Open Access Article
Open Access ArticleLigand engineering of Au44 nanoclusters for NIR-II luminescent and photoacoustic imaging-guided cancer photothermal therapy†
Ge
Yang
a,
Xueluer
Mu
b,
Xinxin
Pan
a,
Ying
Tang
b,
Qiaofeng
Yao
c,
Yaru
Wang
a,
Fuyi
Jiang
d,
Fanglin
Du
a,
Jianping
Xie
 *ce,
Xianfeng
Zhou
*ce,
Xianfeng
Zhou
 *b and
Xun
Yuan
*b and
Xun
Yuan
 *a
*a
aSchool of Materials Science and Engineering, Qingdao University of Science and Technology, Qingdao, 266042, PR China. E-mail: yuanxun@qust.edu.cn
bKey Lab of Biobased Polymer Materials of Shandong Provincial Education Department, School of Polymer Science and Engineering, Qingdao University of Science and Technology, Qingdao, 266042, PR China. E-mail: xianfeng@qust.edu.cn
cJoint School of National University of Singapore and Tianjin University, International Campus of Tianjin University, Binhai New City, Fuzhou, 350207, PR China. E-mail: chexiej@nus.edu.sg
dSchool of Environment and Material Engineering, Yantai University, Yantai, 264005, PR China
eDepartment of Chemical and Biomolecular Engineering, National University of Singapore, 4 Engineering Drive 4, Singapore, 117585, Singapore
First published on 8th March 2023
Abstract
Developing a high-performance noninvasive probe for precise cancer theranostics is very challenging but urgently required. Herein, a novel Au nanoclusters (NCs)-based probe was designed for cancer theranostics via ligand engineering by conjugating photoluminescent (PL) Au44 NCs in the second near-infrared window (NIR-II, 1000–1700 nm) with aromatic photoacoustic (PA)/photothermal molecules through click chemistry. This design bypasses the incompatibility dilemma between photoluminescence (PL) attributes and PA/photothermal properties because the rigidity of the PA/photothermal molecules can lead to aggregation-induced emission (AIE) of the Au(I)-ligand shell of the Au NCs by constraining their nonradiative relaxation. Benefiting from strong NIR-II PL with emissions at 1080 and 1240 nm, high photothermal conversion efficiency (65.12%), low cytotoxicity, appropriate renal clearance, and enhanced permeability and retention (EPR) effect, the as-designed Au NC-based theranostic probe achieves ultradeep NIR-II PL/PA imaging-guided cancer photothermal therapy (PTT). Remarkably, 16 days after photothermal treatment guided by NIR-II PL/PA imaging, mice were all healed without tumor recurrence, while the average life span of the mice in the control groups was only 17–21 days. This study is interesting because it provides a paradigm for designing a metal NC-based theranostics probe, and it may add fundamentally and methodologically to noninvasive imaging-guided disease therapy.
Introduction
Noninvasive yet precise theranostics for cancer (NPTC) is desired for both cancer patients and therapists because of its avoidance of physical trauma, low cost, and real-time feedback-mediated higher therapeutic efficacy.1 However, developing a high-performance NPTC probe is a great challenge due to the following aspects: (1) neither noninvasive disease diagnosis with deep tissue penetration and high spatiotemporal resolution nor tumor therapy with high tumor selectivity and few side effects are easy tasks;2 (2) simply integrating the diagnostic entity and a therapeutic counterpart into one probe usually results in incompatibility of the constructed NPTC probe, such as the mutual energy competition between the photoluminescence (PL) imaging and the photothermal therapy (PTT) based on the Jablonski energy spectrum theory;3 (3) the theranostic probe should also be renal clearable (i.e., a hydrodynamic size of ≤5.5 nm is the kidney filtration threshold) with benign biocompatibility.4 Overall, tackling any one of such issues presents a challenge, let alone simultaneously solving all these issues in designing NPTC probes, which further reflects the difficulties and significance in the construction of high-performance NPTC probes.Recently, ultrasmall Au nanoclusters (NCs) with a core size of ≤3 nm have emerged as a novel class of functional nanomaterials in the biomedical field due to their atomic-precision size,5 rich yet tailorable surface chemistry,6 and unique molecule-like properties, such as strong PL signal,7 chirality,8 and highest occupied molecular orbital-lowest unoccupied molecular orbital (HOMO–LUMO) transitions.9 Such intriguing physicochemical properties allow Au NCs to be biocompatible,10 surface engineerable,6b,11 and renal clearable (i.e., hydrodynamic size of ≤5.5 nm),4a,12 making their application possible for disease diagnosis or therapy.13 For example, several recently developed types of Au NCs with PL emission in the second near-infrared window (NIR-II, 1000–1700 nm) have shown overwhelming advantages in bioimaging in terms of deep tissue penetration (>1 cm) and high spatiotemporal resolution (approximately 10 μm, and 4 ms) compared with NIR-I emitting NC-based probes (650–900 nm), which have a limited in vivo signal-to-noise ratio.14 We thus hypothesize that if we can design atomically precise NIR-II emitting Au NCs as a molecular matrix for delicate engineering, we may develop a high-performance NPTC probe to achieve NIR-II PL imaging-guided tumor therapy. For proof-of-concept purposes, here a noninvasive PTT modality was chosen as the therapy function of the NPTC probe because it is advantageous in cancer treatment with high tumor selectivity and few side effects.2c,15 More importantly, the photothermal attributes may endow the NPTC probes with an additional PA signal for improving diagnostic efficacy due to the thermoelastic expansion-induced PA emission of the treated tissues.2a,16 In addition, the NIR-II PL properties of the NPTC probes may in turn remedy the limitations of the poor light absorption of conventional PTT probes in deep tissues, expectedly achieving dual-mode NIR-II PL/PA imaging-guided cancer PTT. However, it is still a major challenge to design NIR-II emitting Au NCs with atomic precision. Moreover, several other issues may also arise, e.g., how to engineer NIR-II emitting Au NCs to concurrently achieve NIR-II PL/PA imaging with excellent PTT, can the NIR-II PL imaging proceed synchronously with PA imaging, how the NIR-II PL imaging affects the PTT in vivo, and what are the theranostic efficacy and pharmacokinetic behavior of the Au NC-based probe for NPTC like after engineering.
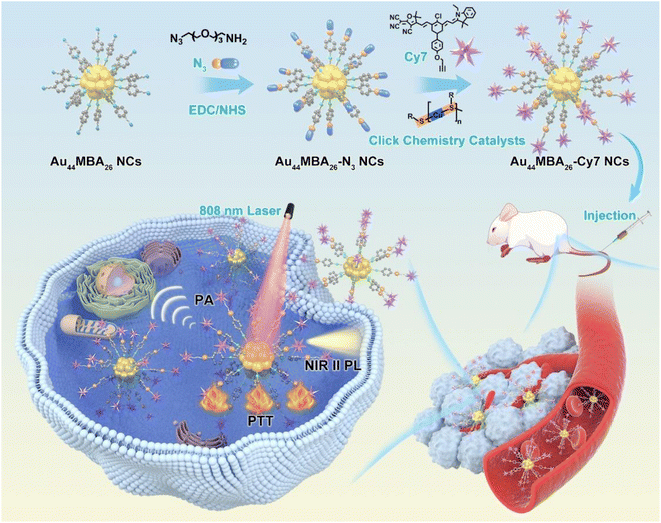
In this work, we report the design of a novel Au NC-based theranostic probe – Au44MBA26-Cy7 (here MBA denotes 4-mercaptobenzoic acid; Cy7 denotes heptamethine dye) for ultradeep NIR-II PL and PA imaging-guided cancer PTT. The Au44MBA26-Cy7 probe comprises NIR-II emitting Au44MBA26 NCs linked with aromatic Cy7 molecules through click chemistry (Scheme 1), which not only enhances the NIR-II PL signal of the Au44 NCs by closing the nonradiative pathway of the inner Au NCs based on AIE,17 but also improves the photothermal conversion efficiency of the outer Cy7 shell. Such intriguing NIR-II PL/photothermal attributes, together with benign biocompatibility, good pharmacokinetics, efficient renal clearance, and enhanced permeability and retention (EPR) effect, make this probe capable of NIR-II PL and PA imaging-guided cancer therapy with high sensitivity and specificity without damaging major organs. To the best of our knowledge, this may be the first successful Au NC probe for NPTC involving simultaneous NIR-II PL/PA imaging-based diagnosis and PTT-based therapy, which would become a promising next-generation noninvasive theranostics technique for cancer.
Results and discussion
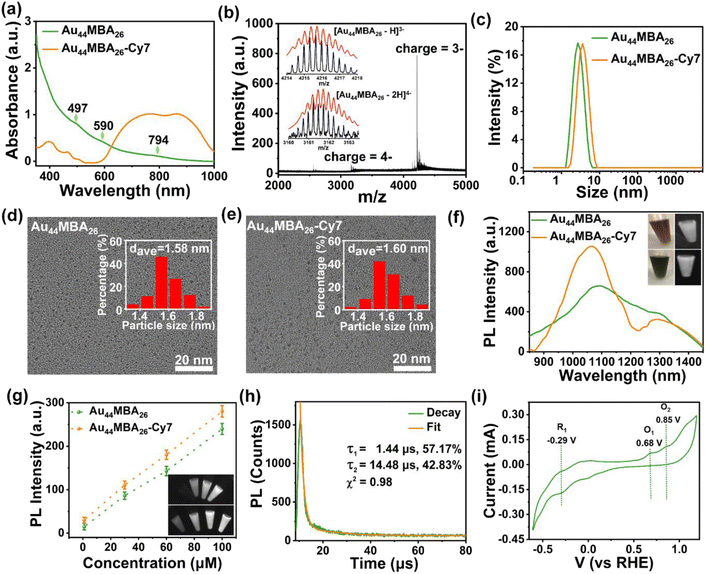
The synthesis of NIR-II emitting metal NCs with atomic precision is a long-standing challenge in the cluster community due to the poor understanding of the correlation between their precise size/structure and PL mechanism. In this study, NIR-II emitting Au44MBA26 NCs were synthesized via an NaOH-mediated NaBH4 reduction method.6b,18 As shown in Fig. 1a, three step-like optical peaks at 497 nm, 590 nm and 794 nm are observed in the UV-vis absorption spectrum of the Au NCs, indicating the successful synthesis of monodisperse Au NCs.19 While the single band in the native polyacrylamide gel electrophoresis (PAGE) results clearly reveals the high monodispersity of the Au NCs (Fig. S1 in the ESI†), the electrospray ionization mass spectrometry (ESI-MS) results disclose the synthesis of high-quality Au44MBA26 NCs, in which two peaks at m/z = ∼3162 and ∼4216 can be ambiguously assigned as [Au44MBA26–2H]4− and [Au44MBA26–H]3− species, respectively (Fig. 1b). In addition, the hydrodynamic diameter of the Au44 NCs was confirmed to be 2.73 nm (Fig. 1c), while their core sizes were all below 2 nm with an average size of 1.58 nm (Fig. 1d). More interestingly, the Au44 NCs display two PL emission peaks at 1080 nm and 1280 nm when excited at 808 nm (Fig. 1f), manifesting that the Au44 NCs are a promising PL probe for NIR-II PL imaging. Taking all the characterization results together, the Au44 NCs exhibit molecular optical absorption, atom-precision formula, good hydrophilicity, ultrasmall hydrodynamic size (<3 nm), and strong NIR-II PL intensity, making them an ideal probe for biomedical applications.Upon acquiring Au44MBA26 NCs, we introduced azide groups on the surface of the Au44 NCs via a simple 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC)/N-hydroxysuccinimide (NHS) crosslinking strategy, and then conjugated the Au44 NCs with aromatic PA/photothermal alkynylated Cy7 molecules via click chemistry to obtain multifunctional Au44MBA26-Cy7 (Scheme 1, Fig. S2–S6 and Supplementary Note I in the ESI†). After conjugation, the as obtained Au44MBA26-Cy7 sample displays broad yet intensive optical absorption in the range of 600–1000 nm in its UV-vis absorption spectrum (Fig. 1a), which is dramatically different from that of pristine Au44MBA26 NCs and was attributed to the contribution of the Cy7 molecules (Fig. S7a†). Furthermore, while the Cy7 modification did not change the core size of the Au44MBA26 NCs (Fig. 1d and e), a slight increase in the hydrodynamic diameter from 2.73 nm to 3.45 nm of the modified Au44MBA26 NCs was observed (Fig. 1c), which ensures the good renal clearance of Au44MBA26-Cy7 NCs because their hydrodynamic diameter is still below the kidney filtration threshold (∼5.5 nm).4a In addition, it was confirmed that around 13 Cy7 molecules were conjugated on each Au44MBA26 NC (Fig. S8 and Supplementary Note II in the ESI†), indicating the good structural symmetry of Au44MBA26-Cy7.
