Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Mycomining: perspective on fungi as scavengers of scattered metal, mineral, and rare earth element resources
Mitchell P.
Jones
 *a and
Alexander
Bismarck
*a and
Alexander
Bismarck
 *ab
*ab
aPolymer & Composite Engineering (PaCE) Group, Institute of Materials Chemistry and Research, Faculty of Chemistry, University of Vienna, Währinger Straße 42, 1090, Vienna, Austria. E-mail: mitchell.jones@univie.ac.at; alexander.bismarck@univie.ac.at
bDivision of Materials Science, Department of Engineering Sciences and Mathematics, Luleå University of Technology, SE-97187 Luleå, Sweden
First published on 15th March 2024
Abstract
Mining provides raw materials critical to our energy, agriculture, infrastructure, and technology but is associated with many environmental challenges. Resource recovery alternatives like urban mining rely on inconsistent supply streams and complicated disassembly and sorting, while extreme mining alternatives such as deep sea and space mining are potentially even less sustainable than traditional mining. This perspective investigates biological mining with emphasis on the potential of fungi for scavenging metals, minerals, and rare earth elements. “Mycomining” produces only biomass-based organic waste and can offer more versatile growth conditions than phytomining using hyperaccumulating plants including substrates ranging from soil, wood, water, and rock to living organisms and dark, space-restricted, or extreme i.e., pH levels, high salt, acidic, radioactive environments. This concept could represent a useful supplement to urban and phytomining to offset demand for traditional mining and is particularly viable when conventional mining may be inefficient or uneconomical i.e., with low-grade ores and sites unsuited to traditional mining for geographical, political, or social reasons.
Sustainability spotlightMining is essential for many sectors but is associated with considerable environmental challenges. Urban mining supply streams are inconsistent and associated disassembly and sorting complicated, while deep sea and space mining pose severe environmental risks. Biological mining using fungi can offset demand for terrestrial mining, representing environmental benefit through this energy- and waste-efficient process and remediate contaminated land. It can be applied in diverse growth environments with low-grade ores and sites unsuited to traditional mining for geographical, political, or social reasons. This work aligns with UN SDG 9 (Industry, Innovation, and Infrastructure), SDG 11 (Sustainable Cities and Communities), SDG 12 (Responsible Consumption and Production) and SDG 15 (Life on Land). |
1. Introduction
Terrestrial mining is a complex process comprising exploration, mine planning and design, site preparation, extraction, ore processing, waste management and transportation of essential raw materials like metals (e.g., iron, gold, copper, and aluminium), minerals (e.g., gypsum, limestone, and salt), and fossil fuels (e.g., coal, oil, and natural gas).1,2 Fossil fuels have historically been primary sources of energy,3 while minerals like potash and phosphate are extracted for use in fertilisers used in food production.4 Metals like iron (for steel) and copper are vital for building infrastructure and much technological advancement in consumer products, medicine, energy storage and other products are reliant on mined metals, such as lithium, cobalt, and rare earth elements (REE).5Traditional mining practices typically produce overburden (soil and rock removed to access ore beneath) and tailings (material left after the minerals are extracted from the ore that can contain chemicals and are usually disposed of in “tailings dams” or ponds).6 They are also associated with many environmental challenges: mine water contaminated with chemicals, heavy metals and other pollutants that can contaminate local waterways and groundwater,7 chemical wastes from mineral extraction, such as cyanide, sulfuric acid and mercury,8 used oil and fuel from mining machinery9 and waste rock that can produce acid when exposed to water and air leading to “acid mine drainage”.10 Smelting can also lead to emissions and dust including sulphur dioxide, heavy metals and other pollutants, and production of slag.11
Alternatives to traditional mining can help to offset demand for extraction of new metals and minerals and consequently improve environmental outcomes. Urban mining constitutes the collection and sorting of e-waste and old infrastructure,12 typically through a process of manual disassembly, shredding, and magnetic separation13 prior to metal recovery using hydro-(metal recovery using aqueous chemistry or dissolving in a strong acid followed by precipitation)14 or pyrometallurgical (melted metals are separated from the slag or dissolved metals in solution are deposited onto a substrate using a current) processes,15 which can then be further refined for reuse. Processing 2 tonnes of spent car catalysts can prevent the mining of 150 kg of platinum group element (PGE) ores.16 That said, urban mining suffers from inconsistent supply streams due to its reliance on discarded products as feedstock,17 requires complicated and intricate sorting processes to separate complex, miniaturized, and fused components18 and requires the handling of toxic substances, such as lead, mercury, cadmium, and brominated fire retardants,19 all of which result in high costs and regulatory and policy challenges.
