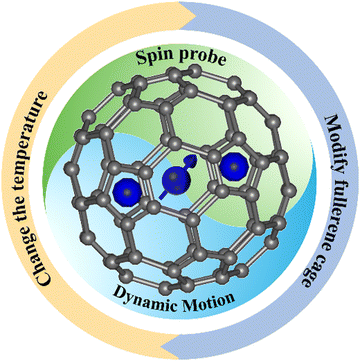
Spin probe for dynamics of the internal cluster in endohedral metallofullerenes
Yingjian
Zhang
and
Taishan
Wang
 *
*
MOE Key Laboratory of Cluster Science, School of Chemistry and Chemical Engineering, Beijing Institute of Technology, Beijing 102488, China. E-mail: wangtais@bit.edu.cn
First published on 19th November 2024
Abstract
Endohedral metallofullerenes (EMFs) are constructed by fullerene cages encapsulating various metal atoms or metal clusters, which usually exhibit some motion. However, due to the fact that the elusive endohedral dynamics are related to many factors, it remains a challenge to image the motion of internal species. Recently, the electron spin was found to be a sensitive probe to detect the motion of internal species in EMFs. Moreover, this technique can be widely applied for many metallofullerenes, i.e., for paramagnetic EMFs, the unpaired electron spin is a natural probe for the endohedral dynamics, and for diamagnetic EMFs, an electron can be introduced to produce spin-active EMF molecules. Based on the analysis of hyperfine coupling constants (hfcc), g-factors, and line patterns of the ESR spectra of EMFs, the spin centers and endohedral dynamics can be deduced. It has been revealed that the spin probes can provide unexpected information about the dynamics of the internal clusters in EMFs. Through changing the temperature, exohedral modification of the EMF, and supramolecular assembly, the motion of the internal species in EMFs can be manipulated, as clearly reflected by the spin probe. These studies revealed that the spin in EMFs exhibits promising applications in quantum sensing and molecular machine technology. In this review, we will address the use of the spin probe in EMFs and attempt to understand the effects in the detection of the endohedral dynamics.
1. Introduction
It is well known that fullerenes possess a nanometer-scale internal space that enables them to encage metal atom(s) and metal clusters to form various endohedral metallofullerenes (EMFs).1–5 To characterize the geometries and electronic structures of EMFs, several characterization techniques, such as XRD, NMR, Raman and IR, have been widely adopted. Based on these characterizations, it has been revealed that the internal species always exhibit motion inside the fullerene cages. For example, the NMR studies revealed that nearly all EMFs with an Ih-C80 cage maintain the high Ih-symmetry of their parent fullerene cages, so the internal species must exhibit free rotation inside the fullerene cages.6–10 Recently, as part of the continuous efforts in metallofullerene research, electron spin resonance (ESR) spectrometry has been found to be a powerful tool in imaging the dynamics of internal species.ESR spectroscopy is a powerful tool for imaging the electronic distributions of molecules, and the electron spin has been widely applied as a sensitive probe in many fields, such as probing the electronic structures of radicals, monitoring labeled biological molecules, developing spin transport electronics, etc.11–13 In the analysis of the ESR spectra, two parameters can generally reveal the electron spin characteristics, namely, the g-factor and hyperfine coupling constants (hfcc). The g-factor gives information about the paramagnetic center, and the hfcc is caused by the interaction between the unpaired electron and a nucleus (or nuclei) with non-zero nuclear spin (I).
So far, dozens of EMFs have been studied using ESR spectroscopy, including mono-metal EMFs, di-metal EMFs, metal carbide EMFs, and metal nitride EMFs.14–17 The spin distributions in EMFs have been classified into three categories according to their locations, namely, unpaired spins residing on the fullerene cages, metal nuclei, or a non-metal unit of the internal species, respectively. ESR spectroscopy has been utilized to image the dynamics of internal species of EMFs through probing the line pattern of their ESR spectra, and can be broadly applied to many EMFs (Fig. 1). For paramagnetic EMFs such as La@C82, Sc@C82, and Sc3C2@C80, the electron spin is a natural probe to obtain the dynamics of the internal species. For diamagnetic EMFs such as La2@C80, Sc3N@C80, and Sc2C2@C82, an electron can be introduced to produce paramagnetic EMFs.
