2D transition metal dichalcogenides (TMDs) are an interesting class of layered materials that have attracted extensive research in various fields such as electrocatalysis, Li–sulfur batteries, and Li–gas batteries, due to their unique physical and chemical properties.84,85 However, the low reactivity of atoms in the basal plane seriously hinders their practical application.86 Therefore, it is necessary to develop a new strategy to minimize the exposure of the TMD basal plane in order to enhance their catalytic activity. Zhou's team developed a vertically grown MoS2 bidirectional catalyst loaded on carbon paper (CP) with Co9S8 (V-MoS2/Co9S8@CP), which had abundant edge active sites and could regulate the electronic transfer path on the basal plane.62 Firstly, Mo–Co(CO3)0.5OH·0.11H2O was vertically loaded on the CP surface by a hydrothermal method. Then, it was placed in a tubular furnace and reacted with gaseous S for 2 h to prepare the MoS2/Co9S8 heterostructure. During the sulfidation process, due to the weak van der Waals interaction between MoS2 layers, MoS2 nanosheets vertically grew on the surface of Co9S8 sheets to form interfaces along the edges of MoS2. SEM and TEM images confirmed that the CP surface was completely covered with nanosheets, and the porous structure formed by the interconnection of these sheets provided rapid CO2 transport channels and space for the deposition of discharge products (Fig. 4a). High-resolution transmission electron microscopy (HRTEM) images show that the size of vertically aligned molybdenum disulfide nanosheets was about 5 nm, as shown in Fig. 4b. XPS spectroscopy results display a negative shift of Mo4+ and a positive shift of Co (Co2+ and Co3+), indicating that a heterojunction was well formed with a strong electronic interaction between Co9S8 and MoS2 (Fig. 4c and d). The LCB with the V–MoS2/Co9S8@CP cathode exhibited an energy efficiency of 81.2% and an extremely low potential difference of 0.68 V at 20 μA cm−2 (Fig. 4e). The essence of the excellent dual-function activity of V–MoS2/Co9S8@CP was obtained through DFT calculations. Compared with the MoS2 basal plane and Co9S8, the MoS2 edge possesses a higher adsorption energy for CO2 and Li, resulting in better catalytic performance for CO2RR (Fig. 4f). Moreover, the fully exposed MoS2 edges and Co9S8 in V–MoS2/Co9S8@CP exhibited a complementary effect, resulting in a significant reduction in the energy barrier of the rate-determining step. The correlation between catalytic properties and exposed facets confirms the chemical essence of morphological effects, guiding the rational design of TMDs with specific crystal facets and offering insights into high-performance cathode catalysts for LCBs.
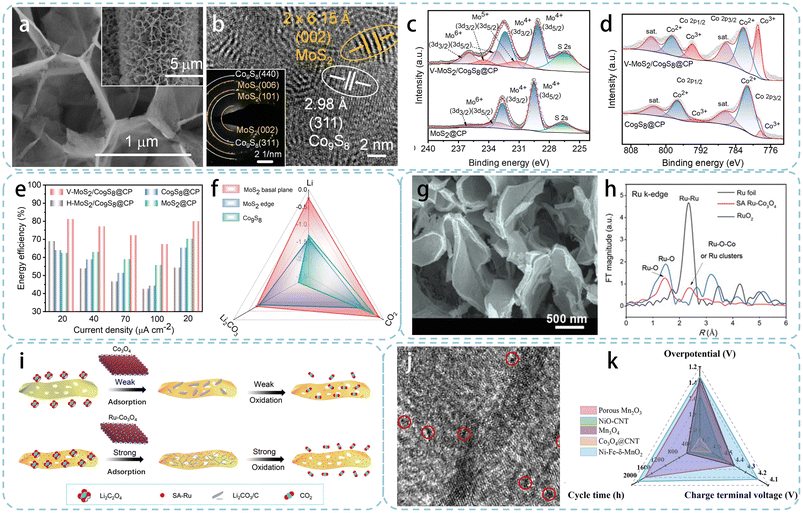 | ||
| Fig. 4 (a) SEM and (b) HRTEM images of V–MoS2/Co9S8@CP, (c) and (d) XPS fine spectra of V–MoS2/Co9S8@CP and MoS2@CP, (e) energy efficiencies at different current densities for four cathodes, (f) adsorption energies of Li, CO2 and Li2CO3 on the different sites.62 Licensed under a Creative Commons Attribution 4.0 Unported International License. (g) SEM image of SA Ru–Co3O4/CC, (h) Ru K-edge EXAFS for SA Ru–Co3O4, RuO2, and Ru foil, (i) schematic illustrations of the working mechanism with different cathodes.63 Licensed under a Creative Commons Attribution 4.0 Unported International License. (j) HRTEM image of Ni–Fe–δ-MnO2 and (k) comparison of the electrochemical properties.64 Licensed under a Creative Commons Attribution 3.0 Unported International License. | ||
To utilize the beneficial properties of SACs and mitigate their drawbacks, high-activity single atoms are loaded onto the surface of planar metal oxides, providing both fully exposed active sites and suitable structures for mass transfer and deposition of discharge products.87,88 Lian et al. ingeniously loaded Ru atoms onto the surface of carbon cloth (CC) supported Co3O4 nanosheets through ion exchange and template replication strategies (referred to as SA Ru–Co3O4/CC).63 The entire preparation process of SA Ru–Co3O4/CC was green and simple, with the potential for large-scale synthesis and application. The SEM image in Fig. 4g showed that the SA Ru–Co3O4/CC obtained after annealing was elliptical and slightly curled. The electronic state and coordination of SA Ru–Co3O4 were further studied using X-ray absorption fine structure (XAFS) spectroscopy. The corresponding spectroscopic results demonstrated that Ru atoms replaced Co nodes in the Co-MOF to form the CoRu-MOF precursor, which subsequently formed Ru–O–Co bonds during the annealing process (Fig. 4h). DFT calculations show that single-atom Ru active sites could significantly enhance the adsorption of key intermediates (Li2C2O4), thereby optimizing the growth pathway of discharge products (Fig. 4i). This work not only presents a green and convenient preparation strategy for SACs, but also provides a new approach for the electrocatalyst morphology study.
Complex 3D nanostructures assembled from 2D nanosheets can effectively promote the transport of ions and electrons and minimize the inactive components. Developing new strategies to construct 3D electrodes with porous structures is important for utilizing nano-materials for energy storage. In a recent study, researchers prepared a nano-flower composite material (referred to as Ni–Fe–δ-MnO2) composed of ultra-thin nanosheets using a combination of co-precipitation and hydrothermal methods.64 The HRTEM image in Fig. 4j revealed numerous micropores on the surface of Ni–Fe–δ-MnO2, which can provide more nucleation sites and oxygen vacancies, facilitating the electron transfer and nucleation of Li2CO3. XPS was further used to investigate the chemical states and electronic structures of Ni–Fe–δ-MnO2. The co-coordination of Ni/Fe with the Mn center and O atoms shifts the Ni 2P and Fe 2P peaks of Ni–Fe–δ-MnO2 to lower binding energies, enhancing metal-intermediate adsorption, thus altering the MnO2 coordination state and creating more oxygen vacancies. Benefiting from the huge surface area and suitable porosity of the nano-flower 3D structure, as well as the synergistic catalytic effect of Ni, Fe and δ-MnO2, the LCB with the Ni–Fe–δ-MnO2 cathode exhibited a high discharge capacity of 8287 mA h g−1 at a current density of 100 mA g−1 with stable cycling over 100 times (Fig. 4k).
In summary, the additional value of higher packing density and volumetric performance of 2D nanomaterials has been widely applied in LCBs. However, the low electron transfer within the substrate and the potential problem of re-aggregation severely hinders their practical performance. Therefore, combining 2D nanomaterials with materials possessing other nanostructures, which facilitates easier access of reactants to surface active sites and exposes more reaction sites, may be an effective strategy to expand the applications.
