Lighting up thiolated Au@Ag nanoclusters via aggregation-induced emission†
Xinyue
Dou‡
,
Xun
Yuan‡
,
Yong
Yu
,
Zhentao
Luo
,
Qiaofeng
Yao
,
David Tai
Leong
and
Jianping
Xie
*
Department of Chemical and Biomolecular Engineering, National University of Singapore, 4 Engineering Drive 4, 117576, Singapore. E-mail: chexiej@nus.edu.sg; Fax: +65 6516 1936; Tel: +65 6516 1067
First published on 23rd October 2013
Abstract
A simple strategy has been developed to synthesize highly luminescent thiolated Au@Ag nanoclusters (NCs) by using Ag(I) ions to bridge small Au(I)-thiolate motifs on the weakly luminescent thiolated Au NCs, leading to the formation of large Au(I)/Ag(I)-thiolate motifs on the NC surface and thus generating strong luminescence via aggregation-induced emission.
Thiolate-protected gold and silver nanoclusters or thiolated Au/Ag NCs are ultrasmall nanoparticles containing up to several hundred Au/Ag atoms, which are stabilized by a certain number of thiolate ligands in solution.1,2 Owing to the strong quantum confinement effects in this sub-2 nm size regime, thiolated Au/Ag NCs have discrete and size-dependent electronic structures3 and therefore show unique molecular-like properties such as quantized charging,4,5 magnetism,6,7 and strong luminescence.8–11 These properties are, however, not observed in their larger counterparts, which are relatively large Au/Ag nanoparticles with core sizes above 2 nm.12–14 The strong luminescence of thiolated Au/Ag NCs is one crucial feature for many of their practical applications. For example, luminescent Au/Ag NCs have recently emerged as a new type of promising luminescent probes for a variety of biomedical applications including bioimaging and biosensing.15–21 These applications have also attracted rapidly growing interest from the research community in developing efficient synthesis strategies for highly luminescent Au/Ag NCs.9,22,23
Very recently, we reported a new type of highly luminescent thiolated Au NCs with a core–shell Au(0)@Au(I)–thiolate nanostructure.24 Such Au NCs showed very strong luminescence in aqueous solution, and their luminescence was generated from the large Au(I)–thiolate complexes on the NC surface via aggregation-induced emission (AIE). We have successfully produced this AIE-type luminescent Au NCs with a high quantum yield (QY) of ∼15%. However, it is still unclear whether the AIE could also be used to synthesize other luminescent metal NCs such as Ag and bimetallic NCs, which is the aim of our current investigation. Here we report a novel and facile synthesis strategy for highly luminescent bimetallic AuAg NCs that can show luminescence via AIE. To the best of our knowledge, this is the first demonstration of an AIE-type luminescent AuAg NC.
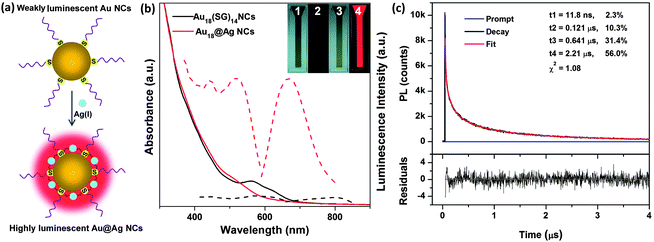
Our target materials are bimetallic AuAg NCs,25–27 which are expected to have synergistic effects in their physicochemical properties compared with their mono-metallic analogues.28–31 There are three major synthesis approaches for bimetallic AuAg NCs in the current development. The first approach is co-reduction (a typical one-pot synthesis method), where Au and Ag precursors (e.g., HAuCl4 and AgNO3) were first mixed, followed by the introduction of a particular reducing agent, leading to the formation of bimetallic AuAg NCs.25,32,33 The second approach is galvanic replacement (a typical two-pot synthesis method), where Ag NCs were first prepared, followed by the addition of Au(III) ions to oxidize the Ag NCs on the basis of the galvanic replacement reaction, resulting in the formation of bimetallic AuAg NCs.27 The third approach is anti-galvanic replacement, where Au NCs were first prepared, followed by the introduction of Ag(I) ions to replace Au atoms in the Au NCs, leading to the formation of bimetallic AuAg NCs.34 These three approaches are efficient for the synthesis of bimetallic AuAg NCs; however, they often produce AuAg NCs with weak luminescence. To address this issue, in this study, we present a new approach to synthesize highly luminescent bimetallic Au@Ag NCs, where a particular weakly luminescent thiolated Au NC species (hereafter referred to as parental Au NCs) was first prepared, followed by the introduction of Ag(I) ions. Interestingly, after ∼15 min, an unexpected strong luminescence was observed from the as-synthesized Au@Ag NCs. The luminescence light-up process is illustrated in Fig. 1a, where, upon addition, the Ag(I) ions immediately link the small Au(I)–thiolate motifs on the parental NC surface, forming a grid network or large Au(I)/Ag(I)–thiolate motifs around the entire NC surface, which can light up the thiolated AuAg NCs via AIE.
We showed this concept by using a well-studied glutathione (GSH)-protected Au NCs35,36 as the parental Au NCs. GSH is a natural tripeptide containing one thiol group in its cysteine (Cys) residue. The GSH-protected Au NCs were prepared according to a reported carbon monoxide (CO)-reduction method.37,38 As a proof-of-concept, Au18(SG)14 was chosen as a model of parental Au NCs in this study. The as-prepared parental Au18(SG)14 NCs were greenish-brown in aqueous solution (Fig. 1b, inset, item 1). No visible luminescence was observed in the NC solution under UV illumination at 365 nm (Fig. 1b, inset, item 2). The weak luminescence of Au18(SG)14 was located at ∼800 nm (Fig. 1b, dashed black line), with a low QY of 0.37% if rhodamine B was used as a reference, which is similar to the observation in a previous report.35 The parental Au NCs also featured two distinct absorption peaks at 560 and 620 nm in the UV-vis region (Fig. 1b, solid black line), which are the absorption characteristics of Au18(SG)14 NCs.38 The well-defined absorption spectrum also suggests high-purity Au18(SG)14 NCs in the as-prepared parental Au NCs.
After the introduction of a certain amount of Ag(I) ions, the initially greenish-brown Au18(SG)14 NCs (Fig. 1b, inset, item 1) instantaneously changed to brown (inset, item 3) in aqueous solution. Accordingly, the characteristic absorption peaks of the parental Au18(SG)14 NCs (560 and 620 nm) disappeared, and a new shoulder peak at ∼520 nm appeared in the reaction solution (Fig. 1b, red solid line). More interestingly, a strong red emission was observed in the reaction solution at ∼15 min (Fig. 1b, inset, item 4), which is in stark contrast to the weak luminescence of the parental Au18(SG)14 NCs (inset, item 2). The emission peak of the as-synthesized Au@Ag NCs was located at 667 nm (Fig. 1b, dashed red line). The QY was ∼6.8% using rhodamine B as a reference. The excitation spectrum (dashed red line, Fig. 1b) of the as-synthesized Au@Ag NCs also matches nicely with its UV-vis absorption spectrum (solid red line, Fig. 1b). Moreover, only one distinct band was observed in the native polyacrylamide gel electrophoresis (PAGE, 30%) of the as-synthesized Au@Ag NCs (Fig. S1,† lane 1), and this band showed strong red emission under UV illumination (lane 2). These data suggest that the strong luminescence observed in the reaction solution was emitted by the as-synthesized Au@Ag NCs, rather than from the impurities or side products in the reaction solution. Transmission electron microscopy (TEM) images also suggest the ultrasmall size feature (<2 nm) of the as-synthesized luminescent Au@Ag NCs (Fig. S2b†), which is similar to the size of the parental Au NCs (Fig. S2a†).
The luminescence property of the as-synthesized Au@Ag NCs was further examined by luminescence lifetime measurements. As shown in Fig. 1c, the luminescence decay response suggests the predominance of long lifetime (in the microsecond scale) components in the luminescent Au@Ag NCs: 2.21 μs (56%), 0.641 μs (31.4%), 0.121 μs (10.3%), and 11.8 ns (2.3%). The microsecond-scale lifetimes in the luminescent Au@Ag NCs were similar to the lifetimes of the previously reported AIE-type luminescent Au NCs.24 These data suggest that the emission of the as-synthesized Au@Ag NCs was generated from the AIE of Au(I)/Ag(I)–thiolate complexes on the NC surface, which is phosphorescence via the metal-centered triplet states. This luminescence pathway is distinctly different from the nanosecond emission from the singlet excited states of previously reported luminescent Ag NCs protected by DNA.15
Matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry was then used to examine the composition of the parental Au NCs and as-synthesized luminescent Au@Ag NCs. It should be noted that extensive fragmentations often occur in the MALDI-TOF measurements.39,40 As shown in Fig. S3,† a broad spectrum in the mass range of 5270 to 7840 Da was observed for the parental Au NCs (top panel), which could be assigned to Au18(SG)14 (the calculated molecular weight of 7834 Da) and its fragmentation to various extents. In contrast, the as-synthesized luminescent Au@Ag NCs showed a broader mass spectrum from 5270 to 8600 Da (Fig. S3,† middle panel), where a molecular weight increase of up to 760 Da (=8600–7840) was seen when compared to the parental Au NCs. This molecular weight difference is most likely contributed by the Ag atoms attached to the parental Au NCs. From the calculation, about one to seven Ag atoms are in the as-synthesized luminescent Au@Ag NCs.
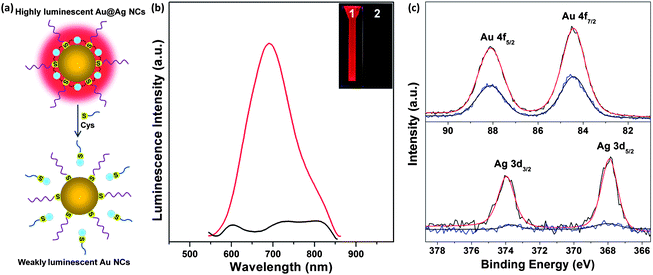
The as-synthesized Au@Ag NCs showed strong luminescence via the AIE of the complexes on the NC surface, where the size/structure of the Au(I)/Ag(I)–thiolate complexes is the key in determining their strong luminescence in the visible to near-infrared region. We hypothesized that the strong red emission in the as-synthesized Au@Ag NCs is generated by connecting the small Au(I)–thiolate motifs on the parental Au NCs via the Ag(I) linkers, forming large Au(I)/Ag(I)–thiolate motifs, which could generate strong luminescence via AIE. Since the Ag(I) ions serve as linkers in forming the large Au(I)/Ag(I)–thiolate complexes on the as-synthesized luminescent Au@Ag NCs, the removal of Ag(I) linkers may break those emission-active species [large Au(I)/Ag(I)–thiolate complexes] and scrub off those Ag(I) ions from the NC surface, which could revert the Au@Ag NCs back to the parental Au NCs, and as a result annul their strong luminescence in solution. The proposed luminescence quenching process is illustrated in Fig. 2a. The Ag(I) linkers on the luminescent Au@Ag NCs are removed by the introduction of a particular thiolate ligand, such as Cys, which can interact strongly with Ag(I) ions via the formation of Ag(I)–thiolate complexes. As expected, the strong red emission of the as-synthesized Au@Ag NCs was immediately quenched upon the addition of Cys to the NC solution (Fig. 2b, item 2). Accordingly, the emission peak of the as-synthesized Au@Ag NCs at 667 nm disappeared after the addition of a certain amount of Cys in the NC solution (Fig. 2b, black line).
X-ray photoelectron spectroscopy (XPS) and inductively coupled plasma-mass spectrometry (ICP-MS) were also used to obtain the composition information of the Au@Ag NCs before and after the addition of Cys. The molar ratio of Au![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ag in the luminescent Au@Ag NCs was found to be 1
Ag in the luminescent Au@Ag NCs was found to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.21 by XPS analysis (Fig. 2c). This value was also confirmed by ICP-MS analysis with a typical value of 1
0.21 by XPS analysis (Fig. 2c). This value was also confirmed by ICP-MS analysis with a typical value of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.24. It should be mentioned that the composition data of the luminescent Au@Ag NCs are also consistent with the number of Ag atoms (1–7 Ag atoms per cluster) in the Au@Ag NCs estimated from their MALDI-TOF mass spectra (Fig. S3†). The Ag species in the luminescent Au@Ag NCs are Ag(I), which is confirmed by the XPS analysis of the binding energies of Ag 3d5/2 in the luminescent Au@Ag NCs. The binding energy of the Ag 3d5/2 in the luminescent Au@Ag NCs is 367.9 eV (Fig. 2c, bottom panel, black line), which is identical to that of the Ag(I) species [using Ag(I)–GSH complexes as a control, Fig. S4†].41 This is an expected result since the added Ag(I) ions only serve as linkers to bridge the small Au(I)–thiolate motifs on the parental Au NC surface via the formation of the strong Ag(I)–thiolate or metallophilic Ag(I)–Au(I) bond. In contrast, no obvious signal of Ag 3d5/2 was observed in the XPS spectrum of the luminescent Au@Ag NCs after the addition of Cys (Fig. 2c, bottom panel, blue line). This information confirms the complete removal of Ag atoms from the luminescent Au@Ag NC surface by Cys. XPS and ICP-MS data provide further evidence on the complete removal of Ag atoms: the molar ratio of Au
0.24. It should be mentioned that the composition data of the luminescent Au@Ag NCs are also consistent with the number of Ag atoms (1–7 Ag atoms per cluster) in the Au@Ag NCs estimated from their MALDI-TOF mass spectra (Fig. S3†). The Ag species in the luminescent Au@Ag NCs are Ag(I), which is confirmed by the XPS analysis of the binding energies of Ag 3d5/2 in the luminescent Au@Ag NCs. The binding energy of the Ag 3d5/2 in the luminescent Au@Ag NCs is 367.9 eV (Fig. 2c, bottom panel, black line), which is identical to that of the Ag(I) species [using Ag(I)–GSH complexes as a control, Fig. S4†].41 This is an expected result since the added Ag(I) ions only serve as linkers to bridge the small Au(I)–thiolate motifs on the parental Au NC surface via the formation of the strong Ag(I)–thiolate or metallophilic Ag(I)–Au(I) bond. In contrast, no obvious signal of Ag 3d5/2 was observed in the XPS spectrum of the luminescent Au@Ag NCs after the addition of Cys (Fig. 2c, bottom panel, blue line). This information confirms the complete removal of Ag atoms from the luminescent Au@Ag NC surface by Cys. XPS and ICP-MS data provide further evidence on the complete removal of Ag atoms: the molar ratio of Au![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ag was 1
Ag was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.02 and 1
0.02 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.027, respectively. Taken together, these data strongly support that the strong red emission of the as-synthesized Au@Ag NCs was generated by connecting the small Au(I)–thiolate motifs on the parental Au NCs via the added Ag(I) linkers, leading to the formation of large Au(I)/Ag(I)–thiolate motifs and thus generating strong red emission via the AIE of such large motifs on the NC surface.
0.027, respectively. Taken together, these data strongly support that the strong red emission of the as-synthesized Au@Ag NCs was generated by connecting the small Au(I)–thiolate motifs on the parental Au NCs via the added Ag(I) linkers, leading to the formation of large Au(I)/Ag(I)–thiolate motifs and thus generating strong red emission via the AIE of such large motifs on the NC surface.
The addition of Cys to the luminescent Au@Ag NCs removed the Ag(I) linkers from the large Au(I)/Ag(I)–thiolate motifs on the NC surface, and as a result the remnant NC species reverted back to thiolated Au NCs. This hypothesis was supported by XPS and MALDI-TOF analyses. It is well-documented that the oxidation states of metal NCs are dictated by their size and structure. However, the binding energies of Au 4f7/2 in the luminescent Au@Ag NCs before and after Ag(I) removal are identical at 84.4 eV (Fig. 2c, top panel), implying that the removal of Ag(I) ions from the NC surface does not destruct the structure/size of the parental Au NCs and thus do not affect the oxidation states of Au in the NCs.35 Furthermore, the MALDI-TOF mass spectrum of the luminescent Au@Ag NCs after the removal of Ag(I) species from the NC surface showed a relatively narrow peak at ∼5800 Da in the mass range of 2000–20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da (Fig. S3,† bottom panel), clearly revealing that Au NCs were preserved after the removal of the Ag(I) linkers despite their mass (∼5800 Da) being smaller than that of the parental Au18(SG)14 NCs. The mass decrease of Au NCs after the addition of Cys could be understood from the possible removal of Au(I) from the NC surface considering that the affinity of Cys with Au(I) is also high.
000 Da (Fig. S3,† bottom panel), clearly revealing that Au NCs were preserved after the removal of the Ag(I) linkers despite their mass (∼5800 Da) being smaller than that of the parental Au18(SG)14 NCs. The mass decrease of Au NCs after the addition of Cys could be understood from the possible removal of Au(I) from the NC surface considering that the affinity of Cys with Au(I) is also high.
