Sustainable pretreatment of blood samples using hydrophobic eutectic solvents to improve the detection of bisphenol A†
Cariny
Polesca
a,
Helena
Passos
bc,
Ana C. A.
Sousa
*de,
Nguyen Minh
Tue
e,
João A. P.
Coutinho
 a,
Tatsuya
Kunisue
e and
Mara G.
Freire
a,
Tatsuya
Kunisue
e and
Mara G.
Freire
 *a
*a
aCICECO – Aveiro Institute of Materials, Department of Chemistry, University of Aveiro, 3810-193 Aveiro, Portugal. E-mail: maragfreire@ua.pt
bLSRE-LCM – Laboratory of Separation and Reaction Engineering – Laboratory of Catalysis and Materials, Faculty of Engineering, University of Porto, Ruo Dr. Roberto Frias, 4200-465 Porto, Portugal
cALiCE – Associate Laboratory in Chemical Engineering, Faculty of Engineering, University of Porto, Ruo Dr. Roberto Frias, 4200-465 Porto, Portugal
dComprehensive Health Research Centre (CHRC), Department of Biology, School of Science and Technology, University of Évora, 7006-554 Évora, Portugal. E-mail: acsousa@uevora.pt
eCenter for Marine Environmental Studies (CMES), Ehime University, Bunkyo-cho 2-5, Matsuyama, 790-8577, Ehime, Japan
First published on 13th November 2024
Abstract
Bisphenols, and mostly bisphenol A (BPA), are widely used in many consumer products. Due to its toxicity, BPA presents a noteworthy risk to the environment and human health. Despite these concerns, monitoring BPA proves challenging, particularly in highly complex matrices such as blood, because extraction and clean-up require multiple steps, the use of volatile organic solvents, and associated high costs. To overcome these limitations, this work discloses a novel, one-step and sustainable pretreatment technique of blood samples using hydrophobic eutectic solvents (HES). Systems composed of different HES, including thymol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) menthol, benzyl alcohol
menthol, benzyl alcohol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cyclohexanol, and decanoic acid
cyclohexanol, and decanoic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
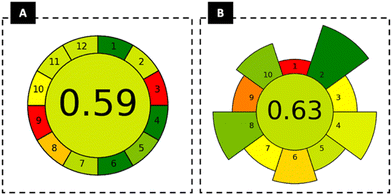
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) trioctylphosphine oxide at various mole ratios, combined with potassium citrate buffer aqueous solutions at different volume ratios, were carefully evaluated as three-phase partitioning (TPP) systems. The high performance of the HES-based systems for the pretreatment of blood samples was confirmed with liquid chromatography–tandem mass spectrometry (LC-MS/MS) analysis, with a BPA recovery of (98 ± 3)% in the HES-rich phase, and with the interfering biological material precipitating at the liquid–liquid interphase. The green nature of the developed method was assessed using the Analytical GREENess Metric (AGREE) and the AGREE metrics of environmental impact of sample preparation (AGREEprep), scoring 0.59 and 0.63, respectively. The high pretreatment performance offered by HES-based TPP systems with respect to blood samples, combined with their greener credentials, paves the way for their application in a variety of biomonitoring studies.
trioctylphosphine oxide at various mole ratios, combined with potassium citrate buffer aqueous solutions at different volume ratios, were carefully evaluated as three-phase partitioning (TPP) systems. The high performance of the HES-based systems for the pretreatment of blood samples was confirmed with liquid chromatography–tandem mass spectrometry (LC-MS/MS) analysis, with a BPA recovery of (98 ± 3)% in the HES-rich phase, and with the interfering biological material precipitating at the liquid–liquid interphase. The green nature of the developed method was assessed using the Analytical GREENess Metric (AGREE) and the AGREE metrics of environmental impact of sample preparation (AGREEprep), scoring 0.59 and 0.63, respectively. The high pretreatment performance offered by HES-based TPP systems with respect to blood samples, combined with their greener credentials, paves the way for their application in a variety of biomonitoring studies.
Introduction
Bisphenols are commonly used in different consumer products (e.g., plastics, toys, cosmetics, and pharmaceuticals), but are being continuously released into the environment. As a result, they have been detected in several environmental compartments, including effluents, surface waters, soils, sediments, air, and many biological samples, from wildlife to humans. Even at low concentrations, their continued exposure has been responsible for several negative effects on animal and human health, such as immunotoxicity, reprotoxicity, metabolic toxicity and antimicrobial resistance.1–5Bisphenol A (BPA), an endocrine-disrupting synthetic chemical, originates from the production of epoxy resins and polycarbonate plastics (e.g., baby bottles, plastic tableware, toys, medical devices, and eyeglass lenses).6 In the last two decades, BPA has been identified in water, air, soil, and human biological fluids (including serum, plasma, placenta, semen, and breast milk).6,7 BPA can cross the placental barrier and be present in the urine and blood of mothers and newborns.8 The presence of BPA in the human body can cause neuronal, physiological and metabolic disorders. It has been associated with reduced fertility, obesity, increased adipose tissue and hormones, irregular glucose concentrations, inhibition of bone metabolism, and modulation of immune responses and signalling pathways.1,4Fig. 1 depicts the matrices in which BPA has been detected and the range of concentrations reported.
