Crystal to non-crystal transformation in a Sn-based MOF anode with long cycle life for lithium storage†
Ruoyu
Wang
a,
Yao
Chen
a,
Zaozao
Wu
a,
Qing
Li
a,
Hao
Wang
a,
Dongming
Li
a,
Zhipeng
Yu
a,
Jianhua
Ding
*a,
Xiaolan
Tong
a and
Jianbo
Xiong
 *ab
*ab
aJiangxi Province Key Laboratory of Functional Organic Polymers, East China University of Technology, Nanchang 330013, China
bState Key Laboratory of Nuclear Resources and Environment, School of Chemistry and Materials, East China University of Technology, Nanchang 330013, China
First published on 16th June 2025
Abstract
A tin-based MOF (Sn(HHTP)) was synthesized for lithium storage by incorporating tin (Sn) nodes with a lithium-active ligand (HHTP = 2,3,6,7,10,11-hexahydroxytriphenylene), which undergoes a transformation from a crystalline to an amorphous state during charge–discharge cycles. The construction of Sn-based MOFs with Sn–O coordination bonds allows them to effectively accommodate the volumetric changes during lithiation/delithiation processes. Concurrently, this transformation process facilitates the exposure of additional active sites. The Sn(HHTP) anode exhibits exceptional cycling stability with a reversible capacity of 239 mA h g−1 at 2 A g−1 after 1000 cycles, which represents a 228% increase in capacity compared to the eleventh-cycle reversible capacity.
Lithium-ion batteries serve as the energy source for electric vehicles and portable devices that rely on battery power.1,2 Commercial graphite anodes, characterized by a comparatively low theoretical capacity (372 mA h g−1), are insufficient to meet the increasing demands of users.3–7 Sn-based compounds, characterized by their comparatively high theoretical capacity and low operating voltage, represent a class of optimal materials for lithium storage.8–10 However, the volume of Sn-based materials varies greatly (∼260%) during lithium alloying and dealloying. The volume expansion can cause problems with mechanical fracture, particle expansion, and instability of the solid–electrolyte interface (SEI) layer, ultimately resulting in reduced cyclic stability.11–16
Metal–organic frameworks (MOFs) are crystalline coordination polymers formed by the connection of metal ions and organic ligands through coordination bonds.17 They usually have adjustable surface areas and abundant pore channels, which can provide active sites and clear lithium-ion transport pathways for lithium storage.18–21 The Sn–O coordination bonds could overcome the drastic volume change of Sn anodes, as the coordination interactions can uniformly anchor and release Sn atoms into the organic matrix, thus hindering particle growth and aggregation.22 The Sn-based MOF with Sn–O coordination bonds can overcome the severe volume changes of the Sn anode during the charge and discharge process.22 However, the pore structure of MOF crystals tends to collapse during lithium ion insertion and extraction, thus leading to poor cycling stability.18 Fortunately, structural collapse that leads to an amorphous state of the electrode materials can sometimes promote the lithium storage performance. For example, Gao et al.18 found that the nanoporous structure of the amorphous MQ cobalt-ZIF-62 glass (ZIF glass) anode exhibits unusual electrochemical properties, especially with a 200% increase in capacity after 1000 charge/discharge cycles at a high current density of 2 A g−1.
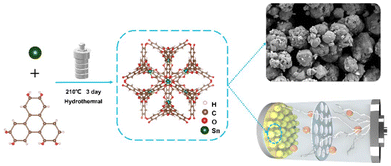
In the present work, we effectively synthesized a tin-based metal–organic framework (Sn(HHTP)), which serves as an anode material for lithium-ion batteries (LIB), utilizing a solvothermal method (Scheme 1). It is a stable 2-fold interpenetrated MOF with isolated distorted octahedral SnO62− active sites.23 In parallel, we synthesized an amorphous coordination polymer (Sn3(HHTP)2) anode at room temperature utilizing different feed ratios of Sn2+ and HHTP. The Sn(HHTP) structure quickly collapsed from crystalline to amorphous during galvanostatic charge–discharge. Remarkably, the Sn(HHTP) anode exhibits unusual electrochemical performance with a 238% capacity enhancement after 870 discharging/charging cycles at a current density of 2 A g−1.
