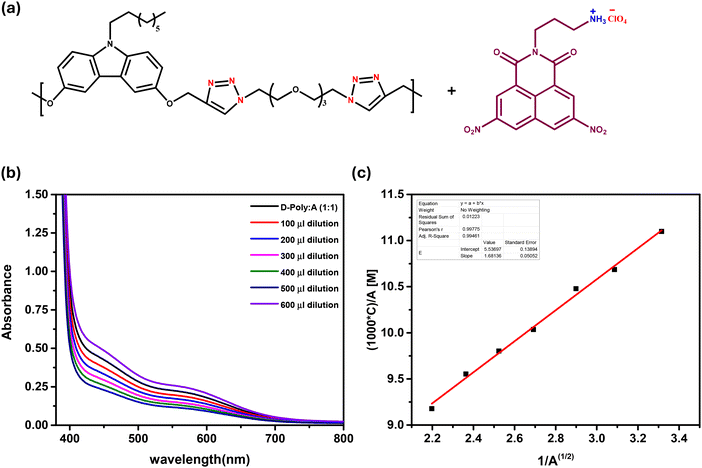
Chain folding of carbazole-donor containing polymers via a two-point interaction with naphthalene monoimide-based acceptors†
Arun Kumar
Gayen
and
S.
Ramakrishnan
 *
*
Department of Inorganic and Physical Chemistry, Indian Institute of Science, Bangalore 560012, India. E-mail: raman@iisc.ac.in
First published on 11th February 2025
Abstract
Linear polymers carrying electron-rich dialkoxy-carbazole (DACBZ) units linked by flexible oligooxyethylene segments were induced into a zigzag folded state by an external folding agent, which carries two subunits: an electron-deficient dinitro-naphthalene monoimide (NMI(NO2)2) acceptor and an ammonium perchlorate unit. The ammonium unit of the folding agent interacts with the backbone oxyethylene segment and, in turn, brings two adjacent CBZ moieties on either side of the electron-deficient NMI(NO2)2 unit of the folding agent to induce a charge-transfer (CT) interaction; this two-point interaction was shown to be crucial for the stability of the pleated chain structure. An interesting, and potentially useful, feature of this system is the possibility to incorporate a pendant unit at the carbazole nitrogen (N) site; a variety of segments, such as linear, branched, or chiral alkyl chains, were installed to examine if the folding of the chain is influenced by the nature of the pendant segment. The formation of the pleated structure was studied by 1H-NMR and UV-visible titration experiments; both these clearly revealed the presence of strong charge transfer (CT) interactions between the donor (D) and acceptor (A) units. The length of the spacer segment linking the NMI(NO2)2 acceptor unit and the ammonium group in the folding agent was varied, and it was seen that a 3-carbon spacer yielded the strongest cooperative interaction. To translate the folded conformation into the solid state, a solution of the donor polymer and the most efficient acceptor, taken in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (D:A) ratio, was cast on a quartz plate. UV-visible studies of the film revealed the retention of the CT band; more importantly, immersing the film into an aqueous NaHCO3 solution neutralized the ammonium group to generate the free amine. This caused an unexpected deepening of the colour, along with a hypsochromic shift of the CT band, suggesting that the free amine acceptor readjusts within the film to improve the CT interaction, exploiting the newly garnered freedom after de-coordination with the oligooxyethylene segment.
1 (D:A) ratio, was cast on a quartz plate. UV-visible studies of the film revealed the retention of the CT band; more importantly, immersing the film into an aqueous NaHCO3 solution neutralized the ammonium group to generate the free amine. This caused an unexpected deepening of the colour, along with a hypsochromic shift of the CT band, suggesting that the free amine acceptor readjusts within the film to improve the CT interaction, exploiting the newly garnered freedom after de-coordination with the oligooxyethylene segment.
Introduction
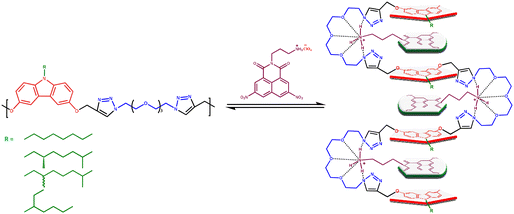
Chemists have long sought to mimic the bio-macromolecular structure and function in synthetic polymers. Controlling the solution conformation of long-chain polymers via intrachain noncovalent interactions is a crucial step in this direction. For synthetic polymers, two distinct strategies have been developed to control their solution conformation: one approach limits the conformational degrees of freedom of the chain either by the choice of repeat unit structure and/or by using H-bonding between adjacent repeat units,1–6 and the other utilizes a combination of multiple weak intra-chain interactions between non-adjacent segments along the chain to stabilize the desired conformation.7–10 Using the latter strategy, Iverson and co-workers demonstrated that charge-transfer interactions between alternatingly located electron-rich dialkoxynaphthalene (DAN) and electron-deficient naphthalene diimide (NDI) units, aided by periodic amphiphilicity, can be utilized to control the solution conformation of oligomeric systems.11–15 In a similar vein, we too showed that polymers carrying alternating donor (D) and acceptor (A) units, linked via an oligooxyethylene segment, undergo a zigzag folding induced by alkali-metal ions; coordination of metal ions with the oxyethylene segment brings the D and A units in close proximity for the onset of charge-transfer (CT) interactions, which further stabilizes the folded form.16,17 Later, we also showed that a polymer chain carrying either a D or A unit can be made to fold via a two-point interaction, one of which was either a podand-like interaction of the oligooxyethylene segment in the polymer with an ammonium group18,19 or a H-bonding interaction between an amine on the backbone and a –COOH group20 in the folding agent.The concept of a small molecule folding agent to control the conformation of a polymer chain is attractive because of its reversibility; thus, this strategy could, in principle, be utilized to translate the control achieved in solution to organize polymer chains in the solid state. To achieve this, however, it is important to design systems where the interactions between the folding agent and the polymer chain are strong, yet reversible. Recently, we reported a new unsymmetrical D–A pair, based on dialkoxy-carbazole (DACBZ) and dinitro-naphthalene monoimide (NMI(NO2)2), which exhibited a relatively strong CT interaction.21 In the present study, we have incorporated the DACBZ donor unit along the polymer backbone linked via oligooxyethylene segments, and the NMI(NO2)2 acceptor was modified to carry a quaternized ammonium perchlorate. The interaction of the ammonium group with the oligooxyethylene segment via a podand-type interaction causes adjacent carbazole donor units to come together and form a CT complex with the NMI(NO2)2 acceptor, as depicted in Scheme 1; this two-point interaction is expected to stabilize the folded conformation.
