Rapid synthesis of fault-free GME zeolite†
Jingqiu
Li
ab,
Caiping
Sheng
b,
Ye
Ma
 c,
Liangni
Ban
b,
Jie
Zhu
b,
Zhongping
Huang
*a,
Hongxia
Shen
b,
Xuebo
Cao
*b and
Longfeng
Zhu
c,
Liangni
Ban
b,
Jie
Zhu
b,
Zhongping
Huang
*a,
Hongxia
Shen
b,
Xuebo
Cao
*b and
Longfeng
Zhu
 *b
*b
aCollege of Chemical Engineering, Zhejiang University of Technology, Hangzhou 310014, China. E-mail: zphuang@zjut.edu.cn
bCollege of Biological, Chemical Science and Engineering, Jiaxing University, Jiaxing 314001, China. E-mail: xbcao@zjxu.edu.cn; zhulf1988@mail.zjxu.edu.cn
cSchool of Physical Science and Technology & Shanghai Key Laboratory of High-resolution Electron Microscopy, ShanghaiTech University, Shanghai 201210, China
First published on 18th March 2025
Abstract
The optimization of the synthesis of known zeolite structures and the discovery of new applications for them are continuously hot topics in the field of zeolite chemistry. One of the typical examples is the GME zeolite structure. Herein, we for the first time report a rapid synthesis of fault-free GME zeolite by the combined strategy of seeding and aging. The fault-free GME zeolite can be obtained at 160 °C in only 2.5 h. Various characterization techniques, such as X-ray diffraction (XRD), scanning electron microscopy (SEM), high-resolution transmission electron microscopy (HRTEM) and nuclear magnetic resonance (NMR) have been used, which show that the obtained product has good crystallinity, perfect hexagonal morphology, fully 4-coordinated Al species and fault-free features. In addition, after the post-treatment of the as-made GME zeolite, Na–GME zeolite with a large BET surface area and high porosity could be successfully obtained. More importantly, the obtained Na–GME zeolite is used for the first time for CO2 capture, giving a high CO2 adsorption of 5.37 mmol g−1 and excellently selective CO2 adsorption from CO2/N2 (15/85, v/v) with a separation coefficient of 58.8. The fault-free GME zeolite used for CO2 capture might be of potential significance for industrial applications.
1. Introduction
Zeolites, particularly aluminosilicate zeolites, have been widely employed in the fields of catalysis, ion-exchange, separation and adsorption because of their high surface area, large pore volume, uniform micropore distribution, and excellent stability.1–10 Currently, over 260 types of zeolite have been given three-letter codes by the International Zeolite Association-Structural Commission (IZA-SC).11 However, only about 20 types of zeolite have been used for industrial applications.12–18 Thus, optimizing the synthesis of known zeolite structures and discovering new applications for them is of great significance, and would be very helpful to promote their practical application in industry. Among them, one of the typical examples is GME zeolite.GME zeolite is composed of double six-membered rings (6-MRs) and GME cages, which form a three-dimensional micropore channel with an aperture size of 12 × 8 × 8-MRs.19,20 Initially, GME zeolite was discovered in nature, where it always exhibits stacking faults and CHA intergrowth, leading to significantly reduced porosity.20–22 Thus, great efforts have been made to reduce the faults and improve the purity.19,22–31 For example, Daniels et al. showed that synthetic GME zeolite could be obtained after crystallization for at least 3 days in the presence of dabco-polymer, giving a greater adsorption capacity of cyclohexane than that of natural GME zeolite.22 Chiyoda et al. showed that GME zeolite could be synthesized from the transformation of Sr–Y zeolite.19 Notably, the crystallization of GME zeolite was successful at 240 °C for 14 d. Mielby et al. displayed that a steam-assisted interzeolite transformation from Y zeolite could be successful achieved to synthesize GME zeolite in 20 h.23 However, these synthetic GME zeolites also have stacking faults, which could still block the micropores and thus reduce the porosity. Recently, Dusselier et al. for the first time showed a fault-free synthesis of GME zeolite in the presence of 3,5-dimethylpiperidinium, which gave a micropore volume of 0.17 cm3 g−1 after ozone-treatment at 150 °C.24 Notably, the crystallization would be completed after at least 110 h. Mitani et al. also reported that GME zeolite with high porosity could be successfully synthesized from the transformation of FAU zeolite using a dual template route, in which it was fully crystallized after 2 d.25 However, despite the great progress that has been made in the successful synthesis of fault-free GME zeolite, it is still not very efficient due to the long crystallization time. Currently, it is still highly desirable to synthesize fault-free GME zeolite in a rapid manner.