The Sn(HHTP) MOF was synthesized according to a reported method;23 a solvothermal reaction of SnCl2·2H2O and HHTP in DMF and water at 210 °C for 72 h affords the earthy yellow microcrystalline powder of Sn(HHTP). As shown in Fig. 1a and b, each SnIV ion is surrounded in a distorted octahedral arrangement by six oxygen atoms, which come from three pairs of phenolic hydroxyl groups belonging to three deprotonated HHTP6− ligands.23 The SnIV metal center and the HHTP ligand are connected to form a regular porous structure in three-dimensional space. The SEM images demonstrate a bulk crystal morphology for Sn(HHTP) with a diameter of hundreds of nanometers (Fig. 1c and Fig. S1a†). The amorphous Sn3(HHTP)2 exhibits a micro-sized particulate powder morphology with a distinctively smaller particle size (Fig. 1d and Fig. S1b†). Elemental mapping analysis demonstrates that C, O and Sn elements are uniformly distributed throughout the composites (Fig. 1e and f). The semi-quantitative results of EDS (energy-dispersive spectroscopy) analysis (Tables S1 and 2†) reveal that the Sn content of Sn(HHTP) (3.26 at%) is lower than that of Sn3(HHTP)2 (9.10 at%), which is consistent with the result of lower initial specific capacity of Sn(HHTP) compared to Sn3(HHTP)2 due to the Sn element playing a key role in the specific capacity for lithium storage.
The PXRD patterns (Fig. 2a) clearly demonstrate the successful synthesis of the target products, with the measured PXRD patterns matching well with the simulated patterns of previously reported Sn(HHTP).23 The unit cell belongs to a P213 cubic space group with lattice parameters a = b = c = 18.271 Å.23 After a few cycles, the crystal of Sn(HHTP) turns into an amorphous state, and the PXRD pattern of the Sn(HHTP) electrode shows the characteristics of only copper foil (Fig. 2a). FT-IR spectra (Fig. 2b) further proved the difference between Sn(HHTP) and the organic ligand (HHTP); no significant signals of –OH stretching vibrations were observed, indicating that the hydroxyl groups of the HHTP linker were fully deprotonated in Sn(HHTP).23,24 The disappearance of the characteristic IR absorptions for –OH stretching vibrations is also observed in the Sn3(HHTP)2 coordination compound (Fig. S4†). Its surface chemical characteristics and elemental states of Sn(HHTP) were further validated through X-ray photoelectron spectroscopy (XPS) analysis (Fig. 2c–f). As illustrated in Fig. 2d, the C 1s spectrum exhibits C–C, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–O bonds at 284.5, 288.9, and 286.6 eV, respectively.25,26 The O 1s spectrum (Fig. 2e) also demonstrates the Sn–O, C–O and C
O and C–O bonds at 284.5, 288.9, and 286.6 eV, respectively.25,26 The O 1s spectrum (Fig. 2e) also demonstrates the Sn–O, C–O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds at 531.9, 532.2 and 533.4 eV, respectively.22,25,26 The Sn 3d spectrum (Fig. 2f) shows the scanning spectrum and fitting results in which two peaks at 487.1 and 495.5 eV correspond to the two chemical states of Sn 3d5/2 and Sn 3d3/2, respectively.25,26
O bonds at 531.9, 532.2 and 533.4 eV, respectively.22,25,26 The Sn 3d spectrum (Fig. 2f) shows the scanning spectrum and fitting results in which two peaks at 487.1 and 495.5 eV correspond to the two chemical states of Sn 3d5/2 and Sn 3d3/2, respectively.25,26
 | ||
| Fig. 2 (a) XRD pattern; (b) FT-IR spectra; (c) wide-scan XPS spectrum of Sn(HHTP); (d) C 1s XPS spectrum; (e) O 1s XPS spectrum; and (f) Sn 3d XPS spectrum. | ||