2. Spin probing of dynamics within fullerene cages
2.1. Probe dynamics within an icosahedral C80 cage
Among the Ih-C80-based EMFs, Sc3C2@Ih-C80 is a paramagnetic molecule with an unpaired spin localized on the C2 unit, which produces a symmetrical 22-line ESR pattern due to the couplings of the unpaired spin with three equivalent Sc nuclei (ISc = 7/2) (Fig. 2a). These highly symmetrical ESR lines indicate a rotational Sc3C2 cluster, which was also revealed by 13C NMR and XRD analyses.18,19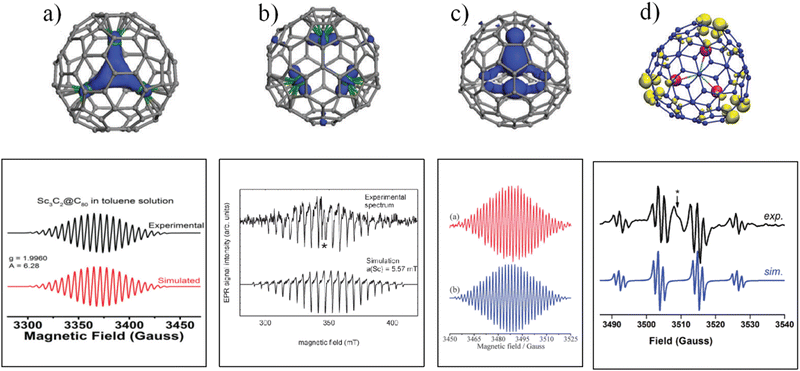 | ||
| Fig. 2 ESR spectra of (a) Sc3C2@C80, (b) Sc3N@C80 anion, (c) Sc3CN@C80 anion, and Y3N@C80 anion. (a) is reproduced with permission.20 Copyright 2018, The Royal Society of Chemistry. (b) is reproduced with permission.21 Copyright 2001, American Chemical Society. (c) is reproduced with permission.22 Copyright 2013, The Royal Society of Chemistry. (d) is reproduced with permission.23 Copyright 2017, The Royal Society of Chemistry. | ||
It should be noted that more Ih-C80-based EMFs have closed-shell electronic structures, making them ESR-silent; thus, spin injection is employed to prepare the corresponding paramagnetic species, such as radicals of the Sc3N@C80 anion, Sc4O2@Ih-C80 anion and cation, Sc3CN@C80 anion, and Y3N@C80 anion.21–24 These EMF radicals show varied ESR parameters, which can illustrate the different dynamics of different internal clusters within the C80 cage.
Sc3CN@C80 was synthesized and characterized as having a planar Sc3CN cluster, and its anion radical was obtained through reduction by potassium.22 The ESR results revealed a highly symmetrical 22-line spectrum with an hfcc value of 3.890 G for two equivalent Sc nuclei, and 1.946 G for the third Sc nucleus (Fig. 2c and Table 1). Undoubtedly, the highly symmetrical 22-line spectrum of the Sc3CN@C80 anion radical is due to the free rotation of the Sc3CN cluster inside the C80 cage, and the small hfcc values are due to the unpaired spin being localized on the non-metal CN moiety. ESR measurements were also performed on the Y3N@C80 anion radical (Fig. 2d).23 The ESR spectrum of the Y3N@C80 anion radical consists of twelve peaks originating from three equivalent Y nuclei with an hfcc value of 11.42 G and one N nucleus with an hfcc of 1.32 G, revealing the free rotation of the Y3N cluster inside the C80 cage as well.
| EMF | a [G] | g | Temperature [K] | Solvent | Ref. |
|---|---|---|---|---|---|
| a o-Dichlorobenzene. b Carbon disulfide. c Tetrahydrofuran. | |||||
| Sc3C2@Ih-C80 | 3 × 6.256 | 2.0006 | RT | o-DCBa | 25 |
| Sc3C2@C80-Ad | 2 × 7.39; 1.99 | 1.99835 | RT | CS2b | 14 |
| Sc3C2@C80 fulleropyrrolidine | 8.602; 2 × 4.822 | 2.0007 | RT | o-DCB | 25 |
| Sc3C2@C80 bisfulleroid | 6.73; 2 × 4.00 | n/a | RT | n/a | 26 |
| Sc3N@C80 anion | 3 × 55.6 | 1.9984 | RT | THFc | 16 |
| Sc3CN@C80 anion | 2 × 3.890; 1.946 | 2.0031 | RT | THF | 17 |
| Sc4O2@C80 anion | 2 × 2.6; 2 × 27.4 | 1.9960 | RT | THF | 18 |
| Sc4O2@C80 cation | 2 × 19.0; 2 × 150.4 | 1.9956 | RT | o-DCB | 18 |
| Sc3N@C68 cation | 3 × 1.289 | 2.0010 | RT | o-DCB | 27 |
| Sc2C2@C72 anion | 2 × 0.77 | 2.0050 | RT | THF | 28 |
| Sc@C2v-C82 | 3.78 | 2.0005 | RT | n/a | 29 |
| Y2@C82 anion | 2 × 34.3 | 2.0025 | RT | THF | 30 |
| Y2C2@C82 anion | 0.4; 0.45 | 2.0038 | RT | THF | 30 |
| Sc2C2@C82 anion | 0.484; 0.968 | 2.0026 | RT | THF | 30 |
| Y2@C79N | 2 × 81.8 | 1.97 | RT | CS2 | 31 |
| Y3N@C80 anion | 11.42; 1.32 (N) | 2.0053 | RT | THF | 19 |
| Y3N@C86 anion | 7.14; 5.10; 4.57; 1.93 (N) | 2.0035 | RT | THF | 19 |
| Y2@C80(CH2Ph) | 81.0 | 1.9733 | RT | Toluene | 32 |
However, the introduction of an extra electron into EMFs changes the electronic structures of the EMFs, and may cause some unprecedented effects on the endohedral dynamics. For example, after introducing an electron into Sc3N@C80, the unpaired spin in the anion radical is localized on three scandium nuclei, and a dozen regularly spaced lines with a large hfcc of 5.56 mT (55.6 G) for three equivalent Sc nuclei were observed in its ESR spectrum at room temperature (Fig. 2b).21 It is well known that the Sc3N cluster exhibits free rotation inside C80, but for the Sc3N@C80 anion, the ESR spectrum not only did not show all of the 22 lines coupled by three Sc nuclei, but also displayed an asymmetric ESR pattern. Popov et al. performed detailed calculations of neutral and anionic Sc3N@C80, and the results showed that for the anionic radical of Sc3N@C80, the cluster rotation is slightly hindered, which is probably due to the increased dSc−πcage interaction induced by the negatively charged metal ions.33
Sc4O2@C80 anion and cation radicals were prepared, their ESR spectra were acquired, and detailed molecular dynamic calculations were performed.24 The Sc4O2@C80 anion and cation radicals have an unpaired spin localized on the metal nuclei. Compared to the Sc3N@C80 anion radical with increased dSc−πcage interaction, the unpaired spin of Sc4O2@C80 anion and cation radicals is mainly localized on the Sc4 cluster with slight metal-cage interactions. The symmetrical ESR patterns in their ESR spectra revealed a rotational Sc4O2 cluster, even though further analyses indicated that the rotation of the Sc4O2 cluster in the anion is more hindered than in the cation.24
2.2. Probe dynamics within cages with low symmetry
Beyond Ih-C80, there are many EMFs with non-spherical carbon cages. Among them, C2v(9)-C82 is a well-known cage for mono-EMFs, e.g., Sc@C2v(9)-C82, Y@C2v(9)-C82, La@C2v(9)-C82, etc.34 As shown by their ESR spectra, Sc@C2v(9)-C82 exhibits a larger hfcc value (3.8 G) than those of La@C2v(9)-C82 (1.2 G) and Y@C2v(9)-C82 (0.49 G).30,35 In Sc@C2v(9)-C82, the unpaired electron resides partially on the carbon cage and partially on the Sc(3d) orbital, which strengthens the metal-cage bonding and strongly influences the motion of Sc atom.36 Combined with other characterizations, it can be concluded that the Sc tends to coordinate strongly with C2v(9)-C82, and that the motion of the encapsulated Sc is thus restricted around the equilibrium point. However, as the ionic radius of the encapsulated metal increases from Sc to Y and La, the electron spin tends to localize on the fullerene cage, and the motion of the endohedral metal accelerates.Among di-metallic EMFs, it was revealed by crystallography that the two Er atoms of Er2@Cs(6)-C82 and Er2@C3v(8)-C82 only circulated along a cage ribbon composed of pure hexagonal rings, revealing a restriction effect of the cage symmetry on the endohedral cluster dynamics.37 When the size of internal species was further increased to metal carbides, e.g., Sc2C2@Cs(6)-C82 and Sc2C2@C3v(8)-C82, their inner clusters were again found to be unable to rotate, as revealed by 45Sc NMR spectrometry, in which the two Sc atoms showed unequal chemical shifts.27,38
We performed a comparative experiment of spin-active anions of Y2@Cs(6)-C82, Y2C2@Cs(6)-C82 and Sc2C2@Cs(6)-C82 to study the size effect of the internal clusters on their dynamics (Fig. 3).28 After electron injection by potassium, the Y2@C82 anion radical displays symmetrical signals coupled by the unpaired spin and two equivalent Y nuclei (IY = 1/2), with hfcc and g-factor values of 34.3 G (for two equivalent nuclei) and 2.0025, respectively. However, for the Y2C2@C82 and Sc2C2@C82 anion radicals, both of their ESR spectra showed asymmetrical patterns, indicating that the free rotation of the internal species was inhibited due to the relatively large cluster size. Moreover, for the Y2C2@C82 anion radical, the hfcc values for the two yttrium nuclei were 0.4 and 0.45 G, and for the Sc2C2@C82 anion radical, the two hfcc values for the two Sc nuclei were 0.484 and 0.968 G, respectively, showing the different dynamics of Y2C2 and Sc2C2. This is because the Sc2C2@C82 anion radical exhibits two very different hfcc values, which are caused by the different chemical environments of the two Sc nuclei resulting from hindered rotation.
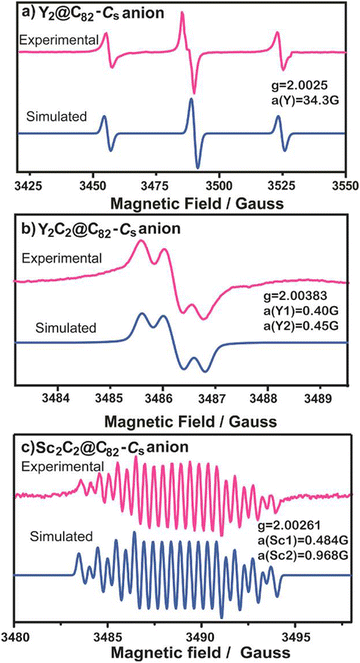 | ||
| Fig. 3 ESR spectra of (a) Y2@Cs(6)-C82, (b) Y2C2@Cs(6)-C82 and (c) Sc2C2@Cs(6)-C82 anion radicals.28 Copyright 2013, American Chemical Society. | ||
We also performed ESR experiments on the spin-active anion of Y3N@C86.23 The Y3N@C86 anion radical shows an unsymmetrical ESR pattern, and the hfcc values for Y are a(Y1) = 7.14 G, a(Y2) = 5.10 G, and a(Y3) = 4.57 G, demonstrating the restricted rotation of the Y3N cluster in the C86 cage. N-Splittings were also observed in the ESR spectrum of the Y3N@C86 anion radical, and the hfcc value was 1.93 G.
