Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis and characterization of bi(metallacycloprop-1-ene) complexes†
Wei
Bai
 ab,
Long Yiu
Tsang
ab,
Long Yiu
Tsang
 a,
Yilun
Wang
bc,
Yang
Li
a,
Yilun
Wang
bc,
Yang
Li
 *bc,
Herman H. Y.
Sung
*bc,
Herman H. Y.
Sung
 a,
Ian D.
Williams
a,
Ian D.
Williams
 *a and
Guochen
Jia
*a and
Guochen
Jia
 *a
*a
aDepartment of Chemistry, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong, P. R. China. E-mail: chjiag@ust.hk; chwill@ust.hk
bState Key Laboratory of Fine Chemicals, Department of Chemistry, School of Chemical Engineering, Dalian University of Technology, Liaoning 116024, P. R. China. E-mail: chyangli@dlut.edu.cn
cSchool of Chemical Engineering, Dalian University of Technology, Panjin, Liaoning 124221, P. R. China
First published on 16th November 2022
Abstract
In all previously reported metallacycloprop-1-ene or η2-vinyl complexes, the metal center bears only one vinyl moiety. We have now successfully synthesized and structurally characterized the first complexes bearing two η2-vinyl moieties or spiro bi(metallacycloprop-1-ene) complexes from reactions of alkynes with rhenium phosphine complexes. Computational studies indicate that the metallacycloprop-1-ene rings are aromatic and the complexes represent a rare σ-type spirometalla-aromatic system.
Introduction
Metallacycloprop-1-enes or η2-vinyl complexes (I, Scheme 1) are compounds with an organic vinyl fragment CR![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CR2 bound asymmetrically to a metal center through both carbon atoms.1 As the smallest metalla-carbocycles with an M
CR2 bound asymmetrically to a metal center through both carbon atoms.1 As the smallest metalla-carbocycles with an M![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and an M–C bond, these complexes are receiving increasing attention for their roles in organometallic synthesis2–9 and catalysis,10–16 as well as for their aromatic properties.17–20
C and an M–C bond, these complexes are receiving increasing attention for their roles in organometallic synthesis2–9 and catalysis,10–16 as well as for their aromatic properties.17–20
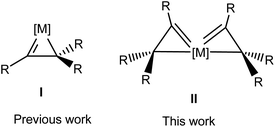 | ||
| Scheme 1 Structures of metallacycloprop-1-ene (I) and spiro bi[metallacycloprop-1-ene] (II) complexes. | ||
Well-defined metallacycloprop-1-ene complexes have been isolated with metals such as tungsten,21 molybdenum,22 rhenium,23 ruthenium,24 and osmium.25 In all these complexes, the metal center bears only one vinyl moiety (I, Scheme 1). In principle, a transition metal fragment may bind two or more η2-vinyl moieties to give interesting spiro metallacycloprop-1-ene complexes. However, such a possibility has not been previously demonstrated. In this work, we report the synthesis and characterization of the first complexes bearing two η2-vinyl moieties or spiro bi(metallacycloprop-1-ene) complexes (II, Scheme 1), a unique σ-type spiro metallaaromatic system.
Results and discussion
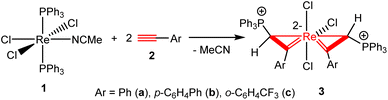
The first spiro bi(metallacycloprop-1-ene) complex was obtained by the reaction of ReCl3(PPh3)2(MeCN) (1) with PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH. Heating a mixture of 1 and four equivalents of phenylacetylene (2a) in dichloromethane at 70 °C for 1 h produced a yellow solution, from which the complex Re{η2-C(Ph)
CH. Heating a mixture of 1 and four equivalents of phenylacetylene (2a) in dichloromethane at 70 °C for 1 h produced a yellow solution, from which the complex Re{η2-C(Ph)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH(PPh3)}2Cl3 (3a) can be isolated as an orange solid in 68.1% yield (Scheme 2). The analogous complexes Re{η2-C(Ar)
CH(PPh3)}2Cl3 (3a) can be isolated as an orange solid in 68.1% yield (Scheme 2). The analogous complexes Re{η2-C(Ar)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH(PPh3)}2Cl3 (Ar = p-C6H4Ph (3b), o-C6H4CF3 (3c)) were obtained from the reactions of 1 with the corresponding aryl alkynes HC
CH(PPh3)}2Cl3 (Ar = p-C6H4Ph (3b), o-C6H4CF3 (3c)) were obtained from the reactions of 1 with the corresponding aryl alkynes HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CAr under similar conditions. In situ NMR experiments indicate that 1 is also reactive with alkyl alkynes such as 1-pentyne and 1-ethynylcyclohexene. The reactions gave mixtures of unidentified species, and the expected bi(metallacycloprop-1-ene) complexes analogous to 3 were not produced.
CAr under similar conditions. In situ NMR experiments indicate that 1 is also reactive with alkyl alkynes such as 1-pentyne and 1-ethynylcyclohexene. The reactions gave mixtures of unidentified species, and the expected bi(metallacycloprop-1-ene) complexes analogous to 3 were not produced.