More recently, we showed for the first time a rapid synthesis of high silica SSZ-13 zeolite by the combined strategy of short aging and zeolite seeding, which gives crystallization in only 4 h.32 The addition of zeolite seeds would produce more basic building units during the short aging, which would lead to the successful synthesis of high silica SSZ-13 zeolite rapidly. However, the rapid synthesis of fault-free GME zeolite at the hour level has still not been reported using this method.
Herein, we demonstrate a rapid synthesis of fault-free GME zeolite for the first time. Notably, GME zeolite with high crystallinity could be fully crystallized at 160 °C in only 2.5 h after the addition of zeolite seeds and then aging at room temperature for 3 h. After the post-treatment of the as-made GME zeolite, a Na–GME zeolite with high porosity could be obtained successfully. More importantly, the obtained Na–GME zeolite gives a high CO2 adsorption of 5.37 mmol g−1 and excellently selective CO2 adsorption from CO2/N2 (15/85, v/v) with a separation coefficient of 58.8.
2. Experimental
Rapid synthesis of GME zeolite at 160 °C for 2.5 h
As a typical example, 1.14 g of deionized water and 0.1156 g of sodium aluminate were mixed in a beaker. After the dissolution of sodium aluminate, 1.7 g of N,N-dimethyl-3,5-dimethylpiperidinium hydroxide solution, 0.48 g of sodium hydroxide, and 3.93 g of silica sol were added into the above solution one by one. After stirring for 1 h at room temperature, 0.024 g of GME seeds was added. After aging for 3 h at room temperature under stirring conditions, the mixture was transferred to an autoclave and sealed. After crystallization at 160 °C for 2.5 h under rotation conditions (50 rpm), a fully crystalline GME zeolite product was obtained with a yield of 22%. Notably, the aging time is very critical for the successful synthesis of the target product with good crystallinity. If the aging time is too short, such as 10 min, the obtained product would always have GIS zeolite as an impurity phase.Synthesis of the conventional GME zeolite
According to the literature report by Mielby et al.,23 the conventional GME zeolite with some faults from interzeolite transformation was successfully synthesized at 120 °C for 3 d, and was named as T–GME.Preparation of K–GME and Na–GME
The as-made GME zeolite was mixed with 2 mol kg−1 KCl solution (solid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) liquid = 1 mg
liquid = 1 mg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 mg) and aged for 12 h at room temperature. The resulting thick paste was calcined at 500 °C for 5 h. The obtained sample was fully washed with warm distilled water to remove excess salt. Then, a second calcination was performed at 580 °C for 6 h, which gave the partially K+-exchanged GME, named as KS–GME. To obtain Na–GME or K–GME, KS–GME was exchanged three times with 1 mol L−1 NaCl or KCl at 80 °C for 2 h.
2 mg) and aged for 12 h at room temperature. The resulting thick paste was calcined at 500 °C for 5 h. The obtained sample was fully washed with warm distilled water to remove excess salt. Then, a second calcination was performed at 580 °C for 6 h, which gave the partially K+-exchanged GME, named as KS–GME. To obtain Na–GME or K–GME, KS–GME was exchanged three times with 1 mol L−1 NaCl or KCl at 80 °C for 2 h.
Performance tests
The CO2 adsorption experiments were carried out using high-purity CO2 (99.999%) at 298 K over a pressure range of 0–101.325 kPa by a JW-ZQ100. The samples were placed inside the balance and outgassed at 473 K under vacuum conditions for 4 h, and then cooled down to the experimental temperature. The experiments started with the gas pressure increasing stepwise until 101.325 kPa. Before achieving the equilibrium condition, the mass variation was recorded constantly.CO2/N2 breakthrough separations were performed in a multi-component adsorption breakthrough curve analyzer (Beishide Instrument Technology (Beijing) Co., Ltd, China). Before the test, each sample (0.4–1.0 g) was pre-processed in a He atmosphere (5 mL min−1) for 2 h at 573 K in quartz tubing (6 mm diameter). The test started when the gas mixture (CO2/N2: 15/85, v/v) was introduced (3 mL min−1) at 298 K after cooling to ambient temperature.
