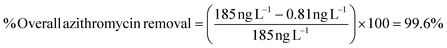Assessing pharmaceutical removal and reduction in toxicity provided by advanced wastewater treatment systems†
Luisa F.
Angeles
 a,
Rachel A.
Mullen
a,
Rachel A.
Mullen
 a,
Irvin J.
Huang
d,
Christopher
Wilson
a,
Irvin J.
Huang
d,
Christopher
Wilson
 b,
Wendell
Khunjar
c,
Howard I.
Sirotkin
e,
Anne E.
McElroy
*d and
Diana S.
Aga
b,
Wendell
Khunjar
c,
Howard I.
Sirotkin
e,
Anne E.
McElroy
*d and
Diana S.
Aga
 *a
*a
aDepartment of Chemistry, University at Buffalo, The State University of New York, Buffalo, NY 14260, USA. E-mail: dianaaga@buffalo.edu
bHampton Roads Sanitation District, Virginia Beach, VA, USA
cHazen and Sawyer, Fairfax, VA, USA
dSchool of Marine and Atmospheric Sciences, Stony Brook University, Stony Brook, NY, USA. E-mail: anne.mcelroy@stonybrook.edu
eDepartment of Neurobiology and Behavior, Stony Brook University, Stony Brook, NY, USA
First published on 4th November 2019
Abstract
Wastewater treatment plants (WWTPs) have been considered as hotspots for pharmaceutical residues, such as antidepressants, antimicrobials and active ingredients of over-the-counter drugs. Despite evidence of ecotoxicological effects of these microcontaminants on exposed fish in the aquatic systems, there is currently no regulation in terms of the levels of pharmaceutical residues allowable for release into the environment. Depending on the extent of treatment employed in WWTPs removal of pharmaceutical residues are highly variable. This study compares the removal efficiencies for several antimicrobials, antidepressants, and other pharmaceuticals at different stages of the wastewater treatment process of seven WWTPs that employ varying treatment technologies including biological, physical, advanced chemical oxidation, and a combination of two or more of these technologies. The concentrations of the pharmaceuticals were measured by liquid chromatography/mass spectrometry at multiple points during the course of treatment. Additionally, the ecotoxicological effects of the WWTP effluents were also evaluated based on the behavioral effects in larval zebrafish. It was found that biological treatment process provided negative to low removal (<50%), while activated carbon and ozonation provided high removal (>95% overall removal) for 14 out of 15, and 9 out of 11 compounds detected in WWTP influents, respectively. Notably, the final effluents of the seven WWTPs in this study did not show any significant behavioral alterations in zebrafish, indicating that despite differences in removal efficiencies all the treatment processes investigated are sufficient in preventing short-term biological effects.
Water impactWastewater effluent contains pharmaceuticals residues due to inefficient removal, and can potentially cause ecotoxicological effects on fish in the aquatic environment. After studying seven WWTPs it was found that advanced treatment, specifically using activated carbon and ozonation, were most effective in removing pharmaceutical contaminants. However, regardless of efficiency of pharmaceutical removal no measurable behavioral alterations were observed in fish larvae exposed to extracts of sewage effluent. |
Introduction
Residues of pharmaceuticals enter the wastewater stream due to improper disposal to water reclamation systems and routine excretion after human consumption. Some of these pharmaceuticals are eliminated or transformed via biological or chemical pathways during the wastewater treatment processes, while others pass through the wastewater treatment plants (WWTPs) relatively unchanged.1–4 Though an overall decrease in concentrations between influent and effluent is typically observed, conventional WWTPs are not designed with the particular focus on the removal of these compounds.5,6 Inefficient removal from WWTPs therefore has the potential to introduce pharmaceutical residues into surface water, resulting in the constant exposure of aquatic organisms to these compounds. The high frequency of detection of pharmaceutical residues in surface waters2,7,8 and their bioaccumulation in fish9 have now been documented in many studies.Advanced WWTP technologies can play an important role in preventing the release of pharmaceutical residues through WWTP effluents. Assessment of the efficiency of pharmaceutical removal in WWTPs has been performed mostly by analyzing the chemical concentrations before and after treatment. In fact, many plants employ multiple treatment processes in series, and information on the relative ability of each process to remove pharmaceuticals could inform efficient design or modifications of WWTP to maximize removal of these compounds. Additionally, it would be beneficial in evaluating the efficacy of different treatment processes to know if they influence the toxicity of the effluents being produced, as there is the possibility that transformation products may have enhanced toxicity as has been observed in some cases.10,11
Animal behavior has been shown to be a sensitive indicator of toxicity, oftentimes demonstrating significant changes before impaired physiological function is measurable.12 Thus, measuring behavioral effects in addition to chemical concentrations provides a more complete ecological assessment of wastewater treatment process efficiency compared to quantifying the pharmaceutical residues alone, particularly for contaminants known to influence behavior at low concentrations such as antidepressants. To date, there have been few published studies examining the impact of WWTP effluents on fish behavior. For instance, alterations in swimming behavior have been reported in juvenile Empire Gudgeons (Hypseleotris compressa) exposed to effluent from a small constructed wetland.13 Another study evaluating the impacts of effluent from a WWTP known to receive inputs from a pharmaceutical formulation facility reported inconsistent results.14 The first study conducted in 2010 failed to yield significant impacts on predator escape response in larval fathead minnows (Pimephales promelas), while the same study repeated in 2012 demonstrated a significant increase in escape performance.14 Effluents from a large secondary WWTP servicing Ontario, Canada were found to cause reductions in aggressive behavior (using a mirror assay) associated with uptake of pharmaceuticals in adult Round Goby (Neogobius melanostomus) after 28 day exposures.15 In contrast, a related study by the same group found no alterations in behavior after in situ exposures of the same species caged within the effluent stream.16 Given the variations in the observed behavioral effects in the different fish species exposed to final WWTP effluents, an examination of the differences in effects resulting from different treatment processes is warranted.
In this study, we compared the efficiencies of various types of advanced treatment systems and conventional activated sludge system (CAS) that are being implemented in one pilot-scale and six full-scale WWTPs. Sampling at multiple points along the treatment train allowed us to determine removal rates associated with a variety of treatment options. In addition to chemical characterization of wastewater samples, we also tested their biological activities by examining behavioral effects in a model vertebrate species to assess the efficacy of these different wastewater treatment processes from an ecotoxicological perspective. We employed the larval zebrafish (Danio rerio) visual motor response (VMR), which is a simple but well-characterized visually evoked swimming response consisting of sustained increased swimming activity after a sudden change in ambient light.17
Since there are currently no regulations in place limiting pharmaceutical dissemination by WWTP effluents, studies to understand the fate of pharmaceuticals in WWTPs are important as they can provide insight into which processes are more effective at pharmaceutical removal. The target analytes selected in this study included antidepressants, antibiotics, and frequently detected active ingredients of over-the-counter drugs. Antidepressants are of particular interest because of their widespread occurrence and persistence in the environment, and their bioaccumulation in fish brain; hence the ecotoxicity endpoint selected in this study to assess WWTP efficiency focused on VMR in fish. We also quantified antibiotics as discharges of these compounds from WWTPs could promote the proliferation of antimicrobial resistance in the environment.18 The knowledge obtained from this study will provide critical information from full-scale WWTP systems on their efficiency of removing antidepressants, antibiotics and other pharmaceuticals. Evaluation of the efficiency of several full-scale WWTPs with varying treatment trains combining advanced technologies, using both chemical and biological assays, will advance our knowledge on the removal of recalcitrant antidepressants and antibiotics that have been lurking in many receiving surface waters.
Materials and methods
Chemicals, solvents, and reference standards used for all the analyses and details on origin are shown in detail in the ESI.†Wastewater treatment plants and sampling
A total of seven WWTPs employing different designs and treatment technologies were investigated. All WWTPs in this study are located in Eastern United States. More precise locations are not provided in order to protect the identity of the WWTPs. Samples were collected between August and December in 2017, and June and July in 2018, and were obtained based on a 24 hour time-proportional collection. While no time shift of sampling was performed, the hydraulic retention time (HRT) of the plants range from 8 to 12 hours, therefore the 24 hour composite sample reflects the average influent and effluent on a common timed basis. This allows us to compare removal across unit processes without having to perform HRT-based sampling. All nitrate levels were below 3 mg L−1 (Table S1†), which is way below the set standard by the U.S. EPA for wastewater effluent discharge of 10 mg L−1, indicating that the WWTPs in this study efficiently remove nitrates.19The sample bottles were either kept on ice or refrigerated during collection. All WWTPs investigated are full-scale serving existing municipalities, except for WWTP 3 that is a pilot plant. The tertiary treatment processes and sampling points for each of the seven WWTPs are shown in Fig. 1. WWTP 3 has a capacity of 1 MGD and employs the most advanced treatment; it was sampled on three different days at five points along the treatment process. The ozone dose applied in WWTP 3 was a O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TOC ratio of 0.8. The WWTP 1, 4, 5, and 6 have capacities ranging from 1.2 to 5.5 MGD, while WWTP 2 and 7 were much larger with capacities ranging from 40 to 60 MGD.
TOC ratio of 0.8. The WWTP 1, 4, 5, and 6 have capacities ranging from 1.2 to 5.5 MGD, while WWTP 2 and 7 were much larger with capacities ranging from 40 to 60 MGD.
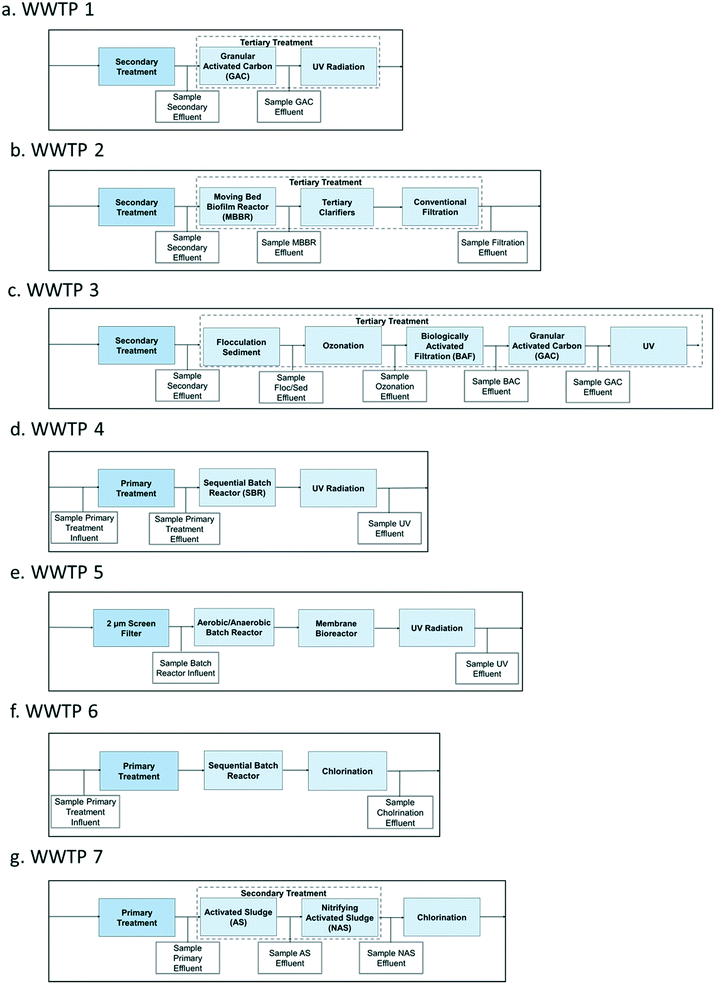 | ||
| Fig. 1 The tertiary treatment processes and location of sampling points for the seven wastewater treatment plants (WWTPs) included in this study. | ||
Sample extraction
Two 500-mL aliquots of each wastewater sample were extracted using solid phase extraction (SPE): one was spiked with 50 μL of 1 mg L−1 surrogate mix for chemical analysis, while the other was reserved for biological tests and was not spiked with any surrogate standard. Both sets of samples were extracted using SPE in order to have a direct comparison between the chemical and biological analyses. The samples were acidified to pH 2 with 85% phosphoric acid prior to SPE using 500 mg HLB Oasis™ cartridges. Each cartridge was conditioned with 6 mL methanol followed by 6 mL NANOpure™ water. The samples were loaded onto the SPE cartridges at an approximate flowrate of 6 mL min−1. After loading the water samples, the SPE cartridges were left to dry under vacuum for about an hour. The set of cartridges that were not spiked with surrogates were sent to Stony Brook University to elute the extracts for fish behavioral evaluation, as described below. The other set of samples spiked with surrogates were eluted with 8 mL methanol, and slowly evaporated to dryness under nitrogen gas. Once dry, the samples were reconstituted in the starting mobile phase of the LC-MS method and spiked with an internal standard (diphenhydramine-d3) to a final concentration of 50 ppb. Overall percent recoveries of all analytes are in Fig. S2.†Liquid chromatography – mass spectrometry
The liquid chromatography – mass spectrometry (LC-MS) method used for pharmaceutical analysis was adapted from our previous publication.20 The selected reaction monitoring (SRM) transitions, retention times, and instrument detection limits are in Table S2 and the instrument parameters in Table S3 of the ESI.† Quantification was performed using isotope dilution and quality assurance parameters for retention time (±5 min) and ion ratio (±30%) were used.21Larval zebrafish behavior screen
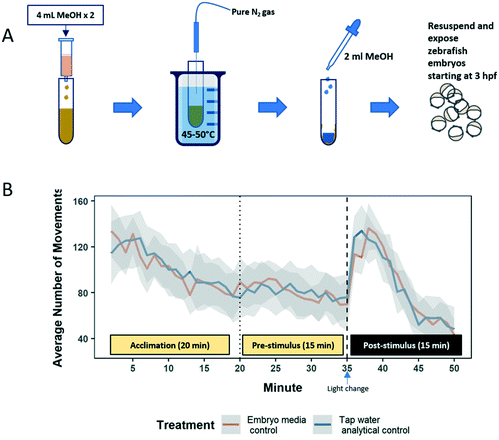
The wastewater samples used for the larval zebrafish behavior screen were prepared following the same SPE protocol for chemical analysis, but without adding the surrogate mix (Fig. 2A). The analytes were eluted with 8 mL HPLC grade methanol, and the eluates were evaporated to dryness using nitrogen gas. Since the SPE protocol involved the addition of acid, 0.01 N NaOH was added to readjust the pH of the samples back to 6.8–7.0 to prevent any potential toxic effects from low pH. The analytes were resuspended in HPLC grade methanol and stored at −20 °C until ready for biological testing. Wastewater extracts were resuspended in zebrafish embryo media (0.3 g L−1 Instant Ocean, 7.5 mg L−1 HCO3−, 1 mL L−1 methylene blue) at concentrations equivalent to the original wastewater samples for embryonic exposures.All animal husbandry and experimental manipulation of embryos and larvae were approved by Stony Brook University's Institutional Animal Care and Use Committee (IACUC). Six to eight pairs of breeding adult wildtype zebrafish (a hybrid of Tubingen Longfin/Brian's wildtype strain) were set up to collect embryos for experimentation. Embryos were dechorionated at 3 hours post fertilization (hpf) using a dilute protease solution (1 mg mL−1 Pronase; Sigma Aldrich) and exposed to resuspended extracts (or control embryo media) in groups of 30 in 100 mm plastic petri dishes lined with 1% agarose. At 24 hpf, embryos were plated on a plastic 48-well plate, with one fish per well containing 1 mL of their respective treatment solutions. Exposures continued statically until the behavior screen at 6 days post fertilization (dpf). Sample sizes ranged from 19–24 replicate fish per treatment.
Behavior was observed in a Zebrabox imaging system (Viewpoint Life Sciences, FR) at 6 dpf following protocols previously developed to screen individual neuroactive pharmaceuticals.22 We monitored the total number of movements made per min during the VMR, which we have divided into three stages: acclimation (first 20 min of recording), pre-stimulus (15 min of spontaneous movement in full light conditions), and post-stimulus (15 min of evoked swimming stimulated by turning off the light). VMR is typically characterized by a large increase in swimming activity after a sudden change in ambient light that is sustained for a couple of minutes before activity returns to baseline levels23 (Fig. 2B). Swimming behavior was analyzed using linear mixed effect models. Wastewater treatment and VMR stage were modeled as fixed factors, while the repeated measures of individual fish were modeled as a random factor. Models were analyzed for significance using a type III analysis of variance (Satterthwaite's method). Models that showed a significant effect of wastewater extract treatment were further analyzed using a Tukey's honest significance post hoc test. Treatments that differed from controls with a p-value less than 0.05 were considered statistically significant. Tap water extracts caused no significant differences in larval zebrafish swimming behavior (Fig. 2B), indicating that the analyte extraction process did not introduce any toxic compounds that might confound the results.