Deep sea mining at depths >200 m can yield gold, silver and copper but primarily targets polymetallic nodules and sulphides, and cobalt-rich ferromanganese crusts.20,21 Potential deep sea mining sites can be identified by autonomous underwater vehicles or remotely operated vehicles, which can then collect polymetallic nodules from the seabed using a vacuum-like system or scrape cobalt-rich crusts from underwater formations.22,23 Polymetallic sulphides occur near hydrothermal vent systems and require more traditional mining techniques, similar to terrestrial mining.24–26 Deep sea mining operations have serious environmental concerns, such as habitat destruction,27 threat to biodiversity, sediment plumes through disruption of the seabed,28 and could release toxic substances trapped in the seabed and impact carbon sequestration.29 Space mining i.e., the harvesting of resources from asteroids, the moon or other celestial bodies has also been proposed but is still in a conceptual phase.30
This perspective focuses on an often-overlooked alternative mining technique: biological mining i.e., the use of plants or microorganisms to recover scattered metals, minerals, and REEs in a more environmentally friendly fashion than traditional mining. We map the research space linking mycoremediation (rehabilitation of a polluted environment through degradation or accumulation and disposal of contaminants e.g., oil, plastics, heavy metals), phytomining and so-called “mycomining” (scavenging dispersed valuable resources using plants and fungi, respectively, and recovery of said resources from the biomass). The perspective serves as a white paper with a broad applied scope based on the similarities, differences, interplay, and ultimately cohesion of these themes. In this vein, we collect, contrast, and extrapolate relevant points from each of these topics, to demonstrate in a single manuscript how principles from each topic can support each other and in doing so provide some insight into the potential of fungi in the active collection and winning of valuable resources, i.e., “mycomining”. Where possible, we also provide vision for how these concepts could be applied and the associated potential benefit and challenges.
2. Phytomining
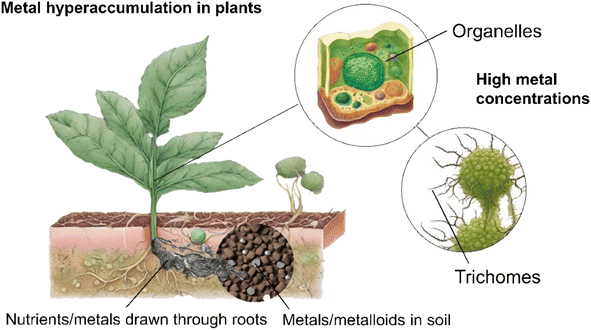
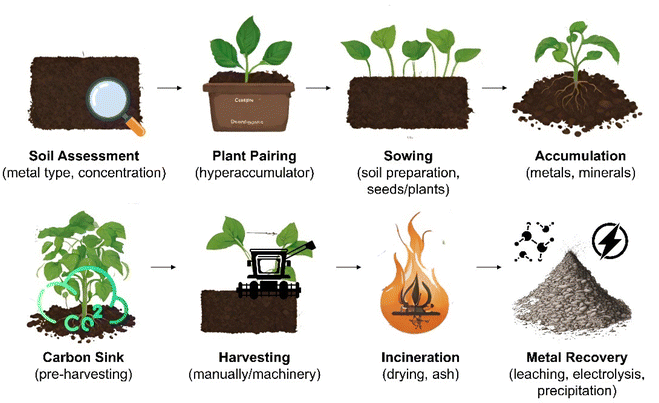
Phytomining is the growth, harvesting and processing of hyperaccumulating plant species to extract valuable metals, such as Ni, Zn, Cd, Mn, As, and Se, and REEs from contaminated soils or subgrade ore bodies.31–34 Metal hyperaccumulation in plants is a genetically controlled trait that is thought to have evolved as a method of preventing herbivores or pests feeding on the plants,35 to alter soil chemistry to provide a competitive advantage over other plants,36 or enhance tolerance to drought or other environmental stresses.37 Over 500 taxa of plants,32 including Berkheya coddii (especially efficient at accumulating Ni)38 possess this ability to absorb high quantities of metals without experiencing detrimental effects to their own health.39 Such plants take specific metals and nutrients from the soil up through their roots using specialised transport proteins40 before binding potentially toxic metals to metal-specific organic molecules called “chelators”.41,42 This prevents the metals from harming the plant's cellular machinery.43 High concentrations of metals are stored in organelles within the cell,44 older tissues,45 or trichomes (hair-like structures on the surface of the plant)46 to prevent metabolic disruption (Fig. 1). Hyperaccumulator plants often have enhanced DNA repair mechanisms,47 detoxification pathways,45 and increased antioxidant production to counteract metal-induced oxidative stress.48The phytomining process commences with a soil composition assessment at the site to determine the type and concentration of metals available followed by pairing with a suitable hyperaccumulating plant e.g., Alyssum spp. for Ni, Brassica juncea for Pb,49 or Fagopyrum esculentum and Cannabis sativa for the simultaneous phytoextraction of potentially toxic and REEs (Fig. 2).50 The soil may then be prepared by tilling, addition of fertilizer or pH adjustment, and traditional agricultural techniques to sow the site with seed or plant seedlings.51,52 While in place these plants act as a carbon sink,53 offsetting the environmental impact associated with their later incineration and making the process close to carbon neutral. The plants are harvested, either manually or using machinery,54 dried using sunlight or industrial dryers, and burned to reduce the plant biomass to ash55 (the accumulated metal compounds have a higher melting point than the combustion temperature and consequently remain in the ash). Incineration typically occurs at 450–850 °C over a period ranging from a few minutes to hours. These temperatures are considerably lower (and hence more energy efficient) than those associated with traditional ore processing, such as smelting, which often occurs at temperatures exceeding 1200 °C but have much lower metal yields.56
Plant biomass ash is then processed to remove the metals through leaching (treated with a suitable solvent, such as an acid, that dissolves the desired metal and can then be separated from the solid residue),57 electrolysis (a current is passed through the solution and the metal accumulates at one of the electrodes),58 or precipitation (chemicals are added to the solution to precipitate the metal out and it is then filtered from the solution).59 Electrolysis is arguably the most environmentally friendly of these processes since it does not introduce new chemicals, can be precise and yield high-purity metal deposits.60 Electrolytes used in this process may, however, degrade over time and need to be replaced and since the process is energy intensive, its environmental footprint depends heavily on the source of the energy.58,61 Precipitation can be efficient in recovering certain metals from solution and does not require as much energy as electrolysis but does result in the addition of new chemicals (precipitating agents) that may pose environmental or disposal risks and may produce sludge or solid wastes that require management.59,62 The precipitated metal may also require further processing to improve purity. Leaching is arguably the least environmentally friendly process due to the use of strong chemicals, the potential for spills or leaks and the environmental burden associated with the production and disposal of these chemicals, which have energy intensive production routes and produce waste that can contaminate water sources.6,8 Refining processes, specific to each metal, may then be used to remove impurities from the extracted metal.