3. Results and discussion
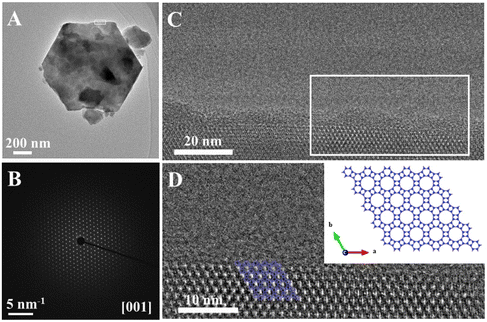
The as-made GME zeolite was firstly characterized in detail by the X-ray diffraction (XRD), scanning electron microscopy (SEM), nuclear magnetic resonance (NMR), and thermogravimetric-differential thermal analysis (TG-DTA) techniques, as displayed in Fig. 1. Fig. 1A shows the XRD pattern of the as-made GME zeolite, giving a series of typical peaks associated with the GME structure, which were in good agreement with the simulated XRD pattern from IZA-SC (Fig. S1†). Fig. 1B shows the SEM image of the as-made GME zeolite with hexagonal features, giving the crystal size at about 1 μm. Fig. 1C displays the 29Si MAS NMR spectrum of the as-made GME zeolite, giving three peaks with chemical shifts at about −109.8, −104.4 and −98.5 ppm, which were reasonably attributed to Si(4Si), Si(3Si) and Si(2Si) species, respectively. The calculated Si/Al ratio of the as-made GME zeolite product was about 3.5 based on the 29Si MAS NMR spectrum, which was very similar to that (Si/Al ratios at 3.6 and 3.1) of the XRF and ICP results, respectively. Fig. 1D displays the 27Al MAS NMR spectrum of the as-made GME zeolite, showing only one peak with the chemical shift at about 55.6 ppm, which means that there are no extra-framework aluminum species. Fig. 1E shows the TG-DTA curve of the as-made GME zeolite, which displays an obvious exothermic peak between 380 and 450 °C, resulting in weight loss associated with the decomposition of the organic template at about 8.74%. The above results also show that the organic template was indeed in the GME zeolite micropores. Fig. 1F displays the 13C MAS NMR spectrum of the as-made GME zeolite and 13C NMR spectrum of the organic template, which showed that the organic template in the GME zeolite was intact, in good agreement with the liquid organic template. The FTIR spectrum of the as-made GME zeolite is shown in Fig. S2,† giving C–H stretching peaks at 2800–3000 cm−1. Thus, according to the results of TG-DTA, NMR, and FTIR, it can be reasonably concluded that the organic template exists in the as-made GME zeolite micropores and directs the formation of the GME zeolite structure.As we known, in the GME zeolite structure (Fig. S3†), faults always appear in the 12-MR channel along the c-axis and thus partially block the micropores.20,23,24,33 Dusselier et al. used rotation electron diffraction to determine the extent of disorder of the GME zeolite (named as CIT-9).24 It could be observed that CIT-9 did not have streaking along the c-axis, indicating fully open 12-MR micropores. Thus, the TEM technique has been used for assessing the extent of the faults in the GME zeolite, as shown in Fig. 2. Fig. 2A and B show the TEM image of the GME zeolite and its corresponding SAED image, where it can be clearly seen that no streaking appeared along the c-axis, indicating that the 12-MR channels were fully open without any faults. In addition, the orderly 12-MR channels could be clearly observed from the HRTEM images and fitted well with the GME structure, which further proves that no faults formed in the GME zeolite product, as shown in Fig. 2C and D. Based on the results of XRD and TEM, it can be concluded that the GME zeolite product synthesized in this work is fault-free.