To understand the electrochemical processes for lithium storage, cyclic voltammetry (CV) was carried out at a sweep rate of 0.1 mV s−1 (Fig. 3a and b). According to the reversible reactions of Sn-based oxides with lithium,22,27–29 the electrochemical process of Sn-MOFs with lithium is expected to undergo a combination of electrochemical conversion and alloying mechanisms, with the following two steps: SnL + 6Li+ + 6e− ↔ Sn + Li6L (L = HHTP6−) and Sn + xLi+ + xe− ↔ LixSn (0 ≤ x ≤ 4.4).22 The first cathodic scan shows two irreversible cathodic peaks in the range of 0.4–0.6 V and 1.39 V, which differ from the subsequent scans of the Sn(HHTP) electrode (Fig. 3a). The activation of Sn(HHTP) results in the formation of an amorphous coordination polymer during the first lithiation process. The peak at 0.4–0.6 V can be assigned to lithium insertion into the ligand, which transforms into a weak broad bulge around 0.75 V, revealing that the lithiation of the HHTP ligand was reduced due to the crystalline to amorphous transformation. The irreversible peak at 1.39 V might be related to the formation of the solid electrolyte interphase (SEI) film.22,30 The sharp peak at 0.01–0.2 V is ascribed to the alloying reaction of lithium with Sn.22,25 Differently, there is one cathodic peak found at 0.62 V during the first cycle for the Sn3(HHTP)2 anode; it is assigned to Li+ intercalation. The 0.62 V cathodic peak then disappeared during the subsequent CV sweep cycles, which may be because the insertion and extraction of the ligand-embedded lithium ions are hindered after the formation of the SEI film. The broad peak at 0.1 to 0.2 V is ascribed to the alloying reaction between lithium and Sn (Fig. 3b).22,25 Both CV curves of the Sn(HHTP) and Sn3(HHTP)2 electrodes overlapped well during the last two scan cycles, confirming the presence of reversible and stable electrochemical reactions.22 Galvanostatic charge–discharge profiles (Fig. 3c and d) reveal that the Sn(HHTP) and Sn3(HHTP)2 anodes possess initial capacities of 731.68 mA h g−1 and 1582.13 mA h g−1 at 0.2 A g−1, respectively. It is worth noting that the discharge plateau of the Sn3(HHTP)2 electrode is lower than that of the Sn(HHTP) electrode. The capacity loss in the first cycle can be attributed to the formation of the SEI film and the irreversible reactions, which are consistent with the CV profiles. The specific capacities of the Sn(HHTP) electrode are lower than those of the Sn3(HHTP)2 electrode before the 100th cycle, probably because the Sn(HHTP) MOF experienced the crystalline-to-amorphous transformation. Subsequently, the specific capacities of the Sn(HHTP) electrode increased gradually, reaching 517 mA h g−1 in the 157th cycle and remaining at 439 mA h g−1 in the 400th cycle (Fig. 3e). The capacity increase and favourable cycling stability can be assigned to the crystalline-to-amorphous transformation of Sn(HHTP), which exposed more active sites and provided elastic strain capacity to adapt to the volume change of Sn alloying with lithium.14Fig. 3f shows the rate performances after cycling at various current densities from 0.2 to 6 A g−1. The rate capacity of Sn(HHTP) is lower compared with Sn3(HHTP)2 due to the lack of adequate lithium storage sites on organic ligands, as well as the lower content of Sn.
The long-term cyclability of the Sn(HHTP) anode further confirmed the excellent stability during repeated lithiation/delithiation processes (Fig. 3g). The long-term cyclability was evaluated by activating the anode at 0.2 A g−1 for the first ten cycles and then running charge–discharge cycles at 2 A g−1 for 2000 cycles. The crystal to noncrystal anode (SnHHTP) maintains a reversible capacity of 239 mAh g−1 at 2 A g−1 after 1000 cycles, representing a 228% capacity increase compared to the eleventh-cycle reversible capacity it even retains a capacity of 167 mA h g−1 after 2000 cycles, with nearly 100% coulombic efficiency, which contrasts with the lithium storage performance of most crystalline MOFs.31 In contrast, the capacity of the amorphous coordination polymer anode (Sn3(HHTP)2) decays faster, demonstrating a lower capacity of 85 mA h g−1 at 2 A g−1 after 2000 cycles.
To further study the difference in the rate performances of Sn(HHTP) and Sn3(HHTP)2 and unprecedented long-term cycling reversible capacity, CV tests at varied sweep rates from 0.1 to 1.5 mV s−1 are carried out. As shown in Fig. 4a, all curves showed similar stable electrochemical behavior during Li+ insertion/extraction. The peak current values increase with the scan rate, which is related to the capacitance control and diffusion control behavior.32 The relationship between peak current values (i) and different scan rates (v) can be described as follows:33,34 log(i) = b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log(v) + log(a) or i = aυb. In general, b = 0.5 indicates a diffusion control process (battery-like behavior) and b value = 1.0 reveals a surface control process (capacitor-like behavior).32
log(v) + log(a) or i = aυb. In general, b = 0.5 indicates a diffusion control process (battery-like behavior) and b value = 1.0 reveals a surface control process (capacitor-like behavior).32
As shown in Fig. 4a and b, the fitted b values of the anodic peak (peak 1) and cathodic peak (peak 2) are 0.6014 and 0.6939, respectively. The result indicates that the lithium storage performance of Sn(HHTP) is synergistically contributed by capacitive and diffusion controlled processes.33 The capacitive and diffusion controlled processes can be quantified using the relationship i(v) = k1v + k2v1/2, where k2v1/2 represents the contribution of the diffusion-controlled reaction, while k1v indicates the contribution of the surface-controlled reaction.33 As shown in Fig. 4c and d, the pseudocapacitance contribution percentages increased with the scan rates varying from 0.1 to 1.5 mV s−1. The pseudocapacitance contribution percentages of Sn(HHTP) are higher than those of Sn3(HHTP)2 at different scan rates from 0.1 to 1.5 mV s−1.
Furthermore, electrochemical impedance spectroscopy (EIS) was carried out to track the lithium-ion diffusion kinetics in various charge–discharge cycles for both the Sn(HHTP) and Sn3(HHTP)2 electrodes (Fig. 4e, f and Fig. S5, S6†). It is noted that the semicircle in the high frequency range is assigned to the charge transfer resistance (Rct) on the surface and bulk of the electrode, and the sloping line in the low-frequency region is related to the Warburg diffusion resistance (σ) for Li+ in the Nyquist plot, which is based on the equation32,34z′ = Re + Rct + σω−1/2. As shown in Fig. 4e, the charge transfer resistance (Rct) of the Sn(HHTP) electrode decreased from 284.2 Ω to 60.44 Ω as the charge–discharge cycles increased from the initial to the 100th cycle. The Rct of the Sn3(HHTP)2 electrode also decreased from 131.5 Ω to 93.59 Ω but was higher than that of the Sn(HHTP) electrode, which indicates that the crystalline-to-amorphous transformation of the Sn(HHTP) electrode during charge–discharge cycles can contribute to reduction in the charge transfer resistance. In addition, the Warburg coefficient σ values of the Sn(HHTP) anode fitted from the low-frequency inclined line also decreased from 187 to 116 Ω s1/2 with the increase in the number of charge–discharge cycles. The corresponding diffusion coefficient of Li+ (DLi+) can be calculated from the inclined lines in the Warburg region using the equation32DLi+ = R2T2/2A2N4F4c2σ2; thus, the calculated corresponding DLi+ of the Sn(HHTP) electrode progressively increased from 7.92 × 10−13 to 1.44 × 10−12 cm2 s−1. However, there is no similar increasing trend in the Warburg coefficient σ and DLi+ values found in the Sn3(HHTP)2 electrodes with the increasing charge–discharge cycles. Lithium-ion storage reaction kinetics analysis reveals that in the process of its conversion from crystalline to amorphous, the Sn(HHTP) anode can not only maintain the impedance characteristics of the MOF anode during the charge–discharge cycle, but also has the low electrochemical impedance of the amorphous materials.
In summary, we prepared a Sn-based MOF (Sn(HHTP)) for use as the anode of Li-ion batteries through a facile hydrothermal method. During the charge–discharge process, Sn(HHTP) undergoes a crystal to non-crystal transformation, which exhibits faster reaction kinetics with a smaller impedance compared with the amorphous electrode (Sn3(HHTP)2). With the breakdown of the framework, the active sites of Sn are released, while the organic ligands suppress Sn aggregation and reversibility of the delithiation and lithium intercalation processes, which jointly endow Sn(HHTP) with stable charge–discharge cycle performance. This work provides a favourable example in the development of promising Sn-based lithium storage materials with a stable and long cycle life.
