Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
General equations to estimate the CO2 production of (bio)catalytic reactions in early development stages†
Pablo
Domínguez de María
 *
*
Sustainable Momentum, SL. Av. Ansite 3, 4-6, 35011 Las Palmas de Gran Canaria, Canary Is., Spain. E-mail: dominguez@sustainable-momentum.net
First published on 24th October 2024
Abstract
Global warming potential (GWP, kg CO2eq per kg product) is a core impact indicator when assessing the greenness of synthetic reactions in life cycle assessments (LCAs). GWP contributions arise from the production and transportation of chemicals, solvents, and catalysts to the chemical plant, from the reaction (upstream), from the purification steps (downstream), and from the energy invested in the process. For (bio)catalysis, water and spent organic solvents are the major waste contributors, from which CO2 is generated through their processing via wastewater treatment or incineration. Assessing GWP in organic synthesis appears wearisome, demanding time, resources and expertise. However, GWP estimations at early process stages would rapidly identify the hotspots to improve the environmental impact. This paper proposes equations that can be combined depending on the reaction, to estimate the GWP by using readily available process parameters (substrate loading, conversion, reaction media, temperature, time, and thermodynamic values). Once equations are chosen for each reaction (e.g. process conducted in water or in organic media, type of downstream, etc.), estimated GWP can be obtained. Scenarios can be simulated by changing parameters, to assist practitioners at process early stages to understand how (bio)catalytic reactions can be established in a greener way.
Sustainability spotlightQuantifying the global warming potential (kg CO2eq per kg product) is fundamental to assessing the greenness of (bio)catalytic reactions. In particular, understanding the impact during early stage research steps may drive the research to more sustainable options, before decisions are made and process implementation is executed. For such purposes, methods that can rapidly but meaningfully provide data on the GWP would be useful. In this work, some combinable equations are provided, to rapidly estimate GWP from available reaction parameters: substrate loading, conversion, temperature, time, reaction media, etc. Furthermore, the GWP of the reaction can be modelled by changing some of the parameters, to determine what are the hotspots for the environmental impact and where efforts should be focused to improve the footprint. |
1. Motivation
Measuring the environmental impact of chemical reactions is gaining increasing importance motivated by the need to provide realistic figures of their (un)greenness, and more importantly, to set timely recommendations to improve their ecological footprint.1–10 In fact, nowadays industrial processes must not only be efficient and economically attractive, but they also need to reach environmental standards to ensure sustainability. Significant debate has emerged on how to measure such a complex aspect, and different metrics have been proposed.1,3,4,6–10 Moreover, there is discussion on where to set the boundaries of the life cycle assessment (LCA), i.e. including the impact of the production and transportation of the solvent, chemicals and (bio)catalyst (“cradle-to-gate”), or approaching narrower “gate-to-gate” strategies to evaluate a particular reaction set-up.6–13 While the holistic vision covering the entire production pipeline would be optimal, the complexity of energy and mass flows in the chemical industry often hampers that approach, due to a lack of time, resources, and expertise. Thus, tools that may serve in process early stages for gate-to-gate assessments11 and that could allow practitioners to validate their lab reactions rapidly and meaningfully would be of high interest.From the impact categories reflected in LCAs, global warming potential (GWP) is prominent when it comes to the chemical industry. Expressed as kg CO2eq per kg product, GWP reflects the contribution of the synthetic procedure to greenhouse gas emissions, which may come from the energy used in the reaction and from the resources consumed and disposed of during the process. Also coined as “C-Factor” to validate the transition from fossil to bio-based feedstock,14 GWP has been measured in LCAs for (bio)catalytic reactions, as some examples illustrate.11,13,15–17 More recently, the Gallou group at Novartis has proposed the TCR concept (total carbon dioxide release), by providing valuable industrial data on the CO2 production when wastewater effluents or organic fractions are treated, either through mild wastewater treatment plants (WWTPs) or through incinerations when recalcitrant wastes or organics are generated.18,19 The meaningfulness and straightforward use of these industrial TCR metrics have stimulated their use by several groups, enabling the comparison between processes, since everything is measured by the same “currency”, the CO2 formation.11,15,16,20–22
Biocatalysis has emerged as a powerful tool to establish efficient and (allegedly) more sustainable industrial processes, and numerous applications have reached a successful industrial implementation.23,24 A reason for that success is the versatility of enzymes, which enable processes not only in aqueous solutions – the natural media for biocatalysts – but also in a myriad of non-aqueous systems (the so-called non-conventional media), such as organic (neoteric) solvents, biphasic systems, micro-aqueous conditions, solvent-free conditions, etc.25,26 This “media-agnostic” skill of many enzymes facilitates their integration within synthetic steps. Likewise, (bio)catalysis intensification to reach economically sound conditions (e.g. high substrate loadings and excellent yields and selectivity) is key to reaching industrial implementation.27,28
When establishing new (bio)catalytic strategies – from laboratory design to scale-up and commercialization – it would be useful to have at hand straightforward methods to determine the GWP of the applied reactions, to timely pinpoint how process development could be driven to create more environment-friendly conditions, before efforts on scale-up are invested. Ideally, it should enable the direct conversion of readily available reaction parameters (e.g. substrate loading, conversion, temperature, reaction time, etc.) to GWP, which could be then modelled depending on the process parameters (e.g. GWP at more or less conversion, higher temperature, etc.). Based on these premises, this paper develops some equations for such estimated GWP values – with a focus on mass- and energy contributions – that enable the assessment of scenarios to rapidly determine the environmental hotspots for improvement. Likewise, such a tool could serve as a training strategy for green chemistry students, who could perform simulation exercises to (better) understand their chemical processes. For synthetic systems in which more data are available, the generic equations can be rapidly adapted to them, to get more accurate GWP figures.
Fig. 1 depicts a generic biotransformation unit (“gate-to-gate”), comprising synthetic (upstream) and purification (downstream) subunits. From both sections, wastewater and spent organic solvents are collected as waste, generating CO2 through their treatment.18,19 Adding to that, the energy invested in the process produces further CO2. Thus, equations for these three CO2 main contributors are established in this paper. In a final section, some notes on the pre-steps (envisaging a “cradle-to-gate” approach) are provided, considering the GWP impact on the production of solvents, chemicals, and enzymes, and on their transportation to the chemical plant.
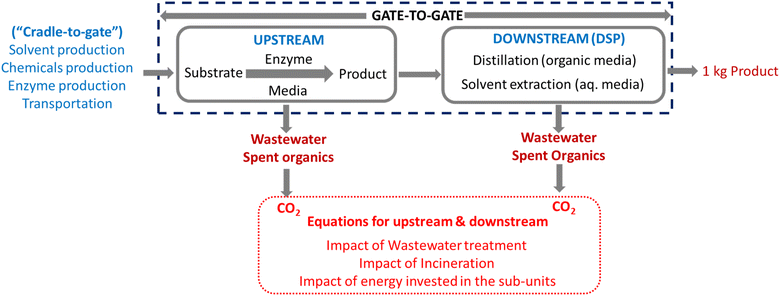 | ||
| Fig. 1 Generic “gate-to-gate” biotransformation, from where the GWP equations are deduced in the following sections. | ||
2. Defining the GWP equations for the upstream part
During the upstream part, an exemplary (bio)catalytic reaction will produce two main waste effluents: the aqueous media – in the form of wastewater – and a pooled organic fraction, where all collected (spent) solvents are sent to incineration (Fig. 1). In addition to that, one should account for the waste generated from the energy invested in the process, to heat the reactor and hold it for the reaction time. Therefore, GWP contributions from the mass and from the energy inputs during the upstream must be considered.2.1. Equations for the upstream part
Eqn (1–7) have been developed for the upstream, providing GWP contributions from mass (1–4) and from energy (5–7) (Table 1). Following the Novartis industrial TCR data,18,19 the equations have been built to enable the introduction of readily available reaction parameters like the “conversion” (conv., in “%”), the substrate loading (SL, in “kg L−1”), and the proportion of the effluent that is sent to treatment (and not recycled), in “%”. In this way, GWP can be rapidly estimated by picking one equation for the mass contribution (1–4) and another one for the energy (5–7), depending on the reaction media (water or non-conventional, Table 1). In the ESI† a detailed development of the equations and approximations is provided. For practitioners or researchers having more data on their actual solvent and systems, the equations can be easily adapted to those real conditions by following the rationale provided in the ESI.†| Metric | Process type | General equation for GWP (upstream) |
|---|---|---|
| a Reaction in organic or aqueous media, heated up to a certain temperature and held for the reaction time. 15% extra energy is assumed for each hour of reaction held at that temperature. 25% extra energy added to the total calculated energy, to account for non-ideal losses. Values of CO2 × kW h−1 from an average current European grid (∼0.25 kg CO2 × kW h−1).29 | ||
| Mass metrics | Reaction in organic media |
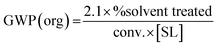 (1) (1) |
| Reaction in aqueous media (recommended, with pre-treatment) |
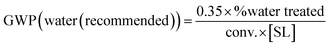 (2) (2) |
|
| Reaction in aqueous media, to mild WWTP treatment without pretreatment (best case) |
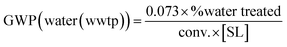 (3) (3) |
|
| Reaction in aqueous media, to incineration due to recalcitrance (worst case) |
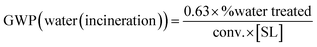 (4) (4) |
|
| Energy metrics | Reaction in organic media, heated up to a temperaturea |
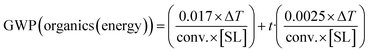 (5) (5) |
| Reaction in water (buffer) without cosolvent, heated up to a temperaturea |
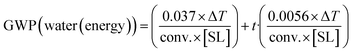 (6) (6) |
|
| Reaction in water (buffer) with cosolvent, heated up to a temperaturea |
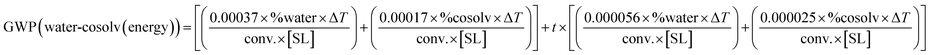
|
|
As stated above, biocatalytic reactions can be conducted in aqueous media – with or without an organic cosolvent – or in non-conventional systems. Following the metrics provided by the Gallou group at Novartis,18,19 different fates can be envisaged for these wastes: (i) direct wastewater treatment plant (WWTP), if the aqueous effluent can be mildly treated (eqn (3)); (ii) wastewater treatment involving some pre-treatment steps before WWTP treatment, to remove hazardous chemicals that hamper the biodegradability (eqn (2)); (iii) water or organic fraction incineration, in the case of recalcitrant effluents (water) (eqn (4) or for organics (eqn (1))). Thus, each strategy leads to different GWPs, e.g. organic fraction incineration generates more CO2 than wastewater incineration (eqn (1) vs. (4)). The best scenario would be an aqueous effluent that can be mildly treated in the WWTP (eqn (3)). However, given the broadness of (intensified) reactions, (co)solvents, and reagents used in (bio)catalysis, eqn (2) is the recommended one for water effluents, as it includes the necessity of some pre-treatment steps to remove hazardous components that hamper the direct WWTP treatment. These pretreatments will generate more CO2 than simple WWTP treatment (eqn (2) vs. (3)).
With respect to the energy contribution to GWP, eqn (5–7) have been deduced from the thermodynamic values of heating organic solvents or water (see the ESI†). As an approximation, an average solvent density of 0.9 g cm−3 has been taken, and an average heat capacity (Cp) for the organic solvents of 2.1 kJ °C−1 kg−1 was considered (Table S1†). For water, its concrete thermodynamic values were taken (1 g cm−3 and a Cp of 4.184 kJ °C−1). A 15% extra energy is added for each hour of reaction in which the system must be held at that temperature. Moreover, a 25% extra energy was added in the equations to account for the losses of ideal behaviour, etc. As stated above, equations can be adapted to more precise figures if data of exact solvent, conditions, etc., are available (see the ESI†).
Therefore, the GWP of the upstream part (Fig. 1) can be rapidly estimated by selecting two equations – one for mass contributions and another one for the energy – that better suit the process (reaction media, type of wastewater treatment, reaction temperature, and time). Scenarios can be defined to compare process conditions. In the following sections, two generic case studies are discussed as examples.
2.2. Case study I: biotransformation in water or in organic media, at different temperatures
In this example, the benchmark reaction to estimate the GWP is a (bio)catalytic process with 50 g substrate per L, at 30 °C for 6 h, and with 100% conversion. It is assumed that 20 °C is room temperature. The benchmark process can be conducted either in an organic solvent or in aqueous media with 10% cosolvent to assist substrate dissolution. No solvent or water is recovered after the reaction (single use). Likewise, for comparison, GWP will also be estimated when the process is conducted at: (i) the same conditions, but with 80% conversion; (ii) the same conditions, but for 16 h (o/n) of reaction; (iii) the same conditions, but at 80 °C; (iv) the same conditions, but recovering 80% of the solvent or the water/cosolvent mixture for reuse.For the reaction in organic solvent, eqn (1) (mass) and (5) (energy) must be taken (Table 1). For the aqueous media with cosolvent, the “recommended” eqn (2), WWTP with pretreatment, is taken for the mass contribution, and eqn (7) is taken for the energy part, as a water-cosolvent system. The results are depicted in Fig. 2.
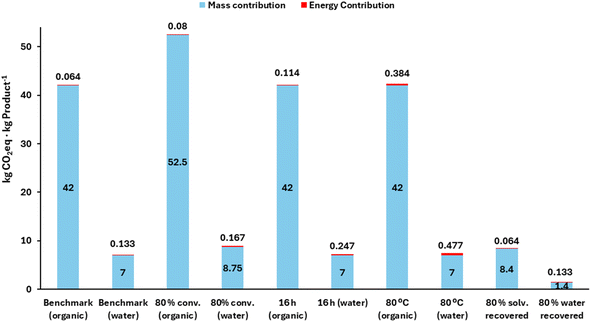 | ||
| Fig. 2 GWP estimations for the (bio)catalytic reaction, either in an organic medium or in an aqueous system with cosolvent and assessing different scenarios of process conditions. | ||
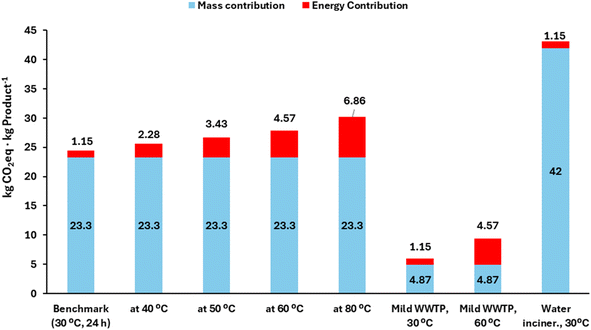
A notable difference is observed in the benchmark if an organic solvent or a water/cosolvent is employed (single use in both), consistent with previous work.20 The incineration of the pooled (spent) organics leads to a 6-fold higher GWP than in the case of the aqueous system, when the “recommended” eqn (2) for wastewater treatment is taken. The energy contribution to GWP remains less relevant, in agreement with the literature.18 It must be noted that the case study considers heating (and holding) the reaction mixture at 30 °C (from r.t. 20 °C) for 6 h, which is a narrow temperature range. Also, the extension of the reaction to 16 h does not have a significant energy impact either. Increasing the temperature to 80 °C results in higher GWP for energy for water (due to its much higher Cp than those of the organic media), but still the energy contribution is not as relevant as the solvent or wastewater treatment. In a different line, when the conversion decreases to 80%, larger liquid fractions are needed to generate one kilogram of product, and this leads to higher GWP from the mass contribution. Connected to that, when 80% of the effluent is recycled – and thus only 20% needs to be treated – more decent GWP values are observed (8.4 for organics and 1.4 for water). Therefore, the simulation shows that the highest GWP impact in the upstream of a biocatalytic reaction at mild temperatures is driven by the treatment of reaction media after use. Organics are more impactful than aqueous systems, and media recycling is mandatory to reach acceptable GWP for the upstream.11,20 Increasing the substrate loadings from 50 g L−1 to higher ranges would also ameliorate the impact, since less water or solvent would be needed.1,11,20
2.3. Case study II: biotransformation in water, at increasing temperatures and with different wastewater treatment options
To evaluate more in-depth the energy contribution and the different options for wastewater treatment (eqn (2) to (4), Table 1), in this second case the benchmark is a biotransformation in water (without cosolvent) at 20 g substrate per L, conducted for 24 h at 30 °C, reaching 75% conversion, and with the “recommended” wastewater path (eqn (2)). Other considered scenarios are: (i) the same process but in a broadened temperature range of 40–80 °C; (ii) the same process at 30 °C or at 60 °C, where mild wastewater treatment is possible (eqn (3)); (iii) the same process at 30 °C, where wastewater must be incinerated due to recalcitrance (eqn (4)). The results are depicted in Fig. 3.In this case, the low substrate loading (20 g L−1) and moderate conversion (75%) penalize the GWP of wastewater treatment in its “recommended” form, eqn (2), leading to more than 23 kg CO2 per kg product. Moreover, given that the reaction needs a large volume fraction and is conducted in water (high Cp) for a long time (24 h) and at increasing temperatures, the GWP from energy is affected more clearly than in the previous case study. At 60–80 °C, the GWP of the energy is in the range of 4.5–6.8 kg CO2 per kg product. In a different line, when a mild WWTP treatment can be implemented for the water effluent (eqn (3)), a much lower GWP is observed, leading to a total GWP of less than 10 kg CO2 per kg product, at 60–80 °C. Conversely, if wastewater contains hazardous components that cannot be pretreated, incineration should be the fate (worst case, eqn (4)), and a considerably high GWP contribution for the upstream is observed.
Beyond the specific obtained values for both case studies, the proposed equations may become a useful tool for lab practitioners and students, to simulate how GWP can change, and how to set greener conditions by adapting the process to the observed environmental hotspots.
3. Incorporating the downstream sub-unit
The above-defined equations focus on the first part of a biotransformation, that is, the enzymatic reaction (“upstream”). However, a complete process must involve a second step – the downstream unit – where the product is purified to reach a marketable form (Fig. 1). There are several methods to perform downstream processing – even involving combined steps, from extraction to crystallization, etc. – depending on the reaction and on the purity needed for the final product (e.g. technical grade vs. pharmaceutical quality). For this work, the downstream part focuses on two widely used strategies: the distillation of the organic solvent for processes conducted under non-aqueous conditions and extraction with an organic solvent when reactions are performed in aqueous media.3.1. Equations incorporating the downstream. Distillation for non-conventional systems and extraction for aqueous media
For (bio)catalytic reactions conducted in organic media, the common downstream is solvent distillation (after filtration to remove cells, suspended enzymes, etc.).26 The GWP of such a downstream unit would include: (i) the GWP of heating the organic solvent from the reaction temperature to its boiling point, and holding it for 1 hour (for distillation); (ii) GWP related to the distillation, based on the enthalpy of vaporization; (iii) GWP related to the final incineration/mineralization of the non-recovered or discarded organic solvent. The total GWP would be:| GWP(org(dsp)) = (GWP(reactemp. − bp)) + (GWTP(dist)) + (GWP(incineration)) |
Following an analogous procedure to the upstream part (see the ESI† for details), eqn (8) can be used for the downstream of a biotransformation in organic media:
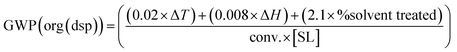 | (8) |
On the other hand, if the reaction is conducted in aqueous media, an organic solvent is typically needed for the extractive downstream. The GWP for the downstream in this case would involve: (i) GWP from heating the extractive phase from the reaction temperature to the boiling point and holding it for 1 hour (distillation); (ii) the GWP of the distillation of the organic solvent, based on the enthalpy of vaporization (kJ kg−1); (iii) the GWP of the incineration/mineralization of the fraction of the organic solvent that is not recovered; (iv) the GWP of the wastewater treatment of the aqueous media, where often the “recommended” eqn (2) should be taken. Following analogous assumptions as mentioned above (see the ESI†), eqn (9) drives the GWP impact of the downstream processing in aqueous solutions:
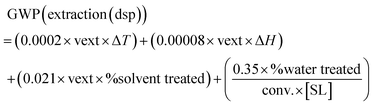 | (9) |
If other scenarios are considered, beyond the “recommended” eqn (2), and wastewater must be incinerated or can be directly sent to the WWTP, the last part of eqn (9) (which comprises eqn (2), see Table 1) can be replaced by eqn (4) or by eqn (3), respectively.
3.2. Case study: biotransformation conducted in water and extracted with organic media or in organic solvent (CPME) followed by distillation
To validate the equations – comprising upstream and downstream – the following case study was considered: a biotransformation performed in CPME as an organic medium or in water without cosolvent, with 60 g substrate per L, 80% conversion, 30 °C, and 8 h. The downstream processing for the reaction in organic media is conducted through solvent distillation (b.p. CPME 106 °C, ΔH = 290 kJ kg−1), and the downstream processing in the aqueous solution is performed with extraction with 2× ethyl acetate and subsequent solvent distillation (b.p. EtOAc 77 °C, ΔH = 366 kJ kg−1). Using eqn (1–9), the GWP of the upstream (energy for the reaction) and that of the downstream are estimated, assuming that: (i) 90% of the solvent (CPME or ethyl acetate) is recovered; (ii) 70% is recovered. In the aqueous phase the water media is not recycled and is sent to wastewater treatment (following the scenario “recommended”, eqn (2), Table 1).Since the wastewater and solvent treatments are now incorporated into the downstream part (eqn (8) or (9)), the upstream only considers the energy needed to heat the reactor to the temperature at which the process will take place (eqn (5–7), Table 1). The downstream GWP comprises the energy of the downstream (heating + distillation) and the GWP of treating the solvent or the wastewater, considering the proportion that is discarded and not recovered. Simulations are depicted in Fig. 4.
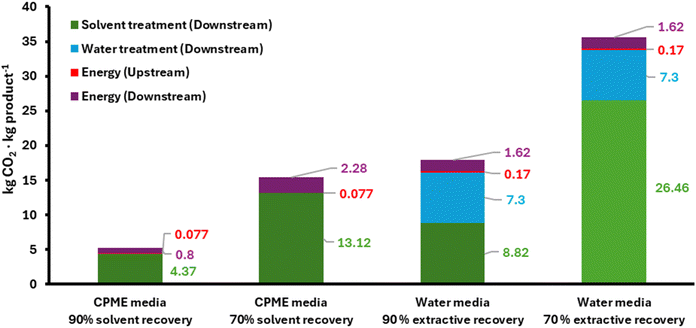 | ||
| Fig. 4 GWP of different biotransformations performed either in organic media with distillation as downstream or in aqueous solutions with extraction with an organic solvent as downstream. | ||
For the organic reaction process, eqn (5) (upstream, energy) and (8) (downstream) are taken, while for the aqueous media reaction, eqn (6) (upstream, energy) and (9) (downstream, with “recommended” wastewater treatment, eqn (2)) are selected. As observed (Fig. 4), processes in non-conventional media are less impactful if an appropriate solvent recovery and reuse is implemented (90 or 70%), consistent with recent literature.16,20 For processes conducted in aqueous media, the solvent surplus – typically used to ensure an optimized product extraction – penalizes the GWP. This is particularly relevant when only 70% of the extractive solvent is recycled, which leads to more than 25 kg CO2 per kg product for the solvent incineration part, and to almost 40 kg CO2 per kg product in total (Fig. 4). The environmental impact of solvents in chemical processes has been addressed before,1,16,30 and recycling is mandatory. It should be noted, though, that industry must follow strict regulations in terms of solvent recycling, which may hamper that option in some cases.21,31 From a different angle, the use of potentially biogenic solvents, such as ethyl acetate or CPME,32 may decrease the environmental burden, since bio-based (neutral) carbons would be fed in the GWP. In another line, for this case study the water treatment (recommended path, eqn (2)) also accounts for a significant proportion of the GWP, due to the low substrate loadings applied (20 g L−1), which necessitates the use of a large volume of effluent. Intensified processes would ameliorate the impact of the aqueous fraction as well, by optimizing the loading-to-volume ratio.20,28
4. Notes on background steps – impact of production of enzymes, solvents, and chemicals and transportation to the chemical plant – and on the energy source
The proposed equations assume a gate-to-gate assessment (Fig. 1), where enzymes, chemicals and solvents are already in the chemical plant, and therefore no environmental impact is considered for their production and transportation.30 The herein presented approach may be useful for lab practitioners, as simulations can be rapidly derived for processes that are under development. However, if a more holistic study is intended (towards a “cradle-to-gate” assessment, Fig. 1), the GWP of the production and transportation of the used chemicals, solvents, and catalysts must be considered as well. With respect to transportation, several estimations can be made based on distances and the means of transportation, train delivery being the most preferred one.30,33 With respect to the enzyme production, some papers reported by industrial research groups provide figures on the GWP for (bio)catalyst biosynthesis, depending on the enzyme and the expression.34,35 Values span from 10 to 25 kg CO2 per kg enzyme, ranging from free to immobilized enzyme forms and from recombinant to wild-type microorganisms. These figures may serve as a basis to estimate the GWP impact of industrial enzyme production, to be added in the assessment (e.g. considering the mass of the enzyme needed to produce one kilogram of product, considering biocatalyst reuse). It must be stressed, though, that more elaborated industrial data on the environmental impact of enzyme production are necessary, to have more accurate figures to benchmark biocatalytic processes.Data on the GWP of solvent production are retrievable from the open literature and available databases, at least from commonly used solvents. However, discrepancies in data can be observed, since the same solvent may have different origins (petro-chemical vs. biogenic) and may follow different synthetic routes, and/or the LCA may set different boundaries. For instance, the reported GWP for the production of ethyl acetate ranges from ∼1.6 kg CO2 per kg EtOAc (petro-chemical) to ∼5 kg CO2 per kg EtOAc when it is produced from switch grass and the study also includes land use and fertilizers (broader boundaries). However, in the latter case one has to keep in mind that, advantageously, the produced solvent is biogenic.36 On the other hand, it is not clear what is included (or what is not) in the petro-chemical synthesis (boundaries), as some previous steps of those processes (e.g. pumping energy of oil, distillation/refine steps, etc.) may add significant CO2 production to the final environmental impact. Some studies have proposed 2.56 kg CO2 per kg EtOAc as the (compromise?) value for GWP estimations of the background (solvent).13 The same accounts for CPME, where GWPs from ∼1.8 kg CO2 per kg CPME to ∼4 kg CO2 per kg product have been given.36 As observed, those data must be taken with caution, and perhaps adopting the worst ones (worst case) or two values (as the best and the worst case scenario, respectively) would be the most appropriate way for performing estimations on GWP when a broader “cradle-to-gate” system is considered.
Finally, it must be noted that the energy included in the equations assumes the impact of using electrical sources (related to the CO2 production per kW h−1), taking the average value applied currently in Europe (0.25 kg CO2 × kW h−1).29 While this may be the case for some chemical plants and processes, other systems may use energy sources based on natural gas, fossil fuels, etc., from which higher CO2 contributions are expected, in the range of 600–700 g CO2 × kW h−1 (ca. 3-fold the average value in Europe today, from electricity).37,38 Conversely, lower emissions are expected, if hydropower renewable energy is used (∼0.04 g CO2 × kW h−1).13 Assuming that the penetration of renewable energy in the chemical industry will make its path in the coming years, the average assumptions made in this paper seem to be a fair trade-off. Nevertheless, if more data on the process to be assessed are available (e.g. knowing which energy sources are actually employed), an adaptation of the equations to reflect the actual impact of the kW h−1 in CO2 production may be considered.
6. Conclusions and outlook
The reported eqn (1–9) enable a rapid estimation of the GWP of different (bio)catalytic reactions, in a gate-to-gate manner. Depending on the process conditions applied (e.g. higher or lower substrate loadings, reaction time, temperature, conversion, solvent vs. water, etc.), the GWP changes, and thus hotspots for improvement can be rapidly monitored. Moreover, the strategy enables the GWP allocation on different units (upstream or downstream) or on different fractions (water, spent organics, etc.). The reported tool appears particularly useful for processes at the early stage, where data are still scarce and more in-depth LCA analyses are not feasible. Moreover, the approach may represent a useful exercise for students, to monitor reactions and define the best processing (environmental) conditions. Making the GWP measurement an early (routine) activity for practitioners may certainly help reaching more sustainable processes in subsequent steps. For those cases, estimated values on the order of magnitude can serve as a basis to put forth mitigation actions. Once more processing data become available (e.g. which specific solvent, actual water or solvent recovery or reuse, conversion, yield, etc.), the equations can be rapidly modulated to be adapted to those more accurate figures. One particular aspect here is the downstream unit, as many combinations of different DSP can be envisioned, depending on the actual process and convenience. In this work, the emphasis has been put on extractions or distillations, as they are arguably the most popular DSP units at the research scale or at early stages. However, equations for other DSP units (or combinations thereof) may be developed as well (e.g. crystallization processes, where anti-solvents are typically used, or chromatographic steps, with large solvent/water volumes used). It is hoped that this work may trigger other groups to adapt the equations to their particular cases, to provide more meaningful environmental assessments.Another important aspect is the energy impact, due to the difficulty in determining the actual energy source in a chemical plant (what will generate different CO2 amounts, as discussed above). In this work, the equations were developed assuming an electrical source with an average European impact (0.25 kg CO2 × kW h−1), but data can be adapted if other energy sources are considered. For “worst-case” scenarios, the values of a truly fossil source (range of 600–700 g CO2 × kW h−1) may be taken as a “conservative” benchmark.
Finally, it must be noted that measuring GWPs enables (bio)synthetic processes to be fairly compared using the same metric, from which the proportion of biogenic carbon in the CO2 can also be estimated (and withdrawn) when bio-based solvents are used.
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support from the European Union's Horizon 2020 research and innovation programme RADICALZ (Grant number: 101000560) is gratefully acknowledged.References
- P. Domínguez de María, Curr. Opin. Green Sustainable Chem., 2021, 31, 100514 CrossRef.
- Y. Wu, C. E. Paul and F. Hollmann, Green Carbon, 2023, 1, 227–241 CrossRef.
- J. P. Lange, Nat. Catal., 2021, 4, 186–192 CrossRef CAS.
- R. A. Sheldon, Catal. Today, 2024, 431, 114571 CrossRef CAS.
- P. Lozano and E. García-Verdugo, Green Chem., 2023, 25, 7041–7057 RSC.
- R. A. Sheldon, Curr. Opin. Green Sustainable Chem., 2022, 33, 100569 CrossRef CAS.
- L. J. Diorazio, P. Richardson, H. F. Sneddon, A. Moores, C. Briddell and I. Martínez, ACS Sustainable Chem. Eng., 2021, 9, 16862–16864 CrossRef CAS.
- R. A. Sheldon, Green Chem., 2023, 25, 1704–1728 RSC.
- C. Jiménez-González, C. Ponder, Q. B. Broxterman and J. B. Manely, Org. Process Res. Dev., 2011, 15, 912–917 CrossRef.
- F. Tieves, F. Tonin, E. Fernández-Fueyo, J. M. Robbins, B. Bommarius, A. S. Bommarius, M. Alcalde and F. Hollmann, Tetrahedron, 2019, 75, 1311–1314 CrossRef CAS.
- P. Domínguez de María, Green Chem., 2022, 24, 9620–9628 RSC.
- M. Becker, A. Zieminska-Stolarska, D. Marlowska, S. Lütz and K. Rosenthal, ChemSusChem, 2023, 8, e202201629 CrossRef PubMed.
- M. A. F. Delgove, A. B. Laurent, J. M. Woodley, S. De Wildeman, K. V. Bernaerts and Y. van der Meer, ChemSusChem, 2019, 12, 1349–1360 CrossRef CAS PubMed.
- C. H. Christensen, J. Rass-Hansen, C. C. Marsden, E. Taarning and K. Egeblad, ChemSusChem, 2008, 1, 283–289 CrossRef CAS PubMed.
- S. Nieto, J. M. Bernal, R. Villa, E. García-Verdugo, A. Donaire and P. Lozano, ACS Sustainable Chem. Eng., 2023, 11, 5737–5747 CrossRef CAS PubMed.
- P. Petermeier, P. Domínguez de María, E. Byström and S. Kara, ACS Sustainable Chem. Eng., 2024, 12, 12869–12878 CrossRef CAS PubMed.
- M. S. Robescu, A. R. Alcántara, C. Calvio, C. F. Morelli, G. Speranza, D. Ubiali and T. Bavaro, ChemSusChem, 2023, 16, e202202108 CrossRef CAS PubMed.
- U. Onken, A. Koettgen, H. Scheidat, P. Schueepp and F. Gallou, Chimia, 2019, 73, 9 CrossRef PubMed.
- C. Krell, R. Schreiber, L. Hueber, L. Sciascera, X. Zheng, A. Clarke, R. Haenggi, M. Parmentier, H. Baguia, S. Rodde and F. Gallou, Org. Process Res. Dev., 2021, 25, 900–915 CrossRef CAS.
- P. Domínguez de María, S. Kara and F. Gallou, Molecules, 2023, 28, 6452 CrossRef PubMed.
- N. Fleck, F. Roschangar and A. Haydl, Org. Process Res. Dev., 2023, 27, 822–830 CrossRef CAS.
- P. Domínguez de María and S. Kara, RSC Sustainability, 2024, 2, 608–615 RSC.
- A. R. Alcántara, P. Domínguez de María, J. A. Littlechild, M. Schürmann, R. A. Sheldon and R. Wohlgemuth, ChemSusChem, 2022, 15, e202102709 CrossRef PubMed.
- S. Wu, R. Snajdrova, J. C. Moore, K. Baldenius and U. T. Bornscheuer, Angew. Chem., Int. Ed., 2021, 4, 88–119 CrossRef PubMed.
- D. Holtmann and F. Hollmann, Mol. Catal., 2022, 517, 112035 CrossRef CAS.
- M. Van Schie M, J. D. Spöring, M. Bocola, P. Domínguez de María and D. Rother, Green Chem., 2021, 23, 3191–3206 RSC.
- B. O. Burek, A. W. H. Dawood, F. Hollmann, A. Liese and D. Holtmann, Front. Catal., 2022, 2, 858706 CrossRef.
- L. E. Meyer, M. Hobisch and S. Kara, Curr. Opin. Biotechnol., 2022, 78, 102835 CrossRef CAS PubMed.
- EEA, Greenhouse gas emission intensity of electricity generation, Data and maps, https://www.eea.europa.eu/data-and-maps/daviz/co2-emission-intensity-14#tab-chart_7, 2024, accessed 12 August 2024 Search PubMed.
- P. Domínguez de María, EFB Bioeconomy J., 2023, 3, 100056 CrossRef.
- US Food and Drug Administration, ICH-Q7 Guideline for GMP-Manufacturing, 2016, pp. 37–38 Search PubMed.
- G. de Gonzalo, A. R. Alcántara and P. Domínguez de María, ChemSusChem, 2019, 12, 2083–2097 CrossRef CAS PubMed.
- L. Leseurre, C. Merea, S. Duprat de Paule and A. Pinchart, Green Chem., 2014, 16, 1139–1148 RSC.
- Y. Hong, A. S. Nizami, M. P. Bafrani, B. A. Saville and H. I. MacLean, Biofuels, Bioprod. Biorefin., 2013, 7, 303–313 CrossRef CAS.
- S. Kim, C. Jiménez-González and B. E. Dale, Int. J. Life Cycle Assess., 2009, 14, 392–400 CrossRef CAS.
- H. H. Khoo, V. Isoni and P. N. Sharratt, Sustain. Prod. Consum., 2018, 16, 68–87 CrossRef.
- P. Gabrielli, L. Rosa, M. Gazzani, R. Meys, A. Bardow, M. Mazzotti and G. Sansavini, One Earth, 2023, 6, 682–704 CrossRef.
- E. G. Hertwich, T. Gibon, E. A. Bouman, A. Arvesen, S. Suh, G. A. Heath, J. D. Bergesen, A. Ramírez, M. I. Vega and L. Shi, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 6277–6282 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4su00535j |
| This journal is © The Royal Society of Chemistry 2024 |