Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Reversible CO2 insertion into the silicon–nitrogen σ-bond of an N-heterocyclic iminosilane†
Jingyan
Wang
,
Gi Byoung
Hwang
 ,
Caroline E.
Knapp
,
Caroline E.
Knapp
 and
Daniel W. N.
Wilson
and
Daniel W. N.
Wilson
 *
*
Department of Chemistry, University College London, 20 Gordon Street, London, UK. E-mail: dan.wilson@ucl.ac.uk
First published on 17th October 2024
Abstract
The reversible insertion of carbon dioxide into the silicon–nitrogen bond of an N-heterocyclic iminosilane is reported. Solution-phase thermodynamic investigations indicate that this process is thermoneutral and reversible, whereas in the solid-phase CO2 can be stored for extended periods and is only released upon heating to 133 °C.
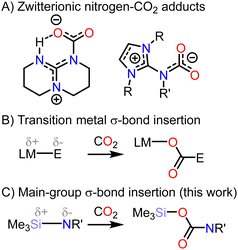
The rising atmospheric concentration of the greenhouse gas carbon dioxide (CO2) is an urgent pressing global concern. New technologies for the capture, storage, or valorization of CO2 from flu gas or directly from the air help mitigate the detrimental effects of CO2 atmospheric accumolation.1,2 The ability to reversibly bind and store CO2 through the formation of weak, easily breakable chemical bonds creates energetically efficient carbon fixation pathways.3 Additionally, such potential energy landscapes are ideal for the development of catalysts which can convert CO2 into industrially useful feedstocks.4 Technologies based on earth abundant elements would provide a sustainable route to CO2 utilization.5 A variety of low-valent transition metal (TM) and p-block Lewis bases can coordinate to the electrophilic carbon of CO2 to form zwitterionic complexes.6 Amongst these, nitrogen bases are particularly desirable due to their low cost and ease of synthetic access (Fig. 1A). Amidine- and guanidine-derived superbases bind CO2 weakly and can catalyse its reduction to a variety of products, including amide and methanol-precursors.6–10 Recently, compounds featuring the imidazolin-2-imino group (N-heterocyclic imines) have been reported which can capture CO2 and release it upon heating or photolysis, highlighting their potential use for CO2 storage.11,12
In addition to Lewis acid–base complexes, CO2 can insert into homo- and hetero-element σ-bonds.13,14 The best studied systems in this class feature late TM–E bonds, where E can be a hydride or p-block element (Fig. 1; B. E = H, OR, NR2, CR3, SiR3), in which CO2 insertion is an elementary step preceding CO2 valorization.
The insertion of CO2 into earth abundant and inexpensive p-block element-element bonds would provide an alternative to TMs, however such insertions typically yield thermodynamically stable products which do not easily release CO2, and requiring catalytic strategies with large driving forces (e.g. high temperatures).6 Examples of reversible CO2 activation by p-block complexes include bonds between divalent group 14 elements, Ge2+–Ge2+ and Sn2+–Sn2+,15 and select Lewis acid–base adducts (including Sn–P, In–P, N–Al complexes) which can dissociate to capture CO2.16–18 In these cases, high temperatures and reduced pressures are needed to induce loss of CO2, rendering them incompatible to thermodynamic study requiring closed systems and limiting the understanding of their mechanisms.
Here, we report the reversible, thermoneutral capture of CO2 by a N–Si bond. The solution phase thermodynamic parameters for the insertion reaction were determined by variable temperature NMR experiments, and the mechanism was further elucidated using density functional theory calculations. Thermogravimetric analysis indicates that CO2 can be stored in the solid state up to 133 °C, at which point reformation of the starting material occurs. Contrasting this, dissolution of the solids results in release of CO2 under ambient conditions, presenting a novel route to molecular CO2 storage.
N-heterocyclic imines typically bind CO2 through coordination of the imine nitrogen to the electrophilic CO2 carbon, forming zwitterionic acid–base adducts.11,12 The steric and electronic properties of the NHC have been shown to affect the binding energy of the N–CO2 bond.11 However, there has been little exploration of how modifying the imine substituent influences reactivity. We hypothesized that modifying the electropositivity, oxophilicity, and/or covalency of the imine subsituent would allow for Sigma-bond insertions analogous to those observed in TM–E compounds.13 Exposure of a benzene solution of IPrNSiMe319 (IPr = 1,3-di(2′,6′-diisopropylphenyl)imidazolin-2-ylidene) to CO2 (1 Bar; Scheme 1) results in the appearance of new resonances in the 1H NMR spectrum alongside the resonances associated with the starting material (Fig. S2, ESI†). Neither addition of further CO2 nor heating resulted in complete conversion to the product, and removal of the CO2 gas from the headspace of the reaction resulted in a loss of intensity of the resonances associated with the product concomitant with an increase in intensity for the IPrNSiMe3 resonances, consistent with a reversible reaction. Performing the reaction in hexane, a low polarity solvent, at −35 °C allowed for the isolation of analytically pure, colourless crystals of IPrNCO2SiMe3 (1, isolated in 76% yield).
