Rare-earth UiO-66 for temperature sensing near room temperature†
Elias
Djanffar‡
a,
Hudson A.
Bicalho‡
bc,
Zvart
Ajoyan
bc,
Ashlee J.
Howarth
 *bc and
Hélène
Serier-Brault
*bc and
Hélène
Serier-Brault
 *a
*a
aNantes Université, CNRS, Institut des Matériaux de Nantes Jean Rouxel, IMN, Nantes F-44000, France. E-mail: helene.brault@cnrs-imn.fr
bDepartment of Chemistry and Biochemistry and Centre of NanoScience Research, Concordia University, 7141 Sherbrooke Street West, Montréal, Quebec H4B 1R6, Canada. E-mail: ashlee.howarth@concordia.ca
cFRQNT Quebec Centre for Advanced Materials (QCAM/CQMF), Montreal, Canada
First published on 14th May 2024
Abstract
In the last decade, rare-earth metal–organic frameworks (RE-MOFs) have received notable interest in luminescence thermometry, which involves correlating the absolute temperature of an object to an optical emission. Numerous investigations that are already reported deal mainly with mixed Eu/Tb coordination polymers and MOFs, and they are based on the Eu3+-to-Tb3+ emission ratio between the transitions 5D4→7F5 and 5D0→7F2 of the Tb3+ and Eu3+ ions, respectively. In this study, we focus our attention on the well-known UiO-66, which can be also built with lanthanoid cations to give RE-UiO-66 analogues. Thus, we investigated the thermometric properties of RE-UiO-66 by synthesizing three different bimetallic Eu/Tb materials, with the aim to develop new sensitive luminescent thermometers.
Introduction
Temperature is a thermodynamic parameter that is crucial to monitor for various domains in fundamental or industry research.1 Compared to conventional thermometry with contact detection, luminescence thermometry has unique and distinct advantages including remote detection, fast response times, high accuracy, and non-invasive character while presenting high spatial resolution (typically submicron scale) where traditional methods are lacking or ineffective.2–4 Among the diverse methods used for RE3+-based luminescence thermometry, one of the most robust methodologies relies on the measurement of the intensity of two transitions of distinct RE3+ emitting centers.2,5–7 Metal–organic frameworks (MOFs) are a class of coordination polymers built up from metallic nodes and organic linkers that have attracted great interest due to their wide range of possible applications,8–12 such as in catalysis13 or gas separation.14 Among the various types of MOFs, rare-earth MOFs (RE-MOFs) are a subclass developed to exploit, among other things, the unique luminescence properties of RE elements, rendering them very attractive for lighting,15–17 chemical sensing,18–21 and luminescence thermometry.2,5,6,22–25 Using Eu-Tb bimetallic RE-MOFs, the temperature of the surrounding environment can thus be determined from the ratio between the intensity of the 5D4→7F5 and of the 5D0→7F2 transitions of Tb3+ and Eu3+, respectively.7,26,27Zr-UiO-66 has been extensively investigated for its robust structure, high surface area, and porosity.28–31 Recently, RE analogues of UiO-66 were designed and synthesized,32 comprising RE6–cluster nodes bridged by 1,4-benzene-dicarboxylate linkers (BDC2−). We previously studied the luminescent properties of some mixed-metal RE-UiO-66 analogues, particularly the white light emitting behaviour of the trimetallic Tb:Gd:Eu-UiO-66.16 Herein, we focus our attention on three different bimetallic Eu-Tb RE-UiO-66 analogues and their potential as luminescent thermometers sensitive near room temperature.