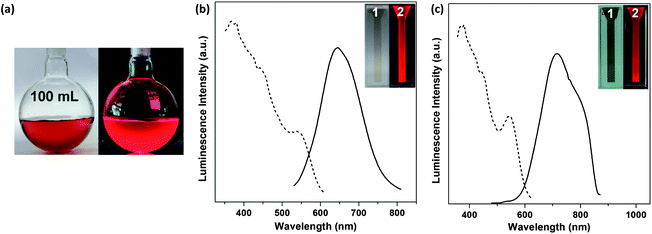
Upscaling production of highly luminescent bimetallic Au@Ag NCs is another salient feature of the synthesis approach developed in this study (e.g., to 100 mL of luminescent Au@Ag NCs in a single batch, Fig. 3a). In addition, our protocol is fairly generic and can be easily extended to synthesize other luminescent Au@Ag NCs by using different sized parental Au NCs. For example, if the weakly luminescent Au15(SG)13 (QY of 0.025%) and Au25(SG)18 (QY of 0.12%) were used as the parental Au NCs (Fig. S5†), the as-synthesized Au15@Ag NCs and Au25@Ag NCs also showed very strong red emission under UV illumination (Fig. 3b and c, inset, item 2). They also have similar photoemission spectra (Fig. 3b and c) as that of luminescent Au@Ag NCs synthesized by using Au18(SG)14 as the parental Au NCs (Fig. 1b, dashed red line). The emission peaks of the as-synthesized luminescent Au15@Ag and Au25@Ag NCs were located at 665 and 730 nm with a QY of 2.1% and 3.2%, respectively.
In summary, we have developed a new approach to synthesize highly luminescent Au@Ag NCs by using Ag(I) ions as linkers to bridge the small Au(I)–thiolate motifs on the weakly luminescent parental Au NCs, leading to the formation of large Au(I)/Ag(I)–thiolate motifs, which can generate strong luminescence via the AIE of the large complexes on the NC surface. The protocols and products developed in this study are important not only because they provide a facile, fast (<15 min), scalable (≥100 mL), and generic approach for highly luminescent Au@Ag NCs, but more importantly because they exemplify that the AIE is a generalized principle in synthesizing luminescent thiolated noble metal NCs.
Acknowledgements
This work is financially supported by the Ministry of Education, Singapore, under Grants R-279-000-327-112 and R-279-000-383-112.Notes and references
- Y. Lu and W. Chen, Chem. Soc. Rev., 2012, 41, 3594 RSC.
- R. Jin, Nanoscale, 2010, 2, 343 RSC.
- O. M. Bakr, V. Amendola, C. M. Aikens, W. Wenseleers, R. Li, L. Dal Negro, G. C. Schatz and F. Stellacci, Angew. Chem., Int. Ed., 2009, 48, 5921 CrossRef CAS PubMed.
- T. Laaksonen, V. Ruiz, P. Liljeroth and B. M. Quinn, Chem. Soc. Rev., 2008, 37, 1836 RSC.
- S. Chen, R. S. Ingram, M. J. Hostetler, J. J. Pietron, R. W. Murray, T. G. Schaaff, J. T. Khoury, M. M. Alvarez and R. L. Whetten, Science, 1998, 280, 2098 CrossRef CAS.
- Y. Negishi, H. Tsunoyama, M. Suzuki, N. Kawamura, M. M. Matsushita, K. Maruyama, T. Sugawara, T. Yokoyama and T. Tsukuda, J. Am. Chem. Soc., 2006, 128, 12034 CrossRef CAS PubMed.
- M. Zhu, C. M. Aikens, M. P. Hendrich, R. Gupta, H. Qian, G. C. Schatz and R. Jin, J. Am. Chem. Soc., 2009, 131, 2490 CrossRef CAS PubMed.
- J. Zheng, C. Zhou, M. Yu and J. Liu, Nanoscale, 2012, 4, 4073 RSC.
- I. Diez and R. H. A. Ras, Nanoscale, 2011, 3, 1963 RSC.
- J. Xie, Y. Zheng and J. Y. Ying, J. Am. Chem. Soc., 2009, 131, 888 CrossRef CAS PubMed.
- Z. Tang, T. Ahuja, S. Wang and G. Wang, Nanoscale, 2012, 4, 4119 RSC.
- J. Yang and J. Y. Ying, Nat. Mater., 2009, 8, 683 CrossRef CAS PubMed.
- C. Gao, J. Vuong, Q. Zhang, Y. Liu and Y. Yin, Nanoscale, 2012, 4, 2875 RSC.
- Z. Jin, M. Xiao, Z. Bao, P. Wang and J. Wang, Angew. Chem., Int. Ed., 2012, 51, 6406 CrossRef CAS PubMed.
- S. Choi, R. M. Dickson and J. Yu, Chem. Soc. Rev., 2012, 41, 1867 RSC.
- W. Guo, J. Yuan and E. Wang, Chem. Commun., 2009, 3395 RSC.
- M. Yu, C. Zhou, J. Liu, J. D. Hankins and J. Zheng, J. Am. Chem. Soc., 2011, 133, 11014 CrossRef CAS PubMed.
- L. Shang, S. Dong and G. U. Nienhaus, Nano Today, 2011, 6, 401 CrossRef CAS PubMed.
- X. Yuan, Z. Luo, Y. Yu, Q. Yao and J. Xie, Chem.–Asian J., 2013, 8, 858 CrossRef CAS PubMed.
- D. M. Chevrier, A. Chatt and P. Zhang, J. Nanophotonics, 2012, 6, 064504 CrossRef PubMed.
- J. Yu, S. A. Patel and R. M. Dickson, Angew. Chem., Int. Ed., 2007, 46, 2028 CrossRef CAS PubMed.
- Y. Yu, Q. Yao, Z. Luo, X. Yuan, J. Y. Lee and J. Xie, Nanoscale, 2013, 5, 4606 RSC.
- P. Maity, S. Xie, M. Yamauchi and T. Tsukuda, Nanoscale, 2012, 4, 4027 RSC.
- Z. Luo, X. Yuan, Y. Yu, Q. Zhang, D. T. Leong, J. Y. Lee and J. Xie, J. Am. Chem. Soc., 2012, 134, 16662 CrossRef CAS PubMed.
- Y. Negishi, T. Iwai and M. Ide, Chem. Commun., 2010, 46, 4713 RSC.
- D. R. Kauffman, D. Alfonso, C. Matranga, H. Qian and R. Jin, J. Phys. Chem. C, 2013, 117, 7914 CAS.
- T. Udayabhaskararao, Y. Sun, N. Goswami, S. K. Pal, K. Balasubramanian and T. Pradeep, Angew. Chem., Int. Ed., 2012, 51, 2155 CrossRef CAS PubMed.
- Y. Song, K. Liu and S. Chen, Langmuir, 2012, 28, 17143 CrossRef CAS PubMed.
- J. Huang, Y. Zhu, M. Lin, Q. Wang, L. Zhao, Y. Yang, K. X. Yao and Y. Han, J. Am. Chem. Soc., 2013, 135, 8552 CrossRef CAS PubMed.
- X. Guo, Q. Zhang, Y. Sun, Q. Zhao and J. Yang, ACS Nano, 2012, 6, 1165 CrossRef CAS PubMed.
- H. Qian, D.-e. Jiang, G. Li, C. Gayathri, A. Das, R. R. Gil and R. Jin, J. Am. Chem. Soc., 2012, 134, 16159 CrossRef CAS PubMed.
- C. Kumara and A. Dass, Nanoscale, 2011, 3, 3064 RSC.
- C. Kumara and A. Dass, Nanoscale, 2012, 4, 4084 RSC.
- Z. Wu, Angew. Chem., Int. Ed., 2012, 51, 2934 CrossRef CAS PubMed.
- Y. Negishi, K. Nobusada and T. Tsukuda, J. Am. Chem. Soc., 2005, 127, 5261 CrossRef CAS PubMed.
- Y. Shichibu, Y. Negishi, H. Tsunoyama, M. Kanehara, T. Teranishi and T. Tsukuda, Small, 2007, 3, 835 CrossRef CAS PubMed.
- Y. Yu, Z. Luo, Y. Yu, J. Y. Lee and J. Xie, ACS Nano, 2012, 6, 7920 CrossRef CAS PubMed.
- Y. Yu, X. Chen, Q. Yao, Y. Yu, N. Yan and J. Xie, Chem. Mater., 2013, 25, 946 CrossRef CAS.
- A. Dass, J. Am. Chem. Soc., 2009, 131, 11666 CrossRef CAS PubMed.
- H. Qian and R. Jin, Chem. Mater., 2011, 23, 2209 CrossRef CAS.
- X. Yuan, Y. Tay, X. Dou, Z. Luo, D. T. Leong and J. Xie, Anal. Chem., 2013, 85, 1913 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details, PAGE analysis, TEM images, MALDI-TOF MS, XPS, UV-vis and photoemission spectra of the samples. See DOI: 10.1039/c3nr04490d |
| ‡ These two authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2014 |