3.2. Nanowires/tubes (1D)
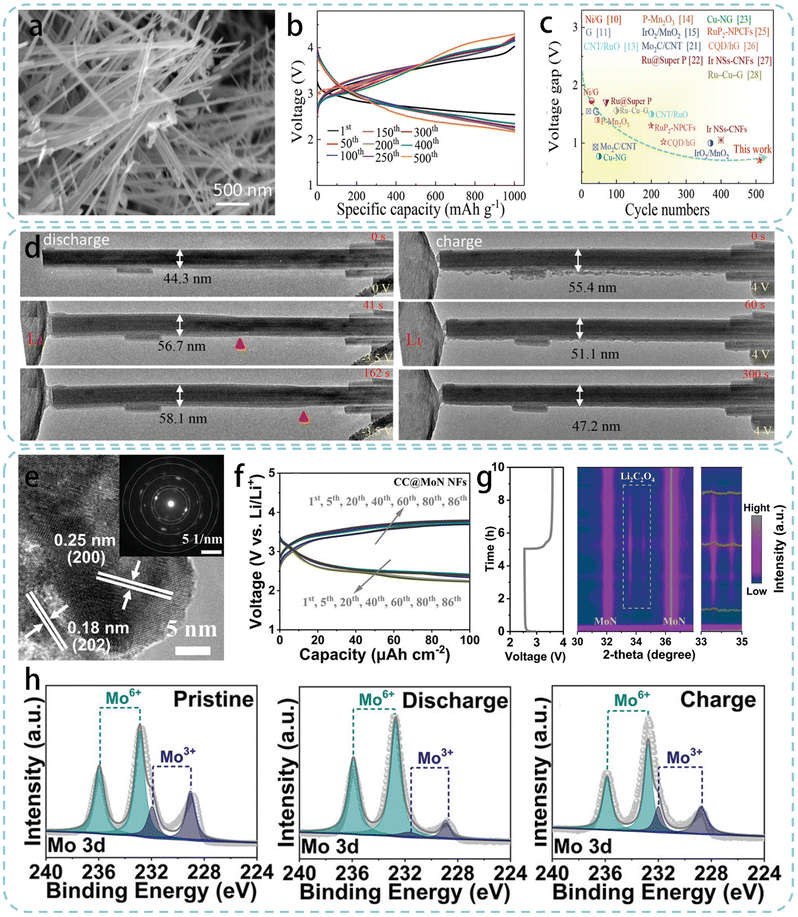
The core characteristic of one-dimensional (1D) structures lies in their ability to construct highly conductive continuous networks, achieving efficient electron transfer by integrating conductive 1D nanomaterials such as carbon nanotubes and metal nanowires on large-area and thick electrodes.89 Moreover, 1D nanomaterials with high mechanical strength can form flexible structures without binders, offering great potential for wearable applications.90 Since 2015, when Zhou's group firstly used carbon nanotubes (CNTs) as cathode catalysts in the LCB, 1D nanomaterials have been considered as a promising strategy for building high-performance LCB cathodes.18 In subsequent work, researchers have also used some strategies (e.g., doping and defect engineering) to further enhance the intrinsic catalytic performance of 1D nanomaterials, aiming to construct practical carbon-based non-metallic catalysts in LCBs. Song et al. developed a simple and scalable method to prepare N, S-doped CNTs, which significantly enhanced the intrinsic activity of CNTs.65 The TEM image of N,S-doped CNTs in Fig. 5a displayed the existing wrinkles on the carbon nanotube channels, which could enhance the active surface and induce defects to enhance the electrocatalytic activity in LCBs. High-resolution XPS spectra confirmed the successful doping of N and S into CNTs (Fig. 5b and c). LCB coin cells were further assembled to investigate the catalytic activity of the N,S-doped CNTs as cathode catalysts. The LCBs with N,S-doped CNTs exhibited higher discharge capacity and cycle life than those with undoped and nitrogen-doped CNTs. Furthermore, when assembled into quasi-solid-state flexible LCBs with a self-developed gel electrolyte, N,S-doped CNTs also enabled an extremely high capacity of 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 560 mA h g−1 and an ultra-long cycle life of over 110 days (538 cycles at 200 mA g−1, Fig. 5d). In another work, researchers introduced a porous structure on CNTs through controlled heat treatment and acid washing steps (Fig. 5e).66 The introduced porous structure not only facilitated the rapid transport of ions/electrolytes but also increased the number of highly active defect sites. Compared to pristine CNTs, the treated porous CNTs exhibited an increased Brunauer–Emmett–Teller (BET) surface area and pore volume, indicating successful opening of the nanotube lumen by pore formation (Fig. 5f and g). These studies imply that CNT catalysts with defect and doping modifications can exhibit more broad application prospects in next-generation high-performance LCBs and catalysis-based fields.
560 mA h g−1 and an ultra-long cycle life of over 110 days (538 cycles at 200 mA g−1, Fig. 5d). In another work, researchers introduced a porous structure on CNTs through controlled heat treatment and acid washing steps (Fig. 5e).66 The introduced porous structure not only facilitated the rapid transport of ions/electrolytes but also increased the number of highly active defect sites. Compared to pristine CNTs, the treated porous CNTs exhibited an increased Brunauer–Emmett–Teller (BET) surface area and pore volume, indicating successful opening of the nanotube lumen by pore formation (Fig. 5f and g). These studies imply that CNT catalysts with defect and doping modifications can exhibit more broad application prospects in next-generation high-performance LCBs and catalysis-based fields.
 | ||
| Fig. 5 (a) TEM images of the N,S-doped CNTs, high-resolution XPS spectra of (b) N 1s and (c) S 2p for the N,S-doped CNTs, (d) discharge/charge profiles of the N,S-doped CNTs.65 Copyright © 2020 Elsevier. Reproduced by permission of copyright owner. (e) Schematic illustration for the synthesis of the holey CNTs, (f) HRTEM image of holey CNTs, (g) N2 adsorption–desorption curves (inset is the corresponding surface area) of holey CNTs and pristine CNTs.66 Copyright © 2022 Elsevier. Reproduced by permission of copyright owner. (h) TEM image of VA-NCNTs, (i) cycle performance and energy efficiency of VA-NCNTs at 250 mA g−1 and 1000 mA h g−1, and (j) schematic diagram and a digital photograph of the flexible quasi-solid-state LCB,67 Copyright © 2021 Elsevier. Reproduced by permission of copyright owner. | ||
Although optimizing CNT catalysts has made progress in capacity and cycle life, LCBs still suffer from inherent issues such as a low effective surface area, disordered aggregation and unreliable mechanical performance. To further enhance the catalytic ability of carbon nanotubes, Wang's group developed a self-supporting cathode of N-doped carbon nanotube arrays grown vertically on titanium wire. Firstly, straight and thick-walled carbon nanotube arrays (VA-NCNT) were prepared through two-stage heating based on a typical floating catalyst chemical vapor deposition technique using titanium wire as the substrate and acetylacetone nickel as the catalyst.67 SEM observation showed that the VA-NCNT array was uniformly anchored on the Ti surface after annealing, providing abundant conductive networks and space for product accommodation. The TEM image in Fig. 5h clearly showed that individual CNTs were linear with an outer wall thickness of about 9 nm. Compared to previously reported CNT-based catalysts, VA-NCNTs had thicker carbon walls and abundant pore space, which helps maintain structural stability during long-term cycling and exposes more active sites, laying a solid foundation for the preparation of high-performance flexible LCBs. Assembled as a self-supporting cathode in button-type LCBs, they could cycle 603 times under harsh conditions of 500 mA g−1 and 500 mA h g−1. It is worth noting that the cathode from a dismantled and cleaned nostalgic battery could still cycle 600 times under the same conditions (total cycling time up to 2406 h), superior in operating time and cycle number to most reported materials (Fig. 5i). To further demonstrate the application potential of VA-NCNTs in flexible devices, VA-NCNTs were assembled with a polymer electrolyte into a flexible fiber-shaped LCB (as shown in Fig. 5j). The battery could cycle over 100 times (200 h) at a current density of 250 mA g−1 and stably power an LED at various bending angles.