Results and discussion
Discovered in 2008, Zr-UiO-6633 is one of the most studied MOFs in the literature due to its permanent porosity, high surface area, and thermal and chemical stabilities. The typical UiO-66 MOF is comprised of 12-connected hexanuclear zirconium clusters [Zr6O4(OH)4]12+ bridged by BDC2− linkers, giving rise to a framework with fcu topology that features accessible pores with apertures of approximately 6 Å. Nearly 13 years after its initial discovery, a method for the synthesis of RE analogues of UiO-66 (Fig. 1) was reported in the literature.32 In this initial study, eight new RE-MOFs (RE = Y3+, Yb3+, Tm3+, Er3+, Ho3+, Tb3+, Gd3+, and Eu3+) were obtained through a solvothermal method, where N,N-dimethylacetamide (DMA) is used as a solvent and 2,6-difluorobenzoic acid (2,6-dFBA) plays the role of modulator in the reaction. It was proposed that the final material presents a chemical formula of [(CH3)2(NH2)]2[RE6X8(BDC)6] (X = OH−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32 and later discovered to also contain F−).34 Different from the typical Zr-UiO-66 that is neutral in charge, RE-UiO-66 is anionic in nature, requiring the presence of counterions for charge balancing purposes, dimethylammonium in this case. This, however, does not seem to impact the most prominent properties of the material, as RE-UiO-66 displays permanent porosity, high surface areas (> 1000 m2 g−1), and chemical and thermal (> 400 °C) stabilities. More recently, new research involving the RE-UiO-66 platform has expanded our knowledge regarding these materials. In one example, two new RE-UiO-66 analogues were reported (Lu- and Sm-UiO-66) and a series of single crystals were synthesized and analysed to relate structural trends to thermal stability in ten RE-UiO-66 analogues.35 In another study, the RE-UiO-66 platform was modified to generate new bimetallic and trimetallic MOFs containing Tb3+, Gd3+, and Eu3+, giving rise to a material with white-light emitting properties.16
32 and later discovered to also contain F−).34 Different from the typical Zr-UiO-66 that is neutral in charge, RE-UiO-66 is anionic in nature, requiring the presence of counterions for charge balancing purposes, dimethylammonium in this case. This, however, does not seem to impact the most prominent properties of the material, as RE-UiO-66 displays permanent porosity, high surface areas (> 1000 m2 g−1), and chemical and thermal (> 400 °C) stabilities. More recently, new research involving the RE-UiO-66 platform has expanded our knowledge regarding these materials. In one example, two new RE-UiO-66 analogues were reported (Lu- and Sm-UiO-66) and a series of single crystals were synthesized and analysed to relate structural trends to thermal stability in ten RE-UiO-66 analogues.35 In another study, the RE-UiO-66 platform was modified to generate new bimetallic and trimetallic MOFs containing Tb3+, Gd3+, and Eu3+, giving rise to a material with white-light emitting properties.16
In the present study, three new bimetallic RE-UiO-66 analogues (RE = Tb3+ and Eu3+) are synthesized and named according to the content of Tb3+ and Eu3+ in the hexanuclear clusters of the MOFs: Tb5.94Eu0.06-UiO-66, Tb5.82Eu0.18-UiO-66, and Tb5.58Eu0.42-UiO-66. As can be seen in Fig. 2a, all Bragg reflections of the new bimetallic MOFs match with the simulated pattern of Tb-UiO-66, confirming the expected UiO-66 structure with fcu topology. N2 sorption measurements were collected (Fig. 2b) and the Brunauer–Emmett–Teller (BET) surface area of the MOFs calculated. Tb5.94Eu0.06-UiO-66, Tb5.82Eu0.18-UiO-66, and Tb5.58Eu0.42-UiO-66 display the expected reversible type I isotherms with surface areas of 1090, 1050, and 1090 m2 g−1, being in accordance with the literature value for Tb-UiO-66.32
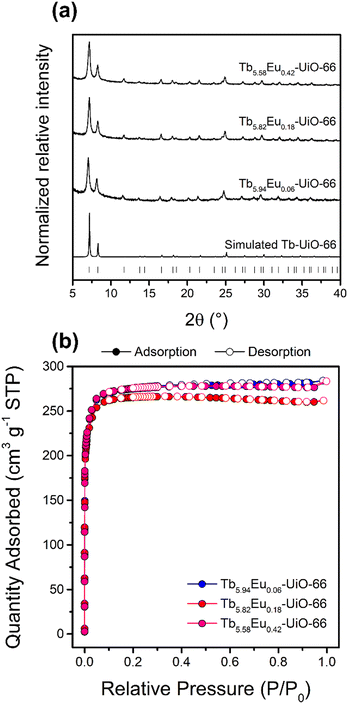 | ||
| Fig. 2 (a) PXRD patterns of simulated Tb-UiO-66 and bimetallic Tb/Eu-UiO-66 MOFs and (b) N2 sorption isotherms of the bimetallic Tb/Eu-UiO-66 MOFs. | ||
Scanning electron microscopy (SEM) images (Fig. S1–S3, ESI†) demonstrate that all MOFs have the expected octahedral morphology but in agglomerates of different sizes. Energy dispersive X-ray spectroscopy (EDS) mapping (Fig. S1–S3, ESI†) was performed to gain a better understanding of the distribution of RE metals in the crystals. In all cases, a homogeneous distribution of Tb3+ and Eu3+ is observed across the crystal agglomerates. Interestingly, the qualitative atomic ratio obtained from EDS data gives almost the exact ratio of Tb:Eu used in the synthesis of these materials (Table S1, ESI†): 98.86 and 1.14% for Tb3+ and Eu3+, respectively, in Tb5.94Eu0.06-UiO-66; 97.09 and 2.91% for Tb3+ and Eu3+, respectively, in Tb5.82Eu0.18-UiO-66; and 93.09 and 6.97% Tb3+ and Eu3+, respectively, in Tb5.58Eu0.42-UiO-66. To confirm these results, inductively coupled plasma mass spectrometry (ICP-MS) measurements were conducted on digested MOF samples. Similar results to the EDS atomic ratio analysis are obtained (Table S1, ESI†), with Tb5.94Eu0.06-UiO-66 displaying a ratio of 99.06![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.94 ± 0.12% (Tb3+:Eu3+), Tb5.82Eu0.18-UiO-66 displaying a ratio of 97.43
0.94 ± 0.12% (Tb3+:Eu3+), Tb5.82Eu0.18-UiO-66 displaying a ratio of 97.43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.57 ± 0.04%, and Tb5.58Eu0.42-UiO-66 displaying a ratio of 93.99
2.57 ± 0.04%, and Tb5.58Eu0.42-UiO-66 displaying a ratio of 93.99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.01 ± 0.14%.
6.01 ± 0.14%.
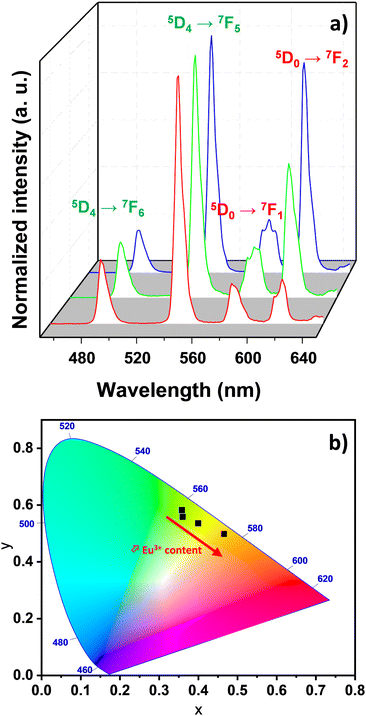
The photophysical properties of Tb6−xEux-UiO-66 were thoroughly investigated at room temperature. First, their emission spectra (Fig. 3a) display the characteristic green and red luminescence of Tb3+ and Eu3+ ions, respectively, with the typical lines at 490, 545, 581, and 619 nm attributed to the Tb3+ 5D4→7F6−3 transitions, and at 587, 611, 652, and 695 nm for the Eu3+ 5D0→7F1−4 transitions. Furthermore, the emission bands of the BDC2− linker are totally absent in the luminescence spectra, proving the efficient sensitization of Ln3+ ions by the organic linker. In the literature,16,36 the lowest-lying triplet energy state for BDC2− is estimated to be around 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 cm−1, which is perfectly suitable to sensitize Eu3+ and Tb3+. Increasing the Eu3+ content, the emission color slightly shifts from pure green emission (related to pure Tb-UiO-66) to orange emission, as evidenced by the CIE chromaticity diagram (Fig. 3b, Table S2, ESI†). In mixed Eu-Tb-UiO-66, the emission color change is often emphasized by the occurrence of a Tb3+-to-Eu3+ energy transfer, which can be evidenced by the presence of 7F6→5D4 Tb3+ transition (at 483 nm) within the 5D0→7F2 Eu3+ transition in the excitation spectra of a mixed compounds (Fig. S4, ESI†). Emission decay times were determined at room temperature for all MOFs. A monoexponential behavior was observed for the 5D4 decay curve monitored at 541 nm (Tb3+ transition) and for the 5D0 decay curve monitored at 617 nm (Eu3+ transition), the decay curves and the decay times being reported in Fig. S5, and Table S3 (ESI†), respectively. The decay times are in good agreement with those reported by Ajoyan et al.16
600 cm−1, which is perfectly suitable to sensitize Eu3+ and Tb3+. Increasing the Eu3+ content, the emission color slightly shifts from pure green emission (related to pure Tb-UiO-66) to orange emission, as evidenced by the CIE chromaticity diagram (Fig. 3b, Table S2, ESI†). In mixed Eu-Tb-UiO-66, the emission color change is often emphasized by the occurrence of a Tb3+-to-Eu3+ energy transfer, which can be evidenced by the presence of 7F6→5D4 Tb3+ transition (at 483 nm) within the 5D0→7F2 Eu3+ transition in the excitation spectra of a mixed compounds (Fig. S4, ESI†). Emission decay times were determined at room temperature for all MOFs. A monoexponential behavior was observed for the 5D4 decay curve monitored at 541 nm (Tb3+ transition) and for the 5D0 decay curve monitored at 617 nm (Eu3+ transition), the decay curves and the decay times being reported in Fig. S5, and Table S3 (ESI†), respectively. The decay times are in good agreement with those reported by Ajoyan et al.16
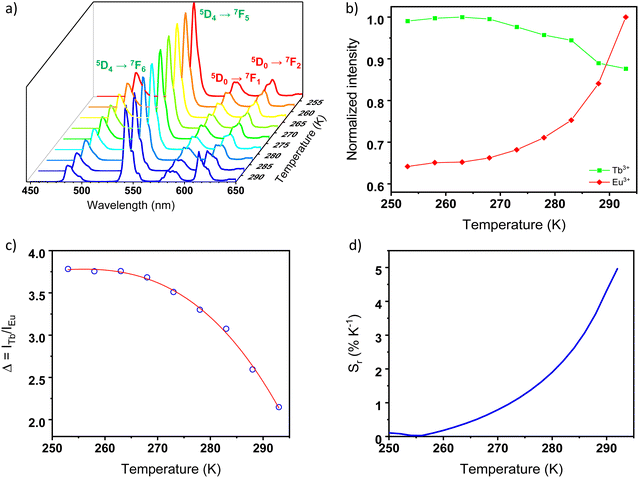
The potential application of the Tb6−xEux-UiO-66 compounds as ratiometric luminescent thermometers was probed using emission spectra collected between 255–295 K. As an illustrative example, Fig. 4a presents the temperature dependent emission spectra of Tb5.94Eu0.06-UiO-66 (similar data for the Tb5.82Eu0.18-UiO-66 and Tb5.58Eu0.42-UiO-66 materials are reported in Fig. S6a and S7a, respectively, ESI†). The temperature dependence of the ITb (green) and IEu (red) parameters is represented in Fig. 4b. The integrated areas of the 5D4→7F5 (ITb) and of the 5D0→7F2 (IEu) emissions were used to define the thermometric parameter as Δ = ITb/IEu, ITb and IEu, obtained using the emission peaks between 532–565 nm and 605–635 nm intervals, respectively. As previously demonstrated, we assumed that the overlap of the Tb3+ 5D4→7F3 and the Eu3+ 5D0→7F2 transitions should not impact substantially the determined temperature dependence of IEu.6 Increasing the temperature from 255 to 295 K causes a thermal quenching of 10% for ITb while the intensity IEu increases by 35%. These moderate thermal evolutions slightly affect the colour of emission, which shifts from green to yellow, as displayed on the CIE chromaticity diagram (Fig. S8, ESI†). Similar tendencies are observed with two other samples of Tb-Eu-UiO-66, i.e., Tb5.82Eu0.18-UiO-66 and Tb5.58Eu0.42-UiO-66, although the IEu increase is slightly weaker (around 20%, and 30% for Tb5.82Eu0.18-UiO-66 and Tb5.58Eu0.42-UiO-66, respectively). The opposite variation of ITb and IEu indicate that the present materials are expected to be promising ratiometric self-calibrated luminescent thermometers.37 Indeed, the temperature can be readily correlated to the thermometric parameter Δ = ITb/IEu, as depicted in Fig. 4c, along with Fig. S5c and S6c (ESI†) for other MOFs. A three-order polynomial curve was arbitrarily used to fit the calibration curve Δ = f(T) as the regular semi-empirical Mott–Seitz7,38 model did not give good reliability factors. All the fitting parameters are reported in ESI† in Table S4, with reliability factors.
The corresponding relative thermal sensitivity, defined as 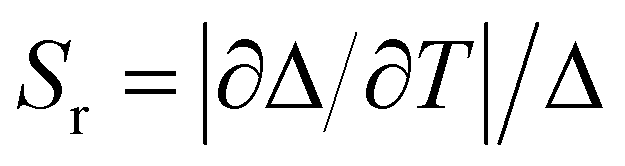 and used as a figure of merit to compare the performance of distinct systems7,38,39 is plotted in Fig. 4d for Tb5.94Eu0.06-UiO-66, along with Fig. S5d and S6d (ESI†) for the other MOFs. Therefore, as luminescent ratiometric thermometers, the compounds Tb5.94Eu0.06-UiO-66, and Tb5.82Eu0.18-UiO-66 are highly sensitive around room temperature with a maximum relative sensitivity (Sm) equal to 4.9 and 3.4% K−1 at 295 K for Tb5.94Eu0.06-UiO-66 and Tb5.82Eu0.18-UiO-66, respectively. Consequently, both materials are very competitive with the most sensitive material in the physiological range (293–313 K) so far, as can be see in Table 1. When increasing the Eu3+ content, a shift of the maximum temperature, where the thermal sensitivity is maximum, to lower temperatures appears. In fact, for the compound Tb5.58Eu0.42-UiO-66, the maximum relative sensitivity Sm is equal to 1.12% K−1 at 255 K, which is 40 degrees lower than others materials. As we have highlighted in previous studies, the Eu/Tb molar ratio has an impact on relative thermal sensitivity and the operating temperature range.6,40 However, here the increase of Eu content leads to a diminution of the relative thermal sensitivity while we obtained an opposite trend in different Eu–Tb coordination polymers.6,40 That could be explained by the structure of RE-UiO-66, which exhibits RE6-clusters with a minimal distance of 11 Å between them, which prevents Tb3+-to-Eu3+ energy transfer between clusters. Thus, when increasing Eu3+ content, the probability to obtain a RE6-cluster totally composed of Eu3+ ions is increased and the Tb3+-to-Eu3+ energy transfer is limited. As this energy transfer seems to dictate the thermometric performances (as supposed by the opposite trend of ITb and IEu with temperature), the relative thermal sensitivity decreases with the increase of Eu3+ content. Others MOFs with cluster-based nodes should be investigated to understand this phenomenon further. With that said, this investigation demonstrates that the Eu/Tb molar ratio is a crucial parameter to tune the thermometric performance of rare-earth cluster-based MOFs.
and used as a figure of merit to compare the performance of distinct systems7,38,39 is plotted in Fig. 4d for Tb5.94Eu0.06-UiO-66, along with Fig. S5d and S6d (ESI†) for the other MOFs. Therefore, as luminescent ratiometric thermometers, the compounds Tb5.94Eu0.06-UiO-66, and Tb5.82Eu0.18-UiO-66 are highly sensitive around room temperature with a maximum relative sensitivity (Sm) equal to 4.9 and 3.4% K−1 at 295 K for Tb5.94Eu0.06-UiO-66 and Tb5.82Eu0.18-UiO-66, respectively. Consequently, both materials are very competitive with the most sensitive material in the physiological range (293–313 K) so far, as can be see in Table 1. When increasing the Eu3+ content, a shift of the maximum temperature, where the thermal sensitivity is maximum, to lower temperatures appears. In fact, for the compound Tb5.58Eu0.42-UiO-66, the maximum relative sensitivity Sm is equal to 1.12% K−1 at 255 K, which is 40 degrees lower than others materials. As we have highlighted in previous studies, the Eu/Tb molar ratio has an impact on relative thermal sensitivity and the operating temperature range.6,40 However, here the increase of Eu content leads to a diminution of the relative thermal sensitivity while we obtained an opposite trend in different Eu–Tb coordination polymers.6,40 That could be explained by the structure of RE-UiO-66, which exhibits RE6-clusters with a minimal distance of 11 Å between them, which prevents Tb3+-to-Eu3+ energy transfer between clusters. Thus, when increasing Eu3+ content, the probability to obtain a RE6-cluster totally composed of Eu3+ ions is increased and the Tb3+-to-Eu3+ energy transfer is limited. As this energy transfer seems to dictate the thermometric performances (as supposed by the opposite trend of ITb and IEu with temperature), the relative thermal sensitivity decreases with the increase of Eu3+ content. Others MOFs with cluster-based nodes should be investigated to understand this phenomenon further. With that said, this investigation demonstrates that the Eu/Tb molar ratio is a crucial parameter to tune the thermometric performance of rare-earth cluster-based MOFs.
| Materials | S m (% K−1) at Tm | Ref. |
|---|---|---|
| Eu0.95Tb0.05(BT) | 1.46 at 300 K | 41 |
| TbMOF@7.3%Eu_tfac | 1.33 at 325 K | 42 |
| Eu0.50Tb0.50DPA.PMO | 1.56 at 360 K | 43 |
| Tb0.8Eu0.2BPDA | 1.19 at 313 K | 44 |
| Tb5.94Eu0.06-UiO-66 | 4.9 at 295 K | This work |
| Tb5.82Eu0.18-UiO-66 | 3.4 at 295 K | This work |
| Tb5.58Eu0.42-UiO-66 | 1.12 at 255 K | This work |
Finally, temperature cycling between 255 and 295 K (Fig. S9, ESI†) demonstrates repeatability better than 95% for Tb5.94Eu0.06-UiO-66.
Conclusions
In conclusion, the first luminescent thermometers based on a Eu3+/Tb3+ mixed UiO-66 are reported. These compounds present a very high thermal sensitivity around room temperature with a maximum relative sensitivity of 4.9% K−1 at 295 K for the most sensitive one. All reported MOFs are very competitive with Eu-Tb hybrid materials already reported in the literature.Experimental
Synthetic procedures
All materials were synthesized following an optimized procedure previously reported by our group.32 Three different MOFs were obtained and named according to the initial amounts of Tb3+ and Eu3+ used in the synthesis. In that way, MOFs containing initial molar amounts of 1, 3, and 7% Eu3+ were named Tb5.94Eu0.06-UiO-66, Tb5.82Eu0.18-UiO-66, and Tb5.58Eu0.42-UiO-66, respectively. For that, Tb(NO3)3·6H2O (0.17226, 0.16878, and 0.16182 mmol), Eu(NO3)3·6H2O (0.00174, 0.00522, and 0.01218 mmol), terephthalic acid (0.171 mmol, 28.5 mg), and 2,6-difluorobenzoic acid (2.78 mmol, 440 mg) were solubilized in 8 mL of N,N-dimethylacetamide in 6-dram vials. This mixture was sonicated until a homogeneous solution was obtained. The reaction vial was then placed on a pre-heated oven at 120 °C and left undisturbed for 72 hours (3 days). The obtained precipitates were collected and washed three times with N,N-dimethylformamide over the course of 24 hours. Then, the samples were washed three times with acetone over the course of 24 hours. Finally, the materials were air dried and activated at 120 °C for 24 hours under vacuum.Materials and methods
Powder X-ray diffraction (PXRD) patterns were obtained on a Rigaku MiniFlex 6G equipped with a Cu-target (λ = 1.54 Å) sealed-tube X-ray source operating at 40 kV/15 mA and a D/tex Ultra detector operating in a range of 3–40° (2θ) at a scan rate of 5° min−1. MOF samples were activated using a Micromeritics SmartVacPrep instrument equipped with a hybrid turbo vacuum pump. Brunauer–Emmett–Teller (BET) apparent surface area measurements were determined by N2 adsorption–desorption isotherms collected at 77 K on a Micromeritics TriStar II Plus equipment. Scanning electron microscopy (SEM) images and energy dispersive X-ray spectroscopy (EDS) elemental mapping were recorded on a Phenom ProX Desktop instrument with acceleration voltage of 15 kV and using a ThermoScientificTM Phenom charge reduction sample holder. Inductively coupled plasma mass spectrometry (ICP-MS) measurements were obtained on a 7500 Agilent 7500ce equipment. Prior to the analysis, approximately 1.0 mg of MOF sample was digested in 1.0 mL of concentrated H2SO4 at 100 °C in a sand bath for 3 hours. Every hour the sample was sonicated for approximately 5 minutes. The volumes of the digested samples were first adjusted to 10 mL using MilliQ water, and then further diluted 30 times. Room-temperature photoluminescence measurements (emission, excitation and lifetimes) were recorded on a Jobin-Yvon Fluorolog 3 fluorimeter equipped with a CCD camera (excitation source: 450 W Xe arc lamp). Emission spectra were corrected for detection and optical spectral response of the spectrofluorimeter and the excitation spectra were weighed for the spectral distribution of the lamp intensity using a photodiode reference detector. The temperature-dependent photoluminescence measurements were recorded on the same spectrometer, controlling the temperature by a Linkam heating stage coupled with the Fluorolog 3 by fibers.Author contributions
Elias Djanffar – conceptualization, methodology, investigation, validation, visualization, writing-original draft; Hudson A. Bicalho – methodology, investigation, validation, visualization, writing-original draft; Zvart Ajoyan – investigation, validation; Ashlee J. Howarth – conceptualization, funding acquisition, project administration, resources, supervision, writing-review & editing; Hélène Serier-Brault – conceptualization, investigation, validation, funding acquisition, project administration, resources, supervision, writing-review & editing.Conflicts of interest
There are no conflicts to declare.Notes and references
- C. D. S. Brites, A. Millán and L. D. Carlos, Handbook on the Physics and Chemistry of Rare Earths, 2016, vol. 49, pp. 339–427 Search PubMed.
- C. D. S. Brites, P. P. Lima, N. J. O. Silva, A. Millan, V. S. Amaral, F. Palacio and L. D. Carlos, Nanoscale, 2012, 4, 4799–4829 RSC.
- O. A. Savchuk, J. J. Carvajal, J. Massons, C. Cascales, M. Aguilo and F. Diaz, Sens. Actuators, A, 2016, 250, 87–95 CrossRef CAS.
- M. Quintanilla, L. M. Liz-Marzán and L. M. Liz-Marzan, Nano Today, 2018, 19, 126–145 CrossRef CAS.
- I. N’Dala-Louika, D. Ananias, C. Latouche, R. Dessapt, L. D. Carlos and H. Serier-Brault, J. Mater. Chem. C, 2017, 5, 10933–10937 RSC.
- V. Trannoy, A. N. Carneiro Neto, C. D. S. Brites, L. D. Carlos and H. Serier-Brault, Adv. Opt. Mater., 2021, 9, 2001938 CrossRef CAS.
- J. Rocha, C. D. S. Brites and L. D. Carlos, Chem. – Eur. J., 2016, 22, 14782–14795 CrossRef CAS PubMed.
- B. F. Hoskins and R. Robson, J. Am. Chem. Soc., 1989, 111, 5962–5964 CrossRef CAS.
- H. Li, M. Eddaoudi, M. O’Keeffe and O. M. Yaghi, Nature, 1999, 402, 276–279 CrossRef CAS.
- M. Kondo, T. Yoshitomi, H. Matsuzaka, S. Kitagawa and K. Seki, Angew. Chem., Int. Ed. Engl., 1997, 36, 1725–1727 CrossRef CAS.
- O. M. Yaghi, G. Li and H. Li, Nature, 1995, 378, 703–706 CrossRef CAS.
- N. L. Rosi, J. Kim, M. Eddaoudi, B. Chen, M. O’Keeffe and O. M. Yaghi, J. Am. Chem. Soc., 2005, 127, 1504–1518 CrossRef CAS PubMed.
- J. Lee, O. K. Farha, J. Roberts, K. A. Scheidt, S. T. Nguyen and J. T. Hupp, Chem. Soc. Rev., 2009, 38, 1450–1459 RSC.
- J. R. Li, R. J. Kuppler and H. C. Zhou, Chem. Soc. Rev., 2009, 38, 1477–1504 RSC.
- V. Trannoy, I. N’Dala-Louika, J. Lhoste, T. Devic and H. Serier-Brault, Eur. J. Inorg. Chem., 2021, 398–404 CrossRef CAS.
- Z. Ajoyan, H. A. Bicalho, P. R. Donnarumma, A. Antanovich and A. J. Howarth, J. Mater. Chem. C, 2023, 11, 8929–8934 RSC.
- L. Qiu, C. Yu, X. Wang, Y. Xie, A. M. Kirillov, W. Huang, J. Li, P. Gao, T. Wu, X. Gu, Q. Nie and D. Wu, Inorg. Chem., 2019, 58, 4524–4533 CrossRef CAS PubMed.
- D. F. Sava Gallis, D. J. Vogel, G. A. Vincent, J. M. Rimsza and T. M. Nenoff, ACS Appl. Mater. Interfaces, 2019, 11, 43270–43277 CrossRef CAS PubMed.
- L. E. Kreno, K. Leong, O. K. Farha, M. Allendorf, R. P. Van Duyne and J. T. Hupp, Chem. Rev., 2012, 112, 1105–1125 CrossRef CAS PubMed.
- N. S. Bobbitt, M. L. Mendonca, A. J. Howarth, T. Islamoglu, J. T. Hupp, O. K. Farha and R. Q. Snurr, Chem. Soc. Rev., 2017, 46, 3357–3385 RSC.
- A. J. Howarth, Y. Liu, J. T. Hupp and O. K. Farha, CrystEngComm, 2015, 17, 7245–7253 RSC.
- A. Kourtellaris, W. Lafargue-Dit-Hauret, F. Massuyeau, C. Latouche, A. J. Tasiopoulos and H. Serier-Brault, Adv. Opt. Mater., 2022, 2200484 CrossRef CAS.
- C. D. S. S. Brites, P. P. Lima, N. J. O. O. Silva, A. Millán, V. S. Amaral, F. Palacio and L. D. Carlos, Adv. Mater., 2010, 22, 4499–4504 CrossRef CAS PubMed.
- Y. Cui, F. Zhu, B. Chen and G. Qian, Chem. Commun., 2015, 51, 7420–7431 RSC.
- B. del Rosal, E. Ximendes, U. Rocha and D. Jaque, Adv. Opt. Mater., 2017, 5, 1600508 CrossRef.
- Y. Cui, H. Xu, Y. Yue, Z. Guo, J. Yu, Z. Chen, J. Gao, Y. Yang, G. Qian and B. Chen, J. Am. Chem. Soc., 2012, 134, 3979–3982 CrossRef CAS PubMed.
- X. Rao, T. Song, J. Gao, Y. Cui, Y. Yang, C. Wu, B. Chen and G. Qian, J. Am. Chem. Soc., 2013, 135, 15559–15564 CrossRef CAS PubMed.
- A. Dhakshinamoorthy, A. Santiago-Portillo, A. M. Asiri and H. Garcia, ChemCatChem, 2019, 11, 899–923 CrossRef CAS.
- G. C. Shearer, S. Chavan, S. Bordiga, S. Svelle, U. Olsbye and K. P. Lillerud, Chem. Mater., 2016, 28, 3749–3761 CrossRef CAS.
- F. Vermoortele, B. Bueken, G. Le Bars, B. Van De Voorde, M. Vandichel, K. Houthoofd, A. Vimont, M. Daturi, M. Waroquier, V. Van Speybroeck, C. Kirschhock and D. E. De Vos, J. Am. Chem. Soc., 2013, 135, 11465–11468 CrossRef CAS PubMed.
- H. Wu, Y. S. Chua, V. Krungleviciute, M. Tyagi, P. Chen, T. Yildirim and W. Zhou, J. Am. Chem. Soc., 2013, 135, 10525–10532 CrossRef CAS PubMed.
- P. R. Donnarumma, S. Frojmovic, P. Marino, H. A. Bicalho, H. M. Titi and A. J. Howarth, Chem. Commun., 2021, 57, 6121–6124 RSC.
- J. H. Cavka, S. Jakobsen, U. Olsbye, N. Guillou, C. Lamberti, S. Bordiga and K. P. Lillerud, J. Am. Chem. Soc., 2008, 130, 13850–13851 CrossRef PubMed.
- J. P. Vizuet, M. L. Mortensen, A. L. Lewis, M. A. Wunch, H. R. Firouzi, G. T. McCandless and K. J. Balkus, J. Am. Chem. Soc., 2021, 143, 17995–18000 CrossRef CAS PubMed.
- P. R. Donnarumma, C. Copeman, M. Richezzi, J. Sardilli, H. M. Titi and A. J. Howarth, Cryst. Growth Des., 2024, 24(4), 1619–1625 CrossRef CAS.
- D. Briones, P. Leo, J. Cepeda, G. Orcajo, G. Calleja, R. Sanz, A. Rodríguez-Diéguez and F. Martínez, CrystEngComm, 2018, 20, 4793–4803 RSC.
- Y. Cheng, Y. Gao, H. Lin, F. Huang and Y. Wang, J. Mater. Chem. C, 2018, 6, 7462–7478 RSC.
- C. D. S. Brites, P. P. Lima, N. J. O. Silva, A. Millán, V. S. Amaral, F. Palacio and L. D. Carlos, New J. Chem., 2011, 35, 1177 RSC.
- M. D. Dramicanin, J. Appl. Phys., 2020, 128, 40902 CrossRef CAS.
- A. Mansar and H. Serier-Brault, J. Solid State Chem., 2022, 312, 123183 CrossRef CAS.
- X. Zhou, L. Chen, Z. Feng, S. Jiang, J. Lin, Y. Pang, L. Li and G. Xiang, Inorg. Chim. Acta, 2018, 469, 576–582 CrossRef CAS.
- A. M. Kaczmarek, Y. Y. Liu, C. Wang, B. Laforce, L. Vincze, P. Van Der Voort and R. Van Deun, Dalton Trans., 2017, 46, 12717–12723 RSC.
- A. M. Kaczmarek, R. Van Deun and P. Van Der Voort, J. Mater. Chem. C, 2019, 7, 4222–4229 RSC.
- D. Zhao, X. Rao, J. Yu, Y. Cui, Y. Yang and G. Qian, Inorg. Chem., 2015, 54, 11193–11199 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: SEM images, EDX mapping, luminescence (emission and excitation) spectra. See DOI: https://doi.org/10.1039/d4tc00781f |
| ‡ E. D and H. A. B contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |