Open Access Article
Open Access ArticleRapid and sensitive determination of etomidate based on colorimetric assays with a chromium(III) complex†
Leo K. B.
Tam‡
 *a,
Weijun
Ma‡
b,
Boyang
Li‡
*a,
Weijun
Ma‡
b,
Boyang
Li‡
 b,
Waygen
Thor
b,
Waygen
Thor
 a,
Ho-Fai
Chau
a,
Ho-Fai
Chau
 *a and
Ka-Leung
Wong
*a and
Ka-Leung
Wong
 *a
*a
aDepartment of Applied Biology and Chemical Technology, The Hong Kong Polytechnic University, Hung Hom, Hong Kong, PR China. E-mail: leo-kb.tam@polyu.edu.hk; ho-fai.chau@polyu.edu.hk; klgwong@polyu.edu.hk
bDepartment of Chemistry, Hong Kong Baptist University, 224 Waterloo Road, Kowloon Tong, Kowloon, Hong Kong, PR China
First published on 17th June 2025
Abstract
Vaping etomidate is currently a red-hot social issue where the drug abuse problem has led to health concerns and even reported deaths for adolescents. Therefore, a fast, sensitive, and cost-effective detection tool for etomidate is urgently needed to support law enforcement. A chromium(III) complex-based sensor, EtoChrom, is presented in this work as an example of a detection method for etomidate based on a colorimetric assay. This low-priced but novel sensor exhibits an eye-catching colour change from purple to yellow and a bathochromic shift in the absorption band upon etomidate binding. Thus, the result can be seen directly by the naked eye. EtoChrom demonstrated a high precision of up to 2 mg mL−1 etomidate with a low detection limit of 0.030 mg mL−1, with the interference from the vape juice and saliva matrices negligible throughout the test. In real etomidate-containing vape juice analysis, EtoChrom can provide accurate results within 1 min, revealing its high potential to serve as an easy-to-handle and cost-effective etomidate detector for rapid on-site screening.
1. Introduction
Etomidate (ET) is an imidazole-based non-barbiturate sedative, which is ordinarily applied for anaesthesia induction and sedation in the form of injection.1 With its strong but ultra-short inhibitory effect on the central nervous system, ET might provide short-term pleasure similar to inebriation for humans.2 However, an overdose of ET can lead to adrenal suppression, myoclonus, respiratory depression, unconsciousness, and even death.3,4 Recently, ET has been found to be a low-cost and easily obtainable substitute for narcotics, leading to a tremendous growth rate in criminal cases of its illegal addition to beverages and vape juice of E-cigarettes in Hong Kong and China in the last two years.5 Consequently, in February 2025, ET and its analogues, including metomediate (MT), propoxate, and isopropoxate (iPrT), were classified as dangerous drugs in Hong Kong.6 Hence, there is an urgent need to develop fast, selective and cost-effective ET sensors to support law enforcement.The current reported analytical methods for ET in human or environmental samples are mainly based on high-performance liquid chromatography (HPLC) or gas chromatography (GC) equipped with a mass detector (MS).7–9 Despite their ability to achieve highly accurate results with a remarkably low limit of detection (LOD), the requirements of long testing time, expensive and large equipment, and professional operators restrict their use under non-laboratory conditions. To overcome these limitations, Peng et al.10 and Ding et al.11 reported two fluorescence sensors that can recognize ET with the LOD in the nanomolar (nM) range, respectively, in 2024. However, using a fluorescence sensing strategy to detect narcotics or drugs heavily relies on the use of spectrofluorometers,12–14 and either the portable instruments are expensive or the signal-to-background ratio is limited, significantly reducing the practical feasibility of on-site ET determination.15 Alternatively, in 2025, the use of lateral-flow immunoassay by Qu et al. provided an ultra-low (ppb level) detection result for both ET and MT in human and environmental samples.16 Yet, the preparation of monoclonal antibodies of both analytes through the development of engineered haptens was costly and time-consuming.17 Apart from the chemical sensor, Zhang et al. in 2025 demonstrated the use of gold-ordered array substrate-engineered surface-enhanced Raman spectrometry to detect ET and its metabolites in vape juice at a concentration of 50 ppm.18 Nevertheless, fabrication of the gold array required tedious and apparatus-assisted synthesis. The benefits and drawbacks of recent and representative detection methods of ET are compared and summarised in Table S2.†
