Open Access Article
Open Access ArticleUnprecedented linear products by a mechanochemically activated Biginelli reaction using lawsone†
Christina L.
Koumpoura
 a,
Laure
Vendier
a,
Laure
Vendier
 a,
Christian
Bijani
a,
Christian
Bijani
 a,
Anne
Robert
a,
Anne
Robert
 a,
Philippe
Carbonnière
a,
Philippe
Carbonnière
 b,
Jean-Marc
Sotiropoulos
b,
Jean-Marc
Sotiropoulos
 *b and
Michel
Baltas
*b and
Michel
Baltas
 *a
*a
aCNRS, LCC (Laboratoire de Chimie de Coordination), Université de Toulouse, UPS, 205 Route de Narbonne, BP 44099, cedex 4, 31077 Toulouse, France. E-mail: michel.baltas@lcc-toulouse.fr
bCNRS-IPREM, UMR 5254 Technopole Helioparc, 2 Avenue du Président Angot, 64053, Pau, France. E-mail: jean-marc.sotiropoulos@cnrs.fr
First published on 2nd April 2024
Abstract
The Biginelli reaction, a crucial multicomponent reaction, was investigated involving 2-hydroxy-1,4-naphthoquinone (lawsone), p-substituted benzaldehydes, and ureas. Surprisingly, the classic Biginelli cyclized DHPM was not observed under various experimental conditions. Mechanochemical conditions, unlike traditional liquid phase conditions, led to the unprecedented formation of a series of ‘Biginelli-linear’ lawsone derivatives with high yields. The observed outcomes were consistent with DFT theoretical predictions, highlighting the preference for the Michael adduct under liquid conditions and the energetically implausible cyclization pathway for the classic DHPM compound. Additionally, the study achieved the novel cyclization of a ‘Biginelli-linear’ lawsone derivative into a cyclic carbamate for the first time.
Introduction
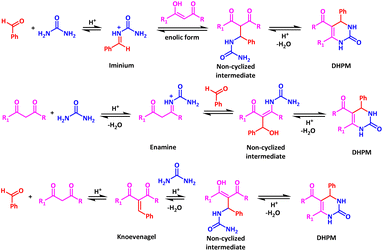
Multicomponent reactions (MCRs) are defined as those employing three or more reagents in a one-pot reaction to form a single product that essentially contains all the reactants.1–4 Due to the simple experimental procedures and one-pot character the MCRs have become excellent tools for the elaboration of libraries of small (essentially heterocyclic) derivatives. In the past decades, several novel and old MCRs have been further developed and nowadays they are widely used in natural product synthesis and drug discovery.5–8Biginelli reaction is a classic amongst the MCRs. Discovered in 1893, this reaction results in the condensation of three components: a β-ketoester, a urea, and an aldehyde to form the 4-aryl-3,4-dihydropyrimidin-2(1H)-one (DHPM). This one-pot multicomponent reaction stands out due to the pharmacological functionality of the produced dihydropyrimidine derivatives,9 showcasing notable biological activities including anticancer,10 antimalarial,11 anti-HIV agents12 and others.13,14 In the last few decades, different variations of the experimental conditions have been proposed for the Biginelli reaction: use of Brønsted or Lewis acid catalysts,15 ionic liquids,16 or excess of one reactant.17,18 After extensive efforts by many groups based on computational studies involving β-ketoesters, urea, and benzaldehyde, three mechanisms with many different intermediates are proposed for the classical Biginelli reaction (Scheme 1): (i) the so-called iminium route,19 implying first condensation of the aldehyde and urea to form a C–N iminium bond, followed by nucleophilic addition of the β-ketoester; (ii) the so-called enamine route,20 implying first reaction of the urea and β-ketoester to form an enamine C–N bond, which consecutively reacts with the aldehyde and (iii) mechanism based on the synthesis of the Knoevenagel intermediate21 where first the aldehyde and the β-ketoester form a C–C bond before reacting with urea (Scheme 1).
In 2015, Puripat et al.22 after using an Artificial Force Induced Reaction (AFIR) calculation scheme concluded that: (1) a second urea molecule can be beneficial in catalysing nearly every step of the reaction; (2) the substitution pattern on the aryl ring of the aldehyde has little influence on the reaction, and (3) the rate determining step (RDS) is the C–N bond formation during the final intramolecular cyclisation step for mechanisms (i) and (ii), and the C–O bond cleavage during the Knoevenagel reaction of the proposed mechanism (iii). The authors concluded that, while these three mechanisms remain the major ones, one could modify the RDS by varying the substitution patterns of the reactants and the acid catalysts.