In addition to 1D carbon-based materials, 1D catalysts composed of transition metal oxides also exhibit excellent electrocatalytic activity in the field of LCBs. In an early report, researchers prepared cobalt-doped MnO2 nanowires loaded on carbon cloth (Co–MnO2/CC) via a simple hydrothermal method.68 The SEM image in Fig. 6a reveals that the CC is completely covered by the thorn-bush nanowires after treatment. LCBs prepared with the optimized Co–MnO2/CC electrode exhibited a low overpotential of about 0.73 V and high stability over 500 cycles, which is the best among the LCBs reported at that time (Fig. 6b and c). In situ environmental TEM was used to investigate the morphology and structural evolution of the Co–MnO2/CC electrode during charge and discharge processes within the potential window of −3.2 V to +4 V, demonstrating the excellent reversibility of the Co–MnO2/CC electrode. The arrow in front of the reaction side indicated that Li ions diffuse along the MnO2 surface and react with CO2 gas to form sheet-like discharge products, facilitating their decomposition during charging (Fig. 6d). Spinel metal oxides have also attracted significant interest in the field of metal–gas batteries due to their unique electron coordination. Thoka et al. synthesized porous ZnCO2O4 nanorods composed of numerous nanoparticles through a combination of conventional hydrothermal treatment and annealing. The nanorods with anisotropic morphologies on the nano scale greatly affected the reaction performance of the CO2 transformation reaction by selectively exposing the desired facets. The cathode assembled with a mixture of these nanorods and CNTs significantly improved the performance of the LCB (maintaining a charging voltage below 4.3 V over 200 cycles).91
 | ||
| Fig. 6 (a) SEM image of Co–MnO2/CC, (b) cycle properties of Co–MnO2/CC under the cut-off capacity of 1000 mA h g−1 at 100 mA g−1, (c) comparison of cycle performance and charge/discharge voltage gap, (d) in situ microstructural features of Co–MnO2/CC under different stages,68 Copyright © 2019 Wiley-VCH reproduced by permission of copyright owner. (e) HRTEM image of CC@MoN NFs with the corresponding SAED pattern, (f) cycle properties of the CC@MoN NFs, (g) in situ XRD spectra of the CC@MoN NFs with the corresponding discharge/charge curves, and (h) XPS spectra of Mo 3d for CC@MoN NFs after charging and discharging.69 Copyright © 2022 Wiley-VCH reproduced by permission of copyright owner. | ||
Transition metal nitrides have great potential in electrocatalysis and energy storage and conversion due to their variable valence caused by the d-orbital overlap and the special half-filled shell effect of valence electrons.92,93 Furthermore, as noted in section 2.1, such catalysts can stabilize intermediate discharge products to reduce overpotential and improve energy efficiency. Therefore, Wang's team designed a non-precious metal self-supporting cathode based on MoN nanofiber on CC, which changed the reaction pathway to improve the overall electrochemical performance of LCBs.69 Firstly, the pretreated CC was immersed in a homogeneous mixture solution of ammonium molybdate tetrahydrate, cetyltrimethylammonium bromide, and concentrated HNO3 for 20 min. Subsequently, the mixture was placed in a Teflon-lined autoclave and treated at 120 °C for 20 h, and the centrifuged precipitate was washed and dried in a vacuum oven at 80 °C for 8 h. Finally, the treated CC was placed in a tube furnace and calcined at 700 °C in an NH3 atmosphere for 3 h to obtain the final CC@MoN NFs. The SAED pattern and HRTEM image revealed lattice spacings of 0.25 and 0.18 nm, which agreed with the (200) and (202) planes of standard MoN, respectively (Fig. 6e). CC@MoN NFs were assembled as a self-supporting cathode in button-type LCBs to evaluate their catalytic activity for CO2RR and CO2ER. Under the conditions of a cut-off capacity of 100 μA h cm−2 and a current density of 10 μA cm−2, the median charging voltage of CC@MoN NFs was only 3.19 V, with an energy density of over 88%. Even when the current density increased to 100 μA cm−2, the charging voltage could be maintained below 3.5 V and the cycle life exceeded 86 cycles (Fig. 6f). Such excellent energy efficiency was mainly due to the formation of more easily decomposable Li2C2O4 as the main discharge product on CC@MoN NFs, controlling the high-energy-efficient battery reaction as a 2-electron process. The in situ XRD spectra shown in Fig. 6g further strongly demonstrated the reversible formation and decomposition of Li2C2O4. The authors further used XPS characterization and DFT calculations to deeply explore the reaction mechanism of Li2C2O4 formation and decomposition. The three unpaired electrons of Mo atoms in MoN and the stronger electronegativity of O atoms in CO2 than N atoms facilitate the transfer of electrons from Mo atoms to O atoms during discharge. This transfer is evidenced by the reversible change in the proportion of Mo6+ and Mo3+ as shown in Fig. 6h. DFT calculations further confirmed that the delocalized electrons of Mo3+ in MoN stabilize Li2C2O4, forming a Mo–O coupling bridge.
In another study by the Wang's group, non-precious metal Mo3N2 with richer delocalized electron density and reversible electron localization structure was chosen as the cathode catalyst for the LCB.70 The preparation method is similar to that of CC@MoN NFs mentioned above, with the difference that there is no loaded substrate in the hydrothermal process and the calcination parameters are changed, and then a carbon-free Mo3N2 independent cathode is prepared through vacuum filtration. Thanks to the fast conductivity and high activity of the 1D structure of Mo3N2, the LCB based on the Mo3N2 cathode has ultra-low overpotential (0.64 V), high energy efficiency (80.46%), and excellent rate performance. Similar to the mechanism of stabilizing intermediate products by MoN, Mo3N2 promotes the transfer of outer electrons to O through MO2+ as the active site, preventing further dismutation of Li2C2O4. As a result, the pouch cell assembled based on the mass of Mo3N2 could provide an energy density of 6350.7 W h kg−1, better than the other flexible energy storage devices reported at that time.
3.3. Nanoparticles (0D)
Nanoparticle (NP) catalysts have some significant characteristics, including a high specific surface area, good dispersibility, controllable size/morphology and rich surface adsorption sites. These characteristics enable nanoparticles to significantly improve reaction rates, conversion rates and selectivity, making them widely applicable in the field of LCBs. Currently, active nanoparticles are typically loaded onto the surfaces of conductive 1D (carbon fibers, carbon nanotubes, etc.) or 2D materials (graphene, MXene, etc.) as composite materials, preventing aggregation/re-aggregation and also helping in charge storage.94 Ru as a widely used precious metal catalyst exhibits excellent catalytic activity in heterogeneous catalysis and electrocatalysis. Zhou's team firstly reported the use of Ru-based catalysts to promote the reversible reaction between Li2CO3 and C, thereby achieving a truly reversible LCB.31 In the same year, the group developed a Ru@Super P cathode catalyst for the LCB and achieved a significant improvement in electrochemical performance (a full discharge capacity of 8229 mA h g−1 and a coulombic efficiency of 86.2% over 70 cycles)43In situ surface-enhanced Raman and XRD results demonstrated the catalytic activity of Ru towards Li2CO3 and C.As one of the most studied precious metals, Ir has high physical/chemical stability and is also expected to effectively improve the overpotential and cycle life issues of LCBs. Wang et al. prepared a highly dispersed Ir nanoparticle composite material (Ir/CNFs) loaded on the surface of carbon nanofiber (CNFs) networks by electrospinning and thermal treatment, and for the first time introduced it as an air cathode into a rechargeable LCB.71 The morphology of the heat-treated Ir/CNFs was characterized by SEM, and the fiber membrane was composed of a complex 3D network of nanofibers with a diameter of about 150 nm. Due to the gas generated by PAN carbonization and thermal decomposition of iridium acetate, some mesopores were introduced on the surface of Ir/CNFs and contributed to a higher surface area. The TEM (HRTEM) image in Fig. 7a displays that the nanoparticles had a regular lattice corresponding to the Ir (111) crystal plane, confirming the formation of Ir NPs. The distribution of Ir NPs was clearly displayed by using high-angle annular dark-field (HAADF) imaging combined with energy dispersive X-ray spectroscopy (EDS) mapping (Fig. 7b). Benefiting from the high catalytic activity of Ir NPs and the unique porous structure of the CNF network, the discharge capacity of the Ir/CNF cathode reached 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 528 mA h g−1 at 50 h with an initial coulombic efficiency of 93.1%, far superior to most reports at that time (Fig. 5c).
528 mA h g−1 at 50 h with an initial coulombic efficiency of 93.1%, far superior to most reports at that time (Fig. 5c).
 | ||
| Fig. 7 (a) TEM and HRTEM (inset) images of Ir/CNFs, (b) HAADF TEM image and EDS mapping of Ir/CNFs, (c) full discharge/charge profiles of CNTs and Ir/CNFs at various current densities,71 Copyright © 2018 Wiley-VCH. Reproduced by permission of copyright owner. (d) DEMS of the LCB with Ni-NG, (e) SEM images of Ni-NG and (f) SAED patterns of Ni-NG in different regions.72 Licensed under a Creative Commons Attribution 4.0 Unported International License. | ||
Besides traditional noble metal-based catalysts, single transition metal Ni has also attracted researchers' attention due to its abundant reserves and non-toxic properties. Zhou's group prepared a highly dispersed Ni nanoparticle composite on N-doped graphene (denoted as Ni-NG) through hydrothermal and thermal treatment.72 The abundant pores and channels provided by N-doped graphene facilitated rapid mass transfer and provided sufficient void volume for the deposition of discharge products, thus forming a stable discharge platform and high discharge capacity. To further understand the reversibility of the LCB, differential electrochemical mass spectrometry and FTIR were used to confirm the reversible evolution of the discharge product Li2CO3 (Fig. 7d). More importantly, SEM and TEM observations revealed that the high dispersion of Ni active sites resulted in the formation of film-like discharge products, aiding in decomposition during the charging process (Fig. 7e and f). This work provides new insights into the rechargeability of the LCB and offers some inspiration for the design of high-performance LCB cathode catalysts.
