Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Atomically precise Au and Ag nanoclusters doped with a single atom as model alloy catalysts
Shinya
Masuda
 ,
Kosuke
Sakamoto
and
Tatsuya
Tsukuda
,
Kosuke
Sakamoto
and
Tatsuya
Tsukuda
 *
*
Department of Chemistry, Graduate School of Science, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033, Japan. E-mail: tsukuda@chem.s.u-tokyo.ac.jp
First published on 11th January 2024
Abstract
Gold and silver nanoclusters (NCs) composed of <200 atoms are novel catalysts because their catalytic properties differ significantly from those of the corresponding bulk surface and can be dramatically tuned by the size (number of atoms). Doping with other metals is a promising approach for improving the catalytic performance of Au and Ag NCs. However, elucidation of the origin of the doping effects and optimization of the catalytic performance are hampered by the technical challenge of controlling the number and location of the dopants. In this regard, atomically precise Au or Ag (Au/Ag) NCs protected by ligands or polymers have recently emerged as an ideal platform because they allow regioselective substitution of single Au/Ag constituent atoms while retaining the size and morphology of the NC. Heterogeneous Au/Ag NC catalysts doped with a single atom can also be prepared by controlled calcination of ligand-protected NCs on solid supports. Comparison of thermal catalysis, electrocatalysis, and photocatalysis between the single-atom-doped and undoped Au/Ag NCs has revealed that the single-atom doping effect can be attributed to an electronic or geometric origin, depending on the dopant element and position. This minireview summarizes the recent progress of the synthesis and catalytic application of single-atom-doped, atomically precise Au/Ag NC catalysts and provides future prospects for the rational development of active and selective metal NC catalysts.
1 Introduction
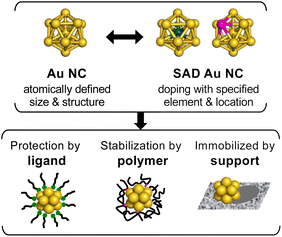
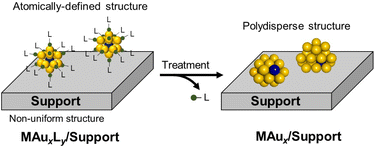
Metal nanoclusters (NCs), composed of <200 metal atoms, are promising catalytic materials because they exhibit novel and size-specific chemical reactivities that are significantly different from those of metal nanoparticles larger than a few nanometers.1–5 Alloying is a straightforward and effective way to further improve their catalytic performance, which is mainly tuned by geometric (or ensemble) and/or electronic (or ligand) effects.6–11 However, the reason for the improved catalytic activity by alloying is not easily explained due to the polydisperse nature of the structural parameters of conventional alloy NCs, such as the size (total number of atoms), composition, and mixing mode (e.g., solid solution, core–shell, and segregated structures). To overcome this situation, it is necessary to precisely control these key parameters without changing other parameters.Single-metal-atom doping is the first, but significant, step toward the optimization of the catalysis of a metal NC, given that it is composed of a countable number of atoms and has a surface-rich structure. Single-atom doping reduces the numerous factors to be controlled in the conventional mixing modes only to control the dopant position. Single-atom-doped (SAD) metal NCs not only lead to understanding the origin of the doping effects on catalysis, but also provide a design principle for alloy catalysts. Nevertheless, it is not a trivial matter to regioselectively dope a single atom without changing the size and structure of the base metal NCs.
Such synthetic problems can be solved by using atomically precise gold or silver (Au/Ag) NCs protected by organic ligands,12–33 the structures of which are determined by single-crystal X-ray diffraction (SCXRD) analysis. For example, single atoms of various transition metals have been regioselectively introduced to an icosahedral Au13 NC while retaining the structure.17,19,23,24 In addition, recent systematic studies have revealed how the energies of the highest-occupied and lowest-unoccupied molecular orbitals (HOMO and LUMO, respectively) are modulated upon doping and how the relaxation dynamics of the electronically excited state are affected by doping. Thus, the SAD Au/Ag NCs and their undoped counterparts (Scheme 1) protected by ligands are ideal platforms for elucidating the doping effects, especially on electrocatalysis and photocatalysis, which are promoted by the electron transfer between reactants through the ligand layer.
On the other hand, for studying the doping effects on thermal catalysis, the SAD NC should be stabilized while exposing a part of the surface. This requirement is met by stabilizing the SAD NC with a polymer or by immobilizing the SAD NC on a solid support (Scheme 1). The dopant located inside the SAD NC modulates the electronic structure of the reaction site on the surface, while the dopant on the surface may directly act as a reaction site for catalytic conversion. The latter type can be viewed as single-atom alloy catalysts34–38 supported by a defined number of base metal atoms. However, achieving atomically precise synthesis and structure determination of these types of SAD NCs is still very difficult.
This minireview surveys the research in the emerging area of the single-atom-doping effects on the catalysis of atomically precise Au/Ag NCs. The catalysts include SAD Au/Ag NCs protected by organic ligands, sparsely coated by polymers, and immobilized on solid supports (Scheme 1). Section 2 describes the atomically precise syntheses and structures of the SAD Au/Ag NCs. The doping effects on the thermal catalysis, electrocatalysis, and photocatalysis are respectively introduced in sections 3–5. Section 6 summarizes the roles of each dopant in various types of catalysis and presents the future prospects for the rational development of active and selective metal NC catalysts.
2 Synthesis and structural characterization
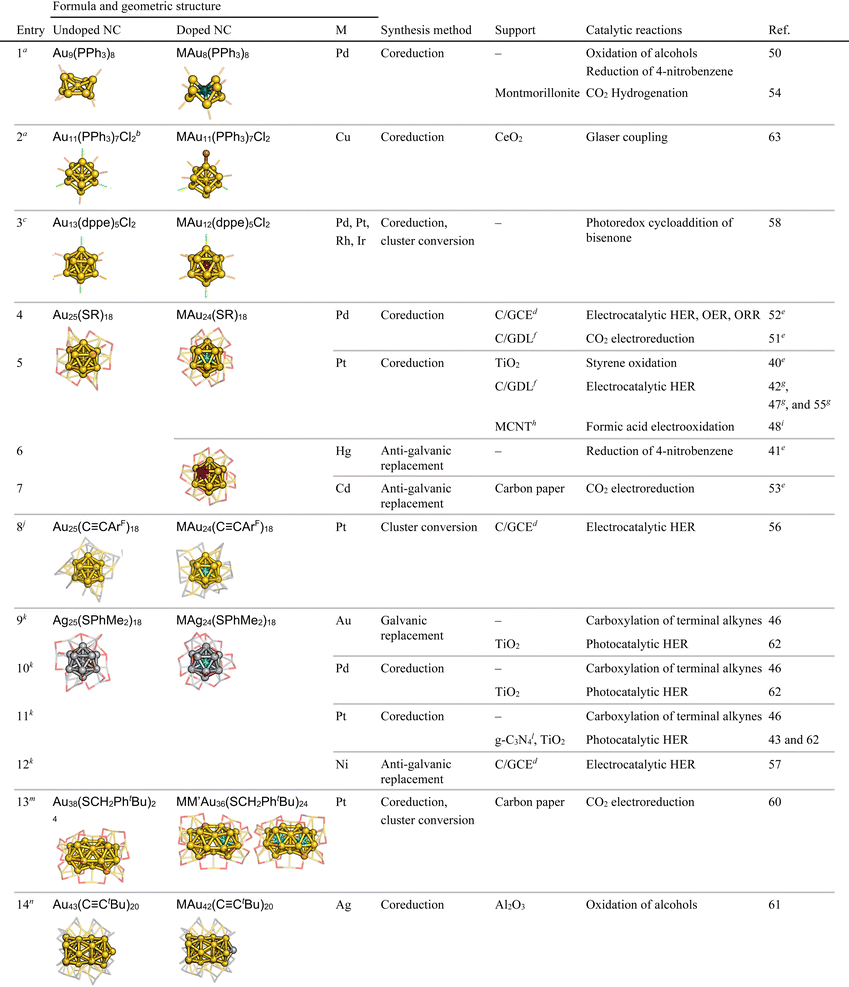
This section presents an overview of the syntheses and structures of three types of SAD Au/Ag NCs as well as the corresponding pure Au/Ag NCs treated in this review (Tables 1–3).39–63 The atomic packing structures and the dopant locations were determined by a combination of methods, including SCXRD,57,58,61,63–69 nuclear magnetic resonance (NMR) spectroscopy,40,70 X-ray absorption fine structure (XAFS) spectroscopy.39,44,45,60,71,72 high-resolution transmission electron microscopy (HRTEM),59,73,74 and density functional theory (DFT) calculations.40,60,75,76
a PPh3: triphenylphosphine.
b Undoped Au cluster: Au11(PPh3)7Cl3.
c dppe: 1,2-bis(diphenylphosphino)ethane.
d GCE: glassy carbon electrode.
e SR = PET: 2-phenylethanethiolate.
f GDL: gas diffusion layer.
g SR = SC6: hexanethiolate.
h MCNT: multi-wall carbon nanotube.
i SR = SC12: dodecanethiolate.
j HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CArF: 3,5-bis(trifluoromethyl)ethynylbenzene.
k SPhMe2: 2,4-dimethylbenzenethiolate.
l g-C3N4: graphitic carbon nitride.
m HSCH2PhtBu: 4-tert-butylbenzyl mercaptan.
n HC CArF: 3,5-bis(trifluoromethyl)ethynylbenzene.
k SPhMe2: 2,4-dimethylbenzenethiolate.
l g-C3N4: graphitic carbon nitride.
m HSCH2PhtBu: 4-tert-butylbenzyl mercaptan.
n HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CtBu: 3,3-dimethyl-1-butyne. Color code: yellow, Au; turquoise, Pd; brown, Cu; purple, Rh; cyan, Pt; pink, Cd; silver, Ag. Protection motifs are in the line-view; orange, P; light green, Cl; red, S; gray, C. Organic moieties of ligands and hydrogen atoms are omitted for clarity. CtBu: 3,3-dimethyl-1-butyne. Color code: yellow, Au; turquoise, Pd; brown, Cu; purple, Rh; cyan, Pt; pink, Cd; silver, Ag. Protection motifs are in the line-view; orange, P; light green, Cl; red, S; gray, C. Organic moieties of ligands and hydrogen atoms are omitted for clarity.
