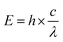The molecular design of and challenges relating to sensitizers for cancer sonodynamic therapy
Yiming
Zhou
,
Mengxuan
Wang
and
Zhifei
Dai
 *
*
Department of Biomedical Engineering, College of Engineering, Peking University, Beijing 100871, China. E-mail: zhifei.dai@pku.edu.cn
First published on 16th June 2020
Abstract
Sonodynamic therapy is a promising non-invasive treatment approach against malignant tumors. It is believed that sonodynamic therapy is advantageous over conventional photodynamic therapy due to its better penetration abilities. However, the efficacy of sonodynamic therapy is limited by the poor reactive oxygen species generation abilities of current sonosensitizers. To fulfill the unreleased potential of sonodynamic therapy, it is reasonable to optimize the properties of the sonosensitizers according to the basic mechanism of sonodynamic therapy. Here, in this review, the most recent research progress relating to the mechanism of sonodynamic therapy will be emphasized and a series of possible principles for the design of effective sonosensitizers will be proposed. Further challenges relating to the clinical translation of sonodynamic therapy will also be discussed to give a clear picture of the problems that must be overcome.
Introduction
Clinical cancer treatment modalities including surgery, chemotherapy and radiotherapy are invasive, non-specific or use radiation.1–5 To increase therapeutic efficacy and reduce adverse effects, non-invasive therapy6 has become an optional choice in treating patients with mild cases or advanced cases that other therapies have failed to treat. Sonodynamic therapy (SDT) has become an emerging non-invasive therapeutic modality for tumor therapy in recent years.7 Similar to the clinically used photodynamic therapy (PDT), sonodynamic therapy applies low intensity focused ultrasound (LIFU) to excite sonosensitizers to generate reactive oxygen species (ROS), which are toxic to tumor cells.8 SDT is advantageous over PDT in several aspects.The efficacy of PDT is hindered by the weak penetration ability of visible light, meaning that only tumor cells receiving light will be damaged whilst others will not be ablated.9 In spite of the development of photosensitizers (PSs) with longer excitation wavelengths,10 near-infrared light can only reach the tissue within several centimeters below the skin.11 As a result, it is not certain whether the light could fully cover the primary tumor or even reach superficial metastases during treatment. Besides, PDT has limitations treating deep tumors such as hepatic carcinoma, renal carcinoma and glioma12 non-invasively. Another “Achilles' heel” of PDT is the phototoxicity of photosensitizers, which is attributed to the systematic distribution of PSs.13 Patients have to avoid direct exposure to light both before and after treatment as visible light could damage healthy skin containing accumulated photosensitizers.14 However, as a routinely applied imaging modality in clinic, ultrasound imaging is capable of monitoring every parenchymal organ.15–19 The penetration ability of ultrasound can be tuned by the modulation of ultrasound frequency and the intensity can be simply regulated with an amplifier.20–22 Therefore, SDT can be applied in the treatment of any kind of solid tumor with distinctive depths non-invasively under proper imaging in theory. Unlike PSs, sonosensitizers with high sonosensitivity and low photosensitivity can be rationally designed to reduce their photoactivity.23 Above all, SDT is a promising non-invasive therapy against tumors, which could be compatible with PDT in the future.
To distinguish SDT from traditional thermal-dependent ultrasound therapy (i.e. high intensity focused ultrasound (HIFU)),24 sonosensitizers are an indispensable component of SDT but not of HIFU. The targeted accumulation of sonosensitizers in the diseased area is the first step of SDT and the therapeutic effect is predominantly determined by the ROS instead of heat. Sonosensitizers can be defined as molecules that absorb ultrasound energy and excite surrounding oxygen molecules or other molecule substrates to release ROS. The earliest sonosensitizer to be investigated was hematoporphyrin by Yumita et al. in 1989.25 For the first time they demonstrated that the combination of ultrasound and drug therapy could cause irreversible damage to tumor cells while neither of them decreased the cell viability alone. Sonosensitizers can be chemotherapeutic drugs and the ROS triggered by ultrasound can further enhance the chemotherapy effect. The antitumor drug doxorubicin is a well-known example.26,27 However, the majority of sonosensitizers have been derived from photosensitizers used for PDT so far.28 Adopting clinically approved photosensitizers can be a double-edged sword for SDT. The drugs involved are more likely to be approved but the defects of the PSs have to be tolerated and they might not be as effective as we thought. To optimize the sonosensitizers used in SDT, it is reasonable to de novo design a sonosensitizer according to the mechanism of SDT as well as taking the pharmacokinetics, biodistribution and toxicity into consideration. Ideally, a good sonosensitizer should (i) be of high sonosensitivity, (ii) be non-toxic in the absence of ultrasound, (iii) specifically accumulate in the tumor site and (iv) be excreted from the body within a short period. Nevertheless, few reviews have extensively discussed the design of sonosensitizers as far as we know.
In this review, we will discuss the principles of the molecular design of sonosensitizers on the basis of the mechanism of sonodynamic therapy. By analyzing recent newly developed sonosensitizers, we outlined the major design considerations of sonosensitizers. At last, the challenges in the clinical translation of SDT will be addressed to point out a pathway that might lead to the successful clinical translation of SDT.
The mechanism of SDT
Since the cytotoxicity of SDT was discovered, lots of researchers devoted their work to reveal the mechanism behind it.8 However, subject to the accuracy and detecting ability of the equipment, part of the proposed mechanism still cannot be verified. Insight into the theory of SDT could provide great help in designing on-demand sonosensitizers. Unlike the wave-particle duality nature of light,29 ultrasound is a kind of mechanical wave.30 As a result, the difference in the energy transfer between molecules and stimulators will be discussed first of all. On the other hand, the unique effects (including cavitation and sonoluminescence) caused by ultrasound should not be ignored.To understand how a sensitizer is triggered by ultrasound, we shall first review how the energy of light is transferred to a photosensitizer during PDT (Fig. 1).9 The energy (E) of a photon can be calculated using Planck's constant (h). The following equation gives the relationship between E and the light wavelength λ, where c represents the speed of light.31
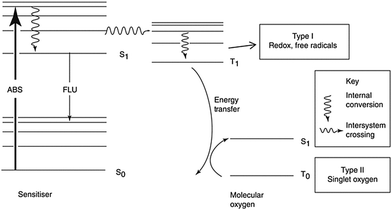 | ||
| Fig. 1 The mechanism of photodynamic therapy. Reproduced with permission ref. 8. | ||
Ultrasound is a kind of mechanical wave with a frequency higher than 20 kHz. To fully describe the properties of ultrasound, acoustic pressure (p) and acoustic intensity (I) should also be included. The relationship between them is listed as the following equation:
| p2 = I × v × ρ |
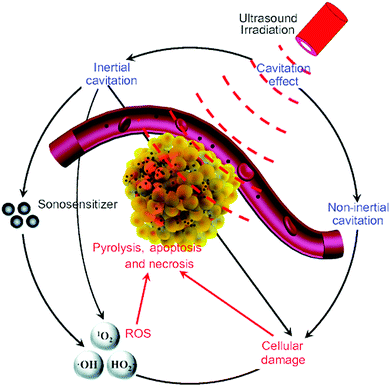 | ||
| Fig. 2 The possible cytotoxic mechanism of sonodynamic therapy. Reproduced with permission ref. 37. | ||
Notably, growing evidence has shown that the emitted light from the non-inertial cavitating bubbles might contribute to the singlet oxygen generation in that the tested sonosensitizers were also photosensitizers.38 Here we can point out the relatively exact mechanism of SDT from the known evidence that is when the acoustic intensity of ultrasound is low, sonoluminescence is the key reason for singlet oxygen generation, while when the acoustic intensity is high, hydroxyl radicals are generated as the result of inertial cavitation. Meanwhile, the antiangiogenesis effect of ultrasound and immunity activation induced by the exposure of antigens on the dead tumor cells also contributes to the mechanism of SDT. Table 1 gives a brief summary of the similarities and differences between the PDT and SDT.
Sonosensitizer design principles
Sonosensitizers can be classified into small molecules and micro/nanoparticles.40 Most of the small molecules are existing photosensitizers or approved drugs. Though plenty of published papers exhibited encouraging results about the so-called multifunctional nanoparticles in SDT, few could provide useful insights into the design of sonosensitizers that are promising for clinical use. Here we try to propose a series of design principles of sonosensitizers on the basis of the fundamental principles of drug design and SDT.ROS generation ability
The ROS generation ability is highly associated with the inherent properties of molecules. In PDT, a concept named singlet oxygen quantum yield (Φ) is introduced to describe the ability of PSs to generate ROS.41 A standard substance with known Φ is used as a reference42 to calculate the Φ of the measured molecule. Different probes are utilized to quantify and distinguish singlet oxygen and OH hydroxyl radicals during the PDT/SDT. Commonly, researchers use the SOSG to semi-quantify the generation of singlet oxygen43 and H2DCF to semi-quantify the general ROS through fluorescence.44Φ is associated with the chemical structure of the sensitizers. Taking the porphyrin class sensitizer as an example, the Φ can be affected by the center atom chelated into the porphyrin ring.45 Usually the element with more than two coordination keys will decrease the Φ as the ligand might block the interaction between the oxygen molecule and the plane of the porphyrin ring.46 A similar principle can be applied to the sonosensitizer because oxygen is indispensable during both PDT and SDT.Besides, to precisely calculate the energy gap of a molecule, the HOMO–LUMO theory has to be introduced to describe the lowest energy needed to excite the molecule to an activated status.47 The lower the value is, the easier it is for the molecule to be excited by external energy. In a recent research report, Ma et al. synthesized a series of metalloporphyrins chelated with different metal elements.48 They firstly calculated the HOMO–LUMO plots of MnTTP, TiOTTP and ZnTTP by density functional theory (Fig. 3). Consistent with the simulation results, MnTTP exhibited the highest ROS generation ability and cytotoxicity in vitro in that it had the lowest excited energy. The MnTTP–HSA complex showed excellent in vivo antitumor effects as well.
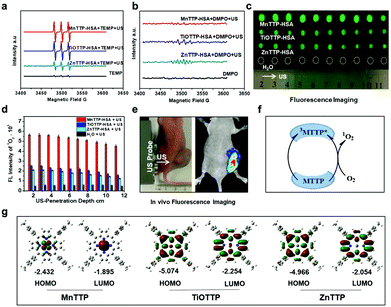 | ||
| Fig. 3 The in vitro ROS generation performances of MnTTP, TiOTTP and ZnTTP (a–d). (e) In vivo ROS fluorescent imaging after ultrasound treatment. (f) A schematic diagram elucidating the mechanism of singlet oxygen generation. (g) HOMO–LUMO plots of these three porphyrins. Reproduced with permission ref. 48. | ||
Another important factor affecting the Φ is the lipid–water partition coefficient (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D), which quantifies the hydrophilicity and hydrophobicity of a molecule.49 Molecules with higher hydrophobicity obtain a larger score. Most naturally existing photosensitizers are very hydrophobic, leading to severe aggregation in the body fluid.50 However, the aggregation state can largely decrease the ROS generated by the sensitizers.51 Introducing polar groups into the molecules can increase the hydrophilicity of the sensitizers preventing them from forming precipitation in solution52 but the aromatic rings of porphyrins or phthalocyanines tend to aggregate, forming the H-aggregates.53 The absorbance peak of the H-aggregates is blue-shifted and the fluorescence is usually quenched.54,55 Axial modification is an efficient way to avoid H-aggregates56 while the synthesis is complex and not all the sensitizers can be modified. Encapsulating the sonosensitizers into the nanoparticles can increase the solubility of the hydrophobic molecules.57 Nevertheless, the aggregation of sonosensitizers in the nanoparticles58 and the potential premature release of the inside drug59 indicate the inherent shortcomings of the nanomedicine. To this end, covalently conjugating the drug onto the nanoparticle surface60 or inside the mesoporous nanoparticle is a promising way to solve these problems. Anchoring the sonosensitizer molecules onto silica nanoparticles reduced the undesired aggregation and enhanced the therapeutic effect.61 The chelated manganese in the porphyrins made the magnetic resonance imaging guided sonodynamic therapy possible. In spite of the extensive evidence showing that mesoporous silica nanoparticles are able to be degraded and excreted from the body within several days,62 the safety concern of these inorganic nanoparticles could be the biggest obstacles hindering them from being translated into clinical use. (Fig. 4).
D), which quantifies the hydrophilicity and hydrophobicity of a molecule.49 Molecules with higher hydrophobicity obtain a larger score. Most naturally existing photosensitizers are very hydrophobic, leading to severe aggregation in the body fluid.50 However, the aggregation state can largely decrease the ROS generated by the sensitizers.51 Introducing polar groups into the molecules can increase the hydrophilicity of the sensitizers preventing them from forming precipitation in solution52 but the aromatic rings of porphyrins or phthalocyanines tend to aggregate, forming the H-aggregates.53 The absorbance peak of the H-aggregates is blue-shifted and the fluorescence is usually quenched.54,55 Axial modification is an efficient way to avoid H-aggregates56 while the synthesis is complex and not all the sensitizers can be modified. Encapsulating the sonosensitizers into the nanoparticles can increase the solubility of the hydrophobic molecules.57 Nevertheless, the aggregation of sonosensitizers in the nanoparticles58 and the potential premature release of the inside drug59 indicate the inherent shortcomings of the nanomedicine. To this end, covalently conjugating the drug onto the nanoparticle surface60 or inside the mesoporous nanoparticle is a promising way to solve these problems. Anchoring the sonosensitizer molecules onto silica nanoparticles reduced the undesired aggregation and enhanced the therapeutic effect.61 The chelated manganese in the porphyrins made the magnetic resonance imaging guided sonodynamic therapy possible. In spite of the extensive evidence showing that mesoporous silica nanoparticles are able to be degraded and excreted from the body within several days,62 the safety concern of these inorganic nanoparticles could be the biggest obstacles hindering them from being translated into clinical use. (Fig. 4).
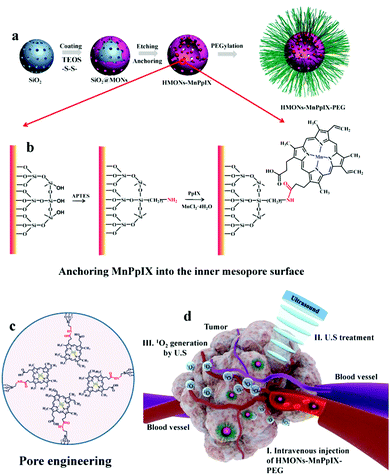 | ||
| Fig. 4 A schematic diagram of how MnPpIX was covalently conjugated onto mesopore silica nanoparticles and the in vivo antitumor effect of SDT. Reproduced with permission ref. 61. | ||
When the yield of 1O2 is limited in the SDT, increasing the hydroxyl radicals can also enhance the SDT effect. Pan and colleagues demonstrated that a metal–organic framework (MOF)-derived carbon nanostructure with a porphyrin-like center had high ROS generation ability under the exposure of ultrasound.63 The ESR results showed that the significant difference in the ROS generation was attributed to the outstanding hydroxyl radicals’ yield (Fig. 5).
