Open Access Article
Open Access ArticleNovel CO2-philic porous organic polymers synthesized in water: a leap towards eco-sustainability†
Riccardo
Mobili‡
 a,
Yue
Wu
a,
Yue
Wu
 b,
Charl Xavier
Bezuidenhout
b,
Charl Xavier
Bezuidenhout
 c,
Sonia
La Cognata
c,
Sonia
La Cognata
 a,
Silvia
Bracco
a,
Silvia
Bracco
 *c,
Mariolino
Carta
*c,
Mariolino
Carta
 *b and
Valeria
Amendola
*b and
Valeria
Amendola
 *a
*a
aDepartment of Chemistry, University of Pavia, Viale Tarquato Taramelli 12, Pavia, 27100, Italy. E-mail: valeria.amendola@unipv.it
bDepartment of Chemistry, Faculty of Science and Engineering, Swansea University, Singleton Park, Swansea, Wales SA2 8PP, UK. E-mail: mariolino.carta@swansea.ac.uk
cDepartment of Materials Science, University of Milano-Bicocca, Via Cozzi 55, Milano, 20125, Italy. E-mail: silvia.bracco@unimib.it
First published on 14th October 2024
Abstract
We introduce two novel keto-enamine-linked porous organic polymers (POPs) distinguished by the presence of methyl or ethyl groups in their triamine precursors. These innovative POPs can be synthesized efficiently in water under mild conditions, utilizing starting materials that can be prepared on a gram scale through well-established procedures. Unlike most CO2-philic POPs, which often require organic solvents, high temperatures, catalysts, additives, or hydrothermal equipment, these new polymers are synthesized in pure water at a relatively low temperature (70 °C) without any catalysts or additives and using common glassware. The N-rich composition of these porous organic polymers also contributes to their high adsorption selectivity for CO2 over N2, as calculated with the IAST method at 298 K. This combination of environmentally friendly synthesis, high yield, and superior adsorption properties positions these novel POPs as promising candidates for greener carbon capture technologies based on solid sorbents.
Sustainability spotlightThe increasing concentration of greenhouse gases in the atmosphere, primarily CO2, is a major concern in our time. Consequently, developing and implementing technologies for efficient carbon capture and reuse is an imperative task. Various technologies have been developed to achieve this goal, including adsorption techniques utilizing CO2-philic sorbents such as porous organic polymers (POPs). In this study, we report the synthesis in water and the gas adsorption properties of two CO2-selective keto-enamine POPs. The reported protocol not only avoids the use of catalysts or additional additives but also directly produces a material with permanent porosity, representing an easy and green process for producing POPs for the selective sorption and separation of CO2 from gas mixtures. Our work emphasizes the importance of the following UN Sustainable Development Goals: Affordable and Clean Energy (SDG 7), Industry, Innovation, and Infrastructure (SDG 9), and Climate Action (SDG 13). |
Introduction
The increasing amount of greenhouse gases in the atmosphere (mostly CO2) is a major concern in our times. With an annual release of more than 32 gigatons (Gt), the accumulation of anthropogenic carbon dioxide in the atmosphere has led to a 0.8–1.2 °C increase in global temperatures compared to pre-industrial levels. Global warming is considered the cause of a myriad of natural calamities, posing threats to the environment and human health.1 Consequently, developing and implementing new technologies for efficient Carbon Capture Utilization and Storage (CCUS) systems are imperative tasks. Various technologies have been developed to selectively remove CO2 from gas mixtures. Examples include chemical absorption in alkanolamine solutions,2 membrane separation,3 and adsorption-based techniques.4 In the latter approach, solid sorbents that can selectively interact with a gas species through physical interactions are employed. A wide range of extended or molecular porous materials have been explored for this purpose, including zeolites and porous carbon,5 Metal–Organic Frameworks (MOFs),6 molecular materials such as Porous Organic Cages (POCs),7 and others.8 Promising alternatives for CO2 capture and separation include Porous Organic Polymers (POPs),9 Covalent Organic Frameworks (COFs),10 Polymers of Intrinsic Microporosity (PIMs),11 and Conjugated Microporous Polymers (CMPs),12 among others.13 POPs comprise organic linkers containing lightweight elements (e.g., C, H, B, N, O) held together by covalent bonds. These materials typically exhibit high porosity, thermal and chemical stability, and allow easy tuning of pore size and functionalities.14 Due to these characteristics, POPs have shown great potential in various applications such as catalysis,15 chemical sensing,16 energy storage,17 and, notably, gas storage and separation.9,18 Furthermore, the presence of heteroatoms in the backbone structure, particularly nitrogen, is well known to promote selective CO2 capture through dipole–quadrupole interactions.19 Schiff base condensation is a well-established process in the synthesis of CO2-philic POPs. Critical issues connected to the susceptibility of Schiff bases to hydrolysis have successfully been addressed by converting imine bonds into more robust linkages, e.g., keto-enamines. For porous organic materials, this is achieved by reacting primary polyamines with 1,3,5-triformylphloroglucinol (TFP).19,20 In fact, the Schiff base formed undergoes an irreversible keto–enol tautomerization, leading to a keto-enamine product that is stabilized by intramolecular H-bonds.19 Apart from a few examples of keto-enamine-based COFs obtained through mechanochemical solid state synthesis,19c most of the currently applied syntheses for CO2-philic POPs involve the use of toxic organic solvents (e.g., dioxane, mesitylene, dimethylformamide, o-dichlorobenzene and other halogenated solvents), acidic or metal catalysts, and high reaction temperatures (100–200 °C).9 It is, therefore, crucial to develop more environmentally friendly procedures for synthesizing POPs in order to facilitate the future industrial applications of these porous materials in CO2 sorption.21 While water represents a greener alternative as a solvent for synthesizing POPs, there are still limited examples of its efficient utilization.9,21–26In this study, we report the syntheses in water and the gas adsorption properties of two novel CO2-philic keto-enamine POPs: POP-W-Et and POP-W-Me (Scheme 1). The optimized protocol for forming these nitrogen-rich polymers involves the condensation between non-planar, water-soluble tris-amines [1,3,5-tris(aminomethyl)mesitylene and 1,3,5-tris(aminomethyl)-2,4,6-triethylbenzene] and 1,3,5-triformylphloroglucinol, conducted without the need for acidic or transition metal-based catalysts, and under mild reaction conditions. The resulting amorphous keto-enamine-based polymers have been comprehensively characterized and assessed for gas adsorption applications, with a particular focus on the selectivity for CO2 over N2. Additionally, the novel POPs have been compared with the same polymers obtained under conventional conditions (using organic solvents and acid catalysts) to determine whether the water-based synthesis affects the physicochemical characteristics and the adsorption properties of the materials.
