The blue shift of fluorescence emission reveals the dsRNA-loading capacity of cationic nanocarriers†
Peng
Li
*ab,
Jiaqi
Ma
b,
Shangxu
Jiang
 b,
Wei
Peng
cd,
Yun
Huang
b,
Wei
Peng
cd,
Yun
Huang
 e,
Run
Zhang
e,
Run
Zhang
 b and
Zhi Ping
Xu
*bf
b and
Zhi Ping
Xu
*bf
aMoganshan Institute, Zhejiang University of Technology, Huzhou, Zhejiang 313200, China
bAustralian Institute for Bioengineering and Nanotechnology, The University of Queensland, St Lucia, QLD 4072, Australia. E-mail: peng.li3@uq.net.au
cQueensland Micro- and Nanotechnology Centre, Griffith University, Nathan, QLD 4111, Australia
dSchool of Engineering and Built Environment, Griffith University, Nathan, QLD 4111, Australia
eAustralian National Fabrication Facility-Queensland Node, St Lucia, QLD 4072, Australia
fInstitute of Systems and Physical Biology, Shenzhen Bay Laboratory, Shenzhen, Guangdong 518107, China. E-mail: gordonxu@szbl.ac.cn
First published on 1st October 2024
Abstract
The loading capacity of dsRNA by nanocarriers is a key parameter in the process of RNAi commercialisation. In this research, a sustainable, simple, and cheap method was developed to determine the dsRNA loading capacity by popular cationic nanocarriers. In this method, the fluorescence emission of the cationic nanocarrier + (dsRNA–DAPI) shows blue shift in comparison to that of dsRNA–DAPI. When dsRNA–DAPI is completely loaded by cationic nanocarriers, the fluorescence peak coincides with the emission wavelength of DNA–DAPI. The samples of nanocarrier + (dsRNA–DAPI) are simply tested in a fluorometer with no damage to the samples. The reported method is simpler, cheaper, and more sustainable than gel electrophoresis and HPLC, and will fulfill the industry need for reliable and quick quality administration/control in the production process.
Introduction
RNA interference-based therapies are promising approaches towards effective and safe treatment in clinics and agriculture.1,2 The specific, efficient, and biocompatible properties could initiate a revolution towards eco-sustainable production in the two major fields. To actualise the practical application of RNA interference, nanocarriers are compulsory in the formulation due to the vulnerability of RNA.3 Among the nanocarriers with significant prospectives of commercialisation, cationic nanocarriers have the advantages of stable properties, consistent loading performance, and simple preparation,4 though excessive positively charged carriers are cytotoxic. Herein, the loading capacity of cationic nanocarriers is an essential criterion for the evaluation of application safety, production cost, and consequent carbon emission. Practically, the reciprocal of the loading capacity, i.e., the lowest nanocarrier dosage for completely loading unit mass of dsRNA, is tested and commonly used, as the amount of dsRNA should be kept constant in the current methods.The most common method for determining the loading capacity is gel electrophoresis.5–7 Free RNA is attracted to the anode by electrostatic force while the nanocarrier–RNA hybrids remain in the sample wells. High-performance liquid chromatography (HPLC) is commonly used for the separation and quantification of RNA and may be adopted as well.8 UV absorbance at 260 nm can indicate the free RNA concentration in the solution, if the nanocarrier + RNA hybrids are removed.9 Quant-iT RiboGreen RNA reagent is one of the most sensitive dyes for RNA quantification and can be used to determine the loading capacity,10 while each kit costs ∼AUD$ 1500. All above techniques have a long time of process and high possibility of operational error, which does not meet the need of rapid, accurate, and cheap quality control for the benefit of industrial production. Moreover, ethidium bromide is commonly used in gel electrophoresis, which is toxic and carcinogenic (GHS06 and 08 in materials safety data sheet (MSDS)), possessing adverse potential to environmental sustainability and occupational health and safety. Fluorescence-based methods are popular to evaluate the encapsulation efficiency by nanocapsules via binding of fluorescent dyes.11 Chali et al. recently reported one application of Cy5-oligo, which might be adopted to test dsRNA encapsulation as dsRNA can also be stained by Cy5.12
To meet industrial needs, we have developed a simple, cheap, and sustainable method to determine the loading capacity of dsRNA by cationic nanocarriers. This method adopts 4′,6-diamidino-2-phenylindole (DAPI) to demonstrate the binding behaviour between dsRNA and DAPI, which would be influenced by cationic nanocarriers. Besides the popular application as fluorescent dye for staining DNA, DAPI can also bind dsRNA with much lower fluorescence intensity. When DAPI binds at AT sites of DNA in the minor groove, DAPI's fluorescence peak is blue shifted. In sharp contrast, DAPI's fluorescence peak is red shifted when DAPI binds at AU sites of dsRNA via intercalation (Fig. S1, ESI†).13 We have found that the addition of cationic nanocarrier to dsRNA–DAPI solution pushed the DAPI's fluorescence emission peak towards lower wavelengths and this peak wavelength reached a similar value to that of DNA–DAPI once dsRNA was completely loaded. This method involves only a fluorometer and DAPI besides dsRNA and cationic nanocarrier, which is simpler, cheaper, and faster than any of the above methods. DAPI is categorised as GHS07 in MSDS, which is much safer than ethidium bromide. According to Regulation (EC) No. 1907/2006 (REACH), DAPI is not considered to have persistent, bioaccumulative and toxic (PBT) or very persistent and very bioaccumulative (vPvB) characteristics. In addition, the samples are not destroyed in the process and can be re-used directly. The fluorescence emission has stable intensity for at least 10 days if stored well (Fig. S2, ESI†), providing the possibility of repeated confirmation. The sustainable and reassuring advantages of this method will have significant merits to the industrial acts of dsRNA applications, which other methods do not have (Table S1, ESI†).
Results and discussion
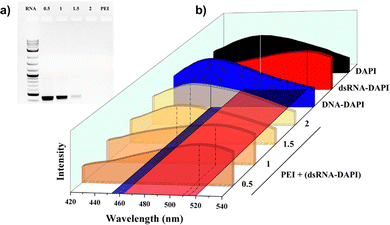
Polyethylene imine (PEI) was adopted to demonstrate how its loading capacity was simply determined. PEI is a typical example of cationic polymers that are popularly adopted to conjugate with silica nanoparticles,14 carbon nanoparticles,15 metal nanoparticles,16 or other polymers17 for loading dsRNA. The dsRNA loading capacities of all the above carriers are essentially determined by the conjugating capacity of the cationic polymers.As shown in Fig. 1a, the conventional gel electrophoresis has clearly indicated that dsRNA was completely loaded by PEI at N/P ratio = 2. In Fig. 1b, the emission wavelengths of PEI + (dsRNA–DAPI) samples shifted to smaller wavelengths (blue shift) with increasing N/P ratios until the wavelength reached a similar value to DNA–DAPI. Notably, PEI, with pKa ranging between 8–10, carried positive charges in the working conditions (pH = ∼7, Table S2, ESI†) while DAPI also had a positive charge. Our hypothesis is that PEI may push DAPI out from the intercalation position to the minor groove of dsRNA, which leads to blue-shifted emission spectra from 520 to 453 nm. The DAPI molecules seemed to be all pushed to the minor groove position when N/P ratio = 2 or greater, showing the same emission wavelength as DNA–DAPI (i.e. 453 nm). The samples with less N/P ratios were actually a mixture of free dsRNA–DAPI and PEI + (dsRNA–DAPI), whose peaks were merged by the emissions from intercalated DAPI at 520 nm and minor-grooved DAPI at 453 nm.
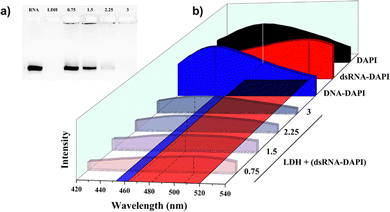
In Fig. 2, another kind of cationic nanocarrier, layered double hydroxide (LDH, Fig. S3, ESI†), was adopted as well to confirm our findings. Fig. 2a confirms that the complete loading occurred at LDH/dsRNA mass ratio = 3, which agreed with our previous report.18Fig. 2b illustrates the same shifting mode of DAPI's emission peak as PEI + (dsRNA–DAPI). The shifting started at 520 nm and stopped at 453 nm as well, corresponding to the DAPI emissions in the intercalation position and minor groove, respectively. The same behaviour of LDH + (dsRNA–DAPI) has thus supported our hypothesis about the essential role of electrostatic forces.
Addition of PEI or LDH into DAPI solution in the same conditions as above did not show any blue shift but little red shift (Fig. S4, ESI†), suggesting that the above-mentioned blue shift was not caused by interactions between cationic nanocarriers and DAPI but the variation of binding modes between dsRNA and DAPI due to the influence from the cationic nanocarrier. The common reasons for the fluorescence blue shift include the variation of the solvent, ionic strength, oxygen content, molecular arrangement, and charge distribution. In our cases, the solvent was always MilliQ water; the working concentrations of DAPI were at the level of ng μL−1, which is too low to induce a significant change of emission wavelength; the oxygen content was not changed in the process. Variation of the charge distribution as well as the molecule configuration would be the main reason of the blue shift in this nanocarrier + (dsRNA–DAPI) system. DAPI, with a positive charge, binds AU sites of dsRNA via intercalation since the minor groove of dsRNA is more positive than DNA (Fig. S1, ESI†).13 Once the cationic nanocarrier was added, the external positive charge would influence the charge distribution and overwhelm the repulsion between the minor groove of dsRNA and DAPI, pushing DAPI out from the intercalated position. Fig. 3 illustrates the proposed mechanism.