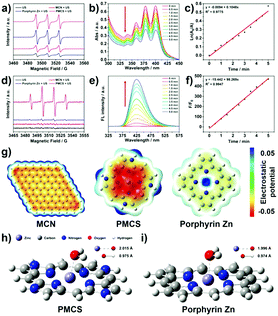 | ||
| Fig. 5 In vitro ROS generation abilities of PMCS upon exposure to ultrasound (a–f). (g) The electrostatic potential profiles of different molecules. (h and i) Molecular models of adsorbed H2O on PMCS and porphyrin Zn. Reproduced with permission ref. 63. | ||
The addition of the external cavitation nucleus is a potential way to enhance the SDT effect by increasing the cavitation effect. TiO2 nanoparticles were found to have an effective ultrasound triggered response.64 Upon the addition of gold nanoparticles as the cavitation nucleus, the ROS was significantly enhanced when using DPBF as the detection probe.65
Sonosensitizer targeting ability
Since the term “magic bullet” was proposed more than 100 years ago,66 much effort has been devoted to realizing accurate drug delivery to a diseased area.67 The theory includes two aspects, one is to enhance drug accumulation in the lesion and the other is to reduce unwanted nonspecific distribution of the drugs. Attaching a targeting ligand to the drug is believed to be an effective strategy68 and the exciting pre-clinical results of nanomedicine indicate its great potential for precise drug delivery.69 However, only several antibody–drug conjugates (ADCs) have been successfully translated into clinic70 and the promise of nanomedicine is discounted due to its poor clinical outcomes.71 Although sonosensitizers are a little bit different from traditional chemo drugs or targeted drugs owing to their mechanism, sonosensitizers with a better targeting ability contribute to enhancement of the SDT effect.Because of the photoactive nature of most sonosensitizers, the fundamental way to protect patients from side effects and to enhance the therapeutic effect is to avoid unwanted accumulation in other organs. Except for a few small molecules with an inherent targeting ability for tumors,72 most porphyrin- or cyanine-based sonosensitizers are not specifically absorbed by tumor cells.73 Modifying the small molecules with an antibody or small peptide which can target the receptors overexpressed on the tumor cell surface is a well-adopted strategy.74 Many antibodies including bevacizumab, cetuximab and panitumumab that have proved effective in patients are being conjugated with fluorescent dyes and are under clinical trial for imaging guided surgery.74 Moreover, the immunophototherapy effects of cetuximab–IRdye700DX conjugate (ASP-1929) has been tested in patients with head and neck cancer, colon cancer, lung cancer and so on.75 The conjugates exhibited long circulation times and enhanced uptake by tumors but their penetration into deep tumor cells was inhibited because of the large volume.76 Nanobodies are believed to have a better penetration ability because they have a smaller volume as well as compatible targeting ability with the traditional antibody.77 However, when it comes to the cost of development, small targeting peptides or small molecules are more accepted than the antibody. Cyclic RGD is a well exploited peptide with great affinity for the integrins on the surface of the neovascularization of tumor cells.78 Peptides targeting c-Met have been conjugated with cy5 for the detection of colon polyps and cancer.79 Besides, plenty of folate acid conjugated drug/fluorescent dyes have entered clinical trials for the treatment of ovarian cancer, lung cancer and other folate acid receptor overexpressed tumors.80 Besides the targeted small molecules, nanoparticles with active targeting ligands are being extensively explored.81 The commonly believed hypothesis was that targeted nanoparticles could directly bind tumor cells, however recent research has overthrown it.82 Upon entry of nanoparticles into the blood vessels, the proteins adsorbed determines their final fate.83 As a result, the exact mechanism of the so called “active targeting” of nanoparticles is still under debate and more research is needed to further elucidate it. The achievement of the pre-clinical results of the targeted nanoparticles cannot guarantee its further success. Based on the enormous efforts toward the development of ADCs and peptide–drug conjugates, we can learn lessons from them to increase the targeting ability of sonosensitizers.
The ideal SDT requires selective control of the location of sonosensitizers and ROS generation. A smart off–on mode of sonosensitizers is crucial for the enhancement of SDT. Precise design for the control of photosensitizers for PDT has been summarized somewhere else.84 These strategies are helpful for reducing undesired phototoxicity but might not be valuable for the enhancement of SDT effects. Few papers explored the relationship between the molecule status and the corresponding sonosensitivity, so it is still unknown to us now whether ROS generation could be tuned with the molecular status change of the sonosensitizers. A deeper understanding of the mechanism of SDT will help us design smarter sonosensitizers.
Oxygen supply
The tumor microenvironment (TME) can be very hypoxic due to the Warburg effect.85 Without a sufficient oxygen supply, SDT cannot fulfill its biggest potential and the consumption of oxygen in tumors might exacerbate the hypoxic status thus leading to poor outcomes.86 To solve these problems, Beguin et al. developed a new kind of oxygen microbubble and the lipid shell was conjugated with sonosensitizer RB87 (Fig. 6). Moreover, to enhance the accumulation of microbubbles in the tumor site, magnetic lipids were incorporated into the shell to achieve magnet guided local SDT. The in vivo antitumor results proved that the alignment of the ultrasound and the magnet could enhance the therapeutic effect of the oxygen microbubble against pancreatic tumors. The enhanced treatment effect could be attributed to several aspects: (i) the sufficient oxygen supply during the SDT, (ii) microbubble assisted cell membrane disruption and improved drug uptake and (iii) the enhanced cavitation effect by the microbubbles.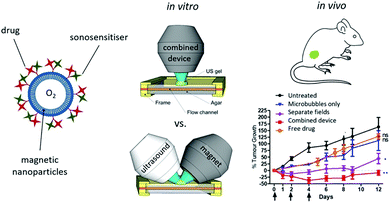 | ||
| Fig. 6 A schematic diagram of magnetic microbubbles conjugated with sonosensitizers and a picture of the combined device involving ultrasound and a magnet, as well as the enhanced tumor inhibiting effects of the oxygen supplied SDT. Reproduced with permission ref. 87. | ||
Since there has been a long-term investigation on overcoming the absence of sufficient oxygen during PDT, it is reasonable to learn from the experience in the molecular design of sonosensitizers.88 Adopting a redox reaction between the endogenous or exogenous H2O2 and catalase, all-in-one nanoparticles were designed to enhance the SDT effect by modulating the hypoxic tumor environment89–92 (Fig. 7). The incorporation of oxygen carriers can significantly ameliorate hypoxia. As a natural carrier of oxygen, engineered red blood cells loaded with sonosensitizers were able to effectively ablate tumors by relieving the hypoxia.93,94 Recently, perfluorocarbon filled nanobubbles carrying the sonosensitizer Ce6 were demonstrated to be able to induce strong anti-tumor immunity in the murine model.95 The natural oxygen concentration ability of the perfluorocarbon might contribute to the enhancement.96
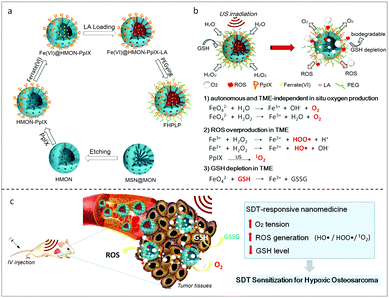 | ||
| Fig. 7 A Fe(VI) loaded porphyrin mesoporous silica nanoparticle (a) and the in situ oxygen production and the ROS generation mechanism of FHPLP in the TME (b). (c) In vivo hypoxia relief of the TME and the mechanism of the enhanced SDT. Reproduced with permission ref. 91. | ||
Ultrasound parameters
When SDT is carried out, the cell membrane could be disrupted by the sono-mechanical force,97 which could enhance the SDT effect. However, there has been a long-standing debate about the cytotoxicity of low intensity focused ultrasound. Recently a research report revealed that low intensity pulsed ultrasound could cause selective ablation of leukemia with little effect on other normal cells such as T cells and red blood cells98 (Fig. 8). The selective killing also applied for the other solid tumor cell lines both from mice and humans. Further mechanism study revealed the increase of immunogenic cell death markers after ultrasonic treatment. This finding was interesting but specific conditions, including a standing wave and a proper reflector, were required. Besides, a gel study used to mimic the solid tumor environment exhibited that there was limited cytotoxicity when no fluid was around the cells as cavitation played an essential part in the selective killing. As a result, the waveform of the ultrasound used in SDT has to be considered when cytotoxicity is referred to as a metric for evaluating sonosensitizers.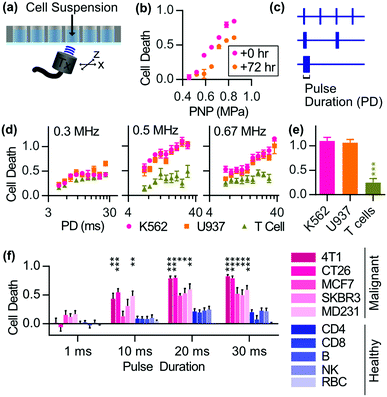 | ||