Similar to most MCRs, the Biginelli reaction is well-suited for the principles of “green chemistry”.23–25 Mal et al.26 reported the first solvent-free ball-milling Biginelli reaction between the classical ethyl-acetoacetate, urea and substituted benzaldehydes. Very recently, Bolm et al. reported the synthesis of cyclic 2,3-dihydro-1,2,6-thiadiazine-1-oxides when NH-free sulfonimidamides react with ethyl acetoacetate and benzaldehydes under mechanochemical conditions.27
The interesting natural product lawsone (2-hydroxy-1,4-naphthoquinone) is unprecedented as a substrate in the Biginelli reaction. In addition, lawsone is an important scaffold in many biologically active compounds and drugs. For example, atovaquone is reported to be a leading drug targeting specifically the mitochondrion of P. falciparum parasite. Many research groups are studying the reactivity of lawsone and potential modifications of atovaquone that could lead to compounds overcoming the strong resistance of Plasmodium to this drug.28 In this field, we have recently reported a domino reaction between lawsone, aldehydes and isocyanides, affording naphthofuroquinones and naphtho-enaminodiones under microwave irradiation. These new series of molecules exhibited interesting activities against both parasites P. falciparum and L. donovani.29
One of the main goals in the area of organic synthesis oriented towards biologically active compounds is the research and development of environmentally safe methods in terms of efficiency, waste management and energy input, issues now addressed and termed “Green Chemistry”.30 Mechanochemistry fulfils this portfolio. According to IUPAC, a mechanochemical reaction is a “Chemical reaction that is induced by the direct absorption of mechanical energy”. Mechanochemistry for organic compounds started to be developed after the pioneering work reported by Toda in the 1980s31 and Kaupp.32 Mechanochemical synthesis has emerged as an efficient approach applicable in different fields,33 and particularly for biologically active molecules. This rapidly increasing field includes nowadays preparation of biologically active compounds,34,35 of Active Pharmaceutical Ingredients (APIs),36 cocrystals and many other aspects that can be entitled “medicinal mechanochemistry”.37
Here, we wish to share our discoveries regarding the unique outcome of a Biginelli MCR involving lawsone, diverse benzaldehydes, and ureas under mechanochemical conditions. Our investigation outlines the unprecedented formation of a “Biginelli-type” reaction resulting in non-cyclized three-component products, termed here ‘Biginelli-linear’.
Results and discussion
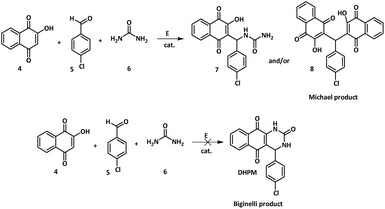
To set up efficient conditions, lawsone (4) was reacted with 4-chlorobenzaldehyde (5) and urea (6) under conventional conditions in common solvents, then in ionic liquids (ILs) and deep eutectic solvents (DES). Next, the reaction was explored by using microwave irradiation and mechanochemical activation. The expected cyclized DHPM Biginelli product was not observed under any of the above conditions. Instead, compound 7 (Biginelli-linear) and the Michael adduct 8 were exclusively observed. The results of the optimization of the reaction conditions are summarized in Table 1.| Entry | Activation/catalyst/temperature | Eq. 4/6/5 | Reaction time | Conversion 5 (%) | Yield 7 (%) | Yield 8 (%) |
|---|---|---|---|---|---|---|
| 1 | EtOH/5% Zn(OAc)2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
15 h | 50 | — | 45 |
| 2 | EtOH/20% pTSA | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
15 h | 50 | Trace | 41 |
| 3 | EtOH/5% Zn(OAc)2 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
15 h | 70 | — | 55 |
| 4 | EtOH (reflux)/10% H3PO2 | 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
15 h | 50 | 35 | 15 |
| 5 | [HNMP]HSO4 (80 °C) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
120 min | 60 | Trace | 47 |
| 6 | BMIM·NTF2 (80 °C) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
300 min | 84 | Trace | 46 |
| 7 | Choline chloride/urea (80 °C) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
60 min | 40 | 20 | 30 |
| 8 | Choline chloride/chloro-acetic acid (70 °C) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
60 min | 35 | — | 30 |
| 9 | μw-irradiation EtOH/10% H3PO2 | 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
120 min | 2 | Trace | — |
| 10 | μw-irradiation CH3CN/20% pTSA | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 1.1 |
60 min | 8 | 5 | — |
| 11 | MM400 (30 Hz)/10% H3PO2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2 × 40 min | 50 | 30 | — |
| 12 | MM400 (30 Hz)/20% pTSA | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2 × 40 min | 50 | 45 | — |
| 13 | MM400 (30 Hz)/20% pTSA | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2 × 40 min | 70 | 55 | — |
| 14 | P7 (800 rpm)/20% pTSA | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2 × 40 min | 95 | 90 | — |
| 15 | P7 (800 rpm)/20% pTSA | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
2 × 40 min | 95 | — | 91 |
Our initial efforts were focused on the conventional synthetic protocols according to literature procedures reported previously. When the reaction was carried out in EtOH catalysed by 5 mol% Zn(OAc)2 at r.t. overnight, in contrast to the results published by Patel,38 only the Michael adduct 8 (entry 1, Table 1) was obtained as a major product in 45% yield after purification (4-Cl-benzaldehyde conversion: 50%).
Surprisingly, no traces of any compound integrating all three Biginelli reactants (DHPM or 7) were found in the crude reaction mixture analysed by 1H NMR and mass spectrometry. The reaction between 2 eq. of lawsone and 1 eq. of 4-chlorobenzaldehyde under the same reaction conditions (entry 3, Table 1) expectedly afforded the Michael adduct 8. Under refluxing conditions in EtOH and catalysed by 10 mol% hypophosphorous acid39 the three-component reaction led to a mixture which upon purification afforded 8 in 15% yield and compound 7 in 35% yield (entry 4, Table 1). Again, no Biginelli-cyclized compound DHPM was observed.
