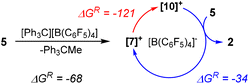Open Access Article
Open Access ArticleSelective synthesis of germasila-adamantanes through germanium–silicon shift processes†
Steffen
Kühn
ab,
Benedikt
Köstler
 c,
Celine
True
b,
Lena
Albers
c,
Celine
True
b,
Lena
Albers
 b,
Matthias
Wagner
b,
Matthias
Wagner
 *c,
Thomas
Müller
*c,
Thomas
Müller
 *b and
Christoph
Marschner
*b and
Christoph
Marschner
 *a
*a
aInstitut für Anorganische Chemie, Technische Universität Graz, Stremayrgasse 9, 8010 Graz, Austria
bInstitut für Chemie, Carl Ossietzky Universität Oldenburg, Carl von Ossietzky-Str. 9-11, 26129 Oldenburg, Germany
cInstitut für Anorganische und Analytische Chemie, Goethe Universität Frankfurt am Main, Max-von-Laue-Str. 7, 60438 Frankfurt am Main, Germany
First published on 31st July 2023
Abstract
The regioselective synthesis of germasila-adamantanes with the germanium atoms in the bridgehead positions is described starting from cyclic precursors by a cationic sila-Wagner–Meerwein (SWM) rearrangement reaction. The SWM rearrangement allows also a deliberate shift of germanium atoms from the periphery and within the cage structures into the bridgehead positions. This opens the possibility for a synthesis of germasila-adamantanes of defined germanium content and controlled regiochemistry. In the same way that sila-adamantane can be regarded as a molecular building block of elemental silicon, the germasila-adamantane molecules represent cutouts of silicon/germanium alloys.
Introduction
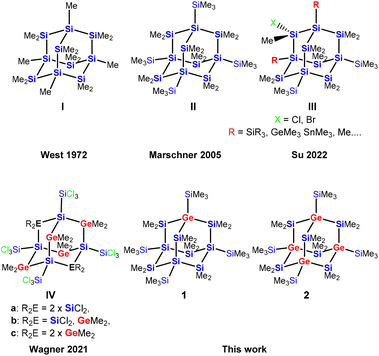
The chemistry of polysilanes has now been studied for more than 100 years.1–5 While their structural chemistry resembles that of alkanes, their property of electron conjugation suggests some similarity to unsaturated hydrocarbons. Structurally, cyclic and polycyclic polysilanes are the higher congeners of cyclo- and polycycloalkanes.6,7Adamantane cage structures of polysilanes are of particular interest as they represent the building blocks of elemental silicon. The synthesis of sila-adamantane cages nicely reflects the development of preparative approaches towards this type of compounds (Fig. 1). The first synthetic attempt, which was reported by West and Indriksons as early as in 1972, employed a Wurtz type condensation of Me2SiCl2 and MeSiCl3 with sodium/potassium alloy.8 As the Wurtz-type approach is not very selective, the formation of the expected permethylsilaadamantane cage I could not unambiguously be proven. Salt elimination reactions between oligosilanides and silyl halides represented a major advancement.9 This method allowed access to a cyclohexasilanyl substituted bicylo[2.2.1]heptasilane, which served as the structural isomer required for sila-Wagner–Meerwein (SWM) rearrangement to the first structurally verified example of a sila-adamantane molecule II.10 Recently, Su and co-workers demonstrated site selective functionalization of both the bridging and the bridgehead positions as in adamantane III.11 Wagner and co-workers presented a surprising simple but high yielding synthesis of germasila-adamantanes. Reactions of Si2Cl6 with Me2GeCl2 in the presence of (nBu4N)Cl form adamantane cages with silicon atoms in the bridgehead and either SiCl2- or GeMe2-units in the bridging positions (compounds IVa–IVc, Fig. 1).12
Our present contribution deals with the selective synthesis of germasila-adamantanes from cyclic germa-oligosilanes, the deliberate formal exchange of a sila-adamantane's bridgehead silicon for an attached germanium atom, and the shift of four germanium atoms from bridging into bridgehead positions, both without affecting the integrity of the adamantane cage. These transformations showcase the high synthetic capacity of SWM reactions for the tailored construction of complex germasila-adamantane structures, which might serve as molecular models for nano-sized silicon/germanium materials. Silicon/germanium nanostructures, SixGe1−x, are extensively used in applications such as advanced transistors, quantum devices and photodetectors, electro-optical modulators, photovoltaics, microelectromechanical systems and thermoelectric generators.13–19 All silicon and germanium atoms of the here investigated Si/Ge cluster molecules are tetracoordinated6 and therefore clearly distinguished from substituent-free silicon and germanium atoms in mixed Si/Ge Zintl ions20–22 and germa-siliconoids.23–26
Oligosilanyl cations are capable of undergoing Wagner–Meerwein rearrangement chemistry in a similar way as alkanes.27 This was discovered by Kumada and Ishikawa, who reported AlCl3-mediated rearrangement of permethylated linear oligosilanes to branched structures and related chemistry of cyclosilanes.28–30 The strictly thermodynamic driving force of this reaction bears some synthetic potential.10,31,32 In addition to structural rearrangements of oligosilanes, the Wagner–Meerwein chemistry of germa-oligosilanes allows also a selective intramolecular shift of germanium atoms into more silylated positions.33 For instance, under the influence of a strong Lewis acid tris(trimethylsilyl)trimethylgermylsilane is quantitatively converted to tetrakis(trimethylsilyl)germane.34,35
Results and discussion
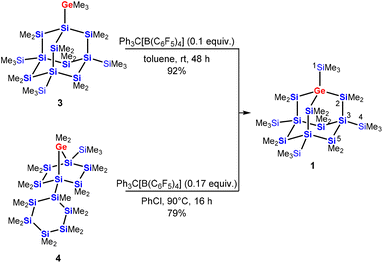
Utilizing this chemistry on a more complex substrate, we treated the trimethylgermylated sila-adamantane 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 with a catalytic amount of Ph3C[B(C6F5)4] in toluene and observed clean conversion to compound 1 with a germa-nonasila-adamantane cage structure in 92% isolated yield (Scheme 1). Adamantane 1 was also obtained from polycyclic starting materials via the established route to all-sila-adamantanes: the tricyclic adamantane precursor 4 with a dimethylgermylene unit rearranges quantitatively and selectively to the germasila-adamantane 1 (79% isolated yield). In this case, slightly enforced reaction conditions had to be applied (Scheme 1).
11 with a catalytic amount of Ph3C[B(C6F5)4] in toluene and observed clean conversion to compound 1 with a germa-nonasila-adamantane cage structure in 92% isolated yield (Scheme 1). Adamantane 1 was also obtained from polycyclic starting materials via the established route to all-sila-adamantanes: the tricyclic adamantane precursor 4 with a dimethylgermylene unit rearranges quantitatively and selectively to the germasila-adamantane 1 (79% isolated yield). In this case, slightly enforced reaction conditions had to be applied (Scheme 1).
The NMR spectrum of 1 exhibits the expected pattern. Five signals in the 1H NMR spectrum at δ = 0.65 (18H), 0.62 (9H), 0.61 (9H), 0.38 (9H), and 0.35 (27H) ppm correspond to the methyl groups at Si2, Si5 (2 × for Meax and Meeq), Si1, and Si4 (Si numbering in accordance with the structural formula of 1 in Scheme 1), respectively. The same number of five signals was found for the methyl groups in the 13C NMR spectrum at 5.5 (Si1), 5.0 (Si4), 4.8 (Si2), 4.2, and 4.1 (Si5) ppm. Also the 29Si NMR spectrum features five signals at 0.8 (Si1), −5.6 (Si4), −20.9 (Si2), −26.3 (Si5), and −118.5 (Si3) ppm (assignment of Si2 and Si5 by 1H–29Si HMBC). Comparison to the 1H NMR spectrum of the all-sila-adamantane II (1H: δ = 0.60) (s, 36H, SiMe2), 0.35 (s, 36H, SiMe3)10 shows the chemical shifts of 1 to be very similar, with all SiMe2 signals around 0.60 ppm and the SiMe3 signals around 0.35 ppm. The 29Si NMR chemical shifts of the cyclohexasilanyl unit of the bottom part of compound 1 (i.e. Si3, Si4, and Si5) are very close to those of the all-sila adamantane II (29Si: δ = −4.8, −26.0, and −118.6), indicating no major influence of the germanium atom.
The recently published synthesis of germanium-containing sila-adamantane IVa describes the use of the Si2Cl6/Me2GeCl2/(nBu4N)Cl system to obtain a germasila-adamantane with two SiCl2 and four GeMe2 units in the bridging positions as well as four SiCl3-substituted silicon atoms as the bridgeheads.12 Exhaustive methylation of this product led to germasila-adamantane 5 (Scheme 2).36
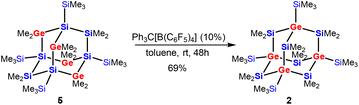 | ||
| Scheme 2 Cationic rearrangement of the tetragermasila-adamantane 5 to the isomeric product 2 with the four germanium atoms of the adamantane cage moving from bridging into bridgehead positions. | ||
Subjecting compound 5 to the rearrangement conditions (Ph3C[B(C6F5)4]/toluene) led to the clean formation of the adamantane cage product 2 with all germanium atoms shifted to the bridgehead positions in 69% isolated yield (Scheme 2).
Due to the higher symmetry of 2 compared to 1, NMR spectroscopic analysis of 2 exhibited a simpler pattern. The two signals in the 1H NMR spectrum at δ = 0.69 (36H) and 0.38 (36H) ppm correspond to the SiMe2 and SiMe3 groups, respectively. The two signals for the methyl groups in the 13C NMR spectrum were observed at 5.6 and 5.4 ppm. Compared to the all-sila-adamantane II (δ = −4.8, −26.0, and −118.6 ppm), the 29Si NMR spectrum of 2 features only the two signals at −0.5 (SiMe3) and −15.7 (SiMe2), with the downfield-shift behavior typical for neighboring germanium atoms.37,38
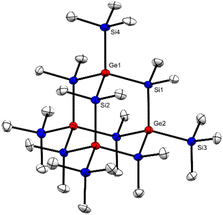
Single crystal XRD analysis of 2 (ref. 39) (Fig. 2) provided a structure isotypic to the all-sila-adamantane II.10 Both compounds crystallize in the space group R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) and the reduced cell parameters of 2 (14.171 Å and 77.13 deg.) give rise to a cell volume of 2659 Å3 compared to the 2636 Å3 observed for the all-sila-adamantane II.10 The Si–Ge bond length in adamantane 2 is 238.7 pm, which is close to the median of the Si–Ge bond lengths in molecular compounds (239.7 pm) and close to the sum of the single bond covalent radii of both elements (237 pm).40,41 Interestingly, it is only 1.7 pm larger than the Si–Si bonds in all-sila-adamantane. The bond angles around the germanium atoms are close to the tetrahedral ideal of 109.5° (108.5–110.1°). Judged from these molecular parameters, the germasila-adamantane 2 appears to be completely unstrained. The UV(vis) spectrum of adamantane 2 in n-hexane shows a strong absorption at λmax = 224 nm (molar absorption ε = 2 × 104 L mol−1 cm−1), also very similar to the UV data reported for all-sila-adamantane II (λmax = 222 nm, ε = 1.2 × 105 L mol−1 cm−1).10
and the reduced cell parameters of 2 (14.171 Å and 77.13 deg.) give rise to a cell volume of 2659 Å3 compared to the 2636 Å3 observed for the all-sila-adamantane II.10 The Si–Ge bond length in adamantane 2 is 238.7 pm, which is close to the median of the Si–Ge bond lengths in molecular compounds (239.7 pm) and close to the sum of the single bond covalent radii of both elements (237 pm).40,41 Interestingly, it is only 1.7 pm larger than the Si–Si bonds in all-sila-adamantane. The bond angles around the germanium atoms are close to the tetrahedral ideal of 109.5° (108.5–110.1°). Judged from these molecular parameters, the germasila-adamantane 2 appears to be completely unstrained. The UV(vis) spectrum of adamantane 2 in n-hexane shows a strong absorption at λmax = 224 nm (molar absorption ε = 2 × 104 L mol−1 cm−1), also very similar to the UV data reported for all-sila-adamantane II (λmax = 222 nm, ε = 1.2 × 105 L mol−1 cm−1).10
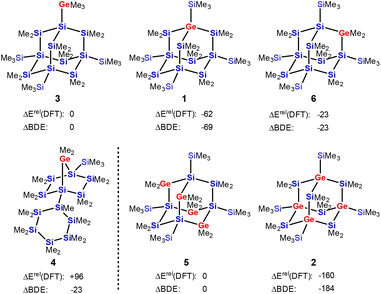
The results of density functional calculations42 at the M06-2X/6-311+G(d,p) level show that the formation of the sila-adamantane cluster is thermodynamically strongly favored, i.e. the isomerization of polycyclic germasilane 4 into germasilaadamantane 1 is exothermic by 158 kJ mol−1 (Fig. 3 and ESI†). Adamantane 1 with the germanium atom in a bridgehead position is also the most stable of the three positional isomers 1, 3, and 6. It is energetically favored by 39 kJ mol−1 over the adamantane isomer 6 with the germanium atom in the bridging position and by 62 kJ mol−1 over germylsila-adamantane 3 with an exocyclic germyl substituent. Similarly, the tetragermasila-adamantane 5 with four bridging GeMe2 units is less stable than its isomer 2 with four germanium atoms in the bridgehead positions by 160 kJ mol−1. The thermodynamic preference of adamantane isomers with germanium in the bridgehead positions is a consequence of the relative strength of the Si–C bond (373 kJ mol−1) versus the Ge–C bond (326 kJ mol−1), which outcompetes the formation of Si–Ge bonds (294 kJ mol−1) on the cost of Si–Si linkages (318 kJ mol−1, see Table S3† for calculated BDEs). For example, the transformation of germasila-adamantane 6 into its isomer 1 needs an exchange of two Ge–C bonds for two stronger Si–C bonds (−94 kJ mol−1) and of two Si–Si bonds for two weaker Ge–Si bonds (+48 kJ mol−1). The estimation of the energy difference 6vs.1 based on these increments results in −46 kJ mol−1 in preference of the germasila-adamantane 1, which is close to the DFT-computed energy difference of −39 kJ mol−1 (Fig. 3). While the transformation of the germasila-adamantane cages 1, 3, 6, and 2, 5 can be understood on this basis, their formation from polycyclic precursors such as 4 is less straightforward. In addition, other effects such as release of ring strain and attractive London dispersion forces between silyl groups add to the thermodynamic preference of the adamantane cluster.43,44 For example, the ring strain of the bicycloheptane part of the tricyclic germasilane 4 is according to an isodesmic reaction 47 kJ mol−1 and the consideration of dispersion energy favors the adamantane 1 over 4 by 37 kJ mol−1 (for details, see ESI, Fig. S33 and S34†).
We investigated the intriguing skeletal rearrangement of the germasila-adamantane cage during the transformation 5 → 2 in detail. Previous experimental and computational studies on related systems demonstrated that the initial step in this type of rearrangement is the formation of silyl- or germyl cations by transfer of a methyl group from the germa-oligosilane to the trityl cation.33 After rearrangement to the more stable silyl or germyl cation, the reaction terminates with a final methyl group transfer. Methyl-anion abstraction from tetragermasila-adamantane 5 allows the formation of three different cations [7]+–[9]+. The secondary germylium cation [7]+ is the most stable one, followed by the secondary silylium cation [8]+ and the primary silylium cation [9]+ with an exo-cage dimethylsilylium substituent is the least stable cation in this series (Fig. 4) (Note: In the context of this manuscript the terms primary or secondary cation are used for cations with either one silyl/germyl substituent or with two such substituents). These cations are significantly higher in energy than the two silyl cations ([10]+ and [11]+) that are possible immediate precursors for tetragermasila-adamantane 2. The reason for the higher stability of the cations [10]+ and [11]+ is the bridgehead position of all four germanium atoms. Therefore, the isomerization of the germylium cation [7]+ to the secondary silyl cation [10]+ is exothermic by 120 kJ mol−1 (Fig. 4). Association of the cations [7]+–[11]+ to the solvent benzene is not of importance as the energy differences between the benzene complexes [7(C6H6)]+–[11(C6H6)]+ are similar to those of the isolated cations and inclusion of entropy effects shows that these complexes do not exist under ambient conditions (free association enthalpy ΔGA,298 > 0 kJ mol−1 ([7]+, [8]+, [10]+)) or are only weakly bonded (ΔGA,298 > −15 kJ mol−1 ([9]+, [11]+), see Table S4†).45
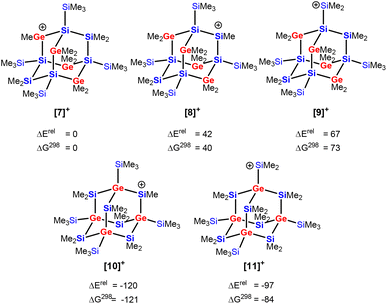 | ||
| Fig. 4 Relative energies ΔErel and free enthalpies ΔG298 of Si10Ge4Me23 cations derived from heteroadamantanes 3 ([7]+–[9]+) and 4 from ([10]+, [11]+, in kJ mol−1, at M06-2X/6-311+G(d,p)). | ||
Based on these relative cation stabilities, we suggest the following mechanistic scenario for the trityl-induced rearrangement 5 → 2. The initial step, the exergonic methyl-anion transfer from a germylene unit of adamantane 5 to the trityl cation and formation of the germylium cation [7]+ (ΔGR = −68 kJ mol−1), is followed by its transformation to the more stable adamantyl cation [10]+ (ΔGR = −121 kJ mol−1). Also the terminating methyl-anion transfer from adamantane 5 to silyl cation [10]+ is thermodynamically favored (ΔGR = −34 kJ mol−1) and closes the catalytic cycle (Scheme 3). The endergonic methyl-anion transfer from Ph3CMe to cation [10]+ (ΔGR = +35 kJ mol−1) suggests that the trityl borate is only the initiating reagent and not the actual catalyst.
The isomerization of secondary germylium cation [7]+ with four germanium atoms in the bridging position of the adamantane cage into the silylium cation [10]+ with all four germanium atoms in the bridgehead position is a multistep process involving several cationic intermediates. To understand this process we investigated the migration of one germanium center of cation [7]+ into a neighboring bridgehead position, i.e. the transformation of cation [7]+ into the germylium cation [12]+ (Scheme 4). This isomerization is thermodynamically favored (by 39 kJ mol−1) and involves three cationic intermediates and the rate determining step (rtds) is a [1,2] methyl-group shift, 159 kJ mol−1 above the initially formed cation [7]+ (see Fig. S32 and Table S2† for details). Finally, three similar consecutive processes transform the germylium cation [12]+ into the more stable cation [10]+.
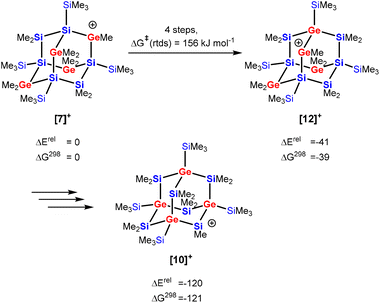 | ||
| Scheme 4 Recapitulation of the isomerization reaction of tetragerma-adamantyl cations [7]+ into [10]+via [12]+ (energies and free enthalpies are given in kJ mol−1 at M06-2X/6-311+G(d,p), for details see Fig. S32†). | ||
Conclusions
Our results indicate that sila-Wagner–Meerwein rearrangement reactions initiated by trityl cation paired with a weakly coordinating anion can be used to prepare a germasila-adamantane from a germa-oligosilane in high yields. The rearrangement reaction is highly selective, only the thermodynamic most stable isomer with the germanium atom in the bridgehead position of the adamantanes is formed. Additionally, we have shown that germanium atoms, installed at the periphery (as in 3) or in the bridging positions of an all-sila-adamantane cage (as in 5), can be shuttled into the bridgehead positions of the cage. The major driving force for these rearrangements is the formation of a maximum of strong Si–C bonds. These results suggest that a stepwise doping of sila-adamantanes with germanium is possible and that adamantanes obtained by different synthetic routes with variable numbers of germanium atoms can be transformed into the most stable isomers without interfering with the cage structure. Therefore, this method allows a rational design of molecular models for mixed silicon germanium alloys. The extension of this method to Sn/Si hetero-adamantanes36 and related main group element hetero-adamantanes is currently investigated in our laboratories and will further broaden the scope of this method.46Data availability
All data we wish to disclose are in the ESI.†Author contributions
Investigation and validation: S. K., C. T., L. A., B. K., T. M. Conceptualisation, methodology, supervision: M. W., C. M., T. M. Writing – original draft: C. M, T. M. Writing – review and editing: M. W., C. M. T. M.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank Dr Judith Baumgartner for crystallographic advice. Computations were done at the HPC Cluster, CARL, University of Oldenburg, funded by the DFG (INST 184/108-1 FUGG) and the Ministry of Science and Culture (MWK) of the Lower Saxony State.References
- R. D. Miller and J. Michl, Chem. Rev., 1989, 89, 1359–1410 CrossRef CAS
.
-
J. Koe and M. Fujiki, in Organosilicon Compounds, ed. V. Y. Lee, Academic Press, 2017, pp. 219–300 Search PubMed
.
-
J. Beckmann, in Comprehensive Organometallic Chemistry III, ed. D. M. P. Mingos and R. H. Crabtree, Elsevier, Oxford, 2007, pp. 409–512 Search PubMed
.
-
C. Marschner, in Functional Molecular Silicon Compounds I: Regular Oxidation States, ed. D. Scheschkewitz, Springer International Publishing, Cham, 2014, pp. 163–228 Search PubMed
.
-
J. Baumgartner and C. Grogger, in Comprehensive Inorganic Chemistry II, ed. J. Reedijk and K. Poeppelmeier, Elsevier, Amsterdam, Second Edition, 2013, pp. 51–82 Search PubMed
.
- Y. Heider and D. Scheschkewitz, Chem. Rev., 2021, 121, 9674–9718 CrossRef CAS PubMed
.
- E. Hengge and R. Janoschek, Chem. Rev., 1995, 95, 1495–1526 CrossRef CAS
.
- R. West and A. Indriksons, J. Am. Chem. Soc., 1972, 94, 6110–6115 CrossRef CAS
.
- C. Marschner, Organometallics, 2006, 25, 2110–2125 CrossRef CAS
.
- J. Fischer, J. Baumgartner and C. Marschner, Science, 2005, 310, 825 CrossRef CAS PubMed
.
- T. C. Siu, M. Imex Aguirre Cardenas, J. Seo, K. Boctor, M. G. Shimono, I. T. Tran, V. Carta and T. A. Su, Angew. Chem., Int. Ed., 2022, 61, e202206877 CrossRef CAS PubMed
.
- B. Köstler, M. Bolte, H.-W. Lerner and M. Wagner, Chem.–Eur. J., 2021, 27, 14401–14404 CrossRef PubMed
.
- J. Aberl, M. Brehm, T. Fromherz, J. Schuster, J. Frigerio and P. Rauter, Opt. Express, 2019, 27, 32009–32018 CrossRef CAS PubMed
.
- G. L. Wang, M. Moeen, A. Abedin, M. Kolahdouz, J. Luo, C. L. Qin, H. L. Zhu, J. Yan, H. Z. Yin, J. F. Li, C. Zhao and H. H. Radamson, J. Appl. Phys., 2013, 114, 123511 CrossRef
.
- D. Marris-Morini, V. Vakarin, J. M. Ramirez, Q. Liu, A. Ballabio, J. Frigerio, M. Montesinos, C. Alonso-Ramos, X. Le Roux, S. Serna, D. Benedikovic, D. Chrastina, L. Vivien and G. Isella, Nano, 2018, 7, 1781–1793 CAS
.
- V. Vakarin, W. N. Ye, J. M. Ramírez, Q. Liu, J. Frigerio, A. Ballabio, G. Isella, L. Vivien, C. Alonso-Ramos, P. Cheben and D. Marris-Morini, Opt. Express, 2019, 27, 9838–9847 CrossRef CAS PubMed
.
- B. Mheen, Y.-J. Song, J.-Y. Kang and S. Hong, ETRI J., 2005, 27, 439–445 CrossRef
.
- S. Sedky, A. Witvrouw and K. Baert, Sens. Actuators, A, 2002, 97–98, 503–511 CrossRef CAS
.
- G. Scappucci, C. Kloeffel, F. A. Zwanenburg, D. Loss, M. Myronov, J.-J. Zhang, S. De Franceschi, G. Katsaros and M. Veldhorst, Nat. Rev. Mater., 2021, 6, 926–943 CrossRef CAS
.
- T. Henneberger, W. Klein and T. F. Fässler, Z. Anorg. Allg. Chem., 2018, 644, 1018–1027 CrossRef CAS
.
- M. Waibel, G. Raudaschl-Sieber and T. F. Fässler, Chem.–Eur. J., 2011, 17, 13391–13394 CrossRef CAS PubMed
.
- M. Waibel and T. F. Fässler, Inorg. Chem., 2013, 52, 5861–5866 CrossRef CAS PubMed
.
- A. Jana, V. Huch, M. Repisky, R. J. F. Berger and D. Scheschkewitz, Angew. Chem., Int. Ed., 2014, 53, 3514–3518 CrossRef CAS PubMed
.
- L. Klemmer, V. Huch, A. Jana and D. Scheschkewitz, Chem. Commun., 2019, 55, 10100–10103 RSC
.
- N. E. Poitiers, V. Huch, B. Morgenstern, M. Zimmer and D. Scheschkewitz, Angew. Chem., Int. Ed., 2022, 61, e202205399 CrossRef CAS PubMed
.
- J. Helmer, J. Droste, M. R. Hansen, A. Hepp and F. Lips, Dalton Trans., 2022, 51, 10535–10542 RSC
.
- H. F. T. Klare, L. Albers, L. Süsse, S. Keess, T. Müller and M. Oestreich, Chem. Rev., 2021, 121, 5889–5985 CrossRef CAS PubMed
.
- M. Ishikawa and M. Kumada, Chem. Commun., 1969, 567b–568b RSC
.
- M. Kumada, Chem. Commun., 1970, 157a Search PubMed
.
- M. Ishikawa, M. Watanabe, J. Iyoda, H. Ikeda and M. Kumada, Organometallics, 1982, 1, 317–322 CrossRef CAS
.
- H. Wagner, A. Wallner, J. Fischer, M. Flock, J. Baumgartner and C. Marschner, Organometallics, 2007, 26, 6704–6717 CrossRef CAS
.
- H. Wagner, J. Baumgartner, C. Marschner and P. Poelt, Organometallics, 2011, 30, 3939–3954 CrossRef CAS PubMed
.
-
T. Müller, in Organogermanium compounds: theory, experiment and applications, ed. Lee V. Y., Wiley, Hoboken N.J., 2023, pp. 299–338 Search PubMed
.
- H. Wagner, J. Baumgartner, T. Müller and C. Marschner, J. Am. Chem. Soc., 2009, 131, 5022–5023 CrossRef CAS PubMed
.
- L. Albers, M. Aghazadeh Meshgi, J. Baumgartner, C. Marschner and T. Müller, Organometallics, 2015, 34, 3756–3763 CrossRef CAS PubMed
.
- B. Köstler, J. Gilmer, M. Bolte, A. Virovets, H.-W. Lerner, P. Albert, F. Fantuzzi and M. Wagner, Chem. Commun., 2023, 59, 2295–2298 RSC
.
- J. Hlina, J. Baumgartner and C. Marschner, Organometallics, 2010, 29, 5289–5295 CrossRef CAS
.
- J. Fischer, J. Baumgartner and C. Marschner, Organometallics, 2005, 24, 1263–1268 CrossRef CAS
.
- Deposition number CCDC 2257841 for 2, contains the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
- P. Pyykkö and M. Atsumi, Chem.–Eur. J., 2009, 15, 12770–12779 CrossRef PubMed
.
-
C. Hemmert and H. Gornitzka, in Organogermanium compounds: theory, experiment and applications, ed. V. Ya. Lee, Wiley, Hoboken, N.J., 2023, pp. 667–743 Search PubMed
.
- The Gaussian-16 program was used. For details, see ESI† material.
- L. Albers, J. Baumgartner, C. Marschner and T. Müller, Chem.–Eur. J., 2016, 22, 7970–7977 CrossRef CAS PubMed
.
- L. Albers, S. Rathjen, J. Baumgartner, C. Marschner and T. Müller, J. Am. Chem. Soc., 2016, 138, 6886–6892 CrossRef CAS PubMed
.
- For an arene complex of a silaadamantyl cation,
see: R. J. Wehmschulte, K. K. Laali, G. L. Borosky and D. R. Powell, Organometallics, 2014, 33, 2146–2149 CrossRef CAS
.
- During the refereeing process of this manuscript, we became aware of strongly related work by Timothy Su and coworkers. M. I. Aguirre Cardenas, T. C. Siu, M. G. Shimono, S. Thai, V. Carta and T. Su, ChemRxiv, 2023, preprint, DOI:10.26434/chemrxiv-2023-dk558.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures analytical data, NMR spectra, computational details, crystallographic information. CCDC 2257841. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3sc03301e |
| This journal is © The Royal Society of Chemistry 2023 |