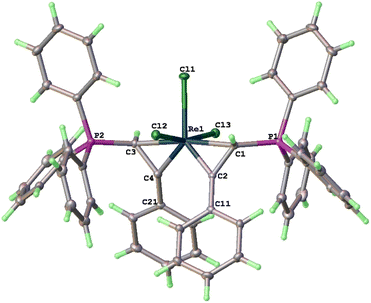
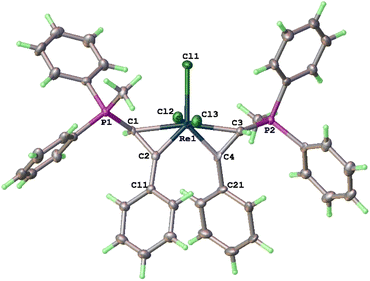
The structure of 3a has been determined by X-ray diffraction. As shown in Fig. 1, complex 3a contains three meridionally bound Cl ligands and two η2-PhC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH(PPh3) moieties. The Re–C(1) (2.211(3) Å) and Re–C(3) (2.205(3) Å) bond distances are typical of Re–C(sp3) bonds, while those of Re–C(2) and Re–C(4) (both are 1.903(3) Å) are typical of Re
CH(PPh3) moieties. The Re–C(1) (2.211(3) Å) and Re–C(3) (2.205(3) Å) bond distances are typical of Re–C(sp3) bonds, while those of Re–C(2) and Re–C(4) (both are 1.903(3) Å) are typical of Re![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C alkylidene double bonds. The C(1)–C(2) (1.467(4) Å) and C(3)–C(4) (1.469(4) Å) are typical of C–C single bonds with some associated π-character. The structural features associated with the Re(η2-CH(PPh3)CPh) fragments are similar to those of reported rhenacycloprop-1-ene complexes.23 Thus, complex 3a can be described as a spiro bi(metallacycloprop-1-ene) complex. The two spiro-connected rhenacycles are nearly co-planar or slightly twisted as evidenced by the twist angle between the MCC planes (25°). The two formal Re
C alkylidene double bonds. The C(1)–C(2) (1.467(4) Å) and C(3)–C(4) (1.469(4) Å) are typical of C–C single bonds with some associated π-character. The structural features associated with the Re(η2-CH(PPh3)CPh) fragments are similar to those of reported rhenacycloprop-1-ene complexes.23 Thus, complex 3a can be described as a spiro bi(metallacycloprop-1-ene) complex. The two spiro-connected rhenacycles are nearly co-planar or slightly twisted as evidenced by the twist angle between the MCC planes (25°). The two formal Re![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds are orthogonal cis to each other (93.19(12)°), while the two Re–C(sp3) single bonds are trans to each other (172.65(10)°). The two PPh3 substituents are oriented trans to each other. The spiro-concept derives from the conformational requirement of coplanarity of the aryl substituent with the metallacycloprop-1-ene ring, which twists either the C11 or C21 phenyl to the front. Both conformations are found in the crystal.
C bonds are orthogonal cis to each other (93.19(12)°), while the two Re–C(sp3) single bonds are trans to each other (172.65(10)°). The two PPh3 substituents are oriented trans to each other. The spiro-concept derives from the conformational requirement of coplanarity of the aryl substituent with the metallacycloprop-1-ene ring, which twists either the C11 or C21 phenyl to the front. Both conformations are found in the crystal.
The presence of the Re{η2-C(Ar)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH(PPh3)}2 moieties in 3 is supported by the NMR data. For example, the 1H NMR spectrum of 3b showed the CH(P) signal at 5.11 ppm. The 31P{1H} NMR spectrum of 3b showed a singlet at 24.5 ppm. The 13C{1H} NMR spectrum of 3b showed the Re
CH(PPh3)}2 moieties in 3 is supported by the NMR data. For example, the 1H NMR spectrum of 3b showed the CH(P) signal at 5.11 ppm. The 31P{1H} NMR spectrum of 3b showed a singlet at 24.5 ppm. The 13C{1H} NMR spectrum of 3b showed the Re![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C signal at 251.4 ppm and the CH(P) signal at 21.3 ppm.
C signal at 251.4 ppm and the CH(P) signal at 21.3 ppm.
In the solid state, complexes 3 are air-stable and can be stored at room temperature for at least 6 months without appreciable decomposition. In solution, they are also air-stable for at least 3 hours. However, decomposition was noted when a solution of complexes 3 in a halogenated solvent was exposed to air for a day.
Complexes 3a–c are interesting as they represent the first examples of spiro bi(metallacycloprop-1-ene) complexes or complexes bearing two η2-vinyl ligands at the same metal center. Complexes 3a–c are also unique mononuclear rhenium complexes with two hydrocarbyl carbene ligands. It is noted that mononuclear complexes with two hydrocarbyl carbene ligands (CHR and CR2) are rare and limited to a few of those of Os,26 W,27 Mo,28 Nb,29 and Ta.30
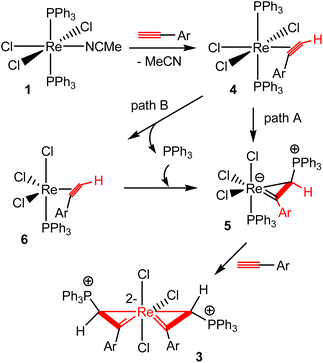
Scheme 3 shows a plausible mechanism for the formation of spiro bi(metallacycloprop-1-ene) complexes 3 from the reactions of 1 with HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CAr. The MeCN ligand in 1 is labile. Thus, complex 1 could initially undergo a ligand substitution reaction with HC
CAr. The MeCN ligand in 1 is labile. Thus, complex 1 could initially undergo a ligand substitution reaction with HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CAr to give an η2-alkyne complex ReCl3(η2-alkyne)(PPh3)2 (4). Complex 4 could evolve to the metallacycloprop-1-ene intermediate 5via the addition of a PPh3 to the terminal carbon of the coordinated aryl alkyne. Further reaction of intermediate 5 with another molecule of ArC
CAr to give an η2-alkyne complex ReCl3(η2-alkyne)(PPh3)2 (4). Complex 4 could evolve to the metallacycloprop-1-ene intermediate 5via the addition of a PPh3 to the terminal carbon of the coordinated aryl alkyne. Further reaction of intermediate 5 with another molecule of ArC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH would give a spiro bi(metallacycloprop-1-ene) complex 3. Both intramolecular migration of PR3 from metal to a coordinated alkyne31 and intermolecular attack of free PR3 on coordinated alkyne ligand32 have been suggested previously for addition reactions of phosphines with alkynes. Similar reaction pathways could be proposed for the addition of PPh3 to the ArC
CH would give a spiro bi(metallacycloprop-1-ene) complex 3. Both intramolecular migration of PR3 from metal to a coordinated alkyne31 and intermolecular attack of free PR3 on coordinated alkyne ligand32 have been suggested previously for addition reactions of phosphines with alkynes. Similar reaction pathways could be proposed for the addition of PPh3 to the ArC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH in the present case. For example, the intermediate 5 could be formed by intramolecular migration of a PPh3 ligand of rhenium in 4 to the terminal carbon of the coordinated aryl alkyne (path A), or dissociation of a PPh3 to give the complex 6, followed by intermolecular nucleophilic attack of the dissociated PPh3 at the terminal alkyne carbon in 6 (path B).