Author contributions
Ruoyu Wang: Data curation, formal analysis, investigation, and formal analysis. Yao Chen: Data curation and investigation. Zaozao Wu: Data curation and investigation. Qing Li: Data curation and investigation. Hao Wang: Data curation and investigation. Dongming Li: Data curation. Zhipeng Yu: Data curation. Xiaolan Tong: data curation and investigation. Jianhua Ding: Data curation and investigation. Jianbo Xiong: Conceptualization, project administration, funding acquisition, and supervision.Conflicts of interest
There are no conflicts to declare.Data availability
All the data supporting this article have been included in the main text and the ESI.†Acknowledgements
This work was supported by the National Natural Science Foundation of China (22101046 and 22261001) and the Jiangxi Provincial Natural Science Foundation (20232BAB213002).References
- Y. Idota, T. Kubota, A. Matsufuji, Y. Maekawa and T. Miyasaka, Science, 1997, 276, 1395–1397 CrossRef CAS.
- M. Zhang, T. Wang and G. Cao, Int. Mater. Rev., 2015, 60, 330–352 CrossRef CAS.
- P. Luo, C. Zheng, J. He, X. Tu, W. Sun, H. Pan, Y. Zhou, X. Rui, B. Zhang and K. Huang, Adv. Funct. Mater., 2021, 32, 2107277 CrossRef.
- J. Xiong, Q. Li, X. Tan, X. Guo, K. Li, Q. Luo, Y. Chen, X. Tong, B. Na and M. Zhong, Dalton Trans., 2024, 53, 11232–11236 RSC.
- L. Zhao, B. Ding, X. Y. Qin, Z. Wang, W. Lv, Y. B. He, Q. H. Yang and F. Kang, Adv. Mater., 2022, 34, 2106704 CrossRef CAS PubMed.
- W. Li, X. Sun and Y. Yu, Small Methods, 2017, 1, 1600037 CrossRef.
- J. Lin, C. Xu, M. Lu, X. Lin, Z. Ali, C. Zeng, X. Xu and Y. Luo, Energy Environ. Mater., 2022, 6, e12284 CrossRef.
- H. Liu, S. Wang, J. Zhao, B. Zhang, L. Liu, R. Bao and Z. Jing, J. Energy Storage, 2024, 80, 109862 CrossRef.
- G.-L. Liu, Z.-H. Zhao, J.-K. Shen, Z.-B. Zhao, N.-T. Wu, D.-L. Guo, W.-W. Yuan, Y. Liu, Y.-H. Su and X.-M. Liu, Rare Met., 2024, 43, 3032–3043 CrossRef CAS.
- K. Liu, D. Guo, D. Zhao, P. Zhao, R. Ma, F. Geng, Q. Zhang, S. Zhang, Y. Song and J. Sun, J. Energy Storage, 2024, 100, 113600 CrossRef.
- J. Liu, J. Jiang, Q. Zhou, Z. Chen, R. Zhang, X. Xu, X. Han, S. Yang, Z. Zhou, P. Cheng and W. Shi, eScience, 2023, 3, 100094 CrossRef.
- H. Pourfarzad, M. Shabani-Nooshabadi and M. R. Ganjali, Composites, Part B, 2020, 193, 108008 CrossRef CAS.
- G. Blugan, N. Kovalska, D. Knozowski, P. V. W. Sasikumar, W. J. Malfait, S. Paz, P. Madajski, M. Leśniewski, M. Sawczak, B. Santhosh, M. Wilamowska-Zawłocka and M. M. Koebel, J. Energy Storage, 2024, 89, 111676 CrossRef.
- A. Jahel, C. M. Ghimbeu, L. Monconduit and C. Vix-Guterl, Adv. Energy Mater., 2014, 4, 1400025 CrossRef.
- B. Li, S. Yang, S. Wang, Q. Yang, Y. Sun, X. Li, Y. Xu, W. Peng and F. Yu, Appl. Surf. Sci., 2025, 701, 163281 CrossRef CAS.
- H. Wang, H. Huang, L. Chen, C. Wang, B. Yan, Y. Yu, Y. Yang and G. Yang, ACS Sustainable Chem. Eng., 2014, 2, 2310–2317 CrossRef CAS.
- K. Liu, C. Li, L. Yan, M. Fan, Y. Wu, X. Meng and T. Ma, J. Mater. Chem. A, 2021, 9, 27234–27251 RSC.
