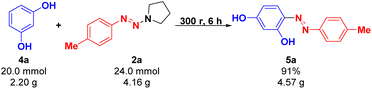Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Solvent-free mechanochemical synthesis of azo dyes†
Lin
Zhang
a,
Qinglang
Song
a,
Yanxian
Wang
a,
Rui
Chen
a,
Yu
Xia
 a,
Bin
Wang
a,
Bin
Wang
 a,
Weiwei
Jin
a,
Weiwei
Jin
 a,
Shaofeng
Wu
a,
Ziren
Chen
a,
Azhar
Iqbal
*b,
Chenjiang
Liu
a,
Shaofeng
Wu
a,
Ziren
Chen
a,
Azhar
Iqbal
*b,
Chenjiang
Liu
 *a and
Yonghong
Zhang
*a and
Yonghong
Zhang
 *a
*a
aState Key Laboratory of Chemistry and Utilization of Carbon Based Energy Resources, Key Laboratory of Oil and Gas Fine Chemicals, Ministry of Education & Xinjiang Uygur Autonomous Region, Urumqi Key Laboratory of Green Catalysis and Synthesis Technology, College of Chemistry, Xinjiang University, Urumqi 830017, P. R. China. E-mail: zhzhzyh@126.com; pxylcj@126.com
bDepartment of Chemistry, Bacha Khan University, Charsadda, Pakistan. E-mail: azhariqbal@bkuc.edu.pk
First published on 9th August 2024
Abstract
An efficient diazotization of phenolic compounds with aryltriazenes is herein demonstrated by employing ball milling under catalyst-, promoter- and solvent-free conditions. The present protocol offers several advantages including mild conditions, good selectivity and high yields, simple operation and practical gram-scale synthesis. Overall, this novel strategy significantly improves the reaction efficiency, simplifies purification procedures of the diazotization reaction and provides potential for the industrial preparation of azo dyes.
Introduction
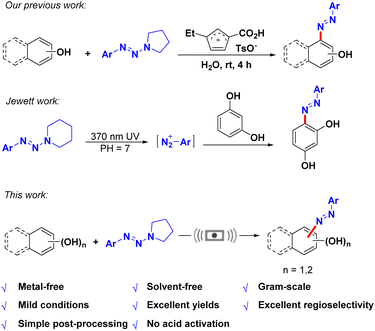
Mechanochemistry with solvent-free and environmentally friendly characteristics is one of the most promising alternatives to traditional liquid-phase-based reactions, demonstrating epoch-making significance in the realization of different types of chemistry.1 Most organic reactions are carried out in volatile organic solvents, which caused a great proportion of waste generation in a laboratory or industry.2 However, poor solubility of the reactants in organic solvents also affects the efficiency of the reaction.2 In addition, solvents may be involved in the reaction to generate byproducts which decrease the efficiency and selectivity of the reaction.3–5 Therefore, the development of environmentally friendly or solvent-free reactions is of great interest. In recent years, ball milling has been widely used in organic synthesis,6 such as Suzuki–Miyaura cross-coupling,7,8 Aza-Morita-Baylis–Hillman reaction,9 azoxy- and azo-arenes synthesis10,11 and so on.Azo dyes are organic compounds that contain –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– groups,12 which were the most common dyes (more than 3000 different varieties) used in the textile industry compared to natural dyes due to their low cost, versatility and colouring ability.13 They account for approximately 70% of the total amount of dyes, and besides the textile industry, azo dyes are also intensively used in the cosmetics, leather, paper, food and pharmaceutical industries.14 Consequently, various methods were developed for synthesis of azo dyes. Typically, most of these compounds were prepared from aromatic primary amine via the diazotization reaction with nitrite to produce aryl diazonium salts, followed by coupling with nucleophiles.14–19 In particular, environmentally friendly protocols for the synthesis of azo dyes via the diazotization and coupling of diazonium salts with phenols or aromatic amines have attracted much attention in recent years, such as: (a) the coupling reaction of resin supported diazonium salts with nucleophiles;20 (b) gas–solid diazotization of 3-aminopyrazolo[3,4-b]pyridine/solid–solid cascade coupling with nucleophiles;21 (c) in situ diazotization of p-nitroaniline with sodium nitrite and coupled with N,N-diethylaniline in supercritical carbon dioxide under mineral acid-free conditions;22 (d) one pot diazo coupling under microwave irradiation;23 (e) the sonochemical method of diazotization and coupling;24 (f) continuous flow diazotization and coupling in a microreactor system,25etc. However, all of the above methods have one or more disadvantages, such as the use of solvents in the reaction. The solvent becomes liquid waste after the reaction, resulting in environmental pollution. In addition, some products formed are poorly soluble in the solvent, thus making the reaction and post-treatment process even more complicated. Nowadays, green chemistry and sustainable approaches for organic synthesis have gained tremendous importance due to environmental and ecosystem based concerns. Mechanochemistry assisted organic reactions are proven to be energy efficient, green,26 fast, solvent-free, and reproducible, show good selectivity, and lead to higher yields.27,28 Although diazonium salts are common reagents for the synthesis of azo dyes, however, their potentially explosive hazards and unstable nature highly restricted their application, especially for the large scale synthesis of azo dyes. Therefore, developing stable and mild alternative reagents to replace aryl diazonium salts is highly desirable. Recently, as the masked surrogates of corresponding diazonium salts, aryltriazenes have been widely applied in a variety of synthetic transformations due to their good stability, multiple reactive sites, and mild reaction conditions.29–31 In particular, they were also used for the efficient synthesis of azo dyes via diazotization of phenolic compounds. In 2018, our group developed an ionic liquid promoted diazotization of naphthols with aryltriazenes for the synthesis of azo dyes.19 In 2021, Jewett's group reported a UV light induced diazotization of phenol with aryltriazenes for protein modification.32 Herein, we developed a novel metal-, promoter-, catalyst- and solvent-free method for the synthesis of various azo dyes in excellent yields and regioselectivity using ball milling with aryltriazenes as diazotization reagents (Scheme 1).
N– groups,12 which were the most common dyes (more than 3000 different varieties) used in the textile industry compared to natural dyes due to their low cost, versatility and colouring ability.13 They account for approximately 70% of the total amount of dyes, and besides the textile industry, azo dyes are also intensively used in the cosmetics, leather, paper, food and pharmaceutical industries.14 Consequently, various methods were developed for synthesis of azo dyes. Typically, most of these compounds were prepared from aromatic primary amine via the diazotization reaction with nitrite to produce aryl diazonium salts, followed by coupling with nucleophiles.14–19 In particular, environmentally friendly protocols for the synthesis of azo dyes via the diazotization and coupling of diazonium salts with phenols or aromatic amines have attracted much attention in recent years, such as: (a) the coupling reaction of resin supported diazonium salts with nucleophiles;20 (b) gas–solid diazotization of 3-aminopyrazolo[3,4-b]pyridine/solid–solid cascade coupling with nucleophiles;21 (c) in situ diazotization of p-nitroaniline with sodium nitrite and coupled with N,N-diethylaniline in supercritical carbon dioxide under mineral acid-free conditions;22 (d) one pot diazo coupling under microwave irradiation;23 (e) the sonochemical method of diazotization and coupling;24 (f) continuous flow diazotization and coupling in a microreactor system,25etc. However, all of the above methods have one or more disadvantages, such as the use of solvents in the reaction. The solvent becomes liquid waste after the reaction, resulting in environmental pollution. In addition, some products formed are poorly soluble in the solvent, thus making the reaction and post-treatment process even more complicated. Nowadays, green chemistry and sustainable approaches for organic synthesis have gained tremendous importance due to environmental and ecosystem based concerns. Mechanochemistry assisted organic reactions are proven to be energy efficient, green,26 fast, solvent-free, and reproducible, show good selectivity, and lead to higher yields.27,28 Although diazonium salts are common reagents for the synthesis of azo dyes, however, their potentially explosive hazards and unstable nature highly restricted their application, especially for the large scale synthesis of azo dyes. Therefore, developing stable and mild alternative reagents to replace aryl diazonium salts is highly desirable. Recently, as the masked surrogates of corresponding diazonium salts, aryltriazenes have been widely applied in a variety of synthetic transformations due to their good stability, multiple reactive sites, and mild reaction conditions.29–31 In particular, they were also used for the efficient synthesis of azo dyes via diazotization of phenolic compounds. In 2018, our group developed an ionic liquid promoted diazotization of naphthols with aryltriazenes for the synthesis of azo dyes.19 In 2021, Jewett's group reported a UV light induced diazotization of phenol with aryltriazenes for protein modification.32 Herein, we developed a novel metal-, promoter-, catalyst- and solvent-free method for the synthesis of various azo dyes in excellent yields and regioselectivity using ball milling with aryltriazenes as diazotization reagents (Scheme 1).
Results and discussion
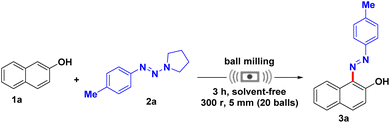
As shown in Table 1, at the outset of the investigation, β-naphthol (1a) (1.0 mmol) and p-methylphenyltriazene (2a) (1.2 mmol) were employed as model substrates, with stainless steel balls (5.0 mm in diameter, 20 balls) in a stainless steel jar (50 mL) to optimize the reaction. To our delight, the desired product was obtained in 92% yield when the reaction was milled at room temperature at 300 rpm for 3 hours (Table 1, entry 1). We then tried to add a small amount of solvents as auxiliary grinding agents, such as acetonitrile, methanol and water, and the results showed that the solvents had almost no effect on the reaction (Table 1, entries 2–4). Then, the influence of the reactant ratio was further studied. A slightly lower yield was afforded when the dosage of p-methylphenyltriazine (2a) was reduced to 1.1 mmol (Table 1, entry 5). The size and number of grinding balls were then investigated; when the number of grinding balls was reduced to 6 and 12, the yield of azo dye was significantly reduced to 40% and 77%, respectively (Table 1, entries 6 and 7). The number of grinding balls with larger size was also examined, such as 1, 3, 6 and 10 large balls which were used to replace 20 small balls in the reaction, and the results showed that 3 large balls with a diameter of 8 mm gave better yields up to 90%, while the others only afforded moderate yields (47–68%) (Table 1, entries 8–11). In the study of the reaction time, decreasing the reaction time resulted in a lower product yield (Table 1, entry 12); however, prolonging the reaction time did not significantly improve yield (Table 1, entry 13). Subsequently, the effect of speed was also studied. Unfortunately, 200 rpm and 400 rpm only afforded a trace amount of the product (Table 1, entries 14 and 15), because low speeds do not provide enough energy to activate the substrate and high speed splashes the substrates onto the lid and walls of the jar. In addition, on replacing stainless steel balls with zirconia balls, 90% of 3a was obtained, which confirmed that the metal was not involved in this transformation (Table 1, entry 16). In order to verify that the ball milling provided the energy, rather than simply mixing the feedstock, we mixed the reactants without providing a rotational speed and found that there was no product formed (Table 1, entry 17). Furthermore, the superiority of the mechanochemical synthesis was demonstrated by comparing it with the reaction in the liquid phase, and only trace 3a was observed when the reaction was conducted in solution, which confirmed that ball milling played an important role in this transformation (Table 1, entry 18). Finally, the optimal reaction conditions are therefore as follows: β-naphthol (1a) (1.0 mmol) and p-methylphenyltriazene (2a) (1.2 mmol) together with stainless steel balls (5 mm in diameter, 20 balls) were introduced into a stainless steel jar (50 mL) and milled in the absence of solvent at 300 rpm in air for 3 h.| Entry | Variation from the “standard conditions” | Yieldb (%) |
|---|---|---|
| a Reactions condition: 1a (1.0 mmol), 2a (1.2 mmol), and 5 mm stainless steel balls were added to a 50 mL stainless steel tank, 300 r for 3 h. b Isolated yield. c 1a (1.0 mmol), 2a (1.2 mmol) and MeCN (1 mL) stirred in a 10 mL glass tube for 3 h at room temperature. | ||
| 1 | None | 92 |
| 2 | 100 μL MeCN as an auxiliary grinding agent | 90 |
| 3 | 100 μL MeOH as an auxiliary grinding agent | 89 |
| 4 | 100 μL H2O as an auxiliary grinding agent | 91 |
| 5 | 1.1 mmol 2a | 89 |
| 6 | 5 mm (6 balls) instead of 5 mm (20 balls) | 40 |
| 7 | 5 mm (12 balls) instead of 5 mm (20 balls) | 77 |
| 8 | 8 mm (1 balls) instead of 5 mm (20 balls) | 47 |
| 9 | 8 mm (3 balls) instead of 5 mm (20 balls) | 90 |
| 10 | 8 mm (6 balls) instead of 5 mm (20 balls) | 68 |
| 11 | 8 mm (10 balls) instead of 5 mm (20 balls) | 63 |
| 12 | 2 hours instead of 3 hours | 47 |
| 13 | 4 hours instead of 3 hours | 93 |
| 14 | 200 rpm instead of 300 rpm | Trace |
| 15 | 400 rpm instead of 300 rpm | Trace |
| 16 | Zirconia balls instead of stainless steel balls | 90 |
| 17 | Mixing without rotational speed | n.r. |
| 18c | Solution reaction | Trace |
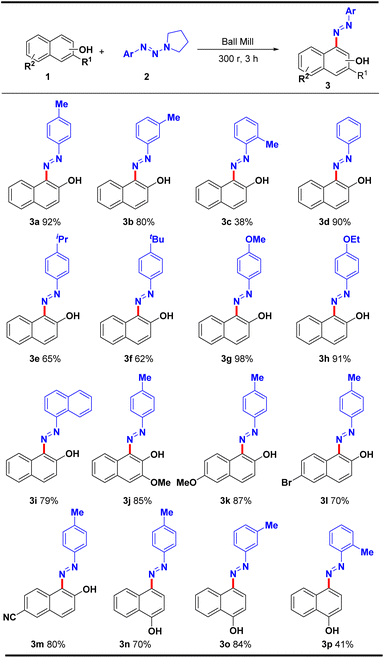
Once the reaction conditions were optimized, we performed substrate generalization studies by using β-naphthol, α-naphthol and resorcinol derivatives with aryltriazenes, respectively. First, the substrate range of β-naphthol with aryltriazenes was examined (Table 2, 3a–3i). The reaction was generally effective for electron-donating substituents, most of which can afford moderate to good yields. Both para- and meta-methyl substituted aryltriazenes are compatible with the reaction, and the desired products can be obtained in 92% and 80% yields (3a and 3b), respectively. However, the ortho-substituted substrate reacted less effectively, with a yield of only 38% (3c), which is possibly due to the steric hindrance effect. To our delight, non-substituted phenyltriazene (2d), 4-iPr (2e) and 4-tBu (2f) substituted aryltriazenes delivered good to excellent yields. Furthermore, excellent yields of up to 98% and 91% were obtained when 4-OMe and 4-OEt substituted aryltriazenes were subjected to the reaction (3g and 3h). Notably, the polycyclic aryltriazene also showed good compatibility, and the target product 3i can be obtained in 79% yield. Then, we examine the universality of substituted β-naphthols, such as 3-methoxy-2-naphthol (1j) and 6-methoxy-2-naphthol (1k), which smoothly reacted and obtained the corresponding products in 85% and 87% yields, respectively. Fortunately, 2-naphthols bearing electron-withdrawing substituents (bromo & cyano) were also tolerated, and target products 3l and 3m can be afforded in good yield. Finally, the reactivity of α-naphthol towards substituted aryltriazenes was investigated, such as p-Me (2n), m-Me (2o) and o-Me (2p) substituted phenyltriazenes, which were also tolerated, and the corresponding products 3n–3p were generated in 41% to 84% yield.
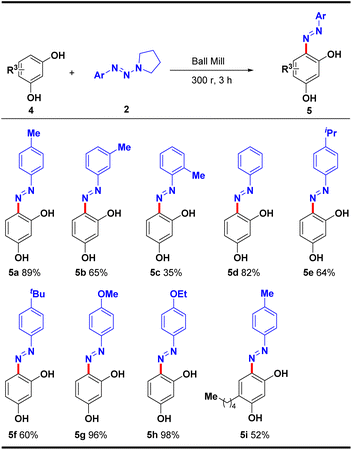
The substrate scope of various aryltriazenes with resorcinol under the established optimal conditions was next evaluated (Table 3). The results showed that various aryltriazenes bearing electron-donating groups (4-Me, 4-iPr, 4-tBu, 4-OMe and 4-OEt) on the benzene ring and non-substituted substrates all reacted smoothly with resorcinol 4a. Besides sterically hindered ortho-methyl substituted triazene (5c) that afforded 35% yield, the desired products 5a–5h were obtained in moderate to good yields (60–98%). In addition, substituted resorcinol compounds such as (E)-4-pentyl-6-(p-tolyldiazenyl)benzene-1,3-diol (4i) also achieved moderate yields. Notably, due to the poor solubility of resorcinol products in low polar solvents such as petroleum ether or hexane, they can be easily isolated and purified by simple filtering and washing with hexane after the reaction.
To demonstrate the practical utility of this mechanochemical synthesis method, the reaction of resorcinol 4a and p-methylphenyltriazene 2a was performed at the 20 mmol scale (Scheme 2). The results showed that this protocol could be successfully performed on a gram scale, and the desired product 5a was obtained smoothly in 91% yield at room temperature in 6 hours of ball milling under solvent-free conditions. It is worth mentioning that the target product (E)-4-(p-tolyldiazen-yl)benzene-1,3-diol (5a) was almost insoluble in petroleum ether (PE) and ethyl acetate (EA), while the unreacted resorcinol 4a and p-methylphenyltriazene 2a are soluble in the above organic solvents. Therefore, as shown in Fig. 1, after the completion of the reaction, the product can be easily isolated from the reaction mixture via washing with petroleum ether (PE) (3 × 15 mL) and water (3 × 15 mL), respectively.
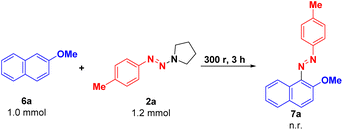
To gain insight into the reaction mechanism, a control experiment was carried out (Scheme 3). Under standard conditions, 2-methoxynaphthalene 6a was used to replace β-naphthol 1a to react with p-methylphenyltriazene 2a. As expected, no corresponding product 7a was obtained, suggesting that the weak acidity of the hydroxyl group of β-naphthol 1a plays a role in activating the aryltriazene to provide an aryldiazonium cation for further diazotization.
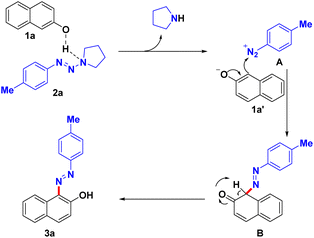
Based on literature reports23,29 and mechanism validation experiments, a plausible reaction mechanism is presented in Scheme 4. First, aryltriazene 2a undergoes N2–N3 cleavage via hydrogen bond activation in the presence of weak acid β-naphthol to form an electron donor–acceptor (EDA) complex of aryl diazonium cation and naphthalen-2-olate. Subsequently, the dearomatized intermediate B was obtained through electrophilic addition of the aryldiazonium cation to naphthalen-2-olate. Finally, product 3a was produced through 1,3-proton transfer and restores aromaticity.
Conclusions
In conclusion, the ball milling method for the synthesis of azo dyes was developed, which avoids heating and the use of any solvents and metal catalysts. The reactions were carried out with aryltriazenes that served as reactive, stable diazotization reagents and phenolic compounds as both nucleophiles and as Brønsted acids to activate aryltriazenes for the release of diazonium cation. The present method is simple, easy to scale up, and environmentally friendly and does not require any solvents. The present strategy provides a new insight into the industrial preparation of azo dyes under mild conditions.Data availability
All experimental and characterization data and detailed experimental procedures are available in the published article and ESI.†Author contributions
Lin Zhang: conceptualization, data curation, formal analysis, investigation, methodology, writing – original draft, writing – review & editing. Qinglang Song: data curation, formal analysis, investigation. Yanxian Wang: data curation, investigation, methodology. Rui Chen: data curation, investigation, methodology. Yu Xia: methodology. Bin Wang: data curation, methodology. Weiwei Jin: conceptualization, methodology. Shaofeng Wu: conceptualization, methodology. Ziren Chen: conceptualization, methodology. Azhar Iqbal: conceptualization, methodology, writing – review & editing. Chenjiang Liu: conceptualization, funding acquisition, methodology. Yonghong Zhang: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, writing – review & editing.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This work was supported by the Tianshan Talents Program for Young Talents in Science and Technology Innovation (No. 2022TSYCCX0024), the Natural Science Foundation for Distinguished Young Scholars of Xinjiang Uyghur Autonomous Region (No. 2021D01E10), the Shanghai Cooperation Organization Science and Technology Partnership Program and International Science and Technology Cooperation Program (No. 2022E01042), the Tianshan Talents Program for Leading Talents in Science and Technology Innovation (No. 2022TSYCLJ0016), the Key Program of Natural Science Foundation of Xinjiang Uygur Autonomous Region (No. 2022D01D06), and the National Natural Science Foundation of China (No. 21861036, 21961037, 22201241 and 22361044).Notes and references
- X. Liu, Y. Li, L. Zeng, X. Li, N. Chen, S. Bai, H. He, Q. Wang and C. Zhang, Adv. Mater., 2022, 34, 2108327–2108356 CrossRef CAS PubMed.
- S. H. Leung and S. A. Angel, J. Chem. Educ., 2004, 81, 1492–1493 CrossRef CAS.
- S. Mateti, M. Mathesh, Z. Liu, T. Tao, T. Ramireddy, A. M. Glushenkov, W. Yang and Y. Chen, Chem. Commun., 2021, 57, 1080–1092 RSC.
- A. R. Pedrazzo, C. Cecone, F. Trotta and M. Zanetti, ACS Sustainable Chem. Eng., 2021, 9, 14881–14889 CrossRef.
- Y. Yang, L. Hansen, A. Fraczek, F. Badalassi and J. Kjellström, Org. Process Res. Dev., 2021, 25, 2090–2099 CrossRef CAS.
- J.-L. Do and T. Friščić, ACS Cent. Sci., 2017, 3, 13–19 CrossRef CAS PubMed.
- R. R. A. Bolt, S. E. Raby-Buck, K. Ingram, J. A. Leitch and D. L. Browne, Angew. Chem., Int. Ed., 2022, 61, e202210508 CrossRef CAS PubMed.
- K. Kubota, K. Kondo, T. Seo and H. Ito, Synlett, 2022, 33, 898–902 CrossRef CAS.
- M. T. J. Williams, L. C. Morrill and D. L. Browne, ACS Sustainable Chem. Eng., 2020, 8, 17876–17881 CrossRef CAS PubMed.
- R. Thorwirth, F. Bernhardt, A. Stolle, B. Ondruschka and J. Asghari, Chem.–Eur. J., 2010, 16, 13236–13242 CrossRef CAS PubMed.
- S. Wada, M. Urano and H. Suzuki, J. Org. Chem., 2002, 67, 8254–8257 CrossRef CAS PubMed.
- H. E. Gaffer, Color. Technol., 2019, 135, 484–500 CAS.
- K.-T. Chung, J. Environ. Sci. Health, Part C: Environ. Carcinog. Ecotoxicol. Rev., 2016, 34, 233–261 CrossRef CAS PubMed.
- B. M. Matijević, Đ. Đ. Vaštag, S. L. Apostolov, F. Assaleh, A. D. Marinković and D. Ž. Mijin, J. Solution Chem., 2016, 45, 885–906 CrossRef.
- A. Vogel, A textbook of practical organic chemistry including qualitative organic analysis, Longman, New York, 4th edn, 1978, p. 718 Search PubMed.
- B. B. F. Mirjalili, A. Bamoniri and A. Akbari, Curr. Chem. Lett., 2012, 1, 109–114 CrossRef CAS.
- N. Noroozi-Pesyan, J. Khalafy and Z. Malekpoor, Prog. Color, Color. Coat., 2009, 2, 61–70 Search PubMed.
- A. Lyčka, A. Koloničný, P. Šimůnek and V. Macháček, Dyes Pigm., 2007, 72, 208–211 CrossRef.
- Y. Zhang, Y. Liu, X. Ma, X. Ma, B. Wang, H. Li, Y. Huang and C. Liu, Dyes Pigm., 2018, 158, 438–444 CrossRef CAS.
- J. Merrington, M. James and M. Bradley, Chem. Commun., 2002, 2, 140–141 RSC.
- G. Kaupp, M. A. Metwally, F. A. Amer and E. Abdel-latif, Eur. J. Org Chem., 2003, 8, 1545–1551 CrossRef.
- J. Hooker, D. Hinks, G. Montero and C. Conlee, Color. Technol., 2002, 118, 273–276 CAS.
- J. Jin, Z. Wen, J. Long, Y. Wang, T. Matsuura and J. Meng, Synth. Commun., 2000, 30, 829–834 CrossRef CAS.
- G. S. Shankarling, P. P. Deshmukh and A. R. Joglekar, J. Environ. Chem. Eng., 2017, 5, 3302 CrossRef CAS.
- F. Wang, J. Huang and J. Xu, Chem. Eng. Process., 2018, 127, 43–49 CrossRef CAS.
- N. Fantozzi, J.-N. Volle, A. Porcheddu, D. Virieux, F. Garcìa and E. Colacino, Chem. Soc. Rev., 2023, 52, 6680–6714 RSC.
- P. Sarma, K. K. Sarmah, D. Kakoti, S. P. Mahanta, N. M. Adassooriya, G. Nandi, P. J. Das, D.-K. Bučar and R. Thakuria, Catal. Commun., 2021, 154, 106304 CrossRef CAS.
- I. C. B. Martins, M. C. Oliveira, H. P. Diogo, L. C. Branco and M. T. Duarte, ChemSusChem, 2017, 10, 1360–1363 CrossRef CAS PubMed.
- Y. Zhang, C. Tang, Y. Liu and C. Liu, Chin. J. Org. Chem., 2021, 41, 2587–2600 CrossRef CAS.
- Y. Zhang, D. Cao, W. Liu, H. Hu, X. Zhang and C. Liu, Curr. Org. Chem., 2015, 19, 151–178 CrossRef CAS.
- T. Liu, H. Wu, Q. Zhang and C. Wang, Org. Biomol. Chem., 2023, 21, 2059–2068 RSC.
- G. J. Davis, J. A. Townsend, M. G. Morrow, M. Hamie, A. J. Shepard, C.-C. Hsieh, M. T. Marty and J. C. Jewett, Bioconjugate Chem., 2021, 32, 2432–2438 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4mr00053f |
| This journal is © The Royal Society of Chemistry 2024 |