Notably, colorimetric analysis, particularly colour variation upon metal ion chelation,19 is the most frequently employed method for on-site inspection of psychoactive drugs and narcotics. For example, transition metals such as Co2+ ions (the Dille–Koppanyi reagent for barbiturates) and Cu2+ ions (the Zwikker reagent for barbiturates) are commonly adopted in preliminary visual examination in the analysis of barbiturates owing to the ease of preparation and rapid reaction kinetics.20,21 Among other transition metal ions, Cr3+ in the form of a [Cr(H2O)6]3+ complex has drawn our attention because of the immediate and clear visual colour change from purple to greenish yellow upon the displacement of one aqua ligand by an electron-rich ligand;22,23 the lone-pair electrons on the imidazole ring and/or the carbonyl of ET are expected to promote this rapid ligand exchange, resulting in ET-specific recognition. Besides, the common source of [Cr(H2O)6]3+, chromium(III) nitrate nonahydrate [Cr(NO3)3·9H2O],24 is inexpensive.25 However, to the best of our knowledge, the potential of applying this Cr3+-based colorimetric analysis in ET detection remains unexplored, although the result can be determined instantly and directly by the naked eye.
In this work, we report an example of using the [Cr(H2O)6]3+ complex to detect ET based on a colorimetric assay. As a proof of concept, EtoChrom, a reagent containing 10 mM [Cr(H2O)6]3+ in 10% (w/v) NaCl solution, is prepared as a low-cost, rapid, sensitive and selective ET sensor which can provide both qualitative and quantitative results. The sensitivity, LOD, selectivity and stability of EtoChrom will be discussed along with its feasibility for on-site detection of ET.
2. Results and discussion
2.1. Molecular design and mechanistic action
Imidazole is an electron-rich field ligand relatively stronger than H2O in the spectrochemical series (i.e. H2O < NCS− < CH3CN < imidazole < py < NH3).26,27 Upon interaction of the [Cr(H2O)6]3+ complex in octahedral geometry (Oh) with ET in the E-cigarette sample, a substitution reaction would occur to displace one water molecule to give [Cr(H2O)5ET]3+ with the preferred dissociative mechanism.28 Owing to the presence of a stronger field ET ligand, an immediate visual colour change from purple (i.e. absorption in the yellow region ca. 580 nm) to greenish yellow (i.e. absorption in the violet region ca. 400 nm) is expected due to the increase in crystal field splitting energy (Δo), resulting in a higher absorbance in the high-energy violet region and a bathochromic shift of maximum absorption wavelength according to the crystal field theory (Fig. 1a).22,23To validate the key principle in the design of our ET sensor, commercially purchased [Cr(H2O)6]3+ was first dissolved in deionized (DI) water to give a solution of 10 mM concentration. As a control, a 10 mM green chromium(III) acetate (CrAc) aqueous solution was also prepared. As shown in Fig. 1b, the absorption spectra of both chromium aqua and acetate complexes exhibited three major absorption bands attributed to the three spin-allowed d–d transitions for the Oh complex in a d3 system, 4A2g → 4T1g(P) (ca. 300 nm), 4A2g → 4T1g(F) (ca. 420 nm), and 4A2g → 4T2g(F) (ca. 580 nm), respectively, which were essentially the same as those reported.23,29 The letters F and P are symbols describing the total orbital angular momentum (L = 1 or 3) of the excited state terms for clarity, respectively, in which their orbital angular momentum may be different from the free ion ground state (4F) of the d3 system.22 Upon the addition of 1 mg mL−1 of ET with 1 min shaking, while the absorption spectrum of the CrAc showed negligible change, bathochromic shifts of maximum absorption wavelength (λabs) for both the 4A2g → 4T1g(F) transition from 412 to 416 nm and the 4A2g → 4T2g(F) transition from 574 to 577 nm were observed in the case of the chromium aqua complex [Fig. 1b and S1a†], leading to a gradient colour change from purple to greenish yellow (Fig. 1c). In addition, the absorbance for 4A2g → 4T1g(F) transition increased from 0.16 to 0.19 (Fig S1b†). These results supported the ET detecting properties of the [Cr(H2O)6]3+ complex as a colorimetric sensor.
