A tetranuclear Pr–W heterometal cluster-imbedded antimotungstate for the catalytic synthesis of benzimidazoles†
Zhijian
Zheng‡
 a,
Song
Jiang‡
a,
Xuejiao
Chen‡
a,
Haoqi
Liu
a,
Yufeng
Liu
a,
Yayu
Dong
b,
Xiaopeng
Sun
a,
Song
Jiang‡
a,
Xuejiao
Chen‡
a,
Haoqi
Liu
a,
Yufeng
Liu
a,
Yayu
Dong
b,
Xiaopeng
Sun
 *c and
Guoping
Yang
*c and
Guoping
Yang
 *a
*a
aSchool of Chemistry and Materials Science, Jiangxi Key Laboratory for Mass Spectrometry and Instrumentation, East China University of Technology, Nanchang, Jiangxi 330013, China. E-mail: erick@ecut.edu.cn
bSchool of Materials Science and Engineering, East China Jiaotong University, Nanchang, Jiangxi 330013, China
cHenan Key Laboratory of Polyoxometalate Chemistry, Institute of Molecular and Crystal Engineering, School of Chemistry and Chemical Engineering, Henan University, Kaifeng, Henan 475004, China. E-mail: xpsun@henu.edu.cn
First published on 14th May 2025
Abstract
A novel organic–inorganic Pr–W heterometal cluster-imbedded antimotungstate, [(CH3)2NH2]4Na20.5{[(OAc)Pr4W6O12(tar)3](SbW9O33)4(H2O)10}Cl0.5·ca. 40.3H2O (Pr-1), was synthesized via a one-step in situ assembly strategy. Pr-1 features a distinctive tetranuclear Pr-based organic–inorganic hybrid structure stabilized by polycarboxylic acid ligands, which efficiently regulate the hydrolysis of Pr3+ to facilitate the assembly of Pr–W heterometallic clusters. Notably, this structure includes abundant Lewis acid sites from Pr3+, making it highly promising for catalytic applications. Pr-1 has been successfully demonstrated as an environmentally friendly, heterogeneous catalyst in synthesizing benzimidazoles from o-phenylenediamines and benzaldehydes. The high catalytic activity, excellent structural stability, and recyclability over five cycles highlight the potential of Pr-1 as an efficient and sustainable catalyst for N-containing heterocyclic organic synthesis.
Introduction
Polyoxometalates (POMs) are an important class of metal–oxygen clusters with excellent oxidative decomposition resistance and thermal stability, enabling extensive applications in pharmaceuticals, catalysis, materials science etc.1–8 Currently, lanthanide metal ions (Ln), owing to their flexible coordination capabilities and high coordination numbers, are increasingly utilized as connection units in the synthesis of novel POMs. The incorporation of Ln ions not only facilitates the assembly of POM building blocks into more intriguing structures but also endows POMs with unexpected properties.9–13 Therefore, Ln-containing POMs (Ln-POMs) have become an important branch of POM chemistry.14–20 Among Ln-POMs, Pr3+ ions can act as metal cores, which play a crucial role in connecting polyoxoanions to generate multinuclear metal clusters, giving rise to diverse and novel structures.21–25 However, the application of Pr3+ ions in constructing novel POMs still suffers from challenges. On one hand, the large spatial steric hindrance of Pr3+ becomes a stumbling block for the effective construction of the corresponding Pr-containing POMs (Pr-POMs). On the other hand, the oxophilic nature of Pr3+ rapidly interacts with oxygen-rich POM building blocks to form amorphous precipitates rather than well-defined crystals during the synthesis of Pr-POMs. This complicates the determination of the crystal structure of the target product, highlighting the necessity of developing effective strategies for the synthesis of Pr-POMs.The introduction of polycarboxylic acid ligands into the reaction for the purpose of regulating the hydrolysis of Ln ions has proven to be a highly effective strategy.9,26–30 In the context of such reactions, Pr3+ initially coordinates with organic ligands to form multinuclear Pr oxide clusters, which subsequently connect the generated WO6 units to produce novel Pr–W heterometallic clusters by adjusting the pH. Therefore, these heterometallic clusters attract surrounding polyoxoanions, consequently leading to the formation of diverse structures. Besides, the metal ions in the clusters furnish abundant Lewis acid activation sites, which exhibit substantial potential within the domain of catalytic organic reactions.