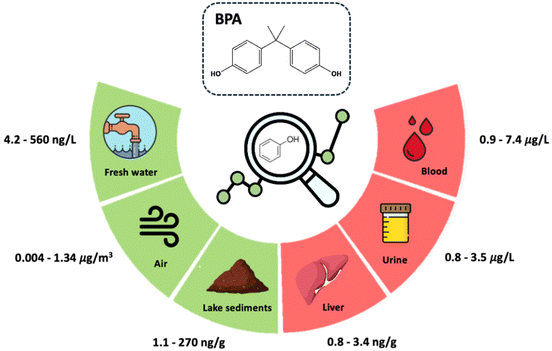 | ||
| Fig. 1 Concentrations of BPA in different environmental and biological samples.1,2,13–16 | ||
Since BPA is a priority substance according to the European Human Biomonitoring Initiative (HBM4EU), currently, monitoring BPA levels in treated water is mandatory, as reported by the Directive (EU) no.2020/2184.9,10 To this end, improved analytical methods have been developed in the last decades for BPA detection, including chromatography and mass spectroscopy-based methods.11 Nevertheless, to reduce interference and improve accuracy before detection, BPA extraction and purification from real samples is necessary. However, current pretreatment methods (e.g., liquid–liquid and solid-phase extraction) demand several steps, are time-consuming and require high-cost solvents and materials/resins. Furthermore, the requirements of green chemistry are generally not followed in these traditional methods since they usually involve applying hazardous volatile solvents and are energy-consuming processes.12
Adhering to the 5th principle of green chemistry (safer solvents),17 alternative solvents such as deep eutectic solvents (DES), if properly selected, may allow the design of more sustainable processes.18,19 DES can be formed from a mixture of biobased and biodegradable compounds20,21 that rely on a hydrogen bond donor (HBD) and a hydrogen bond acceptor (HBA), thus deviating from the ideal solid–liquid phase behaviour.18 These mixtures present melting temperatures significantly lower than the melting temperature of the pure components, which may allow their use in the liquid state at room temperature. Depending on the HBD and HBA compounds, DES can be miscible or immiscible with water, the latter usually being designated as hydrophobic eutectic solvents (HES). Integrating HES into chemical separations has shown great promise in achieving the dual goals of efficiency and environmental consciousness.17
Since several compounds at different mole ratios can be used to prepare HES, their properties can be tailored to suit specific applications. For instance, An and Row22 prepared nine menthol-based HES paired with different carboxylic and hydroxy acids and evaluated the effect of the HES nature on the extraction of BPA from water. Among the HES investigated, formic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) menthol and propionic acid
menthol and propionic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) menthol presented higher extraction efficiencies (up to 98%).22 However, the HBDs (propionic and formic acids) used by these authors exhibit lower log
menthol presented higher extraction efficiencies (up to 98%).22 However, the HBDs (propionic and formic acids) used by these authors exhibit lower log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow values (0.25 and −0.54, respectively),23 meaning that these compounds are less hydrophobic. The use of hydrophilic ES results in higher cross-contamination, with higher losses of the ES to the aqueous phase. Likewise, Florindo et al.24 used natural fatty acids (octanoic/decanoic acid) as the HBD and L-menthol or quaternary ammonium salts (tetraoctylammonium bromide ([N8888]Br), tetraheptylammonium bromide ([N7777]Br), methyltrioctylammonium bromide ([N8881]Br)) as the HBA to extract BPA from aqueous solutions. High BPA extraction efficiencies (85%) were achieved using all HES, which was due to their hydrophobicity and high octanol–water partition coefficient of BPA (log
Kow values (0.25 and −0.54, respectively),23 meaning that these compounds are less hydrophobic. The use of hydrophilic ES results in higher cross-contamination, with higher losses of the ES to the aqueous phase. Likewise, Florindo et al.24 used natural fatty acids (octanoic/decanoic acid) as the HBD and L-menthol or quaternary ammonium salts (tetraoctylammonium bromide ([N8888]Br), tetraheptylammonium bromide ([N7777]Br), methyltrioctylammonium bromide ([N8881]Br)) as the HBA to extract BPA from aqueous solutions. High BPA extraction efficiencies (85%) were achieved using all HES, which was due to their hydrophobicity and high octanol–water partition coefficient of BPA (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow 3.4).23 Still, both these studies only investigated the extraction of BPA from water, not dealing with real matrices or complex biological samples.
Kow 3.4).23 Still, both these studies only investigated the extraction of BPA from water, not dealing with real matrices or complex biological samples.
The aim of this work is to develop a sustainable pre-treatment strategy for blood samples to improve the detection and quantification of BPA, while overcoming the challenges still faced in the analysis of highly complex matrices, where multiple extraction and clean-up steps using volatile organic solvents are usually required all with associated high costs. A novel, one-step and sustainable pretreatment technique for blood samples using hydrophobic eutectic solvents (HES) is herein proposed. The designed HES facilitate the precipitation of the unwanted biological material at the interphase of two liquid phases, thus forming a three-phase partitioning (TPP) system, while extracting and enriching the target analyte (BPA) in the HES-rich phase. Various HES combinations, including thymol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) menthol, benzyl alcohol
menthol, benzyl alcohol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cyclohexanol and decanoic acid
cyclohexanol and decanoic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) trioctylphosphine oxide, at the mole ratios of 0.2
trioctylphosphine oxide, at the mole ratios of 0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8, 0.3
0.8, 0.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.7, 0.5
0.7, 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5, and 0.65
0.5, and 0.65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.35, were tested. Among the studied HES components, thymol is currently recognised as safe by the Environmental Protection Agency (EPA).25 All studied HES are hydrophobic, being immiscible with water at room temperature (Table S1, ESI†), and are of low cost. The optimal conditions for the extraction of BPA were determined, being subsequently evaluated for monitoring BPA in whole blood samples by liquid chromatography–tandem mass spectrometry (LC-MS/MS). Different sources of blood were considered to support reproducibility. Finally, the green nature of the proposed HES–TPP method was demonstrated using the Analytical GREENess Metric (AGREE) and AGREE metrics of environmental impact of sample preparation (AGREEprep).
0.35, were tested. Among the studied HES components, thymol is currently recognised as safe by the Environmental Protection Agency (EPA).25 All studied HES are hydrophobic, being immiscible with water at room temperature (Table S1, ESI†), and are of low cost. The optimal conditions for the extraction of BPA were determined, being subsequently evaluated for monitoring BPA in whole blood samples by liquid chromatography–tandem mass spectrometry (LC-MS/MS). Different sources of blood were considered to support reproducibility. Finally, the green nature of the proposed HES–TPP method was demonstrated using the Analytical GREENess Metric (AGREE) and AGREE metrics of environmental impact of sample preparation (AGREEprep).
Materials and methods
Materials
BPA, 4,4′-(propane-2,2-dial)diphenol (99 wt%), benzyl alcohol (>99.8 wt%), cyclohexanol (>99 wt%), and trioctylphosphine oxide (TOPO) (99 wt%) were purchased from Sigma-Aldrich. Decanoic acid (99 wt%) was obtained from Thermo Scientific. Thymol (>99 wt%) was obtained from TCI America. L(−)menthol (99.5 wt%) and potassium citrate (K3C6H5O7) (99 wt%) were supplied by Acros Organics. Citric acid (99.5 wt%) was purchased from Panreac. LC-MS-grade methanol, acetonitrile, and HPLC-grade ammonium acetate were obtained from Fujifilm Wako Pure Chemical Corp. (Japan). Ultrapure water used in all LC-MS/MS-related experiments was supplied by a Direct-Q UV3 system (Merck). Fresh chicken blood containing water and acidity regulator (E260) produced by Campoaves, S.A. (Oliveira de Frades, Portugal) and from Avicasal, S.A. (Viseu, Portugal), was acquired from a local supermarket in Portugal. The chemical structures of the HES components used in this work and HES mole ratios are presented in Fig. 2.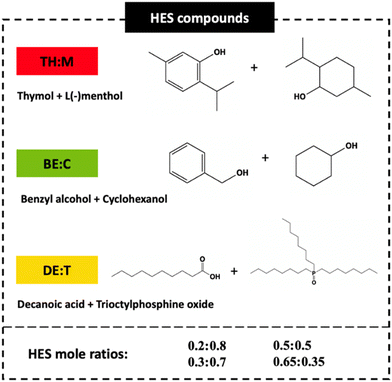 | ||
| Fig. 2 Chemical structures, abbreviations and mole ratios of the compounds used for HES preparation. | ||
Methods
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) aqueous solutions at 1
aqueous solutions at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (500 μL HES
2 (500 μL HES![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 μL buffer), 1
900 μL buffer), 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (500 μL HES
1 (500 μL HES![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 μL buffer), and 2
400 μL buffer), and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w ratios (500 μL HES
1 w/w ratios (500 μL HES![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 150 μL buffer) were spiked with 100 μL of BPA solution (120 mg L−1). The liquid–liquid systems were kept overnight at room temperature to ensure complete analyte partitioning and system equilibrium. Subsequently, the two phases were carefully separated, and the BPA concentration in the water-rich phase (bottom phase) was determined. Then, a mass balance was applied to determine the amount of BPA in the HES-rich phase. Blank control samples were used to eliminate any interference of the buffer or HES towards the BPA quantification. At least 3 independent assays were performed for each sample, and the respective standard deviation was determined.
