Open Access Article
Open Access ArticleA review on the photochemical synthesis of atomically dispersed catalysts
Shaohua
Chen
and
Pengxin
Liu
 *
*
School of Physical Science and Technology, ShanghaiTech University, Shanghai 201210, P. R. China. E-mail: liupx@shanghaitech.edu.cn
First published on 14th December 2023
Abstract
A better world calls for better catalysts. With the increasing demand for low carbon emission processes, both the efficiency and environmentally benign synthesis of catalytic materials should be considered. As an emerging synthetic method for atomically dispersed catalysts, photochemical synthesis has several advantages, including its green process and ability to precisely control the structure of active sites. This review presents the research progress over the last decade, from the very first examples to developed cases on the topic and the aspects of the synthetic strategy, mechanism analysis, and application potential. The key factors affecting the preparation of atomically dispersed catalysts by the photochemical method are thoroughly discussed. More importantly, major challenges in developing the photochemical method, among other methods for synthesizing atomically dispersed catalysts, are highlighted; suggestions are also provided. By keeping abreast of these advances, we foresee the fast and broad development of photochemical methods in the catalysis community.
1. Introduction
Heterogeneous supported catalysts are widely used in the chemical industry owing to their robustness and easy separation, yet their activities and utilization efficiencies of metal atoms are often significantly lower than those of homogeneous molecular catalysts.1–3 To achieve better catalytic performance and higher utilization efficiency of active components, tuning the particle sizes of supported species is a well-documented strategy. Reducing the sizes of supported active components often leads to a boost in catalyst activity, which was first demonstrated in gold-based systems; gold exhibited a high catalytic activity in catalytic CO oxidation when the sizes of the supported gold were reduced to the nanoscale.4 The effect was later confirmed in other systems and was collectively termed as the “size effect”.5,6 One might naturally be curious about the extreme effects in downsizing the supported metal species and its impact on the catalytic performance, but the synthetic methods and characterization techniques in use at that time held back such a curiosity.The concept of single-atom catalysis was proposed for the first time in 2011,7 with the successful synthesis (co-precipitation strategy) and characterization of a Pt1/FeOx catalyst, aided by combined X-ray absorption spectroscopy (XAS), aberration-corrected scanning transmission electron microscopy (AC-STEM), and Fourier-transform infrared (FT-IR) spectroscopy. After over a decade of development, single-atom catalysis has become a central aspect of heterogeneous catalysis and has shown immense potential in areas such as energy,8–10 environment,11,12 and chemical synthesis.13–15 It is now generally accepted that single-atom catalysts have a high atomic utilization efficiency and, therefore, a better catalytic performance in comparison to nanocatalysts.
It should be noted that the use of the term “single-atom catalyst” is not widely accepted in the field. Other terms like “atomically dispersed catalyst”16,17 and “single-atom site catalyst”18 are also in use, with “single-site catalyst” representing another class of catalysts.19–21 “Single-atom catalyst” emphasizes the nuclearity, whereby the active sites of a single-atom catalyst should be mononuclear atoms/ions, but the uniformity of the structures of supported single atoms, especially the coordination structure, does not need to be uniform.2,21–25 Lately, “dual-metal-sites catalyst” was also proposed to describe two different metal species in close proximity, emphasizing the nuclearity but not the uniformity of the structures.26 Alternatively, “single-site catalyst” emphasizes the uniformity of active sites in the pursuit of maintaining the structural uniformity of molecular catalysts (used as precursors for the synthesis of single-site catalysts). We prefer the term “atomically dispersed catalysts” as it includes the information about the atomic dispersion and supported structure. This term is also more general, covering all kinds of “single-atom catalysts” and a part of the “single-site catalysts”.21 In this work, we use the term “atomically dispersed catalysts” to describe the object of the present study.
The synthesis of spatially separated and structurally secured atomically dispersed catalysts is of great interest in both industrial catalysis and scientific research.18,27–32 Significant efforts have been devoted towards achieving this goal. From different viewpoints, the synthesis of atomically dispersed catalysts can be classified into several categories: top-down methods vs. bottom-up methods, or conventional methods vs. emerging methods.29–32 Some examples of bottom-up methods include the wet impregnation method, chemical vapour deposition (CVD), electrochemical method (EM), and atomic layer deposition (ALD), of which the wet impregnation method is also a conventional method to prepare traditional supported catalysts. The advantages and disadvantages of the above-mentioned methods for synthesizing atomically dispersed catalysts have been nicely summarized and reviewed.33–35 Nevertheless, one emerging approach that has attracted some research interests in recent years but that still lacks a progress review is the photochemical method.
Consequently, in this review, we provide such a review, starting from the basic principles of the photochemical method, and then summarizing the research progresses on atomically dispersed catalysts prepared by this method in recent years. The preparation methods are next classified according to the different preparation conditions. Then, the photochemical method for synthesizing atomically dispersed catalysts is compared with the method for synthesizing nanoparticles, focusing on the key factors affecting the synthesis, including the supports, ligands, sacrificial agents, light source, and temperature. Finally, based on recent developments in related fields, the key challenges and opportunities for this method are outlined, and the future development direction is also suggested.
2. Fundamental principles
2.1. Principle behind the photodeposition of nanoparticles
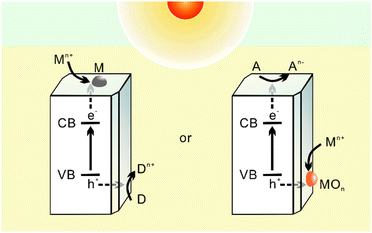
The photochemical method for preparing atomically dispersed catalysts was inspired by the photodeposition of nanoparticles over semiconductors, which is a well-established method to modify photocatalysts to achieve better photocatalytic performance.36 Such a method dates back to 1978, when Kraeutler et al. accomplished the successful loading of well-dispersed Pt nanoparticles onto a TiO2 support, initiating research on the optical deposition of metal (oxide) nanoparticles on semiconductor surfaces.37 After decades of development, photodeposition has also been found to be useful for preparing supported catalysts for other reactions apart from just photocatalysis, as has been reviewed lately.38–40 Due to its mild, simple, green, and safe reaction conditions, photodeposition methods can enable the simple, rapid, and scalable production of metal nanoparticles without deploying dangerous reagents or complex equipment, thereby exhibiting high potential for application purposes.In general, photochemical methods employ light to stimulate chemical reactions and regulate the synthesis of nanomaterials. It is commonly accepted that the reaction process in this method typically involves three steps (Fig. 1a): (i) photoelectric separation occurs after the semiconductor absorbs photons, whereby electrons enter the conduction band (CB) from the valence band (VB), leaving holes in the VB; (ii) photogenerated electrons and holes transfer to the surface, during which some CB electrons and VB holes in the excited state may recombine and dissipate energy in the form of heat; (iii) the residual electrons or holes on the semiconductor surface chemically react with metal ions to deposit them, through reductive or oxidative photodeposition. Here, reductive photodeposition (1) is initiated through the reduction of the metal precursor from n+-valence Mn+ to zero-valence M0 by excited electrons on the semiconductor surface, forming nuclear centres that grow and subsequently aggregate to form metal nanoparticles; while oxidative photodeposition (2) refers to the deposition of metal in the form of oxides on the semiconductor by utilizing the available holes:
| Mn+(aq) + ne− → M(s) | (1) |
| Mn+(aq) + nh+ + nH2O → MOx(s) + 2nH+ | (2) |
Note, these equations are over-simplified and purely theoretical; whereas the true processes are much more complicated, please refer to Part 4.1.
From a mechanism understanding, one can easily conclude the principles of photodeposition: (i) the metal (oxide) being deposited must have a reduction/oxidation potential that is favourable in relation to the semiconductor band position, which means that CB should be more negative than the reduction potential of the metal, and the VB should be more positive than the oxidation potential of the oxidized substance; (ii) the photon energy of the incident light must surpass the band gap energy of the semiconductor; And (iii) the effective separation and migration of photogenerated carriers are necessary.
2.2. Photodeposition of single atoms
Apparently, photodeposition belongs to the category of bottom-up synthetic methods for supported catalysts. For any bottom-up method, decreasing the batching of the metal precursor to an extreme will undoubtedly lead to the formation of atomically dispersed catalysts, if the supported species can be stabilized on the surface of the chosen supports.27 This is the strategy used in the first examples of preparing atomically dispersed catalysts through traditional photodeposition methods. In this regard, the loading amount of active sites would be quite low, the same as with the cases for atomically dispersed catalysts prepared through wet chemistry (impregnation, co-precipitation) or other depositions (CVD, ALD). Several improved methods have been developed since to increase the loading of active atoms, see Part 3 below.In comparison to other bottom-up methods, the photochemical method possesses several advantages: (i) the photodeposition is a surface reaction and no other reductant/oxidant is needed; hence the photodeposition would only happen on the surface of the supports, and no phase separation (as might happen in conventional methods) would occur; (ii) the method typically requires mild reaction conditions, simple operations with a simple equipment (usually only one light source lamp), and minimal energy consumption; (iii) precise regulation of the properties of an atomically dispersed catalyst can be achieved by adjusting the light intensity, illumination time, temperature, reactant concentration, and supports. This regulation allows for customized catalytic performance through manipulation of the type, concentration, and distribution of the active sites. The method is therefore comparably more flexible. Overall, the photochemical method possesses notable advantages in comparison to conventional methods.