|
|---|
|
2.1 Ligand-protected single-atom-doped Au/Ag nanoclusters
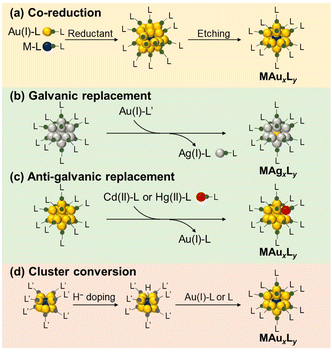
Ligand-protected monometallic Au/Ag NCs with crystallographically determined structures serve as ideal references for studying the doping effects on catalysis.77–89 Atomically precise syntheses of the ligand-protected Au/Ag NCs were achieved by rigorous isolation and fractionation from relatively monodisperse Au/Ag NCs.12,13,22,25 Many examples of ligand-protected Au/Ag NCs have been reported for decades.12–33 The stability of Au/Ag NCs is governed by electronic structures: spherical Au/Ag NCs gain high stability when the total number of valence electrons matches specific numbers such as 2, 8, 18, 20, 34, and so on.90–92 For example, the thiolate (SR)-protected Au NC [Au25(SR)18]− with an icosahedral Au13 core takes a closed electron configuration (1S)2(1P),25 where 1S, 1P, 1D, 2S, 1F, 2P, and so on are molecular orbitals that are distributed over the Au13 core and accommodate the valence electrons. These Au/Ag NCs are viewed as chemically modified Au/Ag superatoms because their hierarchical electronic structures are similar to the conventional atomic orbitals. Table 1 lists the SAD Au/Ag NCs introduced in this minireview: those protected by phosphines (entries 1–3), thiolates (entries 4–7 and 9–13), and alkynyls (entries 8 and 14).The following text introduces four synthetic approaches to doping a single atom to ligand-protected Au/Ag NCs. The most common one is the co-reduction of ions of the base metal (Au/Ag) and dopant M in the presence of ligands (Scheme 2a). This approach is effective for doping transition metals of groups 9 and 10 (Pt, Pd, Ir, Rh, etc.). Simultaneous reduction produces not only SAD NCs, but also undoped NCs, thereby requiring further purification. For example, Negishi et al. isolated PdAu24(SC12)18 (SC12 = SC12H25) via reverse-phase high-performance liquid chromatography.75 Jin et al. employed selective decomposition of undoped Au25(SR)18 with hydrogen peroxide (H2O2) to isolate PtAu24(SR)18.40 Most of the ligand-protected SAD Au/Ag NCs were synthesized by co-reduction (entries 1–5, 10, 11, 13 and 14, Table 1).39,40,42,43,46–52,54,55,58,60–63,93
Galvanic replacement of metal elements driven by a redox reaction is a well-known phenomenon in the bulk surface. It is, for example, used in the synthesis of AuAg24(SR)18 by mixing Ag25(SR)18 and Au(PPh3)Cl (entry 9, Table 1), where Ag0 is oxidized and then dissolved in solution, while Au+ is reduced and deposited on the NC (Scheme 2b).46,57,62 On the other hand, MAu24(SR)18 (M = Cd or Hg) was synthesized by mixing Au25(SR)18 with M(NO3)2 (entries 6 and 7, Table 1).41,53 This process is called anti-galvanic replacement and is specific to small metal NCs, where the most electronegative Au is dissolved as Au+ in solution (Scheme 2c). A group 12 element is usually introduced into pure Au NCs via an anti-galvanic replacement reaction.
SAD NCs can also be obtained via the growth reaction of pure and doped Au NCs as the seed (Scheme 2d). MAu8(PPh3)8 (M = Pd or Pt) was converted to HMAu8(PPh3)8 with eight electrons by doping with hydride (H−).94 This hydride-doped NC was transformed by reacting with metal complexes to a variety of NCs including MAu24(SR)18, MAu24(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CArF)18, and MAu12(dppe)5Cl2 (M = Pd or Pt) (entries 3, 8 and 13, Table 1).56,58,69 The SAD NCs can be synthesized on a large scale (∼100 mg) by taking advantage of the scalability of the MAu8(PPh3)8 precursors and the efficient transformation.
CArF)18, and MAu12(dppe)5Cl2 (M = Pd or Pt) (entries 3, 8 and 13, Table 1).56,58,69 The SAD NCs can be synthesized on a large scale (∼100 mg) by taking advantage of the scalability of the MAu8(PPh3)8 precursors and the efficient transformation.
The structures determined by SCXRD and other methods listed in Table 1 suggest that the location of the dopants depends on the element group. Element M of groups 9 and 10 (Ir, Rh, Pt and Pd) is located at the center of the crown-shaped M@Au8 protected by phosphine (entry 1, Table 1) and the icosahedral M@Au12/M@Ag12 protected by phosphine, thiolate, or alkynyl (entries 3–5 and 8–13, Table 1). The M@Au12 cores become more compact and symmetrical by doping with element M positioned at the lower left of the periodic table due to the reduction of the atomic radius of M. In contrast, element M of groups 11 and 12 (Au, Ag, Cu, Hg and Cd) is attached on the surface of Au@Au10 protected by phosphine (entry 2, Table 1) and incorporated into the surface of the icosahedral Au@MAu11 protected by thiolate (entries 6 and 7, Table 1) and Au12@Au20 protected by alkynyl (entry 14, Table 1). Electrochemical measurements and optical absorption spectroscopy on M@Au12 revealed that the HOMO is energetically upshifted and the HOMO–LUMO gap is expanded by doping with element M positioned at the lower left of the periodic table. The former trend can be understood by a two-step jellium model (Fig. 1): the central part of the jellium potential of the M@(Au+)12 (M = Au+, Pt0, Ir−) superatomic core becomes shallower by replacing Au+ with Pt0 and even shallower with Ir−. The lifetime of the electronically excited state becomes elongated up to the μs range by doping with M positioned at the lower left of the periodic table, such as Pt and Ir (energy gap law).95 The ligand layer acts as a “catalytic poison” for thermal reactions, while ligand-protected SAD Au/Ag NCs act as electrocatalysts by forming active sites through partial removal of the ligand,96–101 and as photocatalysts by electron transfer through the ligand layer.
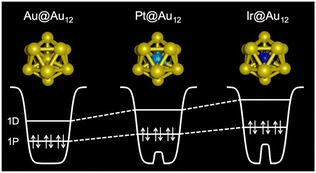 | ||
| Fig. 1 Schematic illustration of the two-step jellium potential and superatomic orbitals of M@(Au+)12 (M = Au+, Pt0, Ir−). Reproduced from ref. 95 with permission from the American Chemical Society, Copyright 2020. | ||
2.2 Polyvinylpyrrolidone-stabilized single-atom-doped Au nanoclusters
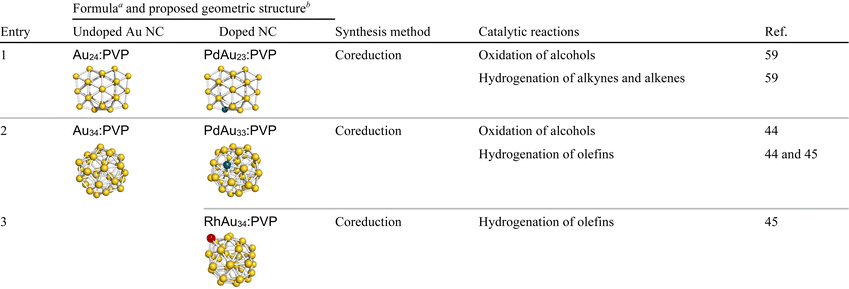
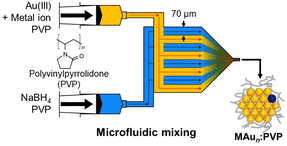
For catalytic application for thermal reactions, NCs should be stabilized by polymers while exposing part of the surface.102,103 Atomically precise synthesis of Aun NCs (n = 24, 34, 38) was recently achieved using polyvinylpyrrolidone (PVP) as a stabilizer.59,73,74 The key factors to this success were: (1) kinetic control of the cluster formation by homogeneous and rapid mixing of Au ions and a strong reducing agent in the presence of an excess amount of PVP (Scheme 3), and (2) application of matrix-assisted laser desorption/ionization mass spectrometry (MALDI-MS) for evaluating the cluster size n and the purities of Aun NCs.44,45,59 This approach has been applied for PVP-stabilized SAD Au NCs: PdAu23:PVP,59 PdAu33:PVP,44 or RhAu34:PVP45 was obtained by the co-reduction of Au ions and a controlled amount of Pd or Rh precursors in the microfluidic mixer (entries 1–3, Table 2). The geometric structures of Au24:PVP and Au23Pd1:PVP were characterized by DFT calculations, aberration-corrected TEM (ACTEM), extended XAFS (EXAFS) analysis, and Fourier transform infrared (FT-IR) spectroscopy of the adsorbed CO. These results showed that Au23Pd1:PVP takes polydisperse structures with all the constituent atoms including the Pd atom on the surface, but all the optimized structures of Au23Pd1:PVP have the same atomic arrangements as the undoped Au24:PVP (entry 1, Table 2).59 Rh–K and Pd–K edge XAFS analysis on PdAu33:PVP44 and RhAu34:PVP45 revealed that Pd is incorporated into the surface of the Au33 NC (entry 2, Table 2), while the Rh atom is attached to the surface of the Au34 NC (entry 3, Table 2).2.3 Solid-supported single-atom-doped Au/Ag nanoclusters
Ligand-protected SAD Au/Ag NCs adsorbed on solid materials in the intact form can be used as catalysts for electrocatalytic and photocatalytic reactions. In contrast, naked SAD Au/Ag NCs immobilized on solid supports exhibit a wider scope of applicability than ligated NCs as the reactants can be activated by direct interaction with the NC surface. However, naked SAD Au/Ag NCs cannot be synthesized with atomic precision on supports by conventional methods, such as impregnation and deposition–precipitation. An alternative synthetic strategy is to remove the ligands from the well-defined SAD Au/Ag NCs on solid supports (Scheme 4).81 Ligand removal was conducted by thermal treatment, H2O2 treatment, or NaBH4 reduction.82,104 The intrinsic challenge is to avoid sintering of the NCs during the ligand removal. This problem was solved by reducing the density of the NCs on the supports by lowering the loading or using supports with a large specific surface area. Suppression of NC aggregation was confirmed by ACTEM and EXAFS. The inherent drawback of the approach shown in Scheme 4 is that the geometric structures of the resulting NCs are not uniform due to the stochastic nature of the ligand desorption process and the non-uniform surface structures of the supports. Nonetheless, EXAFS provides key structural information on whether the dopant is exposed on the NC surface or confined within the NC based on the coordination number of the M–Au(Ag) bonds. The corresponding heterogeneous catalysts together with the dopant positions are listed in Table 3. The position of the dopant seems to be determined by the strength of its bonding to the surrounding Au/Ag atoms and to the solid support. For example, Pd in a PdAu24/carbon nanotube (CNT) is located at the interface with CNT (entry 1, Table 3), but is on the surface when PdAu24 is deposited on BaLa4Ti4O15 (entry 3, Table 3). Barrabés et al. observed by operando diffuse reflectance infrared Fourier transform spectroscopy that the heating of PdAu24(SR)18 at 250 °C under an O2 atmosphere followed by using H2 led to the migration of the Pd atom from the center of the core to the surface.933 Thermal catalysis
3.1 Oxidation reactions
Zhu et al. compared the catalytic activity of [PdAu8(PPh3)8]2+ and its undoped counterpart [Au9(PPh3)8]3+ (entry 1, Table 1) for benzyl alcohol (BnOH) oxidation (eqn (1)).50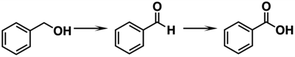 | (1) |
[PdAu8(PPh3)8]2+ showed catalytic activity and high selectivity to benzaldehyde, while [Au9(PPh3)8]3+ did not. The preservation of [PdAu8(PPh3)8]2+ under catalytic conditions was confirmed by ultraviolet-visible (UV-Vis) absorption spectroscopy, TEM, and FT-IR. This result suggested that the exposed Pd site was a reaction site. Mechanistic studies by electron paramagnetic resonance (EPR) spectroscopy showed that the superoxide species was formed only on [PdAu8(PPh3)8]2+. The partial density of states (PDOS) obtained by DFT calculations implied that O2 was more strongly adsorbed to the central Pd dopant of [PdAu8(PPh3)8]2+ than to the central Au of [Au9(PPh3)8]3+. This difference resulted in the preferential formation of the superoxide species by [PdAu8(PPh3)8]2+ for BnOH activation.
Mason et al. recently compared the catalytic activity between AgAu42(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CtBu)20 having an exposed Ag site on the surface and AuAu42(C
CtBu)20 having an exposed Ag site on the surface and AuAu42(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CtBu)20 (entry 14, Table 1) for BnOH oxidation (eqn (1)) using tert-butyl hydroperoxide (TBHP) as an oxidant.61 The conversion decreased from 52% to 18% upon Ag doping. UV-Vis absorption spectroscopy and scanning TEM (STEM) supported the retention of the original cluster during catalysis. Topographic steric hindrance maps indicated that the Ag atom of AgAu42(C
CtBu)20 (entry 14, Table 1) for BnOH oxidation (eqn (1)) using tert-butyl hydroperoxide (TBHP) as an oxidant.61 The conversion decreased from 52% to 18% upon Ag doping. UV-Vis absorption spectroscopy and scanning TEM (STEM) supported the retention of the original cluster during catalysis. Topographic steric hindrance maps indicated that the Ag atom of AgAu42(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CtBu)20 was geometrically more buried in the core than the Au atom at the corresponding position in Au43(C
CtBu)20 was geometrically more buried in the core than the Au atom at the corresponding position in Au43(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CtBu)20, resulting in poorer accessibility of the reactants. The negative doping effect of Ag was also ascribed to the lower ability of the Ag site to abstract hydride from the alkoxide compared to the ability of the Au site.
CtBu)20, resulting in poorer accessibility of the reactants. The negative doping effect of Ag was also ascribed to the lower ability of the Ag site to abstract hydride from the alkoxide compared to the ability of the Au site.
Jin et al. reported that Au25(PET)18/TiO2 and PtAu24(PET)18/TiO2 exhibited 58.9% and 90.8% conversion in styrene oxidation, respectively, using iodobenzene diacetate (PhI(OAc)2) as an oxidant (eqn (2)).40
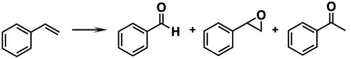 | (2) |
Since the Pt dopant of PtAu24(PET)18 located at the center of the Pt@Au12 core (entry 5, Table 1) cannot serve as a reaction site, modulation of the electronic structure plays a crucial role in promoting catalysis.
Tsukuda et al. found the enhancement of the alcohol oxidation catalysis of PVP-stabilized colloidal Au24 NCs by using a Pd dopant (entry 1, Table 2).59 PdAu23:PVP and Au24:PVP exhibited pseudo-first-order reaction rate constants of 2.3 and 0.87 h−1, respectively, in the aerobic oxidation of BnOH (eqn (1)). UV-Vis absorption spectroscopy and MALDI-MS spectrometry revealed that these clusters are preserved after catalysis. The enhancement was also observed for other para-substituted benzyl alcohols. DFT calculations predicted that hydride adsorbs more strongly on the Pd atom on the surface of PdAu23 than on the Au atom of Au24. Given that the rate-determining step (RDS) of eqn (1) was hydride abstraction from the α-carbon of benzyl alkoxide, the surface Pd atom acted as an efficient adsorption site for hydride, resulting in an enhanced activity (Fig. 2).
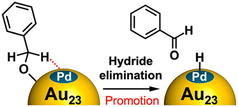 | ||
| Fig. 2 Schematic illustration of the proposed mechanism by Pd doping on Au24:PVP. Reproduced from ref. 59 with permission from the American Chemical Society, Copyright 2022. | ||
Tsukuda et al. reported the effect of single-atom doping on BnOH oxidation (eqn (1)) by using PdAu24/CNT and Au25/CNT (entry 1, Table 3).39 The conversions by PdAu24/CNT and Au25/CNT were 74% and 22%, respectively, indicating that the catalytic activity was significantly enhanced upon Pd atom doping. TEM images after catalysis showed the same average particle size as with the fresh sample, suggesting that the cluster was not aggregated. Given that the Pd dopant atom was buried in the Au NC and located at the interface with the CNT support,71,76 the enhanced catalytic activity was explained by the formation of the anionic Au surface via the electron transfer from Pd to Au, which facilitated the activation of O2 more effectively. Namely, the role of the Pd atom differs from that proposed for PdAu23:PVP.
3.2 Reduction reactions
Wu et al. observed that HgAu24(PET)18 (entry 6, Table 1) showed higher activity than Au25(PET)18 for the reduction of 4-nitrobenzene using NaBH4 as a reductant (eqn (3)).41 The cluster was preserved based on the results of UV-Vis absorption spectroscopy and MALDI-MS spectrometry. | (3) |
Tsukuda compared the catalytic activity of PdAu33:PVP and RhAu34:PVP (entries 2 and 3, Table 2) with respect to undoped Au34:PVP for the hydrogenation of olefins using H2 as a reductant (eqn (4)).44,45
 | (4) |
RhAu34:PVP showed remarkably higher activity in the hydrogenation of olefins compared to that of Au33:PVP, while PdAu33:PVP showed moderate activity. Both Rh and Pd served as reaction sites for H2 activation, while the higher activity of RhAu34:PVP was ascribed to the lower coordination state of the Rh atom.
3.3 CO2 fixation reactions
Zhu et al. compared the catalytic properties of MAg24(SPhMe2)18 (M = Ag, Au, Pd, Pt) (entries 9–11, Table 1) for carboxylation of various terminal alkynes (R–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–H; R = Ph, pMePh, mMePh, pNH2Ph, pClPh, pCHOPh) into the corresponding propiolic acids (eqn (5)).46
C–H; R = Ph, pMePh, mMePh, pNH2Ph, pClPh, pCHOPh) into the corresponding propiolic acids (eqn (5)).46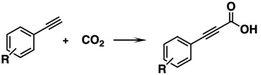 | (5) |
The catalytic activity was enhanced in the order of M = Ag < Pd ≈ Pt < Au. Furthermore, the catalysts could be reused without a significant loss of activity for M = Au, Pd, and Pt, while the undoped NCs (M = Ag) decomposed after the catalytic usage. This result demonstrated that the dopant atom enhanced not only the activity but also the stability of the NCs. UV-Vis absorption spectra of spent clusters showed no drastic change after catalytic cycles.
Zhu et al. intercalated [Au9(PPh3)8]3+ and [PdAu8(PPh3)8]2+ (entry 1, Table 1) into a montmorillonite support to improve the stability during the catalytic usage.54 The catalytic activity of the intercalated [PdAu8(PPh3)8]2+ was much higher than that of [Au9(PPh3)8]3+ for CO2 hydrogenation. More interestingly, the reaction selectivity depended sharply on the central atom: [Au9(PPh3)8]3+ and [PdAu8(PPh3)8]2+ yielded methane and ethane as the major products, respectively. STEM analysis showed that the average diameter of the NCs on the spent catalyst increased to 2.2 nm from ∼1 nm for the fresh catalyst.
3.4 Coupling reactions
Recently, Mandal et al. synthesized a novel Cu-doped Au NC CuAu11(PPh3)7Cl2 (entry 2, Table 1).63 The Cu dopant was fully exposed and thus was expected to work as a reaction site for the catalysis. CuAu11(PPh3)7Cl2 exhibited high activity in the coupling reactions between 1-iodo-4-methylbenzene and various substituted phenylacetylenes (eqn (6)), while the undoped Au11(PPh3)7Cl3 was catalytically inert to these coupling reactions. In addition, CuAu11(PPh3)7Cl2 showed a high selectivity toward the Glaser coupling products, in sharp contrast to the Sonogashira coupling product by using Au25(PET)18. DFT calculations verified that the unsaturated Cu acted as a reaction site like a single-atom catalyst and kinetically favored Glaser coupling over Sonogashira coupling.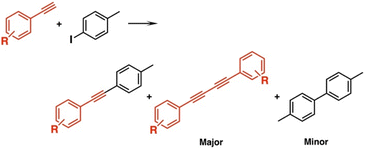 | (6) |
4 Electrocatalysis
4.1 Hydrogen evolution reaction (HER)
The single-atom doping effects on the catalysis of ligated Au/Ag NCs were investigated in the HER. Table 4 compares the catalytic performance under the specified experimental conditions in terms of onset potential (η) and current density.| Entry | Catalyst | η (mV) | Current density (mA cm−2 mg−1) | Electrolyte | Ref. |
|---|---|---|---|---|---|
a SC6: hexanethiolate.
b GDL: gas diffusion layer.
c GCE: glassy carbon electrode.
d PET: 2-phenylethanethiolate.
e HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CArF: 3,5-bis(trifluoromethyl)ethynylbenzene.
f SPhMe2: 2,4-dimethylbenzenethiolate.
g Overpotential at 10 mA cm−2 (η10) CArF: 3,5-bis(trifluoromethyl)ethynylbenzene.
f SPhMe2: 2,4-dimethylbenzenethiolate.
g Overpotential at 10 mA cm−2 (η10)
|
|||||
| 1a,b | PtAu24(SC6)18/C/GDL | 70 | 750 (−0.4 V vs. RHE) | 1.0 M Britton–Robinson buffer solution (pH 3) | 42 |
| 2a,c | Au25(SC6)18/C/GCE | 200 | 239 (−0.6 V vs. RHE) | 1.0 M Britton–Robinson buffer solution and 2.0 M KCl (pH 3) | 47 |
| PdAu24(SC6)18/C/GCE | 70 | 501 (−0.6 V vs. RHE) | |||
| PtAu24(SC6)18/C/GCE | 70 | 870 (−0.6 V vs. RHE) | |||
| 3d,e | Au25(PET)18/C | 562g | 27.3 (−0.5 V vs. RHE) | 0.5 M H2SO4 | 57 |
| PtAu24(PET)18/C | 442g | N.A. | |||
Au25(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CArF)18/C CArF)18/C |
490g | 85.8 (−0.5 V vs. RHE) | |||
PtAu24(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CArF)18/C CArF)18/C |
434g | N.A. | |||
| 4c,f | Ag25(SPhMe2)18/C/GCE | 250 | 2345 (−0.6 V vs. RHE) | 1.0 M KOH | 58 |
| NiAg24(SPhMe2)18/C/GCE | 50 | 4173 (−0.6 V vs. RHE) | |||
Lee et al. reported that PtAu24(SC6)18/C (entry 5, Table 1) showed a much higher reaction rate and turnover frequency (TOF) for the HER in a tetrahydrofuran (THF) solution of trifluoroacetic acid (TFA) than conventional molecular catalysts, and was even more reactive than the benchmark Pt/C (entry 1, Table 4) by depositing on the carbon support.42 They further revealed that PtAu24(SC6)18/C was superior to PdAu24(SC6)18/C (entries 4 and 5, Table 1) as a catalyst for the HER (entry 2, Table 4).47 The onset potentials of PtAu24(SC6)18/C and PdAu24(SC6)18/C were comparable (−0.07 V) (Fig. 3b) and similar to the first reduction potentials, indicating that the reduction potentials of the NCs have a significant impact on the emergence of the activity (Fig. 3a). Contrary to the expectations from this result, the TOF by PtAu24(SC6)18/C was much higher than that by PdAu24(SC6)18/C at any potential (Fig. 3b). The superior activity of PtAu24(SC6)18/C over PdAu24(SC6)18/C was explained by the hydrogen adsorption Gibbs free energy (ΔGH) (Fig. 3c): ΔGH was estimated to be 0.43, 0.36, and 0.31 eV for Au25(SC6)18, PdAu24(SC6)18, and PtAu24(SC6)18, respectively. The DFT-optimized structures for PtAu24(SC1)18 and PdAu24(SC1)18 suggested that hydrogen could be incorporated into the M@Au12 core (M = Pt, Pd), forming a H–M bond (Fig. 3d).
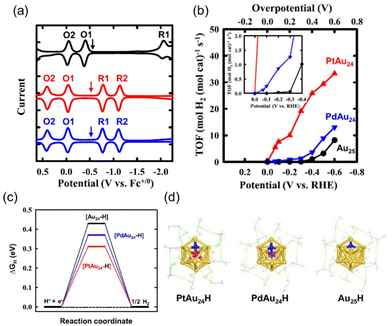 | ||
| Fig. 3 (a) Square-wave voltammetry (SWV) of MAu24(SC6)18 with M = Au (black), Pt (red), and Pd (blue) in dichloromethane (1.0 mM). The arrows denote the open circuit potential of the NCs. (b) Turnover frequency (TOF) against the potential obtained from the constant potential electrolysis of the MAu24(SC6)18/C/gas diffusion layer electrode in 1.0 M Britton–Robinson buffer solution containing KCl (2.0 M). Inset: enlarged view of the low-overpotential region. (c) Hydrogen adsorption Gibbs free energy (ΔGH) diagrams for MAu24(SCH3)18 obtained by theoretical calculations and (d) DFT-optimised structures of H-MAu24(SCH3)18. Color code; pink, Pt; purple, Pd; yellow, Au; blue, adsorbed H. Protection motifs are in the line view; green, S; gray, C; white, H. Reproduced from ref. 47 with permission from the American Chemical Society, Copyright 2018. | ||
The HER in acidic media is known to start with hydrogen adsorption to the active site (*) (Volmer step, eqn (7a)).105
| H+ + e− + * → H* | (7a) |
This step is followed by the production of H2via a heterolytic pathway between the adsorbed H (H*) and H+ (Heyrovsky step, eqn (7b)) or a homolytic reaction between the two H* atoms (Tafel step, eqn (7c)):
| H* + H+ + e− → H2 + * | (7b) |
| H* + H* → H2 + 2* | (7c) |
Lee et al. proposed that the HER pathway was changed by single-atom doping: the HER by Au25(SC6)18/C proceeds via a homoleptic pathway (Scheme 5a), while that by PtAu24(SC6)18/C proceeds via a heterolytic pathway (Scheme 5b).
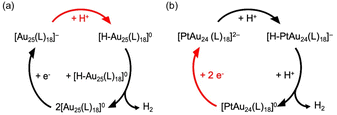 | ||
| Scheme 5 HER cycle catalyzed by (a) Au25(SC6)18 and (b) PtAu24(SC6)18. The RDS is indicated by the red arrow. Reproduced from ref. 56 with permission from the American Chemical Society, Copyright 2021. | ||
Tsukuda et al. revealed that the overpotential at 10 mA cm−2 (η10) of PtAu24(PET)18/C (entry 5, Table 1) was lower compared to Au25(PET)18/C (entry 3, Table 4),56 as observed in the case of PtAu24(SC6)18/C and Au25(SC6)18/C (entry 2, Table 4). The TOF value was more enhanced for Au25(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CArF)18/C (ArF: 3,5-bis(trifluoromethyl)-phenyl) than Au25(PET)18/C (entry 8, Table 1; entry 3, Table 4).56 This remarkable ligand effect is explained in such a manner that the RDS (protonation of [Au25(PET)18]−, Scheme 5a) is promoted by the alkynyl ligand layer having a more electron-rich nature than the thiolate ligand layer. However, the η10 value of PtAu24(C
CArF)18/C (ArF: 3,5-bis(trifluoromethyl)-phenyl) than Au25(PET)18/C (entry 8, Table 1; entry 3, Table 4).56 This remarkable ligand effect is explained in such a manner that the RDS (protonation of [Au25(PET)18]−, Scheme 5a) is promoted by the alkynyl ligand layer having a more electron-rich nature than the thiolate ligand layer. However, the η10 value of PtAu24(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CArF)18/C was not significantly lowered compared to Au25(C
CArF)18/C was not significantly lowered compared to Au25(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CArF)18/C (entry 3, Table 4).56 These results showed that the single-atom doping effects on HER activity depended on the nature of the ligand layer.
CArF)18/C (entry 3, Table 4).56 These results showed that the single-atom doping effects on HER activity depended on the nature of the ligand layer.
Lee et al. reported that NiAg24(SPhMe2)18/C (entry 12, Table 1) in alkaline media showed higher HER activity than Ag25(SPhMe2)18/C (entry 4, Table 4).57 The HER in alkaline media begins with the dissociation of H2O into adsorbed H (H*) and OH− (Volmer step, eqn (8a)).105
| H2O + e− + * → H* + OH− | (8a) |
The adsorbed H* reacts with H2O (Heyrovsky step, eqn (8b)) or recombines with the neighboring H* (Tafel step, eqn (8c)) to produce H2.
| H* + H2O + e− → H2 + OH− + * | (8b) |
| H* + H* → H2 + 2* | (8c) |
The onset potential for NiAg24(SPhMe2)18/C (−0.05 V) shifted to positive with respect to that for Ag25(SPhMe2)18/C (−0.25 V), signifying that the reduction potential is shifted positively upon Ni doping. The Tafel analysis indicated that Ni doping dramatically improved the Volmer step (eqn (8a)). DFT calculations of the dethiolated models NiAg24(SC1)17 and Ag25(SC1)17 further revealed that the hydrogen adsorption energy was substantially reduced upon Ni doping.
4.2 CO2 electroreduction reaction (CO2RR)
Since Au-based catalysts are known to show excellent selectivity in the CO2 electroreduction reaction (CO2RR, eqn (9a)–(9c)) to CO,106,107 Au-based NCs are potential candidates for superior catalysts.| CO2 + H+ + e− + * → COOH* | (9a) |
| COOH* + H+ + e− + * → CO* + H2O | (9b) |
| CO* → CO + * | (9c) |
Table 5 compares the catalytic performance under the specified experimental conditions in terms of faradaic efficiency for CO (FECO) and CO partial current density (jCO).
| Entry | Catalyst | FECO | j CO (mACO cm−2) | Electrolyte | Ref. |
|---|---|---|---|---|---|
| a PET: 2-phenylethanethiolate. b SC6: hexanethiolate. c GCE: glassy carbon electrode. d HSCH2PhtBu: 4-tert-butylbenzyl mercaptan. | |||||
| 1a | Au25(PET)18/C | ∼100% (−0.9 V vs. RHE) | ∼20 (−1.2 V vs. RHE) | 0.1 M aqueous KHCO3 | 52 |
| PdAu24(PET)18/C | ∼100% (−1.2 V vs. RHE) | ∼35.5 (−1.2 V vs. RHE) | |||
| 2b,c | Au25(SC6)18/C/GCE | ∼95% (−0.3–−0.6 V vs. RHE) | 12 (−1.0 V vs. RHE) | 0.1 M aqueous KHCO3 and 0.4 M KCl | 56 |
| PtAu24(SC6)18/C/GCE | ∼50% (−0.6–−0.8 V vs. RHE) | 5 (−1.0 V vs. RHE) | |||
| 3d | Au38(SCH2PhtBu)24/C | 67% (−0.6 V vs. RHE) | 2.5 (−0.6 V vs. RHE) | 0.5 M aqueous KHCO3 | 61 |
| PtAu37(SCH2PhtBu)24/C | 77% (−0.6 V vs. RHE) | 7.0 (−0.6 V vs. RHE) | |||
| Pt2Au36(SCH2PhtBu)24/C | 60% (−0.6 V vs. RHE) | 1.4 (−0.6 V vs. RHE) | |||
| 4a | Au25(PET)18/C | 40–70% (−0.3–−0.8 V vs. RHE) | 7.0 (−0.6 V vs. RHE) | 1 M aqueous KHCO3 | 54 |
| CdAu24(PET)18/C | 83–90% (−0.3–−0.8 V vs. RHE) | 18.1 (−0.6 V vs. RHE) | |||
Jin et al. compared the catalytic activity of PdAu24(PET)18/C and Au25(PET)18/C for the CO2RR. The CO partial mass activity of PdAu24(PET)18/C was ∼2 times larger than that of Au25(PET)18/C at −1.2 V (entry 1, Table 5 and Fig. 4a).51 In addition, PdAu24(PET)18/C maintained ∼100% FECO over a wide range of potential up to −1.2 V by suppressing unfavorable H2 formation via the HER (Fig. 4b), while FECO of Au25(PET)18/C started to decrease at −0.9 V. To identify the active site on PdAu24(PET)18/C, DFT calculations were conducted on two model systems, MAu24(SC1)17 and MAu24S(SC1)17, constructed by removing a CH3S or CH3 from MAu24(SC1)18 (M = Pd, Au; SC1 = SCH3), respectively (Fig. 4c). The formation of a COOH* adsorbate from CO2 was energetically unfavorable on the exposed Au site of MAu24(SC1)17 (blue and green in Fig. 4d), whereas the HER was favorable on such sites in MAu24(PET)17 (blue and green in Fig. 4e). On the other hand, the S site on PdAu24S(SC1)17 stabilized the COOH* intermediate (black in Fig. 4d) and had a large thermodynamic barrier for the HER (black in Fig. 4e), suggesting that the S site on PdAu24S(PET)17 was the reaction site for the selective CO2RR.
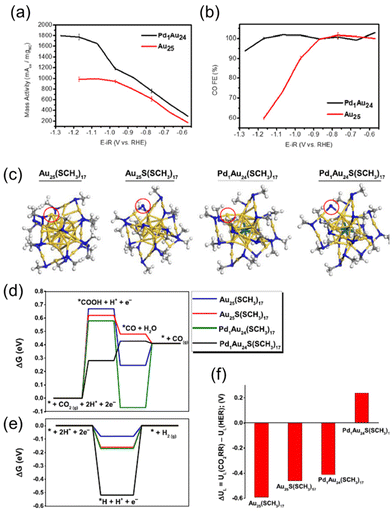 | ||
| Fig. 4 (a) CO mass activity and (b) faradaic efficiency for CO in the CO2RR over Au25(PET)18/C and PdAu24(PET)18/C. (c) DFT-optimized structures of MAu24(SCH3)18 and MAu24S(SCH3)17 (M = Pd, Au). Proposed active sites are circled in red. Colour code: Au, yellow; Pd, turquoise; S, blue; C, gray; H, white. Gibbs free energy diagrams for the (d) CO2RR and (e) HER at U = 0 V vs. RHE. (f) Difference in the predicted limiting potentials for the CO2RR and HER. Reproduced from ref. 51 with permission from the American Chemical Society, Copyright 2020. | ||
Lee et al. studied the CO2RR by using PtAu24(PET)18/C (entry 5, Table 1), which acted as the prominent catalyst for the HER.55 PtAu24(PET)18/C showed a moderate faradaic efficiency for H2 (FEH2) even in the presence of CO2, while Au25(PET)18/C exhibited a high FECO at a lower potential (entry 2, Table 5). Theoretical calculations also supported the experimental trend in terms of thermodynamic stability. In addition, the limiting potential of PtAu24(PET)18/C for the HER (0.09 eV) was much smaller than that for the CO2RR (0.74 eV), whereas the limiting potential of Au25(PET)18/C for the HER (0.32 eV) was higher than that for the CO2RR (0.09 eV). Based on these findings, they demonstrated the production of syngas with controlled ratios of H2/CO using a mixture of Au25(PET)18/C and PtAu24(PET)18/C.
The single-atom or diatomic Pt doping effect on the CO2RR for Au38(PET)24 having a biicosahedral Au23 core (entry 13, Table 1) was reported by Zhu et al. (entry 3, Table 5).60 DFT calculations on model systems revealed that all the orbitals of Pt2Au36(SH)24 and Pt1Au37(SH)24 were stabilized and destabilized, respectively, with respect to those of Au38(SH)24. These results suggested that Pt1Au37(SH)24 with the largest oxidation potential showed the highest activity in the CO2RR. In fact, Pt1Au37(PET)24/C exhibited the best jCO and FECO compared to the others. Gibbs free energy for adsorption of the COOH* intermediate supports the experimental trend: the free energy was estimated by DFT calculations to be 2.37, 2.22, and 2.40 eV for Au38(SH)24, Pt1Au37(SH)24, and Pt2Au36(SH)24, respectively. This study suggested that energy-level tuning had a large impact on the CO2RR activity even if Pt, suitable for the HER, was used as a dopant.
The doping effect of Cd on the CO2RR was investigated by Chen et al. (entry 4, Table 5) using Au25(PET)18/C, CdAu24(PET)18/C, Au19Cd3(S-tol)18/C (S-tol: p-toluenethiolate), and Au38Cd4(d-MBT)30/C (d-MBT: 3,5-dimethylthiophenolate) for comparison (entry 7, Table 1).53 Among these catalysts, CdAu24(PET)18/C exhibited the highest jCO and FECO in all the potential ranges studied. They hypothesized that the difference in the catalysis rose from that of active sites formed by S–C or M–S bond (M: metal) cleavage in the ligand layer. DFT calculations showed that the S–C bond cleavage of CdAu24(SR)18 leads to an open S site, which can efficiently bind CO2 for the CO2RR. They also investigated the effect of the R group on the catalysis of CdAu24(SR)18/C. CdAu24(S-nBu)18/C (S-nBu: 1-butanethiolate) showed a better jCO and FECO than CdAu24(PET)18/C: the DFT calculations predicted that the S–C bond of S-nBu was more easily dissociated than that of PET. Cd doping into Au25(PET)18 promoted the formation of active S sites at lower potentials due to the lower dissociation energy of the S–C bonds close to the Cd atom, thus enhancing the activity and selectivity for the CO2RR.
4.3 Other reactions
The single-atom doping effects were studied for the oxygen evolution reactions (OER, eqn (10)) and oxygen reduction reactions (ORRs, eqn (11a) and (11b)) in an acidic electrolyte.| 2H2O → O2 + 4H+ + 4e− | (10) |
| O2 + 4H+ + 4e− → 2H2O | (11a) |
| O2 + 2H+ + 2e− → H2O2 | (11b) |
Negishi et al. found that PdAu24(PET)18/C showed higher activity than Au25(PET)18/C for the OER and ORR.52
Chen et al. examined the single-atom doping effect for formic acid electrooxidation (FAO, eqn (12)).48
| HCOOH + * → CO2 + * + 2H+ + 2e− | (12) |
Although Au25(SC12)18/MCNT (MCNT = multi-wall carbon nanotube) did not catalyze FAO, PtAu24(SC12)18/MCNT catalyzed FAO without the formation of the unfavorable side product CO, and exhibited a current density about 12 and 34 times higher than that of C12S-protected Pt NC/MCNT and commercial Pt/C, respectively. The enhanced activity by Pt doping was ascribed to the modulation of the electronic structure, and the complete suppression of the formation of the poisoning CO intermediate was due to the protection of the Pt dopant at the center of the PtAu12 core. Based on the DFT-calculated potential-dependent free energies and kinetics, they proposed that the FAO on PtAu24(SC12)18/MCNTs proceeds exclusively via the direct pathway with COOH as the preferred reactive intermediate:
| HCOOH + * → COOH* + H+ + e− | (12a) |
| COOH* → CO2* + H+ + e− | (12b) |
| CO2* → CO2 + * | (12c) |
5 Photocatalysis
5.1 Photocatalytic HER and OER for water splitting
In contrast to mature research on photocatalytic water splitting,108 there have been few applications of Au/Ag NCs in photocatalysis. We herein highlight some examples showing the potential of the Au/Ag NCs as cocatalysts of photocatalytic materials and the improvement of their performance by single-atom doping with other metals.Yang et al. used MAg24(SPhMe2)18 (M = Ag, Pt) (entry 11, Table 1) as a cocatalyst of the visible-light-driven photocatalytic HER by using a graphitic carbon nitride (g-C3N4) with a narrow band gap (2.7–2.9 eV).43 PtAg24(SPhMe2)18/g-C3N4 was 5.2 times superior to Ag25(SPhMe2)18/g-C3N4, while the calcined PtAg24/g-C3N4 (entry 2, Table 3) showed 4.4 times higher activity than Ag25/g-C3N4. These results indicated that single-Pt atom doping was advantageous regardless of annealing. Photoelectrochemical characterization studies revealed that doping with a Pt atom improved the electron transfer efficiency and thus led to high activity.
Negishi et al. investigated water-splitting catalysis by using a Au25 NC supported on a semiconductor BaLa4Ti4O15 under UV light.49 Au24M(PET)18−x(pMBA)x (M = Pd, Pt, Au) (entries 4 and 5, Table 1) supported on BaLa4Ti4O15 was calcined in vacuo at 300 °C for 80 min (Fig. 5a, entries 3 and 4, Table 3) to remove PET and pMBA. Pd–K or Pt–L3 edge XAFS of the calcined catalysts suggested that Pd was located on the surface of the Au24 NC, while Pt was located at the interface between the NC and BaLa4Ti4O15 (Fig. 5a). Au24M/BaLa4Ti4O15 (M = Pt, Pd) showed higher and lower activity than the undoped Au25/BaLa4Ti4O15, respectively, in the water-splitting reaction (Fig. 5b). In contrast, Au24M/BaLa4Ti4O15 (M = Pt, Pd) improved the reaction rates for the photocatalytic HER using methanol as a sacrificial reagent (Fig. 5c). The reaction was slowed to some extent for all the catalysts in the presence of O2 (Fig. 5c). These results suggested that Pd and Pt dopants played different roles in the water-splitting activity: Au24Pd/BaLa4Ti4O15 catalyzed the photocatalytic ORR more than the photocatalytic HER, while the PtAu24 catalyst facilitated the photocatalytic HER more than the photocatalytic ORR.