- C. Gao, Z. Jiang, S. Qi, P. Wang, L. R. Jensen, M. Johansen, C. K. Christensen, Y. Zhang, D. B. Ravnsbæk and Y. Yue, Adv. Mater., 2022, 34, 2110048 CrossRef CAS PubMed.
- Q. Jiang, P. Xiong, J. Liu, Z. Xie, Q. Wang, X. Q. Yang, E. Hu, Y. Cao, J. Sun, Y. Xu and L. Chen, Angew. Chem., Int. Ed., 2020, 59, 5273–5277 CrossRef CAS PubMed.
- A. Han, W. Sun, X. Wan, D. Cai, X. Wang, F. Li, J. Shui and D. Wang, Angew. Chem., Int. Ed., 2023, 62, e202303185 CrossRef CAS PubMed.
- Q. Huang, A. Zeb, Z. Xu, S. Sahar, J.-E. Zhou, X. Lin, Z. Wu, R. C. K. Reddy, X. Xiao and L. Hu, Coord. Chem. Rev., 2023, 494, 215335 CrossRef CAS.
- J. Liu, D. Xie, X. Xu, L. Jiang, R. Si, W. Shi and P. Cheng, Nat. Commun., 2021, 12, 3131 CrossRef CAS PubMed.
- Z. H. Zhao, J. R. Huang, P. Q. Liao and X. M. Chen, Angew. Chem., Int. Ed., 2023, 62, e202301767 CrossRef CAS PubMed.
- N. T. T. Nguyen, H. Furukawa, F. Gándara, C. A. Trickett, H. M. Jeong, K. E. Cordova and O. M. Yaghi, J. Am. Chem. Soc., 2015, 137, 15394–15397 CrossRef CAS PubMed.
- X. Jiang, M. Shao, K. Li, L. Ding and M. Zeng, J. Electroanal. Chem., 2022, 912, 116268 CrossRef CAS.
- X. Zhou, Y. Yu, J. Yang, H. Wang, M. Jia and J. Tang, ChemElectroChem, 2019, 6, 2056–2063 CrossRef CAS.
- S. Gao, N. Wang, S. Li, D. Li, Z. Cui, G. Yue, J. Liu, X. Zhao, L. Jiang and Y. Zhao, Angew. Chem., Int. Ed., 2020, 59, 2465–2472 CrossRef CAS PubMed.
- F. Zhang, Y. Wang, W. Guo, S. Rao and P. Mao, Chem. Eng. J., 2019, 360, 1509–1516 CrossRef CAS.
- Q. Li, M. Yuan, Y. Ji, X. Chen, Y. Wang, X. Gao, H. Li, H. He, H. Chen, Q. Tan, G. Xu, Z. Zhong and F. Su, Chem. Eng. J., 2022, 437, 135340 CrossRef CAS.
- X. Yan, C.-Y. Fan, X. Yang, Y.-Y. Wang, B.-H. Hou, W.-L. Pang and X.-L. Wu, Mater. Today Energy, 2019, 13, 302–307 CrossRef.
- N. Wu, W. Wang, L. Q. Kou, X. Zhang, Y. R. Shi, T. H. Li, F. Li, J. M. Zhou and Y. Wei, Chem. – Eur. J., 2018, 24, 6330–6333 CrossRef CAS PubMed.
- X. Zhang, L. Han, J. Li, T. Lu, J. Li, G. Zhu and L. Pan, J. Mater. Sci. Technol., 2022, 97, 156–164 CrossRef CAS.
- S.-B. Xia, L.-F. Yao, H. Guo, X. Shen, J.-M. Liu, F.-X. Cheng and J.-J. Liu, J. Power Sources, 2019, 440, 227162 CrossRef CAS.
- J. Xiong, Q. Li, K. Li, X. Guo, M. Zhong, R. Wang, Y. Chen, X. Tan, B. Na and X. Tong, J. Colloid Interface Sci., 2025, 681, 182–191 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Details of materials, methods, synthesis and electrochemical measurement, SEM, EDS, IR, CV and Nyquist plots. See DOI: https://doi.org/10.1039/d5dt01226k |
| This journal is © The Royal Society of Chemistry 2025 |