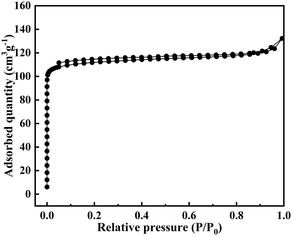
However, the GME zeolite is not resistant to calcination at high temperature, and direct calcination would cause transformation from GME to AFI, as shown in Fig. S4.† After the treatment of the as-made GME zeolite with KCl under suitable conditions, a stable GME zeolite could be successfully obtained, as shown in Fig. S4 and S5.† Moreover, a Na–GME zeolite could also be successfully obtained by ion-exchange of NaCl, as shown in Fig. S6.†Fig. 3 shows the N2 sorption isotherms of the Na–GME zeolite, which displayed the typical Langmuir curves. Simultaneously, the BET surface area and micropore volume were determined to be 341 m2 g−1 and 0.16 cm3 g−1, respectively, which also showed that the obtained GME zeolite was fault-free.
Notably, to obtain the optimized conditions for synthesizing fault-free GME zeolite, various synthetic factors were investigated, including Na2O/SiO2 ratio, OSDA/SiO2 ratio, H2O/SiO2 ratio, Si/Al ratio, and the number of seeds in the initial gel. It was found that the Na2O/SiO2 ratio was very important to synthesize pure GME zeolite with good crystallinity. When the Na2O/SiO2 ratio was 0.34, a GME zeolite with good crystallinity could be obtained successfully (run 4 in Table 1 and Fig. 1A); when the Na2O/SiO2 ratio was lower than 0.31, the products were GME zeolite together with an amorphous phase (run 1–3 in Table 1 and Fig. S7a–c†); when the Na2O/SiO2 ratio was higher than 0.37, the products were GME zeolite together with GIS or ANA zeolites as impurities (run 6–7 in Table 1 and Fig. S7e and f†). Thus, the suitable Na2O/SiO2 ratio for the successful synthesis of the GME zeolite was 0.34. In addition, the most suitable OSDA/SiO2 ratios were 0.096–0.136, which could obtain GME zeolite with high crystallinity (run 4, 9, and 10 in Table 1 and Fig. S8b–d†). If the OSDA/SiO2 ratio was lower than 0.076, the obtained products were GME zeolite with GIS zeolite as an impurity (run 8 in Table 1 and Fig. S8a†); if the OSDA/SiO2 ratio was higher than 0.156, ANA zeolite as an impurity could be observed in the final product (run 11 in Table 1 and Fig. S8e†). Therefore, the OSDA/SiO2 ratio should be carefully adjusted during the whole synthesis. Moreover, when the H2O/SiO2 ratio in the starting gel was 5, the final product was GME zeolite together with an amorphous phase (run 12 in Table 1 and Fig. S9a†); when the H2O/SiO2 ratio was increased to 15, GME zeolite with good quality could be obtained successfully (run 4 in Table 1 and Fig. 1A); further increasing the H2O/SiO2 ratio to 30, the obtained product was GME zeolite together with an amorphous phase (run 13 in Table 1 and Fig. S9c†). Additionally, when the Si/Al ratios were increased from 20 to 40, GME zeolite with high crystallinity was obtained successfully (run 4 and 15 in Table 1 and Fig. S10b and c†); when the Si/Al ratio was lower than 10, the obtained product was GME zeolite together with ANA zeolite as an impurity (run 14 in Table 1 and Fig. S10a†); when the Si/Al ratio increased from 60 to 80, AEI zeolite started to appear in the final product (run 16 and 17 in Table 1 and Fig. S10d and e†). Furthermore, when the zeolite seeds were absent in the starting gel, the product would always be GME zeolite together with GIS zeolite (run 18 in Table 1 and Fig. S11a†). Addition of 2% zeolite seeds was enough to get a GME product with good crystallinity (run 4 in Table 1 and Fig. 1A). Further addition of 4% zeolite seeds would not shorten the complete crystallization time, indicating that the addition of 2% zeolite seeds was enough for complete crystallization of the GME zeolite at 160 °C for 2.5 h (Fig. 4 and 5).
| Runa | Na2O/SiO2 | OSDA/SiO2 | H2O/SiO2 | Si/Al | Seedc (%) | Productd |
|---|---|---|---|---|---|---|
| a The products were synthesized at 160 °C for 4 h. b The products were synthesized at 160 °C for 2.5 h. c Mass ratio of zeolite seeds to the silica source. d The phase that appears first is dominant. | ||||||
| 1 | 0.25 | 0.136 | 15 | 20 | 2 | Amor + GME |
| 2 | 0.28 | 0.136 | 15 | 20 | 2 | GME + Amor |
| 3 | 0.31 | 0.136 | 15 | 20 | 2 | GME + Amor |
| 4 | 0.34 | 0.136 | 15 | 20 | 2 | GME |
| 5b | 0.34 | 0.136 | 15 | 20 | 2 | GME |
| 6 | 0.37 | 0.136 | 15 | 20 | 2 | GME + GIS |
| 7 | 0.40 | 0.136 | 15 | 20 | 2 | GME + ANA |
| 8 | 0.34 | 0.076 | 15 | 20 | 2 | GME + GIS |
| 9 | 0.34 | 0.096 | 15 | 20 | 2 | GME |
| 10 | 0.34 | 0.116 | 15 | 20 | 2 | GME |
| 11 | 0.34 | 0.156 | 15 | 20 | 2 | GME + ANA |
| 12 | 0.34 | 0.136 | 5 | 20 | 2 | Amor + GME |
| 13 | 0.34 | 0.136 | 30 | 20 | 2 | GME + Amor |
| 14 | 0.34 | 0.136 | 15 | 10 | 2 | GME + ANA |
| 15 | 0.34 | 0.136 | 15 | 40 | 2 | GME |
| 16 | 0.34 | 0.136 | 15 | 60 | 2 | GME + AEI |
| 17 | 0.34 | 0.136 | 15 | 80 | 2 | AEI + GME |
| 18 | 0.34 | 0.136 | 15 | 20 | 0 | GIS + GME |
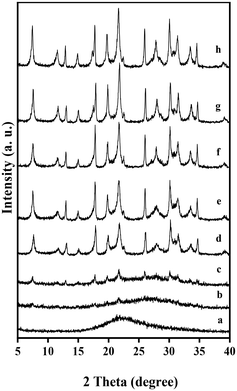
Additionally, the crystallization temperature was also very important for rapid crystallization. When the crystallization temperature was at 140 °C, the complete crystallization of the GME zeolite with good crystallinity would take about 6 h, as shown in Fig. S12.† The corresponding dependence of the zeolite crystallinity on the crystallization time is shown in Fig. S13.† When the crystallization temperature was at 160 °C, the crystallization of the GME zeolite could be completed in 2.5 h. The details are provided in the following text. More importantly, the aging of initial gel was very crucial for the successful synthesis of GME zeolite with good quality. When the aging time after the addition of zeolite seeds was 10 min, the obtained product always contained GIS phase (Fig. S14a†); when the aging time after addition of zeolite seeds was 3 h, pure GME zeolite could be successfully obtained (Fig. 1A). When the aging time was 12 h, the crystallization of the GME zeolite still required 2.5 h (Fig. S14c†). In summary, GME zeolite with good crystallinity could be rapidly synthesized only under appropriate initial ratios, aging and crystallization conditions.
The crystallization process of the GME zeolite was monitored in detail by the XRD and SEM techniques, as shown in Fig. 4 and 5. Before crystallization, almost no peaks associated with the GME structure could be observed from the XRD pattern, even if zeolite seeds were added into the starting gel (Fig. 4a), indicating the dissolution of the zeolite seeds after aging of the starting gel for 3 h. After crystallization for 0.5 h, very weak characteristic peaks associated with the GME structure started to appear (Fig. 4b). Increasing the crystallization time to 1 h, the intensity of the peaks became stronger (Fig. 4c). Correspondingly, small amounts of GME zeolite crystals could be seen via the SEM image, as displayed in Fig. 5b. When the crystallization time was 1.25 h, the characteristic peaks and the hexagonal crystals associated with the GME zeolite structure could be clearly observed, as shown in Fig. 4d and 5c. When the crystallization time increased from 1.5 to 2.5 h, the intensity of the XRD patterns constantly increased (Fig. 4e–g). At the same time, more zeolite crystals could be formed, as shown in Fig. 5d and e. Further increasing the crystallization time to 4 h, the intensity of the XRD pattern and the crystal morphology remained similar (Fig. 4h and 5f), meaning that a GME zeolite with high crystallinity could be already successfully obtained at 2.5 h. The corresponding dependence of the zeolite crystallinity on the crystallization time at 160 °C is displayed in Fig. S13.†
It is well-known that CO2 is a main greenhouse gas, leading to environmental issues such as global warming and ocean acidification.34–38 Thus, great efforts have been devoted to capturing CO2 using efficient technologies. The key to efficiently capturing CO2 is the employment of adsorbents. Current adsorbents, such as ethanol amine, CaO, zeolites and MOFs, have been widely researched.39–47 Among them, zeolites are potentially very important to capture CO2 due to their excellent features, such as large-scale commercialization and large adsorption capacity. Recently, zeolites such as KFI, CHA, FAU, and MOR have been reported for capturing CO2.48–52 For example, Zhou et al. for the first time showed that an Fe-containing MOR zeolite could be efficiently used for capturing CO2 from flue gas.52 However, GME zeolite has never been reported for capturing CO2 efficiently.
Fig. 6 shows the CO2 adsorption isotherms of Na–GME, K–GME, and T–GME. It can be clearly observed that the above three zeolite samples have the ability to capture CO2. Notably, the Na–GME zeolite had a high CO2 adsorption capacity (5.37 mmol g−1), while the K–GME zeolite displayed a low CO2 adsorption capacity (3.73 mmol g−1). The above phenomenon could be reasonably attributed to the fact Na–GME has a larger micropore volume and BET surface area than the K–GME zeolite, as shown in Table S1 and Fig. S15.† However, the T–GME displayed the lowest CO2 adsorption capacity (2.45 mmol g−1), which might be caused by the stacking-faults of the micropore channels (Fig. S16†).
To further explore the potential of the GME zeolite for the separation of a binary gas, a CO2/N2 (15/85, v/v) mixed gas breakthrough experiment was conducted on the above GME zeolite samples. As shown in Fig. 7, the retention time of CO2 for the Na–GME zeolite was 160 min, while N2 would penetrate rapidly. In comparison, the K–GME and T–GME zeolites displayed retention times of only 120 and 80 min, respectively (Fig. S17†), meaning that Na–GME had the best separation performance. In addition, based on the breakthrough curve in Fig. 7, the separation coefficient of Na–GME zeolite for CO2/N2 (15/85, v/v) could be calculated to be about 58.8, also showing the excellent ability of CO2/N2 separation compared with other zeolites reported in the literature (Table S2†).
4. Conclusions
In summary, the rapid synthesis of fault-free GME zeolite has been successfully shown for the first time. By combining both seeding and aging strategies, a GME zeolite with good crystallinity could be synthesized at 160 °C in 2.5 h. The obtained GME zeolite was characterized in detail by HRTEM and N2 sorption techniques, confirming the fault-free feature in the micropore channels. As a result, after the post-treatment of the as-made GME zeolite, a Na–GME zeolite with high porosity could be obtained, giving the abilities of high CO2 adsorption and excellently selective CO2 adsorption for CO2/N2 (15/85, v/v). The rapid synthesis of fault-free GME zeolite, as well as its good CO2 capture performance, might offer a novel door for its practical application in the foreseeable future.Author contributions
This manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by Jiaxing City Public Welfare Research Project of China (2023AY40007), National Natural Science Foundation of China (22302124), and the Qin Shen Scholar Program of Jiaxing University.References
- B. M. Weckhuysen and J. H. Yu, Chem. Soc. Rev., 2015, 44, 7022–7024 CAS.
- Y. Y. Sun, F. Wang and J. Zhang, Inorg. Chem., 2019, 58, 4076–4079 CAS.
- S. Y. Li, J. F. Li, M. Dong, S. B. Fan, T. S. Zhao, J. G. Wang and W. B. Fan, Chem. Soc. Rev., 2019, 48, 885–907 CAS.
- Z. D. Liu, J. Zhu, T. Wakihara and T. Okubo, Inorg. Chem. Front., 2019, 6, 14–31 CAS.
- Q. Zhang, J. H. Yu and A. Corma, Adv. Mater., 2020, 32, 2002927 Search PubMed.
- J. Shin, D. Jo and S. B. Hong, Acc. Chem. Res., 2019, 52, 1419–1427 CAS.
- L. Bui, H. Luo, W. R. Gunther and Y. Román-Leshkov, Angew. Chem. Int. Ed., 2013, 152, 8180–8183 Search PubMed.
- M. Dusselier, P. V. Wouwe, A. Dewaele, P. A. Jacobs and B. F. S. Sels, Science, 2015, 349, 78–80 CAS.
- U. Olsbye, S. Svelle, M. Bjørgen, P. Beato, T. V. W. Janssens, F. Joensen, S. Bordiga and K. P. Lillerud, Angew. Chem., Int. Ed., 2012, 51, 5810–5831 CAS.
- A. M. Beale, F. Gao, I. Lezcano-Gonzalez, C. H. F. Peden and J. Szanyi, Chem. Soc. Rev., 2015, 44, 7371–7405 RSC.
- International Zeolite Association Structure Commission, https://europe.iza-structure.org/IZA-SC/ftc_table.php.
- E. Pérez-Botella, S. Valencia and F. Rey, Chem. Rev., 2022, 122, 17647–17695 CrossRef PubMed.
- C. Martínez and A. Corma, Coord. Chem. Rev., 2011, 255, 1558–1580 CrossRef.
- J. Q. Chen, A. Bozzano, B. Glover, T. Fuglerud and S. Kvisle, Catal. Today, 2005, 106, 103–107 CrossRef CAS.
- F. Collins, A. Rozhkovskaya, J. G. Outram and G. J. Millar, Microporous Mesoporous Mater., 2020, 291, 109667 CAS.
- H. Xu, L. F. Zhu, Q. M. Wu, X. J. Meng and F. S. Xiao, Inorg. Chem. Front., 2022, 9, 1047–1057 CAS.
- S. I. Zones, Microporous Mesoporous Mater., 2011, 144, 1–8 CrossRef CAS.
- Y. Li and J. H. Yu, Nat. Rev. Mater., 2021, 6, 1156–1174 CAS.
- O. Chiyoda and M. E. Davis, Microporous Mesoporous Mater., 1999, 32, 257–264 CAS.
- E. E. Senderov and T. N. Shishakova, Russ. Chem. Bull., 1967, 16, 151–153 Search PubMed.
- P. N. Joshi, A. Thangaraj and V. P. Shiralkar, Zeolites, 1991, 11, 164–168 CAS.
- R. H. Daniels, G. T. Kerr and L. D. Rollmann, J. Am. Chem. Soc., 1978, 100, 3097–3100 CAS.
- J. Mielby, K. H. Møller, D. Iltsiou, F. Goodarzi, K. E. Rasmussen and S. Kegnæs, Microporous Mesoporous Mater., 2022, 330, 111606 CrossRef CAS.
- M. Dusselier, J. H. Kang, D. Xie and M. E. Davis, Angew. Chem., Int. Ed., 2017, 56, 13475–13478 CAS.
- E. Mitani, N. Tsunoji, M. Sadakane and T. Sano, J. Porous Mater., 2019, 26, 1345–1352 CAS.
- H. Holler and U. Wirsching, Fortschr. Mineral., 1985, 63, 21–43 Search PubMed.
- D. Xie and H. S. Lacheen, US Pat9364782, 2016 Search PubMed.
- G. T. Kerr and L. D. Rollman, US Pat4061717, 1977 Search PubMed.
- M. E. Davis and C. Saldarriaga, J. Chem. Soc., Chem. Commun., 1988, 14, 920–921 Search PubMed.
- D. Xie, US Pat9643853, 2017 Search PubMed.
- O. Chiyoda and M. E. Davis, Microporous Mesoporous Mater., 2000, 38, 143–149 CrossRef CAS.
- Y. C. Yang, X. Y. Meng, L. F. Zhu, J. H. Yang, G. L. Zhang, H. X. Shen and X. B. Cao, Inorg. Chem., 2022, 61, 21115–21122 CrossRef CAS PubMed.
- G. M. Fedorova and T. N. Shishakova, Russ. Chem. Bull., 1967, 16, 1782–1784 CrossRef.
- B. G. Guo, Y. Wang, H. Y. Zhou and F. Hu, Environ. Sci. Pollut. Res., 2023, 30, 117037–117049 CrossRef CAS PubMed.
- B. G. Guo, Y. Feng and F. Hu, Environ. Sci. Pollut. Res., 2024, 31, 10119–10132 CrossRef PubMed.
- M. N. Anwar, A. Fayyaz, N. F. Sohail, M. F. Khokhar, M. Baqar, W. D. Khan, K. Rasool, M. Rehan and A. S. Nizami, J. Environ. Manage., 2018, 226, 131–144 CrossRef CAS PubMed.
- C. L. Hurd, A. Lenton, B. Tilbrook and P. W. Boyd, Nat. Clim. Change, 2018, 8, 686–694 CrossRef CAS.
- C. T. A. Chen, H. K. Lui, C. H. Hsieh, T. Yanagi, N. Kosugi, M. Ishii and G. C. Gong, Nat. Clim. Change, 2017, 7, 890–894 CAS.
- F. Barzagli, F. Mani and M. Peruzzini, Environ. Sci. Technol., 2016, 50, 7239–7246 CAS.
- S. X. Chen, X. M. Han, X. Y. Sun, X. Luo and Z. W. Liang, Chem. Eng. J., 2020, 386, 121295 CAS.
- H. J. Yoon, C. H. Lee and K. B. Lee, Chem. Eng. J., 2021, 421, 129584 CAS.
- C. H. Lee, S. W. Choi, H. J. Yoon, H. J. Kwon, H. C. Lee, S. G. Jeon and K. B. Lee, Chem. Eng. J., 2018, 352, 103–109 CAS.
- D. G. Boer, J. Langerak and P. P. Pescarmona, ACS Appl. Energy Mater., 2023, 6, 2634–2656 CAS.
- S. J. Chen, M. Zhu, Y. Fu, Y. X. Huang, Z. C. Tao and W. L. Li, Appl. Energy, 2017, 191, 87–98 CrossRef CAS.
- H. M. He, F. X. Sun, S. Q. Ma and G. S. Zhu, Inorg. Chem., 2016, 55, 9071–9076 CAS.
- W. L. Li, Q. Shuai and J. M. Yu, Small, 2024, 7, 2402783 Search PubMed.
- M. Åhlén, O. Cheung and C. Xu, Dalton Trans., 2023, 52, 1841–1856 Search PubMed.
- T. Remy, S. A. Peter, L. V. Tendeloo, S. V. D. Perre, Y. Lorgouilloux, C. E. A. Kirschhock, G. V. Baron and J. F. M. Denayer, Langmuir, 2013, 29, 4998–5012 CAS.
- M. Debost, P. B. Klar, N. Barrier, E. B. Clatworthy, J. Grand, F. Laine, P. Brazda, L. Palatinus, N. Nesterenko, P. Boullay and S. Mintova, Angew. Chem., Int. Ed., 2020, 59, 23491–23495 CrossRef CAS PubMed.
- J. Madhu, V. M. Ramakrishnan, A. Santhanam, M. Natarajan, B. Palanisamy, D. Velauthapillai, N. T. L. Chi and A. Pugazhendhi, Environ. Res., 2022, 214, 113949 CrossRef CAS PubMed.
- D. Fu, Y. Park and M. E. Davis, Proc. Natl. Acad. Sci. U. S. A., 2022, 119, e2211544119 CrossRef CAS PubMed.
- Y. Zhou, J. L. Zhang, L. Wang, X. L. Cui, X. L. Liu, S. S. Wong, H. An, N. Yan, J. Y. Xie, C. Yu, P. X. Zhang, Y. H. Du, S. B. Xi, L. R. Zheng, X. Z. Cao, Y. J. Wu, Y. X. Wang, C. Q. Wang, H. M. Wen, L. Chen, H. B. Xing and J. Wang, Science, 2021, 373, 315–320 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5dt00508f |
| This journal is © The Royal Society of Chemistry 2025 |