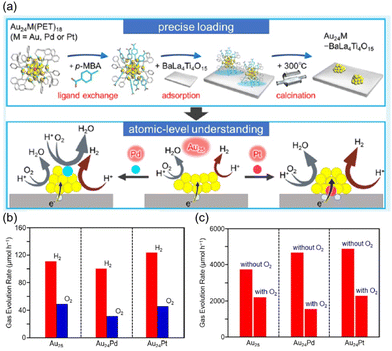 | ||
Fig. 5 (a) Schematic illustration of the synthesis procedure for the Au24M/BaLa4Ti4O15 photocatalyst (M = Au, Pd, or Pt) and the role of the dopant in the HER. (b) Hydrogen and oxygen evolution rates for Au25, Au24Pd and Au24Pt in photocatalytic water splitting under UV light. (c) Difference in the hydrogen evolution rate in the presence of methanol as a sacrificial reagent under a flow of Ar gas (without O2) and a 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 mixture ratio of Ar to air (with O2). Reproduced from ref. 49 with permission from the American Chemical Society, Copyright 2019. 3 mixture ratio of Ar to air (with O2). Reproduced from ref. 49 with permission from the American Chemical Society, Copyright 2019. | ||
Wang et al. compared the photocatalytic HER activity of MAg24(SPhMe2)18/TiO2 (M = Au, Pd, Pt) (entries 9–11, Table 1).62 The undoped Ag25(SPhMe2)18/TiO2 showed the highest activity, which was explained in terms of the M-dependent energy levels of the orbitals. The LUMO levels for M = Au and Pd were located above the conduction band minimum (CBM) of TiO2. Therefore, the photoexcited electron was not transferred from TiO2 to the NCs, leading to the suppression of activity. In contrast, the electron transfer from MAg24(SPhMe2)18 with M = Ag and Pt to TiO2 was suppressed because their LUMO levels were located below the CBM of TiO2. This explains the trend in the photocatalytic HER activity.
5.2 Photoredox catalysts
Successful application of the ligated metal NCs as a photosensitizer109,110 suggests that they can catalyze the photocatalytic reactions. MAu12(dppe)5Cl2 (M = Pt, Ir) in the electronically excited state had a lifetime of the μs timescale, leading to a remarkable photoluminescence quantum yield (PLQY) of >60% under deaerated conditions.95 Recently, Tsukuda et al. applied a series of MAu12(dppe)5Cl2 (M = Au, Pd, Pt, Rh, Ir) (entry 3, Table 1) for photoinduced intermolecular [2 + 2] cycloaddition of bisenone in the presence of LiBF4 (Lewis acid) and i-Pr2NEt (sacrificial reagent) as a model reaction.58 They found that MAu12(dppe)5Cl2 (M = Pt, Ir) with a high PLQY showed higher catalytic activity than MAu12(dppe)5Cl2 (M = Au, Pd, Rh) (Fig. 6a), indicating that the electronically excited state was involved in the catalysis. It was proposed that the reaction proceeded in an oxidative quenching cycle (Fig. 6b): the electronically excited *MAu12(8e) reduced bisenone to form MAu12(7e), which then oxidized i-Pr2NEt to recover the initial MAu12(8e). This mechanism contrasts with the reductive quenching cycle found in the conventional [Ru(bpy)3]2+ (bpy: 2,2′-bipyridine) system.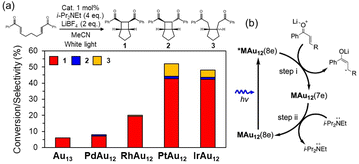 | ||
| Fig. 6 (a) Comparison of the photoredox catalytic properties of MAu12(dppe)5Cl2 (MAu12; M = Au, Pd, Pt, Rh, or Ir). (b) Proposed reaction mechanism for MAu12, a so-called oxidative quenching cycle. Reproduced from ref. 58 with permission from Wiley-VCH, Copyright 2023. | ||
6 Conclusion and outlook
This minireview summarized the synthesis, structural characterization, and catalytic application of SAD Au/Ag NCs. The SAD Au/Ag NCs introduced here have undoped counterparts composed of the same number of atoms. Furthermore, identical geometric structures of these pairs were demonstrated by SCXRD and predicted by DFT calculations. These situations provide novel opportunities to study how the catalysis is affected by replacing a single Au/Ag atom of the NCs with a dopant atom M. All SAD Au/Ag NCs showed significant single-atom doping effects on the catalytic properties of Au/Ag NCs. The role of a particular dopant M in catalysis depends largely on its location, which is governed by its interaction with the surrounding environment of the SAD Au/Ag NC. In many cases, dopants on the NC surface facilitate catalytic reactions by taking advantage of their inherently greater chemical reactivity toward small reacting molecules than that on the Au/Ag surface. In contrast, dopants located inside the NC promote catalytic reactions by modulating the electronic structure (e.g., HOMO–LUMO gap, redox potential, excited state lifetime, etc.). These simple explanations provide practical hints for designing and creating active and selective NC catalysts through doping.Nevertheless, several questions remain to be answered to better understand the doping effects in catalysis. (1) The most important and straightforward question is: Is there any synergistic effect between dopant M and the matrix NCs? In other words, do the chemical properties of the dopant M site and underlying NC surface change through electronic interactions? One way to address such synergistic effects is to compare the catalytic properties of M-doped NCs with those of pure M NCs. We recently reported that the Pd dopant in Au23Pd1 exhibited significantly higher catalytic activity than Pd NPs (1.6 nm) and Pd NPs (2.5 nm), which we attribute to the higher electronegativity of Pd in Pd1Au23 than in Pd NPs. (2) The second question is: How does the structural fluxionality of SAD metal NCs affect their catalytic properties? For example, the structural strain induced by dopants with different atomic radii will affect the electronic structure of the matrix NC. Dynamic structural changes, including M diffusion, induced by the chemisorption of the reactants or thermal excitation, result in the fluctuation of the electronic structure of the mother NC. To answer these challenging questions, in situ structural characterization is necessary.
In addition, the following synthetic challenges for each type of SAD metal NC should be tackled. (1) An inherent problem in applying the ligand-protected SAD metal NCs for thermal reactions is the poor reactivity due to surface poisoning by the ligands. Thus, a challenge to overcome this problem is to design and create open sites. One approach is to reduce the surface coverage via steric hindrance between the adjacent ligands. Successful examples can be found in [Au23(Ph3P)10(dpa)2Cl]2+ (dpa = dipyridylamide)111 and Au44(NHCiPr)9(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CPh)6Br8 (NHCiPr = 1,3-diisopropylbenzimidazolin-2-ylidene),112 which have uncoordinated Au atoms on the surface and show excellent catalytic activity in the alcohol oxidation reaction and hydration of alkynes, respectively. By applying this approach to SAD metal NCs, it would be possible to construct NC catalysts with atomically designed reaction sites for active and selective catalysis. (2) A problem in the linear-polymer-stabilized SAD metal NCs is the uncontrollability of their size and insufficient stability under catalytic conditions. One approach for overcoming these problems is to use dendrimers as a container. The NC size can be controlled by the number of metal ions initially coordinated within the dendrimer. The stability of the resulting NCs in the dendrimer would be higher than that of the NCs stabilized by linear polymers, because the former is sterically encapsulated within the cavity of the dendrimers. Yamamoto et al. successfully synthesized and characterized alloy NCs using their original dendrimers.113 (3) An inherent issue of the solid-supported SAD metal NCs is the uncontrollability of the geometric structures. This problem can be solved by using molecular metal oxides such as polyoxometalates as a support. Suzuki and Yamaguchi successfully obtained atomically precise Ag NCs with well-defined structures and exposed surfaces, such as [Ag7(Si3W27O96)],114 [Ag27(Si6W54O198)],115 and [Ag30(P8W48O184)].116 There is also room for improving robustness under harsh reaction conditions. Masuda and Tsukuda improved the robustness of Au25 catalysts by intentionally and selectively leaving the thiolates located at the interface with the carbon support.117 Multiple van der Waals interactions between the ligands and the carbon support immobilized the Au25 NCs. Application of these approaches may realize supported SAD metal NCs with atomically defined structures.
CPh)6Br8 (NHCiPr = 1,3-diisopropylbenzimidazolin-2-ylidene),112 which have uncoordinated Au atoms on the surface and show excellent catalytic activity in the alcohol oxidation reaction and hydration of alkynes, respectively. By applying this approach to SAD metal NCs, it would be possible to construct NC catalysts with atomically designed reaction sites for active and selective catalysis. (2) A problem in the linear-polymer-stabilized SAD metal NCs is the uncontrollability of their size and insufficient stability under catalytic conditions. One approach for overcoming these problems is to use dendrimers as a container. The NC size can be controlled by the number of metal ions initially coordinated within the dendrimer. The stability of the resulting NCs in the dendrimer would be higher than that of the NCs stabilized by linear polymers, because the former is sterically encapsulated within the cavity of the dendrimers. Yamamoto et al. successfully synthesized and characterized alloy NCs using their original dendrimers.113 (3) An inherent issue of the solid-supported SAD metal NCs is the uncontrollability of the geometric structures. This problem can be solved by using molecular metal oxides such as polyoxometalates as a support. Suzuki and Yamaguchi successfully obtained atomically precise Ag NCs with well-defined structures and exposed surfaces, such as [Ag7(Si3W27O96)],114 [Ag27(Si6W54O198)],115 and [Ag30(P8W48O184)].116 There is also room for improving robustness under harsh reaction conditions. Masuda and Tsukuda improved the robustness of Au25 catalysts by intentionally and selectively leaving the thiolates located at the interface with the carbon support.117 Multiple van der Waals interactions between the ligands and the carbon support immobilized the Au25 NCs. Application of these approaches may realize supported SAD metal NCs with atomically defined structures.
By solving the challenges listed above, we can expand the scope and deepen our understanding of the doping effects on catalysis. The basic knowledge with the help of an AI-based machine learning approach will accelerate the development of on-demand alloy catalysts in the future.
Author contributions
S. M. and T. T. prepared the draft of the manuscript. S. M. and K. S. summarized the related papers and wrote the manuscript. T. T. revised the manuscript prior to submission.Note added in proof
An expression of concern for the chemical composition in Ref. 63 has been issued (S. E. Skrabalak, Chem. Mater., 2023, 35, 9817).Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was financially supported by JST, CREST (grant no. JPMJCR20B2) and a grant-in-aid for Scientific Research (A) (grant no. JP23H00284) and for Early-Career Scientists (grant no. JP23K13617) from the Japan Society for the Promotion of Science (JSPS).References
- N. T. S. Phan, M. Van Der Sluys and C. W. Jones, Adv. Synth. Catal., 2006, 348, 609–679 CrossRef CAS.
- T. Tsukuda, H. Tsunoyama and H. Sakurai, Chem. – Asian J., 2011, 6, 736–748 CrossRef CAS PubMed.
- L. Liu and A. Corma, Chem. Rev., 2018, 118, 4981–5079 CrossRef CAS PubMed.
- C. Mondelli, G. Gözaydın, N. Yan and J. Pérez-Ramírez, Chem. Soc. Rev., 2020, 49, 3764–3782 RSC.
- M. R. Axet and K. Philippot, Chem. Rev., 2020, 120, 1085–1145 CrossRef CAS PubMed.
- V. Ponec, Appl. Catal., A, 2001, 222, 31–45 CrossRef CAS.
- H. Miura and T. Shishido, Chem. Lett., 2021, 50, 346–352 CrossRef CAS.
- M. Zhou, C. Li and J. Fang, Chem. Rev., 2021, 121, 736–795 CrossRef CAS PubMed.
- J. W. M. Crawley, I. E. Gow, N. Lawes, I. Kowalec, L. Kabalan, C. R. A. Catlow, A. J. Logsdail, S. H. Taylor, N. F. Dummer and G. J. Hutchings, Chem. Rev., 2022, 122, 6795–6849 CrossRef CAS PubMed.
- L. Liu and A. Corma, Chem. Rev., 2023, 123, 4855–4933 CrossRef CAS PubMed.
- Y. Nakaya and S. Furukawa, Chem. Rev., 2023, 123, 5859–5947 CrossRef CAS PubMed.
- R. Jin, C. Zeng, M. Zhou and Y. Chen, Chem. Rev., 2016, 116, 10346–10413 CrossRef CAS PubMed.
- I. Chakraborty and T. Pradeep, Chem. Rev., 2017, 117, 8208–8271 CrossRef CAS PubMed.
- J. Yan, B. K. Teo and N. Zheng, Acc. Chem. Res., 2018, 51, 3084–3093 CrossRef CAS PubMed.
- Q. Yao, T. Chen, X. Yuan and J. Xie, Acc. Chem. Res., 2018, 51, 1338–1348 CrossRef CAS PubMed.
- Z. Lei, X.-K. Wan, S.-F. Yuan, Z.-J. Guan and Q.-M. Wang, Acc. Chem. Res., 2018, 51, 2465–2474 CrossRef CAS PubMed.
- A. Ghosh, O. F. Mohammed and O. M. Bakr, Acc. Chem. Res., 2018, 51, 3094–3103 CrossRef CAS PubMed.
- X. Kang, H. Chong and M. Zhu, Nanoscale, 2018, 10, 10758–10834 RSC.
- S. Wang, Q. Li, X. Kang and M. Zhu, Acc. Chem. Res., 2018, 51, 2784–2792 CrossRef CAS PubMed.
- W. W. Xu, X. C. Zeng and Y. Gao, Acc. Chem. Res., 2018, 51, 2739–2747 CrossRef CAS PubMed.
- K. Kwak and D. Lee, Acc. Chem. Res., 2019, 52, 12–22 CrossRef CAS PubMed.
- H. Hirai, S. Ito, S. Takano, K. Koyasu and T. Tsukuda, Chem. Sci., 2020, 11, 12233–12248 RSC.
- X. Kang, Y. Li, M. Zhu and R. Jin, Chem. Soc. Rev., 2020, 49, 6443–6514 RSC.
- T. Kawawaki, Y. Imai, D. Suzuki, S. Kato, I. Kobayashi, T. Suzuki, R. Kaneko, S. Hossain and Y. Negishi, Chem. – Eur. J., 2020, 26, 16150–16193 CrossRef CAS PubMed.
- S. Takano and T. Tsukuda, J. Am. Chem. Soc., 2021, 143, 1683–1698 CrossRef CAS PubMed.
- T. Omoda, S. Takano and T. Tsukuda, Small, 2021, 17, 2001439 CrossRef CAS PubMed.
- T. Kawawaki, A. Ebina, Y. Hosokawa, S. Ozaki, D. Suzuki, S. Hossain and Y. Negishi, Small, 2021, 17, 2005328 CrossRef CAS PubMed.
- Y. Liu, J. Yu, Y. Lun, Y. Wang, Y. Wang and S. Song, Adv. Funct. Mater., 2023, 33, 2304184 CrossRef CAS.
- A. V. Artem'ev and C. W. Liu, Chem. Commun., 2023, 59, 7182–7195 RSC.
- H. Liang, Q. Chen, Q.-L. Mo, Y. Wu and F.-X. Xiao, J. Mater. Chem. A, 2023, 11, 9401–9426 RSC.
- M. F. Matus and H. Häkkinen, Nat. Rev. Mater., 2023, 8, 372–389 CrossRef CAS.
- S. Hossain, D. Hirayama, A. Ikeda, M. Ishimi, S. Funaki, A. Samanta, T. Kawawaki and Y. Negishi, Aggregate, 2023, 4, e255 CrossRef CAS.
- Y. Li and R. Jin, Nanoscale Horiz., 2023, 8, 991–1013 RSC.
- G. Giannakakis, M. Flytzani-Stephanopoulos and E. C. H. Sykes, Acc. Chem. Res., 2019, 52, 237–247 CrossRef CAS PubMed.
- R. T. Hannagan, G. Giannakakis, M. Flytzani-Stephanopoulos and E. C. H. Sykes, Chem. Rev., 2020, 120, 12044–12088 CrossRef CAS PubMed.
- T. Zhang, A. G. Walsh, J. Yu and P. Zhang, Chem. Soc. Rev., 2021, 50, 569–588 RSC.
- T. Shen, S. Wang, T. Zhao, Y. Hu and D. Wang, Adv. Energy Mater., 2022, 12, 2201823 CrossRef CAS.
- J. D. Lee, J. B. Miller, A. V. Shneidman, L. Sun, J. F. Weaver, J. Aizenberg, J. Biener, J. A. Boscoboinik, A. C. Foucher, A. I. Frenkel, J. E. S. van der Hoeven, B. Kozinsky, N. Marcella, M. M. Montemore, H. T. Ngan, C. R. O'Connor, C. J. Owen, D. J. Stacchiola, E. A. Stach, R. J. Madix, P. Sautet and C. M. Friend, Chem. Rev., 2022, 122, 8758–8808 CrossRef CAS PubMed.
- S. Xie, H. Tsunoyama, W. Kurashige, Y. Negishi and T. Tsukuda, ACS Catal., 2012, 2, 1519–1523 CrossRef CAS.
- H. Qian, D.-E. Jiang, G. Li, C. Gayathri, A. Das, R. R. Gil and R. Jin, J. Am. Chem. Soc., 2012, 134, 16159–16162 CrossRef CAS PubMed.
- N. Yan, L. Liao, J. Yuan, Y.-J. Lin, L.-H. Weng, J. Yang and Z. Wu, Chem. Mater., 2016, 28, 8240–8247 CrossRef CAS.
- K. Kwak, W. Choi, Q. Tang, M. Kim, Y. Lee, D.-E. Jiang and D. Lee, Nat. Commun., 2017, 8, 14723 CrossRef PubMed.
- X. L. Du, X. L. Wang, Y. H. Li, Y. L. Wang, J. J. Zhao, L. J. Fang, L. R. Zheng, H. Tong and H. G. Yang, Chem. Commun., 2017, 53, 9402–9405 RSC.
- S. Hayashi, R. Ishida, S. Hasegawa, S. Yamazoe and T. Tsukuda, Top. Catal., 2018, 61, 136–141 CrossRef CAS.
- S. Hasegawa, S. Takano, S. Yamazoe and T. Tsukuda, Chem. Commun., 2018, 54, 5915–5918 RSC.
- Y. Liu, X. Chai, X. Cai, M. Chen, R. Jin, W. Ding and Y. Zhu, Angew. Chem., Int. Ed., 2018, 57, 9775–9779 CrossRef CAS PubMed.
- W. Choi, G. Hu, K. Kwak, M. Kim, D.-E. Jiang, J.-P. Choi and D. Lee, ACS Appl. Mater. Interfaces, 2018, 10, 44645–44653 CrossRef CAS PubMed.
- Y. Lu, C. Zhang, X. Li, A. R. Frojd, W. Xing, A. Z. Clayborne and W. Chen, Nano Energy, 2018, 50, 316–322 CrossRef CAS.
- W. Kurashige, R. Hayashi, K. Wakamatsu, Y. Kataoka, S. Hossain, A. Iwase, A. Kudo, S. Yamazoe and Y. Negishi, ACS Appl. Energy Mater., 2019, 2, 4175–4187 CrossRef CAS.
- J. Xu, S. Xu, M. Chen and Y. Zhu, Nanoscale, 2020, 12, 6020–6028 RSC.
- S. Li, D. Alfonso, A. V. Nagarajan, S. D. House, J. C. Yang, D. R. Kauffman, G. Mpourmpakis and R. Jin, ACS Catal., 2020, 10, 12011–12016 CrossRef CAS.
- B. Kumar, T. Kawawaki, N. Shimizu, Y. Imai, D. Suzuki, S. Hossain, L. V. Nair and Y. Negishi, Nanoscale, 2020, 12, 9969–9979 RSC.
- Y. Sun, X. Liu, K. Xiao, Y. Zhu and M. Chen, ACS Catal., 2021, 11, 11551–11560 CrossRef CAS.
- X. Cai, X. Sui, J. Xu, A. Tang, X. Liu, M. Chen and Y. Zhu, CCS Chem., 2021, 3, 408–420 CrossRef CAS.
- W. Choi, H. Seong, V. Efremov, Y. Lee, S. Im, D.-H. Lim, J. S. Yoo and D. Lee, J. Chem. Phys., 2021, 155, 014305 CrossRef CAS PubMed.
- X. Li, S. Takano and T. Tsukuda, J. Phys. Chem. C, 2021, 125, 23226–23230 CrossRef CAS.
- Y. Jo, M. Choi, M. Kim, J. S. Yoo, W. Choi and D. Lee, Bull. Korean Chem. Soc., 2021, 42, 1672–1677 CrossRef CAS.
- H. Hirai, S. Takano, T. Nakashima, T. Iwasa, T. Taketsugu and T. Tsukuda, Angew. Chem., Int. Ed., 2022, 61, e202207290 CrossRef CAS PubMed.
- S. Hasegawa, S. Masuda, S. Takano, K. Harano, J. Kikkawa and T. Tsukuda, ACS Nano, 2022, 16, 16932–16940 CrossRef CAS PubMed.
- X. Liu, E. Wang, M. Zhou, Y. Wan, Y. Zhang, H. Liu, Y. Zhao, J. Li, Y. Gao and Y. Zhu, Angew. Chem., Int. Ed., 2022, 61, e202207685 CrossRef CAS PubMed.
- Y. Li, H. K. Kim, R. D. McGillicuddy, S.-L. Zheng, K. J. Anderton, G. J. Stec, J. Lee, D. Cui and J. A. Mason, J. Am. Chem. Soc., 2023, 145, 9304–9312 CrossRef CAS PubMed.
- Y. Liu, D. Long, A. Springer, R. Wang, N. Koch, M. Schwalbe, N. Pinna and Y. Wang, Sol. RRL, 2023, 7, 2201057 CrossRef CAS.
- S. Mukherjee, A. Das, A. K. Das, A. Sheriff, K. Sunny, A. S. Nair, S. Bhandary, R. Bhowal, D. Chopra, B. Pathak, S. Yamazoe and S. Mandal, Chem. Mater., 2023, 35, 1659–1666 CrossRef CAS.
- P. D. Jadzinsky, G. Calero, C. J. Ackerson, D. A. Bushnell and R. D. Kornberg, Science, 2007, 318, 430–433 CrossRef CAS PubMed.
- M. W. Heaven, A. Dass, P. S. White, K. M. Holt and R. W. Murray, J. Am. Chem. Soc., 2008, 130, 3754–3755 CrossRef CAS PubMed.
- M. Zhu, C. M. Aikens, F. J. Hollander, G. C. Schatz and R. Jin, J. Am. Chem. Soc., 2008, 130, 5883–5885 CrossRef CAS PubMed.
- J. Yan, H. Su, H. Yang, S. Malola, S. Lin, H. Häkkinen and N. Zheng, J. Am. Chem. Soc., 2015, 137, 11880–11883 CrossRef CAS PubMed.
- M. S. Bootharaju, C. P. Joshi, M. R. Parida, O. F. Mohammed and O. M. Bakr, Angew. Chem., Int. Ed., 2016, 55, 922–926 CrossRef CAS PubMed.
- S. Takano, S. Ito and T. Tsukuda, J. Am. Chem. Soc., 2019, 141, 15994–16002 CrossRef CAS PubMed.
- W. Fei, S. Antonello, T. Dainese, A. Dolmella, M. Lahtinen, K. Rissanen, A. Venzo and F. Maran, J. Am. Chem. Soc., 2019, 141, 16033–16045 CrossRef CAS PubMed.
- S. Yamazoe, T. Yoskamtorn, S. Takano, S. Yadnum, J. Limtrakul and T. Tsukuda, Chem. Rec., 2016, 16, 2338–2348 CrossRef CAS PubMed.
- S. Yamazoe and T. Tsukuda, Bull. Chem. Soc. Jpn., 2018, 92, 193–204 CrossRef.
- S. Hasegawa, S. Takano, K. Harano and T. Tsukuda, JACS Au, 2021, 1, 660–668 CrossRef CAS PubMed.
- S. Hasegawa, S. Masuda, S. Takano, K. Harano and T. Tsukuda, ACS Catal., 2022, 12, 6550–6558 CrossRef CAS.
- Y. Negishi, W. Kurashige, Y. Niihori, T. Iwasa and K. Nobusada, Phys. Chem. Chem. Phys., 2010, 12, 6219–6225 RSC.
- A. Bruma, F. R. Negreiros, S. Xie, T. Tsukuda, R. L. Johnston, A. Fortunelli and Z. Y. Li, Nanoscale, 2013, 5, 9620–9625 RSC.
- S. Zhao, R. Jin and R. Jin, ACS Energy Lett., 2018, 3, 452–462 CrossRef CAS.
- J. Zhao and R. Jin, Nano Today, 2018, 18, 86–102 CrossRef CAS.
- T. Higaki, Y. Li, S. Zhao, Q. Li, S. Li, X.-S. Du, S. Yang, J. Chai and R. Jin, Angew. Chem., Int. Ed., 2019, 58, 8291–8302 CrossRef CAS PubMed.
- Y. Du, H. Sheng, D. Astruc and M. Zhu, Chem. Rev., 2020, 120, 526–622 CrossRef CAS PubMed.
- R. Jin, G. Li, S. Sharma, Y. Li and X. Du, Chem. Rev., 2021, 121, 567–648 CrossRef CAS PubMed.
- T. Kawawaki, Y. Kataoka, M. Hirata, Y. Iwamatsu, S. Hossain and Y. Negishi, Nanoscale Horiz., 2021, 6, 409–448 RSC.
- S. Li, W. Tian and Y. Liu, Nanoscale, 2021, 13, 16847–16859 RSC.
- A. G. Walsh and P. Zhang, Adv. Mater. Interfaces, 2021, 8, 2001342 CrossRef CAS.
- X. Cai, Y. Sun, J. Xu and Y. Zhu, Chem. – Eur. J., 2021, 27, 11539–11547 CrossRef CAS PubMed.
- X. Cai, G. Li, W. Hu and Y. Zhu, ACS Catal., 2022, 12, 10638–10653 CrossRef CAS.
- D. Yang, J. Wang, Q. Wang, Z. Yuan, Y. Dai, C. Zhou, X. Wan, Q. Zhang and Y. Yang, ACS Nano, 2022, 16, 15681–15704 CrossRef CAS PubMed.
- W. Jing, H. Shen, R. Qin, Q. Wu, K. Liu and N. Zheng, Chem. Rev., 2023, 123, 5948–6002 CrossRef CAS PubMed.
- X. Liu, X. Cai and Y. Zhu, Acc. Chem. Res., 2023, 56, 1528–1538 CrossRef CAS PubMed.
- S. N. Khanna and P. Jena, Phys. Rev. Lett., 1992, 69, 1664–1667 CrossRef CAS PubMed.
- S. N. Khanna and P. Jena, Phys. Rev. B: Condens. Matter Mater. Phys., 1995, 51, 13705–13716 CrossRef CAS PubMed.
- P. Jena and Q. Sun, Chem. Rev., 2018, 118, 5755–5870 CrossRef CAS PubMed.
- C. Garcia, V. Truttmann, I. Lopez, T. Haunold, C. Marini, C. Rameshan, E. Pittenauer, P. Kregsamer, K. Dobrezberger, M. Stöger-Pollach, N. Barrabés and G. Rupprechter, J. Phys. Chem. C, 2020, 124, 23626–23636 CrossRef CAS PubMed.
- S. Takano, H. Hirai, S. Muramatsu and T. Tsukuda, J. Am. Chem. Soc., 2018, 140, 12314–12317 CrossRef CAS PubMed.
- H. Hirai, S. Takano, T. Nakamura and T. Tsukuda, Inorg. Chem., 2020, 59, 17889–17895 CrossRef CAS PubMed.
- D. R. Alfonso, D. Kauffman and C. Matranga, J. Chem. Phys., 2016, 144, 184705 CrossRef PubMed.
- N. Austin, S. Zhao, J. R. McKone, R. Jin and G. Mpourmpakis, Catal. Sci. Technol., 2018, 8, 3795–3805 RSC.
- S. Zhao, N. Austin, M. Li, Y. Song, S. D. House, S. Bernhard, J. C. Yang, G. Mpourmpakis and R. Jin, ACS Catal., 2018, 8, 4996–5001 CrossRef CAS.
- H. Seong, V. Efremov, G. Park, H. Kim, J. S. Yoo and D. Lee, Angew. Chem., Int. Ed., 2021, 60, 14563–14570 CrossRef CAS PubMed.
- F. Sun, L. Qin, Z. Tang, G. Deng, M. S. Bootharaju, Z. Wei, Q. Tang and T. Hyeon, Chem. Sci., 2023, 14, 10532–10546 RSC.
- O. López-Estrada, N. Mammen, L. Laverdure, M. M. Melander, H. Häkkinen and K. Honkala, ACS Catal., 2023, 13, 8997–9006 CrossRef.
- T. Imaoka, R. Tanaka, S. Arimoto, M. Sakai, M. Fujii and K. Yamamoto, J. Am. Chem. Soc., 2005, 127, 13896–13905 CrossRef CAS PubMed.
- K. Yamamoto and T. Imaoka, Acc. Chem. Res., 2014, 47, 1127–1136 CrossRef CAS PubMed.
- V. Sudheeshkumar, K. O. Sulaiman and R. W. J. Scott, Nanoscale Adv., 2020, 2, 55–69 RSC.
- N. Mahmood, Y. Yao, J.-W. Zhang, L. Pan, X. Zhang and J.-J. Zou, Adv. Sci., 2018, 5, 1700464 CrossRef PubMed.
- D. R. Kauffman, D. Alfonso, C. Matranga, H. Qian and R. Jin, J. Am. Chem. Soc., 2012, 134, 10237–10243 CrossRef CAS PubMed.
- W. Zhu, R. Michalsky, Ö. Metin, H. Lv, S. Guo, C. J. Wright, X. Sun, A. A. Peterson and S. Sun, J. Am. Chem. Soc., 2013, 135, 16833–16836 CrossRef CAS PubMed.
- Z. Wang, C. Li and K. Domen, Chem. Soc. Rev., 2019, 48, 2109–2125 RSC.
- H. Kawasaki, S. Kumar, G. Li, C. Zeng, D. R. Kauffman, J. Yoshimoto, Y. Iwasaki and R. Jin, Chem. Mater., 2014, 26, 2777–2788 CrossRef CAS.
- K. Isozaki, R. Ueno, K. Ishibashi, G. Nakano, H. Yin, K. Iseri, M. Sakamoto, H. Takaya, T. Teranishi and M. Nakamura, ACS Catal., 2021, 11, 13180–13187 CrossRef CAS.
- S.-F. Yuan, Z. Lei, Z.-J. Guan and Q.-M. Wang, Angew. Chem., Int. Ed., 2021, 60, 5225–5229 CrossRef CAS PubMed.
- H. Shen, Z. Xu, M. S. A. Hazer, Q. Wu, J. Peng, R. Qin, S. Malola, B. K. Teo, H. Häkkinen and N. Zheng, Angew. Chem., Int. Ed., 2021, 60, 3752–3758 CrossRef CAS PubMed.
- K. Yamamoto, T. Imaoka, M. Tanabe and T. Kambe, Chem. Rev., 2020, 120, 1397–1437 CrossRef CAS PubMed.
- K. Yonesato, H. Ito, D. Yokogawa, K. Yamaguchi and K. Suzuki, Angew. Chem., Int. Ed., 2020, 59, 16361–16365 CrossRef CAS PubMed.
- K. Yonesato, H. Ito, H. Itakura, D. Yokogawa, T. Kikuchi, N. Mizuno, K. Yamaguchi and K. Suzuki, J. Am. Chem. Soc., 2019, 141, 19550–19554 CrossRef CAS PubMed.
- K. Yonesato, D. Yanai, S. Yamazoe, D. Yokogawa, T. Kikuchi, K. Yamaguchi and K. Suzuki, Nat. Chem., 2023, 15, 940–947 CrossRef CAS PubMed.
- K. Sakamoto, S. Masuda, S. Takano and T. Tsukuda, ACS Catal., 2023, 13, 3263–3271 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |