Tb(III)-functionalized MOF hybridized bis-crosslinked networked hydrogel luminescent films for arginine and dopamine hydrochloride sensing and anticounterfeiting†
Jiaxuan
Pan
a,
Jiazhen
Lu
a,
Yichen
Shang
a,
Ying
Li
 *a and
Bing
Yan
*a and
Bing
Yan
 *b
*b
aSchool of Materials and Chemistry, University of Shanghai for Science and Technology, Shanghai 200093, P. R. China. E-mail: liying@usst.edu.cn; Tel: +86-21-55270632
bShanghai Key Lab of Chemical Assessment and Sustainability, School of Chemical Science and Engineering, Tongji University, Siping Road 1239, Shanghai 200092, P. R. China. E-mail: byan@tongji.edu.cn; Tel: +86 21-65984663
First published on 11th November 2024
Abstract
Dopamine and arginine are both important substances in the body and are closely related to human health. Timely detection of their concentration abnormalities is of great significance to the prevention of disease. In this work, a Tb3+-functionalized Eu-MOF hybridized double network hydrogel film (Tb@Eu-MOF@PVA/SA) was prepared by a simple synthesis, which can accurately detect arginine (Arg) and dopamine hydrochloride (DH). Meanwhile, the hydrogel acquired good mechanical properties through crosslinking of Tb3+and freeze–thaw cycles. The free carboxyl groups on the surface of the Eu-MOF in this hydrogel film can bind to Tb3+ and sensitize it to emit light through the “antenna effect”, thus forming a fluorescent hydrogel film with dual emission centers. By testing two kinds of detectors and controlling different excitation wavelengths, we can realize the encryption and decryption of information and build an anti-counterfeiting platform to improve the level of information security. The present work not only provides a case study for the preparation of dual-emission Tb3+-functionalized Eu-MOF hybridized dual-network hydrogel films, but also provides ideas for the development of multifunctional luminescent materials.
Introduction
Dopamine hydrochloride (DH) is the hydrochloride state of dopamine, which is more stable in air1 and the lower-cost and more stable DH is generally used in experiments instead of DA for testing. Dopamine is a neurotransmitter that is found in bodily fluids and is crucial to regular physiological processes since it stimulates the circulatory system, human nerve centres, and other systems.2–5 Abnormal concentrations of dopamine in body fluids are thought to be the cause of a variety of diseases, such as Alzheimer's disease, Parkinson's disease, and other geriatric diseases,6–9 as well as depression, schizophrenia, malignant tumors, and other diseases. Timely detection of abnormal dopamine concentration can help in early prevention and control of these diseases. Therefore, it is of great importance to detect its concentration in biological fluids. There are methods such as liquid chromatography,10 capillary electrophoresis,11 and electrochemical analysis,12 but most of these are complicated, costly, require sophisticated instruments and so on. There is an urgent need for a simple means to detect dopamine. Amino acids are essential components of living organisms and affect the health of human beings.13,14 Arginine is an essential amino acid that contains both amino and guanidine groups and is the most alkaline amino acid. Arginine is a precursor for the synthesis of many biomolecules and is involved in the ornithine cycle and the formation of urea.15–18 Excessive levels of arginine in living organisms can lead to a variety of problems and are closely related to cardiovascular and kidney diseases,19–21 for which arginine levels are used as a diagnostic indicator. There is therefore a need for a simple means of detecting arginine levels in organisms.The crystalline materials composed of metal ions/clusters and organic ligands through coordination are known as metal–organic skeletons (MOFs).22,23 MOFs have many outstanding properties, such as high porosity,24 large specific surface area,25 chemical stability,26 and simple synthesis,27 which have important applications in the fields of adsorption/separation, energy storage, catalysis, and drug delivery.28–34 Among them, lanthanide organometallic skeletons (Ln-MOFs) synthesized involving lanthanide ions (Ln3+) have excellent luminescent properties, such as long fluorescence lifetime, narrow emission band, large Stokes shift, etc.35,36 Ln-MOFs are widely used in the fields of fluorescence sensing,37 temperature sensing,38 and anti-counterfeiting.39 Double/multiple luminescence is achieved by encapsulating guest molecules in Ln-MOFs. For example, Guo et al.40 encapsulated carbon quantum dots in Eu-MOFs to achieve radiometric detection of Hg+, which requires high requirements on the size of the synthesized carbon quantum dot particles. Most MOFs have low solubility in aqueous solution and are prone to precipitation, which may lead to insufficient reaction with the detector and uneven luminescence, thus affecting the detection results. Sodium alginate (SA) has good biodegradability and low toxicity, and can be crosslinked by metal cations to prepare hydrogel films,41 which have a wide range of applications in the field of medicine, such as wound dressings,42 drug loading43 and so on. Polyvinyl alcohol (PVA) has good physicochemical properties and can be crosslinked by freeze–thaw cycling to avoid the use of cross-linking agents44 reducing biotoxicity and expanding the field of application. In the polyvinyl alcohol-sodium alginate (PVA-SA) double crosslinking network, many polar groups present on the surface of the MOF can interact with the surrounding polymer chains through hydrogen bonding to form a denser crosslinking network. At the same time, lanthanide metal ions, such as Tb3+, are also metal cations, which can not only cross-link with sodium alginate to improve the mechanical properties but also obtain better compression and tensile strength. It can also be introduced into the luminescent center, which provides ideas for subsequent fluorescence sensing and applications in information anti-counterfeiting.
In recent years, the emergence of counterfeit and substandard products and information leakage has led to more and more theft of important secrets, which has a significant impact on economic development, social stability and even national security.45–48 Therefore, in order to resist the occurrence of such phenomena, there is an urgent need to develop a new type of anti-counterfeiting material to protect information and improve the level of information security. Hydrogel material has the characteristics of biological low toxicity, portability and easy storage and transportation and has a wide range of applications in the field of wound dressings, wearable devices, biological coatings, etc.49,50 Combined with fluorescence sensing technology, fluorescent hydrogels are prepared. Specific external stimuli are used to get different optical response signals, so as to encrypt and decrypt the information, and improve the level of information security. This shows that fluorescent hydrogels have the potential to become a new generation of anti-counterfeiting materials.51,52
In this work, we successfully prepared a hydrogel membrane (Tb@Eu-MOF@PVA/SA) hybridized with a Tb3+ functionalized Eu-MOF, which can detect two substances, Arg and DH. The Eu-MOF was prepared using a simple hydrothermal synthesis method. Eu-MOF powder was doped into a PVA/SA gel solution, placed into a mould and freeze–thaw cycled to obtain Eu-MOF@PVA/SA. In the polyvinyl alcohol-sodium alginate (PVA-SA) bis-crosslinked network, many polar groups present on the surface of MOFs can interact with the surrounding polymer chains through hydrogen bonding to form a denser crosslinked network. By immersing in Tb3+ solution, Tb3+ is substituted with Na ions in SA and the hydrogels get crosslinked. The mechanical properties are improved, resulting in better compressive and tensile strength. Meanwhile, the free carboxyl group of the Eu-MOF provides ligand binding sites for Tb3+ ions, which sensitizes the luminescence of Tb3+ ions through the antenna effect and introduces another emission centre. The dual-network dual-emission hydrogel membrane (Tb@Eu-MOF@PVA/SA) was obtained (Scheme 1). In addition, a series of fluorescent hydrogel membrane platforms for information security storage were prepared based on changes in the fluorescent colour response of the sensors to the two detectors.
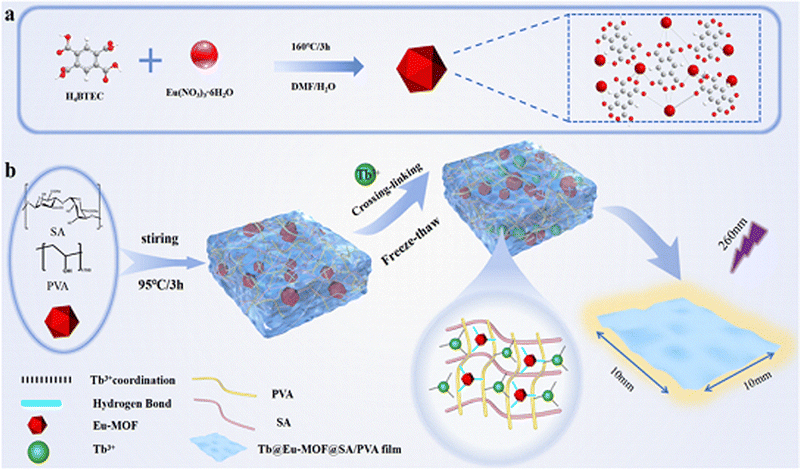 | ||
| Scheme 1 (a) Schematic diagram of the synthesis of Eu-MOF (b) Schematic diagram of the synthesis of Tb@Eu-MOF@PVA/SA film. | ||
Experimental section
Materials
Eu(NO3)3·6H2O, TbCl3·6H2O 1,2,4,5-benzenetetarcarbxylic acid (H4BTEC), and triethylamine were obtained from Adamas Reagent Ltd. N,N-dimethylformamide (DMF) polyvinyl alcohol (1799, MW:∼75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1, >99% hydrolysis) and sodium alginate (SA) were purchased from Sinopharm Chemical Reagent Co. Arginine (Arg), dopamine hydrochloride (DH), aspartic acid (Asp), phenylalanine (Phe), uric acid (UA), alanine (Ala), glycine (Gly), threonine (Thr), urea, glucose, KCl, NaCl, MgCl2, Na2SO4, CaCl2, and ascorbic Acid (AA) were obtained from Aladdin Chemistry Co., Ltd. Deionized water was used for all experiments.
000 g mol−1, >99% hydrolysis) and sodium alginate (SA) were purchased from Sinopharm Chemical Reagent Co. Arginine (Arg), dopamine hydrochloride (DH), aspartic acid (Asp), phenylalanine (Phe), uric acid (UA), alanine (Ala), glycine (Gly), threonine (Thr), urea, glucose, KCl, NaCl, MgCl2, Na2SO4, CaCl2, and ascorbic Acid (AA) were obtained from Aladdin Chemistry Co., Ltd. Deionized water was used for all experiments.
Synthesis of Eu-MOF
Eu-MOF was prepared according to the reported work,53 H4BTEC (63.5 mg) was dissolved in 15 mL DMF, Eu(NO3)3·6H2O (55.75 mg) was dissolved in 5 mL H2O. 133 μL of Triethylamine was added to the organic ligand solution and sonicated for 10 min to deprotonate it. Subsequently, the metal ions solution (5 mL) was quickly poured into it. Then the mixed solution was placed in a Teflon vessel in a steel autoclave and heated at 160 °C for 3 h. Until the reaction system had cooled to room temperature, the white precipitate was collected by centrifugation (6000 rpm, 20 min). To remove residual raw materials completely, the product was washed three times with DMF, water and anhydrous ethanol, separately. Subsequently, it was dried in a vacuum drying oven for 6 h to obtain the Eu-MOF.Synthesis of the Eu-MOF@PVA/SA film
Eu-MOF powder (30 mg) was added to deionized water (25 mL) and sonicated for 30 minutes to disperse it uniformly. Then the sodium alginate (SA) powder (0.25 g) was added and stirred for 30 min at 95 °C, Subsequently, polyvinyl alcohol (PVA) was added and stirred for 3 h to obtain a homogeneous mixed solution. The mixture was dropped into the mould (10 × 10 mm2) after it had cooled to room temperature. Then, they were frozen in a refrigerator for 20 h and thawed for 4 h at room temperature (two cycles). The Eu-MOF@PVA/SA film was obtained after removing the mould.Synthesis of the Tb@Eu-MOF@PVA/SA film
TbCl3·6H2O (0.0373 g) was dispersed uniformly in 50 mL of deionized water. The Eu-MOF@PVA/SA film was immersed in the Tb3+ ion solution for 6 h. After that, deionized water was used to wash the film to remove non-coordinated Tb3+ ions. Then, Tb@Eu-MOF@PVA/SA was prepared.Fluorescence sensing of DH and Arg
The fluorescence emission was recorded using a spectrophotometer after immersing Tb@Eu-MOF@PVA/SA in different concentrations of Arg solution or DH solution. The width of the excitation and emission channels was 5 nm.Simulates the testing of real samples
Arginine and dopamine, as biological analogs, are mainly present in human body fluids such as serum and urine. Tb@Eu-MOF@PVA/SA was immersed in a solution of the major components of body fluids at a concentration of 1 mM (urea, glucose, KCl, NaCl, MgCl2, Na2SO4, and CaCl2), and fluorescence spectroscopy was carried out after 1 h.Tests of mechanical properties
In the tensile test, dumbbell-shaped strips (30 mm × 4 mm × 4 mm) were pulled at a rate of 100 mm min−1. For the compression test, cylindrical samples (13 mm height, 24 mm diameter) were compressed at a rate of 1 mm min−1.Construction of anti-counterfeiting platform
The information was encoded and encrypted by using different types of hydrogel films (Eu-MOF@PVA/SA, Tb@Eu-MOF@PVA/SA) and forming arrays. Subsequently, the information was decrypted by controlling the UV excitation light source (365 nm and 254 nm) and depending on the solution of DH (1 mM) or Arg (2 mM).Results and discussion
Characterization methods of the hybrid material
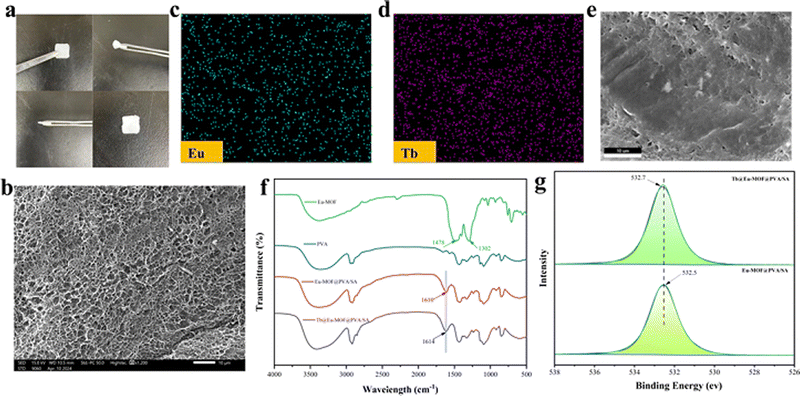
The hydrothermally synthesized Eu-MOF was ultrasonically dispersed in a flask in which SA and PVA were successively dissolved to obtain a homogeneously mixed hydrogel solution, and the solution was poured into a customized polytetrafluoroethylene mold. After two freeze–thaw cycles, the SA polymer chains in the hydrogel and the PVA polymer chains were tightly intertwined through the hydrogen bonding, at the same time, Eu-MOF nanoparticles, which were uniformly dispersed in the hydrogel solution, were formed into a denser hydrogel network by the more compact interlinking between the MOF nanoparticles and the surrounding polymer chains because of the multilayer hollow structure and the polar groups on their surface. After demolding and immersing in TbCl3·6H2O solution, Tb3+ is exchanged with Na+ in SA to obtain a translucent hydrogel film with elasticity and a smooth surface (Fig. 1a). The powder X-ray diffraction (XRD) of the prepared Eu-MOF, which matches the single crystal simulation (CCDC: 862207†) (Fig. S1a, ESI†), indicates that the Eu-MOF was successfully prepared. Since the doping amount of Eu-MOF is very small, the Eu-MOF@PVA/SA mode has only a small fluctuation around 2θ = 10°. It is well known that the PVA has a wide and high peak at 2θ = 19.6° so that this might be masked by the peak of PVA. The peak in the PVA mode is only a small fluctuation around 2θ = 10° (Fig. S1b, ESI†).The microstructures of Eu-MOF and Tb@Eu-MOF@PVA/SA were observed using a scanning electron microscope (SEM), and the Eu-MOF was spindle-shaped, with a size around 200 nm and a rough surface (Fig. S2a, ESI†). The three-dimensional structure of the crystals and crystal cells of the Eu-MOF (CCDC: 862207†) is shown schematically in Fig S3 (ESI†). The distance between the two rare earth atoms is about 1.26 nm. The surface of freeze-dried Tb@Eu-MOF@PVA/SA showed a continuous and uniform reticular structure (Fig. 1b). The EDS spectrum illustrated the presence and content of C, O, Eu, and Tb (Fig. S2b and Table S1, ESI†). As shown in the EDS mapping images (Fig. 1c–e), The elements Eu and Tb were uniformly distributed in Tb@Eu-MOF@PVA/SA, which proved that the Eu-MOF was successfully assembled into the Tb3+ cross-linked hydrogel. However, it can be seen from the table (Table S1, ESI†) that the content of element Eu is poor, which further explains why the distinctive peaks of Eu-MOF in the hydrogel of the XRD pattern were not obvious. The chemical structures of the prepared materials were further explored. As shown in Fig. 1f, the FT-IR spectra of PVA, Eu-MOF, Eu-MOF@PVA/SA, and Tb@Eu-MOF@PVA/SA were collected. It can be clearly seen that 1478 cm−1 and 1304 cm−1 belong to the stretching vibration of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond of the two carboxyl groups in the Eu-MOF, and when the Eu-MOF powder was doped into the hydrogel matrix, the stretching vibration at 1304 cm−1 disappeared. The C
O bond of the two carboxyl groups in the Eu-MOF, and when the Eu-MOF powder was doped into the hydrogel matrix, the stretching vibration at 1304 cm−1 disappeared. The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond at 1478 cm−1 was red-shifted and moved to 1610 cm−1, indicating that the free carboxyl group coordinated with Na+ ions of sodium alginate. After immersion in the TbCl3·6H2O solution, the C
O bond at 1478 cm−1 was red-shifted and moved to 1610 cm−1, indicating that the free carboxyl group coordinated with Na+ ions of sodium alginate. After immersion in the TbCl3·6H2O solution, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O changed to 1614 cm−1, which indicated that substitution between Tb3+ and Na+ and the hydrogel was cross-linked.