Due to the insufficient catalytic ability of single−metal electrocatalysts, their cycling stability is far from meeting the requirements for LCB practical applications. In such cases, multi-component alloys address the deficiencies of single-metal electrocatalysts by exhibiting electronic modulation effects from various metal components and synergistic effects from multiple catalytic sites.95 In 2019, a bimetallic alloy catalyst consisting of single-phase RuCu solid solution nanoparticles was firstly introduced into the LCB electrochemical system.96 The LCB based on RuCu/CNFs showed record-breaking high rate performance with significantly reduced overpotential, proving the powerful catalytic activity of nano-alloy catalysts in LCBs (overpotential as low as 1.56 V at a current density of 2000 mA g−1). Subsequently, Sun's group designed a composite material of ultrafine Ir–Ru alloy nanoparticles anchored on nitrogen-doped carbon nanotubes as an efficient cathode electrocatalyst for LCBs (IrRu/N-CNTs) through a simple solvothermal method.73 The lattice observed in the HRTEM image corresponded to the (101) crystal plane of the IrRu alloy NPs, confirming the high crystallinity of the synthesized IrRu NPs (Fig. 8a). TEM elemental mapping further revealed the uniform distribution of the mixed solid solution IrRu NPs on N-CNTs, consistent with XRD and XPS results (Fig. 8b and c). Due to the abundant high-activity sites and interconnected 3D structure, IrRu/N-CNTs could operate for an astonishing 6000 h (600 cycles) while maintaining a charging potential <4.2 V and a discharge voltage >2.5 V, far surpassing single-metal catalysts (Fig. 8d). To further elucidate the fundamental mechanisms by which IrRu/N-CNTs enhanced LCB electrochemical reactions at the molecular level, DFT was used to study the adsorption behavior of the reactants. The formed Li2CO3 firstly decomposed at Ir-dominated active sites, and then co-decomposed with C at Ru-dominated active sites (as illustrated in the decomposition mechanism in Fig. 8e). This unique two-step decomposition mechanism facilitates the breakdown of discharge products and the overpotential reduction.
 | ||
| Fig. 8 (a) TEM image of IrRu/N-CNTs (the insert is the HRTEM image of alloyed IrRu nanoparticles), (b) Ru 3d XPS spectra and (c) Ir 4f XPS spectra of IrRu/N-CNTs, (d) cycling performance of IrRu/N-CNTs under the cut-off capacity of 500 mA h g−1 at 100 mA g−1, (e) the schematic illustration of the decomposition mechanism on the surface of IrRu nanoalloys,73 licensed under a Creative Commons Attribution 4.0 Unported International License. (f) HAADF-STEM image of Ni/Ru HNPs (inset is the corresponding FFT pattern along the hcp [0001] axis), (g) STEM elemental mapping images of a single Ni/Ru HNP, (h) HRTEM images of Ru NPs (left) and Ni/Ru HNPs (right) in Ru(10–10), as well as (i) Ru(0002) with the aligned bottom atomic layer, (j) calculated surface Ru atomic distance on the (0002) facet in different strain states, and (k) the PDOS of the Ru 4d orbital on Ni/Ru(0002) and pristine Ru(0002).74 Copyright © 2022 Wiley-VCH. Reproduced by permission of copyright owner. | ||
Core–shell nanoparticles are a significant type of electrocatalyst, mainly consisting of catalytically active noble metals on an easily accessible core to enhance noble metal utilization.97 The core material can act as a structural template and adjust the electronic structure of active sites.98 Fan et al. designed unique core–shell structured nickel/ruthenium hexagonal nanoplates (Ni/Ru HNPs), for the first time experimentally and theoretically demonstrating how strain effects adjust the electronic structure of Ru metal and influence the catalytic activity in CO2 conversion reactions.74 The prepared Ni/Ru HNPs were characterized by TEM and HAADF-STEM. Ni/Ru HNPs possessed a hexagonal nanoplate shape with an average lateral size of about 11 nm (Fig. 8f). By analyzing the nanoplates perpendicular to the TEM grid, the thickness of the nanoplates was determined as approximately 2.65 nm (about 12 atomic layers thick). EDS mapping under HAADF-STEM mode more clearly confirmed that the Ru shell was wrapped around the Ni core surface with sidewalls of about 5–6 atomic layers (Fig. 8g). XRD analysis for the crystal facets of Ni/Ru HNPs presented noticeable shifts in diffraction peaks, likely due to inherent compressive strain arising from lattice mismatch between the Ni core and Ru shell. HRTEM images compared the Ru(10–10) and Ru(0002) facets of Ru NPs and Ni/Ru HNPs, showing that after several layers of atomic spacing differences accumulate, the top atomic layer position of Ni/Ru HNPs is significantly lower than that of Ru NPs, providing direct evidence of lattice compression (Fig. 8h and i). Further XPS and DFT analysis demonstrated that lattice compression caused a downward shift in the d-band center, resulting in weaker adsorption capacity on the Ni/Ru surface, which facilitated gas release in Li2CO3 decomposition reactions and thereby led to a lower charging potential (Fig. 8j and k). The unique core–shell structure of Ni/Ru HNPs significantly alters the geometric coordination of surface metal atoms, thereby exhibiting size-dependent catalytic behavior. This work deeply explored the impact of strain effects in electrocatalysis for CO2ER/CO2RR, offering an intriguing research direction for designing efficient LCB catalysts.
3.4. Single-atom catalysts (atom scale)
Due to the high activity, stability and high atomic utilization, SACs have garnered significant interest in their application within LCB electrochemical systems.99 In 2020, Hu and colleagues firstly introduced SACs into the LCB system, successfully loading Fe single atoms onto a 3D interconnected porous N,S co-doped graphene (denoted as Fe-ISA/N,S-HG).75 The Fe-ISA/N,S-HG catalyst was prepared through a two-step process involving complexation and subsequent annealing. In the complexation step, Fe atoms were uniformly and stably fixed on the HG surface through π–π stacking between 1,10-phenanthroline and holey graphene (HG), forming Fe–Nx sites during the subsequent annealing process. The SEM image of Fe-ISA/N,S-HG at different resolutions in Fig. 9a displays the interconnected 3D porous network and clear surface pores. XPS was further used to investigate the chemical composition of Fe-ISA/N,S-HG, confirming the successful incorporation of Fe, N and S into the carbon framework. | ||
| Fig. 9 (a) SEM image of Fe-ISA/N,S-HG, (b) full discharge/charge profiles of three cathodes tested at 100 mA g−1, (c) discharge/charge profiles tested at 1 A g−1 of the Fe-ISA/N,S-HG electrode,75 Copyright © 2020 Wiley-VCH. Reproduced by permission of copyright owner. (d) SEM image of TeAC@NCNS, (e) Fourier-transformed EXAFS spectra for TeAC@NCNS, (f) normalized CO2 TPD profiles of TeAC@NCNS and NCNS samples, (g) the comparison of the full-discharge capacities with different cathodes. Inset: The first deep discharge curves at a current density of 0.05 mA cm−2, (h) performance comparison diagram with different cathodes in terms of overpotential and cycle times, (i) the 3D X-ray tomography plot obtained on the TeAC@NCNS cathode after discharging to 10.0 mA h cm−2, and (j) illustration of the proposed reaction mechanism on different catalysts.76 Licensed under a Creative Commons Attribution 4.0 Unported International License. | ||
To investigate the chemical state of iron atoms in Fe-ISA/N,S-HG, X-ray absorption spectroscopy was employed to obtain the coordination information of Fe–N4. Benefiting from the unique 3D layered porous structure of HG which ensured fast electron/ion transport and exposed active sites, the electrochemical performance of the LCB based on Fe-ISA/N,S-HG was greatly enhanced (23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 174 mA h g−1 full discharge capacity, low overpotential of 1.17 V at 100 mA g−1 and long-term stability of 200 cycles at high current density of 1.0 A g−1, as shown in Fig. 9b and c). DFT calculations were used to delve into the reasons for this enhanced activity. The synergistic effect between N and S co-doping and charge and spin redistribution induced by some Fe–N4 sites effectively catalyses the reduction and evolution reactions of CO2. This work inspires more strategies for designing efficient catalysts for rechargeable LCBs, and also stimulates extensive research and exploration of SACs in LCB applications.
174 mA h g−1 full discharge capacity, low overpotential of 1.17 V at 100 mA g−1 and long-term stability of 200 cycles at high current density of 1.0 A g−1, as shown in Fig. 9b and c). DFT calculations were used to delve into the reasons for this enhanced activity. The synergistic effect between N and S co-doping and charge and spin redistribution induced by some Fe–N4 sites effectively catalyses the reduction and evolution reactions of CO2. This work inspires more strategies for designing efficient catalysts for rechargeable LCBs, and also stimulates extensive research and exploration of SACs in LCB applications.
Tellurium (Te), a typical metalloid element, exhibits excellent conductivity and strong CO2 adsorption capability, making it a promising ideal cathode catalyst for LCBs. Xiao and Wang's team synthesized N-doped carbon nanosheets (NCNS) using a typical method of freeze-drying and pyrolysis, and then fixed Te atoms on the NCNS substrate through chemical vapor deposition (TeAC@NCNS).76 TeAC@NCNS exhibited a porous mesh structure composed of ultrathin nanosheets with surface folds, as shown in Fig. 9d. XPS results confirmed the presence of C and N elements as well as additional Te element in the sample. Specifically, the high-resolution C 1s spectrum displayed a new peak assigned to Te–C bonds. XAS analysis results further provided solid evidence for the presence of Te atom clusters anchored on NCNS nanosheets (Fig. 9e). Additionally, CO2 temperature-programmed desorption was used to explore the chemical interactions between CO2 and the cathode catalyst. Compared to NCNS (279 °C), the enhanced peak of TeAC@NCNS at higher temperatures indicated that the introduction of Te atom clusters could significantly promote the chemical adsorption of CO2 (Fig. 9f). Significantly, this strong interaction is essential for the activation of CO2, implying that TeAC@NCNS could be an effective cathode catalyst for LCBs. LCBs based on TeAC@NCNS provided an unprecedentedly high full discharge capacity of 28.35 mA h cm−2 at a current density of 0.05 mA cm−2 with higher discharge voltage (Fig. 9g). The rate capability of LCBs was studied through constant current charge and discharge at varying current densities from 0.025 to 0.15 mA cm−2. At a high current density of 0.15 mA cm−2, the overpotential of TeAC@NCNS was only 1.74 V, whereas that of NCNS was as high as 2.31 V. Regarding cycling stability, it offers a long-term stable cycling life of 1000 h even at a high cutoff capacity of 1 mA h cm−2 and a current density of 0.05 mA cm−2 (Fig. 9h). X-ray tomography was further used to observe the detailed reconstruction of the TeAC@NCNS cathode at the nanoscale during the entire discharge process. The discharge products (yellow) were widely dispersed covering the entire active part (pink), demonstrating the full utilization of TeAC sites and the vast 3D network space of NCNS (Fig. 9i). DFT calculations confirmed that Te atom clusters could hinder the crystallization of Li2CO3 by modulating the adsorption strength of intermediate products, thereby reducing energy loss during charge and discharge processes (Fig. 9j).