Benzimidazoles represent a significant category in N-containing heterocyclic compounds due to their presence in a variety of natural products and extensive applications in medicinal chemistry.31,32 Therefore, benzimidazoles display a wide array of pharmacological activities, encompassing antifungal, antitumor, anti-tuberculosis, anti-inflammatory, and antibacterial properties.33–36 Currently, the principal approaches for the synthesis of benzimidazoles mainly rely on catalysis utilizing transition metals, noble metals, lanthanide metals, and other Lewis acids accompanied by notable limitations.37,38 As a result, the exploration of efficient and environmentally friendly methods for the synthesis of benzimidazole derivatives holds considerable significance.
In contrast to conventional catalysts mentioned above, Pr-POMs possess distinct advantages such as excellent catalytic activity, easy separation and recycling, and environmental friendliness.39–43 Herein, a novel organic–inorganic Pr–W heterometal cluster-imbedded antimotungstate, [(CH3)2NH2]4Na20.5{[(OAc)Pr4W6O12(tar)3](SbW9O33)4(H2O)10}Cl0.5·ca. 40.3H2O (Pr-1) has been synthesized via a one-step in situ assembly strategy. Pr-1 contains an unprecedented tetranuclear Pr organic–inorganic hybrid structure, {(OAc)Pr4W6(tar)3} (HOAc = CH3COOH, H3tar = tartaric acid). The tetrameric polyoxoanion is aggregated by four {SbW9} units encapsulating a {Pr4W6} unit, which is further expanded to a one-dimensional chain constructed by Na+ cations. As a heterogeneous catalyst, Pr-1 could efficiently catalyze the condensation cyclization of o-phenylenediamine with benzaldehyde to produce benzimidazole. Moreover, Pr-1 still retains high catalytic activity even after five cycles, demonstrating excellent stability.
Experimental
Synthesis of Pr-1
Na2WO4·2H2O (6 mmol, 1.9791 g), SbCl3 (0.8 mmol, 0.1825 g), (CH3)2NH·HCl (12 mmol, 0.9785 g), and DL-tartaric acid (0.3 mmol, 0.0451 g) were sequentially dissolved in a 0.5 M NaAc/HAc buffer solution at pH = 4.8. The solution was adjusted to pH = 4.5 using 6 M HCl and stirred for 10 min. PrCl3·6H2O (0.6 mmol, 0.2132 g) was subsequently added to the solution, and the pH was adjusted to 4.0 after the solids were completely dissolved. The clear green solution was stirred for another 10 min before being filtered. The green filtrate was left to evaporate at room temperature. Colorless stick crystals were collected after about six weeks (yield: 35% based on PrCl3·6H2O). Elemental analysis (%) for C22H140.6N4Cl0.5Na20.5O214.3Pr4Sb4W42: found (calc.): C, 2.05 (2.01); H, 1.01 (1.08), N, 0.39 (0.43); Na, 3.62 (3.58); Pr, 4.28 (4.29); W, 58.72 (58.71). FT-IR (cm−1): 3388 (m), 3155 (m), 2794 (w), 1611 (m), 1538 (m), 1464 (m), 1407 (m), 1370 (m), 1169 (w), 1136 (w), 1071 (w), 1017 (w), 936 (s), 833 (vs), 763 (vs), 680 (vs), 598 (vs).Synthesis of benzimidazoles
Benzaldehyde (1a, 0.2 mmol), o-phenylenediamine (2a, 0.2 mmol), Pr-1 (0.2 mol%), and EtOH (1 mL) were added into a 4 mL reaction vial. Then the reaction was carried out in screw cap vials with a Teflon seal at 100 °C for 1 h under an oxygen atmosphere. After the reaction, the mixture was purified by column chromatography (petroleum ether/EtOAc) or recrystallization to afford the desired products. After the reaction was completed, the catalyst was separated from the reaction solution by filtration and then washed three times with EtOAc and EtOH, respectively, and finally the recovered catalyst was refreshed by drying in a vacuum drying oven at 50 °C for 3 h.Results and discussion
Crystal structure