3. Photochemical synthesis of atomically dispersed catalysts
Since the photochemical method debuted, it has become a highlight in the field of single-atom catalysis. Even long before the term “single-atom catalysis” was proposed, numerous studies have shown that even atomically dispersed species could be catalytically active.41 Yet, whether single atoms were truly responsible for most supported catalysts, other than the supported nanoparticles, was a long-standing debate. It was not until Maria et al. demonstrated that only isolated Au–(OH)x species (photochemically produced) were active for water–gas shift reactions, while Au nanoparticles were inactive, that the question was finally answered.42 We believe Maria's work began the application of the photochemical synthetic method in the field of single-atom catalysis. Other significant milestones in the past decade are illustrated in Fig. 2. We summarize these reports into two groups, depending on the reaction conditions: liquid-phase and solid-phase photochemical syntheses.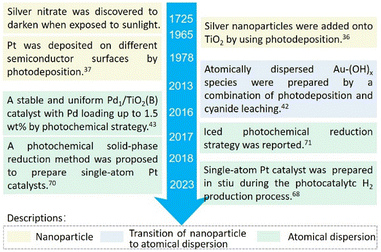 | ||
| Fig. 2 Timeline of the noteworthy milestones in the preparation of atomically dispersed catalysts by the photochemical method. | ||
3.1. Liquid-phase photochemical synthesis
The conventional photodeposition of nanoparticles typically occurs in a liquid medium, which offers a free environment for the precursors’ movement and facilitates a more uniform dispersion of supported nanoparticles in terms of their particle size and distribution. The first examples of the photochemical synthesis of atomically dispersed catalysts were also carried out in the liquid phase. The successful preparation was achieved through a selective etching, metal loading lowering, or using supports with a large surface area. Some atomically dispersed catalysts prepared by photochemical methods in the liquid phase are summarized in Table 1.| Metal/support | Precursor | Ligand | Reagent | Light source | Exposure | Content | Valence state | Ref. |
|---|---|---|---|---|---|---|---|---|
| Au/TiO2 | HAuCl4 | — | Ethanol | 365 nm/10 mW cm−2 | 10 min | 1.16 wt% | Au0 | 42 |
| Pd1/TiO2(B) | H2PdCl4 | EG | — | 365 nm/1.94 mW cm−2 | 10 min | 1.5 wt% | Pd2+ | 43 |
| Pd/TiO2 or P25 | H2PdCl4 | — | — | 365 nm/1.94 mW cm−2 | 10 min | 0.1 wt% | Pd2+ | 44 |
| Pd/TiO2(B) | H2PdCl4 | EG | — | 365 nm/1.94 mW cm−2 | 10 min | 1.0 wt% | Pd2+ | 45 |
| Pd1/TiO2 (anatase) | H2PdCl6 | CH3CN | — | UV | 1 h | 0.4 wt% | Pdδ+ (0 < δ < 2) | 46 |
| Pd1/TiO2(B) | H2PdCl4 | EG | — | 365 nm UV/10 mW cm−2 | 5 min | 0.14 at% | Pdδ+ (0 < δ < 2) | 47 |
| Pd/(GO/TiO2) | K2PdCl6 | — | — | UV | 12 h | 2 wt% | Pd0 + Pd2+ | 48 |
| Pd/CeO2 | H2PdCl4 | — | — | 220–770 nm | — | 0.88 wt% | Pd0 + Pd2+ | 49 |
| Pd–EG–BiOBr | H2PdCl4 | EG | — | UV | 15 min | 0.2 wt% | Pd2+ | 50 |
| PdSCA+NP/SAPO-31 | NaPdCl4 | — | Ethanol | 365 nm/1.92 mW cm−2 | 1 h | 0.38 wt% | Pd0 + Pd2+ | 51 |
| Pd–CdS/SiO2 | PdCl2 | — | Ethanol | λ > 300 nm | 30 min | 1.5 wt% | Pd2+ | 52 |
| Pt1/TiO2 | H2PtCl6 | EG | — | 365 nm | 30 min | 0.77 wt% | Pt0 | 53 |
| Pt/TiO2-A | H2PtCl6 | — | — | 235 nm | 15 min | 0.6 wt% | Ptδ+ (0 < δ < 2) | 54 |
| Pt/ZnIn2S4 | H2PtCl6 | N(C2H5OH)3 | >420 nm | 60 min | 0.26 wt% | Ptδ+ (2 < δ < 4) | 55 | |
| Pt/RuCeOx | H2PtCl6 | — | — | 50 W UV | 3 h | 0.49 wt% | Pt2+ | 56 |
| Pt-PVP/TNR@GC | H2PtCl6 | PVP | Ethanol | 365 nm/4.8 mW cm−2 | 30 min | 1.75 wt% | Ptδ+ (0 < δ < 4) | 57 |
| Pt/MCNN | H2PtCl6 | — | — | UV | 1 h | 3% (nominal) | Ptδ+ | 58 |
| CoSe2−x–Pt | H2PtCl6 | — | — | UV | 10 min | 2.25 wt% | Pt2+ | 59 |
| PA-Ni@PCN/Pt-SAC | H2PtCl6 | Phytate | — | >800 nm | 5 h | 0.22 wt% | Ptδ+ + Pd0 | 60 |
| Ca2Nb3O10–PtSA | H2PtCl6 | TBA+ | — | Sunlight | 5 min | 0.5 wt% | Pt2+ | 61 |
| Ru–N/BC | RuCl3 | Urea | — | UV | 2 h | 0.41 wt% | Ptδ+ (3 < δ < 4) | 62 |
| AgSA-SCN | AgNO3 | Hydramines | — | UV | 30 min | 1.41 wt% | Agδ+ (0 < δ < 1) | 63 |
| Ir–MoSe2@NiCo2Se4 | IrCl3 | — | — | 254 nm | 4 h | 1.5% | Ir4+ | 64 |
| Pd–Bi | Bi(NO3)3 | PVP | — | UV | 0.5–3 h | 2.86 wt% | Bi0 | 65 |
| Ni1/CdS | Ni(CH3COO)2 | — | — | UV | 20 min | 2.85 wt% | Ni(OH)2 | 66 |
| n-Cu/BP | Cu(Ac)2 | — | H2 | λ > 400 nm | 3 h | 11.3 wt% | Cuδ+ (0 < δ < 2) | 67 |
| N–Co/BP | Co(Ac)2 | 5.2 wt% | Coδ+ (0 < δ < 2) |
In 2013, Maria et al. used a combination of UV radiation and cyanide leaching to prepare a Au/TiO2 catalyst, which was the first time observable atomically dispersed Au–(OH)x species were prepared using a photochemical method,42 as depicted in Fig. 3a. They found that the cyanide leaching treatment only removed the Au nanoparticles and did not change the catalytic performance. Therefore, they drew the conclusion that in the reaction only atomically dispersed species were active. From the viewpoint of the catalyst preparation, cyanide leaching is intricate, dangerous, and wasteful of the Au, and so, not aligned with the initial purpose of preparing atomically dispersed catalysts. Also, aggregation after the water–gas shift (WGS) reaction was observed (Fig. 3b).
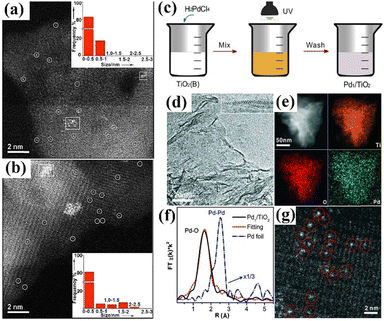 | ||
| Fig. 3 AC-HAADF/STEM images of (a) fresh 1.16 AuG5_UV_L and (b) used 1.16 AuG5_UV_L after reaction in the TPSR mode at 373 K and held steady for 4 h. Reprinted with permission.26 Copyright 2013, American Chemical Society. (c) Scheme for the synthesis and (d)–(g) structural characterizations of Pd1/TiO2(B), in which (d) is a representative TEM image, (e) STEM-EDS elemental mapping, (f) FT-EXAFS spectra and (g) high-resolution HAADF-STEM image. Copyright 2016, Reprinted with permission from AAAS.27 | ||
The next milestone for the photochemical method was performed on a large surface-area support to increase the metal loading, while using surface ligands to stabilize the atomically dispersed species. In 2016, Liu and Zheng et al. reported the synthesis of a Pd1/TiO2(B) system.43 Ultrathin titanium dioxide nanosheets [TiO2(B)] were used as the support. H2PdCl4 was subsequently added to an aqueous dispersion of the nanosheets for the adsorption of Pd species. The mixture was then exposed to low-intensity UV light for 10 min to obtain a Pd1/TiO2(B) catalyst, as shown in Fig. 3c.
The atomic dispersion of Pd over TiO2(B) was evidenced by STEM-EDS, XAS, and AC-HAADF-STEM analyses (Fig. 3d–g). The Pd loading reached as high as 1.5 wt%, on account of the presence of surface ethylene glycolate ligands, which will be discussed below. Furthermore, this strategy enabled the continuous production of atomically dispersed Pd using a peristaltic pump and low-intensity ultraviolet irradiation in a continuous stream. Later on, the same authors found that the method was also feasible using other TiO2 supports, such as anatase and commercial P25.44
Other supports could also be used for supporting single Pd atoms through liquid-phase photodeposition. Using CeO2 with different morphologies, Wang et al. managed to reduce H2PdCl4 to atomically dispersed Pd with photogenerated electrons on the surface of CeO2.49 Liu et al. used EG–BiOBr as the support to fabricate single Pd sites with an unsaturated coordination environment.50 This strategy notably boosted the N2 adsorption capacity of the catalyst and thus elevated its efficiency in photocatalytic nitrogen fixation. Lu et al. prepared a PdSA+C/SAPO-31 catalyst that contained Pd single atoms and clusters.51 They demonstrated that the catalyst performed better than the wet impregnation method-made PdSA/SAPO-31 catalyst in the chemoselective hydrodeoxygenation of vanillin. More recently, Chen et al. obtained atomically dispersed and stable Pd1/TiO2 catalysts by UV treatment of an acetonitrile dispersion of TiO2 (anatase) and H2PdCl4, and then expanded the approach to a range of carriers (CeO2 and WO3) and metal atoms (Ir, Pt, Rh, and Ru).46
Atomically dispersed Pt could also be obtained through photodeposition.68 A catalyst containing atomically dispersed Pt was produced by Qiao et al. via the UV irradiation of a mixed aqueous solution of chloroplatinic acid and a TiO2(B) dispersion.53 The obtained powder was calcined at 350 °C to remove the protective agent, to allow studying the strong metal–support interaction (SMSI) single-atom catalysis. Shi et al. employed ZnIn2S4 as the carrier of an atomically dispersed Pt catalyst for boosting photocatalytic hydrogen production.55 Liu et al. prepared a RuCeOx mixed oxide by a co-precipitation method and used it as a support. In their system, the photogenerated electrons generated in CeO2 transferred to RuO2, so that Pt atoms could be selectively deposited on the surface of RuO2, as shown in Fig. 4a.56 Fourier-transform extended X-ray absorption fine structure (EXAFS) analysis revealed the local atomic structure of Pt atoms in the samples, as shown in Fig. 4b. The Pt in Pt/RuCeOx-PA prepared photochemically was found to be uniformly distributed in a Pt–O–Ru structure, whereas Pt/RuCeOx-CA prepared by chemical activation and Pt/RuCeOx-TA prepared by thermal activation existed in a variety of Pt-bonding units, suggesting that Pt in Pt/RuCeOx-PA was loaded only on RuO2. Furthermore, the high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) images (Fig. 4c and d) also confirmed that the isolated Pt atoms (marked with yellow circles) were selectively deposited on the RuO2 side. This case showed the advantage of photochemical synthesis in comparison to the conventional wet impregnation method to direct the deposition of precious metals, through the utilization of the band structure or heterojunction structures. The photodeposition of non-noble metals as single atoms was also reported; for instance, atomically dispersed Bi on icosahedral Pd nanocrystals, single Cu and Co atoms on black phosphorus, and single Ni atoms on the surface of CdS.65–67
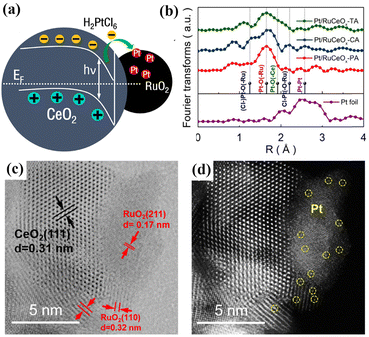 | ||
| Fig. 4 (a) Schematic diagram showing the photogenerated charge separation by an internal electric field at the RuO2/CeO2 heterojunction and the incorporation of platinum atoms on RuCeOx; (b) Fourier transforms of the EXAFS spectra at the Pt L3-edge for Pt/RuCeOx-TA, Pt/RuCeOx-CA, and Pt/RuCeOx-PA catalysts and Pt foil; (c) bright-field and (d) dark-field images of Pt/RuCeOx-PA (atomic Pt atoms are circled in yellow). Reproduced with permission.40 Copyright 2020, Wiley-VCH. | ||
Liquid-phase photochemical synthesis enables reduced energy consumption and waste generation compared to conventional synthesis methods. However, specific types of supports are needed for the method, which limits its universality. Besides, the method also appears to be quite simple and straightforward. However, the real chemical reactions that occur during the photodeposition remain unclear, please see Part 4.1 for details.
3.2. Solid-phase photochemical synthesis
In liquid-phase photochemical reduction, the solution of a metal precursor subjected to direct UV irradiation is easily converted to a dispersion of metal nanoparticles (in the absence of supports), owing to the diffusion, aggregation, and nucleation of atoms.69 Thus, for the purpose of forming single atoms, the task is to avoid atom diffusion and metal core formation, as nucleation of the metal clusters should be avoided. To this end, solid-phase photochemical synthesis was developed, mainly through the pre-adsorption of precursors onto solid supports, or through low-temperature irradiation. Some atomically dispersed catalysts prepared by this method are summarized in Table 2.| Metal/support | Precursor | Ligand | Reagent | Light source | Exposure | Content | Valence state | Ref. |
|---|---|---|---|---|---|---|---|---|
| Pt1/MC | H2PtCl6 | — | — | UV/0.89 mW cm−2 | 1 h | 2.6 wt% | Ptδ+ (1 < δ < 2) | 71 |
| Pt/C3N4 | H2PtCl6 | — | — | 420 nm/300 W Xe lamp | 3 min | 3.45 wt% | Ptδ+ (δ < 4) | 72 |
| Pt–Ru/C3N4 | H2PtCl6 + RuCl3 | — | — | 420 nm/300 W Xe lamp | 10 min | 0.51 wt% | Pt0 | 73 |
| 0.45 wt% | Ru0 | |||||||
| Pd/C3N4 | PdCl2/[PdCl(C3H5)]2/H12N6O6Pd | — | — | 400–780 nm/300 W Xe lamp | 1 h | 0.2855 wt% | Pdδ+ (0 < δ < 2) | 74 |
| Pt@HG | H2PtCl6 | — | C12H8N2O4 | Xe lamp | 1 h | 2.4% | Ptδ+ (0 < δ < 4) | 75 |
| Ir/Ni9FeOOH | IrCl3(H2O)3 | — | — | 0.7 mW cm−2 | 1 h | 2.99 wt% | Ir5.3+ | 76 |
| Pt1/CuO–CeO2 | H2PtCl6 | — | — | 300 W Xe lamp/300 mW cm−2 | 15 min | 0.2 wt% | Pt2+ + Pt4+ | 77 |
| Pd1/TiO2 | K2PdCl4 | — | — | 300 W Xe lamp | 5 min | 0.53 wt% | Pd0 | 78 |
| Ru/TiO2 | RuCl3 | — | Methanol | solar light/50 W m−2 | 2 h | 0.42 wt% | Ruδ+ (0 < δ < 4) | 79 |
| Pt1/NMC | H2PtCl6 | — | Ethanol | 365 nm/0.89 mW cm−2 | 1 h | 2.54% | Pt0 | 80 |
| Au1/In2O3 PNSs | HAuCl4 | — | — | λ = 253.7 nm/0.6 mW cm−2 | 1 h | 0.51 wt% | Auδ+ + Au0 | 81 |
| Pt/NPC | H2PtCl6 | — | — | UV | 1 h | 3.8 wt% | Pt0 | 70 |
| Ag/g-C3N4 | AgNO3 | — | — | 250 W Hg lamp | 1 h | 3.0 wt% | Ag0 | 82 |
Li et al. used nitrogen-doped carbon (NPC) to adsorb [PtCl6]2− ions and irradiated the obtained powder with a certain intensity of ultraviolet light to form an atomically dispersed Pt catalyst with 3.8 wt% loading (Fig. 5).70 AC-STEM and XAS confirmed that the isolated Pt atoms were uniformly dispersed on the carbon at the atomic level and bonded with N atoms, as shown in Fig. 5c–f. This method can effectively avoid the agglomeration of isolated Pt atoms during the process of washing and drying. The key to this method is the uniform adsorption of metal ions in the wet impregnation step. Ligand exchange may also happen during the adsorption.
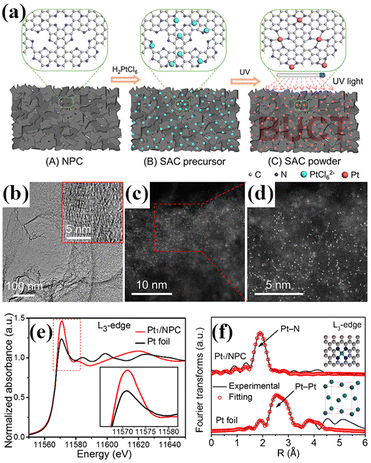 | ||
| Fig. 5 (a) Schematic illustration of the formation of the Pt1/NPC catalyst; (b) HRTEM image of the Pt1/NPC catalyst; insets show enlarged (c) HADDF-STEM and (d) Pt1/NPC catalyst images; (e) normalized XANES spectra at the Pt L3-edge for the Pt1/NPC catalyst and Pt foil; (f) Fourier transform of the EXAFS spectra at the Pt L3-edge and corresponding R-space fitting curves for the Pt1/NPC catalyst and Pt foil. The insets show the atomic structure models for the Pt1/NPC and Pt foil. Reprinted with permission from ref 54. Copyright 2018 American Chemical Society. | ||
To avoid nucleation, an iced-photochemical reduction strategy was developed by Wu et al., opening up new possibilities of frozen photochemistry.71 In this strategy, before exposing the metal precursor to UV, the solution was rapidly frozen by liquid nitrogen (Fig. 6a). The effectiveness of the ice lattice in limiting the diffusion of reactant ions and products allowed the production of confined single atoms, that later melted into an atomic dispersion of metal atoms within the solution. The first-principles molecular dynamics (FPMD) suggested that the produced metal atoms in aqueous solution were coordinated, as H–Pt–OH, by dissociating water molecules. As illustrated in Fig. 6b–h, atomically dispersed Pt could be loaded uniformly and stably on several substrates, except for the graphene support. Additionally, the same method was applied to form isolated Ag and Au atoms on an ultrathin carbon film (Fig. 6i and j). XANES of the Pt single-atom materials (Pt1/MC and Pt1/TiO2) also confirmed the isolated states of Pt atoms (Fig. 6k and l).
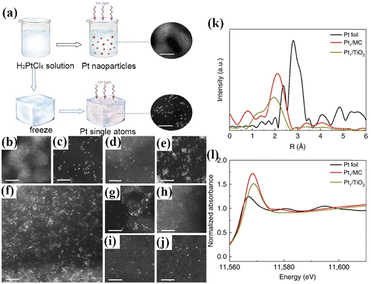 | ||
| Fig. 6 (a) Schematic illustration of the iced-photochemical process; (b)–(g) HAADF-STEM images of Pt species over different supports, with (b) Pt nanocrystals with a size of ∼2 nm formed by the normal photochemical reduction of H2PtCl6 aqueous solution; (c) Pt single atoms dispersed on an ultrathin carbon film. (d) Densely and homogeneously dispersed Pt single atoms on mesoporous carbon; (e) Pt1/MWCNTs, indicating that Pt existed completely as isolated single atoms; (f) Pt1/graphene with concomitant Pt single atoms, nanoclusters, and sub-nanometre clusters. (g) Atomically dispersed Pt on titanium oxide nanoparticles; (h) Pt single atoms attached on the surface of zinc oxide nanowires; (i) Ag and (j) Au single atoms prepared by a similar iced-photochemical route. (scale bar, 2 nm); (k) EXAFS spectra of bulk Pt foil and Pt single atoms absorbed on TiO2 and MC. (l) Normalized XANE structure spectra at the Pt L3-edge.55 Open access. | ||
Combining the above-mentioned two solid-phase strategies, i.e., pre-absorption of the metal precursor and frozen photochemical treatment, Guo et al. mixed a solution of the metal precursor and dispersion of the support, then rapidly froze the mixture in liquid nitrogen and carried out UV treatment on it at 420 nm.72 An atomically dispersed Pt catalyst on g-C3N4 with a metal loading of 3.45 wt% was obtained. The strong coordination bonds allowed high stability at high loading. The presence of Pt–C bonds were proposed based on the EXAFS results, which we tend to disagree with.
Recently, Xu et al. studied the applicability of the frozen photochemical method using three distinct Pd precursors (PdCl2, [PdCl(C3H5)]2, H12N6O6Pd).74 The produced catalysts showed no relevance between the photocatalytic hydrogen production performance and the precursor choice. Some reports on the use of the frozen photochemical method for loading atomically dispersed metals onto oxides are also listed in Table 2.
Solid-phase photochemical deposition is generally highly selective, preventing side reactions, like aggregation. However, the limited availability of photochemical reactions at the solid-phase surfaces can lower the reaction rate. Solid-phase synthesis usually needs a longer duration of irradiation.
3.3. Process control in photochemical synthesis
In this part, we discuss the controlling factors that can impact the photochemical synthesis of atomically dispersed catalysts, from the aspects of both fundamental interests and practical potential.The choice of supports should be adapted to the preparation methods. In photochemical synthesis, the support choice is usually limited to semiconductors; for instance TiO2, CeO2, or C3N4. Still, the structural parameters of the supports are too many to be fully studied and reported here.
First, the polymorphism of inorganic matter is the most commonly considered parameter. Our previous research demonstrated that photochemically prepared Pd1/TiO2 catalysts utilizing two different types of TiO2 supports, namely (001)-exposed anatase and P25, exhibited distinct CO oxidation performances despite their same degrees of atomic dispersion.45 Zhang et al. used a newly developed 1T′-phase MoS2 crystal as a support to produce an atomically dispersed Pt catalyst by a photochemical method, with a Pt loading capacity of up to 10 wt%.83 Although the support was not a free-standing material, the method could still be viewed as a solid-phase photochemical synthesis. The HAADF-STEM image and simulated STEM images revealed three types of atomically dispersed Pt: Ptsub, Ptads-S, and Ptads-Mo. However, using 2H-phase MoS2 as the support only led to the epitaxial growth of platinum (Pt) nanoparticles. In this case, the preferential enrichment of photogenerated carriers onto different facets of different crystal phases was nicely demonstrated. The morphologies of the crystalline supports also have a significant impact on the dispersion and catalytic activity of single atoms. Wang et al. described the photochemical synthesis of atomically dispersed Pd/CeO2 catalysts with two different morphologies of CeO2 (Fig. 7).49 Pd/CeO2-TOP (truncated octahedral CeO2) exhibited higher activity in CO oxidation and better selectivity in the selective oxidation of benzyl alcohol than Pd/CeO2-CP (cubic CeO2), although they had similar structures of Pd, as shown in Fig. 7g and h. Pd/CeO2-TOP exposed a larger number of (111) surfaces, while the higher coordination number of Ce–OL (OL: surface lattice oxygen atoms) for cerium atoms on the CeO2(111) surface could facilitate the reaction by hydrogen removal from Pd.
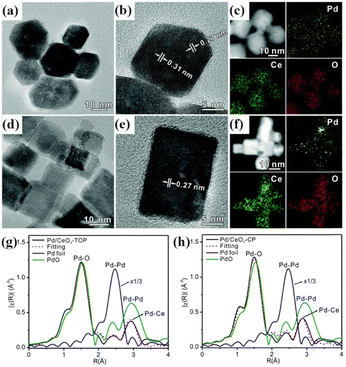 | ||
| Fig. 7 (a) and (d) TEM and (b) and (e) HRTEM images of Pd/CeO2-TOP and Pd/CeO2-CP prepared via a photochemical route. (c) and (f) HAADF-STEM images of Pd/CeO2-TOP and Pd/CeO2-CP, and their corresponding EDX mappings of Pd, Ce, and O; Fourier transforms of EXAFS spectra for (g) Pd/CeO2-TOP and (h) Pd/CeO2-CP as well as the reference materials Pd foil and PdO at the Pd K-edge. Reproduced with permission.33 Copyright 2017, The Royal Society of Chemistry. | ||
For crystalline supports, the surface structures are inevitably different from the bulk structure, being a class of symmetry breaking. The undercoordinated sites and non-stoichiometric composition, usually called defects, are beneficial for anchoring single atoms.84 Wei et al. discovered that atomically dispersed Pt could be firmly attached to the support's edge or at a defect location on the surface of amorphous carbon film, mesoporous carbon (MC), multi-walled carbon nanotubes (MWCNTs), TiO2 nanoparticles, and ZnO nanowires due to their abundant presence of defects.71
Liu and Zheng et al. found that ethylene glycol ligands on the surface of TiO2(B) played a decisive role in the photochemical reaction process during the preparation of atomically dispersed Pd1/TiO2(B).43 As shown in Fig. 8a, when the support was exposed to ultraviolet light, electron–hole pairs were generated. The electrons were trapped in the Ti-3d orbital to form the Ti3+ site (detected), while the holes broke the Ti–O bond between the glycolate and TiO2, resulting in the formation of the –OCH2CH2O˙ radical. A hydrogen transfer occurred between α-H and –OCH2CH2O˙ to form –OCH2˙CHOH radicals (detected). Once PdCl42− was adsorbed on TiO2(B), each PdCl42− released two Cl− ions, producing an intermediate with a separate PdCl2 unit adsorbed on TiO2(B). Subsequently, the –OH group in –OCH2˙CHOH attacked the Pd site nearby by substituting one Cl− to form a PdCl1/TiO2 intermediate. Finally, the residual Cl− on PdCl1/TiO2 could be easily removed by H2 treatment to generate H+ and Cl−. Overall, EG radicals promoted the removal of Cl− on Pd and stabilized the isolated Pd atoms by forming more Pd–O bonds. More importantly, the formed Pd–EG–TiO2 interface facilitated the heterolytic activation of H2, thus improving the catalytic hydrogenation performance, especially in the hydrogenation of polar bonds. Without surface EG ligands, the loading of metal would not exceed 1 wt%, meanwhile the catalytic hydrogenation performance would be lower. The EG-protected TiO2(B) was utilized to synthesize several other atomically dispersed catalysts.47,53,79
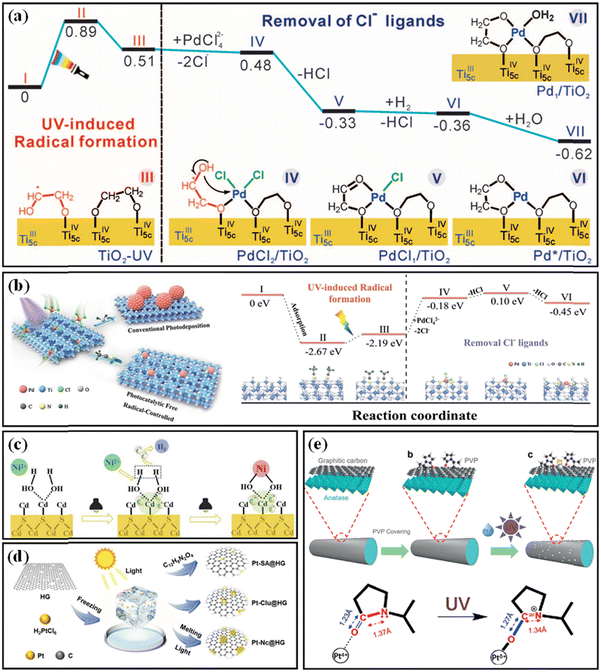 | ||
| Fig. 8 (a) Energies and models of the intermediates and transition states in the stepwise preparation mechanism of Pd1/TiO2(B). From ref. 27. Reprinted with permission from AAAS. (b) Schematic illustrating the photochemical process, and energies and optimized structures in the anchoring process of Pd single atoms in TiO2. Reproduced with permission.30 Copyright 2023, Wiley-VCH. (c) Schematic illustration of the preparation of Ni1/CdS by a photochemical method. Reprinted from Publication ref. 50, Copyright 2020, with permission from Elsevier. (d) Schematic illustration of the preparation of Pt-SA@HG, Pt-Clu@HG, and Pt-Nc@HG. Reproduced with permission.59 Copyright 2022, Wiley-VCH. (e) Illustration of the synthesis of Pt-PVP/TNR@GC catalysts, and change in the local structure of a Pt atom coordinated by PVP before and after UV irradiation.41 Open access. | ||
Very recently, Chen et al. synthesized an atomically dispersed Pd1/TiO2 (commercial anatase) catalyst using a photochemical method in acetonitrile (Fig. 8b).46 Acetonitrile was believed to be activated by oxygen vacancies on TiO2 and then ultraviolet light. The alkyl radical generated by light reacted with [PdCl4]2− ions to dissociate Cl− ions, with the N atom and the lattice oxygen on N/TiO2 serving as anchoring sites for the Pd atom. The role of acetonitrile in this system resembled that of ethylene glycol in the Pd1/TiO2(B) system. Its primary function was to produce radicals that eliminate Cl− with the aid of light, thereby dispersing and stabilizing the Pd atoms.
In a photochemical synthesis of an atomically dispersed Ni1/CdS catalyst, Zhang et al. discovered the significant involvement of hydroxyl groups on the surface of CdS.66Fig. 8c illustrates how CdS gets excited by light, resulting in the formation of electron–hole pairs. Thiourea acts as a sacrificial agent and consumes the holes while the electrons reduce hydrogen ions to H2. The residual hydroxyl ions react with adsorbed Ni2+ to yield isolated Ni(OH)2 species. We believe the coordination role of S in the support should not be overlooked. Sun et al. absorbed 2,2′-bipyridine-4,4′-dicarboxylic acid (C12H8N2O4) ligands onto reduced graphene through electrostatic interaction to anchor Pt ions, and subsequently transformed the structure into Pt–C coordination structures by heat treatment.75 In contrast, in the absence of associated ligands, Pt tended to form clusters, as illustrated in Fig. 8d.
To prevent the aggregation of Pt atoms during photochemical reduction, Li et al. employed pyrrolidone (PVP) as an organic ligand and fixed it to TNR@GC (graphite-carbon encapsulated titanium oxide nanorods) via van der Waals forces (as depicted in Fig. 8e).57 Under ultraviolet radiation, the PVP side chains tended to form stable complexes with photoreduced Pt atoms. This coordination could effectively hinder the aggregation of isolated Pt atoms, allowing a Pt loading of up to 1.75 wt%. The coordination between partially positively charged Pt atoms and amide groups (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) in PVP molecules served as the active centres for the hydrogen production reaction (HER).
O) in PVP molecules served as the active centres for the hydrogen production reaction (HER).
Similarly, sacrificial agents are often used in the photochemical preparation of atomically dispersed catalysts. Maria et al. emphasized the necessity of adding ethanol as an electron acceptor to prepare isolated Au species.42 Otherwise, gold ions would be quickly reduced in H2O2 or H2O solutions and grow into metal particles. Li et al. used ethanol in the preparation of the Pt-PVP/TNR@GC catalyst to eliminate photogenerated holes.57 Wenderich et al. discovered that methanol could generate methyl radicals, hence improving the reducing ability of Pt ions during the photodeposition process.87 Additionally, to suppress photogenerated electron–hole recombination, thiourea and triethanolamine (TEOA) are frequently used as hole scavengers.66,88,89
It is noteworthy that careful selection of the sacrificial agents is crucial in the photochemical preparation of atomically dispersed catalysts, as they can often accelerate the deposition rate of metal ions and may lead to agglomeration.63 Besides, added reagents generally serve multiple purposes in one system, which makes it difficult to understand the photochemical reaction.
Environment-unfriendly sacrificial agents should be avoided. The price of sacrificial agent should not be overlooked too for industrial considerations. Apart from the commonly used alcohols, it is also worth considering novel sacrificial agents for photocatalytic hydrogen production, including biomass and plastic waste, which might also be used to synthesize atomically dispersed catalysts using photochemical techniques.
Gold ions can be readily reduced with visible light.90 To form atomically dispersed Au, it is necessary to either employ an appropriate excitation wavelength or add a sacrificial agent.42 Zhou et al. used UV excitation at a wavelength of 420 nm to avoid the direct reduction of [PtCl6]2− into nanoparticles.72 This method involved selectively exciting electrons solely at the g-C3N4 defect sites, which ensured that only Pt ions adsorbed on the surface could be reduced. This approach could effectively prevent detrimental Pt growth. Our previous work used the 365 nm light of a Xenon lamp, which was weak enough to prevent the photodegeneration of surface EG ligands, but strong enough to allow the formation of EG radicals to anchor Pd single atoms.43
3.4. Catalytic applications
Atomically dispersed catalysts prepared via photochemical synthesis have shown unique functions in several catalytic processes.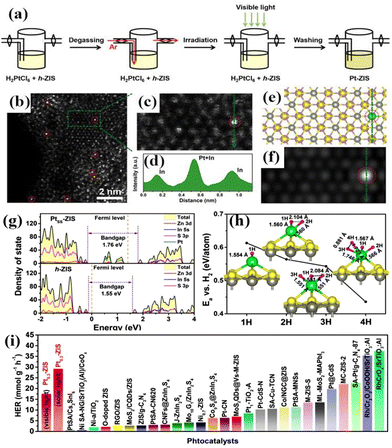 | ||
| Fig. 9 (a) Schematic of the synthesis of Pt-ZIS; (b) HAADF-STEM and (c) magnified HAADF-STEM image of Pt = −ZIS. (d) Strength profiles from the areas labelled by the green line. (e) Optimized structure of Pt0.3-ZIS, where the yellow, grey, pink, and green spheres represent S, Zn, In, and Pt atoms, respectively. (f) Simulated HAADF-STEM image of PtSS-ZIS according to the DFT-optimized structure. (g) Density of states of h-ZIS and PtSS-ZIS. (h) Calculated adsorption energies of H atoms as a function of the H coverage (from one H atom to four H atoms) on single Pt atom photocatalysts with h-ZIS as the support. The H–H and Pt–H distances are shown in the figure; (i) H2 evolution rates for Pt0.3-ZIS in this work compared with representative recently reported photocatalysts.39 Open access. | ||
4. Challenges and perspective
We summarized the advantages of photochemical synthesis for atomically dispersed catalysts in the last paragraph of Part 2. We also presented many examples to show how the method is tuneable and has been well-demonstrated in different systems. Practices have proved that this approach is not only environmentally friendly and safe, but also easy to execute and holds great potential for mass production. However, the method still has several issues that need to be addressed in the future, both from the aspects of science and technology: (1) lack of fundamental understanding of the process; (2) low level of control of the structure and (3) limited number of demonstrated systems. Here, we elaborate the challenges the method faces and give suggestions for further research.4.1. Lack of fundamental understanding of the process
Being a kind of wet-chemistry method, photochemical synthesis inherits both the advantages (for instance, easy scale up for mass production) and disadvantages of wet-chemistry methods. The most profound disadvantage that hinders addressing the other two issues (structure control and extension of the method) is that the basic chemical reactions, especially radical reactions, that happen in photochemical synthesis are not fully understood.Although we point out in Part 2 that the photogenerated carriers are very likely the driving force of single-atoms’ formation and stabilization, the real process has to be much more complicated than that simply laid out eqn (1) and (2). In the presence of solvent and ligands from the metal precursor, photogenerated electrons and holes on the surface can produce extensive kinds of radicals. For instance, it was reported that as many as tens of radical species could be formed in a water dispersion of TiO2 upon photoirradiation.95,96 These radicals may serve as the real intermediates to react with the metal precursors and lead to the photodeposition.
Another evidence on the complexity of the chemical reaction is the oxidation state of the supported metal species. Although usually called “single atoms”, accompanied by the use of terms like “photoreduction” and “photoreducing”, the supported single metal atoms are not likely to be zero-valanced. The major reaction in the photodeposition of metallic nanoparticles is reduction, obviously, but not in the case in photodepositing single atoms. Some reports claimed their products were composed of M0 single atoms, which we tend to disagree with. The coordination bonds between single atoms and the support will lead to charge transfer, hence there will be partial charge on the metal centres (unless the metal was fully coordinated by neutral molecules, like ethene and water). Bearing this in mind, photochemical synthesis is, in principle, not a reduction reaction for the metal. In our previous reports, the supported Pd did not change its oxidation state before and after phototreatment.43 The reaction was more a radical-assisted ligand exchange reaction.
With numerous factors involved in the synthesis, we believe a better understanding of the reactions in photochemical synthesis calls for a systematic study in different systems, or in well-defined model systems.
4.2 Low level of control of the structure
We believe the structures of atomically dispersed catalysts need a higher level of rational design and controllable synthesis, in the following three aspects in particular:Using high surface-area supports, like activated carbon, two-dimensional supports, or porous supports would help increase the metal loading. Using supports that can bind strongly with single metal atoms, or using surface ligands to prevent the surface diffusion is also a practical strategy.
In photochemical synthesis, not only is the surface reaction mechanism unclear, the multiple roles of possible spectators (solvents, added ligands, photogenerated radicals) are also unclear. These species may coordinate with the metal sites and even contribute in catalytic reactions. Li et al. found the amide group (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) in the Pt-PVP/TNR@GC catalyst was coordinated with isolated Pt atoms and donated electrons.57 The partially positively charged Pt atoms located in the –C
O) in the Pt-PVP/TNR@GC catalyst was coordinated with isolated Pt atoms and donated electrons.57 The partially positively charged Pt atoms located in the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O section of the pyrrolidone side chain were found to function as the active centre for the HER. EG ligands were found to significantly contribute to catalytic hydrogenation and photocatalytic nitrogen fixation.50
O section of the pyrrolidone side chain were found to function as the active centre for the HER. EG ligands were found to significantly contribute to catalytic hydrogenation and photocatalytic nitrogen fixation.50
Compared to conventional synthesis via annealing, the photochemical reaction offers gentler and more controllable reaction conditions, thereby largely preserving the supports’ surface structure. In addition, it allows the selective deposition of individual atoms at desired positions on a well-defined surface, with great accuracy. Using highly crystallized materials as the support with well-defined exposed surfaces can serve as model systems to tackle this problem.83 For instance, hexagonal ZnIn2S4 (h-ZIS) nanosheets offer uniform S3 sites for anchoring Pt atoms via photochemical reactions.55
4.3. Limited number of demonstrated systems
The synthesis and application of photochemically produced-atomically dispersed catalysts are both limited in several cases. To the best of our knowledge, we have summarized all the reported cases in Tables 1 and 2.To date, the selected supports are mainly TiO2, carbon-based materials and sulfides. It is essential to evaluate the capability of other supports. It is possible to acquire the desired material by adjusting the input energy of the light source for the photochemical reaction. The limited cases of chosen supports also limit the studied catalytic reactions.
4.4. Summary
As a novel technique for synthesizing atomically dispersed catalysts, the photochemical method possesses substantial promise for future research and application, but with several issues to be addressed. Since 2013 and 2016,42,43 the fast development in the method has given us confidence that the future will witness an increasingly vital role of atomically dispersed catalysts prepared by photochemical methods in energy conversion, environmental protection, and new material synthesis.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the ShanghaiTech University Start-up Funding and the Shanghai Pujiang Talent Program, China (No. 21PJ1410400).References
- X.-F. Yang, A. Wang, B. Qiao, J. Li, J. Liu and T. Zhang, Single-Atom Catalysts: A New Frontier in Heterogeneous Catalysis, Acc. Chem. Res., 2013, 46, 1740–1748 CrossRef CAS PubMed.
- A. Wang, J. Li and T. Zhang, Heterogeneous single-atom catalysis, Nat. Rev. Chem., 2018, 2, 65–81 CrossRef CAS.
- X. Cui, W. Li, P. Ryabchuk, K. Junge and M. Beller, Bridging homogeneous and heterogeneous catalysis by heterogeneous single-metal-site catalysts, Nat. Catal., 2018, 1, 385–397 CrossRef CAS.
- M. Haruta, T. Kobayashi, H. Sano and N. Yamada, Novel Gold Catalysts for the Oxidation of Carbon Monoxide at a Temperature far Below 0 °C, Chem. Lett., 1987, 16, 405–408 CrossRef.
- G. C. Bond, The origins of particle size effects in heterogeneous catalysis, Surf. Sci., 1985, 156, 966–981 CrossRef CAS.
- B. Gao, K. Zhang, Y. Wang, J. Lu and P. Liu, On the Structure Insensitivity of Propane Total Oxidation over Pt/CeO2: A Comparison between Single Atoms, Clusters and Nanoparticles, ChemCatChem, 2023, 15, e202301160 CrossRef CAS.
- B. Qiao, A. Wang, X. Yang, L. F. Allard, Z. Jiang, Y. Cui, J. Liu, J. Li and T. Zhang, Single-atom catalysis of CO oxidation using Pt1/FeOx, Nat. Chem., 2011, 3, 634–641 CrossRef CAS PubMed.
- S. Ding, M. J. Hülsey, J. Pérez-Ramírez and N. Yan, Transforming Energy with Single-Atom Catalysts, Joule, 2019, 3, 2897–2929 CrossRef CAS.
- Z. Liang, J. Shen, X. Xu, F. Li, J. Liu, B. Yuan, Y. Yu and M. Zhu, Advances in the Development of Single-Atom Catalysts for High-Energy-Density Lithium–Sulfur Batteries, Adv. Mater., 2022, 34, 2200102 CrossRef CAS PubMed.
- Z.-H. Xue, D. Luan, H. Zhang and X. W. Lou, Single-atom catalysts for photocatalytic energy conversion, Joule, 2022, 6, 92–133 CrossRef CAS.
- T. Cui, L. Li, C. Ye, X. Li, C. Liu, S. Zhu, W. Chen and D. Wang, Heterogeneous Single Atom Environmental Catalysis: Fundamentals, Applications, and Opportunities, Adv. Funct. Mater., 2022, 32, 2108381 CrossRef CAS.
- Y. Shang, X. Xu, B. Gao, S. Wang and X. Duan, Single-atom catalysis in advanced oxidation processes for environmental remediation, Chem. Soc. Rev., 2021, 50, 5281–5322 RSC.
- J. Gao, L. Feng, R. Ma, B.-J. Su, A. M. Alenad, Y. Liu, M. Beller and R. V. Jagadeesh, Cobalt single-atom catalysts for domino reductive amination and amidation of levulinic acid and related molecules to N-heterocycles, Chem. Catal., 2022, 2, 178–194 CrossRef CAS.
- K. Sun, H. Shan, H. Neumann, G.-P. Lu and M. Beller, Efficient iron single-atom catalysts for selective ammoxidation of alcohols to nitriles, Nat. Commun., 2022, 13, 1848 CrossRef CAS PubMed.
- V. B. Saptal, V. Ruta, M. A. Bajada and G. Vilé, Single-Atom Catalysis in Organic Synthesis, Angew. Chem., Int. Ed., 2023, 62, e202219306 CrossRef CAS PubMed.
- M. Flytzani-Stephanopoulos and B. C. Gates, Atomically Dispersed Supported Metal Catalysts, Annu. Rev. Chem. Biomol., 2012, 3, 545–574 CrossRef CAS PubMed.
- M. Babucci, A. Guntida and B. C. Gates, Atomically Dispersed Metals on Well-Defined Supports including Zeolites and Metal-Organic Frameworks: Structure, Bonding, Reactivity, and Catalysis, Chem. Rev., 2020, 120, 11956–11985 CrossRef CAS PubMed.
- S. Ji, Y. Chen, X. Wang, Z. Zhang, D. Wang and Y. Li, Chemical Synthesis of Single Atomic Site Catalysts, Chem. Rev., 2020, 120, 11900–11955 CrossRef CAS PubMed.
- M. K. Samantaray, V. D’Elia, E. Pump, L. Falivene, M. Harb, S. Ould Chikh, L. Cavallo and J.-M. Basset, The Comparison between Single Atom Catalysis and Surface Organometallic Catalysis, Chem. Rev., 2020, 120, 734–813 CrossRef CAS PubMed.
- M. D. Korzyński and C. Copéret, Single sites in heterogeneous catalysts: separating myth from reality, Trends Chem., 2021, 3, 850–862 CrossRef.
- F. Wu and P. Liu, Surface Organometallic Chemistry for Single-site Catalysis and Single-atom Catalysis, Chem. Res. Chin. Univ., 2022, 38, 1139–1145 CrossRef CAS.
- P. Liu and N. Zheng, Coordination chemistry of atomically dispersed catalysts, Natl. Sci. Rev., 2018, 5, 636–638 CrossRef CAS.
- R. Qin, K. Liu, Q. Wu and N. Zheng, Surface Coordination Chemistry of Atomically Dispersed Metal Catalysts, Chem. Rev., 2020, 120, 11810–11899 CrossRef CAS PubMed.
- M. J. Hülsey, S. Wang, B. Zhang, S. Ding and N. Yan, Approaching Molecular Definition on Oxide-Supported Single-Atom Catalysts, Acc. Chem. Res., 2023, 56, 561–572 CrossRef PubMed.
- J. Resasco, L. DeRita, S. Dai, J. P. Chada, M. Xu, X. Yan, J. Finzel, S. Hanukovich, A. S. Hoffman, G. W. Graham, S. R. Bare, X. Pan and P. Christopher, Uniformity Is Key in Defining Structure–Function Relationships for Atomically Dispersed Metal Catalysts: The Case of Pt/CeO2, J. Am. Chem. Soc., 2020, 142, 169–184 CrossRef CAS PubMed.
- C. Jia, Q. Wang, J. Yang, K. Ye, X. Li, W. Zhong, H. Shen, E. Sharman, Y. Luo and J. Jiang, Toward Rational Design of Dual-Metal-Site Catalysts: Catalytic Descriptor Exploration, ACS Catal., 2022, 12, 3420–3429 CrossRef CAS.
- R. Qin, P. Liu, G. Fu and N. Zheng, Strategies for Stabilizing Atomically Dispersed Metal Catalysts, Small Methods, 2018, 2, 1700286 CrossRef.
- Z.-Y. Wu, P. Zhu, D. A. Cullen, Y. Hu, Q.-Q. Yan, S.-C. Shen, F.-Y. Chen, H. Yu, M. Shakouri, J. D. Arregui-Mena, A. Ziabari, A. R. Paterson, H.-W. Liang and H. Wang, A general synthesis of single atom catalysts with controllable atomic and mesoporous structures, Nat. Synth., 2022, 1, 658–667 CrossRef.
- W. Guo, Z. Wang, X. Wang and Y. Wu, General Design Concept for Single-Atom Catalysts toward Heterogeneous Catalysis, Adv. Mater., 2021, 33, 2004287 CrossRef CAS PubMed.
- S. K. Kaiser, Z. Chen, D. Faust Akl, S. Mitchell and J. Pérez-Ramírez, Single-Atom Catalysts across the Periodic Table, Chem. Rev., 2020, 120, 11703–11809 CrossRef CAS PubMed.
- L. Xing, Y. Jin, Y. Weng, R. Feng, Y. Ji, H. Gao, X. Chen, X. Zhang, D. Jia and G. Wang, Top-down synthetic strategies toward single atoms on the rise, Matter, 2022, 5, 788–807 CrossRef CAS.
- J. Wang, Z. Li, Y. Wu and Y. Li, Fabrication of Single-Atom Catalysts with Precise Structure and High Metal Loading, Adv. Mater., 2018, 30, 1801649 CrossRef PubMed.
- N. Cheng, L. Zhang, K. Doyle-Davis and X. Sun, Single-Atom Catalysts: From Design to Application, Electrochem. Energy Rev., 2019, 2, 539–573 CrossRef.
- F. Zhang, Y. Zhu, Q. Lin, L. Zhang, X. Zhang and H. Wang, Noble-metal single-atoms in thermocatalysis, electrocatalysis, and photocatalysis, Energy Environ. Sci., 2021, 14, 2954–3009 RSC.
- Z. Li, B. Li, Y. Hu, X. Liao, H. Yu and C. Yu, Emerging Ultrahigh-Density Single-Atom Catalysts for Versatile Heterogeneous Catalysis Applications: Redefinition, Recent Progress, and Challenges, Small Struct., 2022, 3, 2200041 CrossRef CAS.
- W. C. Clark and A. G. Vondjidis, An infrared study of the photocatalytic reaction between titanium dioxide and silver nitrate, J. Catal., 1965, 4, 691–696 CrossRef CAS.
- B. Kraeutler and A. J. Bard, Heterogeneous photocatalytic preparation of supported catalysts. Photodeposition of platinum on titanium dioxide powder and other substrates, J. Am. Chem. Soc., 1978, 100, 4317–4318 CrossRef CAS.
- K. Wenderich and G. Mul, Methods, Mechanism, and Applications of Photodeposition in Photocatalysis: A Review, Chem. Rev., 2016, 116, 14587–14619 CrossRef CAS PubMed.
- H. Zhao, Q. Mao, L. Jian, Y. Dong and Y. Zhu, Photodeposition of earth-abundant cocatalysts in photocatalytic water splitting: Methods, functions, and mechanisms, Chin. J. Catal., 2022, 43, 1774–1804 CrossRef CAS.
- G. Chen, R. Li and L. Huang, Advances in photochemical deposition for controllable synthesis of heterogeneous catalysts, Nanoscale, 2023, 15, 13909–13931 RSC.
- J. M. Thomas, Z. Saghi and P. L. Gai, Can a Single Atom Serve as the Active Site in Some Heterogeneous Catalysts?, Top. Catal., 2011, 54, 588–594 CrossRef CAS.
- M. Yang, L. F. Allard and M. Flytzani-Stephanopoulos, Atomically Dispersed Au-(OH)x Species Bound on Titania Catalyze the Low-Temperature Water-Gas Shift Reaction, J. Am. Chem. Soc., 2013, 135, 3768–3771 CrossRef CAS PubMed.
- P. Liu, Y. Zhao, R. Qin, S. Mo, G. Chen, L. Gu, D. M. Chevrier, P. Zhang, Q. Guo, D. Zang, B. Wu, G. Fu and N. Zheng, Photochemical route for synthesizing atomically dispersed palladium catalysts, Science, 2016, 352, 797–800 CrossRef CAS PubMed.
- P. Liu, J. Chen and N. Zheng, Photochemical route for preparing atomically dispersed Pd1/TiO2 catalysts on (001)-exposed anatase nanocrystals and P25, Chin. J. Catal., 2017, 38, 1574–1580 CrossRef CAS.
- P. Liu, Y. Zhao, R. Qin, L. Gu, P. Zhang, G. Fu and N. Zheng, A vicinal effect for promoting catalysis of Pd1/TiO2: supports of atomically dispersed catalysts play more roles than simply serving as ligands, Sci. Bull., 2018, 63, 675–682 CrossRef CAS PubMed.
- X. Chen, S. Guan, J. Zhou, H. Shang, J. Zhang, F. Lv, H. Yu, H. Li and Z. Bian, Photocatalytic Free Radical-Controlled Synthesis of High-Performance Single-Atom Catalysts, Angew. Chem., Int. Ed., 2023, 62, e202312734 CrossRef CAS PubMed.
- X.-L. Ye, S.-J. Lin, J.-W. Zhang, H.-J. Jiang, L.-A. Cao, Y.-Y. Wen, M.-S. Yao, W.-H. Li, G.-E. Wang and G. Xu, Boosting Room Temperature Sensing Performances by Atomically Dispersed Pd Stabilized via Surface Coordination, ACS Sens., 2021, 6, 1103–1110 CrossRef CAS PubMed.
- K. Du, M. Sun, J. Peng, S. Zhou, G. Sheng, R. Shen, L. Deng, C. Hu, Y. Sun and P. Zhang, Mixed-valence palladium single-atom catalyst induced by hybrid TiO2-graphene through a photochemical strategy, Appl. Surf. Sci., 2023, 625, 157115 CrossRef CAS.
- X. Wang, J. Chen, J. Zeng, Q. Wang, Z. Li, R. Qin, C. Wu, Z. Xie and L. Zheng, The synergy between atomically dispersed Pd and cerium oxide for enhanced catalytic properties, Nanoscale, 2017, 9, 6643–6648 RSC.
- J. Liu, F. Li, J. Lu, R. Li, Y. Wang, Y. Wang, X. Zhang, C. Fan and R. Zhang, Atomically dispersed Palladium-Ethylene Glycol- Bismuth oxybromide for photocatalytic nitrogen fixation: Insight of molecular bridge mechanism, J. Colloid Interface Sci., 2021, 603, 17–24 CrossRef CAS PubMed.
- X. Lu, C. Guo, M. Zhang, L. Leng, J. H. Horton, W. Wu and Z. Li, Rational design of palladium single-atoms and clusters supported on silicoaluminophosphate-31 by a photochemical route for chemoselective hydrodeoxygenation of vanillin, Nano Res., 2021, 14, 4347–4355 CrossRef CAS.
- M.-Y. Qi, M. Conte, Z.-R. Tang and Y.-J. Xu, Engineering Semiconductor Quantum Dots for Selectivity Switch on High-Performance Heterogeneous Coupling Photosynthesis, ACS Nano, 2022, 16, 17444–17453 CrossRef CAS PubMed.
- B. Han, Y. Guo, Y. Huang, W. Xi, J. Xu, J. Luo, H. Qi, Y. Ren, X. Liu, B. Qiao and T. Zhang, Strong Metal–Support Interactions between Pt Single Atoms and TiO2, Angew. Chem., Int. Ed., 2020, 59, 11824–11829 CrossRef CAS PubMed.
- Y. Sui, S. Liu, T. Li, Q. Liu, T. Jiang, Y. Guo and J.-L. Luo, Atomically dispersed Pt on specific TiO2 facets for photocatalytic H2 evolution, J. Catal., 2017, 353, 250–255 CrossRef CAS.
- X. Shi, C. Dai, X. Wang, J. Hu, J. Zhang, L. Zheng, L. Mao, H. Zheng and M. Zhu, Protruding Pt single-sites on hexagonal ZnIn2S4 to accelerate photocatalytic hydrogen evolution, Nat. Commun., 2022, 13, 1287 CrossRef CAS PubMed.
- T. Liu, W. Gao, Q. Wang, M. Dou, Z. Zhang and F. Wang, Selective Loading of Atomic Platinum on a RuCeOx Support Enables Stable Hydrogen Evolution at High Current Densities, Angew. Chem., Int. Ed., 2020, 59, 20423–20427 CrossRef CAS PubMed.
- C. Li, Z. Chen, H. Yi, Y. Cao, L. Du, Y. Hu, F. Kong, R. Kramer Campen, Y. Gao, C. Du, G. Yin, I. Y. Zhang and Y. Tong, Polyvinylpyrrolidone-Coordinated Single-Site Platinum Catalyst Exhibits High Activity for Hydrogen Evolution Reaction, Angew. Chem., Int. Ed., 2020, 59, 15902–15907 CrossRef CAS PubMed.
- J. Zhang, X. Xu, Y. Liu, X. Duan, S. Wang and H. Sun, Platinum single atoms anchored on ultra-thin carbon nitride nanosheets for photoreforming of glucose, Surf. Interfaces, 2023, 42, 103423 CrossRef CAS.
- X. Li, Y. Cao, K. Luo, L. Zhang, Y. Bai, J. Xiong, R. N. Zare and J. Ge, Cooperative catalysis by a single-atom enzyme-metal complex, Nat. Commun., 2022, 13, 2189 CrossRef CAS PubMed.
- Y. Huang, D. Li, S. Feng, Y. Jia, S. Guo, X. Wu, M. Chen and W. Shi, Pt Atoms/Clusters on Ni-phytate-sensitized Carbon Nitride for Enhanced NIR-light-driven Overall Water Splitting beyond 800 nm, Angew. Chem., Int. Ed., 2022, 61, e202212234 CrossRef CAS PubMed.
- S. u Haq, M. S. Khan, E. Djatoubai, C.-L. Dong, P. Guo, Y.-C. Huang and S. Shen, A Facile Approach for Pt Single Atoms Deposition on Two-Dimensional Calcium Niobate Nanosheets for Photocatalytic Hydrogen Evolution, ACS Sustainable Chem. Eng., 2022, 10, 9096–9104 CrossRef CAS.
- Y. Yu, S. Yang, M. Dou, Z. Zhang and F. Wang, Photochemically activated atomic ruthenium supported on boron-doped carbon as a robust electrocatalyst for hydrogen evolution, J. Mater. Chem. A, 2020, 8, 16669–16675 RSC.
- M. Liu, X. Liu, D. Fu, Z. Xie, X. Zou, W. Liu, Y. Yu, J. Wang, H. Wang, C. Tong, Z. Cheng, S. Wu, K. Ding and Y. Yu, A facile complexing agent-assistant single atom Ag-N3S1 site photodeposition strategy, Appl. Catal., B, 2022, 318, 121896 CrossRef CAS.
- A. Majumdar, P. Dutta, A. Sikdar, H. Lee, D. Ghosh, S. N. Jha, S. Tripathi, Y. Oh and U. N. Maiti, Impact of Atomic Rearrangement and Single Atom Stabilization on MoSe2@NiCo2Se4 Heterostructure Catalyst for Efficient Overall Water Splitting, Small, 2022, 18, 2200622 CrossRef CAS.
- Q. Chen, Y. Ren, H. Jin, Z. Wang, T. Cheng, T. Sun, J. Tian and Y. Zhu, Icosahedral Pd Nanocrystal Catalysts with Dispersed Bi Atoms via Photochemical Route for Enhanced Formic Acid Oxidation Reaction, ChemNanoMat, 2023, 9, e202300127 CrossRef CAS.
- H. Zhang, Y. Dong, S. Zhao, G. Wang, P. Jiang, J. Zhong and Y. Zhu, Photochemical preparation of atomically dispersed nickel on cadmium sulfide for superior photocatalytic hydrogen evolution, Appl. Catal., B, 2020, 261, 118233 CrossRef CAS.
- W. Fu, J. Wan, H. Zhang, J. Li, W. Chen, Y. Li, Z. Guo and Y. Wang, Photoinduced loading of electron-rich Cu single atoms by moderate coordination for hydrogen evolution, Nat. Commun., 2022, 13, 5496 CrossRef CAS PubMed.
- P. Sharma, M. Sharma, M. Dearg, M. Wilding, T. J. A. Slater and C. R. A. Catlow, Cd/Pt Precursor Solution for Solar H2 Production and in situ Photochemical Synthesis of Pt Single-atom Decorated CdS Nanoparticles, Angew. Chem., Int. Ed., 2023, 62, e202301239 CrossRef CAS PubMed.
- Y. Zhang, J. Zhou, F. Wang, M. Lv and K. Li, Metal-metal oxide synergistic catalysis: Pt nanoparticles anchored on mono-layer dispersed ZrO2 in SBA-15 for high efficiency selective hydrogenation, J. Catal., 2023, 421, 12–19 CrossRef CAS.
- T. Li, J. Liu, Y. Song and F. Wang, Photochemical Solid-Phase Synthesis of Platinum Single Atoms on Nitrogen-Doped Carbon with High Loading as Bifunctional Catalysts for Hydrogen Evolution and Oxygen Reduction Reactions, ACS Catal., 2018, 8, 8450–8458 CrossRef CAS.
- H. Wei, K. Huang, D. Wang, R. Zhang, B. Ge, J. Ma, B. Wen, S. Zhang, Q. Li, M. Lei, C. Zhang, J. Irawan, L.-M. Liu and H. Wu, Iced photochemical reduction to synthesize atomically dispersed metals by suppressing nanocrystal growth, Nat. Commun., 2017, 8, 1490 CrossRef PubMed.
- P. Zhou, F. Lv, N. Li, Y. Zhang, Z. Mu, Y. Tang, J. Lai, Y. Chao, M. Luo, F. Lin, J. Zhou, D. Su and S. Guo, Strengthening reactive metal-support interaction to stabilize high-density Pt single atoms on electron-deficient g-C3N4 for boosting photocatalytic H2 production, Nano Energy, 2019, 56, 127–137 CrossRef CAS.
- P. Zhou, X. Hou, Y. Chao, W. Yang, W. Zhang, Z. Mu, J. Lai, F. Lv, K. Yang, Y. Liu, J. Li, J. Ma, J. Luo and S. Guo, Synergetic interaction between neighboring platinum and ruthenium monomers boosts CO oxidation, Chem. Sci., 2019, 10, 5898–5905 RSC.
- R. Xu, B. Xu, X. You, D. Shao, G. Gao, F. Li, X.-L. Wang and Y.-F. Yao, Preparation of single-atom palladium catalysts with high photocatalytic hydrogen production performance by means of photochemical reactions conducted with frozen precursor solutions, J. Mater. Chem. A, 2023, 11, 11202–11209 RSC.
- Z. Sun, Y. Yang, C. Fang, Y. Yao, F. Qin, H. Gu, Q. Liu, W. Xu, H. Tang, Z. Jiang, B. Ge, W. Chen and Z. Chen, Atomic-Level Pt Electrocatalyst Synthesized via Iced Photochemical Method for Hydrogen Evolution Reaction with High Efficiency, Small, 2022, 18, 2203422 CrossRef CAS PubMed.
- X. Zheng, J. Tang, A. Gallo, J. A. Garrido Torres, X. Yu, C. J. Athanitis, E. M. Been, P. Ercius, H. Mao, S. C. Fakra, C. Song, R. C. Davis, J. A. Reimer, J. Vinson, M. Bajdich and Y. Cui, Origin of enhanced water oxidation activity in an iridium single atom anchored on NiFe oxyhydroxide catalyst, Proc. Natl. Acad. Sci. U. S. A., 2021, 118, e2101817118 CrossRef CAS PubMed.
- Y. Feng, Z. Wang, M. Hua, Y. Liu, L. Jing, L. Wei, Z. Hou, X. Wang, X. Yu, L. Wu, Y. Jiang, J. Deng and H. Dai, Differences between atomically-dispersed and particulate Pt supported catalysts on synergistic photothermocatalytic oxidation of VOCs from cooking oil fumes, Appl. Catal., B, 2023, 339, 123116 CrossRef CAS.
- X. Ge, P. Zhou, Q. Zhang, Z. Xia, S. Chen, P. Gao, Z. Zhang, L. Gu and S. Guo, Palladium Single Atoms on TiO2 as a Photocatalytic Sensing Platform for Analyzing the Organophosphorus Pesticide Chlorpyrifos, Angew. Chem., Int. Ed., 2019, 59, 232–236 CrossRef PubMed.
- Y. Huang, C. Wang, Y. Yu, Y. Yu, W. Wang and B. Zhang, Atomically Dispersed Ru-Decorated TiO2 Nanosheets for Thermally Assisted Solar-Driven Nitrogen Oxidation into Nitric Oxide, CCS Chem., 2021, 4, 1208–1216 CrossRef.
- H. Wei, H. Wu, K. Huang, B. Ge, J. Ma, J. Lang, D. Zu, M. Lei, Y. Yao, W. Guo and H. Wu, Ultralow-temperature photochemical synthesis of atomically dispersed Pt catalysts for the hydrogen evolution reaction, Chem. Sci., 2019, 10, 2830–2836 RSC.
- D. Li, Y. Li, X. Wang, G. Sun, J. Cao and Y. Wang, Surface modification of In2O3 porous nanospheres with Au single atoms for ultrafast and highly sensitive detection of CO, Appl. Surf. Sci., 2023, 613, 155987 CrossRef CAS.
- J. Zhou, F. Duo, C. Jia, Y. Zhou, C. Wang and Z. Wei, Photochemical Solid-Phase In Situ Anchoring of Single Atoms Ag/g-C3N4 for Enhanced Photocatalytic Activity, Environ. Eng. Sci., 2021, 38, 1098–1107 CrossRef CAS.
- Z. Shi, X. Zhang, X. Lin, G. Liu, C. Ling, S. Xi, B. Chen, Y. Ge, C. Tan, Z. Lai, Z. Huang, X. Ruan, L. Zhai, L. Li, Z. Li, X. Wang, G.-H. Nam, J. Liu, Q. He, Z. Guan, J. Wang, C.-S. Lee, A. R. J. Kucernak and H. Zhang, Phase-dependent growth of Pt on MoS2 for highly efficient H2 evolution, Nature, 2023, 621, 300–305 CrossRef CAS PubMed.
- Y. Zhang, L. Guo, L. Tao, Y. Lu and S. Wang, Defect-Based Single-Atom Electrocatalysts, Small Methods, 2019, 3, 1800406 CrossRef.
- P. Liu, R. Qin, G. Fu and N. Zheng, Surface Coordination Chemistry of Metal Nanomaterials, J. Am. Chem. Soc., 2017, 139, 2122–2131 CrossRef CAS PubMed.
- T. Zhang and S. Lu, Sacrificial agents for photocatalytic hydrogen production: Effects, cost, and development, Chem Catal., 2022, 2, 1502–1505 CrossRef CAS.
- K. Wenderich, K. Han and G. Mul, The Effect of Methanol on the Photodeposition of Pt Nanoparticles on Tungsten Oxide, Part. Part. Syst. Char., 2018, 35, 1700250 CrossRef.
- W. Jones, D. J. Martin, A. Caravaca, A. M. Beale, M. Bowker, T. Maschmeyer, G. Hartley and A. Masters, A comparison of photocatalytic reforming reactions of methanol and triethanolamine with Pd supported on titania and graphitic carbon nitride, Appl. Catal., B, 2019, 240, 373–379 CrossRef CAS.
- J. Wang, A. S. Cherevan, C. Hannecart, S. Naghdi, S. P. Nandan, T. Gupta and D. Eder, Ti-based MOFs: New insights on the impact of ligand composition and hole scavengers on stability, charge separation and photocatalytic hydrogen evolution, Appl. Catal., B, 2021, 283, 119626 CrossRef CAS.
- C. Gomes Silva, R. Juárez, T. Marino, R. Molinari and H. García, Influence of Excitation Wavelength (UV or Visible Light) on the Photocatalytic Activity of Titania Containing Gold Nanoparticles for the Generation of Hydrogen or Oxygen from Water, J. Am. Chem. Soc., 2011, 133, 595–602 CrossRef CAS PubMed.
- J.-J. Li, M. Zhang, B. Weng, J. Chen and H. Jia, Zero-degree photochemical synthesis of highly dispersed Pt/TiO2 for enhanced photocatalytic hydrogen generation, J. Alloys Compd., 2020, 849, 156634 CrossRef CAS.
- C. Gao, J. Low, R. Long, T. Kong, J. Zhu and Y. Xiong, Heterogeneous Single-Atom Photocatalysts: Fundamentals and Applications, Chem. Rev., 2020, 120, 12175–12216 CrossRef CAS PubMed.
- Y. Wang, H. Su, Y. He, L. Li, S. Zhu, H. Shen, P. Xie, X. Fu, G. Zhou, C. Feng, D. Zhao, F. Xiao, X. Zhu, Y. Zeng, M. Shao, S. Chen, G. Wu, J. Zeng and C. Wang, Advanced Electrocatalysts with Single-Metal-Atom Active Sites, Chem. Rev., 2020, 120, 12217–12314 CrossRef CAS PubMed.
- R. Qin, Z. Chen, Q. Wu, N. Zheng and P. Liu, Supported atomically dispersed Pd catalyzed direct alkoxylation and allylic alkylation, Chin. J. Chem., 2023 DOI:10.1002/cjoc.202300516.
- J. Schneider, M. Matsuoka, M. Takeuchi, J. Zhang, Y. Horiuchi, M. Anpo and D. W. Bahnemann, Understanding TiO2 Photocatalysis: Mechanisms and Materials, Chem. Rev., 2014, 114, 9919–9986 CrossRef CAS PubMed.
- Y. Nosaka and A. Nosaka, Understanding Hydroxyl Radical (˙OH) Generation Processes in Photocatalysis, ACS Energy Lett., 2016, 1, 356–359 CrossRef CAS.
- P. Liu, X. Huang, D. Mance and C. Copéret, Atomically dispersed iridium on MgO(111) nanosheets catalyses benzene–ethylene coupling towards styrene, Nat. Catal., 2021, 4, 968–975 CrossRef CAS.
| This journal is © the Partner Organisations 2024 |