Phytomining has a lesser physical disruption to the landscape than traditional mining techniques, which commonly cause deforestation, erosion, and contamination of water sources.33 The main waste produced is also organic i.e., plant biomass, which can also be used for energy production through biofuel synthesis63 or anaerobic digestion for biogas production.64,65 Most notably, it has the advantage of simultaneously remediating contaminated soils and harvesting valuable metals, minerals and REEs.55 However, it is not directly comparable with traditional mining processes that extract and process high grade ores, which have high energy requirements and environmental ramifications but also produce high yields.66 It is instead particularly useful in situations where conventional mining may be inefficient or uneconomical, such as when dealing with low-grade ores and sites inaccessible to traditional mining for geographical, political, or social reasons.67
3. Mycoremediation
Like plants, some fungi can also absorb and concentrate metals, minerals and REEs, which they typically bind to proteins, peptides, and other organic molecules within their cellular structures.68 Unlike plants, however, fungi are heterotrophic (not photosynthetic), meaning that they excrete enzymes, such as laccase and peroxidase, which can break down complex organic molecules, including oil spills,69 pesticides,70 and dyes71 into water, carbon dioxide and basic mineral components. Startling examples of fungi being able to degrade or bioaccumulate pollutants are as radical as observations of Cryptococcus neoformans and Cladosporium growing towards radioactive hotspots at Chernobyl in 1986, seemingly feeding on the radiation in something known as “radiosynthesis”.72This ability of fungi to not only accumulate scattered heavy metal contaminants from soils but also to degrade hydrocarbon pollutants inspired the concept of ‘mycoremediation’ as early as 1997, where oyster mushrooms were used to break down hydrocarbons from petroleum products at “The Bioneers” conference.73 Since then, fungi have been employed to break down oil spills and rejuvenate mine soil74 and port sediment health.75 Fungi have also been used to treat run-off from roads,73 often containing toxic chemicals and heavy metals, and to treat soils contaminated with herbicides.
Mycoremediation revolves around the use of mycelium (root-like growth of filamentous fungi), which is introduced at the contaminated site in the form of fully colonised grain, straw or wood chip spawn.70,76 Mycelium can be directly introduced through mixing into the soil using tilling equipment or by hand,70 in the form of a mycelium mat, which is laid directly over surface contaminations,73 as pellets that are spread across the treatment area, as ‘socks’ or ‘baffles’ i.e., mesh tubes filled with colonised substrate that can be placed in contaminated water bodies, as inoculated wood chips or mulch, or can be sprayed over large-scale or uneven terrains as liquid mycelium culture. Species are selected based on the type of contaminant (e.g., hydrocarbons, heavy metals, pesticides), soil type, pH and moisture content, and existing microbial communities.69,77–79 Genera commonly used in mycoremediation include Pleurotus, Phanerochaete, and Trametes.71,80,81 The fungal growth and reduction in contaminants at the site are monitored, with the attainment of acceptable contaminant levels signalling the end of the process. Further restoration, include planting vegetation, soil amendments and reintroduction of native species may also be necessary at this stage.
4. Mycomining
The success of phytomining and mycoremediation hint at the potential of so-called “mycomining” i.e., the intentional scavenging of dispersed valuable resources (e.g., metals, minerals, and REEs) from the environment in a fashion impossible using conventional mining.82 Mycomining is essentially a combination of mycoremediation and phytomining principles. Mycoremediation provides insight into processes that can be used to achieve open environment inoculation of large natural areas but has little emphasis on the recovery of the accumulated material as it focusses on the rehabilitation of a polluted environment and relevant contaminant degradation or accumulation mechanisms. Phytomining processes utilise different bioaccumulation mechanisms but provide insight into recovering valuable resources from biomass.The use of fungal mycelium to scavenge valuable metals, minerals and REEs holds some key advantages over the use of hyperaccumulating plants (Fig. 3). Some fungal species grow very quickly, producing a larger amount of biomass in a shorter time than plants (although this is, of course, species and growth environment dependent with obvious exceptions e.g., bamboo is a plant with a very fast growth rate) and, hence accumulating more metals, minerals or REEs.77,83,84 Fungi can also grow in a wider range of environmental conditions than most plants, including low-light, varied pH levels85 and on a range of substrates from soil, wood, water,86 and rock to living organisms87 and extreme e.g., high salt, acidic,88 radioactive environments89 (although it should be noted that species that exhibit growth versatility in such environments may not necessarily accumulate the desired, or any metal, mineral or REE, in sufficient quantities to be suitable for mycomining). Since fungi do not need light to grow, they may be less affected by pests and diseases and their lower nutrient requirements may make them lower maintenance than plants.90 In space-limited environments, such as underground mines or urban environments, fungi have a smaller physical footprint than plants.68 Some fungi also have a high tolerance for heavy metals and can accumulate metals at concentrations much higher than many plants, and organic acids produced by some fungi can mobilise metals from the environment making them more available for uptake.84,91 It is also worth noting that endophytic fungi live inside plants, and like endophytic bacteria could help them to accumulate metals, and subsequently be implemented into phytomining to enhance yields.92,93