CH in the present case. For example, the intermediate 5 could be formed by intramolecular migration of a PPh3 ligand of rhenium in 4 to the terminal carbon of the coordinated aryl alkyne (path A), or dissociation of a PPh3 to give the complex 6, followed by intermolecular nucleophilic attack of the dissociated PPh3 at the terminal alkyne carbon in 6 (path B).
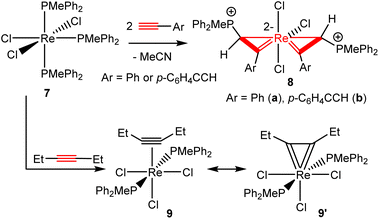
Analogous spiro bi(metallacycloprop-1-ene) complexes could be obtained from reactions of terminal alkynes with other Re(III) phosphine complexes. For example, the tris-phosphine complex ReCl3(PMePh2)3 (7) reacted with phenylacetylene (2a) and p-HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–C6H4–C
C–C6H4–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH in toluene at 100 °C for 2 h to produce the corresponding spiro bi(metallacycloprop-1-ene) complexes 8a and 8b, respectively (Scheme 4). Like complexes 3, complexes 8 are also air-stable in the solid state. Preliminary experiments showed that 7 might also react with alkyl alkynes to give metallacycloprop-1-ene complexes. However, further work is needed to define the structures of the products.
CH in toluene at 100 °C for 2 h to produce the corresponding spiro bi(metallacycloprop-1-ene) complexes 8a and 8b, respectively (Scheme 4). Like complexes 3, complexes 8 are also air-stable in the solid state. Preliminary experiments showed that 7 might also react with alkyl alkynes to give metallacycloprop-1-ene complexes. However, further work is needed to define the structures of the products.
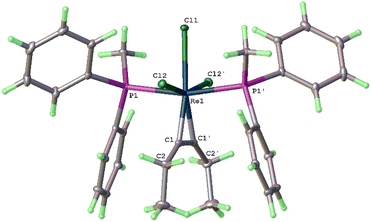
The structure of 8a has also been determined by X-ray diffraction (Fig. 2). In general, complex 8a has structural features similar to those of 3a. The twist angle between the two rhenacycles (30.5°) in 8a is slightly larger than that of 3a (25°). In agreement with the solid-state structure, the 13C{1H} NMR spectrum of 8a shows the Re![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C signal at 254.5 ppm and that of Re–CH(P) at 11.3 ppm. The NMR data of 8b are similar to those of 8a (see ESI†).
C signal at 254.5 ppm and that of Re–CH(P) at 11.3 ppm. The NMR data of 8b are similar to those of 8a (see ESI†).
In the solid-state structures of both 3a and 8a, the two phosphonium substituents are oriented trans to each other. In principle, the two phosphonium substituents could also be oriented cis to each other. To understand the experimental observation, we optimized the structures of 8a (with trans disposed phosphonium substituents) and 8a′ (with cis disposed phosphonium substituents), and calculated their relative energies. As shown in Scheme 5, 8a is thermodynamically more stable than 8a′ by 4.99 kcal mol−1, consistent with the experimental observation that 8a is the only observed isomer.
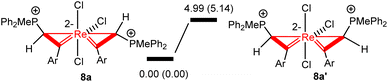 | ||
| Scheme 5 Relative energies of 8a and 8a′ isomers. The relative free energies and electronic energies (in parentheses) are given in kcal mol−1. | ||
We have also explored the possibility of the formation of bis(η2-vinyl) complexes by using internal alkynes. Heating a mixture of 7 and four equivalents of 3-hexyne in toluene at 100 °C for 2 h produced cleanly the alkyne complex Re(η2-EtC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CEt)Cl3(PMePh2)2 (9), which can be isolated as a yellow solid in 89.9% yield (Scheme 4). The complex is diamagnetic and can be readily characterized by NMR spectroscopy. The 31P{1H} NMR in CDCl3 showed a singlet at −53.4 (s). The 1H NMR showed the Et signal at 0.91 (CH3) and 3.91 (CH2) ppm. The 13C{1H} NMR in CDCl3 showed the C
CEt)Cl3(PMePh2)2 (9), which can be isolated as a yellow solid in 89.9% yield (Scheme 4). The complex is diamagnetic and can be readily characterized by NMR spectroscopy. The 31P{1H} NMR in CDCl3 showed a singlet at −53.4 (s). The 1H NMR showed the Et signal at 0.91 (CH3) and 3.91 (CH2) ppm. The 13C{1H} NMR in CDCl3 showed the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C signal at 229.9 ppm. The 13C chemical shift of the alkyne signal (at 229.9 ppm) is characteristic of four-electron alkyne ligands.33
C signal at 229.9 ppm. The 13C chemical shift of the alkyne signal (at 229.9 ppm) is characteristic of four-electron alkyne ligands.33
The structure of 9 has been confirmed by X-ray diffraction. As shown in Fig. 3, it can be described as a distorted octahedral complex with two trans-disposed PMePh2 ligands at the axial positions, three meridionally bound Cl ligands, and an alkyne ligand in the equatorial position. Whereas structures 3a and 8a have 2-fold symmetry, complex 9 has crystallographic 2-fold symmetry, with the axis passing through Cl1 and Re1 and bisecting the coordinated 4e donor alkyne. The alkyne ligand is oriented perpendicular to the P(1)–Re–P(2) axis and bonded to rhenium symmetrically with the Re–C distance of 1.9642(19) Å, and the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C distance of the 1.325(4) Å. The structural parameters are similar to those of Cp*ReCl2(η2-EtC
C distance of the 1.325(4) Å. The structural parameters are similar to those of Cp*ReCl2(η2-EtC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CEt) (Re–C, 1.961(3) and 1.969(3) Å; C
CEt) (Re–C, 1.961(3) and 1.969(3) Å; C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C, 1.326(4) Å).34 The Re–C5 bond length (1.9642(19) Å) is within those reported for typical Re
C, 1.326(4) Å).34 The Re–C5 bond length (1.9642(19) Å) is within those reported for typical Re![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CR2 (R
CR2 (R![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) H or alkyl) carbene bonds (1.850–2.153 Å)11d,14 and shorter than those reported for Re–C bonds of typical rhenium–η2-alkyne (2e donor) complexes (2.118–2.247 Å).35 The NMR and the X-ray data suggest that this structure has contributions from the resonance forms 9 and 9′, with 9′ being dominant (Scheme 4). The successful isolation of 9 provides indirect evidence that complexes 3 and 8 are formed through the η2-alkyne complexes ReCl3(η2-alkyne)(PR3)2.
H or alkyl) carbene bonds (1.850–2.153 Å)11d,14 and shorter than those reported for Re–C bonds of typical rhenium–η2-alkyne (2e donor) complexes (2.118–2.247 Å).35 The NMR and the X-ray data suggest that this structure has contributions from the resonance forms 9 and 9′, with 9′ being dominant (Scheme 4). The successful isolation of 9 provides indirect evidence that complexes 3 and 8 are formed through the η2-alkyne complexes ReCl3(η2-alkyne)(PR3)2.
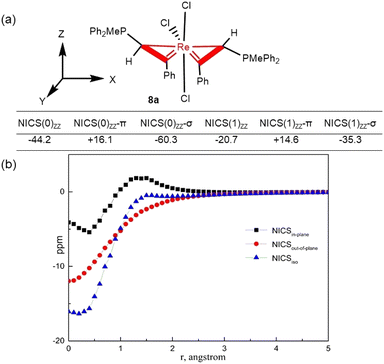
Cyclopropene36,37 and fused metallacycloprop-1-ene complexes17–20,38 have been shown to display σ-aromaticity. We expect that complexes 3 and 8 might also show aromatic character. To verify the hypothesis, we have used a series of theoretical methods to probe the aromatic properties of spiro bi(metallacycloprop-1-ene) complex 8a. We first calculated the values of nucleus-independent chemical shift (NICS), a common index for aromaticity.39 Since 8a has a pseudo 2-fold symmetric structure, the two three-membered rings are chemically equivalent. As shown in Fig. 4a, the NICS(0)ZZ values and NICS(1)ZZ values are −44.2 and −20.7 ppm, respectively, indicating that the metallacycle is aromatic. Canonical molecular orbital (CMO) NICS calculations reveal that the NICS(0)ZZ-π value from π molecular orbitals (+16.1 ppm) has a positive sign, while the NICS(0)ZZ-σ value from the key occupied σ molecular orbitals (−60.3 ppm) has a negative sign, suggesting the possible σ-aromaticity. Consistent with the σ-aromaticity, the NICS(1)ZZ-π value (+14.6 ppm) also has a positive sign, while the NICS(1)ZZ-σ value orbitals (−35.3 ppm) has a negative sign.
To verify the σ-aromaticity, complex 8a was studied by the NICS-scan40 and NICSin–out41 techniques, which can draw inferences about types of aromaticity based on the shape of the curves. To avoid the influence of the ligands in the structure, the NICS values of the metallacycle in 8a were decomposed into atomic contributions in the framework of the “atoms in molecules (AIM)” theory42 using the AIMAll43 program. As shown in Fig. 4b, the NICS out-of-plane curve does not exhibit a pronounced minimum at non-zero r values. The NICS in–out curve is not arc-shaped (Fig. S1, ESI†), as expected for typical π aromatic systems.44 For comparison, NICS-scan and NICSin–out shapes without AIM processing are listed in Fig. S2 and S3.†
The anisotropy of the induced current density (AICD) analysis45 is another commonly used method for the evaluation of aromaticity. As shown in Fig. S4,† the σ-system of the three-membered metallacycles of 8a shows a diatropic ring current, confirming the σ-aromaticity. The σ-aromaticity of 8a is further elucidated by the gauge including magnetically induced currents (GIMIC).46 The GIMIC maps in the plane of a single ring of 8a are shown in Fig. S5,† which vividly display that significant diatropic currents formed in both the inner and outer edges of the three-membered metallacycle. To visualize the induced current of 8a, the induced current modulus surfaces were built up. The resulting Jmod plot (Fig. S6†) clearly shows that the diatropic surfaces (blue) around the single metallacycle are large and closed whereas the paratropic surfaces (red) inside the rings are significantly smaller. The static streamline plot of 8a (Fig. S7†) shows strong net currents circulating the single metallacycle cycle, corresponding to diamagnetic, i.e., aromatic, current. Thus the GIMIC results, both Jmod and streamline plots, confirm the aromatic character of the metallacycle of 8a.
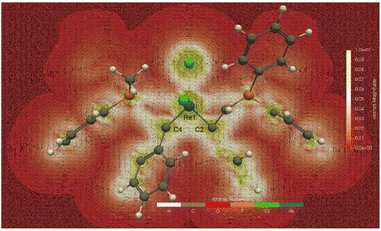
Having confirmed the σ-aromaticity of mono-metallacycle, we have also investigated the global aromaticity of 8a.47 A global π-aromatic system has diatropic ring current circuiting along the outmost periphery. The GIMIC map with the magnetic field direction perpendicular to the plane formed by the three atoms of Re1, C2, and C4 (Fig. 5) shows induced currents generated in 8a connecting the two non-coplanar three-membered metallacycle rings via the unbound C2 and C4 atoms (the calculated C2–C4 Mayer bond order is only 0.05). The induced current between C2 and C4 in 8a is further proved by the current intensity through the integral plane calculated by the GIMIC program, a method that enables one to quantitatively check the aromaticity according to the induced current. As shown in Fig. S8,† the current intensity passing through the bonds of the three-membered ring is greater than 8.6 nA T−1, while that between C2 and C4 atoms is 7.04 nA T−1.
The magnetically induced current can be visualized via the AIMAll program. Fig. S9a and b† show that the magnetically induced current between the two non-coplanar three-membered metallacycle rings in 8a is connected to form an overall diatropic induced current. The current intensity between C2 and C4 atoms is slightly weaker than that on the ring plane, but it cannot be ignored. The NICS-grid (Fig. S9c†) and ICSS plots (Fig. S9d†) based on the NICS method also show that the two non-coplanar three-membered metallacycles of 8a form an overall shielded area. These results suggest that the two three-membered metallacycles in 8a constitute a special 3D global σ-aromaticity, with the induced current flowing via the unbound C2 and C4 atoms.
There has been much interest in the chemistry of metallaaromatics.38,48 Spiro metalla-aromatics, in which two or more aromatic rings are fused by sharing a single metal atom (the spiro atom), are novel metallaaromatic systems introduced recently. For example, Xi and coworkers have realized π-type bis-spirometalla-aromatics with square planar49 and tetrahedral50 geometries, and tris-spiroaromatics with a hexalithio spiro vanadacycle.51 Complexes 3 and 8 represent unique examples of σ-type spiro metallaaromatics.
Conclusions
In summary, we have successfully obtained the first examples of spiro bi(metallacycloprop-1-ene) complexes from reactions of alkynes with rhenium phosphine complexes. Computational studies indicate that the metallacycloprop-1-ene rings are aromatic and thus the complexes represent a rare σ-type spirometallaaromatic system.Data availability
Crystallographic data for 3a (CCDC no. 2205733), 8a (CCDC no. 2205745), and 9 (CCDC no. 2205743) have been deposited at The Cambridge Crystallographic Data Centre.†Author contributions
G. J. conceived the project and supervised the findings of this work. W. B. and L. Y. T. carried out the syntheses and characterizations. Y. W. and Y. L. performed the computations. H. H. Y. S. and I. D. W. performed the XRD. G. J., W. B., I. D. W. and Y. W. wrote the manuscript and all authors contributed to the final manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 22001030, 21903010), and Hong Kong Research Grant Council (No. 16308719, 16308721). We extend our appreciation to the “Supercomputing Center of Dalian University of Technology” for providing access to the supercomputers.Notes and references
- D. S. Frohnapfel and J. L. Templeton, Coord. Chem. Rev., 2000, 206–207, 199–235 CrossRef CAS.
- Selected examples of recent works, see: (a) M. A. Ehweiner, L. M. Peschel, N. Stix, M. Z. Ćorovic, F. Belaj and N. C. Mösch-Zanetti, Inorg. Chem., 2021, 60, 8414–8418 CrossRef CAS; (b) M. J. Byrnes, X. Dai, R. R. Schrock, A. S. Hock and P. Müller, Organometallics, 2005, 24, 4437–4450 CrossRef CAS; (c) W. Zhang, S. Kraft and J. S. Moore, J. Am. Chem. Soc., 2004, 126, 329–335 CrossRef CAS; (d) Y.-C. Tsai, P. L. Diaconescu and C. C. Cummins, Organometallics, 2000, 19, 5260–5262 CrossRef CAS; (e) D. S. Frohnapfel, P. S. White and J. L. Templeton, Organometallics, 2000, 19, 1497–1506 CrossRef CAS.
- (a) S. G. Curto, M. A. Esteruelas, M. Oliván, E. Oñate and A. Vélez, Organometallics, 2018, 37, 1970–1978 CrossRef CAS; (b) R. Gao, D. R. Pahls, T. R. Cundari and C. S. Yi, Organometallics, 2014, 33, 6937–6944 CrossRef CAS; (c) R. J. Pawley, M. A. Huertos, G. C. Lloyd-Jones, A. S. Weller and M. C. Willis, Organometallics, 2012, 31, 5650–5659 CrossRef CAS.
- C. Zhu, J. Wu, S. Li, Y. Yang, J. Zhu, X. Lu and H. Xia, Angew. Chem., Int. Ed., 2017, 56, 9067–9071 CrossRef CAS PubMed.
- M. Luo, Y. Sui, X. Lin, C. Zhu, Z. Yan, Y. Ruan, H. Zhang and H. Xia, Chem. Commun., 2020, 56, 6806–6809 RSC.
- R. Castro-Rodrigo, M. A. Esteruelas, A. M. López, F. López, J. L. Mascareñas, M. Oliván, E. Oñate, L. Saya and L. Villarino, J. Am. Chem. Soc., 2010, 132, 454–455 CrossRef CAS.
- F. Niikura, H. Seino and Y. Mizobe, Organometallics, 2009, 28, 1112–1121 CrossRef CAS.
- T. Kurogi and D. J. Mindiola, Organometallics, 2020, 39, 4474–4478 CrossRef CAS.
- X. Li, T. Vogel, C. D. Incarvito and R. H. Crabtree, Organometallics, 2005, 24, 62–76 CrossRef.
- M. Galiana-Cameo, R. Romeo, A. Urriolabeitia, V. Passarelli, J. J. Pérez-Torrente, V. Polo and R. Castarlenas, Angew. Chem., Int. Ed., 2022, 61, e202117006 CrossRef CAS PubMed.
- (a) A. Fürstner, J. Am. Chem. Soc., 2019, 141, 11–24 CrossRef PubMed; (b) T. Biberger, C. P. Gordon, M. Leutzsch, S. Peil, A. Guthertz, C. Copéret and A. Fürstner, Angew. Chem., Int. Ed., 2019, 58, 8845–8850 CrossRef CAS PubMed; (c) A. Guthertz, M. Leutzsch, L. M. Wolf, P. Gupta, S. M. Rummelt, R. Goddard, C. Farès, W. Thiel and A. Fürstner, J. Am. Chem. Soc., 2018, 140, 3156–3169 CrossRef CAS PubMed; (d) M. Leutzsch, L. M. Wolf, P. Gupta, M. Fuchs, W. Thiel, C. Farès and A. Fürstner, Angew. Chem., Int. Ed., 2015, 54, 12431–12436 CrossRef CAS; (e) B. Sundararaju and A. Fürstner, Angew. Chem., Int. Ed., 2013, 52, 14050–14054 CrossRef CAS PubMed.
- (a) D.-A. Roşca, K. Radkowski, L. M. Wolf, M. Wagh, R. Goddard, W. Thiel and A. Fürstner, J. Am. Chem. Soc., 2017, 139, 2443–2455 CrossRef; (b) S. M. Rummelt and A. Fürstner, Angew. Chem., Int. Ed., 2014, 53, 3626–3630 CrossRef CAS.
- (a) B. Sánchez-Page, J. Munarriz, M. V. Jiménez, J. J. Pérez-Torrente, J. Blasco, G. Subias, V. Passarelli and P. Álvarez, ACS Catal., 2020, 10, 13334–13351 CrossRef; (b) J. J. Pérez-Torrente, D. H. Nguyen, M. V. Jiménez, F. J. Modrego, R. Puerta-Oteo, D. Gómez-Bautista, M. Iglesias and L. A. Oro, Organometallics, 2016, 35, 2410–2422 CrossRef; (c) R. H. Crabtree, New J. Chem., 2003, 27, 771–772 RSC; (d) L. W. Chung, Y.-D. Wu, B. M. Trost and Z. T. Ball, J. Am. Chem. Soc., 2003, 125, 11578–11582 CrossRef CAS PubMed.
- M. Zhang and G. Huang, Chem.–Eur. J., 2016, 22, 9356–9365 CrossRef CAS PubMed.
- Y. Imazaki, E. Shirakawa, R. Ueno and T. Hayashi, J. Am. Chem. Soc., 2012, 134, 14760–14763 CrossRef CAS PubMed.
- (a) J. Jiang, H. Liu, L. Cao, C. Zhao, Y. Liu, L. Ackermann and Z. Ke, ACS Catal., 2019, 9, 9387–9392 CrossRef CAS; (b) S. Warratz, C. Kornhaaß, A. Cajaraville, B. Niepötter, D. Stalke and L. Ackermann, Angew. Chem., Int. Ed., 2015, 54, 5513–5517 CrossRef CAS.
- (a) Y. Huang, C. Dai and J. Zhu, Chem.–Asian J., 2020, 15, 3444–3450 CrossRef CAS PubMed; (b) Y. Hao, J. Wu and J. Zhu, Chem.–Eur. J., 2015, 21, 18805–18810 CrossRef CAS.
- (a) F. Huang, X. Zheng, X. Lin, L. Ding, Q. Zhuo, T. B. Wen, H. Zhang and H. Xia, Chem. Sci., 2020, 11, 10159–10166 RSC; (b) Z. Chu, G. He, X. Cheng, Z. Deng and J. Chen, Angew. Chem., Int. Ed., 2019, 58, 9174–9178 CrossRef CAS; (c) Q. Zhuo, J. Lin, Y. Hua, X. Zhou, Y. Shao, S. Chen, Z. Chen, J. Zhu, H. Zhang and H. Xia, Nat. Commun., 2017, 8, 1912 CrossRef PubMed; (d) C. Zhu, X. Zhou, H. Xing, K. An, J. Zhu and H. Xia, Angew. Chem., Int. Ed., 2015, 54, 3102–3106 CrossRef CAS; (e) F. H. Cui, Q. Li, L. H. Gao, K. Ruan, K. Ma, S. Chen, Z. Lu, J. Fei, Y. M. Lin and H. Xia, Angew. Chem., Int. Ed., 2022, e202211734 CAS; (f) C. Zhu, C. Yang, Y. Wang, G. Lin, Y. Yang, X. Wang, J. Zhu, X. Chen, X. Lu, G. Liu and H. Xia, Sci. Adv., 2016, 2, e1601031 CrossRef PubMed; (g) M. Luo, L. Long, H. Zhang, Y. Yang, Y. Hua, G. Liu, Z. Lin and H. Xia, J. Am. Chem. Soc., 2017, 139, 1822–1825 CrossRef CAS PubMed.
- (a) W. Bai, Y. Sun, Y. Wang, Y. Zhou, Y. Zhao, X. Bao and Y. Li, Chem. Commun., 2022, 58, 6409–6412 RSC; (b) Y. Sun, Y. Zhou, W. Bai, Y. Li and Y. Wang, Chem. Commun., 2022, 58, 435–438 RSC; (c) Y. Wang, Y. Sun, W. Bai, Y. Zhou, X. Bao and Y. Li, Dalton Trans., 2022, 51, 2876–2882 RSC; (d) X. Bao, Y. Li, W. Bai, Y. Zhou, Y. Wang, Y. Sun and J. Jiang, Chem. Commun., 2021, 57, 1643–1646 RSC.
- M. Batuecas, R. Castro-Rodrigo, M. A. Esteruelas, C. García-Yebra, A. M. López and E. Oñate, Angew. Chem., Int. Ed., 2016, 55, 13749–13753 CrossRef CAS PubMed.
- (a) K. C. Stone, G. M. Jamison, P. S. White and J. L. Templeton, Organometallics, 2003, 22, 3083–3095 CrossRef CAS; (b) G. Guillemot, E. Solari, C. Floriani, N. Re and C. Rizzoli, Organometallics, 2000, 19, 5218–5230 CrossRef CAS; (c) D. S. Frohnapfel, A. E. Enriquez and J. L. Templeton, Organometallics, 2000, 19, 221–227 CrossRef CAS.
- (a) D. Buccella, K. E. Janak and G. Parkin, J. Am. Chem. Soc., 2008, 130, 16187–16189 CrossRef CAS PubMed; (b) G. Guillemot, E. Solari, R. Scopelliti and C. Floriani, Organometallics, 2001, 20, 2446–2448 CrossRef CAS; (c) H. Ishino, S. Kuwata, Y. Ishii and M. Hidai, Organometallics, 2001, 20, 13–15 CrossRef CAS; (d) S. R. Allen, R. G. Beevor, M. Green, N. C. Norman, A. G. Orpen and I. D. Williams, J. Chem. Soc., Dalton Trans., 1985, 435–450 RSC.
- (a) G. He, J. Chen, H. H.-Y. Sung, I. D. Williams and G. Jia, Inorg. Chim. Acta, 2021, 518, 120239 CrossRef CAS; (b) G. He, T. Fan, J. Chen, H. H.-Y. Sung, I. D. Williams, Z. Lin and G. Jia, New J. Chem., 2013, 37, 1823–1832 RSC; (c) J. Chen, G. He, H. H.-Y. Sung, I. D. Williams, Z. Lin and G. Jia, Organometallics, 2010, 29, 2693–2701 CrossRef CAS; (d) C. P. Casey, T. M. Boller, S. Kraft and I. A. Guzei, J. Am. Chem. Soc., 2002, 124, 13215–13221 CrossRef CAS.
- M. J. Bernal, O. Torres, M. Martin and E. Sola, J. Am. Chem. Soc., 2013, 135, 19008–19015 CrossRef CAS.
- (a) A. Álvarez-Pérez, C. González-Rodríguez, C. García-Yebra, J. A. Varela, E. Oñate, M. A. Esteruelas and C. Saá, Angew. Chem., Int. Ed., 2015, 54, 13357–13361 CrossRef; (b) P. Barrio, M. A. Esteruelas and E. Oñate, Organometallics, 2003, 22, 2472–2485 CrossRef CAS.
- (a) H.-X. Wang, Q. Wan, K.-H. Low, C.-Y. Zhou, J.-S. Huang, J.-L. Zhang and C.-M. Che, Chem. Sci., 2020, 11, 2243–2259 RSC; (b) Y. Li, J.-S. Huang, Z.-Y. Zhou and C.-M. Che, J. Am. Chem. Soc., 2001, 123, 4843–4844 CrossRef CAS; (c) A. M. LaPointe, R. R. Schrock and W. M. Davis, J. Am. Chem. Soc., 1995, 117, 4802–4813 CrossRef CAS.
- (a) M. J. Benedikter, J. V. Musso, W. Frey, R. Schowner and M. R. Buchmeiser, Angew. Chem., Int. Ed., 2021, 60, 1374–1382 CrossRef CAS; (b) T. Chen, X.-H. Zhang, C. Wang, S. Chen, Z. Wu, L. Li, K. R. Sorasaenee, J. B. Diminnie, H. Pan, I. A. Guzei, A. L. Rheingold, Y.-D. Wu and Z.-L. Xue, Organometallics, 2005, 24, 1214–1224 CrossRef CAS; (c) T. Chen, Z. Wu, L. Li, K. R. Sorasaenee, J. B. Diminnie, H. Pan, I. A. Guzei, A. L. Rheingold and Z.-L. Xue, J. Am. Chem. Soc., 1998, 120, 13519–13520 CrossRef CAS.
- (a) B. Paul, R. R. Schrock and C. Tsay, Organometallics, 2021, 40, 463–466 CrossRef CAS; (b) J. W. Taylor, R. R. Schrock and C. Tsay, Organometallics, 2020, 39, 2304–2308 CrossRef CAS.
- (a) U. J. Kilgore, J. Tomaszewski, H. Fan, J. C. Huffman and D. J. Mindiola, Organometallics, 2007, 26, 6132–6138 CrossRef CAS; (b) J. D. Fellmann, G. A. Rupprecht, C. D. Wood and R. R. Schrock, J. Am. Chem. Soc., 1978, 100, 5964–5966 CrossRef CAS.
- (a) J. B. Diminnie, H. D. Hall and Z. Xue, Chem. Commun., 1996, 2383–2384 RSC; (b) J. D. Fellmann, R. R. Schrock and G. A. Rupprecht, J. Am. Chem. Soc., 1981, 103, 5752–5758 CrossRef CAS; (c) M. R. Churchill and W. J. Youngs, Inorg. Chem., 1979, 18, 1930–1935 CrossRef CAS.
- H. Kuniyasu, T. Nakajima, T. Tamaki, T. Iwasaki and N. Kambe, Organometallics, 2015, 34, 1373–1376 CrossRef CAS.
- A. Allen and W. Lin, Organometallics, 1999, 18, 2922–2925 CrossRef CAS.
- J. L. Templeton and B. C. Ward, J. Am. Chem. Soc., 1980, 102, 3288–3290 CrossRef CAS.
- W. A. Herrmann, R. A. Fischer and E. Herdtweck, Organometallics, 1989, 8, 2821–2831 CrossRef CAS.
- Usually in the range of 2.118−2.247 Å. See, for example: (a) J. J. Kowalczyk, A. M. Arif and J. A. Gladysz, Organometallics, 1991, 10, 1079–1088 CrossRef CAS; (b) C. P. Casey, T. E. Vos, J. T. Brady and R. K. Hayashi, Organometallics, 2003, 22, 1183–1195 CrossRef CAS; (c) C. P. Casey, T. M. Boller, J. S. M. Samec and J. R. Reinert-Nash, Organometallics, 2009, 28, 123–131 CrossRef CAS PubMed; (d) C. P. Casey, A. D. Selmeczy, J. R. Nash, C. S. Yi, D. R. Powell and R. K. Hayashi, J. Am. Chem. Soc., 1996, 118, 6698–6706 CrossRef CAS; (e) C. P. Casey, Y. Ha and D. R. Powell, J. Organomet. Chem., 1994, 472, 185–193 CrossRef CAS; (f) F. W. B. Einstein, K. G. Tyers and D. Sutton, Organometallics, 1985, 4, 489–493 CrossRef CAS.
- J. Wu, X. Liu, Y. Hao, H. Chen, P. Su, W. Wu and J. Zhu, Chem.–Asian J., 2018, 13, 3691–3696 CrossRef CAS PubMed.
- R. R. Aysin, L. A. Leites and S. S. Bukalov, Organometallics, 2020, 39, 2749–2762 CrossRef CAS.
- D. Chen, Y. Hua and H. Xia, Chem. Rev., 2020, 120, 12994–13086 CrossRef CAS PubMed.
- (a) H. Fallah-Bagher-Shaidaei, C. S. Wannere, C. Corminboeuf, R. Puchta and P. v. R. Schleyer, Org. Lett., 2006, 8, 863–866 CrossRef CAS PubMed; (b) Z. Chen, C. S. Wannere, C. Corminboeuf, R. Puchta and P. v. R. Schleyer, Chem. Rev., 2005, 105, 3842–3888 CrossRef CAS; (c) P. v. R. Schleyer, C. Maerker, A. Dransfeld, H. Jiao and N. J. R. van Eikema Hommes, J. Am. Chem. Soc., 1996, 118, 6317–6318 CrossRef CAS PubMed.
- (a) A. J. Stanger, J. Org. Chem., 2006, 71, 883–893 CrossRef CAS PubMed; (b) A. J. Stanger, J. Org. Chem., 2010, 75, 2281–2288 CrossRef CAS PubMed.
- J. J. Torres-Vega, A. Vásquez-Espinal, J. Caballero, M. L. Valenzuela, L. Alvarez-Thon, E. Osorio and W. Tiznado, Inorg. Chem., 2014, 53, 3579–3585 CrossRef CAS PubMed.
- (a) R. F. W. Bader, Atoms in Molecules: a Quantum Theory, Clarendon Press, Oxford, New York, 1990 Search PubMed; (b) T. A. Keith and R. F. W. Bader, Chem. Phys. Lett., 1992, 194, 1–8 CrossRef CAS; (c) T. A. Keith and R. F. W. Bader, Chem. Phys. Lett., 1993, 210, 223–231 CrossRef CAS; (d) T. A. Keith and R. F. W. Bader, J. Chem. Phys., 1993, 99, 3669–3682 CrossRef CAS; (e) T. A. Keith and R. F. W. Bader, Can. J. Chem., 1996, 74, 185–200 CrossRef CAS.
- T. A. Keith, AIMAll, TK Gristmill Software, Overland Park, KS, USA, 2017 Search PubMed.
- (a) L. A. Leites, S. S. Bukalov, R. R. Aysin, A. V. Piskunov, M. G. Chegerev, V. K. Cherkasov, A. V. Zabula and R. West, Organometallics, 2015, 34, 2278–2286 CrossRef CAS; (b) R. R. Aysin, S. S. Bukalov, L. A. Leites and A. V. Zabula, Dalton Trans., 2017, 46, 8774–8781 RSC; (c) R. R. Aysin, L. A. Leites and S. S. Bukalov, Organometallics, 2020, 39, 2749–2762 CrossRef CAS.
- (a) D. Geuenich, K. Hess, F. Köhler and R. Herges, Chem. Rev., 2005, 105, 3758–3772 CrossRef CAS PubMed; (b) R. Herges and D. Geuenich, J. Phys. Chem. A, 2001, 105, 3214–3220 CrossRef CAS.
- (a) H. Fliegl, S. Taubert, O. Lehtonen and D. Sundholm, Phys. Chem. Chem. Phys., 2011, 13, 20500–20518 RSC; (b) D. Sundholm, H. Fliegl and R. J. F. Berger, WIREs Computational Molecular Science, 2016, 6, 639–678 CrossRef CAS.
- For global π-aromaticity/antiaromaticity, see for example: (a) Z. Li, X. Hou, Y. Han, W. Fan, Y. Ni, Q. Zhou, J. Zhu, S. Wu, K.-W. Huang and J. Wu, Angew. Chem., Int. Ed., 2022, 61, e202210697 CAS; (b) O. Dishi, Y. Rahav, R. Carmieli and O. Gidron, Chem.–Eur. J., 2022, 50, e202202082 Search PubMed; (c) T. Xu, Y. Han, Z. Shen, X. Hou, Q. Jiang, W. Zeng, P. W. Ng and C. Chi, J. Am. Chem. Soc., 2021, 143, 20562–20568 CrossRef CAS PubMed; (d) J. Guo, C. Zhou, S. Xie, S. Luo, T. Y. Gopalakrishna, Z. Sun, J. Jouha, J. Wu and Z. Zeng, Chem. Mater., 2020, 32, 5927–5936 CrossRef CAS; (e) Z. Li, T. Y. Gopalakrishna, Y. Han, Y. Gu, L. Yuan, W. Zeng, D. Casanova and J. Wu, J. Am. Chem. Soc., 2019, 141, 16266–16270 CrossRef CAS PubMed; (f) C. Liu, Y. Ni, X. Lu, G. Li and J. Wu, Acc. Chem. Res., 2019, 52, 2309–2321 CrossRef CAS PubMed.
- (a) Y. Zhang, C. Yu, Z. Huang, W.-X. Zhang, S. Ye, J. Wei and Z. Xi, Acc. Chem. Res., 2021, 54, 2323–2333 CrossRef CAS PubMed; (b) D. Chen, Q. Xie and J. Zhu, Acc. Chem. Res., 2019, 52, 1449–1460 CrossRef CAS PubMed; (c) B. J. Frogley and L. J. Wright, Chem.–Eur. J., 2018, 24, 2025–2038 CrossRef CAS PubMed.
- Y. Zhang, J. Wei, Y. Chi, X. Zhang, W.-X. Zhang and Z. Xi, J. Am. Chem. Soc., 2017, 139, 5039–5042 CrossRef CAS PubMed.
- Y. Zhang, J. Wei, M. Zhu, Y. Chi, W.-X. Zhang, S. Ye and Z. Xi, Angew. Chem., Int. Ed., 2019, 58, 9625–9631 CrossRef CAS PubMed.
- Z. Huang, Y. Zhang, W.-X. Zhang, J. Wei, S. Ye and Z. Xi, Nat. Commun., 2021, 12, 1319 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2205733, 2205743 and 2205745. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2sc05378k |
| This journal is © The Royal Society of Chemistry 2023 |