O changed to 1614 cm−1, which indicated that substitution between Tb3+ and Na+ and the hydrogel was cross-linked.
In addition, X-ray photoelectron spectroscopy (XPS) was used to demonstrate the existence of interactions between Tb3+ and Eu-MOF@PVA/SA (Fig. 1g and Fig. S4, ESI†). The binding energy of O 1s changed from 532.5 ev (Eu-MOF@PVA/SA) to 532.7 ev (Tb@Eu-MOF@PVA/SA), indicating that the Tb–O coordination bond was generated in the Tb@Eu-MOF@PVA/SA hydrogel film. Meanwhile, as shown in the XPS spectrum, the characteristic peak of Na 1s originally present in Eu-MOF@PVA/SA disappeared in Tb@Eu-MOF@PVA/SA, which is sufficient to prove that Na+ is exchanged with Tb3+ in SA.54
Luminescence characteristics of the sensor
Fluorescence properties of the synthesis materials were researched at room temperature. As shown in Fig. 2a, the optimal excitation wavelength of Eu-MOF is 307 nm, compared to that of the Eu-MOF@PVA/SA film which remains almost unchanged, with the excitation wavelength red-shifted to blue-shifted to 260 nm (Fig. S5a, ESI†), suggesting that the presence of SA and PVA does not affect the emission properties of Eu-MOF. The emission spectra of Eu-MOF@PVA/SA were also investigated by crosslinking of Tb3+. The characteristic emission of Eu3+ and Tb3+ in Tb@Eu-MOF@PVA/SA is given in Fig. S5b (ESI†), respectively. They exhibited four characteristic emission peaks of Eu3+ at 584, 611, 646, and 692 nm when the excitation wavelength was 260 nm, which corresponded to the jump of 5D0 → 7FJ (J = 1, 2, 3, 4). Similarly, two different Tb3+ characteristic emission peaks corresponding to specific leaps: 5D4 → 7FJ (J = 6, 5) were shown at 485 nm and 540 nm. The Eu-MOF consists of Eu3+ ions and the organic ligand H4BTEC, so the spectral leaps of the Eu-MOF mainly come from the leaps of the ligand molecules (Fig. S6, ESI†). The possible “antenna effect” between Eu-MOF and Tb3+(Fig. S7, ESI†) was similar to that of lanthanide metal–organic skeletons, where there is a slight energy transfer from Eu-MOF to Tb3+ ions. Dopamine and arginine are important biological analogues in the human body, and the determination of their concentrations is an important indicator for monitoring human health. Fluorescent sensors for their detection must be pH tolerant and luminescent stable. The fluorescence intensity of Tb@Eu-MOF@PVA/SA was compared by immersing it in different pH solutions, and it was found that the fluorescence intensity was maximum at pH 7. (Fig. S8, ESI†) Furthermore, the same batch of samples were placed in the air at room temperature, and the fluorescence emission spectra were tested on days 1, 3, 5, and 7 and found to be almost unchanged. This indicates that the material has good stability (Fig. S9, ESI†). In addition, the quantum yield of the material was tested, η = 12.76%.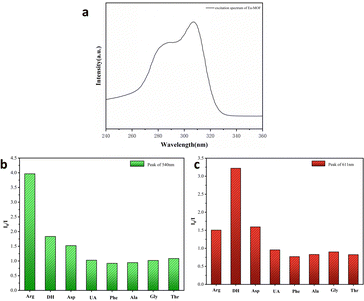 | ||
| Fig. 2 (a) Excitation spectra of Eu-MOF. Histograms of the intensity ratio (I0/I) of Tb@Eu-MOF@PVA/SA at peaks of 540 nm (b), and 611 nm (c) in different amino acid solutions (1 mM). | ||
Sensing Performance for Arg and DH
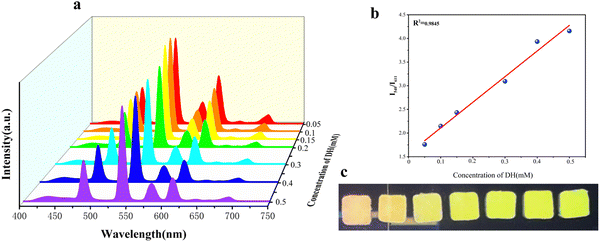
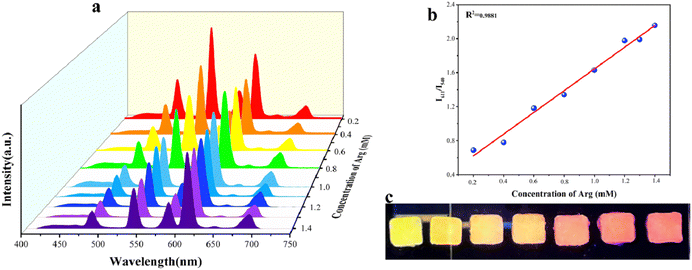
To demonstrate the selectivity of Tb@Eu-MOF@PVA/SA for the Arg and DH detectors, Tb@Eu-MOF@PVA/SA was immersed in different kinds of amino acids and biotics at a concentration of 1 mM and the fluorescence spectra were recorded after 1 h. These substances include arginine (Arg), dopamine hydrochloride (DH), aspartic acid (Asp), phenylalanine (Phe), uric acid (UA), alanine (Ala), glycine (Gly), and threonine (Thr). Compared with the blank sample, except for Arg and DH, the rest of the substances have no significant effect on the emission peaks of Tb@Eu-MOF@PVA/SA (Fig. S10a, ESI†), and it is obvious that Arg significantly reduces the characteristic peaks of Tb at 540 nm by a factor of about 4, and DH reduces the characteristic peaks of Eu at 611 nm by a factor of about 3.3 (Fig. 2b and c). On the CIE coordinate plot (Fig. S10b, ESI†), it is shown that the coordinates of the blank Tb@Eu-MOF@PVA/SA are (0.385, 0.435) in the yellow region, and after immersion in Arg and DH, respectively, the coordinates move to (0.431, 0.388) in the red region and (0.319, 0.486) in the green region, so that the sensor has the potential to quantitatively detect these two substances.In Fig. 3a, the quantitative sensing properties of DH are investigated in detail. After immersing in a range of concentrations of DH solutions (0.05–0.5 mM) for 30 min to determine the fluorescence emission spectra, it was found that the emission intensity of Tb@Eu-MOF@PVA/SA at 611 nm decreased gradually with increasing concentration. The linear regression equation obtained by fitting is expressed as y = 5.42x + 1.56698 with a linear correlation (R2) of 0.9845 (Fig. 3b). According to IUPAC (CDL = 3δ/k) criteria, the detection limit (LOD) is calculated to be 21 μM, where δ is the standard deviation of 5 repeated fluorescence measurements of the blank sample and k is the slope of the fitted linear curve. Subsequently, we investigated Arg and similarly measured the fluorescence spectra after immersing the fluorescence sensing films in a range of concentrations of Arg solution (0.2–1.4 mM) for 1 h (Fig. 4). We found that the concentration of Arg gradually increased, with successive decreases in the emission intensity at 540 nm. The ratio of fluorescence intensity, I0/I, showed a good linear relationship with the concentration gradient, R2= 09881, the linear regression equation was y = 1.276x + 0.3681, and the limit of detection (LOD) was 0.09 mM.
Possible mechanism of Tb@Eu-MOF@PVA/SA response to DH and Arg
The sensing of DH by the fluorescent thin-film sensor (Tb@Eu-MOF@PVA/SA) is mainly dependent on the presence of the Eu-MOF. The prepared Eu-MOF powder was ultrasonically dispersed in DH solution (1 mM), immersed for 30 min and centrifuged. To wash away the residual DH, it was washed three times with deionized water and dried, and the dried product was characterized by XRD. Comparison with the profile of Eu-MOF without DH immersion showed no significant change (Fig. S11, ESI†), indicating that the presence of DH did not destroy the structure of the MOF. Dopamine hydrochloride (DH) is the hydrochloride of dopamine (DA) and is more stable in air than the latter. However, oxidation occurs to produce dopamine quinone (DQ). There may be interactions (van der Waals effects, such as static electricity, π–π*, etc.) between the Eu-MOF surface and these oxides. Density functional theory (DFT) calculations based on the method of Yu et al.53 revealed that the DA oxidation product DQ has an ultra-low unoccupied molecular orbital (LUMO: −3.55 eV), which effectively enhances the photo-induced electron transfer (PET) process from H4BTEC (LUMO: −3.51 eV) to DQ and attenuates the energy transfer from H4BTEC to Eu3+, leading to red fluorescence quench (Fig. S12, ESI†). In addition, the fluorescence lifetime of Tb@Eu-MOF@PVA/SA at 611 nm changed from 556.91 μs to 348.02 μs (Fig. S13, ESI†) after immersion in DH solution (1 mM). This result can indicate that the quenching of Eu-MOF is a dynamic quenching process.Furthermore, the sensing mechanism of Arg was explored in depth. When Arg was added to the Eu-MOF solution, it was found that the fluorescence intensity remained basically unchanged (Fig. S14, ESI†), suggesting that the Eu-MOF would not respond to Arg. Arg is an alkaline amino acid containing carboxyl and amino groups, and the side chains of these two amino acids are protonated in water with a high pKa value.55 We speculate that Arg can form a complex with Tb3+, blocking the energy transfer between Tb3+ and MOF, thus causing the green fluorescence to disappear. In addition, after immersion in Arg solution (1 mM), the fluorescence lifetime of Tb@Eu-MOF@PVA/SA at 540 nm changed from 720.51 μs to 808.26 μs (Fig. S15, ESI†), which can indicate that the quenching is a static quenching process.
Mechanical properties
The hydrogel Tb@Eu-MOF@PVA/SA has excellent tensile and compressive properties, (Fig. S16a and b, ESI†), the stress–strain curves of the Tb@Eu-MOF@PVA/SA hydrogel were tested by tensile experiments, and the tensile strength was as high as 280%, (Fig. S17, ESI†) and the elongation at break was 550 Kpa. The stress–strain curves were tested by compression experiments, and the hydrogel's compressive strength at 80% strain was 1330 Kpa. It is worth noting that after the unloading of compression force, the shape of the hydrogel can be recovered quickly and has excellent elasticity (Fig. S18, ESI†). In summary, the hydrogel has good mechanical properties, which are attributed to the doping of Eu-MOF particles and the crosslinking of Tb3+. Due to the microporosity and large number of carboxyl groups of the MOF nanoparticles, the polymer chains will be crosslinked by the MOF nanoparticles or entangled on the surface by the hydrogen bonding, and the MOF provides more anchorage points for the polymer chains,56 which tightly binds them together and enhances the mechanical stability for the subsequent construction of the anti-counterfeiting platform.Simulation of real samples
Arginine and dopamine are mainly found in human body fluids such as serum and urine, and a variety of complex components also exist in these body fluids to interfere with the detection results. The fluorescence sensor was immersed in the body fluids, which are the main components of these body fluids (urea, glucose, KCl, NaCl, MgCl2, Na2SO4, and CaCl2). The results illustrated in Fig. 5 revealed that compared with the blank samples, the emission spectra of the substances soaked in body fluids were basically unchanged, while the emission peaks in the dopamine and arginine solutions were obviously quenched. Interference experiments in the presence of various other serum components were also performed (Fig. S19, ESI†). After prolonged immersion, the luminescence intensity of Tb@Eu-MOF@PVA/SA was unaffected by the other components as compared to the fluorescence intensity of DH and Arg added alone. The results showed that Tb@Eu-MOF@PVA/SA had good anti-interference properties.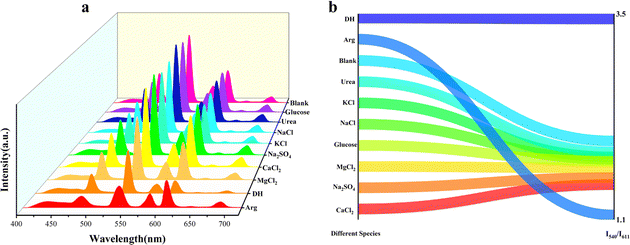 | ||
| Fig. 5 (a) Emission spectra (λex = 260 nm) of 1@PVASA in different species solution (1 mM) (b) I540/I611 of Tb@Eu-MOF@PVA/SA under different species solutions. | ||
Application in anti-counterfeiting and information protection
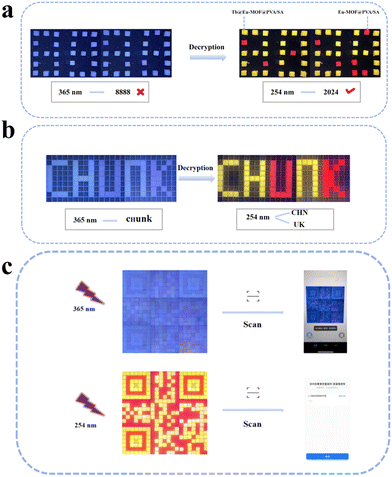
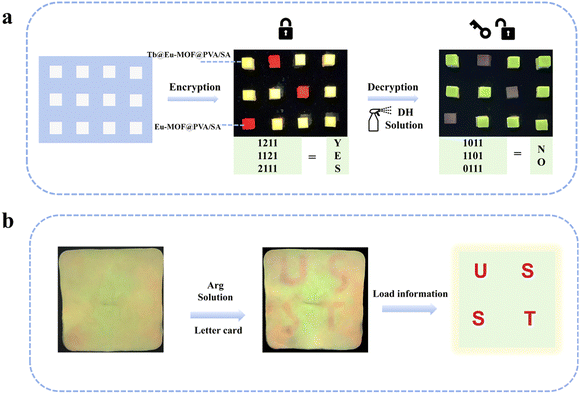
Fluorescence anti-counterfeiting is receiving more and more attention because of its features of not being easy to copy, easy to encrypt and decrypt information, and low cost of raw materials, etc. It is gradually becoming the most secure information encryption technology one. The fluorescence sensing film Tb@Eu-MOF@PVA/SA generates a fluorescence response to the detector DH and Arg, changes the excitation wavelength, encrypts and decrypts the information, and thus realizes the construction of an anti-counterfeiting platform.The Eu-MOF@PVA/SA film shows red emission and the Tb@Eu-MOF@PVA/SA film shows bright yellow under the excitation of 254 nm. An array of hydrogel cubes prepared from a custom-made mould is observed as the number “8888” at 365 nm, while the decrypted message “2024” is obtained at 254 nm (Fig. 6a). Similarly, the word “CHUNK” is visible to the naked eye at 365 nm, and the decrypted messages “CHN” and “UK” are obtained at 254 nm in Fig. 6b. In addition to this, a more complex QR code array (21 × 21) has been formed, which can be scanned using the app to obtain the “USST” information at a specific wavelength of UV light, which is even more encrypted (Fig. 6c).
Furthermore, the two detectors DH and Arg can also play a role in encryption and decoding of information. 4 × 3 film arrays were constructed, and under 254 nm UV irradiation, the yellowish-green and reddish luminescent hydrogels were defined as “1” and “2”, respectively, the green and non-luminescent hydrogels were defined as “1” and “0” after spraying DH solution. Before spraying, the message decoded from the ASCII code is “YES”, but after spraying, the red fluorescence quenched and the correct message is “NO” (Fig. 7a). As shown in Fig. 7b, Tb@Eu-MOF@PVA/SA hydrogel film (10 cm × 10 cm) was prepared, and the letter-shaped cards were cut out of filter paper, soaked with Arg solution and placed on the film, and after a period of time, the red encrypted message “USST” could be clearly seen under 254 nm UV light. The red encryption message “USST” can be clearly seen under 254 nm UV light. The above shows that the fluorescence sensing film has great potential for application in information encryption.
Conclusions
In summary, we prepared a portable dual-emission fluorescence sensing film (Tb@Eu-MOF@PVA/SA) in this work. Tb@Eu-MOF@PVA/SA exhibited excellent selectivity and high sensitivity to Arg and DH. We further explored the sensing mechanism of Tb@Eu-MOF@PVA/SA for the two detectors. In addition, a series of anti-counterfeiting platforms have been built using Tb@Eu-MOF@PVA/SA and Eu-MOF@PVA/SA to encrypt and decrypt information by changing the ultraviolet excitation wavelength and adding detectors (Arg or DH). This work not only provides a simple strategy for the preparation of dual-emission fluorescence sensing films, but also opens a new way for the practical application of the next generation of MOF-based hydrogel films.Data availability
Additional data are made available in the ESI.† The datasets generated or analyzed during the current study are not publicly available due to the nature of the research but are available from the corresponding author on reasonable request.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (21101107 and 22375150) and the State Key Laboratory of Pollution Control and Resource Reuse Foundation (No. PCRRF19017).Notes and references
- C. Zhai, F. Sun, P. Zhang, H. Ma, A. Song and J. Hao, J. Mol. Liq., 2016, 223, 420–426 CrossRef CAS.
- T. T. Rudibaugh, S. R. Stuppy and A. J. Keung, Int. J. Mol. Sci., 2023, 24, 16474 CrossRef CAS PubMed.
- S. M. Matt and P. J. Gaskill, J. Neuroimmune Pharmacol., 2020, 15, 114–164 CrossRef CAS PubMed.
- F. Grill, M. Guitart-Masip, J. Johansson, L. Stiernman, J. Axelsson, L. Nyberg and A. Rieckmann, Nat. Commun., 2024, 15, 59 CrossRef CAS.
- C. A. Ciobanu, L. Ionescu, L. M. Geafer, C. P. Niculae and A. M. Ciobanu, Eur. Psychiatry, 2023, 66, S1067–S1068 CrossRef.
- Y. Suzuki, Sens. Actuators, B, 2017, 239, 383–389 CrossRef CAS.
- C. Yang, K. Hu, D. Wang, Y. Zubi, S. T. Lee, P. Puthongkham, M. V. Mirkin and B. J. Venton, Anal. Chem., 2019, 91, 4618–4624 CrossRef CAS.
- S. J. Park, S. H. Lee, H. Yang, C. S. Park, C.-S. Lee, O. S. Kwon, T. H. Park and J. Jang, ACS Appl. Mater. Interfaces, 2016, 8, 28897–28903 CrossRef CAS PubMed.
- J. C. Munyemana, J. Chen, H. Tang, Y. Han, J. Wang and H. Qiu, ACS Appl. Nano Mater., 2021, 4, 2820–2827 CrossRef CAS.
- T. Li, R. Wang and P. Wang, Molecules, 2022, 28, 184 CrossRef PubMed.
- V. Šolínová, L. Žáková, J. Jiráček and V. Kašička, Anal. Chim. Acta, 2019, 1052, 170–178 CrossRef PubMed.
- C. Zhang, T. Chen, Y. Ying and J. Wu, Sensors, 2024, 24, 2934 CrossRef CAS.
- N. W. C. Campbell, S. H. Patel, P. Ferrandi, S. Couture, D. O. Farino, J. Stout, A. Sabbaghi and C. C. Carroll, Amino Acids, 2023, 55, 777–787 CrossRef CAS.
- R. Elango, Adv. Nutr., 2023, 14, 885–894 CrossRef CAS.
- J. Chi, Y. Song and L. Feng, Biosens. Bioelectron., 2023, 241, 115666 CrossRef CAS.
- J. Huang, H. Zhao, X. Yu, Y. Limeng, C. Fan, P. Liao, B. Zhang, C. Li, G. Du, Q. Dong and C. Zeng, Dyes Pigments, 2023, 217, 111414 CrossRef CAS.
- J. Wu, H. Wang, H. Yang, J. Chen and H. Yang, Anal. Chim. Acta, 2019, 1079, 200–206 CrossRef CAS.
- H. Cai, B. Huang, R. Lin, P. Xu, Y. Liu and Y. Zhao, Talanta, 2018, 189, 353–358 CrossRef CAS.
- J. Xu, F. Cao, C. Lu, Z. Song and Z. Dai, RSC Adv., 2024, 14, 1970–1976 RSC.
- B. M. C. Costa, A. A. Prado, T. C. Oliveira, L. P. Bressan, R. A. A. Munoz, A. D. Batista, J. A. F. Da Silva and E. M. Richter, Talanta, 2019, 204, 353–358 CrossRef CAS.
- B. Zhang, F. Zhou, X. Yu, P. Zhang, X. Sun, J. Su, C. Fan, W. Shu, Q. Dong and C. Zeng, Food Chem., 2024, 455, 139976 CrossRef CAS PubMed.
- Z. Cao, R. Momen, S. Tao, D. Xiong, Z. Song, X. Xiao, W. Deng, H. Hou, S. Yasar, S. Altin, F. Bulut, G. Zou and X. Ji, Nano-Micro Lett., 2022, 14, 181 CrossRef CAS.
- S. Mandal, S. Natarajan, P. Mani and A. Pankajakshan, Adv. Funct. Mater., 2021, 31, 2006291 CrossRef CAS.
- T. Bao, Y. Zou, C. Zhang, C. Yu and C. Liu, Angew. Chem., Int. Ed., 2022, 61, e202209433 CrossRef CAS PubMed.
- F. P. Kinik, A. Ortega-Guerrero, D. Ongari, C. P. Ireland and B. Smit, Chem. Soc. Rev., 2021, 50, 3143–3177 RSC.
- V. Bon, I. Senkovska, J. D. Evans, M. Wöllner, M. Hölzel and S. Kaskel, J. Mater. Chem. A, 2019, 7, 12681–12690 RSC.
- A. G. Zavyalova, D. V. Kladko, I. Y. Chernyshov and V. V. Vinogradov, J. Mater. Chem. A, 2021, 9, 25258–25271 RSC.
- S. Rostami, A. Nakhaei Pour, A. Salimi and A. Abolghasempour, Int. J. Hydrogen Energy, 2018, 43, 7072–7080 CrossRef CAS.
- B. Zhang, Z. Zhu, X. Wang, X. Liu and F. Kapteijn, Adv. Funct. Mater., 2023, 34, 2304788 CrossRef.
- D. Cai, Z. Yang, R. Tong, H. Huang, C. Zhang and Y. Xia, Small, 2024, 20, 2305778 CrossRef CAS.
- Y. Peng, J. Xu, J. Xu, J. Ma, Y. Bai, S. Cao, S. Zhang and H. Pang, Adv. Colloid Interface Sci., 2022, 307, 102732 CrossRef CAS PubMed.
- J. Ren, Y. Huang, H. Zhu, B. Zhang, H. Zhu, S. Shen, G. Tan, F. Wu, H. He, S. Lan, X. Xia and Q. Liu, Carbon Energy, 2020, 2, 176–202 CrossRef CAS.
- X. Li and Q.-L. Zhu, EnergyChem, 2020, 2, 100033 CrossRef.
- M. Shadpour, N. Elham and H. C. Mustansar, Coord. Chem. Rev., 2022, 451, 214262 CrossRef.
- X. Wang, Y. Jiang, A. Tissot and C. Serre, Coord. Chem. Rev., 2023, 497, 215454 CrossRef CAS.
- Y. Zhang, S. Liu, Z.-S. Zhao, Z. Wang, R. Zhang, L. Liu and Z.-B. Han, Inorg. Chem. Front., 2021, 8, 590–619 RSC.
- M. Wu, Z. W. Jiang, P. Zhang, X. Gong and Y. Wang, Sens. Actuators, B, 2023, 383, 133596 CrossRef CAS.
- J.-W. Hu, X.-Y. Wang, J. Xu, X.-Y. Dong and C. Zhang, J. Mater. Chem. C, 2023, 11, 14545–14550 RSC.
- Y. Zhang, X. Xu and B. Yan, J. Mater. Chem. C, 2022, 10, 3576–3584 RSC.
- H. Guo, X. Wang, N. Wu, M. Xu, M. Wang, L. Zhang and W. Yang, Anal. Chim. Acta, 2021, 1141, 13–20 CrossRef CAS PubMed.
- L. Wang, H. J. Zhang, X. Liu, Y. Liu, X. Zhu, X. Liu and X. You, ACS Appl. Polym. Mater., 2021, 3, 3197–3205 CrossRef CAS.
- M. Liao, Y. Zhao, Y. Pan, J. Pan, Q. Yao, S. Zhang, H. Zhao, Y. Hu, W. Zheng, W. Zhou and X. Dong, J. Mater. Sci., 2023, 58, 5756–5772 CrossRef CAS.
- X. Mao, X. Li, W. Zhang, L. Yuan, L. Deng, L. Ge, C. Mu and D. Li, ACS Appl. Bio Mater., 2019, 2, 5810–5818 CrossRef CAS PubMed.
- J. Tavakoli, J. Gascooke, N. Xie, B. Z. Tang and Y. Tang, ACS Appl. Polym. Mater., 2019, 1, 1390–1398 CrossRef CAS.
- Y. Wu, X. Chen, Z. Gong, B. Tian, E. Xue, K. Zheng, J. Liang and W. Wu, J. Mater. Chem. C, 2024, 12, 11497–11505 RSC.
- K. Muthamma, D. Sunil and P. Shetty, Appl. Mater. Today, 2021, 23, 101050 CrossRef.
- L. Guo, T. Wang, Q. Wang, W. Feng, Z. Li, S. Wang, P. Xia, F. Zhao and X. Yu, Chem. Eng. J., 2022, 442, 136236 CrossRef CAS.
- W. Hong, Z. Yuan and X. Chen, Small, 2020, 16, 1907626 CrossRef CAS.
- Y. Liang, J. He and B. Guo, ACS Nano, 2021, 15, 12687–12722 CrossRef CAS.
- C. Owh, D. Ho, X. J. Loh and K. Xue, Trends Biotechnol., 2023, 41, 476–479 CrossRef CAS.
- D. Guo, X. Le, H. Shang, F. Shan, D. Li, C. Ouyang and T. Chen, Chin. Chem. Lett., 2023, 34, 108347 CrossRef CAS.
- J. Wang, P. Du, Y.-I. Hsu and H. Uyama, ACS Sustainable Chem. Eng., 2023, 11, 10061–10073 CrossRef CAS.
- L. Yu, L. Feng, Z. Wei, S. Wang, Y. Feng, Y. Shen, J. Cai, J. Wu and Y. Xiao, Adv. Funct. Mater., 2023, 33, 2300309 CrossRef CAS.
- H. F. Sha and B. Ying, J. Mater. Chem. C, 2022, 10, 7633–7640 RSC.
- Y. Wang, H. Peng, H. Wang, M. Zhang, W. Zhao and Y. Zhang, Chem. Eng. J., 2022, 450, 138216 CrossRef CAS.
- Z. Zhang and Z. Fan, New J. Chem., 2020, 44, 4842 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4tc03444a |
| This journal is © The Royal Society of Chemistry 2025 |