2.2. Optimisation of detection conditions
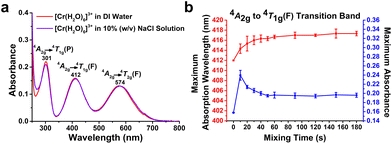
The effects of pH, medium, concentration, and reaction time were further investigated for the optimal ET detection conditions with the [Cr(H2O)6]3+ complex. To study the pH effect, the absorption spectra of 10 mM [Cr(H2O)6]3+ aqueous solution were recorded with the pH adjusted from 1 to 11. As shown in Fig. S2,† under acidic conditions (pH 1.0 and 3.0), while the λabs for three transition bands remained unchanged, their absorbance was slightly reduced compared with that of aqueous [Cr(H2O)6]3+ solution dissolved in DI water. At pH 7.0, a small amount of pale green suspension was noticed, likely chromium(III) hydroxide [Cr(OH)3]. Meanwhile, CrOH2+ and/or [Cr(OH)2]+ complexes were likely formed, which might alter the absorption spectrum of the mixture.30 At pH 9.0, a significant amount of pale green solid precipitated from the solution. It is well known that the presence of precipitates in the cuvette causes radiation scattering, which noticeably increases absorbance.31 Interestingly, at pH 11.0, the pale green precipitate redissolved in this alkaline environment, which changed the absorption profile as [Cr(OH4)]− was expected to be the predominant complex in the solution.30 In particular, at pH 5.0, the absorption profile almost overlapped with that of the[Cr(H2O)6]3+ aqueous solution, suggesting that this pH value provided minimum interference. The buffer solution can resist pH change and thereby the spectral properties of the [Cr(H2O)6]3+ complex were analysed in phosphate-buffered saline (PBS) and sodium acetate (NaAc) at pH 5.0 at different concentrations (Fig. S3a†). At 100 mM, absorption bands at 411 nm showed a bathochromic shift to 420 and 425 nm in PBS and NaAc, respectively (Fig. S3b†). When the concentration of the buffer was reduced to 10 or 1 mM, the change in λabs became marginal but the absorbance was still remarkably higher than that in DI water (Fig. S3c†), implying that the use of buffer solution was not suitable. Instead, we dissolved the [Cr(H2O)6]3+ complex in 10% (w/v) NaCl solution to minimize biodegradation for prolonged storage, since the high salt concentration can effectively inhibit the growth of bacteria and viruses.32 The absorption spectrum of [Cr(H2O)6]3+ in this medium was then measured and found to be very similar to that recorded in DI water (Fig. 2a).To further identify the optimal concentration of [Cr(H2O)6]3+ in this formulation for ET detection, 1 mg mL−1 ET was added to the solution with three different concentrations of [Cr(H2O)6]3+, and the results are depicted in Fig. S4.† It was found that the purple colour at 100 mM was too intense for a positive result reading, while it was difficult to distinguish the colour between the transparent 1 mM testing solution and the ET-chelated mixture by the naked eye. Thus, 10 mM [Cr(H2O)6]3+ in 10% (w/v) NaCl solution at pH 5.0 (EtoChrom) was chosen in the subsequent experiments. The optimal result reading time for EtoChrom was also studied by the change in the absorption profile upon the addition of 1 mg mL−1 ET with different shaking times. As shown in Fig. S5,† the λabs of 4A2g → 4T1g(F) transition increased gradually to 417 nm and reached a plateau at around 1 min. Meanwhile, the absorbance of this band sharply increased to 0.24 in the first 10 s and eventually reduced and stabilized at ca. 0.19 after 1 min. The summarized results in Fig. 2b suggest that EtoChrom can serve as a rapid ET sensor, which is attributed to the rapid equilibrium achieved in Cr-ET complexation within 1 min.