Results and discussion
Occurrence of pharmaceuticals in wastewater treatment plants (WWTPs)
A total of 38 pharmaceuticals, which include antibiotics from 3 classes (sulfonamides, macrolides, quinolones) and antidepressants which include serotonin–norepinephrine reuptake inhibitors (SNRIs), selective serotonin reuptake inhibitors (SSRIs), norepinephrine–dopamine reuptake inhibitors (NDRIs), and anti-psychotics were analyzed in the WWTP samples.The concentrations of each pharmaceutical detected in at least one sample are presented in Tables S3A and S3B† for WWTPs 1–3 and 4–6. A total of 21 and 15 pharmaceuticals were detected in at least one influent sample and effluent sample, respectively. While we are comparing pharmaceutical detections and concentrations, it should be noted that the samples from WWTPs 1–3 are secondary treatment effluent, while the samples from WWTPs 4–7 are primary influent samples, which explains the higher concentrations detected for some compounds in the latter.
The highest concentrations detected for the raw influent samples collected from WWTPs 4–7 were for acetaminophen (550–38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ng L−1), caffeine (13
000 ng L−1), caffeine (13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–100
000–100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ng L−1), and ciprofloxacin (420–2700 ng L−1). Acetaminophen, an analgesic, has also been found at high concentrations in the primary treatment effluents of full-scale WWTPs.24 Sulfamethoxazole and acetyl-sulfamethoxazole were the only detected sulfonamides out of the seven analyzed, consistent with what has been reported in previous studies.25,26 Trimethoprim, which is usually prescribed with sulfamethoxazole was also detected in all the influent samples in this study. Notably, carbamazepine, which is an anti-epileptic drug, and the antibiotic sulfamethoxazole are among the top 5 most frequently detected compounds globally.27 This was reflected in this study, since these two compounds were detected in the influents of all seven WWTPs analyzed. Other antimicrobials detected in the wastewater samples in this study were the macrolides, azithromycin and clarithromycin (22 to 639 ng L−1 for AZI and 13 to 115 ng L−1 for CLA), which were also found in recent studies at concentrations ranging from 2 to 437 ng L−1 for azithromycin, and 88 to 632 ng L−1 for clarithromycin.26,28 Other target macrolides in this study, such as roxithromycin, tylosin, tilmicosin, and spiramycin were not detected; this is consistent with a similar pharmaceutical study that analyzed wastewater and surface waters in Spain.26
000 ng L−1), and ciprofloxacin (420–2700 ng L−1). Acetaminophen, an analgesic, has also been found at high concentrations in the primary treatment effluents of full-scale WWTPs.24 Sulfamethoxazole and acetyl-sulfamethoxazole were the only detected sulfonamides out of the seven analyzed, consistent with what has been reported in previous studies.25,26 Trimethoprim, which is usually prescribed with sulfamethoxazole was also detected in all the influent samples in this study. Notably, carbamazepine, which is an anti-epileptic drug, and the antibiotic sulfamethoxazole are among the top 5 most frequently detected compounds globally.27 This was reflected in this study, since these two compounds were detected in the influents of all seven WWTPs analyzed. Other antimicrobials detected in the wastewater samples in this study were the macrolides, azithromycin and clarithromycin (22 to 639 ng L−1 for AZI and 13 to 115 ng L−1 for CLA), which were also found in recent studies at concentrations ranging from 2 to 437 ng L−1 for azithromycin, and 88 to 632 ng L−1 for clarithromycin.26,28 Other target macrolides in this study, such as roxithromycin, tylosin, tilmicosin, and spiramycin were not detected; this is consistent with a similar pharmaceutical study that analyzed wastewater and surface waters in Spain.26
The antidepressants that were detected in the WWTPs sampled in this study were amitriptyline, bupropion, citalopram, haloperidol, lamotrigine, primidone, sertraline, and venlafaxine, at concentrations ranging from (5 ng L−1 to 2000 ng L−1), with venlafaxine having the highest concentration. Similar findings were reported in a study in the past year in U.S. wastewaters wherein 5 out of 8 of these antidepressants were detected at concentrations around 0.9 ng L−1 to 89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 ng L−1, with bupropion having the highest concentration detected. Haloperidol, lamotrigine, and primidone were not included in their list of analytes.29
500 ng L−1, with bupropion having the highest concentration detected. Haloperidol, lamotrigine, and primidone were not included in their list of analytes.29
In contrast to the primary treatment effluents, acetaminophen was not detected in any of the secondary treatment effluents. Since acetaminophen is negatively charged at neutral pH, it is expected that it will not sorb to the sludge and should be present in the secondary treatment effluents. However, another study25 detected high concentrations of this compound in the sludge and a possible explanation is that other mechanisms of sorption, such as through van der Waals forces, may dominate. The same study showed that diclofenac, an analgesic, was not eliminated in CAS, which is in accordance with this current study where diclofenac was detected in all secondary treatment effluent samples. This reflects global studies wherein diclofenac was found to be the most frequently detected pharmaceutical in environmental samples, being detected in 50 countries in tap, surface, and groundwater.27
The antibiotic erythromycin has been found to have poor removal efficiency in secondary treatment technologies such as CAS,24,25 which is what was observed in the secondary treatment in the WWTPs sampled in this study. The same is true for carbamazepine which was also detected in all secondary treatment effluent samples, and was found to be persistent in wastewater even after CAS.24
Ozonation was found to be effective at removing compounds with activated aromatic moieties, amine functional groups, or double bonds, which usually results in removal rates of >95% for the majority of micropollutants.30,31 In WWTP 3, only two compounds, bupropion and primidone, were detected post-ozonation, suggesting these two pharmaceuticals to be recalcitrant to advanced oxidation, with removal rates of 88% and 35%, respectively. Carbamazepine, diclofenac, and sulfamethoxazole, which appeared to have been eliminated in the effluent after ozonation (below detection limit), were shown to be degraded in a full-scale treatment plant that employs ozonation technology.31 These findings are important because it could mean that with proper ozone dose and contact time, all pharmaceutical residues can be potentially removed from WWTPs prior to discharging treated water into the receiving surface waters.
Removal of pharmaceuticals across secondary and tertiary treatment stages in WWTPs
The % overall removal of the pharmaceuticals in each of the seven WWTPs is shown in Table 1, calculated using the concentrations of the pharmaceuticals in the primary/secondary treatment effluents, which is considered the influent of the secondary/tertiary treatment stages, and the final effluent, as shown in the eqn (1).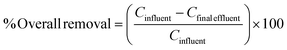 | (1) |
| WWTP 1 | WWTP 2 | WWTP 3 | WWTP 4 | WWTP 5 | WWTP 6 | WWTP 7 | |
|---|---|---|---|---|---|---|---|
| a NA stands for not applicable (meaning compound was not detected in influent sample). | |||||||
| Acetaminophen | NA | NA | NA | 100% | 100% | 100% | 99% |
| Acetylsulfamethoxazole | 100% | 23% | 97% | 100% | NA | 98% | −7% |
| Amitriptyline | 76% | 97% | NA | 97% | 94% | 94% | 21% |
| Anhydro erythromycin | 97% | 97% | 98% | NA | NA | NA | NA |
| Azithromycin | 100% | 96% | 100% | NA | NA | 60% | 45% |
| Bupropion | 100% | 26% | 100% | −42% | 67% | 34% | 23% |
| Caffeine | 100% | 32% | 100% | 100% | 100% | 100% | 99% |
| Carbamazepine | 99% | −10% | 100% | −25% | −21% | 6% | NA |
| Ciprofloxacin | NA | NA | 100% | 93% | 85% | 80% | 70% |
| Citalopram | 100% | 100% | 100% | 11% | 15% | 35% | 11% |
| Clarithromycin | 98% | 91% | 99% | 96% | 100% | 18% | 24% |
| Diclofenac | 100% | 100% | 100% | 40% | −100% | −100% | 13% |
| Haloperidol | NA | NA | NA | NA | NA | 89% | NA |
| Lamotrigine | 100% | 55% | NA | NA | NA | 30% | 32% |
| Primidone | NA | NA | 99% | NA | NA | NA | NA |
| Sertraline | 100% | 99% | 100% | NA | NA | NA | NA |
| Sulfamethoxazole | 95% | 87% | 99% | 100% | −100% | −100% | 21% |
| Trimethoprim | 98% | 99% | 100% | 72% | −20% | 94% | 28% |
| Venlafaxine | 100% | 100% | 100% | 32% | 38% | 24% | −3% |
In cases where a compound was not detected in the effluent, but was detected in the influent, the detection limit of the compound was used as the “effluent concentration” for the purpose of calculating % overall removal. For instance, in WWTP1 azithromycin was detected in the activated carbon filter influent at 185 ng L−1, but not in the effluent. The limit of detection for azithromycin (0.81 ng L−1) was used as “effluent concentration” resulting to a calculated 99.6% overall removal as shown below:
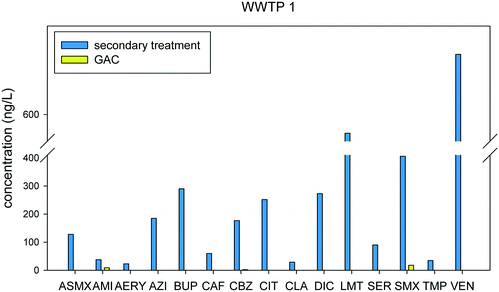
A comparison of the seven WWTPs that provided the highest number of pharmaceuticals being removed by >95% indicate that WWTP 1 and WWTP 3 were the most effective in reducing pharmaceutical concentrations, while WWTP 7 was the least effective. It is notable that both WWTP 1 and 3 employs GAC.