150 μL buffer) were spiked with 100 μL of BPA solution (120 mg L−1). The liquid–liquid systems were kept overnight at room temperature to ensure complete analyte partitioning and system equilibrium. Subsequently, the two phases were carefully separated, and the BPA concentration in the water-rich phase (bottom phase) was determined. Then, a mass balance was applied to determine the amount of BPA in the HES-rich phase. Blank control samples were used to eliminate any interference of the buffer or HES towards the BPA quantification. At least 3 independent assays were performed for each sample, and the respective standard deviation was determined.
The percentage extraction efficiencies of BPA (EBPA), given by eqn (1), is defined as the percentage ratio between the amount of BPA in the HES-rich phase (top phase) and that in the total mixture:
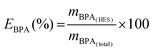 | (1) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) aqueous solution ratios (2
aqueous solution ratios (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 1
1, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 w/w). The samples were prepared in individual Eppendorf tubes, mixed, and centrifuged (13
2 w/w). The samples were prepared in individual Eppendorf tubes, mixed, and centrifuged (13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm). Then, the effect of time (from 2 to 20 min) on BPA extraction efficiency was evaluated. Blank samples were prepared for individual systems using buffer, HES and blood (without BPA). After extraction, the aqueous phase was carefully collected using a syringe, and the BPA concentration was determined using UV spectroscopy, as described before, enabling the determination of the EBPA (%).
000 rpm). Then, the effect of time (from 2 to 20 min) on BPA extraction efficiency was evaluated. Blank samples were prepared for individual systems using buffer, HES and blood (without BPA). After extraction, the aqueous phase was carefully collected using a syringe, and the BPA concentration was determined using UV spectroscopy, as described before, enabling the determination of the EBPA (%).
The most effective HES compositions were then used for the extraction and purification of BPA at lower levels. Aqueous samples (100 μL citrate buffer or blood) were spiked with 5 μL of a methanol-based standard solution containing BPA (1.5 μg) and then sonicated (3 × 5 min, vortexed in between) for equilibration. 900 μL of citrate buffer and 495 μL of HES were then added, and the samples were centrifugated at 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 15 min. After complete phase separation, the HES phases of the prepared systems were diluted in MeOH (10-fold) and analysed using LC-MS/MS without further clean-up.
000 rpm for 15 min. After complete phase separation, the HES phases of the prepared systems were diluted in MeOH (10-fold) and analysed using LC-MS/MS without further clean-up.
Instrumental analysis was performed on a Prominence LC system (Shimadzu, Japan) coupled to an LCMS-8050 mass spectrometer (Shimadzu, Japan) operating in electrospray ionisation (ESI) negative mode with multiple reaction monitoring (MRM). The analytical column was an Extend-C18 (1.8 μm, 100 × 2.1 mm; Agilent, USA). The column temperature was set at 40 °C, and the injection volume was 2 μL. The mobile phases were Milli-Q water containing 10 mM ammonium acetate (A) and methanol (B), and the flow rate was 0.2 mL min−1. The composition of B was 20% at 0–1 min, linearly increased, reaching 95% at 11 min, then was maintained until 15 min and finally returned to the initial condition. BPA was monitored using two MRM transitions: 227 → 211 (collision energy 30 eV) and 227 → 133 (24 eV).
The recovery of BPA (E) was calculated as the ratio of its peak area in the HES-rich phase (AHES) and the peak area in a reference solution (AREF) obtained by spiking 495 μL of the respective HES mixtures with 1.5 μg of BPA (in 5 μL methanol), as described by eqn (2). Duplicate samples were analysed for all systems. BPA was not detected in blank samples obtained using unspiked blood.
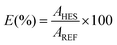 | (2) |
Results and discussion
Performance of HES-based systems to extract BPA
In this work, three HES mixtures were prepared, namely thymol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) menthol (TH
menthol (TH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M), benzyl alcohol
M), benzyl alcohol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cyclohexanol (BE
cyclohexanol (BE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C), and decanoic acid
C), and decanoic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TOPO (DE
TOPO (DE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) T). Different HES mole ratios were evaluated, consisting of 0.2
T). Different HES mole ratios were evaluated, consisting of 0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8, 0.3
0.8, 0.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.7, 0.5
0.7, 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5, and 0.65
0.5, and 0.65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.35 mol mol−1, chosen considering the solid–liquid phase diagrams reported in the literature,26,28 and with the goal of having liquid HES at room temperature. An exception occurs for DE
0.35 mol mol−1, chosen considering the solid–liquid phase diagrams reported in the literature,26,28 and with the goal of having liquid HES at room temperature. An exception occurs for DE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) T, for which only one ratio (0.5
T, for which only one ratio (0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 mol mol−1) was used since the other ratios resulted in cloudy solutions formed during the equilibrium with the citrate buffer.
0.5 mol mol−1) was used since the other ratios resulted in cloudy solutions formed during the equilibrium with the citrate buffer.
The extraction efficiencies of BPA obtained with the different HES at various mole ratios (fixing HES![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) aqueous solution ratio as 1
aqueous solution ratio as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w) are depicted in Fig. 3, with the respective detailed data being provided in Table S2 (ESI†). In this set of experiments, using only aqueous solutions spiked with BPA, only liquid–liquid systems were formed, and no TPP was observed. Generally, good BPA extraction efficiencies (>56%) are achieved with all systems investigated. This trend is due to the higher affinity of BPA to the hydrophobic HES phase, in agreement with its high octanol–water partition coefficient (log
1 w/w) are depicted in Fig. 3, with the respective detailed data being provided in Table S2 (ESI†). In this set of experiments, using only aqueous solutions spiked with BPA, only liquid–liquid systems were formed, and no TPP was observed. Generally, good BPA extraction efficiencies (>56%) are achieved with all systems investigated. This trend is due to the higher affinity of BPA to the hydrophobic HES phase, in agreement with its high octanol–water partition coefficient (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow (3.4)23).
Kow (3.4)23).
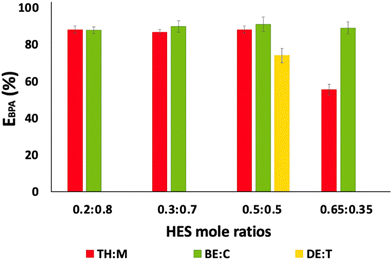 | ||
Fig. 3 Influence of HES type and mole ratios on BPA extraction (fixed HES![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) aqueous solution ratio at 1 aqueous solution ratio at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w). 1 w/w). | ||
When analysing the mole ratio of 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5, it is possible to compare the performances of the three HES mixtures to extract BPA. Similar extraction efficiencies for BPA are observed for TH
0.5, it is possible to compare the performances of the three HES mixtures to extract BPA. Similar extraction efficiencies for BPA are observed for TH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M and BE
M and BE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C HES, which can be explained by the similar molecular structures of their individual compounds (cf.Fig. 2). In contrast, the HES DE
C HES, which can be explained by the similar molecular structures of their individual compounds (cf.Fig. 2). In contrast, the HES DE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) T presents lower efficiency for BPA extraction, despite its higher hydrophobicity (log
T presents lower efficiency for BPA extraction, despite its higher hydrophobicity (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow of 4.09 and 9.76, for decanoic acid and trioctylphosphine oxide, respectively).23 This trend seems to be related to the absence of aromaticity in both DE and T, in contrast with the HES TH
Kow of 4.09 and 9.76, for decanoic acid and trioctylphosphine oxide, respectively).23 This trend seems to be related to the absence of aromaticity in both DE and T, in contrast with the HES TH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M and BE
M and BE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C. Preferential partitioning of BPA from water to phases comprising ionic liquids with aromatic cations was previously observed by Passos et al.6 Furthermore, with long alkyl side chains, the extraction efficiency of BPA decreases, which can be attributed to the weaker dispersive BPA interactions with the HES components. These results suggest that π⋯π interactions between BPA and the aromatic DES components play a significant role. From the data provided in Fig. 3, it is shown that using BE
C. Preferential partitioning of BPA from water to phases comprising ionic liquids with aromatic cations was previously observed by Passos et al.6 Furthermore, with long alkyl side chains, the extraction efficiency of BPA decreases, which can be attributed to the weaker dispersive BPA interactions with the HES components. These results suggest that π⋯π interactions between BPA and the aromatic DES components play a significant role. From the data provided in Fig. 3, it is shown that using BE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C, the BPA extraction efficiencies are higher for all HES mole ratios, with values varying from (88 ± 2)% to (91 ± 4)%.
C, the BPA extraction efficiencies are higher for all HES mole ratios, with values varying from (88 ± 2)% to (91 ± 4)%.
With the goal of increasing the BPA extraction efficiency and concentration in the HES-rich phase, various HES![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) aqueous solution ratios were evaluated, whose results are shown in Fig. 4 and provided in Table S3 (ESI†). Increases of 19%, 53%, and 38% are observed for the systems composed of TH
aqueous solution ratios were evaluated, whose results are shown in Fig. 4 and provided in Table S3 (ESI†). Increases of 19%, 53%, and 38% are observed for the systems composed of TH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M, BE
M, BE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C, and DE
C, and DE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) T, respectively, when the HES
T, respectively, when the HES![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) aqueous solution ratio changed from 1
aqueous solution ratio changed from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 to 2
2 to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w.
1 w/w.
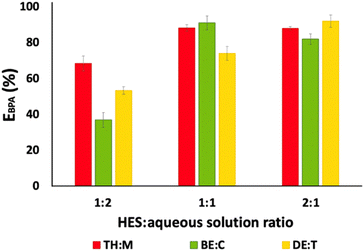 | ||
Fig. 4 Influence of the HES![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) aqueous solution ratio on the BPA extraction using TH aqueous solution ratio on the BPA extraction using TH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M, BE M, BE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C, and DE C, and DE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) T in a mole ratio of 0.5 T in a mole ratio of 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5. 0.5. | ||
Comparing TH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M and BE
M and BE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C at the HES
C at the HES![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) aqueous solution ratios of 1
aqueous solution ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2
1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w, similar BPA extraction efficiencies are obtained, which is due to the similar structures of the individual components in the HES. However, all HES mixtures have equivalent extraction efficiencies for BPA in their best operating conditions, corresponding to (88 ± 2)% and (91 ± 4)% for TH
1 w/w, similar BPA extraction efficiencies are obtained, which is due to the similar structures of the individual components in the HES. However, all HES mixtures have equivalent extraction efficiencies for BPA in their best operating conditions, corresponding to (88 ± 2)% and (91 ± 4)% for TH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M and BE
M and BE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C, respectively, at a HES
C, respectively, at a HES![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) aqueous solution ratio of 1
aqueous solution ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w, and (92 ± 3)% for DE
1 w/w, and (92 ± 3)% for DE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) T at a HES
T at a HES![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) aqueous solution ratio of 2
aqueous solution ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w.
1 w/w.
Pretreatment of whole blood and BPA extraction and detection
Having proved that designed HES-based systems efficiently extract BPA from aqueous samples, we then tested their efficiency as a pretreatment method for blood samples, which remains a challenge for exposure assessment studies. Blood was chosen for its widespread exposure assessment use, while requiring efficient pretreatment techniques for more accurate monitoring due to its complex nature. First, blood precipitation or enrichment at the interface was considered to prevent analytical interference when monitoring BPA in the HES-rich phase. Nevertheless, as shown in Fig. S1 (ESI†), only the HES![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) aqueous solution ratio of 1
aqueous solution ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 w/w allows complete precipitation of the biological material from blood. This is a result of the salting-out ability of potassium citrate buffer, in which an increase of the aqueous phase containing the salt leads to blood matrix “exclusion” by inducing its precipitation at the liquid–liquid interface. Based on this possibility, a one-step clean-up, extraction and purification method for whole blood using the HES–TPP system could be envisaged. The HES–TPP system eliminates blood-interfering compounds by their precipitation at the interphase, while extracting and enriching BPA in the HES-rich phase. When compared with state-of-the-art pretreatment methods, HES–TPP is a simple method that avoids time-consuming steps and allows energy saving. Furthermore, as reviewed by Ballesteros-Gómez et al.,11 clean extracts are desired for LC-MS/MS analysis, aiming to extend the column life and to spend less time on instrument maintenance, in addition to higher confidence in identification and quantification.
