Open Access Article
Open Access ArticleTransition metal chalcogenides for next-generation energy storage
Soubantika
Palchoudhury
 *a,
Karthik
Ramasamy
b,
Jinchen
Han
*a,
Karthik
Ramasamy
b,
Jinchen
Han
 a,
Peng
Chen
a and
Arunava
Gupta
a,
Peng
Chen
a and
Arunava
Gupta
 *c
*c
aChemical and Materials Engineering, University of Dayton, OH, USA. E-mail: spalchoudhury1@udayton.edu
bUbiQD Inc., Los Alamos, NM, USA
cDepartment of Chemistry and Biochemistry, The University of Alabama, AL, USA. E-mail: arunava.gupta@ua.edu
First published on 24th February 2023
Abstract
Transition-metal chalcogenide nanostructures provide a unique material platform to engineer next-generation energy storage devices such as lithium-ion, sodium-ion, and potassium-ion batteries and flexible supercapacitors. The transition-metal chalcogenide nanocrystals and thin films have enhanced electroactive sites for redox reactions and hierarchical flexibility of structure and electronic properties in the multinary compositions. They also consist of more earth-abundant elements. These properties make them attractive and more viable new electrode materials for energy storage devices compared to the traditional materials. This review highlights the recent advances in chalcogenide-based electrodes for batteries and flexible supercapacitors. The viability and structure–property relation of these materials are explored. The use of various chalcogenide nanocrystals supported on carbonaceous substrates, two-dimensional transition metal chalcogenides, and novel MXene-based chalcogenide heterostructures as electrode materials to improve the electrochemical performance of lithium-ion batteries is discussed. The sodium-ion and potassium-ion batteries offer a more viable alternative to lithium-ion technology as they consist of readily available source materials. Application of various transition metal chalcogenides such as MoS2, MoSe2, VS2, and SnSx, composite materials, and heterojunction bimetallic nanosheets composed of multi-metals as electrodes to enhance the long-term cycling stability, rate capability, and structural strength to counteract the large volume expansion during the ion intercalation/deintercalation processes is highlighted. The promising performances of layered chalcogenides and various chalcogenide nanowire compositions as electrodes for flexible supercapacitors are also discussed in detail. The review also details the progress made in new chalcogenide nanostructures and layered mesostructures for energy storage applications.
 Karthik Ramasamy | Karthik Ramasamy is the Vice President of Materials at UbiQD Inc., where he leads research and development and manufacturing of quantum dots and nanocomposites. He received his PhD in chemistry from the University of Manchester, UK. He is a fellow of the Royal Society of Chemistry (FRSC) and an associate editor of Frontiers in Nanotechnology. He has authored/co-authored more than 65 publications and written 8 book chapters and spoken at more than 50 international meetings. He has 6 granted, published and pending patents. He can be reached at E-mail: karthik@ubiqd.com. |
1. Introduction
With increasing energy consumption and the gradual depletion and carbon emission of finite nonrenewable energy sources, energy generation and storage from sustainable sources have become key for several modern technologies.1–4 Modern portable and wearable electronics such as laptops, cell phones, and several health monitoring devices are made possible by electrochemical energy storage technologies including batteries and supercapacitors.5–7 In a supercapacitor, the electrical energy is stored at the electrolyte–electrolyte interface.8 One of the major advantages of a supercapacitor is its high specific energy. They also exhibit promising specific power. Batteries, on the other hand, use electrochemical reactions to store energy and are characterized by exceptional energy density. However, they are limited by low power densities.9,10One of the most effective pathways to realize revolutionary electrochemical energy storage, beyond the scope of existing technologies and to further enhance the electrochemical performance of existing devices is through new electrode materials.11–14 Chalcogenides constitute a promising class of novel electrode materials for both batteries and supercapacitors (Fig. 1).15–18 Chalcogenides are compounds which contain one or more chalcogen anions such as oxygen, sulfur, selenium, and tellurium. However, oxides typically show properties that are distinct from those of other chalcogenides and are treated as a separate class of materials. Various binary and ternary compositions of chalcogenides have been reported in the literature.19–23 Binary chalcogenide compositions are most stable and are easier to synthesize. Fewer reports of quaternary and multinary chalcogenides are available as they require higher levels of control on the synthetic parameters. However, these multinary chalcogenide compositions allow higher levels of flexibility in electrochemical properties due to their hierarchical structure.24,25
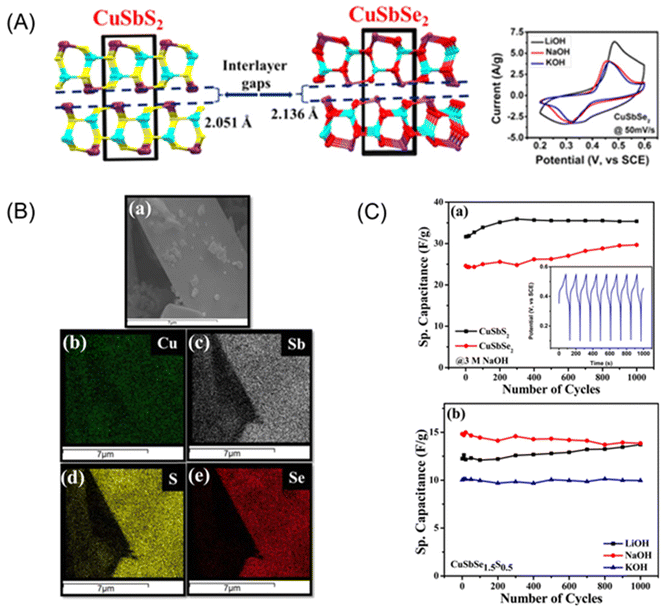 | ||
| Fig. 1 Layered CuSbSexS2−x, x = 1 mesocrystals as electrode materials for supercapacitors. (A) Schematic of the effect of interlayer spacing on the electrochemical properties of these new materials. (B) SEM-EDX mapping images of CuSbSexS2−x, x = 1 mesocrystals showing uniform distribution of Cu, Sb, S and Se, and (C) specific capacitance and cycling performance of the new chalcogenides. (a) Cycling performance of the CuSbS2 and CuSbSe2 electrodes at a constant current of 0.5 mA using NaOH electrolyte. The inset shows the first few cycles of the charge–discharge curves. (b) Cyclic performance of CuSbSe1.5S0.5 using different electrolytes. Reproduced with permission from Ramasamy et al., Chem. Mater., 2015, 27, 379.28 | ||
This review highlights the key role of various chalcogenide-based materials in catalyzing new advances in electrochemical energy storage. Application of chalcogenide nanocrystals and novel hybrid chalcogenide nanoarchitectures for lithium-ion, sodium-ion, and potassium-ion batteries and flexible supercapacitor technologies is discussed. The role of emerging chalcogenide-based electrodes in addressing the existing limitations of these energy storage technologies is highlighted from a materials perspective. The review concludes with a perspective capturing new insights into the fundamental charge transport mechanisms in novel chalcogenide electrodes and the emerging new technologies such as hybrid devices for realizing the full potential of electrochemical energy storage. Industrial sectors such as electric motor vehicles are some of the major worldwide users of battery technology. Recently, there has been a growing interest to partially replace the existing lithium-ion technology with more sustainable battery technologies consisting of raw materials from widely abundant sources e.g., the sodium-ion battery. The Na-ion technology is also more convenient for consumers as it minimizes safety risks and costs related to shipping and transport of the battery when compared to the Li-ion batteries. Therefore, companies such as Faradion in UK, HiNa in China, Natron Energy in USA, and Tiamat and Altris AB in Europe have focused on the development and scalable production of Na-ion batteries.26 However, suitable anode materials like transition metal dichalcogenides with large interlayer spacings are being explored to account for the large volume expansion of the Na-ion intercalation–deintercalation process and to increase the performance efficiency of the Na-ion batteries for large-scale applications. Recently, Pang et al. reported another highly sustainable rechargeable battery technology consisting of aluminum, the most earth-abundant element, as the negative electrode and Se or S as the positive electrode.27 The large-scale application potential of this energy storage technology is huge as all the raw materials are readily available, making the cost of this battery 12–16% that of the existing lithium-ion cells.
2. Lithium-ion batteries
Batteries are one of the key resources for electrochemical energy storage today. Among them, the lithium-ion batteries (LIBs) are most widely used worldwide since their first commercial introduction by SONY Corporation.29 They offer a facile and rechargeable platform for powering major electronic devices used in our daily life including cell phones, laptops, iPads, wearable and portable electronics, modern electric vehicles, and storage devices for clean energy generated from renewable sources such as solar and wind.29,30 The cathode in LIBs typically consists of a Li-intercalation compound like LiCoO2 or LiFePO4 while graphite serves as the traditional anode material for LIBs. The Li ions undergo intercalation and de-intercalation within the layers of the graphite anode during the discharge/charge cycle forming graphite intercalation compounds. The first Li-ion battery, developed by Yoshino used a carbon anode and LiCoO2 cathode with the following electrochemical reaction:31Anode:
| C + xe− + xLi+ = LixC |
Cathode:
| LiCoO2 − xe− − xLi+ = Li1−xCoO2 |
However, the low specific capacity of the graphite anode poses a limitation in terms of achieving the increasing demand for high energy density LIBs. New battery strategies are required to satisfy the energy density needed for realizing affordable and sustainable energy storage and distribution. Therefore, there is a continuous scientific search for suitable active anode materials with enhanced specific capacities for next-generation LIB technologies.32 To this end, layered materials consisting of transition-metal dichalcogenides (TMDs) are promising candidates as they exhibit high specific capacities and enhanced charge/discharge capabilities.
2.1 Transition-metal chalcogenides for anodes
Germanium, being a homologue of carbon is an attractive alternative as an anode material for LIBs as it has a higher capacity and conductivity compared to carbon and facilitates faster diffusion of the Li-ions. However, the large volume changes during lithium alloying/dealloying and low cyclability limit its commercial viability. To this end, doping with chalcogenides such as a zinc chalcogenide doped amorphous Ge compound has been achieved via solvothermal synthesis to realize improved anode materials with high reversible capacity, stable capacity, and increased capacity retention.33 The porous hierarchical morphology of this new material promotes increased ion conductivity and maintains the structural integrity by limiting the volume expansion during Li alloying/dealloying. Transition-metal selenides, having inherent high electrical conductivity and storage capacity, are also one of the most promising candidates as next-generation anode materials for LIBs, and various strategies have been implemented to improve the kinetics of Li-ion transport in these materials. For example, hybrid nanostructures of transition-metal selenides with multiwalled carbon nanotubes (CNTs) have been reported, as the CNTs are both chemically stable and serve as an excellent conductive matrix. CNTs decorated with Co0.85Se and CuCo2Se4 nanocrystals show high specific capacities, good cycling stability, and excellent reversible capacities even at increased current rates.34 The unique morphology of this functionalized anode material facilitates faster transport for Li ions, provides increased active sites for electrochemical reaction, and provides a buffer for the large volume changes during Li+ dealloying/alloying, thereby enhancing the electrochemical performance of the battery.Two-dimensional TMDs offer another attractive platform as anode materials for LIBs as their unique layered structure is similar to that of the traditional graphite electrodes of LIBs, but their interlayer spacing and capacity are significantly larger.35,36 The increased interspacing and higher surface area of these novel materials provide increased active sites for the storage of Li ions and hence facilitate faster transport of Li+. Consequently, the TMD anodes show improved rate performance or capacity to generate high power with minimal voltage loss even at high current loads. They also exhibit increased pseudocapacitance. Both these properties of a TMD anode are attractive for next-generation LIBs.37,38 Layered semiconductor TMDs such as MoS2 and WS2 have been integrated on conductive substrates like graphene to further augment their electrochemical performance for LIB anodes. For example, Wang et al. reported MoS2 nanosheets on an exfoliated graphene substrate, where the graphene layer facilitates enhanced electron transport and provides structural stability during battery operation.39 The morphology of the MoS2 nanostructures plays a key role in the electrochemical performance of these anode materials. MoS2 nanoflakes supported on n-doped carbon nanosheets have also been synthesized via a hydrothermal route and exhibit excellent capacity at high current densities, good cycling stability, as well as increased rate capacity. Different novel three-dimensional structures have been reported with MoS2 for promising application as LIB anodes. In one such example, Chen et al. synthesized hierarchical tubular assembly of MoS2 nanosheets that have been wired with CNTs for enhanced electrochemical performance in terms of specific capacity, rate capability, and cycling lifetime.40,41 Novel hybrid nanoarchitectures have also been synthesized with other chalcogenides including MoSe2, VS2, VSe2, WS2, and WSe2 for improved anode materials for LIBs.36 In one such combination, VS2 has been integrated with CNTs to form a LIB anode.42 Carbon-coated VSe2 nanosheets have also been reported using a solvothermal synthesis.43 These materials exhibit excellent capacity, cycling stability, and rate performance as LIB anodes. Another hybrid aerogel nanoarchitecture composed of WS2 nanosheets on a CNT–rGO substrate has been synthesized via a solvothermal approach and exhibits high specific capacity and attractive electrochemical properties as a LIB anode material.44
Novel heterostructures of layered two-dimensional TMDs offer the advantages of 2D materials while minimizing their limitations, which makes them promising materials for LIB anodes. These materials are synthesized via stacking various two-dimensional TMDs and provide higher levels of flexibility in terms of realizing high performance anodes. For example, MoS2-on-MXene hetero-architectures have been reported using an in situ sulfidation of Mo2TiC2Tx MXene, where the unique MXene component adds excellent conductivity and mechanical support to the heterostructure.45 Based on first principles investigations, the high conductivity of the MXene substrate makes the heterostructure metallic although MoS2 is a semiconductor. The novel MoS2-on-MXene heterostructure promotes strong Li adsorption on the two-dimensional surface and thereby exhibits excellent specific capacity, cycling stability, and rate capability. Heterostructures of intrinsically metallic TMDs like VS2 have also been explored as attractive anode materials for LIBs. The advantages of each component can be integrated in these structures for an enhanced electrochemical performance. For example, the limiting stability of VS2 due to Peierls distortion can be overcome through good cycling stability of the other components in the TiO2-B@VS2 hetero-structured nanowires, while utilizing the high capacity and conductivity of the VS2 chalcogenides.46 These nanostructures exhibit an impressive reversible capacity of 365.4 mA h g−1 after 500 cycles at 335 mA g−1 (1C) and rate capacity (171.2 mA h g−1 @ 10C rate).
Recently, metal–organic frameworks including MIL-96-Al, a chalcogenide-based metal–organic framework has been found to be promising electrodes for Li-based battery technologies.47,48 It has been observed that the smaller sizes and the hexagonal bipyramidal crystal phase of this electrode material significantly improves the cycling performance of the battery by suppressing the shuttle effects. Three-dimensional ordered macroporous materials have also emerged as new and promising active materials for electrochemical energy storage as compared to the one-dimensional and 2D materials because the efficiency of light absorption, dissociation of photoinduced charge pairs, and the surface electron transfer efficiency are all enhanced within the three-dimensional structures.49 Hollow structures can be used to tune the mass and charge transfer and have also been promising materials for energy storage.50
2.2 Li ion cathodes
TMDs have also served as promising cathode materials for LIBs.51 For example, a novel 2D/2D heterostructure-based cathode material has been synthesized with TiS2-decorated VS2 flakes deposited on carbon nanotubes via chemical vapor deposition (CVD).52 A thin layer of TiS2 coating (thickness ∼ 2.5 nm) on the VS2 platelets has been achieved using the atomic layer deposition (ALD) approach in this new cathode material. It protects the VS2 core during the lithiation/delithiation process, which facilitates significant improvement in stability, specific capacity, and rate capacity of the electrode. Therefore, the electrochemical performance of LIBs can be enhanced through 2D heterostructure cathode materials as the capacity retention of VS2 electrodes is 40% that of the novel TiS2/VS2 architectures.52,53 To this end, Fang et al. reported a hierarchical layered structure of VS2 chalcogenides on graphene nanosheets via a one-step hydrothermal synthesis as an effective cathode material for LIBs. The layered architecture of the electrode promotes effective transport of the Li-ions, thereby enhancing the stability and discharge capacity of the electrode as compared to the pristine VS2 electrodes.54Table 1 shows a comparative summary of various lithium ion battery materials reported so far.| Active materials | Synthesis | Specific capacity | Rate | Current density | Cycling stability | Anode/cathode | Ref. |
|---|---|---|---|---|---|---|---|
| Chalcogenides | |||||||
| Zn0.81Ge3.19S8 | Solvothermal | 370 mA h g−1 at 1000 mA g−1 | 100 mA g−1 | 92% after 500 cycles at 1 A g−1 | Anode | 33 | |
| Co0.85Se/CNTs and CuCo2Se4/CNT hybrids | Solvothermal | 1025 and 996 mA h g−1 | 100 mA g−1 | 566 and 479 mA h g−1 after 500 cycles | Anode | 34 | |
| WSe2 nanocrystals | Colloidal hot-injection | 498 mA h g−1 | 100 mA g−1 | 83.28% retention | Anode | 36 | |
| MoS2 nanoplates | Solvothermal | 912 mA h g−1 at 1C (1C = 1.06 A g−1) | 658 mA h g−1 at 30C; 554 mA h g−1 at 50C | 50 mA g−1 | 900 mA h g−1 at 10C after 50 cycles | Anode | 55 |
| Fe3O4/MoS2 composites | Hydrothermal | 1079 mA h g−1 at 100 mA g−1 | 569 at 4 A g−1; 224 at 10 A g−1 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mA g−1 000 mA g−1 |
1033 mA h g−1 at 2 A g−1 after 500 cycles | Anode | 56 |
| WS2/N-doped graphene | Hydrothermal | 905 mA h g−1 at 100 mA g−1 | 700 mA h g−1 at 5 A g−1 | 100 mA g−1 to 5000 mA g−1 | 80% capacity retention; 905 mA h g−1 at 100 mA g−1 after 100 cycles | Anode | 57 |
| MoSx/MWCNTs | Solvothermal | 1549 mA h g−1 at 0.05 A g−1 | ∼200 mA h g−1 at 2 A g−1 | 50 mA g−1 | 60 mA h g−1 at 0.05 A g−1 | Anode | 58 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| Oxides and other 2D materials | |||||||
| Graphene capsules | Controlled peeling of multiwalled graphitic capsules and annealing | 1373 mA h g−1 at 0.5 A g−1 | 750 mA h g−1 at 8 A g−1; 447 mA h g−1 at 20 A g−1 | 20 A g−1 | 1373 mA h g−1 at 0.5 A g−1 after 200 cycles | Anode | 59 |
| B-doped graphene nanosheets | Chemical exfoliation and thermal reduction | 1549 mA h g−1 at 50 mA g−1 | 380 mA h g−1 at 5 A g−1; 235 mA h g−1 at 25 A g−1 | 50 mA g−1 | 1227 mA h g−1 at 50 mA g−1 after 30 cycles | Anode | 60 |
| ZnMn2O4/graphene composites | Modified Hummers method | 730 mA h g−1 at 500 mA g−1 | 568 mA h g−1 at 3.2 A g−1 | 500 mA g−1 | 800 mA h g−1 at 500 mA g−1 for 100 cycles | Anode | 61 |
| Porous ZnO nanosheets | Chemical bath deposition and heat treatment | 750 mA h g−1 at 50 mA g−1 | 195 mA h g−1 at 2 A g−1 | 500 mA g−1 | 400 mA h g−1 at 500 mA g−1 after 100 cycles | Anode | 62 |
| TiO2 nanocrystals/rGO sheets | Sol–gel synthesis; modified Hummers method | 189 mA h g−1 at 0.1 A g−1 | 94 mA h g−1 at 10 A g−1 | 10 A g−1 | 189 mA h g−1 at 0.1 A g−1 after 100 cycles | Anode | 63 |
| Li4Ti5O12 nanosheet arrays | Hydrothermal | 163 mA h g−1 at 20C (1C = 175 mA g−1) | 163 mA h g−1 at 20C; 78 mA h g−1 at 200C | 20–200C | 124 mA h g−1 at 50C after 3000 cycles | Anode | 64 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| Cathode materials | |||||||
| LiFePO4 nanosheets | Solvothermal | 151 mA h g−1 at 0.5C (1C = 170 mA g−1) | 120 mA h g−1 at 5C; 55 mA h g−1 at 30C | 1–30C | 90 mA h g−1 at 10C after 1000 cycles | Cathode | 65 |
| LiFePO4/C nanosheets | Liquid-phase exfoliation and solvothermal lithiation | 164 mA h g−1 at 0.2C | 70 mA h g−1 at 80C | 80C | 163 mA h g−1 at 0.2C after 50 cycles | Cathode | 66 |
| LiMnPO4/C nanosheets | Liquid-phase exfoliation and solvothermal lithiation | 157 mA h g−1 at 0.2C | 40 mA h g−1 at 30C | 30C | 147 mA h g−1 at 0.2C after 50 cycles | Cathode | 66 |
| LiCoPO4/C nanosheets | Liquid-phase exfoliation and solvothermal lithiation | 153 mA h g−1 at 0.2C | 53 mA h g−1 at 20C | 20C | 136 mA h g−1 at 0.2C after 50 cycles | Cathode | 66 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| MXene-based materials | |||||||
| Mo2TiC2 (MXene) | Exfoliation | 269 mA h g−1 at C/10 | 176 mA h g−1 at 1C | 1C | 260 mA h g−1 at C/10 after 25 cycles | Anode | 67 |
| MXene/CNT paper | Ball mill | 420 mA h g−1 at 0.5C | 270 mA h g−1 at 10C; 160 mA h g−1 at 20C | 20C | 430 mA h g−1 at 2.5C after 300 cycles | Anode | 68 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| Materials that have been commercialized | |||||||
| LiTiS2 | CVD-based template synthesis | 210 mA h g−1 | — | 1.5 mA cm−2 | — | Cathode | 69 |
| LiCoO2 | Hydrothermal | 148 mA h g−1 | 140 mA g−1 | 1C (=140 mA g−1) | 138.6 mA g−1 after 20 cycles | Cathode | 70 |
| LiNi0.33Mn0.33Co0.33O2 | Co-precipitation | 160 mA h g−1 | 190 mA g−1 | 280 mA g−1 | — | Cathode | 71 and 72 |
| LiMn2O | Solid-state synthesis and spray drying | 120 mA h g−1 | 1300 mA g−1 @ 10C (1C = 130 mA g−1) | 130 mA g−1 | — | Cathode | 73 |
| LiFePO4 | Solid-state reaction | 165 mA h g−1 | — | >1 mA cm−2 | — | Cathode | 74 and 75 |
LIBs have been the dominant rechargeable battery technology over the last few decades as they exhibit high energy densities along with high power and acceptable cyclability. However, there is a continuous thrust to further improve the energy densities of LIBs and to minimize the safety risks in transport.76 The anode material consisted of metallic Li in the first LIBs, but was subsequently replaced with graphite-based substrates due to the safety risks of metallic Li. However, the replacement also compromised the energy density of the LIBs. The first cathodes for LIBs were composed of TiS2 and provided moderate energy storage capacity as it intercalated the Li ions at a low potential. A major improvement in both the potential and storage capacity of LIBs was brought about by the next phase of anode materials in the form of layered transition metal oxides. This has been accompanied by a 90% drop in the cost of LIBs over the last ten years. TMDs have emerged as promising anode materials for LIBs over the years owing to their high specific capacity, increased charge/discharge capacity, and tunable porous morphology that can promote increased ion transport and compensate the volume expansion during the Li-ion intercalation/deintercalation processes. Among them, the selenides provide higher electrical conductivity and storage capacity than the sulfide-based anodes, but they have been less explored. The two-dimensional layered TMDs and their hybrid nanoarchitectures, such as the new MXene-based materials offer further structural flexibility to achieve enhanced power and cyclability. Recently, hybrid chalcogenides like TiS2/VS2 architectures have also served as promising cathode materials for LIBs.
3. Sodium-ion batteries
Sodium-ion batteries (SIBs) have received widespread attention as an attractive alternative for LIBs, particularly for large-scale energy storage applications as sodium sources are more abundant and cost-effective as compared to lithium.77,78 Though their operation mechanism is similar to that of LIBs, the larger size of Na+ compared to Li+ (1.02 Å vs. 0.76 Å) induces slower ion transport and electrochemical reaction kinetics. There are also considerable volume changes accompanying the charge/discharge process in SIBs. These factors limit the energy density of SIBs.78 Additionally, the larger size and lower standard electrochemical potential of Na-ions may lead to safety issues from explosion or corrosion.79,80 Therefore, high-capacity materials such as transition-metal chalcogenides have been explored as SIB anodes to enhance the cell density and electrochemical performance of SIBs. However, the chalcogenides inherently exhibit poor electrical conductivity and large volume expansion during the de-intercalation of Na ions.81,82 Therefore, various novel modification strategies have been explored to further improve the electrochemical performance of the transition-metal chalcogenide anode materials including carbon doping, metal substitution, and synthesis of hybrid nanoarchitectures.83,84 Transition to nanoscale size increases the surface area, which significantly enhances the electrode–electrolyte interaction surface and compensates for stresses from volume changes during the sodiation/desodiation process.77,85 For example, the three-dimensional hollow nanoarchitecture form of the chalcogenides can immensely improve the electrochemical performance of the anodes as the nanoscale size shortens the Na+ ion transmission distance while the hollow morphology counteracts the volume expansion during the de-intercalation of Na-ions.86 Metal substitution is another key strategy that is known to enhance the rate performance of the chalcogenide anodes, as observed for the case of Co-substituted FeS2.87,88 Similarly, metal-substituted CoS2@CuxS nanostructures exhibit higher capacity (535 mA h g−1 @ 0.1 A g−1), discharge rate (333 mA h g−1 @ 5 A g−1) and cycle capacity (76% capacity retention over 300 cycles) as compared to the parent CoS2 electrode (e.g., 249.0 mA h g−1 at 0.5 A g−1 and 1000 cycles at 1 A g−1).78,89 Other novel hybrid nanostructures have been realized that combine a single layer of MoSe2 onto the surfaces of core–shell Fe7Se8@C structures.90 This hybrid chalcogenide anode material vastly improves the stability and retention rate of the battery.Layered two-dimensional TMDs (e.g., VS2, MoS2, and CoS2), with their unique interlayer spacings and high theoretical storage capacity, provide an ideal platform for rapid intercalation/de-intercalation of Na ions and are attractive anode materials for SIBs.85 For example, hierarchical 2D nanoflake ensembles of VS2 facilitate improved transport of Na+ and significantly improve the electrochemical performance of the battery.91 Anode materials containing VS2 chalcogenides with a flower-like morphology have also been reported and these nanostructures promote superior rate performance as well as excellent long-term cycling stability in SIBs.92 Apart from VS2, various layered MoS2 and MoSe2 chalcogenide nanostructures have been reported as key electrode materials for SIBs (Fig. 2).93,94 Na-ions are stored in these materials via a two-step process, intercalation at higher potential and conversion in a lower potential window as follows:95
| MoS2 + xNa ⇌ NaxMoS2 (>0.4 V, x < 1.5) |
| NaxMoS2 + (4 − x)Na ⇌ Mo + 2Na2S (<0.4 V) |
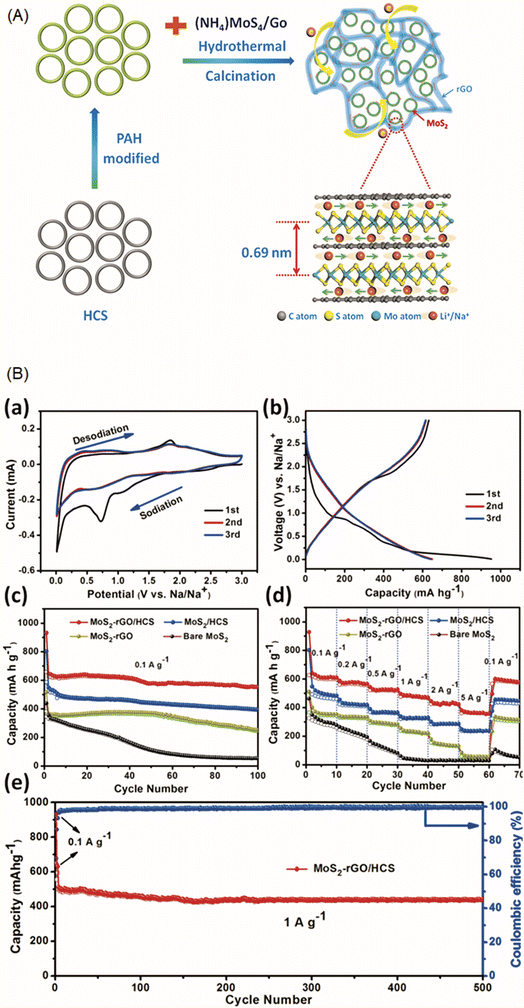 | ||
| Fig. 2 Three-dimensional hybrid architecture of few layer MoS2 on reduced graphene oxide (rGO) cross-linked hollow carbon spheres (HCS). (A) Schematic Illustration of the formation process of MoS2–rGO/HCS and (B) electrochemical performance of the MoS2–rGO/HCS for sodium storage. (a) Representative CV curves of the MoS2–rGO/HCS electrode in a voltage range of 0.01 to 3.0 V and at a scan rate of 0.1 mV s−1. (b) Charge–discharge voltage profiles of the MoS2–rGO/HCS electrode at a current density of 0.1 A g−1. (c) Cycling performance and (d) rate capability of MoS2–rGO/HCS, MoS2/HCS, MoS2–rGO, and bare MoS2. (e) Cycling performance and coulombic efficiency of MoS2–rGO/HCS for 500 cycles at a current density of 1 A g−1. Reproduced with permission from Hu et al., ACS Nano, 2018, 12, 1592.97 | ||
The interlayer spacing of these materials plays a crucial role in improving the electrochemical performance of the battery as the capacity and rate capability of the SIB increases with the interlayer distance in MoS2 nanoflake-based anodes. In addition, electrode materials consisting of hybrid nanoarchitectures of chalcogenides supported on one-dimensional substrates, such as MoS2 decorated n-doped carbon ribbon and core–shell MoSe2@n-doped carbon shell structures also facilitate significant improvement in the cycling stability, reverse capacity, and capacity of the battery. Sun et al. reported novel hierarchical nanohybrid anode materials with MoS2 on a two-dimensional graphene substrate.96 These electrodes have exhibited key enhancements in the current density, rate capability, as well as cycling stability of the SIB. In another report, graphene oxide cross-linked with hollow carbon spheres has been used as a novel 2D scaffold for MoS2 nanoflakes.97 The porous morphology of this novel electrode material offers a unique solution to counteract the volume expansion in SIBs and to promote enhanced transport of electrolytes, ions, and electrons. New SIB anode materials have also been reported with MoS2 nanoflakes vertically aligned on conductive carbon paper.98 The three-dimensional carbon fiber matrix offers increased active sites and ion/electron transfer channels to enhance the reaction kinetics of the electrochemical reactions in SIBs.
SnSx-based anodes are the second most widely researched materials for SIBs due to their key advantages of eco-friendliness, high capacity, and huge natural abundance.99,100 The large interlayer spacing of SnSx allows facile intercalation/de-intercalation of Na ions for improved reaction kinetics. The material has the capacity for storing Na ions during the conversion process as well as the intercalation/de-intercalation process through the following reaction:
| 4Sn + 15Na+ + 15e− ↔ Na15Sn4 |
The storage capacity of SnSx is therefore higher compared to that of the other materials, making it attractive for SIBs. Various novel strategies have been applied to SnSx-based materials to further improve the limiting electrochemical properties including poor stability, high stress from volume changes, and low conductivity. Xiong et al. reported a hybrid nanostructure with SnS nanoparticles on a conducting n-doped graphene scaffold.99 The graphene support minimized aggregation of the nanoparticles and greatly improved the retention rate of the SIB. In another study, a novel nanosheet morphology of SnS2 has facilitated enhanced active sites for the electrochemical reactions while the unique structural features of the rGO substrate allow rapid transport of Na ions and provide structural stability to withstand changes in volume. As a result, the composite anode shows excellent charge capacity (630 mA h g−1 at 0.2 A g−1), rate performance (544 mA h g−1 at 2 A g−1), and cycling stability (500 mA h g−1 at 1 A g−1 for 400 cycles). Liu et al. synthesized a unique composite anode with SnS2 nanoparticles decorated on reduced graphene oxide nanoribbons with a paper-like morphology that has induced remarkable enhancements in the discharge capacity of the battery.101 SnSex-based electrodes, though less widely reported in the literature, have recently been synthesized via novel hydrothermal approaches and exhibit improved rate performance and cycling stability.102–104 Various composite electrodes based on other transition metal chalcogenides such as CoSx/Sex, NiSx/Sex, and WSx/Sex have also been explored as promising materials for SIBs.44,105–115 In a recent report, novel composites of NiSe2 enclosed in boron carbonitride nanotubes have been synthesized via a facile pyrolysis approach.116 These anodes show excellent reversible Na+ storage capacity and long-term cycling stability. In general, the reaction kinetics and decay mechanisms are similar for both S and Se-based electrode materials. Various novel carbonaceous substrates have been reported to overcome the issues of conductivity and volume changes. Se-based electrodes offer a more suitable platform for next-generation SIBs as they possess a larger interlayer spacing and intrinsic conductivity compared to sulfides.
Recently, multi-metal chalcogenides have emerged as highly promising electrode materials for SIBs as the multiple metallic centers provide enhanced adsorption capability and conductivity as well as increased active sites for the electrochemical reactions. Chen et al. synthesized heterojunction bimetallic sulfide nanosheets of the SnS2/FeS2/rGO composite, which have shown highly improved capacities.117 New materials containing multiple transition metals (e.g., NiCo2S4 and Cu2NiSnS4) as well as multi-phase components (e.g., ZnS–Sb2S3, NiS2–CoS2, and Co9S8–ZnS) have been realized. In general, the electrochemical performance of the multi-metal chalcogenides significantly surpasses that of single component transition-metal chalcogenides.118–123 However, these materials are synthetically challenging to achieve with a few reports on sulfide-based structures and limited reports on the selenides. Further insights on a compatible carbonaceous support and avenues to enhance the theoretical capacity will help make these materials viable next-generation electrodes for SIBs.
All solid-state SIBs have been another innovative alternative to LIBs and have emerged as an area of major scientific research in recent years.124 A solid-state electrolyte is used in this battery technology that holds the advantages of being leak-free as well as having high thermal stability as compared to the traditional liquid electrolytes. A vast range of inorganic solid electrolytes for SIBs comprise various chalcogenides such as the Na3MS4 series,125–128 the Na3MSe4 (M = P, Sb) series,129,130 or the Na7P3X11 (X = O, S, Se) series.131 These electrolytes are appealing and cost-effective alternatives to oxides as they can be synthesized at lower temperatures. However, chalcogenide-based electrolytes can be unstable in air and can contain voids that have a detrimental impact on the materials' conductivity. Therefore, several modification strategies including doping of cations at the P site or halogen ions at the S site, forming hybrid organic–inorganic composites like polyethylene oxide-based Na3PS4, high-temperature heat treatment for crystal transformation, and ceramization and vitrification have been effectively explored to increase the stability, electrochemical performance, and mechanical properties of these next-generation solid-state chalcogenide electrolytes.132–138
SIBs contain source materials that are cost-effective, abundantly available on earth, and do not pose any safety concerns. Therefore, they provide an attractive and more sustainable alternative to the existing lithium-ion technology. However, the large volume expansion during the charge/discharge process and the slower ion transport due to the larger size of the Na ion limit the energy density of SIBs in comparison to that of Li-ion batteries. Therefore, transition metal chalcogenides with high specific capacities have been explored as active electrode materials for SIBs. Strategies such as carbon doping, metal substitution, formation of hybrid structures with transition metal chalcogenides, as well as using the nanoscale form of the chalcogenides have been used to enhance the electrical conductivity of these chalcogenide-based SIB electrodes. Furthermore, two-dimensional TMDs such as MoS2 nanoflakes, MoSe2, and VS2 have been used to improve the cycling stability, rate capacity, and current density of SIBs. Various hybrid morphologies of SnSx-based chalcogenides serve as promising electrode materials for SIBs owing to the higher storage capacity of Sn. Finally, all solid-state SIBs are also attractive as next-generation SIB electrodes as they are leak-free and thermally more stable compared to the existing liquid electrolytes.
4. Potassium-ion batteries
Although LIBs have revolutionized battery technology in terms of an uninterrupted energy supply for wearable devices, electric vehicles, and stationary energy storage, the limited global reserves of lithium have been an impediment to realizing affordable technologies. To this end, SIBs and recently, potassium-ion batteries (PIBs) have been explored as alternative materials to achieve a more sustainable, affordable, and high-performance rechargeable battery technology.139 There are a couple key theoretical advantages of PIBs as compared to the LIB and SIB technology. PIBs can ideally attain higher working voltages compared to their Li and Na counterparts as the standard redox potential of K+/K (−2.93 V vs. SHE) is comparable to that of Li+/Li (−3.04 V vs. SHE). K+ is also a weaker Lewis acid and its higher diffusion rate and ionic conductivity facilitate a favorable and reversible intercalation with the graphite anode compared to the irreversible intercalation in SIBs.140–142 A suitable anode material with superior specific capacities, cycling stability, diffusion rate, and reaction kinetics is one of the key aspects for achieving efficient and practically applicable PIB technology.Two-dimensional metal chalcogenides (e.g., MoS2, VS2, SnS2, and Sb2S3) with low energy barriers for alkali metals and large specific capacities are highly attractive as anode materials for PIBs (Fig. 3 and 4).139 These chalcogenide anode materials for PIBs can be primarily classified as the conversion type or conversion/alloying type based on the mechanism of potassium insertion in the anode materials.143–146 The metal and chalcogen are electrochemically inactive for the conversion type materials while the metal atoms in the conversion/alloying anode materials have the ability to store potassium via alloying reactions. In the conversion-type materials (e.g., MoS2, FeS2, ZnS, and V5S8), the chalcogen component is the primary contributor of specific capacity while the inactive metal atom serves to provide a continuously conductive platform.91,140,147,148 Metals from group 14 and 15 constitute the active metallic component in the conversion/alloying type chalcogenide anode materials (e.g., Sb2S3, SnS2, and GeSe) for PIBs. The metal and chalcogen both form reversible alloys with the potassium ion, thereby enhancing the capacity of this type of anode material. The detailed mechanism of potassiation/depotassiation of MoSe2 anodes was first investigated by Lu et al. through a combination of XRD and Raman spectroscopic characterization of the materials at different phases of charge/discharge.148 It was discovered that this electrochemical potassiation proceeds via a two-step process consisting of K+ intercalation and conversion, with the Se chalcogen facilitating the K–Se redox reaction, similar to the reaction mechanism in Li–S batteries. Guan et al. reported that the potassiation of FeS2 based anode materials proceeds through an initial irreversible transformation to KxFeS2, which is followed by reversible intercalation and conversion stages.149 The charge and discharge mechanisms in another anode material, Sb2S3 were investigated using synchrotron XRD. Intercalation of K+ within the Sb2S3 structure first expands the interlayer distances prior to the conversion and alloying reactions. Xia et al. reported the potassiation/depotassiation mechanism in SnS2 materials using XRD and in situ TEM.144 One of the limitations of the conversion/alloying materials is the large volume expansion associated with the potassiation process.
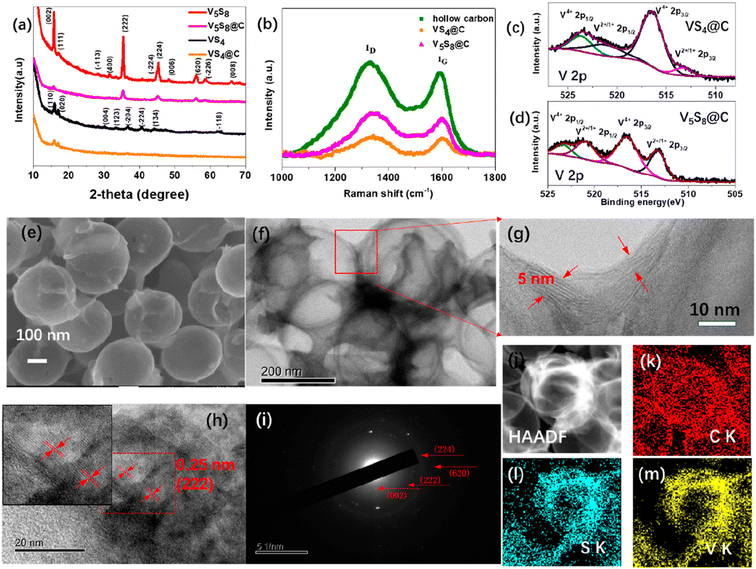 | ||
| Fig. 3 Hollow-carbon-templated few-layered V5S8 nanosheets for PIBs. (a) X-ray diffraction patterns of bare VS4, bare V5S8, VS4@C, and V5S8@C; (b) Raman spectra of hollow carbon, VS4@C, and V5S8@C; X-ray photoelectron spectroscopy of (c) VS4@C: V 2p and (d) V5S8@C: V 2p; SEM image (e); TEM images (f); high-resolution TEM images (g and h); inset of (h) is the enlarged part of the red circle; selected area electron diffraction (i) of V5S8@C; STEM image (j); and elemental mapping analysis of V5S8@C: (k) C, (l) S, and (m) V elements. Reproduced with permission from Li et al., ACS Nano, 2019, 13, 7939.150 | ||
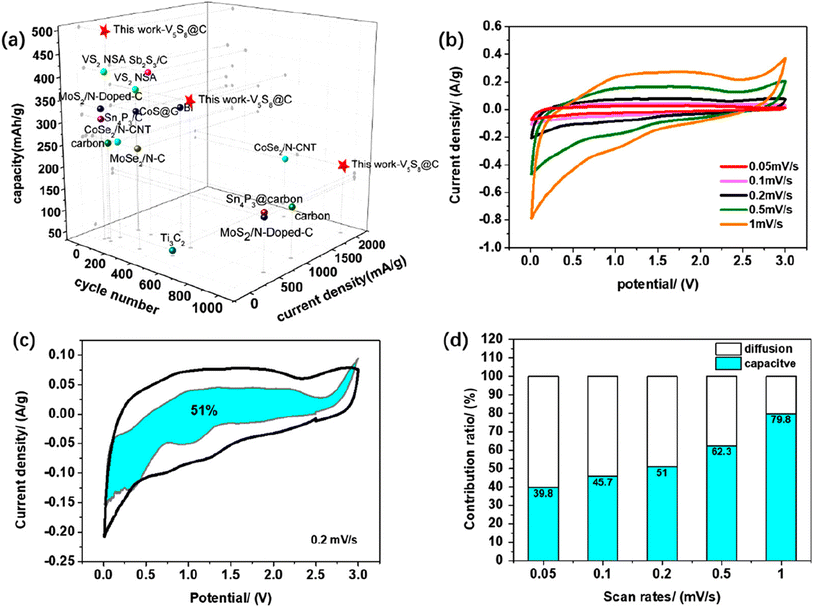 | ||
| Fig. 4 (a) Comparison of the capacity and cycling capabilities of the state-of-the-art KIB anode materials with our work; (b) CV curves of V5S8@C at different scan rates from 0.05 to 1 mV s−1; (c) CV curves of V5S8@C with separation between the total current (black line) and surface capacitive current (blue regions) at 0.2 mV s−1; (d) capacity ratios between diffusion and capacitive contributions of V5S8@C at different scan rates from 0.05 to 1 mV s−1. Reproduced with permission from Li et al., ACS Nano, 2019, 13, 7939.150 | ||
4.1 Challenges
The current state-of-the-art chalcogenide anode materials are highly attractive for potassium storage; however, some limitations still exist in the technology in terms of practical applicability.141,151,152 For example, detrimental side reactions during the deep potassiation phase, lead to formation of potassium polychalcogenide compounds that hinder the diffusion of K+ by forming a film on the metal surfaces and thereby reduce the capacity of the device.143,150,153 Another limitation of the chalcogenide anode materials is the low structural stability caused by the large volume expansion and partially reversible phase transformations during their charge/discharge cycles. The bulk chalcogenides exhibit low electric and ionic conductivity, which detrimentally affect the diffusion of K+ and electron transport mechanisms. Therefore, there is a continued scientific interest to overcome these challenges in the chalcogenide anode materials for achieving practically viable and high-performance PIB technologies.4.2 Technological solutions
To this end, a few key technological strategies have been explored to improve the long-term cycling stability and capacity of chalcogenide anode materials. Wu et al. applied a novel approach of tuning the discharge depth during the K+ intercalation in MoS2 to facilitate the formation of K0.4MoS2 as the degradation product.154 K0.4MoS2 exhibited a lower volume expansion as compared to MoS2 and thereby minimized the detrimental effects on the structural integrity of the anode. Another approach by Shu et al. prevented the capacity loss in TiSe2 materials due to irreversible depotassiation through pretreatment cycles that facilitated the formation of K0.24TiSe2.155 K0.24TiSe2 serves as a suitable host material for K+ intercalation for reversible potassium conversion reactions.Another strategy for improving the chalcogenide anodes is via incorporation of carbon nanophases. The nanophase carbon provides a stable platform for minimizing the aggregation and volume expansion of the chalcogenides during the potassiation and depotassiation processes and enhances the structural integrity of the anode materials. The carbon nanophase–metal chalcogenide hybrid composites also show increased electrical conductivity as the carbon component facilitates a faster electron transfer channel for the chalcogenides. These metal chalcogenide–carbon nanophase hybrid structures can be realized by either embedding the small metal chalcogenides within the carbon matrix or by encapsulation of the chalcogenides with carbon. For example, the electrospinning approach has been effectively used for embedding graphene-coated FeS2 chalcogenide nanoparticles within carbon nanofibers.153 An improved cycling stability of PIBs has been observed with the FeS2–graphene–carbon nanofiber composite anode material.153 Electrospinning-based approaches have also proved effective for other chalcogenide nanoparticles like VSe1.5, where the VSe1.5 embedded carbon nanofiber composite showed stable capacity as the carbon component minimized aggregation and transport of the nanoparticles during cycling.156 In another novel hybrid chalcogenide nanoparticle-based composite anode, CoS nanoparticles have been encapsulated at the ends of carbon nanotubes as well as on the surfaces of n-doped carbon nanofibers via an in situ CVD and electrospinning synthetic strategy.157 This hybrid nanocomposite facilitates enhanced capacity (400 mA h g−1) and good cycling stability (i.e., 130 mA h g−1 at 3.2 A g−1 after 600 cycles) in PIBs owing to the efficient confinement of aggregation and migration of the chalcogenide nanoparticles. Guan et al. reported a unique yolk–shell structure consisting of an FeS2 core with an outer carbon shell to control the volume expansion during potassiation and depotassiation.149 The ability to accommodate volume expansion is a key advantage of the yolk–shell nanostructure as it protects the structural integrity of the anode. The confinement effect within the carbon shell also facilitates enhanced ion and electron transport in the yolk–shell nanostructures.
Ternary alloying is a novel material strategy in which a third cation or anion is used as a key tool to enhance the vacancies and realize tunable electronic and physical properties in the metal chalcogenide anode materials. For example, Guo et al. synthesized MoSSe nanoplates as PIB anode materials via a novel alloying strategy where the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 S/Se stoichiometric ratio facilitates enhanced vacancy concentration.158 The vacancy sites in this novel ternary material increases electron transfer and minimizes K+ ion adsorption during cycling.158 These properties of the MoSSe alloy impart enhanced reversible capacity and cycling stability to the PIB anode. Hybrid PIB anode materials constituting a layered graphene substrate containing ternary chalcogenide nanodots that are prepared via substitution of cations, e.g., SnSb2Te4 have also shown enhanced ionic and electric conductivity.159 A modified ball-milling approach has been used to synthesize these anodes. Wang et al. synthesized ternary Ta2NiSe5 nanosheets via a novel ion-intercalation based exfoliation method as anode materials for PIBs.160 These ternary chalcogenides exhibit a large capacity as well as stable cyclability, which is attractive for potassium storage owing to their multiple Se sites.
1 S/Se stoichiometric ratio facilitates enhanced vacancy concentration.158 The vacancy sites in this novel ternary material increases electron transfer and minimizes K+ ion adsorption during cycling.158 These properties of the MoSSe alloy impart enhanced reversible capacity and cycling stability to the PIB anode. Hybrid PIB anode materials constituting a layered graphene substrate containing ternary chalcogenide nanodots that are prepared via substitution of cations, e.g., SnSb2Te4 have also shown enhanced ionic and electric conductivity.159 A modified ball-milling approach has been used to synthesize these anodes. Wang et al. synthesized ternary Ta2NiSe5 nanosheets via a novel ion-intercalation based exfoliation method as anode materials for PIBs.160 These ternary chalcogenides exhibit a large capacity as well as stable cyclability, which is attractive for potassium storage owing to their multiple Se sites.
Finally, synthesizing the chalcogenide materials at the nanoscale can be used as a niche strategy to address the limited ionic and electrical conductivities of the bulk phases.161,162 A higher flexibility in obtaining a tunable structure–property relationship can be achieved at the nanoscale owing to the available surface area. The nanoparticles offer a shorter diffusion pathway, improved electron transport, and accommodation for the volume expansion during the potassiation/depotassiation cycles, which are key to maintaining the structural integrity of the anode and enhancing the potassiation/depotassiation length. Consequently, these metal chalcogenide nanostructures can serve as a next-generation platform for the anode material of PIBs in terms of enhanced capacity and cycling stability. A few key synthetic approaches used for chalcogenide nanocrystals include chemical vapor deposition, solution-based processes, hydrothermal, calcination, and hard/soft templating.139,140,147,156,163 For example, Wang et al. reported a novel solvothermal synthesis of PIB anode materials containing octahedral CoSe2 nanoparticles that are well-dispersed in n-doped carbon nanotubes to facilitate increased electron transport and structural stability.140 Anodes with novel shape-controlled and hollow MoS2 nanoparticles threaded on n-doped carbon nanotubes have also been reported.147 The abundant active sites, defects, and ion diffusion channels in the expanded MoS2 layers of these hybrid anode materials facilitate enhanced potassium storage. In another modification, rose-shaped MoS2 nanoparticles on reduced graphene oxide (rGO), synthesized via a facile hydrothermal route, have shown significantly improved cycling and capacity retention at high rates due to the stability and minimized nanoparticle aggregation provided by the rGO support and the abundant ion diffusion channels within the structure.164 It has been observed that nanoengineered MoSe2 nanosheets with a flower-like morphology where some of the nanosheets are attached to n-doped carbon layers exhibit a more stable capacity during cycling.148 Layered Sb2S3 nanosheets are also promising as PIB anode materials in terms of enhanced cycling performance and reversible capacity due to the soft layered structure of these materials.143 Hybrid PIB anode nanostructures synthesized with V5S8 nanosheets supported on novel hollow carbon nanospheres have shown the most efficient rate capability among the metal chalcogenide-based anode materials synthesized so far.150,165,166
In summary, PIBs have the potential to achieve higher working voltages than Li and Na-ion batteries. They consist of earth-abundant source materials and facilitate an increased diffusion rate and ion conductivity compared to SIBs. These attributes make PIBs attractive for electrochemical energy storage. Two-dimensional metal chalcogenides (e.g., MoS2, VS2, Sb2S3, SnS2, FeS2, ZnS, and V5S8) have been explored as promising next-generation anode materials for PIBs in both conversion and conversion/alloying types of anodes. One limitation of chalcogenide-based anodes is the formation of potassium polychalcogenide residues during the deep potassiation phase that hinder the transport of K-ions. Therefore, novel approaches like controlling the discharge depth during the K-ion intercalation, using hybrid and nanoscale chalcogenides as anode materials, ternary alloying via adding another cation or anion within the chalcogenide anode material, and adding a nanoscale carbonaceous support have been effectively applied in recent years to further enhance the rate capability and long-term cycling stability of PIBs.
5. Flexible supercapacitors
Wearable and portable electronics such as mobile phones, health-tracking devices, and laptops have become an inherent part of modern life and have revolutionized our living conditions. Flexible energy storage devices like flexible supercapacitors are the key to sustaining these next-generation compact and wearable technologies. They combine the high-power density, rapid charging/discharging capability, and longer lifetime with mechanical flexibility. Each component of a flexible supercapacitor from the case to the electrode is flexible, providing a suitable platform for modern portable electronics.7,167,168 A flexible electrode material greatly influences the overall performance, structural flexibility, and stability and is therefore one of the major components of a supercapacitor. Traditional carbonaceous materials cannot meet the high energy density required for commercial viability of these next-generation electrochemical energy storage devices. On the other hand, transition-metal chalcogenides with their high specific capacitance, excellent storage capacity and electron/ion diffusion, long operation lifetime, and morphology-tunable synthesis strategies are attractive candidates as electrode materials for flexible supercapacitors (Fig. 5). Chalcogenide-based materials can be engineered to have excellent mechanical properties required for high-quality flexible supercapacitors. The morphology of the electrode materials also plays a major role in realizing high-performance flexible supercapacitors, and as a result hollow and linear shapes of the transition-metal chalcogenides have been investigated. One example is a novel nanowire array of Ni3Co6S8 chalcogenides on nitrogen-doped flexible carbon foam prepared via a facile hydrothermal approach.169 The unique morphology of the chalcogenide electrode facilitates enhanced electrochemical performance in terms of specific capacity (243 mA h g−1), rate capability, as well as cycling stability (i.e., 84% specific capacitance after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles) due to the increased efficiency of consumption of the active material. Zhai et al. developed a new chalcogenide-based electrode material combining two transition metal sulfides (i.e., copper sulfide and zinc sulfide) having high pseudo-capacitance with a porous carbon substrate having excellent double-layer capacitance.170 The electrode induced a transformative increase in the energy density of the flexible supercapacitor, which is important for commercial viability and large-scale applicability of the technology. Layered chalcogenides are another class of promising electrode materials for flexible supercapacitors.28,171–174 The limited conductivity in these layered materials has been addressed via various novel strategies. For example, InSe-based electrodes have been incorporated into an interdigitated and flexible micro supercapacitor technology to bring a breakthrough enhancement in the mobility of charge carriers. The structure of these thin layered chalcogenide materials also greatly influences their inherent properties and can therefore be used to tune the electrical conductivity of these materials.173 Hu et al. added hydrogen to Cu2WS4 layered nanosheets and the resulting materials transitioned from semiconductor into metallic, which induced a huge increase in the electrical conductivity.174 In another novel electrode material, ultrathin two-dimensional structures of Ti3C2Tx MXene embedded with FeCo2S4 chalcogenide nanoparticles have been deposited in situ on polyacrylonitrile/polyvinylpyrrolidone-modified carbon nanofibers via electrospinning.175 This flexible device facilitates rapid and continuous transport of electrolyte and provides breakthrough cycling stability and improved sulfide conductivity. Recent studies have also highlighted the remarkable potential of layered MoS2 and MoSe2 chalcogenides for flexible supercapacitor electrodes.176 For example, a detailed investigation of the intercalation properties of MoS2 has revealed its excellent specific capacitance and energy density in both aqueous and organic phases.177 MoS2 synthesized on carbon nanofibers via a microwave-assisted hydrothermal approach has also showed remarkable electrochemical performance.178 Therefore, transition-metal chalcogenides have emerged as attractive components of electrodes that can meet the high energy density and specific capacitance required for next-generation flexible supercapacitors.
000 cycles) due to the increased efficiency of consumption of the active material. Zhai et al. developed a new chalcogenide-based electrode material combining two transition metal sulfides (i.e., copper sulfide and zinc sulfide) having high pseudo-capacitance with a porous carbon substrate having excellent double-layer capacitance.170 The electrode induced a transformative increase in the energy density of the flexible supercapacitor, which is important for commercial viability and large-scale applicability of the technology. Layered chalcogenides are another class of promising electrode materials for flexible supercapacitors.28,171–174 The limited conductivity in these layered materials has been addressed via various novel strategies. For example, InSe-based electrodes have been incorporated into an interdigitated and flexible micro supercapacitor technology to bring a breakthrough enhancement in the mobility of charge carriers. The structure of these thin layered chalcogenide materials also greatly influences their inherent properties and can therefore be used to tune the electrical conductivity of these materials.173 Hu et al. added hydrogen to Cu2WS4 layered nanosheets and the resulting materials transitioned from semiconductor into metallic, which induced a huge increase in the electrical conductivity.174 In another novel electrode material, ultrathin two-dimensional structures of Ti3C2Tx MXene embedded with FeCo2S4 chalcogenide nanoparticles have been deposited in situ on polyacrylonitrile/polyvinylpyrrolidone-modified carbon nanofibers via electrospinning.175 This flexible device facilitates rapid and continuous transport of electrolyte and provides breakthrough cycling stability and improved sulfide conductivity. Recent studies have also highlighted the remarkable potential of layered MoS2 and MoSe2 chalcogenides for flexible supercapacitor electrodes.176 For example, a detailed investigation of the intercalation properties of MoS2 has revealed its excellent specific capacitance and energy density in both aqueous and organic phases.177 MoS2 synthesized on carbon nanofibers via a microwave-assisted hydrothermal approach has also showed remarkable electrochemical performance.178 Therefore, transition-metal chalcogenides have emerged as attractive components of electrodes that can meet the high energy density and specific capacitance required for next-generation flexible supercapacitors.
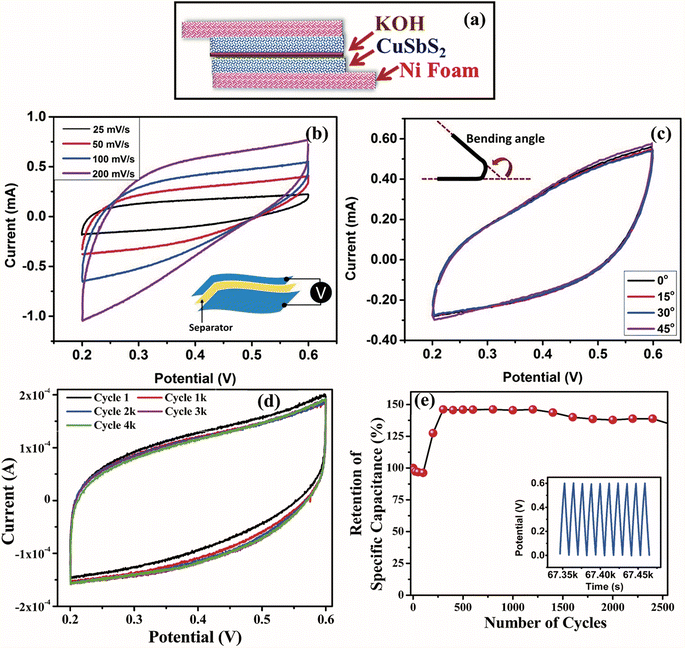 | ||
| Fig. 5 Flexible supercapacitor assembly based on CuSbS2 nanoplates. (a) Schematic of a flexible quasi-solid-state supercapacitor device fabricated using 55 ± 6.5 nm CuSbS2 nanoplates, (b) cyclic voltammogram curves of the CuSbS2 nanoplate supercapacitor device at different scan rates using KOH, (c) cyclic voltammogram curves of the CuSbS2 nanoplate supercapacitor device at different bending angles, (d) cyclic voltammogram curves showing cyclic stability of the CuSbS2 nanoplate device, and (e) cycling performance of the CuSbS2 nanoplate supercapacitor device at a constant current of 1 mA. The inset shows the first few cycles of the charge–discharge curves. Reproduced with permission from Ramasamy et al., J. Mater. Chem. A, 2015, 3, 13263.171 | ||
Therefore, the transition-metal chalcogenides have emerged as attractive electrode materials for high-performance flexible supercapacitors due to their inherently high specific capacitance and storage capacity coupled with their tunable mechanical properties. The electrochemical properties of the chalcogenides including rate, cycling stability, and specific capacity can be further enhanced through shape-control while the mechanical properties are further improved using flexible carbon-based supports. One such example of an improved electrode material for flexible supercapacitors is the Ni3Co6S8 nanowire array supported on nitrogen-doped carbon foams. Layered chalcogenides are also attractive for flexible supercapacitors as the interlayer spacing can be controlled to achieve enhanced electrical conductivity in these materials. For example, MoS2 nanoparticles supported on carbon nanofibers exhibit remarkable electrochemical performance as flexible supercapacitors. A vast increase in cycling stability has also been achieved with hybrid materials containing chalcogenide nanoparticles embedded in MXenes.
6. Future perspectives
Transition-metal chalcogenides and their layered architectures offer a unique class of materials with tunable properties for next-generation electrochemical energy storage devices. For example, our group has realized layered CuSbS2 nanoplates with tunable thickness ranging from six to several layers and CuSbS2 mesobelts that can be exfoliated to a monolayer thickness. These materials offer thickness-dependent electronic and optical properties and are attractive as electrodes for next-generation flexible supercapacitors.28,171,179 Our group has also synthesized a new class of multinary Cu2ZnAS4−x and CuZn2AS4 (A = Al, Ga, In) nanocrystals in the metastable wurtzite phase. The electronic structures of these new chalcogenide semiconductors show a dependence on the ionic radius of the group III cation, which can be tuned to achieve a desired electronic property for electrochemical energy storage. However, the practical viability of transition-metal chalcogenides as standalone electrode materials for batteries and flexible supercapacitors is limited due to their inherent constraints in structural stability and charge transport. Therefore, several novel strategies have been realized to further enhance the electrochemical and structural properties of the chalcogenides. Among them, various organic and inorganic materials for support, hybrid nanostructures with carbonaceous or conducting polymeric materials, doping with heteroatoms, and realizing multinary chalcogenide compositions have paved the way for attractive electrochemical properties and viability of chalcogenide-based electrodes for energy storage. A three-dimensional porous network provides a key material platform for various chalcogenide-based electrodes for electrochemical energy storage, but limited information is currently available on their charge transport dynamics. Understanding the mechanisms of ion/electron diffusion in these new hybrid porous nanostructures through advanced material characterization tools including high-throughput synchrotron X-ray diffraction, small-angle X-ray scattering, and electron energy loss spectroscopy will be the key to realizing practically viable and high-energy density electrodes required for emerging energy storage technologies.180Tellurides have higher volumetric energy density and unique electronic conductivity as compared to sulfides and selenides, offering an attractive alternative for high-throughput electrochemical energy storage in terms of facilitating lightweight and miniaturized next-generation devices.181 The larger size of the Te ion favors enhanced rates of electrochemical energy conversion reaction. The larger interlayer spacing in tellurides provides a platform for increased diffusion of ions. Tellurides in the nanoscale form also offer higher structural stability to withstand volume expansion during the electrochemical energy conversion process. Therefore, these materials could be the key to portable and enhanced energy storage.
Electrosorption in the new layered and porous electrode materials that have been realized for high-throughput energy storage applications involves complex capacitive mechanisms beyond the traditional non-faradaic electric double-layer capacitance and faradaic charge transfer via pseudocapacitance. The unique electrolyte confinement in these materials involves new phenomena at the interface of the electric double layer and pseudocapacitive charge transfer, which open the scope for rich innovations in future energy storage technologies.182
One of the niche future directions for electrochemical energy storage is realizing hybrid devices that synergize multiple desirable properties such as high energy and power densities, long cycle-life, and rapid self-charging within a single device. For example, an asymmetric supercapacitor combines a battery-type electrode with a supercapacitor electrode to exploit the different potential windows of the electrodes for achieving a four-fold higher energy density as compared to regular supercapacitors.183–190 However, the specific power and cycling life can be limiting metrics for these hybrid platforms. Therefore, different battery technologies and supercapacitors have been hybridized to achieve an alternative class of energy storage devices that can meet both the high energy and high power density requirements.191–193 With the increasing application of portable and wearable electronics, next-generation energy storage devices in the form of self-charging supercapacitors have been developed that integrates energy harvesting from sustainable sources (e.g., wind, water, and solar) and supercapacitor technologies within the same device.194–196 These hybrid technologies synergize energy harvesting from renewable sources with the advantages of supercapacitors to achieve the next frontier of electrochemical energy storage devices.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
SP acknowledges the AFRL/DAGSI Ohio-Student Faculty fellowship RX-2210, University of Dayton's (UD) Research Council Summer grant, and UD Chemical and Materials Engineering for support. SP and AG acknowledge ORNL NTI and ORNL STEM, respectively, as this research was conducted as part of a user project at the Center for Nanophase Materials Sciences (CNMS), which is a US Department of Energy, Office of Science User Facility at Oak Ridge National Laboratory.References
- A. Bielecki, S. Ernst, W. Skrodzka and I. Wojnicki, Environ. Sci. Pollut. Res., 2020, 27, 11506 CrossRef PubMed.
- K. Mehta, M. Ehrenwirth, C. Trinkl, W. Zorner and R. Greenough, Energies, 2021, 14, 2805 CrossRef.
- R. Marks-Bielska, S. Bielski, K. Pik and K. Kurowska, Energies, 2020, 13, 4624 CrossRef CAS.
- K. Lukasiewicz, P. Pietrzak, J. Kraciuk, E. Kacperska and M. Cieciora, Energies, 2022, 15, 8284 CrossRef.
- K. Mensah-Darkwa, D. N. Ampong, E. Agyekum, F. M. de Souza and R. K. Gupta, Energies, 2022, 15, 4052 CrossRef CAS.
- M. Dai and R. Wang, Small, 2021, 17, 2006813 CrossRef CAS PubMed.
- S. Palchoudhury, K. Ramasamy, R. K. Gupta and A. Gupta, Front. Mater., 2019, 5, 83 CrossRef.
- P. Galek, A. Mackowiak, P. Bujewska and K. Fic, Front. Energy Res., 2020, 8, 139 CrossRef.
- P. Simon and Y. Gogotsi, Nat. Mater., 2008, 7, 845 CrossRef CAS PubMed.
- H. Budde-Meiwes, J. Drillkens, B. Lunz, J. Muennix, S. Rothgang, J. Kowal and D. U. A. Sauer, Proc. Inst. Mech. Eng., Part D, 2013, 227, 761 CrossRef CAS.
- J. Theerthagiri, K. Karuppasamy, G. Durai, A. S. Rana, P. Arunachalam, K. Sangeetha, P. Kuppusami and H. S. Kim, Nanomater, 2018, 8, 256 CrossRef PubMed.
- G. A. Muller, J. B. Cook, H. S. Kim, S. H. Tolbert and B. Dunn, Nano Lett., 2015, 15, 1911 CrossRef CAS PubMed.
- Y. Zhang, L. Zhang, T. A. Lv, P. K. Chu and K. F. Huo, ChemSusChem, 2020, 13, 1114 CrossRef CAS PubMed.
- T. Lv, G. Zhu, S. Dong, Q. Kong, Y. Peng, S. Jiang, G. Zhang, Z. Yang, S. Yang, X. Dong, H. Pang and Y. Zhang, Angew. Chem., Int. Ed., 2022, 62, e202216089 Search PubMed.
- M. F. Sajjad, F. Cheng and W. Lu, RSC Adv., 2021, 11, 25450 RSC.
- J. Cherusseri, N. Choudhary, K. S. Kumar, Y. Jung and J. Thomas, Nanoscale Horiz., 2019, 4, 840 RSC.
- M. A. Bissett, S. D. Worrall, I. A. Kinloch and R. A. W. Dryfe, Electrochim. Acta, 2016, 201, 30 CrossRef CAS.
- H. Xia, Q. Xu and J. Zhang, Nano-Micro Lett., 2018, 10, 66 CrossRef PubMed.
- S. Song, Y. Sim, S. Kim, J. Hwa, I. Oh, W. Na, D. Lee, J. Wang, S. Yan, Y. Liu, J. Kwak, J. Chen, H. Cheong, J. Yoo, Z. Lee and S. Kwon, Nat. Electron., 2020, 3, 207 CrossRef CAS.
- Y. Sun, Y. Wang, J. Chen, K. Fujisawa, C. Holder, J. Miller, V. Crespi, M. Terrones and R. Schaak, Nat. Chem., 2020, 12, 284 CrossRef CAS PubMed.
- J. Fenton, B. Steimle and R. Schaak, Science, 2018, 360, 513 CrossRef CAS PubMed.
- H. Lim, Y. Nakanishi, Z. Liu, J. Pu, M. Maruyama, T. Endo, C. Ando, H. Shimizu, K. Yanagi, S. Okada, T. Takenobu and Y. Miyata, Nano Lett., 2021, 21, 243 CrossRef CAS PubMed.
- W. Li, X. Wei, H. Dong, Y. Ou, S. Xiao, Y. Yang, P. Xiao and Y. Zhang, Front. Chem., 2020, 8, 189 CrossRef CAS PubMed.
- S. Palchoudhury, K. Ramasamy and A. Gupta, Nanoscale Adv., 2020, 2, 3069 RSC.
- A. Ghosh, S. Palchoudhury, T. Rajalingam, Z. Zhou, N. Naghibolashrafi, K. Ramasamy and A. Gupta, Chem. Commun., 2016, 52, 264 RSC.
- N. Tapia-Ruiz, A. Armstrong, H. Alptekin, M. Amores, H. Au, J. Barker, R. Boston, W. Brant, J. Brittain and Y. Y. Chen, et al. , J. Phys.: Energy, 2021, 3, 031503 CAS.
- Q. Pang, J. Meng, S. Gupta, X. Hong, C. Kwok, J. Zhao, Y. Jin, L. Xu, O. Karahan, Z. Wang, S. Toll, L. Mai, L. Nazar, M. Balasubramanium, B. Narayan and D. R. Sadoway, Nature, 2022, 608, 704 CrossRef CAS PubMed.
- K. Ramasamy, R. K. Gupta, S. Palchoudhury, S. Ivanov and A. Gupta, Chem. Mater., 2015, 27, 379 CrossRef CAS.
- J. Goodenough and K. Park, J. Am. Chem. Soc., 2013, 135, 1167 CrossRef CAS PubMed.
- K. Turcheniuk, D. Bondarev, V. Singhal and G. Yushin, Nature, 2018, 559, 467 CrossRef CAS PubMed.
- J. Xie and Y. Lu, Nat. Commun., 2020, 11, 2499 CrossRef CAS PubMed.
- Y. Sun, N. Liu and Y. Cui, Nat. Energy, 2016, 1, 16071 CrossRef CAS.
- X. Chen, J. Zhou, J. Li, H. Luo, L. Mei, T. Wang, J. Zhu and Y. Zhang, RSC Adv., 2019, 9, 35045 RSC.
- R. Jin, W. Cui and R. Li, Solid State Ionics, 2021, 366, 115660 CrossRef.
- Q. Wang, K. Kalantar-Zadeh, A. Kis, J. Coleman and M. Strano, Nat. Nanotechnol., 2012, 7, 699 CrossRef CAS PubMed.
- P. Zhou, G. Collins, Z. Hens, K. Ryan, H. Geaney and S. Singh, Nanoscale, 2020, 12, 22307 RSC.
- L. Peng, Y. Zhu, D. Chen, R. Ruoff and G. Yu, Adv. Energy Mater., 2016, 6, 1600025 CrossRef.
- B. Zhang, X. Ji, K. Xu, C. Chen, X. Xiong, J. Xiong, Y. Yao, L. Miao and J. Jiang, Electrochim. Acta, 2016, 217, 1–8 CrossRef CAS.
- C. Wei, Z. Hou, H. Sun and J. Wang, Front. Chem., 2021, 9, 802788 CrossRef CAS PubMed.
- L. Chen, H. Jiang, H. Jiang, H. Zhang, S. Guo, Y. Hu and C. Li, Adv. Energy Mater., 2017, 7, 1602782 CrossRef.
- H. Xu, L. Sun, W. Li, M. Gao, Q. Zhou, P. Li, S. Yang and J. Lin, Chem. Eng. J., 2022, 435(PT 2), 135129 CrossRef CAS.
- X. Li, J. Fu, Y. Sun, M. Sun, S. Cheng, K. Chen, X. Yang, Q. Lou, T. Xu, Y. Shang, J. Xu, Q. Chen and C. Shan, Nanoscale, 2019, 11, 13343 RSC.
- F. Ming, H. Liang, Y. Lei, W. Zhang and H. Alshareef, Nano Energy, 2018, 53, 11–16 CrossRef CAS.
- Y. Wang, D. Kong, W. Shi, B. Liu, G. Sim, Q. Ge and H. Yang, Adv. Energy Mater., 2016, 6, 1601057 CrossRef.
- C. Chen, X. Xie, B. Anasori, A. Sarycheva, T. Makaryan, M. Zhao, P. Urbankowski, L. Miao, J. Jiang and Y. Gogotsi, Angew. Chem., Int. Ed., 2018, 57, 1846 CrossRef CAS PubMed.
- M. Cao, L. Gao, X. Lv and Y. Shen, J. Power Sources, 2017, 350, 87 CrossRef CAS.
- P. Geng, L. Wang, M. Du, Y. Bai, W. Li, Y. Liu, S. Chen, P. Braunstein, Q. Xu and H. Pang, Adv. Mater., 2022, 34, 2107836 CrossRef CAS PubMed.
- S. Zheng, Y. Sun, H. Xue, P. Braunstein, W. Huang and H. Pang, Natl. Sci. Rev., 2022, 9, nwab197 CrossRef CAS PubMed.
- Y. Ding, C. Wang, R. Zheng, S. Maitra, G. Zhang, T. Barakat, S. Roy, B. Su and L. Chen, EnergyChem, 2022, 4, 100081 CrossRef CAS.
- L. Guo, Z. Mu, P. Da, Z. Weng, P. Xi and C. Yan, Hollow structures with rare earths: Synthesis and electrocatalytic applications, EnergyChem, 2022, 4, 100088 CrossRef CAS.
- J. Broadhead, F. A. Trumbore and S. Basu, J. Electroanal. Chem., 1981, 118, 241 CrossRef CAS.
- L. Li, Z. Li, A. Yoshimura, C. Sun, T. Wang, Y. Chen, Z. Chen, A. Littlejohn, Y. Xiang, P. Hundekar, S. Bartolucci, J. Shi, S. Shi, V. Meunier, G. Wang and N. Koratkar, Nat. Commun., 2019, 10, 1764 CrossRef PubMed.
- J. Zhao, R. Huang, P. Ramos, Y. Yue, Q. Wu, M. Pavanello, J. Zhou, X. Kuai, L. Gao, H. He and Y. Wang, ACS Appl. Mater. Interfaces, 2017, 9, 31181 CrossRef CAS PubMed.
- W. Fang, H. Zhao, Y. Xie, J. Fang, J. Xu and Z. Chen, ACS Appl. Mater. Interfaces, 2015, 7, 13044 CrossRef CAS PubMed.
- H. Hwang, H. Kim and J. Cho, Nano Lett., 2011, 11, 4826 CrossRef CAS PubMed.
- Y. Chen, B. Song, X. Tang, L. Lu and J. Xue, Small, 2014, 10, 1536 CrossRef CAS PubMed.
- D. Chen, G. Ji, B. Ding, Y. Ma, B. Qu, W. Chen and J. Lee, Nanoscale, 2013, 5, 7890 RSC.
- Y. Shi, Y. Wang, J. Wong, A. Tan, C. Hsu, L. Li, Y. Lu and H. Yang, Sci. Rep., 2013, 3, 2169 CrossRef PubMed.
- C. Hu, L. Lv, J. Xue, M. Ye, L. Wang and L. Qu, Chem. Mater., 2015, 27, 5253 CrossRef CAS.
- Z. Wu, W. Ren, L. Xu, F. Li and H. Cheng, ACS Nano, 2011, 5, 5463 CrossRef CAS PubMed.
- P. Xiong, B. Liu, V. Teran, Y. Zhao, L. Peng, X. Wang and G. Yu, ACS Nano, 2014, 8, 8610 CrossRef CAS PubMed.
- X. Huang, X. Xia, Y. Yuan and F. Zhou, Electrochim. Acta, 2011, 56, 4960 CrossRef CAS.
- W. Li, F. Wang, S. Feng, J. Wang, Z. Sun, B. Li, Y. Li, J. Yang, A. Elzatahry, Y. Xia and D. Zhao, J. Am. Chem. Soc., 2013, 135, 18300 CrossRef CAS PubMed.
- S. Chen, Y. Xin, Y. Zhou, Y. Ma, H. Zhou and L. Qi, Energy Environ. Sci., 2014, 7, 1924 RSC.
- Y. Zhao, L. Peng, B. Liu and G. Yu, Nano Lett., 2014, 14, 2849 CrossRef CAS PubMed.
- X. Rui, X. Zhao, Z. Lu, H. Tan, D. Sim, H. Hng, R. Yazami, T. Lim and Q. Yan, ACS Nano, 2013, 7, 5637 CrossRef CAS PubMed.
- M. Naguib, M. Kurtoglu, V. Presser, J. Lu, J. Niu, M. Heon, L. Hultman, Y. Gogotsi and M. Barsoum, Adv. Mater., 2011, 23, 4248 CrossRef CAS PubMed.
- M. Naguib, J. Halim, J. Lu, K. Cook, L. Hultman, Y. Gogotsi and M. Barsoum, J. Am. Chem. Soc., 2013, 135, 15966 CrossRef CAS PubMed.
- G. Che, K. Jirage, E. Fisher, C. Martin and H. Yoneyama, J. Electrochem. Soc., 1997, 144, 4296 CrossRef CAS.
- J. Cho, Y. Kim, B. Kim, J. Lee and B. Park, Angew. Chem., Int. Ed., 2003, 42, 1618 CrossRef CAS PubMed.
- F. Lin, I. Markus, D. Nordlund, T. Weng, M. Asta, H. Xin and M. Doeff, Nat. Commun., 2014, 5, 3529 CrossRef PubMed.
- C. Gong, Z. Xue, S. Wen, Y. Ye and X. Xie, J. Power Sources, 2016, 318, 93 CrossRef CAS.
- M. Lee, S. Lee, P. Oh, Y. Kim and J. Cho, Nano Lett., 2014, 14, 993 CrossRef CAS PubMed.
- A. Yamada, S. Chung and K. Hinokuma, J. Electrochem. Soc., 2001, 148, A224 CrossRef CAS.
- N. Nitta, F. Wu, J. Lee and G. Yushin, Mater. Today, 2015, 18, 252 CrossRef CAS.
- M. Fichtner, K. Edstrom, E. Ayerbe, M. Berecibar, A. Bhowmik, I. Castelli, S. Clark, R. Dominko, M. Erakca, A. Franco, A. Grimaud, B. Horstmann, A. Latz, H. Lorrman, M. Meeus, R. Narayan, F. Pammer, J. Ruhland, H. Stein, T. Vegge and M. Weil, Adv. Energy Mater., 2022, 12, 2102904 CrossRef CAS.
- Z. Ali, T. Zhang, M. Asif, L. Zhao, Y. Yu and Y. Hou, Mater. Today, 2020, 35, 131 CrossRef CAS.
- Y. Fang, D. Luan, Y. Chen, S. Gao and X. Lou, Angew. Chem., Int. Ed., 2020, 59, 2644 CrossRef CAS PubMed.
- G. Eshetu, G. Elia, M. Armand, M. Forsyth, S. Komaba, T. Rojo and S. Passerini, Adv. Energy Mater., 2020, 10, 2000093 CrossRef CAS.
- J. Ma, Y. Li, N. S. Grundish, J. B. Goodenough, Y. Chen, L. Guo, Z. Peng, X. Qi, F. Yang, L. Qie, C. Wang, B. Huang, Z. Huang, L. Chen, D. Su, G. Wang, X. Peng, Z. Chen, J. Yang, S. He, X. Zhang, H. Yu, C. Fu, M. Jiang, W. Deng, C. Sun, Q. Pan, Y. Tang, X. Li, X. Ji, F. Wan, Z. Niu, F. Lian, C. Wang, G. G. Wallace, M. Fan, Q. Meng, S. Xin, Y. Guo and L. Wan, The 2021 battery technology roadmap, J. Phys. D: Appl. Phys., 2021, 54, 183001 CrossRef CAS.
- S. Li, S. Luo, L. Rong, L. Wang, Z. Xi, Y. Liu, Y. Zhou, Z. Wan and X. Kong, Molecules, 2022, 27, 3989 CrossRef CAS PubMed.
- S. Dai, L. Wang, M. Cao, Z. Zhong, Y. Shen and M. Wang, Mater. Today Energy, 2019, 12, 114 CrossRef.
- Y. Fang, X. Yu and X. Lou, Angew. Chem., Int. Ed., 2019, 58, 7744 CrossRef CAS PubMed.
- Z. Li, Y. Fang, J. Zhang and X. Lou, Adv. Mater., 2018, 30, 1800525 CrossRef PubMed.
- E. Yang, H. Ji and Y. Jung, J. Phys. Chem. C, 2015, 119, 26374 CrossRef CAS.
- Y. Fang, X. Yu and X. Lou, Adv. Mater., 2018, 30, 1706668 CrossRef PubMed.
- Y. Huang, M. Xie, Z. Wang, Y. Jiang, G. Xiao, S. Li, L. Li, F. Wu and R. Chen, Energy Storage Mater., 2018, 11, 100 CrossRef.
- K. Zhang, M. Park, L. Zhou, G. Lee, J. Shin, Z. Hu, S. Chou, J. Chen and Y. Kang, Angew. Chem., Int. Ed., 2016, 55, 12822 CrossRef CAS PubMed.
- Z. Li, W. Feng, Y. Lin, X. Liu and H. Fei, RSC Adv., 2016, 6, 70632 RSC.
- S. Chen, S. Huang, Y. Zhang, S. Fan, D. Yan, Y. Shang, M. Pam, Q. Ge, Y. Shi and H. Yang, Nano Energy, 2020, 69, 104389 CrossRef CAS.
- J. Zhou, L. Wang, M. Yang, J. Wu, F. Chen, W. Huang, N. Han, H. Ye, F. Zhao, Y. Li and Y. Li, Adv. Mater., 2017, 29, 1702061 CrossRef PubMed.
- D. Yu, Q. Pang, Y. Gao, Y. Wei, C. Wang, G. Chen and F. Du, Energy Storage Mater., 2018, 11, 1–7 CrossRef CAS.
- Y. Pang, S. Zhang, L. Liu, J. Liang, Z. Sun, Y. Wang, C. Xiao, D. Ding and S. Ding, J. Mater. Chem. A, 2017, 5, 17963 RSC.
- D. Xie, X. Xia, Y. Zhong, Y. Wang, D. Wang, X. Wang and J. Tu, Adv. Energy Mater., 2021, 11, 2003595 CrossRef CAS.
- N. Mahmood, T. Tang and Y. Hou, Adv. Energy Mater., 2016, 6, 1600374 CrossRef.
- D. Sun, D. Ye, P. Liu, Y. Tang, J. Guo, L. Wang and H. Wang, Adv. Energy Mater., 2018, 8, 1702383 CrossRef.
- X. Hu, Y. Li, G. Zeng, J. Jia, H. Zhan and Z. Wen, ACS Nano, 2018, 12, 1592 CrossRef CAS PubMed.
- X. Xie, T. Makaryan, M. Zhao, K. Van Aken, Y. Gogotsi and G. Wang, Adv. Energy Mater., 2016, 6, 1502161 CrossRef.
- X. Xiong, C. Yang, G. Wang, Y. Lin, X. Ou, J. Wang, B. Zhao, M. Liu, Z. Lin and K. Huang, Energy Environ. Sci., 2017, 10, 1757 RSC.
- B. Qu, C. Ma, G. Ji, C. Xu, J. Xu, Y. Meng, T. Wang and J. Lee, Adv. Mater., 2014, 26, 3854 CrossRef CAS PubMed.
- Y. Liu, Y. Yang, X. Wang, Y. Dong, Y. Tang, Z. Yu, Z. Zhao and J. Qiu, ACS Appl. Mater. Interfaces, 2017, 9, 15484 CrossRef CAS PubMed.
- Z. Wei, L. Wang, M. Zhuo, W. Ni, H. Wang and J. Ma, J. Mater. Chem. A, 2018, 6, 12185 RSC.
- R. Chen, S. Li, J. Liu, Y. Li, F. Ma, J. Liang, X. Chen, Z. Miao, J. Han, T. Wang and Q. Li, Electrochim. Acta, 2018, 282, 973 CrossRef CAS.
- X. Yang, R. Zhang, N. Chen, X. Meng, P. Yang, C. Wang, Y. Zhang, Y. Wei, G. Chen and F. Du, Chem.–Eur. J., 2016, 22, 1445 CrossRef CAS PubMed.
- Y. Dong, W. Shi, P. Lu, J. Qin, S. Zheng, B. Zhang, X. Bao and Z. Wu, J. Mater. Chem. A, 2018, 6, 14324 RSC.
- L. Zhou, K. Zhang, J. Sheng, Q. An, Z. Tao, Y. Kang, J. Chen and L. Mai, Nano Energy, 2017, 35, 281 CrossRef CAS.
- Z. Shadike, M. Cao, F. Ding, L. Sang and Z. Fu, Chem. Commun., 2015, 51, 10486 RSC.
- J. Deng, Q. Gong, H. Ye, K. Feng, J. Zhou, C. Zha, J. Wu, J. Chen, J. Zhong and Y. Li, ACS Nano, 2018, 12, 1829 CrossRef CAS PubMed.
- J. Li, J. Li, D. Yan, S. Hou, X. Xu, T. Lu, Y. Yao, W. Mai and L. Pan, J. Mater. Chem. A, 2018, 6, 6595 RSC.
- D. Zhang, W. Sun, Y. Zhang, Y. Dou, Y. Jiang and S. Dou, Adv. Funct. Mater., 2016, 26, 7479 CrossRef CAS.
- C. Dong, J. Liang, Y. He, C. Li, X. Chen, L. Guo, F. Tian, Y. Qian and L. Xu, ACS Nano, 2018, 12, 8277 CrossRef CAS PubMed.
- B. Dong, W. Li, X. Huang, Z. Ali, T. Zhang, Z. Yang and Y. Hou, Nano Energy, 2019, 55, 37 CrossRef CAS.
- D. Su, S. Dou and G. Wang, Chem. Commun., 2014, 50, 4192 RSC.
- Y. Wang, D. Kong, S. Huang, Y. Shi, M. Ding, Y. Lim, T. Xu, F. Chen, X. Li and H. Yang, J. Mater. Chem. A, 2018, 6, 10813 RSC.
- Y. Liu, N. Zhang, H. Kang, M. Shang, L. Jiao and J. Chen, Chem.–Eur. J., 2015, 21, 11878 CrossRef CAS PubMed.
- Z. Liang, H. Tu, K. Zhang, Z. Kong, M. Huang, D. Xu, S. Liu, Y. Wu and X. Hao, Chem. Eng. J., 2022, 437, 135421 CrossRef CAS.
- Y. Chen, H. Liu, X. Guo, S. Zhu, Y. Zhao, S. Iikubo and T. Ma, ACS Appl. Mater. Interfaces, 2021, 13, 39248 CrossRef CAS PubMed.
- X. Yu and X. Lou, Adv. Energy Mater., 2018, 8, 1701592 CrossRef.
- L. Shen, L. Yu, H. Wu, X. Yu, X. Zhang and X. Lou, Nat. Commun., 2015, 6, 6694 CrossRef CAS PubMed.
- J. Li, B. Liu, M. Li, Q. Zhou, Q. Chen, C. Guo and L. Zhang, Eur. J. Inorg. Chem., 2018, 2018, 3036 CrossRef CAS.
- H. Geng, J. Yang, Z. Dai, Y. Zhang, Y. Zheng, H. Yu, H. Wang, Z. Luo, Y. Guo, H. Fan, X. Wu, J. Zheng, Y. Yang, Q. Yan and H. Gu, Small, 2017, 13, 1603490 CrossRef PubMed.
- S. Dong, C. Li, X. Ge, Z. Li, X. Miao and L. Yin, ACS Nano, 2017, 11, 6474 CrossRef CAS PubMed.
- X. Liu, Y. Wang, Z. Wang, T. Zhou, M. Yu, L. Xiu and J. Qiu, J. Mater. Chem. A, 2017, 5, 10398 RSC.
- H. Dai, W. Xu, Z. Hu, Y. Chen, X. Wei, B. Yang, Z. Chen, J. Gu, D. Yang, F. Xie, W. Zhang, R. Guo, G. Zhang and W. Wei, Front. Energy Res., 2020, 8, 97 CrossRef.
- N. Tanibata, K. Noi, A. Hayashi and M. Tatsumisago, RSC Adv., 2014, 4, 17120 RSC.
- T. Krauskopf, S. P. Culver and W. G. Zeier, Inorg. Chem., 2018, 57, 4739 CrossRef CAS PubMed.
- S. Takeuchi, K. Suzuki, M. Hirayama and R. Kanno, J. Solid State Chem., 2018, 265, 353 CrossRef CAS.
- Q. Zhang, C. Zhang, Z. Hood, M. Chi, C. Liang, N. H. Jalarvo, M. Yu and H. Wang, Chem. Mater., 2020, 32, 2264 CrossRef CAS.
- S. Bo, Y. Wang, J. Kim, W. Richards and G. Ceder, Chem. Mater., 2016, 28, 252 CrossRef CAS.
- M. M. A. Mahmoud, D. P. Joubert and M. P. Molepo, Eur. Phys. J. B, 2019, 92, 214 CrossRef CAS.
- Y. Wang, W. Richards, S. Bo, L. Miara and G. Ceder, Chem. Mater., 2017, 29, 7475 CrossRef CAS.
- B. Ma, Q. Jiao, Y. Zhang, X. Sun, G. Yin, X. Zhang, H. Ma, X. Liu and S. Dai, Ceram. Int., 2020, 46, 6544 CrossRef CAS.
- K. Park, D. Kim, H. Kwak, S. Jung, H. Lee, A. Banerjee, J. Lee and Y. Jung, J. Mater. Chem. A, 2018, 6, 17192 RSC.
- F. Tsuji, N. Tanibata, A. Sakuda, A. Hayashi and M. Tatsumisago, Chem. Lett., 2018, 47, 13 CrossRef CAS.
- Z. Zhu, I. Chu, Z. Deng and S. Ong, Chem. Mater., 2015, 27, 8318 CrossRef CAS.
- N. J. J. de Klerk and M. Wagemaker, Chem. Mater., 2016, 28, 3122 CrossRef CAS.
- H. Wan, L. Cai, W. Weng, J. Mwizerwa, J. Yang and X. Yao, J. Power Sources, 2020, 449, 227515 CrossRef CAS.
- S. Yubuchi, A. Ito, N. Masuzawa, A. Sakuda, A. Hayashi and M. Tatsumisago, J. Mater. Chem. A, 2020, 8, 1947 RSC.
- J. Zhou, Y. Liu, S. Zhang, T. Zhou and Z. Guo, InfoMat, 2020, 2, 437 CrossRef CAS.
- Q. Yu, B. Jiang, J. Hu, C. Lao, Y. Gao, P. Li, Z. Liu, G. Suo, D. He, W. Wang and G. Yin, Adv. Sci., 2018, 5, 1800782 CrossRef PubMed.
- W. Zhang, Y. Liu and Z. Guo, Sci. Adv., 2019, 5, eaav741 Search PubMed.
- Y. Xu, C. Zhang, M. Zhou, Q. Fu, C. Zhao, M. Wu and Y. Lei, Nat. Commun., 2018, 9, 1720 CrossRef PubMed.
- Y. Liu, Z. Tai, J. Zhang, W. Pang, Q. Zhang, H. Feng, K. Konstantinov, Z. Guo and H. Liu, Nat. Commun., 2018, 9, 3645 CrossRef PubMed.
- L. Fang, J. Xu, S. Sun, B. Lin, Q. Guo, D. Luo and H. Xia, Small, 2019, 15, 1804806 CrossRef PubMed.
- C. He, J. Zhang, W. Zhang and T. Li, J. Phys. Chem. C, 2019, 123, 5157 CrossRef CAS.
- Z. Yi, Y. Qian, J. Tian, K. Shen, N. Lin and Y. Qian, J. Mater. Chem. A, 2019, 7, 12283 RSC.
- B. Jia, Q. Yu, Y. Zhao, M. Qin, W. Wang, Z. Liu, C. Lao, Y. Liu, H. Wu, Z. Zhang and X. Qu, Adv. Funct. Mater., 2018, 28, 1803409 CrossRef.
- J. Ge, L. Fan, J. Wang, Q. Zhang, Z. Liu, E. Zhang, Q. Liu, X. Yu and B. Lu, Adv. Energy Mater., 2018, 8, 1801477 CrossRef.
- Y. Zhao, J. Zhu, S. Ong, Q. Yao, X. Shi, K. Hou, Z. Xu and L. Guan, Adv. Energy Mater., 2018, 8, 1802565 CrossRef.
- L. Li, W. Zhang, X. Wang, S. Zhang, Y. Liu, M. Li, G. Zhu, Y. Zheng, Q. Zhang, T. Zhou, W. Pang, W. Luo, Z. Guo and J. Yang, ACS Nano, 2019, 13, 7939 CrossRef CAS PubMed.
- Y. Wu, H. Huang, Y. Feng, Z. Wu and Y. Yu, Adv. Mater., 2019, 31, 1901414 CrossRef CAS PubMed.
- M. Zhang, M. Shoaib, H. Fei, T. Wang, J. Zhong, L. Fan, L. Wang, H. Luo, S. Tan, Y. Wang, J. Zhu, J. Hu and B. Lu, Adv. Energy Mater., 2019, 9, 1901663 CrossRef CAS.
- C. Chen, Y. Yang, X. Tang, R. Qiu, S. Wang, G. Cao and M. Zhang, Small, 2019, 15, 1804740 CrossRef PubMed.
- X. Ren, Q. Zhao, W. McCulloch and Y. Wu, Nano Res., 2017, 10, 1313 CrossRef CAS.
- H. Yu, X. Cheng, M. Xia, T. Liu, W. Ye, R. Zheng, N. Long, M. Shui and J. Shu, Energy Storage Mater., 2019, 22, 154 CrossRef.
- L. Xu, P. Xiong, L. Zeng, Y. Fang, R. Liu, J. Liu, F. Luo, Q. Chen, M. Wei and Q. Qian, Nanoscale, 2019, 11, 16308 RSC.
- W. Miao, Y. Zhang, H. Li, Z. Zhang, L. Li, Z. Yu and W. Zhang, J. Mater. Chem. A, 2019, 7, 5504 RSC.
- H. He, D. Huang, Q. Gan, J. Hao, S. Liu, Z. Wu, W. Pang, B. Johannessen, Y. Tang, J. Luo, H. Wang and Z. Guo, ACS Nano, 2019, 13, 11843 CrossRef CAS PubMed.
- Z. Wu, G. Liang, W. Pang, T. Zhou, Z. Cheng, W. Zhang, Y. Liu, B. Johannessen and Z. Guo, Adv. Mater., 2020, 32, 1905632 CrossRef CAS PubMed.
- H. Tian, X. Yu, H. Shao, L. Dong, Y. Chen, X. Fang, C. Wang, W. Han and G. Wang, Adv. Energy Mater., 2019, 9, 1901560 CrossRef.
- F. Cheng, J. Liang, Z. Tao and J. Chen, Adv. Mater., 2011, 23, 1695 CrossRef CAS PubMed.
- X. Cao, C. Tan, M. Sindoro and H. Zhang, Chem. Soc. Rev., 2018, 47, 5997 RSC.
- S. Mishra, Chem. Commun., 2022, 58, 10136 RSC.
- S. Chong, L. Sun, C. Shu, S. Guo, Y. Liu, W. Wang and H. Liu, Nano Energy, 2019, 63, 103868 CrossRef CAS.
- S. Liu, H. Zhang, M. Zhou, X. Chen, Y. Sun and Y. Zhang, J. Electroanal. Chem., 2021, 903, 115841 CrossRef CAS.
- C. Yang, X. Ou, X. Xiong, F. Zheng, R. Hu, Y. Chen, M. Liu and K. Huang, Energy Environ. Sci., 2017, 10, 107 RSC.
- Q. Xue, J. Sun, Y. Huang, M. Zhu, Z. Pei, H. Li, Y. Wang, N. Li, H. Zhang and C. Zhi, Small, 2017, 13, 1701827 CrossRef PubMed.
- C. Zhang, X. Cai, Y. Qian, H. Jiang, L. Zhou, B. Li, L. Lai, Z. Shen and W. Huang, Adv. Sci., 2018, 5, 1700375 CrossRef PubMed.
- Y. Li, J. Gong, X. Xing, J. Du, P. Xu, K. Cheng, K. Ye, K. Zhu, J. Yan, D. Cao and G. Wang, Int. J. Energy Res., 2021, 45, 5517 CrossRef CAS.
- S. Zhai, Z. Fan, K. Jin, M. Zhou, H. Zhao, Y. Zhao, F. Ge, X. Li and Z. Cai, J. Colloid Interface Sci., 2020, 575, 306 CrossRef CAS PubMed.
- K. Ramasamy, R. K. Gupta, H. Sims, S. Palchoudhury, S. Ivanov and A. Gupta, J. Mater. Chem. A, 2015, 3, 13263 RSC.
- Z. Zhai, W. Yan, L. Dong, J. Wang, C. Chen, J. Lian, X. Wang, D. Xia and J. Zhang, Nano Energy, 2020, 78, 105193 CrossRef CAS.
- C. Mu, X. Sun, Y. Chang, F. Wen, A. Nie, B. Wang, J. Xiang, K. Zhai, T. Xue and Z. Liu, J. Power Sources, 2021, 482, 228987 CrossRef CAS.
- X. Hu, W. Shao, X. Hang, X. Zhang, W. Zhu and Y. Xie, Angew. Chem., Int. Ed., 2016, 55, 5733 CrossRef CAS PubMed.
- S. Yan, S. Luo, Q. Wang, Y. Zhang and X. Liu, Composites, Part B, 2021, 224, 109246 CrossRef CAS.
- S. Balasingam, J. Lee and Y. Jun, Dalton Trans., 2015, 44, 15491 RSC.
- N. Feng, R. Meng, L. Zu, Y. Feng, C. Peng, J. Huang, G. Liu, B. Chen and J. Yang, Nat. Commun., 2019, 10, 1372 CrossRef PubMed.
- F. Sari and J. Ting, Sci. Rep., 2017, 7, 5999 CrossRef PubMed.
- K. Ramasamy, H. Sims, W. Butler and A. Gupta, J. Am. Chem. Soc., 2014, 136, 1587 CrossRef CAS PubMed.
- Y. Yang, T. Zhu, L. Shen, Y. Liu, D. Zhang, B. Zheng, J. Gong, X. Zheng and X. Gong, SmartMat, 2022, 3, 349 CrossRef CAS.
- M. Han, Z. Zhou, Y. Li, Q. Chen and M. Chen, ChemElectroChem, 2021, 8, 4412 CrossRef CAS.
- S. Fleischmann, Y. Zhang, X. Wang, P. Cummings, J. Wu, P. Simon, Y. Gogotsi, V. Presser and V. Augustyn, Nat. Energy, 2022, 7, 222 CrossRef CAS.
- D. Gao, Z. Luo, C. Liu and S. Fan, Green Energy Environ., 2022 DOI:10.1016/j.gee.2022.02.002.
- F. Zhang, T. Zhang, X. Yang, L. Zhang, K. Leng, Y. Huang and Y. Chen, Energy Environ. Sci., 2013, 6, 1623 RSC.
- V. Aravindan, J. Gnanaraj, Y. Lee and S. Madhavi, Chem. Rev., 2014, 114, 11619 CrossRef CAS PubMed.
- V. Pushparaj, M. Shaijumon, A. Kumar, S. Murugesan, L. Ci, R. Vajtai, R. Linhardt, O. Nalamasu and P. Ajayan, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 13574 CrossRef CAS PubMed.
- L. Lam and R. Louey, J. Power Sources, 2006, 158, 1140 CrossRef CAS.
- M. Park, Y. Lim, J. Kim, Y. Kim, J. Cho and J. Kim, Adv. Energy Mater., 2011, 1, 1002 CrossRef CAS.
- M. Salanne, B. Rotenberg, K. Naoi, K. Kaneko, P. Taberna, C. Grey, B. Dunn and P. Simon, Nat. Energy, 2016, 1, 16070 CrossRef CAS.
- F. Wang, S. Xiao, Y. Hou, C. Hu, L. Liu and Y. Wu, RSC Adv., 2013, 3, 13059 RSC.
- D. Dubal, O. Ayyad, V. Ruiz and P. Gomez-Romero, Chem. Soc. Rev., 2015, 44, 1777 RSC.
- L. Kouchachvili, W. Yaici and E. Entchev, J. Power Sources, 2018, 374, 237 CrossRef CAS.
- B. Culpin and D. Rand, J. Power Sources, 1991, 36, 415 CrossRef CAS.
- F. Fan, Z. Tian and Z. Wang, Nano Energy, 2012, 1, 328 CrossRef CAS.
- Z. Wang, ACS Nano, 2013, 7, 9533 CrossRef CAS PubMed.
- F. Fan, J. Luo, W. Tang, C. Li, C. Zhang, Z. Tian and Z. Wang, J. Mater. Chem. A, 2014, 2, 13219 RSC.
| This journal is © The Royal Society of Chemistry 2023 |