In order to demonstrate the potential of SACs with metal-NX moieties for improving LCB reaction kinetics, Liu et al. computationally screened SACs on N-doped graphene (SAMe@NG, Me = Cr, Mn, Fe, Co, Ni, Cu) through DFT calculations for CO2 reduction and evolution reactions.100 Combined with the experimental results, it is confirmed that the SACr@NG/PCF cathode has the highest catalytic activity among SAMe@NG/PCF cathodes and can effectively improve the LCB rate and cycling performance. In another typical instance, the authors developed Cd–N4 sites and anchored them on N-doped carbon (Cd SAs/NC) via a highly simple and scalable calcination process.77 The HAADF-STEM image showed uniformly distributed bright spots attributed to Cd species, with no nanoparticles or clusters observed (Fig. 10a). XRD, XPS and XAS revealed the chemical composition and coordination state of Cd SAs/NC, confirming the presence of Cd as a single-atom metal center without metal–metal coordination. The LCB based on Cd SAs/NC exhibited an impressive full cell discharge capacity of 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 045 mA h g−1 at a current density of 500 mA g−1 and a cutoff voltage of 2 V, with a coulombic efficiency of 96.6% (Fig. 10b). At current densities of 1 A g−1 and 2 A g−1, the full cell capacities were 125
045 mA h g−1 at a current density of 500 mA g−1 and a cutoff voltage of 2 V, with a coulombic efficiency of 96.6% (Fig. 10b). At current densities of 1 A g−1 and 2 A g−1, the full cell capacities were 125![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 222 mA h g−1 and 60
222 mA h g−1 and 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 743 mA h g−1, respectively, reflecting excellent high-current tolerance. Cd SAs/NC maintained a terminal discharge voltage above 2 V even at an extreme current density of 10 A g−1, which was significant for its application in some extreme scenarios (Fig. 10c). Furthermore, the LCB with Cd SAs/NC exhibited excellent long-term stability (cycling over 1685 times at 1 A g−1 and 500 mA h g−1, and 669 times at 1 A g−1 and 1000 mA h g−1). At a more practical current density of 2 A g−1, it cycled 625 times at 500 mA h g−1 and 380 times at 1 A h g−1. DFT calculations of the Gibbs free energy changes during charge and discharge proved the lowest free energy barrier of the limiting step on Cd SAs/NC, confirming the crucial role of Cd SA in enhancing LCB performance.
743 mA h g−1, respectively, reflecting excellent high-current tolerance. Cd SAs/NC maintained a terminal discharge voltage above 2 V even at an extreme current density of 10 A g−1, which was significant for its application in some extreme scenarios (Fig. 10c). Furthermore, the LCB with Cd SAs/NC exhibited excellent long-term stability (cycling over 1685 times at 1 A g−1 and 500 mA h g−1, and 669 times at 1 A g−1 and 1000 mA h g−1). At a more practical current density of 2 A g−1, it cycled 625 times at 500 mA h g−1 and 380 times at 1 A h g−1. DFT calculations of the Gibbs free energy changes during charge and discharge proved the lowest free energy barrier of the limiting step on Cd SAs/NC, confirming the crucial role of Cd SA in enhancing LCB performance.
 | ||
| Fig. 10 (a) HAADF-STEM image of Cd SAs/NC, (b) full GDC profiles at 500 mA g−1 with a voltage and the inset is the coulombic efficiency, (c) rate performance of Cd SAs/NC at varying high current densities from 4 to 100 A g−1.77 Copyright © 2023 Wiley-VCH. Reproduced by permission of copyright owner. (d) Schematic illustrating the synthesis procedure for RuAC+SA@NCB, (e) HAADF-STEM image of RuAC+SA@NCB, (f) the normalized Ru K-edge XANES curves of RuO2, Ru powder, RuAC+SA@NCB, and RuSA@NCB, (g) and (h) EXAFS fitting curves at R space for RuAC+SA@NCB and RuSA@NCB samples, respectively. Insets: schematic atom configurations for the fitting of EXAFS spectra, (i) rate performance of RuAC+SA@NCB tested at current densities from 100 to 2000 mA g−1, (j) Gibbs free energy diagrams during the CO2ER process, and (k) the atomic configuration of *Li2C2O4 intermediate adsorption on RuAC+SA@NCB and RuSA@NCB.78 Copyright © 2022 Wiley-VCH. Reproduced by permission of copyright owner. | ||
Furthermore, in exploring the mechanism of SAC catalytic activity, the synergistic effects among active sites have shown a notably significant correlation compared to the direct effects of single atomic sites.99,101 Lin et al. developed a novel catalyst with Ru atom clusters (RuAC) and single-atom Ru–N4 (RuSA) composite sites on N-doped carbon nanoboxes (NCB) to investigate the impact of interacting sites in LCBs.78 Using chemical vapor deposition technology, nitrogen-doped carbon nanoboxes (NCB) were prepared using cubic magnesium oxide as a template and acetonitrile carried by nitrogen gas as the precursor. Subsequently, the template was removed by acid washing with the assistance of amine-functionalized carbon quantum dots (NH2-CQD). RuAC and Ru–N4 groups (RuSA) were successfully loaded onto the NCB. Fig. 10d shows the schematic of the RuAC+SA@NCB preparation process. In this process, RuAC and RuSA substances were respectively formed by controlling the complexation of Ru groups with different substrates (amine groups on CQDs and nitrogen groups on NCB). The catalyst samples prepared using NCB as a template inherited the nanobox morphology of NCB, with a shell thickness of about 5 nm and highly graphitized carbon structure. HAADF-STEM images indicate that composite sites containing RuAC and RuSA could be formed on NCB by controlling the synthesis conditions, as shown by the dashed circles in Fig. 10e. Without the assistance of NH2-CQD, only Ru atom clusters (RuAC@NCB) can be formed, while selective removal of Ru atom clusters by acid leaching results in samples containing only RuSA (RuSA@NCB). The electronic structure and atomic configuration of active sites were deeply analyzed using synchrotron X-ray absorption spectroscopy. Compared to RuSA@NCB, the near-edge absorption threshold of RuAC+SA@NCB shifted to lower energy, suggesting an interaction between Ru atom clusters and positively charged Ru single atoms (Fig. 10f). Additionally, the weighted EXAFS characteristic peaks of Ru–N and Ru–Ru bonds indicated the coexistence of nitrogen-coordinated Ru single atoms and Ru atom clusters, with smaller cluster sizes which led to relatively weaker Ru–Ru bond peaks (Fig. 10g and h). RuAC+SA@NCB exhibited significant enhancement in activity towards CO2RR and CO2ER, markedly improving LCB electrochemical performance with reduced overpotentials of 1.65 V and 1.86 V at 1 A g−1 and 2 A g−1, respectively (Fig. 10i).
DFT calculations uncovered the unique properties of RuAC+SA as active sites. It was revealed that adjacent RuAC components substantially altered the electronic structure of Ru–N4 sites, significantly lowering the energy barriers of the rate-determining steps in CO2ER and CO2RR (Fig. 10j). Furthermore, due to the weakening of the Li–O bond, the *Li2C2O4 bond dissociation step in the CO2ER process occurring at RuAC+SA composite sites became easier (Fig. 10k). In summary, this method that establishes electronic synergistic effects among different metal atom components opens up new possibilities for future novel electrocatalysts of LCBs.