The influent and effluent for each stage in all three WWTPs were collected and analyzed in order to assess the efficiency of removal for each stage. To evaluate the contributions of each of the treatment stage to the overall pharmaceutical removal, % removal was calculated using the concentrations in the influent and effluent of each particular stage. For instance, the % removal by GAC in WWTP 1 is shown below (eqn (2)).
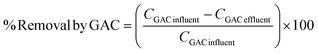 | (2) |
As shown in the calculation for % overall removal, the detection limit of the compound is used as the “effluent concentration” if the compound was not detected in the effluent. Table S5 in the ESI† shows the % removal for each stage in the seven WWTPs.
The GAC, which is the treatment technology employed in WWTP 1, works by the adsorption of organic micropollutants from wastewater. Some limited biological activity is also present as the GAC becomes saturated. The use of GAC in WWTPs as a tertiary treatment process has been investigated in recent studies and was found to be highly effective towards the removal of pharmaceuticals and other micropollutants.32–35 Electrostatic interaction plays a role in the process of adsorption as stated in previous studies. Activated carbon is known to adsorb natural organic matter (NOM) present in wastewater, which gives the surface of the activated carbon a net negative charge34,36–38 in addition to the presence of phenolic groups in NOM that provides additional lipophilic and hydrogen bonding interactions with micropollutants. This allows for the high affinity of the positively charged compounds in the wastewater effluent. As seen in Fig. 3, all of the pharmaceuticals that were detected were highly removed (>90% removal rate) in GAC. At the pH of wastewater which is around 7, 11 compounds are either neutral or positively charged. Acetyl sulfamethoxazole, diclofenac, and sulfamethoxazole are negatively charged, however, they were still found to have high adsorption to the activated carbon. The hydrophobicity of diclofenac may have contributed to its high adsorption, while other factors such as the formation of H-bonds and pi–pi interactions with NOM and GAC may have increased the adsorption of sulfamethoxazole and acetyl sulfamethoxazole.38 Biological activity may also play a role in the fate of these chemicals; however, it was not possible to concretely determine the precise mechanism dictating removal of pharmaceuticals in the full-scale and pilot scale WWTPs.
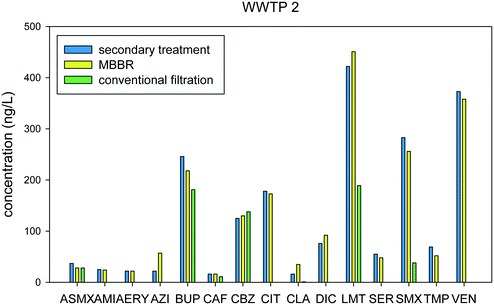
A tertiary moving bed biofilm reactor (MBBR), tertiary clarifiers, and conventional filtration were employed in WWTP 2. The MBBR is used to help achieve nitrate removal at the treatment facility downstream of the secondary treatment processes. Out of the 15 compounds detected, five were found to have negative % removal and the remaining 10 compounds had low removal rates, ranging from 1% to 25%, as shown in Fig. 5 and Table S4.†
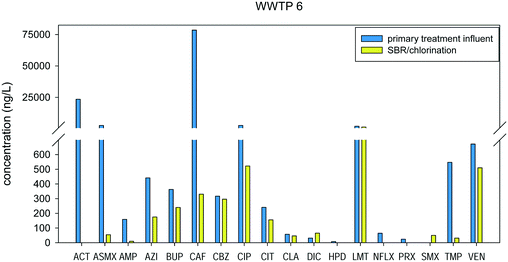
Negative % removal was observed for WWTPs 2–7, which could be attributed to the transformation of some human or microbiological metabolites (e.g. conjugated pharmaceuticals) that are present in the influent or generated within the collection system or biological process, to their original parent compound.24,25,39 Deconjugation of pharmaceutical metabolites or transformation back to their parent compounds in activated sludge systems have been observed in other studies. For instance, the negative removal for carbamazepine in this study may be due to the deconjugation of its hydroxylated human metabolites to the free form due to microbial activity in the BAC, as previously observed.40 Sulfamethoxazole was also found to have negative removal, which can be explained by the presence of its excreted human metabolite, acetyl-sulfamethoxazole, in the influent, which is then transformed back to sulfamethoxazole after biological treatment.25,39,41 For instance in WWTP 6, it can be observed that sulfamethoxazole has negative removal, with the compound not being detected in the influent but was found to be 50 ng L−1 in the effluent. In the same samples, the concentration of N-acetylsulfamethoxazole was found to decrease from 2656 ng L−1 to 54 ng L−1, which supports the hypothesis that some of it might be converting to the parent molecule (Fig. 4).
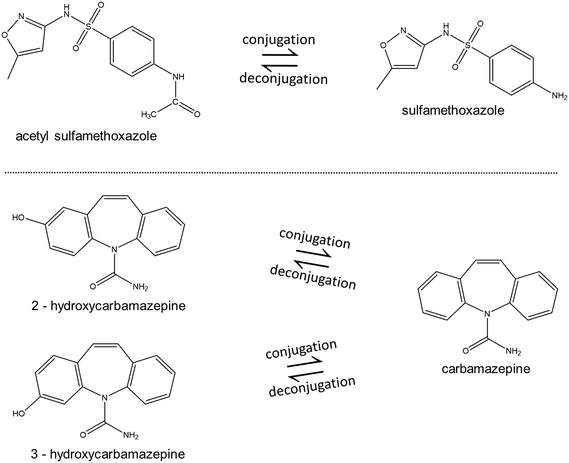 | ||
| Fig. 4 Deconjugation of the human metabolites of sulfamethoxazole and carbamazepine to the parent compounds. | ||
Desorption of pharmaceuticals from biological materials could also contribute to negative % overall removal. Azithromycin, anhydro-erythromycin, clarithromycin, trimethoprim, and venlafaxine exhibited negative % removals in biological treatment processes. It is possible that previously sorbed parent compounds desorbed from MBBR biomass and sloughed off the media, causing the increase in the concentration after the process.
The media filter bed (MFB) gave higher removal rates compared to the MBBR and was responsible for the overall removal rates of WWTP 2, indicating that effective solids removal was critical for helping to control the constituents monitored in this study. Results showed detection of 15 compounds wherein 8 were found to have greater than 95% removal. The remaining 7 compounds had % removal values ranging from −7% to 91%.
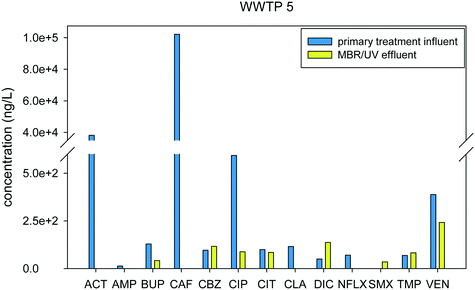
WWTP 5 is a membrane bioreactor (MBR), where primary influent is passed through a 2-μm screen prior to treatment in a batch reactor alternating between aerobic and anoxic conditions, through pump aeration followed by a membrane bioreactor using hollow fiber filtration as the secondary treatment stage, prior to a final disinfection step. MBR is a biological treatment technology that is similar to the CAS, but uses membrane filtration as the liquid–solid separation process, which allows the elimination of secondary clarifiers.42Fig. 6 shows the removal of the compounds from the primary influent to the final effluent. Only 4 out of 13 pharmaceuticals detected in WWTP was removed by >95%.