| Fig. 8 The selective antitumor effects of low intensity pulsed focused ultrasound on malignant tumor cell lines. Reproduced with permission ref. 98. | ||
Challenges
Sonosensitizer screening
There is no standard procedure to screen sonosensitizers compared with other mature targeted drugs yet. The conventional method is time- and resource-consuming. Most papers focused on the validation of the SDT effect of the photosensitizers108 while a few papers verified other drugs such as non-steroidal anti-inflammatory drugs (Table 2). With the help of computer aided drug design (CADD), we can first predict the associated properties of the designed molecules in silicon without the necessity to synthesize them. Besides, it is reasonable to simulate the interaction between oxygen and the molecules in solution to predict the ROS generating ability. Considering the oxygen-dependent nature of the SDT, conjugating a respiratory depression drug with a sonosensitizer is a reasonable strategy to relieve hypoxia and make up for the deficiency of the dissolved oxygen.| Sonosensitizer | Tumor | Frequency | Sound intensity | Time | Treatment cycles | Dose | Ref. | |
|---|---|---|---|---|---|---|---|---|
| Porphyrin | SF1 | S180 | 1 MHz | 1.2 W cm−2 | 3 min | 1 | 20 mg kg−1 (i.p.) | 99 |
| ATX-70 | Colon adenocarcinoma | 2 MHz | 3 W cm−2 | 15 min | 1 | 2.5 mg kg−1 (i.v.) | 100 | |
| DVDMS | S180 | 1.9 MHz | 4 W | 3 min | 3 | 2 mg kg−1 (i.v.) | 101 and 102 | |
| PpIX | Oral squamous cell carcinoma | 1 MHz | 0.89 W cm−2 | 15 min | — | — | 103 | |
| DCPH-P-Na | MKN-45 | 1 MHz | 1–2 W cm−2 | 10 min | 1 | 144 μg/kg (i.v.) | 23 | |
| DEG | MKN-74 | 1 MHz | 2 W cm−2 | 10 min | 6 | 1 mg kg−1 | 104 | |
| Phthalocyanine | AlPcTS | Colon adenocarcinoma | 1.92 MHz | 3 W cm−2 | 15 min | 1 | 2.5 mg kg−1 | 105 |
| Cyanine | IR780 | Breast cancer | 1 MHz | 2 W cm−2 | 4 min | 1 | 80 μg (i.t.) | 106 |
| Xanthene | Bengal Rose | Glioma | 1 MHz | 25 W cm−2 | 5 min | 1 | 50 mg kg−1 (i.v.) | 107 |
Sonosensitizer delivery
To avoid side effects to the maximum extent possible, healthy tissues should absorb the sonosensitizer to the lowest extent possible. Nanomedicine has been a promising way to precisely deliver drugs to tumors109–112 based on the enhanced permeability and retention effect (EPR effect) but the delivery efficacy is very low and non-specific distribution is difficult to avoid.113 Moreover, the excretion time of nanoparticles costs several times more than small molecules. Only a small portion of nanomedicines were successfully translated into clinical use. To come closer to clinical applications, we believe that rationally designed targeted small molecules should be mainstream in the development of sonosensitizers because of the following reasons: (i) ligand modified molecules exhibit a higher targeting ability than nanoparticles relying on the EPR effect, (ii) small molecules are capable of penetrating into deep tumor sites and (iii) small molecules with a proper log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D exhibit better pharmacokinetics. All these features determine that small molecules are feasible to be approved by the regulatory agency.
D exhibit better pharmacokinetics. All these features determine that small molecules are feasible to be approved by the regulatory agency.
Safety
From the in vitro data we have determined that the SDT requires a higher concentration of drugs to achieve a comparable cytotoxicity by PDT.102 So, the toxicity of the high dose of sonosensitizer in vivo is a big concern. There is still limited research studying the dose-dependent toxicity of sonosensitizers but it is essential because we cannot simply believe that the PSs or other clinical approved drugs are also safe when they are used as sonosensitizers at a much higher dose. Another safety concern is the intensity of ultrasound. We cannot ignore the attenuations of ultrasound as it travels through tissues. It is still hard to evaluate the accurate ultrasound focus intensity in vivo, though it is not hard to achieve in vitro by using a hydrophone. As a result, this will raise the question of whether the ultrasound intensity is enough to excite the sonosensitizer in vivo to achieve therapeutic effects. In order to improve the treatment efficacy, the sound intensity should be well adjusted to meet the treatment requirements of tumors in different tissues. It is highly recommended that the acoustic pressure should also be reported for the purpose of evaluating the bioeffects of the ultrasound. At the same time, the tolerance of ultrasound in different healthy organs has to be taken into consideration to avoid undesirable damage.Effectiveness of SDT
Strictly, current clinical data on SDT has not provided convincing evidence for the effectiveness of SDT. A more common conclusion is that ultrasound could enhance the treatment effect of PDT after patients received light exposure.114 A lot of pre-clinical research has demonstrated that photo-sonodynamic therapy or sono-photodynamic therapy surpassed monotherapy but the mechanism behind this is far from being elucidated.39 Plenty of tumor bearing mice have been cured by SDT, but we are eager to see its effect on bigger animals and even on humans.Conclusions
Sonodynamic therapy holds great potential for the non-invasive treatment of tumors. However, the lack of effective sonosensitizers hinders its progress to clinical trials. When we optimize sonosensitizers, the ROS generation ability, pharmacokinetics and even the match with the unique ultrasound parameters are affected (Fig. 9). There still is no established relationship between the molecular chemical structure and the ROS generating ability, as well as the ultrasound parameters. Moreover, the ultrasound–cell interactions under exposure to distinctive molecules can be quite diverse. For clinical cancer treatment, a combination of SDT and other modalities, including chemotherapy and immunotherapy, could enhance the therapeutic effects of monotherapy. We believe that elucidating the fundamental principles of SDT and the actual synergistic mechanism of ultrasound and drug therapy could pave the way for the final clinical translation of SDT.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
This contribution was financially supported by the National Project for Research and Development of Major Scientific Instruments (No. 81727803), National Key Research and Development Program of China (No. 2016YFA0201400), State Key Program of National Natural Science of China (No. 81930047), Projects of International Cooperation and Exchanges NSFC-PSF (No. 31961143003), Beijing Natural Science Foundation, Haidian, original innovation joint fund (No. 17L20170), and Foundation for Innovative Research Groups of the National Natural Science Foundation of China (No. 81421004).References
- A. L. Vahrmeijer, M. Hutteman, J. R. Van Der Vorst, C. J. Van De Velde and J. V. Frangioni, Image-guided cancer surgery using near-infrared fluorescence, Nat. Rev. Clin. Oncol., 2013, 10(9), 507 CrossRef CAS PubMed.
- V. T. DeVita and E. Chu, A history of cancer chemotherapy, Cancer Res., 2008, 68(21), 8643–8653 CrossRef CAS PubMed.
- P. G. Rose, B. N. Bundy, E. B. Watkins, J. T. Thigpen, G. Deppe, M. A. Maiman, D. L. Clarke-Pearson and S. Insalaco, Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer, N. Engl. J. Med., 1999, 340(15), 1144–1153 CrossRef CAS PubMed.
- L. Yu, R. Zhao, X. Han, J. Shou, L. You, H. Jiang, X. Zhou, Z. Liu, H. Pan and W. Han, Lymph node involvement in pancreatic neuroendocrine tumors: significance as a predictor of survival, J. Bio-X Res., 2019, 2(1), 25–33 Search PubMed.