Single-crystal X-ray diffraction analyses reveal that Pr-1 crystallizes in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (Table S1†). The average bond valence sum (BVS) values for W, Pr, and Sb atoms are 5.97 (within the range of 5.53–6.28), 3.30 (within the range of 3.26–3.34) and 2.80 (within the range of 2.77–2.85), indicating that they are in +6, +3 and +3 oxidation states, respectively (Table S2†). In the polyanionic skeleton of Pr-1, four {SbW9O33} units are interconnected via a unique organic–inorganic hybrid linking fragment (OAc)Pr4W6(tar)3 (Fig. 1A and S1†), which consists of a single acetic anion, three tartrate anions, four Pr3+ cations and six {WO6} octahedra. The organic–inorganic hybrid fragment features two sets of corner-sharing W and Pr centers, denoted as {Pr2W3O29}, which are interconnected by three tartrate anions and one acetic anion (Fig. 2). This linkage is facilitated by a Pr–O–C–O–Pr bridge emanating from the acetic anion, along with one W–O⋯C⋯O–W bridge and two Pr–O⋯C⋯O–W bridges established by the tartrate anions. In detail, two of the four praseodymium ions, specifically Pr1 and Pr3, exhibit a distorted mono-capped square-antiprism geometry, being coordinated by nine oxygen atoms (Fig. S2†). In this configuration, the Pr1 ion is bonded to five μ2-O atoms (O7 and O8 from the {SbW9O33} subunit and O3, O35, and O68 from the {WO6} fragment) with the bond distances ranging from 2.436 Å to 2.581 Å. Additionally, it forms a bond with one water molecule (O1W) at a distance of 2.589 Å, one oxygen atom (O1) from the acetic acid ligand at a distance of 2.354 Å, and two oxygen atoms (O1L and O2L) from the tartaric acid ligand with distances of 2.572 Å and 2.587 Å. On the other hand, the remaining two praseodymium ions, Pr2 and Pr4, adopt an eight-coordinate distorted double-capped triangular prism geometry (Fig. S2†). In this arrangement, Pr2 is coordinated by five μ2-O atoms (O39 and O40 from the {SbW9O33} subunit and O4, O6, and O67 from the {WO6} fragment) with the bond distances varying from 2.323 Å to 2.495 Å. Furthermore, it forms bonds with three water molecules (O2W, O3W, and O4W) with distances ranging from 2.452 Å to 2.589 Å. It is noteworthy that the tetrameric polyanions (Fig. 1B and C) are organized into a one-dimensional (1D) architecture through the interconnection of W–O–Na–O–W and W–O–Na–O–C bridging units (Fig. 1D), in which the six-coordinate Na+ cation is coordinated by O7L, O17L, O9W, O12W, O134 and the Cl1 anion with a Na–Cl bond length of 2.718 Å. These 1D structures are arranged in parallel along the a-axis direction (Fig. S2†).
(Table S1†). The average bond valence sum (BVS) values for W, Pr, and Sb atoms are 5.97 (within the range of 5.53–6.28), 3.30 (within the range of 3.26–3.34) and 2.80 (within the range of 2.77–2.85), indicating that they are in +6, +3 and +3 oxidation states, respectively (Table S2†). In the polyanionic skeleton of Pr-1, four {SbW9O33} units are interconnected via a unique organic–inorganic hybrid linking fragment (OAc)Pr4W6(tar)3 (Fig. 1A and S1†), which consists of a single acetic anion, three tartrate anions, four Pr3+ cations and six {WO6} octahedra. The organic–inorganic hybrid fragment features two sets of corner-sharing W and Pr centers, denoted as {Pr2W3O29}, which are interconnected by three tartrate anions and one acetic anion (Fig. 2). This linkage is facilitated by a Pr–O–C–O–Pr bridge emanating from the acetic anion, along with one W–O⋯C⋯O–W bridge and two Pr–O⋯C⋯O–W bridges established by the tartrate anions. In detail, two of the four praseodymium ions, specifically Pr1 and Pr3, exhibit a distorted mono-capped square-antiprism geometry, being coordinated by nine oxygen atoms (Fig. S2†). In this configuration, the Pr1 ion is bonded to five μ2-O atoms (O7 and O8 from the {SbW9O33} subunit and O3, O35, and O68 from the {WO6} fragment) with the bond distances ranging from 2.436 Å to 2.581 Å. Additionally, it forms a bond with one water molecule (O1W) at a distance of 2.589 Å, one oxygen atom (O1) from the acetic acid ligand at a distance of 2.354 Å, and two oxygen atoms (O1L and O2L) from the tartaric acid ligand with distances of 2.572 Å and 2.587 Å. On the other hand, the remaining two praseodymium ions, Pr2 and Pr4, adopt an eight-coordinate distorted double-capped triangular prism geometry (Fig. S2†). In this arrangement, Pr2 is coordinated by five μ2-O atoms (O39 and O40 from the {SbW9O33} subunit and O4, O6, and O67 from the {WO6} fragment) with the bond distances varying from 2.323 Å to 2.495 Å. Furthermore, it forms bonds with three water molecules (O2W, O3W, and O4W) with distances ranging from 2.452 Å to 2.589 Å. It is noteworthy that the tetrameric polyanions (Fig. 1B and C) are organized into a one-dimensional (1D) architecture through the interconnection of W–O–Na–O–W and W–O–Na–O–C bridging units (Fig. 1D), in which the six-coordinate Na+ cation is coordinated by O7L, O17L, O9W, O12W, O134 and the Cl1 anion with a Na–Cl bond length of 2.718 Å. These 1D structures are arranged in parallel along the a-axis direction (Fig. S2†).
 | ||
| Fig. 2 Schematic representation of the polyhedron and the topology of organic–inorganic hybrid fragments in the polyanionic skeleton of Pr-1. | ||
In terms of packing efficiency, the unit cell exhibits a void volume of approximately 14.6%, as calculated using the solvent-accessible surface. This significant void contributes to highly disordered solvent molecules (Fig. S4†), which are removed using the SQUEEZE routine, and the molecular formula is finally determined through comprehensive consideration of crystallographic data, elemental analyses, and thermogravimetric measurements.
Catalytic activity for benzimidazole synthesis
To evaluate the catalytic performance of Pr-1, a model reaction was conducted using benzaldehyde (1a, 0.2 mmol) and o-phenylenediamine (2a, 0.2 mmol) in ethanol (EtOH) under an oxygen atmosphere (Table 1). Product 3a was obtained with a 45% yield when catalyzed by Pr-1 (0.2 mol%) at room temperature for 0.5 h. To enhance the reaction yield, various solvents including dimethyl carbonate (DMC), propylene carbonate (PC), isopropanol (i-PrOH), and ethyl acetate (EA) were investigated. Although the reaction could proceed in these solvents, the highest yield was achieved in EtOH. Additionally, the reaction was significantly influenced by temperature, with the yield of 3a increasing as the temperature rose, reaching an optimal 89% yield at 100 °C. Finally, extending the reaction time to 1 h resulted in a maximum yield of 93%.| Entry | Solvent | Temperature (°C) | Time (h) | Yieldb [%] |
|---|---|---|---|---|
| a Reaction conditions: benzaldehyde 1a (0.2 mmol), o-phenylenediamine 2a (0.2 mmol), Pr-1 (0.2 mol%), solvent (1 mL), O2, rt, 0.5 h. b The reaction yields were determined by gas chromatography using biphenyl as an internal standard. | ||||
| 1 | EtOH | rt | 0.5 | 45 |
| 2 | EA | rt | 0.5 | 19 |
| 3 | DMC | rt | 0.5 | 18 |
| 4 | PC | rt | 0.5 | 29 |
| 5 | EtOH | 60 | 0.5 | 71 |
| 6 | EtOH | 80 | 0.5 | 80 |
| 7 | EtOH | 90 | 0.5 | 85 |
| 8 | EtOH | 100 | 0.5 | 89 |
| 9 | EtOH | 100 | 1 | 93 |
| 10 | EtOH | 100 | 1.25 | 93 |
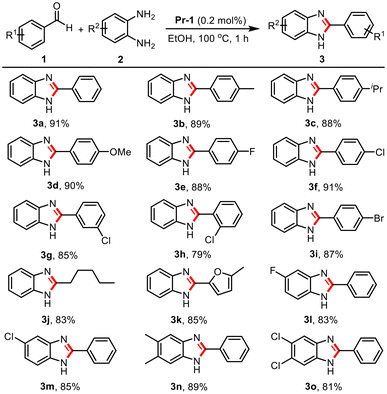