We examined the folding using the evolution of the CT band in the UV-visible spectra and by following the variation in the proton NMR signals of the D and A units. Furthermore, we have examined the solid-state properties of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex of one selected donor polymer and the most effective acceptor folding agent; it was seen that the CT complex-induced folded state is retained in thin films as well. Importantly, neutralizing the ammonium group by treatment of the film with an aqueous NaHCO3 solution releases the free amine and weakens the two-point interaction. Unexpectedly, this treatment led to a deepening of the colour, along with a small hypsochromic shift of the CT band, suggesting that the loosening of the podand-type interaction led to a slight rearrangement of the D and A units, causing a dramatic change in the absorption spectrum.
1 complex of one selected donor polymer and the most effective acceptor folding agent; it was seen that the CT complex-induced folded state is retained in thin films as well. Importantly, neutralizing the ammonium group by treatment of the film with an aqueous NaHCO3 solution releases the free amine and weakens the two-point interaction. Unexpectedly, this treatment led to a deepening of the colour, along with a small hypsochromic shift of the CT band, suggesting that the loosening of the podand-type interaction led to a slight rearrangement of the D and A units, causing a dramatic change in the absorption spectrum.
Results and discussion
3,6-Dihydroxycarbazole derivatives, bearing different substituents (R), were readily synthesized from dibromocarbazole in a few steps, as described earlier;22 reactions with propargyl bromide yielded 3,6-dipropargyloxycarbazole, which served as one of the monomers. This monomer was reacted with the diazide prepared from tetraethylene glycol (see Scheme 2), under standard Cu-catalyzed alkyne-azide click reaction conditions.23 Depending on the lateral substituent on the carbazole, the nature of the polymer obtained varied from a tacky substance to an elastomeric one. In a typical procedure, the dipropargyloxy-carbazole monomer was reacted with TEG-diazide in the presence of CuI and diisopropylethylamine (DIPEA) under N2 sealed conditions for 5 days at 60 °C, using a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1(v/v) THF and CH3CN solvent mixture. The polymer was isolated and purified by dissolution in chloroform and reprecipitation in methanol, multiple times.
1(v/v) THF and CH3CN solvent mixture. The polymer was isolated and purified by dissolution in chloroform and reprecipitation in methanol, multiple times.
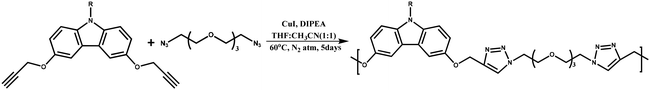 | ||
| Scheme 2 Synthesis of a donor-bearing polymer by a reaction of 3,6-dipropargyloxy-9-alkyl-carbazole and tetraethylene glycol diazide, using a Cu-catalyzed alkyne-azide click reaction. | ||
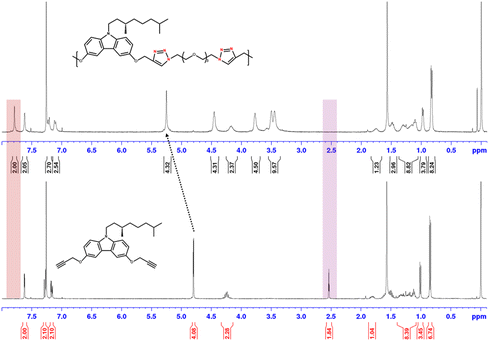
Comparison of the 1H-NMR spectra (Fig. 1) of the carbazole monomer with that of a representative polymer, poly-D(β-cit-S) (where R = (S)-β-citronellyl), confirms its structure; the disappearance of the propargyl proton peak at 2.54 ppm, the appearance of a new peak at 7.78 ppm corresponding to the triazole ring proton, and the downfield shift of the propargyl CH2 peak are in accordance with the structure of the expected polymer. Similarly, 3 other donor-containing polymers with different pendant units, namely, poly-D(C8-linear), poly-D(C8-branched), and poly-D(β-cit-rac), were synthesized; poly-D(β-cit-S) and poly-D(β-cit-rac) represent polymers carrying enantiomerically pure and racemic β-citronellyl units, respectively. The molecular weights of all the polymers were estimated by SEC using a triple detector system; three of the polymers had moderately high molecular weights (Mn > 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), whereas one of them had a lower Mn value of ∼14
000), whereas one of them had a lower Mn value of ∼14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, as seen in Table 1. An interesting observation was that the Mark–Houwink exponents, estimated using the in-line differential viscometer, reveal that the polymers bearing branched pendant units exhibit comparatively higher chain stiffness in THF (compare entries 1 and 2), while the other polymers adopt a random coil configuration in solution.
000, as seen in Table 1. An interesting observation was that the Mark–Houwink exponents, estimated using the in-line differential viscometer, reveal that the polymers bearing branched pendant units exhibit comparatively higher chain stiffness in THF (compare entries 1 and 2), while the other polymers adopt a random coil configuration in solution.
| Entry | Polymer | M n(gm mol−1) | Đ | α (M-H) |
|---|---|---|---|---|
| a Đ = Mw/Mn as determined by SEC. | ||||
| 1 | Poly-D (C8-linear) | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
2.40 | 0.557 |
| 2 | Poly-D (C8-branched) | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 350 350 |
1.27 | 1.027 |
| 3 | Poly-D (β-cit-S) | 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
1.55 | 0.732 |
| 4 | Poly-D (β-cit-rac) | 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
1.65 | 0.748 |
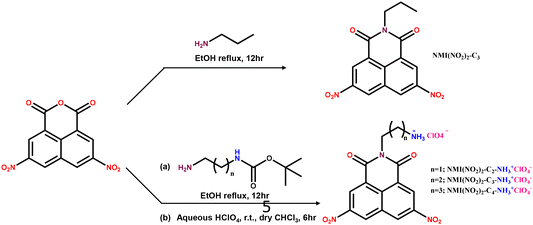
Having prepared the DACBZ donor-containing polymer, the synthesis of naphthalene monoimide-based external folding agents was undertaken; here, one of the objectives was to optimize the length of the spacer, linking the NMI(NO2)2 aromatic core and the ammonium group, for achieving maximum folding efficiency. Thus, 3,6-dinitronaphthalene anhydride was reacted with an excess of singly BOC-protected diamines, namely 1,2 diaminoethane, 1,3 diaminopropane, and 1,4-diaminobutane, as depicted in Scheme 3; the resulting naphthalene monoimides were then deprotected using perchloric acid to directly yield the required ammonium perchlorate salt.
For a comparative study of the folding process, a model n-propyl 3,6-dinitronaphthalene monoimide, which does not carry the ammonium unit (Scheme 3), was also prepared. The 1H-NMR spectra of all three folding agents (Fig. S1†) confirmed their structures.
Interaction between poly-D and the NMI(NO2)2 folding agent – NMR titrations
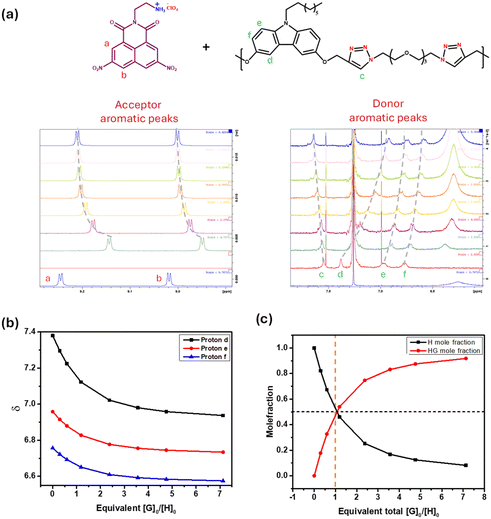
To examine the interaction between the folding agents and the carbazole-containing polymers, both 1H-NMR and UV-visible spectroscopy were utilized. The NMR titration experiments were carried out by varying the concentration of the folding agent, while retaining a fixed concentration of the polymer, whereas in the UV-visible titration experiments, a solution containing equimolar amounts of the polymer (considering repeating unit molecular weight) and acceptor was prepared and the resulting solution was then subjected to serial dilution; in each step, 100 μL was pipetted out from the cuvette, and 100 μL of solvent was added to it, and its spectrum was recorded.The formation of the CT complex is well known to influence the chemical shift of the aromatic donor and acceptor protons;24 typically, since the CT complex undergoes rapid exchange on the NMR time scale, the observed chemical shift values represent a weighted average of the free (δD or δA) and complexed species (δDA). In Fig. 2, the proton NMR spectra of poly-D-C8-linear (aromatic regions of the donor and acceptor), in CDCl3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3CN (1
CH3CN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), with increasing concentrations of the acceptor, NMI(NO2)2-C2-NH3+ClO4−, are shown; in all cases, the two aromatic protons of the acceptor unit are seen to undergo a significant up-field shift with increasing formation of the CT complex species. It is important to note that, in the experimental protocol, the ratio of D
2), with increasing concentrations of the acceptor, NMI(NO2)2-C2-NH3+ClO4−, are shown; in all cases, the two aromatic protons of the acceptor unit are seen to undergo a significant up-field shift with increasing formation of the CT complex species. It is important to note that, in the experimental protocol, the ratio of D![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A is highest after the first aliquot of the acceptor is added; at this stage, most of the added acceptor will be complexed and the weighted average δ-value would be closest to that expected for the pure CT complex. Hence, the acceptor protons appear at a large up-field shifted value after the first addition, which then continuously moves down-field with increasing amounts of acceptor, as evident in the spectra (Fig. 2a, left panel). Likewise, all the donor aromatic protons also experience a gradual up-field shift upon CT complex formation (Fig. 2a, right panel); the extent of the shift is clearly not the same for all the protons, which is a reflection of the geometry of the CT complex.
A is highest after the first aliquot of the acceptor is added; at this stage, most of the added acceptor will be complexed and the weighted average δ-value would be closest to that expected for the pure CT complex. Hence, the acceptor protons appear at a large up-field shifted value after the first addition, which then continuously moves down-field with increasing amounts of acceptor, as evident in the spectra (Fig. 2a, left panel). Likewise, all the donor aromatic protons also experience a gradual up-field shift upon CT complex formation (Fig. 2a, right panel); the extent of the shift is clearly not the same for all the protons, which is a reflection of the geometry of the CT complex.
The chemical shift variation of the three different carbazole aromatic protons, for this representative titration, with increasing amounts of added external acceptor molecule, is shown in Fig. 2b. To calculate the association constants (Ka), a binding isotherm (Fig. 2c), namely, the plot of the concentration of the CT complex as a function of the guest/host ratio, [G0]/[H0], was first generated from the chemical shift variation, using the online tool provided by Thordarson and co-workers, which uses a non-linear regression approach.25,26 From such fitted isotherms, the association constants were estimated, assuming a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 D–A complex. It is evident that the extent of folding that the polymer chain undergoes upon interaction with a folding agent will depend on the association constant (Ka) between them; here, note that the expected final structure will be one where the acceptor (A) would be sandwiched between two adjacent carbazole donors, as depicted in Scheme 1. Thus, the expected complexation ratio between D and A will be 1
1 D–A complex. It is evident that the extent of folding that the polymer chain undergoes upon interaction with a folding agent will depend on the association constant (Ka) between them; here, note that the expected final structure will be one where the acceptor (A) would be sandwiched between two adjacent carbazole donors, as depicted in Scheme 1. Thus, the expected complexation ratio between D and A will be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, when the entire chain adopts a folded conformation.
1, when the entire chain adopts a folded conformation.
The values of association constants, estimated from the NMR titrations using the carbazole proton shifts, are listed in Table 2 (column 4); these values represent the average of the values obtained using the three different carbazole protons. Given that the typical percentage error in such a non-linear regression analysis, in our systems, is between 10 and 15%, one may conclude that the Ka values generally fall in the 1000–1500 M−1 range for different donor polymers, using the folding agent with a C2 spacer (compare entries 2–5). It is important to recognize that, here, we are not dealing with a simple D and A pair, where the product is a single D–A charge-transfer complex. In our systems, the donors are strung together in a polymer chain and the complexation is aided by the interaction of the ammonium group with the oligooxyethylene segment; this in turn would generate a D–A–D type of complex, in the early stages, where A gets sandwiched between two D units. In our earlier studies,17 using model compounds, we showed that the chemical shifts of the D protons of a simple D–A molecule are significantly different from those in the ADA trimer, but similar to those in the DAD trimer. In other words, when D lies between two A units, it experiences a larger shift than that in the simple DA case. Thus, as the chain folds, we would have a larger fraction of sandwiched D units. Therefore, it is evident that a fitting process, which optimizes for a single δDA value, would provide only a reasonable estimate of Ka values. Nevertheless, from these estimated values, it is evident that the nature of the lateral substituent does not affect the CT complexation very significantly, and consequently, the folding propensity is largely unaffected by the nature of the lateral substituent. On the other hand, variation in the spacer length in the folding agent would be expected to influence the folding, as was shown earlier;18 here, a flexible oligooxyethylene segment in the backbone of the polymer was specifically incorporated to coerce the folding via a podand-type interaction16–18 between the oligooxyethylene segment and ammonium of the acceptor folding molecule. To further examine this, the three folding agents, carrying spacer segments with two (C2), three (C3), and four (C4) methylene carbons, were compared; additionally, a model folding agent carrying a C3 segment without the ammonium unit was also studied. All the titration experiments were carried out with only a single donor polymer, namely poly-D(C8-linear); as earlier, titrations were carried out in a solvent mixture, namely CDCl3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3CN (1
CH3CN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). The 1H-NMR spectra as a function of varying amounts of different folding agents are shown in Fig. S5,† along with the variation of δ of different carbazole ring protons (Fig. S6†) and the calculated binding isotherms (Fig. S7†); the expected up-field shift of all the aromatic protons is seen with increasing CT complex formation. As earlier, we see that one of the three carbazole peaks (d) undergoes a significantly larger shift upon CT complex formation. Of the four folding agents, the change in the δ-value was maximum in the case of the C3 spacer, followed by C2 and then the C4 spacer (Fig. S6†). On the other hand, the model folding agent without the ammonium unit exhibited a much smaller change in the δ-values; as earlier, the nonlinear regression analysis yielded the association constant values, which are listed in Table 2 (entries 2, 6 and 7). It is evident that the spacer does play a very significant role; the folding agent with a C3 spacer exhibits a Ka value that is almost 75% greater than that of C2 and ∼100% greater than that of C4. Such an effect of the spacer segment in a two-point interaction was also seen earlier by Ghosh et al.18 On the other hand, the association constant value for the model folding agent (entry 1), with no ammonium group, was far smaller (264 M−1); thus, revealing the importance of the two-point interaction.
2). The 1H-NMR spectra as a function of varying amounts of different folding agents are shown in Fig. S5,† along with the variation of δ of different carbazole ring protons (Fig. S6†) and the calculated binding isotherms (Fig. S7†); the expected up-field shift of all the aromatic protons is seen with increasing CT complex formation. As earlier, we see that one of the three carbazole peaks (d) undergoes a significantly larger shift upon CT complex formation. Of the four folding agents, the change in the δ-value was maximum in the case of the C3 spacer, followed by C2 and then the C4 spacer (Fig. S6†). On the other hand, the model folding agent without the ammonium unit exhibited a much smaller change in the δ-values; as earlier, the nonlinear regression analysis yielded the association constant values, which are listed in Table 2 (entries 2, 6 and 7). It is evident that the spacer does play a very significant role; the folding agent with a C3 spacer exhibits a Ka value that is almost 75% greater than that of C2 and ∼100% greater than that of C4. Such an effect of the spacer segment in a two-point interaction was also seen earlier by Ghosh et al.18 On the other hand, the association constant value for the model folding agent (entry 1), with no ammonium group, was far smaller (264 M−1); thus, revealing the importance of the two-point interaction.
| Entry | Polymer | Folding agent | K a carbazole protons (M−1) |
|---|---|---|---|
| NMR titration method | |||
| NE = not estimated (the CT band intensity is too low to estimate the Ka). | |||
| 1 | Poly-D (C8-linear) | NMI(NO2)2-C2-CH3 | 264 |
| 2 | Poly-D (C8-linear) | NMI(NO2)2-C2-NH3+ClO4− | 1316 |
| 3 | Poly-D (C8 branched) | NMI(NO2)2-C2-NH3+ClO4− | 1549 |
| 4 | Poly-D (β-cit-S) | NMI(NO2)2-C2-NH3+ClO4− | 1179 |
| 5 | Poly-D (β-cit-rac) | NMI(NO2)2-C2-NH3+ClO4− | 929 |
| 6 | Poly-D (C8-linear) | NMI(NO2)2-C3-NH3+ClO4− | 2032 |
| 7 | Poly-D (C8-linear) | NMI(NO2)2-C4-NH3+ClO4− | 1104 |
| UV-visible titration method | K a (UV-visible; λCT) | ||
| 8 | Poly-D (C8-linear) | NMI(NO2)2-C2-CH3 | NE |
| 9 | Poly-D (C8-linear) | NMI(NO2)2-C3-NH3+ClO4− | 1959 |
| 10 | Poly-D (C8 branched) | NMI(NO2)2-C3-NH3+ClO4− | 2325 |
| 11 | Poly-D (β-cit-S) | NMI(NO2)2-C3-NH3+ClO4− | 3395 |
| 12 | Poly-D (β-cit-rac) | NMI(NO2)2-C3-NH3+ClO4− | 4013 |
UV-visible titration studies
The charge-transfer interactions between the carbazole donors in the polymer and the acceptor in the folding agent generated a CT band, which was examined using UV-visible spectroscopy. Unlike in the NMR titration experiments, here, a solution containing equimolar amounts of the polymer (repeat unit) and the acceptor folding agent (guest) was prepared; the resulting solution was then serially diluted, and the absorption spectrum was recorded after each dilution step. Since the folding agent with the C3 spacer segment, namely NMI(NO2)2-C3-NH3+, exhibited the highest binding affinity, the UV-visible titration experiments were carried out only with this folding agent; however, all the carbazole donor polymers, bearing different pendant N-alkyl segments, were tested; one of the reasons for doing so was to examine if the inclusion of a chiral alkyl segment can induce a twist in the folded (pleated) polymer chain, which would be reflected by a CD signal associated with the CT absorption band. Fig. 3b shows the variation in the UV-visible spectra (region showing the CT band) of poly D(C8-linear) and NMI(NO2)2-C3-NH3+ as a function of dilution. Unlike in the NMR titrations, here, the intensity of the CT band is directly proportional to the concentration of the CT complex; hence, the analysis utilized a different protocol. The data obtained from the dilution experiments were used to generate a Benesi–Hildebrand plot,27 and from the slope and intercept of this plot, the association constant Ka was calculated. UV-visible spectral variation for all other polymers is shown in Fig. S8;† and the linearly fitted Benesi–Hildebrand plots used to estimate Ka values are shown in Fig. S9.† The Ka value estimated from this linearization method for poly D(C8-linear) and NMI(NO2)2-C3-NH3+ matched well with that estimated from the NMR titration experiment (see Table 2, entries 6 and 9). Furthermore, all the other donor polymers yielded significantly higher values with NMI(NO2)2-C3-NH3+ than those with NMI(NO2)2-C2-NH3+; the latter were estimated from the NMR titrations (Table 2; compare entries 2–5 and 9–12). The CD spectra of the donor polymers bearing a chiral alkyl substituent, poly-D(β-cit-S), and that with the racemic segments, poly-D(β-cit-rac), were recorded in the presence of increasing amounts of NMI(NO2)2-C3-NH3+ClO4−; unfortunately, no CD signal in the CT absorption region, which reveals the formation of a helically twisted folded chain, was observed.Retention of charge-transfer induced folded chains in the solid state
The donor–acceptor CT interactions are dynamic in nature; hence, in solution, the bound acceptor molecules continuously exchange with the free species. The appearance of NMR peaks at a weighted-average chemical shift of the free and complexed species reveals this dynamic behaviour. In the solid state, of course, one would expect the dynamics to be significantly arrested. With the objective of probing the formation of folded chain conformation in the solid state, the carbazole-donor polymer, poly-D(C8-linear), and the acceptor, NMI(NO2)2-C3-NH3+ClO4−, were taken in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mole ratio (w.r.t. polymer repeat unit) and dissolved in a mixture of CHCl3-CH3CN (1
1 mole ratio (w.r.t. polymer repeat unit) and dissolved in a mixture of CHCl3-CH3CN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), and cast on a quartz plate; a brownish film (see the inset of Fig. 5a) was formed reflecting the occurrence of charge transfer. Additionally, a solid sample was also prepared by evaporating a solution of this 1
2), and cast on a quartz plate; a brownish film (see the inset of Fig. 5a) was formed reflecting the occurrence of charge transfer. Additionally, a solid sample was also prepared by evaporating a solution of this 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
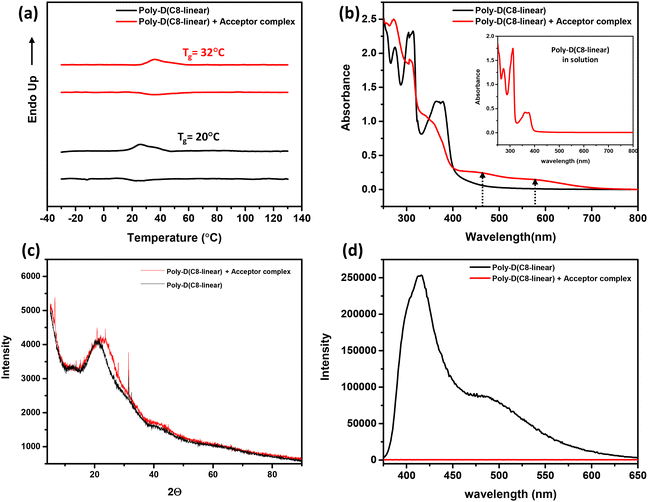
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture, first under a flow of N2 gas, followed by thorough drying under reduced pressure; this sample was used for differential scanning calorimetric (DSC) studies. The DSC thermograms, shown in Fig. 4a, reveal that the Tg of poly-D(C8-linear) increases from ∼20 °C to about ∼32 °C, upon complexation with NMI(NO2)2-C3-NH3+ClO4−, suggesting a slight stiffening of the chain.
1 mixture, first under a flow of N2 gas, followed by thorough drying under reduced pressure; this sample was used for differential scanning calorimetric (DSC) studies. The DSC thermograms, shown in Fig. 4a, reveal that the Tg of poly-D(C8-linear) increases from ∼20 °C to about ∼32 °C, upon complexation with NMI(NO2)2-C3-NH3+ClO4−, suggesting a slight stiffening of the chain.
The UV-visible spectra of thin films of pure poly-D(C8-linear) and that of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 polymer complex are compared in Fig. 4b; it is evident that the spectrum of a thin film of the CT-induced folded chains is similar to that in solution, with two CT absorption peaks at ∼455 nm and ∼590 nm. Likewise, the spectrum of the pure poly-D(C8-linear) film is essentially identical to that in solution (see the inset in Fig. 4b). To further understand the ordering present in the solid state, the WAXS profiles of thicker drop-cast films were compared (Fig. 4c); the films were evidently not highly ordered, but the broad peak at 20.98° in poly-D(C8-linear) and that at 22.58° in the CT complex film correspond to an average distance between the stacked aromatic rings of 4.2 and 3.9 Å, respectively. The small reduction in the average interplanar distance, in the presence of the aromatic acceptor, is consistent with the hypothesis that CT interaction-induced folding leads to stronger interactions and consequently a smaller average distance between the aromatic chromophores. As carbazole and its derivatives are known to be fluorescent,28–30 emission spectra were recorded for both thin films; it was seen that, while poly-D(C8-linear) was highly fluorescent, upon complexation with the acceptor molecules, the fluorescence was completely quenched (Fig. 4d), as expected.31,32 Together, these observations demonstrate that poly-D(C8-linear) retains the CT complexation with NMI(NO2)2-C3-NH3+ClO4− in thin films, presumably translating the folded structure via the two-point interaction.
1 polymer complex are compared in Fig. 4b; it is evident that the spectrum of a thin film of the CT-induced folded chains is similar to that in solution, with two CT absorption peaks at ∼455 nm and ∼590 nm. Likewise, the spectrum of the pure poly-D(C8-linear) film is essentially identical to that in solution (see the inset in Fig. 4b). To further understand the ordering present in the solid state, the WAXS profiles of thicker drop-cast films were compared (Fig. 4c); the films were evidently not highly ordered, but the broad peak at 20.98° in poly-D(C8-linear) and that at 22.58° in the CT complex film correspond to an average distance between the stacked aromatic rings of 4.2 and 3.9 Å, respectively. The small reduction in the average interplanar distance, in the presence of the aromatic acceptor, is consistent with the hypothesis that CT interaction-induced folding leads to stronger interactions and consequently a smaller average distance between the aromatic chromophores. As carbazole and its derivatives are known to be fluorescent,28–30 emission spectra were recorded for both thin films; it was seen that, while poly-D(C8-linear) was highly fluorescent, upon complexation with the acceptor molecules, the fluorescence was completely quenched (Fig. 4d), as expected.31,32 Together, these observations demonstrate that poly-D(C8-linear) retains the CT complexation with NMI(NO2)2-C3-NH3+ClO4− in thin films, presumably translating the folded structure via the two-point interaction.
To further probe the structure of the polymer in thin films, we carried out a few additional experiments. First, it is important to recognize that the two-point interaction of the acceptor with the polymer chain is crucial for reinforcing the formation of the folded structure, which in turn is formed only in the presence of the ammonium group in the acceptor, as was discussed earlier. Hence, releasing the free base of the acceptor by treatment of the film with aqueous NaHCO3 solution should weaken the interaction between the polymer and the acceptor; however, since the polymer chain has restricted mobility in the solid state, one may expect the pleated conformation to remain largely intact. Furthermore, since the acceptor-amine is not highly soluble in the aqueous medium, it will remain within the film.
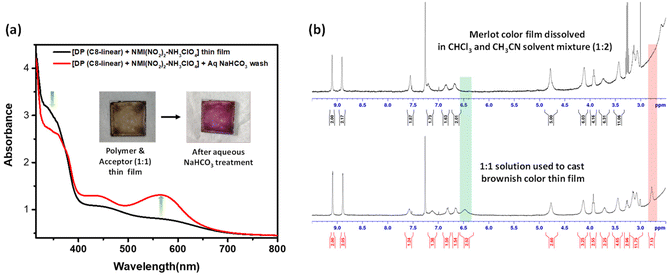
To test these hypotheses, the film of the CT complex, coated on a quartz plate, was dipped in an aqueous solution of NaHCO3; effervescence due to the release of CO2 gas was noticed on the film surface during neutralization, and the colour of the film changed dramatically, as evident in Fig. 5a. The UV-visible spectra of the film, before and after bicarbonate treatment, revealed substantial changes; although the two peaks in the CT region remained intact, there was a significant hypsochromic shift of one peak from 455 nm to 436 nm and the second from 590 nm to 565 nm. In addition, there was a substantial increase in the intensity of both CT bands, with a slight decrease in the intensity of the shoulder in the UV region; this change apparently reflects a change in the relative position/orientation of the D and A units, once the interaction with the oxyethylene segment is destroyed, upon deprotonation. Hypsochromism in the UV region has been observed in biopolymers33 and aedamers,15 where the extended face-centered stacking of aromatic units induces this effect, and this has been used to estimate the extent of stacking. To reveal the changes in composition that have occurred, upon treatment with aqueous NaHCO3 solution, the 1H-NMR spectra of solutions prepared by dissolving the two films were compared (Fig. 5b); the most prominent change noticed was the disappearance of the peaks at 6.48 and 2.77 ppm, due to the –NH3+ and the –CH2 unit adjacent to the ammonium salt, respectively. This clearly reveals that treatment with NaHCO3 solution indeed transforms the ammonium salt into the free amine, while both components remain intact within the film. Thus, the change observed upon NaHCO3 treatment primarily reflects a change in the nature of interaction between the donor polymer and the acceptor, and the colour and spectral changes noticed are due to a change in the geometry of the CT complex. It may be noted that, since the polymer carries two triazole rings in each repeat unit, the acceptor-amine can also form H-bonds with the two triazole rings, providing an alternate, possibly weaker, two-point interaction.
Conclusions
In conclusion, novel carbazole-based donor-containing polymers that undergo folding in the presence of an external folding agent by a two-point interaction were designed and synthesized. The folding agents carried an electron-deficient acceptor unit based on dinitronaphthalene monoimide, linked to an ammonium group via a short alkylene spacer of varying lengths. The folding of the polymer was induced by two synergistic interactions: one is a charge-transfer (CT) interaction between electron-rich donor units in the polymer backbone and electron-deficient acceptor units in the folding agent, and the second is the podand-type interaction between the oligooxyethylene segment and the ammonium group in the folding agents. The latter interaction of the ammonium unit with the flexible oxyethylene segment brings the adjacent carbazole units in close proximity, so as to establish the CT between the D and A units, which in turn causes the polymer chain to adopt a pleated folded structure. The interaction between the donor polymer and the small-molecule acceptor folding agent was examined by 1H-NMR and UV-visible titration experiments, both of which revealed that the folding agent carrying a trimethylene (C3) spacer segment between the acceptor and the ammonium unit exhibited the highest association constant with the polymer; the lateral substituent linked to the carbazole N-atom did not influence the binding significantly. Furthermore, we were able to translate the folded chain conformation of one carbazole donor-polymer into the solid state by casting a film using a solution of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex with the most effective acceptor-folding agent, namely NMI(NO2)2-C3-NH3+; interestingly, the neutralization of the ammonium perchlorate unit by dipping the film in an aqueous NaHCO3 solution led to an unexpected deepening of the colour, along with a small hypsochromic shift of the CT band. It is suggested that, while the two-point interaction with the ammonium-containing acceptor is essential for stabilizing the folded conformation in solution, once immobilized in thin films, the release of the podand-type binding permits the chromophore to reorient to further optimize the CT interaction. This concept of utilizing two interactions under dynamic conditions in solution, one of which can be disengaged in the solid state to relieve the compromised arrangement and attain the most preferred geometry, we believe, could be applied in a more general context to elicit interesting functions. Another important outcome of this study is the potential to install other functional units as pendant segments using the carbazole N-atom; the CT-induced folding of the polymer chain would in turn result in the ordering of these pendant units, which could be designed to impart specific interesting functions to such systems.
1 complex with the most effective acceptor-folding agent, namely NMI(NO2)2-C3-NH3+; interestingly, the neutralization of the ammonium perchlorate unit by dipping the film in an aqueous NaHCO3 solution led to an unexpected deepening of the colour, along with a small hypsochromic shift of the CT band. It is suggested that, while the two-point interaction with the ammonium-containing acceptor is essential for stabilizing the folded conformation in solution, once immobilized in thin films, the release of the podand-type binding permits the chromophore to reorient to further optimize the CT interaction. This concept of utilizing two interactions under dynamic conditions in solution, one of which can be disengaged in the solid state to relieve the compromised arrangement and attain the most preferred geometry, we believe, could be applied in a more general context to elicit interesting functions. Another important outcome of this study is the potential to install other functional units as pendant segments using the carbazole N-atom; the CT-induced folding of the polymer chain would in turn result in the ordering of these pendant units, which could be designed to impart specific interesting functions to such systems.
Author contributions
AKG carried out all the synthesis and characterization studies, analysed the data and contributed to writing the manuscript; AKG and SR designed the problem, developed the strategies, analysed the data and wrote the manuscript.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
We would like to thank DST, New Delhi, for the research grant (CRG/2021/005211) and awarding the J C Bose fellowship (2016-2021) to SR. AKG would like to thank CSIR-HRDG for the fellowship. The NMR facility at the IPC department, procured using the FIST grant from DST, New Delhi, is gratefully acknowledged.References
- E. Berni, C. Dolain, B. Kauffmann, J. M. Léger, C. Zhan and I. Huc, J. Org. Chem., 2008, 73, 2687–2694 CrossRef CAS.
- B. B. Ni, Q. Yan, Y. Ma and D. Zhao, Coord. Chem. Rev., 2010, 254, 954–971 CrossRef CAS.
- C. F. Wu, Z. M. Li, X. N. Xu, Z. X. Zhao, X. Zhao, R. X. Wang and Z. T. Li, Chem. – Eur. J., 2014, 20, 1418–1426 CrossRef CAS.
- R. Guo, L. Zhang, H. Wang, D. W. Zhang and Z. T. Li, Polym. Chem., 2015, 6, 2382–2385 RSC.
- M. Lago-Silva, M. Fernández-Míguez, R. Rodríguez, E. Quiñoá and F. Freire, Chem. Soc. Rev., 2023, 53, 793–852 RSC.
- V. Damodara, H. Sardana and S. Ramakrishnan, Eur. Polym. J., 2024, 207, 112818 CrossRef CAS.
- B. Tuemmler, G. Maass, F. Voegtle, H. Sieger, U. Heimann and W. Edwin, J. Am. Chem. Soc., 1979, 101, 2588–2598 CrossRef CAS.
- J. Hou, M. Jia, X. Jiang, Z. Li and G. Chen, J. Org. Chem., 2004, 69, 6228–6237 CrossRef CAS PubMed.
- H. Juwarker, J. M. Suk and K. S. Jeong, Chem. Soc. Rev., 2009, 38, 3316–3325 RSC.
- C. Zhou, Y. Wang, M. Muthukumar, R. Zhang, J. Zhao and D. Jia, Macromolecules, 2022, 55, 8133–8142 CrossRef CAS.
- J. Q. Nguyen and B. L. Iverson, J. Am. Chem. Soc., 1999, 121, 2639–2640 CrossRef CAS.
- A. J. Zych and B. L. Iverson, J. Am. Chem. Soc., 2000, 122, 8898–8909 CrossRef CAS.
- G. J. Gabriel and B. L. Iverson, J. Am. Chem. Soc., 2002, 124, 15174–15175 CrossRef CAS.
- G. J. Gabriel, S. Sorey and B. L. Iverson, J. Am. Chem. Soc., 2005, 127, 2637–2640 CrossRef CAS PubMed.
- V. J. Bradford and B. L. Iverson, J. Am. Chem. Soc., 2008, 130, 1517–1524 CrossRef CAS PubMed.
- S. Ghosh and S. Ramakrishnan, Angew. Chem., Int. Ed., 2004, 43, 3264–3268 CrossRef CAS PubMed.
- S. Ghosh and S. Ramakrishnan, Macromolecules, 2005, 38, 676–686 CrossRef CAS.
- S. Ghosh and S. Ramakrishnan, Angew. Chem., Int. Ed., 2005, 44, 5441–5447 CrossRef CAS PubMed.
- S. G. Ramkumar and S. Ramakrishnan, J. Chem. Sci., 2008, 120, 187–194 CrossRef CAS.
- S. De, D. Koley and S. Ramakrishnan, Macromolecules, 2010, 43, 3183–3192 CrossRef CAS.
- A. K. Gayen, P. Huilgol, S. V. Kailas and S. Ramakrishnan, Langmuir, 2024, 40, 26103–26113 CrossRef CAS PubMed.
- J. B. Harlé, S. Mine, T. Kamegawa, V. T. Nguyen, T. Maeda, H. Nakazumi and H. Fujiwara, J. Phys. Chem. C, 2017, 121, 15049–15062 CrossRef.
- V. V. Rostovtsev, L. G. Green, V. V. Fokin and K. B. Sharpless, Angew. Chem., 2002, 114, 2708–2711 CrossRef.
- T. Ackermann and K. A. Connors, Binding constants-the measurement of molecular complex stability, John Wiley & Sons, Ltd, New York, 1987 Search PubMed.
- D. Brynn Hibbert and P. Thordarson, Chem. Commun., 2016, 52, 12792–12805 RSC.
- Supramolecular, https://supramolecular.org.
- H. A. Benesi and J. H. Hildebrand, J. Am. Chem. Soc., 1949, 71, 2703–2707 CrossRef CAS.
- J. Chen, X. Zhang, Z. Xie and B. Liu, Aggregate, 2024, e638, 2692–4560 Search PubMed.
- G. Srikanth, D. Devadiga, B. M. Samrudhi and T. N. Ahipa, J. Fluoresc., 2024, 1, 1053–0509 Search PubMed.
- A. Afrin and P. Chinna Ayya Swamy, J. Mater. Chem. C, 2024, 12, 1923–1944 RSC.
- S. Thazhathethil, T. Muramatsu, N. Tamaoki, C. Weder and Y. Sagara, Angew. Chem., Int. Ed., 2022, 61, e202209225 CrossRef CAS PubMed.
- Y. Sagara, M. Karman, A. Seki, M. Pannipara, N. Tamaoki and C. Weder, ACS Cent. Sci., 2019, 5, 874–881 CrossRef CAS PubMed.
- H. Devoe, Ann. N. Y. Acad. Sci., 1969, 158, 298–307 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4py01234h |
| This journal is © The Royal Society of Chemistry 2025 |