- H. Xu, Y. Huang, Q. Lan, Y. Zhang, Y. Zeng, T. Zhang, C. Chen, P. Su, Z. Chu and W. Lai, The mechanisms of colorectal cancer cell mesenchymal–epithelial transition induced by hepatocyte exosome-derived miR-203a-3p, J. Bio-X Res., 2018, 1(2), 62–72 Search PubMed.
- N. Watkin, S. Morris, I. Rivens and C. Woodhouse, A feasibility study for the non-invasive treatment of superficial bladder tumours with focused ultrasound, Br. J. Urol., 1996, 78(5), 715–721 CAS.
- I. Rosenthal, J. Z. Sostaric and P. Riesz, Sonodynamic therapy–a review of the synergistic effects of drugs and ultrasound, Ultrason. Sonochem., 2004, 11(6), 349–363 CAS.
- A. P. McHale; J. F. Callan; N. Nomikou; C. Fowley and B. Callan, Sonodynamic Therapy: Concept, Mechanism and Application to Cancer Treatment, Therapeutic Ultrasound, 2016, pp. 429–450 Search PubMed.
- H. I. Pass, Photodynamic therapy in oncology: mechanisms and clinical use, JNCI, J. Natl. Cancer Inst., 1993, 85(6), 443–456 CrossRef CAS PubMed.
- E. Lukyanets, NIR photosensitizers in photodynamic therapy, Near-Infrared Dyes for High Technology Applications, Springer, 1998, pp. 307–324 Search PubMed.
- L. A. Sordillo, Y. Pu, S. Pratavieira, Y. Budansky and R. R. Alfano, Deep optical imaging of tissue using the second and third near-infrared spectral windows, J. Biomed. Opt., 2014, 19(5), 056004 CrossRef PubMed.
- S. J. Madsen, C. H. Sun, B. J. Tromberg, V. P. Wallace and H. Hirschberg, Photodynamic Therapy of Human Glioma Spheroids Using 5-Aminolevulinic Acid, Photochem. Photobiol., 2000, 72(1), 128–134 CrossRef CAS PubMed.
- T. J. Dougherty, M. T. Cooper and T. S. Mang, Cutaneous phototoxic occurrences in patients receiving Photofrin®, Lasers Surg. Med., 1990, 10(5), 485–488 CrossRef CAS PubMed.
- L. E. Rhodes, M. M. Tsoukas, R. R. Anderson and N. Kollias, Iontophoretic delivery of ALA provides a quantitative model for ALA pharmacokinetics and PpIX phototoxicity in human skin, J. Invest. Dermatol., 1997, 108(1), 87–91 CrossRef CAS PubMed.
- E. J. Halpern, Contrast-enhanced ultrasound imaging of prostate cancer, Rev. Urol., 2006, 8(Suppl 1), S29 Search PubMed.
- R. Liu, J. Tang, Y. Xu and Z. Dai, Bioluminescence imaging of inflammation in vivo based on bioluminescence and fluorescence resonance energy transfer using nanobubble ultrasound contrast agent, ACS Nano, 2019, 13(5), 5124–5132 CrossRef CAS PubMed.
- M. Chen, X. Liang, C. Gao, R. Zhao, N. Zhang, S. Wang, W. Chen, B. Zhao, J. Wang and Z. Dai, Ultrasound triggered conversion of porphyrin/camptothecin-fluoroxyuridine triad microbubbles into nanoparticles overcomes multidrug resistance in colorectal cancer, ACS Nano, 2018, 12(7), 7312–7326 CrossRef CAS PubMed.
- Y. You, X. Liang, T. Yin, M. Chen, C. Qiu, C. Gao, X. Wang, Y. Mao, E. Qu and Z. Dai, Porphyrin-grafted lipid microbubbles for the enhanced efficacy of photodynamic therapy in prostate cancer through ultrasound-controlled in situ accumulation, Theranostics, 2018, 8(6), 1665 CrossRef CAS PubMed.
- N. Zhang, Y. Fei, L. Xiaolong, W. Manxiang, S. Yuanyuan, C. Min, X. Yunxue, Z. Guangyang, J. Peng and T. Caiyun, Localized delivery of curcumin into brain with polysorbate 80-modified cerasomes by ultrasound-targeted microbubble destruction for improved Parkinson's disease therapy, Theranostics, 2018, 8(8), 2264–2277 CrossRef CAS PubMed.
- F. d'Astous and F. Foster, Frequency dependence of ultrasound attenuation and backscatter in breast tissue, Ultrasound Med. Biol., 1986, 12(10), 795–808 CrossRef.
- J. H. Demmink, P. J. Helders, H. Hobæk and C. Enwemeka, The variation of heating depth with therapeutic ultrasound frequency in physiotherapy, Ultrasound Med. Biol., 2003, 29(1), 113–118 CrossRef PubMed.
- S. Schäfer, S. Kliner, L. Klinghammer, H. Kaarmann, I. Lucic, U. Nixdorff, U. Rosenschein, W. G. Daniel and F. A. Flachskampf, Influence of ultrasound operating parameters on ultrasound-induced thrombolysis in vitro, Ultrasound Med. Biol., 2005, 31(6), 841–847 CrossRef PubMed.
- K. Hachimine, H. Shibaguchi, M. Kuroki, H. Yamada, T. Kinugasa, Y. Nakae, R. Asano, I. Sakata, Y. Yamashita and T. Shirakusa, Sonodynamic therapy of cancer using a novel porphyrin derivative, DCPH-P-Na (I), which is devoid of photosensitivity, Cancer Sci., 2007, 98(6), 916–920 CrossRef CAS PubMed.
- R. Illing, J. Kennedy, F. Wu, G. Ter Haar, A. Protheroe, P. Friend, F. Gleeson, D. Cranston, R. Phillips and M. Middleton, The safety and feasibility of extracorporeal high-intensity focused ultrasound (HIFU) for the treatment of liver and kidney tumours in a Western population, Br. J. Cancer, 2005, 93(8), 890–895 CrossRef CAS PubMed.
- N. Yumita, R. Nishigaki, K. Umemura and S. i. Umemura, Hematoporphyrin as a sensitizer of cell-damaging effect of ultrasound, Jpn. J. Cancer Res., 1989, 80(3), 219–222 CrossRef CAS PubMed.
- H. Chen, X. Zhou, Y. Gao, B. Zheng, F. Tang and J. Huang, Recent progress in development of new sonosensitizers for sonodynamic cancer therapy, Drug Discovery Today, 2014, 19(4), 502–509 CrossRef CAS PubMed.
- K. Takemae, J. Okamoto, Y. Horise, K. Masamune and Y. Muragaki, Function of Epirubicin-Conjugated Polymeric Micelles in Sonodynamic Therapy, Front. Pharmacol., 2019, 10, 546 CrossRef CAS PubMed.
- M. Lafond, S. Yoshizawa and S. I. Umemura, Sonodynamic Therapy: Advances and Challenges in Clinical Translation, J. Ultrasound Med., 2019, 38(3), 567–580 CrossRef PubMed.
- M. Minnaert, The nature of light and colour in the open air, Courier Corporation, 2013 Search PubMed.
- M. H. Repacholi, Ultrasound: Characteristics and biological action, 1981 Search PubMed.
- A. Pais, Einstein and the quantum theory, Rev. Mod. Phys., 1979, 51(4), 863 CrossRef CAS.
- M. Klaper, W. Fudickar and T. Linker, Role of distance in singlet oxygen applications: a model system, J. Am. Chem. Soc., 2016, 138(22), 7024–7029 CrossRef CAS PubMed.
- M. Platte, A polyvinylidene fluoride needle hydrophone for ultrasonic applications, Ultrasonics, 1985, 23(3), 113–118 CrossRef CAS.