2.3. Stability of EtoChrom
The stability of EtoChrom was then examined by monitoring the change in the absorption spectrum of EtoChrom (10 mM) at room temperature over a period of 90 days (Fig. 3a). It can be seen that the changes in both λabs and absorbance for 4A2g → 4T1g(F) and 4A2g → 4T2g(F) bands were inconsequential throughout the whole period (Fig. S6a and b†). To further understand the ET detection properties of EtoChrom stored for a long time, a detection assay was performed with EtoChrom incubated with ET (1 mg mL−1) for 28, 56, and 90 days. As shown in Fig. S7,† the absorption spectra at these time points were comparable to those recorded at day 0. All of these results confirmed the high stability and retained ET recognition ability of EtoChrom over a period of storage.2.4. Sensitivity of EtoChrom
To investigate the correlation between the λabs and absorbance of EtoChrom and the concentration of ET, the absorption spectra of EtoChrom upon the addition of ET from 0 to 40 mg mL−1 with 1 min of shaking were recorded (Fig. 3b). As expected, a bathochromic shift in λabs and enhanced absorbance can be observed for both the 4A2g → 4T1g(F) and 4A2g → 4T2g(F) transition bands, along with a notable visual colour change from purple to greenish yellow when the concentration of ET increased (Fig. 3c). As shown in Fig. 3d and S8a–c,† the λabs of the 4A2g → 4T1g(F) band exhibited a good linear relationship with the concentration of ET among three other parameters (i.e. λabs of 4A2g → 4T2g(F) and the absorbance of two transition bands) within 2 mg mL−1, indicating the colorimetric nature of EtoChrom in ET sensing. However, when the ET concentration was >2 mg mL−1, the λabs at 424 nm reached a plateau and it is believed that the concentration was saturated and precipitation of ET was observed, losing the linear relationship with the λabs or absorbance. The LOD was also determined to be 0.030 mg mL−1, while the colour change can be visualized by the human eye at ca. 1 mg mL−1.2.5. Selectivity of EtoChrom
It is important to understand if EtoChrom is specific toward etomidate and unaffected by other common analytes in the matrices during the analysis. Since our target is to develop a specific on-site ET detection means, various potential interfering species, including glycerol (EG), propylene glycol (PG), flavouring chemicals and nicotine in the vape juice,33 and electrolytes (i.e. Na+, K+, Ca2+, Mg2+, Cl−, HCO3−, PO42−), enzymes and proteins in the saliva,34 were treated with EtoChrom and the changes in the absorption spectra were monitored. To simplify the assay, 20 mmol L−1 NaHCO3, 30 mmol mL−1 KCl, 2.5 mmol L−1 CaCl2, 0.25 mmol L−1 MgCl2, and 30 mmol Na2PO4 were tested in this study while 2 mg mL−1 of human serum albumin (HSA) was used to mimic the total amount of enzymes and proteins, making both ion and protein concentrations similar to those in the saliva of a normal adult.34 Because of the numerous flavours of E-cigarettes available commercially, three common and representative flavouring chemicals found in vape juice, namely diacetyl, 2,3-pentanedione, and acetoin (all 500 μg mL−1), were examined in this assay.35 In addition, the concentration of nicotine to be examined was 18 mg mL−1, which is similar to the typical content of nicotine in commercially available vape juice (i.e. 1.8%). As shown in Fig. 3e, the change in λabs of the 4A2g → 4T1g(F) band was negligible after treatment with most species, except for nicotine, which showed a slightly hypsochromic shift for 2 nm. It is noteworthy that the absorbance at ca. 412 nm was higher in the cases of PO42− and nicotine, with the difference being marginal for the former but considerable for the latter (Fig. S9†).Therefore, the absorption spectrum of EtoChrom was recorded and subjected to comparison in the presence of nicotine (18 mg mL−1) and ET (1 mg mL−1) (Fig. S10†). Interestingly, the absorption features and change in λabs of the 4A2g → 4T1g(F) band of EtoChrom upon addition of nicotine and ET were similar to those for ET addition only. Furthermore, EtoChrom was incubated with three ratios of PG/EG [i.e. 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, 7
4, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 8
3, 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (v/v)] that mimic commercial vape juice to understand its compatibility in this fundamental matrix for future real sample analysis.36 Similar to the individual components, the absorption spectra of EtoChrom in all of these ratios remained almost unchanged, which suggests that our probe is inert to the major composition of vape juice in common formulations (Fig. S11†). All of the above results reveal that the bathochromic change in λabs and yellow colour formation by EtoChrom were highly specific to ET in the presence of the aforementioned interfering species.