4. Conclusion and perspective
In summary, LCBs have the dual functions of electrochemically consuming greenhouse gases and converting them into energy storage, which is significant for addressing fossil energy shortages and global warming. However, research on LCBs is still in the initial stages, facing challenges such as the thermal stability of Li2CO3, inadequate catalyst activity and stability and side reactions caused by H2O and O2 in air. Thus, developing affordable, highly active and stable electrocatalysts is essential for enhancing battery performance and achieving commercialization. Among these, metal-based catalysts, with their superior catalytic activity and tunability, are promising candidates for high-performance LCB cathode catalysts. In the past decade, significant progress has been made in the synthesis techniques of metal and metal compound nanomaterials, as well as advanced in situ characterization, enabling us to deeply study the interaction between catalytic performance and chemical and structural factors. Numerous studies have shown that changes in the particle size of nanoparticles affect the coordination environment and the electronic state of surface atoms for the adsorption of molecules, dissociated substances or intermediates, thereby directly influencing the catalytic activity of specific reactions. Moreover, nanocatalysts with specific morphologies can optimize catalytic activity by exposing specific crystal surfaces, thereby regulating the chemical adsorption or dissociation of reactant molecules. Therefore, systematically summarizing previous work and exploring the impact of nanocatalyst morphology and size on catalytic performance is highly instructive for developing high-performance LCB electrocatalysts.The first part of this work details the charge and discharge reaction mechanisms of LCBs. During discharge, various reaction pathways may occur based on specific reaction conditions, resulting in different discharge products such as Li2CO3/C, Li2CO3/CO, Li2O/C and Li2C2O4. During the reverse charging process, these discharge products serve as initial reactants and primarily decompose into Li+ and CO2. Detailed electrochemical mechanism exploration is essential for designing and developing suitable high-performance electrocatalysts. Subsequently, we clarified the significant influence of nano-sized catalysts with various sizes and morphologies on catalytic performance (coordination number, electronic state, adsorption energy, etc.) and summarized the practical applications of metal-based catalysts with different morphologies (from 2/3D to atom-scale) in LCBs. Although metal-based catalysts have been extensively studied, the specific effects of their morphology and size on the electrochemical reactions of LCBs remain unclear. Therefore, for the scale and morphology design of metal catalysts in LCBs, we have looked into the future development directions from the following aspects.
(1) Currently, SACs emerge as the most promising nanocatalysts for LCBs due to the superior catalytic performance and notable cost advantages. However, the commercialization of SACs necessitates substantial efforts in researching practical methods for large-scale synthesis. In short, the various nanostructures of catalysts have both advantages and limitations in the electrochemical reactions of LCBs. To better utilize the useful properties of unique structures and mitigate their drawbacks, the advantages of various structures can be combined through nano-reassembly. For example, to enhance the activity of nanoparticles, highly active nanoparticles can be decorated on 1D or 2D materials, which prevents the re-aggregation of particles and facilitates rapid electron transfer. Moreover, highly open nanostructures (mesoporous structures, nano-cages, and nano-frameworks), which exhibit significant potential in the field of electrocatalysis, have become an emerging research focus due to the order assembly of small nanoparticles in 3D space. This open structure not only provides rich surface area and active sites for heterogeneous catalytic reactions but also significantly enhances the stability of the catalysts by inhibiting particle aggregation and the Ostwald ripening process. Therefore, it is worthwhile for researchers to conduct in-depth exploration of the application of open structure catalysts in the field of LCB electrocatalysis.
(2) Tandem catalysis is a technology that directly performs cascade reactions on a single nanostructured catalyst with multiple active sites, providing plenty of opportunities for improving chemical conversions. This technology, by eliminating the steps of intermediate separation and transport, is particularly advantageous for reactions involving unstable intermediates like LCBs. By integrating nano-components into composite nanostructures, it is possible to effectively control size and apparent morphology, and at the same time, provide unprecedented opportunities for the formation of multiple catalytic interfaces. Performing complex sequential reactions in a single nanostructured catalyst through careful spatial arrangement, and utilizing the synergistic effects between these catalytic interfaces to achieve higher reaction selectivity and efficiency, may be one of the important approaches to solve the slow kinetics problem of CO2.
(3) In the past decade, with the continuous advancement of in situ/operando research instruments and techniques, researchers have been able to delve into the chemical and structural properties of numerous catalysts under reaction conditions or during the catalytic process. With the help of such in situ characterization methods, we can establish the intrinsic relationship between the chemical properties and structure in LCB electrochemical reactions, thus accurately evaluating the performance of cathode catalysts. The integration of in situ characterization and theoretical calculations into the fundamental understanding of size and shape-dependent catalytic performance is crucial, as it provides us with a true and accurate reaction for the impact of size or shape on catalytic performance.
(4) In addition to cathode catalysts, other components of the LCBs (anode and electrolyte) also require optimization prior to practical application. For instance, the issues associated with lithium metal anodes, such as dendrite formation, electrolyte/CO2 corrosion, and high polarization, significantly impair the cycle life of LCBs. Consequently, elucidating the working mechanisms on the anode side holds crucial guiding significance. One potential strategy involves modifying the lithium metal anode with a stable artificial protective layer that exhibits high ionic conductivity while shielding H2O and other undesired molecules dissolved in the electrolyte. Another approach is to optimize the electrolyte to achieve desirable properties. According to the Nernst equation, the potential of a chemical reaction can be determined by the concentrations of the reactants. In most cases, increasing the concentration of CO2 in the electrolyte is beneficial. Furthermore, the composition of the electrolyte (salts and solvents) is closely related to the formation of the solid–electrolyte interphase (SEI) layer on the anode, thereby significantly influencing anode performance. Meanwhile, since CO2RR and CO2ER occur at the three-phase interface, the solvation structure of ions and the diffusion layer at the interface are of paramount importance. Issues such as electrolyte leakage, flammability, and volatility also need to be considered.
Based on current understanding, addressing the challenges in the commercial application of LCBs still requires significant effort. Studying the behavior of LCBs through in situ characterization and theoretical calculations can help us understand the intrinsic relationship between the catalytic mechanisms and size-catalytic performance during discharge and charging processes, thus maximizing the efficient use of active materials. Through continuous research on the electrochemical mechanisms of LCBs and the activity of heterogeneous catalysts (including cascade catalysis, synergistic effects among active sites, and complex nanostructures), it is believed that metal-based catalysts will have broader application prospects in the future, and LCBs will become the mainstream technology in the next generation of green electrochemical systems.
Data availability
No data was used for the research described in the article.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
The authors greatly thank the financial support from the National Natural Science Foundation of China (52172097), New Energy Material Innovation Consortium Projects of Yunnan Province (202302AB080018), and the Royal Society International Exchanges 2021 Cost Share (NSFC) scheme (IEC\NSFC\211074).References
- B. Obama, Science, 2017, 355, 126–129 CrossRef CAS PubMed.
- E. T. A. Mitchard, Nature, 2018, 559, 527–534 CrossRef CAS PubMed.
- L. A. Magruder, S. L. Farrell, A. Neuenschwander, L. Duncanson, B. Csatho, S. Kacimi and H. A. Fricker, Nat. Rev. Earth Environ., 2024, 5, 120–136 CrossRef.
- N. Zeng, K. Jiang, P. Han, Z. Hausfather, J. Cao, D. Kirk-Davidoff, S. Ali and S. Zhou, Adv. Atmos. Sci., 2022, 39, 1229–1238 CrossRef.
- A. Huovila, H. Siikavirta, C. Antuña Rozado, J. Rökman, P. Tuominen, S. Paiho, Å. Hedman and P. Ylén, J. Cleaner Prod., 2022, 341, 130912 CrossRef CAS.
- Y. Zhao, B. P. Setzler, J. Wang, J. Nash, T. Wang, B. Xu and Y. Yan, Joule, 2019, 3, 2472–2484 CrossRef CAS.
- A. Mustafa, B. G. Lougou, Y. Shuai, S. Razzaq, Z. Wang, E. Shagdar and J. Zhao, J. Electrochem. Sci. Technol., 2022, 13, 148–158 CrossRef CAS.
- R. Zhao, P. Ding, P. Wei, L. Zhang, Q. Liu, Y. Luo, T. Li, S. Lu, X. Shi, S. Gao, A. M. Asiri, Z. Wang and X. Sun, Adv. Funct. Mater., 2021, 31, 2009449 CrossRef CAS.
- C. Chen, H. Jin, P. Wang, X. Sun, M. Jaroniec, Y. Zheng and S.-Z. Qiao, Chem. Soc. Rev., 2024, 53, 2022–2055 RSC.
- J. Lin, W. Song, C. Xiao, J. Ding, Z. Huang, C. Zhong, J. Ding and W. Hu, Carbon Energy, 2023, 5, e313 CrossRef CAS.
- Y.-F. Wang, L.-N. Song, L.-J. Zheng, Y. Wang, J.-Y. Wu and J.-J. Xu, Angew. Chem., Int. Ed., 2024, e202400132 Search PubMed.
- J. Chen, X.-Y. Chen, Y. Liu, Y. Qiao, S.-Y. Guan, L. Li and S.-L. Chou, Energy Environ. Sci., 2023, 16, 792–829 RSC.
- A. Sarkar, V. R. Dharmaraj, C. H. Yi, K. Iputera, S. Y. Huang, R. J. Chung, S. F. Hu and R. S. Liu, Chem. Rev., 2023, 123, 9497–9564 CrossRef CAS.
- H. Gao and B. M. Gallant, Nat. Rev. Chem., 2020, 4, 566–583 CrossRef.