- T. Boutkedjirt and R. Reibold, Reconstruction of ultrasonic fields by deconvolving the hydrophone aperture effects: I. Theory and simulation, Ultrasonics, 2002, 39(9), 631–639 CrossRef CAS PubMed.
- J. Wu and W. L. Nyborg, Ultrasound, cavitation bubbles and their interaction with cells, Adv. Drug Delivery Rev., 2008, 60(10), 1103–1116 CrossRef CAS PubMed.
- V. Mišík and P. Riesz, Free radical intermediates in sonodynamic therapy, Ann. N. Y. Acad. Sci., 2000, 899(1), 335–348 CrossRef PubMed.
- X. Pan, H. Wang, S. Wang, X. Sun, L. Wang, W. Wang, H. Shen and H. Liu, Sonodynamic therapy (SDT): a novel strategy for cancer nanotheranostics, Sci. China: Life Sci., 2018, 61(4), 415–426 CrossRef PubMed.
- E. Beguin, S. Shrivastava, N. V. Dezhkunov, A. P. McHale, J. F. Callan and E. Stride, Direct Evidence of Multibubble Sonoluminescence Using Therapeutic Ultrasound and Microbubbles, ACS Appl. Mater. Interfaces, 2019, 11(22), 19913–19919 CrossRef CAS PubMed.
- Z. Huang, H. Moseley and S. Bown, Rationale of combined PDT and SDT modalities for treating cancer patients in terminal stage: the proper use of photosensitizer, Integr. Cancer Ther., 2010, 9(4), 317–319 CrossRef PubMed.
- X. Qian, Y. Zheng and Y. Chen, Micro/nanoparticle-augmented sonodynamic therapy (SDT): Breaking the depth shallow of photoactivation, Adv. Mater., 2016, 28(37), 8097–8129 CrossRef CAS PubMed.
- E. Gandin and Y. Lion, Van de Vorst, A., Quantum yield of singlet oxygen production by xanthene derivatives, Photochem. Photobiol., 1983, 37(3), 271–278 CrossRef CAS.
- N. A. Kuznetsova, N. S. Gretsova, V. M. Derkacheva, O. L. Kaliya and E. A. Lukyanets, Sulfonated phthalocyanines: aggregation and singlet oxygen quantum yield in aqueous solutions, J. Porphyrins phthalocyanines, 2003, 7(03), 147–154 CrossRef CAS.
- X. Ragàs, A. Jiménez-Banzo, D. Sánchez-García, X. Batllori and S. Nonell, Singlet oxygen photosensitisation by the fluorescent probe Singlet Oxygen Sensor Green®, Chem. Commun., 2009, 2920–2922 RSC.
- K. A. Kristiansen, P. E. Jensen, I. M. Møller and A. Schulz, Monitoring reactive oxygen species formation and localisation in living cells by use of the fluorescent probe CM-H2DCFDA and confocal laser microscopy, Physiol. Plant., 2009, 136(4), 369–383 CrossRef CAS PubMed.
- R. Venkatesan; N. Periasamy and T. Srivastava, Singlet molecular oxygen quantum yield measurements of some porphyrins and metalloporphyrins, Proceedings of the Indian Academy of Sciences-Chemical Sciences, Springer, 1992, pp. 713–722 Search PubMed.
- T. L. Figueiredo, R. A. Johnstone, A. M. S. Sørensen, D. Burget and P. Jacques, Determination of Fluorescence Yields, Singlet Lifetimes and Singlet Oxygen Yields of Water-Insoluble Porphyrins and Metalloporphyrins in Organic Solvents and in Aqueous Media, Photochem. Photobiol., 1999, 69(5), 517–528 CrossRef CAS.
- J.-i. Aihara, Reduced HOMO–LUMO gap as an index of kinetic stability for polycyclic aromatic hydrocarbons. The, J. Phys. Chem. A, 1999, 103(37), 7487–7495 CrossRef CAS.
- A. Ma, H. Chen, Y. Cui, Z. Luo, R. Liang, Z. Wu, Z. Chen, T. Yin, J. Ni, M. Zheng and L. Cai, Metalloporphyrin Complex-Based Nanosonosensitizers for Deep-Tissue Tumor Theranostics by Noninvasive Sonodynamic Therapy, Small, 2019, 15(5), e1804028 CrossRef PubMed.
- W. H. Oldendorf, Lipid solubility and drug penetration of the blood brain barrier, Proc. Soc. Exp. Biol. Med., 1974, 147(3), 813–816 CrossRef CAS PubMed.
- R.-M. Ion, Spectral analysis of the porphyrins incorporation into human blood, J. Biomed. Opt., 1999, 4(3), 319–327 CrossRef CAS PubMed.
- X.-F. Zhang and H.-J. Xu, Influence of halogenation and aggregation on photosensitizing properties of zinc phthalocyanine (ZnPC), J. Chem. Soc., Faraday Trans., 1993, 89(18), 3347–3351 RSC.
- J. M. Dąbrowski, M. M. Pereira, L. G. Arnaut, C. J. Monteiro, A. F. Peixoto, A. Karocki, K. Urbańska and G. Stochel, Synthesis, photophysical studies and anticancer activity of a new halogenated water-soluble porphyrin, Photochem. Photobiol., 2007, 83(4), 897–903 CrossRef PubMed.
- N. C. Maiti, S. Mazumdar and N. Periasamy, J- and H-aggregates of porphyrin− surfactant complexes: time-resolved fluorescence and other spectroscopic studies, J. Phys. Chem. B, 1998, 102(9), 1528–1538 CrossRef CAS.
- H. Sternlicht, G. Nieman and G. Robinson, Triplet—triplet annihilation and delayed fluorescence in molecular aggregates, J. Chem. Phys., 1963, 38(6), 1326–1335 CrossRef CAS.
- S. Mo, X. Zhang, S. Hameed, Y. Zhou and Z. Dai, Glutathione-responsive disassembly of disulfide dicyanine for tumor imaging with reduction in background signal intensity, Theranostics, 2020, 10(5), 2130 CrossRef PubMed.
- C. Jing, R. Wang, H. Ou, A. Li, Y. An, S. Guo and L. Shi, Axial modification inhibited H-aggregation of phthalocyanines in polymeric micelles for enhanced PDT efficacy, Chem. Commun., 2018, 54(32), 3985–3988 RSC.
- K. A. Carter, S. Shao, M. I. Hoopes, D. Luo, B. Ahsan, V. M. Grigoryants, W. Song, H. Huang, G. Zhang and R. K. Pandey, Porphyrin–phospholipid liposomes permeabilized by near-infrared light, Nat. Commun., 2014, 5(1), 1–11 Search PubMed.
- F. Postigo, M. Mora, M. De Madariaga, S. Nonell and M. Sagrista, Incorporation of hydrophobic porphyrins into liposomes: characterization and structural requirements, Int. J. Pharm., 2004, 278(2), 239–254 CrossRef CAS PubMed.
- E. Chen, B.-M. Chen, Y.-C. Su, Y.-C. Chang, T.-L. Cheng, Y. Barenholz and S. R. Roffler, Premature Drug Release from Polyethylene Glycol (PEG)-Coated Liposomal Doxorubicin via Formation of the Membrane Attack Complex, ACS Nano, 2020 DOI:10.1021/acsnano.9b07218.
- X. Liang, X. Li, L. Jing, X. Yue and Z. Dai, Theranostic porphyrin dyad nanoparticles for magnetic resonance imaging guided photodynamic therapy, Biomaterials, 2014, 35(24), 6379–6388 CrossRef CAS PubMed.
- P. Huang, X. Qian, Y. Chen, L. Yu, H. Lin, L. Wang, Y. Zhu and J. Shi, Metalloporphyrin-Encapsulated Biodegradable Nanosystems for Highly Efficient Magnetic Resonance Imaging-Guided Sonodynamic Cancer Therapy, J. Am. Chem. Soc., 2017, 139(3), 1275–1284 CrossRef CAS PubMed.
- L. Yu, Y. Chen, H. Lin, W. Du, H. Chen and J. Shi, Ultrasmall mesoporous organosilica nanoparticles: Morphology modulations and redox-responsive biodegradability for tumor-specific drug delivery, Biomaterials, 2018, 161, 292–305 CrossRef CAS PubMed.
- X. Pan, L. Bai, H. Wang, Q. Wu, H. Wang, S. Liu, B. Xu, X. Shi and H. Liu, Metal-Organic-Framework-Derived Carbon Nanostructure Augmented Sonodynamic Cancer Therapy, Adv. Mater., 2018, 30(23), e1800180 CrossRef PubMed.
- K. Ninomiya, K. Noda, C. Ogino, S.-i. Kuroda and N. Shimizu, Enhanced OH radical generation by dual-frequency ultrasound with TiO2 nanoparticles: its application to targeted sonodynamic therapy, Ultrason. Sonochem., 2014, 21(1), 289–294 CrossRef CAS PubMed.
- V. G. Deepagan, D. G. You, W. Um, H. Ko, S. Kwon, K. Y. Choi, G. R. Yi, J. Y. Lee, D. S. Lee, K. Kim, I. C. Kwon and J. H. Park, Long-Circulating Au-TiO2 Nanocomposite as a Sonosensitizer for ROS-Mediated Eradication of Cancer, Nano Lett., 2016, 16(10), 6257–6264 CrossRef CAS PubMed.
- K. Strebhardt and A. Ullrich, Paul Ehrlich's magic bullet concept: 100 years of progress, Nat. Rev. Cancer, 2008, 8(6), 473–480 CrossRef CAS PubMed.
- Y. H. Bae and K. Park, Targeted drug delivery to tumors: myths, reality and possibility, J. Controlled Release, 2011, 153(3), 198 CrossRef CAS PubMed.
- A. Mullard, Maturing antibody–drug conjugate pipeline hits 30, Nature Publishing Group, 2013 Search PubMed.
- B. Y. Kim, J. T. Rutka and W. C. Chan, Nanomedicine, N. Engl. J. Med., 2010, 363(25), 2434–2443 CrossRef CAS PubMed.
- B. E. de Goeij and J. M. Lambert, New developments for antibody-drug conjugate-based therapeutic approaches, Curr. Opin. Immunol., 2016, 40, 14–23 CrossRef CAS PubMed.
- M. L. Etheridge, S. A. Campbell, A. G. Erdman, C. L. Haynes, S. M. Wolf and J. McCullough, The big picture on nanomedicine: the state of investigational and approved nanomedicine products, Nanomedicine, 2013, 9(1), 1–14 CrossRef CAS PubMed.
- S. Pushpan, S. Venkatraman, V. Anand, J. Sankar, D. Parmeswaran, S. Ganesan and T. Chandrashekar, Porphyrins in photodynamic therapy-a search for ideal photosensitizers, Curr. Med. Chem.: Anti-Cancer Agents, 2002, 2(2), 187–207 CrossRef CAS PubMed.
- S. Hameed, H. Chen, M. Irfan, S. Z. Bajwa, W. S. Khan, S. M. Baig and Z. Dai, Fluorescence guided sentinel lymph node mapping: from current molecular probes to future multimodal nanoprobes, Bioconjugate Chem., 2018, 30(1), 13–28 CrossRef PubMed.
- R. R. Zhang, A. B. Schroeder, J. J. Grudzinski, E. L. Rosenthal, J. M. Warram, A. N. Pinchuk, K. W. Eliceiri, J. S. Kuo and J. P. Weichert, Beyond the margins: real-time detection of cancer using targeted fluorophores, Nat. Rev. Clin. Oncol., 2017, 14(6), 347 CrossRef CAS PubMed.
- M. A. Biel; A. M. Gillenwater; D. M. Cognetti; J. M. Johnson; A. Argiris and M. Tahara, A global phase III multicenter, randomized, double-arm, open label trial of ASP-1929 photoimmunotherapy versus physician's choice standard of care for the treatment of patients with locoregional, recurrent head and neck squamous cell carcinoma (rHNSCC), American Society of Clinical Oncology, 2019 Search PubMed.
- C. Vasalou, G. Helmlinger and B. Gomes, A mechanistic tumor penetration model to guide antibody drug conjugate design, PLoS One, 2015, 10(3), e0118977 CrossRef PubMed.
- V. Cortez-Retamozo, N. Backmann, P. D. Senter, U. Wernery, P. De Baetselier, S. Muyldermans and H. Revets, Efficient cancer therapy with a nanobody-based conjugate, Cancer Res., 2004, 64(8), 2853–2857 CrossRef CAS PubMed.
- H. S. Choi, S. L. Gibbs, J. H. Lee, S. H. Kim, Y. Ashitate, F. Liu, H. Hyun, G. Park, Y. Xie and S. Bae, Targeted zwitterionic near-infrared fluorophores for improved optical imaging, Nat. Biotechnol., 2013, 31(2), 148 CrossRef CAS PubMed.
- J. Burggraaf, I. M. Kamerling, P. B. Gordon, L. Schrier, M. L. De Kam, A. J. Kales, R. Bendiksen, B. Indrevoll, R. M. Bjerke and S. A. Moestue, Detection of colorectal polyps in humans using an intravenously administered fluorescent peptide targeted against c-Met, Nat. Med., 2015, 21(8), 955–961 CrossRef CAS PubMed.
- J. Sudimack and R. J. Lee, Targeted drug delivery via the folate receptor, Adv. Drug Delivery Rev., 2000, 41(2), 147–162 CrossRef CAS PubMed.
- J. D. Byrne, T. Betancourt and L. Brannon-Peppas, Active targeting schemes for nanoparticle systems in cancer therapeutics, Adv. Drug Delivery Rev., 2008, 60(15), 1615–1626 CrossRef CAS PubMed.
- C. H. J. Choi, C. A. Alabi, P. Webster and M. E. Davis, Mechanism of active targeting in solid tumors with transferrin-containing gold nanoparticles, Proc. Natl. Acad. Sci. U. S. A., 2010, 107(3), 1235–1240 CrossRef CAS PubMed.
- M. Hadjidemetriou and K. Kostarelos, Nanomedicine: evolution of the nanoparticle corona, Nat. Nanotechnol., 2017, 12(4), 288 CrossRef CAS PubMed.
- X. Li, S. Kolemen, J. Yoon and E. U. Akkaya, Activatable Photosensitizers: Agents for Selective Photodynamic Therapy, Adv. Funct. Mater., 2017, 27(5), 1604053 CrossRef.
- M. G. Vander Heiden, L. C. Cantley and C. B. Thompson, Understanding the Warburg effect: the metabolic requirements of cell proliferation, Science, 2009, 324(5930), 1029–1033 CrossRef CAS PubMed.
- J. M. Brown and W. R. Wilson, Exploiting tumour hypoxia in cancer treatment, Nat. Rev. Cancer, 2004, 4(6), 437–447 CrossRef CAS PubMed.
- E. Beguin, M. D. Gray, K. A. Logan, H. Nesbitt, Y. Sheng, S. Kamila, L. C. Barnsley, L. Bau, A. P. McHale and J. F. Callan, Magnetic microbubble mediated chemo-sonodynamic therapy using a combined magnetic-acoustic device, J. Controlled Release, 2020, 317, 23–33 CrossRef CAS PubMed.
- Z. Zhou, J. Song, L. Nie and X. Chen, Reactive oxygen species generating systems meeting challenges of photodynamic cancer therapy, Chem. Soc. Rev., 2016, 45(23), 6597–6626 RSC.
- W. P. Li, C. H. Su, Y. C. Chang, Y. J. Lin and C. S. Yeh, Ultrasound-Induced Reactive Oxygen Species Mediated Therapy and Imaging Using a Fenton Reaction Activable Polymersome, ACS Nano, 2016, 10(2), 2017–2027 CrossRef CAS PubMed.