To investigate the substrate scope of the catalytic system, various aldehydes and phenylenediamines were employed to synthesize benzimidazoles under optimized conditions (Table 2). Benzaldehydes bearing electron-donating substituents such as –Me, –iPr, and –OMe were successfully converted into the corresponding benzimidazoles with yields of 89%, 88%, and 90%, respectively (3b–3d). The electron-withdrawing groups including –F, –Cl, and –Br were also well tolerated, affording the desired products in good yields (3e–3i). The electronic properties of substituents have little effect on the reaction yield. Notably, the reaction yields of 3f and 3g were higher than that of 3h, indicating that steric hindrance had a certain effect on the reaction efficiency. Furthermore, this catalytic system was also compatible with aliphatic and heterocyclic aldehydes, affording the corresponding products 3j and 3k in 83% and 85% yields, respectively. Subsequently, we also explored the substrate range of o-phenylenediamines. The o-phenylenediamines containing –F and –Cl groups could react smoothly with benzaldehyde, producing the corresponding products (3l, 3m) in 83% and 85% yields, respectively. The disubstituted o-phenylenediamines with 5,6-dimethyl and 5,6-dichlorine groups were also successfully converted into the corresponding products in good yields (3n, 3o).
| a Reaction conditions: benzaldehyde 1 (0.2 mmol), o-phenylenediamine 2 (0.2 mmol), Pr-1 (0.2 mol%), EtOH (1 mL), O2, 100 °C, 1 h. |
|---|
|
Finally, a scaled-up reaction and cycling experiments were conducted to evaluate the practicality of this method. 10 mmol of 1a and 10 mmol of 2a reacted smoothly under optimal conditions, yielding the desired product 3a in 86% yield, indicating its potential for larger-scale synthesis (Fig. 3A). Reusability tests revealed that the catalytic performance of Pr-1 remained consistent after five runs, indicating that the catalytic activity of Pr-1 remained stable during the catalytic process (Fig. 3B). Furthermore, no significant changes in the PXRD spectra before and after catalysis further confirm the stability of the Pr-1 structure (Fig. 3C).
Conclusions
In summary, a novel Pr–W heterometallic cluster-imbedded antimotungstate, Pr-1, has been successfully synthesized. Polycarboxylic acid ligands were introduced to modulate the hydrolysis of Pr3+, effectively overcoming its oxophilic nature and preventing the formation of amorphous precipitates. As an environmentally friendly and heterogeneous catalyst, Pr-1 exhibited exceptional performance in the synthesis of benzimidazoles from o-phenylenediamines and benzaldehydes. It exhibited high efficiency and stability, maintaining its catalytic activity over five cycles. This endows Pr-1 with significant potential for sustainable organic synthesis. This work highlights the promising role of Ln-POMs in advancing environmentally friendly and economically viable catalytic processes, paving the way for future exploration in POM chemistry.Author contributions
Zhijian Zheng, Yayu Dong, and Xiaopeng Sun wrote the draft; Haoqi Liu collected the data; Zhijian Zheng, Song Jiang and Xuejiao Chen performed the IR, TG and XRD experiments; Song Jiang and Yufeng Liu investigated the catalytic activities; and Guoping Yang contributed to conceiving the idea of the study. All authors contributed to the writing and revisions.Data availability
The data supporting this article have been included as part of the ESI.† Crystallographic data for Pr-1 have been deposited at the CCDC under 2373492.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (22371040) and the Jiangxi Provincial Natural Science Foundation (20232ACB213005).References
- Q.-S. Lai, X.-X. Li and S.-T. Zheng, Coord. Chem. Rev., 2023, 482, 215077 CrossRef CAS.
- N. Li, J. Liu, B.-X. Dong and Y.-Q. Lan, Angew. Chem., Int. Ed., 2020, 59, 20779–20793 CrossRef CAS PubMed.
- H. Liu, L. Bai, L. Ai, W. Dai, D. Zhang, Q. Wu and R. Zhang, J. Rare Earths, 2019, 37, 617–621 CrossRef CAS.
- J.-C. Liu, J.-W. Zhao, C. Streb and Y.-F. Song, Coord. Chem. Rev., 2022, 471, 214734 CrossRef CAS.
- H. Lv, Y. V. Geletii, C. Zhao, J. W. Vickers, G. Zhu, Z. Luo, J. Song, T. Lian, D. G. Musaev and C. L. Hill, Chem. Soc. Rev., 2012, 41, 7572–7589 RSC.
- H. N. Miras, J. Yan, D.-L. Long and L. Cronin, Chem. Soc. Rev., 2012, 41, 7403–7430 RSC.
- D.-Q. Qian, Y.-D. Lin, H.-P. Xiao, B. Wu, X.-X. Li and S.-T. Zheng, Polyoxometalates, 2024, 3, 9140040 CrossRef.
- H. Zhang, A. Li, K. Li, Z. Wang, X. Xu, Y. Wang, M. V. Sheridan, H.-S. Hu, C. Xu, E. V. Alekseev, Z. Zhang, P. Yan, K. Cao, Z. Chai, T. E. Albrecht-Schönzart and S. Wang, Nature, 2023, 616, 482–487 Search PubMed.
- C. Ritchie, E. G. Moore, M. Speldrich, P. Kögerler and C. Boskovic, Angew. Chem., Int. Ed., 2010, 49, 7702–7705 CrossRef CAS PubMed.
- S. Li, Y. Wang, P. Ma, J. Wang and J. Niu, CrystEngComm, 2014, 16, 10746–10749 RSC.
- B. Nohra, P. Mialane, A. Dolbecq, E. Rivière, J. Marrot and F. Sécheresse, Chem. Commun., 2009, 19, 2703–2705 RSC.
- S. Zhang, Y. Wang, J. Zhao, P. Ma, J. Wang and J. Niu, Dalton Trans., 2012, 41, 3764–3772 RSC.
- Y. Wang, X. Sun, S. Li, P. Ma, J. Wang and J. Niu, Dalton Trans., 2015, 44, 733–738 RSC.
- F. Hussain, R. W. Gable, M. Speldrich, P. Kögerler and C. Boskovic, Chem. Commun., 2009, 3, 328–330 RSC.
- W.-W. Ju, H.-T. Zhang, X. Xu, Y. Zhang and Y. Xu, Inorg. Chem., 2014, 53, 3269–3271 CrossRef CAS PubMed.
- J. Niu, K. Wang, H. Chen, J. Zhao, P. Ma, J. Wang, M. Li, Y. Bai and D. Dang, Cryst. Growth Des., 2009, 9, 4362–4372 CrossRef CAS.
- M. Ibrahim, S. S. Mal, B. S. Bassil, A. Banerjee and U. Kortz, Inorg. Chem., 2010, 50, 956–960 CrossRef PubMed.
- P. Mialane, A. Dolbecq, J. Marrot and F. Sécheresse, Inorg. Chem. Commun., 2005, 8, 740–742 CrossRef CAS.
- N. Lotfian, M. Mirzaei, H. Eshtiagh-Hosseini, M. Löffler, M. Korabik and A. Salimi, Eur. J. Inorg. Chem., 2014, 2014, 5908–5915 CrossRef CAS.
- F. Li, W. Guo, L. Xu, L. Ma and Y. Wang, Dalton Trans., 2012, 41, 9220–9226 RSC.
- J.-Q. Geng, Y. Lu, R. Zhang, Y.-X. Lv, S.-T. Huang, Y.-Y. Yang, X. Qu, H.-F. Shi, H. Jin and X.-Y. Yu, J. Mol. Struct., 2024, 1302, 137502 CrossRef CAS.
- Y.-X. Li, S. Liu, Y.-H. Fan, S. Andra, D.-B. Dang, Y.-M. Li and Y. Bai, Inorg. Chem., 2024, 63, 3637–3641 CrossRef CAS PubMed.
- Q. Shang, K. Shen, D. Zhao, X. Wang, Y. Wei, H. Wang, G. Wang, D. Zhang and J. Niu, Polyoxometalates, 2025, 4, 9140077 CrossRef.
- Y.-N. Gu, D. Zhao, H. Yu, R. Ge, Z. Li, C.-B. Tian, X.-X. Li, Y.-Q. Sun and S.-T. Zheng, RSC Adv., 2019, 9, 13543–13549 RSC.
- H. Naruke, J. Iijima and T. Sanji, Inorg. Chem., 2011, 50, 7535–7539 CrossRef CAS PubMed.
- T. Gong, S. Yang, Z. Wang, M. Li, S. Zhang, J. Liu, L. Chen and J. Zhao, Inorg. Chem. Front., 2023, 10, 2799–2810 RSC.
- X. Jia, J. Jiang, L. Liu, L. Meng, L. Chen and J. Zhao, Inorg. Chem., 2022, 61, 10965–10976 CrossRef CAS PubMed.
- J. Liu, D. Wang, X. Xu, H. Li, J. Zhao and L. Chen, Inorg. Chem., 2021, 60, 2533–2541 CrossRef CAS PubMed.
- Y. Liu, G. Liu, B. Zeng, Y. Li, L. Chen and J. Zhao, Inorg. Chem., 2024, 63, 7858–7868 Search PubMed.
- B. Niu, M. Zhang, L. Yan, A. Yu, P. Ma, J. Wang and J. Niu, Inorg. Chem., 2023, 62, 20153–20161 CrossRef CAS PubMed.
- Y. Bansal and O. Silakari, Bioorg. Med. Chem., 2012, 20, 6208–6236 CrossRef CAS PubMed.
- C. G. Arya, R. Gondru, Y. Li and J. Banothu, Eur. J. Med. Chem., 2022, 227, 113921 CrossRef PubMed.
- M. Fontecha-Barriuso, D. Martín-Sanchez, J. M. Martinez-Moreno, D. Cardenas-Villacres, S. Carrasco, M. D. Sanchez-Niño, M. Ruiz-Ortega, A. Ortiz and A. B. Sanz, Redox Biol., 2020, 32, 101464 CrossRef CAS PubMed.
- K. Jana, T. Bandyopadhyay and B. Ganguly, J. Phys. Chem. B, 2018, 122, 5765–5775 CrossRef CAS PubMed.
- C. Kang, Drugs, 2022, 82, 335–340 CrossRef CAS PubMed.
- Q. Li, S. Demir, Á. Del Río-Álvarez, R. Maxwell, A. Wagner, J. Carrillo-Reixach, C. Armengol, C. Vokuhl, B. Häberle, D. von Schweinitz, I. Schmid, S. Cairo and R. Kappler, Cancers, 2022, 14, 4196 CrossRef CAS PubMed.
- M. I. García-Aranda, J. E. Gonzalez-Padilla, C. Z. Gómez-Castro, Y. M. Gómez-Gómez, M. C. Rosales-Hernández, E. V. García-Báez, M. O. Franco-Hernández, J. L. Castrejón-Flores and I. I. Padilla-Martínez, Bioorg. Med. Chem., 2020, 28, 115427 CrossRef PubMed.
- K. Gong, C. Li, D. Zhang, H. Lu, Y. Wang, H. Li and H. Zhang, Mol. Catal., 2022, 519, 112139 CrossRef CAS.
- D. Y. Vandyshev, K. S. Shikhaliev, A. V. Kokonova, A. Y. Potapov, M. G. Kolpakova, A. L. Sabynin and F. I. Zubkov, Chem. Heterocycl. Compd., 2016, 52, 493–497 CrossRef CAS.
- D. Singla, V. Luxami and K. Paul, Eur. J. Org. Chem., 2023, e202300531 CrossRef CAS.
- Y.-T. Lee, F.-Y. Chiu, I. J. Barve and C.-M. Sun, Adv. Synth. Catal., 2018, 360, 502–512 CrossRef CAS.
- P. Sunani, P. Thiruvengetam and D. K. Chand, Dalton Trans., 2025, 54, 3704–3713 RSC.
- S. Anand, A. Muthusamy, S. Dineshkumar and J. Chandrasekaran, J. Mol. Struct., 2017, 1147, 351–363 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2373492. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5dt00386e |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |



