Open Access Article
Open Access ArticleEmerging polymeric materials for treatment of oral diseases: design strategy towards a unique oral environment
Bo
Jia†
 a,
Beibei
Zhang†
a,
Beibei
Zhang†
 bc,
Jianhua
Li†
bc,
Jianhua
Li†
 c,
Jinlong
Qin
c,
Jinlong
Qin
 bc,
Yisheng
Huang
bc,
Yisheng
Huang
 a,
Mingshu
Huang
a,
Mingshu
Huang
 a,
Yue
Ming
a,
Yue
Ming
 a,
Jingjing
Jiang
c,
Ran
Chen
c,
Yufen
Xiao
*bc and
Jianzhong
Du
a,
Jingjing
Jiang
c,
Ran
Chen
c,
Yufen
Xiao
*bc and
Jianzhong
Du
 *bc
*bc
aDepartment of Oral and Maxillofacial Surgery, Stomatological Hospital, School of Stomatology, Southern Medical University, Guangdong, China
bDepartment of Gynaecology and Obstetrics, Shanghai Key Laboratory of Anesthesiology and Brain Functional Modulation, Clinical Research Center for Anesthesiology and Perioperative Medicine, Translational Research Institute of Brain and Brain-Like Intelligence, Shanghai Fourth People's Hospital, School of Medicine, Tongji University, Shanghai 200434, China. E-mail: yufenxiao@alumni.tongji.edu.cn; jzdu@tongji.edu.cn
cDepartment of Polymeric Materials, School of Materials Science and Engineering, Key Laboratory of Advanced Civil Engineering Materials of Ministry of Education, Tongji University, 4800 Caoan Road, Shanghai 201804, China
First published on 20th March 2024
Abstract
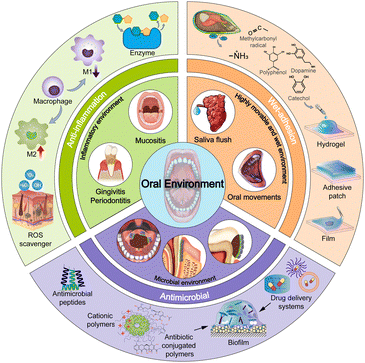
Oral diseases are prevalent but challenging diseases owing to the highly movable and wet, microbial and inflammatory environment. Polymeric materials are regarded as one of the most promising biomaterials due to their good compatibility, facile preparation, and flexible design to obtain multifunctionality. Therefore, a variety of strategies have been employed to develop materials with improved therapeutic efficacy by overcoming physicobiological barriers in oral diseases. In this review, we summarize the design strategies of polymeric biomaterials for the treatment of oral diseases. First, we present the unique oral environment including highly movable and wet, microbial and inflammatory environment, which hinders the effective treatment of oral diseases. Second, a series of strategies for designing polymeric materials towards such a unique oral environment are highlighted. For example, multifunctional polymeric materials are armed with wet-adhesive, antimicrobial, and anti-inflammatory functions through advanced chemistry and nanotechnology to effectively treat oral diseases. These are achieved by designing wet-adhesive polymers modified with hydroxy, amine, quinone, and aldehyde groups to provide strong wet-adhesion through hydrogen and covalent bonding, and electrostatic and hydrophobic interactions, by developing antimicrobial polymers including cationic polymers, antimicrobial peptides, and antibiotic-conjugated polymers, and by synthesizing anti-inflammatory polymers with phenolic hydroxy and cysteine groups that function as immunomodulators and electron donors to reactive oxygen species to reduce inflammation. Third, various delivery systems with strong wet-adhesion and enhanced mucosa and biofilm penetration capabilities, such as nanoparticles, hydrogels, patches, and microneedles, are constructed for delivery of antibiotics, immunomodulators, and antioxidants to achieve therapeutic efficacy. Finally, we provide insights into challenges and future development of polymeric materials for oral diseases with promise for clinical translation.
1. Introduction
Oral diseases are the most prevalent diseases with severe health and economic burdens, affecting over 3.5 billion people globally. The oral and maxillofacial region is an open and closed structure composed of soft and hard tissues such as teeth, periodontium, oral mucosa, maxilla, and salivary gland. Its complex physical and chemical environments, such as food, microbiota, saliva, and functional movement, lead to the wide existence of oral diseases and challenges in treatment.1 The complex oral environment not only drives the progression of many oral diseases, but is also responsible for the failure of dental materials.2,3 Current clinically used biomaterials in the treatment of oral diseases include metal and alloys as components of dentures, wires and cast restorations, polymer composite resin for dental restorations and filling, inorganic salts as dental cement, elastomers as impression materials, fibres for wound healing and dressing, and scaffolds as bone substitutes for guided bone regeneration.4–7 Owing to their advantages and wide clinical applications, advanced biomaterials have significantly promoted the diagnosis and treatment of oral diseases.8,9Polymers are macromolecules composed of a large number of repeat units. The diversity in monomers, initiators, polymerization and post-modification methods endows polymers with multifunctionality.10–15 Polymeric materials such as nanoparticles (NPs), nanofibers, hydrogels, and microneedles manufactured by advanced nanotechnology and engineering show great potential in biomedical applications.16–21 Owing to their advantages in flexible design and facile preparation, an increasing number of polymeric materials have been developed and applied in oral diseases, making them a very hot topic in the cutting edge of oral disease treatment.22–25
Over the past five years, several reviews have been published focusing on polymeric materials for treatment of oral diseases.26–31 For example, Guo and colleagues have focused on mandibular tissue engineering related to hydrogels with better mechanical properties, antibacterial ability, injectability, and three-dimensional bio-printed hydrogel structure.26 Alqurashi and co-workers have reviewed thermoplastic polymer polyetherketoneketone (PEKK), which has a wide range of dental applications, including dental restorations, crowns, bridges, internal columns, denture frames, implant-supported fixed prostheses and dental implants.27 Kalelkar et al. have summarized biomaterial-based antibacterial therapies.32 Pooyan has described the advance in antibacterial polymer fillers used in dental materials and evaluated the structure, preparation, and application of antibacterial polymers and inorganic fillers and compounds.29 Mao has emphasized the oral and maxillofacial wet-adhesive materials with wet environment retention, internal stability and plasticity that can be used for oral mucosal administration, oral vaccination, wound healing and bone defect treatment.31 However, previous reviews have focused on certain properties of the materials. Nevertheless, oral diseases associated with the highly movable and wet, microbial and inflammatory environment are barely discussed. To the best of our knowledge, there is no review on polymeric materials for treatment of oral diseases by overcoming the important challenges from the unique oral environment. Considering that oral cancer is one of the most prevalent cancers, reviews are continuously presented concomitant with the progress in the targeted treatment of oral cancer, including exosomes,33 intraoral polymeric delivery systems,34 and mucoadhesive polymers.35 Therefore, this review will not include a specific discussion on the treatment of oral cancer.
In this review, we aim to highlight the latest progress of emerging polymeric materials for the treatment of oral diseases (Fig. 1). We summarize the oral environments that are conducive to the pathogenesis of various oral diseases and the key factors in the treatment before emphasizing the design strategies towards the specific oral environment and their applications in the treatment of oral diseases. Finally, challenges and perspectives are discussed to provide insight and inspiration for the development of future materials in this field.
2. Oral environment
The oral cavity is the entrance of the digestive and respiratory systems, making it important in human physiology. It has many critical anatomical structures, including teeth, periodontal tissue, oral mucosa, maxilla, and salivary glands. The open and closed structure of the oral cavity, accompanied by a highly movable and wet, microbial, and inflammatory environment, significantly affects the diagnosis, treatment, and prognosis of various oral diseases. Therefore, understanding the complex oral environment and the existing challenges is of great importance for the design of novel polymeric materials towards oral diseases.2.1. Highly movable and wet environment
Fundamental oral movements include breathing, chewing, swallowing, and speaking. The mainly involved hard tissues are the mandible, temporomandibular joint, and dentition.36 These movements and the resulting functional signals regulate tissue differentiation, occlusal establishment, remodeling, and decomposition during the growth and development.Fundamental oral movements also affect the occurrence and development of oral diseases. Therefore, oral materials are required to meet the requirements for oral movement. For example, mouth breathing, which results in abnormal tooth and maxillofacial development, increases the risk of bad breath, bruxism, and periodontal diseases.37 Mouth breathing and other malocclusion can be corrected by orthodontic clear aligner materials, mainly made of polymers, which require long-term resistance to mechanical stress related to oral functional movements as well as the viscoelastic property of polymers.25 Regarding dental materials, long-term excessive masticatory movement and food impact can lead to the wear and tear of teeth and dental materials, resulting in dentin allergy and damage to dental materials.38,39 Hence, in the area of future dental filling composites and dental bonding agents, more attention should be paid to the viscosity and flexibility before solidification, and the elastic properties and mechanical strength after solidification.40 In drug delivery and tissue repair areas, the continuous change of saliva, oral movement, and involuntary swallowing hinder the stable adhesion of drug-loaded and tissue repair materials, resulting in a short retention time of biomaterials. Therefore, developing biological macromolecules with high cohesion and adhesion strength can achieve controlled drug delivery or improve the efficiency of tissue repair.41,42 Overall, polymeric materials for treating oral diseases are immersed in an environment of long-term activity, cyclic contact force, and wear, which should be particularly taken into consideration in the design of the materials.
Another character of the oral cavity is the wet environment induced by saliva and fluid. As the primary contributor to a wet environment, saliva is crucial in maintaining oral health.43 It encompasses a spectrum of inorganic elements, including sodium, chloride, potassium, calcium, magnesium, phosphorus, and bicarbonate.44 Additionally, saliva comprises various protein classes, such as acidic and basic proline-rich proteins, α-amylases, mucins, cystatins, and approximately 200 other proteins and peptides.45 The complex composition helps maintain the oral pH within a relatively stable range of 6.5–7.0. Notably, alterations in the concentration of several molecules presented in saliva have proven valuable in diagnosing and monitoring oral diseases, including dental caries, periodontitis, oral squamous cell carcinoma, and Sjögren's syndrome.46,47
The interaction between saliva and polymeric materials used in oral rehabilitation or tissue regeneration is inherent, which may lead to chemical–physical–biological changes in polymeric materials, such as hydrophilicity, degradation, morphology, surface properties, and biocompatibility.48,49 The acidic conditions can lead to phase/morphology change and even degradation of polymeric materials that are sensitive to pH.50 In addition, the abundant proteins may be adsorbed on the surface of polymeric materials, which results in a subsequent change in surface properties, such as alteration in size, variation in zeta potential, and loss of targeting ability.48 These changes then affect the fate and efficacy of polymeric materials. For example, saliva can damage the super-hydrophilic nano-textured surface of dental implants, which may affect bone healing.51 Furthermore, the high wetness of saliva and the presence of various proteins and mucins prevent the strong and stable adhesion of drugs and biomaterials to the oral mucosa and dentoidin.52
Besides saliva, the wet environment caused by fluid from food and drink intake hampers the retention of polymeric materials at the lesion site. The flashing fluid-induced shearing force quickly interrupts the integrity of polymeric materials and leads to the exfoliation of materials. Therefore, much effort has been made to maintain the adhesive and mechanical strength between polymeric materials and oral cavity in a wet environment.42
2.2. Microbial environment
The oral microbes is the most important biological factor affecting the oral environment.53 It is also one of the most complex and dense microbial ecosystems in the human body, containing bacteria, archaea, fungi, protozoa, and viruses.53 These oral microbes can escape the antibacterial defense of saliva and adapt to living in warm and humid environments, and are exposed to oxygen regularly.54 Complex biological signal systems and interactions with the host regulate the microbes. Once the oral microbes is out of balance, the microbes will produce virulence factors and metabolites, leading to dental caries, periodontal diseases, etc.55,56Biofilm is an important mediator for the pathogenicity and therapeutic resistance of many bacterial species, and is an important obstacle to the treatment of many infections. The three-dimensional biofilm network serves as a natural defense barrier that protects enclosed bacteria from the influence of antibiotic drugs and other antibacterial mechanisms.57 Oral biofilms are usually formed on the tooth surface and embedded in a matrix of extracellular polymeric substances (EPS) with diverse microbiota inside.58 Among them, Streptococcus mutans (S. mutans) in the oral microbiota is considered to be an important pathogen in biofilm formation and dysbiosis. With frequent carbohydrate exposure, continuous metabolism by S. mutans produces glucan-rich EPS and acidic by-products. The dense EPS protect microbiota from fluid flashing and therapeutic agent penetration. Moreover, the EPS provide nutrition and facilitate colonization of microbiota, which in turn strengthens the biofilm. Aciduric microbiota prosper with the increasing acidity of the biofilm. The local acidity further demineralizes adjacent teeth and exacerbates the biofilm growth. In addition, with the aggregation of the biofilm, the anaerobic microenvironment facilitates the growth of aerobic and facultative anaerobic Gram-negative bacteria. All these factors finally contribute to oral diseases such as dental caries and create a barrier to dental treatment. Therefore, removing or interrupting the biofilm is a new opportunity for the effective treatment of oral diseases.59
2.3. Inflammatory environment
Inflammatory diseases in the oral cavity mainly include periodontitis and oral mucosal diseases. The complex relationships among bacterial infection, immune response disorder, and environmental risk factors induce periodontitis.60 In recent years, more and more evidence reveals that periodontitis is an inflammatory disease of oxidative stress. Reactive oxygen species (ROS) induce the over-expression of pro-inflammatory cytokines (interleukin-1 (IL-1), IL-6, tumour necrosis factor (TNF) family members, etc.), which directly or indirectly lead to connective tissue destruction and bone resorption.61 For example, Porphyromonas gingivalis (P. gingivalis), a key pathogen of periodontitis, secretes peptidyl arginine deiminase that prolongs neutrophil inflammatory response with sustainable secretion of pro-inflammatory cytokines and ROS production.62 In addition, macrophage polarization, a variety of matrix metalloproteinases and signal pathways are pathological molecular processes of periodontitis.60 Therefore, improving the level and activity of antioxidants, regulating macrophage polarization, enzyme regulation, and signal pathway targeted therapy may be feasible supplements for the prevention and treatment of periodontitis and other oxidative stress diseases.Oral mucosa is a barrier to protect oral health, but it is easily affected by periodontitis.63 Oral mucosa is the intimal tissue of the oral cavity, composed of two main parts: the physical barrier, which is composed of layered epithelial cells and cell–cell junction, and the microbial immune barrier, which keeps the internal environment in a steady state.64 The physical barrier of the oral mucosa in the mouth has been subjected to persistent tissue damage caused by mastication.65 The immune barrier is constantly exposed to inflammation signals, including affluent oral microbes and their metabolites and antigens produced by food and air.66–68 All these factors pose challenges to the homeostasis of oral mucosa, and result in oral mucosal diseases, such as oral candidiasis, oral lichen planus, recurrent aphthous ulcer, oral leukoplakia, and a series of conditions that affect oral mucosa and soft tissue.69 For example, recurrent aphtha is the most common disease observed by clinicians. However, the specific etiology is unclear, but many factors, including trauma, eating habits, and mouth microbes, may affect the progress of the disease by regulating immunity.70 Therefore, it is important for successful prevention and treatment of oral mucosal diseases to understand the root causes of disorders or diseases by investigating the relationship between physical, microbial, and immune barriers.71
The inflammatory environment is also related to the occurrence and progression of oral cancer.72–74 It is identified that certain microbe components, such as P. gingivalis, Fusobacterium nucleatum, and Candida albicans, are associated with an increased risk of oral cancer.75–78 The microbe induce chronic inflammation, produce carcinogenic byproducts, and modulate the host immune response, creating an environment conducive to cancer development. The role of microbe in cancer development has been reviewed in detail.53,79,80 It has been explored to understand the host–microbiota interaction and manipulate the microbiota for cancer therapy.81–84
One key problem is the dysregulation of the immune system.85 In the oral inflammatory environment, immune cells, such as T lymphocytes, natural killer cells, macrophages, and neutrophils, and ROS have been found to play a vital role in the development and progression of oral cancer. Strategies of immunomodulation, such as down-regulating inflammatory cytokines by anti-inflammatory materials86–88 and inducing anti-tumor response by activating T cells, are explored.89,90 Unfortunately, there are few studies on the immune regulation of oral cancer by polymeric materials.91 Future research deserves an eye on probing the clinical correlation between immune cells and related molecules and oral cancer to improve the accurate medical treatment of oral cancer patients, as well as developing biomaterials including polymeric materials to regulate the inflammatory tumor microenvironment.
Despite the growing body of research on the relationship between the oral environment and oral cancer, direct evidence indicating that a specific increase or decrease in certain microorganisms or inflammatory factors is a direct cause of oral cancer is limited. It is crucial to understand the impact of the oral environment on the molecular and cellular processes involved in cancer progression, which could provide guidance for therapeutic strategies.
3. Wet-adhesive polymeric materials to overcome the highly movable and wet environment
The oral cavity, characterized by its highly movable and wet environment, presents notable obstacles in achieving effective adhesion and keeping the structural stability of biomaterials.92 The oral mucosa lining on the gingiva and palate is elastic and mobile which is undesirable for the retention of adhesive materials. Moreover, the continuous endogenous salivation and exogenous water flushing from food and beverages can rupture the cross-linking networks, chemical bonds and interactions to hamper adhesion at the tissue surfaces. On the other hand, the movements by chewing and speaking not only deteriorate the mechanical strength and tensile strength, but also shorten the retention of adhesive materials. To address these challenges associated with the highly movable and wet environment of the oral cavity, efforts have been devoted to a range of wet-adhesive materials.93,94 Herein, we start with the adhesion mechanism, followed by design strategies of wet-adhesive polymeric materials towards wet and highly movable environments, as well as their therapeutic applications in wound healing.3.1. Adhesion mechanism
Bioadhesive materials can promote wound healing and reduce complications associated with the inflammation of open wounds. However, common adhesives face challenges in the wet environment and even can be eliminated once in contact with water. Therefore, it is urgent to develop wet-adhesive materials with robust adhesion, applicable viscoelasticity and excellent retention capability to adapt to the oral wet environment.The adhesive mechanisms of materials in the oral cavity depend on the compositions of materials and their interaction with the surrounding environment.93,95 These mechanisms include mechanical locking and chemical bonding (Fig. 2). Biomaterials can form physical connections with the surrounding tissue or matrix through mechanical forces such as porous structures and topological entanglement, which interlock geometries or surface textures to enhance the adhesive strength.96,97 Functional groups presented on the surface of oral tissues, such as primary amines, carboxylic acids, hydroxyls, and thiols, can interact with reactive groups of adhesives, forming chemical bonds through activated chemical reactions.98 These chemical bonds involve hydrogen bonds, dynamic covalent bonds, electrostatic interactions, hydrophobic interactions, etc.99,100 Moreover, cell–substrate affinity can be achieved by transmembrane proteins like integrins, cell adhesion molecules such as calreticulin and bridges.101 Incorporating bioactive molecules into biomaterials or creating micro- or nanoscale surface structures that mimic the natural extracellular matrix can facilitate the cell adhesion and tissue integration.
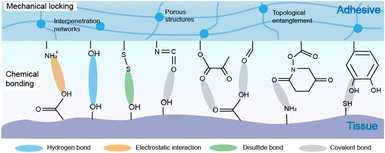 | ||
| Fig. 2 Adhesion mechanisms between polymeric materials and the oral cavity: mechanical locking and chemical bonding. | ||
To meet the requirements of wet-adhesion, polymers are equipped with functional groups for forming mechanical locking and chemical bonding with the oral tissue. Typically, mucoadhesive polymers such as chitosan, cellulose, hyaluronic acid, polyelectrolytes, and polyacrylates are used as polymer backbones. Then, functional groups are attached to the polymer backbones and side chains. Hydrogen bonds between primary amines and hydroxy groups, dynamic covalent bonds including metal coordination bonds between Ca2+ and hydroxy groups, Michael addition and Schiff base reaction between quinone and ammonia, electrostatic interactions between positively charged amine groups, quaternary ammonium, and carboxy groups, etc. are introduced to the adhesive materials to enrich the adhesion strength. Detailed modification strategies are discussed in Section 3.2.
In addition to modifying functional groups with strong wet-adhesive properties, there is research aiming to transform the disadvantages of the moist environment into advantages. The existing water hinders interactions between adhesive and tissue; therefore, removing interfacial water can facilitate the adhesion. Strategies have been developed to perform gelation under water using powders and dry double-sided tapes that form cross-linked hydrogels after absorbing water.102–105
3.2. Polymeric materials for wet-adhesive wound healing
Wound healing involves four phases including haemostasis, inflammation, proliferation, and remodelling.106 As the first step of wound healing, haemostasis with the formation of blood clotting is important. Polymers with different haemostatic mechanisms are employed as haemostatic materials.31,93,107,108 For example, most natural polymers like chitosan function in promoting adhesion and aggregation of platelets, while synthetic polymers like polyethylene glycol (PEG) are excellent vehicles for delivering active components with increased coagulation factor concentrations.106 In the following two subsections, in addition to discussing the role and mechanism of polymeric materials in haemostasis (Table 1), we focus on the design strategies for polymeric materials with enhanced wet-adhesion strength to overcome the wet oral environment and promote wound healing.| Strategy | Materials | Adhesive group | Adhesive chemistry | Ref. |
|---|---|---|---|---|
| Abbreviations: DOPA: 3,4-dihydroxy-D-phenylalanine, PVA: polyvinyl alcohol, PLEL: PDLLA-b-PEG-b-PDLLA triblock copolymers, nBG: nano-scaled bioactive glass, QCS-C: catechol modified quaternized chitosan, QCS: quaternized chitosan, TA: tannic acid, SA-BA: benzeneboronic acid-modified sodium alginate, CNB-HA: cyclic o-nitrobenzyl-modified hyaluronic acid. | ||||
| DOPA modification | PVA-DOPA hydrogel |
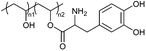
|
Hydrogen bonding | 113 |
| Catechol modification | Chitoral sponge |
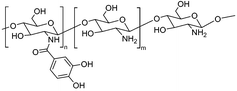
|
Electrostatic interaction | 42 |
| PLEL-nBG-QCS-C hydrogel |
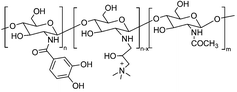
|
Hydrophobic interaction | 117 | |
| Polyphenol modification | QCS/TA hydrogel |
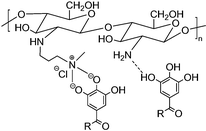
|
π–π interaction | 118 |
| Cation-π interaction | ||||
| Michael addition and Schiff base reaction between quinone and ammonia | ||||
| Polysaccharide modification | SA-BA/QCS-C powder |
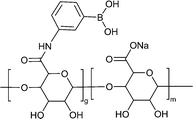
|
Hydrogen bonding Electrostatic interaction | 119 |
| Metal coordination | ||||
| Oxidized pectin coated chitosan nanofiber membranes |
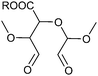
|
Hydrogen bonding Electrostatic interaction | 119 | |
| Metal coordination | ||||
| Schiff base dynamic covalent bond between aldehydes and ammonia | ||||
| UV-generated adhesion | CNB-HA hydrogel |
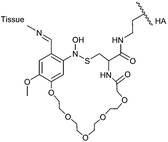
|
Schiff base dynamic covalent bond between aldehydes and ammonia | 121 |
A range of biomaterials that can enhance adhesion and promote wound healing were developed based on the adhesive mechanisms observed in mussels. mfp-6, an acidic substance rich in thiols, acted as a reducing agent to revert the oxidized dopaquinone to DOPA, maintaining the latter's reduced state.110 Leveraging this reduction mechanism, the oxidized dopaquinone can be reduced through Michael addition reaction between thiols and amines. Accordingly, the secretion of mfps was promoted and the adhesion or cohesion of DOPA was enhanced upon low-concentration periodate treatment.111,112 Based on this theory, films of buccal tissue adhesives have been proposed, providing a strategy for achieving mucosal adhesive capacity by modifying DOPA to polymer chains (Fig. 3).113 The accomplishment of surface modification between polymer chains and DOPA can be proven by the vibration absorption peak of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond (1734 cm−1). The adhesion tests on freshly excised porcine buccal mucosa (the flow-through method and rotating disc method) and several mechanical tests (lap-shear tests, tensile tests, and peel tests) demonstrated that DOPA-modified polymers displayed better adhesion to tissues than the unmodified ones. The difference in the UV-vis spectra and the shift of amide II to 1528 cm−1 under FTIR revealed the covalent binding of catechol groups in DOPA to mucin in tissues. Moreover, thermodynamic analysis (differential scanning calorimeter, DSC) showed that the heat of fusion (ΔHm) of the DOPA–mucin blend increases with the DOPA content, possibly caused by interactions of large molecules including hydrogen bonds and chain entanglement. A similar wavelength shift of hydroxy groups (–OH) from 3254 cm−1 for unmodified polymers to 3277 cm−1 was observed in the binding process of polymers with DOPA, which indicates that the hydrogen bonding has formed between the matrix and catechol groups. In general, the mucosal adhesive property of DOPA might originate from the interpenetration and entanglement of polymer chains with the mucus in tissues, as well as the formation of hydrogen bonds and covalent bonds between DOPA and mucin in tissues.
O bond (1734 cm−1). The adhesion tests on freshly excised porcine buccal mucosa (the flow-through method and rotating disc method) and several mechanical tests (lap-shear tests, tensile tests, and peel tests) demonstrated that DOPA-modified polymers displayed better adhesion to tissues than the unmodified ones. The difference in the UV-vis spectra and the shift of amide II to 1528 cm−1 under FTIR revealed the covalent binding of catechol groups in DOPA to mucin in tissues. Moreover, thermodynamic analysis (differential scanning calorimeter, DSC) showed that the heat of fusion (ΔHm) of the DOPA–mucin blend increases with the DOPA content, possibly caused by interactions of large molecules including hydrogen bonds and chain entanglement. A similar wavelength shift of hydroxy groups (–OH) from 3254 cm−1 for unmodified polymers to 3277 cm−1 was observed in the binding process of polymers with DOPA, which indicates that the hydrogen bonding has formed between the matrix and catechol groups. In general, the mucosal adhesive property of DOPA might originate from the interpenetration and entanglement of polymer chains with the mucus in tissues, as well as the formation of hydrogen bonds and covalent bonds between DOPA and mucin in tissues.
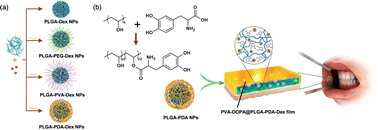 | ||
| Fig. 3 Synthesis of the PVA-DOPA@NPs-Dex mucoadhesive film with enhanced mucoadhesion for buccal drug delivery. Reprinted from Hu et al.113 with permission from Springer Nature. Copyright (2021). | ||
However, the adjacent phenolic hydroxyl structure of DOPA is highly unstable and prone to oxidative reactions with active hydrogen on hydroxy, amino, or thiol groups, yielding adjacent quinones.114 The adjacent quinones and DOPA moieties further undergo complex chemical reactions, which leads to the failure of adhesion. One strategy to enhance the stability of DOPA is the combination of stabilizers or antioxidants with adhesives to prevent oxidation.115,116 Despite the drawbacks associated with DOPA, it remains a noteworthy component in the fields of biomedicine and materials science, particularly in the study of biomimetic adhesives. Efforts have been continuously devoted to overcoming these limitations to enhance its feasibility and performance in clinical applications.
Catechol modification endows polymers with wet-adhesion properties, extending their applications in haemostasis and wound healing.123,124 For example, Zheng et al. developed a thermo-responsive hydrogel based on poly(D,L-lactide)-b-poly(ethylene glycol)-b-poly(D,L-lactide). Catechol-modified quaternary chitosan was incorporated into the hydrogel. The incorporation led to a 4-fold increase in the gel adhesion strength compared to that without incorporation, reaching 16.98 ± 0.84 kPa at 37 °C (Fig. 4).117 This enhanced adhesion can be attributed to multiple interactions between catechol-modified quaternary chitosan and the substrate surface, including electrostatic interactions, hydrogen bonding, hydrophobic interactions, and interfacial bonding formed between the oxidized quinone and primary amine groups on the tissue surface. This contributes to excellent adhesion, which is crucial for treating infected wounds in the oral cavity.
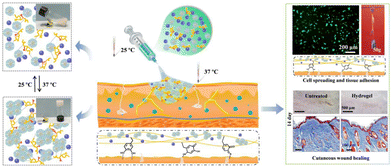 | ||
| Fig. 4 Schematic illustration of a catechol-modified quaternary chitosan incorporated hydrogel with wet-adhesive and antibacterial function. This thermo-responsive hydrogel underwent in situ gelation at 37 °C and facilitated wound healing. Reprinted from Zheng et al.117 with permission from Elsevier. Copyright (2020). | ||
Some natural polymers possess inherent adhesive properties and can form strong bonds in wet environments, making them ideal for use in the oral cavity. Chitosan with abundant –OH and –NH2 groups is capable of forming hydrogen and covalent bonds, enabling interactions with reactive groups on the surface of oral tissues and triggering various chemical reactions.125 For instance, Ryu et al. developed a catechol modified chitosan-adhesive hydrogel (Chitoral) that overcame the wet oral environment (Fig. 5).42 When attached to the oral tissues, Chitoral immediately interacted with saliva, forming intermolecular interactions with mucins. Moreover, a salivary-induced cross-linked network and physical entanglement formed with time and further enhanced the adhesive strength. Catechol modification can further enhance the adhesive strength with a detachment force increased from 4.1 kPa to 10.3 kPa on porcine tongues. This strong adhesion enhanced by both chemical and physical interactions endows Chitoral with long-lasting therapeutic effects in the oral ulcer site. Collagen, with a large number of cationic amino acids, enables strong electrostatic interactions with mucins. Modifying catechol onto collagen chains enhanced the adhesion strength by mimicking the adhesion mechanism of mussel proteins. In addition, using Ca2+ as a cross-linking agent, mucoadhesive hydrogels were developed with an adhesion strength of 60 kPa.126 Given the fact that the hard tissues in the oral cavity have a large amount of CaCO3 and Ca2+, this strategy may be suitable for applications of self-healing hydrogels in the treatment of oral diseases.
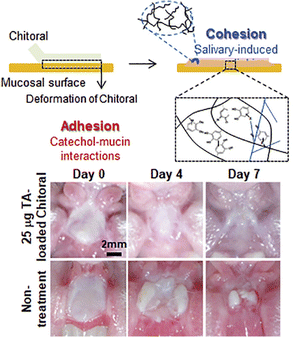 | ||
| Fig. 5 Schematic diagram of the adhesive hydrogel formed by the combined effect of covalent crosslinking and physical entanglement of Chitoral. Chitoral loaded with triamcinolone acetonide can expedite the healing process of oral ulcers. Adapted from Ryu et al.42 with permission from Elsevier. Copyright (2020). | ||
Guo et al. employed TA as an additive to facilitate the rapid and reversible formation of ionic and hydrogen bonds between quaternary chitosan and TA, thereby creating a hydrogel network for in situ wound repair.118 The presence of polyphenol groups in TA resulted in a strong binding affinity for thiol and amino groups presented on the peptides and proteins at the tissue surface. The enriched ionic and hydrogen bonds thereby enhanced the cross-linking degree of the TA-modified quaternary chitosan-based hydrogel. As a result, the mechanical properties of the hydrogel were improved, and the self-healing efficiency was more than 80%. In addition to the inherent haemostatic activity of chitosan, the robust adhesion of the modified hydrogel acted as a barrier, leading to improved haemostasis and wound healing.
To address the limited wet-adhesive issue of highly water-soluble TA, self-polymerized poly(tannic acid) (PTA) was prepared via oxidation polymerization. Chen et al. developed a matrix-independent adhesive bonding material for oral wound healing, considering the variation in wound pH in the oral cavity.128 A polymer–monomer complex was formed and the hydrogen and π–π bonding between PTA and TA resulted in a composite coating named MPTA. The adhesive material exhibited pH-dependent behaviour. At high pH, TA experienced electron loss, leading to the formation of a quinone structure and oxidative cross-linking of TA. Conversely, at low pH, protonation of most hydroxy groups destabilized the PTA cross-links. As a result, the degradation rate was affected by the pH at the wound site, leading to varying healing time at different stages of wound healing. The strong adhesive capacity of PTA allowed for the immobilization of other substances such as growth factors and antibiotics. This capability extended the application of the material by enabling the incorporation of additional therapeutic agents. Furthermore, this polymeric material could mimic the extracellular matrix and provide a scaffold for cell growth, thereby promoting the formation of new tissue, making it a potentially viable option for next-generation haemostatic materials in oral wound healing.
In addition to traditional dressing forms, sprayable hydrogels appear to be more suitable for therapeutic applications with improved patient compliance. To achieve this goal, Seung-Woo Cho et al. employed pyrogallol in pectin polymers to construct mucoadhesive polymer hydrogels.129 Mucoadhesive strength was achieved by covalent bonding including Michael addition and Schiff reactions, and non-covalent interactions including hydrogen bonding, hydrophobic interactions and π–π stacking between pyrogallol and mucin proteins. After spraying onto a rat tongue, hydrogels formed in situ and adhered onto the tongue tightly regardless of vigorous PBS wash. Beyond the oral cavity, theoretically, this sprayable form is applicable to other organs containing mucosal tissues, such as the nose, lungs, stomach, and intestines.
Alginate is a polysaccharide that possesses abundant hydroxy and carboxy groups. These functional groups enable cross-linking with multivalent cations, such as calcium, resulting in the formation of a solid network structure.132 This cross-linking can be further enhanced by incorporating methacrylate and dopamine, which improves the adhesion properties of alginate-based materials. Moreover, when calcium-cross-linked alginate adhesives are exposed to blood, the release of calcium ions can facilitate the coagulation process.133 In a study conducted by Li et al., phenyl boron-alginate/quaternized chitosan-catechol powders were developed for haemostasis of non-compressible haemorrhage.119 These powders not only absorbed plenty of blood but also formed a stable physical barrier of hydrogel at the site of bleeding due to their self-gelation character upon hydration. Particularly, in cases of post-tooth extraction wounds, these powders offer the advantage of circumventing the complexities associated with traditional suture removal. This finding underscores the future commercial viability of haemostatic powders that undergo in situ self-gelation and exhibit efficient adhesion to wet tissues. Dou et al. also developed a calcium alginate/fucoidan hydrogel with robust wet-adhesion.134 Cross-linking between calcium and alginate formed a wet-adhesive hydrogel which could be maintained against flush water.
Other polysaccharides, such as fucoidan, β-glucan, and carrageenan, also exhibit mucoadhesive ability. In the complex oral environment with varying pH, ion composition, and enzymes, the structure of polysaccharides can be reshaped. Taking this advantage, fucoidan-based adhesive materials were developed.135 Interpenetrating polymer networks between fucoidan and mucins allow polymer chains to easily form hydrophobic and hydrogen interactions. The abundant functional groups resulted in strong supramolecular interactions including disulfide bonding, hydrogen bonding, and hydrophobic interactions that enhanced the wet-adhesive strength in the intestinal tract.
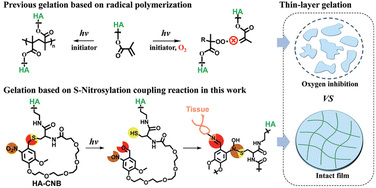 | ||
| Fig. 6 Schematic depicting the photo-responsive behaviour of cyclic o-nitrobenzyl-modified hyaluronic acid (CNB-HA) in the context of periodontitis treatment. Upon irradiation with 395 nm light, CNB-HA undergoes a photochemical reaction that simultaneously generates three distinct reactive groups: thiol, nitroso, and aldehyde. Through rapid non-radical cross-linking via S-nitrosylation, the generated thiols and nitroso groups form a thin gel within 5 s. Concurrently, the aldehyde selectively binds the amino groups of the tissue, facilitating in situ adhesion and establishing a sealed environment that prevents bacterial infiltration into the wound site. Reproduced from ref. 121 with permission from Wiley. Copyright (2021). | ||
Overall, the wet adhesivity of polymeric materials represents an important direction of development for their clinical translation in the oral environment. Apart from the adhesive modification strategies mentioned above, polymers can also be synthesized to form multiple interactions with oral tissues to further increase their wet-adhesion strength. Synthetic polymers with enriched amine and amide groups are capable of forming strong hydrogen bonding with the tissue matrix.137,138 Our group recently reported several dual-amide containing zwitterionic polymers with cell repulsion and cell adhesion functions.139 The poly(dual-amide) backbone provides strong amide–amide hydrogen bonding interactions with cells, therefore endowing polymers with excellent adhesive properties. These self-healing hydrogels hold promise in accelerating wound healing with promoted cell proliferation and vascularization. In the field of wet-adhesive polymeric materials design, several tips are suggested: (1) catechol based polymers exhibit wet-adhesive activity; however, their sensitivity to pH, oxidation, and temperature is an issue in their applications. Polymers are expected to address these issues by the involvement of an antioxidant agent, hydrophobic groups apart from catechol, borate protected catechol, etc. (2) In addition to catechol-based adhesives, polymers armed with multiple hydroxy and carboxy groups, polyelectrolytes, and cross-linked polymers with topological entanglement are all candidates for wet-adhesive materials with strong mechanical strength.
3.3. Polymeric materials with enhanced mechanical properties for wound healing
As discussed before, the oral cavity is characterized by a highly movable environment. Therefore, in addition to achieving strong adhesive capacity, it is crucial to ensure that polymeric materials used in oral diseases are resistant to deformation and can withstand various mechanical forces. Currently, common approaches include the employment of composite materials, surface coatings, and structural design modifications.140–143 Reinforcing materials like carbon fibres, glass fibres, or ceramic particles are often incorporated into composites to enhance their mechanical properties. The addition of NPs or fibres to polymer matrices can improve their strength, stiffness, and toughness, making them suitable to overcome the highly movable environment and maintain resistance to shear forces.To that end, Zhu et al. developed a low-swelling viscous hydrogel by incorporating nanoclay into a TA based hydrogel using a dissipative mechanism (Fig. 7).144 This formulation improved the tensile strength and extensibility of the hydrogel. The hydrogel exhibited a wide range of noncovalent bonding interactions and abundant hydrogen bonding that enabled strong adhesion in the humid and dynamic oral environment. The nanoclay with a strong surface charge was used as a cross-linker to enhance the physical cross-linking of hydrogels. This approach resulted in hydrogels with remarkable elasticity and self-healing properties, surpassing the performance of chemically cross-linked gels. In comparison to the control group, the hydrogel modified with nanoclay could withstand stretching, maintaining its stable appearance, and adhere to the mucous membrane for over 10 h.
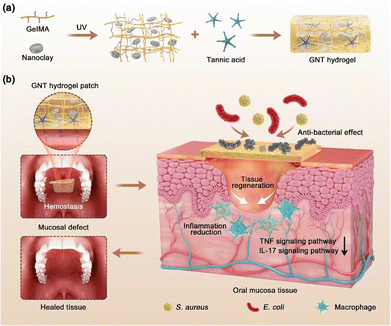 | ||
| Fig. 7 Illustration of the routes for the preparation of hydrogels and the effect of low-swelling viscous hydrogels on full-thickness oral mucosal defects. (a) Schematic diagram of the fabrication of a hydrogel. (b) The hydrogel had synergistic haemostatic, antibacterial, and anti-inflammatory properties, thus accelerating the wound healing of full-thickness mucosal defects. Reproduced from ref. 144 with permission from American Chemical Society. Copyright (2022). | ||
Apart from reinforcing material incorporation, polymers can also be designed to possess high mechanical properties through multiple chemical and covalent bonds. Gao et al. employed poly(N-isopropylacrylamide) (PNIPAAm)/chitosan hydrogels and polyethylene terephthalate (PET) surgical mesh.145 Through the use of a biocoupling agent, the chitosan formed covalent bonds with the tissue, resulting in adhesion energy surpassing 100 J m-2. The tensile strength of the adhered samples was found to be approximately 270 kPa owing to the topological entanglement of polymer networks, which aids in wound closure against blood pressure.
To enhance the mechanical properties of biomaterials, one common biological approach involves dissipating deformation energy through noncovalent reversible attraction by introducing secondary networks.146 During tensile deformation, ionic cross-links between chains undergo reversible breakage, while covalently cross-linked networks offer additional support by dynamically rearranging molecules to bridge cracks. Similarly, the incorporation of ionically cross-linked alginate into a PEG network enhanced the tensile properties of the hydrogels from approximately 300% to 400% compared to the control group.147 Yang et al. successfully enhanced the compressive stress, elastic modulus, elasticity, and toughness of hydrogels by exposing them to UV light.148 This improvement was achieved by a dynamic thiol-aldehyde addition reaction between the thiol of poly(γ-glutamic acid) and the aldehyde of glycidyl methacrylate-conjugated oxidized HA. Moreover, upon UV irradiation, a non-dynamic thiol-Michael addition occurred, resulting in a transition of polymer interactions from dynamic covalent bonding to stable covalent bonding. Introduction of thiol-aldehyde and thiol-Michael addition endowed the hydrogel with a compression stress of 80 kPa and an elastic modulus of 1010 Pa. Additionally, recent research has demonstrated the emerging potential of ultrasound-induced cavitation in hydrogels for enhancing polymer–tissue entanglement, leading to the development of strong bioadhesive properties.149 This technique harnesses the formation and subsequent collapse of microscopic bubbles induced by ultrasound, thereby facilitating improved interactions between the hydrogel and surrounding tissues.
Conventional mono-cross-linked hydrogels typically have limited mechanical properties. For example, physically cross-linked hydrogels may be too soft, while covalently cross-linked hydrogels may be too brittle. Physicochemical double cross-linking is able to address this issue and endows the hydrogel with improved mechanical strength, adhesion strength, and adjustable self-healing property. For example, Yuan and co-workers employed this strategy to construct a double cross-linked hydrogel based on Schiff base bonds and catechol-Fe3+ chelation bonds (Fig. 8).150 Dually cross-linked hydrogels can be created by combining covalent/covalent, noncovalent/noncovalent, or covalent–noncovalent bonds. The covalent cross-linking enhances the mechanical properties of the hydrogel system, improving its strength and stability with 535 kPa. Once applied to the wound sites, the hydrogel absorbed blood, rapidly gelled, and adhered to the tissue to perform haemostatic functions and accelerated the healing ability in deep second-degree burn wound. Introducing reversible non-covalent interactions enhances the dynamic properties and recyclability of the gels, which makes these hydrogels particularly effective in treating dynamic irregular wounds and provides insights for the design of materials used in the oral cavity which is characterized by a wet and highly movable environment.
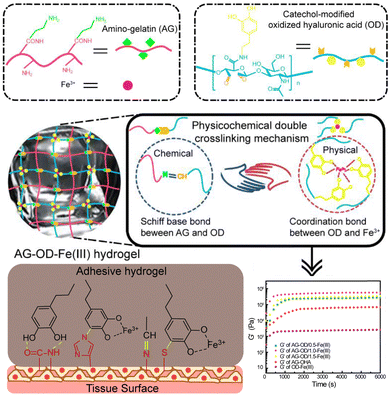 | ||
| Fig. 8 Schematic representation of the design strategy of the physicochemical double cross-linked multifunctional hydrogel capable of healing deep second-degree burn wounds. The amino and the aldehyde undergo Schiff base bonding, while the catechol groups form coordinate bonds with Fe3+ in the hydrogel network. The introduction of Schiff base bonding enhances the mechanical properties of the hydrogels formed by a single coordination bond. Reproduced from ref. 150 with permission from Elsevier. Copyright (2021). | ||
Combinatorial design of oral adhesives with both reinforcing materials and secondary networks further enhances the tough strength.151 Physical and chemical cross-linking of hydrogels by Michael addition and Schiff base reaction between gelatin and polydopamine increased the storage modulus from 150 to 800 Pa. Further applying nanoclay into the hydrogel increased the modulus to 7000 Pa. As a result, the toughness was improved by 9 fold, reaching 1026 J m−2 after cross-linking network formation and nanoclay reinforcement.
Although most attention is paid to the adhesive strength in oral care, the mechanical properties of polymeric materials are also important. The mechanical strength allows materials to combat the oral movements and assist in haemostasis against blood pressure. The oral cavity consists of hard tissues and soft tissues. Hard tissues such as teeth usually have a tensile strength ranging from 44 to 98 MPa, whereas soft tissues like mucosa exhibit weaker strength.152 The tensile strength of oral mucosa depends on the specific location, varying from 1 to 5 MPa.153 Therefore, materials for different use need to optimize their strength to adapt to oral tissues. Oral adhesive materials require adequate mechanical strength to resist forces during normal oral functions and tough strength to accommodate the natural movements and flexures of oral tissues. Nevertheless, in applications such as dental fillers or coatings, the increased resistance to chewing force reduces the possibility of microleakage caused by polymerization shrinkage, which greatly prolongs the life of the restoration. For future oral care materials, more efforts should be devoted to materials with both high adhesive and mechanical strength. Materials with strong covalent bonds, multi-cross-linking networks and topological entanglements are ideal candidates to meet these requirements.
4. Antimicrobial polymeric materials to combat the oral biofilm
There are over 700 different microorganisms present in the human oral cavity.154 The majority of bacteria in the oral cavity that adhere to teeth and mucosal surfaces are in the form of biofilms. Imbalances in the oral microbiota can lead to a range of oral diseases, including periodontal diseases, dental caries, and oral implant infections. To address these challenges associated with dysbiosis caused by pathogenic bacteria in the oral cavity, various biomaterials have been developed. Furthermore, there are some reviews that summarize existing antimicrobial biomaterials in the context of this topic.8,32,155 Here, we summarize the design concept of antimicrobial polymeric biomaterials targeting the specific oral microbial environment and the pathology of oral diseases (Fig. 9).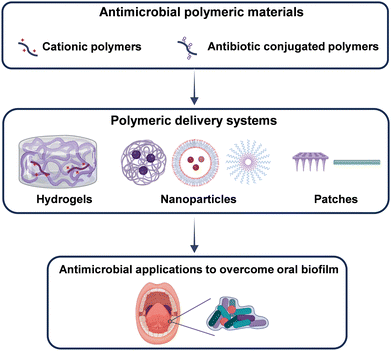 | ||
| Fig. 9 Schematic illustration of strategies for constructing antimicrobial polymeric materials and their application in disrupting the oral biofilm. Created with BioRender.com. | ||
4.1. Antimicrobial polymers adapting to oral microorganisms
From the perspective of designing antimicrobial biomaterials, various antimicrobial strategies have been extensively explored, and their mechanisms of action have gradually been elucidated. For example, cationic polymers have garnered significant attention owing to their distinctive antimicrobial properties and mechanisms.156,157 The pronounced antimicrobial effects of metals such as silver have been extensively investigated.158–161 Furthermore, polymeric materials loaded with antibiotics for the treatment of bacterial infections have been the subject of widespread research.162 In this subsection, we primarily review the antimicrobial components that are considered when designing antimicrobial polymer materials for the treatment of oral diseases.Cationic polymeric materials have been prepared for treatment of oral diseases as they can overcome complex challenges in oral microbial environments.165–168 For example, natural cationic dextrans were employed to disrupt the EPS of bacteria.169 A phase transition occurred in the EPS of the P. gingivalis induced biofilm, leading to the decrease in the biofilm thickness from 18 μm to 3 μm. Further exposure to bacteria enhanced the antibacterial activity both in vitro and in vivo. In addition, cationic polymers can be synthesized by advanced polymerization methods such as atom transfer radical polymerization (ATRP) and reversible addition-fragmentation chain transfer (RAFT) polymerization. These techniques are capable of introducing cationic segments, such as N,N′-dimethylamino-2-ethyl methacrylate (DMAEMA), 2-(tert-butylamino)ethyl methacrylate, and amino butyl methacrylate, into the polymer backbone to realize antibacterial activities.170 Takahashi synthesized a cationic methacrylate copolymer with amino butyl methacrylate as the antibacterial segment (Fig. 10a).166 The randomly distributed amino butyl methacrylate and methacrylate endowed the copolymer with both hydrophobicity and positive charge to interact with the bacterial cell membrane. Therefore, the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) values decreased from 52.1 to 7.8 and 62.5 to 10.4 μg mL−1, respectively. The copolymer was also capable of eradicating the S. mutans biofilm in 2 h by rinsing and swishing, demonstrating potential in oral care.
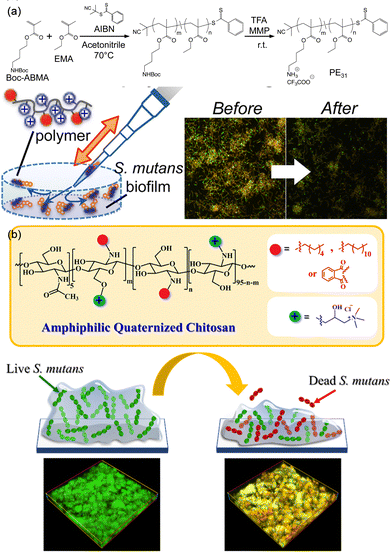 | ||
| Fig. 10 (a) Synthetic cationic copolymer with antimicrobial activities that could eradicate the S. mutans biofilm. Reproduced from ref. 166 with permission from American Chemical Society. Copyright (2017). (b) Diagram of amphiphilic quaternary chitosan derivatives employed as an environmentally friendly antimicrobial agent for oral care that exhibited comparable anti-biofilm efficacy to chlorhexidine. Reproduced from ref. 167 with permission from Elsevier. Copyright 2022. | ||
Quaternary ammonium polymers are common antimicrobial agents due to their permanent positive charge.171 Fu et al. have harnessed quaternary ammonium salts to modify polyurethane acrylates to afford antimicrobial polymeric dental restorative materials.172 Hoven et al. prepared quaternized chitosan with anti-biofilm effectiveness comparable to that of chlorhexidine when combating the dental caries-causing pathogen S. mutans (Fig. 10b).167 Both the hydrophobic alkyl tails and the hydrophilic quaternary ammonium domains contributed to the enhanced biofilm eradiation efficacy and inhibited bacterial viability within the biofilm.
Antimicrobial peptides (AMPs) are a class of natural substances composed of positive charged and hydrophobic amino acids.173 Although the specific mechanisms are not fully understood, the positive charges carried by AMPs, along with their distinctive secondary structures, constitute the structural basis for their antimicrobial activity.174,175 Antimicrobial peptides have been rated as a generation of antibiotics due to their less risk to induce antimicrobial resistance (AMR) and unique antimicrobial properties.176 However, applications of natural AMPs are limited by their antimicrobial activity, toxicity and high cost. Recently, advanced synthetic methods have been employed to develop mimics of AMPs. Our group devoted much effort in synthesizing AMPs along with understanding their mechanisms.177–186 We synthesized an effective AMP poly[lysine11-stat-phenylalanine10] (Poly(Lys11-stat-Phe10)) on a large scale in 2013.180 The random distribution of Lys and Phe in the polymer side chain plays a vital role in the antibacterial activity.182 The positively charged amino groups of Lys assist in sticking to the bacterial membrane by strong electrostatic interactions. Penetration was then achieved by hydrophobic interactions between Phe and the bacterial membrane, and by electrostatic interactions between Lys and the bacterial membrane. The disruption of the membrane further leads to the leakage of bacterial cell contents to realize a broad-spectrum antibacterial effect. By constructing this AMP with other functional polymer segments, a variety of antimicrobial polymers have been developed with a wide range of applications. For example, by introducing adhesive DOPA, we prepared Poly(Phe10-stat-Lys12)-DOPA to form coatings for preventing biofilms on implants.186 Apart from this key AMP, we designed another antimicrobial-antioxidative peptide Trp-Arg-Trp-Arg-Try-Tyr to in situ self-assemble into NPs on the skin, promoting the healing of wounds.185
Antimicrobial peptides can also be used for the treatment of oral diseases.187,188 Several strategies were developed to improve the antimicrobial activity of AMPs. Zhang et al. reported an AMP–polymer conjugate (Fig. 11).189 A natural AMP lysozyme (LYS) was designed as an initiator to polymerize cationic DMAEMA via photo-ATRP, obtaining an AMP–polymer conjugate named LYS-PDMAEMA. Compared to LYS alone, MIC and MBC of LYS-PDMAEMA were reduced by 1–2 orders. The enhanced antimicrobial activity was attributed to the increased positive charge that assisted in adsorption to the negatively charged bacterial membrane. In addition, polymerization protected the stability of LYS, which could effectively hydrolyze the peptidoglycan layer of the bacterial cell wall and lead to bacterial death, showing in vivo antimicrobial activity with less bone damage in a rat periodontitis model. The antimicrobial activity can be enhanced by regulating the acidic environment to prevent dental caries. Wang and co-workers developed an AMP mimic β-peptide polymer with a MIC of 3.13 μg mL−1 against S. mutans.186 This β-peptide polymer adsorbed to the bacterial surface, reduced the hydrophobicity of S. mutans, and impaired biofilm formation. Moreover, the β-peptide polymer down-regulated metabolic related genes, inhibited bacterial metabolism, and reduced acid production. To adapt to the acidic condition induced by microbial metabolism, Jiang et al. designed a pH-sensitive peptide.190 The in vitro antimicrobial experiments using S. mutans as a model showed that the peptide inhibited biofilm formation and decreased the acidogenicity in S. mutans, displaying MIC and MBC of 12 and 16 μg mL−1, respectively. The acidic environment led to the protonation of the peptide, which enhanced its attachment to bacterial cell membranes through electrostatic attraction and random coil to α-helix structure transition that enhanced interactions with membranes. As a result, the bacterial membranes were effectively disrupted, leading to increased bacterial permeability. Overall, the concept of material design tailored to the characteristics of oral diseases contributes to the development of more targeted advanced biomaterials for the treatment of oral diseases.
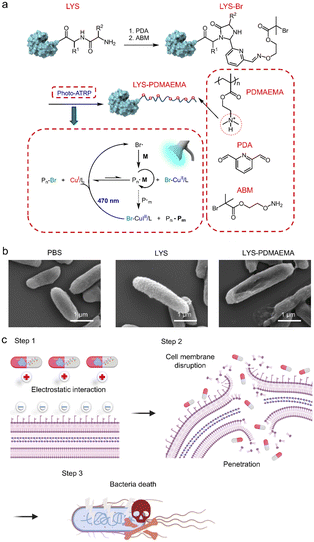 | ||
| Fig. 11 Preparation of LYS-PDMAEMA with improved antimicrobial activity. LYS-PDMAEMA killed bacteria through a membrane disruption mechanism. The positively charged PDMAEMA assisted in adsorption to the surface of the bacterial membrane, followed by LYS-catalysed hydrolysis of the peptidoglycan layer of the bacterial cell wall. The cell membrane disruption resulted in bacterial death. Reproduced from ref. 189 with permission from American Chemical Society. Copyright (2022). | ||
Polymers can conjugate with antibiotics via ester, amide, or ether bonds to improve the solubility and stability of antibiotics.192,193 For instance, Zhang and co-workers designed a polymer conjugated with antibiotics that performs excellently as an antibacterial dental resin (Fig. 12).194 The antibiotic ciprofloxacin (Cip) was first chemically modified with a polymerizable monomer 2-hydroxyethyl methacrylate (HEMA), affording a Cip containing monomer. This monomer was polymerized with HEMA to generate an antibacterial copolymer. After incorporating into a commercially available dental resin, this antibiotic-conjugated polymer containing resin displayed long-term inhibition against S. mutans with sustainable release of Cip. Conjugation of antibiotics with functional polymers is further developed to respond to the local environment. To that end, Ye et al. designed a pH-responsive cationic polymer-antibiotic conjugate that efficiently penetrated biofilms and inhibited bacterial growth in antimicrobial resistance infections.195 This synergistic antibacterial strategy can also be achieved by conjugating cationic polymers or antimicrobial peptides with antibiotics to further improve antibacterial efficacy.196
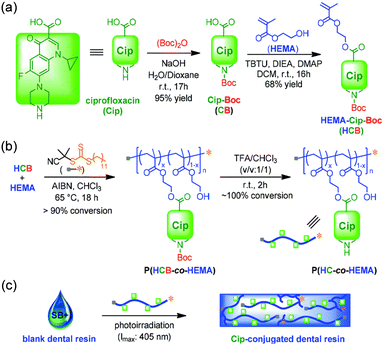 | ||
| Fig. 12 Preparation of antibacterial dental resins containing polymer–antibiotic conjugates via RAFT polymerization and deprotection. The polymer–antibiotic conjugates were further incorporated into dental resins by photocuring. Reproduced from ref. 194 with permission from Royal Society of Chemistry. Copyright (2019). | ||
Although antibiotics are essential for treating oral diseases, the development of composite therapeutic approaches involving polymeric materials and small-molecular antibacterial agents is equally indispensable. For the future direction of the antimicrobial polymer tailored oral environment, the following design strategies may be valuable for enhanced antimicrobial activities and reduced antimicrobial resistance: (1) inspired by PEGylated therapeutics, polymer modification should be helpful in improving the stability and compatibility of natural AMPs; (2) optimization of hydrophobicity and electro-positivity of cationic polymers might be a way to mimic the structure of natural AMPs. The polymer structure, i.e., homopolymers, block copolymers, and star polymers, etc. might also be factors affecting antimicrobial activities.
4.2. Oral microenvironment-adaptive delivery systems
Polymeric drug delivery systems are designed to protect the potency of therapeutics, maintain therapeutic levels, and reduce side effects.197 The therapeutic levels of oral drugs are hindered by the oral microenvironment. The highly movable and wet environment hampers the retention of therapeutics. The mucosa, EPS, and biofilms impede the direct interaction of drugs with bacteria, leading to a low antimicrobial activity. Therefore, an oral microenvironment-adaptive delivery system is crucial to deliver antimicrobial ingredients in a controlled manner to combat the oral physical and chemical barriers to treat oral diseases.For instance, Xie et al. developed injectable alginate microspheres loaded with silver-TiO2 NPs, achieving synergistic antimicrobial activity through the sustained release of Ag+ and the photothermal effect of TiO2.202 The alginate microspheres prevented silver-TiO2 NPs from quick flushing out and extended the retention time. Therefore, the antimicrobial activity was enhanced. They conducted bacterial viability staining and laser confocal scanning of bacterial biofilms on dentin slices. In the experimental group treated with silver NP-loaded microspheres, the antimicrobial activities against biofilms induced by S. gordonii and P. gingivalis reached 83% and 88%, respectively.
Xin et al. developed a non-invasive method for removing biofilms, which combines the acoustic cavitation effect induced by ultrasound and microbubbles with antibacterial compounds.203 They used a dual emulsion method to encapsulate the convertible perfluoropentane and antibiotic meropenem in poly(lactic acid-co-glycolic acid) (PLGA) NPs, and then chemically coupled them to Pseudomonas aeruginosa specific monoclonal antibodies. Targeted delivery of antibiotic meropenem improved the antimicrobial efficacy and reduced the risk of antimicrobial resistance due to off-target effects. The in vivo and in vitro biofilm clearance and antibacterial efficacy can be further improved by sonication. On the one hand, ultrasonic cavitation disrupted the biofilm and enhanced penetration of NPs. On the other hand, sonication triggered the release of antibiotics to eliminate bacteria and promote wound healing. Due to bacterial resistance and biofilm protection, it is difficult for conventional measures to achieve satisfactory therapeutic efficacy in open wounds with bacterial infection. Therefore, Liu et al. constructed a photothermal cascade nanoreactor (CPNC@GOx-Fe2+) based on a supramolecular strategy through hydrogen bonding and coordination of chitosan-modified palladium nano-cubes (CPNCs), glucose oxidase (GOx) and ferrous diiron (Fe2+).204 CPNC@GOx-Fe2+ has favorable photothermal effects and supports a GOx-assisted cascade to produce hydroxyl radicals to achieve combined photothermal and chemodynamic therapy. In the biofilm-associated extraction wound model, synergistic antibacterial activity and wound protection were achieved in the context of accelerated wound healing of infected tooth extraction wounds without affecting the oral symbiotic microbiota. This study also provides an avenue for multifunctional supramolecular systems for open wound infections.
Recently, polymer vesicles (also known as polymersomes) have been widely used for the treatment of diseases caused by various bacteria.205,206 Compared with commonly used liposomes in clinical practice, polymer vesicles have advantages such as diverse chemical structures, easy synthesis, and easy post-functionalization.206 Therefore, designing functionalized polymersomes can effectively destroy biofilms. What's more, the fluid membrane of polymersomes can adhere to the surface of bacteria, thereby increasing the contact area with the bacteria during the sterilization process, finally, making them easier to entry into the bacterial biofilms. This further enhances the effectiveness of anti-biofilm strategies.
For example, our group designed a dual corona polymer vesicle with inherent antibacterial activity and enhanced antibiotic delivery function to achieve effective treatment of biofilm-induced periodontitis.24,207 The dual corona polymer vesicles with two different hydrophilic coronas can be easily endowed with multiple functions. Two block copolymers, poly(ε-caprolactone)-block-poly(lysine-stat-phenylalanine) [PCL-b-P(Lys-stat-Phe)] and poly(ethylene oxide)-block-poly(ε-caprolactone) [PEO-b-PCL], were synthesized and co-assembled to form dual corona polymer vesicles (Fig. 13). PEO endows the polymer vesicle with protein-resistant ability, enabling it to penetrate EPS in biofilms. P(Lys-stat-Phe) endows the polymer vesicle with positive charge and broad-spectrum intrinsic antibacterial activity. It is noteworthy that such polymer vesicles can effectively reduce the antibiotic dosage by 50% when eradicating biofilms. It has been confirmed that the dual corona polymer vesicles effectively improve the anti-biofilm efficiency of antibiotics.
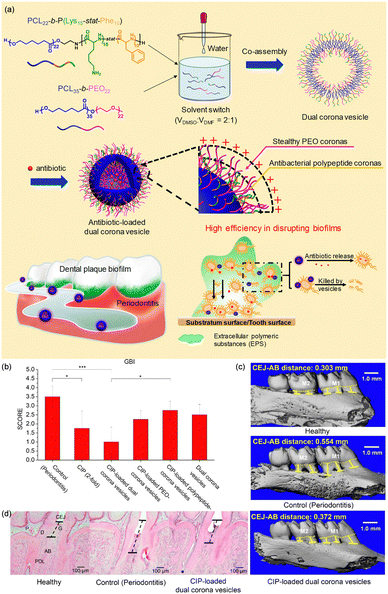 | ||
| Fig. 13 (a) Dual corona polymer vesicles with intrinsic antibacterial and enhanced antibiotic delivery capabilities for effective treatment of biofilm-induced periodontitis. Application of Cip-loaded dual corona polymer vesicles achieves a good therapeutic effect in periodontitis in vivo. (b) Quantitative results of the gingival bleeding index (GBI, n = 5). (c) 3D micro-CT reconstructed images of the maxillary molar area and the distance between the cementoenamel junction (CEJ, yellow dotted line) and the crest of alveolar bone (AB, yellow line) measured along three buccal roots for the first molar M1 and two of the second molar M2. (d) Tissue sections from rat's periodontal tissue processed for hematoxylin and eosin staining. P, pulp; D, dentin; G, gingiva; PDL, periodontal ligament. The dotted lines indicate the distance between CEJ and AB. Reproduced from ref. 24 with permission from American Chemical Society. Copyright 2019. | ||
Microneedle patches can simultaneously load multiple drugs and penetrate the epidermis to form micropores.211 Therefore, in oral diseases with mucosa and biofilms that hinder drug penetration, microneedle patches are promising strategies to improve the efficacy along with patient compliance. The mechanical strength, adhesive strength, waterproof ability and dissolvability of microneedles are key design factors. HA and polyvinylpyrrolidone (PVP) are ideal candidates due to their water solubility. Double layered microneedles were prepared with HA tips and PVP base to obtain mechanical strength and water solubility.212 A waterproof layer was further coated to overcome the wet oral environment. To control the drug release profile, hyaluronic acid methacryloyl was introduced to HA-PVP microneedles to mediate hydrophilicity.213 The microneedle was able to penetrate oral mucosa with an insert depth of 480 μm. Controlled and stepwise drug release was achieved as evidenced by in vivo dye delivery study. Delivery of multi-drugs could achieve promoted and synergistic therapeutic efficacy. For example, Yin et al. reported an HA microneedle encapsulating basic fibroblast growth factor (bFGF) and cetylpyridinium chloride.214 This microneedle exhibited antimicrobial activity, increased the growth and proliferation of fibroblasts, and facilitated the healing effect when attached to the oral ulcer site. Constructing microneedles with antimicrobial materials may benefit the intrinsic antibacterial properties and promote oral ulcer healing. Xie et al. reported a multifunctional polysaccharide composite microneedle patch for effectively promoting oral ulcer healing (Fig. 14).210 This patch was composed of HA and hydroxypropyl trimethylammonium chloride chitosan, loaded with dexamethasone and bFGF. Hydroxypropyl trimethylammonium chloride chitosan functioned as an oral antimicrobial agent, while dexamethasone inhibited oral inflammation, promoting oral ulcer healing in 6 days.
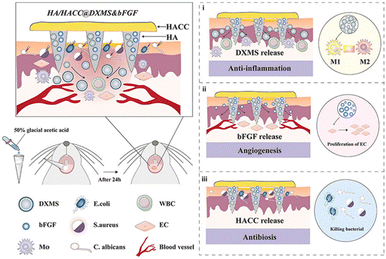 | ||
| Fig. 14 Multifunctional polysaccharide composite microneedle patch loaded with dexamethasone and basic fibroblast growth factor (bFGF) promotes healing of oral ulcer. Reproduced from ref. 210 with permission from Elsevier. Copyright 2023. | ||
Overall, polymeric delivery systems offer several advantages to adapt to the oral microenvironment. The following tips could be adopted when designing such oral microenvironment-adaptive delivery systems:
(1) At the molecular level, dynamic cross-linking would be beneficial to the release of loaded active ingredients, so a range of dynamic chemistry could be applied to design such hydrogels and patches.
(2) The off-target effect is crucial for AMR and side effects. Therefore, targeted drug delivery achieved by stimuli-responsive systems and in situ delivery systems should be considered.
(3) At the material level, various factors related to oral disease treatment should be considered, such as biocompatibility, wet-adhesion, and antibacterial activity.
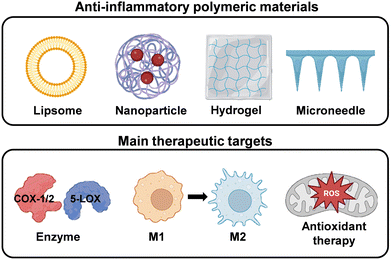
5. Anti-inflammatory polymeric materials to overcome the oral immune microenvironment
Under normal physiological conditions, the inflammatory response is widely recognized as a crucial process for maintaining tissue homeostasis under various unfavourable conditions. However, persistent and chronic inflammation accompanied by infection inevitably leads to increased production of inflammatory chemokines and cytokines, thereby impeding the tissue healing process and exacerbating inflammatory diseases.215 Common oral diseases such as oral candidiasis, oral lichen planus, and recurrent aphthous ulcers are associated with local immune dysfunction. Moreover, immune dysregulation may lead to precancerous lesions and tumor development.69 One characteristic feature of most oral diseases is chronic exposure to inflammation.216 The inflammation and host responses play vital roles in the pathogenesis of these diseases. Therefore, modulation of immune-inflammatory responses is key to the treatment of oral inflammatory diseases. In this section, we provide various design strategies for polymeric materials with different pathways for the effective suppression of inflammatory responses to treat oral diseases (Fig. 15).5.1. Anti-inflammatory treatment
The oral immune microenvironment is composed of pro-inflammatory enzymes, inflammatory cells, and inflammatory mediators, playing a crucial role in the pathological processes.217,218 In this subsection, we discuss three anti-inflammatory treatment approaches for oral inflammatory diseases based on the composition of the immune microenvironment.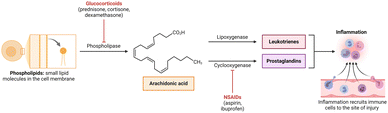 | ||
| Fig. 16 Arachidonic acid pathway in inflammation. Reprinted from “Arachidonic acid pathway in inflammation”, by BioRender.com (2023). Retrieved from https://app.biorender.com/biorender-templates. | ||
5.1.1.1. Phospholipase A2 inhibitor. Phospholipase A2 releases arachidonic acid by hydrolysing phospholipids such as phosphatidylcholine. Glucocorticoids can inhibit the activity of phospholipase A2, thereby suppressing the release of the inflammatory mediator precursor arachidonic acid, achieving the anti-inflammatory effect.221 Triamcinolone acetonide is a type of glucocorticosteroid that is commonly utilized in clinical settings due to its anti-inflammatory properties.222 However, the hydrophobic nature of triamcinolone acetonide and the complex oral microenvironment contribute to its low bioavailability and inconsistent therapeutic efficacy in treating ulcerative wounds. Therefore, several drug delivery systems have been developed.223–225 For example, Hao et al. prepared a hyaluronic acid-based dissolving microneedle patch to facilitate the delivery of triamcinolone acetonide-loaded mesoporous polydopamine NPs for the treatment of oral mucosal inflammation. Obvious reduction in the ulcer area and levels of inflammatory factors such as TNF-α and CD31 was observed.226
Glucocorticoids which inhibit phospholipase A2 are still less studied due to multifaceted immunosuppressive mechanisms.227 Gui et al. designed a core–shell nanostructure for the treatment of osteoporosis by loading glucocorticoids.228 Glucocorticoids interfered with arachidonic acid metabolism and suppressed cytokine synthesis to rapidly inhibit inflammation and immune responses. These results suggest that drug delivery systems loaded with glucocorticoids may be applied for treatment of inflammatory diseases.
5.1.1.2. Cyclooxygenase inhibitor. Non-steroidal anti-inflammatory drugs (NSAID) are the most frequently recommended medications for treating inflammatory diseases,229 which function by inhibiting the production of the pro-inflammatory mediator prostaglandin E2 through the inhibition of cyclooxygenase enzymes.230 Diclofenac is the most widely prescribed NSAID worldwide.231 Yan et al. developed a poly(ionic liquid)-based diclofenac sodium (DS)-loaded patch (PIL-DS) for the treatment of oral aphthous ulcers (Fig. 17).232 Through electrostatic interactions, ionic liquid cations could bind diclofenac anions, which greatly improved the bioavailability, biological properties and solubility of the drug. Furthermore, the ionic liquid used for polymerization contained catechol groups, which allowed the patch to adhere to mucosa and efficiently deliver DS to the mucosa ulcer sites. PIL-DS displayed anti-inflammatory activities, accelerating the healing process of the ulcers. In vitro study in RAW 264.7 cells stimulated with lipopolysaccharides (LPS) showed an overexpression of pro-inflammatory cytokines IL-6 and IL-1β without treatment. However, the expression of these pro-inflammatory cytokines was suppressed in cells treated with PIL-DS, indicating that the release of DS within the PIL-DS formulation could effectively protect cells from inflammation.
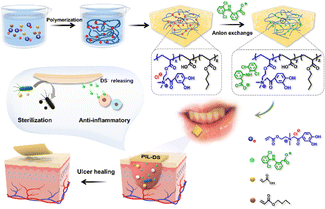 | ||
| Fig. 17 Synthesis of PIL-DS oral tissue patches and their schematic representation for the electrostatic loading of the nonsteroidal anti-inflammatory drug diclofenac sodium. Reproduced from ref. 232 with permission from Elsevier. Copyright 2023. | ||
5.1.1.3. Lipoxygenase inhibitor. Amlexanox (AMX) is a widely recognized anti-inflammatory and immunomodulatory agent used for the treatment of recurrent aphthous stomatitis.233 AMX exerts its anti-inflammatory effects by inhibiting 5-lipoxygenase, blocking the activity of Hsp90, as well as the non-classical IKK-ε and Tank-binding kinase. However, it was discontinued in 2014 due to reported adverse reactions including transient pain, stinging and burning sensation, contact mucositis, nausea, and diarrhea. To address these issues, Elsawy et al. loaded AMX into liposomes. These liposomes could serve as a local delivery system, thereby enhancing selective cellular uptake in the ulcerated area of the oral mucosa and augmenting the anti-inflammatory activity of AMX while minimizing its adverse reactions. They demonstrated the potential of AMX-loaded liposomes to effectively target macrophages and modulate their inflammatory response. As the concentration of AMX liposomes increased, a dose-dependent decrease in TNF-α expression in macrophages was observed, further confirming the anti-inflammation role of AMX liposomes.234
5.1.2.1. Inhibition of the M1 phenotype by releasing antibodies targeting TNF-α to alleviate inflammation. TNF-α, produced by M1 macrophages, serves as an inducer of the M1 phenotype and mediates excessive immune and inflammatory responses in various pathological conditions.239 As a result, it is an important target for anti-inflammatory drugs.240 TNF-α induces neighbouring oral keratinocytes and fibroblasts to release chemotactic factors, further recruiting lymphocytes and other immune cells. Ultimately, cytotoxic T cells within the inflammatory infiltrate, in conjunction with TNF-α, trigger apoptosis of basal keratinocytes in the oral mucosa, leading to epithelial loss and ulcer formation. Therefore, theoretically delivering anti-TNF-α biologics directly to the site of oral inflammation is effective in suppressing the inflammatory response.241–243
It is known that the progression of tissue ulceration is mediated by TNF-α. Edmans et al. developed and evaluated a mucoadhesive electrospun patch for the localized delivery of anti-TNFα biologics to treat oral mucosal ulcers (Fig. 18).244 Poly(vinyl pyrrolidone) (PVP) and Eudragit® RS100 polymer fibers were prepared by electrospinning. The anti-TNF-α biotinylated antibody fragment (F(ab)) was then encapsulated into the fibers, forming an electrospun patch. The patch protected the integrity of the biotinylated antibody fragment (F(ab)) from the complex oral environment and effectively released in 3 h to the ulcers without compromising its antigen-binding functionality. Moreover, treatment with the anti-TNF-α patch showed inhibition of TNF-α actions in a 3D tissue-engineered immunocompetent in vitro model of oral mucosal ulcers, resulting in reduced levels of immunogenic TNF-α and TNF-α-sensitive chemokines. Nevertheless, the organic solvents used in the electrospinning process may cause the denaturation of antibodies, and residual solvents may also be toxic to cells and tissues.
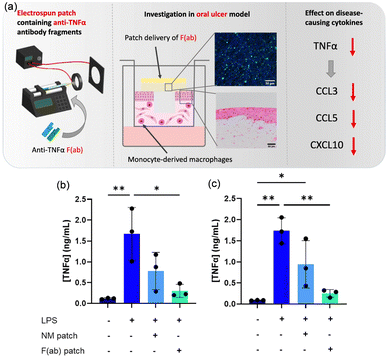 | ||
| Fig. 18 (a) Schematic representation of the preparation of an adhesive patch loaded with anti-TNF-α biologics during electrospunning for the treatment of inflammatory oral mucosal diseases. The patch leads to a decrease in active TNF-α levels, as well as a reduction in the levels of disease-associated T cell chemokines (CCL3, CCL5, and CXCL10) to baseline concentrations. ELISA data showed that the electrospun adhesive patch loaded with anti-TNF-α biologics applied to the oral ulcer model can neutralize LPS-mediated TNFα release. TNF-α concentrations (b) 6 h and (c) 24 h following LPS stimulation. Reproduced from ref. 244 with permission from Elsevier. Copyright 2022. | ||
5.1.2.2. Promotion of the M2 phenotype by releasing cytokines to alleviate inflammation. IL-4 is renowned as the most potent and extensively utilized factor for polarization towards the M2 phenotype that successfully induces a shift from M1 to M2 macrophages.245,246 Chen et al. loaded IL-4 and stromal cell-derived factor-1α onto high-stiffness transglutaminase-crosslinked gelatin for the regeneration of complex periodontal tissues in cases of periodontitis. They found that the presence of IL-4 induced the development of anti-inflammatory M2 macrophage polarization, thereby promoting osteogenic effects of bone marrow-derived mesenchymal stem cells (BMSCs) in vitro and improving periodontal regeneration. This suggested that supplementing with IL-4 was beneficial for regulating macrophage polarization and achieving immune modulation.247 However, directly encapsulating bioactive factors in hydrogels may lead to an explosive release. Therefore, it is an urgent issue to find a solution for achieving the programmable release of IL-4 from hydrogels, allowing it to continuously exhibit its reprogramming function and enhance the efficacy of implantable hydrogels in the oral cavity.
To overcome the complex oral microenvironment and achieve programmable release of IL-4, Cui et al. designed an immune microsphere-engineered hydrogel membrane that enabled effective wound coverage, improved drug encapsulation, and controlled the release of IL-4.248 As displayed in Fig. 19, they employed the physical blending method to prepare a hydrophilic soybean lecithin (SL) and IL-4 complex (SL/IL-4), which was then encapsulated into hydrophobic PLGA microspheres using microfluidic technology. The obtained PLGA microspheres loaded with SL/IL-4 complexes exhibited sustained release properties for IL-4. Lastly, the microsphere system was suspended in a GelMA solution, and through the adjustment of GelMA concentration and blue light irradiation time, an immune microsphere-engineered hydrogel membrane with adhesive properties for mucosal wounds was created. This hydrogel membrane achieved stable tri-phasic controlled release of IL-4 and possessed an elastic modulus similar to that of mucosal tissue, making it more suitable for oral applications. Additionally, it enabled stable macrophage reprogramming and upregulation of platelet-derived growth factor expression in vitro. In a rabbit model with oral mucosal defects, it also demonstrated the ability to effectively suppress wound inflammation, promote blood vessel generation, and facilitate tissue repair.
 | ||
| Fig. 19 Preparation and mechanism of immune-engineering microsphere hydrogels. (a) The preparation process involved creating PLGA microspheres, P/S/IL-4 complexes, and G/P/S/IL-4 complexes. (b) The immunized microsphere engineered hydrogel membrane was assembled using a specific mechanism. (c) The G/P/S/IL-4 complexes reprogrammed macrophages and HUVECs, leading to the secretion of paracrine factors and initiation of cascade signalling. This regulated the immune microenvironment, promoting angiogenesis and facilitating mucosal healing. Reproduced from ref.248 with permission from Wiley. Copyright 2023. | ||
5.1.2.3. Release of other anti-inflammatory substances to control M1–M2 dynamics. In addition to the aforementioned anti-inflammatory effect through the inhibition of phospholipase A2, glucocorticoids are capable of suppressing inflammatory reactions by interfering with the signalling pathways involved in the transcription of pro-inflammatory cytokine genes.249 Glucocorticoids can inhibit the pro-inflammatory response of macrophages, reduce the release of inflammatory mediators, and promote the polarization of macrophages towards an anti-inflammatory (M2) phenotype.230,250 The glucocorticoid drug prednisolone effectively inhibits inflammatory activation signalling, such as the NF-κB pathway, which can lead to the suppression of various pro-inflammatory cytokines.251 pH-responsive PMPC-b-PDPA diblock copolymer nanocarriers have been prepared for the controlled delivery of prednisolone disodium phosphate to inflamed target cells.252 This approach aimed to enhance the anti-inflammatory properties of prednisolone while minimizing the risk of inflammation and reducing off-target effects. By inhibiting the NF-κB pathway, which is responsible for inflammatory activation signalling, the expression of pro-inflammatory cytokines such as IL1β, IL6, and TNF-α could be decreased. This strategy holds promise in treating inflammatory diseases, addressing drug resistance, and promoting inflammation resolution in vitro.
In addition to biologics and dedicated anti-inflammatory drugs, various other compounds exhibit anti-inflammatory properties. These immunomodulatory molecules differ in chemical complexity and origin, ranging from proteins isolated from diverse sources to small molecules derived from plants.253
Nanozymes can also regulate macrophage polarization from M1 phenotype to M2 phenotype through Nrf1/NF-κB pathways, which reduces pro-inflammatory cytokines and increases anti-inflammatory cytokines, thereby alleviating inflammation. For example, Xu et al. encapsulated copper tannic acid (CuTA) coordination nanosheets in the glyceryl monostearate/triethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid (TM/BHT) hydrogel, and found that TM/BHT/CuTA could promote the expression of Nrf2, inhibit the expression of p65, and regulate the Nrf2/NF-κB pathway, thereby achieving immunomodulatory effects (Fig. 20).254 Furthermore, due to the regulation of the Nrf2/NF-κB pathway, M1 macrophages could be polarized into M2 macrophages, decreasing the expression of pro-inflammatory cytokines and relieving inflammation. In a periodontitis rat model, TM/BHT/CuTA facilitated the expression of transforming growth factor-β (TGF-β), and restored alveolar bone (AB) with decreased distance between AB and CEJ.
 | ||
| Fig. 20 Schematic illustration of the synthesis of the TM/BHT/CuTA hydrogel and its application in periodontitis. The CuTA nanozyme can promote the transformation of M1 macrophages into M2 macrophages by modulating the Nrf1/NF-κB pathway, thereby reducing pro-inflammatory factors, upregulating anti-inflammatory factors and osteogenic gene expression, and further accelerating periodontal tissue regeneration. Reproduced from ref. 254 with permission from American Chemical Society. Copyright 2023. | ||
Crocin is a natural carotenoid with excellent anti-inflammatory properties.255 For example, Haeri et al. developed a mucoadhesive hydrogel containing thiolated chitosan NPs as a saffron delivery system for aphthous stomatitis. The thiolated chitosan hydrogel improved ulcer healing, reduced the expression of TNF-α and p53, and increased the expression of vascular endothelial growth factor (VEGF) and alpha-smooth muscle actin (α-SMA). Overall, the thiolated chitosan hydrogel-encapsulated crocin loaded with thiolated chitosan NPs represents an option for treatment of aphthous stomatitis.256
In summary, polymeric biomaterials serve as carriers for inflammation-related enzyme inhibitors and immunomodulators for regulating the balance between pro-inflammatory and anti-inflammatory activities of immune cells. The immunomodulatory activity of biomaterials is intricately linked to their chemical properties, thereby allowing customization of distinct anti-inflammatory strategies. As the concept of biomaterial-mediated immune-inflammatory balance regulation advances, future research emphasis should pivot towards applications, taking into account comprehensive interactions between biomaterials and the inflammatory microenvironment. Optimization of drug delivery and tissue engineering should be directed towards providing the foundational basis for the clinical translation of biomaterials in the treatment of oral inflammation.
5.2. Reactive oxygen species (ROS) scavenger
In oral diseases, there is a close relationship between ROS and inflammation.257 While inflammation is a self-protective response of the body to damaged tissues or external stimuli, prolonged and excessive inflammation can actually damage tissues. For example, ROS are released in large quantities by inflamed cells when stimulated. ROS not only eliminate pathogens through oxidative reactions, but also promote the exacerbation of inflammation, leading to cell damage and apoptosis.258,259 This imbalance in ROS levels can easily lead to the occurrence and exacerbation of oral diseases. Therefore, regulating ROS levels to a normal state has become a focus in the treatment of oral diseases. There are two major strategies for oral disease treatment using biomaterials (Fig. 21). Firstly, biomaterials can serve as effective carriers for antioxidants, facilitating the safe and efficient delivery of ROS-removing drugs. Secondly, biomaterials can directly interact with ROS, exerting antioxidant effects.260–263Antioxidants are molecules that reduce radicals to prevent oxidative damage. Phenolic derivatives are commonly used antioxidants that act as hydrogen donors to radicals through single electron transfer.265 Resveratrol is a natural phenol that possesses antioxidant activities. Shi et al. developed a liposome to deliver resveratrol, addressing the challenge of its poor water solubility. This liposome demonstrated effective clearance of ROS and inhibition of pro-inflammatory signalling pathways.266 Curcumin is another phenol derivative that is capable of ROS scavenging.267 Xu et al. proposed antioxidant curcumin-loaded biodegradable NPs.268 The NPs were further embedded in an antibacterial hydrogel based on chitosan and AMP-conjugated PEG for both antimicrobial and antioxidant applications. The hydrogel can effectively alleviate periodontitis and promote tissue repair. Once this hydrogel was applied in bone marrow-derived macrophages, the anti-inflammatory macrophage phenotype increased with the related mRNA expression and cytokines, and the intracellular ROS levels apparently decreased.
Enzymes such as glutathione peroxidase and superoxide dismutase can regulate cellular ROS levels through catalysis of toxic ROS into O2 or H2O.269N-Acetyl-l-cysteine (NAC) is a precursor of the endogenous antioxidant glutathione. Qiu et al. designed PEG-ss-PCL NPs, a polymeric nanocarrier that responded to ROS by a thioketal bond and encapsulated NAC for ROS scavenging (Fig. 22).264 At the inflammation site, PEG-ss-PCL NPs were able to responsively disassemble and release NAC. The cellular ROS level induced by LPS apparently decreased after the treatment of PEG-ss-PCL NPs. This method not only reduces the cytotoxicity of NAC, but also reduces the activity of osteoclasts.
 | ||
| Fig. 22 Schematic diagram of the preparation process of the ROS-responsive polymer carrier loaded with NAC. Reproduced from ref. 264 with permission from Elsevier. Copyright (2021). | ||
In addition to loading antioxidants, polymeric biomaterials can be used as ROS scavengers to treat oral diseases. To that end, various antioxidant polymers were developed. Phenolic-based polymeric nanomaterials are widely used for scavenging ROS due to their function as electron donors to ROS. The enriched intermolecular interactions allow for the easy incorporation into the polymer backbone, side chain, and end group units through condensation polymerization, enzyme polymerization, or metal chelation polymerization, enabling the synthesis of effective polymeric antioxidants capable of removing ROS. Polydopamine is well known for its ability to scavenge ROS and function as an antioxidant material. Bao et al. developed biodegradable polydopamine (PDA) NPs as smart ROS scavengers for oxidative stress-induced periodontal diseases.270 Leveraging the inherent properties of PDA in reducing functional groups, the PDA NPs exhibited strong antioxidant capabilities, effectively removing ROS and reducing periodontal inflammation without any adverse effects. In an LPS-induced mouse periodontal disease model, administration of PDA NPs successfully eliminated ROS in a dose dependent manner. Recently, Tian et al. prepared functionalized epigallocatechin-3-gallate (EGCG) NPs for combating oxidative stress and reducing inflammation by clearing excessive ROS.271 The linear polyphenol oligomers in the EGCG NPs were synthesized through condensation reactions of EGCG, formaldehyde, and amine. Through hydrogen bonding and π–π stacking effects between the molecules of linear polyphenol oligomers, EGCG self-assembled into NPs with an average size of 72.4 ± 9.7 nm. This preparation method addresses the issues of poor stability and unsatisfactory therapeutic effects of tea polyphenols. The obtained polyphenol-based NPs can serve as ROS scavengers. Direct injection into the lesion site of chronic periodontitis could induce the consumption of ROS, downregulate the expression of inflammatory cytokines, and reshape the inflammatory microenvironment of chronic periodontitis. Therefore, EGCG NPs provide a simple and effective antioxidant defense platform for the treatment of chronic periodontitis.
Inspired by various methods for evaluating the ability of antioxidants to scavenge ROS, free radical traps were developed as free radical scavengers. These traps capture free radicals, preventing them from further reactions, thereby helping to reduce oxidative damage and cellular injury. Nagaski et al. synthesized poly[4-(2,2,6,6-tetramethylpiperidine-N-oxyl)aminomethylstyrene]-b-poly(ethylene glycol)-b-poly(4-(2,2,6,6-tetramethylpiperidine-N-oxyl)aminomethylstyrene) (PMNT-b-PEG-b-PMNT) that possessed an ROS scavenging nitroxide radical at the side chain of the PMNT segment.272,273 PMNT-b-PEG-b-PMNT was able to self-assemble into flower-like micelles and further combine with hydrogels to reduce ROS levels both in vitro and in vivo. Liu et al. constructed NAC-derived red fluorescent carbonized polymer dots (NAC-CPDs) with high ROS scavenging capacity (Fig. 23).170 Cysteine is a widely used antioxidant. By constructing NAC-CPDs through formamide-assisted solvothermal reaction, the stability of NAC was greatly improved than when it was encapsulated into carriers. Furthermore, the ROS scavenging efficacy was satisfied in cells and mice. NAC-CPDs not only eliminated ROS and promoted osteogenic differentiation, but also reduced alveolar bone resorption. In a ligature-induced periodontitis mouse model, NAC-CPD treatment reduced inflammatory cells and pro-inflammatory cytokine secretion, which finally contributed to the relief of tissue damage. Very recently, α-lipoic acid (LA), a coenzyme that has antioxidant ability by ROS scavenging, was employed in an oral adhesive patch for oral mucosal regeneration.274 Polymerized lipoic acid and sodium α-lipoate were mixed to form an elastomer patch (PolyLA-Na/PolyLA). The hydrogen bonding and electrostatic interactions between carboxy groups in the polymers and amino groups in the tissue provided wet-adhesion strength, whereas water-dissociating PolyLA-Na continuously released LA to remove excess ROS.
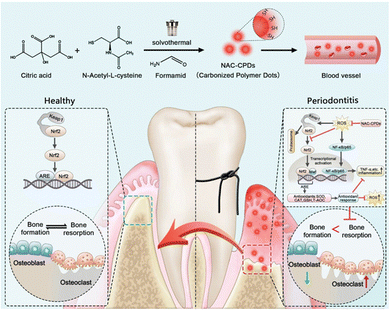 | ||
| Fig. 23 Synthesis of the polymer dots NAC-CPDs and the mechanism of clearing ROS in the treatment of periodontitis. Reproduced from ref. 170 with permission from John Wiley and Sons, copyright 2023. | ||
Overall, the use of biomaterials as antioxidant defense platforms provides a safe and efficient approach to the treatment of oral diseases. This opens diverse avenues for therapeutic interventions and holds great promise for the future of oral healthcare. From the viewpoint of antioxidant polymer design, polyphenol and its derivatives possess both wet-adhesive and antioxidant properties; therefore, incorporating catechol chemistry in polymer design is of great promise to build ROS scavengers effectively in oral diseases. In addition, multifunctional polymers are expected to build a bridge with inorganic antioxidant materials such as ceria for synergistic antioxidant–antibiotic therapy.
6. Conclusions and perspectives
In this review we have summarized the recent progress of polymeric materials tailored to overcome unique oral environments. The oral environment was first discussed from highly movable and wet, microbial, and inflammatory environments along with their impact on oral diseases. Design strategies to overcome these barriers were then discussed. To that end, polymeric materials with high adhesivity, high mechanical modulus, antimicrobial and anti-inflammatory properties were developed through chemical engineering and advanced nanotechnology. Combining the oral environment and disease pathogens with materials, as well as other advances in cell biology, molecular biology, materials science, and nanotechnology, will enable further development and attainment in the treatment of oral diseases. Despite the achievements, the complex oral environment remains an important challenge in oral healthcare in clinical translation.First, the oral environment is constantly subjected to mechanical forces generated by chewing, temperature variations, and the influence of saliva and oral fluids. The design of biomaterials should be able to withstand these challenges, possessing not only strong adhesive properties but also long-term structural integrity. This helps prevent microleakage and bacterial infiltration, and reduces the risk of secondary caries or reinfection. The focus of future research lies in balancing the adhesive and mechanical properties of biomaterials without compromising their porosity or degradation performance, particularly in designing smart materials that can respond to local chemical signals during oral disease repair processes to maintain a stable oral environment.
Second, drug resistance remains a huge challenge in antimicrobial applications. Microbes naturally develop resistance. The use of antibiotics accelerates the resistance development. Narrow spectrum antibiotics are preferred to slow down the resistance than broad spectrum antibiotics. However, considering the complex genericity of oral microbiota, broad-spectrum antibacterial materials are potentially more effective for the treatment of oral microbiota induced diseases. Therefore, it is urgent to find a balance between antimicrobial resistance and therapeutic efficacy. Disrupting the bacterial cell membrane without interaction with intracellular organelles might be a future direction to develop next-generation antibacterial materials.
Third, from molecular and histological aspects, oral diseases are subject to several transcription factors. For example, wounds are related to sex-determining region Y-box 2 and paired-like homeodomain 1, which assist in the wound healing process.275 Thus, improving these transcription factors with an oral specific delivery system, such as nucleic acid encapsulated mucus penetrating polymeric materials, might be interesting in oral wound healing. In addition, a novel gene editing system targeting bacterial pathogens and antibiotic resistance might also be interesting.276
Finally, biocompatibility should always be considered when turning into clinical translation. Various biomaterials, as well as modifications with bioactive ions and loading of bioactive molecules, may serve as effective strategies for improving oral diseases treatment. Therefore, the safety related to biomaterials needs to be monitored, particularly in terms of long-term stability within the oral cavity. Biomaterials can interact with oral tissues, promoting desired biological responses. The combination of maintaining the homeostasis of the oral microenvironment and bioactivity may provide new research perspectives for oral disease treatment.
Author contributions
B. J., Y. X., and J. D. created the outline of this review. B. J., B. Z., J. L., J. Q., Y. H., M. H., Y. M., J. J., R. C. and Y. X. wrote the manuscript and designed the figures. B. J. wrote Section 1, B. Z. and R. C. wrote Section 5, J. L. and J. J. wrote Section 4, J. Q., Y. H., M. H., and Y. M. wrote Sections 2 and 3, Y. X. wrote Section 6. Y. X. and J. D. revised the manuscript. All authors discussed, edited, and reviewed the manuscript before submission.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Key R&D Program of China (2022YFC2402900), National Natural Science Foundation of China (21925505 and 22335005), Innovation Program of Shanghai Municipal Education Commission (2023ZKZD28) and the International Scientific Collaboration Fund of Science and Technology Commission of Shanghai Municipality (21520710100). Jianzhong Du is a recipient of the National Science Fund for Distinguished Young Scholars.Notes and references
- W. G. Wade, J. Periodontol., 2021, 86, 113–122 CrossRef PubMed.
- G. Hajishengallis, R. J. Lamont and H. Koo, Cell Host Microbe, 2023, 31, 528–538 CrossRef CAS PubMed.
- Z. Chen, Z. Chu, Y. Jiang, L. Xu, H. Qian, Y. Wang and W. Wang, Mater. Today Bio., 2023, 20, 100635 CrossRef CAS PubMed.
- M. Montazerian, F. Baino, E. Fiume, C. Migneco, A. Alaghmandfard, O. Sedighi, A. V. DeCeanne, C. J. Wilkinson and J. C. Mauro, Prog. Mater. Sci., 2023, 132, 101023 CrossRef CAS.
- M. Arseneault, V. Granskog, S. Khosravi, I. M. Heckler, P. Mesa Antunez, D. Hult, Y. Zhang and M. Malkoch, Adv. Mater., 2018, 30, e1804966 CrossRef PubMed.
- Z. Ye, T. Sang, K. Li, N. G. Fischer, I. Mutreja, C. Echeverría, D. Kumar, Z. Tang and C. Aparicio, Acta Biomater., 2022, 140, 338–349 CrossRef CAS PubMed.
- D. Xia, F. Yang, Y. Zheng, Y. Liu and Y. Zhou, Bioact. Mater., 2021, 6, 4186–4208 CAS.
- H. Cui, Y. You, G. W. Cheng, Z. Lan, K. L. Zou, Q. Y. Mai, Y. H. Han, H. Chen, Y. Y. Zhao and G. T. Yu, Sci. Technol. Adv. Mater., 2023, 24, 2156257 CrossRef PubMed.
- M. Huang, Y. Huang, H. Liu, Z. Tang, Y. Chen, Z. Huang, S. Xu, J. Z. Du and B. Jia, Biomater. Sci., 2022, 10, 6413–6446 RSC.
- M. Rother, M. G. Nussbaumer, K. Renggli and N. Bruns, Chem. Soc. Rev., 2016, 45, 6213–6249 RSC.
- M. Haktaniyan and M. Bradley, Chem. Soc. Rev., 2022, 51, 8584–8611 RSC.
- H. Mutlu, L. M. de Espinosa and M. A. R. Meier, Chem. Soc. Rev., 2011, 40, 1404–1445 RSC.
- P. Murugan, M. Krishnamurthy, S. N. Jaisankar, D. Samanta and A. B. Mandal, Chem. Soc. Rev., 2015, 44, 3212–3243 RSC.
- J. C. Worch, H. Prydderch, S. Jimaja, P. Bexis, M. L. Becker and A. P. Dove, Nat. Rev. Chem., 2019, 3, 514–535 CrossRef CAS.
- D. T. Gentekos, R. J. Sifri and B. P. Fors, Nat. Rev. Mater., 2019, 4, 761–774 CrossRef.
- Z. Wang, H. Wei, Y. Huang, Y. Wei and J. Chen, Chem. Soc. Rev., 2023, 52, 2992–3034 RSC.
- S. Nemati, S. J. Kim, Y. M. Shin and H. Shin, Nano Convergence, 2019, 6, 36 CrossRef PubMed.
- Y. Ye, J. Yu, D. Wen, A. R. Kahkoska and Z. Gu, Adv. Drug Delivery Rev., 2018, 127, 106–118 CrossRef CAS PubMed.
- A. Kirillova, T. R. Yeazel, D. Asheghali, S. R. Petersen, S. Dort, K. Gall and M. L. Becker, Chem. Rev., 2021, 121, 11238–11304 CrossRef CAS PubMed.
- Q. Huang, Y. Zou, M. C. Arno, S. Chen, T. Wang, J. Gao, A. P. Dove and J. Z. Du, Chem. Soc. Rev., 2017, 46, 6255–6275 RSC.
- S. A. Dilliard and D. J. Siegwart, Nat. Rev. Mater., 2023, 8, 282–300 CrossRef CAS PubMed.
- M. Toledano Osorio, R. Osorio, F. S. Aguilera, A. L. Medina Castillo, M. Toledano, E. Osorio, S. Acosta, R. Chen and C. Aparicio, Acta Biomater., 2020, 111, 316–326 CrossRef CAS PubMed.
- J. Jin, R. Bhat, U. Mangal, J. Y. Seo, Y. Min, J. Yu, D. E. Kim, K. Kuroda, J. S. Kwon and S. H. Choi, Biomater. Sci., 2022, 10, 2224–2236 RSC.
- Y. J. Xi, Y. Wang, J. Y. Gao, Y. F. Xiao and J. Z. Du, ACS Nano, 2019, 13, 13645–13657 CrossRef CAS PubMed.
- Y. M. Bichu, A. Alwafi, X. Liu, J. Andrews, B. Ludwig, A. Y. Bichu and B. Zou, Bioact. Mater., 2023, 22, 384–403 Search PubMed.
- J. Guo, H. Yao, X. Li, L. Chang, Z. Wang, W. Zhu, Y. Su, L. Qin and J. Xu, Bioact. Mater., 2023, 21, 175–193 Search PubMed.
- H. Alqurashi, Z. Khurshid, A. U. Y. Syed, S. Rashid Habib, D. Rokaya and M. S. Zafar, J. Adv. Res., 2021, 28, 87–95 CrossRef CAS PubMed.
- K. Kasza, P. Gurnani, K. R. Hardie, M. Cámara and C. Alexander, Adv. Drug Delivery Rev., 2021, 178, 113973 CrossRef CAS PubMed.
- P. Makvandi, J. T. Gu, E. N. Zare, B. Ashtari, A. Moeini, F. R. Tay and L. N. Niu, Acta Biomater., 2020, 101, 69–101 CrossRef CAS PubMed.
- D. Rokaya, V. Srimaneepong, J. Sapkota, J. Qin, K. Siraleartmukul and V. Siriwongrungson, J. Adv. Res., 2018, 14, 25–34 CrossRef CAS PubMed.
- Q. Zeng, X. Qi, G. Shi, M. Zhang and H. Haick, ACS Nano, 2022, 16, 1708–1733 CrossRef CAS PubMed.
- P. P. Kalelkar, M. Riddick and A. J. García, Nat. Rev. Mater., 2021, 7, 39–54 CrossRef PubMed.
- H. Liu, Y. Huang, M. Huang, Z. Huang, Q. Wang, L. Qing, L. Li, S. Xu and B. Jia, Int. J. Nanomed., 2022, 17, 2679–2705 CrossRef PubMed.
- K. G. H. Desai, J. Biomed. Mater. Res., Part B, 2018, 106, 1383–1413 CrossRef CAS PubMed.
- S. Das, K. Bhattacharya, J. J. Blaker, N. K. Singha and M. Mandal, Biopolymers, 2023, 114, e23556 CrossRef CAS PubMed.
- D. C. Hatcher, Neuroimaging Clin. N. Am., 2022, 32, 749–761 CrossRef PubMed.
- L. Lin, T. Zhao, D. Qin, F. Hua and H. He, Front. Public Health, 2022, 10, 929165 CrossRef PubMed.
- A. Tsujimoto, W. W. Barkmeier, N. G. Fischer, K. Nojiri, Y. Nagura, T. Takamizawa, M. A. Latta and M. Miazaki, Jpn. Dent. Sci. Rev., 2018, 54, 76–87 CrossRef PubMed.
- M. Cadenaro, U. Josic, T. Maravić, C. Mazzitelli, G. Marchesi, E. Mancuso, L. Breschi and A. Mazzoni, J. Dent. Res., 2023, 102, 254–262 CrossRef CAS PubMed.
- X. Zhang, Q. Zhang, X. Meng, Y. Ye, D. Feng, J. Xue, H. Wang, H. Huang, M. Wang and J. Wang, Polymers, 2021, 13, 2975 CrossRef CAS PubMed.
- Y. Zhou, Y. Yang, R. Liu, Q. Zhou, H. Lu and W. Zhang, Int. J. Nanomed., 2023, 18, 2623–2645 CrossRef CAS PubMed.
- J. H. Ryu, J. S. Choi, E. Park, M. R. Eom, S. Jo, M. S. Lee, S. K. Kwon and H. Lee, J. Controlled Release, 2020, 317, 57–66 CrossRef CAS PubMed.
- C. Dawes and D. T. W. Wong, J. Dent. Res., 2019, 98, 133–141 CrossRef CAS PubMed.
- F. Romano, A. Castiblanco, F. Spadotto, F. Di Scipio, M. Malandrino, G. N. Berta and M. Aimetti, Biomedicines, 2020, 8, 354 CrossRef CAS PubMed.
- P. D. Marsh, T. Do, D. Beighton and D. A. Devine, J. Periodontol., 2016, 70, 80–92 CrossRef PubMed.
- P. Ahmad, A. Hussain, A. Carrasco-Labra and W. L. Siqueira, Caries Res., 2022, 56, 385–398 CrossRef PubMed.
- M. P. Gomez Hernandez, E. E. Starman, A. B. Davis, M. H. H. Withanage, E. Zeng, S. M. Lieberman, K. A. Brogden and E. A. Lanzel, Rheumatology, 2021, 60, 4765–4777 CrossRef PubMed.
- D. Zuanazzi, Y. Xiao and W. L. Siqueira, Clin. Oral Investig., 2021, 25, 2281–2296 CrossRef PubMed.
- Y. Jinno, M. Stocchero, M. Toia, E. Papia, M. Ahmad and J. P. Becktor, Clin. Oral. Implants Res., 2023, 34, 254–262 CrossRef CAS PubMed.
- P. Vervliet, S. De Nys, R. C. Duca, I. Boonen, L. Godderis, M. Elskens, K. L. Van Landuyt and A. Covaci, Dent. Mater., 2022, 38, 19–32 CrossRef CAS PubMed.
- M. F. Kunrath, A. Correia, E. R. Teixeira, R. Hubler and C. Dahlin, Nanomaterials, 2022, 12, 757–766 CrossRef PubMed.
- J. Wu, Z. Pan, Z. Y. Zhao, M. H. Wang, L. Dong, H. L. Gao, C. Y. Liu, P. Zhou, L. Chen, C. J. Shi, Z. Y. Zhang, C. Yang, S. H. Yu and D. H. Zou, Adv. Mater., 2022, 34, e2200115 CrossRef PubMed.
- J. L. Baker, J. L. M. Welch, K. M. Kauffman, J. S. Mclean and X. S. He, Nat. Rev. Microbiol., 2024, 22, 89–104 CrossRef CAS PubMed.
- K. Campbell, Nature, 2021 DOI:10.1038/d41586-021-02920-w.
- L. Sedghi, V. DiMassa, A. Harrington, S. V. Lynch and Y. L. Kapila, J. Periodontol., 2021, 87, 107–131 CrossRef PubMed.
- R. J. Lamont, H. Koo and G. Hajishengallis, Nat. Rev. Microbiol., 2018, 16, 745–759 CrossRef CAS PubMed.
- L. Hall-Stoodley, J. W. Costerton and P. Stoodley, Nat. Rev. Microbiol., 2004, 2, 95–108 CrossRef CAS PubMed.
- P. E. Kolenbrander, R. J. Palmer, S. Periasamy and N. S. Jakubovics, Nat. Rev. Microbiol., 2010, 8, 471–480 CrossRef CAS PubMed.
- D. S. W. Benoit, K. R. Sims and D. Fraser, ACS Nano, 2019, 13, 4869–4875 CrossRef CAS PubMed.
- B. G. Loos and T. E. Van Dyke, J. Periodontol., 2020, 83, 26–39 CrossRef PubMed.
- F. S. C. Sczepanik, M. L. Grossi, M. Casati, M. Goldberg, M. Glogauer, N. Fine and H. C. Tenenbaum, J. Periodontol., 2020, 84, 45–68 CrossRef PubMed.
- Z. Prucsi, A. Zimny, A. Płonczyńska, N. Zubrzycka, J. Potempa and M. Sochalska, Int. J. Mol. Sci., 2023, 24, 12922 CrossRef CAS PubMed.
- D. W. Williams, T. Greenwell-Wild, L. Brenchley, N. Dutzan, A. Overmiller, A. P. Sawaya, S. Webb, D. Martin, G. Hajishengallis, K. Divaris, M. Morasso, M. Haniffa and N. M. Moutsopoulos, Cell, 2021, 184, 4090–4104 CrossRef CAS PubMed.
- S. Şenel, Int. J. Mol. Sci., 2021, 22, 7821 CrossRef PubMed.
- S. Groeger and J. Meyle, Front. Immunol., 2019, 10, 208 CrossRef CAS PubMed.
- M. Ptasiewicz, E. Grywalska, P. Mertowska, I. Korona Głowniak, A. Poniewierska Baran, P. Niedźwiedzka Rystwej and R. Chałas, Int. J. Mol. Sci., 2022, 23, 882 CrossRef CAS PubMed.
- A. Barbour, O. Elebyary, N. Fine, M. Oveisi and M. Glogauer, FEMS Microbiol. Rev., 2022, 46, fuab039 CrossRef CAS PubMed.
- H. E. Ho, Y. Chun, S. Jeong, O. Jumreornvong, S. H. Sicherer and S. Bunyavanich, J. Allergy Clin. Immunol., 2021, 148, 627–632 CrossRef CAS PubMed.
- D. Lin, L. Yang, L. Wen, H. Lu, Q. Chen and Z. Wang, Mucosal Immunol., 2021, 14, 1247–1258 CrossRef CAS PubMed.
- Z. H. Wang, H. B. Cao, J. Q. Xiong, Y. L. Lu, Y. X. Deng, H. Nan, S. T. Zheng, H. Ye and Z. S. Cao, Postgrad. Med. J., 2022, 98, 57–66 CrossRef PubMed.
- N. M. Moutsopoulos and J. E. Konkel, Trends Immunol., 2018, 39, 276–287 CrossRef CAS PubMed.
- Y. J. Shin, H. W. Choung, J. H. Lee, I. C. Rhyu and H. D. Kim, J. Dent. Res., 2019, 98, 526–533 CrossRef CAS PubMed.
- G. Baima, M. Minoli, D. S. Michaud, M. Aimetti, M. Sanz, B. G. Loos and M. Romandini, J. Periodontol., 2023, 00, 1–12 Search PubMed.
- J. E. Tota, E. A. Engels, M. W. Lingen, N. Agrawal, A. R. Kerr, Z. S. Zumsteg, L. C. Cheung, H. A. Katki, C. C. Abnet and A. K. Chaturvedi, J. Clin. Oncol., 2023 DOI:10.1200/JCO.23.00729.
- M. Stasiewicz and T. M. Karpiński, Semin. Cancer Biol., 2022, 86, 633–642 CrossRef CAS PubMed.
- R. J. Lamont, Z. R. Fitzsimonds, H. Wang and S. Gao, J. Periodontol., 2022, 89, 154–165 CrossRef PubMed.
- A. I. Mäkinen, V. Y. Pappalardo, M. J. Buijs, B. W. Brandt, A. A. Mäkitie, J. H. Meurman and E. Zaura, Microbiome, 2023, 11, 171 CrossRef PubMed.
- G. D. Sepich-Poore, L. Zitvogel, R. Straussman, J. Hasty, J. A. Wargo and R. Knight, Science, 2021, 371, eabc4552 CrossRef CAS PubMed.
- G. El Tekle and W. S. Garrett, Nat. Rev. Cancer, 2023, 23, 600–618 CrossRef CAS PubMed.
- N. Mohamed, J. Litlekalsoy, I. A. Ahmed, E. M. H. Martinsen, J. Furriol, R. Javier-Lopez, M. Elsheikh, N. M. Gaafar, L. Morgado, S. Mundra, A. C. Johannessen, T. A. H. Osman, E. S. Nginamau, A. Suleiman and D. E. Costea, Front. Cell. Infect. Microbiol., 2021, 11 Search PubMed.
- Y. Zong, Y. Zhou, B. Liao, M. Liao, Y. Shi, Y. Wei, Y. Huang, X. Zhou, L. Cheng and B. Ren, Front. Cell. Infect. Microbiol., 2021, 11 Search PubMed.
- B. A. Helmink, M. A. W. Khan, A. Hermann, V. Gopalakrishnan and J. A. Wargo, Nat. Med., 2019, 25, 377–388 CrossRef CAS PubMed.
- W. Song, A. C. Anselmo and L. Huang, Nat. Nanotechnol., 2019, 14, 1093–1103 CrossRef CAS PubMed.
- J. Sun, F. Chen and G. Wu, ISME J., 2023, 17, 1535–1551 CrossRef CAS PubMed.
- E. Elinav, R. Nowarski, C. A. Thaiss, B. Hu, C. Jin and R. A. Flavell, Nat. Rev. Cancer, 2013, 13, 759–771 CrossRef CAS PubMed.
- J. Hou, M. Karin and B. Sun, Nat. Rev. Clin. Oncol., 2021, 18, 261–279 CrossRef PubMed.
- S. Mignani, J. Rodrigues, H. Tomas, M. Zablocka, X. Shi, A.-M. Caminade and J.-P. Majoral, Chem. Soc. Rev., 2018, 47, 514–532 RSC.
- T. Li, T. Akinade, J. Zhou, H. Wang, Q. Tong, S. He, E. Rinebold, L. E. Valencia Salazar, D. Bhansali, Y. Zhong, J. Ruan, J. Du, P. Dalerba and K. W. Leong, Adv. Sci., 2022, 9, 2203949 CrossRef CAS PubMed.
- I. Sequeira, J. F. Neves, D. Carrero, Q. Peng, N. Palasz, K. Liakath-Ali, G. M. Lord, P. R. Morgan, G. Lombardi and F. M. Watt, Nat. Commun., 2018, 9, 3437 CrossRef PubMed.
- J. Nam, S. Son, L. J. Ochyl, R. Kuai, A. Schwendeman and J. J. Moon, Nat. Commun., 2018, 9, 1074 CrossRef PubMed.
- P. Zhao, M. Wang, M. Chen, Z. Chen, X. Peng, F. Zhou, J. Song and J. Qu, Biomaterials, 2020, 254, 120142 CrossRef CAS PubMed.
- Y. Mao, Z. Xu, Z. He, J. Wang and Z. Zhu, Chin. Chem. Lett., 2023, 34, 107461 CrossRef CAS.
- S. Nam and D. Mooney, Chem. Rev., 2021, 121, 11336–11384 CrossRef CAS PubMed.
- C. Cui and W. Liu, Prog. Polym. Sci., 2021, 116, 101388 CrossRef CAS.
- Z. Xiao, Q. Li, H. Liu, Q. Zhao, Y. Niu and D. Zhao, Eur. Polym. J., 2022, 173, 111277 CrossRef CAS.
- J. Yang, R. Bai, B. Chen and Z. Suo, Adv. Funct. Mater., 2019, 30, 1901693 CrossRef.
- M. Wuttig, V. L. Deringer, X. Gonze, C. Bichara and J. Y. Raty, Adv. Mater., 2018, 30, e1803777 CrossRef PubMed.
- H. Y. Yuen, H. P. Bei and X. Zhao, Chem. Eng. J., 2022, 431, 133372 CrossRef CAS.
- S. Chen, K. Zhang, Z. Li, Y. Wu, B. Zhu and J. Zhu, Supramol. Mater., 2023, 2, 100032 Search PubMed.
- S. Ma, Y. Wu and F. Zhou, Curr. Opin. Colloid Interface Sci., 2020, 47, 84–98 CrossRef CAS.
- Y. Chen, J. Meng, Z. Gu, X. Wan, L. Jiang and S. Wang, Adv. Funct. Mater., 2019, 30, 1905287 CrossRef.
- X. Peng, X. Xia, X. Xu, X. Yang, B. Yang, P. Zhao, W. Yuan, P. W. Y. Chiu and L. Bian, Sci. Adv., 2021, 7, eabe8739 CrossRef CAS PubMed.
- H. Yuk, C. E. Varela, C. S. Nabzdyk, X. Mao, R. F. Padera, E. T. Roche and X. Zhao, Nature, 2019, 575, 169–174 CrossRef CAS PubMed.
- Y. Tang, G. Gong, X. He, M. Dai, M. Chen, B. Wang, Y. Wang, X. Wang and J. Guo, Adv. Healthcare Mater., 2023, 12, 2201578 CrossRef CAS PubMed.
- Z. Pan, Q. Q. Fu, M. H. Wang, H. L. Gao, L. Dong, P. Zhou, D. D. Cheng, Y. Chen, D. H. Zou, J. C. He, X. Feng and S. H. Yu, Nat. Commun., 2023, 14, 5378 CrossRef CAS PubMed.
- B. Guo, R. Dong, Y. Liang and M. Li, Nat. Rev. Chem., 2021, 5, 773–791 CrossRef CAS PubMed.
- C. Cui, C. Fan, Y. Wu, M. Xiao, T. Wu, D. Zhang, X. Chen, B. Liu, Z. Xu, B. Qu and W. Liu, Adv. Mater., 2019, 31, 1905761 CrossRef CAS PubMed.
- C. Cui, B. Liu, T. Wu, Y. Liu, C. Fan, Z. Xu, Y. Yao and W. Liu, J. Mater. Chem. A, 2022, 10, 1257–1269 RSC.
- B. K. Ahn, J. Am. Chem. Soc., 2017, 139, 10166–10171 CrossRef CAS PubMed.
- J. Yu, W. Wei, E. Danner, R. K. Ashley, J. N. Israelachvili and J. H. Waite, Nat. Chem. Biol., 2011, 7, 588–590 CrossRef CAS PubMed.
- Y. Li and Y. Cao, Nanoscale Adv., 2019, 1, 4246–4257 RSC.
- B. Wang, S. Wang, Q. Zhang, Y. Deng, X. Li, L. Peng, X. Zuo, M. Piao, X. Kuang, S. Sheng and Y. Yu, Acta Biomater., 2019, 96, 55–67 CrossRef CAS PubMed.
- S. Hu, X. Pei, L. Duan, Z. Zhu, Y. Liu, J. Chen, T. Chen, P. Ji, Q. Wan and J. Wang, Nat. Commun., 2021, 12, 1689 CrossRef CAS PubMed.
- A. B. Asha, Y. Chen and R. Narain, Chem. Soc. Rev., 2021, 50, 11668–11683 RSC.
- L. Xia, L. Yuan, K. Zhou, J. Zeng, K. Zhang, G. Zheng, Q. Fu, Z. Xia and Q. Fu, ACS Appl. Mater. Interfaces, 2023, 15, 22493–22505 CrossRef CAS PubMed.
- H. Li, Y. Jia, H. Peng and J. Li, Adv. Colloid Interface Sci., 2018, 252, 1–20 CrossRef CAS PubMed.
- Z. Zheng, S. Bian, Z. Li, Z. Zhang, Y. Liu, X. Zhai, H. Pan and X. Zhao, Carbohydr. Polym., 2020, 249, 116826 CrossRef CAS PubMed.
- S. Guo, Y. Ren, R. Chang, Y. He, D. Zhang, F. Guan and M. Yao, ACS Appl. Mater. Interfaces, 2022, 14, 34455–34469 CrossRef CAS PubMed.
- Y. Du, X. Chen, L. Li, H. Zheng, A. Yang, H. Li and G. Lv, Carbohydr. Polym., 2023, 318, 121049 CrossRef CAS PubMed.
- S. K. Boda, N. G. Fischer, Z. Ye and C. Aparicio, Biomacromolecules, 2020, 21, 4945–4961 CrossRef CAS PubMed.
- W. J. Zhang, B. K. Bao, F. Jiang, Y. Q. Zhang, R. J. Zhou, Y. Z. Lu, S. H. Lin, Q. N. Lin, X. Q. Jiang and L. Y. Zhu, Adv. Mater., 2021, 33, 2105667 CrossRef CAS PubMed.
- C. Zhang, B. Wu, Y. Zhou, F. Zhou, W. Liu and Z. Wang, Chem. Soc. Rev., 2020, 49, 3605–3637 RSC.
- F. Awaja, M. Gilbert, G. Kelly, B. Fox and P. J. Pigram, Prog. Polym. Sci., 2009, 34, 948–968 CrossRef CAS.
- C. Heinzmann, C. Weder and L. M. de Espinosa, Chem. Soc. Rev., 2016, 45, 342–358 RSC.
- A. Moeini, P. Pedram, P. Makvandi, M. Malinconico and G. Gomez d'Ayala, Carbohydr. Polym., 2020, 233, 115839 CrossRef CAS PubMed.
- W. Yang, X. Kang, X. Gao, Y. Zhuang, C. Fan, H. Shen, Y. Chen and J. Dai, Adv. Funct. Mater., 2023, 33, 2211340 CrossRef CAS.
- X. Zhang, Z. Li, P. Yang, G. Duan, X. Liu, Z. Gu and Y. Li, Mater. Horiz., 2021, 8, 145–167 RSC.
- Y. G. Chen, C. X. Li, Y. Zhang, Y. D. Qi, X. H. Liu, J. Feng and X. Z. Zhang, Mater. Horiz., 2022, 9, 2824–2834 RSC.
- S. Choi, J. Jeon, Y. Bae, Y. Hwang and S.-W. Cho, Adv. Funct. Mater., 2023, 33, 2303043 CrossRef CAS.
- M. Goldberg, A. Manzi, A. Birdi, B. Laporte, P. Conway, S. Cantin, V. Mishra, A. Singh, A. T. Pearson, E. R. Goldberg, S. Goldberger, B. Flaum, R. Hasina, N. R. London, G. L. Gallia, C. Bettegowda, S. Young, V. Sandulache, J. Melville, J. Shum, S. E. O’Neill, E. Aydin, A. Zhavoronkov, A. Vidal, A. Soto, M. J. Alonso, A. J. Rosenberg, M. W. Lingen, A. D’Cruz, N. Agrawal and E. Izumchenko, Nat. Commun., 2022, 13, 4829 CrossRef CAS PubMed.
- D. W. Zheng, W. W. Deng, W. F. Song, C. C. Wu, J. Liu, S. Hong, Z. N. Zhuang, H. Cheng, Z. J. Sun and X. Z. Zhang, Nat. Biomed. Eng., 2022, 6, 32–43 CrossRef CAS PubMed.
- A. Karim, A. Rehman, J. Feng, A. Noreen, E. Assadpour, M. S. Kharazmi, Z. Lianfu and S. M. Jafari, Adv. Colloid Interface Sci., 2022, 307, 102744 CrossRef CAS PubMed.
- L. Wang, F. Hao, S. Tian, H. Dong, J. Nie and G. Ma, Carbohydr. Polym., 2022, 291, 119574 CrossRef CAS PubMed.
- X. Dou, G. Li, S. Wang, D. Shao, D. Wang, X. Deng, Y. Zhu, P. Gao, J. Liu, N. Deng, C. Yuan and Q. Zhou, Int. J. Biol. Macromol., 2023, 244, 125273 CrossRef CAS PubMed.
- A. Chen, Y. Liu, T. Zhang, Y. Xiao, X. Xu, Z. Xu and H. Xu, Carbohydr. Polym., 2023, 304, 120460 CrossRef CAS PubMed.
- M. Okada, A. Nakai, E. S. Hara, T. Taguchi, T. Nakano and T. Matsumoto, Acta Biomater., 2017, 57, 404–413 CrossRef CAS PubMed.
- J. Yu, K. Wang, C. Fan, X. Zhao, J. Gao, W. Jing, X. Zhang, J. Li, Y. Li, J. Yang and W. Liu, Adv. Mater., 2021, 33, 2008395 CrossRef CAS PubMed.
- X. Dai, Y. Zhang, L. Gao, T. Bai, W. Wang, Y. Cui and W. Liu, Adv. Mater., 2015, 27, 3566–3571 CrossRef CAS PubMed.
- Q. M. Sun, K. Hong, L. Fan, X. Y. Zhang, T. Wu, J. Z. Du and Y. Q. Zhu, Chem. Mater., 2023, 35, 9208–9224 CrossRef CAS.
- S. Y. Lee, S. Jeon, Y. W. Kwon, M. Kwon, M. S. Kang, K. Y. Seong, T. E. Park, S. Y. Yang, D. W. Han, S. W. Hong and K. S. Kim, Sci. Adv., 2022, 8, eabn1646 CrossRef CAS PubMed.
- Z. Bao, C. Xian, Q. Yuan, G. Liu and J. Wu, Adv. Healthcare Mater., 2019, 8, 1900670 CrossRef PubMed.
- Z. Jiang, A. Bhaskaran, H. M. Aitken, I. C. G. Shackleford and L. A. Connal, Macromol. Rapid Commun., 2019, 40, 1900038 CrossRef PubMed.
- P. Song and H. Wang, Adv. Mater., 2020, 32, 1901244 CrossRef CAS PubMed.
- J. Zhu, Y. Li, W. Xie, L. Yang, R. Li, Y. Wang, Q. Wan, X. Pei, J. Chen and J. Wang, ACS Appl. Mater. Interfaces, 2022, 14, 53575–53592 CrossRef CAS PubMed.
- Y. Gao, X. Han, J. Chen, Y. Pan, M. Yang, L. Lu, J. Yang, Z. Suo and T. Lu, Proc. Natl. Acad. Sci. U. S. A., 2021, 118, e2103457118 CrossRef CAS PubMed.
- H. Montazerian, E. Davoodi, A. Baidya, M. Badv, R. Haghniaz, A. Dalili, A. S. Milani, M. Hoorfar, N. Annabi, A. Khademhosseini and P. S. Weiss, Chem. Soc. Rev., 2022, 51, 9127–9173 RSC.
- R. E. Webber, C. Creton, H. R. Brown and J. P. Gong, Macromolecules, 2007, 40, 2919–2927 CrossRef CAS.
- R. Yang, J. Huang, W. Zhang, W. Xue, Y. Jiang, S. Li, X. Wu, H. Xu, J. Ren and B. Chi, Carbohydr. Polym., 2021, 273, 118607 CrossRef CAS PubMed.
- Z. Ma, C. Bourquard, Q. Gao, S. Jiang, T. De, R. Huo, X. Li, Z. He, Z. Yang, G. Yang, Y. Wang, E. Lam, Z. H. Gao, O. Supponen and J. Li, Science, 2022, 377, 751–755 CrossRef CAS PubMed.
- Y. Yuan, S. Shen and D. Fan, Biomaterials, 2021, 276, 120838 CrossRef CAS PubMed.
- H. An, Z. Gu, L. Zhou, S. Liu, C. Li, M. Zhang, Y. Xu, P. Zhang and Y. Wen, Acta Biomater., 2022, 149, 126–138 CrossRef CAS PubMed.
- M. Staninec, G. W. Marshall, J. F. Hilton, D. H. Pashley, S. A. Gansky, S. J. Marshall and J. H. Kinney, J. Biomed. Mater. Res., 2002, 63, 342–345 CrossRef CAS PubMed.
- J. J. E. Choi, J. Zwirner, R. S. Ramani, S. Ma, H. M. Hussaini, J. N. Waddell and N. Hammer, Clin. Exp. Dent. Res., 2020, 6, 602–611 CrossRef PubMed.
- R. Huang, Z. Zhou, X. Lan, F. K. Tang, T. Cheng, H. Sun, K. Cham Fai Leung, X. Li and L. Jin, Mater. Today Bio., 2023, 18, 100507 CrossRef CAS PubMed.
- Z. Yun, D. Qin, F. Wei and L. Xiaobing, Nanotechnol. Rev., 2022, 11, 2433–2450 CrossRef.
- S. Wu, C. Xu, Y. Zhu, L. Zheng, L. Zhang, Y. Hu, B. Yu, Y. Wang and F. J. Xu, Adv. Funct. Mater., 2021, 31, 2103591 CrossRef CAS.
- J. Wei, L. Zhu, Q. Lu, G. Li, Y. Zhou, Y. Yang and L. Zhang, J. Controlled Release, 2023, 354, 337–353 CrossRef CAS PubMed.
- S. Tang and J. Zheng, Adv. Healthcare Mater., 2018, 7, 1701503 CrossRef PubMed.
- A. Ivask, A. ElBadawy, C. Kaweeteerawat, D. Boren, H. Fischer, Z. Ji, C. H. Chang, R. Liu, T. Tolaymat, D. Telesca, J. I. Zink, Y. Cohen, P. A. Holden and H. A. Godwin, ACS Nano, 2014, 8, 374–386 CrossRef CAS PubMed.
- B. Le Ouay and F. Stellacci, Nano Today, 2015, 10, 339–354 CrossRef CAS.
- P. Makvandi, C. Y. Wang, E. N. Zare, A. Borzacchiello, L. N. Niu and F. R. Tay, Adv. Funct. Mater., 2020, 30, 1910021 CrossRef CAS.
- N. Abed and P. Couvreur, Int. J. Antimicrob. Agents, 2014, 43, 485–496 CrossRef CAS PubMed.
- Z. Si, W. Zheng, D. Prananty, J. Li, C. H. Koh, E. T. Kang, K. Pethe and M. B. Chan Park, Chem. Sci., 2022, 13, 345–364 RSC.
- Y. Yang, Z. Cai, Z. Huang, X. Tang and X. Zhang, Polym. J., 2017, 50, 33–44 CrossRef.
- H. Fu, J. Yang, Z. Shen, Y. Zhang, S. Kuang, L. Li, Z. Lin and X. Shi, Biomater. Sci., 2023, 11, 3214–3226 RSC.
- H. Takahashi, E. T. Nadres and K. Kuroda, Biomacromolecules, 2017, 18, 257–265 CrossRef CAS PubMed.
- T. Phuangkaew, N. Booranabunyat, S. Kiatkamjornwong, P. Thanyasrisung and V. P. Hoven, Carbohydr. Polym., 2022, 277, 118882 CrossRef CAS PubMed.
- E. Escamilla-García, A. G. Alcázar-Pizaña, J. C. Segoviano-Ramírez, C. Del Angel-Mosqueda, A. P. López-Lozano, E. Cárdenas-Estrada, M. A. De La Garza-Ramos, C. E. Medina-De La Garza and M. Márquez, Int. J. Microbiol., 2017, 5924717 Search PubMed.
- Y. Li, Z. Xing, S. Wang, Y. Wang, Z. Wang and L. Dong, Acta Biomater., 2023, 158, 759–768 CrossRef CAS PubMed.
- X. Liu, Y. Hou, M. Yang, X. Xin, Y. Deng, R. Fu, X. Xiang, N. Cao, X. Liu, W. Yu, B. Yang and Y. Zhou, Adv. Healthcare Mater., 2023, 12, 2300890 CrossRef CAS PubMed.
- Y. Jiao, L. N. Niu, S. Ma, J. Li, F. R. Tay and J. H. Chen, Prog. Polym. Sci., 2017, 71, 53–90 CrossRef CAS PubMed.
- Z. Zhou, W. Hu, Z. Zhao and H. Fu, ACS Appl. Mater. Interfaces, 2022, 14, 46313–46323 CrossRef CAS PubMed.
- C. D. Fjell, J. A. Hiss, R. E. W. Hancock and G. Schneider, Nat. Rev. Drug Discovery, 2011, 11, 37–51 CrossRef PubMed.
- M. Zasloff, Nature, 2002, 415, 389–395 CrossRef CAS PubMed.
- L. T. Nguyen, E. F. Haney and H. J. Vogel, Trends Biotechnol., 2011, 29, 464–472 CrossRef CAS PubMed.
- J. Xuan, W. Feng, J. Wang, R. Wang, B. Zhang, L. Bo, Z. S. Chen, H. Yang and L. Sun, Drug Resist. Updates, 2023, 68, 100954 CrossRef CAS PubMed.
- H. Sun, Y. Hong, Y. Xi, Y. Zou, J. Gao and J. Z. Du, Biomacromolecules, 2018, 19, 1701–1720 CrossRef CAS PubMed.
- K. X. Yang, D. Q. Liu, R. X. Teng, C. Li, Z. Fan and J. Z. Du, ACS Biomater. Sci. Eng., 2023, 9, 1900–1908 CrossRef CAS PubMed.
- Y. Y. Yang, L. S. Chen, M. Sun, C. Y. Wang, Z. Fan and J. Z. Du, Chinese J. Polym. Sci., 2021, 39, 1412–1420 CrossRef CAS.
- C. Zhou, M. Wang, K. Zou, J. Chen, Y. Zhu and J. Z. Du, ACS Macro Lett., 2013, 2, 1021–1025 CrossRef CAS PubMed.
- J. Gao, M. Wang, F. Wang and J. Z. Du, Biomacromolecules, 2016, 17, 2080–2086 CrossRef CAS PubMed.
- Y. J. Xi, T. Song, S. Y. Tang, N. S. Wang and J. Z. Du, Biomacromolecules, 2016, 17, 3922–3930 CrossRef CAS PubMed.
- C. Zhou, Y. Yuan, P. Zhou, F. Wang, Y. Hong, N. Wang, S. Xu and J. Z. Du, Biomacromolecules, 2017, 18, 4154–4162 CrossRef CAS PubMed.
- L. Chen, Y. Hong, S. He, Z. Fan and J. Z. Du, Acta Phys.-Chim. Sin., 2021, 37, 1910059 Search PubMed.
- R. Teng, Y. Yang, Z. Zhang, K. Yang, M. Sun, C. Li, Z. Fan and J. Z. Du, Adv. Funct. Mater., 2023, 33, 2214454 CrossRef CAS.
- Y. Yang, Y. Qian, M. Zhang, S. Hao, H. Wang, Y. Fan, R. Liu, D. Xu and F. Wang, J. Mater. Sci. Technol., 2023, 133, 77–88 CrossRef CAS.
- M. Zasloff, Lancet, 2002, 360, 1116–1117 CrossRef PubMed.
- S. Mai, M. T. Mauger, L. Niu, J. B. Barnes, S. Kao, B. E. Bergeron, J. Ling and F. R. Tay, Acta Biomater., 2017, 49, 16–35 CrossRef CAS PubMed.
- T. Zhang, W. An, J. Sun, F. Duan, Z. Shao, F. Zhang, T. Jiang, X. Deng, C. Boyer and W. Gao, Nano Lett., 2022, 22, 8294–8303 CrossRef CAS PubMed.
- W. Jiang, Z. Xie, S. Huang, Q. Huang, L. Chen, X. Gao and Z. Lin, J. Oral Microbiol., 2023, 15, 2159375 CrossRef PubMed.
- P. J. Baker, R. T. Evans, J. Slots and R. J. Genco, J. Clin. Periodontol., 1985, 12, 201–208 CrossRef CAS PubMed.
- X. Ding, A. Wang, W. Tong and F. J. Xu, Small, 2019, 15, e1900999 CrossRef PubMed.
- W. Qiu, Y. Zhou, Z. Li, T. Huang, Y. Xiao, L. Cheng, X. Peng, L. Zhang and B. Ren, Biomed Res. Int., 2020, 5658212 CAS.
- R. Zhang, M. M. Jones, H. Moussa, M. Keskar, N. Huo, Z. Zhang, M. B. Visser, C. Sabatini, M. T. Swihart and C. Cheng, Biomater. Sci., 2019, 7, 287–295 RSC.
- M. Ye, Y. Zhao, Y. Wang, N. Yodsanit, R. Xie and S. Gong, Adv. Funct. Mater., 2020, 30, 2002655 CrossRef CAS.
- J. Tian, J. Zhang, J. Yang, L. Du, H. Geng and Y. Cheng, ACS Appl. Mater. Interfaces, 2017, 9, 18512–18520 CrossRef CAS PubMed.
- S. Borandeh, B. van Bochove, A. Teotia and J. Seppälä, Adv. Drug Delivery Rev., 2021, 173, 349–373 CrossRef CAS PubMed.
- Y. P. Liang, J. H. He and B. L. Guo, ACS Nano, 2021, 15, 12687–12722 CrossRef CAS PubMed.
- X. Zhao, H. Wu, B. L. Guo, R. N. Dong, Y. S. Qiu and P. X. Ma, Biomaterials, 2017, 122, 34–47 CrossRef CAS PubMed.
- W. X. Qi, N. J. Dong, L. L. Wu, X. Q. Zhang, H. Li, H. Wu, N. Ward, J. Yu, H. Liu, J. Wang, X. Y. Deng and R. C. Zhao, Bioact. Mater., 2023, 23, 53–68 CAS.
- W. Zheng, Y. Hao, D. Wang, H. Huang, F. Guo, Z. Sun, P. Shen, K. Sui, C. Yuan and Q. Zhou, Carbohydr. Polym., 2021, 272, 118493 CrossRef CAS PubMed.
- X. Tan, S. Liu, X. Hu, R. Zhang, X. Su, R. Qian, Y. Mai, Z. Xu, W. Jing, W. Tian and L. Xie, ACS Appl. Mater. Interfaces, 2022, 15, 391–406 CrossRef PubMed.
- L. Xin, C. Zhang, J. Chen, Y. Jiang, Y. Liu, P. Jin, X. Wang, G. Wang and P. Huang, ACS Appl. Mater. Interfaces, 2022, 14, 47420–47431 CrossRef CAS PubMed.
- L. Chen, M. Peng, J. Zhou, X. Hu, Y. Piao, H. Li, R. Hu, Y. Li, L. Shi and Y. Liu, Adv. Mater., 2023, 35, 2301664 CrossRef CAS PubMed.
- S. Kansiz and Y. M. Elcin, Adv. Colloid Interface Sci., 2023, 317, 102930 CrossRef CAS PubMed.
- Y. Q. Zhu, B. Yang, S. Chen and J. Z. Du, Prog. Polym. Sci., 2017, 64, 1–22 CrossRef CAS.
- D. B. Wright, J. P. Patterson, A. Pitto-Barry, A. Lu, N. Kirby, N. C. Gianneschi, C. Chassenieux, O. Colombani and R. K. O’Reilly, Macromolecules, 2015, 48, 6516–6522 CrossRef CAS.
- L. Bonetti, A. Caprioglio, N. Bono, G. Candiani and L. Altomare, Biomater. Sci., 2023, 11, 2699–2710 RSC.
- A. Nasajpour, S. Ansari, C. Rinoldi, A. S. Rad, T. Aghaloo, S. R. Shin, Y. K. Mishra, R. Adelung, W. Swieszkowski, N. Annabi, A. Khademhosseini, A. Moshaverinia and A. Tamayol, Adv. Funct. Mater., 2018, 28, 1703437 CrossRef.
- Y. Zeng, Y. Gao, L. He, W. Ge, J. Liu, Y. Yu and X. Xie, Mater. Today Bio, 2023, 22, 100782 CrossRef CAS PubMed.
- G. Guan, Q. Zhang, Z. Jiang, J. Liu, J. Wan, P. Jin and Q. Lv, Small, 2022, 18, 2203064 CrossRef CAS PubMed.
- X. J. Li, Y. Li, Y. Meng, X. Q. Pu, J. W. Qin, R. Xie, W. Wang, Z. Liu, L. Jiang, X. J. Ju and L. Y. Chu, Biomater. Adv., 2022, 139, 213001 CrossRef CAS PubMed.
- Y. Meng, X. J. Li, Y. Li, T. Y. Zhang, D. Liu, Y. Q. Wu, F. F. Hou, L. Ye, C. J. Wu, X. D. Feng, X. J. Ju and L. Jiang, ACS Appl. Mater. Interfaces, 2023, 15, 13892–13906 CAS.
- Z. Yin, X. Zhang, Y. Bai, Y. Yang, B. Liu and Z. Fan, New J. Chem., 2022, 46, 7279–7289 RSC.
- S. A. Eming, T. A. Wynn and P. Martin, Science, 2017, 356, 1026–1030 CrossRef CAS PubMed.
- I. Tabas and C. K. Glass, Science, 2013, 339, 166–172 CrossRef CAS PubMed.
- L. Ferrucci and E. Fabbri, Nat. Rev. Cardiol., 2018, 15, 505–522 CrossRef CAS PubMed.
- G. Zhang, L. Ma, L. Bai, M. Li, T. Guo, B. Tian, Z. He and Q. Fu, J. Controlled Release, 2021, 334, 114–126 CrossRef CAS PubMed.
- E. G. Eltay and T. Van Dyke, Pharmacol. Ther., 2023, 247, 108453 CrossRef CAS PubMed.
- M. O. Freire and T. E. Van Dyke, J. Periodontol., 2013, 63, 149–164 CrossRef PubMed.
- B. P. Wallner, R. J. Mattaliano, C. Hession, R. L. Cate, R. Tizard, L. K. Sinclair, C. Foeller, E. P. Chow, J. L. Browning, K. L. Ramachandran and R. B. Pepinsky, Nature, 1986, 320, 77–81 CrossRef CAS PubMed.
- S. Shahinfar and H. Maibach, Clin. Pharmacokinet., 2023, 62, 1189–1199 CrossRef CAS PubMed.
- C. Padula, I. Telò, A. Di Ianni, S. Pescina, S. Nicoli and P. Santi, Eur. J. Pharm. Sci., 2018, 115, 233–239 CrossRef CAS PubMed.
- H. S. Seon Woo, H. J. Kim, J. Y. Roh and J. H. Park, Macromol. Res., 2019, 27, 282–289 CrossRef CAS.
- H. Hamishehkar, A. Nokhodchi, S. Ghanbarzadeh and M. Kouhsoltani, Adv. Pharm. Bull., 2015, 5, 277–282 CrossRef CAS PubMed.
- X. Qu, X. Guo, T. Zhu, Z. Zhang, W. Wang and Y. Hao, Front. Bioeng. Biotech., 2023, 11, 1203709 CrossRef PubMed.
- D. W. Cain and J. A. Cidlowski, Nat. Rev. Immunol., 2017, 17, 233–247 CrossRef CAS PubMed.
- J. Li, M. Zhao, X. Xiang, Q. He and R. Gui, J. Nanobiotechnol., 2021, 19, 417 CrossRef CAS PubMed.
- Z. Ju, M. Li, J. Xu, D. C. Howell, Z. Li and F. E. Chen, Acta Pharm. Sin. B, 2022, 12, 2790–2807 CrossRef CAS PubMed.
- J. Luan, R. Li, W. Xu, H. Sun, Q. Li, D. Wang, S. Dong and J. Ding, Acta Pharm. Sin. B, 2023, 13, 2310–2333 CrossRef CAS PubMed.
- R. Altman, B. Bosch, K. Brune, P. Patrignani and C. Young, Drugs, 2015, 75, 859–877 CrossRef CAS PubMed.
- Z. Zhang, Q. Zhang, S. Gao, H. Xu, J. Guo and F. Yan, Acta Biomater., 2023, 166, 254–265 CrossRef CAS PubMed.
- J. Fu, X. Zhu, H. Dan, Y. Zhou, C. Liu, F. Wang, Y. Li, N. Liu, Q. Chen, Y. Xu, X. Zeng and L. Jiang, Oral Surg., Oral Med., Oral Pathol. Oral Radiol., 2012, 113, 638–643 CrossRef PubMed.
- A. Abouzid, A. Y. Moustafa, N. Allcock, M. Najlah, A. Elhissi, C. W. Stanley, W. Ahmed, P. Seville, S. Crean, R. T. Forbes and M. A. Elsawy, J. Drug Delivery Sci. Technol., 2023, 79, 104052 CrossRef CAS.
- R. Mata, Y. Yao, W. Cao, J. Ding, T. Zhou, Z. Zhai and C. Gao, Research, 2021, 2021, 4189516 CrossRef CAS PubMed.
- K. L. Spiller, S. Nassiri, C. E. Witherel, R. R. Anfang, J. Ng, K. R. Nakazawa, T. Yu and G. Vunjak-Novakovic, Biomaterials, 2015, 37, 194–207 CrossRef CAS PubMed.
- F. O. Martinez and S. Gordon, F1000Prime Rep., 2014, 6, 13 Search PubMed.
- N. Wang, H. Liang and K. Zen, Front. Immunol., 2014, 5, 614 Search PubMed.
- A. Mantovani, A. Sica, S. Sozzani, P. Allavena, A. Vecchi and M. Locati, Trends Immunol., 2004, 25, 677–686 CrossRef CAS PubMed.
- D. Brenner, H. Blaser and T. W. Mak, Nat. Rev. Immunol., 2015, 15, 362–374 CrossRef CAS PubMed.
- E. E. Friedrich, L. T. Sun, S. Natesan, D. O. Zamora, R. J. Christy and N. R. Washburn, J. Biomed. Mater. Res., Part A, 2014, 102, 1527–1536 CrossRef PubMed.
- V. Andretto, S. Dusi, S. Zilio, M. Repellin, D. Kryza, S. Ugel and G. Lollo, Adv. Drug Delivery Rev., 2023, 201, 115080 CrossRef CAS PubMed.
- S. Iqbal, X. Du, J. Wang, H. Li, Y. Yuan and J. Wang, Nano Res., 2018, 11, 2872–2884 CrossRef CAS.
- J. G. Edmans, B. Ollington, H. E. Colley, M. E. Santocildes-Romero, L. Siim Madsen, P. V. Hatton, S. G. Spain and C. Murdoch, J. Controlled Release, 2022, 350, 146–157 CrossRef CAS PubMed.
- X. Ge, J. Hu, Y. Peng, Z. Zeng, D. He, X. Li, Y. Chen, G. Luo, J. Deng, Z. Xu and S. He, Biomaterials, 2023, 301, 122254 CrossRef CAS PubMed.
- L. A. J. O’Neill and D. G. Hardie, Nature, 2013, 493, 346–355 CrossRef PubMed.
- X. T. He, X. Li, Y. Xia, Y. Yin, R. X. Wu, H. H. Sun and F. M. Chen, Acta Biomater., 2019, 88, 162–180 CrossRef CAS PubMed.
- X. Wen, K. Xi, Y. Tang, J. Bian, Y. Qin, W. Xiao, T. Pan, X. Cheng, Z. Ge and W. Cui, Small, 2023, 19, 2207030 CrossRef CAS PubMed.
- E. Oppong and A. C. B. Cato, in Glucocorticoid Signaling: From Molecules to Mice to Man, ed. J. C. Wang and C. Harris, Springer, New York, NY, 2015, pp. 217–233 Search PubMed.
- S. Duarah, M. Sharma, S. Chen, T. K. Proft, J. Loh and J. Wen, Int. J. Pharm., 2023, 635, 122690 CrossRef CAS PubMed.
- R. Hayashi, H. Wada, K. Ito and I. M. Adcock, Eur. J. Pharmacol., 2004, 500, 51–62 CrossRef CAS PubMed.
- V. M. Gouveia, L. Rizzello, C. Nunes, A. Poma, L. Ruiz-Perez, A. Oliveira, S. Reis and G. Battaglia, Pharmaceutics, 2019, 11, 614 CrossRef CAS PubMed.
- M. M. Alvarez, J. C. Liu, G. Trujillo de Santiago, B. H. Cha, A. Vishwakarma, A. M. Ghaemmaghami and A. Khademhosseini, J. Controlled Release, 2016, 240, 349–363 CrossRef CAS PubMed.
- Y. Y. Xu, Y. F. Luo, Z. Z. Weng, H. C. Xu, W. Zhang, Q. Li, H. J. Liu, L. B. Liu, Y. M. Wang, X. X. Liu, L. Liao and X. L. Wang, ACS Nano, 2023, 17, 18732–18746 CrossRef CAS PubMed.
- M. Hashemzaei, C. Mamoulakis, K. Tsarouhas, G. Georgiadis, G. Lazopoulos, A. Tsatsakis, E. Shojaei Asrami and R. Rezaee, Food Chem. Toxicol., 2020, 143, 111521 CrossRef CAS PubMed.
- F. Taghizadeh, F. Mehryab, S. A. Mortazavi, S. Rabbani and A. Haeri, Carbohydr. Polym., 2023, 318, 121068 CrossRef CAS PubMed.
- E. C. Cheung and K. H. Vousden, Nat. Rev. Cancer, 2022, 22, 280–297 CrossRef CAS PubMed.
- B. N. Salman, S. Darvish, A. Goriuc, S. Mazloomzadeh, M. Hossein Poor Tehrani and I. Luchian, Antioxidants, 2021, 10, 1540 CrossRef CAS PubMed.
- R. Waddington, R. Moseley and G. Embery, Oral Dis., 2000, 6, 138–151 CrossRef CAS PubMed.
- E. Chen, T. Wang, Y. Tu, Z. Sun, Y. Ding, Z. Gu and S. Xiao, J. Mater. Chem. B, 2023, 11, 482–499 RSC.
- X. He, J. Xue, L. Shi, Y. Kong, Q. Zhan, Y. Sun, Q. Zhang, S. Ramakrishna and Y. Dai, Mater. Today Nano, 2022, 17, 100149 CrossRef CAS.
- J. Zhang, Y. Fu, P. Yang, X. Liu, Y. Li and Z. Gu, Adv. Mater. Interfaces, 2020, 7, 2000632 CrossRef CAS.
- Z. Zhang, Y. Pan, Z. Guo, X. Fan, Q. Pan, W. Gao, K. Luo, Y. Pu and B. He, Bioact. Mater., 2024, 33, 71–84 CAS.
- X. Qiu, Y. Yu, H. Liu, X. Li, W. Sun, W. Wu, C. Liu and L. Miao, Acta Biomater., 2021, 135, 593–605 CrossRef CAS PubMed.
- M. C. Foti, J. Pharm. Pharmacol., 2010, 59, 1673–1685 CrossRef PubMed.
- J. Shi, Y. Zhang, X. Zhang, R. Chen, J. Wei, J. Hou, B. Wang, H. Lai and Y. Huang, J. Nanobiotechnol., 2021, 19, 1–13 CrossRef PubMed.
- R. Yuan, Y. Li, S. Han, X. Chen, J. Chen, J. He, H. Gao, Y. Yang, S. Yang and Y. Yang, ACS Cent. Sci., 2022, 8, 10–21 CrossRef CAS PubMed.
- S. Xu, B. Hu, T. Dong, B. Y. Chen, X. J. Xiong, L. J. Du, Y. L. Li, Y. L. Chen, G. C. Tian, X. B. Bai, T. Liu, L. J. Zhou, W. C. Zhang, Y. Liu, Q. F. Ding, X. Q. Zhang and S. Z. Duan, Adv. Healthcare Mater., 2023, 12, 2203337 CrossRef CAS PubMed.
- J. M. MatÉs, C. Pérez-Gómez and I. N. De Castro, Clin. Biochem., 1999, 32, 595–603 CrossRef PubMed.
- X. Bao, J. Zhao, J. Sun, M. Hu and X. Yang, ACS Nano, 2018, 12, 8882–8892 CrossRef CAS PubMed.
- M. Tian, G. Chen, J. Xu, Y. Lin, Z. Yi, X. Chen, X. Li and S. Chen, Chem. Eng. J., 2022, 433, 132197 CrossRef CAS.
- S. Ishii, J. Kaneko and Y. Nagasaki, Macromolecules, 2015, 48, 3088–3094 CrossRef CAS.
- M. Saita, J. Kaneko, T. Sato, S. S. Takahashi, S. Wada Takahashi, R. Kawamata, T. Sakurai, M. C. Lee, N. Hamada, K. Kimoto and Y. Nagasaki, Biomaterials, 2016, 76, 292–301 CrossRef CAS PubMed.
- C. Cui, L. Mei, D. Wang, P. Jia, Q. Zhou and W. Liu, Nat. Commun., 2023, 14, 7707 CrossRef CAS PubMed.
- R. Iglesias-Bartolome, A. Uchiyama, A. A. Molinolo, L. Abusleme, S. R. Brooks, J. L. Callejas-Valera, D. Edwards, C. Doci, M. L. Asselin-Labat, M. W. Onaitis, N. M. Moutsopoulos, J. Silvio Gutkind and M. I. Morasso, Sci. Transl. Med., 2018, 10, eaap8798 CrossRef PubMed.
- Y. K. Kang, K. Kwon, J. S. Ryu, H. N. Lee, C. Park and H. J. Chung, Bioconjugate Chem., 2017, 28, 957–967 CrossRef CAS PubMed.
Footnote |
| † These authors equally contributed to this work. |
| This journal is © The Royal Society of Chemistry 2024 |