2 w/w allows complete precipitation of the biological material from blood. This is a result of the salting-out ability of potassium citrate buffer, in which an increase of the aqueous phase containing the salt leads to blood matrix “exclusion” by inducing its precipitation at the liquid–liquid interface. Based on this possibility, a one-step clean-up, extraction and purification method for whole blood using the HES–TPP system could be envisaged. The HES–TPP system eliminates blood-interfering compounds by their precipitation at the interphase, while extracting and enriching BPA in the HES-rich phase. When compared with state-of-the-art pretreatment methods, HES–TPP is a simple method that avoids time-consuming steps and allows energy saving. Furthermore, as reviewed by Ballesteros-Gómez et al.,11 clean extracts are desired for LC-MS/MS analysis, aiming to extend the column life and to spend less time on instrument maintenance, in addition to higher confidence in identification and quantification.
Focusing on achieving the exclusion of biological material (for complete sample purification, thus enabling accurate BPA extraction without interference), the HES![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) aqueous solution ratio of 1
aqueous solution ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 w/w was selected. Using a fixed HES composition (BE
2 w/w was selected. Using a fixed HES composition (BE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C 0.5
C 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5), the extraction efficiency of BPA was evaluated over varying time intervals, as shown in Fig. 5. A noticeable increase in BPA recovery was observed from 10 min to 15 min, with the extraction yield rising from (29 ± 4)% to (54 ± 4)%, and then reaching a plateau corresponding to the system equilibrium.
0.5), the extraction efficiency of BPA was evaluated over varying time intervals, as shown in Fig. 5. A noticeable increase in BPA recovery was observed from 10 min to 15 min, with the extraction yield rising from (29 ± 4)% to (54 ± 4)%, and then reaching a plateau corresponding to the system equilibrium.
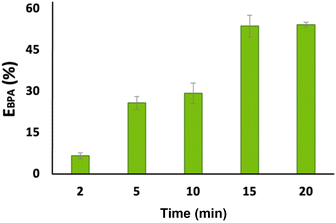 | ||
Fig. 5 Effect of time on BPA extraction from a system with blood (HES![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) aqueous solution ratio of 1 aqueous solution ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 w/w) using BE:C in a mole ratio of 0.5 2 w/w) using BE:C in a mole ratio of 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5. 0.5. | ||
Based on the optimal identified time (15 min) to reach equilibrium, all HES mixtures were then investigated in systems comprising blood. The corresponding results are shown in Fig. 6. Among the studied HES, TH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M leads to a higher BPA extraction yield, with thymol being recognised as safe by the EPA.25 Therefore, TH
M leads to a higher BPA extraction yield, with thymol being recognised as safe by the EPA.25 Therefore, TH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M can be considered as a more environmentally friendly solvent with high potential for extraction of bisphenols. However, considering the similarities between TH
M can be considered as a more environmentally friendly solvent with high potential for extraction of bisphenols. However, considering the similarities between TH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M and BE
M and BE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C, both HES were employed in subsequent studies. All obtained data are detailed in the ESI (Table S5†), including the use of a different blood sample, to support the reproducibility of the proposed process.
C, both HES were employed in subsequent studies. All obtained data are detailed in the ESI (Table S5†), including the use of a different blood sample, to support the reproducibility of the proposed process.
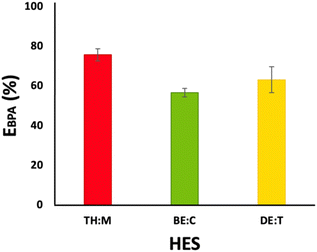 | ||
Fig. 6 Recovery of BPA from systems with blood (HES![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) aqueous solution ratio of 1 aqueous solution ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 w/w and HES mole ratios of 0.2 2 w/w and HES mole ratios of 0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8 for TH 0.8 for TH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M and 0.5 M and 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 for BE 0.5 for BE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C and DE C and DE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) T). T). | ||
As pointed out before, one of the major concerns related to the analysis of bisphenols at low levels is their quantification in complex matrices, which requires several steps involving first protein precipitation and then analyte extraction. After fine-tuning the best conditions of the tested systems (HES mole ratios 0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8 and 0.5
0.8 and 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 for TH
0.5 for TH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M and BE
M and BE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C, respectively), we evaluated their applicability as a novel one-step extraction and purification technique for BPA directly from blood samples and LC-MS/MS monitoring. BPA recovery results are shown in Fig. 7.
C, respectively), we evaluated their applicability as a novel one-step extraction and purification technique for BPA directly from blood samples and LC-MS/MS monitoring. BPA recovery results are shown in Fig. 7.
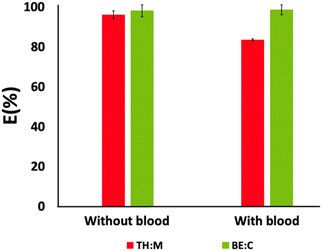 | ||
Fig. 7 Recovery of BPA from systems without blood and with blood (HES![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) aqueous solution ratio of 1 aqueous solution ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 w/w and HES mole ratios of 0.2 2 w/w and HES mole ratios of 0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8 and 0.5 0.8 and 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 for TH 0.5 for TH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M and BE M and BE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C, respectively). C, respectively). | ||
The results obtained prove the efficiency of the proposed one-step extraction and purification system using HES, even when the BPA concentration is decreasing and it is present in a complex matrix. For the model system without blood, BPA extraction yields in the top phase (HES-rich phase) correspond to (96 ± 2)% and (98 ± 3)% for TH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M and BE
M and BE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C, respectively. Nevertheless, for those systems with blood, BE
C, respectively. Nevertheless, for those systems with blood, BE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C presents the best performance, achieving a BPA recovery of (99 ± 3)%, while the TH
C presents the best performance, achieving a BPA recovery of (99 ± 3)%, while the TH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M system can recover (84 ± 1)% of BPA. Additionally, it is interesting to highlight that when present in blood the partitioning of BPA by the HES-rich phase when BE
M system can recover (84 ± 1)% of BPA. Additionally, it is interesting to highlight that when present in blood the partitioning of BPA by the HES-rich phase when BE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C is applied is favoured. The high extraction efficiency of BPA from complex systems is a result of the strong salting-out ability of the salt used (high-charge density anion with an improved ability to create hydration complexes), leading to the exclusion of BPA from the salt-rich phase to the HES-rich phase. It is important to emphasise that the method herein proposed is a simple and efficient one-step extraction and purification method validated for water and blood without using common volatile organic or toxic solvents.
C is applied is favoured. The high extraction efficiency of BPA from complex systems is a result of the strong salting-out ability of the salt used (high-charge density anion with an improved ability to create hydration complexes), leading to the exclusion of BPA from the salt-rich phase to the HES-rich phase. It is important to emphasise that the method herein proposed is a simple and efficient one-step extraction and purification method validated for water and blood without using common volatile organic or toxic solvents.
Generally, the first step in biological sample treatment is protein precipitation (usually using acetonitrile), aiming to eliminate any protein interference, and the second step is the analyte extraction.29 According to Tarafdar et al.,30 the liquid–liquid extraction method for BPA is the preferred technique for treating samples. Yet, despite its benefits, it is not eco-friendly due to the high consumption of volatile organic solvents (e.g., hexane, acetonitrile). Using HES can be a safer and more sustainable approach for bisphenol extraction by achieving biological matrix precipitation in a single-step procedure.
Evaluation of the HES–TPP pretreatment method by green metrics
The greenness of the proposed HES–TPP method was assessed using the GREENess Metric (AGREE) and the AGREE metrics of environmental impact of sample preparation (AGREEprep). AGREE evaluates the entire analytical procedure, including material requirements (considering quality and quantity), waste generation, energy consumption, safety, and the general approach of the process. For that, the 12 principles of green analytical chemistry are considered, as defined by Pena-Pereira et al.31 AGREEprep evaluates the environmental impact of sample preparation methods and considers 10 parameters associated with the 10 green sample preparation (GSP) principles.32 For both metrics, user-friendly software was used to calculate and compile the graphs, with the results being presented in coloured diagrams. Each assessed parameter has a different colour depending on its respective score. The green colour represents the maximum (1.0), and the red colour represents the minimum score (0.0).31,32 The graphs obtained for the developed HES–TPP method by applying AGREE and AGREEprep metrics are represented in Fig. 8.Regarding AGREE, the greener aspects include the direct analytical techniques (principle 1), the involvement of only 3 steps (principle 4), and the non-existence of a derivatisation step (principle 6). Principle 1 addresses the reduction of sample preparation steps, achieved in this study by developing a one-step extraction and purification technique, therefore reducing the environmental, and health and safety impacts of the process. This is also linked to principle 4, which is to decrease the number of analytical steps to save material, energy, and time. In our study, a simple procedure was successfully developed despite the high complexity of the biological sample. Most of the principles obtained an intermedium score, such as sample size (principle 2), automated and miniaturised methods (principle 5), generation of analytical waste (principle 7), toxic reagents (principle 11) and safety of the operator (principle 12). Non-green scores are related principally to offline measurements (principle 3) and energy consumption (principle 9). Regarding principle 3, field-portable instruments and miniaturized analytical systems could offer great potential for advancing greener chemical analyses, offering advantages such as enhancing operator safety and eliminating the costs of sample preservation.31 For principle 9, the high energy consumption is due to the analytical LC-MS/MS analysis, which also requires the use of hazardous solvents (methanol is used for sample dilution). Nevertheless, this analysis is more accurate and appropriate than UV-Vis (initially used) for detecting lower concentrations of BPA. Thus, the energy consumption estimates need to be calculated more precisely by considering the power consumption listed in the technical specifications of the analytical systems used, along with the duration of each analytical step (e.g. run time and the number of samples processed).31
While the AGREE metric is an effective tool for evaluating the greenness of the entire analytical process, it does not consider the environmental impact of the production route of the chemicals involved. This underscores the importance of incorporating a life-cycle assessment (LCA) in future studies to obtain a more comprehensive view of the sustainable characteristics of pre-treatment strategies. For instance, Zaib et al.33 presented a comparative LCA analysis of choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) urea DES with traditional organic solvents (e.g. methanol, ethanol, dichloromethane, and ethyl acetate). Their findings reveal that while the DES demonstrates lower environmental impacts than dichloromethane and ethyl acetate, its impacts exceed those of methanol and ethanol. This result is due to the effect of the chemical constituents used for the synthesis of the DES components, highlighting the need for careful selection of precursor materials to achieve a more sustainable process.
urea DES with traditional organic solvents (e.g. methanol, ethanol, dichloromethane, and ethyl acetate). Their findings reveal that while the DES demonstrates lower environmental impacts than dichloromethane and ethyl acetate, its impacts exceed those of methanol and ethanol. This result is due to the effect of the chemical constituents used for the synthesis of the DES components, highlighting the need for careful selection of precursor materials to achieve a more sustainable process.
For AGREEprep, the greenest aspect is related to using non-hazardous materials (criterion 2). The waste (criterion 4) is related to the analysis of a small volume (∼1.5 mL) and also promotes a green score in the size economy of the sample (criterion 5). The energy consumption (criterion 8) is only related to the centrifugation step (∼25 W h). Focusing on criterion 2, despite the safety of the proposed HES, its recovery and reuse were not investigated in this study but are strongly encouraged in future work to achieve a more sustainable process and obtain a greener score. Prices for each compound were obtained to assess the profitability of the HES mixtures, corresponding to $0.19 g−1 and $0.11 g−1 for thymol and L(−)menthol, respectively.34,35 Likewise, the procedure for HES preparation is simple and does not require any purification step after its preparation. In criterion 1 (sample preparation), a minimum score is related to the necessity of the samples to be collected and transported to the laboratory for sample preparation and analysis, which is inevitable if the goal of the developed process is to analyse real biological samples. Moreover, a low score for criterion 9 (post-sample preparation configuration for analysis) can be observed since the LC-MS/MS system is required.
For comparison purposes, diagrams of both green metrics were accessed for representative works published in the literature (Fig. S2 given in the ESI†).13,36 Wiraagni et al.36 developed a simple procedure for BPA extraction from human plasma, followed by LC-MS/MS analysis. The authors obtained plasma from human whole blood, resulting in a simpler sample, while in our work, a successful method was developed for the more complex matrix – blood. Despite the minimal sample size and small number of steps reported by these authors, similar to the positive effects of our method, acetonitrile was used for extraction, resulting in lower scores for both metrics (0.52 for AGREE and 0.47 for AGREEprep). Nevertheless, the similarity of these scores with those obtained with our method seems to be linked to the lower volume sample used by the authors (∼0.4 mL) compared to the volume used in our process (∼1.5 mL). To better address the effect of sample size on green metrics scores, a simulation of the HES–TPP system with a smaller sample size (the same used by these authors) was performed. According to the obtained results (Fig. S2, ESI†), higher scores (0.65 and 0.69 for AGREE and AGREEprep, respectively) were obtained, highlighting the best performance of the HES–TPP method compared to the method reported by these authors. It reveals the importance of reducing sample size in accordance with principle 5 of sample preparation (minimise sample, chemical and material amounts), which is linked to the amount of waste generation (principle 4 of sample preparation). Furthermore, downscaled sample sizes are associated with faster procedures (principle 6) that minimise exposure risks for operators (principle 10), resulting in a greener analytical methodology.37 Geens et al.13 investigated BPA extraction from human adipose tissue, liver and brain; however, the developed method involves several steps, many toxic solvents (e.g., acetonitrile and hexane), ultrasonication for extraction, and derivatisation. In the end, GC/MS was used for the analytical quantification, resulting in low scores of 0.26 and 0.2 for AGREE and AGREEprep, respectively. This method has a negative score compared to our method and the previous one, resulting from the overconsumption of solvents and large waste generation, in addition to the requirement of time-consuming steps and high energy input in the overall sample preparation stage.
In summary, the proposed HES–TPP method has higher green scores in AGREE and AGREEprep than previous pretreatment methods used to analyse BPA reported in the literature. The assessment of green metrics indicates, in general, greener credentials of the HES–TPP method, paving the way for its extended use in monitoring studies.
Conclusions
The extraction and concentration of bisphenol contaminants from aqueous solutions and biological samples is generally carried out by organic solvents and applying multiple steps. Dealing with biologically complex matrices brings additional challenges, requiring several clean-up steps and the use of hazardous volatile solvents. In this work, a strategy comprising the simultaneous clean-up, extraction and purification of BPA was successfully developed, using HES-based TPP systems. This approach offers a greener, time-saving, and simpler solution, demonstrating the noteworthy ability of HES to efficiently extract BPA when used to pretreat blood samples.The blood sample clean-up was achieved by forming an interphase, leading to the first reported formation of HES–TPP systems. This behaviour enabled BPA extraction into the HES-rich phase without blood material contamination. The salting-out effect allowing blood precipitation was confirmed, revealing remarkable BPA extraction efficiencies (up to (98 ± 3)%) to the HES-rich phase, as confirmed by LC-MS/MS analysis. Finally, the proposed HES–TPP approach was evaluated through different analytical greenness metrics, where scores of 0.59 and 0.63 were obtained, thus supporting its greener credentials compared to other previously reported methods. The versatility and sustainability of the HES-based TPP system as a pretreatment strategy paves the way for its use in the monitoring of other relevant compounds in biological fluids and tissues.
Author contributions
Conceptualisation, H. P., A. C. A. S., N. M. T., M. G. F.; methodology C. P. and N. M. T; writing – original draft preparation, C. P.; writing – review and editing, C. P., H. P., A. C. A. S., N. M. T., J. A. P. C., T. K., M. G. F.; supervision, H. P., A. C. A. S., N. M. T., J. A. P., T. K., M. G. F; funding acquisition, T. K., M. G. F.; project administration, T. K, M. G. F. All authors listed have made a substantial, direct, and intellectual contribution to the work and agreed to the published version of the manuscript.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was developed within the scope of the project CICECO-Aveiro Institute of Materials, UIDB/50011/2020 (https://doi.org/10.54499/UIDB/50011/2020), UIDP/50011/2020 (https://doi.org/10.54499/UIDP/50011/2020) & LA/P/0006/2020 (https://doi.org/10.54499/LA/P/0006/2020), financed by national funds through the FCT/MCTES (PIDDAC). C. Polesca acknowledges FCT – Fundação para a Ciência e a Tecnologia for the PhD grant with the reference UI/BD/151282/2021 (https://doi.org/10.54499/UI/BD/151282/2021). This work was supported by national funds through FCT/MCTES (PIDDAC): LSRE-LCM, UIDB/50020/2020 (https://doi.org/10.54499/UIDB/50020/2020), UIDP/50020/2020 (https://doi.org/10.54499/UIDP/50020/2020),and ALiCE, LA/P/0045/2020 (https://doi.org/10.54499/LA/P/0045/2020). This work was also supported by national funds through CHRC, UIDP/04923/2020. Further financial support was provided by the Leading Academia in Marine and Environment Pollution Research (LaMer) project, a Joint Usage/Research Center project funded by the Japanese Ministry of Education, Culture, Sports, Science, and Technology (MEXT).References
- J. L. Torres-García, M. Ahuactzin-Pérez, F. J. Fernández and D. V. Cortés-Espinosa, Chemosphere, 2022, 303, 134940 CrossRef.
- S. M. Colorado-Yohar, A. C. Castillo-González, J. Sánchez-Meca, M. Rubio-Aparicio, D. Sánchez-Rodríguez, E. Salamanca-Fernández, E. Ardanaz, P. Amiano, M. F. Fernández, J. Mendiola, F. Navarro-Mateu and M. D. Chirlaque, Sci. Total Environ., 2021, 775, 145755 CrossRef CAS PubMed.
- M. H. Lin, C. Y. Lee, Y. S. Chuang and C. L. Shih, Environ. Res., 2023, 237, 116900 CrossRef CAS PubMed.
- C. Lambré, J. M. B. Baviera, C. Bolognesi, A. Chesson, P. S. Cocconcelli, R. Crebelli, D. M. Gott, K. Grob, E. Lampi, M. Mengelers, A. Mortensen, G. Rivière, V. Silano, I. L. Steffensen, C. Tlustos, L. Vernis, H. Zorn, M. Batke, M. Bignami, E. Corsini, R. FitzGerald, U. Gundert-Remy, T. Halldorsson, A. Hart, E. Ntzani, E. Scanziani, H. Schroeder, B. Ulbrich, D. Waalkens-Berendsen, D. Woelfle, Z. Al Harraq, K. Baert, M. Carfì, A. F. Castoldi, C. Croera and H. Van Loveren, EFSA J., 2023, 21, 6857 Search PubMed.
- Y. Onundi, B. A. Drake, R. T. Malecky, M. A. DeNardo, M. R. Mills, S. Kundu, A. D. Ryabov, E. S. Beach, C. P. Horwitz, M. T. Simonich, L. Truong, R. L. Tanguay, L. J. Wright, N. Singhal and T. J. Collins, Green Chem., 2017, 19, 4234–4262 RSC.
- H. Passos, A. C. A. Sousa, M. R. Pastorinho, A. J. A. Nogueira, L. P. N. Rebelo, J. A. P. Coutinho and M. G. Freire, Anal. Methods, 2012, 4, 2664–2667 RSC.
- M. D. Garrison, P. J. Storch, W. S. Eck, V. H. Adams, P. W. Fedick and B. G. Harvey, Green Chem., 2021, 23, 8016–8029 RSC.
- A. G. C. Guimarães, V. L. Coutinho, A. Meyer, P. C. Lisboa and E. G. de Moura, Environ. Toxicol. Pharmacol., 2023, 97, 104040 CrossRef PubMed.
- European parliament and of the council, directive (EU) 2020/2184, https://eur-lex.Europa.eu/eli/dir/2020/2184/oj?locale=pt, (accessed 2 February 2024).
- E. Govarts, L. Gilles, L. R. Martin, T. Santonen, P. Apel, P. Alvito, E. Anastasi, H. R. Andersen, A. M. Andersson, L. Andryskova, J. P. Antignac, B. Appenzeller, F. Barbone, Z. Barnett-Itzhaki, R. Barouki, T. Berman, W. Bil, T. Borges, J. Buekers, A. Cañas-Portilla, A. Covaci, Z. Csako, E. Den Hond, D. Dvorakova, L. Fabelova, T. Fletcher, H. Frederiksen, C. Gabriel, C. Ganzleben, T. Göen, T. I. Halldorsson, L. S. Haug, M. Horvat, P. Huuskonen, M. Imboden, M. J. Hudobivnik, B. Janasik, N. J. Holcer, S. Karakitsios, A. Katsonouri, J. Klanova, V. Kokaraki, T. K. Jensen, J. Koponen, M. Laeremans, F. Laguzzi, R. Lange, N. Lemke, S. Lignell, A. K. Lindroos, J. L. Vicente, M. Luijten, K. C. Makris, D. Mazej, L. Melymuk, M. Meslin, H. Mol, P. Montazeri, A. Murawski, S. Namorado, L. Niemann, S. Nübler, B. Nunes, K. Olafsdottir, L. P. Murinova, N. Papaioannou, S. Pedraza-Diaz, P. Piler, V. Plichta, M. Poteser, N. Probst-Hensch, L. Rambaud, E. Rauscher-Gabernig, K. Rausova, S. Remy, M. Riou, V. Rosolen, C. Rousselle, M. Rüther, D. Sarigiannis, M. J. Silva, Z. Šlejkovec, J. S. Tratnik, A. Stajnko, T. Szigeti, J. V. Tarazona, C. Thomsen, Ž. Tkalec, H. Tolonen, T. Trnovec, M. Uhl, A. Van Nieuwenhuyse, E. Vasco, V. J. Verheyen, S. Viegas, A. M. Vinggaard, N. Vogel, K. Vorkamp, W. Wasowicz, T. Weber, S. Wimmerova, M. Woutersen, P. Zimmermann, M. Zvonar, H. Koch, M. Kolossa-Gehring, M. E. López, A. Castaño, L. Stewart, O. Sepai and G. Schoeters, Int. J. Hyg. Environ. Health, 2023, 249, 114119 CrossRef CAS PubMed.
- A. Ballesteros-Gómez, S. Rubio and D. Pérez-Bendito, J. Chromatogr. A, 2009, 1216, 449–469 CrossRef PubMed.
- F. Sun, L. Kang, X. Xiang, H. Li, X. Luo, R. Luo, C. Lu and X. Peng, Anal. Bioanal. Chem., 2016, 408, 6913–6927 CrossRef CAS PubMed.
- T. Geens, H. Neels and A. Covaci, Chemosphere, 2012, 87, 796–802 CrossRef CAS PubMed.
- M. Zielińska, K. Bułkowska, A. Cydzik-Kwiatkowska, K. Bernat and I. Wojnowska-Baryła, Int. J. Environ. Sci. Technol., 2016, 13, 2239–2248 CrossRef.
- N. González, S. C. Cunha, C. Monteiro, J. O. Fernandes, M. Marquès, J. L. Domingo and M. Nadal, Environ. Res., 2019, 176, 108576 CrossRef PubMed.
- S. Shekhar, S. Sood, S. Showkat, C. Lite, A. Chandrasekhar, M. Vairamani, S. Barathi and W. Santosh, Gen. Comp. Endocrinol., 2017, 241, 100–107 CrossRef CAS PubMed.
- P. Anastas and N. Eghbali, Chem. Soc. Rev., 2010, 39, 301–312 RSC.
- A. Maletta, A. Gutiérrez, P. J. Tan, J. Springstead, S. Aparicio and M. Atilhan, J. Mol. Liq., 2023, 381, 121806 CrossRef CAS.
- O. G. Sas, M. Castro, Á. Domínguez and B. González, Sep. Purif. Technol., 2019, 227, 115703 CrossRef CAS.
- T. Hložek, T. Bosáková, Z. Bosáková and P. Tůma, Sep. Purif. Technol., 2022, 303, 122310 CrossRef.
- S. E. Hooshmand, R. Afshari, D. J. Ramón and R. S. Varma, Green Chem., 2020, 22, 3668–3692 RSC.
- Y. An and K. H. Row, Anal. Lett., 2021, 54, 1533–1545 CrossRef CAS.
- ChemSpider, https://www.chemspider.com/, (accessed 3 November 2023).
- C. Florindo, N. V. Monteiro, B. D. Ribeiro, L. C. Branco and I. M. Marrucho, J. Mol. Liq., 2020, 297, 111841 CrossRef CAS.
- EPA, U.S. Environmental Protection Agency, https://www.epa.gov/, (accessed 1 April 2024).
- N. Schaeffer, D. O. Abranches, L. P. Silva, M. A. R. Martins, P. J. Carvalho, O. Russina, A. Triolo, L. Paccou, Y. Guinet, A. Hedoux and J. A. P. Coutinho, ACS Sustainable Chem. Eng., 2021, 9, 2203–2211 CrossRef CAS.
- S. J. R. Vargas, G. Pérez-Sánchez, N. Schaeffer and J. A. P. Coutinho, Green Chem., 2021, 23, 4540–4550 RSC.
- D. O. Abranches, M. A. R. Martins, L. P. Silva, N. Schaeffer, S. P. Pinho and J. A. P. Coutinho, Chem. Commun., 2019, 55, 10253–10256 RSC.
- I. Moscoso-Ruiz, Y. Gálvez-Ontiveros, S. Cantarero-Malagón, A. Rivas and A. Zafra-Gómez, Microchem. J., 2022, 175, 107122 CrossRef CAS.
- A. Tarafdar, R. Sirohi, P. A. Balakumaran, R. Reshmy, A. Madhavan, R. Sindhu, P. Binod, Y. Kumar, D. Kumar and S. J. Sim, J. Hazard. Mater., 2022, 423, 127097 CrossRef CAS PubMed.
- F. Pena-Pereira, W. Wojnowski and M. Tobiszewski, Anal. Chem., 2020, 92, 10076–10082 CrossRef CAS PubMed.
- F. Pena-Pereira, M. Tobiszewski, W. Wojnowski and E. Psillakis, Adv. Sample Prep., 2022, 3, 100025 CrossRef.
- Q. Zaib, M. J. Eckelman, Y. Yang and D. Kyung, Green Chem., 2022, 24, 7924–7930 RSC.
- TCI America, TCI AMERICA, https://www.tcichemicals.com/CA/en/p/M0410, (accessed 2 April 2024).
- Merck, Sigma-Aldrich, https://www.sigmaaldrich.com/PT/en/product/aldrich/w266590, (accessed 2 April 2024).
- I. A. Wiraagni, M. A. Mohd, R. b. A. Rashid and D. E. b. M. Haron, PLoS One, 2019, 14, 1–13 CrossRef PubMed.
- Á. I. López-Lorente, F. Pena-Pereira, S. Pedersen-Bjergaard, V. G. Zuin, S. A. Ozkan and E. Psillakis, TrAC, Trends Anal. Chem., 2022, 148, 116530 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4gc03396e |
| This journal is © The Royal Society of Chemistry 2025 |