- E. R. Ezeigwe, L. Dong, R. Manjunatha, Y. Zuo, S.-Q. Deng, M. Tan, W. Yan, J. Zhang and D. P. Wilkinson, Nano Energy, 2022, 95, 106964 CrossRef CAS.
- Y. Liu, R. Mao, B. Chen, B. Lu, Z. Piao, Y. Song, G. Zhou and H.-M. Cheng, Mater. Today, 2023, 63, 120–136 CrossRef CAS.
- Z. Zhang, Q. Zhang, Y. Chen, J. Bao, X. Zhou, Z. Xie, J. Wei and Z. Zhou, Angew. Chem., Int. Ed., 2015, 54, 6550–6553 CrossRef CAS PubMed.
- X. Zhang, Q. Zhang, Z. Zhang, Y. Chen, Z. Xie, J. Wei and Z. Zhou, Chem. Commun., 2015, 51, 14636–14639 RSC.
- L. Liu, Y. Qin, K. Wang, H. Mao, H. Wu, W. Yu, D. Zhang, H. Zhao, H. Wang, J. Wang, C. Xiao, Y. Su and S. Ding, Adv. Energy Mater., 2022, 12, 2103681 CrossRef CAS.
- P.-F. Zhang, T. Sheng, Y. Zhou, Y.-J. Wu, C.-C. Xiang, J.-X. Lin, Y.-Y. Li, J.-T. Li, L. Huang and S.-G. Sun, Chem. Eng. J., 2022, 448, 137541 CrossRef CAS.
- Y. Xing, Y. Yang, D. Li, M. Luo, N. Chen, Y. Ye, J. Qian, L. Li, D. Yang, F. Wu, R. Chen and S. Guo, Adv. Mater., 2018, 30, e1803124 CrossRef PubMed.
- A. Ahmadiparidari, R. E. Warburton, L. Majidi, M. Asadi, A. Chamaani, J. R. Jokisaari, S. Rastegar, Z. Hemmat, B. Sayahpour, R. S. Assary, B. Narayanan, P. Abbasi, P. C. Redfern, A. Ngo, M. Voros, J. Greeley, R. Klie, L. A. Curtiss and A. Salehi-Khojin, Adv. Mater., 2019, 31, e1902518 CrossRef.
- X. Zhang, T. Wang, Y. Yang, X. Zhang, Z. Lu, J. Wang, C. Sun, Y. Diao, X. Wang and J. Yao, ACS Energy Lett., 2021, 6, 3503–3510 CrossRef CAS.
- S. Li, Y. Dong, J. Zhou, Y. Liu, J. Wang, X. Gao, Y. Han, P. Qi and B. Wang, Energy Environ. Sci., 2018, 11, 1318–1325 RSC.
- X. Li, H. Wang, Z. Chen, H. S. Xu, W. Yu, C. Liu, X. Wang, K. Zhang, K. Xie and K. P. Loh, Adv. Mater., 2019, 31, e1905879 CrossRef PubMed.
- R. Peng, S. Li, X. Sun, Q. Ren, L. Chen, M. Fu, J. Wu and D. Ye, Appl. Catal., B, 2018, 220, 462–470 CrossRef CAS.
- R. T. Hannagan, G. Giannakakis, M. Flytzani-Stephanopoulos and E. C. H. Sykes, Chem. Rev., 2020, 120, 12044–12088 CrossRef CAS.
- M. Shao, A. Peles and K. Shoemaker, Nano Lett., 2011, 11, 3714–3719 CrossRef CAS.
- J. Lu, L. Cheng, K. C. Lau, E. Tyo, X. Luo, J. Wen, D. Miller, R. S. Assary, H. H. Wang, P. Redfern, H. Wu, J. B. Park, Y. K. Sun, S. Vajda, K. Amine and L. A. Curtiss, Nat. Commun., 2014, 5, 4895 CrossRef CAS.
- Y. Hou, J. Wang, L. Liu, Y. Liu, S. Chou, D. Shi, H. Liu, Y. Wu, W. Zhang and J. Chen, Adv. Funct. Mater., 2017, 27, 1700564 CrossRef.
- Y. Qiao, J. Yi, S. Wu, Y. Liu, S. Yang, P. He and H. Zhou, Joule, 2017, 1, 359–370 CrossRef CAS.
- C. Yang, K. Guo, D. Yuan, J. Cheng and B. Wang, J. Am. Chem. Soc., 2020, 142, 6983–6990 CrossRef CAS.
- Z. Zhao, L. Pang, Y. Su, T. Liu, G. Wang, C. Liu, J. Wang and Z. Peng, ACS Energy Lett., 2022, 7, 624–631 CrossRef CAS.
- S. Xu, S. K. Das and L. A. Archer, RSC Adv., 2013, 3, 6656 RSC.
- Y. Liu, R. Wang, Y. Lyu, H. Li and L. Chen, Energy Environ. Sci., 2014, 7, 677 RSC.
- Z. Zhang, W.-L. Bai, Z.-P. Cai, J.-H. Cheng, H.-Y. Kuang, B.-X. Dong, Y.-B. Wang, K.-X. Wang and J.-S. Chen, Angew. Chem., Int. Ed., 2021, 60, 16404–16408 CrossRef CAS PubMed.
- J. Zhou, X. Li, C. Yang, Y. Li, K. Guo, J. Cheng, D. Yuan, C. Song, J. Lu and B. Wang, Adv. Mater., 2019, 31, 1804439 CrossRef.
- J. Xie, Q. Liu, Y. Huang, M. Wu and Y. Wang, J. Mater. Chem. A, 2018, 6, 13952–13958 RSC.
- Z. Xie, X. Zhang, Z. Zhang and Z. Zhou, Adv. Mater., 2017, 29, 1605891 CrossRef.
- B. Lu, X. Wu, X. Xiao, B. Chen, W. Zeng, Y. Liu, Z. Lao, X. X. Zeng, G. Zhou and J. Yang, Adv. Mater., 2024, 36, e2308889 CrossRef PubMed.
- Z. Zhao, J. Huang and Z. Peng, Angew. Chem., Int. Ed., 2018, 57, 3874–3886 CrossRef CAS PubMed.
- N. Mahne, S. E. Renfrew, B. D. McCloskey and S. A. Freunberger, Angew. Chem., Int. Ed., 2018, 57, 5529–5533 CrossRef CAS PubMed.
- S. Yang, Y. Qiao, P. He, Y. Liu, Z. Cheng, J.-J. Zhu and H. Zhou, Energy Environ. Sci., 2017, 10, 972–978 RSC.
- S. Chen, K. Yang, H. Zhu, J. Wang, Y. Gong, H. Li, M. Wang, W. Zhao, Y. Ji, F. Pan, S. R. P. Silva, Y. Zhao and L. Yang, Nano Energy, 2023, 117, 108872 CrossRef CAS.
- F. Wang, Y. Li, X. Xia, W. Cai, Q. Chen and M. Chen, Adv. Energy Mater., 2021, 11, 2100667 CrossRef CAS.
- Y. Liu, Z. Zhang, J. Tan, B. Chen, B. Lu, R. Mao, B. Liu, D. Wang, G. Zhou and H.-M. Cheng, Nat. Commun., 2024, 15, 2167 CrossRef CAS PubMed.
- F. Zhang, W. Zhang, J. A. Yuwono, D. Wexler, Y. Fan, J. Zou, G. Liang, L. Sun and Z. Guo, Nat. Commun., 2024, 15, 3393 CrossRef CAS.
- C. Xie, Z. Niu, D. Kim, M. Li and P. Yang, Chem. Rev., 2020, 120, 1184–1249 CrossRef CAS PubMed.
- A. Hu, C. Shu, C. Xu, R. Liang, J. Li, R. Zheng, M. Li and J. Long, J. Mater. Chem. A, 2019, 7, 21605–21633 RSC.
- F. Tao, S. Dag, L.-W. Wang, Z. Liu, D. R. Butcher, H. Bluhm, M. Salmeron and G. A. Somorjai, Science, 2010, 327, 850–853 CrossRef CAS.
- M. Chen and D. W. Goodman, Chem. Soc. Rev., 2008, 37, 1860–1870 RSC.
- C. L. Cleveland, U. Landman, T. G. Schaaff, M. N. Shafigullin, P. W. Stephens and R. L. Whetten, Phys. Rev. Lett., 1997, 79, 1873–1876 CrossRef CAS.
- M. S. Chen and D. W. Goodman, Science, 2004, 306, 252–255 CrossRef CAS.
- G. A. Somorjai and A. S. Mujumdar, Drying Technol., 1994, 13, 507–508 CrossRef.
- F. Calle-Vallejo, D. Loffreda, M. T. M. Koper and P. Sautet, Nat. Chem., 2015, 7, 403–410 CrossRef CAS PubMed.
- H. M. Lu and X. K. Meng, J. Phys. Chem. C, 2010, 114, 1534–1538 CrossRef CAS.
- H. Zhang, M. Jin, Y. Xiong, B. Lim and Y. Xia, Acc. Chem. Res., 2013, 46, 1783–1794 CrossRef CAS.
- J. Chen, B. Lim, E. P. Lee and Y. Xia, Nano Today, 2009, 4, 81–95 CrossRef CAS.
- Y. Xing, K. Wang, N. Li, D. Su, W.-T. Wong, B. Huang and S. Guo, Matter, 2020, 2, 1494–1508 CrossRef.
- Y. Xu, H. Gong, H. Ren, X. Fan, P. Li, T. Zhang, K. Chang, T. Wang and J. He, Small, 2022, 18, e2203917 CrossRef PubMed.
- Y. Wang, X. Wang, B. Ge, J. Guo, C. Fernandez and Q. Peng, ACS Appl. Energy Mater., 2021, 4, 9961–9968 CrossRef CAS.
- B. Lu, B. Chen, D. Wang, C. Li, R. Gao, Y. Liu, R. Mao, J. Yang and G. Zhou, Proc. Natl. Acad. Sci. U. S. A., 2023, 120, e2216933120 CrossRef CAS PubMed.
- Z. Lian, Y. Lu, C. Wang, X. Zhu, S. Ma, Z. Li, Q. Liu and S. Zang, Adv. Sci., 2021, 8, e2102550 CrossRef PubMed.
- X. Chen, J. Chen, Y. Qiao, Y. Gao, S. Fan, Y. Liu, L. Li, Y. Liu and S. Chou, Chem. Sci., 2024, 15, 2473–2479 RSC.
- L. Song, C. Hu, Y. Xiao, J. He, Y. Lin, J. W. Connell and L. Dai, Nano Energy, 2020, 71, 104595 CrossRef CAS.
- H. Xie, B. Zhang, C. Hu, N. Xiao and D. Liu, Electrochim. Acta, 2022, 417, 140310 CrossRef CAS.
- X. Li, J. Zhang, G. Qi, J. Cheng and B. Wang, Energy Storage Mater., 2021, 35, 148–156 CrossRef.
- B. Ge, Y. Sun, J. Guo, X. Yan, C. Fernandez and Q. Peng, Small, 2019, 15, e1902220 CrossRef PubMed.
- G. Qi, J. Zhang, L. Chen, B. Wang and J. Cheng, Adv. Funct. Mater., 2022, 32, 2112501 CrossRef CAS.
- G. Qi, J. Zhang, J. Cheng and B. Wang, SusMat, 2023, 3, 276–288 CrossRef CAS.
- C. Wang, Q. Zhang, X. Zhang, X. G. Wang, Z. Xie and Z. Zhou, Small, 2018, 14, e1800641 CrossRef PubMed.
- Z. Zhang, X. G. Wang, X. Zhang, Z. Xie, Y. N. Chen, L. Ma, Z. Peng and Z. Zhou, Adv. Sci., 2018, 5, 1700567 CrossRef PubMed.
- Z. Wang, B. Liu, X. Yang, C. Zhao, P. Dong, X. Li, Y. Zhang, K. Doyle-Davis, X. Zeng, Y. Zhang and X. Sun, Adv. Funct. Mater., 2023, 33, 2213931 CrossRef CAS.
- L. Fan, H. Shen, D. Ji, Y. Xing, L. Tao, Q. Sun and S. Guo, Adv. Mater., 2022, 34, e2204134 CrossRef PubMed.
- C. Hu, L. Gong, Y. Xiao, Y. Yuan, N. M. Bedford, Z. Xia, L. Ma, T. Wu, Y. Lin, J. W. Connell, R. Shahbazian-Yassar, J. Lu, K. Amine and L. Dai, Adv. Mater., 2020, 32, e1907436 CrossRef PubMed.
- K. Wang, D. Liu, L. Liu, X. Li, H. Wu, Z. Sun, M. Li, A. S. Vasenko, S. Ding, F. Wang and C. Xiao, Adv. Sci., 2023, 10, e2205959 CrossRef PubMed.
- K. Zhu, X. Li, J. Choi, C. Choi, S. Hong, X. Tan, T. S. Wu, Y. L. Soo, L. Hao, A. W. Robertson, Y. Jung and Z. Sun, Adv. Funct. Mater., 2023, 33, 2213841 CrossRef CAS.
- J. Lin, J. Ding, H. Wang, X. Yang, X. Zheng, Z. Huang, W. Song, J. Ding, X. Han and W. Hu, Adv. Mater., 2022, 34, e2200559 CrossRef PubMed.
- J.-H. Wang, S. Li, Y. Chen, L.-Z. Dong, M. Liu, J.-W. Shi, S.-L. Li and Y.-Q. Lan, Adv. Funct. Mater., 2022, 32, 2210259 CrossRef CAS.
- B. Anasori, M. R. Lukatskaya and Y. Gogotsi, Nat. Rev. Mater., 2017, 2, 16098 CrossRef CAS.
- F. Bonaccorso, L. Colombo, G. Yu, M. Stoller, V. Tozzini, A. C. Ferrari, R. S. Ruoff and V. Pellegrini, Science, 2015, 347, 1246501 CrossRef PubMed.
- X. Zhuang, S. Zhang, Y. Tang, F. Yu, Z. Li and H. Pang, Coord. Chem. Rev., 2023, 490, 215208 CrossRef CAS.
- T. Bashir, S. Zhou, S. Yang, S. A. Ismail, T. Ali, H. Wang, J. Zhao and L. Gao, Electrochem. Energy Rev., 2023, 6, 5 CrossRef CAS.
- D. Yuan, Y. Dou, Z. Wu, Y. Tian, K.-H. Ye, Z. Lin, S. X. Dou and S. Zhang, Chem. Rev., 2022, 122, 957–999 CrossRef CAS PubMed.
- G. Babu, N. Masurkar, H. Al Salem and L. M. R. Arava, J. Am. Chem. Soc., 2017, 139, 171–178 CrossRef CAS PubMed.
- P. Kumbhakar, C. Chowde Gowda, P. L. Mahapatra, M. Mukherjee, K. D. Malviya, M. Chaker, A. Chandra, B. Lahiri, P. M. Ajayan, D. Jariwala, A. Singh and C. S. Tiwary, Mater. Today, 2021, 45, 142–168 CrossRef CAS.
- X. Cui, W. Li, P. Ryabchuk, K. Junge and M. Beller, Nat. Catal., 2018, 1, 385–397 CrossRef CAS.
- F. Chen, X. Jiang, L. Zhang, R. Lang and B. Qiao, Chin. J. Catal., 2018, 39, 893–898 CrossRef CAS.
- G. Zhou, L. Xu, G. Hu, L. Mai and Y. Cui, Chem. Rev., 2019, 119, 11042–11109 CrossRef CAS PubMed.
- Q. Wei, F. Xiong, S. Tan, L. Huang, E. H. Lan, B. Dunn and L. Mai, Adv. Mater., 2017, 29, 1602300 CrossRef PubMed.
- S. Thoka, C. J. Chen, A. Jena, F. M. Wang, X. C. Wang, H. Chang, S. F. Hu and R. S. Liu, ACS Appl. Mater. Interfaces, 2020, 12, 17353–17363 CrossRef CAS PubMed.
- M.-S. Balogun, Y. Huang, W. Qiu, H. Yang, H. Ji and Y. Tong, Mater. Today, 2017, 20, 425–451 CrossRef CAS.
- K. N. Dinh, Q. Liang, C.-F. Du, J. Zhao, A. I. Y. Tok, H. Mao and Q. Yan, Nano Today, 2019, 25, 99–121 CrossRef CAS.
- E. Pomerantseva, F. Bonaccorso, X. Feng, Y. Cui and Y. Gogotsi, Science, 2019, 366, 6468 CrossRef PubMed.
- J. Yi, Q. Deng, H. Cheng, D. Zhu, K. Zhang and Y. Yang, Small, 2024, 2401146 CrossRef CAS PubMed.
- Y. Jin, F. Chen, J. Wang and R. L. Johnston, Chem. Eng. J., 2019, 375, 121978 CrossRef CAS.
- W. Xiao, W. Lei, M. Gong, H. L. Xin and D. Wang, ACS Catal., 2018, 8, 3237–3256 CrossRef CAS.
- W. Lu, X. Guo, Y. Luo, Q. Li, R. Zhu and H. Pang, Chem. Eng. J., 2019, 355, 208–237 CrossRef CAS.
- Y. Wang, F. Chu, J. Zeng, Q. Wang, T. Naren, Y. Li, Y. Cheng, Y. Lei and F. Wu, ACS Nano, 2021, 15, 210–239 CrossRef CAS PubMed.
- Y. Liu, S. Zhao, D. Wang, B. Chen, Z. Zhang, J. Sheng, X. Zhong, X. Zou, S. P. Jiang, G. Zhou and H. M. Cheng, ACS Nano, 2022, 16, 1523–1532 CrossRef CAS PubMed.
- T. Bai, D. Li, S. Xiao, F. Ji, S. Zhang, C. Wang, J. Lu, Q. Gao and L. Ci, Energy Environ. Sci., 2023, 16, 1431–1465 RSC.
| This journal is © The Royal Society of Chemistry 2025 |