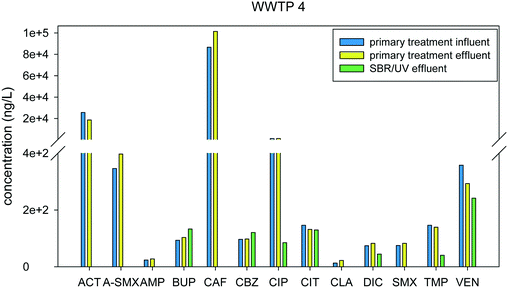
The treatment processes in WWTPs 4 and 6 are similar and consist of a primary treatment, a sequencing batch reactor (SBR), and a disinfection step. WWTP 4 has a UV treatment step, while WWTP 6 employed chlorination/de-chlorination with sodium hypochlorite/sodium bisulfite. In the primary treatment, the wastewater influent passes through clarifiers to remove suspended solids. Fig. 7 shows little to no removal in the influent and effluent of the primary treatment stage for WWTP 4, which is to be expected as this process is not intended to remove small molecules, but only to remove bulk organic carbon and suspended solids. Pharmaceutical removal at the primary treatment stage may be attributed to the sorption of compounds to the suspended solids that are settled out in the process. Biological activity may also be present in this stage, although not to the same extent in CAS, or other biologically based treatments. SBRs are activated sludge systems that facilitate biological reactions over several cycles. It is a fill-and-draw process, wherein equalization, biological treatment, and secondary clarification occurs in the tank followed by effluent discharge.43 Since this treatment involves a biological process, some compounds were found to have negative removal, as seen in Fig. S6 and S7† for WWTPs 4 and 6, respectively (Fig. 7 and 8).
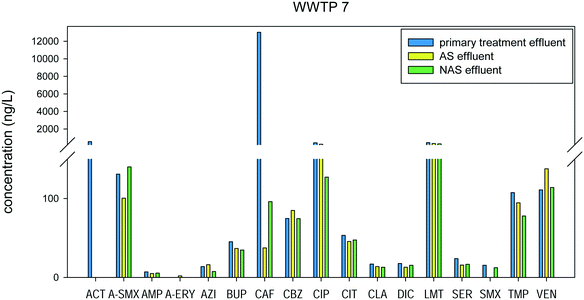
The secondary treatment of WWTP 7, which has activated sludge (AS) and nitrifying activated sludge (NAS), was also evaluated. AS is a biological treatment process, which makes use of microorganisms to degrade organic contaminants in wastewater. NAS converts the nitrogen contaminants in wastewater by converting ammonia to nitrate, in the presence of nitrifying bacteria. It can be observed from Fig. 9 that in the AS process, 4 out of the 17 compounds detected had negative removal, and 5 had low removal (0–25%). The four compounds with negative removal, anhydro-erythromycin, azithromycin, carbamazepine, and venlafaxine, also had negative removal in the other WWTPs with biological treatment processes.
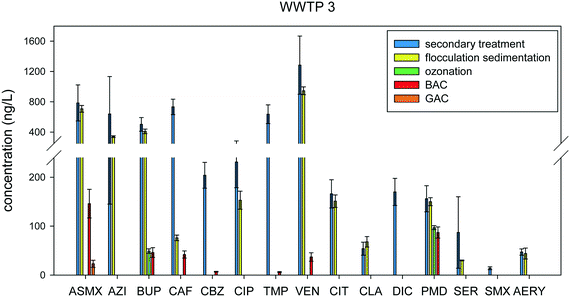
WWTP 3 is a pilot plant and it provides the most advanced treatment system out of all the WWTPs sampled in this study. WWTP 3 has four tertiary treatment technologies: flocculation sedimentation, ozonation, BAF, and GAC. Fig. 10 shows the concentrations of the compounds detected in WWTP 3 at each treatment stage. Combined flocculation and sedimentation work by making small suspended particles in the water clump together, which subsequently settle. Pharmaceutical removal in flocculation sedimentation range from 0% to 100% removal, depending on the compound. Of the 15 compounds detected, four compounds were strongly removed: carbamazepine, diclofenac, sulfamethoxazole, and trimethoprim. At this stage, removal is mainly driven by the sorption affinity of the compounds to the suspended particles being removed in the process. Carbamazepine and diclofenac are both hydrophobic compounds with relatively high octanol–water partition coefficient (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow) values of 2.45 and 4.51, showing that removal most likely occurred via adsorption to the biological solids or other suspended particles. Trimethoprim and sulfamethoxazole, on the other hand, are both relatively more hydrophilic compounds with log
Kow) values of 2.45 and 4.51, showing that removal most likely occurred via adsorption to the biological solids or other suspended particles. Trimethoprim and sulfamethoxazole, on the other hand, are both relatively more hydrophilic compounds with log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow values of 1.13 and 0.95, respectively. For these compounds, the mechanism of removal is most likely through the electrostatic interactions with the functional groups present on the surface of the particles in wastewater.
Kow values of 1.13 and 0.95, respectively. For these compounds, the mechanism of removal is most likely through the electrostatic interactions with the functional groups present on the surface of the particles in wastewater.
Ozonation degrades most organic micropollutants through the production of hydroxyl radicals and through its oxidative properties, as shown in previous studies.31,34,44 Out of the 11 pharmaceuticals, 9 were removed at high efficiencies ranging from 98–100% post-ozonation. This is expected since these compounds have electron rich moieties, which are known to have high reactivities with ozone.34,45 Bupropion and primidone, however, only had 88% and 35% removal, respectively (Table S5†). The low removal of primidone with ozone may be due to its low ozone reactivity (kO3 = 1.0 M−1 s−1).46 Compounds with ozone reactivities <104 M−1 s−1 are not easily degraded with ozone, making the reaction with the hydroxyl radical the main pathway for oxidation.47,48 The second-order rate constants for reactions with ozone and hydroxyl radical are shown in Table S7.† Additionally, other factors that occur in wastewater such as the possible sorption of pharmaceuticals to colloidal particles, making them unavailable for ozone attack,34,49 may have also contributed. Following the ozonation step are BAF and GAC, which are activated carbon processes that remove compounds through adsorption. BAF, unlike GAC, has strains of bacteria on its surface, in order to achieve biodegradation together with adsorption.50 Since BAF followed the oxidation step, only seven compounds were detected because most of the compounds were already fully removed or transformed by the oxidation process. In general, GAC and BAF are expected to be more efficient in removing compounds with higher log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow values. However, this may not always be the case since more hydrophobic compounds may also be eliminated through this process due to adsorption brought about by electrostatic interactions, hydrogen bonding, and van der Waals interaction.51 GAC gave excellent removal for the detected compounds. Out of the seven pharmaceuticals, five were removed at either 98% and 99%, while two of them, acetylsulfamethoxazole, and trimethoprim, were removed at 85% and 87%, respectively.
Kow values. However, this may not always be the case since more hydrophobic compounds may also be eliminated through this process due to adsorption brought about by electrostatic interactions, hydrogen bonding, and van der Waals interaction.51 GAC gave excellent removal for the detected compounds. Out of the seven pharmaceuticals, five were removed at either 98% and 99%, while two of them, acetylsulfamethoxazole, and trimethoprim, were removed at 85% and 87%, respectively.
WWTP 3 was the only plant where we had the opportunity to sample on multiple days, providing three determinations for each analyte for all five steps of the treatment process. As can be seen in Fig. 10, variations in multiple samples was relatively low, averaging 19% of the mean value. Although variation throughout the season was not assessed in this study, the consistency of these measurements from samples collected within the same week indicates these removal efficiencies are indicative of the performance of this facility. A t-test assuming unequal variance at α = 0.05 (Table S6†) was performed in order to provide a comparison between samples from different treatment stages. Notably, there was no significant difference between the concentrations in the samples before and after the flocculation sedimentation stage for azithromycin and sertraline, even if their % removal values were 47% and 65%, respectively. Looking at the detected concentrations for these two compounds, it was found that their concentrations in the first day of sampling were higher compared to the two other days of sample collection. For azithromycin, the concentration detected on the first day was 1200 ng L−1, while in the two other days, the concentrations were 462 and 256 ng L−1. The same trend was found for sertraline where the concentrations detected for the multiple days of sampling were 170, 50, and 38 ng L−1. A limitation of this study is that we are unable to perform statistical analysis on the data for the other WWTPs in the study since only one set of samples were collected for these treatment plants. However, these data are still representative of what would be expected, and most importantly given the consistent sampling and analysis techniques employed provides a good first step comparison between the plants and their respective treatment approaches.
Behavioral changes in larval zebrafish
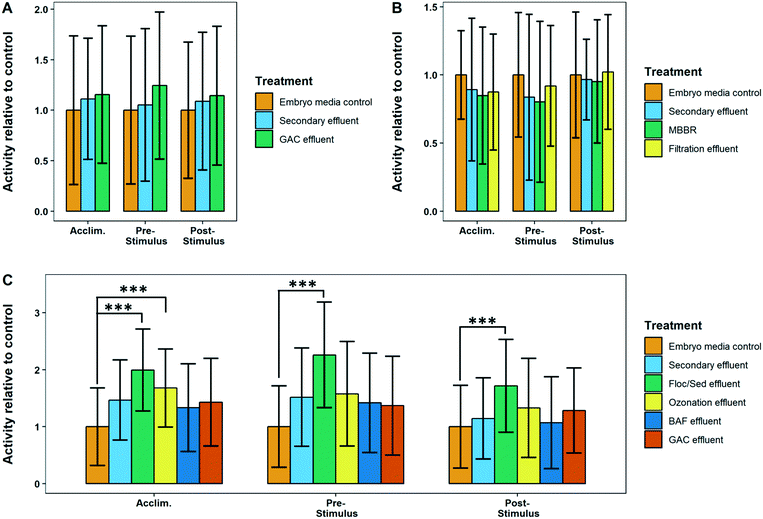
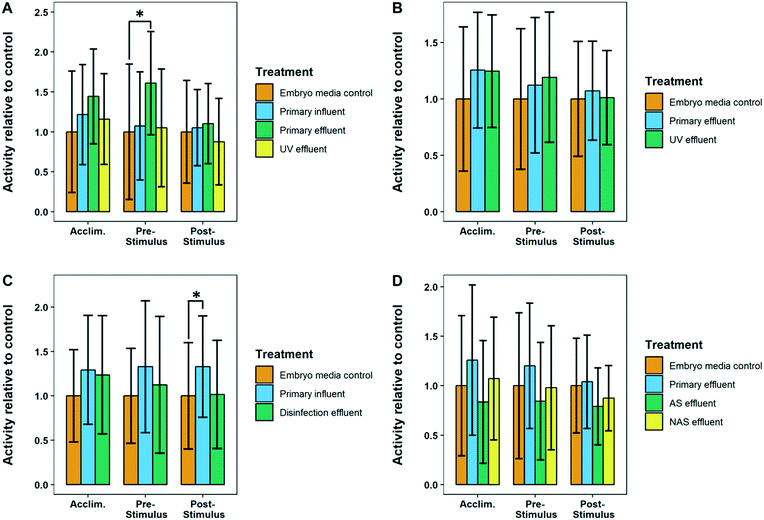
Many of the treatment plants sampled had final effluents that caused slight hyperactivity in larval zebrafish however none were statistically significant from control fish, likely due to the high variance of our data. This suggests that all the plants sampled remove contaminants at a high enough efficiency to prevent behavioral effects from acute exposures. This is particularly important considering that this study used dechorionated embryos, thus maximizing exposure to toxic compounds as these embryos lack the protective outer membrane that fish naturally have during early development.Most of the effluents of the mid treatment processes did not have concentrations of pharmaceuticals high enough to impact the swimming ability of zebrafish larvae.22 However, despite a large margin of error, we found that some of the mid treatment process wastewater samples did cause a significant change in the visual motor response. WWTP 4 primary effluent and WWTP 6 primary influent caused transient effects in zebrafish locomotion, with significant hyperactivity observed in only the pre-stimulus and post-stimulus periods, respectively (Fig. 12). This is not entirely surprising as WWTP 4 primary effluent and WWTP 6 primary influent had some of the highest concentrations of total analytes measured in this study (Table S4B†). Furthermore, WWTP 6 influent had the highest total concentration of psychiatric drugs (3847 ng L−1), which are specifically designed to affect neurological systems, which previous studies have shown can influence fish swimming behavior.15,22,52
Additionally, WWTP 3 flocculation–sedimentation effluent caused a consistent hyperactive response in all three stages of the VMR behavior (Fig. 11). Ozonation effluent from WWTP 3 also caused significant hyperactivity in comparison to control fish, however only in the acclimation phase. The elevated response in WWTP 3 flocculation–sedimentation effluent is likely due to the chemical addition applied immediately upstream of the sampling location (i.e. pre-formed monochloramine at a dosage of 5 mg Cl2 L−1) to mitigate formation of bromate from background bromide during immediately downstream ozonation. The addition of monochloramine may have contributed to the behavioral changes, due to the possible transformation of some of the pharmaceuticals or other nitrogen containing organic matter into potentially toxic by-products.53
WWTP 3 flocculation–sedimentation effluent was found to have a lower concentration of nearly all measured analytes compared to secondary effluent, which did not cause a significant change in fish swimming behavior. Additionally, the ozonation effluent has no detectable levels of all but two of the target analytes. However, residues of the toxic by-products from the addition of monochloramine from the flocculation–sedimentation may have contributed to the changes in swimming behavior since the samples that were obtained post-ozonation were not yet quenched. Also, ozonation introduces new compounds, such as degradation by-products of either specific organic compounds of interest or natural organic matter, that are not measured but are potentially toxic and at high enough concentrations to cause biological effects.54,55 For example, low molecular weight aldehydes such as formaldehyde, acetaldehyde, and chloroacetaldehyde, produced during ozonation of natural water, have been demonstrated to be toxic to different systems such as bacteria, fungi, mammalian cells and rodents.56–58 In the ozone-biofiltration process, biofiltration downstream of ozonation is relied upon for the removal of these breakdown products (thus achieving significant removal of these pharmaceuticals and total organic carbon).59 It is nevertheless important for us to study these intermediate processes in more detail to understand what other intermediate products that may be produced, as well as the potential for their release into the environment.
Due to the complex and temporally variable composition of wastewater effluent, studies of biological effects of wastewater effluent exposure often have varying and conflicting results. Nevertheless our results reported here are consistent with several previous studies. Larval fathead minnows exposed to wastewater effluent samples showed no changes in predator escape behaviors despite changes in larval growth.14 Similarly, round gobies caged within a wastewater treatment plant had significant decreases in survival but no significant changes in aggression, startle response, or spontaneous swimming activities.16 The high variation in spontaneous swimming behavior observed here is not uncommon as other similar studies have noted a wide variance in spontaneous swimming behaviors in both baseline control and toxicant exposure scenarios.13,16,60,61 Additionally, other studies have found that individual factors like sex or parental lineage can greatly influence their response to toxicant exposures and can contribute significantly to inter-individual variability in behavioral endpoints.14,60 Given that we cannot control for many of these factors (e.g. zebrafish larvae cannot be sexed at 6 dpf), we are forced to include multiple sources of variation that could have been controlled for in studies using older test subjects.
The short-term laboratory assay used in this study was conducted to provide a consistent method to rapidly screen effluents among these different plants and the individual treatment processes they employed. Despite the lack of effect with exposure to final effluents, we cannot confidently conclude that there is no potential adverse effect of exposure to these complex mixtures for wild fish populations. Individual behaviors represent the manifestation of signals, stimuli, and inputs from a variety of physiological systems and it is entirely possible that other important behaviors, or other biological endpoints, may be significantly impacted even when spontaneous swimming is not.12 Additionally, field experiments paired with laboratory behavioral assays have shown that in situ conditions can lead to significant changes in fish behavior despite having no significant differences in laboratory experiments, further suggesting that differences in the sensitivities of behavioral endpoints are a major contributor to the variation in effects observed over many different studies.62 Another major limitation of many biological assays is the timeframe considered. Most studies use relatively short acute exposure scenarios due to practical demands, however long-term impacts from sublethal exposure are rarely studied and represent a large blind spot in our understanding of ecological risk of emerging contaminants. Thus, short-term experiments like the ones conducted in this study should be considered only a first step in assessing biological impacts of wastewater effluents. Further studies, particularly those that use a variety of behavioral endpoints, longer exposure periods and additional species, will be required to fully understand the potential impacts these pharmaceuticals may have on the aquatic ecosystem.