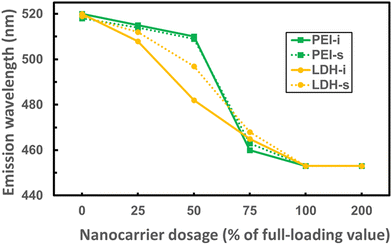
To confirm the recycling of the sample, the experiments were repeated by adding PEI or LDH into the same cuvette of dsRNA–DAPI sample stepwise (detailed in the ESI†) with the same N/P or LDH/dsRNA ratios used in Fig. 1 and 2. Fig. 4 summarises the trends of emission wavelengths with increasing PEI or LDH dosages. Data from Fig. 1 and 2 were denoted with “−i” (isolated samples) while the new data were denoted with “−s” (stepwise samples). The stepwise path consumes much less dsRNA and has less chance of operational error than the isolated path. The coincidence at 520 and 453 nm of all 4 curves suggests that complete loading of dsRNA by PEI and LDH was demonstrated correctly regardless of the addition method and nanocarrier type, confirming the validity of our method. In addition, the emission remained at 453 nm even when the nanocarrier dosages were doubled, suggesting that no further shift was induced by excessive nanocarrier. In comparison, the stepwise curves could have longer emission wavelengths, i.e., more unbound dsRNA–DAPI, than the isolated ones before reaching complete loading. This may be due to the insufficient mixing during stepwise addition at the observation timepoint.
 | ||
| Fig. 4 Comparison between stepwise addition of PEI or LDH suspension into the dsRNA–DAPI solution and isolated samples. | ||
Interestingly, we noticed that the emission wavelengths of nanocarrier + (dsRNA–DAPI) with intermediate N/P or LDH/dsRNA ratios varied case by case. The difference might be caused by the different types of nanocarriers in the suspension. In the ideal situation, adding cationic nanocarrier with 25% of complete-loading dosage would load 25% dsRNA molecules and push 25% DAPI from intercalation to the minor groove, resulting in emission combined with 25% of the spectrum peaked at 520 nm and 75% of that peaked at 453 nm. This combination would be similar for the cases with 50%, 75%, and 100% of the complete-loading dosage. However, both PEI trends in Fig. 4 suggest that the amount of minor-grooved DAPI molecules did not match the PEI dosage. The absence of minor-grooved DAPI could be attributed to less loaded portion of dsRNA or less movement of DAPI. The loaded portion of dsRNA may be further determined by HPLC. If the loaded dsRNA matches the PEI dosage whereas minor-grooved DAPI is less, it could be attributed to the change of charge distribution or even the conformation of PEI due to the repulsion between PEI and DAPI.19
Thus, our method can be used to simply, cheaply, and quickly determine the dsRNA loading capacity of cationic nanocarriers, as compared with other methods (Table S1, ESI†). Further study may be carried out to compare different cationic nanocarriers. For instance, the charge strength and density may vary with the cations in LDH nanosheets. The structure of the cationic polymer, i.e., linear or branched, substitution on amine/imine, or any modification that can influence the charge distribution, could be investigated to amend the design of the polymer for optimal dsRNA loading. Simulation from the data of emission peaks could be conducted to quickly predict the loaded portion of dsRNA at any nanocarrier/dsRNA ratio by combining HPLC results of dsRNA quantification.
Conclusions
A simple, fast, cheap, and sustainable method was developed to determine the dsRNA loading capacity of cationic nanocarriers, which will significantly benefit the industrial production. After binding dsRNA with DAPI, complete loading of dsRNA–DAPI by cationic nanocarriers can be readily identified by the fluorescence emission reaching the wavelength of DNA–DAPI. This phenomenon is attributed to the electrostatic repulsion between DAPI and the cationic nanocarrier, which overwhelms the electrostatic “threshold” so that the DAPI binding mode is forced to change from intercalation to that in the minor groove of dsRNA. The method involves simple facility and reagent, straightforward operation, and reusable samples, and thus has promising prospects to prompt the commercialisation of RNAi therapies.Author contributions
P. L. and Z. P. X. conceived the project. P. L. conceptualized, supervised, and designed the research. J. M. and S. J. performed the PEI-relevant experiments and analysed the data. S. J. and W. P. carried out the LDH-relevant experiments and analysed the data. P. L. and J. M. wrote the paper. W. P. contributed in the application of software and figure visualisation. Y. H. and R. Z. participated and contributed to the methodology and revision of the manuscript. All authors reviewed and approved the manuscript before submission.Data availability
Data for this paper, including EXCEL files of original data for plotting the figures, are available at Open Science Framework at https://osf.io/qphw5/?view_only=b119dfb6dbd240c59595751e197671e4.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This research was supported by the Australian Research Council Industrial Transformation Research Hub for Sustainable Crop Protection (IH190100022) and funded by the Hub Scholarship.References
- D. Baulcombe, Nature, 2004, 431, 356–363 CrossRef CAS PubMed.
- B. L. Davidson and P. B. McCray Jr, Nat. Rev. Genet., 2011, 12, 329–340 CrossRef CAS.
- P. Li, Y. Huang, C. Fu, S. X. Jiang, W. Peng, Y. Jia, H. Peng, P. Zhang, N. Manzie, N. Mitter and Z. P. Xu, EcoMat, 2021, 3, e12132 CrossRef CAS.
- I. Lostalé-Seijo and J. Montenegro, Nat. Rev. Chem., 2018, 2, 258–277 CrossRef.
- D. Guo, X. Ji, F. Peng, Y. Zhong, B. Chu, Y. Su and Y. He, Nano-Micro Lett., 2019, 11, 27 CrossRef CAS.
- J. Huang, H. Huang, Y. Wang, B. Xu, M. Lin, S. Han, Y. Yuan, Y. Wang and X. Shuai, Biomaterials, 2023, 299, 122134 CrossRef CAS PubMed.
- C. O. Franck, A. B. Popov, I. Ahmed, R. E. Hewitt, L. Franslau, P. Tyagi and L. Fruk, Nanoscale Horiz., 2023, 8, 1588–1594 RSC.
- O. Amsalem, T. Nassar, S. Benhamron, P. Lazarovici, S. Benita and E. Yavin, J. Controlled Release, 2017, 257, 144–155 CrossRef CAS PubMed.
- T. Tieu, S. Dhawan, V. Haridas, L. M. Butler, H. Thissen, A. Cifuentes-Rius and N. H. Voelcker, ACS Appl. Mater. Interfaces, 2019, 11, 22993–23005 CrossRef CAS PubMed.
- N. Veiga, M. Goldsmith, Y. Granot, D. Rosenblum, N. Dammes, R. Kedmi, S. Ramishetti and D. Peer, Nat. Commun., 2018, 9, 4493 CrossRef.
- S. Seneca, S. K. Pramanik, L. D’Olieslaeger, G. Reekmans, D. Vanderzande, P. Adriaensens and A. Ethirajan, Mater. Chem. Front., 2020, 4, 2103–2112 RSC.
- S. P. Chali, J. Kang, M. Fichter, K. R. Speth, V. Mailänder and K. Landfester, Adv. Sci., 2024, 11, 2403668 CrossRef CAS PubMed.
- F. A. Tanious, J. M. Veal, H. Buczak, L. S. Ratmeyer and W. D. Wilson, Biochemistry, 1992, 31, 3103–3112 CrossRef CAS PubMed.
- J. Liu, B. Wang, S. Budi Hartono, T. Liu, P. Kantharidis, A. P. J. Middelberg, G. Q. (Max) Lu, L. He and S. Z. Qiao, Biomaterials, 2012, 33, 970–978 CrossRef CAS PubMed.
- R. Li, F. Wei, X. Wu, P. Zhou, Q. Chen, Y. Cen, G. Xu, X. Cheng, A. Zhang and Q. Hu, Carbon, 2021, 177, 403–411 CrossRef CAS.
- Y. Ding, Z. Jiang, K. Saha, C. S. Kim, S. T. Kim, R. F. Landis and V. M. Rotello, Mol. Ther., 2014, 22, 1075–1083 CrossRef CAS.
- H. Wang, S. Zhang, J. Lv and Y. Cheng, View, 2021, 2, 20200026 CrossRef CAS.
- N. Mitter, E. A. Worrall, K. E. Robinson, P. Li, R. G. Jain, C. Taochy, S. J. Fletcher, B. J. Carroll, G. Q. (Max) Lu and Z. P. Xu, Nat. Plants, 2017, 3, 1–10 Search PubMed.
- Y. Shin, J. E. Roberts and M. M. Santore, J. Colloid Interface Sci., 2002, 247, 220–230 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4tb01393j |
| This journal is © The Royal Society of Chemistry 2024 |