Results and discussion
Synthesis of the novel keto-enamine POPs
POP-W-Et and POP-W-Me were synthesized in aqueous solution (‘W’ indicates that the reaction was conducted in water) through one to one Schiff base condensation between 1,3,5-triformylphloroglucinol (TFP) and two distinct non-planar, water-soluble tris-amines, 1,3,5-tris(aminomethyl)mesitylene and 1,3,5-tris(aminomethyl)-2,4,6-triethylbenzene (named MeNH2 and EtNH2 for simplicity). All the starting compounds (EtNH2, MeNH2, TFP) were synthesized on a gram scale following procedures already reported in the literature (see the ESI section†).Our study started by optimizing the synthetic conditions: reaction time, temperature, and presence/absence of acetic acid as a catalyst. EtNH2 was chosen as the model amine at this stage, given that its homologue (i.e., MeNH2) demonstrated comparable reactivity and water solubility. Five polymer samples (s1–s5) were obtained for POP-W-Et, in diverse conditions reported in Table 1. This investigation pointed out that variations in reaction temperature (room temperature vs. 70 °C) and reaction time (24 hours vs. 72 hours) had low impact on reaction yields and surface areas. On the other hand, the presence of an acid catalyst negatively impacted the porosity of the materials. This observation aligns with the findings of J. Lu et al.,27 who reported that azine-linked COFs could be efficiently synthesized in water (72 hours, 120 °C) in the absence of acetic acid as a catalyst. Compared to most of the previously reported examples,9a the polymers synthesized in this work were obtained in larger yield (90%) by reacting in water without any catalyst (or other additives) at a lower reaction temperature (70 °C). The sample featuring the highest surface area is POP-W-Et_s4 (Table 1).
| Sample (S) | Solvent | Catalyst | Temperature (°C) | Time (h) | Yield (%) | SABETa (m2 g−1) | SABETb (m2 g−1) |
|---|---|---|---|---|---|---|---|
| a The SABET was calculated from CO2 adsorption curves at 273 K. b The SABET was calculated from CO2 adsorption curves at 195 K. | |||||||
| POP-W-Et_s1 | H2O | — | r.t. | 24 | 93 | 321 | — |
| POP-W-Et_s2 | H2O | AcOH | r.t. | 24 | 87 | 176 | — |
| POP-W-Et_s3 | H2O | — | 70 | 24 | 83 | 344 | — |
| POP-W-Et_s4 | H2O | — | 70 | 72 | 90 | 344 | 389 |
| POP-W-Et_s5 | H2O | AcOH | 70 | 24 | 73 | 182 | — |
| POP-O-Et_s1 | Dioxane/mesitylene 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— | 70 | 72 | 70 | 253 | — |
| POP-O-Et_s2 | Dioxane/mesitylene 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
AcOH | 70 | 72 | 85 | 454 | 440 |
| POP-O-Me | Dioxane/mesitylene 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
AcOH | 70 | 72 | 92 | 227 | 302 |
| POP-W-Me | H2O | — | 70 | 72 | 93 | 257 | 348 |
We also synthesized the POPs using more conventional conditions, such as a 1,4-dioxane/mesitylene 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) mixture, to compare and validate the efficacy of the water-based synthesis. The results for the two polymer samples, POP-O-Et_s1 and POP-O-Et_s2, are shown in Table 1 (‘O’ indicates the use of the organic solvent mixture). On the contrary of our previous findings in water, using acetic acid as a catalyst proved crucial for achieving high specific surface areas, such as for POP-O-Et_s2 synthesized at 70 °C for 72 hours with an 85% yield.
1 (v/v) mixture, to compare and validate the efficacy of the water-based synthesis. The results for the two polymer samples, POP-O-Et_s1 and POP-O-Et_s2, are shown in Table 1 (‘O’ indicates the use of the organic solvent mixture). On the contrary of our previous findings in water, using acetic acid as a catalyst proved crucial for achieving high specific surface areas, such as for POP-O-Et_s2 synthesized at 70 °C for 72 hours with an 85% yield.
The optimized reaction conditions (reported in the experimental section), corresponding to those employed for samples POP-W-Et_s4 and POP-O-Et_s2, were subsequently applied in the reactions of MeNH2 with TFP, leading to the corresponding POP-W-Me and POP-O-Me polymers (Table 1). Before undergoing chemical and morphological characterization, the crude POPs were thoroughly washed with water, acetone, and methanol to eliminate any residual unreacted monomers or small oligomers. Subsequently, they were dried under vacuum at 80 °C for 24 hours.
Physico-chemical characterization
The novel POPs have been characterized by elemental analysis (Table S1†), Fourier Transform Infrared (FT-IR) and solid-state NMR spectroscopies, Powder X-ray diffraction (PXRD) and scanning electron microscopy (SEM). Moreover, thermal stability was assessed using Thermogravimetric analysis (TGA). POP-W-Et/POP-O-Et and POP-W-Me/POP-O-Me exhibited similar decomposition patterns, with a first mass loss initiating around 300 °C and a second starting at 400 °C, leading to complete sample degradation at approximately 650 °C. The patterns also display a slight mass loss below 100 °C, which can be attributed to residual solvent or adsorbed humidity (see Fig. S4–S7†), as observed in previously reported β-keto-enamine-based organic materials.28 Differential Scanning Calorimetry (DSC) measurements on the POPs confirmed this hypothesis, showing that endothermic peaks at 55 °C have no counterpart in the cooling cycle (Fig. S8–S11†).The FT-IR spectra exhibited similarities for all the investigated samples, regardless of whether they were synthesized in water or organic media.