2 (v/v)] that mimic commercial vape juice to understand its compatibility in this fundamental matrix for future real sample analysis.36 Similar to the individual components, the absorption spectra of EtoChrom in all of these ratios remained almost unchanged, which suggests that our probe is inert to the major composition of vape juice in common formulations (Fig. S11†). All of the above results reveal that the bathochromic change in λabs and yellow colour formation by EtoChrom were highly specific to ET in the presence of the aforementioned interfering species.
The performance of EtoChrom with MT and iPrT (all at 1 mg mL−1) was also examined because of the growing trend of the illegal addition of these ET analogues to vape juice to avoid the detection of ET.37Fig. 3f shows that the absorption spectra of EtoChrom were commensurate in the presence of ET or iPrT, while the change in λabs with MT was minimal, which might be attributed to the electron donating effect of the substituents.
Still, EtoChrom has the potential to recognize some other analogues of ET with a similar structure, implying its high capability to recognize illegally added ET derivatives in actual scenarios.
2.6. Calculation for etomidate–EtoChrom binding
In light of the concerns about the binding geometry between ET and EtoChrom, computational modelling and calculation were employed using density functional theory (DFT) with the PBE0/def2-SVP level of theory to stimulate the optimized structure of the ET–EtoChrom complex.38,39 There are three possible binding modes between the Cr3+ ion and the ET ligand, including (1) monodentate N-coordination with the imidazole ring; (2) monodentate C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O coordination with the ester moiety; and (3) bidentate N– and C
O coordination with the ester moiety; and (3) bidentate N– and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O coordination. As shown in Fig. 4a, the change in the single point energy (ΔSPE) with reference to [Cr(H2O)6]3+ for N-coordination (−32.90 kcal mol−1) was calculated to be much lower than that of C
O coordination. As shown in Fig. 4a, the change in the single point energy (ΔSPE) with reference to [Cr(H2O)6]3+ for N-coordination (−32.90 kcal mol−1) was calculated to be much lower than that of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O coordination (−17.94 kcal mol−1), suggesting that the ET ligand with N-coordination to the Cr3+ ion is preferred. Nonetheless, bidentate chelation was unlikely to be seen due to the steric hindrance and the large distance between the two binding sites (Fig. S12†). For the number of ligands coordinated onto EtoChrom, the calculated ΔSPE of the bi-coordinated complex (−33.64 kcal mol−1) was marginally lower than that of the mono-coordinated complex (−32.90 kcal mol−1) (Fig. 4b). However, with reference to our experimental results given in Fig. 3d, the maximum concentration of ET with a good linear relationship detected by 10 mM EtoChrom was 2 mg mL−1 (i.e. 8.2 mM), while ET precipitation was observed at >4 mg mL−1 (i.e. 16.4 mM), revealing that the bi-coordination was kinetically not favoured due to the ET solubility. Hence, we believe that a 1
O coordination (−17.94 kcal mol−1), suggesting that the ET ligand with N-coordination to the Cr3+ ion is preferred. Nonetheless, bidentate chelation was unlikely to be seen due to the steric hindrance and the large distance between the two binding sites (Fig. S12†). For the number of ligands coordinated onto EtoChrom, the calculated ΔSPE of the bi-coordinated complex (−33.64 kcal mol−1) was marginally lower than that of the mono-coordinated complex (−32.90 kcal mol−1) (Fig. 4b). However, with reference to our experimental results given in Fig. 3d, the maximum concentration of ET with a good linear relationship detected by 10 mM EtoChrom was 2 mg mL−1 (i.e. 8.2 mM), while ET precipitation was observed at >4 mg mL−1 (i.e. 16.4 mM), revealing that the bi-coordination was kinetically not favoured due to the ET solubility. Hence, we believe that a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding between ET and EtoChrom dominated in the reaction mixture although the energy of the bi-coordinated complex is lower than that of the mono-coordinated complex. All the above results suggest a strong, monodentate, and mono-coordinated binding between the Cr3+ ion and the ET ligand, which is consistent with our above experimental results.
