High open-circuit voltage ternary organic solar cells based on ICBA as acceptor and absorption-complementary donors
Jin
Fang
 ab,
Dan
Deng
a,
Jianqi
Zhang
a,
Yajie
Zhang
ab,
Dan
Deng
a,
Jianqi
Zhang
a,
Yajie
Zhang
 a,
Kun
Lu
a,
Kun
Lu
 a and
Zhixiang
Wei
a and
Zhixiang
Wei
 *a
*a
aKey Laboratory of Nanosystem and Hierarchical Fabrication, CAS Center for Excellence in Nanoscience, National Center for Nanoscience and Technology, Beijing 100190, P. R. China. E-mail: weizx@nanoctr.cn
bUniversity of Chinese Academy of Sciences, Beijing 100049, P. R. China
First published on 13th January 2017
Abstract
Ternary organic solar cells (OSCs) are fabricated with indene-C60 bisadduct (ICBA) as an electron acceptor and the low-band-gap polymer PBDTTT-C-T and the highly crystalline small molecule n-BDT-3T-CNCOO as electron donors. A high open-circuit voltage of 0.98 V is achieved, which is 0.2 V higher than that of ternary OSCs based on phenyl-C71-butyric acid methyl ester (PC71BM) as the acceptor. Incorporation of n-BDT-3T-CNCOO promotes the power conversion efficiency (PCE) from 5.01% for polymer binary devices to 5.51% for ternary devices. The improved PCE is attributed to the nanofibrous morphology with enhanced crystallinity of the donors and improved aggregation of the ICBA acceptor, which facilitate charge separation and charge transport. This work reveals that the ternary strategy of blending highly crystalline small molecules enhances PCEs of OSCs based on ICBA and other non-fullerene acceptors.
1. Introduction
Organic solar cells (OSCs) have attracted significant interest in the past few decades because of their potential in terms of ease of processing, low cost, light weight and high flexibility.1–3 To achieve high power conversion efficiencies (PCEs), researchers have focused on synthesizing low-band-gap polymers to maximize the short-circuit current (Jsc), while lowering the highest occupied molecular orbital (HOMO) level to get a higher open-circuit voltage (Voc).4 An optimized polymer should balance Jsc and Voc. An electron acceptor with a higher lowest unoccupied molecular orbital (LUMO) level than the widely used phenyl-C60-butyric acid methyl ester (PCBM), for instance, indene-C60 bisadduct (ICBA), may increase Voc, and hence, improve the PCE of OSC devices.5 Instead of the highly crystalline polymer poly(3-hexylthiophene) (P3HT) and a few other large-band-gap polymers,6–10 OSC devices based on a low-band-gap polymer/ICBA as the active layers usually present low Jsc or fill factor (FF).11–15 To obtain a high-performance in the polymer/ICBA blend, forming of relatively pure, crystalline polymer domains is necessary for good donor/acceptor phase separation, which is beneficial to charge separation and charge transport. Meanwhile, fullerene crystallization was reported as another key energetic driving force for charge separation and charge transport in polymer/fullerene bulk heterojunction solar cells.16,17 Less crystalline ICBA achieves lower electron mobilities than PCBM when blended with disordered low-band-gap polymers.18 Disordered polymers could not act as templates to guide fullerene aggregation to form suitable mesoscopic heterojunctions.19 Hence, crystals of the donor and aggregation of the acceptor in the active layers, which consist of a low-band-gap polymer as the donor and ICBA as the acceptor, are both important factors that can improve the PCEs of related OSC devices.Recently, ternary OSCs were developed as promising candidates for high-performance OSCs, which can simultaneously or individually increase Voc, Jsc, and FF by carefully selecting third molecules.20–24 In the literature, mechanisms such as cascade charge transfer, energy transfer, parallel-linkage, and alloy models were used to explain improvement of ternary OSCs.20,25 For instance, Ameri et al.26 produced a ternary OSC by blending a low-band-gap polymer, Si-PCPDTBT, into the binary-blend P3HT/ICBA. This ternary system overcame limitations of Jsc and FF in the low-band-gap polymer/ICBA. However, improvement of the ternary devices still relies on the properties of P3HT. On the other hand, ICBA is usually used as a third component in one-donor two-acceptor ternary-blend devices.27–31 A blend of two acceptors, called an alloy acceptor, combines the advantages of two acceptors and forms a cascade energy level to facilitate charge transfer.29 However, the ICBA content is limited because of the influence of excess ICBA on the morphology and recombination dynamics of the ternary-blend film. Thus, the enhancement degree of Voc is limited. We reported that blending conjugated small molecules into a polymer donor/PCBM system exerted synergistic effects in optimizing the Jsc and especially increasing the FF of ternary OSCs.32–34 Highly crystalline small molecules provided a driving force for the ternary-blend films to form favourable nanostructures. A low-band-gap polymer/ICBA binary blend usually yields lower PCEs.29 Studies should check whether blending a conjugated small molecule into a low-band-gap polymer/ICBA improves the performance of ICBA-based ternary OSCs.
In this work, we present ternary OSCs based on ICBA as the acceptor and a conjugated small molecule and a low-band-gap polymer as donors. Owing to the higher LUMO level of ICBA, Voc increases from 0.77 V to 0.98 V when the PC71BM acceptor is replaced by ICBA. Nanofibrous structures were observed in the ternary OSCs, which benefits charge separation and transport. Interestingly, the crystallinity of the donor and acceptor phases was increased by incorporation of highly crystalline small molecules. The PCE was enhanced from 5.01% in binary OSCs to 5.51% in ternary OSCs through enhanced Jsc and FF in the ternary OSCs.
2. Experimental section
2.1 Fabrication of the ternary solar cells
PBDTTT-C-T and ICBA were obtained from Solarmer Energy, Inc. and used as received. n-BDT-3T-CNCOO was synthesized as described previously.35,36 Pre-patterned indium-tin oxide (ITO)-coated glass with a sheet resistance of 15 Ω sq−1 was used as the substrate. ITO glass was cleaned by successive sonication in deionized water, acetone, and isopropanol thrice for 10 min, and dried by compressed N2 gas. After UV/ozone treatment (Jelight) for 5 min, an electron transport layer, ZnO (approximately 30 nm), was prepared by spin-coating a ZnO precursor solution (0.45 M zinc acetate dehydrate and 0.45 M ethanolamine in 2-methoxy ethanol). Then the ZnO-coated ITO glass substrates were immediately baked at 200 °C for 30 min in air. Active layer solutions (donor/acceptor ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were dissolved in chloroform with 3% (volume fraction) 1,8-diodooctane (DIO). Typically, the content of n-BDT-3T-CNCOO in the overall donor was 0%, 25%, 50%, 75%, and 100%, respectively, and the overall donor concentration was 10 mg mL−1. The active layers were spin-coated from the solution onto the substrate in a N2 glove box at 3000 rpm to obtain a thickness of about 100 nm. At approximately 2 × 10−4 Par vacuum level, MoOx (5 nm) and Ag (160 nm) were thermally evaporated using a shadow mask. The active area of the OSC devices was 0.04 cm2.
1) were dissolved in chloroform with 3% (volume fraction) 1,8-diodooctane (DIO). Typically, the content of n-BDT-3T-CNCOO in the overall donor was 0%, 25%, 50%, 75%, and 100%, respectively, and the overall donor concentration was 10 mg mL−1. The active layers were spin-coated from the solution onto the substrate in a N2 glove box at 3000 rpm to obtain a thickness of about 100 nm. At approximately 2 × 10−4 Par vacuum level, MoOx (5 nm) and Ag (160 nm) were thermally evaporated using a shadow mask. The active area of the OSC devices was 0.04 cm2.
2.2 Measurements and characterization
Photovoltaic performance was determined using a Keithley 2400 source meter at AM 1.5 G (100 mW cm−2) simulated by a solar simulator (Enli Technology Ltd). The light intensity was calibrated with a standard photovoltaic cell with a KG5 filter window. External quantum efficiency (EQE) spectra were characterized by a solar cell spectral response measurement system (Enli technology Ltd) in the glove box. Film thickness was obtained through a surface profilometer (Kla-Tencor). Transmission electron microscopy (TEM) specimens were prepared by floating an active layer film onto the surface of deionized water and then transferred to copper grids. Measurements were performed using a Tecnai G2 U-TWIN (FEI) transmission electron microscope under proper defocus conditions. Two-dimensional grazing incidence wide-angle X-ray scattering (2D-GIWAXS) measurements were performed in a Xeuss SAXS/WAXS system with a wavelength of λ = 1.5418 Å. The carrier mobilities of the blend films were measured with a hole-only and electron-only device structure of ITO/PEDOT:PSS/active layer/MoOx/Ag and ITO/ZnO/active layer/Ca/Al, respectively.3. Result and discussion
3.1 Molecular structure and photovoltaic properties
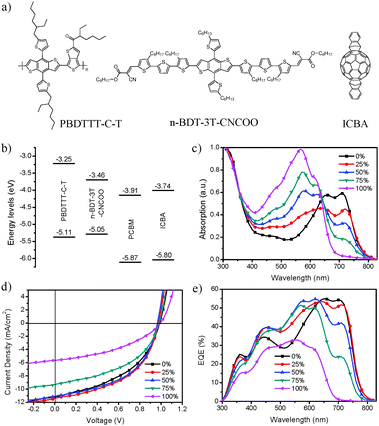
In this study, a ternary system was formed by blending the low-band-gap polymer PBDTTT-C-T, the wide-band-gap small molecule n-BDT-3T-CNCOO with linear alkylthienyl substituted benzo[1,2-b:4,5-b]dithiophene units, and the acceptor ICBA. Fig. 1a shows the chemical structures of the materials used in the active layer of the ternary OSCs. Fig. 1b presents the HOMO and LUMO energy levels of the donor and acceptor materials, respectively. Between the LUMO levels of n-BDT-3TCNCOO and ICBA, the energy offset was only 0.28 eV, whereas n-BDT-3T-CNCOO and PC71BM exhibited an offset of 0.45 eV. The low-energy offset increased Voc whereas reduction was noted in the driving force for charge transfer and the probability of exciton dissociation into free charges.11,15Fig. 1c displays the ultraviolet-visible (UV-vis) absorption spectra of the ternary active layer with different n-BDT-3T-CNCOO weight ratios. The maximum absorption coefficient of n-BDT-3T-CNCOO was higher than that of PBDTTT-C-T, hence, blending small molecules gradually increased the light absorption from 380 nm to 640 nm. However, reduction was observed in the absorption strength between 640 nm and 780 nm. Interestingly, in the ternary-blend films, the shoulder absorption peak of PBDTTT-C-T and n-BDT-3T-CNCOO red-shifted about 10 nm relative to that of the binary-blend films, which indicates the formation of well-ordered aggregation in the ternary-blend films.An inverted structure of ITO/ZnO/PBDTTT-C-T:n-BDT-3T-CNCOO:ICBA/MoOx/Ag was fabricated to study the photovoltaic performance of the ternary OSCs. The ratio of overall donor to the ICBA acceptor was maintained at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The thickness of the photovoltaic active layers with different n-BDT-3T-CNCOO weight ratios was about 100 nm. Fig. 1d shows the current density versus voltage (J–V) characteristics of the devices with different weight ratios of n-BDT-3T-CNCOO (0%, 25%, 50%, 75%, and 100% of overall donor) under simulated A.M. 1.5 G illumination at 100 mV cm−2. Table 1 summarizes the photovoltaic performance parameters of the OSC devices. As expected, the Voc of the devices increased to near 1 V; this increase represents a 0.2 V increment with respect to that of PC71BM-based devices. The polymer (PBDTT-C-T/ICBA) binary device exhibited a PCE of 5.01% with Voc of 0.99 V, Jsc of 11.19 mA cm−2, and an FF of 45.3%. By contrast, the small molecule (n-BDT-3T-CNCOO/ICBA) binary device revealed a PCE of 2.31% with a Voc of 1.0 V, Jsc of 5.65 mA cm−2, and FF of 41.1%. The ternary devices with 25% and 50% ratios of n-BDT-3T-CNCOO presented better performances than the polymer binary devices. The ternary device incorporated with 75% n-BDT-3T-CNCOO attained almost twice the PCE compared with that of the small molecule binary device. The optimum ternary OSC device achieved a Voc of 0.98 V, Jsc of 11.43 mA cm−2, FF of 49.4%, and PCE of 5.51%. Compared with the polymer binary device, Voc slightly decreased, but Jsc slightly increased and FF was improved by 9%. Improvement of the PCE of the ternary device was mainly due to the increased FF.
1. The thickness of the photovoltaic active layers with different n-BDT-3T-CNCOO weight ratios was about 100 nm. Fig. 1d shows the current density versus voltage (J–V) characteristics of the devices with different weight ratios of n-BDT-3T-CNCOO (0%, 25%, 50%, 75%, and 100% of overall donor) under simulated A.M. 1.5 G illumination at 100 mV cm−2. Table 1 summarizes the photovoltaic performance parameters of the OSC devices. As expected, the Voc of the devices increased to near 1 V; this increase represents a 0.2 V increment with respect to that of PC71BM-based devices. The polymer (PBDTT-C-T/ICBA) binary device exhibited a PCE of 5.01% with Voc of 0.99 V, Jsc of 11.19 mA cm−2, and an FF of 45.3%. By contrast, the small molecule (n-BDT-3T-CNCOO/ICBA) binary device revealed a PCE of 2.31% with a Voc of 1.0 V, Jsc of 5.65 mA cm−2, and FF of 41.1%. The ternary devices with 25% and 50% ratios of n-BDT-3T-CNCOO presented better performances than the polymer binary devices. The ternary device incorporated with 75% n-BDT-3T-CNCOO attained almost twice the PCE compared with that of the small molecule binary device. The optimum ternary OSC device achieved a Voc of 0.98 V, Jsc of 11.43 mA cm−2, FF of 49.4%, and PCE of 5.51%. Compared with the polymer binary device, Voc slightly decreased, but Jsc slightly increased and FF was improved by 9%. Improvement of the PCE of the ternary device was mainly due to the increased FF.
| n-BDTT-3T-CNCOO weight ratio (%) | V oc (V) | J sc (mA cm−2) | FF (%) | PCE (PCEave) (%) |
|---|---|---|---|---|
| 0 | 0.99 | 11.19 | 45.3 | 5.01 (4.82) |
| 25 | 0.98 | 11.43 | 49.4 | 5.51 (5.28) |
| 50 | 0.97 | 11.10 | 50.2 | 5.38 (5.19) |
| 75 | 0.98 | 9.33 | 46.3 | 4.24 (4.14) |
| 100 | 1.00 | 5.65 | 41.1 | 2.31 (2.17) |
The EQE spectra of the corresponding devices were measured and are shown in Fig. 1e. The calculated Jsc was obtained by integrating the EQE data convoluted with A.M. 1.5 G solar spectra and was consistent with the result from the J–V curve with about 5% error. The decrease in EQE from 300 nm to 500 nm can be ascribed to the poor absorption of C60-based fullerenes compared with the C70-based ones. As n-BDT-3T-CNCOO was incorporated, the EQE increased from 440 nm to 610 nm but decreased from 640 nm to 800 nm where PBDTTT-C-T was mainly absorbed. The decrease in EQE from 640 nm to 800 nm was attributed to the low absorption coefficient of the polymer and decreased content in the films. The EQE of the 25% n-BDT-3T-CNCOO ternary device showed a slight redshift, which was consistent with the UV-vis spectra. The EQE decrease between 640 nm to 800 nm did not linearly change with the weight ratio of polymer in the films. At 710 nm, the EQE values of the 25%, 50%, and 75% BDT-3T-CNCOO weight ratio devices were 96%, 78%, and 44% of that of the PBDTTT-C-T/ICBA binary-blend device, respectively. Therefore, the EQE value reached a maximum value for the ternary device with 25% n-BDT-3T-CNCOO.
3.2 Charge generation, recombination and transport
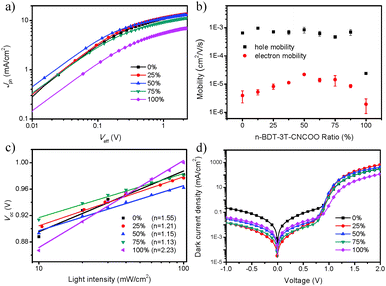
To study the effects of n-BDT-3T-CNCOO on charge generation and dissociation, we analyzed the photocurrent density (Jph) versus effective voltage (Veff) characteristics37,38 of the PBDTTT-C-T:n-BDT-3T-CNCOO:ICBA devices with a 0%, 25%, 50%, 75%, and 100% weight ratio of n-BDT-3T-CNCOO (Fig. 2a). Jph is defined as Jph = JL − JD, where JL and JD represent light and dark current density, respectively. Veff is defined as Veff = V0 − V, where V0 is the voltage at which Jph = 0 and V is the applied voltage. It is assumed that all generated electron–hole pairs dissociated and were collected by electrodes at high Veff (typically 2 V) and saturation current (Jsat) is limited by the total amount of absorbed incident photos. The corresponding exciton dissociation probability (Pdiss) is defined as the ratio of Jph to Jsat. The Jsat values for the 0%, 25%, 50%, 75%, and 100% weight ratio devices were 13.36, 13.41, 12.83, 11.09, and 6.86 mA cm−2, respectively. The Pdiss values under short-circuit conditions for the five devices were 83.7%, 85.2%, 86.5%, 84.1%, and 82.3% respectively, whereas the Pdiss values at Veff = 0.2 V for the five devices were 39.7%, 48.2%, 48.7%, 42.5%, and 35.0%, respectively. The results suggest that incorporation of 25% and 50% of the small molecule facilitated charge dissociation, leading to the improvement of the ternary-blend devices. Notably, the Pdiss values were lower than the data reported in the literature.38 This discrepancy may be ascribed to a lower LUMO offset, which caused the lower Jsc and FF of the ICBA-based ternary devices relative to that of PCBM-based ternary devices.The hole and electron mobilities of PBDTTT-C-T:n-BDT-3T-CNCOO:ICBA with different weight ratios of n-BDT-3T-CNCOO were measured using the space-charge-limited current (SCLC) mode. Zero-field mobilities were fitted with hole-only and electron-only devices under the Mott–Gurney law.38 The mobility values are plotted in Fig. 2b. The hole mobility of the n-BDT-3T-CNCOO binary blend is 2.4 × 10−5 cm2 V−1 s−1, an order of magnitude lower than those of the polymer binary and the ternary-blend devices. We observed that adding ICBA influenced the donor film structure and lowered the hole mobilities below those of PC71BM-based ternary-blend films. However, the hole mobilities are still maintained. The electron mobilities of the ternary-blend films based on ICBA were two orders of magnitude lower than those of the ternary-blend film based on PC71BM.33 The electron mobilities of the ternary blend first increased with the weight ratio of n-BDT-3T-CNCOO and then decreased. Improvement of the electron mobilities of the ternary-blend films was consistent with the increased FF of the ternary-blend photovoltaic devices when PBDTTT-C-T dominated in the films.
To study charge recombination, we measured J–V curves under different light intensities for the 0%, 25%, 50% 75%, and 100% devices. Under Voc conditions, the net current is zero, indicating that all photogenerated charge carriers recombine within the cell. Thus, the recombination type can be identified from the slope of Vocversus the natural logarithm of light intensity.39 Slope is written as nkT/q, where n is the ideality factor under illumination, k is Boltzmann's constant, T is temperature, and q is elementary charge.40 For bimolecular recombination dominating in the cell, n is equal to 1, whereas n is 2 for trap-assisted recombination.39 As shown in Fig. 2c, the slopes of 1.55, 1.21, 1.15, 1.13, and 2.23 kT/q were obtained for the five devices, respectively. The result indicates that incorporation of n-BDT-3T-CNCOO into the PBDTTT-C-T/ICBA blend suppressed trap-assisted recombination. In parallel, the ideality factors of the five devices under dark conditions were found to be 2.48, 1.65, 1.70, 1.76, and 2.42, respectively, as determined from the slope of the exponential part of the dark J–V characteristics,40 as shown in Fig. 2d. The results of ideality factors of the devices in the dark and under illumination imply that incorporation of n-BDT-3T-CNCOO reduced the degree of trap-assisted recombination and consequently decreased monomolecular recombination.
3.3 Morphology characterization
TEM was conducted to study the morphology of the PBDTTT-C-T:n-BDT-3T-CNCOO:ICBA ternary-blend films. As shown in Fig. 3, the PBDTTT-C-T/ICBA binary film displayed a short nanofibrous structure, and adding n-BDT-3T-CNCOO induced the formation of additional such nano-size structures. The fibril structure is beneficial to exciton separation and charge transfer,21 which is consistent with the result from the Jph–Veff curve and SCLC analysis. The n-BDT-3T-CNCOO/ICBA binary film possessed a large domain size, which explains the low EQE value of the BDT-3T-CNCOO/ICBA binary device. Images based on ICBA as an acceptor showed a less ordered structure compared with those of previous images based on PC71BM as an acceptor at each ratio of small molecule.33 A mesoscopic-phase heterogeneity model was proposed to explain the difference in morphology between ICBA and PCBM in the same polymer.19 A low-crystalline polymer cannot self-assemble into an effective fibrous structure to serve as a stream guide and a space restrictor for ICBA aggregation. Incorporating highly crystalline n-BDT-3T-CNCOO induced PBDTTT-C-T to form a longer nanofibrous structure, which in turn facilitated crystallization of ICBA.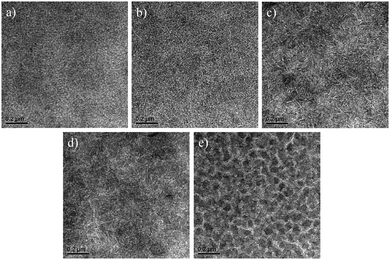 | ||
| Fig. 3 TEM images of ternary-blend films with different contents of n-BDT-3T-CNCOO: (a) 0%, (b) 25%, (c) 50%, (d) 75%, and (e) 100%. Scale bar is 200 nm in each image. | ||
3.4 Donor and acceptor crystals
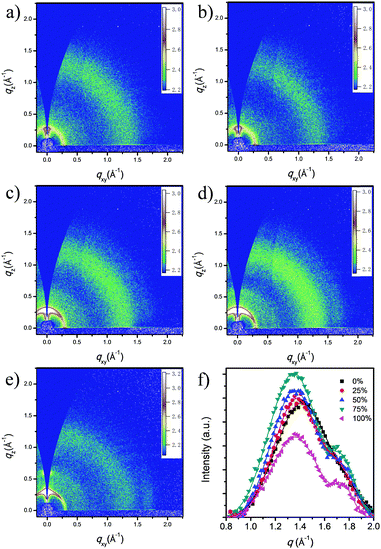
To confirm the crystallization of ICBA in ternary-blend films, 2D-GIWAXS was performed to study the crystallinity of the donors and acceptors in ternary-blend films. As shown in Fig. 4, arc-like scattering arising from the PBDTTT-C-T and n-BDT-3T-CNCOO lamellar stacking (100) layers was observed in the out-of-plane direction around 0.32 Å−1. And the scattering intensity gradually increased with the increasing of the n-BDT-3T-CNCOO weight ratio. This result indicates that adding the small molecule n-BDT-3T-CNCOO promotes crystallization of the donors in the films. The π–π stacking (010) peak at ≈1.75 Å−1 was very weak in both directions. All films exhibited a ring-like layering peak at ≈1.35 Å−1, and this layering peak was ascribed to ICBA aggregation.To obtain additional information on π–π stacking and the aggregation of ICBA, we integrated the full range of data from 2D-GIWAXS. Fig. 4f presents the integrated data after baseline processing and fitting of peaks of ICBA aggregation and π–π stacking with a Gaussian function. The stacking coherence lengths of the peaks were calculated using the Scherrer equation. The peak heights for both ICBA aggregation and π–π stacking of the 25%, 50%, and 75% weight ratio ternary films were higher than both the PBDTTT-C-T and n-BDT-3T-CNCOO binary films, indicating an enhanced crystallinity degree for the ternary-blend films. The coherence lengths of ICBA aggregation of the 0%, 25%, 50%, 75%, and 100% weight ratio films are 11.5, 13.7, 12.8, 12.4, and 12.9 Å, respectively. The π–π stacking coherence lengths for the five films are 20.2, 22.8, 21.7, 25.3, and 33.7 Å, respectively. The results suggest that incorporating n-BDT-3T-CNCOO promotes both ICBA aggregation and π–π stacking of the ternary-blend films. Overall, the higher crystallinity of the donors and the better aggregation of the acceptor constitute the advantages of the ternary-blend films over polymer binary films. These superior properties can be used to explain the better charge transport and lower degree of trap-assisted recombination in ternary-blend devices than in binary devices.
3.5 Generality of the ternary strategy
According to above discussion, incorporation of a highly crystalline small molecule into the low-band-gap polymer promoted the photovoltaic performance of the ICBA-based ternary-blend devices. The ternary-blend films presented a more highly ordered structure than that of the polymer/ICBA binary film. To check the generality of the ternary strategy, we plotted photovoltaic parameters (Voc, Jsc, FF, and PCE) that were normalized with polymer binary device data(Fig. 5). Fig. 5a shows the data of the PBDTTT-C-T:n-BDT-3T-CNCOO:ICBA system under different weight ratios of n-BDT-3T-CNCOO. Improvement of the PCEs of the ternary-blend devices was mainly due to increased FF. To examine whether the ternary-blend strategy can be used in other acceptors, for example non-fullerene molecule ITIC,41 we tested the photovoltaic property of the PBDTTT-C-T:n-BDT-3T-CNCOO:ITIC system. The best ternary PBDTTT-C-T:n-BDT-3T-CNCOO:ITIC device demonstrated a PCE of 5.73% with a Voc of 0.84 V, Jsc of 12.64 mA cm−2, and FF of 54.2%. By contrast, binary-blend PBDTTT-C-T/ITIC gave a PCE of 5.39% with a Voc of 0.83 V, Jsc of 12.65 mA cm−2, and FF of 51.3%. The photovoltaic parameter of the ternary devices based on ITIC as the acceptor was normalized and plotted in Fig. 5b. Except for that of the binary-blend n-BDT-3T-CNCOO/ITIC, the Voc of the ternary devices increased with the weight ratio of small molecule. Jsc tended to decrease with the weight ratio of small molecule, whereas FF improved from 51.3% to 54.2% and then dropped as the content of n-BDT-3T-CNCOO increased. PCE showed a similar tendency to Jsc, and the improved PCE was mainly because of the FF. Overall, the ternary-blend strategy of adding a small molecule into the polymer could be used in different acceptor systems. Moreover, the incorporation of a highly crystalline small molecule can improve the FF of ternary-blend devices at a proper weight ratio.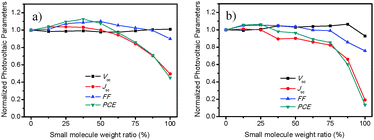 | ||
| Fig. 5 Photovoltaic parameters normalized with that of polymer binary device. (a) PBDTTT-C-T:n-BDT-3T-CNCOO:ICBA ternary system. (b) PBDTTT-C-T:n-BDT-3T-CNCOO:ITIC ternary system. | ||
4. Conclusions
In summary, a ternary strategy was used in ICBA-based OSCs, and a potential method for improving the performance of low-band-gap polymer/ICBA binary devices was revealed. The PCE of the ICBA-based ternary blend device is 5.51%, which is 10% higher than the binary blend device. Especially, a Voc as high as ca. 1 V was obtained, which was mainly contributed by the higher LUMO level of ICBA. In the present system, a highly crystalline small molecule was incorporated into the polymer to promote donor crystallization to form nanofibrous morphologies. Such addition also enhanced aggregation of the acceptor, and resulted in enhanced charge carrier dissociation, improved charge transport, and a decreased degree of trap-assisted recombination. The ternary strategy can be used in a non-fullerene system, in which increased FF is also obtained.Acknowledgements
We acknowledge the financial support by the Ministry of Science and Technology of China (No. 2016YFA0200700), the National Natural Science Foundation of China (Grant No. 21534003, 91427302, and 21504066) and the Chinese Academy of Sciences (Grant No. XDA09040200).Notes and references
- A. J. Heeger, Adv. Mater., 2014, 26, 10–28 CrossRef CAS PubMed.
- B. Kippelen and J.-L. Bredas, Energy Environ. Sci., 2009, 2, 251–261 CAS.
- F. C. Krebs, N. Espinosa, M. Hosel, R. R. Sondergaard and M. Jorgensen, Adv. Mater., 2014, 26, 29–39 CrossRef CAS PubMed.
- Y. Liang, Z. Xu, J. Xia, S.-T. Tsai, Y. Wu, G. Li, C. Ray and L. Yu, Adv. Mater., 2010, 22, E135–E138 CrossRef CAS PubMed.
- Y. J. He, H. Y. Chen, J. H. Hou and Y. F. Li, J. Am. Chem. Soc., 2010, 132, 5532 CrossRef CAS.
- G. J. Zhao, Y. J. He and Y. F. Li, Adv. Mater., 2010, 22, 4355–4358 CrossRef CAS PubMed.
- Y. F. Li, Chem. – Asian J., 2013, 8, 2316–2328 CrossRef CAS PubMed.
- J. Min, Z.-G. Zhang, M. Zhang and Y. Li, Polym. Chem., 2013, 4, 1467–1473 RSC.
- X. Guo, M. Zhang, L. Huo, C. Cui, Y. Wu, J. Hou and Y. Li, Macromolecules, 2012, 45, 6930–6937 CrossRef CAS.
- H. Xin, S. Subramaniyan, T.-W. Kwon, S. Shoaee, J. R. Durrant and S. A. Jenekhe, Chem. Mater., 2012, 24, 1995–2001 CrossRef CAS.
- S. Albrecht, K. Vandewal, J. R. Tumbleston, F. S. U. Fischer, J. D. Douglas, J. M. J. Fréchet, S. Ludwigs, H. Ade, A. Salleo and D. Neher, Adv. Mater., 2014, 26, 2533–2539 CrossRef CAS PubMed.
- C. Li, Y. Ding, M. Soliman, J. Lorenzo, N. Dhasmana, P. Chantharasupawong, A. V. Ievlev, A. J. Gesquiere, L. Tetard and J. Thomas, ACS Appl. Mater. Interfaces, 2016, 8, 4730–4738 CAS.
- J. Wang, F. J. Zhang, Q. S. An, Q. Q. Sun, J. Zhang and B. Hu, Phys. Chem. Chem. Phys., 2015, 17, 29671–29678 RSC.
- Q. S. An, F. J. Zhang, L. L. Li, Z. L. Zhuo, J. Zhang, W. H. Tang and F. Teng, Phys. Chem. Chem. Phys., 2014, 16, 16103–16109 RSC.
- T. E. Kang, H.-H. Cho, C.-H. Cho, K.-H. Kim, H. Kang, M. Lee, S. Lee, B. Kim, C. Im and B. J. Kim, ACS Appl. Mater. Interfaces, 2013, 5, 861–868 Search PubMed.
- F. C. Jamieson, E. B. Domingo, T. McCarthy-Ward, M. Heeney, N. Stingelin and J. R. Durrant, Chem. Sci., 2012, 3, 485–492 RSC.
- S. Shoaee, S. Subramaniyan, H. Xin, C. Keiderling, P. S. Tuladhar, F. Jamieson, S. A. Jenekhe and J. R. Durrant, Adv. Funct. Mater., 2013, 23, 3286–3298 CrossRef CAS.
- M. A. Faist, S. Shoaee, S. Tuladhar, G. F. A. Dibb, S. Foster, W. Gong, T. Kirchartz, D. D. C. Bradley, J. R. Durrant and J. Nelson, Adv. Energy Mater., 2013, 3, 744–752 CrossRef CAS.
- H. Yan, D. H. Li, C. He, Z. X. Wei, Y. L. Yang and Y. F. Li, Nanoscale, 2013, 5, 11649–11656 RSC.
- T. Ameri, P. Khoram, J. Min and C. J. Brabec, Adv. Mater., 2013, 25, 4245–4266 CrossRef CAS PubMed.
- L. Lu, T. Xu, W. Chen, E. S. Landry and L. Yu, Nat. Photonics, 2014, 8, 716–722 CrossRef CAS.
- Q. S. An, F. J. Zhang, J. Zhang, W. H. Tang, Z. B. Deng and B. Hu, Energy Environ. Sci., 2016, 9, 281–322 Search PubMed.
- P. Cheng and X. W. Zhan, Mater. Horiz., 2015, 2, 462–485 RSC.
- L. Y. Lu, M. A. Kelly, W. You and L. P. Yu, Nat. Photonics, 2015, 9, 491–500 CrossRef CAS.
- L. Q. Yang, L. Yan and W. You, J. Phys. Chem. Lett., 2013, 4, 1802–1810 CrossRef CAS PubMed.
- T. Ameri, T. Heumuller, J. Min, N. Li, G. Matt, U. Scherf and C. J. Brabec, Energy Environ. Sci., 2013, 6, 1796–1801 CAS.
- P. Cheng, Y. F. Li and X. W. Zhan, Energy Environ. Sci., 2014, 7, 2005–2011 CAS.
- P. P. Khlyabich, B. Burkhart and B. C. Thompson, J. Am. Chem. Soc., 2011, 133, 14534–14537 CrossRef CAS PubMed.
- P. Cheng, C. Yan, Y. Wu, J. Wang, M. Qin, Q. An, J. Cao, L. Huo, F. Zhang, L. Ding, Y. Sun, W. Ma and X. Zhan, Adv. Mater., 2016, 28, 8021–8028 CrossRef CAS PubMed.
- Y. J. Kim, J. Hong and C. E. Park, ACS Appl. Mater. Interfaces, 2015, 7, 21423–21432 CAS.
- T. Y. Huang, D. Patra, Y. S. Hsiao, S. H. Chang, C. G. Wu, K. C. Ho and C. W. Chu, J. Mater. Chem. A, 2015, 3, 10512–10518 CAS.
- Y. J. Zhang, D. Deng, K. Lu, J. Q. Zhang, B. Z. Xia, Y. F. Zhao, J. Fang and Z. X. Wei, Adv. Mater., 2015, 27, 1071–1076 CrossRef CAS PubMed.
- J. Fang, Z. Y. Wang, J. Q. Zhang, Y. J. Zhang, D. Deng, Z. Wang, K. Lu, W. Ma and Z. X. Wei, Adv. Sci., 2015, 2, 1500250 CrossRef PubMed.
- J. Q. Zhang, Y. J. Zhang, J. Fang, K. Lu, Z. Y. Wang, W. Ma and Z. X. Wei, J. Am. Chem. Soc., 2015, 137, 8176–8183 CrossRef CAS PubMed.
- D. Deng, Y. Zhang, L. Yuan, C. He, K. Lu and Z. Wei, Adv. Energy Mater., 2014, 4, 1400538 CrossRef.
- D. Deng, Y. Zhang, L. Zhu, J. Zhang, K. Lu and Z. Wei, Phys. Chem. Chem. Phys., 2015, 17, 8894–8900 RSC.
- V. D. Mihailetchi, L. J. A. Koster, J. C. Hummelen and P. W. M. Blom, Phys. Rev. Lett., 2004, 93, 216601 CrossRef CAS PubMed.
- Z. He, C. Zhong, X. Huang, W.-Y. Wong, H. Wu, L. Chen, S. Su and Y. Cao, Adv. Mater., 2011, 23, 4636–4643 CrossRef CAS PubMed.
- S. R. Cowan, A. Roy and A. J. Heeger, Phys. Rev. B: Condens. Matter Mater. Phys., 2010, 82, 245207 CrossRef.
- L. J. A. Koster, V. D. Mihailetchi, R. Ramaker and P. W. M. Blom, Appl. Phys. Lett., 2005, 86, 123509 CrossRef.
- Y. Lin, J. Wang, Z.-G. Zhang, H. Bai, Y. Li, D. Zhu and X. Zhan, Adv. Mater., 2015, 27, 1170–1174 CrossRef CAS PubMed.
| This journal is © the Partner Organisations 2017 |