Notably, the disappearance of the N–H stretching frequency for the tris-amines, the shift of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O peak to lower energy (from 1639 cm−1 in TFP to 1598 cm−1 in both POPs) and the emergence of prominent C
O peak to lower energy (from 1639 cm−1 in TFP to 1598 cm−1 in both POPs) and the emergence of prominent C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C peaks (at 1545 cm−1 for POP-W-Et/POP-O-Et; 1541 cm−1 for POP-W-Me/POP-O-Me) are consistent with the formation of the β-keto-enamine frameworks. This is further endorsed by the absence of stretching peaks corresponding to O–H and C
C peaks (at 1545 cm−1 for POP-W-Et/POP-O-Et; 1541 cm−1 for POP-W-Me/POP-O-Me) are consistent with the formation of the β-keto-enamine frameworks. This is further endorsed by the absence of stretching peaks corresponding to O–H and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds (Fig. S12–S13†), and by the presence of peaks due to C–N bonds at 1303 and 1309 cm−1 for POP-W-Et/POP-O-Et and POP-W-Me/POP-O-Me, respectively.20
N bonds (Fig. S12–S13†), and by the presence of peaks due to C–N bonds at 1303 and 1309 cm−1 for POP-W-Et/POP-O-Et and POP-W-Me/POP-O-Me, respectively.20
Solid-state NMR spectroscopy confirmed the formation of the β-keto-enamine polymer frameworks. In particular, the 1H–13C Cross Polarization Magic Angle Spinning (CP MAS) spectra collected with the contact time of 2 ms exhibit several signals in complete agreement with the chemical structure of the monomeric unit, both for POP-W-Et and POP-W-Me (Fig. 1 and S14–S18†). Notably, the spectra do not display peaks around 192 ppm (ref. 20) due to residual or terminal aldehyde groups. Single peaks are observed for each species, except for the carbonyl (keto) group (ca. 185 ppm), which shows a more complex line shape, suggesting alternative configurations of the nearby enamine double bonds. Signal assignments were established by comparison to 1H–13C CPMAS experiments performed at shorter contact time (50 μs), which allows only the observation of carbon atoms directly bonded to hydrogen atoms. Additionally, 13C Single Pulse Experiment spectra (SPE MAS) performed with a recycle delay of 60 s confirmed the formation of the expected chemical structures (Fig. S15, S17 and Table S2†).
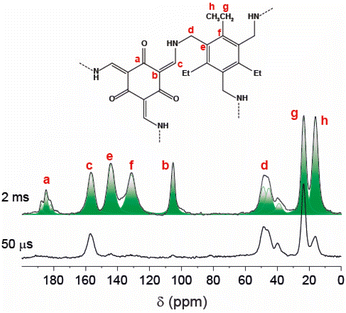 | ||
| Fig. 1 1H–13C CP MAS NMR spectra of POP-W-Et performed at variable contact time and a spinning speed of 12.5 kHz. | ||
Notably, no discernible differences have been found between materials synthesized in water and their counterparts produced in organic solvents, demonstrating that both synthetic strategies are perfectly feasible. PXRD analysis revealed that all the employed synthetic procedures yield amorphous materials, as demonstrated by the presence of two broad features at 14° (d = 6.3 Å) and 22° (d = 4.0 Å) for both POP-W-Et/POP-O-Et and POP-W-Me/POP-O-Me (Fig. 2 and S20–S23†). Scanning Electron Microscopy (SEM) investigations unveiled similar morphologies for the four POPs, characterized by aggregated globular-shaped particles with average dimensions of 0.5–1 μm. Remarkably, even in this case we found no significant differences between materials synthesized in water and those produced in organic media (Fig. 2 and S24–S25†).
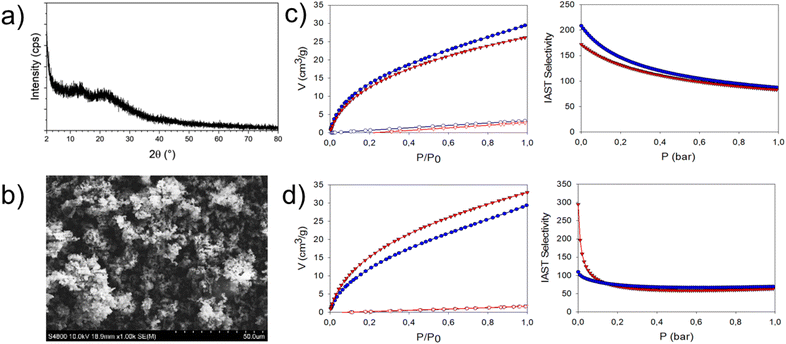 | ||
| Fig. 2 (a) and (b), PXRD and SEM picture of POP-W-Et (see additional material in the ESI†). c) POP-W-Me (blue) and POP-O-Me (red): adsorption curves at 298 K of CO2 and N2 (full and empty symbols, respectively), and calculated IAST selectivity. (d) POP-W-Et (blue) and POP-O-Et (red): adsorption curves at 298 K of CO2 and N2 (full and empty symbols, respectively), and calculated IAST selectivity. The units for the adsorption plots are cm3 g−1 STP. | ||
The elemental composition of the two polymers was analysed, with the results presented in Table S1.† From these data, we can conclude that, although POP-Me exhibits a slightly higher nitrogen content (in both water and organic solvent), this does not appear to significantly impact the CO2 uptake. Interestingly, the highest CO2/N2 selectivity (Table 2 and Fig. 2) was observed for POP-Et, despite the slightly greater presence of nucleophilic nitrogen in POP-Me, which would theoretically enhance CO2 binding due to its Lewis acidity. In both cases (Et and Me), the polymers synthesised in water exhibited a slightly higher nitrogen content compared to those synthesized in the organic solvent, which may suggest a greater abundance of free amine end groups in the water-based synthesis and, potentially, indicating a lower degree of polymerization.
| POP | CO2 uptake @273 K cm3 g−1 (mmol g−1) | CO2 uptake @298 K (cm3 g−1; mmol g−1 STP) | CO2/N2 (IAST) |
|---|---|---|---|
| POP-W-Et | 38 (1.70) | 29 (1.29) | 88 |
| POP-W-Me | 34 (1.52) | 30 (1.34) | 69 |
| POP-O-Et | 39 (1.74) | 33 (1.47) | 83 |
| POP-O-Me | 30 (1.34) | 26 (1.16) | 63 |
Nevertheless, the strong performance of the water-based synthesis yields results that are highly comparable to the organic solvent method and, in addition, offers a greener alternative.
Gas adsorption studies
As earlier mentioned, the optimization of synthesis was carried out considering the best balance between reaction yield and surface area of the individual polymer samples.Before determining the surface area, the samples in Table 1 were activated by outgassing overnight at 350 K under dynamic vacuum to eliminate any traces of water in the materials. Gas adsorption studies were carried out on the activated materials using CO2 and N2 as probe gases. Initial efforts to determine the Brunauer–Emmett–Teller surface areas (SABET) using N2 at 77 K proved unsuccessful due to very low nitrogen adsorption. The surface area of the materials was thus determined from the CO2 adsorption isotherms collected at 273 K and 195 K. The latter, in particular, is considered a reliable method given that the saturation pressure of carbon dioxide at this temperature is 1 atm, which is the same as for N2 at 77 K.29 The surface areas calculated using the isotherms at these two temperatures yield congruent results, as shown in Table 1.
At 195 K, the CO2 uptakes for the POPs synthesized in water, POP-W-Me and POP-W-Et, were found to be 113 cm3 g−1 and 145 cm3 g−1, respectively, accompanied by calculated BET surface areas on the Type-I adsorption curves of 348 m2 g−1 for POP-W-Me and 389 m2 g−1 for POP-W-Et (Fig. S26 and 27†). Consistent results were observed for the samples synthesized in organic solvent, POP-O-Me and POP-O-Et, with SABET values of 302 m2 g−1 and 440 m2 g−1, respectively (Fig. S28 and S29†). The CO2 adsorption at 195 K confirms the porous nature of the materials, albeit the small and less accessible pores pose challenges for N2 measurements. Additionally, the flexibility of the building units may result in framework swelling, allowing higher and faster adsorption kinetics of CO2 compared to N2.29,30
Adsorption experiments with CO2 at 273 K (Fig. S30–S33†) allowed for the assessment of pore size distribution (PSD) using nonlocal density functional theory (NLDFT).31 The assessment of PSD by CO2 adsorption at 273 K over N2 at 77 K is considered more reliable because nitrogen cannot penetrate pores smaller than 5 Å, on the contrary of carbon dioxide that can enter pores in the ultra-microporous region, i.e., ∼3.5 Å.31,32 PSD assessment revealed the coexistence of ultra-micropores of 3.5 Å and micropores of 5.0 Å and 8.0 Å in all the materials (Fig. S38 and S39†), which is typical of microporous polymers.33
To evaluate the affinity for CO2 and the adsorption selectivity over N2, the uptakes of both gases were measured at 298 K and 1 bar (see e.g.Fig. 2 for POP-W-Et) to mimic Vacuum-Swing-Adsorption carbon capture (VSA),34 which is one of the most common and mildest way to approach CCUS. The CO2 uptakes at 298 K were lower than that at 195 K but remained consistent for all the investigated materials (30 cm3 g−1, 26 cm3 g−1 for POP-W-Me and POP-O-Me; 29 cm3 g−1, 33 cm3 g−1 for POP-W-Et and POP-O-Et, see Fig. S38–S41† and Table 2).
The observed difference in adsorption compared to nitrogen suggests the potentially high CO2 selectivity of these materials over nitrogen. The analysis of CO2 isotherms at 273 and 298 K allows for the calculations of the isosteric heats of adsorption (Qst). The isotherms were fitted with the Langmuir–Freundlich equation, and the Qst was determined using the Clausius–Clapeyron equation.35 The Qst for each material were in the 30–35 kJ mol−1 range at low coverage, thus following a physisorption-based model (see Fig. S40†). The Qst of CO2 can be ascribed to the interactions between its quadrupole moment and the POPs, which results in its selective adsorption compared to nitrogen. These values are indicative of a high affinity of the investigated materials for CO2 but, at the same time, show that the carbon dioxide is not adsorbed too tightly in the pores, making the subsequent desorption less energetically demanding.36
Since the amount of CO2 adsorbed largely exceeds the amount of N2 adsorbed at the same temperature, we calculated the selectivity of these materials for their potential use in post-combustion separation processes.37 The fit of the adsorption curves with the IAST method using the IAST++ software38 allowed us to calculate the ideal selectivity, assuming a 15% CO2 and 85% N2 gas mixture, which simulates the typical flue gas composition during post-combustion carbon capture.39 At pressure equal to 1 bar, POP-W-Et is slightly more selective than POP-W-Me (88 vs. 69). It has to be noted that the selectivity of POP-O-Et and POP-O-Me (83 and 63, respectively) are very close to those obtained with the materials synthesized in aqueous media (Fig. 2 and Table 2).
As a summary and analysis of the reported textural properties we can assess that the bulkier ethyl group in POP-Et, compared to the methyl group in POP-Me, likely acts as a more effective spacer between polymer chains, leading to higher BET surface areas (440 and 302 m2 g−1 for POP-O-Et and POP-O-Me, respectively; Table 1). POP-Et exhibits superior CO2 uptake than POP-Me (1.74 vs. ∼1.34 mmol g−1 at 273 K, and 1.47 vs. ∼1.16 mmol g−1 at 298 K), and this is combined with a higher CO2/N2 selectivity (83 vs. 63, as shown in Table 2 and Fig. 2c and d). In terms of solvent effects, no significant differences in textural properties were observed when synthesizing the same polymer in water versus an organic solvent. The slight improvement in CO2 uptake in the organic medium (see 1.47 vs. 1.16 mmol g−1 for POP-O-Et vs. POP-O-Me, Table 2) is expected due to the organic nature of the polymer backbone. However, the water-synthesized polymers display similar textural properties, making the water-based synthesis advantageous due to its greener and more sustainable nature. The pore size distribution, calculated via NLDFT from CO2 adsorption data at 273 K, supports these findings. Fig. S34–S35† demonstrate that both POP-W-Et and POP-O-Et have a higher fraction of ultramicropores centred at 3.5 Å compared to POP-W-Me and POP-O-Me, easily correlating with the higher surface area of the ethyl-substituted polymers. This observation holds for polymers synthesized in both aqueous and organic media and also helps explaining the enhanced CO2 selectivity of the POP-Et polymers. A detailed analysis of the CO2 adsorption and desorption isotherms at different temperatures (195–298 K, Fig. S26–S39†) reveals that both POP-Et and POP-Me polymers, regardless of the synthesis medium, exhibit significant hysteresis during CO2 desorption, particularly evident at 195 K. This consistency across synthesis conditions confirms the reproducibility of both preparation methods. POP-Et displays a more pronounced hysteresis compared to POP-Me, highlighting its enhanced affinity for CO2. This observation is further supported by the higher isosteric heat of adsorption (Qst) of POP-Et (37 kJ mol−1) compared to POP-Me (31 kJ mol−1), as shown in Fig. S40.† The stronger affinity for CO2 in POP-Et suggests that the polymer swells more during adsorption, making the desorption in vacuum conditions more difficult. While this hysteresis effect is more prominent at 195 K, it is also observable at higher temperatures (273 and 298 K), albeit to a lesser degree.
Experimental
Details on materials, methods, and characterization techniques are reported in the ESI material.†Synthesis in water (POP-W-Me/POP-W-Et)
0.4 mmol of the starting tris-amine (i.e. 84 mg of MeNH2 and 100 mg of EtNH2 for POP-W-Me and POP-W-Et, respectively) were dissolved in 10 mL of distilled water in a glass scintillation vial (20 mL) provided with screw cap. The mixture was sonicated for 5 minutes and then 0.4 mmol (84 mg) of TFP were added. The suspension was sonicated for additional 5 minutes to form a homogenous dispersion. The suspension was stirred for 72 h at 70 °C in the closed vial. The formed solid was filtered on a glass fritted funnel, washed with water and ethanol and then dried under vacuum at 80 °C for 24 hours. The yield of polymerization was 93% for POP-W-Me (136 mg), and 90% POP-W-Et (174 mg).Synthesis in 1,4-dioxane/mesitylene (POP-O-Me/POP-O-Et)
0.4 mmol of the starting tris-amine (i.e. 84 mg of MeNH2 and 100 mg of EtNH2 for POP-W-Me and POP-W-Et, respectively) and 0.4 mmol (84 mg) of TFP were suspended in 7.5 mL of a mixture of 1,4-dioxane/mesitylene 4/1 in a glass scintillation vial (20 mL) provided with screw cap. The mixture was sonicated for 5 minutes, and then 2.5 mL of aqueous AcOH 10.5 M were added. The suspension was then sonicated for an additional 5 minutes to form a homogenous dispersion and then stirred for 72 h at 70 °C. The formed solid was filtered on a glass-fitted funnel, washed with water and ethanol and then dried under vacuum at 80 °C for 24 hours. The yield of the polymerization was 92% for POP-O-Me (135 mg) and 85% for POP-O-Et (164 mg).Conclusions
In this manuscript, we report two new keto-enamine-linked POPs, which differ only in the presence of methyl or ethyl groups in the triamine precursors. The reported method represents an important step forward, as the synthesis of comparable porous organic polymers or covalent organic frameworks requires catalysts such as acetic acid21,40 or hydrochloric acid in combination with additional reagents such as sodium nitrite.41 The protocol not only avoids the use of catalyst or additional additives, but also directly produces (without supercritical drying42) a material with permanent porosity, representing an easy and green process that allows to produce CO2-philic POPs. Moreover, the physico-chemical and CO2 adsorption properties of the obtained materials have proven to be unaffected by the synthesis in water. This was demonstrated by thorough characterization of the POPs synthesised in water (POP-W-Me/POP-W-Et) and from the classical mixture of organic solvents (POP-O-Me/POP-O-Et), combining gas adsorption experiments, SEM, FTIR spectroscopy, PXRD, solid-state magic angle spinning (MAS) NMR spectroscopy, DSC and TGA. Compared to other CO2-philic POPs synthesized in aqueous media (see Table S3†),21–26 POP-W-Me and POP-W-Et are highly competitive, being synthesized in pure water at relatively low temperature (70 °C), without addition of catalysts or other additives, in simple glass vials. Most of the CO2-philic POPs reported in the literature are synthesized in organic solvents or mixtures, while the syntheses in water are performed at high temperatures (>100 °C), in presence of catalysts or other additives, and/or require sophisticate equipment (e.g., for hydrothermal synthesis).The reported results turn out to be advantageous also compared to the solvent-free mechanochemical synthesis. The porous materials produced through mechanochemical synthesis, in fact, often exhibit a low performance due to the entrapment of oligomeric impurities within the pores during the grinding process.19
The slight decrease in surface areas and the quantity of CO2 adsorbed in the POP-Me family, compared to that of the POP-Et materials, seems to correlate to the presence of a shorter alkyl chain in the amine precursor. Less bulky groups (Me vs. Et) in the polymer structure, in fact, can favour the establishment of π–π and H-bonds interactions within the polymer network. This can promote the partial closure of the polymer pores, making them less accessible to gas penetration, as seen in previous works where aliphatic side chains produced the same effect.43
The N-/O-rich POP-W-Me and POP-W-Et also show a high adsorption selectivity for CO2 over N2 (up to 88) as calculated with the IAST method at 298 K. The obtained results make these novel POPs promising candidates for greener carbon capture technologies based on solid sorbents. However, there are areas that require refinement and will be central to our future studies. Specifically, we aim to develop more sustainable methods for synthesizing building blocks and to optimize the scaling-up of polymer synthesis with a focus on minimizing water consumption.
Data availability
The data supporting the article Novel CO2-philic porous organic polymers synthesised in water: a leap towards eco-sustainability have been included as part of the ESI.†Author contributions
RM and SLC: synthesis and characterization, TGA and DSC. YW, MC: gas adsorption studies, data analysis. CXB, SB: CPMAS, PXRD, elemental analyses. VA: supervision, writing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
VA, SLC, RM acknowledge the Cariplo Foundation (MOCA project, grant no. 2019-2090) for financial support. VA and SLC also acknowledge the support from the Ministero dell’Università e della Ricerca (MUR) and the University of Pavia through the program “Dipartimenti di Eccellenza 2023–2027”. SB acknowledges Lombardy Region for ‘‘Enhancing Photosynthesis’’ grant (2021–2024) for the financial support.Notes and references
- (a) R. L. Siegelman, E. J. Kim and J. R. Long, Nat. Mater., 2021, 20, 1060 CrossRef CAS; (b) H. Chu, Z. Huang, Z. Zhang, X. Yan, B. Qiu and N. Xu, Sep. Purif. Technol., 2024, 343, 127153 CrossRef CAS; (c) S. Nagireddi, J. R. Agarwal and D. Vedapuri, ACS Eng. Au, 2024, 4, 22–48 CrossRef CAS.
- (a) F. Meng, Y. Meng, T. Ju, S. Han, L. Lin and J. Jiang, Renew. Sustain. Energy Rev., 2022, 168, 112902 CrossRef CAS; (b) P. Wang, Z. Liu, Z. Pan, J. González-Arias, L. Shang, Y. Wang and Z. Zhang, Sep. Purif. Technol., 2024, 346, 127252 CrossRef CAS.
- (a) E. Khaki, H. Abyar, M. Nowrouzi, H. Younesi, M. Abdollahi and M. G. Enderati, Environ. Technol. Innovat., 2021, 22, 101507 CrossRef CAS; (b) A. Katare, S. Kumar, S. Kundu, S. Sharma, L. Mohan Kundu and B. Mandal, ACS Omega, 2023, 8, 17511–17522 CrossRef CAS PubMed; (c) S. Kanehashi and C. A. Scholes, Front. Chem., 2020, 14, 460 CAS; (d) Z. Dai and L. Deng, Sep. Purif. Technol., 2024, 335, 126022 CrossRef CAS; (e) M. Monteleone, R. Mobili, C. Milanese, E. Esposito, A. Fuoco, S. La Cognata, V. Amendola and J. C. Jansen, Molecules, 2021, 26, 5557 CrossRef CAS PubMed; (f) R. Mobili, S. La Cognata, M. Monteleone, M. Longo, A. Fuoco, S. A. Serapian, B. Vigani, C. Milanese, D. Armentano, J. C. Jansen and V. Amendola, Chem.–Eur. J., 2023, 29, e202301437 CrossRef CAS PubMed.
- (a) R. Ben-Mansour, M. A. Habib, O. E. Bamidele, M. Basha, N. A. A. Qasem, A. Peedikakkal, T. Laoui and M. Ali, Appl. Energy, 2016, 161, 225 CrossRef CAS; (b) L. B. Hamdy, C. Goel, J. A. Rudd, A. R. Barron and E. Andreoli, Adv. Mater., 2021, 2, 5843–5880 RSC; (c) B. Gea, M. Zhanga, B. Hua, D. Wua, X. Zhua, U. Eickerb and R. Wang, Appl. Energy, 2024, 355, 122229 CrossRef.
- R. Gonzalez-Olmos, A. Gutierrez-Ortega, J. Sempere and R. Nomen, J. CO2 Util., 2022, 55, 101791 CrossRef CAS.
- (a) K. Sumida, D. L. Rogow, J. A. Mason, T. M. McDonald, E. D. Bloch, Z. R. Herm, T.-H. Bae and J. R. Long, Chem. Rev., 2012, 112(2), 724 CrossRef CAS; (b) Y. Lin, C. Kong, Q. Zhang and L. Chen, Adv. Energy Mater., 2017, 7, 1601296 CrossRef; (c) H. Demir, G. Onder Aksu, H. Can Gulbalkan and S. Keskin, Carbon Capture Sci. Technol., 2022, 2, 100026 CrossRef CAS.
- (a) K. Krishnan, J. M. Crawford, P. K. Thallapally and M. A. Carreon, Ind. Eng. Chem. Res., 2022, 61, 10547–10553 CrossRef CAS; (b) W. Wang, K. Su and D. Yuan, Mater. Chem. Front., 2023, 7, 5247 RSC; (c) S. La Cognata, R. Mobili, C. Milanese, M. Boiocchi, M. Gaboardi, D. Armentano, J. C. Jansen, M. Monteleone, A. R. Antonangelo, M. Carta and V. Amendola, Chem.–Eur. J., 2022, 28, e202201631 CrossRef CAS PubMed; (d) V. Amendola, M. Boiocchi, L. Fabbrizzi and N. Fusco, Eur. J. Org Chem., 2011, 32, 6434–6444 CrossRef; (e) S. La Cognata and V. Amendola, Chem. Commun., 2023, 59, 13668–13678 RSC.
- (a) J. Baltrusaitis, J. Schuttlefield, E. Zeitler and V. H. Grassian, Chem. Eng. J., 2011, 170(2–3), 471 CrossRef CAS; (b) G. Landeta Avellaneda, R. Denoyel and I. Beurroies, Microporous Mesoporous Mater., 2024, 363, 112801 CrossRef; (c) Y. Abdullatif, A. Sodiq, N. Mir, Y. Bicer, T. Al-Ansari, M. H. El-Naasc and A. I. Amhamed, RSC Adv., 2023, 13, 5687–5722 RSC.
- (a) K. S. Song, P. W. Fritz and A. Coskun, Chem. Soc. Rev., 2022, 51, 9831 RSC; (b) W. Wang, M. Zhouab and D. Yuan, J. Mater. Chem. A, 2017, 5, 1334 RSC; (c) S. Fajal, S. Dutta and S. K. Ghosh, Mater. Horiz., 2023, 10, 4083 RSC; (d) S. Bracco, D. Piga, I. Bassanetti, J. Perego, A. Comotti and P. Sozzani, J. Mater. Chem. A, 2017, 5, 10328–10337 RSC; (e) A. Comotti, F. Castiglioni, S. Bracco, J. Perego, A. Pedrini, M. Negroni and P. Sozzani, Chem. Commun., 2019, 5, 8999–9002 RSC; (f) W. Song, Y. Tang, B. Y. Moon, Q. Liao, H. Xu, Q. Hou, H. Zhang, D.-G. Yu, Y. Liao and I. Kim, Green Chem., 2024, 26, 2476–2504 RSC.
- (a) H. Li, A. Dilipkumar, S. Abubakar and D. Zhao, Chem. Soc. Rev., 2023, 52, 6294 RSC; (b) H. Mabuchi, T. Irie, J. Sakai, S. Das and Y. Negishi, Chem.–Eur. J., 2024, 30, e202303474 CrossRef CAS; (c) H. Lyu, H. Li, N. Hanikel, K. Wang and O. M. Yaghi, J. Am. Chem. Soc., 2022, 144(28), 12989 CrossRef CAS.
- (a) Z. Hu, Y. Wang, X. Wang, L. Zhai and D. Zhao, AIChE J., 2018, 64, 3376 CrossRef CAS; (b) S. H. Pang, M. L. Jue, J. Leisen, C. W. Jones and R. P. Lively, ACS Macro Lett., 2015, 4(12), 1415 CrossRef CAS PubMed.
- (a) Y. Xie, T.-T. Wang, X.-H. Liu, K. Zou and W.-Q. Deng, Nat. Commun., 2013, 4, 1960 CrossRef PubMed; (b) D. Dai, J. Yang, Y.-C. Zou, J.-R. Wu, L.-L. Tan, Y. Wang, B. Li, T. Lu, B. Wang and Y.-W. Yang, Angew. Chem., Int. Ed., 2021, 60, 8967 CrossRef CAS.
- (a) W. Chen, P. Chen, G. Zhang, G. Xing, Y. Feng, Y.-W. Yang and L. Chen, Chem. Soc. Rev., 2021, 50, 11684 RSC; (b) Z. L and Y.-W. Yang, Adv. Mater., 2022, 34, 2107401 CrossRef.
- (a) J. L. Wu, F. Xu, S. M. Li, P. W. Ma, X. C. Zhang, Q. H. Liu, R. W. Fu and D. C. Wu, Adv. Mater., 2019, 31, 1802922 CrossRef PubMed; (b) A. R. Antonangelo, N. Hawkins, E. Tocci, C. Muzzi, A. Fuoco and M. Carta, J. Am. Chem. Soc., 2022, 144(34), 15581 CrossRef CAS.
- (a) D.-H. Yang, Y. Tao, X. Ding and B.-H. Han, Chem. Soc. Rev., 2022, 51, 761 RSC; (b) G. Ji, Y. Zhao and Z. Liu, Green Chem., 2022, 3(2), 96 CrossRef; (c) T. Zhang, G. Xing, W. Chen and L. Chen, Mater. Chem. Front., 2020, 4, 332 RSC.
- (a) Z. Li and Y.-W. Yang, Adv. Mater., 2022, 34, 2107401 CrossRef CAS PubMed; (b) S. Wang, H. Li, H. Huang, X. Cao, X. Chen and D. Cao, Chem. Soc. Rev., 2022, 51, 2031 RSC.
- (a) X. Liu, C.-F. Liu, W.-Y. Lai and W. Huang, Adv. Mater. Technol., 2020, 5, 2000154 CrossRef CAS; (b) H. Liao, G. Liao, Q. Yang, Y. Xiao, B. Cheng and S. Lei, J. Energy Storage, 2024, 82, 110520 CrossRef.
- (a) P. Bhanja, A. Modak and A. Bhaumik, ChemCatChem, 2019, 11, 244 CrossRef CAS; (b) J. Deng, Z. Dai and L. Deng, J. Membr., 2020, 610, 118262 CrossRef CAS.
- (a) S. Kandambeth, A. Mallick, B. Lukose, M. V. Mane, T. Heine and R. Banerjee, J. Am. Chem. Soc., 2012, 134, 19524–19527 CrossRef CAS; (b) S. Kandambeth, K. Dey and R. Banerjee, J. Am. Chem. Soc., 2019, 141(5), 1807–1822 CrossRef CAS; (c) B. P. Biswal, S. Chandra, S. Kandambeth, B. Lukose, T. Heine and R. Banerjee, J. Am. Chem. Soc., 2013, 135, 5328–5331 CrossRef CAS; (d) J. Perego, D. Piga, S. Bracco, P. Sozzani and A. Comotti, Chem. Commun., 2018, 54, 9321–9324 RSC.
- (a) D. Kaleeswaran, P. Vishnoi and R. Murugavel, J. Mater. Chem. C, 2015, 3, 7159–7171 RSC; (b) R. Gomes and A. Bhaumi, RSC Adv., 2016, 6, 28047–28054 RSC.
- D. Luo, T. Shi, Q.-H. Li, Q. Xu, M. Strømme, Q.-F. Zhang and C. Xu, Angew. Chem., Int. Ed., 2023, 62, e202305225 CrossRef CAS.
- J. Thote, H. B. Aiyappa, R. R. Kumar, S. Kandambeth, B. P. Biswal, D. B. Shinde, N. C. Royd and R. Biswaee, IUCrJ, 2016, 3, 402 CrossRef CAS.
- J. A. Martìn-Illàn, D. Rodrìguez-San-Miguel, C. Franco, I. Imaz, D. Maspoch, J. Puigmartì-Luis and F. Zamora, Chem. Commun., 2020, 56, 6704 RSC.
- L. Guo, Q. Y. Zhang, Z. Yu, R. Krishna and F. Luo, Chem. Mater., 2023, 35(14), 5648 CrossRef CAS.
- (a) M. Lahnsteiner, M. Caldera, H. M. Moura, D. A. Cerron-Infantes, J. Roeser, T. Konegger, A. Thomas, J. Menche and M. M. Unterlass, J. Mater. Chem. A, 2021, 9, 19754–19769 RSC; (b) B. Baumgartner, M. Puchberger and M. M. Unterlass, Polym. Chem., 2015, 6, 5773–5781 RSC.
- G. Ji, Z. Yang, H. Zhang, Y. Zhao, B. Yu, Z. Ma and Z. Liu, Angew. Chem., Int. Ed., 2016, 55, 9685–9689 CrossRef CAS PubMed.
- J. Lu, F. Lin, Q. Wen, Q.-Y. Qi, J.-Q. Xu and X. Zhao, New J. Chem., 2019, 43, 6116 RSC.
- S. Chandra, S. Kandambeth, B. P. Biswal, B. Lukose, S. M. Kunjir, M. Chaudhary, R. Babarao, T. Heine and R. Banerjee, J. Am. Chem. Soc., 2013, 135, 17853–17861 CrossRef CAS PubMed.
- K. C. Kim, T. U. Yoon and Y. S. Bae, Microporous Mesoporous Mater., 2016, 224, 294 CrossRef CAS.
- M. Chen, B. Coasne, R. Guyer, D. Derome and J. Carmeliet, Nat. Commun., 2018, 9, 3507 CrossRef PubMed.
- (a) M. Thommes, K. Kaneko, A. V. Neimark, J. P. Olivier, F. Rodriguez-Reinoso, J. Rouquerol and K. S. W. Sing, Pure Appl. Chem., 2015, 87, 1051 CrossRef CAS; (b) M. Thommes and K. A. Cychosz, Adsorption, 2014, 20, 233 CrossRef CAS.
- (a) D. Lozano-Castelló, D. Cazorla-Amorós and A. Linares-Solano, Carbon, 2004, 42, 1233 CrossRef; (b) K. A. Cychosz and M. Thommes, Engineering, 2018, 4, 559 CrossRef CAS.
- (a) R. Tan, A. Wang, R. Malpass-Evans, R. Williams, E. W. Zhao, T. Liu, C. Ye, X. Zhou, B. P. Darwich, Z. Fan, L. Turcani, E. Jackson, L. Chen, S. Y. Chong, T. Li, K. E. Jelfs, A. I. Cooper, N. P. Brandon, C. P. Grey, N. B. McKeown and Q. Song, Nat. Mater., 2020, 19, 195 CrossRef CAS; (b) C. Soto, N. Cicuttin, F. J. Carmona, M. de la Viuda, A. Tena, Á. E. Lozano, A. Hernández, L. Palacio and P. Prádanos, J. Membr., 2023, 683, 121841 CrossRef CAS.
- (a) H. Zhang, Z. Zhou and Y. Yin, et al. , EcoEnergy, 2023, 1(2), 217 CrossRef; (b) M. Chawla, H. Saulat, M. Masood Khan, M. Mahmood Khan, S. Rafiq, L. Cheng, T. Iqbal, M. I. Rasheed, M. Z. Farooq, M. Saeed, N. M. Ahmad, M. B. Khan Niazi, S. Saqib, F. Jamil, A. Mukhtar and N. Muhammad, Chem. Eng. Technol., 2020, 43, 184 CrossRef CAS; (c) K. A. Fayemiwo, G. T. Vladisavljević, S. A. Nabavi, B. Benyahia, D. P. Hanak, K. N. Loponov and V. Manović, Chem. Eng. J., 2018, 334, 2004 CrossRef CAS.
- K. Wang, S. Qiao and X. Hu, Sep. Purif. Technol., 2004, 34, 165–176 CrossRef CAS.
- L. W. Bruch, Surf. Sci., 1983, 125(1), 194 CrossRef CAS.
- H. Zhou, C. Rayer, A. R. Antonangelo, N. Hawkins and M. Carta, ACS Appl. Mater. Interfaces, 2022, 14, 20997–21006 CrossRef CAS.
- S. Lee, J. H. Lee and J. Kim, Korean J. Chem. Eng., 2018, 35, 214–221 CrossRef CAS.
- K. T. Leperi, R. Q. Snurr and F. You, Ind. Eng. Chem. Res., 2016, 55, 3338–3350 CrossRef CAS.
- I. Martínez-Visus, M. Ulcuango, B. Zornoza, J. Coronas and C. Téllez, Chem.–Eur. J., 2023, 29, e202203907 CrossRef.
- (a) L. Huang, M. He, B. Chen, Q. Cheng and B. Hu, ACS Sustainable Chem. Eng., 2017, 5, 4050–4055 CrossRef CAS; (b) Y. Shen, W.-X. Ni and B. Li, ACS Omega, 2021, 6, 3202–3208 CrossRef CAS.
- M. Avais and S. Chattopadhyay, ACS Appl. Polym. Mater., 2021, 3, 789–800 CrossRef CAS.
- (a) B. S. Ghanem, M. Hashem, K. D. M. Harris, K. J. Msayib, M. Xu, P. M. Budd, N. Chaukura, D. Book, S. Tedds, A. Walton and N. B. McKeown, Macromolecules, 2010, 43(12), 5287 CrossRef CAS; (b) R. Swaidan, M. Al-Saeedi, B. Ghanem, E. Litwiller and I. Pinnau, Macromolecules, 2014, 47(15), 5104 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Materials and methods; synthetic procedures; details on physico-chemical characterization and spectra of the synthesized polymers; gas adsorption studies. See DOI: https://doi.org/10.1039/d4su00479e |
| ‡ Present address: Sorbonne Université, CNRS, Institut Parisien de Chimie Moléculaire, 4 Place Jussieu, 75005 Paris, France. |
| This journal is © The Royal Society of Chemistry 2024 |