1 binding between ET and EtoChrom dominated in the reaction mixture although the energy of the bi-coordinated complex is lower than that of the mono-coordinated complex. All the above results suggest a strong, monodentate, and mono-coordinated binding between the Cr3+ ion and the ET ligand, which is consistent with our above experimental results.
2.7. In situ detection of etomidate
Recently, an increasing number of criminal cases involving the illegal addition of etomidate to vape juice in E-cigarettes.5,6 Hence, we would like to verify the feasibility of using EtoChrom to detect ET in real samples, which can potentially assist police inspections in the future. The concentrations of ET in four real samples, including three suspected ET-containing samples (samples 1–3) and one commercially available nicotine-containing vape juice sample (sample 4), were first determined via LC/MS analysis (Fig. S13†). EtoChrom was then used to treat with these samples and their absorption spectra were monitored and compared (Fig. S14†). The corresponding ET concentrations in real samples determined by both methods are summarized in Table 1. From the LC/MS results, samples 1–3 contained ca. 147, 168, and 117 mg mL−1 ET, respectively, while sample 4 was ET-free. EtoChrom exhibited similar results and a pale-yellow colour could also be observed visually for the positive result (Fig. S15†). For quantitative analysis, using the calibration curve shown in Fig. 3d and considering the dilution factor, samples 1–3 were found to contain ca. 139, 162, and 116 mg mL−1 ET, respectively, with the results lower than those of the LC/MS analysis but within an acceptable error range of ca. 5%. Nevertheless, it is illegal to possess any amount of this dangerous drug,6 and thereby EtoChrom can still provide important information despite its upper and lower detection limits being relatively narrow when compared with other reported non-chromatographic methods (Table S2†).10,11,16 Accordingly, EtoChrom can still be considered as an acceptable tool for rapid on-site ET detection because of its advantages such as low cost, no requirement for instruments, matrix treatment-free operation, and easy and fast result reading.| Real sample | LC/MS | EtoChrom |
|---|---|---|
| Concentration of ET (mg mL−1) | ||
| 1 | 147.0 ± 0.4 | 138.7 ± 1.1 |
| 2 | 168.3 ± 0.7 | 161.7 ± 0.9 |
| 3 | 118.3 ± 0.2 | 115.7 ± 1.7 |
| 4 | 0 | 0 |
3. Conclusions
In summary, we have developed a novel colorimetric etomidate sensor, EtoChrom, based on the [Cr(H2O)6]3+ complex, utilizing the properties of a bathochromic shift in absorption wavelength and thus the colour change from purple to greenish yellow upon etomidate binding and aqua ligand displacement on the chromium(III) ion as the key principle supported by computational calculation of the monodentate and mono-coordinated geometry. EtoChrom can detect etomidate within 1 min with a low detection limit of 0.030 mg mL−1, can provide qualitative and quantitative results up to 2 mg mL−1, unaffected by the common interference species in the vape juice and saliva matrices, and is highly stable for up to 90 days. The highly accurate results with easy-to-read colour change by the naked eye in etomidate-containing real sample analysis renders EtoChrom a cost-effective, rapid, easy-to-handle, highly sensitive and etomidate-specific testing kit for practical on-site etomidate detection in vape juice samples.Author contributions
Leo K. B. Tam: writing – original draft, investigation, formal analysis, data curation, and conceptualization. Weijun Ma: data curation. Boyang Li: data curation. Waygen Thor: investigation, formal analysis, and data curation. Ho-Fai Chau: writing – review & editing. Ka-Leung Wong: writing – review & editing, supervision, project administration, funding acquisition, and conceptualization.Conflicts of interest
There are no conflicts to declare.Data availability
The data that support the findings of this study are available in the ESI† of this article.Acknowledgements
All experiments were performed in compliance with the relevant laws and institutional guidelines of Hong Kong Polytechnic University, according to which human serum albumin (HSA) was purchased and the samples were supplied by Sigma-Aldrich. Financial assistance from the HK PolyU Research Grant No. P0054517 and 4-68AS is gratefully acknowledged (K.-L. W). We thank the Hong Kong Hospital Authority, Pamela Youde Nethersole Eastern Hospital, Narcotics Bureau, Hong Kong Police Force, and the Hong Kong Government Laboratory for their assistance. Inspiring discussions with Dr Chen Xie and Dr Yan-Ho Fung are also highly appreciated.References
- G. Zheng, Y. Chen, G. Wu, T. Song, X. Zou, Q. Nie and P. Zhang, Int. J. Toxicol., 2024, 1–9 Search PubMed.
- L. C. McPhee, O. Badawi, G. L. Fraser, P. A. Lerwick, R. R. Riker, I. H. Zuckerman, C. Franey and D. B. Seder, Crit. Care Med., 2013, 41, 774–783 CrossRef CAS PubMed.
- Y. Feng, X. B. Chen, Y. L. Zhang, P. Chang and W. S. Zhang, Eur. Rev. Med. Pharmacol. Sci., 2023, 27, 1322–1335 CAS.
- H. Zhang, A. Wu, X. Nan, L. Yang, D. Zhang, Z. Zhang and H. Liu, Mol. Pharmaceutics, 2024, 21, 5989–6006 CrossRef CAS PubMed.
- Notice from the National Medical Products Administration and the National Health Commission of China on Strengthening the Management of Etomidate and Modafinil Pharmaceuticals. Available at: https://www.nmpa.gov.cn/xxgk/fgwj/gzwj/gzwjyp/20231007154014186.html.
- Press release from the Government of the Hong Kong Special Administrative Region on Orders to amend the Dangerous Drugs Ordinance and Control of Chemicals Ordinance to be gazetted on February 14 and etomidate to become a dangerous drug on the same date. Available at: https://www.info.gov.hk/gia/general/202502/12/P2025021100159.htm.
- Y.-K. Jung, S. Y. You, S.-Y. Kim, J. Y. Kim and K.-J. Paeng, Molecules, 2019, 24, 4459 CrossRef CAS PubMed.
- Y. J. Park, E. Cho, S. H. Kim, H. Lee, H. Jegal, M. Park, S. Choe, Y. E. Sim, S.-H. Baek, K. M. Kim and J. Pyo, J. Forensic Sci., 2022, 67, 2479–2486 CrossRef CAS PubMed.
- T.-F. He, H.-H. Zhu, X.-W. Lin, Y.-Y. Tian, L.-M. Sun, X. Guan, H.-Y. Zhang, L. Tan and S.-C. Wang, J. Pharmacol. Toxicol. Methods, 2024, 125, 107490 CrossRef CAS PubMed.
- Y. Zhao, Y. Guo, Z. Xu, T. Lv, L. Wang, M. Li, X. Chen, B. Liu and X. Peng, Chem. Commun., 2024, 60, 4691–4694 RSC.
- J. Li, J. Ling, Z. Cai, Y. Liao, P. Xing, W. Liu and Y. Ding, Forensic Sci. Int., 2024, 361, 112136 CrossRef CAS PubMed.
- E. Garrido, L. Pla, B. Lozano-Torres, S. El Sayed, R. Martínez-Máñez and F. Sancenón, ChemistryOpen, 2018, 7, 401–428 CrossRef CAS PubMed.
- H. Chu, L. Yang, L. Yu, J. Kim, J. Zhou, M. Li and J. S. Kim, Coord. Chem. Rev., 2021, 449, 214208 CrossRef CAS.
- V. Kumar, P. Kumar, A. Pournara, K. Vellingiri and K.-H. Kim, TrAC, Trends Anal. Chem., 2018, 106, 84–115 CrossRef CAS.
- Y.-H. Shin, M. T. Gutierrez-Wing and J.-W. Choi, J. Electrochem. Soc., 2021, 168, 017502 CrossRef CAS.
- Q. Liu, X. Xu, L. Liu, A. Qu, C. Xu and H. Kuang, Analyst, 2025, 150, 542–551 RSC.
- S. Dengl, C. Sustmann and U. Brinkmann, Immunol. Rev., 2016, 270, 165–177 CrossRef CAS PubMed.
- Y. Mo, X. Zhang, K. Zou, W. Xing, X. Hou, Y. Zeng, Y. Cai, R. Xu, H. Zhang and W. Cai, Nanomaterials, 2024, 14, 1958 CrossRef CAS PubMed.
- S. Rasheed, M. Ikram, D. Ahmad, M. N. Abbas and M. Shafique, Microchem. J., 2024, 206, 111438 CrossRef CAS.
- T. Koppanyi, J. M. Dille, W. S. Murphy and S. Krop, J. Am. Pharm. Assoc., 1934, 23, 1074–1079 CAS.
- M. J. de Faubert Maunder, Analyst, 1975, 100, 878–883 RSC.
- D. F. Shriver, P. W. Atkins and C. H. Langford, Inorganic Chemistry, W. H. Freeman and Company, New York, 1994 Search PubMed.
- M. Uchikoshi, D. Akiyama, K. Kimijima and K. Shinodac, RSC Adv., 2022, 12, 32722–32736 RSC.
- D. Lazar, B. Ribár, V. Divjakovic and Cs. Mészáros, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1991, 47, 1060–1062 CrossRef.
- Price of chromium(III) nitrate nonahydrate offered by Dieckmann (Hong Kong) Chemical Industry Co. Ltd. Available at: https://www.dkmchem.hk/product/213963.html?goodsno=MC01913-500g.
- R. J. Sundberg and R. B. Martin, Chem. Rev., 1974, 74, 471–517 CrossRef CAS.
- J. Reedjik, Recl. Trav. Chim. Pays-Bas, 2010, 88, 1451–1570 CrossRef.
- J. F. Perez-Benito and X. Julian-Millan, React. Kinet., Mech. Catal., 2019, 128, 1–22 CrossRef CAS.
- M. J. Bjerrum and J. Bjerrum, Acta Chem. Scand., 1990, 44, 358–363 CrossRef CAS.
- D. Rai, B. M. Sass and D. A. Moore, Inorg. Chem., 1987, 26, 345–349 CrossRef CAS.
- S. J. Leach and H. A. Scheraga, J. Am. Chem. Soc., 1960, 82, 4790–4792 CrossRef CAS.
- A. J. Muñoz, R. V. Alasino, A. G. Garro, V. Heredia, N. H. García, D. C. Cremonezzi and D. M. Beltramo, Pharmaceuticals, 2018, 11, 47 CrossRef PubMed.
- E. J. Z. Krüsemann, A. Havermans, J. L. A. Pennings, K. de Graaf, S. Boesveldt and R. Talhout, Tob. Control, 2021, 30, 185–191 CrossRef PubMed.
- S. P. Humphrey and R. T. Williamson, J. Prosthet. Dent., 2001, 85, 162–169 CrossRef CAS PubMed.
- J. G. Allen, S. S. Flanigan, M. LeBlanc, J. Vallarino, P. MacNaughton, J. H. Stewart and D. C. Christiani, Environ. Health Perspect., 2016, 124, 733–739 CrossRef CAS PubMed.
- P. Kubica, Molecules, 2023, 28, 4425 CrossRef CAS PubMed.
- M. Li, B. Lin and B. Zhu, Toxics, 2024, 12, 884 CrossRef CAS PubMed.
- F. Neese, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2012, 2, 73–78 CAS.
- F. Neese, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2018, 8, e1327 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental, UV-vis absorption spectra and LC/MS chromatograms. See DOI: https://doi.org/10.1039/d5dt00849b |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2025 |