The NIR-II PL emission peaks of the Au44MBA26 NCs at 1080 nm and 1280 nm were retained after Cy7 modification, accompanied by partial enhancement in the emission intensity (Fig. 1f). Indeed, surface engineering has been proven to be an efficient strategy by which to enhance the PL of metal NCs. For example, Pyo et al. reported that the PL of Au22GSH18 NCs (here GSH denotes glutathione) was enhanced by more than five times in water upon surface engineering via conjugating benzyl chloroformate (CBz-Cl) and pyrene (Py), attributed to the ligand shell rigidification effect as well as the promoted energy transfer from the ligands to the metal core.20 Inspired by this, we speculated that the enhanced NIR-II PL of Au44MBA26-Cy7 could be due to the stronger absorption in the range of 600–1000 nm, the promoted charge transfer of the Cy7 molecules, and the resulting aggregation-induced emission (AIE) effect. Specifically, upon conjugating the Au44MBA26 NCs with rigid Cy7 molecules, the high π-electronic delocalization of the 2-(3-cyano-4,5,5-trimethylfuran-2(5H)-ylidene) malononitrile (TCF) group in the Cy7 molecules enhanced the charge transfer from the ligands to the metal core of the NCs.15a Moreover, the as-obtained Au44MBA26-Cy7 NCs showed remarkable AIE effect (Fig. S9†), i.e., the Au44MBA26-Cy7 NCs displayed weak PL emission in N,N-dimethylformamide (DMF) with good solubility, but a more intense PL signal in mixed DMF/H2O with relatively poor solubility, which is due to the rigidity of the Cy7 shell endowing the Au44MBA26-Cy7 NCs with AIE properties by closing the nonradiative pathways of the inner NCs.21 It should be emphasized that the acquisition of AIE properties by conjugating the Au44MBA26 NCs with rigid Cy7 molecules is the key to the design, which not only enhances the NIR-II PL intensity via AIE, but also ensures the maintenance of excellent PA/photothermal signals of the outer Cy7 shell (discussed later), bypassing the dilemma of the incompatibility between the PA/photothermal properties and PL attributes. Meanwhile, as shown in Fig. 1g, both Au44MBA26 and Au44MBA26-Cy7 show concentration-dependent NIR-II PL imaging contrast, allowing their usage in NIR-II PL imaging at an extremely low concentration (e.g., 30 μM).
Considering that the NIR-II PL signal of the Au44MBA26-Cy7 NCs originates from the Au44MBA26 NCs instead of the Cy7 molecules that exhibit emission at 931 nm (Fig. S7b†), we may uncover the NIR-II PL mechanism of the Au44MBA26-Cy7 NCs by investigating Au44MBA26. On this basis, both the NIR-II PL lifetime and the highest unoccupied molecular orbital-lowest unoccupied molecular orbital (HOMO–LUMO) transition of the Au44MBA26 NCs were analyzed. As shown in Fig. 1h, the NIR-II PL decay profile of the Au44MBA26 NCs reveals that the NCs are composed of microsecond lifetime components (1.44 μs: 57.17%; 14.48 μs: 42.83%). The long PL lifetime and a large Stokes shift (>200 nm) indicate that the NIR-II PL emission of the Au44MBA26 NCs most probably results from ligand-to-metal charge transfer or ligand-to-metal-metal charge transfer and subsequent radiative relaxation. In addition, we conducted cyclic voltammetry (CV) tests to assess the HOMO–LUMO gaps of the Au44MBA26 NCs that may correlate to their PL properties22 (Fig. 1i). The results show a reduction potential of 0.29 V and two oxidation potentials at 0.68 V and 0.85 V (vs. the reversible hydrogen electrode, RHE). Of note, such reduction and oxidation peaks have been used to determine the positions of the LUMO and HOMO.22 On this basis, two energy transition gaps of the Au44MBA26 NCs were identified to be 0.97 eV (i.e., the first transition) and 1.14 eV (i.e., the second transition), which are consistent with the NIR-II PL emissions at 1080 nm and 1280 nm shown in Fig. 1f. However, it is still difficult to establish the precise correlation between the NIR-II PL properties and the structure of the Au44MBA26 NCs, although the Au44MBA26 NCs may share the same crystal structure as the reported Au44(2,4-dimethylbenzenethiol)26 NCs.23 Such correlation may be established with additional efforts on theoretical simulations.
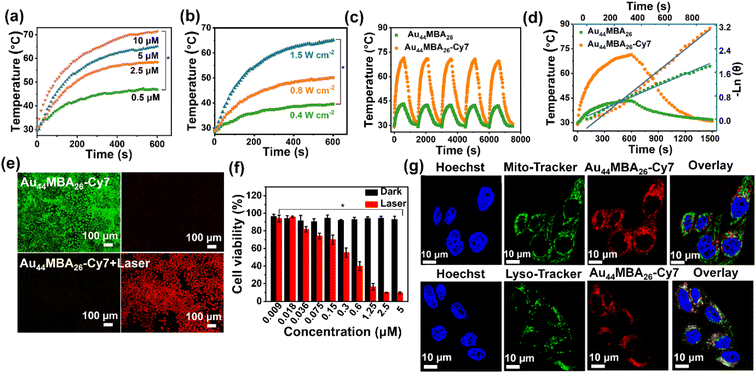
The Au44MBA26-Cy7 NCs exhibit excellent photothermal properties. As shown in Fig. 2a, the temperature of the aqueous Au44MBA26-Cy7 solution (10 μM) elevates dramatically from 30 °C to 70 °C within 10 min of 808 nm laser irradiation, outperforming the photothermal efficacy of pristine Au44MBA26 NCs, which only cause a temperature increase of up to 43 °C under the same conditions (Fig. S10†). It should be emphasized that such an excellent photothermal trait of the Au44MBA26-Cy7 NCs guarantees their high-efficiency killing of tumor cells, and could overcome the challenges caused by the high expression of heat shock proteins (HSPs).24 HSPs are a class of heat-stress proteins that are widespread, from bacteria to mammals, and could modulate the tumor cell response to heat-induced cytotoxicity.24 In addition, the temperature response of the Au44MBA26-Cy7 NCs under NIR laser irradiation exhibits good correlation with their concentration (Fig. 2a) and laser power (Fig. 2b). In order to study the photothermal stability of the Au44MBA26-Cy7 NCs, we carried out repeated radiation-cooling experiments. As shown in Fig. 2c, while both the Au44MBA26-Cy7 NCs and pristine Au44MBA26 NCs show good photothermal stability in these five radiation-cooling cycles, the photothermal activity of the Au44MBA26-Cy7 NCs is much higher than that of pristine Au44MBA26 NCs with the same concentration. In terms of the photothermal conversion efficiency,15a,25 the Au44MBA26-Cy7 NCs (65.12%) also perform better than pristine Au44MBA26 NCs (34.96%) based on the heat transfer time constant and maximum steady-state temperature results (Fig. 2d), which reveal the major role of Cy7 conjugation in enhancing the photothermal conversion efficiency of the Au44MBA26-Cy7 NCs. Of note, the conjugation of rigid Cy7 with the Au44MBA26 NCs not only endows the Au44MBA26-Cy7 NCs with AIE properties to avoid the possible energy competition between the PL attributes and PA/photothermal signals, but also creates a highly localized concentration of Cy7 molecules that is more favorable for high photothermal efficiency than that of unconjugated analogues, providing a paradigm in the design of a multifunctional probe for NPTC.
Naturally, we assessed the in vitro PTT effect as well as cytotoxicity of the Au44MBA26-Cy7 NCs by conducting both live/dead staining and 3-[4,5-dimethylthiazole-2-yl]-2,5-diphenyl tetrazolium bromide (MTT) assays using HeLa cells as a model. As shown in Fig. 2e, the live/dead staining results show that the HeLa cells treated with the Au44MBA26-Cy7 NCs but without exposure to laser irradiation are almost all alive (green color), which suggests that the Au44MBA26-Cy7 NCs exhibit very low cytotoxicity. By contrast, upon laser irradiation for 6 min, the HeLa cells treated with the Au44MBA26-Cy7 NCs were all dead (red color, Fig. 2e), which is very different from those treated with the pristine Au44MBA26 NCs, where only a small number of dead HeLa cells stained in red were observed (Fig. S11a†). These results indicate that the Au44MBA26-Cy7 NCs have excellent in vitro PTT effect. In addition, MTT assay provides quantitative data on the PTT effect. As shown in Fig. 2f, the Au44MBA26-Cy7 NCs display neglectable cytotoxicity, which is evidenced by the fact that no apparent reduction in the HeLa cell viability is observed even upon increasing their concentration to 5 μM without laser irradiation. In comparison, obvious necrosis of HeLa cells with 55.2% viability could be detected for the sample treated with as low as 0.3 μM Au44MBA26-Cy7 NCs under 808 nm laser irradiation, and <10% HeLa cells survived when treated with ≥2.5 μM of the Au44MBA26-Cy7 NCs under the same conditions, while the pristine Au44MBA26 NCs began to show PTT activity for cell killing when their concentration was higher than 10 μM (Fig. S11b†). Taken together, both live/dead staining and MTT assays clearly demonstrate that the Au44MBA26-Cy7 NCs not only exhibit good biocompatibility, but also show excellent in vitro PTT effect. Besides this, we also performed confocal PL microscopy to uncover the uptake behavior of the HeLa cells for the Au44MBA26-Cy7 NCs. As shown in Fig. 2g, the HeLa cells show red PL in the cytoplasm after incubation with the Au44MBA26-Cy7 NCs. To determine the subcellular distribution, the lysosome-specific staining probe LysoTracker Green and mitochondria-specific staining probe MitoTracker Green were used for co-staining the Au44MBA26-Cy7 NCs. The green PL of LysoTracker green is strongly correlated with the red PL channel (Rr = 0.8), indicating that most of the Au44MBA26-Cy7 NCs are located within the lysosome (Fig. 2g). Therefore, the above results manifest that the Au44MBA26-Cy7 NCs enter the cells through endocytosis.
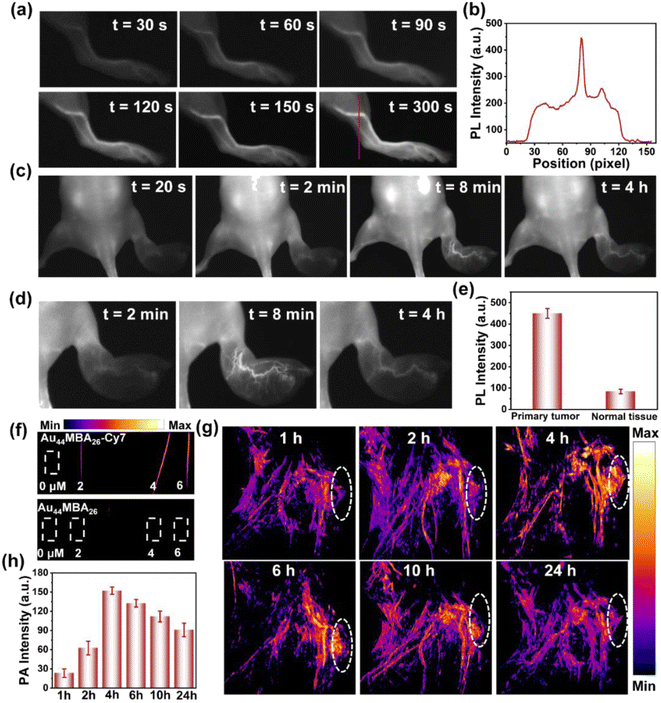
In addition to the remarkable in vitro photothermal properties, the Au44MBA26-Cy7 NCs also display prominent performance in noninvasive in vivo NIR-II PL and PA imaging. The strong NIR-II PL signal of the Au44MBA26-Cy7 NCs allowed high-contrast, real-time NIR-II vessel imaging under 808 nm laser irradiation to be achieved. As shown in Fig. 3a, the hindlimb vessels in mice could be observed with an ultrahigh contrast in the NIR-II window. Specifically, the vessels of the mice were visible at 30 s from the intravenous injection of the Au44MBA26-Cy7 NCs, and extremely clear at 120 s. The cross-sectional intensity profile of the mice's hindlimb at 300 s also revealed the intense NIR-II PL signal in the vessels (Fig. 3b). Taken together, one may conclude that the Au44MBA26-Cy7 NCs with ultrasmall size exhibit molecule-like pharmacokinetics, possessing great potential in ultradeep NIR-II PL imaging for medical diagnosis.
To test the possibility of NIR-II PL imaging-guided tumor therapy, we further established a metastatic tumor model in which 4T1 cells were inoculated into the right hind paw of nude mice, and checked the usefulness of the Au44MBA26-Cy7 NCs for the real-time NIR-II PL imaging of tumors. As shown, after intravenously injecting Au44MBA26-Cy7 NCs into the 4T1 tumor-bearing mice, the NIR-II PL signal under 808 nm laser irradiation could be recognized at 20 s (Fig. 3c). Benefiting from the continuous accumulation of the Au44MBA26-Cy7 NCs at the tumor site via the EPR effect, the NIR-II PL intensity in the primary tumor tissues gradually increased within 8 min, and was five times greater than that in the healthy tissue (Fig. 3c and d), indicating that the Au44MBA26-Cy7 NCs have tumor targeting ability and possess significant advantages in NIR-II PL imaging for tumor diagnosis with deep-tissue penetration (Fig. 3e). Furthermore, the NIR-II PL signal of the Au44MBA26-Cy7 NCs in primary tumor tissues remained after 4 h with a high resolution (Fig. 3c and d), which suggests their long-lasting imaging ability, supporting their application in long-period NIR-II PL imaging-guided tumor therapy.
One salient feature of the Au44MBA26-Cy7 NCs is their dual-mode noninvasive imaging of deep tissues with mutual signal correction. Aside from NIR-II PL imaging, the Au44MBA26-Cy7 NCs could simultaneously achieve in vivo PA imaging. It should be mentioned that the generation of the PA signal is related to thermal expansion. While an Au44MBA26-Cy7 NC solution with a low concentration of 2 μM showed strong PA signals (Fig. 3f), the same was not realized by the pristine Au44MBA26 NC solution, although it generated a PA signal when the concentration was increased by ≥10 times (Fig. S12†). This preliminary result implies the capability of the Au44MBA26-Cy7 NCs of real-time PA imaging. To further check the applicability for in vivo PA imaging, the Au44MBA26-Cy7 NCs were injected into 4T1 tumor-bearing mice via the tail vein. Owing to the EPR effect, the Au44MBA26-Cy7 NCs accumulated continuously at the tumor site, enabling the successful PA imaging of the tumor with a rapidly increasing signal intensity until a maximum was reached at 4 h (Fig. 3g and h), which demonstrated the excellent in vivo PA imaging ability of the Au44MBA26-Cy7 NCs. Based on the quantitative analysis of the time-related relative PA intensities of the Au44MBA26-Cy7 NCs at the tumor sites (Fig. 3h), we can deduce that 4 h from NCs injection is the optimal time point for in vivo PTT. More importantly, the Au44MBA26-Cy7 NCs could simultaneously implement NIR-II PL and PA imaging for tumors with both deep-tissue penetration and high spatial resolution at 4 h from NC injection, which may guide cancer PTT in terms of providing precise tumor location and real-time feedback. It is worth noting that the PL and PA attributes with a competitive relationship were successfully integrated into one material in this study, highlighting the strong designability of the Au44MBA26-Cy7 NCs via ligand engineering.
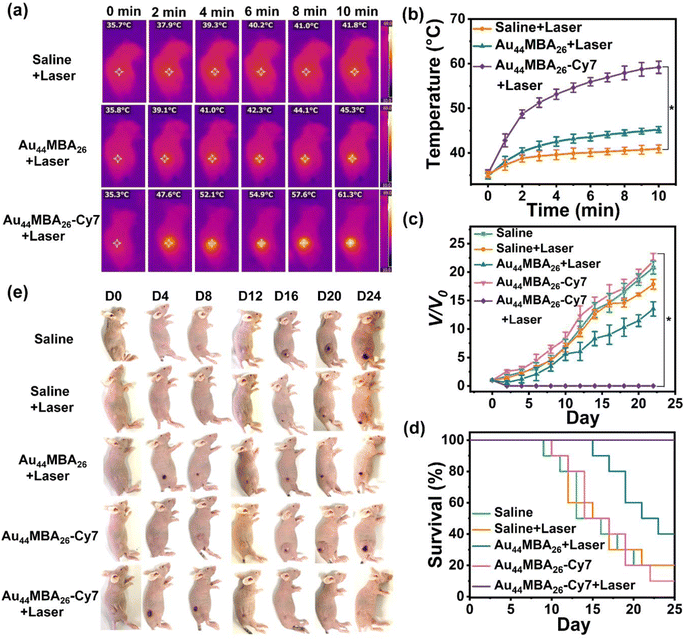
With the guidance of in vivo NIR-II PL and PA imaging, we further investigated the in vivo PTT of the Au44MBA26-Cy7 NCs for 4T1 tumor-bearing mice. As shown in Fig. 4a and b, the infrared radiation (IR) thermal mapping images of 4T1 tumor-bearing mice treated with the Au44MBA26-Cy7 NCs show that the tumor temperature increases rapidly, and reaches the highest temperature of 61.3 °C with 808 nm laser irradiation for 10 min. This result is better than the treatment of the Au44MBA26 NCs and saline with temperature elevations of 9.5 °C and 6 °C, respectively. Consequently, the tumors in mice treated with the Au44MBA26-Cy7 NCs were successfully ablated upon laser irradiation, and burn scars were generated at the tumor site.
After 16 days, 4T1 tumor-bearing mice treated with the Au44MBA26-Cy7 NCs were all healed without tumor recurrence (Fig. 4c and e). In addition, all mice in this group were alive and had to be euthanized (Fig. 4d). By contrast, the tumor of mice treated with the Au44MBA26 NCs initially showed a slight decrease, but then began to grow rapidly from the 4th day (Fig. 4c and e). A worse situation was observed in the control groups (i.e., tumor-bearing mice treated with only laser irradiation or Au44MBA26-Cy7 NCs) where tumors continued to grow rapidly (Fig. 4c and e). In comparison to the recovered health status of mice treated with PTT using the Au44MBA26-Cy7 NCs, the average life span of mice in the other four groups was only 17–21 days (Fig. 4d). Such excellent PTT efficacy of the Au44MBA26-Cy7 NCs is expected considering their outstanding photothermal efficiency, decent molecular pharmacokinetics, good tumor selectivity (due to the EPR effect), and benign biocompatibility. On this basis, we can conclude that the Au44MBA26-Cy7 NCs have been demonstrated to be efficient in NIR-II PL and PA imaging-guided cancer PTT, realizing the first successful attempt in designing an Au NC-based molecular theranostic probe for noninvasive NIR-II PL and PA imaging-guided cancer PTT.
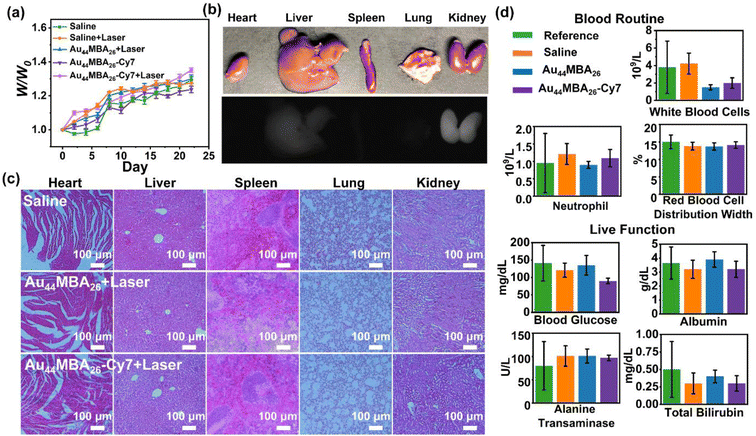
In addition, the possible adverse effects of the Au44MBA26-Cy7 NCs on mice were also examined. Firstly, we checked the body weights of mice in each group during the whole PTT process. The results show that no remarkable weight variation was observed between the different groups of the mice (Fig. 5a), indicating that Au44MBA26-Cy7 NCs-directed PTT had almost no adverse effects on the mice. In addition, after 24 h from the intravenous injection of the Au44MBA26-Cy7 NCs, the mice were sacrificed and dissected in order to evaluate the influence of the Au44MBA26-Cy7 NCs on their major organs (i.e., heart, liver, spleen, lung and kidney). As shown in Fig. 5b, no NIR-II PL signal was perceived from the heart, spleen, and lung, while a moderate PL signal emanated from the kidney and a weak PL signal from the liver was detected. These results suggest the good renal clearance of the ultrasmall Au44MBA26-Cy7 NCs, further reflecting their good biosafety in in vivo dual-mode imaging-guided PTT. Moreover, the haematoxylin and eosin (H & E) histopathological assessment of the major organs of mice in each group disclosed that there is no significant difference in the organs after PTT (Fig. 5c), which indicates that the dual-mode imaging-guided PTT with the Au44MBA26-Cy7 NCs did not damage the major organs of the mice. Besides this, the blood and liver function of the tumor-bearing mice treated with saline, Au44MBA26 NCs, and Au44MBA26-Cy7 NCs were further evaluated. Routine blood tests revealed that the white blood cell, neutrophil and red blood cell counts were all unaffected by the PTT, corroborating the safety and low toxicity of the Au44MBA26-Cy7 NCs and PTT (Fig. 5d). Liver function tests show no significant differences between the three mice groups, and all parameters, including blood glucose, albumin, alanine, and total bilirubin, were within the reference range, indicating that the treated mice exhibited basically normal liver function (Fig. 5d). All of these results exemplify the perfect biosafety of PTT using the Au44MBA26-Cy7 NCs.
Conclusions
In this study, we developed a novel Au NC-based theranostics probe for ultradeep NIR-II PL and PA imaging-guided cancer PTT. The key to this design is the ligand engineering of NIR-II emitting Au44MBA26 NCs by rigid PA/photothermal Cy7 molecules via click chemistry. This not only enhanced the NIR-II PL intensity of the Au44 NCs via AIE, but also elevated their photothermal efficiency to 65.12% by offering a highly localized Cy7 concentration on the NC surface, contradicting the Jablonski energy spectrum theory that strong PL signal and excellent photothermal properties cannot be attained simultaneously. Owing to the EPR effect, the as-synthesized Au44MBA26 NCs-Cy7 NCs successfully achieved in vivo NIR-II PL and PA imaging of tumors in real time with deep tissue penetration, high sensitivity and specificity, which enabled them to be capable of guiding cancer PTT by offering accurate tumor location and real-time feedback. In particular, the 4T1 tumor-bearing mice could be healed without tumor recurrence upon PTT using the Au44MBA26-Cy7 NCs by efficiently ablating tumors. In addition, comprehensive studies revealed that the Au44MBA26-Cy7 NCs exhibit neglectable cytotoxicity and good biosafety in terms of good renal clearance, no damage to major organs, and unaffected blood and liver functions, confirming the feasible application of the Au44MBA26-Cy7 NCs for noninvasive NIR-II PL and PA imaging-guided cancer PTT. This study is interesting because it provides a paradigm in the design of metal NCs-based multifunctional biomedicine for disease theranostics. We envision that the Au44MBA26-Cy7 NCs may have great potential for future clinical translation, which may stimulate additional research activities in noninvasive imaging-guided disease therapy.Data availability
All experimental supporting data and procedures are available in the ESI.†Author contributions
J. P. X., X. F. Z., and X. Y. conceived and supervised the project. G. Y. performed the experiments, and collected and analyzed the data. X. L. M., X. X. P., Y. T., Q. F. Y., Y. R. W., F. Y. J., and F. L. D. helped with the data collection and analysis of animal experiments. G. Y. and X. Y. co-wrote the manuscript. All authors discussed the results and commented on the manuscript. All authors approved the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (22071127, 22071174), the Taishan Scholar Foundation (tsqn201812074), the Natural Science Foundation of Shandong Province (ZR2019YQ07), and the Academic Research Grants from the Ministry of Education of Singapore (R-279-000-580-112 and R-279-000-538-114). This study was performed in strict accordance with the NIH guidelines for the care and use of laboratory animals (NIH Publication No. 85–23 Rev. 1985) and was approved by the Biomedical Ethics Committee of Qingdao Zhong Hao Biological Engineering Co., Ltd. (Qingdao, China).References
- (a) B. Chen, Y. Yan, Y. Yang, G. Cao, X. Wang, Y. Wang, F. Wan, Q. Yin, Z. Wang, Y. Li, L. Wang, B. Xu, F. You, Q. Zhang and Y. Wang, Nat. Nanotechnol., 2022, 17(7), 788–798 CrossRef CAS PubMed; (b) J. Zhang, L. Ning, J. Huang, C. Zhang and K. Pu, Chem. Sci., 2020, 11, 618–630 RSC; (c) N. Niu, Y. Yu, Z. Zhang, M. Kang, L. Wang, Z. Zhao, D. Wang and B. Z. Tang, Chem. Sci., 2022, 13, 5929–5937 RSC; (d) H.-T. Feng, S. Zou, M. Chen, F. Xiong, M.-H. Lee, L. Fang and B. Z. Tang, J. Am. Chem. Soc., 2020, 142, 11442 CrossRef CAS PubMed; (e) J. K. Tee, L. X. Yip, E. S. Tan, S. Santitewagun, A. Prasath, P. C. Ke, H. K. Ho and D. T. Leong, Chem. Soc. Rev., 2019, 48, 5381 RSC; (f) D. Y. Oh, S. S. Kwek, S. S. Raju, T. Li, E. McCarthy, E. Chow, D. Aran, A. Ilano, C.-C. S. Pai, C. Rancan, K. Allaire, A. Burra, Y. Sun, M. H. Spitzer, S. Mangul, S. Porten, M. V. Meng, T. W. Friedlander, C. J. Ye and L. Fong, Cell, 2020, 181, 1612 CrossRef CAS PubMed.
- (a) L. V. Wang and S. Hu, Science, 2012, 335, 1458 CrossRef CAS PubMed; (b) A. L. Antaris, H. Chen, K. Cheng, Y. Sun, G. Hong, C. Qu, S. Diao, Z. Deng, X. Hu, B. Zhang, X. Zhang, O. K. Yaghi, Z. R. Alamparambil, X. Hong, Z. Cheng and H. Dai, Nat. Mater., 2016, 15, 235 CrossRef CAS PubMed; (c) Y. Liu, P. Bhattarai, Z. Dai and X. Chen, Chem. Soc. Rev., 2019, 48, 2053 RSC; (d) X. Li, T. Yong, Z. Wei, N. Bie, X. Zhang, G. Zhan, J. Li, J. Qin, J. Yu, B. Zhang, L. Gan and X. Yang, Nat. Commun., 2022, 13, 2794 CrossRef CAS PubMed.
- J. Qi, C. Chen, X. Zhang, X. Hu, S. Ji, R. T. K. Kwok, J. W. Y. Lam, D. Ding and B. Z. Tang, Nat. Commun., 2018, 9, 1848 CrossRef PubMed.
- (a) B. Du, X. Jiang, A. Das, Q. Zhou, M. Yu, R. Jin and J. Zheng, Nat. Nanotechnol., 2017, 12, 1096 CrossRef CAS PubMed; (b) J. Huang, X. Chen, Y. Jiang, C. Zhang, S. He, H. Wang and K. Pu, Nat. Mater., 2022, 21, 598 CrossRef CAS PubMed.
- (a) P. N. Duchesne, Z. Y. Li, C. P. Deming, V. Fung, X. Zhao, J. Yuan, T. Regier, A. Aldalbahi, Z. Almarhoon, S. Chen, D.-e. Jiang, N. Zheng and P. Zhang, Nat. Mater., 2018, 17, 1033 CrossRef CAS PubMed; (b) M. Han, M. Guo, Y. Yun, Y. Xu, H. Sheng, Y. Chen, Y. Du, K. Ni, Y. Zhu and M. Zhu, Adv. Funct. Mater., 2022, 32, 2202820 CrossRef CAS; (c) X. Kang, Y. Li, M. Zhu and R. Jin, Chem. Soc. Rev., 2020, 49, 6443 RSC; (d) I. Chakraborty and T. Pradeep, Chem. Rev., 2017, 117, 8208 CrossRef CAS PubMed; (e) C. Yao, N. Guo, S. Xi, C.-Q. Xu, W. Liu, X. Zhao, J. Li, H. Fang, J. Su, Z. Chen, H. Yan, Z. Qiu, P. Lyu, C. Chen, H. Xu, X. Peng, X. Li, B. Liu, C. Su, S. J. Pennycook, C.-J. Sun, J. Li, C. Zhang, Y. Du and J. Lu, Nat. Commun., 2020, 11, 4389 CrossRef CAS PubMed.
- (a) M. R. Narouz, K. M. Osten, P. J. Unsworth, R. W. Y. Man, K. Salorinne, S. Takano, R. Tomihara, S. Kaappa, S. Malola, C.-T. Dinh, J. D. Padmos, K. Ayoo, P. J. Garrett, M. Nambo, J. H. Horton, E. H. Sargent, H. Häkkinen, T. Tsukuda and C. M. Crudden, Nat. Chem., 2019, 11, 419 CrossRef CAS PubMed; (b) X. Yuan, B. Zhang, Z. Luo, Q. Yao, D. T. Leong, N. Yan and J. Xie, Angew. Chem., Int. Ed., 2014, 53, 4623 CrossRef CAS PubMed; (c) T. Kawawaki, Y. Kataoka, M. Hirata, Y. Akinaga, R. Takahata, K. Wakamatsu, Y. Fujiki, M. Kataoka, S. Kikkawa, A. S. Alotabi, S. Hossain, D. J. Osborn, T. Teranishi, G. G. Andersson, G. F. Metha, S. Yamazoe and Y. Negishi, Angew. Chem., Int. Ed., 2021, 60, 21340 CrossRef CAS PubMed; (d) H. Shen, Q. Wu, S. Malola, Y.-Z. Han, Z. Xu, R. Qin, X. Tang, Y.-B. Chen, B. K. Teo, H. Häkkinen and N. Zheng, J. Am. Chem. Soc., 2022, 144, 10844 CrossRef CAS PubMed.
- (a) M. Zhou, T. Higaki, G. Hu, Y. Sfeir Matthew, Y. Chen, D.-e. Jiang and R. Jin, Science, 2019, 364, 279 CrossRef CAS PubMed; (b) M. R. Narouz, S. Takano, P. A. Lummis, T. I. Levchenko, A. Nazemi, S. Kaappa, S. Malola, G. Yousefalizadeh, L. A. Calhoun, K. G. Stamplecoskie, H. Häkkinen, T. Tsukuda and C. M. Crudden, J. Am. Chem. Soc., 2019, 141, 14997 CrossRef CAS PubMed; (c) C. Zhu, J. Xin, J. Li, H. Li, X. Kang, Y. Pei and M. Zhu, Angew. Chem., Int. Ed., 2022, e202205947 CAS; (d) J.-J. Li, C.-Y. Liu, Z.-J. Guan, Z. Lei and Q.-M. Wang, Angew. Chem., Int. Ed., 2022, 61, e202201549 CAS.
- (a) X.-Q. Liang, Y.-Z. Li, Z. Wang, S.-S. Zhang, Y.-C. Liu, Z.-Z. Cao, L. Feng, Z.-Y. Gao, Q.-W. Xue, C.-H. Tung and D. Sun, Nat. Commun., 2021, 12, 4966 CrossRef CAS PubMed; (b) J.-Q. Wang, Z.-J. Guan, W.-D. Liu, Y. Yang and Q.-M. Wang, J. Am. Chem. Soc., 2019, 141, 2384 CrossRef CAS PubMed; (c) K. R. Krishnadas, L. Sementa, M. Medves, A. Fortunelli, M. Stener, A. Fürstenberg, G. Longhi and T. Bürgi, ACS Nano, 2020, 14, 9687 CrossRef CAS PubMed.
- (a) Y. Li, M. Zhou, Y. Song, T. Higaki, H. Wang and R. Jin, Nature, 2021, 594, 380 CrossRef CAS PubMed; (b) X. Cai, W. Hu, S. Xu, D. Yang, M. Chen, M. Shu, R. Si, W. Ding and Y. Zhu, J. Am. Chem. Soc., 2020, 142, 4141 CrossRef CAS PubMed; (c) H. Seong, V. Efremov, G. Park, H. Kim, J. S. Yoo and D. Lee, Angew. Chem., Int. Ed., 2021, 60, 14563 CrossRef CAS PubMed; (d) X. Yuan, L. L. Chng, J. Yang and J. Y. Ying, Adv. Mater., 2020, 32, 1906063 CrossRef CAS PubMed; (e) L.-J. Liu, Z.-Y. Wang, Z.-Y. Wang, R. Wang, S.-Q. Zang and T. C. W. Mak, Angew. Chem., Int. Ed., 2022, e202205626 CAS; (f) X. Wang, L. Zhao, X. Li, Y. Liu, Y. Wang, Q. Yao, J. Xie, Q. Xue, Z. Yan, X. Yuan and W. Xing, Nat. Commun., 2022, 13, 1596 CrossRef CAS PubMed.
- (a) M. Yu, J. Xu and J. Zheng, Angew. Chem., Int. Ed., 2019, 58, 4112 CrossRef CAS PubMed; (b) K. Zheng and J. Xie, Trends Chem., 2020, 2, 665 CrossRef CAS.
- (a) X. Jiang, B. Du and J. Zheng, Nat. Nanotechnol., 2019, 14, 874 CrossRef CAS PubMed; (b) J. Liu, M. Yu, X. Ning, C. Zhou, S. Yang and J. Zheng, Angew. Chem., Int. Ed., 2013, 52, 12572 CrossRef CAS PubMed; (c) C.-Y. Liu, S.-F. Yuan, S. Wang, Z.-J. Guan, D.-e. Jiang and Q.-M. Wang, Nat. Commun., 2022, 13, 2082 CrossRef CAS PubMed.
- (a) C. N. Loynachan, A. P. Soleimany, J. S. Dudani, Y. Lin, A. Najer, A. Bekdemir, Q. Chen, S. N. Bhatia and M. M. Stevens, Nat. Nanotechnol., 2019, 14, 883 CrossRef CAS PubMed; (b) B. Du, M. Yu and J. Zheng, Nat. Rev. Mater., 2018, 3, 358 CrossRef.
- (a) X. Zhang, Z. Zhang, Q. Shu, C. Xu, Q. Zheng, Z. Guo, C. Wang, Z. Hao, X. Liu, G. Wang, W. Yan, H. Chen and C. Lu, Adv. Funct. Mater., 2021, 31, 2008720 CrossRef CAS; (b) K. Zheng, K. Li, T.-H. Chang, J. Xie and P.-Y. Chen, Adv. Funct. Mater., 2019, 29, 1904603 CrossRef CAS; (c) Y. Hua, J.-H. Huang, Z.-H. Shao, X.-M. Luo, Z.-Y. Wang, J.-Q. Liu, X. Zhao, X. Chen and S.-Q. Zang, Adv. Mater., 2022, 2203734 CrossRef CAS PubMed; (d) O.-V. Pham-Nguyen, J. Shin, Y. Park, S. Jin, S. R. Kim, Y. M. Jung and H. S. Yoo, Biomacromolecules, 2022, 23, 3130 CrossRef CAS PubMed; (e) S. M. van de Looij, E. R. Hebels, M. Viola, M. Hembury, S. Oliveira and T. Vermonden, Bioconjugate Chem., 2022, 33, 4 CrossRef CAS PubMed.
- (a) H. Liu, G. Hong, Z. Luo, J. Chen, J. Chang, M. Gong, H. He, J. Yang, X. Yuan, L. Li, X. Mu, J. Wang, W. Mi, J. Luo, J. Xie and X.-D. Zhang, Adv. Mater., 2019, 31, 1901015 CrossRef CAS PubMed; (b) X. Song, W. Zhu, X. Ge, R. Li, S. Li, X. Chen, J. Song, J. Xie, X. Chen and H. Yang, Angew. Chem., Int. Ed., 2021, 60, 1306 CrossRef CAS PubMed; (c) W. Wang, Y. Kong, J. Jiang, Q. Xie, Y. Huang, G. Li, D. Wu, H. Zheng, M. Gao, S. Xu, Y. Pan, W. Li, R. Ma, M. X. Wu, X. Li, H. Zuilhof, X. Cai and R. Li, Angew. Chem., Int. Ed., 2020, 59, 22431 CrossRef CAS PubMed; (d) D. Li, Q. Liu, Q. Qi, H. Shi, E.-C. Hsu, W. Chen, W. Yuan, Y. Wu, S. Lin, Y. Zeng, Z. Xiao, L. Xu, Y. Zhang, T. Stoyanova, W. Jia and Z. Cheng, Small, 2020, 16, 2003851 CrossRef CAS PubMed; (e) Y. Chen, D. M. Montana, H. Wei, J. M. Cordero, M. Schneider, X. Le Guével, O. Chen, O. T. Bruns and M. G. Bawendi, Nano Lett., 2017, 17, 6330 CrossRef CAS PubMed.
- (a) X. Mu, Y. Lu, F. Wu, Y. Wei, H. Ma, Y. Zhao, J. Sun, S. Liu, X. Zhou and Z. Li, Adv. Mater., 2020, 32, 1906711 CrossRef CAS PubMed; (b) J. Chen, M. Gong, Y. Fan, J. Feng, L. Han, H. L. Xin, M. Cao, Q. Zhang, D. Zhang, D. Lei and Y. Yin, ACS Nano, 2022, 16, 910 CrossRef CAS PubMed; (c) G. Xu, C. Li, C. Chi, L. Wu, Y. Sun, J. Zhao, X.-H. Xia and S. Gou, Nat. Commun., 2022, 13, 3064 CrossRef CAS PubMed.
- (a) X. Jiang, B. Du, S. Tang, J.-T. Hsieh and J. Zheng, Angew. Chem., Int. Ed., 2019, 58, 5994 CrossRef CAS PubMed; (b) M. Y. Lucero and J. Chan, Nat. Chem., 2021, 13, 1248 CrossRef CAS PubMed; (c) C. A. Wood, S. Han, C. S. Kim, Y. Wen, D. R. T. Sampaio, J. T. Harris, K. A. Homan, J. L. Swain, S. Y. Emelianov, A. K. Sood, J. R. Cook, K. V. Sokolov and R. R. Bouchard, Nat. Commun., 2021, 12, 5410 CrossRef CAS PubMed.
- (a) M. Zhang, W. Wang, M. Mohammadniaei, T. Zheng, Q. Zhang, J. Ashley, S. Liu, Y. Sun and B. Z. Tang, Adv. Mater., 2021, 33, 2008802 CrossRef CAS PubMed; (b) Z. Wu, Q. Yao, O. J. H. Chai, N. Ding, W. Xu, S. Zang and J. Xie, Angew. Chem., Int. Ed., 2020, 59, 9934 CrossRef CAS PubMed; (c) D. Yan, M. Wang, Q. Wu, N. Niu, M. Li, R. Song, J. Rao, M. Kang, Z. Zhang, F. Zhou, D. Wang and B. Z. Tang, Angew. Chem., Int. Ed., 2022, e202202614 CAS; (d) Z. Luo, X. Yuan, Y. Yu, Q. Zhang, D. T. Leong, J. Y. Lee and J. Xie, J. Am. Chem. Soc., 2012, 134, 16662 CrossRef CAS PubMed.
- X. Zhang, Z. Wang, S. Qian, N. Liu, L. Sui and X. Yuan, Nanoscale, 2020, 12, 6449 RSC.
- Q. Yao, X. Yuan, V. Fung, Y. Yu, D. T. Leong, D.-e. Jiang and J. Xie, Nat. Commun., 2017, 8, 927 CrossRef PubMed.
- K. Pyo, V. D. Thanthirige, S. Y. Yoon, G. Ramakrishna and D. Lee, Nanoscale, 2016, 8, 20008–20016 RSC.
- H. Deng, K. Huang, L. Xiu, W. Sun, Q. Yao, X. Fang, X. Huang, H. A. A. Noreldeen, H. Peng, J. Xie and W. Chen, Nat. Commun., 2022, 13, 3381 CrossRef CAS PubMed.
- Y.-S. Chen and P. V. Kamat, J. Am. Chem. Soc., 2014, 136, 6075 CrossRef CAS PubMed.
- L. Liao, S. Zhuang, C. Yao, N. Yan, J. Chen, C. Wang, N. Xia, X. Liu, M.-B. Li, L. Li, X. Bao and Z. Wu, J. Am. Chem. Soc., 2016, 138, 10425 CrossRef CAS PubMed.
- (a) C. Iserman, C. Desroches Altamirano, C. Jegers, U. Friedrich, T. Zarin, A. W. Fritsch, M. Mittasch, A. Domingues, L. Hersemann, M. Jahnel, D. Richter, U.-P. Guenther, M. W. Hentze, A. M. Moses, A. A. Hyman, G. Kramer, M. Kreysing, T. M. Franzmann and S. Alberti, Cell, 2020, 181, 818 CrossRef CAS PubMed; (b) C. Spiess, A. Beil and M. Ehrmann, Cell, 1999, 97, 339 CrossRef CAS PubMed.
- X. Cai, X. Jia, W. Gao, K. Zhang, M. Ma, S. Wang, Y. Zheng, J. Shi and H. Chen, Adv. Funct. Mater., 2015, 25, 2520 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: The native PAGE and ESI mass of Au44MBA26 NCs; synthetic route and characteristics of the alkynylated Cy7 molecules; FTIR spectra and UV-vis absorption spectra of Au44 NCs; NIR-II PL emission spectra of Au44MBA26-Cy7 NCs in mixed solvents; PTT of Au44MBA26 NCs in vitro; PA images of Au44MBA26 NCs; supplementary notes. See DOI: https://doi.org/10.1039/d2sc05729h |
| This journal is © The Royal Society of Chemistry 2023 |