- J. Chen, H. Luo, Y. Liu, W. Zhang, H. Li, T. Luo, K. Zhang, Y. Zhao and J. Liu, Oxygen-Self-Produced Nanoplatform for Relieving Hypoxia and Breaking Resistance to Sonodynamic Treatment of Pancreatic Cancer, ACS Nano, 2017, 11(12), 12849–12862 CrossRef CAS PubMed.
- J. Fu, T. Li, Y. Zhu and Y. Hao, Ultrasound-Activated Oxygen and ROS Generation Nanosystem Systematically Modulates Tumor Microenvironment and Sensitizes Sonodynamic Therapy for Hypoxic Solid Tumors, Adv. Funct. Mater., 2019, 29(51), 1906195 CrossRef CAS.
- P. Zhu, Y. Chen and J. Shi, Nanoenzyme-Augmented Cancer Sonodynamic Therapy by Catalytic Tumor Oxygenation, ACS Nano, 2018, 12(4), 3780–3795 CrossRef CAS PubMed.
- B. Du, X. Yan, X. Ding, Q. Wang, Q. Du, T. Xu, G. Shen, H. Yao and J. Zhou, Oxygen Self-Production Red Blood Cell Carrier System for MRI Mediated Cancer Therapy: Ferryl-Hb, Sonodynamic, and Chemical Therapy, ACS Biomater. Sci. Eng., 2018, 4(12), 4132–4143 CrossRef CAS.
- C. Li, X.-Q. Yang, J. An, K. Cheng, X.-L. Hou, X.-S. Zhang, Y.-G. Hu, B. Liu and Y.-D. Zhao, Red blood cell membrane-enveloped O(2) self-supplementing biomimetic nanoparticles for tumor imaging-guided enhanced sonodynamic therapy, Theranostics, 2020, 10(2), 867–879 CrossRef PubMed.
- W. Um, H. Ko, D. G. You, S. Lim, G. Kwak, M. K. Shim, S. Yang, J. Lee, Y. Song, K. Kim and J. H. Park, Necroptosis-Inducible Polymeric Nanobubbles for Enhanced Cancer Sonoimmunotherapy, Adv. Mater., 2020, e1907953 CrossRef PubMed.
- Y. Cheng, H. Cheng, C. Jiang, X. Qiu, K. Wang, W. Huan, A. Yuan, J. Wu and Y. Hu, Perfluorocarbon nanoparticles enhance reactive oxygen levels and tumour growth inhibition in photodynamic therapy, Nat. Commun., 2015, 6(1), 1–8 Search PubMed.
- L. B. Feril and T. Kondo, Biological effects of low intensity ultrasound: the mechanism involved, and its implications on therapy and on biosafety of ultrasound, J. Radiat. Res., 2004, 45(4), 479–489 CrossRef PubMed.
- D. R. Mittelstein, J. Ye, E. F. Schibber, A. Roychoudhury, L. T. Martinez, M. H. Fekrazad, M. Ortiz, P. P. Lee, M. G. Shapiro and M. Gharib, Selective ablation of cancer cells with low intensity pulsed ultrasound, Appl. Phys. Lett., 2020, 116(1), 013701 CrossRef.
- X. Wang, T. J. Lewis and D. Mitchell, The tumoricidal effect of sonodynamic therapy (SDT) on S-180 sarcoma in mice, Integr. Cancer Ther., 2008, 7(2), 96–102 CrossRef CAS PubMed.
- N. Yumita, K. Sasaki, S. i. Umemura and R. Nishigaki, Sonodynamically induced antitumor effect of a gallium-porphyrin complex, ATX-70, Jpn. J. Cancer Res., 1996, 87(3), 310–316 CrossRef CAS PubMed.
- J. Hu, X. Wang, K. Zhang, P. Wang, X. Su, Y. Li, Z. Huang and Q. Liu, Sinoporphyrin sodium: a novel sensitizer in sonodynamic therapy, Anti-Cancer Drugs, 2014, 25(2), 174–182 CrossRef CAS PubMed.
- W. Xiong, P. Wang, J. Hu, Y. Jia, L. Wu, X. Chen, Q. Liu and X. Wang, A new sensitizer DVDMS combined with multiple focused ultrasound treatments: an effective antitumor strategy, Sci. Rep., 2015, 5, 17485 CrossRef PubMed.
- Y. Lv, J. Zheng, Q. Zhou, L. Jia, C. Wang, N. Liu, H. Zhao, H. Ji, B. Li and W. Cao, Antiproliferative and apoptosis-inducing effect of exo-protoporphyrin IX based sonodynamic therapy on human oral squamous cell carcinoma, Sci. Rep., 2017, 7(1), 1–12 CrossRef CAS PubMed.
- H. Tsuru, H. Shibaguchi, M. Kuroki, Y. Yamashita and M. Kuroki, Tumor growth inhibition by sonodynamic therapy using a novel sonosensitizer, Free Radicals Biol. Med., 2012, 53(3), 464–472 CrossRef CAS PubMed.
- N. Yumita and S. Umemura, Sonodynamic antitumour effect of chloroaluminum phthalocyanine tetrasulfonate on murine solid tumour, J. Pharm. Pharmacol., 2004, 56(1), 85–90 CrossRef CAS PubMed.
- Y. Li, Q. Zhou, Z. Deng, M. Pan, X. Liu, J. Wu, F. Yan and H. Zheng, IR-780 Dye as a Sonosensitizer for Sonodynamic Therapy of Breast Tumor, Sci. Rep., 2016, 6, 25968 CrossRef CAS PubMed.
- M. Nonaka, M. Yamamoto, S. Yoshino, S.-I. Umemura, K. Sasaki and T. Fukushima, Sonodynamic therapy consisting of focused ultrasound and a photosensitizer causes a selective antitumor effect in a rat intracranial glioma model, Anticancer Res., 2009, 29(3), 943–950 Search PubMed.
- A. E. O'Connor, W. M. Gallagher and A. T. Byrne, Porphyrin and nonporphyrin photosensitizers in oncology: preclinical and clinical advances in photodynamic therapy, Photochem. Photobiol., 2009, 85(5), 1053–1074 CrossRef PubMed.
- H. Xu, X. Zhang, R. Han, P. Yang, H. Ma, Y. Song, Z. Lu, W. Yin, X. Wu and H. Wang, Nanoparticles in sonodynamic therapy: state of the art review, RSC Adv., 2016, 6(56), 50697–50705 RSC.
- Z. Gong, M. Chen, Q. Ren, X. Yue and Z. Dai, Fibronectin-targeted dual-acting micelles for combination therapy of metastatic breast cancer, Signal Transduction Targeted Ther., 2020, 5(1), 1–11 CrossRef PubMed.
- C. Gao, P. Bhattarai, M. Chen, N. Zhang, S. Hameed, X. Yue and Z. Dai, Amphiphilic drug conjugates as nanomedicines for combined cancer therapy, Bioconjugate Chem., 2018, 29(12), 3967–3981 CrossRef CAS PubMed.
- S. Hameed, P. Bhattarai, X. Liang, N. Zhang, Y. Xu, M. Chen and Z. Dai, Self-assembly of porphyrin-grafted lipid into nanoparticles encapsulating doxorubicin for synergistic chemo-photodynamic therapy and fluorescence imaging, Theranostics, 2018, 8(19), 5501 CrossRef CAS PubMed.
- S. Wilhelm, A. J. Tavares, Q. Dai, S. Ohta, J. Audet, H. F. Dvorak and W. C. Chan, Analysis of nanoparticle delivery to tumours, Nat. Rev. Mater., 2016, 1(5), 1–12 Search PubMed.
- Z. Liu, J. Li, W. Chen, L. Liu and F. Yu, Light and sound to trigger the Pandora's box against breast cancer: A combination strategy of sonodynamic, photodynamic and photothermal therapies, Biomaterials, 2020, 232, 119685 CrossRef CAS PubMed.
| This journal is © the Partner Organisations 2020 |