Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Concentration, distribution, and fractionation of metals in the filter material of 29 bioretention facilities: a field study†
Robert
Furén
 *ad,
Heléne
Österlund
a,
Ryan J.
Winston
*ad,
Heléne
Österlund
a,
Ryan J.
Winston
 bc,
R. Andrew
Tirpak
b,
Jay D.
Dorsey
b,
Joseph
Smith
b,
Maria
Viklander
a and
Godecke-Tobias
Blecken
a
bc,
R. Andrew
Tirpak
b,
Jay D.
Dorsey
b,
Joseph
Smith
b,
Maria
Viklander
a and
Godecke-Tobias
Blecken
a
aUrban Water Engineering, Department of Civil, Environmental and Natural Resources Engineering, Luleå University of Technology, 971 87 Luleå, Sweden. E-mail: rober.furen@associated.ltu.se
bDepartment of Food, Agricultural, and Biological Engineering, Ohio State University, Agricultural Engineering Building AE, Building 298, 590 Woody Hayes Dr, Columbus, 43210, OH, USA
cDepartment of Civil, Environmental, and Geodetic Engineering, Ohio State University, 470 Hitchcock Hall, 2070 Neil Avenue, Columbus, 43210, OH, USA
dNCC Sverige AB, Department of Research and Innovation, Herrjärva Torg 4, 17080 Solna, Sweden
First published on 17th May 2023
Abstract
Pollutant loads stemming from anthropogenic activities conveyed in urban stormwater runoff contribute to the impairment of downstream water bodies. Cities and municipalities are increasingly turning toward green infrastructure stormwater control measures to treat pollutants at the source of runoff. One example of these technologies is bioretention, which is commonly applied for stormwater treatment in urban areas due to its demonstrated effectiveness in removing various pollutants from water, including sediment, nutrients (e.g., N and P), and metals. As metals are mainly removed by filtration or adsorption to soil particles, the filter media is important for metal removal in bioretention. However, the capacity to remove metals through adsorption by bioretention media is finite; thus, the media may need to be replaced and disposed of after maintenance or at the end of its operational lifespan. Pollutant accumulation in bioretention media has the potential to approach toxicity thresholds, which may introduce complexities for safe handling and disposal. To fully capture the potential challenges associated with metals accumulation in media over time, it is important to understand the accumulation processes and mobility of metals in bioretention facilities as they age. Although several studies have investigated metal accumulation and distribution in bioretention media, few have assessed metal mobility by fractionation using sequential extraction methods in older (i.e., >7 years) facilities. In November 2019, we conducted a comprehensive field study of older facilities in Ohio, Michigan, and Kentucky (USA) to improve the understanding of the accumulation processes and metal mobility in bioretention. In this study, concentrations of several metals (i.e., Cr, Cu, Ni, Pb, and Zn) were analyzed in samples of filter material from 29 bioretention sites in operation for 7–16 years. Except for Cd, all metals were found in all samples. Metals accumulation was clear with highest concentrations found in the top (0–5 cm) layer of the filter material, attributable to the filtration of particles percolating through the media profile. Lower concentrations were observed in deeper (i.e., >10 cm) layers of the bioretention media. The fractionation showed that the metals of interest were present at high levels with a risk of leaching over time, among which Cd, Zn, and Pb were suggested to be mobile from the filter material during precipitation. Thus, there is a potential risk of leakage from filter material or sediment removed from biofilters, e.g., during maintenance and disposal. The results of principal component analysis indicated specifically correlations between metal concentrations and the filter material soil texture including the organic matter content. These results contribute to improved design and operation and suggest regular maintenance to reduce long-term risks associated with the accumulation of metals in bioretention and similar urban stormwater treatment facilities. Since most metals are trapped in the top layer of the filter it may be enough to remove only the top layer. However, metal fractionation should be considered when handling the material.
Water impactThese results contribute towards improving maintenance and, thus, long-term functionality of stormwater bioretention facilities. To maintain treatment function and reduce risk of metal leakage, regular replacement of the filter material's top layer is recommended. When removing filter material, one has to consider the material as potentially dangerous waste, especially material from close to surface and inlets. |
Introduction
Urban stormwater runoff conveys significant loads of anthropogenic pollutants which lead to the impairment of receiving waters.1 Metals (e.g., Cd, Cu, Pb, Zn) are commonly regarded as pollutants of concern in stormwater that contribute to the degradation of aquatic habitats.2 The Nationwide Urban Runoff Program3 identified metals, especially Cu, Pb, and Zn, as being toxic in road runoff, while Cd, Cr, Cu, Ni, Pb, and Zn have been identified as contaminants of concern for human and aquatic life in stormwater.4 Thus, stormwater should be treated to remove metals before being discharged to surface waters.5Bioretention is a commonly applied low impact development practice for stormwater treatment in urban areas.6–8 In these systems, also referred to as biofilters, biofiltration systems or raingardens, stormwater from a contributing watershed is treated vertically through a filter before being released to downstream systems. Bioretention, also known as biofiltration or stormwater biofilters, typically consists of a filter media of sand, silt, clay, and organic matter and/or soil often topped with mulch and/or topsoil and planted with a variety of plant species and can, depending on design, be equipped with a perforated underdrain pipe (Fig. 1).9–11 Research has demonstrated that bioretention provides effective removal of various pollutants such as total suspended solids and metals.12 As most metals are removed by filtration or adsorption to soil particles,12 the filter media is critically important for metal removal in bioretention. Bioretention normally has a filter depth of 0.7–1 m,13 although metals are primarily trapped in the upper (0–10 cm) media layers in a fast adsorption process.14–16 This has implications for bioretention maintenance, wherein media could potentially become contaminated such that disposal becomes more difficult and costly. Davis et al.17 estimated that regulatory limits for biosolids application in the top layer could be reached after 20, 77, 16, and 16 years of bioretention operation for Cd, Cu, Pb, and Zn, respectively.18 Moreover, based on laboratory results, Hatt et al.19 estimated a life span of 12–15 years of operation before the levels of Cd, Cu, and Zn in filter material would exceed guideline values20 for human and ecological health and would require special disposal. Al-Ameri et al.14 found for highly polluted catchment areas (e.g., industrial areas) that filter material after 9–16 years of operation had high Zn concentrations (Zn >200 mg kg−1) and could be classified as contaminated material according to the Victorian EPA criteria's for classification of waste,21 meaning it would require special disposal if removed. Al-Ameri et al.14 also suggested that clogging (e.g., reduced hydraulic function) may be the primary limit to bioretention function for less contaminated areas, rather than high concentrations of metals.
Pollutants captured in the filter material create a pollutant depot17 which carries a risk of metal leaching. To mitigate leaching, Kluge et al.22 recommend removal of the top 10 cm of the filter media (with accumulated sediments and associated metals) and replacement after 20–25 years. Further, there is a potential risk of leaching when disposing removed filter material from bioretention. It is essential to better understand the characteristics and behaviors of accumulated metals in the filter media to evaluate and reduce associated risks during the bioretention lifespan or when material is removed during maintenance or decommissioning. Metal fractionation by sequential extraction is one method that can provide information about the mobility and leachability of metals from filter material and may support the evaluation of the risk of metal release during operation, maintenance, and disposal. Other studies14,23,24 have examined metal behavior in bioretention; however, only a few have assessed the availability of metals in mature bioretention facilities using sequential extraction methods.
Many previous studies on bioretention have used the sequential extraction method; indeed, Wang et al.,25 used the sequential extraction method to study Cd in a laboratory-scale bioretention column experiment, while Søberg et al.16 used the same method to evaluate the characteristics of adsorbed dissolved metals on different bioretention filter materials. In field-scale bioretention, Li and Davis,15 and Jones and Davis26 used a five step sequential extraction when studying a quantitative theory for metal capture (Cu, Pb, and Zn) and to evaluate the environmental availability of metals. A recent study by Rommel et al.27 involved the use of sequential extraction to assess the mobility of metals (Cr, Cu, Ni, and Zn) in road run-off from roadside bioretention cells. However, the large sample size of this study, which involved field sampling of 29 older (7–16 years of operation) bioretention sites, laboratory analyses of total concentrations and a 5-step sequential extraction of six metals (Cd, Cr, Cu, Ni, Pb, and Zn) make these results and conclusions more impactful as it relates to the potential to glean design and maintenance recommendations for bioretention systems.
Here, we conducted a major field study to increase the knowledge surrounding metal mobility and availability and to improve the understanding and risks associated with metal release from bioretention filter material. The study included filter media sampling and lab analyses of Cd, Cr, Cu, Ni, Pb, and Zn, including fractionation by sequential extraction. The results will assist with improving filter design, operation, and maintenance, which will serve to reduce the long-term risks associated with accumulated metals in bioretention or similar facilities in the context of urban stormwater treatment.
Method
Field sites
Metals accumulation was studied in 29 bioretention facilities, mainly those treating runoff from urban catchments with different land use characteristics, including parking lots, roads, downtown urban areas, and industrial, commercial, and residential areas. The bioretention facilities were located in Ohio, Michigan, and Kentucky (USA). They varied in age from 7 to 16 years old at the time of sampling (2019) and the filter surface areas ranged from approximately 10 m2 to 1900 m2. The contributing catchment areas varied in size from approximately 50 m2 to 125 ha, which results in a variation in the ratio of the filter areas and catchment areas of 0.1% to 20%. All evaluated bioretention systems were equipped with an underdrain pipe. The weather and climate in these areas are described as hot-summer humid continental, humid subtropical, and warm-summer humid continental climate with annual precipitation roughly around 760 mm to 1100 mm. Further details are presented in Table 1.| Site | Age [year] | Location | Catchment area usage | Catchment area [m2] | Filter area [m2] | Ratio [%] | Filter materiala | LOIa [%] |
|---|---|---|---|---|---|---|---|---|
| a Soil type and loss on ignition (LOI) for location 1 and depth 1. Data for all sampling points (e.g., used in the PCA) are attached in the ESI† material, Table S1. | ||||||||
| 1 | 9 | Upper Arlington, OH | Residential | 318![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
950 | 0.3 | Sandy loam | 5.8 |
| 2 | 9 | Upper Arlington, OH | Residential | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1200 | 0.1 | Sand | 7.0 |
| 3 | 9 | Upper Arlington, OH | Residential | 224![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
900 | 0.4 | Sandy loam | 19 |
| 4 | 9 | Upper Arlington, OH | Residential | 146![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1900 | 1.3 | Loamy sand | 6.6 |
| 5 | 8 | Upper Arlington, OH | Commercial | 750 | 40 | 5.3 | Silt loam | 23 |
| 6 | 10 | Columbus, OH | Industrial | 6000 | 300 | 5.0 | Sand | 3.3 |
| 7 | 8 | Westerville, OH | Parking/roads | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
600 | 5.0 | Sandy loam | 30 |
| 8 | 8 | Westerville, OH | Parking/roads | 2000 | 50 | 2.5 | Sandy loam | 13 |
| 9 | 7 | Westerville, OH | Commercial | 4000 | 170 | 4.3 | Sandy loam | 7.7 |
| 10 | 9 | Columbus, OH | Parking/roads | 4500 | 580 | 13 | Loamy sand | 11 |
| 11 | 9 | Columbus, OH | Downtown urban | 300 | 40 | 13 | NA | 23 |
| 12 | 8 | Columbus, OH | Downtown urban | 50 | 10 | 20 | Sandy loam | 15 |
| 13 | 12 | Hamilton, OH | Industrial | 4500 | 200 | 4.4 | Sand | 7.4 |
| 14 | 12 | Hamilton, OH | Industrial | 4500 | 300 | 6.7 | Sand | 32 |
| 15 | 12 | Hamilton, OH | Industrial | 4500 | 200 | 4.4 | Sand | 9.3 |
| 16 | 16 | Fort Wright, KY | Commercial | 3000 | 190 | 6.3 | Sandy loam | 28 |
| 17 | 9 | Toledo, OH | Residential | 250 | 50 | 20 | Loamy sand | 15 |
| 18 | 12 | Lansing, MI | Downtown urban | 600 | 50 | 8.3 | Sandy loam | 19 |
| 19 | 11 | Lansing, MI | Downtown urban | 500 | 50 | 10 | Loamy sand | 17 |
| 20 | 14 | Ann Arbor, MI | Parking/roads | 2250 | 156 | 6.9 | Sand | 14 |
| 21 | 11 | Seven Hills, OH | Commercial | 1200 | 200 | 17 | Sandy loam | 25 |
| 22 | 8 | Parma, OH | Fueling station | 2500 | 200 | 8.0 | Sandy loam | 15 |
| 23 | 13 | Twinsburg, OH | Fueling station | 2000 | 70 | 3.5 | Sandy loam | 12 |
| 24 | 10 | Orange Village, OH | Residential | 250 | 20 | 8.0 | Loamy sand | 18 |
| 25 | 10 | Orange Village, OH | Residential | 250 | 20 | 8.0 | Sand | 12 |
| 26 | 11 | Kent, OH | Fueling station | 800 | 70 | 8.8 | Silt loam | 21 |
| 27 | 13 | Akron, OH | Parking/roads | 6500 | 180 | 2.8 | Loamy sand | 12 |
| 28 | 12 | North Canton, OH | Fueling station | 1250 | 180 | 14 | Loamy sand | 11 |
| 29 | 12 | North Canton, OH | Fueling station | 1000 | 100 | 10 | Loamy sand | 14 |
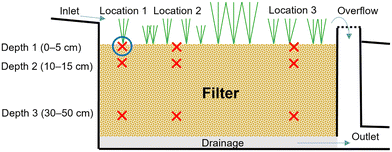
Nine samples were collected from each of the 29 bioretention facilities (Fig. 1), except for the smaller sites (24 and 25), in which only three samples each were collected; thus, a total of 249 samples were collected. The methodology was a hypothesis-guided sampling similar to that used by Tedoldi et al.,28 which included three sampling locations along each bioretention filter (i.e., three distances from the inlet) located approximately 1 m, 3 m, and 6 m from the inlet at three different depths. However, for sites 5, 8, 12, which were smaller, these distances were scaled down to fit the three sampling locations within the site and for sites 24 and 25, only one sample point was included. Further, some filters had multiple inlets (sites 5, 16, 23, and 26) or received diffuse flow along one edge (sites 13, 14, 15, 24, and 25); for these sites, the sampling locations were positioned based on the most likely flow path through the system. Therefore, the field work for each site started with mapping and examination of the local site hydrology and topography, before the catchment areas, inlets, deposited sediments, and erosion were studied to define a main inlet from which the sampling points were then measured out. At each of the three sampling locations, samples were taken at three depths (0–5 cm, 10–15 cm, and 30–50 cm from the surface) as illustrated in Fig. 1 except for sites 4, 7 and 23, which were sampled to 20–30 cm maximum depth due to shallow filter depth. The filter materials show great variation between the different sites including sand, loamy sand, sandy loam to silt loam (classification according to the USDA soil textural triangle29). The content of organic matter (loss on ignition (LOI)) varies between 1% and 46% with a median of 10% of dry matter (DM). Some maintenance has been performed at the sites (e.g. vegetation pruning, removal of trash), but to our best knowledge the filter materials had not been replaced recently.
Sampling
Samples in the field were collected by digging a core (approximately 5 cm × 15 cm × 15 cm for layer 1 and 10 cm × 10 cm × 10 cm for layers 2 and 3), with approximately 1–1.5 kg of filter material collected from each of the nine sampling points. The filter material was stored in diffusion-tight plastic bags (18 cm × 35 cm), which were sealed shut with cable ties. The outdoor temperature during sampling was between −12 and +6 °C and the samples were refrigerated before laboratory analysis, which was conducted within 3 months of sampling.Laboratory analysis
All samples were sent to an accredited laboratory (ALS Scandinavia AB) for pre-treatment and analysis. To determine the total metal concentration, the samples were dried (50 °C) and sieved (2 mm) according to the Swedish standards.30,31 Drying at 105 °C was conducted in parallel with sample analysis to correct to a dry matter (DM) concentration. Microwave-assisted digestion was performed on the dried samples in 5 ml of concentrated HNO3 + 0.5 ml H2O2.To assess the bioavailability of the six metal species of interest (i.e., Cd, Cr, Cu, Ni, Pb, and Zn) in the filter material and to determine to what extent the metals were leachable, a fractionation with a 5-step sequential extraction method was performed in one sample from each sampling site, corresponding to location 1 and depth 1 (cf.Fig. 1), probably the most polluted location. This analysis was informed by methodology developed by Hall et al.32,33 for laboratory simulations of leaching.
Analysis of metal leachate water was performed on samples acidified with 1 ml concentrated HNO3 (Suprapur for trace analysis) per 100 ml. Analysis was performed with Inductively Coupled Plasma Sector Field Mass Spectrometry (ICP-SFMS) according to Swedish standards34,35 and U.S. EPA method 2008.36 Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES or ICP-AES) was also performed according to Swedish standards37 and U.S. EPA methods.38 The detection limits (DLs) were affected in one sample (site 16, step 2 for Cd, Cr, and Ni) e.g., extra dilution was necessary to reduce matrix effects (For DL see Table 2, Fig. 3). Determination of pH was performed according to Swedish standards39 after suspension in water. Loss on ignition (LOI) was measured using gravimetric analysis based on CSN EN 12879,40 CSN 72 0103 (ref. 41) and CSN 46 5735.42
| Metal | Fraction | Median | Min | Max | DL | Occurence > DL |
|---|---|---|---|---|---|---|
| [mg kg−1, DM] | ||||||
| a Indicates for detection limits (DL) that for Cd, Cr, and Ni there was one sample from the analysis with a higher detection limit (double the typical detection limit) than all of the other detection limits (28 samples: CDDL = 0.3 for 28 samples and CdDL = 0.6 for one sample; 27 samples: CrDL = 3; 1 sample: CrDL = 6; and for Ni, where 26 samples had NiDL = 3 and one sample had NiDL = 6). The values for the total concentrations are taken from lab analyses of total concentration. | ||||||
| Cd | Total | 0.35 | 0.10 | 1.58 | 0.10 | 90% |
| 1 | 0.20 | 0.06 | 0.42 | — | 100% | |
| 2 | — | — | — | 0.3a | 0% | |
| 3 | 0.05 | 0.01 | 0.10 | — | 100% | |
| 4 | 0.05 | 0.02 | 0.10 | — | 100% | |
| 5 | 0.02 | 0.01 | 0.06 | — | 100% | |
| Cr | Total | 8.75 | 2.66 | 60.9 | — | 100% |
| 1 | 1.02 | 0.47 | 3.63 | — | 100% | |
| 2 | <3 | <3 | 3.19 | 3a | 3% | |
| 3 | 0.41 | 0.16 | 4.53 | — | 100% | |
| 4 | 5.27 | 1.84 | 27.1 | — | 100% | |
| 5 | 6.40 | 1.44 | 26.8 | — | 100% | |
| Cu | Total | 20.7 | 4.89 | 93.6 | — | 100% |
| 1 | 1.26 | 0.19 | 5.81 | — | 100% | |
| 2 | <8.65 | <6 | 32.8 | 6 | 66% | |
| 3 | 0.42 | 0.03 | 9.33 | — | 100% | |
| 4 | 13.3 | 5.22 | 87.6 | — | 100% | |
| 5 | 4.95 | 2.21 | 100 | — | 100% | |
| Ni | Total | 14.8 | 3.67 | 64.0 | — | 100% |
| 1 | 1.37 | 0.33 | 3.90 | — | 100% | |
| 2 | <3 | <3 | 4.99 | 3a | 7% | |
| 3 | 1.25 | 0.08 | 6.78 | — | 100% | |
| 4 | 6.97 | 3.35 | 25.8 | — | 100% | |
| 5 | 4.28 | 1.27 | 31.4 | — | 100% | |
| Pb | Total | 16.0 | 2.89 | 122 | — | 100% |
| 1 | 3.51 | 0.59 | 22.5 | — | 100% | |
| 2 | <2.50 | <1 | 29.0 | 1 | 76% | |
| 3 | 4.57 | 0.32 | 33.8 | — | 100% | |
| 4 | 9.24 | 2.06 | 36.4 | — | 100% | |
| 5 | 1.16 | 0.32 | 3.40 | — | 100% | |
| Zn | Total | 84.6 | 16.9 | 813 | — | 100% |
| 1 | 53.9 | 4.48 | 304 | — | 100% | |
| 2 | <13.8 | <11 | 78.6 | 11 | 66% | |
| 3 | 33.6 | 2.63 | 330 | — | 100% | |
| 4 | 55.9 | 13.0 | 214 | — | 100% | |
| 5 | 14.5 | 3.69 | 26.1 | — | 100% | |
Fraction 1 included adsorbed and exchangeable metals and carbonates; this fraction reflects metals that would potentially leach under acidifying conditions. The leaching was conducted with 1.0 M sodium acetate buffer at pH 5, following which, easily soluble and weakly adsorbed substances are released from material surfaces, including those that are bound to carbonate phases. The exchangeable fractions are released by ion exchange. Easily leached forms that are mobilized during precipitation represent a direct threat to the environment.43
Fraction 2 measures the potential for metals bound to labile organic forms to leach with 0.1 M Na-pyrophosphate at pH 9, which releases metals bound in humic and fulvic acids. This leaching step serves to simulate what metals will leach and could be bioavailable under oxidizing conditions.43
Fraction 3 included leaching of metals from amorphous Fe/Mn-oxides and indicates the proportion that can be released if the redox potential in the soil is significantly reduced, and anoxic conditions prevail in the filter material; conditions which might spur this to occur include elevated water levels or at high oxygen consumption due to high levels of organic material. Metals are leached with 0.25 M NH2OH·HCl in 0.10 M HCl at 60 °C, pH 1. To some extent, the release of metals in hydroxide form may be due to the acidic environment as opposed to the altered redox potential.43
Fraction 4 includes metals in crystalline Fe-oxides. Filter material is leached under greatly reduced conditions with 1.0 M NH2OH·HCl in 25% acetic acid at 90 °C; this reduces crystalline iron oxides such as ingot, hematite, and magnetite, and releases the metals bound to these phases. The pH of the leachate solution was approximately 1.43
Fraction 5 quantifies metals in stable organic forms and sulfides by leaching with KClO3 in 12 M HCl, 4 M HNO3 at 90 °C. Upon exposure to air and water, sulfides dissolve to form sulfuric acid and release metals.43
After every fractionation step, the leachate was analyzed and the extracted concentrations of metals were calculated as mg kg−1, DM. Specific surface area (SSA) was measured according to BS ISO 9277:201044 gas adsorption – Brunauer, Emmett and Teller (BET) method.
Statistical analysis
For data analyses and to illustrate the metal distribution and concentration in the bioretention filter material, boxplots and stacked bar charts were created in Minitab 18 and principal component analysis (PCA) was performed using Simca 17. As parts of the data were non-normally distributed, the nonparametric Kruskal–Wallis test was used to identify statistically significant differences between examined parameters (i.e., metal concentrations, depth, location, land usage, bioretention age). Censoring of data at the highest reporting limit was performed according to Helsel method45 for the boxplot of Cd in Fig. 2.Results and discussion
Concentrations
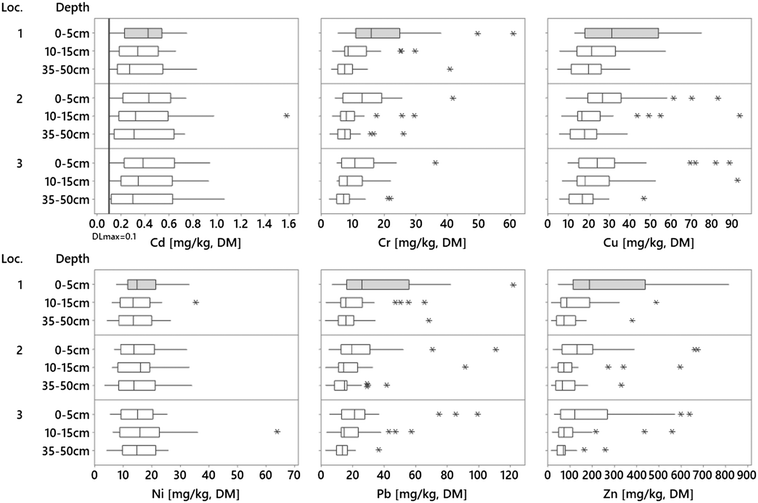
All analyzed metals (Cr, Cu, Ni, Pb, and Zn) were found in all 249 analyzed samples except for Cd, which was detected in 225 of the samples. The concentration ranges (Fig. 2) observed in the filter materials were lower for Cr, Cu, Ni, Pb, and Zn compared to other similar studies while comparable or slightly higher concentrations were found for Cd herein. Indeed, Al-Ameri et al.14 reported comparable concentrations for Cu (29 mg kg−1), higher concentrations for Pb (30 mg kg−1) and Zn (170 mg kg−1), and lower concentrations for Cd (0.1 mg kg−1) in a study of vegetated biofilters (9–16 years old) with a sandy loam or loamy sand filter medium. Rommel et al.27 reported concentrations in filter material from the top layer (0–5 cm) of bioretention cells (<3 years old) treating road runoff from a highly trafficked road in the Munich area, with comparable ranges for Pb (0.7 times higher) and slightly higher ranges for Ni (2.9 times higher), and 8.0–8.9 times higher for Cu, Cr, and Zn.Moreover, a comparison of the soil background concentrations in England (Cd = 0.29, Cr = 29.2 Cu = 17.3, Ni = 15.8, Pb = 37.4, Zn = 65.9 [mg kg−1])46 showed similar background levels as in the filter materials in the deeper filter layers in this study, indicating that the background metals concentrations in the filters are relatively low; thus, to assess the filter accumulation, it is important to determine the original levels of metals in the filter media. This also confirms the importance of the top layer as a metal's accumulator in bioretention since that is where the investigated metals are concentrated (Fig. 2 and Table 2, top layer medians; Cd = 0.43, Cr = 13.1 Cu = 26.8, Ni = 14.8, Pb = 23.3, Zn = 146 [mg kg−1] and top layer max; Cd = 0.94, Cr = 60.9, Cu = 88.5, Ni = 33, Pb = 122, Zn = 813 [mg kg−1]). One study of Cu, Pb, and Zn also showed a high surface accumulation in soil profiles (top 10 cm), while the lower layer concentrations were reported as low as background concentrations.15 In the current study, the metal concentrations in the deeper layers were similar to “possible” background concentrations,47 however, the actual original background concentrations in the filter materials are unknown.
Depth profiles
Metal concentrations tended to decrease with depth in the bioretention media (Fig. 2). The concentrations of Cr, Cu, Pb, and Zn were significantly higher (Kruskal–Wallis, p < 0.05) in the top layer and decreased with increased depth in the filter. In contrast to the other metals, Ni showed no such trend (p = 0.635). Additionally, median Cd concentrations decreased with depth; however, this trend was not statistically significant (p = 0.29), likely due to the large variation between sites. For all metals, the concentrations varied considerably between different facilities (4 to 15 times, Fig. 2), which is due to the relatively high variation of the data, which affected the statistical significance of the different concentrations between the layers. However, studying all 29 sites separately (ESI† Fig. S3.1), the highest site individual concentration was observed in the top layer at 17 sites for Cd, 25 sites for Cr, 23 sites for Cu, 15 sites for Ni, and 26 sites for both Pb and Zn. The same trend of decreasing metal concentration with depth in bioretention filter material has been shown previously.12,15,48 This can be explained by the fact that the accumulated metals are associated with particles, which are then removed in the upper soil layers by filtration.49 Additionally, Al-Ameri et al.14 showed that 70% of dissolved metals were trapped in the top 7 cm of the filter media, mainly explained by metal association to the substrate, which may be caused by fast adsorption of dissolved metals onto the filter material.16As for Cr, significantly higher concentrations of Cu were also observed in the top layer, which may be related to a higher content of organic matter in this layer (Fig. 5, LOI). This is similar to the background concentrations of Cu in soils, which is normally correlated with the texture and content of organic matter and explains why soils with high amounts of clay minerals and organic matter generally have higher Cu concentrations.50 One reason for the higher content of organic matter in the top layer could be the breakdown of the mulch layer often placed on top for the vegetation.
Length profiles
A trend of reduced concentrations with increased distance from the bioretention inlets was also observed for Cr, Cu, and Zn, mainly in the upper layers, although these trends were not statistically significant (Kruskal–Wallis, p > 0.05). A similar tendency was observed for Pb, but only in the top layer of the filter. Previous studies have reported variations in concentrations along the top layer of biofilters, which has been explained by hydrology, where the filter media treats more of the runoff closer to the inlets and thus has higher metal concentrations.26 Additionally, Al-Ameri et al., 2018 conducted a study of stormwater bioretention media and reported that 11 of 19 filters had decreased metal concentrations with increased distance from inlet; 5 of 19 were higher in the middle, while seven filters had lower concentrations close to the inlet. Al-Ameri et al.14 and Jones and Davis26 suggested that stormwater pathways are not always uniform across a filter meaning that sediments could be carried further into the filter during high flow rates. Furthermore, as was the case in this study, filter designs may vary, resulting in different flow paths into and along each filter. To mitigate this, we conducted an onsite visual investigation in each filter to determine the main inlet and the likely primary flow direction for the filter. However, this approach involves some uncertainty and may explain the insignificant correlation between concentration and distance from the inlet in this study. Some sites (e.g., 5, 10, 13, 14, 15, 16, 22, 23, and 26) had multiple inlets and not one clear main flow path; in these cases, the probable main inlet was defined. Also, local hydrology and filter design, including different shapes, and large variation in the ratio of the catchment to filter areas are factors that could affect the flow patterns in the filter such that the strategic sampling pattern with three sampling points from the inlet may not always describe the actual variation in surface metal concentrations.Fractionation
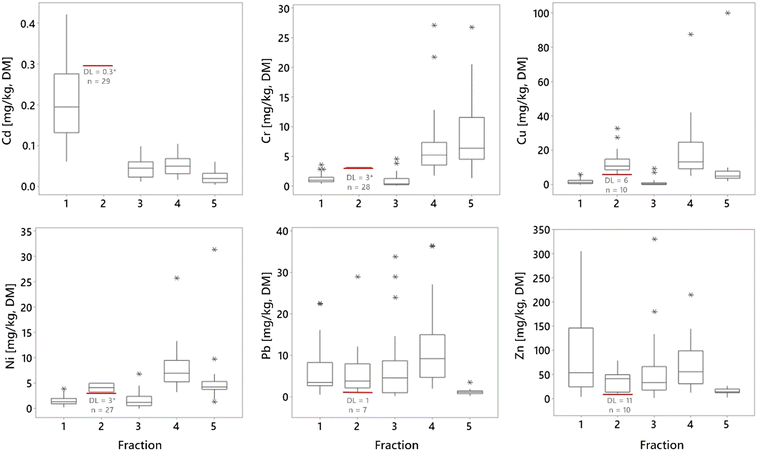
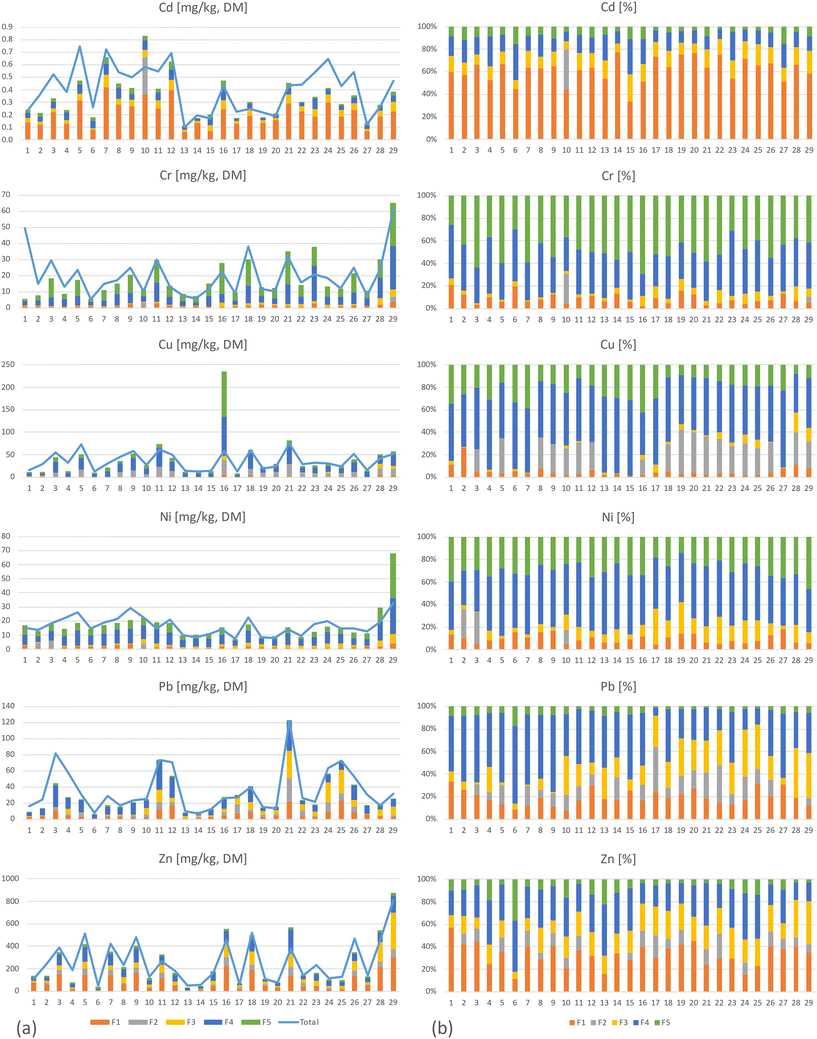
The fractionation of metals performed on samples at location 1, depth 1 (Fig. 1) showed that all metals appeared in all five fractions with the exception of fraction 2, where several of the 29 analyzed samples were below the DL (Cd<DL = 29, Cr<DL = 28, Cu<DL = 10, Ni<DL = 27, Pb<DL = 7 and Zn<DL = 10, Table 2 and Fig. 3). The reason for the non-detection in fraction 2 could be either a result of low concentrations in this fraction and/or due to the relatively high DLs for this fraction. The average distribution between the fractions in this study was as follows: fraction 4 = 36% > fraction 1 = 23% > fraction 5 = 20% > fraction 3 = 13% > fraction 2 = 8% (were fraction 1 to fraction 4 are potential available), although there were numerous samples below DL in fraction 2. For Cd, Pb, and Zn, most of the detected mass was in the first four fractions (Fig. 2, 4, and ESI† Table S5.1), while the contents of Cr, Cu, and Ni were greatest in fractions 4 and 5. Cr was the only metal with the highest content in fraction 5 (Fig. 3 and 4).Several studies have used sequential extraction methods to assess metal availability in stormwater. However, these studies have either focused on sediments,51,52 been performed as laboratory experiments,16,25 focused on other applications for stormwater treatment such as coarse surface particles,53 or used other sequential extraction methods,15,26,54 based on those of Ahnstrom and Parker.55 The current study was based on field sampling of a material as a mixture of filter material and accumulated sediments analyzed with a sequential extraction based on that described by Hall et al.32 and therefore, there are few other comparable studies. As the extent of extraction is method dependent,55 one must be aware of these differences when comparing and assessing results from studies using different extraction methods and rather focus on the main trends of mobility rather than the concentrations.
Cd was mainly present in fraction 1 but also in fractions 3, 4, and 5 (Fig. 3 and 4). The high presence in fraction 1 indicates that the sampled filter media have low affinity for Cd; the accumulated Cd is easily soluble and may be leached or mobilized from the filter material during normal rainfall or snowfall. Of all the included metals, Cd seemed to be the most mobile, with the highest proportion in fraction 1, as well as from fractions 1 to 4 (Cd∑F1−F4 = 93%). For Cd, although all 29 samples in fraction 2 were below the DL, this does not indicate that Cd was not present in this fraction. With low content in the higher fractions and higher content in the lower fractions, it is most likely that some Cd is present in fraction 2, albeit at concentrations below the DL (DLF2 = 0.3 mg kg−1). These results are in line with those of previous studies that indicate, despite the high removal of total Cd by bioretention,25,56 that metals primarily are adsorbed to exchangeable forms rather than permanent, and therefore pose a delayed threat to water resources rather than an immediate.16 Lange et al.57 also indicated that salt could have a negative impact on the metal treatment and increase the truly dissolved fractions which then could result in release of Cd from the filter media over time. Cr was found at the highest levels in fraction 5, followed by fraction 4, and to a lesser extent in fractions 1 and 3, while it was only found above the DL in one sample in fraction 2. Of the studied metals, Cr comprised the highest proportion in fraction 5 and the lowest sum in fractions 1 to 4 (i.e., potential available fractions; Cr∑F1−F4 = 52%). The high Cr content found in fraction 5 indicates that Cr in the filter material is associated with stable organic forms and may be mobile and bioavailable only under more extreme conditions. Fraction 5 is also associated with sulphides. Which, in contact with air, oxygen, and/or water dissolves to form sulfuric acid, which could result in release of metals; however, these conditions are unlikely to occur in bioretention.16
The high Cr content in fraction 4 indicates that Cr is also is potentially mobile under long-term anoxic conditions, which serve to reduce crystalline iron oxides, releasing the Cr bound to these phases. These conditions are unlikely to occur in the surface layers of a bioretention (i.e., where most metals are captured) but are possible in deeper layers in a saturated zone often implemented in designs to target nitrogen removal via denitrification. The behavior of Cr in soil is complex, controlled by various processes (e.g., biological and chemical redox, sorption, and precipitation) and external conditions (e.g., pH, soil aeration, presence of reductants and oxidants).58,59 However, as Cr(VI) is soluble in soil, while Cr(III) is more easily adsorbed,60,61 and with a median pH of 7.2 in the sampled filter material, Cr in fractions 4 and 5 is most likely Cr(III). Taken together, the high Cr content in fractions 4 and 5 (Cr∑F4+F5 = 89%) indicated that Cr was the most stable and least mobile of the studied metals.
Cu was found at the highest levels in fractions 4 and 2, followed by fraction 5, while only low levels were found in fractions 1 and 3. The distribution of Cu in soil is strongly influenced by Mn and Fe oxides (total median Mn = 344 mg kg−1 and Fe = 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 mg kg−1, ESI† Fig. S3.2) and Cu has a strong affinity to soil organic matter;58 indeed, the ability to form strong complexes with soluble organic matter62 is a known mechanism for effective Cu retention in soils. Fraction 2 in the sequential extraction is associated with soil organic matter and described the extraction of metals bound in labile organic forms, such as humic and fulvic acids, which may leach over time if the organic matter in the filter breaks down. These conditions may occur in bioretention, where organic matter (a component of filter media), the top mulch layers, or vegetation degrades over time.9 Therefore, Cu should be regarded as a potentially mobile metal in bioretention filter media and sediments. Fraction 4 indicated that Cu is related to Fe oxides, which also have strong influence on Cu mobility, meaning that Cu may be mobile also after a longer period under anoxic conditions.
300 mg kg−1, ESI† Fig. S3.2) and Cu has a strong affinity to soil organic matter;58 indeed, the ability to form strong complexes with soluble organic matter62 is a known mechanism for effective Cu retention in soils. Fraction 2 in the sequential extraction is associated with soil organic matter and described the extraction of metals bound in labile organic forms, such as humic and fulvic acids, which may leach over time if the organic matter in the filter breaks down. These conditions may occur in bioretention, where organic matter (a component of filter media), the top mulch layers, or vegetation degrades over time.9 Therefore, Cu should be regarded as a potentially mobile metal in bioretention filter media and sediments. Fraction 4 indicated that Cu is related to Fe oxides, which also have strong influence on Cu mobility, meaning that Cu may be mobile also after a longer period under anoxic conditions.
Ni was found at the highest levels in fraction 4, followed by fraction 5, with lower levels detected in fractions 1, 2, and 3. In fraction 2, only two of the 29 samples had concentrations above the DL (3.28 and 4.99 mg kg−1 with DLNi = 3 mg kg−1 for all samples but one with DLNi = 6 mg kg−1). Compared to the other metals in the study, after Cr, Ni had the highest content in fraction 5, and the lowest content in the sum of fractions 1 to 4 (Ni∑(F1−F4) = 68%). Ni content was spread across all 5 fractions, indicating that Ni appears to have mobility and bioavailability in the filter medias under study, but was stable compared to the other metals (Cd, Cu, Pb, and Zn) except for Cr.
Pb was found at the highest levels in fractions 4, 3, and 1, while less was found in fractions 2 and 5. Pb had the highest sum of content from fractions 1 to 4 (Pb∑(1−4) = 96%) and the lowest content in fraction 5. The distribution of the Pb content (Fig. 3 and 4) indicated that Pb has high potential mobility from bioretention. Many processes and factors affect Pb adsorption or release; these include humic matter, which plays an important role in adsorption in organic rich soil, and Fe oxides, which are more important in mineral soil, while many bioretention facilities contain both. The Pb in fraction 1 is associated with carbonates but also the hydrolysis process that easily adsorbs Pb in the bioretention filter; however, Pb seems to be less mobile than Cd and Zn given the lower content in fraction 1 (Fig. 3). This is consistent with the results of previous studies, including Sansalone and Buchberger,63 who also reported that Pb was primarily particulate bound in urban roadway stormwater run-off while mainly dissolved for Zn. Also Alloway,64 described the tendency of Pb to concentrate on smaller particle fractions in soil. The content of Pb in fraction 3, and particularly the high content in fraction 4, are most likely effects of Pb-adsorption to Fe- and Mn oxides.
The Zn distribution was relatively evenly spread between fractions 1 and 4, with the highest content found in fraction 4 and the lowest in fraction 5. After Pb, Zn had the highest sum of content in fractions 1 to 4 (Zn∑(1−4) = 94%) and the second highest content in fraction 1 (36%) after Cd. The Zn sorption process is mainly influenced by pH, clay mineral content (and clay SSA), cation-exchange capacity, soil organic matter, and soil type, where in alkaline soils Zn sorption easily occurs to carbonates but undergoes wetting or water logging with increased mobility.58,65 This could explain the even spread of Zn observed between fractions 1–4, as well as the high content in fraction 1. Moreover, the sorption of carbonates in the alkaline filter material may explain why Zn seems to have a high potential level of mobility in the filter media.
The metals distribution between fractions in this study is spread over all five fractions in varying degrees (Fig. 4). Fig. 4 also shows that Cd is mainly present in fraction 1, while Cr is mainly present in fractions 4 and 5, but also that there is a large variation between the different field sites. Despite this local variation, the main characteristic for the different metals is clear. If assessing mobility between the metals in the filter material based on their content in fraction 1 and then ranking them from high to low mobility, the rank will be Cd > Zn > Pb > Ni > Cr > Cu, meaning that Cd is the most mobile, while Cu is the least.63 conducted a study of stormwater and reported that Pb and Cr were primarily particulate bound, while Zn, Cd, and Cu were major concerns due to their propensity to be mainly dissolved, bioavailable, and highly mobile. Moreover, Jones and Davis,26 found metals as strongly bound to the filter media and to remain immobile since fractionation showed low content in the soluble–exchangeable fraction while the majority of metals was detected in the sorbed-carbonate, oxidizable, reducible and residual fractions. Additionally, Li and Davis,15 using the same fractionation method, reported low soluble–exchangeable fractions for Zn, Pb, and Cu, where Zn had the highest mobility followed by Cu and Pb. Thus, a comparison of results herein to previous research shows both similarities and differences. However, even in the same study, depending on the expected environmental impact on the filter material, one could also assess the mobility differences. If assessing the sum of fractions 1 to 2, or that of fractions 1 to 3, then the order of mobility would be Cd > Zn > Pb > Cu > Ni > Cr, while if assessing the sum of fractions 1 to 4, the rank would be Pb > Zn > Cd > Cu > Ni > Cr. Therefore, the approach for assessing the potential environmental risks according to the fractionation results is important, and in one way, all five metals are potentially mobile in the filter material depending on which environmental factors (lack of oxygen, changes in pH or decomposition of organic matter) to which the filter material is exposed. Therefore, a risk assessment and or filter sampling is recommended before removing filter material after many years of stormwater treatment.
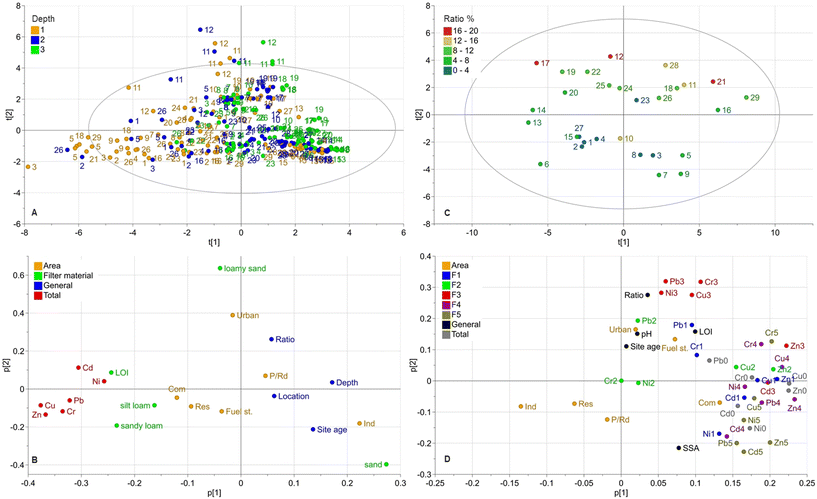
Principle components analysis
To further assess how the different biofilter materials, sizes and ages, depth and location of the sampling points in the filters, and catchment characteristics affect the accumulation, distribution, and fractionation of metals, a PCA with two models were performed.Model 1 was generated with data from the analysis of total metal concentrations from all nine samples (Fig. 1) and the variables land use type, filter material (classification according to the USDA soil textural triangle29), filter ratio (between catchment and filter surface areas), site age, depth, and location. Model 1 (total concentrations) had two components, with R2Xcum = 0.357 (cumulative X-variation modeled after all seven components) and Q2(cum) = 0.192 (cumulative overall cross-validated R2X). Most variations were explained in the first component (R2X(p1) = 0.249).
Model 2 consisted of data from the metal fractionation analysis and from the total concentrations in the corresponding sample (location 1 and depth 1 at each filter, Fig. 1), together with variables for catchment area land use, filter ratio, site age, pH, and LOI. Model 2 (fractionation) had three components, with R2Xcum = 0.622 (cumulative X-variation modeled after all seven components) and Q2(cum) = 0.4 (cumulative overall cross-validated R2X). However, most variations were explained in the first two components (R2X(p1) = 0.364, R2X(p2) = 0.154). A summary of the results of both PCA models is shown in the score and loading plots in Fig. 5.
In model 1, the score plot for total concentrations (Fig. 5A) showed a trend with observations clustered to the lower right of origin and then stretched out in two major directions, to the left negative component 1 (p[1]) and to the top in positive component 2 (p[2]). The strongest trend towards the left in component 1 contains observations mainly from the top layer (depth 1) while observations clustered to the lower right mainly are from the deeper layers (depth 2 and 3). As most metals are found in the top layer (depth 1), this correlates with the metal concentrations placed towards the left of component 1 in the loading plot. Further, the loading plot indicates positive correlations between these total metal concentrations, organic matter (LOI) and finer graded particles (silt loam and sandy loam). In contrast, the coarser filter materials are negatively correlated to metal concentrations. Similar applies to depth, site age, location and (on the second component) also to area ratio.
Previous research has shown that the fine graded fractions and organic material support metal adsorption.66,67 The correlation between high metal concentrations in the top layer, LOI, and finer graded soils is likely due to mulch present in that layer (by design), finer soils often used in the top layer, and accumulation of fine stormwater sediment in that layer (which affects the particle size distribution in this study towards the finer fractions). Thus, these factors interact with each other leading to higher metal concentrations in this layer.
However, filter materials in biofilter do also have to fulfil other requirements. While fine graded filter materials containing organic matter promote metal adsorption, on the other hand they do not favor infiltration and/or increase the risk for clogging,11 (i.e. increasing the amount of high flow bypass of non-treated water). Organic matter which increases the risk of especially phosphorus leaching.13 Further, the degradation of organic matter over time could lead to leaching of metals, e.g., Cu–organic matter-complexes.68 Thus, due to these competing requirements, the results of this study do not imply to use as fine and organic rich materials as possible, but to choose a compromise between these different requirements is necessary, as in detail discussed by.11
Another observation in model 1 (loading plot, Fig. 5) was that metal concentrations were strongly negatively correlated with depth, meaning that the highest concentrations are located near the surface with lower concentrations at deeper levels in the filter, as also confirmed in the boxplots in Fig. 2. Distance from inlet shows a negative correlation to metal concentrations indicating higher concentrations close to inlet. However, this trend is less pronounced than the depth related trend. Biofilter age seems to have some significance according to the loading plots in model 1 (less in model 2), where the total concentration is negatively correlated with site age. One would rather assume the opposite as pollutants accumulated in bioretention over time. However, since most facilities were around 10 years old (Table 1), there was relatively little variation of age and newly built facilities were completely lacking (as the aim of this study was to evaluate aged facilities). Model 1 also shows that LOI and SSA are positively correlated with the total concentrations. The loading plots in Fig. 5 indicate some correlation of catchment area land use with metal concentrations. However, given the relatively large variation of metal concentrations and filter characteristics, few investigated facilities per land use type and an only weak correlation, no general conclusions can be drawn.
In the loading plot of model 2 (Fig. 5D) the content from all fractions (fraction 1–5) and total concentrations are located to the right of the origin, in origin (Cr in fraction 2 with only 2 values above the DL or not included as Cd in fraction 2 with no values above the DL). This pattern indicates in the first component (p[1]) that concentrations are positively correlated with each other and to commercial land use, fueling stations and down town urban areas, as well as to LOI, SSA, pH, filter area ratio and site age. In contrast the Industrial, Residential and Parking/Roads land uses were negatively correlated with all concentrations and fractions. Fractions 4 and 5 seem to be most strongly correlated with the total concentrations for all metals. Fraction 1 also showed a strong correlation with total concentrations especially for Zn1 and Cu1 but less strong for Cr1 and Pb1. Fraction 2 showed a weaker correlation, especially for Cr and Ni, although this could partly be explained by the many non-detects in fraction 2, resulting in a weaker model and correlations. However, for Cu and Zn there is a stronger observed correlation between fraction 2 and total concentrations. Fraction 3 is positively correlated with the total concentrations for Cu and Zn, while Cr, Cu, Ni, and Pb are grouped and correlated to filter area ratio in the second component (p[2]); however, the second component is not as well described by the model as p[1]. The model also shows that for Zn, all fractions (1–5) were positively correlated with the total concentrations, which was also true for Cu and Cd, with the exception of fraction 2. In the score plot (Fig. 5C), there is a tendency to group in the second component t[2] according to filter area ratio where the observations with higher area ratios are in the upper part of the plot, while those with lower area ratios are in the lower part, which indicated that the area ratio has some impact in p[2].
Environmental implication
As an example of the practical implications of this work, the metal concentrations measured herein were compared to the Swedish national guidance limits for the classification of soil, “soil for sensitive land use” (abbreviated KM) and “soil for less sensitive land use” (abbreviated MKM), published by the Swedish Environmental protection agency69 and the UK CL:AIRE (UK charity committed for sustainable land reuse) “soil guideline values” (SGVs) for Cd70 and Ni.71 Soil contaminated above the Swedish EPA class KM and MKM means that if material is removed, special permits are required for transport and disposal. All concentrations were below the UK CL:AIRE SGVs and all metals concentration (Cd, Cr, Cu, Ni and Pb) except Zn were below the Swedish soil guidelines MKM (as illustrated in boxplot in ESI† Fig. S4.1). Cr was the only metal below the KM, Ni had one outlier above the KM, while Cd, Cu, Pb, and Zn were more frequently detected above the KM.Assessing metal mobility and potential environmental risks from old bioretention media is likely to depend on the choice of analysis methods55 and method of data interpretation. If assessing the risk according to detected concentrations above the Swedish SGVs69 (ESI† Fig. S4.1 and Table S5.1), the order would be Zn > Pb > Cd >Cu > Ni > Cr. However, if assessing the environmental risk as mobility according to the detected metal content in fraction 1, the order would be Cd > Zn > Pb > Ni > Cr > Cu; as the sum of fractions 1 to 2 (and fractions 1 to 3), the order would be Cd > Zn > Pb > Cu > Ni > Cr; and according to the sum of fractions 1 to 4 the rank would be Pb > Zn > Cd > Cu > Ni > Cr. Irrespective of the approach used to assess the risks with accumulated metals in the bioretention filter material, the local environmental sensibility, legislation, and metal mobility should be considered if the filter material and/or sediments are being removed or replaced from a facility.
As the top layer of the filter media is likely to retain the most pollutants and sediments, as a long-term maintenance measure of bioretention technology, the top layer of the filter material could be regularly replaced to restore infiltration capacity of filter materials, reduce surface concentrations of metals, or reduce risk of metal pollutants. However, despite a clear general trend, the large variation in concentration and pollutant load between the different facilities a generally valid recommendation for a specific maintenance frequency cannot be give but is site specific. In a previous laboratory study, Hatt et al. (2011) estimated that during 12–15 years of operation, the levels of Cd, Cu, and Zn in the filter material would most likely exceed the guidelines for human and ecological health20 and therefore may be classified as contaminated soil requiring special disposal. Moreover, Al-Ameri et al.14 suggested clogging, rather than high concentrations to limit bioretention function if regularly maintained, given that replacing the top 10 cm of the filter will also remove most accumulated metals. Additionally Hatt et al.19 recommended a 2–3 year interval for replacement of top layer to reduce clogging, while Kluge et al.22 recommended replacement after 20–25 years as a maintenance routine considering leaching potential. Davis et al.,17 also estimated that according to US EPA standards18 and considering the highest concentrations in the top layer, the accumulation limits could be reached after 20, 77, 16, and 16 years for Cd, Cu, Pb, and Zn, respectively. Thus, filter material removed from older bioretention facilities should be managed and prioritized given that it may have to be treated as a hazardous waste.
Conclusions
All the study metals (Cd, Cr, Cu, Ni, Pb, and Zn) were found in all bioretention filter media samples, except for Cd, which was found in 90% of the samples. The highest metal concentrations were generally found in the top layer (top 5 cm) of the filter material. A comparison of metal concentrations in the filter material using the Swedish national guidance limits for classification of soil showed that Zn is the most significant pollutant in the bioretention filter media, while Cd, Cu, Ni, and Pb were detected at concentration levels of restricted use, suggesting special disposal techniques would be necessary for filter media.The metal fractionation shows that all study metals (Cd, Cr, Cu, Ni, Pb, Zn) in the top layer of the filter material were present at large extents, with a potential risk of leaching over time. The risk of leaching according to mobility in fraction 1 was highest for Cd, Zn, and Pb, all of which are potentially mobile during precipitation, while Cr followed by Cu and Ni were most stable.
The studied metals are also at a potential risk of leaching from filter material or sediments if removed from the bioretention sites. In that case, the conditions at the new location are of great importance for the risk of metal leaching. For instance, if they are deposited under lack of oxygen the risk of leaching over time would increase for Cr, Cu, and Ni; this may have practical implications for bioretention operators given that removing material from the top layer of media, e.g., in order to reduce clogging, not only carries a risk when handling the material on site but also if the material is placed in a landfill.
The results of PCA indicated a strong correlation between high metal concentrations and low ratio between the filter area and catchment area (filter area ratio). Additionally, the various land uses show correlation with concentrations, which may be useful for predicting the degree of metal pollution at bioretention sites given that a lower filter area ratio could indicate an increased risk of a highly polluted bioretention site. However, the catchment area land use, operation time, and quality of maintenance are likely to be the most important factors for these predictions. In order to maintain function in biofilters over time and reduce the risk of leakage of metals, regular maintenance at site specific intervals, including replacement of the top layer, can be recommended.
Author contributions
Robert Furén: conceptualization, methodology, formal analysis, investigation, data curation, writing – original draft, funding acquisition. Helene Österlund: methodology, formal analysis, writing – review & editing. Ryan J. Winston: conceptualization, methodology, investigation, writing – review & editing. R. Andrew Tirpak: investigation, writing – review & editing. Jay D. Dorsey: investigation. Joseph Smith: investigation, formal analysis. Maria Viklander: conceptualization, methodology, writing – review & editing supervision, project administration, funding acquisition. Godecke-Tobias Blecken: conceptualization, methodology, formal analysis, investigation, writing – review & editing, supervision, project administration, funding acquisition.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The financial support for this work was provided by VINNOVA (Swedish Governmental Agency for Innovation Systems, Grant No. 2016 – 05176, DRIZZLE Centre for Stormwater Management) and SBUF (The Development Fund of the Swedish Construction Industry, Grant No. 13623).We thank Peter Rosander and Kerstin Nordqvist at LTU for their help with the laboratory work and discussion on the data.
References
- A. Müller, H. Österlund, J. Marsalek and M. Viklander, The pollution conveyed by urban runoff: A review of sources, Sci. Total Environ., 2020, 709, 136125 CrossRef PubMed.
- P. Göbel, C. Dierkes and W. G. Coldewey, Storm water runoff concentration matrix for urban areas, J. Contam. Hydrol., 2007, 91(1–2), 26–42 CrossRef PubMed.
- US EPA, US-EPA, 1983, Final Report of the Nationwide Urban Runoff Program, U.S. Environmental Protection Agency, Water Planning Division, Washington DC, USA [Internet], 1983, Available from: https://nepis.epa.gov/Exe/ZyNET.exe/9100JH5T.txt?ZyActionD=ZyDocument&Client=EPA&Index=1981%20Thru%201985&Docs=&Query=&Time=&EndTime=&SearchMethod=1&TocRestrict=n&Toc=&TocEntry=&QField=&QFieldYear=&QFieldMonth=&QFieldDay=&UseQField=&IntQFieldOp=0&ExtQFieldOp=0&XmlQuery=&File=D%3A%5CZYFILES%5CINDEX%20DATA%5C81THRU85%5CTXT%5C00000018%5C9100JH5T.txt&User=ANONYMOUS&Password=anonymous&SortMethod=h%7C-&MaximumDocuments=1&FuzzyDegree=0&ImageQuality=r75g8/r75g8/x150y150g16/i425&Display=hpfr&DefSeekPage=x&SearchBack=ZyActionL&Back=ZyActionS&BackDesc=Results%20page&MaximumPages=1&ZyEntry=1# Search PubMed.
- E. Eriksson, A. Baun, L. Scholes, A. Ledin, S. Ahlman and M. Revitt, et al. Selected stormwater priority pollutants — a European perspective, Sci. Total Environ., 2007, 383(1–3), 41–51 CrossRef CAS PubMed.
- A. E. Barbosa, J. N. Fernandes and L. M. David, Key issues for sustainable urban stormwater management, Water Res., 2012, 46(20), 6787–6798 CrossRef CAS PubMed.
- J. P. Johnson and W. F. Hunt, Field Assessment of the Hydrologic Mitigation Performance of Three Aging Bioretention Cells, J. Sustain. Water Built Environ., 2020, 6(4), 04020017 CrossRef.
- H. Kratky, Z. Li, Y. Chen, C. Wang, X. Li and T. Yu, A critical literature review of bioretention research for stormwater management in cold climate and future research recommendations, Front. Environ. Sci. Eng., 2017, 11(4), 16 CrossRef.
- S. J. McGrane, Impacts of urbanisation on hydrological and water quality dynamics, and urban water management: a review, Hydrol. Sci. J., 2016, 61(13), 2295–2311 CrossRef.
- K. Lange, M. Viklander and G. T. Blecken, Effects of plant species and traits on metal treatment and phytoextraction in stormwater bioretention, J. Environ. Manage., 2020, 276, 111282 CrossRef CAS PubMed.
- G. H. LeFevre, K. H. Paus, P. Natarajan, J. S. Gulliver, P. J. Novak and R. M. Hozalski, Review of Dissolved Pollutants in Urban Storm Water and Their Removal and Fate in Bioretention Cells, J. Environ. Eng., 2015, 141(1), 04014050 CrossRef CAS.
- R. A. Tirpak, A. N. Afrooz, R. J. Winston, R. Valenca, K. Schiff and S. K. Mohanty, Conventional and amended bioretention soil media for targeted pollutant treatment: A critical review to guide the state of the practice, Water Res., 2021, 189, 116648 CrossRef CAS PubMed.
- G. T. Blecken, Y. Zinger, A. Deletić, T. D. Fletcher and M. Viklander, Influence of intermittent wetting and drying conditions on heavy metal removal by stormwater biofilters, Water Res., 2009, 43(18), 4590–4598 CrossRef CAS PubMed.
- A. P. Davis, W. F. Hunt, R. G. Traver and M. Clar, Bioretention Technology: Overview of Current Practice and Future Needs, J. Environ. Eng., 2009, 135(3), 109–117 CrossRef CAS.
- M. Al-Ameri, B. Hatt, S. Le Coustumer, T. Fletcher, E. Payne and A. Deletic, Accumulation of heavy metals in stormwater bioretention media: A field study of temporal and spatial variation, J. Hydrol., 2018, 567, 721–731 CrossRef CAS.
- H. Li and A. P. Davis, Heavy Metal Capture and Accumulation in Bioretention Media, Environ. Sci. Technol., 2008, 42(17), 6776 CrossRef CAS.
- L. C. Søberg, R. Winston, M. Viklander and G. T. Blecken, Dissolved metal adsorption capacities and fractionation in filter materials for use in stormwater bioretention facilities, Water Res. X, 2019, 4, 100032 CrossRef PubMed.
- A. P. Davis, M. Shokouhian, H. Sharma, C. Minami and D. Winogradoff, Water Quality Improvement through Bioretention: Lead, Copper, and Zinc Removal, Water Environ. Res., 2003, 75(1), 73–82 CrossRef CAS PubMed.
- U.S. EPA, Standards for the Use or Disposal of Sewage Sludge (40 CFR Part 503). Fed. Regist., 1993, vol. 58, No. 32 [Internet], Available from: https://www.epa.gov/biosolids/biosolids-laws-and-regulations#standards Search PubMed.
- B. E. Hatt, A. Steinel, A. Deletic and T. D. Fletcher, Retention of heavy metals by stormwater filtration systems: breakthrough analysis, Water Sci. Technol., 2011, 64(9), 1913–1919 CrossRef CAS PubMed.
- NEPC, NEPC 1999 Guideline of the Investigation Levels for Soil and Groundwater, Schedule B(1), National Environment Protection Measure [Internet], 1999, Available from: http://www.nepc.gov.au/system/files/resources/93ae0e77-e697-e494-656f-afaaf9fb4277/files/schedule-b1-guideline-investigation-levels-soil-and-groundwater-sep10.pdf Search PubMed.
- EPA Victoria, Epa Classification of Wastes (May ed.), Victorian Environmental Protection Agency, Victoria, Australia [Internet], 2007, Available from: https://scholar-google-com.proxy.lib.ltu.se/scholar_lookup?title=Classification%20of%20Wastes&publication_year=2007&author=Epa Search PubMed.
- B. Kluge, A. Markert, M. Facklam, H. Sommer, M. Kaiser and M. Pallasch, et al. Metal accumulation and hydraulic performance of bioretention systems after long-term operation, J. Soils Sediments, 2018, 18(2), 431–441 CrossRef CAS.
- D. M. Costello, E. W. Hartung, J. T. Stoll and A. J. Jefferson, Bioretention cell age and construction style influence stormwater pollutant dynamics, Sci. Total Environ., 2020, 712, 135597 CrossRef CAS PubMed.
- J. P. Johnson and W. F. Hunt, Evaluating the spatial distribution of pollutants and associated maintenance requirements in an 11 year-old bioretention cell in urban Charlotte, NC, J. Environ. Manage., 2016, 184, 363–370 CrossRef CAS PubMed.
- J. Wang, P. Zhang, L. Yang and T. Huang, Cadmium removal from urban stormwater runoff via bioretention technology and effluent risk assessment for discharge to surface water, J. Contam. Hydrol., 2016, 185–186, 42–50 CrossRef CAS PubMed.
- P. S. Jones and A. P. Davis, Spatial Accumulation and Strength of Affiliation of Heavy Metals in Bioretention Media, J. Environ. Eng., 2013, 139(4), 479–487 CrossRef CAS.
- S. H. Rommel, P. Stinshoff and B. Helmreich, Sequential extraction of heavy metals from sorptive filter media and sediments trapped in stormwater quality improvement devices for road runoff, Sci. Total Environ., 2021, 782, 146875 CrossRef CAS.
- D. Tedoldi, G. Chebbo, D. Pierlot, Y. Kovacs and M. C. Gromaire, Assessment of metal and PAH profiles in SUDS soil based on an improved experimental procedure, J. Environ. Manage., 2017, 202, 151–166 CrossRef CAS PubMed.
- C. Ditzler, K. Scheffe and H. C. Monger, Soil Science Division Staff, 2017, Soil survey manual, USDA Handbook 18, ed. C. Ditzler, K. Scheffe and H. C. Monger, Government Printing Office, Washington, D.C. [Internet], 2017 (USDA Handbook), Available from: https://www.nrcs.usda.gov/resources/guides-and-instructions/soil-survey-manual Search PubMed.
- SS, Determination of dry matter and ignition residue in water, sludge and sediment SWEDISH STANDARD, SS 28113 [Internet], 2004, Available from: https://www.sis.se/produkter/miljo-och-halsoskydd-sakerhet/vattenkvalitet/avloppsvatten/ss28113/ Search PubMed.
- SS, Determination of dry matter and ignition residue in water, sludge and sediment SWEDISH STANDARD, SS 28113-1 [Internet], 2004, Available from: https://www.sis.se/produkter/miljo-och-halsoskydd-sakerhet/vattenkvalitet/avloppsvatten/ss28113/ Search PubMed.
- G. E. M. Hall, J. E. Vaive, R. Beer and M. Hoashi, Selective leaches revisited, with emphasis on the amorphous Fe oxyhydroxide phase extraction, J. Geochem. Explor., 1996, 56(1), 59–78 CrossRef CAS.
- G. E. M. Hall, J. E. Vaive and A. I. MacLaurin, Analytical aspects of the application of sodium pyrophosphate reagent in the specific extraction of the labile organic component of humus and soils, J. Geochem. Explor., 1996, 56(1), 23–36 CrossRef CAS.
- SS-EN ISO, Water quality – Application of inductively coupled plasma mass spectrometry (ICP-MS) – Part 2: Determination of selected elements including uranium isotopes (ISO 17294-2:2016) [Internet], 2016, Available from: https://www.sis.se/produkter/miljo-och-halsoskydd-sakerhet/vattenkvalitet/undersokning-av-vatten-efter-kemiska-substanser/sseniso1729422016/ Search PubMed.
- SS-EN ISO, Water quality – Application of inductively coupled plasma mass spectrometry (ICP-MS) – Part 1: General guidelines (ISO 17294-1:2004) SS-EN ISO 17294-1:2006 [Internet], SIS swedish institute for standards, 2006, Available from: https://www.sis.se/en/produkter/environment-health-protection-safety/water-quality/examination-of-water-for-chemical-substances/sseniso1729412006/ Search PubMed.
- U.S. EPA, Method 200.8: Determination of Trace Elements in Waters and Wastes by Inductively Coupled Plasma-Mass Spectrometry Revision 5.4., Cincinnati, OH [Internet], 1994, Available from: https://www.epa.gov/esam/epa-method-2008-determination-trace-elements-waters-and-wastes-inductively-coupled-plasma-mass Search PubMed.
- SS-EN ISO, Water quality – Determination of selected elements by inductively coupled plasma optical emission spectrometry (ICP-OES) (ISO 11885:2007) [Internet], 2009, Available from: https://www.sis.se/en/produkter/environment-health-protection-safety/water-quality/general/sseniso118852009/ Search PubMed.
- U.S. EPA, Method 200.7: Determination of Metals and Trace Elements in Water and Wastes by Inductively Coupled Plasma-Atomic Emission Spectrometry Revision 4.4., Cincinnati, OH [Internet], 1994, Available from: https://www.epa.gov/esam/method-2007-determination-metals-and-trace-elements-water-and-wastes-inductively-coupled-plasma Search PubMed.
- SS-EN ISO, Water quality – Determination of pH [Internet], SIS swedish institute for standards, 2012, Available from: https://www.sis.se/api/document/preview/88264/ Search PubMed.
- CSN EN, ČSN EN 12879 (758005) Sludge characterization – Determination of loss by annealing (Sludge characterization – Determination of loss by annealing) [Internet], 2014, Available from: https://www.technicke-normy-csn.cz/csn-en-12879-758005-226751.html Search PubMed.
- CSN, ČSN 72 0103 (720103) Základní postup rozboru silikátů – Stanovení ztráty žíháním (Basic procedure for the analysis of silicates – Determination of loss by annealing) [Internet], 2009, Available from: https://www.technicke-normy-csn.cz/csn-72-0103-720103-218183.html Search PubMed.
- CSN, ČSN 46 5735 (465735) Průmyslové komposty (CSN 46 5735 (465735) Industrial composts) [Internet], 1991, Available from: https://www.technicke-normy-csn.cz/csn-46-5735-465735-206692.html Search PubMed.
- ALS, Sequential extraction information sheet [Internet], ALS Global, 2018 [cited 2021 Nov 8], Available from: https://www.alsglobal.se/miljoanalys/laktester/sekventiella-lakningar Search PubMed.
- BS ISO, BS ISO 9277: 2010 Determination of the specific surface area of solids by gas adsorption. BET method [Internet], 2010, Available from: https://www.en-standard.eu/bs-iso-9277-2010-determination-of-the-specific-surface-area-of-solids-by-gas-adsorption-bet-method/ Search PubMed.
- D. R. Helsel, Statistics for censored environmental data using Minitab and R, Wiley, Hoboken, N.J, 2nd edn, 2012, p. 324 (Wiley series in statistics in practice) Search PubMed.
- Heavy Metals in Soils Trace Metals and Metalloids in Soils and their Bioavailability, Table 2.8 p. 38. [Internet], ed. B. J. Alloway, Department of Soil Science University of Reading United Kingdom: Springer, Dordrecht, 3rd edn, 2013, XVIII, p. 614 (Environmental Pollution), Available from: https://doi.org/10.1007/978-94-007-4470-7 Search PubMed.
- Heavy Metals in Soils Trace Metals and Metalloids in Soils and their Bioavailability, Table 17.1 p. 467. with reference (Holmgren et al, 1993) [Internet], ed. B. J. Alloway, Department of Soil Science University of Reading United Kingdom: Springer, Dordrecht, 3rd edn, 2013, XVIII, p. 614 (Environmental Pollution), Available from: https://doi.org/10.1007/978-94-007-4470-7 Search PubMed.
- T. M. Muthanna, M. Viklander, G. Blecken and S. T. Thorolfsson, Snowmelt pollutant removal in bioretention areas, Water Res., 2007, 41(18), 4061–4072 CrossRef CAS PubMed.
- D. Tedoldi, G. Chebbo, D. Pierlot, Y. Kovacs and M. C. Gromaire, Impact of runoff infiltration on contaminant accumulation and transport in the soil/filter media of Sustainable Urban Drainage Systems: A literature review, Sci. Total Environ., 2016, 569–570, 904–926 CrossRef CAS PubMed.
- Heavy Metals in Soils Trace Metals and Metalloids in Soils and their Bioavailability, Table 13.2 p. 371. [Internet], ed. B. J. Alloway, Department of Soil Science University of Reading United Kingdom: Springer, Dordrecht, 3rd edn, 2013, XVIII, p. 614 (Environmental Pollution), Available from: https://doi.org/10.1007/978-94-007-4470-7 Search PubMed.
- S. Gavrić, G. Leonhardt, H. Österlund, J. Marsalek and M. Viklander, Metal enrichment of soils in three urban drainage grass swales used for seasonal snow storage, Sci. Total Environ., 2021, 760, 144136 CrossRef PubMed.
- K. Karlsson, G. T. Blecken, B. Öhlander and M. Viklander, Environmental Risk Assessment of Sediments Deposited in Stormwater Treatment Facilities: Trace Metal Fractionation and Its Implication for Sediment Management, J. Environ. Eng., 2016, 142(11), 04016057 CrossRef.
- M. Borris, H. Österlund, J. Marsalek and M. Viklander, Contribution of coarse particles from road surfaces to dissolved and particle-bound heavy metal loads in runoff: A laboratory leaching study with synthetic stormwater, Sci. Total Environ., 2016, 573, 212–221 CrossRef CAS PubMed.
- G. Rauret, J. F. López-Sánchez, A. Sahuquillo, R. Rubio, C. Davidson and A. Ure, et al. Improvement of the BCR three step sequential extraction procedure prior to the certification of new sediment and soil reference materials, J. Environ. Monit., 1999, 1(1), 57–61 RSC.
- Z. S. Ahnstrom and D. R. Parker, Development and Assessment of a Sequential Extraction Procedure for the Fractionation of Soil Cadmium, Soil Sci. Soc. Am. J., 1999, 63(6), 1650–1658 CrossRef CAS.
- G. T. Blecken, J. Marsalek and M. Viklander, Laboratory Study of Stormwater Biofiltration in Low Temperatures: Total and Dissolved Metal Removals and Fates, Water, Air, Soil Pollut., 2011, 219(1–4), 303–317 CrossRef CAS.
- K. Lange, H. Österlund, M. Viklander and G. T. Blecken, Metal speciation in stormwater bioretention: Removal of particulate, colloidal and truly dissolved metals, Sci. Total Environ., 2020, 724, 138121 CrossRef CAS PubMed.
- H. B. Bradl, Adsorption of heavy metal ions on soils and soils constituents, J. Colloid Interface Sci., 2004, 277(1), 1–18 CrossRef CAS PubMed.
- S. E. Fendorf, Surface reactions of chromium in soils and waters, Geoderma, 1995, 67(1–2), 55–71 CrossRef CAS.
- K. Cederkvist, S. T. Ingvertsen, M. B. Jensen and P. E. Holm, Behaviour of chromium(VI) in stormwater soil infiltration systems, Appl. Geochem., 2013, 35, 44–50 CrossRef CAS.
- D. E. Kimbrough, Y. Cohen, A. M. Winer, L. Creelman and C. Mabuni, A Critical Assessment of Chromium in the Environment, Crit. Rev. Environ. Sci. Technol., 1999, 29(1), 1–46 CrossRef CAS.
- S. P. McGrath, J. R. Sanders and M. H. Shalaby, The effects of soil organic matter levels on soil solution concentrations and extractabilities of manganese, zinc and copper, Geoderma, 1988, 42(2), 177–188 CrossRef CAS.
- J. J. Sansalone and S. G. Buchberger, Partitioning and First Flush of Metals in Urban Roadway Storm Water, J. Environ. Eng., 1997, 123(2), 134–143 CrossRef CAS.
- Heavy Metals in Soils Trace Metals and Metalloids in Soils and their Bioavailability, p. 397. [Internet], ed. B. J. Alloway, Department of Soil Science University of Reading United Kingdom: Springer, Dordrecht, 3rd edn, 2013, XVIII, p. 614 (Environmental Pollution), Available from: https://doi.org/10.1007/978-94-007-4470-7 Search PubMed.
- Heavy Metals in Soils Trace Metals and Metalloids in Soils and their Bioavailability, p. 478. with reference (Olivie-Lauquet et al., 2001) [Internet], ed. B. J. Alloway, Department of Soil ScienceUniversity of ReadingReadingUnited Kingdom: Springer, Dordrecht, 3rd edn, 2013, XVIII, p. 614 (Environmental Pollution), Available from: https://doi.org/10.1007/978-94-007-4470-7 Search PubMed.
- Heavy Metals in Soils Trace Metals and Metalloids in Soils and their Bioavailability [Internet], ed. B. J. Alloway, Department of Soil Science University of Reading United Kingdom: Springer, Dordrecht, 3rd edn, 2013, XVIII, p. 614 (Environmental Pollution), Available from: https://doi.org/10.1007/978-94-007-4470-7 Search PubMed.
- H. Li, X. Qian and Q. Wang, Heavy Metals in Atmospheric Particulate Matter: A Comprehensive Understanding Is Needed for Monitoring and Risk Mitigation, Environ. Sci. Technol., 2013, 47(23), 13210–13211 CrossRef CAS PubMed.
- G. T. Blecken, Y. Zinger, A. Deletić, T. D. Fletcher and M. Viklander, Impact of a submerged zone and a carbon source on heavy metal removal in stormwater biofilters, Ecol. Eng., 2009, 35(5), 769–778 CrossRef.
- Swedish EPA, Riktvärden för förorenad mark Modellbeskrivning och vägledning [Internet], Sweden, 2009, Report No.: 5976, Available from: https://www.naturvardsverket.se/978-91-620-5976-7 Search PubMed.
- I. Martin, H. Morgan and E. Waterfall, Soil Guideline Values for cadmium in soil [Internet], CL:AIRE, 2009p. 11, Report No.: SC050021, Available from: https://webarchive.nationalarchives.gov.uk/ukgwa/20140328084622/http://www.environment-agency.gov.uk/static/documents/Research/SCHO0709BQRO-e-e.pdf Search PubMed.
- I. Martin, H. Morgan, C. Jones, E. Waterfall and J. Jeffries, Soil Guideline Values for nickel in soil [Internet], CL:AIRE, 2009p. 11, Report No.: SC050021, Available from: https://webarchive.nationalarchives.gov.uk/ukgwa/20140328084622/http://www.environment-agency.gov.uk/static/documents/Research/SCHO0709BQRO-e-e.pdf Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ew00823h |
| This journal is © The Royal Society of Chemistry 2023 |