It is well known that the metal atoms are usually bonded to the fullerene cage in non-isolated pentagon rule (non-IPR) EMFs, and the rotation of the internal species is thus strongly restricted. For example, the typical non-IPR EMF Sc3N@D3(6140)-C68 has three pentalene motifs that are separately bonded to three Sc ions.39 Since Sc3N@C68 is a diamagnetic molecule, its cation radical was generated by electrochemical oxidation.40,41 Surprisingly, the ESR study of the Sc3N@C68 cation radical showed a 22-line pattern with high symmetry, suggesting three equivalent Sc nuclei with hfcc values of 1.289 G. Detailed ESR analysis and theoretical calculations revealed that the unpaired spin is mainly delocalized on the C68 cage, unlike in the case of the Sc3N@C80 anion radical, in which the spin is mainly distributed on the Sc nuclei. Obviously, although the Sc3N cluster is seriously restricted inside the C68 cage, the delocalized electron spin on the D3-C68 cage allows sufficient averaging of the g factor and hyperfine tensors. In addition, the D3 symmetry of the C68 cage is another reason for its highly symmetrical ESR pattern. Therefore, ESR spectroscopy has limitations in probing the dynamics of internal species in EMFs, as in some cases, even though the metal nuclei are restricted in the EMFs, the EMFs can exhibit isotropic paramagnetic properties if the unpaired spin is mainly delocalized on the symmetrical fullerene cages. Decreasing the temperature may be a possible way to detect the true endohedral dynamics of Sc3N@C68.
In addition, another non-IPR, Sc2C2@Cs(10528)-C72, with two pentalene motifs was reported.42 The crystallographic results revealed the motion of the Sc2C2 cluster inside the non-IPR Cs-C72 cage. Spin ejection was thus performed to obtain the anion radical of Sc2C2@Cs-C72 by chemical reduction of potassium, and a highly symmetrical 15-line ESR spectrum with an hfcc value of 0.77 G for the two equivalent Sc nuclei was observed. Similar to the Sc3N@C68 anion radical, the delocalized electron spin on the fullerene cage allows sufficient averaging of the g factor and hyperfine tensors. On one hand, the symmetry of Sc2C2@Cs-C72 is the reason for its highly symmetrical ESR pattern. As disclosed by single-crystal XRD results, the Sc atoms surrounding the C2 carbide unit have twelve Sc sites in total generated via crystallographic mirror plane. On the other hand, the Sc2C2@Cs-C72 shows motional behavior of the carbide cluster within a non-IPR cage, as disclosed by single-crystal XRD. Consequently, the EPR spectrum of Sc2C2@Cs-C72 anion radical was determined by the molecular symmetry as well as the internal dynamics.
3. Manipulation of the dynamics of the internal cluster and spin probe
3.1. Changing the temperature
The motion of internal species in EMFs is due to the π-conjugated fullerene cage forming a static electron field inside the cage. Under certain conditions, the internal cluster may overcome the energetic barrier to move freely inside the cage. However, once the fullerene symmetry is broken, or the moving energy of the internal species is increased, the motion of the internal species will be inhibited. In these processes, the spin probe has been found to be an effective tool to detect and analyze the motion of internal species in EMFs.An easy way to manipulate the rotation of internal species is by decreasing the temperature. As discussed above, the ESR spectrum of Sc@C2v(9)-C82 exhibits eight highly symmetrical lines at room temperature due to the motion of the scandium atom, but with the decrease of the temperature, the intensity of the ESR signals at higher magnetic field appears reduced, and paramagnetic anisotropy emerges (Fig. 4a).43 This kind of anisotropy could be due to insufficient rotational averaging of the g and hyperfine tensors as well as the spin–rotation coupling interaction, which are caused by the restricted motion of the Sc.
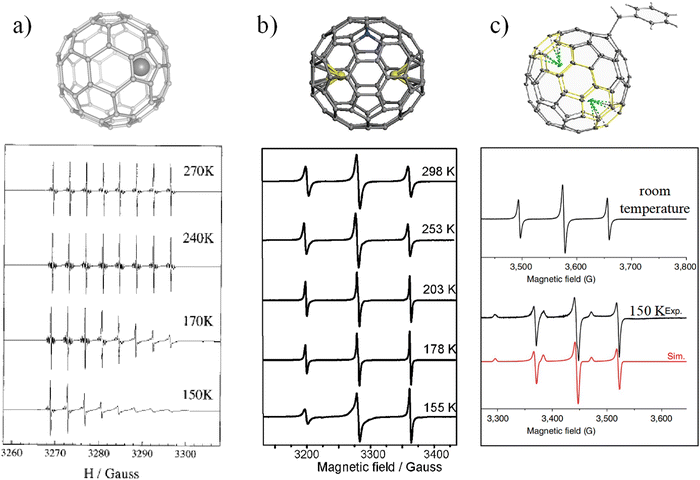 | ||
| Fig. 4 ESR spectra of (a) Sc@C82, (b) Y2@C79N and (c) Y2@C80(CH2Ph) at various temperatures. (a) is reproduced with permission.39 Copyright 2000, American Chemical Society. (b) is reproduced with permission.42 Copyright 2012, The Royal Society of Chemistry. (c) is reproduced with permission.32 | ||
Using electrochemical reduction, Kato studied the ESR spectra of the La2@C80 anion radical, which shows anisotropic paramagnetism at 80 K.31 The very large hfcc tensors (greater than 300 G) of the two La nuclei (ILa = 7/2) indicated that the unpaired spin was almost completely localized on the La–La dimer. Although the pristine La2@Ih-C80 has a rotational La2 moiety, in its anion radical, the La2 dimer bearing the electron spin exhibits decreased motion at 80 K, as indicated by the obvious anisotropy.
Before metal carbide EMFs were proposed, Sc3C2@Ih-C80 was long mistakenly assigned as Sc3@C82, whose temperature-dependent ESR properties were studied early.25,32 In CS2 solution at 220 K, Sc3C2@Ih-C80 exhibits an ESR spectrum with an hfcc of 6.51 G and a linewidth (ΔH) of 0.77 G. With increasing temperature, the linewidths (ΔH) of all 22 signals increase. However, when the temperature is decreased to 217 K, the ESR linewidth at the central part of the spectrum is different from those at either side. This feature was naturally ascribed to the decelerating rotation of the Sc3C2 cluster, which induces slightly different hfcc values for the three Sc nuclei at low temperature.
Azafullerene is produced by substituting one or more carbon atoms of a fullerene cage with nitrogen atoms. In 2008, the preparation of M2@C79N (M = Y, Tb) in monomer form was reported.26 In light of the fact that [C79N]5− is iso-electronic with [Ih-C80]6−, it would not be surprising to observe high stability if only the unpaired electron was located on the inner M2 moiety. Recently, we reported the temperature-dependent ESR properties of Y2@C79N (Fig. 4b).29 It was observed that the ESR spectrum of Y2@C79N shows a symmetrical pattern at room temperature. However, as the temperature is decreased, the intensity of the ESR signals at higher magnetic field gradually increases, which was ascribed to the paramagnetic anisotropy and insufficient averaging of the g and hyperfine tensors caused by restricted rotation of the Y2 moiety.
A benzyl monoadduct of the Y2@C80(CH2Ph) derivative with an unpaired spin was studied using ESR (Fig. 4c). The EPR spectrum of the Y2@C80(CH2Ph) solution at room temperature shows isotropic ESR signals with an hfcc of 81.0 G. DFT-based Born–Oppenheimer molecular dynamics (BOMDs) simulations of Y2@C80(CH2Ph) were performed to analyze the dynamics of the metal atoms, and it was found that the Y2 atoms can rotate within one plane within the cage. However, in frozen toluene solution at 150 K, Y2@C80(CH2Ph) exhibits a rhombic ESR pattern, revealing the restricted motion of the internal Y2 cluster at low temperature.44
3.2. Exohedral modification of fullerene cages
EMFs can undergo many chemical reactions, such as carbene reaction, Prato reaction, Bingel–Hirsh reaction, etc., to be modified by various adduct groups.34 These exohedral adducts usually break the molecular symmetry and change the static electron field inside the cage, thus strongly affecting the endohedral dynamics of the internal species.Through the use of different adducts, the motion of the internal species in EMFs can be manipulated easily, and the unpaired spin acts as a sensitive probe to image the internal dynamics (Fig. 5). The cycloadduct derivative of Sc3C2@Ih-C80 with adamantylidene carbene (Sc3C2@C80-Ad) shows an ESR spectrum with hfcc values of 7.39 G (two nuclei) and 1.99 G (one nucleus).18 In 2010, we reported that the unpaired spin of Sc3C2@C80 fulleropyrrolidine is localized inhomogeneously on the Sc3C2 with hfcc values of 8.602 G (one nucleus) and 4.822 G (two nuclei).45 Recently, a bisfulleroid derivative of Sc3C2@C80 was reported to show an ESR pattern with hfcc values of 6.73 G (one nucleus) and 4.00 G (two nuclei).46 These ESR spectra are all different from that of pristine Sc3C2@Ih-C80 with three equal nuclei, indicating the considerable influence of the exohedral modification on the paramagnetism. Combining these results with theoretical calculations, the hindered rotation of the endohedral Sc3C2 cluster of Sc3C2@C80 fulleropyrrolidine has been analyzed in detail, and the oscillation modes around the equilibrium position were clearly revealed. Additionally, the ESR study of these Sc3C2@C80 derivatives showed that different adduct sites clearly manipulated the ESR properties. For Sc3C2@C80-Ad, the adamantylidene carbene was added to a [6, 6] site of the cage, and the unpaired spin was tended to the Sc nucleus adjacent to the exohedral adduct. On the contrary, for both Sc3C2@C80 fulleropyrrolidine and Sc3C2@C80 bisfulleroid, the exohedral groups were added on [5, 6] sites on the fullerene cage, and the unpaired spin was inclined to the Sc nuclei far away from the exohedral adducts. In the future, the influences of different adduct sites on endohedral dynamics should be investigated further using ESR experiments and theoretical calculations.
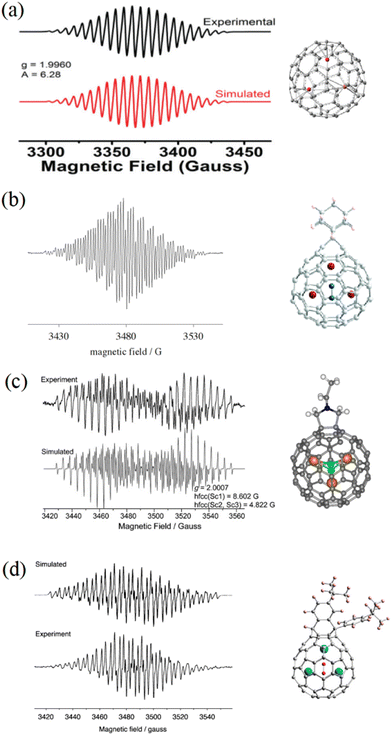 | ||
| Fig. 5 ESR spectra of (a) Sc3C2@C80, (b) carbene derivative of Sc3C2@C80, (c) Sc3C2@C80 fulleropyrrolidine, and (d) bisfulleroid derivative of Sc3C2@C80. (a) is reproduced with permission.20 Copyright 2018, The Royal Society of Chemistry. (b) is reproduced with permission.18 Copyright 2005, American Chemical Society. (c) is reproduced with permission.45 Copyright 2010, John Wiley & Sons, Inc. (d) is reproduced with permission.46 Copyright 2012, American Chemical Society. | ||
The derivatives of low-symmetry EMFs have more isomers than those of Ih-C80-based EMFs, and each isomer leads to a different geometry and electronic structure. These isomers will result in various spin probes and more complicated endohedral dynamics. For example, four monoadduct isomers of Sc@C82-Ad were obtained with different adduct sites, inducing four groups of different hfcc parameters and g-factors in their ESR spectra.47 Recently, paramagnetic isomers of Y2@C79N fulleropyrrolidines were isolated and characterized using ESR spectroscopy.29 As mentioned above, the Y2@C79N has anisotropic paramagnetism at low temperature. Again, paramagnetic anisotropy was observed on these Y2@C79N fulleropyrrolidines at room temperature, and each isomer exhibits a unique hfcc parameter and g-factor.
3.3. Host–guest interactions
Endohedral metallofullerenes have curved π-systems, and this spherical structure makes metallofullerenes usable as guest molecules in supramolecular chemistry. In supramolecular systems, the host–guest interaction can influence the orientation of the guest molecules. Recently, our group found that the motion of the internal clusters in metallofullerenes can also be influenced by a nanoring host, and more importantly, the spin probe can sense these changes.48–51 In a supramolecular complex of [12]CPP (cycloparaphenylene) and Y2@C79N, it was found that the nanoring can induce the orientation of Y2@C79N and modulate its ESR signals.48 Temperature-dependent ESR spectra and theoretical calculations revealed that the nanoring can attract the internal cluster and results in anisotropic EPR signals due to insufficient rotational averaging of electron spin. In detail, the optimized structure of Y2@C79N⊂[12]CPP shows that the Y2 cluster of Y2@C79N tends to adhere to the wall of the nanoring and shows restricted rotation within C79N cage. Furthermore, for another supramolecular complex, Y2@C79N⊂[4]CHBC, the ESR results and theoretical calculations also showed that the Y2 cluster tends to adhere to the wall of the [4]CHBC host, which results in anisotropic EPR signals as well.494. Conclusions
The motion of internal species inside the fullerene cage is a universal behavior in EMFs. Due to the fact that the elusive endohedral dynamics are related to many factors, such as the symmetry of the fullerene cage, the interaction between the internal species and its parent fullerene cage, the environmental temperature etc., it is still a challenge to image the motion of internal species. The electron spin has been found to be a sensitive probe to detect the motion of internal species in EMFs. When the internal species exhibits free rotation inside the fullerene cage, the ESR lines of the EMFs usually display isotropic character; while a restricted internal species would induce the paramagnetic anisotropy.For paramagnetic EMFs, the unpaired spin is a natural probe to study the motion of the internal species inside fullerenes, and for diamagnetic EMFs, an electron spin can be injected using an alkali metal or electrochemistry technique. The spin probe has been found to be a powerful tool to observe the motion of internal species upon changing the temperature, modifying the EMF, and supramolecular assembly. Therefore, this is a universal technique for EMFs, although this technique still has some shortcomings. For example, if the unpaired spin is mainly delocalized on the fullerene cage, sufficient averaging of the paramagnetic tensors can also be observed for some endohedral fullerenes without motion of the internal species. Therefore, the use of ESR measurements to detect the endohedral dynamics still requires further improvements to image the dynamics of internal species in EMFs.
Data availability
No primary research results, software, or code has been included and no new data were generated or analyzed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (52472041), and the Beijing National Laboratory for Condensed Matter Physics (2023BNLCMPKF004).Notes and references
- M. N. Chaur, F. Melin, A. L. Ortiz and L. Echegoyen, Angew. Chem., Int. Ed., 2009, 48, 7514 CrossRef CAS PubMed.
- X. Lu, T. Akasaka and S. Nagase, Acc. Chem. Res., 2012, 46, 1627 CrossRef.
- H. Shinohara, Rep. Prog. Phys., 2000, 63, 843 CrossRef CAS.
- A. A. Popov, S. Yang and L. Dunsch, Chem. Rev., 2013, 113, 5989 CrossRef CAS PubMed.
- J. Zhang, S. Stevenson and H. C. Dorn, Acc. Chem. Res., 2013, 46, 1548 CrossRef CAS PubMed.
- T. Akasaka, S. Nagase, K. Kobayashi, M. Walchli, K. Yamamoto, H. Funasaka, M. Kako, T. Hoshino and T. Erata, Angew. Chem., Int. Ed. Engl., 1997, 36, 1643 CrossRef CAS.
- S. F. Yang, A. A. Popov and L. Dunsch, Angew. Chem., Int. Ed., 2008, 47, 8196 CrossRef CAS PubMed.
- T. S. Wang, L. Feng, J. Y. Wu, W. Xu, J. F. Xiang, K. Tan, Y. H. Ma, J. P. Zheng, L. Jiang, X. Lu, C. Y. Shu and C. R. Wang, J. Am. Chem. Soc., 2010, 132, 16362 CrossRef CAS PubMed.
- T. S. Wang, N. Chen, J. F. Xiang, B. Li, J. Y. Wu, W. Xu, L. Jiang, K. Tan, C. Y. Shu, X. Lu and C. R. Wang, J. Am. Chem. Soc., 2009, 131, 16646 CrossRef CAS PubMed.
- S. Stevenson, G. Rice, T. Glass, K. Harich, F. Cromer, M. R. Jordan, J. Craft, E. Hadju, R. Bible, M. M. Olmstead, K. Maitra, A. J. Fisher, A. L. Balch and H. C. Dorn, Nature, 1999, 401, 55 CrossRef CAS.
- X. Hu, Adv. Mater., 2012, 24, 294 CrossRef CAS.
- K. V. Raman, A. M. Kamerbeek, A. Mukherjee, N. Atodiresei, T. K. Sen, P. Lazic, V. Caciuc, R. Michel, D. Stalke, S. K. Mandal, S. Blugel, M. Munzenberg and J. S. Moodera, Nature, 2013, 493, 509 CrossRef CAS PubMed.
- L. Bogani and W. Wernsdorfer, Nat. Mater., 2008, 7, 179 CrossRef CAS PubMed.
- F. Liu, L. Spree, D. S. Krylov, G. Velkos, S. M. Avdoshenko and A. Popov, Acc. Chem. Res., 2019, 52, 2981 CrossRef CAS.
- W. Xiang, Z. Hu, J. Xin, H. Jin, Z. Jiang, X. Han, M. Chen, Y.-R. Yao and S. Yang, J. Am. Chem. Soc., 2023, 145, 22599 CrossRef CAS.
- J. Qiu, A. Laura, X. Du, Z. Cao, Z. He, Q. Meng, Y. Yan, M. Josep, L. Sun, R.-F. Antonio and N. Chen, J. Am. Chem. Soc., 2024, 146, 24310 CrossRef CAS PubMed.
- Y. Yan, L. Abella and R. Sun, Nat. Commun., 2023, 14, 6637 CrossRef CAS.
- Y. Iiduka, T. Wakahara, T. Nakahodo, T. Tsuchiya, A. Sakuraba, Y. Maeda, T. Akasaka, K. Yoza, E. Horn, T. Kato, M. T. Liu, N. Mizorogi, K. Kobayashi and S. Nagase, J. Am. Chem. Soc., 2005, 127, 12500 CrossRef CAS PubMed.
- E. Nishibori, I. Terauchi, M. Sakata, M. Takata, Y. Ito, T. Sugai and H. Shinohara, J. Phys. Chem. B, 2006, 110, 19215 CrossRef CAS PubMed.
- H. Meng, C. Zhao, Y. Li, M. Nie, C. Wang and T. Wang, Nanoscale, 2018, 10, 3291 RSC.
- P. Jakes and K. P. Dinse, J. Am. Chem. Soc., 2001, 123, 8854 CrossRef CAS PubMed.
- Y. Q. Feng, T. S. Wang, J. Y. Wu, Y. H. Ma, Z. X. Zhang, L. Jiang, C. H. Ge, C. Y. Shu and C. R. Wang, Chem. Commun., 2013, 49, 2148 RSC.
- C. Zhao, T. Wang, Y. Li, H. Meng, M. Nie, J. Tian and C. Wang, Phys. Chem. Chem. Phys., 2017, 19, 26846 RSC.
- A. A. Popov, N. Chen, J. R. Pinzon, S. Stevenson, L. A. Echegoyen and L. Dunsch, J. Am. Chem. Soc., 2012, 134, 19607 CrossRef CAS.
- T. Kato, S. Bandou, M. Inakuma and H. Shinohara, J. Phys. Chem., 1995, 99, 856 CrossRef CAS.
- T. M. Zuo, L. S. Xu, C. M. Beavers, M. M. Olmstead, W. J. Fu, D. Crawford, A. L. Balch and H. C. Dorn, J. Am. Chem. Soc., 2008, 130, 12992 CrossRef CAS.
- Y. Iiduka, T. Wakahara, K. Nakajima, T. Nakahodo, T. Tsuchiya, Y. Maeda, T. Akasaka, K. Yoza, M. T. H. Liu, N. Mizorogi and S. Nagase, Angew. Chem., Int. Ed., 2007, 46, 5562 CrossRef CAS.
- Y. H. Ma, T. S. Wang, J. Y. Wu, Y. Q. Feng, H. Li, L. Jiang, C. Y. Shu and C. R. Wang, J. Phys. Chem. Lett., 2013, 4, 464 CrossRef CAS.
- Y. H. Ma, T. S. Wang, J. Y. Wu, Y. Q. Feng, L. Jiang, C. Y. Shu and C. R. Wang, Chem. Commun., 2012, 48, 11570 RSC.
- K. Kikuchi, Y. Nakao, S. Suzuki, Y. Achiba, T. Suzuki and Y. Maruyama, J. Am. Chem. Soc., 1994, 116, 9367 CrossRef CAS.
- T. Kato, J. Mol. Struct., 2007, 838, 84 CrossRef CAS.
- H. Shinohara, M. Inakuma, N. Hayashi, H. Sato, Y. Saito, T. Kato and S. Bandow, J. Phys. Chem., 1994, 98, 8597 CrossRef CAS.
- A. A. Popov and L. Dunsch, J. Am. Chem. Soc., 2008, 130, 17726 CrossRef CAS.
- M. Yamada, T. Akasaka and S. Nagase, Acc. Chem. Res., 2010, 43, 92 CrossRef CAS PubMed.
- T. Kato, S. Suzuki, K. Kikuchi and Y. Achiba, J. Phys. Chem., 1993, 97, 13425 CrossRef CAS.
- E. Y. Misochko, A. V. Akimov, V. A. Belov, D. A. Tyurin, V. P. Bubnov, I. E. Kareev and E. B. Yagubskii, Phys. Chem. Chem. Phys., 2010, 12, 8863 RSC.
- M. M. Olmstead, A. de Bettencourt-Dias, S. Stevenson, H. C. Dorn and A. L. Balch, J. Am. Chem. Soc., 2002, 124, 4172 CrossRef CAS PubMed.
- X. Lu, K. Nakajima, Y. Iiduka, H. Nikawa, N. Mizorogi, Z. Slanina, T. Tsuchiya, S. Nagase and T. Akasaka, J. Am. Chem. Soc., 2011, 133, 19553 CrossRef CAS PubMed.
- S. Stevenson, P. W. Fowler, T. Heine, J. C. Duchamp, G. Rice, T. Glass, K. Harich, E. Hajdu, R. Bible and H. C. Dorn, Nature, 2000, 408, 427 CrossRef CAS PubMed.
- S. F. Yang, P. Rapta and L. Dunsch, Chem. Commun., 2007, 189 RSC.
- P. Rapta, A. A. Popov, S. Yang and L. Dunsch, J. Phys. Chem. A, 2008, 112, 5858 CrossRef CAS PubMed.
- Y. Q. Feng, T. S. Wang, J. Y. Wu, L. Feng, J. F. Xiang, Y. H. Ma, Z. X. Zhang, L. Jiang, C. Y. Shu and C. R. Wang, Nanoscale, 2013, 5, 6704 RSC.
- M. Inakuma and H. Shinohara, J. Phys. Chem. B, 2000, 104, 7595 CrossRef CAS.
- F. Liu, D. S. Krylov, L. Spree, S. M. Avdoshenko, N. A. Samoylova, M. Rosenkranz, A. Kostanyan, T. Greber, A. U. B. Wolter, B. Büchner and A. A. Popov, Nat. Commun., 2017, 8, 16098 CrossRef CAS.
- T. S. Wang, J. Y. Wu, W. Xu, J. F. Xiang, X. Lu, B. Li, L. Jiang, C. Y. Shu and C. R. Wang, Angew. Chem., Int. Ed., 2010, 49, 1786 CrossRef CAS PubMed.
- H. Kurihara, Y. Iiduka, Y. Rubin, M. Waelchli, N. Mizorogi, Z. Slanina, T. Tsuchiya, S. Nagase and T. Akasaka, J. Am. Chem. Soc., 2012, 134, 4092 CrossRef CAS PubMed.
- M. Hachiya, H. Nikawa, N. Mizorogi, T. Tsuchiya, X. Lu and T. Akasaka, J. Am. Chem. Soc., 2012, 134, 15550 CrossRef CAS PubMed.
- C. Zhao, H. Meng, M. Nie, X. Wang, Z. Cai, T. Chen, D. Wang, C. Wang and T. Wang, J. Phys. Chem. C, 2019, 123, 12514 CrossRef CAS.
- C. Zhao, H. Meng, M. Nie, Q. Huang, P. Du, C. Wang and T. Wang, Chem. Commun., 2019, 55, 11511 RSC.
- A. Xu, X. Zhou and R. Zheng, Sci. China: Chem., 2022, 65, 1607 CrossRef CAS.
- Y. Lu, C. Zhao, J. Zhang, W. Li, L. Liu, C. Zheng, C. Wang and T. Wang, J. Phys. Chem. C, 2023, 127, 4660 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |