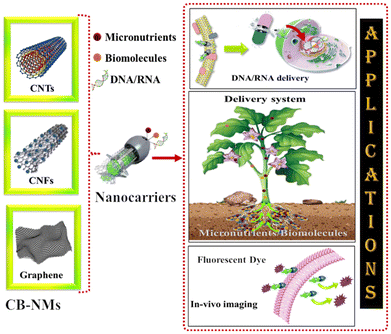
Carbon-based nanocarriers for plant growth promotion: fuelling when needed
Mohammad
Ashfaq
 a,
Govind
Gupta
a,
Govind
Gupta
 *b and
Nishith
Verma
*b and
Nishith
Verma
 *cd
*cd
aDepartment of Biotechnology, University Centre for Research & Development (UCRD), Chandigarh University, Gharaun, Mohali, Punjab 140413, India
bLaboratory for Particles-Biology Interactions, Swiss Federal Laboratories for Materials Science and Technology (Empa), Lerchenfeldstrasse 5, St Gallen, 9014 Switzerland. E-mail: govindgupta.iitk@gmail.com
cCenter for Environmental Science and Engineering, Indian Institute of Technology Kanpur, Kanpur 208016, India. E-mail: vermanishith@gmail.com
dDepartment of Chemical Engineering, Indian Institute of Technology Kanpur, Kanpur 208016, India
First published on 7th November 2024
Abstract
Climate change (i.e., rising temperature and precipitation) due to global warming is affecting soil fertility, thereby significantly causing a decrease in agriculture production worldwide. At the same time, increasing demands for food supplies with the growing global population puts extra pressure to improve agricultural production. Indeed, chemical fertilizers and pesticides are a great help in fuelling agro-production, but their excess use could deteriorate the environment and human health. Nevertheless, nanomaterials, especially carbon-based nanostructured materials (CB-NMs), have revolutionized the agricultural sector in various ways including the on-demand supply of essential nutrients, biomolecules, and growth factors to plants. Carbon nanofibers (CNFs) are one such example that can be tuned to carry essential nutrients (i.e., Fe, Cu, Zn, and Mo) and deliver to plants when and what is in need. As a result, it not only improves the crop yield but also maintains the nutritional quality (protein, carbohydrate, and mineral contents) of plant products. This review discusses the most innovative development in CB-NM-based carriers (CNFs, carbon nanotubes (CNTs), and graphene as well as its derivatives) for plant growth applications including the approaches being used for their lab-scale synthesis. In addition, their application as the carrier of micronutrients and biomolecules and the successful delivery (and the underlying mechanism) of genes, nucleic acids, microbes, and their components in plants are discussed.
1. Introduction
Climate change is an emerging and unprecedented natural threat for agro-farming, lowering overall crop productivity by affecting soil health, for example, altered nutrient cycling, the carbon–nitrogen (C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N) ratio, and unregulated microbial community dynamics in the rhizosphere.1–4 The loss of crop productivity due to natural threats (i.e., flood, high temperature, drought) and excessive use of chemical fertilizers over the years and at the same time increasing food demands with the growing worldwide population have exerted considerable pressure on the agricultural sector to develop novel and sustainable strategies for combating the impending alarming situation and fulfill the food requirement of society. Indeed, there have been considerable technological developments in recent years to combat such a situation which include novel methods for food preservation and storage, more effective and sustainable fertilizers (including biofertilisers) and pesticides, and micronutrient supplements for deprived soil. Sustainable production has also taken center stage in the development of new technologies, especially in the agricultural sector. It plays a significant role in reducing the release of agricultural pollutants, thereby improving environmental and human health.5–7 The availability of micronutrients and a biomolecule delivery system can potentially improve sustainable crop production by enhancing the nutritional value, uptake, high tolerance ability of biotic stress, and protection against pathogens. Researchers continue exploring different strategies for micronutrients (i.e., Fe, Zn, Cu, and Mo) and biomolecule delivery to the plant, as it not only improves their productivity but also the nutritional quality (high protein, antioxidants, and mineral contents) of the product. However, the delivery of micronutrients and biomolecules, including genes and nucleic acid, in the plant cell is rather challenging, since they have to pass through the rhizosphere and then cross the plant cell wall to reach the cells. In this process, a large part of the administered material is either degraded or trapped in the soil, and can never reach the plant cell. Therefore, repetitive supplementation of the material at relatively higher doses is required to achieve optimal effects on plant growth and yield.8,9 On the other hand, frequent use of such micronutrients and fertilizers at high doses could significantly pollute the terrestrial and aquatic ecosystems and pose adverse effects potentially by altering the resident microbial community (and the microbiome) and its functions that subsequently affect soil-nutrient recycling, humus content, and ultimately, plant productivity.10–12 Additionally, the positively charged surface of the micronutrients decreases the translocation ability and increases the accumulation on the root surface, subsequently causing high phytotoxicity.13–17 To tackle such issues sustainably, novel material-based approaches specifically targeting the applications related to the delivery of micronutrients, bioactive molecules, and nucleic acids in the rhizosphere and then in the crops have been pursued.18,19 Researchers have also explored new avenues for enhancing crop production by genetic/cellular modification using the delivery of biomolecules within the plant. However, the major challenges in biomolecule and nucleic acid delivery for agricultural applications are their instability in complex environments due to rapid degradation in the presence of nucleases and proteases or their unavailability to plants. In this context, numerous delivery systems such as biolistic particle/gene guns, electroporation, agrobacterium, and pollen tube pathways have been used to deliver biomolecules in plants.20–23 However, there are a few hindrances to the efficient application of such delivery systems, such as host limitation, the inevitable DNA integration to the host genome, cellular damage, and toxicity. Nanocarriers, especially carbon-based nanostructured materials (CB-NMs) have emerged as key materials in agricultural applications owing to their high surface area, light weight, biocompatibility, long-term stability, and low phytotoxicity.24–30 In addition, the high stiffness, tunable surface properties, and surface charge of CB-NMs render them a potentially efficient carrier of micronutrients and biomolecules.31–33 The surface characteristics of CB-NMs, such as functional groups and charge, can also be easily modified, making them suitable for attachment/binding with micronutrients and biomolecules.34–40 Once applied in seeds, CB-NMs can penetrate the seed coat and improve water uptake ability, thereby improving the germination efficiency of the seeds.32,41 Overall, CB-NMs offer a new paradigm shift towards a safe and sustainable option for agricultural applications, especially, the carrier and delivery agents of micronutrients and biomolecules. As illustrated in Fig. 1, different types of CB-NMs have been developed and exploited as (nano)carriers for delivering a range of substances (chemical and biological) related to improving plant growth and resistance to diseases.
N) ratio, and unregulated microbial community dynamics in the rhizosphere.1–4 The loss of crop productivity due to natural threats (i.e., flood, high temperature, drought) and excessive use of chemical fertilizers over the years and at the same time increasing food demands with the growing worldwide population have exerted considerable pressure on the agricultural sector to develop novel and sustainable strategies for combating the impending alarming situation and fulfill the food requirement of society. Indeed, there have been considerable technological developments in recent years to combat such a situation which include novel methods for food preservation and storage, more effective and sustainable fertilizers (including biofertilisers) and pesticides, and micronutrient supplements for deprived soil. Sustainable production has also taken center stage in the development of new technologies, especially in the agricultural sector. It plays a significant role in reducing the release of agricultural pollutants, thereby improving environmental and human health.5–7 The availability of micronutrients and a biomolecule delivery system can potentially improve sustainable crop production by enhancing the nutritional value, uptake, high tolerance ability of biotic stress, and protection against pathogens. Researchers continue exploring different strategies for micronutrients (i.e., Fe, Zn, Cu, and Mo) and biomolecule delivery to the plant, as it not only improves their productivity but also the nutritional quality (high protein, antioxidants, and mineral contents) of the product. However, the delivery of micronutrients and biomolecules, including genes and nucleic acid, in the plant cell is rather challenging, since they have to pass through the rhizosphere and then cross the plant cell wall to reach the cells. In this process, a large part of the administered material is either degraded or trapped in the soil, and can never reach the plant cell. Therefore, repetitive supplementation of the material at relatively higher doses is required to achieve optimal effects on plant growth and yield.8,9 On the other hand, frequent use of such micronutrients and fertilizers at high doses could significantly pollute the terrestrial and aquatic ecosystems and pose adverse effects potentially by altering the resident microbial community (and the microbiome) and its functions that subsequently affect soil-nutrient recycling, humus content, and ultimately, plant productivity.10–12 Additionally, the positively charged surface of the micronutrients decreases the translocation ability and increases the accumulation on the root surface, subsequently causing high phytotoxicity.13–17 To tackle such issues sustainably, novel material-based approaches specifically targeting the applications related to the delivery of micronutrients, bioactive molecules, and nucleic acids in the rhizosphere and then in the crops have been pursued.18,19 Researchers have also explored new avenues for enhancing crop production by genetic/cellular modification using the delivery of biomolecules within the plant. However, the major challenges in biomolecule and nucleic acid delivery for agricultural applications are their instability in complex environments due to rapid degradation in the presence of nucleases and proteases or their unavailability to plants. In this context, numerous delivery systems such as biolistic particle/gene guns, electroporation, agrobacterium, and pollen tube pathways have been used to deliver biomolecules in plants.20–23 However, there are a few hindrances to the efficient application of such delivery systems, such as host limitation, the inevitable DNA integration to the host genome, cellular damage, and toxicity. Nanocarriers, especially carbon-based nanostructured materials (CB-NMs) have emerged as key materials in agricultural applications owing to their high surface area, light weight, biocompatibility, long-term stability, and low phytotoxicity.24–30 In addition, the high stiffness, tunable surface properties, and surface charge of CB-NMs render them a potentially efficient carrier of micronutrients and biomolecules.31–33 The surface characteristics of CB-NMs, such as functional groups and charge, can also be easily modified, making them suitable for attachment/binding with micronutrients and biomolecules.34–40 Once applied in seeds, CB-NMs can penetrate the seed coat and improve water uptake ability, thereby improving the germination efficiency of the seeds.32,41 Overall, CB-NMs offer a new paradigm shift towards a safe and sustainable option for agricultural applications, especially, the carrier and delivery agents of micronutrients and biomolecules. As illustrated in Fig. 1, different types of CB-NMs have been developed and exploited as (nano)carriers for delivering a range of substances (chemical and biological) related to improving plant growth and resistance to diseases.
Indeed, there are plenty of novel findings in the existing literature supporting the role of CB-NMs in improving crop yield and health by delivering substances that are either deficient in soil or required as supplements. CB-NMs, mainly carbon nanotubes (CNTs), carbon nanofibers (CNFs), and graphene, have been tested for agricultural applications, mainly as micronutrient and biomolecule carriers, delivery agents, and rhizomicrobiome stimulators, improving crop production, yield, and nutritional quality, and protecting against pathogens and abiotic stress.32,42–44 These CB-NMs have been synthesized using various techniques such as arc discharge, laser ablation, chemical vapor deposition (CVD), mechanical exfoliation, oxidation–reduction, and hydrothermal treatment.24,45,46 Moreover, CVD, exfoliation, and oxidation–reduction techniques are commonly used for synthesizing CB-NMs for agricultural applications owing to their ability to produce materials with tunable properties.
Here, we discuss the most significant developments in the synthesis and design of CB-NM-based nanocarriers for delivery applications in crops. In addition, the potential underlying mechanism of their interaction with plant systems and its downstream effects (i.e., translocation, bioaccumulation, molecular changes, plant growth, and stress response) including safety and sustainability have been discussed in this review.
In the end, we also provide recommendations for the potential future applicability of CB-NMs for sustainable agricultural production, considering that CB-NM-based agriculture is yet to develop wholly, and the interaction between CB-NMs and plants is not fully understood.
2. Lab-scale production of CB-NM-based carriers
Several synthesis procedures have been developed for CB-NMs, especially for CNTs, CNFs, and two-dimensional (2D) graphene and its derivatives i.e., graphene oxide (GO) and reduced-graphene oxide (rGO). Below, we summarised the most commonly applied methods in the lab-scale synthesis of CB-NMs and discussed their uniqueness in relation to agricultural applications.Chemical vapor deposition (CVD)
This is an emerging approach for the synthesis of CB-NMs (CNTs, CNFs, and graphene) at the scale mainly due to its reproducibility that allows a relatively easy transition of synthesis parameters to large-scale industrial productions. In CVD, the hydrocarbon gases (acetylene, methane, ethylene, benzene, etc.) are used as a source of carbon to produce CB-NMs.34,47–50 The temperature and gas flow rate during CVD are optimized based on the type of transition metal catalyst (i.e., Cu, Zn, Fe, and Ni) and hydrocarbon gases used for forming carbon nanostructures. For agricultural applications, CB-NMs can either be loaded with specific nutrients or the surface can be tuned to achieve specific functionalization for targeted applications.24 This can be achieved during the catalytic reaction. For example, CNFs grown using transition elements (Cu, Zn, Fe, and Mn) as catalysts for CVD can themselves serve as micronutrients for plants, particularly in mineral-deficient soil.34,50 Moreover, the incorporation of agrochemicals (i.e., fertilizers, plant growth regulators, and pesticides) and heteroatoms such as nitrogen (N), phosphorus (P), potassium (K), and boron (another essential element for plant growth) could also be achieved, which increases their applicability as plant fertilizers.51,52In addition, the production of CB-NMs using the CVD process allows tailoring the physicochemical properties (size, shape, and crystallinity) of particles based on needs.53,54 Finally, the CVD approach is scalable and it ensures the purity of the material with the possibility of easy functionalization post-synthesis due to its chemical reactivity, surface defects, high surface area, and π–π interaction.55 On the negative side, high production cost, substrate compatibility, and safety considerations (since high-temperature synthesis under pressure can increase surface reactivity) are the major challenges that need to be resolved in the future.
Mechanical exfoliation
It is one of the most commonly used methods in 2D materials synthesis. Controlled exfoliation of graphite is the most widely used process to synthesize high-quality few-layer graphene by tuning the auxiliary, standard, and lateral forces.56–58 The mechanical exfoliation process is categorized as (1) micromechanical cleavage, in which scotch tape is applied to the graphite surface and is peeled off (Fig. 2a). This process is repeated several times to exfoliate single/multi-layered graphene of high quality and large surface area.56,59–61 However, it is a relatively labor-intensive and time-consuming process, thus challenging to scale up. (2) Sonication: liquid phase exfoliation using bath sonication under optimized conditions of time and energy is a simple, effective, and scalable method (Fig. 2b). The exfoliation of graphite in organic solvents such as N-methyl pyrrolidone (NMP), dimethylformamide (DMF), ortho-dichlorobenzene (o-DCB), and pluronics has been extensively used to produce graphene. The main conditions that can affect the quality including the stability of graphene are the concentration of graphite, sonication times, surfactants, and polymers.62–64 (3) Ball milling is conducted in two steps: (a) exfoliation of graphite and then (b) fragmentation, both involving shear forces and collision applied by metal balls during milling (Fig. 2c). The ball-milling process destroys the crystallinity and produces good-quality graphene “but not the best”.65–67 In general, high-quality and low-cost graphene was synthesized using an exfoliation process. On the negative side, low yield, high energy requirements, and potential metal impurities from milling balls limit the use of this method in the large-scale production of high-purity graphene.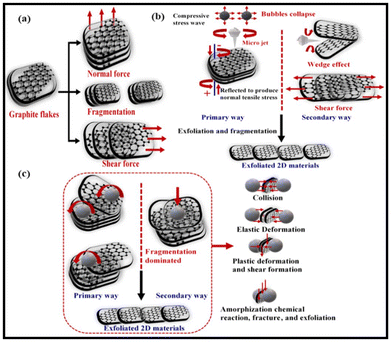 | ||
| Fig. 2 A schematic representation of the synthesis processes of graphene. (a) Mechanical exfoliation by fragmentation of graphite flakes by applying normal or shear force. (b) Sonication by introducing high-frequency ultrasound waves passing through solvents that produce cavitational bubbles. These cavitational bubbles eventually collapse and release energy in the form of microjets, shock waves, and shear forces to influence the reduction of size. (c) The ball-milling process allows the mechanical fragmentation of graphene due to the collision of the metal balls under high force. Reproduced from ref. 56 with permission from Elsevier, copyright 2022. | ||
Oxidative exfoliation–reduction
In general, graphene derivatives mainly GO and rGO are synthesized via oxidative exfoliation followed by the reduction of graphite. There are four common procedures for the synthesis of graphene derivatives, namely: (1) Staudenmaier, (2) Brodie, (3) Hofmann, and (4) Hummers methods.68–72 Strong acids mainly nitric and sulfuric acids, and neutral salts such as potassium permanganate are used for oxidation. At present, Hummers’ method is extensively used for the synthesis of GO, as the method is faster than the Hofmann, Bordie, and Staudenmaier methods.73 Once GO is synthesized, it can be further reduced chemically using ethanol, propanol, and acetone to produce rGO. However, it is important to note that compared to GO, rGO has higher conductivity74 and a higher surface area75, but has reduced dispersibility in water because of the higher C/O ratio,76 limiting its biological applications.In recent years, significant progress has also been seen in the development of green and sustainable approaches such as electrolytic oxidation of graphite77 using gelatin as the reducing agent78 and recycled sulfuric acid79 for the production of GO, and using green reducing agents for the production of rGO.80 The synthesis at low temperatures is anticipated to reduce the production cost.81
On the other hand, the evolution of hazardous gases such as chlorine dioxide (ClO2), dinitrogen tetroxide (N2O4), and nitrogen dioxide (NO2) during the synthesis could pose a threat to humans and the environment and should be considered a major challenge with regard to the production of GO at the industrial level.82 The oxidation–reduction process has certain advantages such as facile, scalable, and easy functionalization owing to chemical reactivity. However, chemical processes can decrease the material conductivity and quality, and produce hazardous gases.83 Overall, the use of CVD and sustainable exfoliation methods for the production of GO could mitigate environmental safety concerns as compared to the oxidation–reduction process.
3. Characterization of CB-NM-based carriers
For the successful development of CB-NMs for the intended application in agro-products, it is crucial to characterize the synthesized particles for their physicochemical properties, such as surface texture and chemistry, porosity, shape, and size. These characteristic parameters would then be used to ensure the quality control of the product if successfully transitioned to industrial-scale production. High-resolution microscopy (TEM, AFM & SEM) and spectroscopy techniques, such as X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), Fourier transform infrared (FTIR) spectroscopy, and Raman spectroscopy, have been applied in the morphological and chemical characterization of CB-NMs (Fig. 3).84 SEM and TEM imaging studies were performed to elucidate the primary size and morphology (i.e., fibrous, tubular, or 2D sheets) of CB-NMs (i.e., CNFs, CNTs (single or multi-walled), and graphene) (Fig. 3a and b). However, atomic force microscopy (AFM) was used to investigate the lateral dimensions of 2D (i.e., graphene) and 1D (CNT) materials. In addition, SEM coupled with EDX allows elemental analyses of CB-NMs (Fig. 3c). Raman spectroscopy is extensively applied to understand the graphitic characteristics of CB-NMs (especially graphene-based 2D materials) and hence, one of the crucial techniques to ensure the successful of CB-NMs (Fig. 3d). The Raman spectra show the D and G bands confirming the defects and graphitic characteristics of CB-NMs, which in turn confirm the purity of the materials. The hydrodynamic size is also an important parameter to measure especially when working with colloidal suspensions since it allows measuring the actual size distribution of particles at the nano level with the potential to agglomerate in different aqueous media in the long term (Fig. 3e). In fact, the average hydrodynamic size of particles in the treatment medium is a key determinant of their translocation ability in plants, considering that the smaller the size the higher the translocation of particles can be expected. Fig. 3f shows the XRD spectra of CNFs, revealing the crystallographic indices and crystalline size of the CB-NMs. Fig. 3g shows the AFM image of 2D graphene which provides the information related to particle thickness and surface texture (i.e., smooth or rough, spikes, needles, etc.).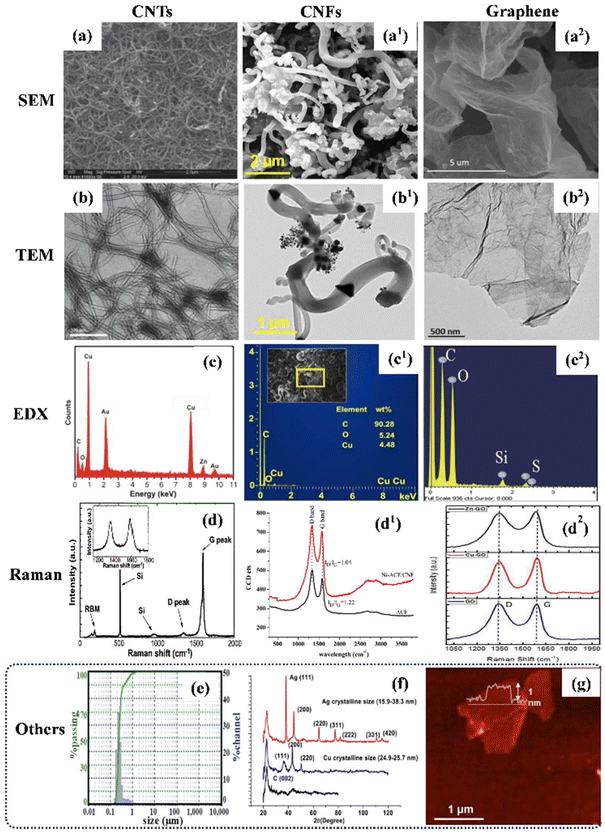 | ||
| Fig. 3 Characterization of CB-NMs. (a–a2) SEM images of CB-NMs, (a) CNTs,85 (a1) CNFs,86 and (a2) graphene.87 (b–b2) TEM images of CB-NMs, (b) CNTs,85 (b1) CNFs,86 and (b2) graphene.87 (c–c2) EDX spectra of CB-NMs, (c) CNTs,85 (c1) CNFs,86 (c2) graphene.88 (d–d2) Raman spectra of the CB-NMs (d) CNTs,89 (d1) CNFs,90 and (d2) graphene.87 (e–g) Other characterization techniques, (e) particle size distribution of CNFs,34 and (f) XRD spectra of CNFs,91 and (g) AFM image of graphene.88 (a, b and c) Reproduced from ref. 85 with permission from the Royal Society of Chemistry, copyright 2011. (a1, b1 and c1) Reproduced from ref. 86 with permission from Wiley, copyright 2024. (a2, b2 and d2) Reproduced from ref. 87 with permission from American Chemical Society, copyright 2017. (c2 and g) Reproduced from ref. 88 with permission from Springer Nature, copyright 2011. (d) Reproduced from ref. 89 with permission from AIP copyright 2004. (d1) Reproduced from ref. 90 with permission from Elsevier, copyright 2014. (e) Reproduced from ref. 34 with permission from the Royal Society of Chemistry, copyright 2017. (f) Reproduced from ref. 91 with permission from American Chemical Society, copyright 2013. | ||
These characterization techniques can also be deployed to understand the interactions of CB-NMs with plant cells including their translocation from the root to shoot to leaf or vice versa. For example, Safdar et al. (2022)32 used TEM or Raman microscopy to investigate the uptake and translocation of CNTs in broccoli and maize plants (Fig. 4a and b). Fig. 4a shows the TEM images indicating CNT uptake in broccoli plant roots and stems after 7 days of exposure at a dose of 10 mg L−1. The presence of CNTs in both the root and stems represents the upward translocation of CNTs. In addition, the authors could show the accumulation of CNTs in plant vacuoles, the intercellular space, and the cytoplasm of the stem and root.32Fig. 4b shows the large-area mapping of the selected tissue section of the CNT-exposed maize plants, using Raman microscopy. Raman point mapping confirms the translocation of CNTs within the root, shoot, and leaf of the maize plant.32 These characterization techniques efficiently analyzed the interactions of CB-NMs within the plants. However, these techniques are not able to quantify the CB-NMs within the plants and their fruits/vegetables.
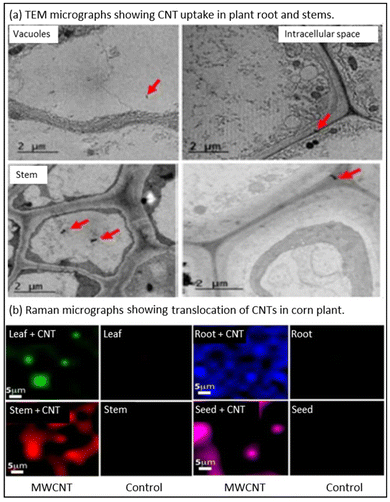 | ||
| Fig. 4 TEM and Raman spectroscopy images of the uptake and translocation of the CNTs in plants. (a) TEM images of the uptake of CNTs in broccoli plants after 7 days of exposure at 10 mg L−1. The red arrow indicates the movement of CNTs in vacuole, intracellular space, and cytoplasm in roots and stems. (b) Translocation of CNTs into maize plants in leaf, stem, root, and shoot.32 Reproduced from ref. 32 under Creative Commons Attribution 4.0 International License. | ||
In a comprehensive review, Petersen et al.92 summarised the most used techniques for the detection and quantification of CB-NMs in plants. Briefly, microwave or radio frequency (RF) assisted heating, and analysis of elemental carbon (EC)/organic carbon (OC) ratios are being used to detect and quantify CB-NMs in different parts of the plants (roots, shoots, leaves, fruits, and flowers). In the microwave-assisted heating process, CNTs in the plant tissues efficiently absorb microwave energy compared to that of other organic materials, which leads to localized heating that enables the detection of CNTs by thermal imaging.93–95 For measurement of EC/OC ratios to quantify CB-NMs (corresponds to the EC content), the plant tissue is first heated at ∼600 °C under an inert atmosphere (helium), where OC is released and detected followed by heating at ∼900 °C under an oxidizing atmosphere (a mixture of helium and oxygen) to detect EC.96 In a previous study, Ashfaq et al.34 successfully quantified CNFs in the plant roots by measuring EC/OC ratios, where the EC content corresponds to the CNF level and OC indicates background carbon from plant tissue. Their results showed a significantly higher EC content in plants grown with CNFs than in the controls (plants supplemented with water only).34
4. Delivery on-demand using CB-NM nanocarriers to improve soil quality, plant growth, and health
The rising earth's temperature due to global climate change is alarming not only for humans but also for the environmental food web and ecosystem services. For example, an impact on soil microbial diversity due to high intensities of heat waves, drought, or even excessive precipitation can affect biogeochemical cycling, nutrient recycling, and the invasions of plant pathogens leading to nutrient scarcity and rotting of plants that eventually impact plant growth and overall crop production.97,98 In addition, such effects on crops are intended to lower the nutritional quality of plant-based foods and grains, which eventually affect human health. In recent years, considerably advanced technological approaches have been presented in different studies which can directly (via entering plants) or indirectly (via improving agricultural soil quality) enhance crop production and protection against diseases.99,100 Major advancements have also been seen in the development of nano-delivery agents for plant micronutrients, bioactive molecules, pesticides, genes, or nucleic acids in the plants or rhizosphere.101–104The use of nanomaterials, mainly CB-NMs, for agricultural applications is an emerging research area due to their extraordinary properties i.e., light weight, high adsorption ability, large surface area, biodegradability, and biocompatibility for rhizospheric microbes.25 CB-NMs have unique physical and chemical properties that render them attractive for various applications in plant science, including nutrient uptake, plant growth, stress tolerance, and disease resistance. Interestingly, one of the most promising applications of CB-NMs in plant systems is the delivery systems for various molecules including micronutrients and biomolecules (nutrients and genetic materials).25,31,105–107 CB-NMs can also be functionalized with various molecules for targeted delivery to specific plant tissues.
Micronutrient delivery
Micronutrients such as Cu, Zn, Fe, Mo, and Mn are essential elements for the growth and development of plants. The micronutrients are first taken up by plants through roots and then translocated from the root to shoot to leaf through the xylem and phloem.108 The delivery of the micronutrients mainly depends on soil conditions and types of plants.109 It is important to mention here that an excessive dose of micronutrients can be phytotoxic, whereas a lesser amount affects the growth and development of plants. It is, therefore, necessary to ensure an appropriate dose of micronutrients with controlled release.Various studies have shown that CB-NMs can efficiently penetrate plant systems as growth stimulants.32,35,43,93,110–116 For instance, CNTs penetrate tomato seed coats and augment the growth of plants.35 Another study shows that the surface factionalized MWCNTs are effectively dispersed in plant shoots, improving the material's translocation and plant growth. Interestingly, germination and plant growth are shown to increase because MWCNTs are capable of regulating water channel proteins.114 Begum et al.117 used MWCNTs to assess the translocation ability in different plants and found the materials effectively translocated in rice, lettuce, and red spinach. However, MWCNTs can be toxic to plants, if used beyond certain threshold concentrations.117 The oxidized MWCNTs (O-MWCNTs) have also been shown to be effectively translocated in the plant vascular tissue. Such materials show higher water adsorption ability than the pristine MWCNTs, indicating that surface functionalization can be beneficial for crops.118 Similarly, sparingly water-soluble CNTs are translocated in the plants, augmenting the growth of chickpea plants.119 Some studies have also shown that GO and rGO are translocated, augmenting plant growth by increasing water transport and protection against climatic changes as well as plant pathogens.120–124
CB-NMs can deliver micronutrients to plants to overcome nutrient deficiency. Kumar et al. synthesized ZnO-MWCNTs and delivered Zn micronutrients in onion seed.125 The authors could show that MWCNTs penetrate the seed coat of onion and deliver Zn micronutrients in a controlled manner, increasing the germination rate even under unfavorable arid conditions compared with that of control (water). Kabiri et al.87 synthesized the Cu/Zn-GO-based fertilizers that allow slow-release micronutrients (Cu and Zn) in the agricultural soil, samples without Cu/Zn served as controls. The authors could show that the Cu-GO- and Zn-GO-based pellets are initially robust for 5 h but later release Cu/Zn micronutrients in the soil in a controlled manner for 72 h.87
CVD-synthesized CNFs have lately transformed the field of nanotechnology and materials science because of the materials’ versatile applicability in environmental remediation, energy, sensor, antibiotic materials, and agricultural fields. During CNF production, we can choose transition metals as micronutrients (Cu, Zn, and Fe) as well as the catalysts for CNF growth. The transition metal nutrients can then fulfill the nutrient deficiency of agricultural soil. Remarkably, the tip-growth mechanism of CNF production is beneficial for biological applications, especially agriculture. The metals are tightly held at the tips of the fibers, which move along with CNFs and are released in a controlled manner. For example, Cu-CNFs are synthesized by the CVD process and used as growth stimulants in chickpea plants, while plants grown without Cu-CNFs serve as a control.34 Cu-CNFs are translocated from the root to shoot to leaf, slowly releasing Cu-micronutrients and significantly improving plant growth and development.34 Similarly, the other study has successfully synthesized the bimetal Cu-Zn-grown CNFs via the CVD process and then encapsulated them in a polymeric composite, which is then used as a fertilizer to improve the growth of chickpea plants compared with that of control (soil without the Cu-Zn-CNFs-polymeric composite).50 Similar to Cu-CNFs, Cu-Zn-CNFs are also efficiently translocated, releasing micronutrients in a controlled manner. Additionally, the polymeric composite is supported as a carrier of Cu-Zn-CNFs improving on-demand delivery, as the material is first dissolved under moist conditions in the soil, releasing Cu-Zn-CNFs in a controlled manner. This way, the abrupt release of the micronutrients is impeded. The Cu-Zn bimetals-CNFs not only enhanced the plant growth but also retained antioxidant properties, reducing the production of reactive oxygen species (ROS), which otherwise would pose negative effects on plant cells. Fig. 5 shows the SEM images of the Cu-Zn-CNF-treated plants and the elemental mapping of the plant samples, confirming the successful translocation of CNFs with metal micronutrients. In addition, the high-resolution SEM images of plant tissues indicate the presence of intact cells with no sign of toxicity in Cu-Zn-CNF-treated plants as compared to the control (no treatment) (Fig. 5a–c). The uniform distribution of the Cu micronutrient indicates effective translocation (Fig. 5d). Another study has used Cu-CNFs as growth stimulants in chromium (Cr)-contaminated soil. Interestingly, Cu-CNFs not only delivered the micronutrients to the plants, but the materials impeded the uptake of Cr from the soil, thus clearly indicating that Cu-CNFs can augment the growth of the plants even in Cr-contaminated or stressed soil.126 The aforementioned studies provide enough evidence that CB-NMs are promising carriers for the delivery of micronutrients.
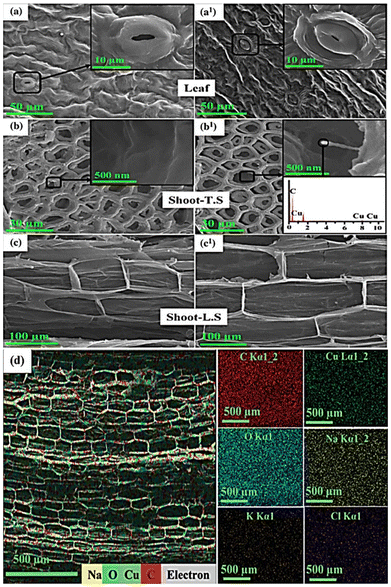 | ||
| Fig. 5 (a–c) SEM images of leaf, shoot, and root (without exposure of control), respectively, (a1–c1) SEM images of Cu-CNF-exposed leaf, shoot (transverse section), and shoot (longitudinal section),50 and (d) the elemental mapping50 of the Cu-Zn-CNF-exposed chickpea. Reproduced from ref. 50 with permission from Springer Nature, copyright 2018. | ||
Usually, such micronutrient-loaded CB-NMs are synthesized either by the surface functionalization of CB-NMs with the ligands/chelating agents that can bind micronutrients onto their surface or use the transition metals as the CVD catalyst to grow CNFs, viz. micronutrient-based CNFs such as Cu-CNFs, Fe-CNFs, and Cu-Zn-CNFs.
Biomolecule delivery
Biomolecules such as phytohormones, proteins, lipids, enzymes, nucleic acids (DNA and RNA), and genes are essential for plant growth and development. These biomolecules also protect plants against climatic changes and pathogens.127 Plant cells are enclosed by cell walls that serve as a physiological barrier against foreign molecules and pathogens.128 CB-NMs have been shown to penetrate the physiological barriers and deliver the biomolecules.35 For instance, cup stacked-CNTs-cellulase (CS-CNTs-C) could efficiently deliver the cellulase enzyme within the Arabidopsis thaliana (A. thaliana) cells. Another study developed pyraclostrobin-GO (GO-P)-based composite materials to combat pathogenic fungi Fusarium graminearum (F. graminearum) and Sclerotinia sclerotiorum (S. sclerotiorum) infection in rape seed (Brassica rapa L.).129 GO-P was demonstrated to effectively deliver pyraclostrobin from the composites that inhibited the growth of fungi and protected the plants from infection. Similarly, pyraclostrobin-MWCNTs have been successfully synthesized and demonstrated for the delivery of pyraclostrobin in model rice plants for the protection against the fungal pathogen, Pyricularia oryzae (P. oryzae). The authors could also show controlled release of pyraclostrobin from MWCNTs-P in soil for ∼360 h, which effectively inhibited the fungal growth and its subsequent infection in model plants without any adverse effects on seed germination.130 Thus, these studies clearly show that CB-NMs such as CNTs and GO are capable of delivering biomolecules in plants and protecting against pathogens.Indeed, there is plenty of evidence and promising results in the existing literature that indicate that CB-NMs can potentially be used as carriers for delivering essential elements and biomolecules without losing their biological functions. We believe that the simultaneous delivery of micronutrients as well as biomolecules could be the salient benefit of using CB-NMs. Few successful attempts have been made so far using CNFs that can simultaneously deliver biomolecules and micronutrients to plants.131,132 The advantage of this innovative delivery system is to protect against pathogens and other stressful conditions, as well as enhance the growth and nutritional quality of crops. For instance, the acylated homoserine lactone (AHL)-coated Fe-CNFs (AHL/Fe-CNFs) have been successfully synthesized and used for enhancing the growth as well as the nutritional quality of plants including protection against abiotic and abiotic stress conditions.131 The study showed that CNFs efficiently delivered the Fe-micronutrient and AHL biomolecules simultaneously. The study also showed that the AHL/Fe-CNF-treated chickpea plants developed resistance against stress (oxidative and salinity), and the materials protected the plants from fungal pathogens, resulting in enhanced plant growth and development. Another study has developed a mixture of AHL/Fe-CNFs and the endospore-immobilized activated carbon beads (E-ACBs) (AHL-Fe-CNFs-E-ACBs) and successfully tested the materials for plant-growth-promoting activity in chickpea (Cicer arietinum) and wheat (Triticum aestivum) crops.132 As depicted in Fig. 6, seed germination and radical growth were significantly improved in both crops when supplemented with AHL-Fe-CNFs/ACBs. AHL-Fe-CNFs were shown to translocate in the plants, delivering Fe micronutrients and AHL biomolecules. On the other hand, ACBs remained in the soil and augmented the plant growth by improving soil-nitrogen fixation and the production of the plant growth hormone, viz. indole acetic acid (IAA). The enhanced IAA production in plants further helped in improving defense against fungal and bacterial pathogens.132 It is important to mention that micronutrients develop resilience in plants against abiotic (heavy metal, drought, and salinity) and biotic (bacteria, fungi, and viruses) stress through the activation of plant defense mechanisms. Thus, managing the equilibrium of soil micronutrients and microbiomes is of utmost importance to maintain soil-plant health.133 The study shows the interactions between micronutrients such as Fe, Zn, Ni, Cu, Mo, and Mn, and the soil microbiome, indicating that micronutrients can affect the soil microbiome and functional genes.
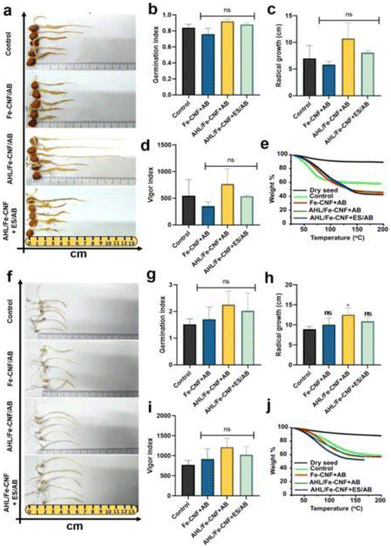 | ||
| Fig. 6 Effect of an AHL-Fe-CNFs-E-ACB-based innovative delivery system on seed germination and radical growth of the chickpea and Triticum aestivum plants. Chickpea: (a) photographic images of seed germination, (b) germination rate, (c) radical growth, (d) vigor index, and (e) water holding capacity. Triticum aestivum: (f) photographic images of seed germination, (g) germination rate, (h) radical growth, (i) vigor index, and (j) water holding capacity. Data presented are mean ± SD. Statistical significance was calculated by applying one-way ANOVA and Tukey's post hoc test. ns, p > 0.05 was considered statistically insignificant with respect to the control.132 Reproduced from ref. 132 with permission from American Chemical Society, copyright 2021. | ||
The delivery of micronutrients mainly contributes to plant growth in two ways. (1) It provides nutrients in the plants and (2) it impacts the soil microbiome to some extent.134 Thus, it is clear that a smart delivery system based on CNFs may become next-generation fertilizers that can efficiently deliver the plant micronutrients and biomolecules, besides controlling the growth of pathogens, inhibiting the translocation of other pollutants, and suppressing environmental stress. Mechanistically, the π-π electrons at the surface of CB-NMs enhance the adsorption of biomolecules, thereby acting as their efficient carrier.
Gene and nucleic acid delivery
Plant genetic engineering can improve the quality and production of crops by delivering genes and DNA/RNA molecules to plants. The plant cell wall is one of the major physiological barriers that often limit the delivery of genes and DNA/RNA biomolecules to plants. Usually, delivery systems such as biolistic particles, electroporation, PEG-mediation, and agrobacterium-mediation are effectively used as delivery agents. These delivery systems have, however, some drawbacks such as the limited host range, low transformation efficiency, instability, unavoidable DNA integration, and toxicity. With the understanding of the conventional gene delivery system, genetic engineering might benefit from a simple, robust, and cost-effective delivery of genes in all plant species. In this aspect, NM-based delivery systems have shown promising results for plant medication at the gene level. There are, however, challenges due to the potential phytotoxic effects of the nanoparticles and the unclear mechanism of translocation, for example, how NMs interact with the membrane and cell wall of the plant.135–137 Moreover, external mechanical support such as a biolistic (DNA fragment shot into the cells using a gene gun) approach is required to deliver the nanoparticles containing genes.138 CB-NMs can combat such associated issues because these particles can easily penetrate seed coats and also enter into plant cells after crossing the cell wall and associated plasma membrane attributed to their exceptional translocation ability, which makes them promising carriers to deliver genes in plants.32 For instance, PEI-DNA-CNTs have been used to deliver DNA in intact plants without transgene integration.139 Another study shows that the SWCNT-DNA efficiently crosses the plant cell wall and reaches the cells.140Fig. 7 shows the internalization of SWCNTs-DNA within the plant cell including successful delivery of foreign DNA.140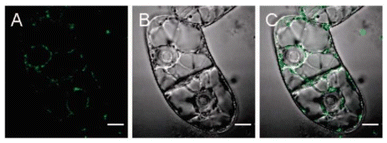 | ||
| Fig. 7 Nucleic acid delivery in plant cells using single-walled carbon nanotubes conjugated with DNA. (A–C) Confocal microscopy images of BY-2 plant cells after exposure to DNA-SWCNTs; (A) fluorescent, (B) bright field, and (C) overlay images (scale bar = 10 μm).140 Reproduced from ref. 140 with permission from American Chemical Society, copyright 2009. | ||
Another study has also demonstrated the successful delivery of DNA using the SWCNT conjugated with a polymer hybrid modified with mitochondria-specific functional peptides (cytochrome c oxidase IV (Cytox)-KH9 peptide) (CK-CNTs) in the intact plant (A. thaliana) with almost 30 times higher efficacy than the other existing methods.141 However, the localization of CNTs was majorly observed within the nucleus of plant cells defying their applications for the mitochondria-specific delivery of DNA or other related substances. Fig. 8 shows the schematic representation of CK-CNT delivery in plant cells. Ali et al.142 synthesized a polyethyleneimine (PEI)-CNTs-DNA delivery agent to ascertain transcription and translation efficiency in intact plants. The results indicated that only partially condensed DNA was accessible to cellular transcription; thus, low transcription and translation efficiency were observed.142 Similarly, CNTs were bound with DNA, and the yellow fluorescent protein (YFP) gene expression was observed in the protoplast.143 Another study reported the synthesis and use of polyethylene glycol (PEG)-chitosan (CS)-DNA-SWCNTs(PEG-CS-DNA-SWCNTs) for the selective delivery of genes into the plant chloroplast.144 Overall, these studies indicate that CB-NMs can efficiently deliver genes and DNA into plant cells (with some obvious limitations as discussed below) that subsequently alter gene expression patterns to achieve intended functions in cells without posing stable transformation of the plant. Thus, significant developments have been made so far in building efficient carriers for genes and DNA to deliver in plant cells. However, the mechanistic understanding of DNA–protein association/dissociation has not yet been fully explored, and releasing DNA from complexes is inefficient, as it lowers gene expression. In addition, the internalization of DNA and tracking mechanisms also remain unclear, which limits the effectiveness of the gene delivery system.
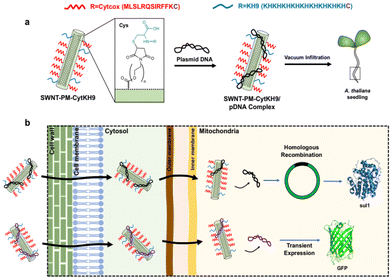 | ||
| Fig. 8 Schematic representation of the CK-CNT-based delivery system for plants. (a) Cytcox and KH9 peptide Cys residues on C-terminus are functionalized to the polymer layer on to the CNTs. (b) CK-CNTs deliver DNA into mitochondria.141 Reproduced from ref. 141 under Creative Commons Attribution 4.0 International License. | ||
The recent intervention of RNAi technology in plant systems is considered to be a significant advancement in genetic engineering. With the help of small interfering RNA (siRNA), the gene function, signaling pathways, breeding of crops, and developing resistance against biotic/abiotic stress in plants can be studied. However, the introduction of siRNA in plants having intact cell walls is limited because of the absence of an efficient delivery pathway.145–148 The siRNA delivery mainly depends on viral vectors and agrobacterium-mediated delivery which are constrained by genotypes. However, such siRNA delivery might produce anonymous genomic integration and, subsequently, an uncontrollable siRNA expression in the host genome. Therefore, an effective and simple siRNA delivery system is essential for crop improvement.149–152 Also, the delivery system should be able to deliver siRNA into the intact cell wall of the plants without any external support. This review has already discussed the suitability of CB-NMs for the delivery of biomolecules, including DNA/RNA and genes. For instance, the GO nanosheet incorporated siRNA (GO-siRNA) could efficiently deliver siRNA through the cell wall into an intact plant cell by forming a spheroidal complex. The complex formation resulted in efficient gene silencing (∼97.2%) after one day of exposure and was restored to a normal state after 5 days of exposure.153Fig. 9 illustrates the GO-mediated delivery of siRNA in a plant cell by crossing the intact plant cell wall. Another study has reported the use of ultrasmall size carbon dots to deliver small interfering (si)-RNAs in Nicotiana benthamiana (N. benthamiana) and tomato (Solanum lycopersicum). The efficacy of siRNA delivery was demonstrated by successfully achieving the silencing of endogenous genes that encode magnesium chelatase, an enzyme required for chlorophyll synthesis in plants.154 Demirer et al. (2020) also successfully demonstrated the use of SWCNTs to deliver siRNA in plant cells, with excellent efficacy (∼95%) achieved in the silencing of endogenous genes.155 The authors could also show that the use of nanotubes significantly protected siRNAs from nuclease-mediated degradation in plant cells. The overview presented in the text above and the comparative data summarised in Table 1 support the use of CB-NM-based siRNA delivery in the plant system. Overall, CB-NM-mediated gene manipulation could significantly improve small-molecule production and lead to the onset of cellular pathways involved in managing abiotic stress or pathogen resistance. CB-NM-mediated gene silencing is also used as an efficient and DNA-free delivery system. Therefore, CB-NMs (CNTs, CNFs, and graphene) can be considered to be a strategic delivery agent for biomolecules as well as nucleic acids and genes.
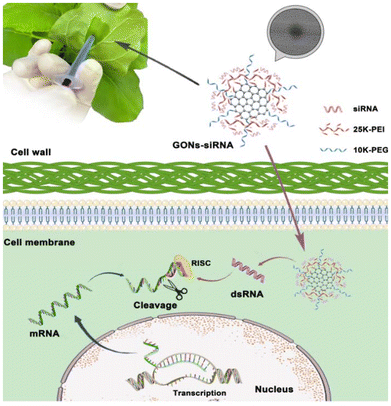 | ||
| Fig. 9 A schematic representation of the siRNA-GO-based delivery system for siRNA delivery and gene silencing. GO was used to deliver siRNA through the cell wall to an intact plant by producing a spherical siRNA-GO-based complex to accomplish efficient gene silencing.153 Reproduced from ref. 153 with permission from Wiley, copyright 2022. | ||
| S. no. | CB-NMs | Micronutrients/Biomolecules | Size diameter (nm) | Plants | Remarks | Ref. |
|---|---|---|---|---|---|---|
| 1 | ZnO-MWCNTs | Zn | MWCNT- 20–30; ZnO- ∼4.25 | Onion seed | Zn micronutrient is delivered in plants in a controlled manner that enhances seedling growth | 125 |
| 2 | Zn-GO and Cu-GO | Zn and Cu | Few layers of GO | Wheat | The oxygen binding site and edge of GO bind the Cu and Zn nutrients. The initially robust and later controlled release might be beneficial for plant growth | 87 |
| 3 | Cu-CNFs | Cu | ∼230 | Chickpea | Effectively translocate Cu-CNFs in plants and release them in a controlled manner | 34 |
| 4 | Cu-Zn-CNF-based polymeric composites | Cu and Zn | ∼34 | Chickpea | Initially, Cu-Zn-CNFs exhibit controlled release within the soil, and then Cu-Zn-CNFs translocate within the plant and release slowly | 50 |
| 5 | Cu-CNFs | Cu | ∼70–338 | Chickpea | Cu-CNFs simultaneously inhibit the uptake of Cr and release Cu within the plant | 126 |
| 6 | CS-CNTs-C | Cellulase | 60–100 | Arabidopsis thaliana | CS-CNTs-C effectively penetrates thick cellulosic plant cells and transports them | 156 |
| 7 | GO-P | Pyraclostrobin | 1–100 | Pathogenic fungi Fusarium graminearum and Sclerotinia sclerotiorum | Inhibit the pathogenic fungi in plants and release them in a controlled manner | 129 |
| 8 | MWCNTs-P | Pyraclostrobin | — | P. oryzae | Synergetic effects of drug and MWCNTs with high antifungal activity | 130 |
| 9 | AHL-Fe-CNFs | AHL and Fe | ∼3–60 | Chickpea | Simultaneous delivery of Fe micronutrients and AHL biomolecules in a controlled manner. AHL-Fe-CNFs develop resistance to salinity stress, oxidative stress, and pathogenic fungi | 131 |
| 10 | AHL-Fe-CNFs-E-ACBs | AHL, Fe, and bacterial endospore | ∼825 | Chickpea and Triticum aestivum | Simultaneous delivery of Fe micronutrients, AHL, and bacterial endospore biomolecules | 132 |
| 11 | SWCNTs-DNA | DNA | ∼500 | N. tobbacum | SWCNTs-DNA effectively penetrates within the cell wall | 140 |
| 12 | CK-CNTs | DNA | ∼3.2 | Arabidopsis thaliana | CK-CNTs deliver DNA into mitochondria with 30-fold higher expression than the existing method | 141 |
| 13 | PEI-CNTs-DNA | DNA | ∼3.2 | N. benthamiana | The transcription and translation efficiency is low | 142 |
| 14 | CNTs | DNA | ∼1–2 | N. tobbacum | Delivered DNA in the protoplast and expression of the yfp gene | 143 |
| 15 | PEG-CS-SWCNTs | Gene | ∼0.8–1.2 | Eruca sativa, Nasturtium officinale, N. tabacum, & Spinacia oleracea | Chloroplast selective gene delivery without any external support | 144 |
| 16 | siRNA-GO | siRNA | ∼15–50 | N. benthamiana | Efficient delivery of siRNA with gene silencing (97.2% efficacy) | 153 |
| 17 | Carbon-dots | siRNA | ∼8.7 | N. benthamiana and tomato | Exogenous gene GFP silencing in both plants | 157 |
| 18 | SWCNTs | siRNA | ∼1.567 | Transgenic mGFP5 Nb seeds | Delivering siRNA and silencing endogenous genes | 155 |
CNTs and CNFs are effective in enhancing root absorption and translocating micronutrients/biomolecules via the xylem from the root to shoot to leaf because of their tubular structure, which makes them a suitable choice for the delivery of micronutrients or biomolecules.158 On the other hand, the sheet-like structure and high surface area of 2D graphene and GO may allow higher and more efficient loading of micronutrients or biomolecules than the CNTs or CNFs, but there could be limitations in translocation ability in plants. Overall, CNTs and CNFs may offer fast translocation and delivery of nutrients in plants, while 2D-graphene and GO are effective in achieving higher loading and controlled release of nutrients.156 Remarkably, CNFs provide the best of both aspects, as the tubular structure facilitates rapid nutrient uptake and augments their ability to tightly hold micronutrients, thus making them next-generation nanocarriers for both the transport and control delivery of micronutrients.
5. Mechanisms of CB-NM mediated deliveries in plants
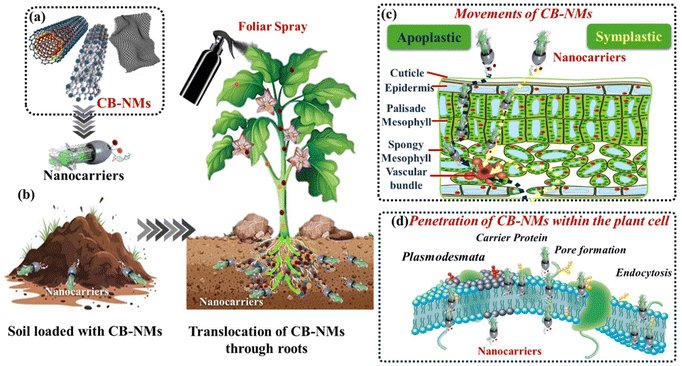
The emergence of innovative technologies and the use of nanomaterials in agricultural sciences could be a key step in overcoming challenges in crop production and fulfilling the exceeding demands of global food requirements. Indeed, there are a significant number of studies to showcase the applicability of nanomaterials or more specifically CB-NMs in agricultural sciences that can improve plant growth by enhancing the micronutrient/biomolecule uptake and high-stress tolerance against biotic/abiotic stresses. Although different strategies have been used to deliver micronutrients and biomolecules to plants, understanding the delivery mechanism is, however, crucial for improving their effectiveness as well as safe and sustainable production. CB-NMs could efficiently and effectively deliver micronutrients/biomolecules in plants either via the soil-to-root or foliar application (through stomata) of materials.159–161 The delivery of CB-NMs via plant roots mainly depends on the physicochemical properties of materials, plant physiology, and the interaction of CB-NMs with the surrounding environment (soil and its components).162,163 Specifically, a relatively smaller particle size (nanometer range) is essential for translocation in plants considering that the particles have to move from the epidermis of roots to the cortex and xylem and phloem. Therefore, CB-NMs such as CNTs and CNFs of ∼500 nm size can easily translocate within the plant system.34,164–166 Moreover, the osmosis or capillary forces also facilitate the translocation of CB-NMs via plasmodesmata, apoplast, and symplast pathways to the cellular wall to shoot to the leaves. As far as the foliar uptake is concerned, CB-NMs can penetrate the cuticle and epidermis of leaves through stomata, and then reach mesophyll cells, eventually the phloem to move throughout the plants.167 On the other hand, translocation of CB-NMs in plants involves various routes such as endocytosis, carrier proteins, and plasmodesmata. The endocytosis uptake of the CB-NMs in plant cells involves navigating through the plant cell wall and plasma membrane. Once they reach the plasma membrane, CB NMs can be engulfed either via a receptor (known as receptor-mediated endocytosis) or by endosome formation, subsequently delivering cargo (filled with CB) into the cell. The carrier protein assists CB-NMs throughout the cell membrane, whereas plasmodesmata act as channels for intracellular transport, which allows smaller-sized CB-NMs (less than 50 nm) to move between cells. The efficiency of the uptake depends on the plant species and types of CB-NMs.34,168Fig. 10 shows a schematic representation of the uptake and translocation of CB-NMs within the plants. The surface charge on the particles is also important for translocating CB-NMs in plants.169 For instance, plant cells have an overall negatively charged surface, and if the CB-NMs are positively charged, they could interact with plant roots via electrostatic forces and adhere therein, whereas the negatively charged CB-NMs can efficiently translocate from the root to shoot to leaf, with a relatively less accumulation onto the root surface because of electrostatic repulsion.17,34,169–171 Surface functional groups and the surface coating of nanomaterials have also been shown to be affecting their uptake and translocation ability.172–174The smaller-sized and negatively charged nanomaterials translocate within plants via two types of pathways. The apoplastic movement occurs outside the plasma membrane via extracellular spaces, cell walls, and xylem. The symplastic movement involves the cytoplasm through plasmodesmata. The apoplastic movement allows the nanomaterials to reach the vascular tissue of the roots and the aerial parts of plants through the xylem. The symplastic movement enables the nanomaterials to translocate via phloem and distribute toward the plant organs. Indeed, the translocation of nanomaterials within plants is a complex process, and their distribution is affected by the types of nanomaterials and plant species having different physiology.170,173,175–177 Further studies are required to understand the exact mechanism related to nanomaterial distribution in different plant tissues and cells.
6. Safety considerations for CB-NMs
Inhalation and dermal contact are the two most pertinent exposure routes of CB-NMs during production and use in agricultural settings (e.g. farming). However, consumers are mainly exposed through the oral route via the intake of contaminated food or other agroproducts. One of the first clinical trial studies of GO in human volunteers showed negligible acute toxicity after inhalation exposure.178 The other studies have also shown negligible or mild effects (only if used at a high dose) of purified (without surfactants or additives) graphene and graphene oxide in skin cells (based on the skin irritation test).179,180 In addition, a recent study performed to study a human hazard assessment of the reinforced-GO nanocomposites (mimicking the end-of-life scenario of graphene-based products) did not reveal any alarming effects after inhalation and dermal exposure.181 However, not all CB-NMs are equally safe. For instance, an inhalation exposure to high aspect ratio CNTs (long and rigid morphology) can cause lung fibrosis during long-term exposure, supporting the fiber paradigm of lung toxicity.182,183 On the other hand, 90 days of subchronic exposure to CNFs in rats resulted in a lack of fiber paradigm (as compared to CNTs) with no major chronic toxicities.184In contrast, other studies support the potential role of CB-NMs (especially CNTs) as plant growth regulators without causing severe toxicity in mammalian cells in vitro and in vivo at the concentrations detected in edible parts of the plants.185–187 Lahiani et al. showed that exposure of T-84 human intestinal epithelial cells to CNT-containing fruits or to an equal residual concentration (0.001 μg mL−1) of pristine CNTs had no effect either on the epithelial barrier integrity of cell cultures or gene expression profiles.188 However, harmful effects were observed at an extremely high concentration (10 μg mL−1), which is 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 times higher than the residual concentrations detected in edible parts of the plants.188 Moreover, Rezaei-Cherati et al.189 showed the absence of any pronounced organ toxicity of CNTs in mice once fed with CNT-contaminated tomatoes, primarily because of negligible accumulation of CNTs in the animal tissue.189
000 times higher than the residual concentrations detected in edible parts of the plants.188 Moreover, Rezaei-Cherati et al.189 showed the absence of any pronounced organ toxicity of CNTs in mice once fed with CNT-contaminated tomatoes, primarily because of negligible accumulation of CNTs in the animal tissue.189
Overall, these studies suggest CNTs as a promising material for agricultural applications with the least toxicity at low doses. However, one should also investigate the bioaccumulative behavior of CNTs in recipient tissue, which may otherwise lead to long-term persistence and chronic toxicities. In the case of CNFs, there is limited literature on hazard assessment and a thorough investigation is recommended to conclude their safety aspects in humans.
It is also of utmost importance to discuss the environmental safety implications of CB-NMs from a sustainability standpoint of view to fully utilize the benefits of CB-NMs. Few studies have been performed so far focusing on soil health, non-targeted organisms, the soil microbiome, and bioaccumulation.190–192 For instance, Nepal et al.190 used carbon nanoparticles to enhance the effectiveness of sandy soils and their impact on lettuce plants. The study showed that the incorporation of carbon nanoparticles in sandy soils significantly improved the fertility of the soil, thereby augmenting the growth of crops.190 Another study also involved the use of carbon nanoparticles to improve the soil health and growth of the Zea mays L. plant. The study demonstrates that the carbon nanoparticles in soil significantly improved soil health by increasing the ammonium, nitrate, total nitrogen, and phosphorus contents, which significantly improved the nutrient uptake and, subsequently, the growth of the Zea mays plant.191 Wu et al.192 investigated the potential effects of SWCNTs and MWCNTs on the soil microbiome (bacterial community) and showed that MWCNTs trigger relatively greater changes in soil bacterial abundance and diversity, whereas SWCNTs only affected soil bacterial composition.192 Similarly, another study shows that GO and graphite exposure in soil could influence bacterial abundance; however, no effects were observed in bacterial diversity.193 Yang et al.194 investigated the potential toxicity of MWCNTs in earthworms and their results showed no major effects on the growth and reproductive behavior of earthworms up to a dose of ∼100 mg kg−1 MWCNTs tested in the study.194 Petersen et al.92 reviewed the available literature on the bioaccumulation and biomagnification potential of CNTs, fullerenes, and graphene in plants, which resulted in no or limited bioaccumulation,92 of CB-NMs in plants. Other studies indicated a lower absorption of CNTs at the epithelial cell surface and low persistence in environmental organisms.195–198
7. Economic implications and regulatory challenges
The economic consequences of using CB-NMs as nanofertilizers/nanocarriers in agriculture are of utmost importance. Although CB-NMs require a higher investment in production by offsetting their original cost, they would be inexpensive compared to traditional fertilizers.199,200 The cost-effectiveness of CB-NMs, when compared with traditional fertilizers, involves several aspects. (1) The on-demand micronutrient/biomolecule delivery and higher bioavailability of CB-NMs in plants can significantly reduce the quantity of the required fertilizers, thereby lowering the overall cost.201,202 (2) Multifunctional roles, e.g., fertilizer and biocontrol activities, can be achieved in the same formulation of the material. (3) It improves soil health and the soil microbiome, as well as reduces nutrient runoff that can eventually lead to long-term savings in the management of soil, and also, in compliance with regulatory standards.203–205To date, there are no adequate and specific international guidelines for using nanotechnology-based agriproducts.206–208 Nevertheless, there are ongoing efforts to synthesize nanomaterials with certain specific guidelines for their safe use in the agricultural field. Moreover, continual advancement in nanomaterials with alteration of the surface functionality makes it impractical to apply a collective set of assessments for emerging nanofertilizers.206,209 Therefore, it is strongly recommended that studies should incorporate a comparative analysis of nanofertilizers and traditional fertilizers under different environmental conditions so that necessary regulatory guidelines can be formed globally, which might be beneficial for the commercialization of CB-NMs as nanofertilizers/nanocarriers.
8. Summary and outlook
The advent of CB-NMs mainly CNTs, CNFs, and graphene has revolutionized the agriculture sector by augmenting crop yield and sustainability even under adverse conditions such as climate changes and stressed soil. Indeed, CB-NMs have translocation ability because of their distinct physiochemical characteristics, especially size and surface charge, making them suitable carriers for micronutrients, biomolecules, and genes. The development of CB-NMs as carriers, as well as fertilizers and insecticides, is even more an advanced step and should be vigorously explored. There are plenty of benefits in using CB-NMs, for example, controlled release (in soil) or delivery (in plants) of micronutrients/biomolecules/nucleic acid, prevention of pathogen growth, sequestering pollutants, and reducing abiotic and biotic stresses, all of these benefits leading to a significant enhancement in the crop production and nutritional quality of agro-products. Furthermore, this review has determined that CNFs can be easily translocated in plants, which tightly hold metal-NPs/micronutrients and release them in the soil or plants in a controlled manner. CNFs can also simultaneously deliver micronutrients and biomolecules.CNTs have been extensively studied for nucleic acid and gene deliveries in plant cells, but considering the established risk of CNT exposure in humans an alternative approach needs to be explored in the future. In this context, CNFs could be a promising material, to begin with since they present similarities in physicochemical properties to CNTs and are relatively safer for humans as shown at least in initial studies.184 CNFs are also considered to be biocompatible in plant tissues.34
Some limitations exist when delivering biomolecules using CB-NMs since it will require low-temperature storage to keep biomolecules intact and functional until delivered to plants. Therefore, a cold chain setup needs to be established at the grassroots level to achieve real outcomes, which includes an extra cost to use these materials at the consumer level. Therefore, future research will be needed to develop novel materials that can deliver biomolecules to plants at ambient temperature without affecting their functionality. Moreover, CB-NMs could behave differently when changing geographical soil conditions, such as extremely acidic or alkaline soil. This needs to be studied in the future in order to define the actual usability of CB-NM-based carriers in diverse geographical settings. Finally, lowering the production cost and shifting the production from lab-scale to industrial-scale without hampering their properties is another major challenge for CB-NM-based carriers that needs to be addressed in the future.
Author contributions
All authors envisioned the structure of the manuscript and contributed to writing and editing.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
The authors acknowledge the financial support received from the Council of Scientific and Industrial Research (CSIR) (New Delhi, India) in the form of a research grant (Grant No. 22/0860/23).References
- P. Datta, B. Behera and D. B. Rahut, Environ. Challenges, 2022, 8, 100543 CrossRef.
- B. Praveen and P. Sharma, J. Public Aff., 2019, 19, e1960 CrossRef.
- A. Ullah, A. Bano and N. Khan, Front. Sustainable Food Syst., 2021, 5, 618092 CrossRef.
- M. van der Sloot, D. Kleijn, G. B. De Deyn and J. Limpens, Crop Environ., 2022, 1, 161–167 CrossRef.
- F. Pulizzi, Nat. Nanotechnol., 2019, 14, 507–507 CrossRef CAS PubMed.
- I. Vågsholm, N. S. Arzoomand and S. Boqvist, Front. Sustainable Food Syst., 2020, 4, 16 CrossRef.
- S. J. Davis, K. Alexander, J. Moreno-Cruz, C. Hong, M. Shaner, K. Caldeira and I. McKay, Nat. Sustainability, 2024, 7, 90–95 CrossRef.
- P. S. Bindraban, C. Dimkpa, L. Nagarajan, A. Roy and R. Rabbinge, Biol. Fertil. Soils, 2015, 51, 897–911 CrossRef CAS.
- M. Mng'ong'o, L. K. Munishi, W. Blake, S. Comber, T. H. Hutchinson and P. A. Ndakidemi, Heliyon, 2021, 7, e07745 CrossRef.
- B.-B. Li, S. S. Roley, D. S. Duncan, J. Guo, J. F. Quensen, H.-Q. Yu and J. M. Tiedje, Soil Biol. Biochem., 2021, 160, 108349 CrossRef CAS.
- Y. Pan, N. Cassman, M. de Hollander, L. W. Mendes, H. Korevaar, R. H. E. M. Geerts, J. A. van Veen and E. E. Kuramae, FEMS Microbiol. Ecol., 2014, 90, 195–205 CrossRef CAS.
- T. Ma, X. He, S. Chen, Y. Li, Q. Huang, C. Xue and Q. Shen, Front. Microbiol., 2022, 13, 890712 CrossRef PubMed.
- C. Tarafder, M. Daizy, M. M. Alam, M. R. Ali, M. J. Islam, R. Islam, M. S. Ahommed, M. Aly Saad Aly and M. Z. H. Khan, ACS Omega, 2020, 5, 23960–23966 CrossRef CAS PubMed.
- K. Lubkowski, Pol. J. Chem. Technol., 2016, 18, 72–79 CrossRef CAS.
- P. J. White and P. H. Brown, Ann. Bot., 2010, 105, 1073–1080 CrossRef CAS.
- C. O. Dimkpa and P. S. Bindraban, Agron. Sustainable Dev., 2016, 36, 7 CrossRef.
- M. Liu, S. Feng, Y. Ma, C. Xie, X. He, Y. Ding, J. Zhang, W. Luo, L. Zheng, D. Chen, F. Yang, Z. Chai, Y. Zhao and Z. Zhang, ACS Appl. Mater. Interfaces, 2019, 11, 16905–16913 CrossRef CAS PubMed.
- D. Garg, K. Sridhar, B. Stephen Inbaraj, P. Chawla, M. Tripathi and M. Sharma, Bioengineering, 2023, 10(9), 10 CrossRef PubMed.
- E. E. Elemike, I. M. Uzoh, D. C. Onwudiwe and O. O. Babalola, Appl. Sci., 2019, 9(3), 499 CrossRef CAS.
- Z. Lv, R. Jiang, J. Chen and W. Chen, Plant J., 2020, 104, 880–891 CrossRef CAS.
- S. Rustgi, S. Naveed, J. Windham, H. Zhang and G. S. Demirer, Front. Genome Ed., 2022, 4, 1011934 CrossRef.
- B. Lacroix and V. Citovsky, Methods Mol. Biol., 2020, 2124, 125–139 CrossRef CAS.
- J.-Y. Cho, P. Bhowmik, P. L. Polowick, S. G. Dodard, M. El-Bakkari, G. Nowak, H. Fenniri and U. D. Hemraz, ACS Omega, 2020, 5, 24422–24433 CrossRef CAS.
- O. Zaytseva and G. Neumann, Chem. Biol. Technol. Agric., 2016, 3, 17 CrossRef.
- A. Mukherjee, S. Majumdar, A. D. Servin, L. Pagano, O. P. Dhankher and J. C. White, Front. Plant Sci., 2016, 7, 172 CrossRef PubMed.
- J. Jampílek and K. Kráľová, in Nanotechnology: An Agricultural Paradigm, ed. R. Prasad, M. Kumar and V. Kumar, Springer Singapore, Singapore, 2017, pp. 177–226. DOI:10.1007/978-981-10-4573-8_9.
- S. Afreen, N. Talreja, M. Ashfaq and D. Chauhan, in Nanotechnology-Based Sustainable Alternatives for the Management of Plant Diseases, ed. G. M. Balestra and E. Fortunati, Elsevier, 2022, pp. 287–300. DOI:10.1016/B978-0-12-823394-8.00004-4.
- K. Aggrawal, V. Dixit, A. K. Pal, K. K. Chaubey, S. Barman, S. Pandey, S. Rajawat, T. Khandelwal and M. Gangwar, in Carbon-Based Nanomaterials: Synthesis, Agricultural, Biomedical, and Environmental Interventions, ed. A. Bachheti, R. K. Bachheti and A. Husen, Springer Nature Singapore, Singapore, 2024, pp. 123–144. DOI:10.1007/978-981-97-0240-4_7.
- M. Chandel, K. Kaur, B. K. Sahu, S. Sharma, R. Panneerselvam and V. Shanmugam, Carbon, 2022, 188, 461–481 CrossRef CAS.
- G. V. Lowry, J. P. Giraldo, N. F. Steinmetz, A. Avellan, G. S. Demirer, K. D. Ristroph, G. J. Wang, C. O. Hendren, C. A. Alabi, A. Caparco, W. da Silva, I. González-Gamboa, K. D. Grieger, S.-J. Jeon, M. V. Khodakovskaya, H. Kohay, V. Kumar, R. Muthuramalingam, H. Poffenbarger, S. Santra, R. D. Tilton and J. C. White, Nat. Nanotechnol., 2024, 19, 1255–1269 CrossRef CAS.
- L. Zhu, L. Chen, J. Gu, H. Ma and H. Wu, Carbon-Based Nanomaterials for Sustainable Agriculture: Their Application as Light Converters, Nanosensors, and Delivery Tools, Plants, 2022, 11, 511 CrossRef CAS PubMed.
- M. Safdar, W. Kim, S. Park, Y. Gwon, Y.-O. Kim and J. Kim, J. Nanobiotechnol., 2022, 20, 275 CrossRef CAS PubMed.
- M. Rashid, Q. Hussain, K. S. Khan, M. I. Alwabel, R. Hayat, M. Akmal, S. S. Ijaz, S. Alvi and Obaid-ur-Rehman, J. Soil Sci. Plant Nutr., 2021, 21, 1144–1169 CrossRef CAS.
- M. Ashfaq, N. Verma and S. Khan, Environ. Sci.: Nano, 2017, 4, 138–148 RSC.
- M. Khodakovskaya, E. Dervishi, M. Mahmood, Y. Xu, Z. Li, F. Watanabe and A. S. Biris, ACS Nano, 2009, 3, 3221–3227 CrossRef CAS PubMed.
- J. Chen, L. Yang, S. Li and W. Ding, Molecules, 2018, 23, 1104 CrossRef PubMed.
- P. Wang, E. Lombi, F.-J. Zhao and P. M. Kopittke, Trends Plant Sci., 2016, 21, 699–712 CrossRef CAS PubMed.
- N. Majeed, K. C. S. Panigrahi, L. B. Sukla, R. John and M. Panigrahy, Mater. Today: Proc., 2020, 30, 340–345 CAS.
- I. Santana, S.-J. Jeon, H.-I. Kim, M. R. Islam, C. Castillo, G. F. H. Garcia, G. M. Newkirk and J. P. Giraldo, ACS Nano, 2022, 16, 12156–12173 Search PubMed.
- A. Husen, in Nanomaterials for Agriculture and Forestry Applications, ed. A. Husen and M. Jawaid, Elsevier, 2020, pp. 199–218. DOI:10.1016/B978-0-12-817852-2.00008-1.
- A. Pourkhaloee, M. Haghighi, M. J. Saharkhiz, H. Jouzi and M. M. Doroodmand, Seed Technol., 2011, 33, 155–169 Search PubMed.
- L. Zhu, L. Chen, J. Gu, H. Ma and H. Wu, Plants, 2022, 11, 511 CrossRef CAS PubMed.
- K. Pandey, M. Anas, V. K. Hicks, M. J. Green and M. V. Khodakovskaya, Sci. Rep., 2019, 9, 19358 Search PubMed.
- Y. Luo, W. Zeng, G. Lei, Y. Hou, C. Ao, H. Chen, T. Gaiser and A. K. Srivastava, Front. Plant Sci., 2022, 13, 1093529 CrossRef PubMed.
- Z. Liu, Q. Ling, Y. Cai, L. Xu, J. Su, K. Yu, X. Wu, J. Xu, B. Hu and X. Wang, Nanoscale Adv., 2022, 4, 1246–1262 RSC.
- S. Rathinavel, K. Priyadharshini and D. Panda, Mater. Sci. Eng., B, 2021, 268, 115095 CrossRef CAS.
- H. Villagarcia, E. Dervishi, K. de Silva, A. S. Biris and M. V. Khodakovskaya, Small, 2012, 8, 2328–2334 CrossRef CAS PubMed.
- S. Mathew, D. K. Tiwari and D. Tripathi, Carbon Lett., 2021, 31, 167–176 CrossRef.
- Q. Liu, B. Chen, Q. Wang, X. Shi, Z. Xiao, J. Lin and X. Fang, Nano Lett., 2009, 9, 1007–1010 CrossRef CAS PubMed.
- R. Kumar, M. Ashfaq and N. Verma, J. Mater. Sci., 2018, 53, 7150–7164 CrossRef CAS.
- J. Yang, Z. Yan, D. Xu and X. Wang, ACS Sustainable Chem. Eng., 2021, 9, 16437–16449 CrossRef CAS.
- H. Hamdi, R. De La Torre-Roche, J. Hawthorne and J. C. White, Nanotoxicology, 2015, 9, 172–180 CrossRef CAS PubMed.
- L. Sun, G. Yuan, L. Gao, J. Yang, M. Chhowalla, M. H. Gharahcheshmeh, K. K. Gleason, Y. S. Choi, B. H. Hong and Z. Liu, Nat. Rev. Methods Primers, 2021, 1, 5 CrossRef CAS.
- C. Mattevi, H. Kim and M. Chhowalla, J. Mater. Chem., 2011, 21, 3324–3334 RSC.
- J. In-Yup, C. Dong Wook, K. Nanjundan Ashok and B. Jong-Beom, in Carbon Nanotubes, ed. Y. Siva, IntechOpen, Rijeka, 2011, ch. 5. DOI:10.5772/18396.
- M. Ashfaq, N. Talreja, D. Chauhan, S. Afreen, A. Sultana and W. Srituravanich, J. Drug Delivery Sci. Technol., 2022, 70, 103268 CrossRef CAS.
- V. Sasidharan, D. Sachan, D. Chauhan, N. Talreja and M. Ashfaq, Sci. Rep., 2021, 11, 7708 CrossRef CAS.
- Y. Huang, Y.-H. Pan, R. Yang, L.-H. Bao, L. Meng, H.-L. Luo, Y.-Q. Cai, G.-D. Liu, W.-J. Zhao, Z. Zhou, L.-M. Wu, Z.-L. Zhu, M. Huang, L.-W. Liu, L. Liu, P. Cheng, K.-H. Wu, S.-B. Tian, C.-Z. Gu, Y.-G. Shi, Y.-F. Guo, Z. G. Cheng, J.-P. Hu, L. Zhao, G.-H. Yang, E. Sutter, P. Sutter, Y.-L. Wang, W. Ji, X.-J. Zhou and H.-J. Gao, Nat. Commun., 2020, 11, 2453 CrossRef CAS.
- M. Yi and Z. Shen, J. Mater. Chem. A, 2015, 3, 11700–11715 RSC.
- K. Parvez, S. Yang, X. Feng and K. Müllen, Synth. Met., 2015, 210, 123–132 CrossRef CAS.
- R. C. Sinclair, J. L. Suter and P. V. Coveney, Phys. Chem. Chem. Phys., 2019, 21, 5716–5722 RSC.
- J. N. Coleman, Acc. Chem. Res., 2013, 46, 14–22 CrossRef CAS.
- K. R. Paton, E. Varrla, C. Backes, R. J. Smith, U. Khan, A. O'Neill, C. Boland, M. Lotya, O. M. Istrate, P. King, T. Higgins, S. Barwich, P. May, P. Puczkarski, I. Ahmed, M. Moebius, H. Pettersson, E. Long, J. Coelho, S. E. O'Brien, E. K. McGuire, B. M. Sanchez, G. S. Duesberg, N. McEvoy, T. J. Pennycook, C. Downing, A. Crossley, V. Nicolosi and J. N. Coleman, Nat. Mater., 2014, 13, 624–630 CrossRef CAS PubMed.
- C. Vacacela Gomez, M. Guevara, T. Tene, L. Villamagua, G. T. Usca, F. Maldonado, C. Tapia, A. Cataldo, S. Bellucci and L. S. Caputi, Appl. Surf. Sci., 2021, 546, 149046 CrossRef CAS.
- H. Zhu, Y. Cao, J. Zhang, W. Zhang, Y. Xu, J. Guo, W. Yang and J. Liu, J. Mater. Sci., 2016, 51, 3675–3683 CrossRef CAS.
- W. Zhao, M. Fang, F. Wu, H. Wu, L. Wang and G. Chen, J. Mater. Chem., 2010, 20, 5817–5819 RSC.
- F. M. C. Caicedo, E. Vera López, A. Agarwal, V. Drozd, A. Durygin, A. F. Hernandez and C. Wang, Diamond Relat. Mater., 2020, 109, 108064 CrossRef.
- C. K. Chua and M. Pumera, Chem. Soc. Rev., 2014, 43, 291–312 RSC.
- O. Jankovský, Š. H. Kučková, M. Pumera, P. Šimek, D. Sedmidubský and Z. Sofer, New J. Chem., 2014, 38, 5700–5705 RSC.
- T. Somanathan, K. Prasad, K. Ostrikov, A. Saravanan and V. M. Krishna, Nanomaterials, 2015, 5, 826–834 CrossRef CAS PubMed.
- M. Ashfaq, T. Wongpakham, N. Talreja, D. Chauhan, T. Tharasanit and W. Srituravanich, Mater. Today Commun., 2022, 33, 104786 CrossRef CAS.
- M. Ashfaq, N. Talreja, D. Chauhan, C. A. Rodríguez, A. C. Mera and M. Ramalinga Viswanathan, J. Ind. Eng. Chem., 2022, 110, 447–455 CrossRef CAS.
- D. C. Marcano, D. V. Kosynkin, J. M. Berlin, A. Sinitskii, Z. Sun, A. Slesarev, L. B. Alemany, W. Lu and J. M. Tour, ACS Nano, 2010, 4, 4806–4814 CrossRef CAS PubMed.
- Y. Tian, N. Guo, W.-Y. Wang, W. Geng, L.-C. Jing, T. Wang, X.-T. Yuan, Z. Zhu, Y. Ma and H.-Z. Geng, Sci. Rep., 2021, 11, 9891 CrossRef CAS PubMed.
- S. Nasir, M. Z. Hussein, N. A. Yusof and Z. Zainal, Nanomaterials, 2017, 7, 182 CrossRef.
- D. Konios, M. M. Stylianakis, E. Stratakis and E. Kymakis, J. Colloid Interface Sci., 2014, 430, 108–112 CrossRef CAS.
- S. Pei, Q. Wei, K. Huang, H.-M. Cheng and W. Ren, Nat. Commun., 2018, 9, 145 CrossRef PubMed.
- D. Zhang, X. Liu and X. Wang, J. Inorg. Biochem., 2011, 105, 1181–1186 Search PubMed.
- W. K. Park, Y. Yoon, S. Kim, S. Y. Choi, S. Yoo, Y. Do, S. Jung, D. H. Yoon, H. Park and W. S. Yang, ACS Omega, 2017, 2, 186–192 Search PubMed.
- J. Singh, N. Jindal, V. Kumar and K. Singh, Chem. Phys. Impact, 2023, 6, 100185 Search PubMed.
- J. Kauppila, L. Lund, T. Laiho, M. Salomäki, J. Kankare and J. Lukkari, J. Mater. Chem. C, 2014, 2, 3602–3609 RSC.
- M. Al Kausor and D. Chakrabortty, Inorg. Chem. Commun., 2021, 129, 108630 Search PubMed.
- A. A. Moosa and M. S. Abed, Turk. J. Chem., 2021, 45, 493–519 CrossRef CAS PubMed.
- S. Mourdikoudis, R. M. Pallares and N. T. K. Thanh, Nanoscale, 2018, 10, 12871–12934 Search PubMed.
- S. Tripathi, S. K. Sonkar and S. Sarkar, Nanoscale, 2011, 3, 1176–1181 Search PubMed.
- S. Mishra, Y. Budania, A. Tyagi, S. Singh, P. Kumar and S. Singh, Chem. – Asian J., 2024, 19(10), e202400241 Search PubMed.
- S. Kabiri, F. Degryse, D. N. H. Tran, R. C. da Silva, M. J. McLaughlin and D. Losic, ACS Appl. Mater. Interfaces, 2017, 9, 43325–43335 Search PubMed.
- L. Feng, G. Gao, P. Huang, X. Wang, C. Zhang, J. Zhang, S. Guo and D. Cui, Nanoscale Res. Lett., 2011, 6, 551 Search PubMed.
- R. G. Lacerda, K. B. K. Teo, A. S. Teh, M. H. Yang, S. H. Dalal, D. A. Jefferson, J. H. Durrell, N. L. Rupesinghe, D. Roy, G. A. J. Amaratunga, W. I. Milne, F. Wyczisk, P. Legagneux and M. Chhowalla, J. Appl. Phys., 2004, 96, 4456–4462 Search PubMed.
- S. Singh, A. Singh, V. S. S. Bais, B. Prakash and N. Verma, Mater. Sci. Eng., C, 2014, 38, 46–54 CAS.
- M. Ashfaq, S. Singh, A. Sharma and N. Verma, Ind. Eng. Chem. Res., 2013, 52, 4672–4682 CAS.
- E. Petersen, A. C. Barrios, R. Bjorkland, D. G. Goodwin, J. Li, G. Waissi and T. Henry, Environ. Int., 2023, 173, 107650 CAS.
- M. H. Lahiani, J. Chen, F. Irin, A. A. Puretzky, M. J. Green and M. V. Khodakovskaya, Carbon, 2015, 81, 607–619 CAS.
- F. Irin, B. Shrestha, J. E. Cañas, M. A. Saed and M. J. Green, Carbon, 2012, 50, 4441–4449 CrossRef CAS.
- A. Vashisth, S. T. Upama, M. Anas, J.-H. Oh, N. Patil and M. J. Green, Nanoscale Adv., 2021, 3, 5255–5264 RSC.
- L. Hong, G. Liu, L. Zhou, J. Li, H. Xu and D. Wu, Particuology, 2017, 31, 181–190 CrossRef CAS.
- R. A. Duchenne-Moutien and H. Neetoo, J. Food Prot., 2021, 84, 1884–1897 CrossRef CAS.
- S. I. Zandalinas, F. B. Fritschi and R. Mittler, Trends Plant Sci., 2021, 26, 588–599 CrossRef CAS PubMed.
- S. Gouda, R. G. Kerry, G. Das, S. Paramithiotis, H.-S. Shin and J. K. Patra, Microbiol. Res., 2018, 206, 131–140 CrossRef.
- A. Yadav, K. Yadav, R. Ahmad and K. A. Abd-Elsalam, Agrochemicals, 2023, 2, 220–256 CrossRef.
- S. Dutta, S. Pal, P. Panwar and R. K. Sharma, ACS Omega, 2022, 7, 25909–25920 CrossRef CAS PubMed.
- C. Thagun, Y. Horii, M. Mori, S. Fujita, M. Ohtani, K. Tsuchiya, Y. Kodama, M. Odahara and K. Numata, ACS Nano, 2022, 16, 3506–3521 CrossRef CAS PubMed.
- M. Wang and H. Jin, Trends Microbiol., 2017, 25, 4–6 CrossRef CAS.
- Q. Zhang, Y. Ying and J. Ping, Adv. Sci., 2022, 9(2), 2103414 CrossRef CAS.
- F. J. Cunningham, N. S. Goh, G. S. Demirer, J. L. Matos and M. P. Landry, Trends Biotechnol., 2018, 36, 882–897 CrossRef CAS.
- I. Santana, H. Wu, P. Hu and J. P. Giraldo, Nat. Commun., 2020, 11, 2045 CrossRef CAS PubMed.
- S. K. Debnath and R. Srivastava, Front. Nanotechnol., 2021, 3, 644564 CrossRef.
- T. C. de Bang, S. Husted, K. H. Laursen, D. P. Persson and J. K. Schjoerring, New Phytol., 2021, 229, 2446–2469 CrossRef CAS.
- J. Che, F. K. Ricachenevsky and F. Deng, Front. Plant Sci., 2023, 14, 1179674 CrossRef PubMed.
- M. V. Khodakovskaya, B.-S. Kim, J. N. Kim, M. Alimohammadi, E. Dervishi, T. Mustafa and C. E. Cernigla, Small, 2013, 9, 115–123 CrossRef CAS PubMed.
- M. V. Khodakovskaya, K. de Silva, A. S. Biris, E. Dervishi and H. Villagarcia, ACS Nano, 2012, 6, 2128–2135 CrossRef CAS PubMed.
- M. Alimohammadi, Y. Xu, D. Wang, A. S. Biris and M. V. Khodakovskaya, Nanotechnology, 2011, 22, 295101 CrossRef PubMed.
- M. H. Lahiani, E. Dervishi, J. Chen, Z. Nima, A. Gaume, A. S. Biris and M. V. Khodakovskaya, ACS Appl. Mater. Interfaces, 2013, 5, 7965–7973 CrossRef CAS PubMed.
- H. Villagarcia, E. Dervishi, K. de Silva, A. S. Biris and M. V. Khodakovskaya, Small, 2012, 8, 2328–2334 CAS.
- M. H. Lahiani, E. Dervishi, I. Ivanov, J. Chen and M. Khodakovskaya, Nanotechnology, 2016, 27, 265102 Search PubMed.
- M. H. Lahiani, Z. A. Nima, H. Villagarcia, A. S. Biris and M. V. Khodakovskaya, J. Agric. Food Chem., 2018, 66, 6654–6662 CrossRef CAS PubMed.
- P. Begum, R. Ikhtiari, B. Fugetsu, M. Matsuoka, T. Akasaka and F. Watari, Appl. Surf. Sci., 2012, 262, 120–124 Search PubMed.
- A. Mondal, R. Basu, S. Das and P. Nandy, J. Nanopart. Res., 2011, 13, 4519–4528 CrossRef CAS.
- S. Tripathi, S. K. Sonkar and S. Sarkar, Nanoscale, 2011, 3, 1176–1181 RSC.
- M. Kazlauskas, Ž. Jurgelėnė, S. Šemčuk, K. Jokšas, N. Kazlauskienė and D. Montvydienė, Chemosphere, 2023, 312, 137221 CrossRef CAS PubMed.
- Q. Wang, S. Zhao, Y. Zhao, Q. Rui and D. Wang, RSC Adv., 2014, 4, 60891–60901 RSC.
- L. Chen, C. Wang, S. Yang, X. Guan, Q. Zhang, M. Shi, S.-T. Yang, C. Chen and X.-L. Chang, Environ. Sci.: Nano, 2019, 6, 1077–1088 RSC.
- S. Park, K. S. Choi, S. Kim, Y. Gwon and J. Kim, Nanomaterials, 2020, 10(4), 758 Search PubMed.
- Y. He, R. Hu, Y. Zhong, X. Zhao, Q. Chen and H. Zhu, Nano Res., 2018, 11, 1928–1937 CrossRef CAS.
- V. Kumar, D. Sachdev, R. Pasricha, P. H. Maheshwari and N. K. Taneja, ACS Appl. Mater. Interfaces, 2018, 10, 36733–36745 CrossRef CAS.
- A. Kumar, P. Gahoi and N. Verma, Chemosphere, 2020, 239, 124760 CrossRef CAS.
- P. Li, Y. Huang, C. Fu, S. X. Jiang, W. Peng, Y. Jia, H. Peng, P. Zhang, N. Manzie, N. Mitter and Z. P. Xu, EcoMat, 2021, 3, e12132 CrossRef CAS.
- J. Wan, M. He, Q. Hou, L. Zou, Y. Yang, Y. Wei and X. Chen, Stress Biol., 2021, 1, 3 CrossRef CAS.
- F. Peng, X. Wang, W. Zhang, X. Shi, C. Cheng, W. Hou, X. Lin, X. Xiao and J. Li, Nanomaterials, 2022, 12, 1112 CrossRef CAS PubMed.
- Y. Wang, J. Tian, Z. Wang, C. Li and X. Li, ACS Agric. Sci. Technol., 2022, 2, 534–545 CrossRef CAS.
- G. S. Gupta, A. Kumar and N. Verma, Environ. Sci.: Nano, 2019, 6, 1246–1258 RSC.
- P. Gahoi, R. A. Omar, N. Verma and G. S. Gupta, ACS Agric. Sci. Technol., 2021, 1, 240–252 CrossRef CAS.
- M. Noman, T. Ahmed, J. Wang and J. C. White, Trends Microbiol., 2024, 32(4), 319–320 CrossRef CAS PubMed.
- Z. Dai, X. Guo, J. Lin, X. Wang, D. He, R. Zeng, J. Meng, J. Luo, M. Delgado-Baquerizo, E. Moreno-Jiménez, P. C. Brookes and J. Xu, Nat. Commun., 2023, 14, 8456 CrossRef CAS PubMed.
- H. Wang, S. Mukherjee, J. Yi, P. Banerjee, Q. Chen and S. Zhou, ACS Appl. Mater. Interfaces, 2017, 9, 18639–18649 Search PubMed.
- X. Deng, M. Cao, J. Zhang, K. Hu, Z. Yin, Z. Zhou, X. Xiao, Y. Yang, W. Sheng, Y. Wu and Y. Zeng, Biomaterials, 2014, 35, 4333–4344 CrossRef CAS.
- D. K. Tripathi, Shweta, S. Singh, S. Singh, R. Pandey, V. P. Singh, N. C. Sharma, S. M. Prasad, N. K. Dubey and D. K. Chauhan, Plant Physiol. Biochem., 2017, 110, 2–12 CrossRef CAS PubMed.
- F. Torney, B. G. Trewyn, V. S. Lin and K. Wang, Nat. Nanotechnol., 2007, 2, 295–300 CrossRef CAS PubMed.
- G. S. Demirer, H. Zhang, N. S. Goh, E. González-Grandío and M. P. Landry, Nat. Protoc., 2019, 14, 2954–2971 CrossRef CAS.
- Q. Liu, B. Chen, Q. Wang, X. Shi, Z. Xiao, J. Lin and X. Fang, Nano Lett., 2009, 9, 1007–1010 CrossRef CAS.
- S. S. Y. Law, G. Liou, Y. Nagai, J. Giménez-Dejoz, A. Tateishi, K. Tsuchiya, Y. Kodama, T. Fujigaya and K. Numata, Nat. Commun., 2022, 13, 2417 CrossRef CAS PubMed.
- Z. Ali, M. F. Serag, G. S. Demirer, B. Torre, E. di Fabrizio, M. P. Landry, S. Habuchi and M. Mahfouz, ACS Appl. Nano Mater., 2022, 5, 4663–4676 CrossRef CAS.
- O. M. Burlaka, Y. V. Pirko, A. I. Yemets and Y. B. Blume, Cytol. Genet., 2015, 49, 349–357 CrossRef.
- S.-Y. Kwak, T. T. S. Lew, C. J. Sweeney, V. B. Koman, M. H. Wong, K. Bohmert-Tatarev, K. D. Snell, J. S. Seo, N.-H. Chua and M. S. Strano, Nat. Nanotechnol., 2019, 14, 447–455 CrossRef CAS.
- A. Kamthan, A. Chaudhuri, M. Kamthan and A. Datta, Front. Plant Sci., 2015, 6, 208 Search PubMed.
- G. J. Hannon, Nature, 2002, 418, 244–251 CrossRef CAS PubMed.
- J. M. Casacuberta, Y. Devos, P. du Jardin, M. Ramon, H. Vaucheret and F. Nogué, Trends Biotechnol., 2015, 33, 145–147 CrossRef CAS PubMed.
- T. Muhammad, F. Zhang, Y. Zhang and Y. Liang, Cells, 2019, 8, 38 CrossRef CAS.
- M. R. Joga, M. J. Zotti, G. Smagghe and O. Christiaens, Front. Physiol., 2016, 7, 553 Search PubMed.
- B. Mamta and M. V. Rajam, Physiol. Mol. Biol. Plants, 2017, 23, 487–501 CAS.
- J. K. Bharathi, R. Anandan, L. K. Benjamin, S. Muneer and M. A. S. Prakash, Plant Physiol. Biochem., 2023, 194, 600–618 CrossRef CAS PubMed.
- R. Majumdar, K. Rajasekaran and J. W. Cary, Front. Plant Sci., 2017, 8, 200 Search PubMed.
- S. Li, J. Li, M. Du, G. Deng, Z. Song and H. Han, Angew. Chem., Int. Ed., 2022, 61, e202210014 CrossRef CAS PubMed.
- S. H. Schwartz, B. Hendrix, P. Hoffer, R. A. Sanders and W. Zheng, Plant Physiol., 2020, 184, 647–657 CrossRef CAS.
- G. S. Demirer, H. Zhang, N. S. Goh, R. L. Pinals, R. Chang and M. P. Landry, Sci. Adv., 2020, 6, eaaz0495 CrossRef CAS.
- M. F. Serag, N. Kaji, M. Tokeshi and Y. Baba, RSC Adv., 2012, 2, 398–400 RSC.
- S. H. Schwartz, B. Hendrix, P. Hoffer, R. A. Sanders and W. Zheng, Plant Physiol., 2020, 184, 647–657 CrossRef CAS PubMed.
- M. C. Martinez-Ballesta, N. Chelbi, A. Lopez-Zaplana and M. Carvajal, Plant Physiol. Biochem., 2020, 146, 23–30 CrossRef CAS PubMed.
- Z. Chen, J. Zhao, J. Cao, Y. Zhao, J. Huang, Z. Zheng, W. Li, S. Jiang, J. Qiao, B. Xing and J. Zhang, Crop Des., 2022, 1, 100006 Search PubMed.
- P. Das, C. R. Penton, P. Westerhoff and F. Perreault, Environ. Sci.: Nano, 2023, 10, 2936–2956 RSC.
- F. Aslani, S. Bagheri, N. Muhd Julkapli, A. S. Juraimi, F. S. G. Hashemi and A. Baghdadi, Sci. World J., 2014, 2014, 641759 Search PubMed.
- S. Lin, J. Reppert, Q. Hu, J. S. Hudson, M. L. Reid, T. A. Ratnikova, A. M. Rao, H. Luo and P. C. Ke, Small, 2009, 5, 1128–1132 CrossRef CAS PubMed.
- C. M. Rico, S. Majumdar, M. Duarte-Gardea, J. R. Peralta-Videa and J. L. Gardea-Torresdey, J. Agric. Food Chem., 2011, 59, 3485–3498 CrossRef CAS PubMed.
- S. Lin, J. Reppert, Q. Hu, J. S. Hudson, M. L. Reid, T. A. Ratnikova, A. M. Rao, H. Luo and P. C. Ke, Small, 2009, 5, 1128–1132 CrossRef CAS PubMed.
- G. Chichiriccò and A. Poma, Nanomaterials, 2015, 5, 851–873 CrossRef.
- A. Pérez-de-Luque, Front. Environ. Sci., 2017, 5, 12 Search PubMed.
- Y. Zhang, M. R. Martinez, H. Sun, M. Sun, R. Yin, J. Yan, B. Marelli, J. P. Giraldo, K. Matyjaszewski, R. D. Tilton and G. V. Lowry, Environ. Sci. Technol., 2023, 57, 8269–8279 CrossRef CAS.
- P. N. Yaron, B. D. Holt, P. A. Short, M. Lösche, M. F. Islam and K. N. Dahl, J. Nanobiotechnol., 2011, 9, 45 CrossRef CAS PubMed.
- E. Spielman-Sun, A. Avellan, G. D. Bland, R. V. Tappero, A. S. Acerbo, J. M. Unrine, J. P. Giraldo and G. V. Lowry, Environ. Sci.: Nano, 2019, 6, 2508–2519 Search PubMed.
- Z. J. Zhu, H. Wang, B. Yan, H. Zheng, Y. Jiang, O. R. Miranda, V. M. Rotello, B. Xing and R. W. Vachet, Environ. Sci. Technol., 2012, 46, 12391–12398 Search PubMed.
- A. Milewska-Hendel, M. Zubko, D. Stróż and E. U. Kurczyńska, Int. J. Mol. Sci., 2019, 20(7), 1650 Search PubMed.
- J. D. Judy, J. M. Unrine, W. Rao, S. Wirick and P. M. Bertsch, Environ. Sci. Technol., 2012, 46, 8467–8474 CrossRef CAS PubMed.
- R. Raliya, C. Franke, S. Chavalmane, R. Nair, N. Reed and P. Biswas, Front. Plant Sci., 2016, 7, 1288 Search PubMed.
- C. M. Rico, S. Majumdar, M. Duarte-Gardea, J. R. Peralta-Videa and J. L. Gardea-Torresdey, J. Agric. Food Chem., 2011, 59, 3485–3498 CrossRef CAS PubMed.
- D. Sun, H. I. Hussain, Z. Yi, R. Siegele, T. Cresswell, L. Kong and D. M. Cahill, Plant Cell Rep., 2014, 33, 1389–1402 CrossRef CAS.
- S. Zafar, M. Bilal, M. F. Ali, A. Mahmood, J. Kijsomporn, L. S. Wong, M. Harshini, V. Kumar and S. S. Alotaibi, Environ. Sustainability Indic., 2024, 24, 100470 CrossRef.
- J. Lv, P. Christie and S. Zhang, Environ. Sci.: Nano, 2019, 6, 41–59 RSC.
- J. P. M. Andrews, S. S. Joshi, E. Tzolos, M. B. Syed, H. Cuthbert, L. E. Crica, N. Lozano, E. Okwelogu, J. B. Raftis, L. Bruce, C. A. Poland, R. Duffin, P. H. B. Fokkens, A. J. F. Boere, D. L. A. C. Leseman, I. L. Megson, P. D. Whitfield, K. Ziegler, S. Tammireddy, M. Hadjidemetriou, C. Bussy, F. R. Cassee, D. E. Newby, K. Kostarelos and M. R. Miller, Nat. Nanotechnol., 2024, 19, 705–714 CrossRef CAS PubMed.
- L. Fusco, M. Garrido, C. Martín, S. Sosa, C. Ponti, A. Centeno, B. Alonso, A. Zurutuza, E. Vázquez, A. Tubaro, M. Prato and M. Pelin, Nanoscale, 2020, 12, 610–622 RSC.
- M. Pelin, L. Fusco, V. León, C. Martín, A. Criado, S. Sosa, E. Vázquez, A. Tubaro and M. Prato, Sci. Rep., 2017, 7, 40572 CrossRef CAS PubMed.
- S. Chortarea, O. C. Kuru, W. Netkueakul, M. Pelin, S. Keshavan, Z. Song, B. Ma, J. Gómes, E. V. Abalos, L. A. V. d. Luna, T. Loret, A. Fordham, M. Drummond, N. Kontis, G. Anagnostopoulos, G. Paterakis, P. Cataldi, A. Tubaro, C. Galiotis, I. Kinloch, B. Fadeel, C. Bussy, K. Kostarelos, T. Buerki-Thurnherr, M. Prato, A. Bianco and P. Wick, J. Hazard. Mater., 2022, 435, 129053 CrossRef CAS PubMed.
- G. Vietti, D. Lison and S. van den Brule, Part. Fibre Toxicol., 2016, 13, 11 CrossRef.
- N. Kobayashi, H. Izumi and Y. Morimoto, J. Occup. Health, 2017, 59, 394–407 CrossRef CAS.
- M. P. DeLorme, Y. Muro, T. Arai, D. A. Banas, S. R. Frame, K. L. Reed and D. B. Warheit, Toxicol. Sci., 2012, 128, 449–460 CrossRef CAS.
- Z. Liu, S. Tabakman, K. Welsher and H. Dai, Nano Res., 2009, 2, 85–120 CrossRef CAS.
- S. K. Verma, A. K. Das, S. Gantait, V. Kumar and E. Gurel, Sci. Total Environ., 2019, 667, 485–499 CrossRef CAS.
- R. Tarrahi, S. Mahjouri and A. Khataee, Ecotoxicol. Environ. Saf., 2021, 208, 111697 CrossRef CAS.
- M. H. Lahiani, S. Khare, C. E. Cerniglia, R. Boy, I. N. Ivanov and M. Khodakovskaya, Nanoscale, 2019, 11, 3639–3655 RSC.
- S. R. Cherati, M. Anas, S. Liu, S. Shanmugam, K. Pandey, S. Angtuaco, R. Shelton, A. N. Khalfaoui, S. V. Alena, E. Porter, T. Fite, H. Cao, M. J. Green, A. G. Basnakian and M. V. Khodakovskaya, ACS Nano, 2022, 16, 12061–12072 CrossRef.
- J. Nepal, X. Xin, G. Maltais-Landry, W. Ahmad, J. Pereira, S. Santra, A. L. Wright, A. Ogram, P. J. Stofella and Z. He, NanoImpact, 2023, 31, 100480 CrossRef CAS.
- F. Zhao, X. Xin, Y. Cao, D. Su, P. Ji, Z. Zhu and Z. He, Nanomaterials, 2021, 11, 2717 CrossRef CAS.
- F. Wu, Y. You, X. Zhang, H. Zhang, W. Chen, Y. Yang, D. Werner, S. Tao and X. Wang, Environ. Sci. Technol., 2019, 53, 5707–5716 CrossRef CAS.
- C. Forstner, T. G. Orton, A. Skarshewski, P. Wang, P. M. Kopittke and P. G. Dennis, Sci. Total Environ., 2019, 671, 140–148 Search PubMed.
- X. Yang, X. Zhang, X. Shu, W. Zhang, J. Kai, M. Tang, J. Gong, J. Yang, J. Lin, Y. Chai and J. Liu, Ecotoxicol. Environ. Saf., 2022, 246, 114158 CrossRef CAS.
- J. H. Bisesi Jr., J. Merten, K. Liu, A. N. Parks, A. R. M. N. Afrooz, J. B. Glenn, S. J. Klaine, A. S. Kane, N. B. Saleh, P. L. Ferguson and T. Sabo-Attwood, Environ. Sci. Technol., 2014, 48, 1973–1983 CrossRef.
- J. H. Bisesi, T. Ngo, S. Ponnavolu, K. Liu, C. M. Lavelle, A. R. M. N. Afrooz, N. B. Saleh, P. L. Ferguson, N. D. Denslow and T. Sabo-Attwood, Nanomaterials, 2015, 5, 1066–1086 Search PubMed.
- R. Bjorkland, D. A. Tobias and E. J. Petersen, Environ. Sci.: Nano, 2017, 4, 747–766 RSC.
- P. Jackson, N. R. Jacobsen, A. Baun, R. Birkedal, D. Kühnel, K. A. Jensen, U. Vogel and H. Wallin, Chem. Cent. J., 2013, 7, 154 CrossRef.
- A. Gade, P. Ingle, U. Nimbalkar, M. Rai, R. Raut, M. Vedpathak, P. Jagtap and K. A. Abd-Elsalam, Agrochemicals, 2023, 2, 257–278 CrossRef.
- A. Nongbet, A. K. Mishra, Y. K. Mohanta, S. Mahanta, M. K. Ray, M. Khan, K. H. Baek and I. Chakrabartty, Plants, 2022, 11(19), 2587 CrossRef CAS PubMed.
- C. An, C. Sun, N. Li, B. Huang, J. Jiang, Y. Shen, C. Wang, X. Zhao, B. Cui, C. Wang, X. Li, S. Zhan, F. Gao, Z. Zeng, H. Cui and Y. Wang, J. Nanobiotechnol., 2022, 20, 11 CrossRef CAS.
- Y. Su, X. Zhou, H. Meng, T. Xia, H. Liu, P. Rolshausen, C. Roper, J. E. McLean, Y. Zhang, A. A. Keller and D. Jassby, Nat. Food, 2022, 3, 1020–1030 CrossRef.
- E. A. Alsherif, O. Almaghrabi, A. M. Elazzazy, M. Abdel-Mawgoud, G. T.S. Beemster and H. AbdElgawad, Plant Physiol. Biochem., 2023, 194, 29–40 CrossRef CAS.
- S. Soni, A. B. Jha, R. S. Dubey and P. Sharma, Sci. Total Environ., 2024, 925, 171433 CrossRef CAS.
- Y. González-García, G. Cadenas-Pliego, Á. G. Alpuche-Solís, R. I. Cabrera and A. Juárez-Maldonado, Nanomaterials, 2021, 11, 1080 CrossRef.
- A. Jain and S. Das, in Advances in Nano-Fertilizers and Nano-Pesticides in Agriculture, ed. S. Jogaiah, H. B. Singh, L. F. Fraceto and R. d. Lima, Woodhead Publishing, 2021, pp. 145–152. DOI:10.1016/B978-0-12-820092-6.00006-9.
- V. Amenta, K. Aschberger, M. Arena, H. Bouwmeester, F. B. Moniz, P. Brandhoff, S. Gottardo, H. J. P. Marvin, A. Mech, L. Q. Pesudo, H. Rauscher, R. Schoonjans, M. V. Vettori, S. Weigel and R. J. Peters, Regul. Toxicol. Pharmacol., 2015, 73, 463–476 CrossRef.
- J. H. E. Arts, M. Hadi, A. M. Keene, R. Kreiling, D. Lyon, M. Maier, K. Michel, T. Petry, U. G. Sauer, D. Warheit, K. Wiench and R. Landsiedel, Regul. Toxicol. Pharmacol., 2014, 70, 492–506 CrossRef CAS PubMed.
- R. Kumari, K. Suman, S. Karmakar, V. Mishra, S. G. Lakra, G. K. Saurav and B. K. Mahto, Front. Genome Ed., 2023, 5, 1200987 Search PubMed.
| This journal is © The Royal Society of Chemistry 2025 |