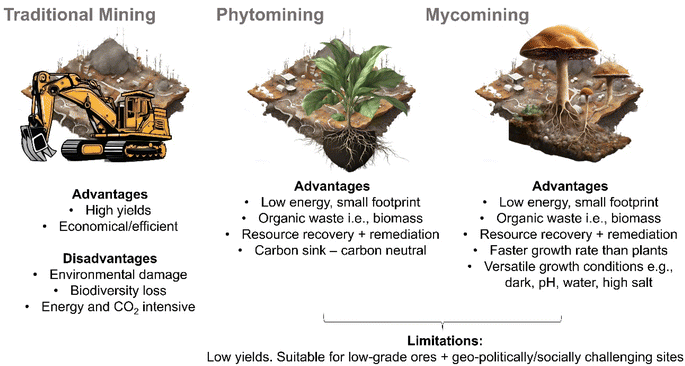 | ||
| Fig. 3 Key advantages, disadvantages, and limitations of traditional (terrestrial) mining, phytomining and “mycomining”. | ||
The environmental impact of mycomining compared to alternatives, such as terrestrial mining, urban mining and phytomining is challenging to accurately predict, however, Folchi matrices (a common environmental impact assessment in mining operations) can provide broad insight into the relative environmental impact of the different mining approaches (Table 1).94,95 Mycomining would likely improve soil quality and positively impact local flora and fauna through the bioaccumulation process. This is opposed to terrestrial mining, which, as discussed, can severely degrade soil quality, lead to considerable water, and air pollution, and disrupt local flora and fauna. Mycomining likely would not require use of fertilisers or pesticides that can lead to water pollution and soil degradation in phytomining, and is not associated with the manual disassembly or disposal of non-recoverable e-waste residues of urban mining, or the excavation and ore processing stages of terrestrial mining, both of which can release toxic materials into the environment and expose workers to health hazards. Like other mining methods, mycomining also offers socio-economic benefits, including job opportunities and the promotion of green technology. However, it is worth noting that the extraction process associated with mycomining might release spores or dust, affecting air quality and posing health risks.
| Mining activities | Soil quality | Water quality | Air quality | Flora and fauna | Human health | Landscape | Socio-economic |
|---|---|---|---|---|---|---|---|
| Terrestrial mining | |||||||
| Excavation | − | − | − | − | − | − | − |
| Ore processing | − | − | − | − | − | − | + |
| Waste disposal | − | − | − | − | − | − | − |
| Tailings management | − | − | − | − | − | − | − |
| Transportation | − | − | − | + | |||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| Urban mining | |||||||
| E-waste collection | − | + | |||||
| Disassembly | − | − | − | − | − | + | |
| Processing | − | − | − | − | − | + | |
| Material recovery | + | + | + | + | + | + | |
| Residue disposal | − | − | − | − | − | − | − |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| Phytomining | |||||||
| Planting | + | − | − | − | + | + | |
| Maintenance | + | − | − | − | + | + | |
| Harvesting | + | − | − | − | + | + | |
| Processing | − | − | − | − | + | ||
| Waste disposal | − | − | − | − | − | − | − |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| Mycomining | |||||||
| Inoculation | + | − | + | − | + | + | |
| Maintenance | + | − | + | − | + | + | |
| Harvesting | + | + | − | + | + | ||
| Processing | − | − | − | − | − | − | + |
| Waste disposal | − | − | − | − | − | − | − |
It is challenging to accurately predict how viable mycomining would be commercially, however, it could be effective in bioaccumulation of metals including Au,96,97 Cu,98,99 Ni,97 Pt97 and Zn.100 Metal recovery potential of fungi cultivated on soil or rock substrates from former mine sites is challenging to estimate as a dearth in the literature exists for fungi-based metal bioaccumulation on such substrates. However, phytomining is known to accumulate up to 304 mg kg−1 Au from gold-enriched silica sand using Brassica campestris and 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg kg−1 Ni from Ni-contaminated soil using Alyssum spp.101 Mycomining is also likely suitable for use with REEs: ectomycorrhizal and saprobic macrofungi grown in “unpolluted sites with differing bedrock chemistry” i.e., normal sites not especially conducive to mycomining, can exhibit concentrations as high as 276 and 357 μg kg−1, respectively, of 14 REEs (i.e., La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, and Lu).102 These values are competitive with available data on phytoextraction of La, Ce and Nd using Phytolacca americana plant biomass grown on mine tailings from a former mining site i.e., a much more favourable substrate for bioaccumulation, suggesting that mycomining at a similar site could potentially recover similar or even greater concentrations of REEs.103 The described phytomining example recovers up to 16.6% of the REE concentration associated with e-waste (urban mining), while the described “mycomining” example (on poorly suited substrate) recovers up to 6.6% (Table 2).102–104 Selective accumulation of specific REEs is also likely possible with Mucor javanicus, accumulating approximately three times more Sm than it does Y, La, Er and Lu.105