Faced with these surprising results, we turned to ionic liquids (ILs) and deep eutectic solvents (DESs), as multicomponent Biginelli reactions have also been reported in such a medium. Among various possible ionic liquids, the homemade 1-methyl-2-pyrrolidonium hydrogen sulfate {[HNMP]HSO4} and the commercially available 1-butyl-3-methylimidazolium bis (trifluoro-methanesulfonyl) imide {BMIM·NTF2} were chosen.40,41 Reactions in these ILs, frequently used in the litterature for a classical Biginelli reaction (entries 5 and 6, Table 1), did not afford any Biginelli-type compound. Instead, the Michael adduct 8 was found to be the major product, obtained in 46–47% yield in both cases. Two DES media were also selected and prepared according to literature procedures. When the reaction was carried out in choline chloride/urea,42 the linear Biginelli-type compound 7 was obtained along with compound 8 in 20% and 30% yields respectively (entry 7, Table 1). When choline chloride/chloroacetic acid was used as DES (entry 8, Table 1),43 only compound 8 was obtained in 35% yield. The reaction was also tested under microwave irradiation (two examples presented in entries 9 and 10, Table 1) but disappointedly no meaningful reaction occurred as there was only 2% and 8% of aldehyde conversion and only traces (entry 9, Table 1) and <5% (entry 10, Table 1) of compound 7 were detected. Many modifications of the reaction conditions, including the catalyst variation (CH3COOH, p-TSA, H3PO2), the reaction time and solvent (EtOH, CH3CN, IL), failed to improve the reaction, all leading to compound 7 at a maximum of 5% yield.
Finally, the reaction was performed under mechanochemical activation. When operating an equimolar mixture of all three components with 10% of H3PO2 as the catalyst in a vibrator ball mill (MM400) working at frequency 30 Hz, for 2 successive runs of 40 min each, compound 7 was obtained in 30% yield, along with the non-reacted starting material (entry 11, Table 1). Modification of the catalyst to p-TSA (20%)44 or increase of the urea amount (1.5 equiv. instead of 1 equiv.) afforded compound 7 in 55% or 45% yield respectively (entries 12 and 13, Table 1) which was obtained by precipitation in a mixture of dichloromethane/diethyl ether (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). Gratifyingly, when operating with the planetary ball mill Pulverisette 7 (P7), and under the same conditions (entry 14, Table 1), compound 7 was obtained with an excellent 90% yield. Noteworthily, compound 7 was conveniently obtained by a simple wash with water to remove the urea excess and the catalyst. Surprisingly, under mechanochemical activation, the Michael adduct 8 was not formed, and the linear Biginelli-type compound 7 was in all cases the unique product of the multicomponent reaction. This result was further investigated by two additional experiments. The three-component reaction in solution (entry 2, Table 1) afforded only the Michael adduct 8 with 41% yield. The reaction between 2 eq. of lawsone and 1 eq. of 4-chlorobenzaldehyde under mechanochemical activation (entry 15, Table 1) afforded the Michael adduct 8 with an excellent 91% yield.
2). Gratifyingly, when operating with the planetary ball mill Pulverisette 7 (P7), and under the same conditions (entry 14, Table 1), compound 7 was obtained with an excellent 90% yield. Noteworthily, compound 7 was conveniently obtained by a simple wash with water to remove the urea excess and the catalyst. Surprisingly, under mechanochemical activation, the Michael adduct 8 was not formed, and the linear Biginelli-type compound 7 was in all cases the unique product of the multicomponent reaction. This result was further investigated by two additional experiments. The three-component reaction in solution (entry 2, Table 1) afforded only the Michael adduct 8 with 41% yield. The reaction between 2 eq. of lawsone and 1 eq. of 4-chlorobenzaldehyde under mechanochemical activation (entry 15, Table 1) afforded the Michael adduct 8 with an excellent 91% yield.
Scope of the mechanochemical activation when using different aldehydes and ureas
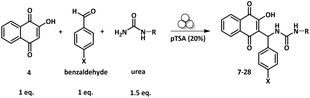
Having in hand the optimal reaction conditions to obtain compound 7 in high yield (entry 12, Table 1), we explored the substrate scope of this reaction, by modifying the para-benzaldehyde substituent and by using N-mono substituted urea derivatives. The results are summarized in Table 2.| Entry | Compound | X | R | Yielda (%) |
|---|---|---|---|---|
| a Isolated yield after filtration and isolation of the target product. | ||||
| 1 | 7 | Cl | H | 90 |
| 2 | 9 | F | Et | 63 |
| 3 | 10 | Cl | Et | 62 |
| 4 | 11 | Br | Et | 70 |
| 5 | 12 | I | Et | 70 |
| 6 | 13 | NO2 | Et | 85 |
| 7 | 14 | CF3 | Et | 90 |
| 8 | 15 | CH3 | Et | 70 |
| 9 | 16 | F | n-Bu | 81 |
| 10 | 17 | Cl | n-Bu | 85 |
| 11 | 18 | Br | n-Bu | 95 |
| 12 | 19 | NO2 | n-Bu | 90 |
| 13 | 20 | CF3 | n-Bu | 80 |
| 14 | 21 | F | CH2C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH CH |
83 |
| 15 | 22 | Cl | CH2C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH CH |
73 |
| 16 | 23 | Br | CH2C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH CH |
88 |
| 17 | 24 | NO2 | CH2C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH CH |
70 |
| 18 | 25 | CF3 | CH2C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH CH |
82 |
| 19 | 26 | Cl | (CH2)2OH | 80 |
| 20 | 27 | NO2 | (CH2)2OH | 82 |
| 21 | 28 | CF3 | (CH2)2OH | 80 |
All reactions were conducted in a P7 planetary ball mill (the number of runs was studied and optimized for each reaction) catalysed by 20 mol% pTSA, while the ratio of the three reactants was lawsone/urea/aldehyde 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5
1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
First, N-ethylurea was chosen to undergo the reaction with lawsone and various para-substituted benzaldehydes (entries 2–8, Table 2). In all cases where the benzaldehyde was substituted by a halogen atom (entries 2–5, Table 2) or a methyl group (entry 8, Table 2), the non-cyclized target products 9, 10, 11, 12 and 15 were obtained in a good range of 62–70% yields.