Conclusion
This study provides an analysis of the effectiveness of a number of conventional and more advanced treatment methods for removal of pharmaceuticals from municipal WWTPs, including a highly advanced pilot plant. The results of this study can help inform practitioners about selection and design of unit processes with higher potential for pharmaceutical removal via biological and/or chemical pathways. Primary treatment did not provide significant reductions in the pharmaceutical concentrations, while secondary and tertiary processes resulted in significant removal of several of the pharmaceuticals monitored. It was found that GAC in WWTP 1 removed 14 out of 15 compounds with >95% efficiency, while GAC in WWTP 3 removed 5 out of 7 compounds with >95% efficiency, indicating the effectiveness of GAC in removing pharmaceuticals from WWTPs. Ozonation also provided excellent removal for 9 out of 11 compounds at >95% efficiency. Despite the efficiency of ozonation to remove the pharmaceuticals analyzed here, removal may not necessarily mean complete degradation, since oxidation processes are known to form transformation products. WWTP 3, which was the highly advanced treatment plant having four tertiary treatment stages, was found to be the most efficient in removing pharmaceuticals, with all compounds having an overall % removal ranging from 97% to 100%. The behavioral effects seen in larval zebrafish exposed to ozonation effluent and some other intermediate process samples is consistent with the formation of toxic transformation products which should be further evaluated. While 3 out of 7 plants had early or intermediate process samples that caused behavioral effects in zebrafish, none of the final effluents caused behavioral effects, suggesting that these treatment processes are sufficient to prevent short-term biological effects. Long-term chronic toxicity studies still need to be conducted to assess the impact of constant, sublethal exposures to these pharmaceuticals.Funding
This work was supported by the New York Sea Grant Project (Project no. 1139881 (McElroy) and 1130883 (Aga) under Award no. R/CTP-54).Ethical statement
All animal procedures were performed in accordance with the Guidelines for the Care and Use of Laboratory Animals of Stony Brook University and are approved by their Institutional Animal Care and Use Committee (IACUC) under protocol 269492-15 to Howard I. Sirotkin.Conflicts of interest
There are no conflicts of interest to declare.References
- Z. Li, et al., High-throughput evaluation of organic contaminant removal efficiency in a wastewater treatment plant using direct injection UHPLC-Orbitrap-MS/MS, Environ. Sci.: Processes Impacts, 2018, 20(3), 561–571 RSC.
- B. Subedi and K. Kannan, Occurrence and fate of select psychoactive pharmaceuticals and antihypertensives in two wastewater treatment plants in New York State, USA, Sci. Total Environ., 2015, 514, 273–280 CrossRef CAS.
- V. L. Borova, et al., Highly sensitive determination of 68 psychoactive pharmaceuticals, illicit drugs, and related human metabolites in wastewater by liquid chromatography–tandem mass spectrometry, Anal. Bioanal. Chem., 2014, 406(17), 4273–4285 CrossRef CAS.
- E. N. Evgenidou, I. K. Konstantinou and D. A. Lambropoulou, Occurrence and removal of transformation products of PPCPs and illicit drugs in wastewaters: A review, Sci. Total Environ., 2015, 505, 905–926 CrossRef CAS.
- D. W. Kolpin, et al., Pharmaceuticals, Hormones, and Other Organic Wastewater Contaminants in U.S. Streams, 1999−2000: A National Reconnaissance, Environ. Sci. Technol., 2002, 36(6), 1202–1211 CrossRef CAS.
- E. Gracia-Lor, et al., Occurrence and removal of pharmaceuticals in wastewater treatment plants at the Spanish Mediterranean area of Valencia, Chemosphere, 2012, 87(5), 453–462 CrossRef CAS.
- M. S. Kostich, A. L. Batt and J. M. Lazorchak, Concentrations of prioritized pharmaceuticals in effluents from 50 large wastewater treatment plants in the US and implications for risk estimation, Environ. Pollut., 2014, 184, 354–359 CrossRef CAS.
- A. Puckowski, et al., Bioaccumulation and analytics of pharmaceutical residues in the environment: A review, J. Pharm. Biomed. Anal., 2016, 127, 232–255 CrossRef CAS.
- P. Arnnok, et al., Selective Uptake and Bioaccumulation of Antidepressants in Fish from Effluent-Impacted Niagara River, Environ. Sci. Technol., 2017, 51(18), 10652–10662 CrossRef CAS.
- C. Prasse, et al., Oxidation of the Antiviral Drug Acyclovir and Its Biodegradation Product Carboxy-acyclovir with Ozone: Kinetics and Identification of Oxidation Products, Environ. Sci. Technol., 2012, 46(4), 2169–2178 CrossRef CAS.
- L. B. Stadler, et al., Micropollutant Fate in Wastewater Treatment: Redefining “Removal”, Environ. Sci. Technol., 2012, 46(19), 10485–10486 CrossRef CAS.
- G. R. Scott and K. A. Sloman, The effects of environmental pollutants on complex fish behaviour: integrating behavioural and physiological indicators of toxicity, Aquat. Toxicol., 2004, 68(4), 369–392 CrossRef CAS.
- S. D. Melvin, Short-term exposure to municipal wastewater influences energy, growth, and swimming performance in juvenile Empire Gudgeons (Hypseleotris compressa), Aquat. Toxicol., 2016, 170, 271–278 CrossRef CAS.
- H. L. Schoenfuss, et al., Complex mixtures, complex responses: Assessing pharmaceutical mixtures using field and laboratory approaches, Environ. Toxicol. Chem., 2016, 35(4), 953–965 CrossRef CAS.
- E. S. McCallum, et al., Exposure to wastewater effluent affects fish behaviour and tissue-specific uptake of pharmaceuticals, Sci. Total Environ., 2017, 605–606, 578–588 CrossRef CAS.
- E. S. McCallum, et al., In situ exposure to wastewater effluent reduces survival but has little effect on the behaviour or physiology of an invasive Great Lakes fish, Aquat. Toxicol., 2017, 184, 37–48 CrossRef CAS.
- C. E. Burton, et al., Spectral properties of the zebrafish visual motor response, Neurosci. Lett., 2017, 646, 62–67 CrossRef CAS.
- P. J. Vikesland, et al., Toward a Comprehensive Strategy to Mitigate Dissemination of Environmental Sources of Antibiotic Resistance, Environ. Sci. Technol., 2017, 51(22), 13061–13069 CrossRef CAS.
- National Pollutant Discharge Elimination System (NPDES) Permit Limits, United States Environmental Protection Agency, 2016 Search PubMed.
- R. R. Singh, et al., Towards a harmonized method for the global reconnaissance of multi-class antimicrobials and other pharmaceuticals in wastewater and receiving surface waters, Environ. Int., 2019, 124, 361–369 CrossRef CAS.
- L. F. Angeles and D. S. Aga, Establishing Analytical Performance Criteria for the Global Reconnaissance of Antibiotics and Other Pharmaceutical Residues in the Aquatic Environment Using Liquid Chromatography-Tandem Mass Spectrometry, J. Anal. Methods Chem., 2018, 2018, 9 Search PubMed.
- I. J. Huang, H. I. Sirotkin and A. E. McElroy, Varying the exposure period and duration of neuroactive pharmaceuticals and their metabolites modulates effects on the visual motor response in zebrafish (Danio rerio) larvae, Neurotoxicol. Teratol., 2019, 72, 39–48 CrossRef CAS.
- Y. Gao, et al., A High-Throughput Zebrafish Screening Method for Visual Mutants by Light-Induced Locomotor Response, IEEE/ACM Trans. Comput. Biol. Bioinf., 2014, 11(4), 693–701 Search PubMed.
- J. Radjenović, M. Petrović and D. Barceló, Fate and distribution of pharmaceuticals in wastewater and sewage sludge of the conventional activated sludge (CAS) and advanced membrane bioreactor (MBR) treatment, Water Res., 2009, 43(3), 831–841 CrossRef.
- A. Göbel, et al., Fate of sulfonamides, macrolides, and trimethoprim in different wastewater treatment technologies, Sci. Total Environ., 2007, 372(2), 361–371 CrossRef.
- M. Gros, S. Rodríguez-Mozaz and D. Barceló, Rapid analysis of multiclass antibiotic residues and some of their metabolites in hospital, urban wastewater and river water by ultra-high-performance liquid chromatography coupled to quadrupole-linear ion trap tandem mass spectrometry, J. Chromatogr. A, 2013, 1292, 173–188 CrossRef CAS.
- T. aus der Beek, et al., Pharmaceuticals in the environment—Global occurrences and perspectives, Environ. Toxicol. Chem., 2016, 35(4), 823–835 CrossRef CAS.
- K. Grabicova, et al., Presence of pharmaceuticals in benthic fauna living in a small stream affected by effluent from a municipal sewage treatment plant, Water Res., 2015, 72, 145–153 CrossRef CAS.
- T.-M. Scott, et al., Pharmaceutical manufacturing facility discharges can substantially increase the pharmaceutical load to U.S. wastewaters, Sci. Total Environ., 2018, 636, 69–79 CrossRef CAS.
- M. M. Huber, et al., Oxidation of Pharmaceuticals during Ozonation and Advanced Oxidation Processes, Environ. Sci. Technol., 2003, 37(5), 1016–1024 CrossRef CAS PubMed.
- J. Hollender, et al., Elimination of Organic Micropollutants in a Municipal Wastewater Treatment Plant Upgraded with a Full-Scale Post-Ozonation Followed by Sand Filtration, Environ. Sci. Technol., 2009, 43(20), 7862–7869 CrossRef CAS.
- V. Kårelid, G. Larsson and B. Björlenius, Pilot-scale removal of pharmaceuticals in municipal wastewater: Comparison of granular and powdered activated carbon treatment at three wastewater treatment plants, J. Environ. Manage., 2017, 193, 491–502 CrossRef PubMed.
- R. Mailler, et al., Study of a large scale powdered activated carbon pilot: Removals of a wide range of emerging and priority micropollutants from wastewater treatment plant effluents, Water Res., 2015, 72, 315–330 CrossRef CAS.
- J. Margot, et al., Treatment of micropollutants in municipal wastewater: Ozone or powdered activated carbon?, Sci. Total Environ., 2013, 461–462, 480–498 CrossRef CAS.
- F. Meinel, et al., Pilot-Scale Investigation of Micropollutant Removal with Granular and Powdered Activated Carbon, Water, Air, Soil Pollut., 2014, 226(1), 2260 CrossRef.
- J. Yu, et al., Effect of effluent organic matter on the adsorption of perfluorinated compounds onto activated carbon, J. Hazard. Mater., 2012, 225-226, 99–106 CrossRef CAS.
- G. Newcombe, Activated Carbon and Soluble Humic Substances: Adsorption, Desorption, and Surface Charge Effects, J. Colloid Interface Sci., 1994, 164(2), 452–462 CrossRef CAS.
- D. J. de Ridder, et al., Modeling equilibrium adsorption of organic micropollutants onto activated carbon, Water Res., 2010, 44(10), 3077–3086 CrossRef CAS.
- N. H. Tran, et al., Occurrence and removal of multiple classes of antibiotics and antimicrobial agents in biological wastewater treatment processes, Water Res., 2016, 104, 461–472 CrossRef CAS.
- X.-S. Miao, J.-J. Yang and C. D. Metcalfe, Carbamazepine and Its Metabolites in Wastewater and in Biosolids in a Municipal Wastewater Treatment Plant, Environ. Sci. Technol., 2005, 39(19), 7469–7475 CrossRef CAS PubMed.
- L. B. Stadler, et al., Effect of redox conditions on pharmaceutical loss during biological wastewater treatment using sequencing batch reactors, J. Hazard. Mater., 2015, 282, 106–115 CrossRef CAS PubMed.
- O. T. Iorhemen, R. A. Hamza and J. H. Tay, Membrane Bioreactor (MBR) Technology for Wastewater Treatment and Reclamation: Membrane Fouling, Membranes, 2016, 6(2), 33 CrossRef.
- EPA, U, Wastewater Technology Fact Sheet: Sequencing Batch Reactors, U.E.P. Agency, Editor, 1999 Search PubMed.
- J. Reungoat, et al., Removal of micropollutants and reduction of biological activity in a full scale reclamation plant using ozonation and activated carbon filtration, Water Res., 2010, 44(2), 625–637 CrossRef CAS.
- Y. Lee and U. von Gunten, Quantitative structure–activity relationships (QSARs) for the transformation of organic micropollutants during oxidative water treatment, Water Res., 2012, 46(19), 6177–6195 CrossRef CAS.
- M. C. Dodd, M.-O. Buffle and U. von Gunten, Oxidation of Antibacterial Molecules by Aqueous Ozone: Moiety-Specific Reaction Kinetics and Application to Ozone-Based Wastewater Treatment, Environ. Sci. Technol., 2006, 40(6), 1969–1977 CrossRef CAS PubMed.
- F. J. Real, et al., Kinetics of the Chemical Oxidation of the Pharmaceuticals Primidone, Ketoprofen, and Diatrizoate in Ultrapure and Natural Waters, Ind. Eng. Chem. Res., 2009, 48(7), 3380–3388 CrossRef CAS.
- M. M. Huber, et al., Oxidation of Pharmaceuticals during Ozonation of Municipal Wastewater Effluents: A Pilot Study, Environ. Sci. Technol., 2005, 39(11), 4290–4299 CrossRef CAS PubMed.
- K. Maskaoui, A. Hibberd and J. L. Zhou, Assessment of the Interaction between Aquatic Colloids and Pharmaceuticals Facilitated by Cross-Flow Ultrafiltration, Environ. Sci. Technol., 2007, 41(23), 8038–8043 CrossRef CAS PubMed.
- U. Ghosh, et al., Granular Activated Carbon and Biological Activated Carbon Treatment of Dissolved and Sorbed Polychlorinated Biphenyls, Water Environ. Res., 1999, 71(2), 232–240 CrossRef CAS.
- J. Reungoat, et al., Ozonation and biological activated carbon filtration of wastewater treatment plant effluents, Water Res., 2012, 46(3), 863–872 CrossRef CAS.
- S. V. Nielsen, et al., The psychoactive drug Escitalopram affects swimming behaviour and increases boldness in zebrafish (Danio rerio), Ecotoxicology, 2018, 27(4), 485–497 CrossRef CAS.
- C. M. M. Bougeard, et al., Comparison of the disinfection by-product formation potential of treated waters exposed to chlorine and monochloramine, Water Res., 2010, 44(3), 729–740 CrossRef CAS.
- A. Lajeunesse, et al., Ozone oxidation of antidepressants in wastewater -Treatment evaluation and characterization of new by-products by LC-QToFMS, Chem. Cent. J., 2013, 7, 15 CrossRef CAS.
- D. Stalter, et al., Toxication or detoxication? In vivo toxicity assessment of ozonation as advanced wastewater treatment with the rainbow trout, Water Res., 2010, 44(2), 439–448 CrossRef CAS PubMed.
- S. D. Richardson, et al., Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection by-products in drinking water: A review and roadmap for research, Mutat. Res., 2007, 636(1), 178–242 CAS.
- J. Nawrocki, et al., Influence of Ozonation Conditions on Aldehyde and Carboxylic Acid Formation, Ozone: Sci. Eng., 2003, 25(1), 53–62 CrossRef CAS.
- A. Dąbrowska, J. Nawrocki and E. Szeląg-Wasielewska, Appearance of aldehydes in the surface layer of lake waters, Environ. Monit. Assess., 2014, 186(7), 4569–4580 CrossRef.
- M. Bourgin, et al., Evaluation of a full-scale wastewater, treatment plant upgraded with ozonation and biological post-treatments: Abatement of micropollutants, formation of transformation products and oxidation by-products, Water Res., 2018, 129, 486–498 CrossRef CAS.
- H. Mehdi, et al., Impacts of wastewater treatment plant effluent on energetics and stress response of rainbow darter (Etheostoma caeruleum) in the Grand River watershed, Comp. Biochem. Physiol. B, Biochem. Mol. Biol., 2018, 224, 270–279 CrossRef CAS PubMed.
- T.-H. Chen, et al., Endocrine disrupting effects of domestic wastewater on reproduction, sexual behavior, and gene expression in the brackish medaka Oryzias melastigma, Chemosphere, 2016, 150, 566–575 CrossRef CAS PubMed.
- E. S. McCallum, et al., Investigating tissue bioconcentration and the behavioural effects of two pharmaceutical pollutants on sea trout (Salmo trutta) in the laboratory and field, Aquat. Toxicol., 2019, 207, 170–178 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ew00559e |
| This journal is © The Royal Society of Chemistry 2020 |