000 mg kg−1 Ni from Ni-contaminated soil using Alyssum spp.101 Mycomining is also likely suitable for use with REEs: ectomycorrhizal and saprobic macrofungi grown in “unpolluted sites with differing bedrock chemistry” i.e., normal sites not especially conducive to mycomining, can exhibit concentrations as high as 276 and 357 μg kg−1, respectively, of 14 REEs (i.e., La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, and Lu).102 These values are competitive with available data on phytoextraction of La, Ce and Nd using Phytolacca americana plant biomass grown on mine tailings from a former mining site i.e., a much more favourable substrate for bioaccumulation, suggesting that mycomining at a similar site could potentially recover similar or even greater concentrations of REEs.103 The described phytomining example recovers up to 16.6% of the REE concentration associated with e-waste (urban mining), while the described “mycomining” example (on poorly suited substrate) recovers up to 6.6% (Table 2).102–104 Selective accumulation of specific REEs is also likely possible with Mucor javanicus, accumulating approximately three times more Sm than it does Y, La, Er and Lu.105
| Source | Rare earth element (μg kg−1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| La | Ce | Nd | Pr | Sm | Eu | Gd | Tb | Dy | |
| Saprobic macrofungi | 207 | 357 | 139 | 40 | 37 | 13 | 30 | 9 | 58 |
| P. americana plant biomass | 522 | 224 | 322 | — | — | — | — | — | — |
| Ground sieved undersize e-waste | 5144 | 7851 | 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 235 235 |
9475 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 558 558 |
4061 | 4061 | 4602 | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 719 719 |
| Ground un-sieved e-waste | 4286 | 9286 | 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 714 714 |
5000 | 7857 | 3571 | 3571 | 3571 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 286 286 |
| Ground sieved oversize e-waste | 3134 | 5445 | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 643 643 |
2942 | 3026 | 1653 | 2830 | 1462 | 4899 |
Other notable factors hinting at the viability of mycomining include cost estimations competitive with biohydrometallurgy (i.e., 10–70 US $ per t) based on an example of Ni accumulation using Trichoderma harzianum with a soil penetration depth of 1 m and yield of 1.1% Ni per kg dry weight (11 kg Ni per t biomass)86 and the fact that the accumulated resources e.g., metals, tend to be concentrated in the fruiting body of the fungus rather than the young or old mycelium;106,107 harvesting of protruding fruiting bodies rather than interconnected mycelial sheets may be more straightforward.
5. Conclusion
Bioprocesses, including bioreduction, bioleaching, bioprecipitation and similar, based on plants and fungi are well established and documented and have wide industry utilization. On-location, open environment bioprocesses, such as the use of plants to scavenge dispersed valuable resources e.g., metals, minerals, and rare earth elements, which are then recovered from the biomass (phytomining), and fungi to degrade or accumulate environmental contaminants e.g., oil, plastics, heavy metals to then be disposed of with the biomass (mycoremediation), have also received considerable interest. The success of phytomining and mycoremediation hint at the potential of so-called “mycomining”, which could offer key benefits, such as faster and more versatile growth conditions, including in substrates ranging from soil, wood, water, and rock to living organisms and dark, space-restricted, or extreme i.e., pH levels, high salt, acidic, radioactive environments (although not all species that exhibit growth versatility in such environments may necessarily be suitable for mycomining). The existing field of mycoremediation can provide insight into biological processes that can be used to achieve open environment inoculation of large natural areas, such as the tilling of fully colonised grain, straw or wood chip spawn into soil, application as a surface mat, pellets, or spraying of uneven terrains using liquid spore medium. Mycoremediation also provides insight into bioaccumulation mechanisms and achieved concentrations, albeit for environmental contaminants rather than valuable resources, such as metals, minerals, and REEs, and with limited emphasis on resource recovery. Conversely, established phytomining processes utilise different bioaccumulation mechanisms but provide insight into recovering valuable resources from biomass through ashing followed by leaching, electrolysis, or precipitation and the relative environmental impact of these processes. Contrasting and extrapolating on phytomining and mycoremediation bioaccumulation processes, “mycomining” would seem conceptually viable, with broad estimates suggesting comparable recovery potential and environmental impact to phytomining. Although not directly comparable with traditional mining processes, mycomining could be a useful supplement to urban mining to offset demand for mining or used in situations where conventional mining may be inefficient or uneconomical.Conflicts of interest
The authors have no conflicts of interest to declare.References
-
H. L. Hartman and J. M. Mutmansky, Introductory Mining Engineering, John Wiley & Sons, 2002 Search PubMed
.
-
P. Darling, SME Mining Engineering Handbook, SME, 2011 Search PubMed
.
-
V. Smil, Energy at the Crossroads: Global Perspectives and Uncertainties, Cambridge, Mass, MIT Press, 2003 Search PubMed
.
-
J. Havlin, S. L. Tisdale, W. L. Nelson and J. D. Beaton, Soil Fertility and Fertilizers: An Introduction to Nutrient Management, Pearson, 2014 Search PubMed
.
- S. H. Ali, D. Giurco, N. Arndt, E. Nickless, G. Brown, A. Demetriades, R. Durrheim, M. A. Enriquez, J. Kinnaird and A. Littleboy, Nature, 2017, 543, 367–372 CrossRef CAS PubMed
.
-
B. Lottermoser and B. G. Lottermoser, Mine Wastes: Characterization, Treatment and Environmental Impacts, 2010, 205–241 Search PubMed
.
-
P. L. Younger, S. A. Banwart, R. S. Hedin, P. L. Younger, S. A. Banwart and R. S. Hedin, Mine Water Hydrology, Springer, 2002 Search PubMed
.
-
A. G. Smith and T. Mudder, The Chemistry and Treatment of Cyanidation Wastes, Mining journal books limited, 1991 Search PubMed
.
-
G. Kodilinye, Commonwealth Caribbean Tort Law, Routledge-Cavendish, 2009 Search PubMed
.
- A. Akcil and S. Koldas, J. Cleaner Prod., 2006, 14, 1139–1145 CrossRef
.
- T. Norgate and N. Haque, J. Cleaner Prod., 2010, 18, 266–274 CrossRef CAS
.
- V. Balaram, Geosci. Front., 2019, 10, 1285–1303 CrossRef CAS
.
- C. Hagelüken and C. W. Corti, Gold Bull., 2010, 43, 209–220 CrossRef
.
- A. Mecucci and K. Scott, J. Chem. Technol. Biotechnol., 2002, 77, 449–457 CrossRef CAS
.
- J. Cui and L. Zhang, J. Hazard. Mater., 2008, 158, 228–256 CrossRef CAS PubMed
.
- A. Fornalczyk and M. Saternus, Metalurgija, 2009, 48, 133 CAS
.
- X. Zeng, J. A. Mathews and J. Li, Environ. Sci. Technol., 2018, 52, 4835–4841 CrossRef CAS PubMed
.
- S. Gupta, G. Modi, R. Saini and V. Agarwala, Int. Refereed J. Eng. Nat. Appl. Sci., 2014, 3, 05–17 Search PubMed
.
-
K. Lundgren, The Global Impact of E-Waste: Addressing the Challenge, International Labour Organization, 2012 Search PubMed
.
- J. R. Hein, K. Mizell, A. Koschinsky and T. A. Conrad, Ore Geol. Rev., 2013, 51, 1–14 CrossRef
.
- J. R. Hein, T. A. Conrad and R. E. Dunham, Mar. Georesour. Geotechnol., 2009, 27, 160–176 CrossRef CAS
.
-
R. Sharma, Deep-sea Mining: Resource Potential, Technical and Environmental Considerations, Springer, 2017 Search PubMed
.
- J. Halfar and R. M. Fujita, Science, 2007, 316, 987 CrossRef CAS PubMed
.
- P. M. Herzig and M. D. Hannington, Ore Geol. Rev., 1995, 10, 95–115 CrossRef
.
- S. Petersen, A. Krätschell, N. Augustin, J. Jamieson, J. R. Hein and M. D. Hannington, Mar. Pol., 2016, 70, 175–187 CrossRef
.
- P. Hoagland, S. Beaulieu, M. A. Tivey, R. G. Eggert, C. German, L. Glowka and J. Lin, Mar. Pol., 2010, 34, 728–732 CrossRef
.
- L. A. Levin, K. Mengerink, K. M. Gjerde, A. A. Rowden, C. L. Van Dover, M. R. Clark, E. Ramirez-Llodra, B. Currie, C. R. Smith and K. N. Sato, Mar. Pol., 2016, 74, 245–259 CrossRef
.
- D. O. Jones, S. Kaiser, A. K. Sweetman, C. R. Smith, L. Menot, A. Vink, D. Trueblood, J. Greinert, D. S. Billett and P. M. Arbizu, PLoS One, 2017, 12, e0171750 CrossRef PubMed
.
- D. J. Amon, J. Gobin, C. L. Van Dover, L. A. Levin, L. Marsh and N. A. Raineault, Front. mar. sci., 2017, 4, 342 CrossRef
.
- I. A. Crawford, Prog. Phys. Geogr., 2015, 39, 137–167 CrossRef
.
- V. Sheoran, A. S. Sheoran and P. Poonia, J. Geochem. Explor., 2013, 128, 42–50 CrossRef CAS
.
- A. Van der Ent, A. J. Baker, R. D. Reeves, A. J. Pollard and H. Schat, Plant Soil, 2013, 362, 319–334 CrossRef CAS
.
- R. R. Brooks, M. F. Chambers, L. J. Nicks and B. H. Robinson, Trends Plant Sci., 1998, 3, 359–362 CrossRef
.
- T. Dinh, Z. Dobo and H. Kovacs, Chemosphere, 2022, 297, 134259 CrossRef CAS PubMed
.
- N. Rascio and F. Navari-Izzo, Plant Sci., 2011, 180, 169–181 CrossRef CAS PubMed
.
- A. J. Pollard, R. D. Reeves and A. J. Baker, Plant Sci., 2014, 217, 8–17 CrossRef PubMed
.
- G. Potters, T. P. Pasternak, Y. Guisez, K. J. Palme and M. A. Jansen, Trends Plant Sci., 2007, 12, 98–105 CrossRef CAS PubMed
.
- J. Mesjasz-Przybyłowicz, M. Nakonieczny, P. Migula, M. Augustyniak, M. Tarnawska, W. Reimold, C. Koeberl, W. Przybyłowicz and E. Głowacka, Acta Biol. Cracov., Ser. Bot., 2004, 46, 75–85 Search PubMed
.
- A. van Der Ent, A. J. Baker, R. D. Reeves, R. L. Chaney, C. W. Anderson, J. A. Meech, P. D. Erskine, M.-O. Simonnot, J. Vaughan and J. L. Morel, Environ. Sci. Technol., 2015, 49(8), 4773–4780 CrossRef CAS PubMed
.
- T. Kobayashi and N. K. Nishizawa, Annu. Rev. Plant Biol., 2012, 63, 131–152 CrossRef CAS PubMed
.
- J. á. Hall, J. Exp. Bot., 2002, 53, 1–11 CrossRef CAS PubMed
.
- S. Clemens, M. G. Palmgren and U. Krämer, Trends Plant Sci., 2002, 7, 309–315 CrossRef CAS PubMed
.
-
A. Manara, in Plants and Heavy Metals, ed. A. Furini, Springer Netherlands, Dordrecht, 2012, pp. 27–53, DOI:10.1007/978-94-007-4441-7_2
.
- N. Verbruggen, C. Hermans and H. Schat, New Phytol., 2009, 181, 759–776 CrossRef CAS PubMed
.
- U. Krämer, Annu. Rev. Plant Biol., 2010, 61, 517–534 CrossRef PubMed
.
- D. E. Salt, I. Baxter and B. Lahner, Annu. Rev. Plant Biol., 2008, 59, 709–733 CrossRef CAS PubMed
.
- G. DalCorso, S. Farinati and A. Furini, Plant Signaling Behav., 2010, 5, 663–667 CrossRef CAS PubMed
.
- P. Sharma, A. B. Jha, R. S. Dubey and M. Pessarakli, J. Bot., 2012, 2012, 217037 Search PubMed
.
- P. N. Kumar, V. Dushenkov, H. Motto and I. Raskin, Environ. Sci. Technol., 1995, 29, 1232–1238 CrossRef CAS PubMed
.
- P. U. Okoroafor, C. O. Ogunkunle, H. Heilmeier and O. Wiche, Int. J. Phytorem., 2022, 24, 1310–1320 CrossRef CAS PubMed
.
- B. Robinson, A. Chiarucci, R. Brooks, D. Petit, J. Kirkman, P. Gregg and V. De Dominicis, J. Geochem. Explor., 1997, 59, 75–86 CrossRef CAS
.
-
I. Raskin and B. D. Ensley, Phytoremediation of Toxic Metals, John Wiley and Sons, 2000 Search PubMed
.
- R. Lal, Science, 2004, 304, 1623–1627 CrossRef CAS PubMed
.
- V. Sheoran, A. Sheoran and P. Poonia, Miner. Eng., 2009, 22, 1007–1019 CrossRef CAS
.
- A. J. Baker and R. Brooks, Biorecovery, 1989, 1, 81–126 CAS
.
-
W. J. Rankin, Minerals, Metals and Sustainability: Meeting Future Material Needs, CSIRO publishing, 2011 Search PubMed
.
-
J. O. Marsden and C. I. House, Chemistry of Gold Extraction, Society for Mining, Metallurgy, and Exploration, Colorado, USA, 2006 Search PubMed
.
-
M. L. Free, Hydrometallurgy: Fundamentals and applications, John Wiley and Sons, Hoboken, New Jersey, 2013, vol. 199, p. 222 Search PubMed
.
-
F. Habashi, Principles of Extractive Metallurgy, Routledge, 2017 Search PubMed
.
- G. Chen, Sep. Purif. Technol., 2004, 38, 11–41 CrossRef CAS
.
- A. P. Abbott, G. Frisch, J. Hartley and K. S. Ryder, Green Chem., 2011, 13, 471–481 RSC
.
- F. Fu and Q. Wang, J. Environ. Manage., 2011, 92, 407–418 CrossRef CAS PubMed
.
- S. N. Naik, V. V. Goud, P. K. Rout and A. K. Dalai, Renewable Sustainable Energy Rev., 2010, 14, 578–597 CrossRef CAS
.
- N. Zaffar, E. Ferchau, U. Feuerstein, H. Heilmeier, C. Moschner and O. Wiche, Carpathian J. Earth Environ. Sci., 2020, 15, 43–48 CrossRef
.
- Y. Tian and H. Zhang, Int. J. Green Energy, 2016, 13, 1556–1563 CrossRef
.
- R. L. Chaney, J. S. Angle, C. L. Broadhurst, C. A. Peters, R. V. Tappero and D. L. Sparks, J. Environ. Qual., 2007, 36, 1429–1443 CrossRef CAS PubMed
.
- C. W. Anderson, R. R. Brooks, R. B. Stewart and R. Simcock, Nature, 1998, 395, 553–554 CrossRef CAS
.
- G. M. Gadd, Mycol. Res., 2007, 111, 3–49 CrossRef CAS PubMed
.
- A. Haritash and C. Kaushik, J. Hazard. Mater., 2009, 169, 1–15 CrossRef CAS PubMed
.
-
H. Singh, Mycoremediation: Fungal Bioremediation, John Wiley & Sons, 2006 Search PubMed
.
- D. Wesenberg, I. Kyriakides and S. N. Agathos, Biotechnol. Adv., 2003, 22, 161–187 CrossRef CAS PubMed
.
- E. Dadachova and A. Casadevall, Curr. Opin. Microbiol., 2008, 11, 525–531 CrossRef CAS PubMed
.
-
P. Stamets, Mycelium Running: How Mushrooms Can Help Save the World, Ten Speed Press, Berkeley, CA, 2005 Search PubMed
.
- T. M. Palanivel, B. Pracejus and L. A. B. Novo, Chemosphere, 2023, 314, 137688 CrossRef CAS PubMed
.
- G. Cecchi, G. Vagge, L. Cutroneo, G. Greco, S. Di Piazza, M. Faga, M. Zotti and M. Capello, Environ. Sci. Pollut. Res., 2019, 26, 35602–35609 CrossRef CAS PubMed
.
- V. Šašek, T. Cajthaml and M. Bhatt, Water, Air, Soil Pollut.: Focus, 2003, 3, 5–14 CrossRef
.
- G. M. Gadd, Geoderma, 2004, 122, 109–119 CrossRef CAS
.
-
R. M. Atlas and J. Philp, Bioremediation. Applied Microbial Solutions for Real-World Environmental Cleanup, ASM press, 2005 Search PubMed
.
- S. Kuppusamy, P. Thavamani, K. Venkateswarlu, Y. B. Lee, R. Naidu and M. Megharaj, Chemosphere, 2017, 168, 944–968 CrossRef CAS PubMed
.
- S. Pointing, Appl. Microbiol. Biotechnol., 2001, 57, 20–33 CrossRef CAS PubMed
.
- P. Baldrian, FEMS Microbiol. Ecol., 2004, 50, 245–253 CrossRef CAS PubMed
.
- X. Liang and G. M. Gadd, Microb. Biotechnol., 2017, 10, 1199–1205 CrossRef PubMed
.
- B. Volesky, Water Res., 2007, 41, 4017–4029 CrossRef CAS PubMed
.
- M. Fomina and G. M. Gadd, Bioresour. Technol., 2014, 160, 3–14 CrossRef CAS PubMed
.
-
G. M. Gadd, Fungi in Biogeochemical Cycles, Cambridge University Press, 2006 Search PubMed
.
- G. Cecchi, E. Roccotiello, S. Di Piazza, A. Riggi, M. G. Mariotti and M. Zotti, J. Environ. Sci. Health, Part B, 2017, 52, 166–170 CrossRef CAS PubMed
.
- G. Strobel, B. Daisy, U. Castillo and J. Harper, J. Nat. Prod., 2004, 67, 257–268 CrossRef CAS PubMed
.
- G. Cecchi, A. Ceci, P. Marescotti, A. M. Persiani, S. Di Piazza and M. Zotti, Mycol. Prog., 2019, 18, 415–423 CrossRef
.
- S. Onofri, L. Selbmann, G. S. de Hoog, M. Grube, D. Barreca, S. Ruisi and L. Zucconi, Adv. Space Res., 2007, 40, 1657–1664 CrossRef
.
-
G. N. Agrios, Plant Pathology, Elsevier, 2005 Search PubMed
.
- P. Baldrian, Enzyme Microb. Technol., 2003, 32, 78–91 CrossRef CAS
.
- M. Rajkumar, N. Ae and H. Freitas, Chemosphere, 2009, 77, 153–160 CrossRef CAS PubMed
.
- Y. Ma, M. Rajkumar, Y. Luo and H. Freitas, J. Hazard. Mater., 2011, 195, 230–237 CrossRef CAS PubMed
.
-
R. Folchi, Environmental Impact Statement for Mining with Explosives: A Quantitative Method, I.S.E.E. 29th Annual Conference on Explosives and Blasting Technique, Nashville, Tennessee, U.S.A., 2003 Search PubMed
.
- M. Monjezi, K. Shahriar, H. Dehghani and F. Samimi Namin, Environ. Geol., 2009, 58, 205–216 CrossRef CAS
.
- F. Reith, M. F. Lengke, D. Falconer, D. Craw and G. Southam, ISME J., 2007, 1, 567–584 CrossRef CAS PubMed
.
- B. A. Moore, J. R. Duncan and J. E. Burgess, Miner. Eng., 2008, 21, 55–60 CrossRef CAS
.
- K. Bosecker, FEMS Microbiol. Rev., 1997, 20, 591–604 CrossRef CAS
.
- S. Zapotoczny, A. Jurkiewicz, G. Tylko, T. Anielska and K. Turnau, Microbiol. Res., 2007, 162, 219–228 CrossRef CAS PubMed
.
- E. Mohammadian, A. Babai Ahari, M. Arzanlou, S. Oustan and S. H. Khazaei, Chemosphere, 2017, 185, 290–296 CrossRef CAS PubMed
.
-
LAB Novo, P. M. L. Castro, P. Alvarenga and E. F. da Silva, in Phytoremediation: Management of Environmental Contaminants, ed. A. A. Ansari, S. S. Gill, R. Gill, G. R. Lanza and L. Newman, Springer International Publishing, Cham, 2017, vol. 5, pp. 469–486, DOI:10.1007/978-3-319-52381-1_18
.
- J. Borovička, J. Kubrová, J. Rohovec, Z. Řanda and C. E. Dunn, BioMetals, 2011, 24, 837–845 CrossRef PubMed
.
- W.-S. Liu, Y.-Y. Chen, H. Huot, C. Liu, M.-N. Guo, R.-L. Qiu, J. L. Morel and Y.-T. Tang, J. Cleaner Prod., 2020, 275, 122959 CrossRef CAS
.
- A. Yuksekdag, B. Kose-Mutlu, B. Zeytuncu-Gokoglu, M. Kumral, M. R. Wiesner and I. Koyuncu, Environ. Sci. Pollut. Res., 2022, 1–10 Search PubMed
.
- T. Tsuruta, J. Rare Earths, 2007, 25, 526–532 CrossRef
.
- H. Ghahremani-Majd and F. Dashti, Hortic., Environ. Biotechnol., 2015, 56, 376–382 CrossRef CAS
.
- J. Falandysz, A. R. Fernandes and D. Meloni, Trends Food Sci. Technol., 2022, 119, 338–347 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2024 |