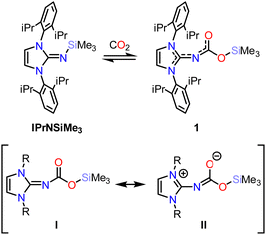 | ||
| Scheme 1 Top: Equilibrium between IPrNSiMe3 and 1. Bottom: Neutral (I) and zwitterionic (II) resonance structures of 1 (R = 2,6-diisopropylphenyl). | ||
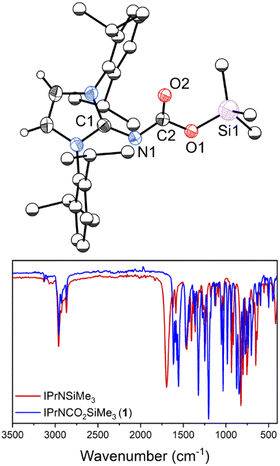
The crystal structure of 1 (Fig. 2; top) revealed CO2 insertion into the N–Si bond of IPrNSiMe3, rather than formation of an acid–base adduct as observed in previous studies on alkyl-substituted N-heterocyclic imines.11,12 The C1–N1 distance is 1.319(2) Å, elongated with respect to the precursor (cf. 1.265(3) Å)19 indicating reduced double bond character with respect to IPrNSiMe3. The N1–C2 distance is 1.3434(15) Å, falling within the expected distance for single (1.46 Å)20 and double bonds (1.27 Å), in line with contribution from both the neutral (Scheme 1; I) and zwitterionic (II) resonance structures of 1. The C2–O1 and C2–O2 bonds are C2 1.3636(14) and 1.2272(15) Å, respectively, slightly contracted with respect to the expected distance for single (1.38 Å) and double (1.24 Å) bonds. Despite significant delocalization throughout the conjugated atoms, which would benefit from planarity to maximise overlap between π-orbitals, the NCO2SiMe3 moiety is twisted out of the NHC plane by 47°. This is likely due to steric hinderance of the diisopropylphenyl groups. Notably, 1 is sensitive to hydrolysis and liberates CO2 upon exposure to water to yield IPrNH.
Fourier-transform infrared (FTIR) analysis performed on crystalline 1 was consistent with complete consumption of IPrNSiMe3, evidenced by the loss of the diagnostic carbonic CNHC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretch at 1695 cm−1 (Fig. 2). New stretches at 1613 and 1558 cm−1 were present in the spectrum of 1, which we assign as the CNHC
N stretch at 1695 cm−1 (Fig. 2). New stretches at 1613 and 1558 cm−1 were present in the spectrum of 1, which we assign as the CNHC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretch and the CO2 asymmetric stretch, respectively, the former is in keeping with the reduction of CNHC–N bond order observed in the solid state, and further supports contribution from the zwitterionic resonance structure. Dissolving the crystals of 1 resulted in the reappearance of IPrNSiMe3 in the 1H NMR spectrum, and leaving the system open to atmosphere or applying vacuum converts the mixture to IPrNSiMe3, indicating that the two species are in equilibrium. While p-block systems capable of CO2 capture are known,21–24 there are few examples of reversible CO2 capture,15–17 and fewer still are amenable to mechanistic study.25,26 Because of the reversibility of CO2 binding under ambient conditions, IPrNSiMe3 presents an ideal platform for such study.
N stretch and the CO2 asymmetric stretch, respectively, the former is in keeping with the reduction of CNHC–N bond order observed in the solid state, and further supports contribution from the zwitterionic resonance structure. Dissolving the crystals of 1 resulted in the reappearance of IPrNSiMe3 in the 1H NMR spectrum, and leaving the system open to atmosphere or applying vacuum converts the mixture to IPrNSiMe3, indicating that the two species are in equilibrium. While p-block systems capable of CO2 capture are known,21–24 there are few examples of reversible CO2 capture,15–17 and fewer still are amenable to mechanistic study.25,26 Because of the reversibility of CO2 binding under ambient conditions, IPrNSiMe3 presents an ideal platform for such study.
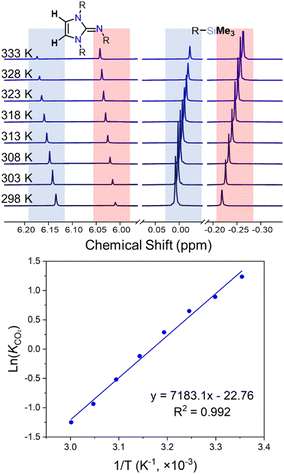
The presence of well-defined and unbroadened 1H NMR resonances for IPrNSiMe3 and 1 indicates slow chemical exchange with respect to the NMR timescale (500 MHz). Variable temperature (VT) NMR studies performed on a solution of IPrNSiMe3 under 1 bar of CO2 allowed for the determination of thermodynamic parameters for the equilibrium (ΔH°, ΔS°, and ΔG°) by linear regression of the van’t Hoff plot of ln(KCO2) vs. 1/T (Fig. 3). Giving value of ΔH° = −61 ± 1 kJ mol−1, ΔS° = −194 ± 4 J mol−1, and ΔG° = −3.3 ± 0.1 kJ mol−1. The reaction is overall slightly endergonic, with a relatively large negative enthalpy, consistent with the formation of new N–C and O–Si bonds, and a negative entropy as expected from a condensation reaction.
Self-exchange rates were extracted from the VT NMR analysis.27 Due to the slow rate of equilibrium, no line broadening was observed and the rate of exchange between 1 and IPrNSiMe3 was determined from the integration of NMR peaks. We propose the mechanism of interconversion of 1 + IPrNSiMe3 ⇌ IPrNSiMe3 + 1 occurs via dissociation of CO2 from 1, followed by association of CO2 to IPrNSiMe3 (Fig. S7, ESI†), where the rate limiting step is the dissociation of CO2 (kloss) (see ESI† Section S4.0). Eyring plots of ln(kloss/T) vs. 1/T (Fig. S8, ESI†) allowed for the determination of activation parameters of CO2 loss: ΔH‡loss = +32 ± 0.6 kJ mol−1, ΔS‡loss = –95 ± 24 J mol−1 and ΔG‡loss = +61 ± 7 kJ mol−1. Further, from the relationship ΔG‡loss = ΔG‡bind – ΔG°, ΔG‡bind can be estimated as +64 ± 7 kJ mol−1.
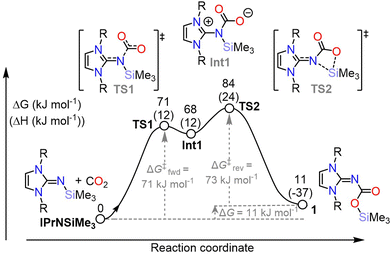
Density functional theory (DFT) calculations were performed to gain further insight into the mechanism of CO2 binding. A variety of basis sets and functional combinations were screened (see ESI,† 8.0).28,29 Notably, the thermodynamics of the reaction were highly dependent on the method employed. Pople basis sets resulted in highly exergonic reactions with reverse barriers too large to be reversible at room temperature (e.g. for PBE/6311++g-D3 ΔGcalc = −55 kJ mol−1, ΔG‡reverse = +131 kcal mol−1). The method BP86/def2-TZVP-D3 gave forward (+71 kJ mol−1) and reverse (+73 kJ mol−1) energetic barriers which were slightly overestimated with respect to the experimentally determined barrier (cf. +64 ± 7 kJ mol−1) however qualitatively reproduces the reaction energetics (Fig. 4). An energetically reasonable mechanism involves initial coordination of CO2 to the imine to form the zwitterionic intermediate (Int1). Contrasting previously reported and isolable N-heterocyclic imine–CO2 adducts, Int1 is significantly higher in energy than the starting molecules (+68 kcal mol−1), possibly due to steric clash between the diisopropylphenyl groups and the CO2 moiety.11 The energetic barrier between Int1 and 1 is small (12 kJ mol−1) and proceeds via a 4-membered transition state (TS2) in which the silyl group migrates from the nitrogen to the oxygen. The overall reaction, IPrNSiMe3 + CO2→1, is slightly endergonic (ΔGcalc = +11 kJ mol−1), contrasting the experimentally determined ΔG° = −3.3 ± 0.1 kJ mol−1. The difference between experimental and computational free energy is small and is likely due to errors associated with the methodology employed, which even in the best case can be as much as 8 kJ mol−1,28 and the limitations of DFT in accurately accounting for the entropy associated with solvating gas-phase molecules, in this case CO2.30
Reducing the steric bulk at the N-heterocyclic carbene moiety in the calculated models (Dipp → Me; PBE//6311g++/D3) did not impact the qualitative reaction profile, with Int1Me significantly higher in energy in comparison to reactants and products (ΔG‡ = +44 kcal mol−1, ΔGcalc = +6 kJ mol−1). This indicates that the electropositivity and propensity for migration of silane in comparison to carbon substituents facilitates the migration, rather than the reaction being driven by release of steric clash.
Having established the solution-phase behaviour of 1, we sought to assess if 1 could store and release CO2 in the solid state. Thermogravimetric analysis on crystalline 1 revealed two features, a sharp decrease at 133 °C followed by a broad feature beginning after 200 °C and centered at 347 °C (Fig. 5). Plotting the first derivative of the curve revealed two separate events, with the area under the first peak integrating as 8.7% of the total sample mass, in agreement with the theoretical mass of CO2 in the sample (8.5%). FTIR analysis of crystals of 1 heated to 140 °C showed loss of the absorbances associated with the CO2 stretch and reappearance of the peak at 1695 cm−1. Dissolving the resultant solids displayed the diagnostic 1H NMR resonances associated with IPrNSiMe3. Therefore, in the solid-state CO2 is released and IPrNSiMe3 can be reformed. The temperature at which 1 releases CO2 is higher than those reported for N-heterocyclic imine–CO2 adducts, which decarboxylate between 30 and 100 °C, depending on the identity of the NHC moiety and its substituents.11
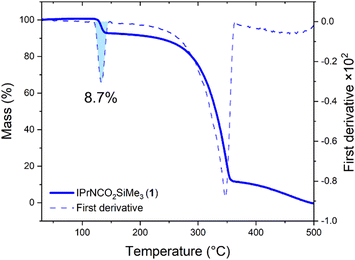 | ||
| Fig. 5 Thermogravimetric plot for 1 (solid line), and its first derivative (dashed line). Shaded blue area shows the area integrating as 8.7% of total sample mass. | ||
In summary, we have demonstrated the capture of CO2 by an N-heterocyclic iminosilane. In the solution-phase, NMR experiments demonstrate that this reaction is almost thermoneutral and fully reversible, while in the solid-state CO2 can be stored for extended periods up to 133 °C. Computations indicate that the reversibility of this reaction is due to the oxophilicity of the silane substituent.
D. W. N. W. thanks the Royal Commission for the Exhibition 1851 (RF2023DW) and the Royal Society of Chemistry (R23-4527710839) for funding. We thank Dr Abil Aliev (UCL) for assistance with NMR measurements.
Data availability
Data for this article, including experimental procedures, computational details, crystallographic data and NMR spectra. The data supporting this article have been included as part of the ESI.† Crystallographic data for 1 has been deposited at the CCDC under 2384340.Conflicts of interest
There are no conflicts to declare.Notes and references
- M. Aresta, A. Dibenedetto and A. Angelini, Chem. Rev., 2014, 114, 1709–1742 CrossRef CAS PubMed.
- E. S. Sanz-Pérez, C. R. Murdock, S. A. Didas and C. W. Jones, Chem. Rev., 2016, 116, 11840–11876 CrossRef PubMed.
- L. J. Murphy, K. N. Robertson, R. A. Kemp, H. M. Tuononen and J. A. C. Clyburne, Chem. Commun., 2015, 51, 3942–3956 RSC.
- L. Zhao, H.-Y. Hu, A.-G. Wu, A. O. Terent’ev, L.-N. He and H.-R. Li, J. CO2 Util., 2024, 82, 102753 CrossRef CAS.
- K. M. P. Wheelhouse, R. L. Webster and G. L. Beutner, Org. Process Res. Dev., 2023, 27, 1157–1159 CrossRef CAS.
- P. Sreejyothi and S. K. Mandal, Chem. Sci., 2020, 11, 10571–10593 RSC.
- C. Villiers, J.-P. Dognon, R. Pollet, P. Thuéry and M. Ephritikhine, Angew. Chem., Int. Ed., 2010, 49, 3465–3468 CrossRef CAS PubMed.
- C. Das Neves Gomes, O. Jacquet, C. Villiers, P. Thuéry, M. Ephritikhine and T. Cantat, Angew. Chem., Int. Ed., 2012, 51, 187–190 CrossRef CAS PubMed.