When using electron withdrawing p-nitrobenzaldehyde or p-trifluoromethylbenzaldehyde, the yields of the expected products 13 and 14 rose up to 85–90% (entries 6 and 7, respectively, Table 2). For all cases studied, no side-products were observed and the selectivity of the reaction to provide the Biginelli-linear derivative was over 97%. The target products were conveniently isolated from the reaction mixtures as powders, by simply washing with water and crystallizing in the minimum volume of dichloromethane/diethyl ether. No column chromatography was used for purifications of any reaction mixture mentioned herein.
When N-ethylurea was changed to N-butylurea the conversion of both starting materials in deficit (benzaldehyde and lawsone) was complete under the same mechanochemical conditions. All target compounds 16, 17, 18, 19 and 20 (entries 9–13, Table 2) were obtained with excellent yields ranging from 80 to 95%. Analogous to N-ethylurea, a unique major product was obtained, and no side-products were formed. Similar results were obtained with N-propargylurea affording compounds 21–25 with yields in the range of 73–88% (entries 14–18, Table 2). Finally, 2-hydroxyethylurea was also chosen to undergo this three-component reaction. The expected products 26–28 were obtained in 80–82% yield, whatever the benzaldehyde substituent was, –Cl, –NO2 or –CF3 (entries 19–20, Table 2). Together, a series of 20 Biginelli-linear derivatives were obtained in isolated yields varying between a good value of 62% and an excellent value of 95%. While there are no other side products and the aldehyde conversion is complete, the discrepancies in yields might result from the solubility differences of the formed compounds in the mixture of organic solvents used during the crystallization process.
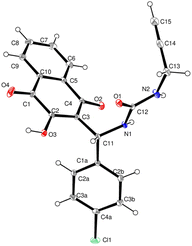
Among all compounds, 22 was crystallized and used for X-ray diffraction analysis also confirming our structure proposal. Crystallization was achieved in the solvent mixture of dichloromethane/methanol (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The obtained single crystals appeared as yellow platelets.
1). The obtained single crystals appeared as yellow platelets.
The compound exhibited a triclinic P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group (find crystallographic data in the ESI†) under Mo-Kα radiation (λ = 0.71073 Å). Inspection of the structure exhibited dihedral angles of 19.7° for O3/O2/C3/C11, of 9.2° for C2/C3/C11/N1, of 83.6° for C4/C3/C11/N1 and of 79.5° for C3/C11/N1/C12, indicating that none of the three pi system components of the molecule (urea, naphthoquinone and 4-chlorophenyl rings) share coplanarity. All hydrogen bonds N1–H1, N2–H2, O3–H3 and C11–H11 had consistent values. Finally, it's noteworthy to highlight the significance of the very long distance between the atoms N2–C2 (d = 5.3 Å) and N2–O3 (d = 5.8 Å) for assessing any potential interactions that might favor the formation of the cyclized Biginelli DHPM (Fig. 1).Considering all experimental data, we conclude that when reacting under mechanochemical conditions, lawsone with aryl aldehydes and ureas, the unique ‘Biginelli-linear’ compounds containing all three starting reagents were obtained in good to excellent yields. To the best of our knowledge, this is the first time that a linear Biginelli compound is isolated. Considering the three routes proposed for a classical Biginelli reaction (Scheme 1), two of them, namely the iminium and the Knoevenagel ones, go through a Biginelli-linear intermediate before cyclizing.
space group (find crystallographic data in the ESI†) under Mo-Kα radiation (λ = 0.71073 Å). Inspection of the structure exhibited dihedral angles of 19.7° for O3/O2/C3/C11, of 9.2° for C2/C3/C11/N1, of 83.6° for C4/C3/C11/N1 and of 79.5° for C3/C11/N1/C12, indicating that none of the three pi system components of the molecule (urea, naphthoquinone and 4-chlorophenyl rings) share coplanarity. All hydrogen bonds N1–H1, N2–H2, O3–H3 and C11–H11 had consistent values. Finally, it's noteworthy to highlight the significance of the very long distance between the atoms N2–C2 (d = 5.3 Å) and N2–O3 (d = 5.8 Å) for assessing any potential interactions that might favor the formation of the cyclized Biginelli DHPM (Fig. 1).Considering all experimental data, we conclude that when reacting under mechanochemical conditions, lawsone with aryl aldehydes and ureas, the unique ‘Biginelli-linear’ compounds containing all three starting reagents were obtained in good to excellent yields. To the best of our knowledge, this is the first time that a linear Biginelli compound is isolated. Considering the three routes proposed for a classical Biginelli reaction (Scheme 1), two of them, namely the iminium and the Knoevenagel ones, go through a Biginelli-linear intermediate before cyclizing.
Without having the aim to undergo an extensive theoretical study concerning all possible mechanisms and potential intermediates that could be in equilibrium, we envisaged to consider only the fact that we obtain either starting compounds, either the Michael adduct or the Biginelli-linear derivative.
In that respect, in order to compare our findings regarding Michael adduct vs. Biginelli-linear we considered the reaction mechanism starting from the addition of lawsone to the aldehyde.
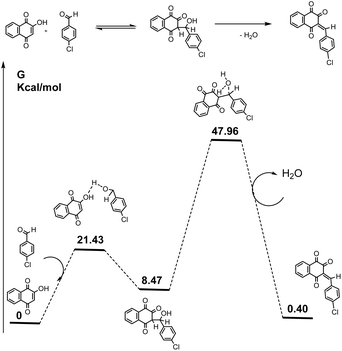
A preliminary modelling in the gas phase, using the B3LYP functional coupled with a 6-31+G(d,p) Gaussian basis set and accounting for dispersion (D3), when using p-chlorobenzaldehyde, revealed the energy profile shown in Scheme 2. The nucleophilic attack of lawsone to the carbonyl group of the aldehyde, led to the formation of an initial adduct with rapid kinetics. Specifically, the calculated activation energy for the intermediate formation was 21.43 kcal mol−1, while the energy level of the intermediate is situated at 8.47 kcal mol−1. At this stage, a slower elimination of a water molecule with an energy barrier of 47.96 kcal mol−1 and an energy difference from the first intermediate of 39.49 kcal mol−1, can lead to formation of the Knoevenagel intermediate.
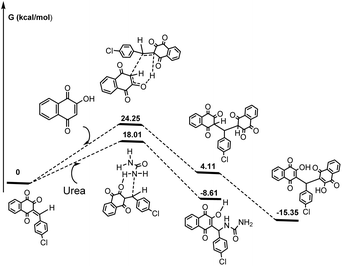
Two pathways emerged starting from the Knoevenagel intermediate (Scheme 3). The first one involved a Michael addition between the Knoevenagel intermediate and lawsone, with an activation energy of 24.25 kcal mol−1. This resulted in the formation of a significantly stable enolic derivative through a keto form. This process represented the driving force of this step. Next, we looked on the reaction coordinates when a urea was present along with the Knoevenagel intermediate, leading to the formation of a three-component Biginelli-linear adduct. This reaction exhibited a more favorable energy profile compared to the previous Michael one. Its activation energy was at least 6.24 kcal lower than that leading to the Michael adduct (Scheme 3).
In addition, the Michael triketone derivative was energetically less favorable than the final linear adduct by 12.72 kcal. It is the dienolic form of the Michael adduct that can be extremely stable, this presumably mostly occurring under protic conditions.
It should be noted that calculations considering ethanol as the solvent (solvent effects were taken into account using the conductor-like polarizable continuum model (CPCM)) did not modify in a significant manner both barriers leading to a Michael adduct (around 25 kcal mol−1) and to a Biginelli-linear derivative (around 20 kcal mol−1). This could be probably more pronounced when using catalysts like Zn(OAc)2, ILs or DES (choline chloride/chloroacetic acid) leading more favourably to the Michael adduct, than when using H3PO2 which was a weak monobasic acid or DES choline chloride/urea where the Biginelli linear compound can also be obtained. We believe that these findings were in complete agreement with our experimental results and the consequent observations.
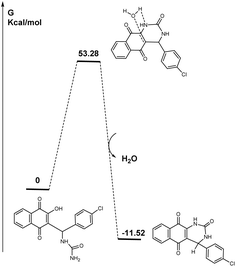
Finally, concerning the cyclization impossibility for the DHPM obtention in the solid-state reaction, it is interesting to note two points. The first one relates to the activation energy required to cyclize the compound. In fact, it is necessary to overcome a barrier of 53.28 kcal mol−1, which was not at all favourable. The second point concerns the structure. In fact, the calculated structure aligns with the resulting structure obtained from X-ray resolution. Here, we have a geometric structure where the “amido” part is positioned opposite to the carbon it is supposed to attack. As a result, even if we can consider that by mechanochemical friction we can transfer energy to the Biginelli-linear derivative, the amount is not sufficent for the cyclization process to take place even if the cyclized structure is thermodynamically favoured (−11.52 kcal mol−1) (Scheme 4).
Even if we cannot directly transpose the calculation results in the gas or by using a simple liquid continuum phase to solid state issues, the results obtained can give an idea (are representative) of the energies engaged in the process and the energy gaps. DFT calculations have already been reported to be used before undergoing theoretical fundamental studies on solid state organic mechanochemical reactions.45
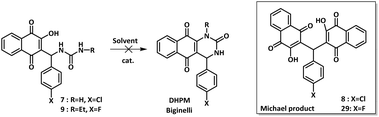
Attempts for cyclization of Biginelli-linear derivatives 7 and 9
As the spontaneous Biginelli-type cyclization was found to be challenging in solid state and solution, attempts enforcing cyclization of the ‘Biginelli-linear’ products were carried out in protic solvent and IL and DES media. Both compounds 7 and 9 were chosen to undergo the cyclization studies in order to ensure that urea substitution was not affecting the cyclization step. For compound 9 all attempts are summarized in Table 3, while for compound 7 three attempts have been made, namely in EtOH and {[HNMP]HSO4} IL and choline chloride/chloroacetic acid DES.| Entry | Activation/catalyst/temperature (°C) | Time (min) | Yield of Michael adduct 29 (8)a |
|---|---|---|---|
| a Isolated yield after filtration and isolation of the target product. | |||
| 1 | EtOH/5% Zn(OAc)2/rt | Overnight | — |
| 2 | {[HNMP]+[HSO4]−}/80 °C | 60 | 40% (43%) |
| 3 | Choline chloride/chloroacetic acid/70 °C | 180 | <5% |
| 4 | CH3COOH (glacial)/μw/160 °C | 30 | <5% |
| 5 | P2O5/MeSO3H/rt | 180 | — |
When compound 7 or 9 was allowed to react in ethanol catalyzed by Zn(OAc)2 as described for other Biginelli cyclizations,39 no reaction occurred and the starting compounds were fully recovered (entry 1, Table 3). When submitting compound 7 or 9 to cyclization in homemade {[HNMP]HSO4} IL media40 no cyclization was observed either. To our surprise, the corresponding Michael adducts were isolated as main products in 40% and 43% yields respectively after crushing the reaction mixture into ice and collecting the provided precipitates (entry 2, Table 3). Disappointingly, when conducting the cyclization attempts in choline chloride/chloroacetic acid DES media,43 less than 5% of Michael adducts 29 and 8 were observed (estimated by 1H NMR) along with the non-reacted starting material (entry 3, Table 3). When compound 7 was chosen to undergo the same cyclization, it also provided similar results. Stronger reaction conditions, glacial acetic acid under microwave irradiation applied to compound 9 led to similar Michael product formation, compound 29 was obtained in <5% yield (entry 4, Table 3) along with the decomposed starting compound in a quite sluggish and unstable reaction mixture. Finally, the same reaction catalysed by Eaton's reagent (phosphorus pentoxide in methanesulfonic acid 1/10)46 also afforded a complex mixture that was unstable under various purification conditions. For these two latter assays, all three counterparts (lawsone, urea, benzaldehyde) were identified in the reaction media by spectroscopic techniques.
Given these findings, the transformation of the ‘Biginelli-linear’ products into Michael adducts in solution serves as direct evidence for the generation of the Knoevenagel intermediate through equilibria originating from both the iminium and Knoevenagel mechanisms (Scheme 1). Furthermore, it underscores that the rate-determining step (RDS) involves the cleavage of the C–O bond during the Knoevenagel reaction.
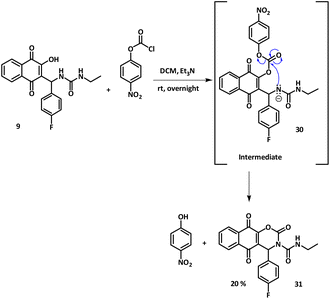
After all these attempts, new conditions/reactants needed to be elaborated in order to succeed in a cyclization having in mind direct activation of the lawsone enol functional group. In that respect and after a few attempts, compound 9 was submitted to react with para-nitrophenyl chloroformate in dichloromethane at rt, overnight, with Et3N as a base. The cyclized derivative 31 was obtained in 20% yield after purification (Scheme 5). To the best of our knowledge, this is the first time that this type of cyclized carbamate structure is obtained: 2-hydroxy-1,4-naphthoquinone derivatives bearing a urea fragment, paving the way for developing this synthetic approach.
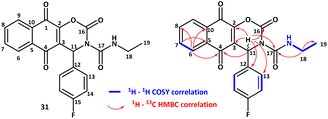
The structure of compound 31 was confirmed by 2D-NMR analyses at 298 K in CDCl3. All 1H and 13C signals were assigned based on the chemical shifts, spin–spin coupling constants, splitting patterns, and signal intensities by using 1H–1H COSY, 1H–13C HSQC, and 1H–13C HMBC experiments (see the ESI†) 2D 1H and 13C NMR correlation experiments in CDCl3 (298 K) for compound 31 (Scheme 6).
1H NMR spectrum displayed some important characteristics: the NH resonance appeared as a broad triplet at 8.41 ppm indicative of a coupling to the neighbouring CH2 group as proved by the COSY experiment, while H-11 was found at 6.84 as a sharp singlet, as it had no neighbouring protons. Interestingly, protons Ha-18 and Hb-18 appeared as diastereotopic because of structure rigidity and they exhibited coupling with both neighbouring CH3 and NH. The 13C resonance data revealed 19 signals in total for each type of carbon, permitting the non-ambiguous structural identification of 31. Among them, the signals at 176.3 and 180.9 ppm were attributed to the two carbonyl groups of the naphthoquinone system while the urea carbon C-17 resonated at 151.3 ppm and the newly formed carbamate carbonyl C-16 signal appeared at 148.5 ppm. Finally, the 13C–1H HMBC experiment allowed the identification of all long-range couplings between protons and carbons: the most important ones are depicted in Scheme 6. In addition, the fact that H-11 was correlated with all carbons C-2, C-3, C-4, C-16, C-12, C-13, C-14 and C-17 and that H-11 and C-11 chemical shifts were found dislocated compared to the starting compound 9 confirmed that the cyclization occurred, leading to the formation of a stable carbamate ring.
Conclusion
Our investigation focused on the three-component Biginelli-type reaction utilizing the 2-hydroxy-1,4-naphthoquinone compound (lawsone) 4 as the active methylene system in conjunction with aryl aldehydes and ureas. Notably, classical Biginelli cyclized DHPM was consistently elusive under all explored experimental conditions. While “liquid conditions,” particularly under weak acid catalysis, predominantly yielded the Michael adduct, successful isolation of ‘Biginelli-linear’ derivatives was achieved under mechanochemical conditions with good to excellent yields. This marks the first instance where a three-component Biginelli reaction resulted in Biginelli-type linear adducts, assumed to be intermediates in the classical reaction rather than cyclized DHPMs. All attempts to cyclize the latter Biginelli-linear compound in a final step proved unsuccessful, aligning with both experimental and theoretical results highlighting the energetically improbable pathway for final cyclization. The formation of Biginelli-linear products is attributed to the iminium and/or Knoevenagel mechanisms.Remarkably, we achieved the novel cyclization of a Biginelli-linear derivative into a cyclic carbamate, presenting an innovative development. We believe that our findings have opened new and promising perspectives.
Perspectives
Under the reaction conditions examined here, two out of three classical Biginelli mechanisms are apparently operating with the lawsone reagent. In future work it is important to explore ways to selectively conduce the reaction through the enamine-mechanism. It is noteworthy to point out the work of Cook and Clemmer47 concerning a microdroplet-accelerated Biginelli reaction providing evidence for two competing pathways, the enamine and the Knoevenagel one. The scope of the carbamate cyclisation reaction is underway in order to find the optimal conditions and the scope of the Biginelli-linear derivatives that can efficiently undergo cyclization. Finally, the outcome of this multicomponent reaction with other cyclic β-dicarbonyl systems (dimedone, syncarpic acid…) will be investigated.Data availability
Data for the crystal structure reported in this paper have been deposited at the Cambridge Crystallographic Centre (CCDC) under the deposition 2268819. All other data supporting the findings of this study, including experimental procedures, characterization of compounds and computational data are available within the paper and its ESI files.†Author contributions
C. L. K. performed all experiments and analysed data, prepared the experimental part of the ESI file and was implicated in all draft changes. C. B. supervised all 2D NMR analyses. L. V. performed the X-ray structure and analysis. A. R. was implicated for initial discussions on the project and for draft ameliorations. P. C. performed the computational study. J. M. S. performed the computational data, wrote the computational part of the manuscript and prepared the corresponding ESI. M. B. conceived, led the project, analysed all data and prepared the final draft along with C. L. K. and J. M. S. All authors discussed the results and commented on the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge the Centre National de la Recherche Scientifique (CNRS) and the Université Paul Sabatier for financial support. We (C. L. K. and M. B.) also thank the Institut National de la Santé et de la Recherche Médicale (Inserm) and the Fondation pour la Recherche Médicale, FRM “EQU202103012596” for financial support. We also thank the “Institut de Chimie de Toulouse” (ICT, UAR2599) for all the MS spectra and for providing access to HPLC equipment, “PICT” for UPLC equipment and Isabelle Fabing, engineer in charge of all this equipment, for technical assistance and purification of the final cyclized carbamate derivative. The authors also gratefully acknowledge Professor Alexander Dömling (ERA Chair of Innovative Chemistry Palackӯ University, Olomouc, Czech Republic) for numerous and fruitful discussions on the multicomponent reactions and on the obtention of the final draft version.Notes and references
- J. Y. Zhu and H. Bienaymé, Multicomponent Reactions, Wiley-VCH, Weinheim, 2005 and references cited therein Search PubMed.
- T. J. J. Müller, Multicomponent Reactions, Georg Thieme, Stuttgart, Germany, 2014, vol. 1 and 2 Search PubMed.
- A. Dömling and I. Ugi, Angew. Chem., Int. Ed., 2000, 39, 3168–3210 CrossRef.
- S. Garbarino, D. Ravelli, S. Protti and A. Basso, Angew. Chem., Int. Ed., 2016, 55, 15476–15484 CrossRef PubMed.
- B. Ganem, Acc. Chem. Res., 2009, 42, 463–472 CrossRef CAS.
- B. B. Touré and D. G. Hall, Chem. Rev., 2009, 109, 4439–4486 CrossRef.
- M. D. Sanchez Duque, O. Basle, Y. Genisson, J. C. Plaquevent, X. Bugaut, T. Constantieux and J. Rodriguez, Angew. Chem., Int. Ed., 2013, 52, 14143–14146 CrossRef CAS PubMed.
- Y. Mendez, K. Perez-Labrada, J. Gonzalez-Bacerio, G. Valdes, M. A. de los Chaves, J. Osuna, J. L. Charli, I. Pascual and D. G. Rivera, ChemMedChem, 2014, 9, 2351–2359 CrossRef CAS PubMed.
- G. Tron, A. Minassi and G. Appendino, Eur. J. Org. Chem., 2011, 5541–5550 CrossRef CAS.
- B. C. Raju, R. N. Rao, P. Suman, P. Yogeeswari, D. Sriram, T. B. Shaik and S. V. Kalivendi, Bioorg. Med. Chem. Lett., 2011, 21(10), 2855–2859 CrossRef.
- A. N. Chiang, J.-C. Valderamos, R. Balachandran, R. J. Chovatiya, B. P. Mead, C. Schneider, S. L. Bell, M. G. Klein, D. M. Hyrun, X. S. Chen, B. W. Day, D. A. Fidock, P. Wipf and J. L. Brodsky, Bioorg. Med. Chem., 2009, 17, 1527–1533 CrossRef CAS PubMed.
- A. D. Patil, N. V. Kumar, W. C. Kokke, M. F. Bean, A. J. Freyer, C. De Brosse, S. Mai, A. Truneh and B. Carte, J. Org. Chem., 1995, 60(5), 1182–1188 CrossRef CAS.
- P. A. Barsanti, W. Wang, Z. J. Ni, D. Duhl, N. Brammeier, E. Martin, D. Bussiere and A. O. Walter, Bioorg. Med. Chem. Lett., 2010, 20(1), 157–160 CrossRef CAS PubMed.
- O. Alam, S. A. Khan, N. Siddiqui, W. Ahsan, S. P. Verma and S. J. Gilani, Eur. J. Med. Chem., 2010, 45(11), 5113–5119 CrossRef CAS PubMed.
- H. Cho, Y. Nishimura, Y. Yasui, S. Kobayashi, S. Yoshida, E. Kwon and M. Yamagushi, Tetrahedron, 2011, 52, 2661–2669 CrossRef.
- L. M. Ramos, A. Y. Ponce, D. Leon, M. R. Santos, H. C. B. Oliveira, A. F. De Gomes, F. C. Gozzo, A. L. Oliveira and B. A. D. De Neto, J. Org. Chem., 2012, 77, 10184–10193 CrossRef CAS PubMed.
- M. L. Ramos, B. C. Guido, C. C. Nobrega, J. R. Correa, R. G. Silva, C. B. de Oliveira Heibbe, A. F. Gomes, F. C. Gozzo and A. D. Neto, Eur. J. Chem., 2013, 19, 4156–4168 CrossRef PubMed.
- H. G. O. Alvim, T. B. Lima, A. L. de Oliveira, H. C. B. de Oliveira, F. M. Silva, F. C. Gozzo, R. Y. Souza, V. A. da Silva and B. A. D. Neto, J. Org. Chem., 2014, 79, 3383–3397 CrossRef CAS PubMed.
- C. O. Kappe, J. Org. Chem., 1997, 62, 7201–7204 CrossRef CAS PubMed.
- K. Folkers and T. B. Johnson, J. Am. Chem. Soc., 1933, 55, 3784–3791 CrossRef CAS.
- F. Sweet and J. D. Fissekis, J. Am. Chem. Soc., 1973, 95, 8741–8749 CrossRef CAS.
- M. Puripat, R. Ramozzi, M. Hatanaka, W. Parasuk, V. Parasuk and K. Morokuma, J. Org. Chem., 2015, 80(14), 6959–6967 CrossRef CAS PubMed.
- P. T. Anastas and J. C. Warner, Green Chemistry, Theory and Practice, Oxford University Press, Oxford, UK, 2000, ISBN 0198502346/9780198502340 Search PubMed.
- N. K. Ganesh, D. Zhang, S. J. Miller, K. Rossen, P. J. Chirik, M. C. Kozlowski, J. B. Zimmerman, B. W. Brooks, P. E. Savage, D. T. Allen and A. M. Voutchkova-Kostal, Org. Process Res. Dev., 2021, 25(7), 1455–1459 CrossRef.
- T. Schaub, Chem.–Eur. J., 2021, 27, 1865–1869 CrossRef CAS PubMed.
- P. K. Sahoo, A. Bose and P. Mal, Eur. J. Org Chem., 2015, 6994–6998 CrossRef CAS.
- F. Krauskopf, K.-N. Truong, K. Rissanen and K. Bolm, Org. Lett., 2021, 23, 2699–2703 CrossRef CAS PubMed.
- B. A. Akhoon, K. P. Singh, M. Varshney, S. K. Gupta, Y. Shukla and S. K. Gupta, PLoS One, 2014, 9(10), e110041 CrossRef PubMed.
- C. L. Koumpoura, M. Nguyen, C. Bijani, L. Vendier, E. G. Salina, S. Buroni, G. Degiacomi, S. Cojean, P. M. Loiseau, F. Benoit-Vical, A. T. García-Sosa and M. Baltas, ACS Omega, 2022, 7(40), 35635–35655 CrossRef CAS PubMed.
- P. T. Anastas and J. C. Warner, Green Chemistry, Oxford University Press, 1998, ISBN 0198502346 Search PubMed.
- F. Toda, K. Tanaka and S. Iwata, J. Org. Chem., 1989, 54, 3007–3009 CrossRef CAS.
- G. Kaupp, CrystEngCom, 2009, 11, 388–403 RSC.
- G. W. Wang, Chem. Soc. Rev., 2013, 42, 7668–7700 RSC.
- O. Bento, F. Luttringer, T. M. El Dine, N. Petry, X. Bantreil and F. Lamaty, Eur. J. Org. Chem., 2022, e202101516 CrossRef CAS.
- P. F. M. Oliveira, et al , Molecules, 2017, 22, 1457 CrossRef PubMed.
- I. Sovic, et al , ACS Omega, 2020, 5, 28663–286672 CrossRef CAS PubMed.
- D. Tan, L. Loots and T. Friscic, Chem. Commun., 2016, 52, 7760–7781 RSC.
- P. N. Patel, N. C. Patel and D. H. Desai, Russ. J. Org. Chem., 2022, 58, 536–540 CrossRef CAS.
- Z. Moutaoukil, C. Ronco and R. Benhida, J. Saudi Chem. Soc., 2022, 26, 101398 CrossRef CAS.
- S. R. Patil, A. S. Choudhary, V. S. Patil and N. Sekar, Fibers Polym., 2015, 16, 2349–2358 CrossRef CAS.
- H. G. O. Alvim, T. B. de Lima and H. C. B. de Oliveira, ACS Catal., 2013, 3, 1420–1430 CrossRef CAS.
- S. Lakshmanan, D. Govindaraj, N. Ramalakshmi and S. A. Antony, J. Mol. Struct., 2017, 1150, 88–95 CrossRef CAS.
- A. R. Momeni, H. A. Samimi and H. Vaezzadeh, Chem. Methodol., 2018, 253–261 CAS.
- A. K. Bose, S. Pednekar, S. N. Ganguly, G. Chakraborty and M. S. Manhas, Tetrahedron Lett., 2004, 45, 8351–8353 CrossRef CAS.
- W. Sakai, L. Gonnet, N. Haruta, T. Sato and M. Baron, J. Phys. Chem. A, 2023, 127, 5790–5794 CrossRef CAS.
- A. Borse, M. Patil, N. Patil and R. Shinde, ISRN Org. Chem., 2012, 1–6 Search PubMed.
- N. Sahota, D. I. AbuSalim, M. L. Wang, C. J. Brown, Z. Zhang, El-T. J. Baba, S. P. Cook and D. E. Clemmer, Chem. Sci., 2019, 10(18), 4822–4827 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2268819. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3mr00032j |
| This journal is © The Royal Society of Chemistry 2024 |