- H. Zhou, W. Chen, J.-H. Liu, W.-Z. Zhang and X.-B. Lu, Green Chem., 2020, 22, 7832–7838 RSC.
- J. K. Mannisto, L. Pavlovic, T. Tiainen, M. Nieger, A. Sahari, K. H. Hopmann and T. Repo, Catal. Sci. Technol., 2021, 11, 6877–6886 RSC.
- L. F. B. Wilm, T. Eder, C. Mück-Lichtenfeld, P. Mehlmann, M. Wünsche, F. Buß and F. Dielmann, Green Chem., 2019, 21, 640–648 RSC.
- L. F. B. Wilm, M. Das, D. Janssen-Müller, C. Mück-Lichtenfeld, F. Glorius and F. Dielmann, Angew. Chem., Int. Ed., 2022, 61, e202112344 CrossRef CAS PubMed.
- N. Hazari and J. E. Heimann, Inorg. Chem., 2017, 56, 13655–13678 CrossRef CAS PubMed.
- A. P. Deziel, M. R. Espinosa, L. Pavlovic, D. J. Charboneau, N. Hazari, K. H. Hopmann and B. Q. Mercado, Chem. Sci., 2022, 13, 2391–2404 RSC.
- A. Caise, L. P. Griffin, C. McManus, A. Heilmann and S. Aldridge, Angew. Chem., Int. Ed., 2022, 61, e202117496 CrossRef CAS PubMed.
- D. A. Dickie, E. N. Coker and R. A. Kemp, Inorg. Chem., 2011, 50, 11288–11290 CrossRef CAS PubMed.
- D. A. Dickie, M. T. Barker, M. A. Land, K. E. Hughes, J. A. C. Clyburne and R. A. Kemp, Inorg. Chem., 2015, 54, 11121–11126 CrossRef CAS PubMed.
- T. W. Yokley, H. Tupkar, N. D. Schley, N. J. DeYonker and T. P. Brewster, Eur. J. Inorg. Chem., 2020, 2958–2967 CrossRef CAS PubMed.
- M. Tamm, S. Randoll, E. Herdtweck, N. Kleigrewe, G. Kehr, G. Erker and B. Rieger, Dalton Trans., 2006, 459–467 RSC.
- P. Pyykkö, J. Phys. Chem. A, 2015, 119, 2326–2337 CrossRef PubMed.
- D. Sarkar, L. Groll, D. Munz, F. Hanusch and S. Inoue, ChemCatChem, 2022, 14, e202201048 CrossRef CAS.
- F. Gründler, H. Scholz, M. Herbig, S. Schwarzer, J. Wagler and E. Kroke, Euro. J. Inorg. Chem., 2021, 2211–2224 CrossRef.
- K. Kraushaar, C. Wiltzsch, J. Wagler, U. Böhme, A. Schwarzer, G. Roewer and E. Kroke, Organometallics, 2012, 31, 4779–4785 CrossRef CAS.
- M. Reißmann, A. Schäfer, S. Jung and T. Müller, Organometallics, 2013, 32, 6736–6744 CrossRef.
- C. M. Mömming, E. Otten, G. Kehr, R. Fröhlich, S. Grimme, D. W. Stephan and G. Erker, Angew. Chem., Int. Ed., 2009, 48, 6643–6646 CrossRef PubMed.
- F. Buß, P. Mehlmann, C. Mück-Lichtenfeld, K. Bergander and F. Dielmann, J. Am. Chem. Soc., 2016, 138, 1840–1843 CrossRef PubMed.
- R. C. Cammarota, J. Xie, S. A. Burgess, M. V. Vollmer, K. D. Vogiatzis, J. Ye, J. C. Linehan, A. M. Appel, C. Hoffmann, X. Wang, V. G. Young and C. C. Lu, Chem. Sci., 2019, 10, 7029–7042 RSC.
- M. Bursch, J.-M. Mewes, A. Hansen and S. Grimme, Angew. Chem., Int. Ed., 2022, 61, e202205735 CrossRef CAS PubMed.
- B. A. Shiekh, ACS Omega, 2019, 4, 15435–15443 CrossRef CAS PubMed.
- S.-C. Liu, X.-R. Zhu, D.-Y. Liu and D.-C. Fang, Phys. Chem. Chem. Phys., 2023, 25, 913–931 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Data for this article, including experimental procedures, computational details, crystallographic data and NMR spectra. The data supporting this article have been included as part of the ESI. Crystallographic data for 1 has been deposited at the CCDC 2384340 (1). For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4cc04798b |
| This journal is © The Royal Society of Chemistry 2024 |