Ionic-liquid/metal–organic-framework composites: synthesis and emerging sustainable applications
Maiyong
Zhu

School of Materials Science & Engineering, Jiangsu University, Zhenjiang, 212013, P. R. China. E-mail: maiyongzhu@ujs.edu.cn
First published on 6th November 2024
Abstract
Ionic liquids (ILs) as emerging solvents have demonstrated a significant promise for green chemistry application and can be used as green solvents, high-efficiency catalysts, electrolytes, and so on. Metal –organic frameworks (MOFs), also called coordination polymers, are crystalline solids comprising metal ions and organic linkers linked through coordination bonds, which are characterized by high porosity. Both ILs and MOFs have been extensively investigated by many groups all over the world, and their applications cover a wide range of fields, including separation, catalysis and energy devices. IL/MOF composites, integrating the advantages of ILs and MOFs in one moiety, are a new class of functional materials that have attracted increasing attention during the past decades. This study offers an extensive overview of the design, synthesis and sustainable applications of IL/MOF composites and provides some critical viewpoints on the present challenges and future development directions. Firstly, we briefly introduce the background of ILs, MOFs, and the combination of ILs and MOFs. Thereafter, we systematically discuss the design and methodology for the synthesis of IL/MOF composites. For each method, the basic chemical or operation principle and benefits/drawbacks are elaborated. Later, an overview of the sustainable applications of IL/MOF composites is provided by highlighting numerous recent representative studies reported in the literature, covering carbon capture and conversion, chemical catalysis, adsorptive removal of pollutants, and utilization as electrolytes and membranes in energy storage devices. Finally, research trends and insights into IL/MOF composites are outlined to extend the family of IL/MOF composites and their application scope.
Introduction
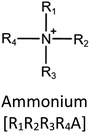
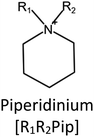
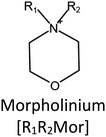
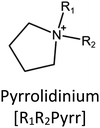
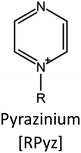
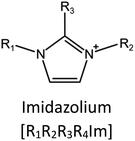
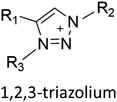
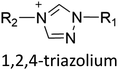
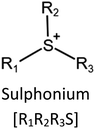
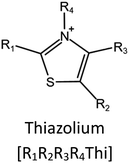
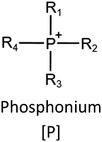
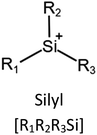
Ionic liquids (ILs) are a type of particular ionic compounds that are generally in the liquid state at near-room temperature (below 100 °C). Usually, ILs consist of asymmetric organic cations paired with inorganic or organic anions, which endow ILs with tunable physical and chemical properties. Table 1 lists the chemical structures of most common IL cations and IL anions. Owing to the large size of organic cations, many properties of ILs are dramatically different from those of traditional ionic compounds and organic solvents, such as a wide electrochemical window, good thermal stability, nonflammability, lack of measurable vapor pressure, adjustable acidity, and high gas solubility. As a consequence, ILs have been regarded as multifunctional green solvents and widely used in processes such as the synthesis of functional materials,1–3 catalysis,4–6 extraction,7–9 electrolysis,10–12 and gas separation.13,14 Despite these exciting prospects, ILs still suffer from several drawbacks. First, their intrinsic liquid state in most cases may cause difficulties in storage, packaging, and transportation. Second, other drawbacks such as high viscosity and low diffusion coefficients are not beneficial for purifying products, causing tedious handling procedures and difficulty in recycling.Immobilization of ILs onto a solid support (particularly porous one) is an effective strategy to circumvent these issues, which may cause improvement in mechanical integrity, ionic conductivity, and processibility. Among various porous materials, metal organic frameworks (MOFs), comprising metal ions/clusters and organic linkers linked through coordination bonds, are emerging as an innovative class of porous crystalline materials. To date, there are many types of MOFs reported in the literature, and Fig. 1 shows examples of most common MOFs and their corresponding metal nodes and linkers. The flexibility in the combination of metal nodes and organic linkers offers great space to adjust the properties of MOFs. Besides, MOFs are versatile precursors for preparing various structural/functional materials.15–19 Nowadays, IL/MOF composites stand the front of academic research, bringing many new insights. First of all, the intrinsic physicochemical properties of ILs present chances to adjust the number/characteristics of the available active sites in MOFs. Meanwhile, the introduction of IL is capable of inducing defects in MOFs, modulating the structure and properties of MOFs.20 Furthermore, by accommodating ILs, the pore nature of the parent MOF may be regulated, including size, shape, and acidity/basicity. The available pore space plays a significant role in gas separation/uptake and catalysis processes.21 At last, the IL loading level on MOFs also has significant impacts on the properties of IL/MOF composites. For example, at low IL loadings, the ions drift in the pores along the electric field. This case is beneficial for improving the conductivity of the composite. Nevertheless, it is not the same case for high IL loading. Owing to the collective field-induced interactions of the cations and anions, the ionic transport may be blocked. As a result, the ionic mobility is suppressed and the conductivity will be decreased.22
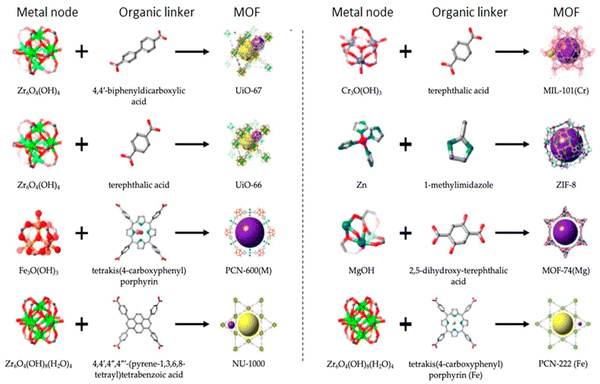 | ||
| Fig. 1 Examples of different MOFs with their corresponding metal nodes and linkers. Reproduced from ref. 23 with permission. Copyright 2023 American Chemical Society. | ||
To date, exciting research activities have emerged in IL/MOF composites, whose development has lasted for more than two decades, covering many areas (Fig. 2). The first IL/MOF composite refers to [Cu(I)(bpp)]BF4, which was synthesized by Jin et al. in 2002.24 From then on till 2010, only several groups were devoted to the synthesis of IL/MOF composites. In 2011, theory studies predicted the potential of IL/MOF composites as adsorbents for carbon capture.25 One year later, the capability of IL/MOF-supported membranes for CO2 capture were evidenced experimentally. It is from 2011 that the research of IL/MOF composite toward various practical applications became increasingly more, covering catalysis,26 separation,27–33 sensing,34,35 ionic conductivity,36,37 and phase change materials.38
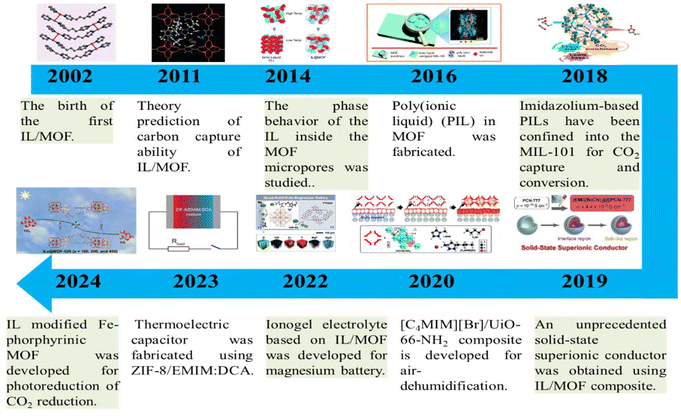 | ||
| Fig. 2 Timeline illustrating the key progresses in IL/MOF composites achieved to date. Reproduced from ref. 24 with permission. Copyright 2002 the Royal Society of Chemistry. Reproduced from ref. 25 with permission. Copyright 2011 American Chemical Society. Reproduced from ref. 39 with permission. Copyright 2014 Wiley-VCH. Reproduced from ref. 40 with permission. Copyright 2016 the Royal Society of Chemistry. Reproduced from ref. 41 with permission. Copyright 2018 American Chemical Society. Reproduced from ref. 42 with permission. Copyright 2019 Wiley-VCH. Reproduced from ref. 43 with permission. Copyright 2020 the Royal Society of Chemistry. Reproduced from ref. 44 with permission. Copyright 2022 Wiley-VCH. Reproduced from ref. 45 with permission. Copyright 2023 Wiley-VCH. Reproduced from ref. 46 with permission. Copyright 2024 American Chemical Society. | ||
There have been several excellent reviews on IL/MOF composites. Early in 2016, the first review on ILs supported on MOFs was provided by Fujie et al.,47 in which the plausible applications of IL/MOF composites, including gas absorption, catalysis, templates for synthesizing porous nanocarbons, and ionic conductors, were also summarized. One year later, Kinik et al.48 provided a comprehensive overview on the synthesis of IL/MOF composites and their emerging applications in catalysis, ionic conduction, gas separation/adsorption and liquid adsorption/separation. Li et al.49 supplied a comprehensive review on IL/MOF composites for adsorption application. Ali et al.23 also summarized the application of IL/MOF composites in CO2 uptake. Chen et al.50 established a primary database in CO2 capture and conversion by highlighting the recently emerging IL-MOFs. In viewpoint of ion mobility, Yoshida et al.51 gave a general discussion on interactions and the distribution of component ions of the ILs within the MOFs. Recently, Song et al.52 have provided a comprehensive overview on proton conductivity of IL/MOF composites and corresponding mixed matrix membranes for practical usability. Last but not least, some groups have demonstrated that a computational approach is a versatile supplement to experimental investigation, which may provide assistance in screening and simulate the interaction between ILs and MOFs for tailoring their properties to satisfy the desired application requirements.53,54
Considering the rapid development and lack of a systematic review, in this study, we present rational synthesis techniques for IL/MOF composites, which are categorized into wet impregnation, post chemical modification, ship-in-bottle and in situ synthesis. Furthermore, the possible applications of IL/MOF composites in CO2 capture, CO2 conversion, organic catalysis, liquid adsorption removal of pollutants, and energy storage and conversion will be discussed. Finally, it summarizes the outstanding problems and challenges in the field, and then discusses future directions to overcome these existing issues.
Synthesis methods
Wet impregnation process
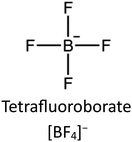
Wet impregnation is one of the easiest methods to prepare IL/MOF composites. Fig. 3a presents a typical procedure to synthesize IL/MOF composites, in which the pre-synthesized MOF is poured into a solution containing IL molecules. In order to ensure the complete contact between MOF and IL molecules, subsequent stirring or ultrasonic irradiation may be applied. Finally, a drying treatment may also be needed to remove the solvent.55 Owing to the ionic nature of IL molecules, many polar and nonpolar solvents are available. However, MOFs are usually sensitive to some solvents. For example, ethanol has a destructive effect on the structure of CuBTC MOFs. Sezginel et al.56 incorporated 1-butyl-3-methylimidazolium tetrafluoroborate ([BMIM][BF4]) into a CuBTC MOF, wherein they used acetone as the solvent. The resulting [BMIM][BF4]/CuBTC composite exhibits excellent gas separation performance, delivering ideal selectivity for CO2/CH4, CO2/N2, CO2/H2, CH4/N2, CH4/H2, and N2/H2 separation. Particularly, compared to pristine CuBTC, the [BMIM][BF4]/CuBTC composite delivers high selectivity for CO2. Kinik et al.57 prepared a [BMIM][PF6]/ZIF-8 composite via a similar process, which demonstrates excellent separation performance of CO2/CH4, CO2/N2, and CH4/N2. Considering formates coordinating with Zr, Jia et al.58 prepared an MOF-808-IL composite using ethanol as a reaction medium and utilized MOF-808 (Zr6O4(OH)4(BTC)2(HCOO)5(H2O)1(OH)1, BTC = benzenetricarboxylate) as the initial material and 1-(propyl-3-sulfonate)imidazolium hydrosulfate ([(CH2)3SO3H-HIM]HSO4) as the functional molecule. The resulting MOF-808-IL composite exhibits a high intrinsic proton conductivity of 1.28 × 10−1 S cm−1 at 60 °C and 100% relative humidity (RH). Sun et al.59 prepared an EIMS-HTFSA@MIL-100 composite by impregnating the binary IL, which demonstrates good proton conductivity. By making use of the IL characteristics, EIMS-HTFSA@MIL is a potential candidate for use as a safe electrolyte in proton conduction above 100 °C.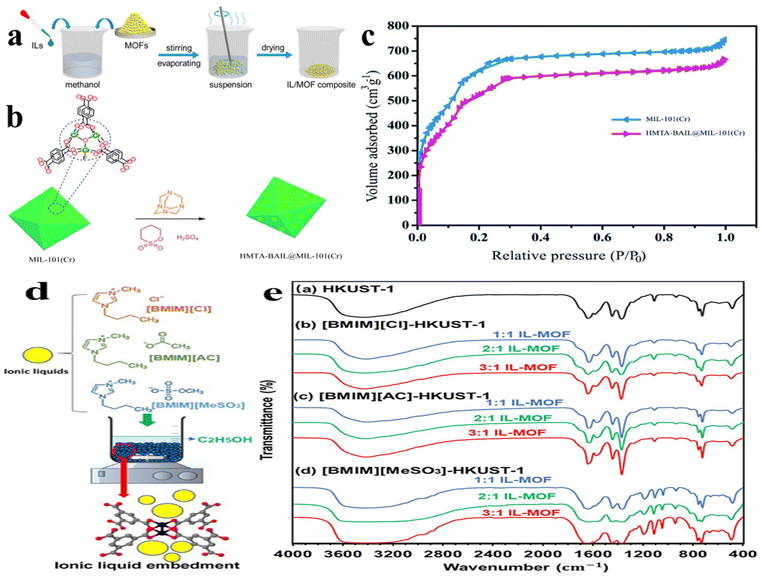 | ||
| Fig. 3 (a) Synthesis of the IL/MOF composite via a wet impregnation process. Reproduced from ref. 55 with permission. Copyright 2019 American Chemical Society. (b) Procedure for the synthesis of HMTA-BAIL@MIL-101(Cr). (c) N2 adsorption–desorption curves of HMTA-BAIL@MIL-101(Cr) and MIL-101(Cr). Reproduced from ref. 60 with permission. Copyright 2020, published by the Royal Society of Chemistry under a CC-BY license. (d) Schematic of the synthesis of the IL/HKUST-1 composite. (e) FTIR results of HKUST-1 based MOFs. Reproduced from ref. 63 with permission. Copyright 2024 American Chemical Society. | ||
Mirhosseini-Eshkevari et al.60 reported a wet impregnation strategy to graft a hexamethylenetetramine (HMTA)-based IL into MIL-101(Cr). As illustrated in Fig. 3b, the synthesis is operated in toluene. Owing to the grafting of HMTA-BAIL, the specific area of HMTA-BAIL@MIL-101(Cr) demonstrates a slight decline compared to MIL-101(Cr), as presented in Fig. 3c. Zou et al.61 reported the immobilization of Zn-containing imidazolium bromide IL onto MIL-101(Cr) by a similar wet impregnation process. The structural and formation mechanism characterization indicates that the formation is based on the coordination bonding between ILs and Cr3+. From this conclusion, it is easy to confer that the unsaturated metal sites may be necessary to prepare stable IL/MOF composites. Considering that the interaction between MOF and IL in a wet impregnation process mainly lies on the defects in MOFs. That is to say the unsaturated metal sites are responsible for affinity to IL molecules. In fact, the number of unsaturated metal sites is usually very limited. As a result, it may be better to introduce extra metal sites. Reminding this point, Chen et al.62 forwarded a phosphotungstic acid (HPW)-assisted wet impregnation approach to synthesize a –SO3H-containing IL immobilized on MIL-101(Cr). In their work, HPW was first impregnated into the cages of MIL-101(Cr), and then reacted with a SO3H-functionalized IL via anion exchange, generating SIL-PW/MIL-101(Cr). Han et al.63 proposed IL/HKUST-1 to boost water transfer for transforming waste heat into work. In their work, HKUST-1 should be treated with an acetone solvent followed by thermal activation to create coordinatively unsaturated sites (CUSs). By dispersing HKUST-1 with CUSs in ethanol, the addition of IL will lead to the formation of IL/HKUST-1 composites (Fig. 3d). Crystal structure analysis reveals that the embedment of ILs did not change the overall integrity of HKUST-1. Fig. 3e presents the FTIR profiles of HKUST-1 with different embedded IL contents. All samples share the similar infrared absorption peaks, reflecting the core functional groups of the structure.
Luo et al.64 reported a wet impregnation process to introduce amino-functionalized basic ILs (ABIL-OH) into HKUST-1 (Cu3(BTC)2, H3(BTC) = 1,3,5 benzenetricarboxylic acid). The process is schematically shown in Fig. 4a, which involves several steps. Initially, ABIL-OH IL is dissolved in ethanol, which is followed by adding the powder of HKUST-1. Afterwards, owing to the presence of CUSs, HKUST-1 will interact with ABIL-OH ions by their Lewis acidity. Then the excess ABIL-OH will be removed by filtration. Finally, ABIL-OH@HKUST-1 can be obtained by removing the solvents by drying under vacuum. Yin et al.65 also forwarded a direct wet impregnation process to prepare UiO-66-T3PILs, which represents type 3 porous liquid (Fig. 4b). The resulting UiO-66-T3PILs deliver high catalytic activity for oxidative desulfurization. Bunge et al.66 used ionic liquid based fiber welding to attach UiO-66-NH2 to cotton fibers through the similar process. The surface of the resulting IL/UiO-66-NH2 fibers can be adjusted by controlling the extent of the welding process. For optimal samples, it may reach approximately 50–100 m2 g−1. Furthermore, the ionic liquid/UiO-66-NH2 welding solution can be applied by directly placing the fabric in the welding solution or by utilizing an airbrushing technique.
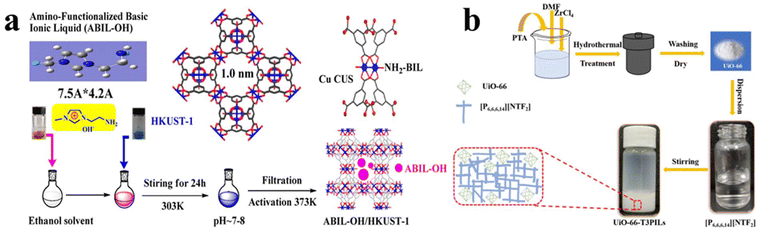 | ||
| Fig. 4 (a) Procedures of synthesizing ABIL-OH@HKUST-1 via wet impregnation. Reproduced from ref. 64 with permission. Copyright 2014 Elsevier. (b) Schematic illustration of the preparation of UiO-66-T3PILs. Reproduced from ref. 65 with permission. Copyright 2023 Elsevier. | ||
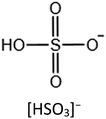
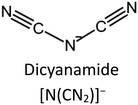
The stability of the IL/MOF composite is dramatically dependent on both the IL structure and the MOF structure. Zeeshan et al.67 investigated the stability of IL/MOF composites, in which they screened two different MOFs, CuBTC and ZIF-8, and 29 imidazolium-based ILs. Their result indicates that many structural differences in ILs, such as the length of the substitutional groups (determined by the number of carbon atoms in the alkyl chain in an imidazole ring), methylation on the C2 site, and the nature of composition ions in ILs, are verified to affect the thermal stability limits of the composites, and the corresponding results are presented in Fig. 5. Specifically, the imidazole rings with a long alkyl chain are demonstrated to be beneficial for enhancing the temperature tolerance of IL/MOF composites. Similarly, the substitution alkyl groups in the imidazole ring also produce the same effect; the larger the substitution group, the better the temperature tolerance of the IL/MOF composites. The effect of the chain length and the substitution groups can be ascribed to the increase in molecular weight. The higher the molecular weight, the more excellent the thermal stability. As for anion effects, it is demonstrated that the anions containing fluorine are more stable than others. Such result can be ascribed to their thermal stability limits caused by the presence of fluorine. Furthermore, compared to their bulk counterparts, those CuBTC samples incorporated with dicyanamide, acetate, and phosphate anion also delivered an increase in their maximum tolerable temperatures. These conclusions can be verified by thermal analysis and DFT calculations. In other studies reported in the literature, spectroscopy methods such as FTIR and NMR are also versatile techniques to uncover the interaction between ILs and MOFs, providing indirect evidence to the stability of IL/MOF composites.68
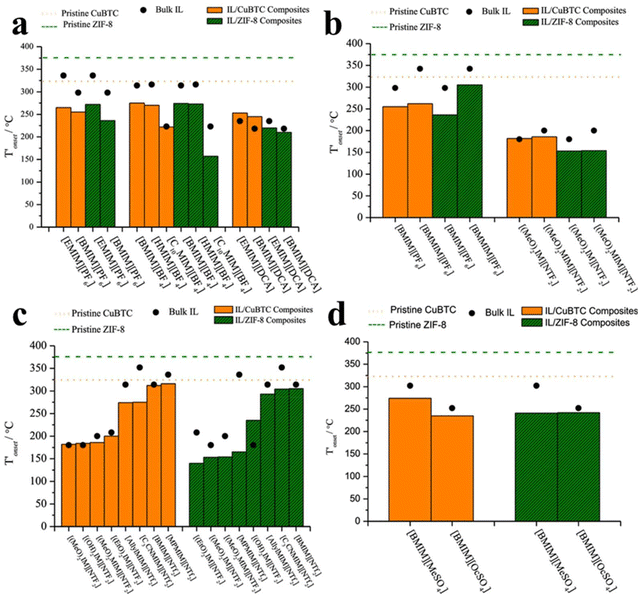 | ||
| Fig. 5 Factors for the stability of various IL/MOF composites. (a) Length of the alkyl chain in the imidazolium ring. (b) Methylation of the C2 position in the imidazolium ring. (c) Changes in the functional groups of the substitutional alkyl groups in the imidazolium ring. (d) Size of anions in ILs. Reproduced from ref. 67 with permission. Copyright 2019 American Chemical Society. | ||
The wet impregnation method is widely used for constructing IL/MOF composites. In most cases, no chemical bonds are formed between ILs and MOFs when wet impregnation process is applied to synthesize IL/MOF composites. As a result, the distribution of IL molecules in the composite may be difficult to control. The IL molecules either enter into the pore channel of MOFs or cover the surface of MOFs, which is dependent on the suitability of IL molecule's size to the pore size. Meanwhile, the hydrophobic properties of the (pore channel) surface are also critical to accommodate IL molecules. A drawback common to wet impregnation processes lies in consuming a solvent or a relatively large volume of the precursor or ILs. Furthermore, after incorporation into the pores of MOFs, IL cations may lose their bulk properties. Meanwhile, the specific area and pore volume of MOFs will certainly decrease, which may not be beneficial for some applications. In addition, it is difficult to load high amounts of ILs onto MOFs.
Post chemical modification
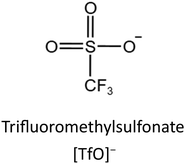
In order to anchor IL into MOFs firmly, it is necessary to introduce some functional group into MOFs, which may react with IL molecules. For example, Zhang et al.69 selected UiO-66-NH2 as a carrier. By reacting with 3-amino-1,2,4-triazole using the HCHO molecule as a bridging agent, UiO-66-NH2-AT can be obtained. Afterwards, ionic exchanges are carried out between UiO-66-NH2 or UiO-66-NH2-AT and CF3SO3H, and the IL grafted solid acids can be generated, designated as UiO-66-NH2-[SO3CF3] and UiO-66-NH2-AT-[SO3CF3], respectively (Fig. 6a). Sadjadi et al.70 forwarded a post modification process to synthesise bicationic IL/MIL-101(Fe) (IL-MIL-101(Fe)). As illustrated in Fig. 6b, their synthesis process started from the hydrothermal formation of NH2-MIL-101(Fe), which is followed by sequentially reacting with 2,4,6-trichloro-1,3,5-triazine (TCT) and 1,4-diazabicyclo[2.2.2]octane (DABCO) to form ILs on metal–organic frameworks. The coordinately unsaturated metal sites in MOFs exhibit Lewis acidity, and it is necessary to maintain such sites when modifying MOFs. Taking this key point in mind, using the similar post modification approach, Xiang et al.71 prepared a ZIF-supported IL (IL-ZIF-8), in which the IL is grafted onto a dual-ligand ZIF. As presented in Fig. 6c, the ZIF-8 is modified by aldehyde before introducing IL. Owing to the introduction of an aldehyde group, it can react with amine groups in the IL molecules. In this way, coordinately unsaturated Zn may act as a Lewis acid site to promote the catalytic performance in some cases.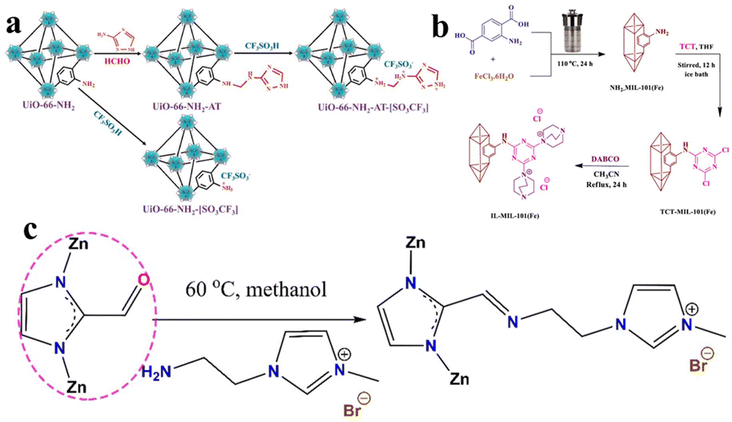 | ||
| Fig. 6 (a) Synthetic routes for UiO-66-NH2-[SO3CF3] and UiO-66-NH2-AT-[SO3CF3]; CF3SO3− denotes the provided anion. Reproduced from ref. 69 with permission. Copyright 2024 Elsevier. (b) Schematic of the synthesis of IL-MIL-101(Fe) via a post modification process. Reproduced from ref. 70 with permission. Copyright 2022 Springer. (c) Post-synthetic modification of ZIF-8(x) with 1-aminoethyl-3-methylimidazolium bromide. Reproduced from ref. 71 with permission. Copyright 2021 Elsevier. | ||
Luo et al.72 developed an organic chemical bridging process to incorporate Brønsted acidic ionic liquid (BAIL) inside MIL-101 nanocages. Their process is based on the tandem post-synthetic strategy (Fig. 7a). The resulting BAIL/MIL-101 material combines the merits of acid IL catalyst and MIL-101 nanocage confinement effects, exhibiting catalytic performance for the acetalization of benzaldehyde with glycol. In another work, Han et al.73 also modified MIL-101(Cr) with MBIAILs (MIL-101(Cr)@MBIAILs). As shown in Fig. 7b, their synthesis relies on the S–Cr coordination bond since 2-mercaptobenzimidazole ILs with electron-rich single bond –SH groups can be immobilized onto the surface of the well-defined MIL-101(Cr) frameworks. Tharun et al.74 also grafted a pyridinium-based IL onto ZIF-90 by the covalent post functionalization, generating IL-supported ZIF-90 (IL-ZIF-90).
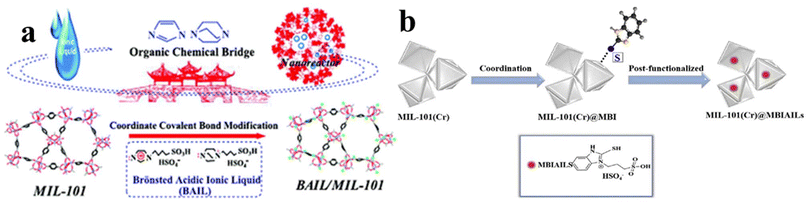 | ||
| Fig. 7 (a) Organic chemical bridging construction of the BAIL/MIL-101 composite. Reproduced from ref. 72 with permission. Copyright 2013 the Royal Society of Chemistry. (b) Schematic of the synthesis of MIL-101(Cr)@MBIAILs. Reproduced from ref. 73 with permission. Copyright 2018 Elsevier. | ||
The post modification process to synthesize IL/MOF composites is based on the reaction of ILs (precursor) with groups in MOFs. Different from wet impregnation, the interaction between ILs and parent MOFs is covalent bond instead of π⋯metal interaction. As a result, IL/MOF composites prepared by this approach can maintain the coordinately unsaturated metal sites, which are desired for the construction of multi-functional catalysts. This method is very attractive for the cases that the size of ILs is larger than that of the aperture of MOFs. Compared to the wet impregnation approach, post modification methods fix IL firmly in the cavity of MOFs. Meanwhile, owing to the dispersive characteristic of groups in MOFs, the ILs are difficult to aggregate in the final composite. The drawback of the post modification approach may be the tedious operation steps.
Ship-in-bottle approach
A “Ship-in-bottle” approach used for encapsulating guest species into MOFs has been well established in the past few decades. In this method, MOFs should be prepared (as “bottles”) first and then the adsorbed precursors should be introduced into them to form guest species (“ships”). To date, many guest species have been successfully incorporated inside MOFs by this method, such as metal nanocrystals75 and polymers.76 Mortazavi et al.77 entrapped hexamethylenetetramine (HMTA) and 1,3-propane sultone-derived –SO3H-functionalized ILs inside the pores of MIL-100(Fe). As presented in Fig. 8, HMTA molecules initially diffused into the nanocages of MIL-100(Fe), where MIL-100(Fe) serves as a boat. This process can be assured by the size characteristic of the MIL-100(Fe) windows (diameters of 5.5 and 8.6 Å for pentagonal and hexagonal windows, respectively). In order to accommodate more HMTA molecules in the nanocages, MIL-100(Fe) should be first subject to a heat treatment to remove any residual solvent in the nanocages. Once the concentration of HMTA inside the nanocages is higher enough, the formation of multi –SO3H-functionalized ILs may be possible, which is based on the nucleophilic reaction between 1,3-propane sultone and HMTA inside the cavities.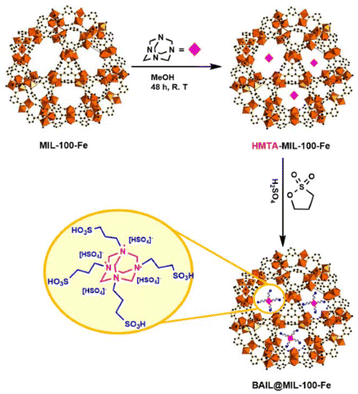 | ||
| Fig. 8 Ship-in-bottle approach for the synthesis of the BAIL@ MIL-100(Fe) composite. Reproduced from ref. 77 with permission. Copyright 2021 Wily-VCH. | ||
A vapor-phase process demonstrates the advantages of thoroughly reacting among reactants, involving no solvent, and high compatibility with other workflows.78 By choosing volatile precursors, the high loading of guest species into MOF can be simply achieved, which has been experimentally evidenced by anthracene as well as some organometallic compounds.79 Unfortunately, it is difficult to directly load IL into MOFs since ILs usually have a very low vapor pressure (<10−3 mbar at moderate temperatures). Taking these viewpoints in mind, Obst et al.80 proposed a vapor-phase approach to synthesize IL/ZIF-8 composites, wherein they turn to IL precursors. As illustrated in Fig. 9a and b, this process is based on the vaporability of IL precursors and available in a Schlenk flask setup. In operation, the MOF is placed between two precursors of ILs to avoid forming ILs outside of the MOF pores. In their work, the effect of ZIF-8 crystal size on the IL molecule loading was investigated. As presented in Fig. 9c, a small crystal is beneficial for high loading of IL molecules. This result can be ascribed to the high surface area. In their work, ZIF-8 with two different crystal sizes were applied, as shown in Fig. 9d and e. Jiang et al.81 reported the incorporation of dicationic IL 1,1′-(hexane-1,6-diyl)-bis(3-methylimidazolium)dibromide inside MIL-101 via a ship-in-bottle technique (Fig. 9f). In their work, 1,6-dibromohexane and N-methylimidazole were used as dicationin IL precursors reacting inside MIL-101. Different from Obst et al., these precursors are input in liquid state. N-Methylimidazole itself is liquid, whereas 1,6-dibromohexane is dissolved in methanol. Following a similar principle, Khan et al.82 also prepared a monocationic imidazolium IL inside MIL-101, which acts as a stable adsorbent to eliminate benzothiophene from the liquid fuel.
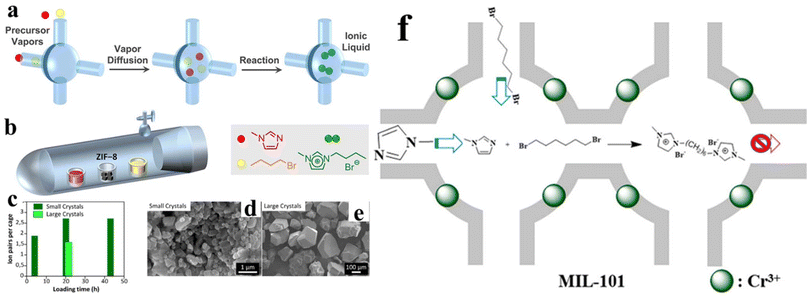 | ||
| Fig. 9 (a) Schematic of the principles of the vapor-phase ship-in-bottle approach to load IL into a MOF pore. (b) Experimental setup used for preparing IL/MOF composites. The two precursors of IL are separated by ZIF-8 to avoid forming ILs outside of the ZIF-8 pores. The influence of the ZIF-8 crystal size on IL loading. (c) Loading time-dependent IL pair number obtained per cage of ZIF-8. SEM images of small (d) and large ZIF-8 crystals (e) used in VSIB experiments. Reproduced from ref. 80 with permission. Copyright 2022 American Chemical Society. (f) Schematic of the fabrication of Mim-6@MIL-101 via ship-in-bottle technique. Reproduced from ref. 81 with permission. Copyright 2021 American Chemical Society. | ||
The difference between wet impregnation and ship-in-bottle approaches is obvious. The former involves no chemical reactions since both MOF and IL are pre-synthesized. The latter involves a chemical reaction for the formation of ILs inside the pores of MOFs. The merits of ship-in-bottle process include high loading of IL molecules and environment-friendliness (since no solvent is needed). However, the sizes of MOF cavity and IL precursor molecules should be well matched to ensure the formation of ILs inside the pore. Furthermore, the wettability among the pore wall and IL precursor molecules should also be seriously considered.
In situ synthesis
In situ synthesis is a well-known approach to prepare an IL/MOF composite, which is based on the reacting precursors (metal ions/clusters and organic linkers) of MOFs in IL solvents or a solution containing ILs. It is well known that many freshly prepared MOFs usually contain solvents either on the surface or inside the pores of MOFs. Even in some cases, the solvent may coordinate with metal centers, becoming building blocks in the MOF structure. Inspired by this phenomenon, by using ILs as the solvent to synthesize MOFs, it is possible to embed IL molecules into the structure of MOFs. For example, Zhang et al.83 forwarded an in situ self-assembly method to prepare MIL-101-IL (Cr) composites. As illustrated in Fig. 10a, the self-assembly process is carried out in an aromatic-anion-functionalized ILs, which act as both reaction media and competitive ligands to modulate the structure of the final product. Structural analysis indicates that the presence of an aromatic moiety allows IL molecules to coordinate with Metal ions (Cr3+). As a result, the cavity of MIL-101-Phe (5–8 nm, characteristic of mesopores) is much larger than that of MIL-101 (1.9 nm, characteristic of micropores). The specific surface area of MIL-101-Phe reaches 441.9–624.9 cm2 g−1. By varying the aromatic moiety, the distribution of electrons for ILs may be changed, as presented in Fig. 10b. Specially, an increase in electronegativity is beneficial to enhance the ability of an IL to coordinate with metal ions by donating electrons. By analyzing the electronic distribution, the bonding ability to metal ions can be expected, which in turn can predict the portion of the initial ligand and IL ligand in the IL/MOF system.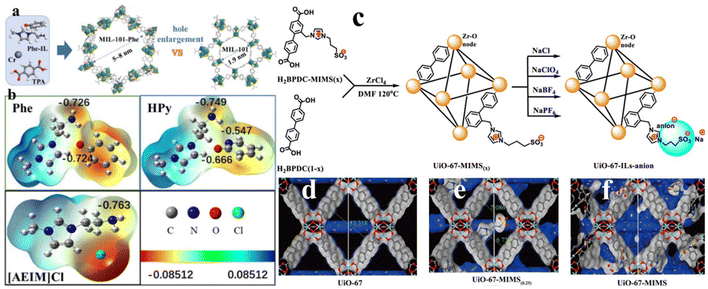 | ||
| Fig. 10 (a) Synthesis of MIL-101-Phe as well as its structural comparison with MIL-101. (b) Charge density distribution of Phe-IL, HPy-IL, and [AEIM]Cl. Reproduced from ref. 83 with permission. Copyright 2023 American Chemical Society. (c) Schematic of the synthesis UiO-67-MIMS(x) and UiO-67-IL-anion (anion = ClO4−, Cl−, BF4−, and PF6−). Molecular models of (d) UiO-67, (e) UiO-67-MIMS(0.25), and (f) UiO-67-MIMS. Reproduced from ref. 84 with permission. Copyright 2020 American Chemical Society. | ||
In another work, Xue et al.84 forwarded a strategy to synthesize UiO-67-IL-anion composites, which contains a binary IL moiety. As presented in Fig. 10c, a reticular synthesis method is involved in their process to introduce the zwitterionic group 1-(1-mthyl-3-imidazolio)propane-3-sulfonate (MIMS) into the cages of UiO-67, where two mixed linkers, namely H2BPDC (4,4′-biphenyl-dicarboxylate acid) and H2BPDC-MIMS, are used. By changing the ratio of two ligands, the pore size can be adjusted, which will be further used to accommodate sodium salts (anion = ClO4−, Cl−, BF4−, and PF6−). Due to the linker containing the pendant MIMS, the atom distances between MIMS and the benzene rings of the nearest linkers would lie within the 6.5–7.0 Å range in UiO-67-MIMS(0.25), as shown in Fig. 10d–f. Following a similar principle, Peng et al.85 prepared various acid IL-grafted UiO-66 composites from the quaternization of UiO-66 with 1,3-propane sultone, which is followed by ion-exchanging with HSO3CF3 or H2SO4. Benefiting from the super-acidity of H2SO4 or HSO3CF3, the resulting IL-grafted UiO-66 composites exhibit catalytic activity toward biodiesel production toward transesterification.
Compared to the other methods, the largest merit of in situ synthesis may be its advantages in operation since it integrates the MOF formation and compositing with ILs in one system. In in situ synthesis, ILs serve at least two roles in the formation of IL/MOF composites. On the one hand, ILs act as solvents providing reaction media. On the other hand, ILs possessing surfactant characteristics may act as structure-directing agents to control the morphology/size of the final products. In most cases, the in situ formed MOFs are generally negative in charge, assuring IL cations entering to link with the MOF structure and provide electrical neutrality. The driven force for the ILs bonding with MOFs can be ascribed to either host–guest interactions or covalent bonding. As for drawbacks, the IL and MOF precursors should be highly matched in the in situ synthesis, which constitutes main limits in extending this method as a general technique. Fortunately, there have been a few reports on synthesizing IL/MOF composites by this approach to date. Bearing it in mind that in situ synthesis generates IL/MOF composites with benign structural stability and exceptional functionalities, it is believed that the prospect of this method in designing novel IL/MOF composites will be promising.
Application
CO2 capture
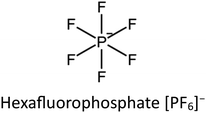
Owing to the huge consumption of fossil fuels, greenhouse gases, especially CO2, are still on the rise. As a result, CO2 capture and storage become one of the most direct and effective way to alleviate CO2 emissions.86 As an emerging functional material, IL/MOF composites deliver great promise in gas separation.87 When solely used, the former usually suffers from the nature of liquid states, which limits transportation and operation, whereas the latter has the drawback of low diffusion kinetics. In order to leverage their combined advantages, increasing attention has been paid to designing novel systems comprising an IL immobilized onto a porous structure, either on the surface or inside the pores. Obviously, the pores of MOFs not only confine ILs but also provide paths for CO2 diffusion/migration. The presence of functional ILs is expected to selectively bond to CO2, thus ensuring efficient capture of CO2.88 In many cases, the MOF is expected to offer confinement effects, whereas ILs in the composite exhibit an ordered structure as observed from radial distribution functions. Early in 2011, computational simulation has predicted the potential of IL/MOF composites in CO2 capture. As presented in Fig. 11a, the cations of ILs are usually confined in the cavity of MOFs. Owing to the confinement effect, [PF6]− anions of IL molecules are observed to preferentially locate in the metal cluster corner of IRMOF-1, resulting in a poor mobility of ILs in the composite. Benefiting from the strong interaction between CO2 and ions, the number of CO2 molecules adsorbed in the composite is much larger than that of N2. Meanwhile, the CO2 molecules are proximal to the ions, as presented in Fig. 11b.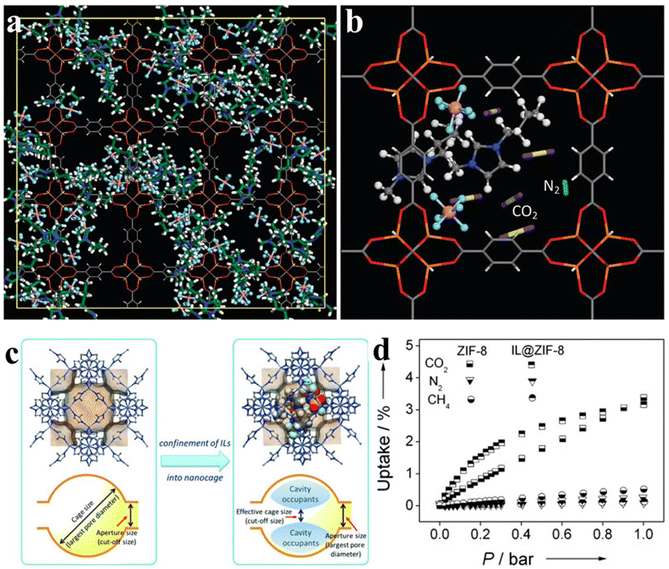 | ||
| Fig. 11 (a) [BMIM][PF6]/IRMOF-1 composite at a weight ratio WIL/IRMOF-1 = 0.4. N, blue; C in [BMIM]+, green; P, pink; F, cyan; Zn, orange; O, red; C in IRMOF-1, gray; H, white. (b) Simulation snapshot of CO2/N2 mixture (Ptotal = 1000 kPa) in IL/IRMOF-1 at WIL/IRMOF-1 = 0.4. Reproduced from ref. 25 with permission. Copyright 2011 American Chemical Society. (c) Illustration of the cavity-occupying concept for tailoring the molecular sieving properties of ZIF-8 by the incorporation of ILs. (d) Adsorption isotherms of CO2, N2 and CH4 on ZIF-8 and IL@ZIF-8 at 298 K. Reproduced from ref. 89 with permission. Copyright 2015 Wiley-VCH. | ||
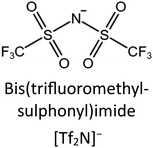
Ban et al.89 forwarded a cavity-occupying concept, which is capable of tailoring the properties of porous materials. As a state-of-the-art strategy, they filled IL molecules into the cavity of MOF. As presented in Fig. 11c, the anion [Tf2N](−) in ([bmim][Tf2N]) is favorable for CO2 adsorption, whereas the cation [bmim](+), owing to the bulky size, will efficiently occupy the cavity of ZIF-8. The reason for choosing ZIF-8 as the host MOF is its cage-type microporous structure as well as its high stability. After incorporating ILs, an interconnected network will be formed by linking the large SOD cages (1.12 nm) through small apertures (0.34 nm). It is well known that the size of CO2 molecules is 0.33 nm, matching the small apertures. As a result, the interconnected network is capable of sieving CO2. Nevertheless, those gas molecules whose sizes are larger than that of the cages, such as N2 (0.364 nm) and CH4 (0.38 nm), are expected to be excluded, leading to a high selectivity for capturing CO2.
A critical issue, the blocking of channels by loaded AAILs into MOFs, may occur, though the IL/MOF composites in most cases maintain the features of parent MOFs in topological shape as well as porosity (including the size and distribution). As revealed by both experimental and theoretical investigation, such channel blocking is not beneficial for CO2 uptake. The reduction of CO2 uptake performance can be ascribed to the filling pore effect of ILs in MOFs, which causes a decrease in pore volume and active sites. Take the composites consisting of amino acid ILs (AAILs) and MOFs as example. As shown in Fig. 12, complicated blockage may occur when mixing AAILs and MOFs in different ratios. Generally, a larger number of IL molecules in the composite lead to a higher blockage effect, resulting in a lower CO2 uptake amount.
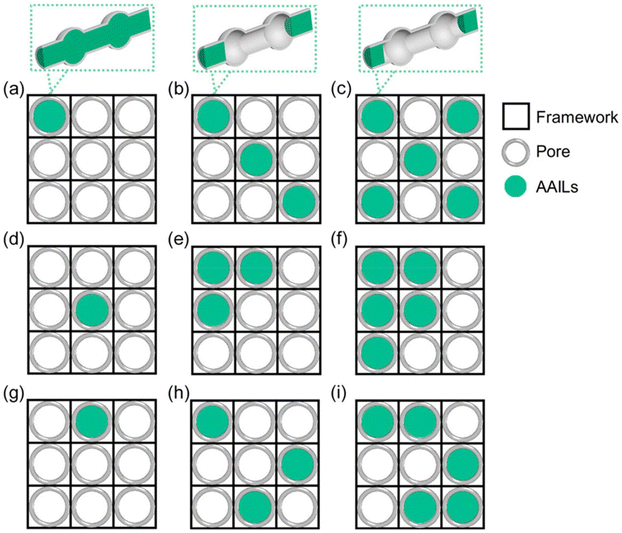 | ||
| Fig. 12 Plausible pore blocking caused by filling AAIL into MOFs with same AAIL loading amount: (a, d, and g) a single channel blocked, (b, e, and h) three channels blocked, and (c, f, and i) five channels blocked by ILs with varying distributions in supercell of MOFs. Reproduced from ref. 55 with permission. Copyright 2019 American Chemical Society. | ||
ZIF is one of the most commonly investigated MOFs, which has a framework of fused [4668] sodalite cages (Fig. 13a). The void volume of ZIF-8 is calculated to be 2.500 Å3 per unit cell.90–92 Such high volume ensures its high capacity to accommodate guest molecules. It has been demonstrated that guest molecules are capable of fully occupying the pores of ZIF-8. Such advantageous characteristic, in turn, constitutes poor selectivity for gas uptake. Koyuturk et al.93 reported that the gas separation of ZIF-8 can be enhanced by incorporating [BMIM][BF4]. Their result indicates that the morphology of ZIF-8 remains intact upon [BMIM][BF4] incorporation up to 28 wt%, at which the selectivity in gas capture is dramatically improved at 0.1 bar (CO2/CH4 selectivity from 2.2 to 4.0; CO2/N2 selectivity from 6.5 to 13.3, and CH4/N2 selectivity from 3.0 to 3.4). Despite the blocking effect of IL molecules on the pore of parent MOFs, Zeeshan et al.94 realized that ZIF-8 shows no capacity to CO2 at low pressures, which presents a good basis for demonstrating its utility as the porous substrate for providing a large surface area for the reactive IL. Taking this point in mind, they incorporated CO2-reactive IL, 1-ethyl-3-methylimidazolium 2-cyanopyrolide ([EMIM][2-CNpyr]), into ZIF-8. As revealed by Fig. 13b, the resulting IL/ZIF composites produce an increasing trend in CO2 uptake with the number of IL molecules. Interestingly, this IL/ZIF composite demonstrates excellent selectivity in CO2 uptake (0.5 mmol g−1 at 30 °C), which is experimentally verified by dynamic breakthrough measurements with synthetic air (500 ppm of CO2). The captured CO2 can further rapidly release (2–4 min) by dielectric heating at 60 °C, presenting opportunities for recycling use. Casado-Coterillo et al.95 developed a mixed-matrix membrane consisting of [emim][Ac] (5% wt%), chitosan, and ZIF-8 or HKUST-1, which showed great potential of developing high-temperature waterproof membranes for CO2 separation. For both MOF-based mixed-matrix membranes, 10 wt% ZIF-8 and 5 wt% HKUST-1 deliver the best CO2 permeability and CO2/N2 selectivity performance. Ferreira et al.96 systematically investigated the effect of cation/anion in ILs on the IL@ZIF-8 performance of the sorption capacity and ideal CO2/CH4 selectivity. Habib et al.97 incorporated an ionic liquid (IL) (1-methyl-1-propyl pyrrolidinium dicyanamide, [MPPyr][DCA]) into MIL-101(Cr), which offers remarkably high CO2/N2 and CH4/N2 selectivity.
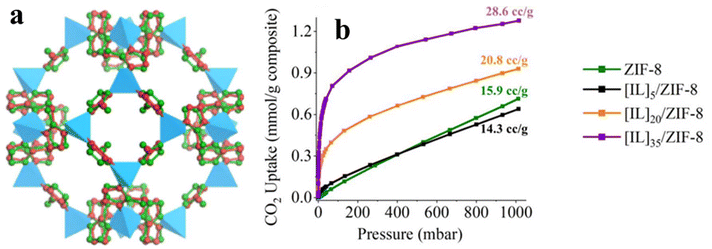 | ||
| Fig. 13 (a) Overlay of sodalite cages in conventional ZIF-8. Reproduced from ref. 91 with permission. Copyright 2019 American Chemical Society. (b) Isothermal curves of CO2 adsorption on various IL/ZIF-8 adsorbents. Reproduced from ref. 94 with permission. Copyright 2024 American Chemical Society. | ||
Zeeshan et al.98 also prepared a core–shell structured IL/ZIF-8 composite. Such core–shell structure is obtained by depositing the IL molecules on the external surface of the MOF. The resulting IL/ZIF-8 composite shows approximately 5.7 times higher CO2 uptake and 45 times higher CO2/CH4 selectivity at 1 mbar and 25 °C than those of the parent MOF (Fig. 14a–c). Mechanism investigation reveals that the IL acts as a smart gate for the guest molecules. Buddin et al.99 fabricated a polyethersulfone/poly(dimethylsiloxane)/ZIF-L (PES/PDMS/ZIF-L) composite membrane, noted as SILM. Fig. 14d illustrates the fabrication process of SILM, where ZIF-L and [BMIM][BF4] are chosen as MOF and IL candidates, respectively. Benefiting from the good flexibility of ZIF, the resulting SILM shows excellent response to temperature. Specifically, thermal treatment leads to the activation of the SILM to expose the IL to the external environment, whereas the IL will be entrapped into ZIF-L frameworks upon cooling. Such thermal responsive property effectively prevents the loss of ILs from SILM. The high aspect ratio of ZIF-L constitutes a highly tortuous path allowing for fast permissions of the gas molecules. As a result, this SILM shows high affinity toward CO2 and excellent recycling performance (Fig. 14e and f).
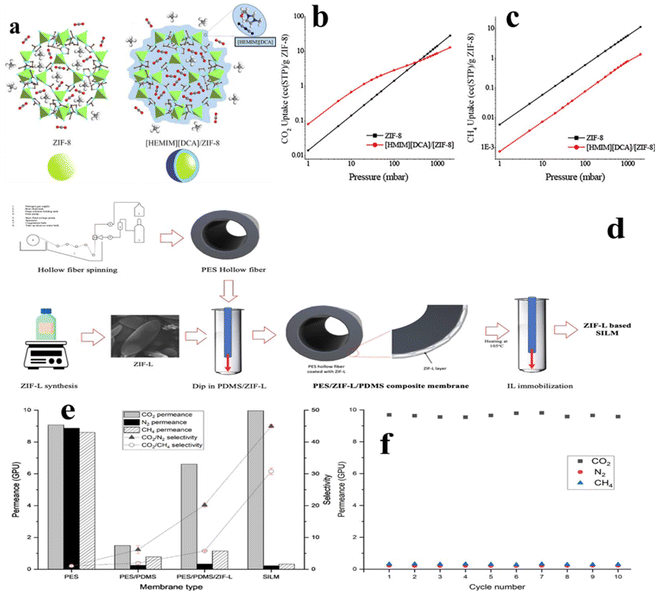 | ||
| Fig. 14 (a) Schematic of the core–shell-type IL/MOF structure. (b) CO2 and (c) CH4 adsorption isotherms of ZIF-8 and [HEMIM][DCA]/ZIF-8 at room temperature. Reproduced from ref. 98 with permission. Copyright 2018 published by American Chemical Society under a CC-BY license. (d) Fabrication process of SILMs. (e) Separation performance of pristine, PES/PDMS, optimized PES/PDMS/ZIF-L, and SILM for CO2/N2 and CO2/CH4 gas pairs. (f) SILM long-term performance test. Reproduced from ref. 99 with permission. Copyright 2023 American Chemical Society. | ||
Yang et al.100 developed a mixed matrix membrane by covalent grafting of poly(ionic liquid) (PIL) brushes on the surface of UiO-66-(OH)2, wherein KH560 is used as coupling agent (Fig. 15a). Owing to the hydrophilicity of PIL and the formed hydrogen bonds between the MOF fillers and Pebax matrix, the grafting of PIL brushes results in an improvement in the dispersity of as-synthesized UiO-66-(OH)2@PIL in the solvent media and Pebax matrix. When used for CO2 uptake, both the selectivity and permeability for CO2/N2 separation are simultaneously increased by the obtained UiO-66-(OH)2@PIL. As shown in Fig. 15b and c, the CO2 permeability in UiO-66-(OH)2 was 128 Barrer and the CO2/N2 selectivity was 59 with the addition of 5 wt% of UiO-66-(OH)2. For UiO-66-(OH)2@PIL, the optimal selectivity/permeability for CO2/N2 separation occurs at 20 wt% MOF loading (Fig. 15c). In another work, Ma et al.101 fabricated a type 3 porous liquid via a wet impregnation process, wherein UiO-66-NH2 and a specific hydrophilic ionic liquid (IL, [HEMIM][DCA]) are used as porous materials and the liquid host. The obtained UIO-66-PL is evidenced to be a kind of stable porous liquid possessing a core–shell structure with well-maintained permanent porosity and liquid-like behavior, as illustrated in Fig. 15d. Owing to the introduction of ILs into UIO-66-NH2, the zeta potentials of UiO-66-NH2 demonstrate a dramatically positive shift, as displayed in Fig. 15e. Furthermore, a significant improvement in hydrophilicity can also be obtained for UiO-66-NH2, which is confirmed by the contact angles (Fig. 15f). When evaluated as an adsorbent for CO2 capture, this UIO-66-PL delivers favorable performance. As presented in Fig. 15g, UIO-66-PL always uptakes more CO2 than IL throughout the gravimetric CO2 absorption–desorption isotherms, and the CO2 absorption capacity of UIO-66-PL is about 5 times that of IL at 1 bar, i.e., 0.72 wt% and 0.14 wt%, respectively. Such excellent performance can be ascribed to the increased CO2 solubility in UIO-66-PL since the well-maintained permanent porosity is capable of providing free space to adsorb and store gas. Additionally, ILs are expected to act as a selective gate for target gas to the permanent porosity within UIO-66-PL.
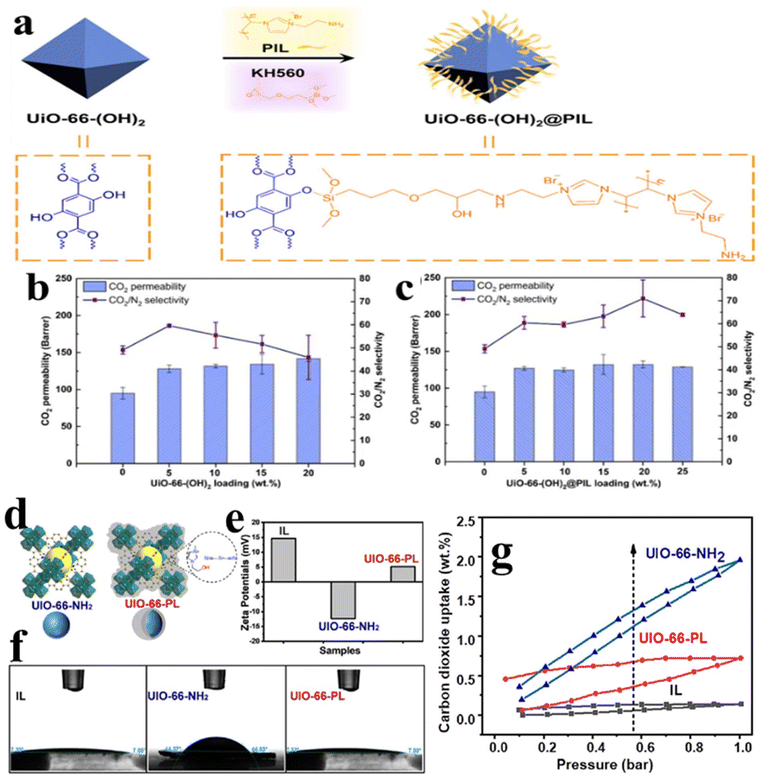 | ||
| Fig. 15 (a) Schematic illustration of the preparation of UiO-66-(OH)2@PIL. CO2/N2 separation performance of Pebax-based MMMs with different loadings of (b) UiO-66-(OH)2 and (c) UiO-66-(OH)2@PIL. Reproduced from ref. 100 with permission. Copyright 2022 American Chemical Society. (d) Schematic diagram of the core–shell structure of UIO-66-PL, (e) zeta potentials, (f) contact angles of IL, UIO-66-NH2 and UIO-66-PL, (g) Gravimetric CO2 absorption–desorption isotherms of UIO-66-NH2, IL and UIO-66-PL. Reproduced from ref. 101 with permission. Copyright 2023 Elsevier. | ||
In some cases, CO2 along with other acidic gases (e.g. H2S) co-exists with natural gas, hindering the exploitation and utilization of natural gas. As a result, it is necessary to develop effective separation techniques to remove the disturbing acid impurities.102,103 Bearing this task in mind, Gao et al.104 systematically investigated the adsorption and separation of CO2/H2S/CH4 on a series of IL/MOF-108 composites by theoretical methods. The results indicate that the incorporation of ILs into MOF-108 do not destroy or severely distort the structure of MOF-108. Owing to the strong affinity between the IL/MOF composite and CO2 molecule, the CO2 capture ability demonstrates significant boosting accompanied by weakening the undesired methane adsorption. Among various examined adsorbents, the choline-based amino acid IL/MOF composites display excellent separation performance, which can be ascribed to the intrinsic polarizable nature of ILs. Moreover, the combination of the IL and MOF-108 produces suitable annular space, which is also demonstrated to be beneficial for CO2 capture. The DFT analysis suggests that ILs can act as new adsorption sites for H2S and CO2. The IL in MOF-108 greatly boosts the framework attraction to H2S, thus making H2S more competitive for the adsorption sites of the IL/MOF frameworks than CO2. Finally, the results show that 15 wt% [Chl][Ala]/MOF composite exhibits excellent (H2S and CO2)/CH4 selectivity of about 735% higher than that of the original MOF (Fig. 16).
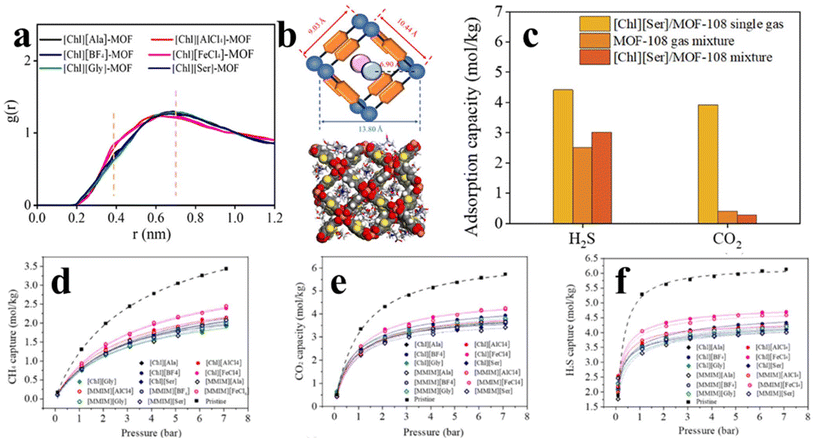 | ||
| Fig. 16 (a) RDF between ILs and MOF-108. (b) Schematic diagram of the IL arrangement in MOF-108, and 10 wt% [Chl][Ser]/MOF-108 composite. (c) Adsorption performance of MOF-108 and [Chl][Ser]/MOF-108. (d–f) H2S, CO2, and CH4 adsorption isotherms of the IL/MOF-108 composites with 10 wt% MOF loading. Reproduced from ref. 104 with permission. Copyright 2022 American Chemical Society. | ||
In this section, we discuss the CO2 capture performances of representative IL-MOF composites. Besides the cases discussed above in detail, there have been a large of reports on ILMOF composites for CO2 capture (Table 2), which may be beneficial to offer a database about IL-MOF composites for CO2 capture from different systems. Compared to pristine MOFs, the IL/MOF composite adsorbents are expected to provide new absorption sites. In addition to the flexibility of choosing ILs, the hydrophilic properties of the resulting IL/MOF composites can be adjusted, resulting in selective capture of CO2. Furthermore, the coverage of ILs onto MOFs provides a protective shell to improve the stability of MOFs. Instead of completely incorporating ILs into the porous structure of MOFs, target molecules are partially capable of entering the MOF porous structure channel at the interface, thus preserving inner permanent porosity and imparting liquid-like behavior to the resulting porous liquid MOFs. Compared to conventional gas capture materials (such as metal oxide, zeolite), IL/MOF composites have emerged as promising candidates for CO2 capture and storage. The advantages of such adsorbents include low recovery temperatures (25 to 100 °C), low regeneration energy, good cyclability, and growing technology. The disadvantage of IL/MOF composites lies in their high production cost and water/solvent waste generation.123
| Ionic liquid | MOF | CO2 capture performance | Ref. |
|---|---|---|---|
| [BMIM][BF4] | CuBTC | Unexpected improvements are obtained in gas separation through incorporation of ILs into MOFs | 56 |
| [EMIM+][SCN−], [EMIM+][Tf2N−], [EMIM+][NO3−], [EMIM+][BF4−], [EMIM+] [PF6−] | CuBTC | The incorporation of RTIL into the pores of MOFs demonstrates enhancement in low temperature adsorption of CO2 but minor influence on methane and nitrogen adsorption, showing great promise in the selective uptake of CO2 | 105 |
| [Bmim+][PF6−], [Bmim+][Tf2N−] | MIL-100 (Fe) | Both CO2 adsorbed concentration and selectivities (CO2/CH4 and CO2/N2) are dramatically improved through impregnating IL into the pores of MIL-100(Fe) | 106 |
| [EMIM]+[SCN]− | IRMOF-1, HMOF-1, MIL-47, and MOF-1 | An increase trend is obtained in adsorption selectivity (CO2/CH4) with increase of incorporated IL amount | 107 |
| Superbase-derived ionic liquid | Ni-MOF | SIL-derived sorbents are prepared, delivering an exceptional capability in carbon capture from ambient air. Excitingly, the capture process exhibits fast kinetics, ease releasing and good recycling usage performance | 108 |
| [TRIEN]L | ZIF-8 | Core–shell IL/MOF composite adsorbent is developed, which shows an overwhelming adsorption of CO2 over C2H2 | 109 |
| [APTMS][AC] | UiO-66-NH2 | The combination of RTIL with UiO-66 enhanced the separation efficiency of the membrane | 110 |
| [OMIM][Tf2N], [OMIM][PF6] | ZIF-7, ZIF-8 | A synergistic effect is obtained in increasing the total CO2 solubility between ZIF-7 or ZIF-8 and screened ILS | 111 |
| [BMIM][PF6], [BMIM][PF6] | CuBTC | It is demonstrated that methylation of ILs in IL/MOF has a slight negative influence on CO2/N2 and CH4/N2 selectivities | 112 |
| [C3NH2bim][Tf2N] | NH2-MIL-101(Cr) | TSIL@NH2-MIL-101(Cr)/PIM-1 membrane is developed, which exhibits a dramatic improvement in gas permeability and selectivity for CO2/N2 separation | 113 |
| [C3NH2Mim][Cl] | ZIF-7–8 | ZIF-7–8-IL (1%)/PI membrane is developed, exhibiting a dramatically improved CO2 permeability within the investigated pressures | 114 |
| [Bmim][Tf2N] | ZIF-67 | [Bmim][Tf2N]@ZIF-67 nanoparticle on PSf selective layer provided better CO2 permeance and selectivity | 115 |
| [Bmim][Tf2N] | ZIF-8 | Mechanical and gas separation properties of IL@ZIF-8/Pebax membrane were improved simultaneously | 116 |
| [Emim]2[IDA] | ZIF-8 | A remarkable CO2 adsorption capacity of 14 cm3 g−1 with the ideal selectivity of CO2/N2 and CO2/CH4 is achieved | 117 |
| [H2Pi][HSO4]2 | Mg-HKUST-1 | The resulting IL/HKUST composite demonstrated 44% and 318% improvement in CO2 adsorption capacity and CO2/N2 selectivity | 118 |
| [Emim][Ac] | ZIF-8 | Compared to pristine ZIF-8, ZIF-8 was impregnated with [Emim][Ac] delivers an increase of 700% in CO2 capture capacity | 119 |
| philic IL | MOF-808 | The mixed matrix membrane of PIM-philic IL/MOF-808 delivers fast and selective transport channels for CO2 | 120 |
| [BMIM][Ac] | HKUST-1 | Compared to HKUST-1, [BMIM][Ac]@HKUST-5% exhibited a double CO2 adsorption capacity and better cycling use performance | 121 |
| TMGHIM, TMGHPhO | ZIF-8 | Two task-specific ionic liquids (TSILs) impregnated in ZIF-8 demonstrate high adsorption capacity and selectivity for CO2 | 122 |
Conversion of CO2
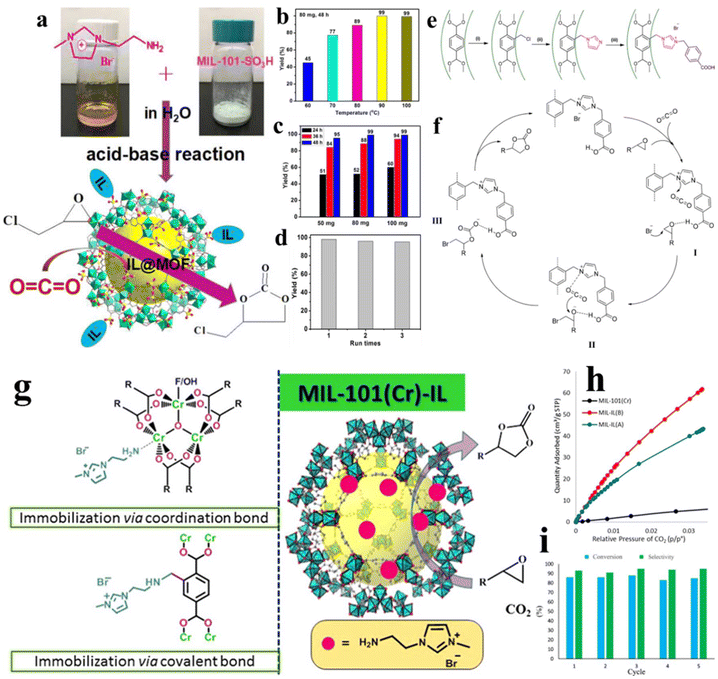
Besides the capture and storage of CO2, the catalytic conversion of CO2 into valuable chemicals also demonstrates significance in carbon neutrality.124,125 During the last years, the catalytic cycloaddition of CO2 with epoxides, generating cyclic carbonates, has demonstrated one of the green approaches to utilize CO2 in the viewpoint of atom economy. Besides, the cyclic carbonates are important chemical intermediates, showing versatile applications such as pharmaceuticals, fine chemicals, and functional materials. Owing to the high thermodynamic and kinetic stability of CO2, traditionally the conversion of CO2 requires harsh reaction conditions and consumes a large energy supply to cleavage the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond. In order to perform the conversion under mild conditions, it is necessary to develop highly efficient catalysts. Liu et al.126 developed a multi-functional site catalyst by immobilizing imidazolium-based ILs on MIL-101 (MIL-101-IMBr). In this composite catalyst, the ILs play dual roles. On the one hand, the basic acidity of the imidazole ring functions as active sites to adsorb CO2, which is critical for the afterward reaction. On the other hand, the halide anions can be contributed as a nucleophile in cycloaddition reactions. Experimentally, MIL-101-IMBr delivers high catalytic activity. A high conversion of propylene oxide of 95.8% is achieved accompanied by a high selectivity of cyclic carbonate (97.6%). Meanwhile, the catalyst shows no obvious activity loss after five reuse times. Using ship-in-bottle and post polymerization approaches, Liu et al.127 immobilized imidazole IL into MIL-101, affording IL@MIL-101 and polyILs@MIL-101. Both IL@MIL-101 and polyILs@MIL-101 demonstrate catalytic performance for CO2 cycloaddition with epoxide. Sun et al.128 introduced an amino-functionalized imidazolium-based IL into sulfonate-modified MIL-101 by the acid–base attraction. The produced IL@MIL-101–SO3H exhibits a remarkable catalytic performance for CO2 cycloaddition reaction with epichlorohydrin (Fig. 17a). The screening of experimental conditions reveals that the optimum product yield is offered at 90 °C, 1 atm CO2, and 48 h reaction time using 80 mg of catalysts per g of epichlorohydrin (Fig. 17b and c). Despite the slight increase in the yield of products upon extending the reaction time from 24 to 48 h, there was no significant increase in activity when the catalyst amount is increased from 50 to 100 mg. Significantly, IL@MIL-101–SO3H demonstrates excellent reusability but also stability. After three consecutive runs, no obvious decrease in the product yield is observed (Fig. 17d). In another work, Bao et al.129 immobilized aminoethylimidazole IL@MIL-101-NH2. They also evidenced the presence of multiple active sites containing Cr, –NH2 and Br−, delivering 93.5% of propylene carbonate via catalyzing the cycloaddition reaction of CO2 and epoxide. Bahadori et al.130 proposed a covalent grafting approach to introduce specific task ILs (STILs) onto MIL-101 (Cr). As shown in Fig. 17e, the preparation process starts by treating MIL-101(Cr) with methoxyacetyl chloride, generating MIL-101(Cr)-CH2Cl. Afterwards, the chloride atom is very active to reacting with imidazole to form MIL-101(Cr)-CH2-imi. Finally, MIL-101(Cr)-CH2-imi will be converted into MIL-101(Cr)-TSIL by reacting with 4-(bromomethyl)benzoic acid. The resulting MIL-101(Cr)-TSIL exhibits excellent catalytic performance toward the cycloaddition of CO2 with many epoxides. Under the optimized conditions, for most substrates, both the conversion of CO2 and selectivity of the corresponding cyclic carbonates are above 90%. Mechanism investigation indicates that the carboxylic acid group and IL form hydrogen bond, which produces a synergetic effect to boost the catalytic activity. As presented in Fig. 17f, the reaction occurs through the following steps. Initially, the reactants (CO2 and epoxide) are activated by the catalyst. The driving force for activating epoxide is ascribed to the hydrogen bond formed between the epoxide and the carboxylic acid group oxygen, generating intermediate I. The imidazolium cations may react with CO2 to activate the latter. Afterwards, the less sterically hindered carbon atom of intermediate I will be attacked by Br− to generate intermediate II. At the same time, the oxygen atom in the epoxide will be attacked by CO2, forming intermediate III. Finally, the intramolecular ring-closing reaction will occur, accompanied by the release of Br−. As a result, the corresponding cyclic carbonate is yielded and the catalyst is regenerated. Besides high catalytic activity, the MIL-101(Cr)-TSIL catalyst displays good thermal stability, allowing for reuse without a significant decrease in its catalytic activity. Bahadori et al.131 compared the performance of imidazolium IL immobilized on MIL-101(Cr) in CO2 capture and CO2 cycloaddition to epoxides, noted as MIL-IL(A) via coordination to Cr centers and MIL-IL(A) via covalent bonds (Fig. 17g). These two materials are capable of adsorbing CO2 at p/p0 = 0.033 and 0 °C. The adsorbing capacity conforms to the order of MIL-IL(B) > MIL-IL(A) > parent MIL (Fig. 17h). Besides absorbing CO2, both materials are capable of promoting the conversion of CO2 into cyclic carbonates via reacting with epoxides. Owing to the firm bond between the IL and the MOF, MIL-IL(B) demonstrates advantageous structural stability compared with MIL-IL(A), endowing it a recyclable heterogeneous catalyst. As presented in Fig. 17i, MIL-IL(B) remained intact after 5 cycles in the cycloaddition of CO2 to styrene epoxide.
O bond. In order to perform the conversion under mild conditions, it is necessary to develop highly efficient catalysts. Liu et al.126 developed a multi-functional site catalyst by immobilizing imidazolium-based ILs on MIL-101 (MIL-101-IMBr). In this composite catalyst, the ILs play dual roles. On the one hand, the basic acidity of the imidazole ring functions as active sites to adsorb CO2, which is critical for the afterward reaction. On the other hand, the halide anions can be contributed as a nucleophile in cycloaddition reactions. Experimentally, MIL-101-IMBr delivers high catalytic activity. A high conversion of propylene oxide of 95.8% is achieved accompanied by a high selectivity of cyclic carbonate (97.6%). Meanwhile, the catalyst shows no obvious activity loss after five reuse times. Using ship-in-bottle and post polymerization approaches, Liu et al.127 immobilized imidazole IL into MIL-101, affording IL@MIL-101 and polyILs@MIL-101. Both IL@MIL-101 and polyILs@MIL-101 demonstrate catalytic performance for CO2 cycloaddition with epoxide. Sun et al.128 introduced an amino-functionalized imidazolium-based IL into sulfonate-modified MIL-101 by the acid–base attraction. The produced IL@MIL-101–SO3H exhibits a remarkable catalytic performance for CO2 cycloaddition reaction with epichlorohydrin (Fig. 17a). The screening of experimental conditions reveals that the optimum product yield is offered at 90 °C, 1 atm CO2, and 48 h reaction time using 80 mg of catalysts per g of epichlorohydrin (Fig. 17b and c). Despite the slight increase in the yield of products upon extending the reaction time from 24 to 48 h, there was no significant increase in activity when the catalyst amount is increased from 50 to 100 mg. Significantly, IL@MIL-101–SO3H demonstrates excellent reusability but also stability. After three consecutive runs, no obvious decrease in the product yield is observed (Fig. 17d). In another work, Bao et al.129 immobilized aminoethylimidazole IL@MIL-101-NH2. They also evidenced the presence of multiple active sites containing Cr, –NH2 and Br−, delivering 93.5% of propylene carbonate via catalyzing the cycloaddition reaction of CO2 and epoxide. Bahadori et al.130 proposed a covalent grafting approach to introduce specific task ILs (STILs) onto MIL-101 (Cr). As shown in Fig. 17e, the preparation process starts by treating MIL-101(Cr) with methoxyacetyl chloride, generating MIL-101(Cr)-CH2Cl. Afterwards, the chloride atom is very active to reacting with imidazole to form MIL-101(Cr)-CH2-imi. Finally, MIL-101(Cr)-CH2-imi will be converted into MIL-101(Cr)-TSIL by reacting with 4-(bromomethyl)benzoic acid. The resulting MIL-101(Cr)-TSIL exhibits excellent catalytic performance toward the cycloaddition of CO2 with many epoxides. Under the optimized conditions, for most substrates, both the conversion of CO2 and selectivity of the corresponding cyclic carbonates are above 90%. Mechanism investigation indicates that the carboxylic acid group and IL form hydrogen bond, which produces a synergetic effect to boost the catalytic activity. As presented in Fig. 17f, the reaction occurs through the following steps. Initially, the reactants (CO2 and epoxide) are activated by the catalyst. The driving force for activating epoxide is ascribed to the hydrogen bond formed between the epoxide and the carboxylic acid group oxygen, generating intermediate I. The imidazolium cations may react with CO2 to activate the latter. Afterwards, the less sterically hindered carbon atom of intermediate I will be attacked by Br− to generate intermediate II. At the same time, the oxygen atom in the epoxide will be attacked by CO2, forming intermediate III. Finally, the intramolecular ring-closing reaction will occur, accompanied by the release of Br−. As a result, the corresponding cyclic carbonate is yielded and the catalyst is regenerated. Besides high catalytic activity, the MIL-101(Cr)-TSIL catalyst displays good thermal stability, allowing for reuse without a significant decrease in its catalytic activity. Bahadori et al.131 compared the performance of imidazolium IL immobilized on MIL-101(Cr) in CO2 capture and CO2 cycloaddition to epoxides, noted as MIL-IL(A) via coordination to Cr centers and MIL-IL(A) via covalent bonds (Fig. 17g). These two materials are capable of adsorbing CO2 at p/p0 = 0.033 and 0 °C. The adsorbing capacity conforms to the order of MIL-IL(B) > MIL-IL(A) > parent MIL (Fig. 17h). Besides absorbing CO2, both materials are capable of promoting the conversion of CO2 into cyclic carbonates via reacting with epoxides. Owing to the firm bond between the IL and the MOF, MIL-IL(B) demonstrates advantageous structural stability compared with MIL-IL(A), endowing it a recyclable heterogeneous catalyst. As presented in Fig. 17i, MIL-IL(B) remained intact after 5 cycles in the cycloaddition of CO2 to styrene epoxide.
 | ||
| Fig. 17 (a) Schematic diagram of the fabrication of IL@MIL-101–SO3H as well as the catalyzed CO2 cycloaddition reaction with epichlorohydrin. (b) Temperature-dependent activity of IL@MIL-101–SO3H. (c) Catalytic reaction efficiency at different catalyst dosages. (d) Recycle test of IL@MIL-101–SO3H catalyst. Reproduced from ref. 128 with permission. Copyright 2017 American Chemical Society. (e) Synthesis procedure for MIL-101(Cr)–TSIL catalyst. (f) Mechanism of cycloaddition of CO2 with epoxides catalyzed by MIL-101(Cr)–TSIL. Reproduced from ref. 130 with permission. Copyright 2019 American Chemical Society. (g) Schematic diagram of the structure of MIL-IL(A) and MIL-IL(B). (h) Adsorption isotherm of CO2 in relative pressure for MIL-101(Cr), MIL-IL(A), and MIL-IL(B). (i) Recycling of MIL-IL(B) in the cycloaddition of CO2 with styrene epoxide under optimized conditions. Reproduced from ref. 131 with permission. Copyright 2020 American Chemical Society. | ||
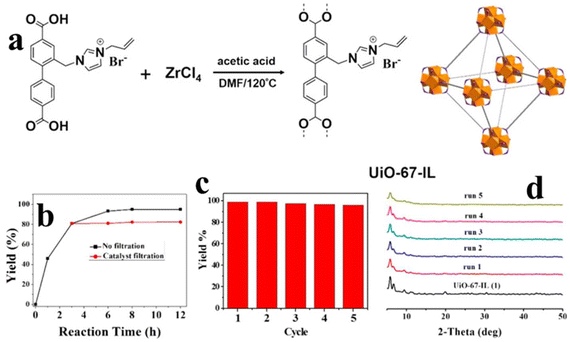
Liang et al.132 developed (I−)Meim-UiO-66 from the imidazole-containing Zr-MOF Im-UiO-66, which showed efficiency in promoting the reaction between CO2 and epoxides. In some cases, it is difficult to graft an IL moiety onto MOFs owing to the steric effect. In other words, the grafting amount of ILs may affect the performance of IL/MOF composite. In order to ensure the IL loading amount, Ding et al.133 forwarded a ligand modification process to synthesize UiO-67-IL catalysts. As presented in Fig. 18a, the IL cation 1-propeneyl-imidazolium is covalently modified onto 1,4-DBC, which is followed by coordination with Zr4+ to generate UiO-67-IL. Owing to the covalent bond interaction between IL and UiO-67, the IL is difficult to leach out when used as the catalyst for liquid-phase reactions. As seen from Fig. 18b, the reaction can suddenly stop without an observable progress once filtration is performed to isolate the catalyst. Furthermore, such structural stability endows UiO-67-IL with excellent recycling performance. As shown in Fig. 18c, the yield of cyclic carbonate is maintained at 96%. The powder XRD patterns of fresh UiO-67-IL and those after each cycle run are measured, and the result (Fig. 18d) indicates the well-preserved structure.
 | ||
| Fig. 18 (a) Synthesis of the UiO-67-IL catalyst. (b) Reaction time-dependent yield of product. (c) Recycling performance of the UiO-67-IL catalyst. (d) PXRD patterns of the catalyst for each cycle. Reproduced from ref. 133 with permission. Copyright 2017 American Chemical Society. | ||
Ding et al.41 reported a polyILs/MIL-101 composite synthesized via in situ polymerization of encapsulated monomers, which exhibits good CO2 capture capability for further cycloaddition of CO2 with epoxides (Fig. 19a). Besides delivering excellent CO2 enrichment capacity, the polyILs@MIL-101 simultaneously provides Lewis acid sites, which originated from MIL-101, as well as the Lewis base sites, which originated from PILs. Owing to the synergy among these factors, a significantly enhanced activity of polyILs@MIL-101 is achieved, which is much superior to either MIL-101 or polyILs (Fig. 19b). Significantly, polyILs@MIL-101 demonstrates good structural stability since no significant loss of crystallinity and structural integrity for polyILs@MIL-10 is observed in powder X-ray diffraction patterns. As a result, its catalytic activity throughout multiple use cycles is well maintained (Fig. 19c). In another work, Dong et al.134 fabricated core–shell PILs@ZIF-8 composites and investigated the structure–activity relationship, reusability, and versatility for the cycloaddition reaction between CO2 and epoxide. Among screened the scope of catalysts, PIL-Br@ZIF-8(2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) demonstrates the best activity and selectivity with an 88% PC yield under the given conditions, as presented in Fig. 19d. By screening a wide range of epoxide substrates, it is concluded that PIL-Br@ZIF-8(2
1) demonstrates the best activity and selectivity with an 88% PC yield under the given conditions, as presented in Fig. 19d. By screening a wide range of epoxide substrates, it is concluded that PIL-Br@ZIF-8(2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) is a general catalyst efficient for various substrates. As shown in Fig. 19e, the yields vary with the steric hindrance of the epoxide substrates; the lower the steric hindrance, the higher the product yield. In particular, owing to the electron-withdrawing effect, halogenated epoxides are favorable for obtaining a higher yield. Such result can be ascribed to the diminished electron cloud density of β-C in epoxides, facilitating nucleophilic attack by nucleophilic Br− sites in the catalyst. Remarkably, the catalytic activity and selectivity of PIL-Br@ZIF-8(2
1) is a general catalyst efficient for various substrates. As shown in Fig. 19e, the yields vary with the steric hindrance of the epoxide substrates; the lower the steric hindrance, the higher the product yield. In particular, owing to the electron-withdrawing effect, halogenated epoxides are favorable for obtaining a higher yield. Such result can be ascribed to the diminished electron cloud density of β-C in epoxides, facilitating nucleophilic attack by nucleophilic Br− sites in the catalyst. Remarkably, the catalytic activity and selectivity of PIL-Br@ZIF-8(2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) are well maintained even after reuse for 8 runs of the CO2 cycloaddition reaction (Fig. 19f).
1) are well maintained even after reuse for 8 runs of the CO2 cycloaddition reaction (Fig. 19f).
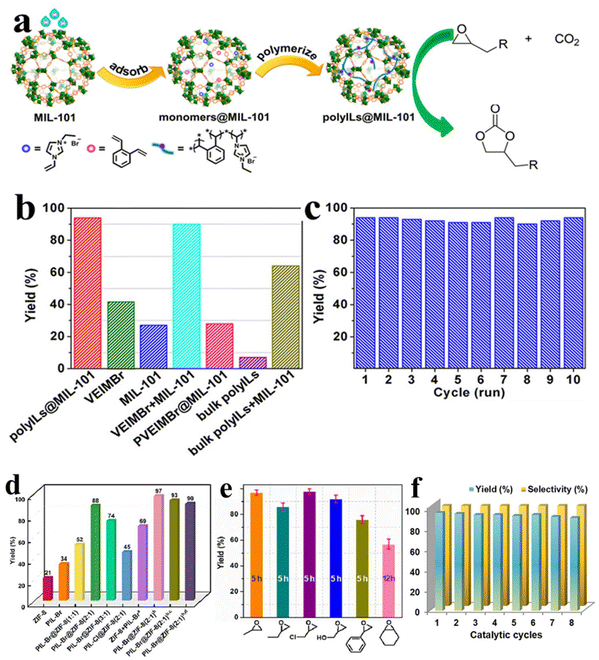 | ||
Fig. 19 (a) Schematic illustration of the synthetic process of the PIL/MOF composite and the catalytic conversion of CO2 into cyclic carbonate. (b) Catalytic comparison of various catalysts. (c) Recycling performance of PIL@MOF catalysts. Reproduced from ref. 41 with permission. Copyright 2018 American Chemical Society. (d) Catalytic comparison of various PIL/ZIF catalysts. (e) Substrate scope and (f) reusability of PIL-Br@ZIF-8(2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Reproduced from ref. 134 with permission. Copyright 2024 Elsevier. 1). Reproduced from ref. 134 with permission. Copyright 2024 Elsevier. | ||
Wang et al.135 reported that MIL-101-NH2-supported carboxyl-functionalized ILs have dual functions in catalyzing CO2 cycloaddition reactions, which deliver a great activity under mild conditions. The yield reached up to 91%. They concluded that the improved reactivity is ascribed to the cooperative activation of CO2 and epoxides. Clark et al.136 investigated the solvation environment of the IL/MOF composite catalyst upon CO2 cycloaddition to cyclic carbonate. As for mechanism, they concluded that ring-opening is involved.
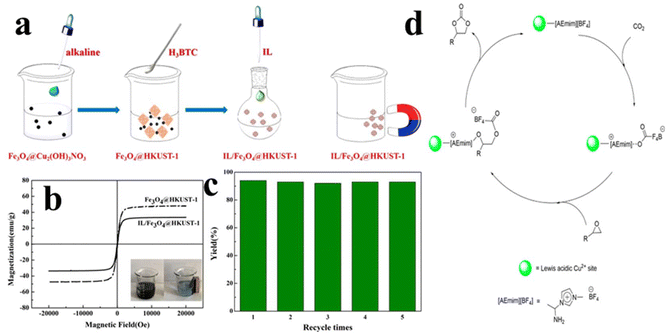
Although the combination of ILs and MOFs shows great promise in the cycloaddition of CO2, the separation of products often requires filtration or centrifugation, restricting their commercial application. During the past few decades, magnetic recyclable catalysts demonstrate particular attention owing to the high separation efficiency and ease of operation.137,138 Bearing this concept in mind, Wang et al.139 introduced Fe3O4 nanoparticles, which act as supports to immobilize IL/MOF catalysts. As shown in Fig. 20a, IL/Fe3O4@HKUST-1 was synthesized via grafting the ionic liquid (IL) [AEMIm]BF4 onto a magnetically functionalized metal–organic framework, Fe3O4@HKUST-1, which is achieved in a water–ethanol media. Magnetic measurement (Fig. 20b) reveals that IL/Fe3O4@HKUST-1 maintains superparamagnetic property of F3O4 nanoparticles. The coating of IL/HKUST-1 results in an obvious decline in saturated magnetization, which is still enough to ensure the separation of the catalyst using an external magnet. The catalytic activity evaluation was carried out using the cycloaddition of CO2 with epoxides as a model reaction. It is demonstrated that the resulting IL/Fe3O4@HKUST-1 delivered a higher activity than that of pristine IL or KHUST-1. Such high activity is probably ascribed to the synergistic effect of the Lewis acid and base sites in IL/Fe3O4@HKUST-1. Furthermore, the catalyst can be easily separated from the reaction mixture, and the recycled catalyst maintained high performance for several cycles (Fig. 20c). Through a series of experimental investigations, the plausible mechanism is further proposed. As presented in Fig. 20d, there are three steps involved. Initially, a CO2 molecule is inserted into the catalyst. This is achieved by static electronic interaction. In CO2, the carbon atom is positively charged, attacking with anions of ILs, whereas the oxygen atom is negatively charged, linking to the cation of ILs. Then, the ring-opening of epoxide induces the carbon atom possessing a positive charge and oxygen possessing a negative charge. Simultaneously, the ring-opened epoxide will be inserted into the intermediate formed in the last step. Finally, the ring closure of cyclic carbonate leads to the formation of cyclic carbonates and the regeneration of catalysts.
 | ||
| Fig. 20 (a) Procedures for the synthesis of IL/Fe3O4/HKUST-1. (b) Magnetization curves of Fe3O4@HKUST-1 and IL/Fe3O4@HKUST-1. (c) Recycling catalytic performance of IL/Fe3O4/HKUST-1. (d) Plausible mechanism of CO2 reacting with epoxides over the IL/Fe3O4/HKUST-1 catalyst. Reproduced from ref. 139 with permission. Copyright 2018 American Chemical Society. | ||
In order to further improve the activity and extend the application of the IL/MOF catalyst, noble nanoparticle demonstrates a versatile co-catalyst. For example, Zhao et al.140 designed a multi-functional catalyst (named IL-Au@UiO-66-NH2/CMC), which consists of integrated acids (Zr4+), bases (ionic liquid (IL)), and Au nanoparticles (Fig. 21a). Owing to these active sites, the produced IL-Au@UiO-66-NH2/CMC is capable of catalyzing CO2 by directly reacting with styrene derivatives to generate cyclic carbonates. The whole catalyzing mechanism is schemed in Fig. 21b. Initially, both styrene and TBHP are activated by the Au nanoparticles. Owing to the high reactivity of t-C4H9OO˙ and H˙ radicals, a carbon-centered radical will be generated from the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond of styrene. Afterward, the epoxide intermediate will be formed via oxygen migrating to the carbon-centered radical, which is a common conversion reaction.141,142 Then, the Lewis Zr4+ sites in the UiO-66 will induce the cycloaddition step, which is accompanied by SO adsorption and polarization, facilitating bromide anions attacking the epoxide carbon. As a result, a ring-opened intermediate will be formed. At last, the ring-opened intermediate will react with CO2 activated by IL to produce an alkylcarbonate anion, resulting in the formation of SC. Obviously, the whole process includes two sequential reactions, namely, epoxidation of styrene and CO2 cycloaddition reaction. Each component in IL-Au@UiO-66-NH2/CMC activates one species. Au nanoparticle activates styrene, whereas IL activates CO2 and UiO-66 activates the epoxide intermediate. Compared to conventional stepwise synthetic routes, such catalytic system shares advantages in avoiding the isolation of intermediates. Fig. 21c displays the conversion of styrene and the selectivity of products. One can see that the conversion of styrene can reach nearly 100% within a short time. However, owing to several steps involved in the whole reaction, as discussed in the mechanism stage, the yield of the final product (SC) shows an increasing trend with reaction time accompanied by the decrease in intermediate products. It should be noted that the byproduct and SO intermediate cannot be completely eliminated by prolonging the reaction time. Remarkably, compared to a series of controlled samples, IL-Au@UiO-66-NH2/CMC exhibits superior selectivity despite the slightly lower conversion of styrene than the controlled catalyst without ILs (Fig. 21d).
C bond of styrene. Afterward, the epoxide intermediate will be formed via oxygen migrating to the carbon-centered radical, which is a common conversion reaction.141,142 Then, the Lewis Zr4+ sites in the UiO-66 will induce the cycloaddition step, which is accompanied by SO adsorption and polarization, facilitating bromide anions attacking the epoxide carbon. As a result, a ring-opened intermediate will be formed. At last, the ring-opened intermediate will react with CO2 activated by IL to produce an alkylcarbonate anion, resulting in the formation of SC. Obviously, the whole process includes two sequential reactions, namely, epoxidation of styrene and CO2 cycloaddition reaction. Each component in IL-Au@UiO-66-NH2/CMC activates one species. Au nanoparticle activates styrene, whereas IL activates CO2 and UiO-66 activates the epoxide intermediate. Compared to conventional stepwise synthetic routes, such catalytic system shares advantages in avoiding the isolation of intermediates. Fig. 21c displays the conversion of styrene and the selectivity of products. One can see that the conversion of styrene can reach nearly 100% within a short time. However, owing to several steps involved in the whole reaction, as discussed in the mechanism stage, the yield of the final product (SC) shows an increasing trend with reaction time accompanied by the decrease in intermediate products. It should be noted that the byproduct and SO intermediate cannot be completely eliminated by prolonging the reaction time. Remarkably, compared to a series of controlled samples, IL-Au@UiO-66-NH2/CMC exhibits superior selectivity despite the slightly lower conversion of styrene than the controlled catalyst without ILs (Fig. 21d).
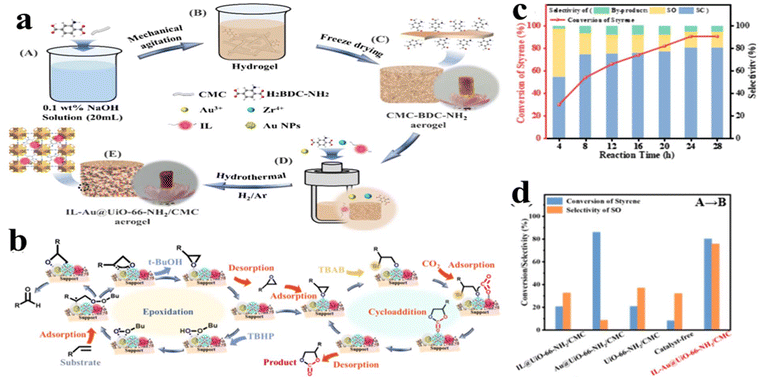 | ||
| Fig. 21 (a) Synthesis of IL-Au@UiO-66-NH2/CMC. (b) Catalytic mechanism of the tandem reaction from styrene to form SC. (c) Catalytic performance of IL-Au@UiO-66-NH2/CMC. (d) Catalytic comparison of various catalysts. Reproduced from ref. 140 with permission. Copyright 2024 American Chemical Society. | ||
Besides conversion into cyclic carbonates, the value-added formylation product is a promising approach to fix CO2, which can also be achieved using IL/MOC catalysts. For example, He et al.143 developed a porous metal–organic cage (MOC) liquid (Im-PL-Cage). As presented in Fig. 22a, the utilized MOC is prepared by coordination between calixarene molecule and Zn2+ ions. Owing to the unique features of calixarene, a long polyethylene glycol (PEG)-imidazolium chain functional linker can be further self-assembled onto the MOC. Benefiting from the permanent porosity and fluidity, the produced Im-PL-Cage possesses a high capacity of CO2 adsorption. Interestingly, CO2 stored in an Im-PL-Cage can be further efficiently converted into a formylation product. As presented in Fig. 22b, a high yield of 96.7% with 7.74 mmol morpholine conversion is achieved, which is much superior to controlled samples. Such high performance is achieved because the Im-PL-Cage has permanent pores, capable of storing CO2 in a quantity almost 15 times more than that of pure PEG-Im-H2BDC. Recently, Wang et al.144 have developed a IL-functionalized Cu-based catalyst, Cu2O-HKUST-1/IL1/PTFE, by a wet impregnation process (Fig. 22c). Thanks to the high adsorption and multiple active sites of HKUST-1, the modified Cu2O-HKUST-1/IL1/PTFE exhibits excellent electrocatalytic activity for converting CO2 into C1 compounds (mainly CH4 and CO). As presented in Fig. 22d, experimentally a synergistic effect between IL and HKUST-1 is obtained. In particular, the Cu2O-HKUST-1/IL1/PTFE catalyst manifested a high C1 faradaic efficiency (FE) up to 96.5%, and in particular, 92.7% for FECO at −1.7 V vs. RHE (Fig. 22e). Besides the intrinsic catalytic activity of Cu2O, ILs synergistically regulating and stabilizing the intermediates (*HOCO and *CO) also make positive contribution to the excellent CO selectivity.
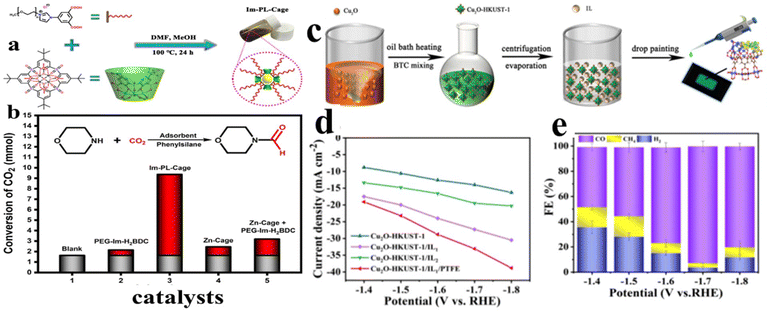 | ||
| Fig. 22 (a) Schematic illustration of the synthesis of the Im-PL-Cage composite. (b) Conversion of CO2 to N-formylmorpholine with different catalysts. Reproduced from ref. 143 with permission. Copyright 2023 published by Springer Nature under a CC-BY license. (c) Preparation of Cu2O-HKUST-1/IL/PTFE. (d) Comparison of the current density of samples. (e) Histogram of FE for the sample Cu2O-HKUST-1/IL1/PTFE. Reproduced from ref. 144 with permission. Copyright 2024 American Chemical Society. | ||
In this section, we discuss the recent advancement of the utilization of IL-MOFs as catalysts for CO2 conversion, particularly highlighting the conversion of CO2 into a cyclic carbonate using some representative IL/MOF composites. It is noted that the CO2 conversion is a large topic. The target product covers a wide range, which are not discussed here, including alcohols and hydrocarbons. Expectedly, these findings are expected to provide new guidance and insights for developing high-performance catalysts in these fields.
Organic transformation
From the standpoint of creating new compounds and industrial manufacturing, organic transformations, such as reduction/oxidation of organic molecules, coupling two organic compounds, and so on, play a vital role in both environmental and synthetic processes. In many cases, organic transformations can only process with the assistance of catalysts. Nowadays, increasing attention is paid to developing greener catalytic organic transformations, in which no byproduct/waste is generated, small amount of catalysts are consumed, operating conditions are mild (at ambient temperature and atmospheric pressure), and multi-functions are integrated into one system. As a result, the potential applications of IL/MOF composites as catalysts for organic transformations have gained particular interest from chemists and chemical engineers. Great progress has been achieved in IL/MOF-assisted high-efficiency organic transformations during the past decade. When used in catalysis, the combination of ILs and MOFs may provide new insights. For example, Takashima et al.145 developed an ion-exchange approach to immobilize an IL in an MOF. Interestingly, they found that either pristine IL or the physical mixture of ILs and MOFs delivers no catalytic activity toward the ring-opening polymerization reaction of propene epoxide. It is not the case for the resulting IL/MOF composite, which efficiently promotes the reaction, as presented in Fig. 23. In this section, we will summarize the application of IL/MOF-based catalysts in the reduction/oxidation of organic molecules, bicomponent reactions, and multicomponent/cascade reactions. | ||
| Fig. 23 Illustration of IL/MOF-catalyzed ring-opening polymerization. Reproduced from ref. 145 with permission. Copyright 2017 the Royal Society of Chemistry. | ||
 | ||
| Fig. 24 Synthesis of (a) MOF-5, (b) [DEIm][PF6], and (c) [DEIm][PF6]@MOF-5 composite. (d) Conversion mechanism of 4-nitrophenol into 4-aminophenol over the [DEIm][PF6]@MOF-5 catalyst. (e) Recycling performance of the [DEIm][PF6]@MOF-5 catalyst. Reproduced from ref. 146 with permission. Copyright 2023 published by American Chemical Society under a CC-BY license. | ||
Selective oxidation of alcohols to aldehydes is a significant transformation in organic chemistry. Ma et al.150 prepared a Co@C–N catalyst by the pyrolysis of [Bmim][CoCl3]@ZIF-8 as precursors, which show excellent catalytic activity for the aerobic oxidation of alcohols (Fig. 25). Both the mole fraction of ILs in the precursor and temperature have dramatic impacts on the performance of the resulting catalysts. The optimal condition is experimentally determined to be 900 °C and 0.1 mole fraction, at which the sample is noted as Co@C–N(0.1)-900. The catalytic performance of the resulting Co@C–N(0.1)-900 was evaluated by the oxidation of alcohol with molecular oxygen under base-free conditions. The selectivity for the oxidation of alcohols to aldehydes reaches high with up to 99% yield under optimal conditions. The mechanism investigation indicates that such high activity is ascribed to both the highly dispersed CoCx sites and the strong interaction between Co and N species in the Co@C–N materials.
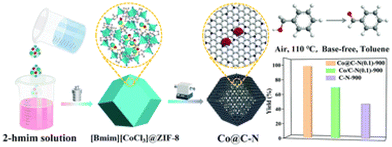 | ||
| Fig. 25 Schematic illustration of the preparation of Co@C–N as well as its catalytic aerobic oxidation of alcohols. Reproduced from ref. 150 with permission. Copyright 2022 the Royal Society of Chemistry. | ||
Nowadays, organic pollutants such as dyes and antibiotics have become increasingly more serious issues. Photocatalytic oxidation degradation is a versatile approach to eliminate organic dyes, in which the organic dyes may be completely degraded into CO2 and H2O.151–154 Asgari et al.155 fabricated a nanofibrous piezo-photocatalyst (ZrU@IL/PAN) composite consisting of UiO-66, HMIM+TFSI− IL, and PAN nanofiber. As shown in Fig. 26a, the preparation of this ZrU@IL/PAN piezo-photocatalyst starts from the solvothermal synthesis of UiO-66, which is followed by the incorporation of HMIM+TFSI− IL. Afterward, blend electrospinning is carried out. Piezo-photocatalytic activity is evaluated by the degradation of Rhodamine B (RhB), and the result indicates that the degradation performance follows the order of HMIM+TFSI− < ZrU < purePAN NFs < IL/PAN NFs < ZrU@IL < ZrU/PAN NFs < ZrU@IL/PAN NFs under given conditions. The controlled experiment measurement indicates a synergistic effect among piezo- and photo-catalytic processes since the combination catalysis offers a much higher degradation efficiency (2.2%–95.4%) compared to individual photodegradation processes (0.0%–21.3%) and individual piezodegradation efficiencies (2.3%–84.2%), as presented in Fig. 26b. Remarkably, ZrU@IL-1/PAN delivers stable activity, displaying high recycling after four cycles of usage (Fig. 26c).
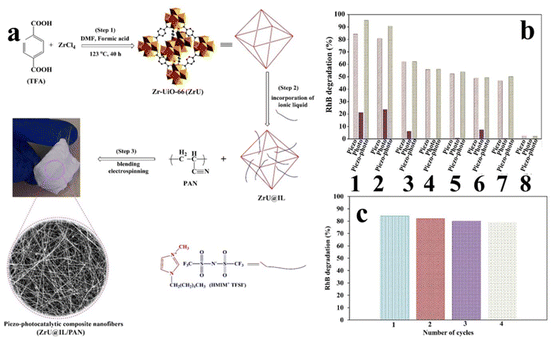 | ||
| Fig. 26 (a) Synthesis of the ZrU@IL@PAN catalyst. (b) RhB piezo-/photo/piezo-photodegradation graphs over (1) ZrU@IL-1/PAN NFs, (2) ZrU/PAN NFs, (3) ZrU@IL-1, (4) IL/PAN NFs, (5) pure PAN NFs, (6) ZrU, (7) PAN powder, and (8) HMIM+TFSI−. (c) Recycling performance of the ZrU@IL@PAN catalyst. Reproduced from ref. 155 with permission. Copyright 2024 Elsevier. | ||
In the petroleum industry, the combustion of sulfur-containing diesel emits sulfur oxides (SOx), raising serious environmental concerns. As a result, much effort has been devoted to the desulfurization of fuel. To date, various techniques such as hydrodesulfurization (HDS), extraction desulfurization (EDS), and oxidative desulfurization (ODS) have been developed for desulfurization.156–159 Among these methods, ODS has been regarded as a promising one since it is capable of offering a high desulfurization degree under mild conditions. In general, the ODS requires acidic active sites to induce the oxidation reaction. Taking this task in mind, Yin et al.160 fabricated a bifunctional catalyst consisting of UiO-66 and Brønsted acidic porous ionic liquids (BAPILs), denoted as UiO-66-BAPILs. As presented in Fig. 27a, this bifunctional catalyst is prepared via a simple direct blending procedure. Both experimental and theoretical studies reveal that the desulfurization mechanism conforms to the sequential steps, and DBT was extracted into UiO-66-BAPILs and then oxidized (Fig. 27b). Excitingly, a desirable sulfur removal of 99.5% is experimentally achieved under optimal conditions, demonstrating the great potential of such IL/MOF composite catalysts.
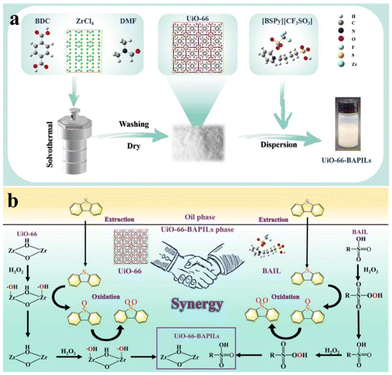 | ||
| Fig. 27 (a) Synthesis of UiO-66-BAPILs. (b) Catalytic mechanism of oxidative desulfurization over UiO-66-BAPILs. Reproduced from ref. 160 with permission. Copyright 2024 Elsevier. | ||
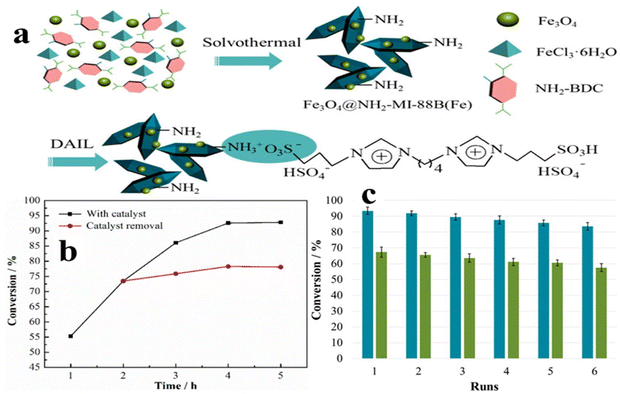 | ||
| Fig. 28 (a) Synthesis of the DAIL-Fe3O4@NH2-MIL-88B(Fe) catalyst. (b) Leaching test of DAIL-Fe3O4@NH2-MIL-88B(Fe). (c) Recycling of DAIL-Fe3O4@NH2-MIL-88B(Fe) (blue) and AIL-Fe3O4@NH2-MIL-88B(Fe) (green) for the esterification of oleic acid with ethanol. Reproduced from ref. 161 with permission. Copyright 2016 American Chemical Society. | ||
Chong et al.164 developed a novel heterogeneous catalyst (denoted as IL(OAc−)-MIL-101-NH2), in which the IL of BmimOAc is dispersed in the MIL-101-NH2 nanocages primordially. As presented in Fig. 29a, this catalyst is prepared using a traditional “ship-in-a-bottle” strategy. The immobilization of BmimOAc IL is achieved through a condensation reaction of MIL-101-NH2's amine group with 1,1′-carbonyldiimidazole (CDI) reacting with 1-bromo butane, which is followed by the intermediate exchanged with potassium acetate. Structural analysis indicated that BmimOAc IL was anchored in the MIL-101-NH2 skeleton. This was achieved via the acylamino group and confined to the nanocages in the form of a single molecule. When evaluated as the catalyst for synthesizing 3-aryl-2-oxazolone by reacting propylene carbonate with phenylamine, the resulting IL(OAc−)-MIL-101-NH2 composite material demonstrated an outstanding catalytic activity. Under optimal conditions, the yield reached high up to 92%. By screening a wide range of substrates, it is concluded that the IL(OAc−)-MIL-101-NH2 catalyst works for many reactants. Mechanism investigation reveals that the catalytic reaction follows the following procedures (Fig. 29b). Initially, the high electronegativity of N atoms endows C-2 hydrogen of the imidazolium cation with Brønsted acidity. The hydrogen atoms can lose when meeting electron-rich groups. Thus, the C-2 site will react with the carbonyl oxygen of propylene carbonate through hydrogen donating-accepting interaction. At the same time, an intermediate will also be formed by phenylamine reacting with the acetate anion from IL(OAc−)-MIL-101-NH2. By these two procedures, both phenylamine and propylene carbonate are activated. These processes are closely followed by a nucleophilic reaction occurring between these two activated intermediates. Finally, the system will undergo dehydration accompanied by intramolecular cyclization, so the final product is obtained. Those amines containing electron-withdrawing groups can be activated more easily, and then converted into the intermediate. As for recycling usage, the IL(OAc−)-MIL-101-NH2 catalyst shows no noteworthy loss of its activity after six times, as presented by Fig. 29c. Hassan et al.165 immobilized N-methyl-2-pyrrolidonium methyl sulfonate ([NMP]+ CH3SO3−) on MIL-101(Cr), which delivered catalytic activity for the esterification of acetic acid with amyl alcohol and the Friedel–Crafts acylation of anisole. Taking advantage of a tandem post-synthetic strategy, Luo et al.166 introduced Brønsted acidic IL (BAIL) inside the cages of well-defined MIL-101. The resulting BAIL/MIL-101 shows efficient catalytic performance for the acetalization of benzaldehyde with glycol. The outstanding catalytic performance is probably ascribed to the synergistic effect of the IL catalyst and MOF support. The former in a highly dispersive state is expected to expose more active sites, whereas the latter provides a desirable microenvironment for the reaction. Ali et al.167 reported a series of heterogeneous catalysts, namely, [HMIm]3[PW12O40]@MOF-Fe, [HMIm]3[PMo12O40]@MOF-Fe, and [HMIm]4[SiW12O40]@MOF-Fe, by a simple impregnation method. These catalysts show excellent catalytic conversion of glycerol into solketal. In particular, three important indexes, the glycerol conversion, solketal selectivity and solketal yield, all reach high up to 100% at room temperature within 1 h when 5 wt% [HMIm]3[PW12O40]@MOF-Fe acts as the catalyst, as presented in Fig. 29d.
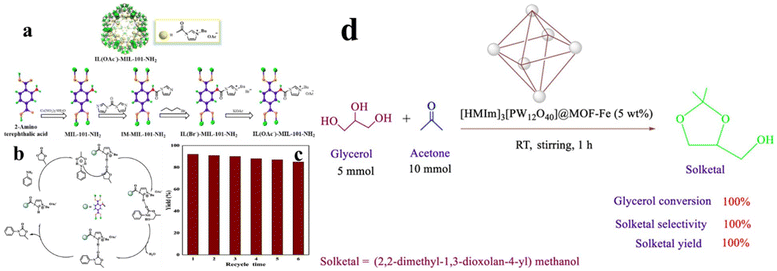 | ||
| Fig. 29 (a) Synthesis of IL(OAc−)-MIL-101-NH2. (b) Formation mechanism of 3-aryl-2-oxazolidinones by reacting propylene carbonate with phenylamine catalyzed by IL(OAc-)-MIL-101-NH2. (c) Recycling catalytic performance of IL(OAc-)-MIL-101-NH2. Reproduced from ref. 164 with permission. Copyright 2019 American Chemical Society. (d) Schematic illustration of the selective acetalization of glycerol to solketal. Reproduced from ref. 167 with permission. Copyright 2023, published by the American Chemical Society under a CC-BY license. | ||
In another work, Ye et al.168 developed an efficient Brønsted–Lewis acidic catalyst [(CH2COOH)2IM]HSO4@H-UiO-66 via bidentate coordination between one –COO– group of [(CH2COOH)2IM]HSO4 and two portions of unsaturated Zr ion defects of the hierarchically porous H-UiO-66. The obtained material exhibits high chemical stability and generic thermal stability. When evaluated as the heterocatalyst for biodiesel synthesis, the obtained [(CH2COOH)2IM]HSO4@H-UiO-66 delivers efficient activity toward the esterification between methanol and oleic acid (Fig. 30a). As presented in Fig. 30b, the reaction has only a minor influence on the activity of the resulting catalyst. Under the optimal temperature, the product yield reaches high up to 93.82%. Moreover, the catalyst demonstrates good stability. As presented in Fig. 30c, the yield of biodiesel shows only a minor decrease (from 93.82 to 90.95%) after five runs. As for the catalytic mechanism, it is described in Fig. 30d. The catalytic process may be explained by two paths. Path 1 includes the dissociation of the catalyst to generate H+, which can attack the oxygen atom of the carbonyl group in oleic acid. Owing to accepting H+, carbocations will be generated. Finally, the carbocation with excellent electrophilicity will be attacked by methanol, generating a tetrahedral intermediate. The formed tetrahedral intermediate can be decomposed into biodiesel and H+, so the catalyst returns to its original state. In path II, the H-UiO-66 part produces the unsaturated Zr atoms, which may offer an empty orbital to accept an electron pair from the carbonyl group of oleic acid. Owing to the presence of protons, the carbon atoms of the carbonyl group will become carbocation. The carbocation will then be attacked by methanol to give a tetrahedral intermediate. In the end, the decomposition of the intermediate will generate biodiesel and unsaturated Zr atoms.
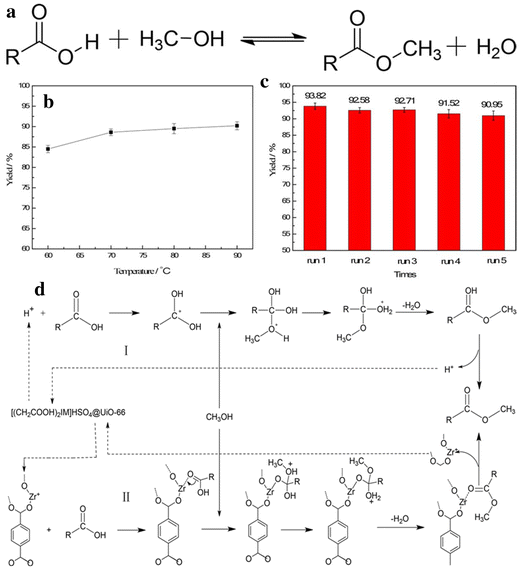 | ||
| Fig. 30 (a) Esterification of oleic acid with methanol. (b) Temperature-dependent yield. (c) Recycling performance of the catalyst. (d) Catalytic mechanism. Reproduced from ref. 168 with permission. Copyright 2019 American Chemical Society. | ||
Besides acting as active species, IL/MOF composites can serve as supports to immobilize other catalysts. For example, Askari et al.169 reported a composite heterogeneous catalyst comprising dibutylimidazolium bromide IL-modified UiO-66-NH2 and guanidine (UiO-66-NH2-ILPF6–guanidine), which is efficient for promoting aldehydes reacting with dimedone to generate xanthene derivatives (Fig. 31a and b). As presented in Fig. 31a, the preparation process of the UiO-66-NH2-ILPF6–guanidine catalyst involves three steps. The first step refers to the modification of UiO-66-NH2 using the mentioned IL, which is realized by a post-synthesis modification method, generating UiO-66-NH2-ILBr−. The second step is the attachment of guanidine hydrochloride to UiO-66-NH2-ILBr−. Finally, an anion exchange reaction is performed between Br− and PF6− to generate a UiO-66-NH2-ILPF6–guanidine catalyst. In order to evaluate the catalytic performance of the resulting UiO-66-NH2-ILPF6–guanidine catalyst, a reaction of aldehydes with dimedone is performed, yielding xanthene derivatives. Their results indicate that UiO-66-NH2-ILPF6–guanidine is a general catalyst applicable to various types of aromatic aldehydes with medium to high yields. Catalytic mechanism investigation indicates that a tandem reaction of Knoevenagel, Michael, and intramolecular cyclization is involved in this system, as illustrated in Fig. 31c which is in good agreement with other reports.170 In this composite catalyst, each component is expected to play a special role in the catalytic reaction. Guanidine, as the main active species, offers basic sites for deprotonation of the acidic proton of the dimedone. The main support material, UiO-66-NH2, contains metallic nodes, activating the carbonyl atom of aldehydes. As for the supported IL, it may polarize the area near the surface of the catalyst, facilitating the contact of the starting materials and the catalyst surface.
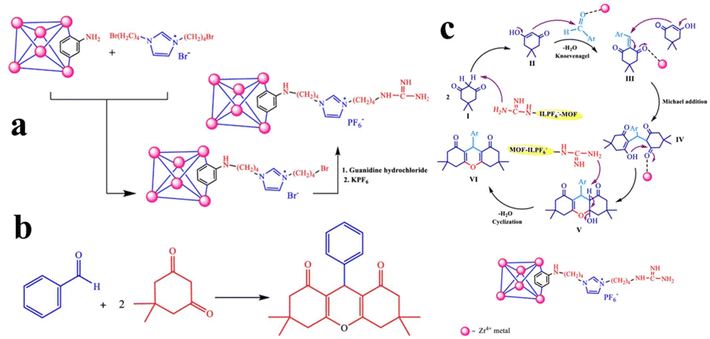 | ||
| Fig. 31 (a) Preparation of ILBr– and UiO-66-NH2-ILPF6–guanidine. (b) Reaction of aldehydes with dimedone generating xanthene derivatives. (c) Plausible reaction mechanism. Reproduced from ref. 169 with permission. Copyright 2021 Springer. | ||
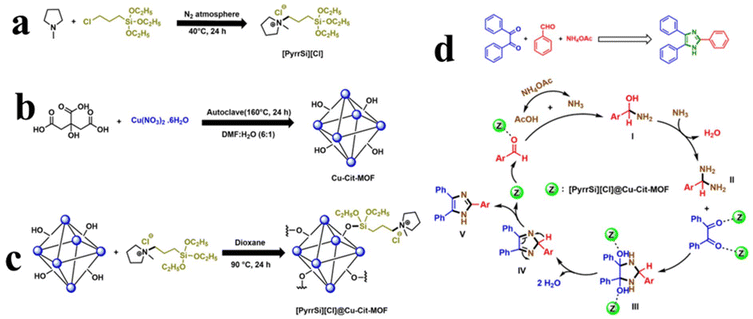 | ||
| Fig. 32 Synthesis of the [PyrrSi][Cl] ionic liquid (a), Cu-Cit-MOF (b), and [PyrrSi][Cl]@Cu-Cit-MOF (c). (d) Synthesis of 2,4,5-triphenyl-1H-imidazole as well as the reaction mechanism. Reproduced from ref. 174 with permission. Copyright 2023 published by Elsevier under a CC-BY license. | ||
Similarly, Hu et al.175 unveiled an approach by leveraging premodified chiral IL-decorated UiO-68 assembled onto TiO2 nanoparticles via tandem reactions, generating TiO2@UiO-68-CIL. This inventive approach capitalizes on the distinctive attributes of CIL and TiO2 nanoparticles, which integrates the chiral catalytic properties of CIL and the photocatalytic ability of TiO2 in one moiety. As a result, the resulting TiO2@UiO-68-CIL demonstrates effectiveness in the one-pot Morita–Baylis–Hillman reaction starting from aromatic alcohols in a tandem way (Fig. 33a). The resulting composite catalysts exhibit good photocurrent responses, which are ascribed to the presence of TiO2 (Fig. 33b). Remarkably, TiO2@UiO-68-CIL shows good stability and activity. As shown in Fig. 33c, once the catalyst is filtrated out, the reaction may stop. This phenomenon indicates no leaching of the catalytically active sites. Furthermore, the steady product yield and high ee (%) selectivity also verify the stability of the catalyst, which is also directly evidenced by XRD and SEM (Fig. 33d–f).
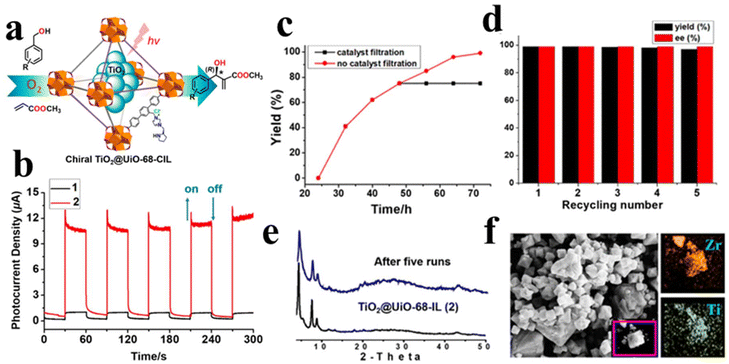 | ||
| Fig. 33 (a) Structural diagram of the TiO2@UiO-68-CIL composite as well as catalytic principle. (b) Transient photocurrents of 1 (black line) and 2 (red line). (c) Time-dependent product yield (red line) and leaching test (black line) for the catalyzed one-pot tandem model reaction. (d) Recycling performance of the prepared catalyst for the one-pot stepwise reaction based on 4-methoxybenzyl alcohol and methyl acrylate. (e) PXRD characterization of as-synthesized 2 before and after five catalyzing runs. (f) SEM image and the corresponding EDS elemental mapping of the catalyst after five recycle runs. Reproduced from ref. 175 with permission. Copyright 2019 American Chemical Society. | ||
Energy storage
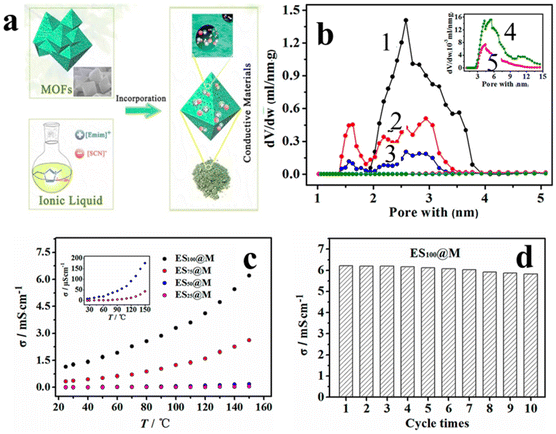 | ||
| Fig. 34 (a) Synthesis of IL/MIL-101 composite. (b) DFT predicted pore size distribution of various samples, (1) MIL-101, (2) ES25@MIL-101, (3) ES50@MIL-101, (4) ES75@MIL-101, and (5) ES100@MIL-101. (c) Temperature dependent ionic conductivity of samples. (d) Cycle testing of the temperature-dependent conductivity of ES100@MIL-101. Reproduced from ref. 179 with permission. Copyright 2019 American Chemical Society. | ||
By filling a guest electrolyte into ZIF-8, Sun et al.180 reported a ZIF-8-based quasi-solid-state electrolyte (QSSE). The resulting ZIF-8 QSSE exhibits good ionic conductivity (1.05 × 10−4 S cm−1). The lithium ion mobility investigation reveals that the lithium-ion transference number is very high, reaching up to 0.52. In order to verify the potential of the availability of the ZIF-8 QSSE, they further assembled a prototype lithium battery, in which LiCoO2 is utilized as the cathode and the ZIF-8 QSSE acts as the electrolyte. The device shows good electrochemical performances. Fujie et al.181 demonstrate the ionic conductivity of an IL inside the micropores of a MOF. Their investigated system consists of EMI-TFSA (1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)amide) and ZIF-8 (Zn(MeIM)2, H(MeIM) = 2-methylimidazole) as the IL and the MOF, respectively. Different from the bulk EMI-TFSA, which produces a dramatically decreasing trend in ionic conductivity upon freezing, the EMI-TFSA@ZIF-8 showed no marked decrease. This result can be ascribed to no phase transition occurring in EMI-TFSA@ZIF-8. Qi et al.182 designed a IPPZ solid-like electrolyte through a facile in situ growth method, in which the Li-[EMIM][TFSI] IL is impregnated in ZIF-8/PP porous membrane (Fig. 35a). By integral distribution of ZIF-8 nanoparticles in the whole PP membrane, the affinition to Li-[EMIM][TFSI] is dramatically improved compared to pristine PP. As a result, there will be more transportation pathways for lithium-ion migration, leading to an enhanced ionic conductivity. The ionic conductivity and lithium-ion transference number reach up to 2.09 × 10−4 S cm−1 and 0.45 at 25 °C, respectively. What is more, the SILM demonstrates superior mechanical properties over the PP separator owing to the consequence of “crack pinning”. For the ZIF-8/PP composite membrane, both the tensile strength and elongation at break are demonstrated to be dramatically improved, reaching 131 MPa and 205.2%, respectively. Such performances are much superior over those of commercial PP separator (127 MPa and 128%). When used in a Lu symmetrical cell, a prominent Li plating/stripping properties can be delivered with long duration (550 h at 0.1 mA cm−2 and 0.1 mA h cm−2). A full LiFePO4/Li battery was further constructed using the solid-like electrolyte. The rate capability is determined to be 157.9 mA h g−1 at the initial stage. Meanwhile, the device also demonstrates excellent cycling stability with maintaining 91.23% of the initial capacity after 450 cycles at 0.2 C (Fig. 35b). The time dependent voltage profiles of the symmetrical cells (Li/IPPZ/Li and Li/IPP/Li) are shown in Fig. 35c. For the IPPZ solid-like electrolyte, a smooth and steady curve is manifested, which reaches up to 550 h with negligible polarization, demonstrating excellent cycling stability of the Li/IPPZ/Li symmetrical cell. Wang et al.183 constructed Li-IL@HKUST-1 ion conductive membrane by filling the pores of HKUST-1(Cu) with Li-IL, which can be further used to construct flexible composite polymer electrolytes (CPEs) via the incorporation of Li-IL@HKUST-1 with PEO. An enhanced ionic conductivity (1.20 × 10−4 S cm−1) is obtained for the as-synthesized CPE membrane compared to the PEO-only electrolyte (9.76 × 10−6 S cm−1). Furthermore, the combination of Li-IL@HKUST-1 and the PEO matrix demonstrates an effective approach to reinforce the polymer matrix and facilitate the lithium ion transformation. As a result, a LiFePO4/Li solid-state battery is further assembled, which delivers a stable reversible capacity of 136.2 mA h g−1 with a capacity retention of 92% after 100 cycles at a high current density of 1 C (60 °C). Wu et al.184 developed a quasi-solid electrolyte by incorporating two types of ILs into MOFs. As depicted in Fig. 35d, the ionic transport channels are continuous, which are connected owing to the presence of the functional sulfonic acid groups serving as lithium ion hopping sites. Obviously, such continuous channels are beneficial for accelerating the Li+ transport both in the bulk and at the interfaces.
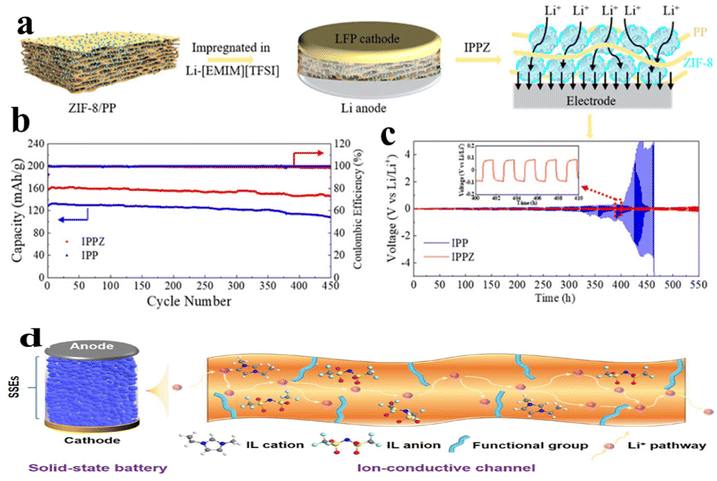 | ||
| Fig. 35 (a) Schematic of the IPPZ solid-like electrolyte and lithium-ion transportation mechanism. (b) Cycling performances of LFP/IPPZ/Li and LFP/IPP/Li batteries. (c) Voltage profiles of Li stripping/plating for Li/IPPZ/Li and Li/IPP/Li symmetrical cells. The inset is the magnified curve between 400 and 410 h of Li/IPPZ/Li. Reproduced from ref. 182 with permission. Copyright 2019 American Chemical Society. (d) Illustration of the Assembly and Design of the Solid-State Battery Based on the UN-LiM-IL SE. Reproduced from ref. 184 with permission. Copyright 2023 American Chemical Society. | ||
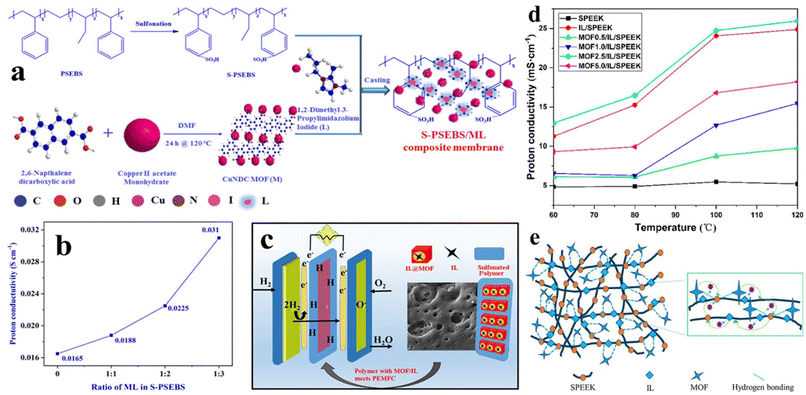 | ||
| Fig. 36 (a) Synthesis of the S-PSEBA/ML composite membrane. (b) Proton conductivities of S-PSEBS and composite membranes. (c) Profile of a fuel cell based on S-PSEBA/ML composite membrane. Reproduced from ref. 185 with permission. Copyright 2022 American Chemical Society. (d) Temperature dependent proton conductivity of various SPEEK membranes. (e) Mechanism diagram of proton conduction in composite membranes. Reproduced from ref. 187 with permission. Copyright 2022 American Chemical Society. | ||
Sulfonated poly(ether ether ketone) (SPEEK) represents another proton-conducting polymer with certain thermodynamic properties. Similar to other polymer membranes, its efficiency is also restricted by its poor proton conductivity.186 In order to improve its performance, Sun et al.187 prepared ternary composite membrane of Co-MOF-74/phosphate-4-phenylimidazole/SPEEK (Co-MOF-74/[IM2][H2PO4]/SPEEK) via a solution casting method. In this ternary composite membrane, the IL is encapsulated by MOF, which is achieved through hydrogen bonding. Fig. 36d compares the proton conductivity of various SPEEK membranes modified by either MOF or IL. The proton conductivities of all membranes produce increase trend with increasing temperature. This result conforms well to molecular dynamics since a high temperature is favorable for polymer chains rendering fluidity, facilitating proton transport in the membrane. Compared to the pristine SPEEK membrane, the composite demonstrates much better performance when used in batteries. Particularly, an ultrahigh proton conductivity of 25.96 mS cm−1 is observed for the optimal composite sample (MOF2.5/IL/SPEEK). This value is improved at least by five times compared with the SPEEK membrane. Fig. 36e presents the preliminary proton conduction mechanism. Such high proton conductivity can be ascribed to the following aspects. On the one hand, the composite produces a “ship-in-bottle” structure, where the ILs act as ship and the MOF act as bottle. The encapsulation of ILs in MOF is achieved by hydrogen bonding. Benefiting from such confinement effect, the ILs can only move along the cage of MOF, limiting their escape from the MOF. Thus, for the composite membrane an improvement in the proton conductivity can be obtained. On the other hand, the interaction between the electron-withdrawing groups (benzimidazole) on [IM2][H2PO4−] also makes contribution to the increase in membrane conductivity. Simultaneously, the interaction between benzimidazole and the MOF on [IM2][H2PO4−] leads to more free H2PO4− ions, both of which are beneficial for proton conductivity. By incorporating an IL-impregnated MOF inorganic nanofiller (IL@NH2-MIL-101), Ru et al.188 designed a hybrid proton exchange membrane based on SPAEK with pendent carboxyl groups. The resulting membrane possessed a high proton conductivity of 0.184 S cm−1, a low methanol permeability of 7.53 × 10−7 cm2 s−1, and an outstanding selectivity of 2.44 × 105 S cm−3, demonstrating practical application in direct methanol fuel cells.
Adsorption
The water environment is polluted mainly by the rapid development of industry and agriculture, posing a new threat to humans, animals, and the ecosystem. To date, many treatment methods, advanced oxidation, adsorption, reverse osmosis, electrolysis, and so on, have already been developed for the purification of sewage. Among all available methods, adsorption is regarded as one of the most encouraging innovations for the removal of pollutants. The key is to develop low-cost, highly selective, and reusable adsorbents. In this regard, IL/MOF composites have been regarded as one of the most efficient adsorbents, because of their flexible combination of metal ions/clusters and organic linkers, which provides large space to adjust their physicochemical properties and structural features such as porosity, specific surface area and so on.Oily pollutants originating from the oil exploitation and various human activities represent one of the serious environmental issues. Many oily pollutants may form emulsion in water. In order to remove oily pollutants, Wang et al.189 presented a demulsifier through the creation of an innovative composite, Amim@MIL-100(Fe) (Fig. 37a). This composite is designed with in situ encapsulation of the IL (1-allyl-3-methylimidazolium, Amim) into the micropore of MIL-100(Fe). The encapsulated ILs demonstrate to increase the positive charge density of MIL-100(Fe), which has been verified by zeta potential. Importantly, the study reports an impressive and recyclable adsorption capacity for purification of sodium dodecyl sulfate (SDS) stabilized oil-in-water model emulsion by the Amim@MIL-100(Fe) composite. The demulsification efficiency (DE) under optimal condition reaches up to 94% within 30 s, which is much superior over the pristine MIL-100(Fe). By prolonging the time to 5 min, the maximum DE of Amim@MIL-100(Fe) can reach 98% (Fig. 37b). Notably, the DE retention by MIL-100(Fe) at the third run was improved from 64 to 83% after encapsulating Amim+ (Fig. 37c). Of significance is the role of cations in ILs encapsulated in MIL-100(Fe), which could be explained by the enhanced nonbonded interaction (π⋯Fe interaction) of electrostatic attraction and van der Waals.
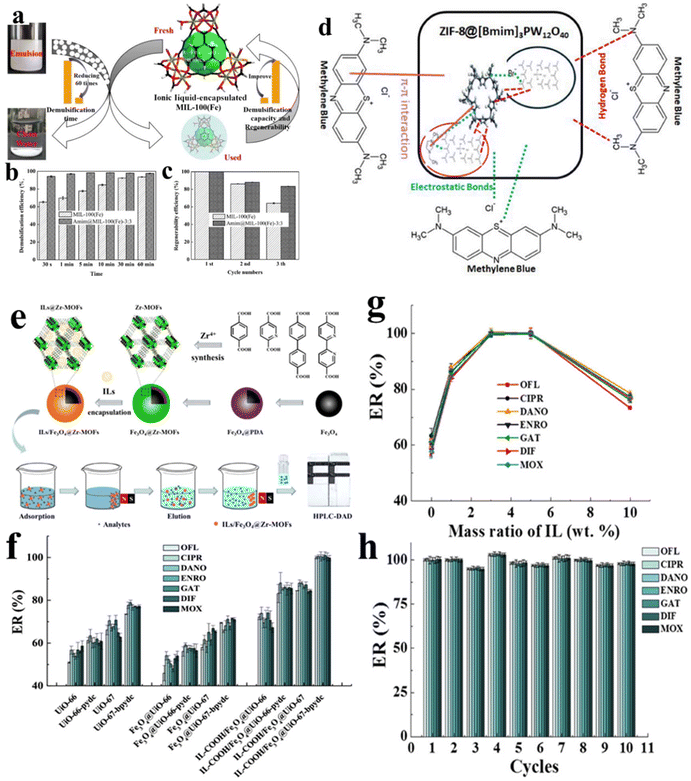 | ||
Fig. 37 (a) Schematic of IL/MIL-100 (Fe) for oil–water separation. Demulsification efficiencies for SDS-stabilized model emulsion over MIL-100(Fe) and Amim@MIL-100(Fe)-3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 at different times (b) and regenerability of MIL-100(Fe) and Amim@MIL-100(Fe)-3 3 at different times (b) and regenerability of MIL-100(Fe) and Amim@MIL-100(Fe)-3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 for demulsification (c). Reproduced from ref. 189 with permission. Copyright 2021 American Chemical Society. (d) Possible mechanism of adsorption of MB onto ZIF-8-[Bmim]3PW12O4. Reproduced from ref. 190 with permission. Copyright 2021 American Chemical Society. (e) Schematic illustration for preparing ILs/Fe3O4@Zr-MOFs and MSPE. (f) Extraction rates of FQs on various adsorbents. (g) Reproduced from ref. 196 with permission. Copyright 2021 American Chemical Society. 3 for demulsification (c). Reproduced from ref. 189 with permission. Copyright 2021 American Chemical Society. (d) Possible mechanism of adsorption of MB onto ZIF-8-[Bmim]3PW12O4. Reproduced from ref. 190 with permission. Copyright 2021 American Chemical Society. (e) Schematic illustration for preparing ILs/Fe3O4@Zr-MOFs and MSPE. (f) Extraction rates of FQs on various adsorbents. (g) Reproduced from ref. 196 with permission. Copyright 2021 American Chemical Society. | ||
Ranjbari et al.190 investigated the adsorption of MB on [BmIm]3PW12O40/ZIF-8, in which the polyoxometalate-based IL is encapsulated within ZIF-8. This encapsulation is achieved through the reaction between the imidazolate groups from ZIF-8 with polyoxometalate. Various factors are systematically investigated when evaluating the adsorption of MB onto the [BmIm]3PW12O40/ZIF-8, including the adsorbent dosage, acidity of MB solution, and contact time. Their result indicates that the optimal adsorption performance is obtained at a pH of 11, an adsorbent dosage of 4 g L−1, and a contact time of 45 min. The removal efficiency gives an increase trend with the pH of solution (from 7 to 11). Specifically, the removal rate of MB can reach up to 95% within the first 45 min at pH = 11. The maximum removal percentage is found to be 95.75%. The adsorption kinetics conforms to the pseudo-second-order kinetic model. The high adsorption performance can be corresponding to the multiple interactions between MB molecules and ZIF-8-[Bmim]3PW12O40 electrostatic interactions, hydrogen bonds, and π–π interactions (Fig. 37d). Khan et al.191 supported acidic IL supported on MIL-101 and demonstrated that the produced IL/MIL-101 shows a remarkable improvement in adsorptive desulfurization compared to the pristine MIL-101. Mechanism investigation reveals that the improved adsorptive performance is probably ascribed to the acid–base interactions between the acidic IL and basic benzothiophene (BT). Li et al.192 investigated the adsorption of Congo red onto imidazole/pyridine-based ILs modified MOF. The adsorption capability reaches high up to 544.56 mg g−1, while the removal rate is 83.28%. Such adsorption performance is much superior over the pure MOF, 407.37 mg g−1 and a removal rate of 65.46%. The adsorption kinetic data conforms to Langmuir model well, which is mainly derived from π–π interactions, electrostatic interactions, and hydrogen bonding between IL/MOF and Congo red.
Nowadays, it is desirable to develop recyclable adsorbents for avoiding secondary pollution. Among various separation techniques, magnetic separation shares advantages of high isolation efficiency and easy operation.193–195 As such, Lu et al.196 developed a composite adsorbent consisting of IL containing carboxyl groups supported on Fe3O4@Zr-MOF (denoted as IL-COOH/Fe3O4@Zr-MOFs). Fig. 37e presents the synthetic procedures of such composite adsorbents. As one sees that PDA-functionalized Fe3O4 is introduced to construct the core–shell structure via layer-by-layer modification. The PDA demonstrates several roles in this occasion. For one thing, PDA allows to endow the Fe3O4@Zr-MOFs with positive charge, which is beneficial for affinity toward FQs. For another thing, the acid-proofing properties of Fe3O4 may be improved. This role is very similar to functionalize Fe3O4 with carbon and other polymers. In addition, the core of magnetic Fe3O4 ensures excellent response of the resulting adsorbent to an external magnet, allowing recovery for recycling use. Afterwards, the hydrophobic carboxyl-functionalized IL (IL-COOH) is encapsulated into Fe3O4@Zr-MOFs. In this way, the selectivity of the adsorbent toward FQs demonstrates improvement, leading to a high removal efficiency. In their work, a series of Zr-MOFs are examined, among which IL-COOH/Fe3O4@UiO-67-bpydc delivers the most excellent adsorption efficiency, when evaluated as an adsorbent for the extraction of residual FQs in environmental water samples (Fig. 37f–h) in terms of adsorption capacity, recovery rate, and recycling performance. A high adsorption capacity of ofloxacin is obtained with the maximum value reaching up to 438.5 mg g−1. The recoveries of environmental water were ranging from 90.0 to 110.0%, and the detection limits were lower than 0.02 μg L−1. Excitingly, the composite adsorbent shows good reusability and regeneration capacity with no significant ER loss for 10 cycles with the reloading of IL after every three times.
Utilizing a ship-in-bottle method, Yohannes et al.197 prepared three IL/MOF composites, where the a tropine-based IL (N-propyl-substituted tropine hexafluorophosphate, [C3tr][PF6]) is used to composite with three MOFs (MIL-101, HKUST-1 and ZIF-8). These IL/MOF composites shown excellent adsorption performance toward enrichment of tropane alkaloids, followed by chromatographic analysis. Among measured samples, [C3tr][PF6]@MIL-101 delivers excellent adsorption capacity. The extraction results showed satisfactory recoveries in the range of 91.5–104.7% (RSD < 5%). Further, the linear range from 100–500 μg L−1 with a very low detection limit (10–20 μg L−1). Rong et al.198 prepared a series of PIL/DUT composites via combining in situ polymerization and quaternization. The adsorption of some pollutants (including permanganate, methyl blue and methyl orange) onto the resulting PIL/DUT composite is investigated in detail. Under optimal conditions, the screened pollutant can be removed efficiently by the optimized adsorbent, UT-5-(60)I. The removal rate reaches up to 90% within 10 min such high adsorption performance is ascribed to the large surface area and abundant exchangeable free iodide ions.
Besides organic pollutants, the rapid development of chemical industry caused an increased extent of heavy metal contamination in water sources, resulting in serious threaten to the health of human beings through food chain.199–202 IL/MOF composites can also be used for eliminating heavy metal ions from wastewater. Luo et al.203 developed a composite adsorbent comprising chitosan/ZIF-8/algal powder (ZIF-8/CP/CT), delivering efficient adsorption of uranyl ions in seawater. As presented in Fig. 38a, the composite adsorbent is synthesized via in situ formation of ZIF-8 on Chlorella pyrenoidosa powder immobilized by quaternary phosphorus salt ILs with chitosan. In their work, tannic acid is utilized as crosslinker to construct chitosan network, enhancing its stability. When evaluated as adsorbent for adsorptive removal of uranium from water, the resulting composite adsorbent demonstrates outstanding performance. A series of experimental parameters are systematically considered to investigate the adsorption process, including the dosage of adsorbent, the pH of the solution, and so on. Their result indicates that 5 mg dosage of the adsorbent offers a high uranium removal efficiency of 98%, which can be increased to 99% at a dosage of 10 mg. The adsorption capacity under optimal conditions reaches873 mg g−1 (308 K, pH = 6.0). Furthermore, the resulting ZIF-8/CP/CT demonstrates high selectivity toward U(VI) removal from seawater, which is evidenced by the distribution coefficients of different metal ions after adsorption (Fig. 38b). Kinetic investigation reveals the adsorption process is a spontaneous exothermic reaction. As for the adsorption mechanism, the hydroxyl, amino, and P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds on the surface of ZIF-8/CP/CT and the C
O bonds on the surface of ZIF-8/CP/CT and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond within ZIF-8 demonstrate a significant role in facilitating uranium adsorption. Furthermore, the resulting ZIF-8/CP/CT shows good recycling stability. As shown in Fig. 38c, the infrared spectrum of ZIF-8/CP/CT after three desorption cycles shows no discernible uranium peak, resembling its preadsorptive state, demonstrating negligible damage to its components.
N bond within ZIF-8 demonstrate a significant role in facilitating uranium adsorption. Furthermore, the resulting ZIF-8/CP/CT shows good recycling stability. As shown in Fig. 38c, the infrared spectrum of ZIF-8/CP/CT after three desorption cycles shows no discernible uranium peak, resembling its preadsorptive state, demonstrating negligible damage to its components.
 | ||
| Fig. 38 (a) Schematic of the synthesis of the ZIF-8/CP/CT composite adsorbent. (b) Distribution coefficients (Kd) of ZIF-8/CP/CT to competing ions. (c) Infrared spectra of ZIF-8/CP/CT, ZIF-8/CP/CT-D, and ZIF-8/CP/CT-U. Reproduced from ref. 203 with permission. Copyright 2024 Elsevier. | ||
Besides acting adsorbent, IL/MOFs can be converted into carbon-based adsorbents. Ahmed et al.204 synthesized a porous carbon from the calcination of the IL@MOF composite (Fig. 39a). The obtained carbon material (noted as IMDC, ionic liquids@MOF-derived carbons) was equipped with high nitrogen content, prominent mesoporosity, and high pore volume, as determined by comprehensive characterization results. The batch adsorption experiments indicated IMDC exhibited efficient adsorptive removal ability of contaminants from both aqueous and organic solutions. Significantly, the adsorption performance of the resulting IMDC was much superior over commercially activated carbon and MDC (pristine MOF derived carbon), as verified by Fig. 39b and c. A plausible mechanism for the adsorption is illustrated in Fig. 39d. The high adsorption performance might be through hydrogen bond for all of the adsorbates.
 | ||
| Fig. 39 (a) Schematic illustration for synthesizing IMDC for adsorptive removal of pollutants in water. (b and c) Adsorption performances of several pollutant onto IMDC. (d) Plausible adsorption mechanism of several pollutants onto IMDC. Reproduced from ref. 204 with permission. Copyright 2017 American Chemical Society. | ||
Utilizing IL/MOF composites as adsorbents to remove pollutants has several advantages. First, the environmentally friendly ILs and versatile MOFs can produce a synergistic adsorption performance, sparking considerable interest among researchers. Second, the combination of ILs and MOFs is capable of offering a novel and captivating avenue, promising the creation of adsorbent materials, which allows for the tuning of the target pollutant affinity of MOFs, resulting in adsorption improvement in capacity ad selectivity. Third, benefiting from the obvious linkages among the pore walls of MOFs and ILs, ILs could be properly penetrated into MOFs, enhancing the pollutant capture capacity of MOFs with low IL content. Finally, the incorporation of ILs into MOFs provides more heteroatoms, which is beneficial for preparing doped carbon materials, as we know that carbon materials also represent a great family of advanced functional materials, whose applications cover many fields. In a word, composites comprising ILs and MOF have become emerging hybrid materials with adaptable characteristics and prospective uses in adsorptive removal of pollutants.
Conclusion
The study provided a comprehensive overview of IL/MOF composites, covering the history, synthetic methodology, and sustainable applications. In this review, we started from the advantageous characteristics of ILs and MOFs, highlighting the necessary trend of the combination of these two fascinating components. We also briefly looked back to the history of IL/MOF composites. Afterwards, we systematically summarized the synthesis methodology for preparing IL/MOF composites, emphasizing each chemical principle and interaction between the IL guest and MOF host. Furthermore, we focused on the sustainable applications of IL/MOF composites, including CO2 capture, CO2 conversion, organic catalysis, energy storage devices, and adsorption removal of water pollutant. Lastly, the research progress of IL/MOF composites for sustainable applications was outlined.(1) As far as their synthesis is concerned, despite a huge group of MOFs and ILs available, most of reported IL/MOF composites are focused on only a minor fraction of MOFs (e.g., ZIF-8, CuBTC, HKUST-1, UiO-66, and MIL-101) and ILs (particularly imidazolium-based ILs). Thus, it is necessary to enlarge the family of both MOFs and ILs for promoting the development of IL-MOF composites by exploring more advantageous properties.
(2) Followed up with the synthetic methodology, it is impractical and unnecessary to experimentally combine each of MOFs and ILs for the extensive diversity of MOFs and ILs. No matter what stage this area locates, we are always desiring for new computation methods to identify the most matchable combination of ILs and MOFs for obtaining specific properties, optimizing experimental efforts and resource allocation.
(3) Moving forward, it is also necessary to develop new techniques to disclose the fine structural information of IL/MOF composites. In most of the existing literature reports on IL/MOF composites, comprehensive insights into atomic-level structure information seems to be lost. Surely the traditional characterization techniques such as powder XRD, NMR, FTIR, BET, SEM/TEM, and XPS can assist in identifying fundamental phase/crystal structures, bonding information between IL and MOF, porosity, morphologies, and valence states of elements in IL/MOF composites. Nevertheless, they may fall short in achieving the necessary resolution to untangle ILs’ precious configuration as well as their real distribution in IL/MOF composites. Furthermore, in situ characterization is urgent to reveal the property evolution upon working for special applications.
(4) Apart from the design and synthesis, the compatibility of IL/MOF systems should be taken seriously into account. Take catalysis application as an example in case, most current systems are carried out in organic solvent, which seems not to be environmentally friendly. The reason can be partly ascribed to the instability of IL/MOF systems in water. As a result, it is necessary to develop IL/MOF systems which can be used in aqueous media.
(5) In future, much effort should be devoted to the structure–performance relationship of IL-MOF composites. As demonstrated in the above sections, extensive experimental research has been carried out on a wide range of application areas. Unfortunately, few work attempted to vary the type of MOF or IL to uncover the relationship between the structure and application performance of IL/MOF composites. Meanwhile, the corresponding theory investigation should also be carried out. Currently, most simulation works for IL/MOFs nearly focus on gas capture.
Data availability
The data and materials used in this study are available upon reasonable request.Conflicts of interest
The authors declare no competing interest.References
- X. Sang, J. Zhang, J. Xiang, J. Cui, L. Zheng, J. Zhang, Z. Wu, Z. Li, G. Mo, Y. Xu, J. Song, C. Liu, X. Tan, T. Luo, B. Zhang and B. Han, Ionic liquid accelerates the crystallization of Zr-based metal–organic frameworks, Nat. Commun., 2017, 8, 175 CrossRef.
- V. Nozari, C. Calahoo, J. M. Tuffnell, D. A. Keen, T. D. Bennett and L. Wondraczek, Ionic liquid facilitated melting of the metal-organic framework ZIF-8, Nat. Commun., 2021, 12, 5703 CrossRef CAS PubMed.
- H. Cheng and J. Ouyang, Soret Effect of Ionic Liquid Gels for Thermoelectric Conversion, J. Phys. Chem. Lett., 2022, 13(46), 10830–10842 CrossRef CAS.
- M. Zhu and Y. Yang, Poly(ionic liquid)s: an emerging platform for green chemistry, Green Chem., 2024, 26, 5022–5102 RSC.
- W. S. He, L. Zhao, H. Yang, J. Rui, J. Li and Z. Y. Chen, Novel Synthesis of Phytosterol Ferulate Using Acidic Ionic Liquids as a Catalyst and Its Hypolipidemic Activity, J. Agric. Food Chem., 2024, 72(4), 2309–2320 CrossRef CAS.
- R. Kore, A. M. Scurto and M. B. Shiflett, Review of Isobutane Alkylation Technology Using Ionic Liquid-Based Catalysts—Where Do We Stand?, Ind. Eng. Chem. Res., 2020, 59(36), 15811–15838 CrossRef CAS.
- A. Zhu, T. Jiao, S. Ali, Y. Xu, Q. Ouyang and Q. Chen, Dispersive micro solid phase extraction based ionic liquid functionalized ZnO nanoflowers couple with chromatographic methods for rapid determination of aflatoxins in wheat and peanut samples, Food Chem., 2022, 391, 133277 CrossRef CAS PubMed.
- I. D. Boateng, X. M. Yang, H. Yin and W. Liu, Separation and purification of polyprenols from Ginkgo biloba leaves by silver ion anchored on imidazole-based ionic liquid functionalized mesoporous MCM-41 sorbent, Food Chem., 2024, 450, 139284 CrossRef CAS PubMed.
- K. R. Baca, K. Al-Barghouti, N. Wang, M. G. Bennett, L. M. Valenciano, T. L. May, I. V. Xu, M. Cordry, D. M. Haggard, A. G. Haas, A. Heimann, A. N. Harders, H. G. Uhl, D. T. Melfi, A. D. Yancey, R. Kore, E. J. Maginn, A. M. Scurto and M. B. Shiflett, Ionic Liquids for the Separation of Fluorocarbon Refrigerant Mixtures, Chem. Rev., 2024, 124(9), 5167–5226 CrossRef CAS PubMed.
- T. Zhou, C. Gui, L. Sun, Y. Hu, H. Lyu, Z. Wang, Z. Song and G. Yu, Energy Applications of Ionic Liquids: Recent Developments and Future Prospects, Chem. Rev., 2023, 123(21), 12170–12253 CrossRef CAS PubMed.
- B. A. Rosen, A. Salehi-Khojin, M. R. Thorson, W. Zhu, D. T. Whipple, P. J. A. Kenis and R. I. Masel, Ionic Liquid–Mediated Selective Conversion of CO2 to CO at Low Overpotentials, Science, 2011, 334, 643–644 CrossRef CAS PubMed.
- J. Wang, L. Xu, G. Jia and J. Du, Challenges and Opportunities of Ionic Liquid Electrolytes for Rechargeable Batteries, Cryst. Growth Des., 2022, 22(9), 5770–5784 CrossRef.
- S. Zeng, X. Zhang, L. Bai, X. Zhang, H. Wang, J. Wang, D. Bao, M. Li, X. Liu and S. Zhang, Ionic-Liquid-Based CO2 Capture Systems: Structure, Interaction and Process, Chem. Rev., 2017, 117(14), 9625–9673 CrossRef CAS PubMed.
- S. Yan, F. Han, Q. Hou, S. Zhang and S. Ai, Recent Advances in Ionic Liquid-Mediated SO2 Capture, Ind. Eng. Chem. Res., 2019, 58(31), 13804–13818 CrossRef CAS.
- G. Cai, P. Yan, L. Zhang, H. C. Zhou and H. L. Jiang, Metal–Organic Framework-Based Hierarchically Porous Materials: Synthesis and Applications, Chem. Rev., 2021, 121(20), 12278–12326 CrossRef CAS PubMed.
- X. An, X. Gao, J. Yin, L. Xu, B. Zhang, J. He, H. Li, H. Li and W. Jiang, MOF-derived amorphous molybdenum trioxide anchored on nitrogen-doped porous carbon by facile in situ annealing for efficient oxidative desulfurization, Chem. Eng. J., 2024, 480, 147879 CrossRef CAS.
- S. Jia, F. Ma, R. Lin, M. Yan and Y. Wu, MOF-initiated imprinted self-supporting basswood membranes for precise recognition and separation of artemisinin, J. Environ. Chem. Eng., 2023, 11, 110316 CrossRef CAS.
- J. Li, J. Jia, D. Wang, H. Dong and M. Zhu, Recent research progress of MOFs-based heterostructures for photocatalytic hydrogen evolution, Chem. Eng. J., 2024, 498, 155194 CrossRef CAS.
- P. Chen, J. Hou and L. Wang, Metal-organic framework-tailored perovskite solar cells, Microstructures, 2022, 2, 2022014 CAS.
- Y. Wang, Y. Shi, Z. Li, H. Wang, J. Qiu, X. Xuan and J. Wang, Defects in a Metal–Organic Framework Fabricated by Carboxy-Functionalized Ionic Liquids for Enhancing NH3 Uptake, ACS Sustainable Chem. Eng., 2022, 10(37), 12457–12465 CrossRef CAS.
- S.-G. Koh, T. Koide, A. A. Ohira and K. Kinoshita, Structural Strengthening of Metal–Organic Frameworks Owing to the Confinement Effect of Ionic Liquids in the Nanopores, J. Phys. Chem. C, 2022, 126(15), 6736–6744 CrossRef CAS.
- A. B. Kanj, R. Verma, M. Liu, J. Helfferich, W. Wenzel and L. Heinke, Bunching and Immobilization of Ionic Liquids in Nanoporous Metal–Organic Framework, Nano Lett., 2019, 19(3), 2114–2120 CrossRef CAS PubMed.
- S. A. Ali, A. U. Khan, W. U. Mulk, H. Khan, S. N. Shah, A. Zahid, K. Habib, M. U. H. Shah, M. H. D. Othman and S. Rahman, An Ongoing Futuristic Career of Metal–Organic Frameworks and Ionic Liquids, A Magical Gateway to Capture CO2; A Critical Review, Energy Fuels, 2023, 37(20), 15394–15428 CrossRef CAS.
- K. Jin, X. Huang, L. Pang, J. Li, A. Appel and S. Wherland, [Cu(i)(bpp)]BF4: the first extended coordination network prepared solvothermally in an ionic liquid solvent, Chem. Commun., 2002, 2872–2873 RSC.
- Y. Chen, Z. Hu, K. M. Gupta and J. Jiang, Ionic Liquid/Metal–Organic Framework Composite for CO2 Capture: A Computational Investigation, J. Phys. Chem. C, 2011, 115(44), 21736–21742 CrossRef CAS.
- R. Babu, J. F. Kurisingal, J.-S. Chang and D.-W. Park, Bifunctional Pyridinium-Based Ionic-Liquid-Immobilized Diindium Tris(diphenic acid) Bis(1,10-phenanthroline) for CO2 Fixation, ChemSusChem, 2018, 11, 924–932 CrossRef CAS.
- A. Aijaz, T. Akita, H. Yang and Q. Xu, From ionic-liquid@metal–organic framework composites to heteroatom-decorated large-surface area carbons: superior CO2 and H2 uptake, Chem. Commun., 2014, 50, 6498–6501 RSC.
- Z. Li, Y. Xiao, W. Xue, Q. Yang and C. Zhong, Ionic Liquid/Metal–Organic Framework Composites for H2S Removal from Natural Gas: A Computational Exploration, J. Phys. Chem. C, 2015, 119(7), 3674–3683 CrossRef CAS.
- J. Wang, D. Xie, Z. Zhang, Q. Yang, H. Xing, Y. Yang, Q. Ren and Z. Bao, Efficient adsorption separation of acetylene and ethylene via supported ionic liquid on metal-organic framework, AIChE J., 2017, 63, 2165–2175 CrossRef CAS.
- K. M. Gupta, Y. Chen, Z. Hu and J. Jiang, Metal–organic framework supported ionic liquid membranes for CO2 capture: anion effects, Phys. Chem. Chem. Phys., 2012, 14, 5785–5794 RSC.
- C. Li, W. Zhang, Q. Meng, H. Xu, C. Shen and G. Zhang, Ionic-liquid-modified MOFs incorporated in a mixed-matrix membrane by metal-site anchoring for gas separation, Chem. Commun., 2024, 60, 4100–4103 RSC.
- Q. Dai, J. Ma, S. Ma, S. Wang, L. Li, X. Zhu and X. Qiao, Cationic Ionic Liquids Organic Ligands Based Metal–Organic Frameworks for Fabrication of Core–Shell Microspheres for Hydrophilic Interaction Liquid Chromatography, ACS Appl. Mater. Interfaces, 2016, 8(33), 21632–21639 CrossRef CAS.
- Y. Lan, T. Yan, M. Tong and C. Zhong, Large-scale computational assembly of ionic liquid/MOF composites: synergistic effect in the wire-tube conformation for efficient CO2/CH4 separation, J. Mater. Chem. A, 2019, 7, 12556–12564 RSC.
- E. Fernandez, P. G. Saiz, N. Peřinka, S. Wuttke and R. F. de Luis, Printed Capacitive Sensors Based on Ionic Liquid/Metal-Organic Framework Composites for Volatile Organic Compounds Detection, Adv. Funct. Mater., 2021, 31, 2010703 CrossRef CAS.
- P. Qin, S. Okur, Y. Jiang and L. Heinke, A MOF-based electronic nose for carbon dioxide sensing with enhanced affinity and selectivity by ionic-liquid embedment, J. Mater. Chem. A, 2022, 10, 25347–25355 RSC.
- Z. Liu, Z. Hu, X. Jiang, X. Wang, Z. Li, Z. Chen, Y. Zhang and S. Zhang, Metal-Organic Framework Confined Solvent Ionic Liquid Enables Long Cycling Life Quasi-Solid-State Lithium Battery in Wide Temperature Range, Small, 2022, 18, 2203011 CrossRef CAS.
- S. Zhang, Y. Xie, R. J. Somerville, F. F. Tirani, R. Scopelliti, Z. Fei, D. Zhu and P. J. Dyson, MOF-Based Solid-State Proton Conductors Obtained by Intertwining Protic Ionic Liquid Polymers with MIL-101, Small, 2023, 19, 2206999 CrossRef CAS.
- V. Nozari, A. N. V. Azar, R. Sajzew, C. Castillo-Blas, A. Kono, M. Oschatz, D. A. Keen, P. A. Chater, G. P. Robertson, J. M. A. Steele, L. León-Alcaide, A. Knebel, C. W. Ashling, T. D. Bennett and L. Wondraczek, Observation of a Reversible Order-Order Transition in a Metal-Organic Framework-Ionic Liquid Nanocomposite Phase-Change Material, Small, 2024, 2303315 CrossRef CAS PubMed.
- K. Fujie, T. Yamada, R. Ikeda and H. Kitagawa, Introduction of an Ionic Liquid into the Micropores of a Metal–Organic Framework and Its Anomalous Phase Behavior, Angew. Chem., Int. Ed., 2014, 53, 11302–11305 CrossRef CAS PubMed.
- Z. Li, W. Wang, Y. Chen, C. Xiong, G. He, Y. Cao, H. Wu, M. D. Guiver and Z. Jiang, Constructing efficient ion nanochannels in alkaline anion exchange membranes by the in situ assembly of a poly(ionic liquid) in metal–organic frameworks, J. Mater. Chem. A, 2016, 4, 2340–2348 RSC.
- M. Ding and H.-L. Jiang, Incorporation of Imidazolium-Based Poly(ionic liquid)s into a Metal-Organic Framework for CO2 Capture and Conversion, ACS Catal., 2018, 8(4), 3194–3201 CrossRef CAS.
- Y. Yoshida, K. Fujie, D.-W. Lim, R. Ikeda and H. Kitagawa, Superionic Conduction over a Wide Temperature Range in a Metal–Organic Framework Impregnated with Ionic Liquids, Angew. Chem., Int. Ed., 2019, 59, 10909–10913 CrossRef.
- S. Park and H.-K. Jeong, Highly H2O permeable ionic liquid encapsulated metal–organic framework membranes for energy-efficient air-dehumidification, J. Mater. Chem. A, 2020, 8, 23645–23653 RSC.
- Z. Wei, R. Maile, L. M. Riegger, M. Rohnke, K. Müller-Buschbaum and J. Janek, Ionic Liquid-Incorporated Metal-Organic Framework with High Magnesium Ion Conductivity for Quasi-Solid-State Magnesium Batteries, Batteries Supercaps, 2022, 5, e202200318 CrossRef CAS.
- Q. Qian, H. Cheng, Q. Le and J. Ouyang, Great Enhancement in the Thermopower of Ionic Liquid by a Metal-Organic Framework, Adv. Funct. Mater., 2023, 33, 2303311 CrossRef CAS.
- X. Zhao, Q. Xu, J. Han, W. Zhang, H. Rao, D.-Y. Du, P. She and J.-S. Qin, Ionic Liquid Modified Fe-Porphyrinic Metal–Organic Frameworks as Efficient and Selective Photocatalysts for CO2 Reduction, ACS Appl. Mater. Interfaces, 2024, 16(20), 26272–26279 CrossRef CAS PubMed.
- K. Fujie and H. Kitagawa, Ionic liquid transported into metal–organic frameworks, Coord. Chem. Rev., 2016, 307, 382–390 CrossRef CAS.
- F. P. Kinik, A. Uzun and S. Keskin, Ionic Liquid/Metal–Organic Framework Composites: From Synthesis to Applications, ChemSusChem, 2017, 10, 2842–2863 CrossRef CAS.
- X. Li, K. Chen, R. Guo and Z. Wei, Ionic Liquids Functionalized MOFs for Adsorption, Chem. Rev., 2023, 123(16), 10432–10467 CrossRef CAS.
- S. Chen, N. Wang, H. Zhang, M. Qiu, L. Shi, Y. Xia, J. Zhang, Y. Huang, F. Cheng, P. Gu, X. Zhang and Q. Yi, Ionic Liquid-Functionalized Metal–Organic Frameworks/Covalent–Organic Frameworks for CO2 Capture and Conversion, Ind. Eng. Chem. Res., 2024, 63(8), 3443–3464 CrossRef CAS.
- Y. Yoshida and H. Kitagawa, Ionic Conduction in Metal–Organic Frameworks with Incorporated Ionic Liquids, ACS Sustainable Chem. Eng., 2019, 7(1), 70–81 CrossRef CAS.
- J. H. Song, S. Koo and D. W. Kang, Ionic liquid-functionalized metal organic frameworks and their composite membranes for enhanced proton transport, CrystEngComm, 2024, 26, 2450–2458 RSC.
- M. Zeeshan, H. C. Gulbalkan, O. Durak, Z. P. Haslak, U. Unal, S. Keskin and A. Uzun, An Integrated Computational–Experimental Hierarchical Approach for the Rational Design of an IL/UiO-66 Composite Offering Infinite CO2 Selectivity, Adv. Funct. Mater., 2022, 32, 2204149 CrossRef CAS.
- O. Durak, A. S. Aydogdu, N. Habib, H. C. Gulbalkan, Z. Ozerdem, S. S. Bayazit, S. Keskin and A. Uzun, In Silico-Directed Design and Experimental Validation of an IL/UiO-66 Nanocomposite with Exceptional CO2 Selectivity across a Wide Pressure Range, ACS Appl. Nano Mater., 2024, 7(18), 21705–21716 CrossRef.
- X. Xia, G. Hu, W. Li and S. Li, Understanding Reduced CO2 Uptake of Ionic Liquid/Metal–Organic Framework (IL/MOF) Composites, ACS Appl. Nano Mater., 2019, 2(9), 6022–6029 CrossRef CAS.
- K. B. Sezginel, S. Keskin and A. Uzun, Tuning the Gas Separation Performance of CuBTC by Ionic Liquid Incorporation, Langmuir, 2016, 32(4), 1139–1147 CrossRef CAS.
- F. P. Kinik, C. Altintas, V. Balci, B. Koyuturk, A. Uzun and S. Keskin, [BMIM][PF6] Incorporation Doubles CO2 Selectivity of ZIF-8: Elucidation of Interactions and Their Consequences on Performance, ACS Appl. Mater. Interfaces, 2016, 8(45), 30992–31005 CrossRef CAS.
- J. Jia, X.-M. Li, H. Wu, Q. Huang and J. Gao, Achieving High Intrinsic Proton Conductivity via Functionalization of MOF-808 by Ionic Liquid, ACS Sustainable Chem. Eng., 2023, 11(36), 13502–13507 CrossRef CAS.
- X.-L. Sun, W.-H. Deng, H. Chen, H.-L. Han, J. M. Taylor, C.-Q. Wan and G. Xu, A Metal–Organic Framework Impregnated with a Binary Ionic Liquid for Safe Proton Conduction above 100 °C, Chem. – Eur. J., 2017, 23, 1248–1252 CrossRef CAS PubMed.
- B. Mirhosseini-Eshkevari, M. A. Ghasemzadeh, M. Esnaashari and S. T. Ganjali, Hexamethylenetetramine-based ionic liquid/MIL-101(Cr) metal–organic framework composite: a novel and versatile tool for the preparation of pyrido[2,3-d:5,6-d′]dipyrimidines, RSC Adv., 2020, 11, 364–373 RSC.
- M. Zou, W. Dai, P. Mao, B. Li, J. Mao, S. Zhang, L. Yang, S. Luo, X. Luo and J. Zou, Integration of multifunctionalities on ionic liquid-anchored MIL-101(Cr): A robust and efficient heterogeneous catalyst for conversion of CO2 into cyclic carbonates, Microporous Mesoporous Mater., 2021, 312, 110750 CrossRef CAS.
- C. Chen, F. Wang, Q. Li, Y. Wang and J. Ma, Embedding of SO3H-functionalized ionic liquids in mesoporous MIL-101(Cr) through polyoxometalate bridging: A robust heterogeneous catalyst for biodiesel production, Colloids Surf., A, 2022, 648, 129432 CrossRef CAS.
- B. Han and A. Chakraborty, Synthesis and Characteristics of Ionic Liquid-Implanted HKUST-1 Metal–Organic Frameworks for Transforming Heat into Extraordinary Water Transfer, ACS Sustainable Chem. Eng., 2024, 12(21), 8115–8127 CrossRef CAS.
- Q. Luo, X. Song, M. Ji, S. Park, C. Hao and Y. Li, Molecular size- and shape-selective Knoevenagel condensation over microporous Cu3(BTC)2 immobilized amino-functionalized basic ionic liquid catalyst, Appl. Catal., A, 2014, 478, 81–90 CrossRef CAS.
- J. Yin, W. Fu, J. Zhang, X. Liu, X. Zhang, C. Wang, J. He, W. Jiang, H. Li and H. Li, UiO-66(Zr)-based porous ionic liquids for highly efficient extraction coupled catalytic oxidative desulfurization, Chem. Eng. J., 2023, 470, 144290 CrossRef CAS.
- M. A. Bunge, E. Pasciak, J. Choi, L. Haverhals, W. M. Reichert and T. G. Glover, Ionic Liquid Welding of the UIO-66-NH2 MOF to Cotton Textiles, Ind. Eng. Chem. Res., 2020, 59(43), 19285–19298 CrossRef CAS.
- M. Zeeshan, V. Nozari, S. Keskin and A. Uzun, Structural Factors Determining Thermal Stability Limits of Ionic Liquid/MOF Composites: Imidazolium Ionic Liquids Combined with CuBTC and ZIF-8, Ind. Eng. Chem. Res., 2019, 58(31), 14124–14138 CrossRef CAS.
- V. Nozari, S. Keskin and A. Uzun, Toward Rational Design of Ionic Liquid/Metal–Organic Framework Composites: Effects of Interionic Interaction Energy, ACS Omega, 2017, 2(10), 6613–6618 CrossRef CAS PubMed.
- Y. Zhang, X. Gao, N. Ma, H. Zhou and L. Feng, Efficient alcoholysis of total saponins in D. zingiberensis to diosgenin using ionic liquid-grafted MOF superacids, Mater. Chem. Phys., 2024, 319, 129389 CrossRef CAS.
- S. Sadjadi and F. Koohestani, Synthesis and catalytic activity of a novel ionic liquid-functionalized metal–organic framework, Res. Chem. Intermed., 2022, 48, 291–306 CrossRef CAS.
- W. Xiang, C. Shen, Z. Lu, S. Chen, X. Li, R. Zou, Y. Zhang and C. Liu, CO2 cycloaddition over ionic liquid immobilized hybrid zeolitic imidazolate frameworks: Effect of Lewis acid/base sites, Chem. Eng. Sci., 2021, 233, 116429 CrossRef CAS.
- Q. Luo, M. Ji, M. Lu, C. Hao, J. Qiu and Y. Li, Organic electron-rich N-heterocyclic compound as a chemical bridge: building a Brønsted acidic ionic liquid confined in MIL-101 nanocages, J. Mater. Chem. A, 2013, 1, 6530–6534 RSC.
- M. Han, Y. Li, Z. Gu, H. Shi, C. Chen, Q. Wang, H. Wan and G. Guan, Immobilization of thiol-functionalized ionic liquids onto the surface of MIL-101(Cr) frameworks by S-Cr coordination bond for biodiesel production, Colloids Surf., A, 2018, 553, 593–600 CrossRef CAS.
- J. Tharun, K.-M. Bhin, R. Roshan, D. W. Kim, A. C. Kathalikkattil, R. Babu, H. Y. Ahn, Y. S. Won and D.-W. Park, Ionic liquid tethered post functionalized ZIF-90 framework for the cycloaddition of propylene oxide and CO2, Green Chem., 2016, 18, 2479–2487 RSC.
- Y. Wang, L. Ling, W. Zhang, J. Guo, K. Ding, W. Duan and B. Liu, “Ship-in-Bottle” Strategy to Encapsulate Shape-Controllable Metal Nanocrystals into Metal–Organic Frameworks: Internal Space Matters, Chem. Mater., 2019, 31(22), 9546–9553 CrossRef CAS.
- N. A. Khan, D. K. Yoo and S. H. Jhung, Polyaniline-Encapsulated Metal–Organic Framework MIL-101: Adsorbent with Record-High Adsorption Capacity for the Removal of Both Basic Quinoline and Neutral Indole from Liquid Fuel, ACS Appl. Mater. Interfaces, 2018, 10(41), 35639–35646 CrossRef CAS PubMed.
- S.-S. Mortazavi, M. Masteri-Farahani and A. Abbasi, Ship-in-bottle preparation of multi-SO3H functionalized ionic liquid@MIL-100(Fe) for acid-catalyzed ring-opening of epoxides, Appl. Organomet. Chem., 2021, 35, e6424 CrossRef CAS.
- M. Obst, G. Arnauts, A. J. Cruz, M. C. Gonzalez, K. Marcoen, T. Hauffman and R. Ameloot, Chemical Vapor Deposition of Ionic Liquids for the Fabrication of Ionogel Films and Patterns, Angew. Chem., Int. Ed., 2021, 60(49), 25668–25673 CrossRef CAS PubMed.
- P. Su, M. Tu, R. Ameloot and W. Li, Vapor-Phase Processing of Metal–Organic Frameworks, Acc. Chem. Res., 2022, 55(2), 186–196 CrossRef CAS PubMed.
- M. Obst, M. L. Tietze, A. Matavž, S. Rodriguez-Hermida, K. Marcoen, T. Hauffman and R. Ameloot, Vapor-Phase Loading of an Ionic Liquid into a Zeolitic Imidazolate Framework, Inorg. Chem., 2022, 61(43), 17137–17143 CrossRef CAS PubMed.
- Y. Jiang, Z. Wang, P. Xu and J. Sun, Dicationic Ionic Liquid @MIL-101 for the Cycloaddition of CO2 and Epoxides under Cocatalyst-free Conditions, Cryst. Growth Des., 2021, 21(7), 3689–3698 CrossRef CAS.
- N. A. Khan, Z. Hasan and S. H. Jhung, Ionic liquid@MIL-101 prepared via the ship-in-bottle technique: remarkable adsorbents for the removal of benzothiophene from liquid fuel, Chem. Commun., 2016, 52, 2561–2564 RSC.
- Y. Zhang, Y. Duan, H. Wu, H. Xu, F. Pei, L. Shi, J. Wang and Q. Yi, Ionic-Liquid-Assisted One-Step Construction of Mesoporous Metal–Organic Frameworks, Langmuir, 2023, 39(7), 2491–2499 CrossRef CAS PubMed.
- W.-L. Xue, L. Wang, Y. K. Li, H. Chen, K. X. Fu, F. Zhang, T. He, Y. H. Deng, J. R. Li and C.-Q. Wan, Reticular Chemistry for Ionic Liquid-Functionalized Metal–Organic Frameworks with High Selectivity for CO2, ACS Sustainable Chem. Eng., 2020, 8(50), 18558–18567 CrossRef CAS.
- W.-L. Peng, J. Mi, F. Liu, Y. Xiao, W. Chen, Z. Liu, X. Yi, W. Liu and A. Zheng, Accelerating Biodiesel Catalytic Production by Confined Activation of Methanol over High-Concentration Ionic Liquid-Grafted UiO-66 Solid Superacids, ACS Catal., 2020, 10(20), 11848–11856 CrossRef CAS.
- S. Shao, Y. Wang, L. Ma, Z. Huang and X. Li, Sustainable preparation of hierarchical porous carbon from discarded shells of crustaceans for efficient CO2 capture, Fuel, 2024, 355, 129287 CrossRef CAS.
- A. M. Sampaio, A. R. Nabais, L. C. Tomé and L. A. Neves, Impact of MOF-5 on Pyrrolidinium-Based Poly(ionic liquid)/Ionic Liquid Membranes for Biogas Upgrading, Ind. Eng. Chem. Res., 2020, 59(1), 308–317 CrossRef CAS.
- Z. Tian, S. Dai and D. Jiang, Confined Ionic Liquid in an Ionic Porous Aromatic Framework for Gas Separation, ACS Appl. Polym. Mater., 2019, 1(1), 95–102 CrossRef CAS.
- Y. Ban, Z. Li, Y. Li, Y. Peng, H. Jin, W. Jiao, A. Guo, P. Wang, Q. Yang, C. Zhong and W. Yang, Confinement of Ionic Liquids in Nanocages: Tailoring the Molecular Sieving Properties of ZIF-8 for Membrane-Based CO2 Capture, Angew. Chem., Int. Ed., 2015, 54, 15483–15487 CrossRef CAS PubMed.
- D. Fairen-Jimenez, S. A. Moggach, M. T. Wharmby, P. A. Wright, S. Parsons and T. Duren, Opening the gate: framework flexibility in ZIF-8 explored by experiments and simulations, J. Am. Chem. Soc., 2011, 133, 8900–8902 CrossRef CAS PubMed.
- J. Choi, J. Im, K. Noh, J. Kim, T. Vogt and Y. Lee, Universal Gas-Uptake Behavior of a Zeolitic Imidazolate Framework ZIF-8 at High Pressure, J. Phys. Chem. C, 2019, 123(42), 25769–25774 CrossRef CAS.
- A. Phan, C. J. Doonan, F. J. Uribe-Romo, C. B. Knobler, M. O'Keeffe and O. M. Yaghi, Synthesis, structure, and carbon dioxide capture properties of zeolitic imidazolate frameworks, Acc. Chem. Res., 2010, 43, 58–67 CrossRef CAS PubMed.
- B. Koyuturk, C. Altintas, F. P. Kinik, S. Keskin and A. Uzun, Improving Gas Separation Performance of ZIF-8 by [BMIM][BF4] Incorporation: Interactions and Their Consequences on Performance, J. Phys. Chem. C, 2017, 121(19), 10370–10381 CrossRef CAS.
- M. Zeeshan, A. Klemm, J. T. Damron, K. A. Unocic, M. K. Kidder and B. Gurkan, Ionic Liquid Functionalizes the Metal Organic Framework for Microwave-Assisted Direct Air Capture of CO2, ACS Mater. Lett., 2024, 6(8), 3854–3861 CrossRef CAS.
- C. Casado-Coterillo, A. Fernández-Barquín, B. Zornoza, C. Téllez, J. Coronas and Á. Irabien, Synthesis and characterisation of MOF/ionic liquid/chitosan mixed matrix membranes for CO2/N2 separation, RSC Adv., 2015, 5, 102350–102361 RSC.
- T. J. Ferreira, R. P. P. L. Ribeiro, J. P. B. Mota, L. P. N. Rebelo, J. M. S. S. Esperança and I. A. A. C. Esteves, Ionic Liquid-Impregnated Metal–Organic Frameworks for CO2/CH4 Separation, ACS Appl. Nano Mater., 2019, 2(12), 7933–7950 CrossRef CAS.
- N. Habib, O. Durak, H. C. Gulbalkan, A. S. Aydogdu, S. Keskin and A. Uzun, Composite of MIL-101(Cr) with a Pyrrolidinium-Based Ionic Liquid Providing High CO2 Selectivity, ACS Appl. Eng. Mater., 2023, 1(6), 1473–1481 CrossRef CAS PubMed.
- M. Zeeshan, V. Nozari, M. B. Yagci, T. Isık, U. Unal, V. Ortalan, S. Keskin and A. Uzun, Core–Shell Type Ionic Liquid/Metal Organic Framework Composite: An Exceptionally High CO2/CH4 Selectivity, J. Am. Chem. Soc., 2018, 140(32), 10113–10116 CrossRef CAS PubMed.
- M. M. H. Shah Buddin and A. L. Ahmad, Performance Evaluation of Supported Ionic Liquid Membranes (SILMs) Derived from Optimized PES/PDMS/ZIF-L Composites for CO2 Separation, Ind. Eng. Chem. Res., 2023, 62(12), 5199–5215 CrossRef CAS.
- Z. Yang, Y. Ying, Y. Pu, D. Wang, H. Yang and D. Zhao, Poly(ionic liquid)-Functionalized UiO-66-(OH)2: Improved Interfacial Compatibility and Separation Ability in Mixed Matrix Membranes for CO2 Separation, Ind. Eng. Chem. Res., 2022, 61(22), 7626–7633 CrossRef CAS.
- J. Ma, L. He, R. Yang, D. Wang, D. Qu, C. Su, H. Pang, W. Wu, P. Li, L. Zhang and X. Liu, Porous liquid metal-organic frameworks with selectively high gas solubility, Fuel, 2023, 344, 128051 CrossRef CAS.
- Q. Qian, A. M. Wright, H. Lee, M. Dincă and Z. P. Smith, Low-Temperature H2S/CO2/CH4 Separation in Mixed-Matrix Membranes Containing MFU-4, Chem. Mater., 2021, 33(17), 6825–6831 CrossRef CAS.
- P. Jin, C. Huang, Y. Shen, X. Zhan, X. Hu, L. Wang and L. Wang, Simultaneous Separation of H2S and CO2 from Biogas by Gas–Liquid Membrane Contactor Using Single and Mixed Absorbents, Energy Fuels, 2017, 31(10), 11117–11126 CrossRef CAS.
- W. Gao, W. Zheng, W. Sun and L. Zhao, Understanding the Effective Capture of H2S/CO2 from Natural Gas Using Ionic Liquid@MOF Composites, J. Phys. Chem. C, 2022, 126(46), 19872–19882 CrossRef CAS.
- J. M. Vicent-Luna, J. J. Gutiérrez-Sevillano, J. A. Anta and S. Calero, Effect of Room-Temperature Ionic Liquids on CO2 Separation by a Cu-BTC Metal–Organic Framework, J. Phys. Chem. C, 2013, 117(40), 20762–20768 CrossRef CAS.
- L. T. Oliveira, R. V. Gonçalves, D. V. Gonçalves, D. C. S. Azevedo and S. M. P. Lucena, Superior Performance of Mesoporous MOF MIL-100 (Fe) Impregnated with Ionic Liquids for CO2 Adsorption, J. Chem. Eng. Data, 2019, 64(5), 2221–2228 CrossRef CAS.
- J. M. Vicent-Luna, J. J. Gutiérrez-Sevillano, S. Hamad, J. Anta and S. Calero, Role of Ionic Liquid [EMIM]+[SCN]− in the Adsorption and Diffusion of Gases in Metal–Organic Frameworks, ACS Appl. Mater. Interfaces, 2018, 10(35), 29694–29704 CrossRef CAS PubMed.
- L. Qiu, L. Peng, D. Moitra, H. Liu, Y. Fu, Z. Dong, W. Hu, M. Lei, D. Jiang, H. Lin, J. Hu, K. A. McGarry, I. Popovs, M. Li, A. S. Ivanov, Z. Yang and S. Dai, Harnessing the Hybridization of a Metal-Organic Framework and Superbase-Derived Ionic Liquid for High-Performance Direct Air Capture of CO2, Small, 2023, 19, 2302708 CrossRef CAS PubMed.
- J. Yang, M. Tong, G. Han, M. Chang, T. Yan, Y. Ying, Q. Yang and D. Liu, Solubility-Boosted Molecular Sieving-Based Separation for Purification of Acetylene in Core–Shell IL@MOF Composites, Adv. Funct. Mater., 2023, 33, 2213743 CrossRef CAS.
- H. M. Khalid, A. Mujahid, A. Ali, A. L. Khan, M. Saleem and R. M. Santos, Development of Mixed Matrix Membranes by Using NH2-Functionalized UiO-66 and [APTMS][AC] Ionic Liquid for the Separation of CO2, Int. J. Energy Res., 2024, 2107340 CrossRef.
- Z. Lei, C. Dai and W. Song, Adsorptive Absorption: A Preliminary Experimental and Modeling Study on CO2 Solubility, Chem. Eng. Sci., 2015, 127, 260–268 CrossRef CAS.
- V. Nozari, M. Zeeshan, S. Keskin and A. Uzun, Effect of methylation of ionic liquids on the gas separation performance of ionic liquid/metal–organic framework composites, CrystEngComm, 2018, 20, 7137–7143 RSC.
- J. Ma, Y. Ying, X. Guo, H. Huang, D. Liu and C. Zhong, Fabrication of mixed-matrix membrane containing metal–organic framework composite with task-specific ionic liquid for efficient CO2 separation, J. Mater. Chem. A, 2016, 4, 7281–7288 RSC.
- L. Gong, Z. Cai, Q. Wu, L. Liu, C. Wang, L. Shan, X. Meng, S. Luo, Z. Liu and S. Zhang, Ionic liquid enhancement of interface compatibility in mixed-linker ZIF-based mixed matrix membranes for advanced CO2/CH4 separation, J. Mater. Chem. A, 2022, 10, 24975–24984 RSC.
- B. Sasikumar and G. Arthanareeswaran, Concurrent enhancement of CO2-philic pathway and interfacial compatibilization in ZIF-67 based polysulfone membranes through [Bmim][Tf2N] for CO2 separation, Appl. Surf. Sci., 2022, 606, 154900 CrossRef CAS.
- H. Li, L. Tuo, K. Yang, H.-K. Jeong, Y. Dai, G. He and W. Zhao, Simultaneous enhancement of mechanical properties and CO2 selectivity of ZIF-8 mixed matrix membranes: Interfacial toughening effect of ionic liquid, J. Membr. Sci., 2016, 511, 130–142 CrossRef CAS.
- S. Nie, E. Liu, F. Chen, Y. Ma, K. Chen and J. Gao, Enhancement of CO2 adsorption and separation in basic ionic liquid/ZIF-8 with core–shell structure, Chem. Commun., 2024, 60, 3559–3562 RSC.
- S. Kuchekar, S. Gaikwad and S. Han, Design and synthesis of Mg-HKUST-1/solid ionic liquid composites for CO2 capture with improved water stability, J. Environ. Chem. Eng., 2024, 12, 113379 CrossRef CAS.
- M. Mohamedali, H. Ibrahim and A. Henni, Incorporation of acetate-based ionic liquids into a zeolitic imidazolate framework (ZIF-8) as efficient sorbents for carbon dioxide capture, Chem. Eng. J., 2018, 334, 817–828 CrossRef CAS.
- W. Chen, Z. Zhang, C. Yang, J. Liu, H. Shen, K. Yang and Z. Wang, PIM-based mixed-matrix membranes containing MOF-801/ionic liquid nanocomposites for enhanced CO2 separation performance, J. Membr. Sci., 2021, 636, 119581 CrossRef CAS.
- M. Mohamedali, A. Henni and H. Ibrahim, Markedly improved CO2 uptake using imidazolium-based ionic liquids confined into HKUST-1 frameworks, Microporous Mesoporous Mater., 2019, 284, 98–110 CrossRef CAS.
- S. Hussain, H. Dong, H. Duan, X. Ji, H. M. Asif, W. Liu and X. Zhang, Efficient Selective Carbon Dioxide Separation via Task-Specific Ionic Liquids Incorporated in ZIF-8, Langmuir, 2024, 40(16), 8636–8644 CrossRef CAS PubMed.
- E. Martínez-Ahumada, A. López-Olvera, V. Jancik, J. E. Sánchez-Bautista, E. González-Zamora, V. Martis, D. R. Williams and I. A. Ibarra, MOF Materials for the Capture of Highly Toxic H2S and SO2, Organometallics, 2020, 39(7), 883–915 CrossRef.
- W. Wang, S. Gong, H. Wang, Y. Tan, X. Zhu, X. Wang, J. Liu, W. Yu, G. Zhu and X. Lv, Surface-modified silver aerogels combining interfacial regulation for electrocatalytic CO2 reduction under large current density, Chem. Eng. J., 2024, 490, 151849 CrossRef CAS.
- H. Chen, J. Li, Q. Fan, T. Zheng, Y. Zhang, Y. C. Yong and Z. Fang, A feasible strategy for microbial electrocatalytic CO2 reduction via whole-cell-packed and exogenous-mediator-free rGO/Shewanella biohydrogel, Chem. Eng. J., 2023, 460, 141863 CrossRef CAS.
- D. Liu, G. Li and H. Liu, Functionalized MIL-101 with imidazolium-based ionic liquids for the cycloaddition of CO2 and epoxides under mild condition, Appl. Surf. Sci., 2018, 428, 218–225 CrossRef CAS.
- X. Liu, M. Ding, P. Ma and J. Yao, Optimizing the mobility of active species in ionic liquid/MIL-101 composites for boosting carbon dioxide conversion, New J. Chem., 2022, 46, 44–48 RSC.
- Y. Sun, H. Huang, H. Vardhan, B. Aguila, C. Zhong, J. A. Perman, A. M. A.-E. Nafady and S. Ma, Facile Approach to Graft Ionic Liquid into MOF for Improving the Efficiency of CO2 Chemical Fixation, ACS Appl. Mater. Interfaces, 2018, 10(32), 27124–27130 CrossRef CAS PubMed.
- C. Bao, Y. Jiang, L. Zhao, D. Li, P. Xu and J. Sun, Aminoethylimidazole ionic liquid-grafted MIL-101-NH2 heterogeneous catalyst for the conversion of CO2 and epoxide without solvent and cocatalyst, New J. Chem., 2021, 45, 13893–13901 RSC.
- M. Bahadori, S. Tangestaninejad, M. Bertmer, M. Moghadam, V. Mirkhani, I. Mohammadpoor-Baltork, R. Kardanpour and F. Zadehahmadi, Task-Specific Ionic Liquid Functionalized–MIL–101(Cr) as a Heterogeneous and Efficient Catalyst for the Cycloaddition of CO2 with Epoxides Under Solvent Free Conditions, ACS Sustainable Chem. Eng., 2019, 7(4), 3962–3973 CrossRef CAS.
- M. Bahadori, A. Marandi, S. Tangestaninejad, M. Moghadam, V. Mirkhani and I. Mohammadpoor-Baltork, Ionic Liquid-Decorated MIL-101(Cr) via Covalent and Coordination Bonds for Efficient Solvent-Free CO2 Conversion and CO2 Capture at Low Pressure, J. Phys. Chem. C, 2020, 124(16), 8716–8725 CrossRef CAS.
- J. Liang, R.-P. Chen, X.-Y. Wang, T.-T. Liu, X.-S. Wang, Y.-B. Huang and R. Cao, Postsynthetic ionization of an imidazole-containing metal–organic framework for the cycloaddition of carbon dioxide and epoxides, Chem. Sci., 2017, 8, 1570–1575 RSC.
- L.-G. Ding, B.-J. Yao, W.-L. Jiang, J.-T. Li, Q.-J. Fu, Y.-A. Li, Z.-H. Liu, J.-P. Ma and Y.-B. Dong, Bifunctional Imidazolium-Based Ionic Liquid Decorated UiO-67 Type MOF for Selective CO2 Adsorption and Catalytic Property for CO2 Cycloaddition with Epoxides, Inorg. Chem., 2017, 56(4), 2337–2344 CrossRef CAS PubMed.
- J. Dong, H. Zhang, J. Ma, K. Gao, F. Liu, Y. Li and M. Liu, Synergistic effects of core–shell poly(ionic liquids)@ZIF-8 nanocomposites for enhancing additive-free CO2 conversion, J. Colloid Interface Sci., 2024, 661, 1000–1010 CrossRef CAS PubMed.
- T. T. Wang, X. D. Song, Q. X. Luo, X. D. Yang, S. Y. Chong, J. Zhang and M. Ji, Acid-base bifunctional catalyst: Carboxyl ionic liquid immobilized on MIL-101-NH2 for rapid synthesis of propylene carbonate from CO2 and propylene oxide under facile solvent-free conditions, Microporous Mesoporous Mater., 2018, 267, 84–92 CrossRef CAS.
- R. Clark, J. Ávila, M. C. Gomes and A. A. H. Padua, Solvation Environments in Porous Ionic Liquids Determine Selectivity in CO2 Conversion to Cyclic Carbonates, J. Phys. Chem. B, 2023, 127(14), 3266–3277 CrossRef CAS PubMed.
- M. Zhu, C. Wang, D. Meng and G. Diao, In Situ Synthesis of Silver Nanostructures on Magnetic Fe3O4@C Core/Shell Nanocomposites and Their Application in Catalytic Reduction Reactions, J. Mater. Chem. A, 2013, 1, 2118–2125 RSC.
- M. Zhu and G. Diao, Magnetically Recyclable Pd Nanoparticles Immobilized on Magnetic Fe3O4@C Nanocomposites: Preparation, Characterization and Their Catalytic Activity toward Suzuki and Heck Coupling Reactions, J. Phys. Chem. C, 2011, 115, 24743–24749 CrossRef CAS.
- T. Wang, X. Song, H. Xu, M. Chen, J. Zhang and M. Ji, Recyclable and Magnetically Functionalized Metal–Organic Framework Catalyst: IL/Fe3O4@HKUST-1 for the Cycloaddition Reaction of CO2 with Epoxides, ACS Appl. Mater. Interfaces, 2021, 13(19), 22836–22844 CrossRef CAS PubMed.
- X. Zhao, G. Chang, H. Xu, Y. Yao, D. Dong, S. Yang, G. Tian and X. Yang, A Hierarchical Metal–Organic Framework Composite Aerogel Catalyst Containing Integrated Acid, Base, and Metal Sites for the One-Pot Catalytic Synthesis of Cyclic Carbonates, ACS Appl. Mater. Interfaces, 2024, 16(6), 7364–7373 CrossRef CAS PubMed.
- C. Xiong, C. Xue, X. Yu, Y. He, Y. Liang, X. Zhou and H. Ji, Tuning the olefin-VOCs epoxidation performance of ceria by mechanochemical loading of coinage metal, J. Hazard. Mater., 2023, 441, 129888 CrossRef CAS PubMed.
- S. R. Leandro, A. C. Mourato, U. Łapińska, O. C. Monteiro, C. I. Fernandes, P. D. Vaz and C. D. Nunes, Exploring bulk and colloidal Mg/Al hydrotalcite-Au nanoparticles hybrid materials in aerobic olefin epoxidation, J. Catal., 2018, 358, 187–198 CrossRef CAS.
- C. He, Y.-H. Zou, D.-H. Si, Z.-A. Chen, T.-F. Liu, R. Cao and Y.-B. Huang, A porous metal-organic cage liquid for sustainable CO2 conversion reactions, Nat. Commun., 2023, 14, 3317 CrossRef CAS PubMed.
- J. Wang, M. Shi, L. P. Tang, S. N. Ruan, Y. Y. Chao, P. Chen and F. C. Shen, Customizing Ionic Liquids Functionalized MOFs Composites with Hydrophobic Interface for Electrochemical CO2 Reduction, ACS Appl. Mater. Interfaces, 2024, 16(40), 53775–53784 CrossRef PubMed.
- Y. Takashima, M. Yokoyama, A. Horikoshi, Y. Sato, T. Tsuruoka and K. Akamatsu, Ionic liquid/metal–organic framework hybrid generated by ion-exchange reaction: synthesis and unique catalytic activity, New J. Chem., 2017, 41, 14409–14413 RSC.
- A. A. Q. Ali and Z. N. Siddiqui, Ionic Liquid Functionalized Metal–Organic Framework ([DEIm][PF6]@MOF-5): Synthesis, Characterization, and Catalytic Application in the Reduction of 4-Nitrophenol, ACS Omega, 2023, 8(4), 3785–3797 CrossRef CAS PubMed.
- J. Rong, F. Qiu, T. Zhang, Y. Zhu, J. Xu, Q. Guo and X. Peng, Non-Noble Metal@carbon Nanosheet Derived from Exfoliated MOF Crystal as Highly Reactive and Stable Heterogeneous Catalyst, Appl. Surf. Sci., 2018, 447, 222–234 CrossRef CAS.
- S. R. Thawarkar, B. Thombare, B. S. Munde and N. D. Khupse, Kinetic Investigation for the Catalytic Reduction of Nitrophenol Using Ionic Liquid Stabilized Gold Nanoparticles, RSC Adv., 2018, 8, 38384–38390 RSC.
- H. Yang, S. Li, X. Zhang, X. Wang and J. Ma, Imidazolium Ionic Liquid-Modified Fibrous Silica Microspheres Loaded with Gold Nanoparticles and Their Enhanced Catalytic Activity and Reusability for the Reduction of 4-Nitrophenol, J. Mater. Chem. A, 2014, 2, 12060–12067 RSC.
- X. Ma, F. Chen, X. Zhang, T. Wang, S. Yuan, X. Wang, T. Li and J. Gao, Hierarchical Co@C-N synthesized by the confined pyrolysis of ionic liquid@metal–organic frameworks for the aerobic oxidation of alcohols, New J. Chem., 2022, 46, 7528–7536 RSC.
- G. Zhou, Y. Xu, P. Wang, L. Tang, Y. Cheng, J. Jin, Z. Ma, X. Liu, C. Li and Z. Lu, Homogenization spin coating strategy for synthesizing IM-BTO photocatalytic membrane aims to tetracycline selectively degradation, Chem. Eng. J., 2024, 486, 150163 CrossRef CAS.
- W. Sun, F. Chen, P. Huang, Y. Sun, Y. Song and Y. Tang, The significantly boosted photodegradation of tetracycline over a novel hierarchical 0D/3D S-scheme heterojunction ZnSnO3/CdIn2S4, J. Environ. Chem. Eng., 2023, 11, 111293 CrossRef CAS.
- J. Liu, L. Huang, Y. Li, L. Yang, C. Wang, J. Liu, Y. Song, Q. Tian and H. Li, Fabrication oxygen defect-mediated double Z-scheme BiOI/BiO2−x/BiOBr photocatalyst for pollutions degradation and bacteria inactivation, J. Environ. Chem. Eng., 2022, 10, 106668 CrossRef CAS.
- M. Liu, P. Ye, M. Wang, L. Wang, C. Wu, J. Xu and Y. Chen, 2D/2D Bi-MOF-derived BiOCl/MoS2 nanosheets S-scheme heterojunction for effective photocatalytic degradation, J. Environ. Chem. Eng., 2022, 10, 108436 CrossRef CAS.
- S. Asgari, G. M. Ziarani, A. Badiei and Y. Vasseghian, Zr-UiO-66, ionic liquid (HMIM+TFSI−), and electrospun nanofibers (polyacrylonitrile): All in one as a piezo-photocatalyst for degradation of organic dye, Chem. Eng. J., 2024, 487, 150600 CrossRef CAS.
- T. Huang, X. Qiu, L. Zhu, C. Wang, H. Li, Y. Fan, M. Zhang, H. Li and W. Zhu, Engineering high specific surface area over poly (ionic liquids)-derived molybdenum-silica hybrid materials for enhanced oxidative desulfurization, J. Environ. Chem. Eng., 2023, 11, 111487 CrossRef CAS.
- J. Xiong, H. Huang, M. Zhang, P. Song, H. Li and J. Di, Incorporating carbon quantum dots into phosphotungstic acid ionic liquid materials for enhanced catalytic oxidative desulfurization, Fuel, 2024, 365, 131168 CrossRef CAS.
- S. Xun, R. Le, C. Hu, D. Liu, S. Wang, M. He, W. Zhu and H. Li, Ionic Liquid Promotes High Dispersion of V2O5 on 3D Porous g-C3N4 Carrier to Enhance Catalytic Oxidative Desulfurization Performance, Energy Fuels, 2023, 37(8), 6276–6280 CrossRef CAS.
- D. Liu, S. Xun, B. Yang, M. He, J. Liu, W. Jiang, H. Li and W. Zhu, Phosphotungstic-Acid-Based Ionic Liquid Catalyst for Efficient Photocatalytic Oxidative Desulfurization under Visible Light, Energy Fuels, 2024, 38(7), 6553–6559 CrossRef CAS.
- J. Yin, W. Fu, J. Zhang, X. Zhang, W. Qiu, W. Jiang, L. Zhu, H. Li and H. Li, Bifunctional pyridinium-based Brønsted acidic porous ionic liquid for deep oxidative desulfurization, Chem. Eng. J., 2024, 492, 152349 CrossRef CAS.
- Z. Wu, C. Chen, H. Wan, L. Wang, Z. Li, B. Li, Q. Guo and G. Guan, Fabrication of Magnetic NH2-MIL-88B (Fe) Confined Brønsted Ionic Liquid as an Efficient Catalyst in Biodiesel Synthesis, Energy Fuels, 2016, 30(12), 10739–10746 CrossRef CAS.
- H. Wan, C. Chen, Z. Wu, Y. Que, Y. Feng, W. Wang, L. Wang, G. Guan and X. Liu, Encapsulation of Heteropolyanion-Based Ionic Liquid within the Metal–Organic Framework MIL-100(Fe) for Biodiesel Production, ChemCatChem, 2015, 7, 441–449 CrossRef CAS.
- P. Lu, H. Li, M. Li, J. Chen, C. Ye, H. Wang and T. Qiu, Ionic liquid grafted NH2-UiO-66 as heterogeneous solid acid catalyst for biodiesel production, Fuel, 2022, 324, 124537 CrossRef CAS.
- S. Y. Chong, T. T. Wang, L. C. Cheng, H. Y. Lv and M. Ji, Metal–Organic Framework MIL-101-NH2-Supported Acetate-Based Butylimidazolium Ionic Liquid as a Highly Efficient Heterogeneous Catalyst for the Synthesis of 3-Aryl-2-oxazolidinones, Langmuir, 2019, 35(2), 495–503 CrossRef CAS PubMed.
- H. M. A. Hassan, M. A. Betiha, S. K. Mohamed, E. A. El-Sharkawy and E. A. Ahmed, Stable and recyclable MIL-101(Cr)–Ionic liquid based hybrid nanomaterials as heterogeneous catalyst, J. Mol. Liq., 2017, 236, 385–394 CrossRef CAS.
- Q. Luo, M. Ji, M. Lu, C. Hao, J. Qiu and Y. Li, Organic electron-rich N-heterocyclic compound as a chemical bridge: building a Brønsted acidic ionic liquid confined in MIL-101 nanocages, J. Mater. Chem. A, 2013, 1, 6530–6534 RSC.
- A. A. Q. Ali and Z. N. Siddiqui, Heteropoly Ionic Liquid Functionalized MOF-Fe: Synthesis, Characterization, and Catalytic Application in Selective Acetalization of Glycerol to Solketal as a Fuel Additive at Room Temperature, Solvent-Free Conditions, Precis. Chem., 2023, 1(8), 485–496 CrossRef CAS.
- C. Ye, Z. Qi, D. Cai and T. Qiu, Design and Synthesis of Ionic Liquid Supported Hierarchically Porous Zr Metal–Organic Framework as a Novel Brønsted–Lewis Acidic Catalyst in Biodiesel Synthesis, Ind. Eng. Chem. Res., 2019, 58(3), 1123–1132 CrossRef CAS.
- S. Askari, M. M. Khodaei and M. Jafarzadeh, Basic ionic liquid anchored on UiO-66-NH2 metal–organic framework: a stable and efficient heterogeneous catalyst for synthesis of xanthenes, Res. Chem. Intermed., 2021, 47, 2881–2899 CrossRef CAS.
- S. N. K. Mogha, K. Chaudhary, G. Kumar and D. T. Masram, Fur-Imine-Functionalized Graphene Oxide-Immobilized Copper Oxide Nanoparticle Catalyst for the Synthesis of Xanthene Derivatives, ACS Omega, 2018, 3(11), 16377–16385 CrossRef PubMed.
- B. Mirhosseini-Eshkevari, M. Esnaashari and M. A. Ghasemzadeh, Novel Brönsted Acidic Ionic Liquids Confined in UiO-66 Nanocages for the Synthesis of Dihydropyrido[2,3-d]Pyrimidine Derivatives under Solvent-Free Conditions, ACS Omega, 2019, 4(6), 10548–10557 CrossRef CAS PubMed.
- B. Mirhosseini-Eshkevari, M. A. Ghasemzadeh, M. Esnaashari and S. T. Ganjali, Introduction of a Novel Brønsted Acidic Ionic Liquid Incorporated in UiO-66 Nanocages for the Efficient Synthesis of Pyrimido[4,5-d]Pyrimidines, ChemistrySelect, 2019, 4, 12920–12927 CrossRef CAS.
- Z. Mahmoudi, M. A. Ghasemzadeh, H. Kabiri-Fard and S. T. Ganjali, Multicomponent Synthesis of Pyrimidoquinolinetriones and Pyridodipyrimidines in the Presence of Triethylenediamine-Based Ionic Liquid/MIL-101(Cr) Metal-Organic Framework Composite, Polycyclic Aromat. Compd., 2022, 43, 7526–7545 CrossRef.
- N. Zameer, A. Mustafa, N. Khan and Z. N. Siddiqui, Silica-based pyrrolidinium ionic liquid immobilised on copper-citric acid metal-organic framework, ([PyrrSi][Cl]@Cu-Cit-MOF): Heterogeneous catalysis to the multicomponent reaction for the synthesis of imidazoles, J. Ionic Liquids, 2024, 4, 100074 CrossRef.
- Y.-H. Hu, C.-X. Liu, J.-C. Wang, X.-H. Ren, X. Kan and Y.-B. Dong, TiO2@UiO-68-CIL: A Metal–Organic-Framework-Based Bifunctional Composite Catalyst for a One-Pot Sequential Asymmetric Morita–Baylis–Hillman Reaction, Inorg. Chem., 2019, 58(8), 4722–4730 CrossRef CAS PubMed.
- T. Hou, W. Xu, X. Pei, L. Jiang, O. M. Yaghi and K. A. Persson, Ionic Conduction Mechanism and Design of Metal–Organic Framework Based Quasi-Solid-State Electrolytes, J. Am. Chem. Soc., 2022, 144(30), 13446–13450 CrossRef CAS PubMed.
- V. Nozari, C. Calahoo, J. M. Tuffnell, P. Adelhelm, K. Wondraczek, S. E. Dutton, T. D. Bennett and L. Wondraczek, Sodium Ion Conductivity in Superionic IL-impregnated Metal-organic Frameworks: Enhancing Stability Through Structural Disorder, Sci. Rep., 2020, 10, 3532 CrossRef CAS PubMed.
- X. Yu, N. S. Grundish, J. B. Goodenough and A. Manthiram, Ionic Liquid (IL) Laden Metal–Organic Framework (IL-MOF) Electrolyte for Quasi-Solid-State Sodium Batteries, ACS Appl. Mater. Interfaces, 2021, 13(21), 24662–24669 CrossRef CAS PubMed.
- Q. Xu, X. Zhang, S. Zeng, L. Bai and S. Zhang, Ionic Liquid Incorporated Metal Organic Framework for High Ionic Conductivity over Extended Temperature Range, ACS Sustainable Chem. Eng., 2019, 7(8), 7892–7899 CrossRef CAS.
- C. Sun, J. Zhang, X. Yuan, J. Duan, S. Deng, J. Fan, J.-K. Chang, M. Zheng and Q. Dong, ZIF-8-Based Quasi-Solid-State Electrolyte for Lithium Batteries, ACS Appl. Mater. Interfaces, 2019, 11(50), 46671–46677 CrossRef CAS PubMed.
- K. Fujie, K. Otsubo, R. Ikeda, T. Yamada and H. Kitagawa, Low temperature ionic conductor: ionic liquid incorporated within a metal–organic framework, Chem. Sci., 2015, 6, 4306–4310 RSC.
- X. Qi, D. Cai, X. Wang, X. Xia, C. Gu and J. Tu, Ionic Liquid-Impregnated ZIF-8/Polypropylene Solid-like Electrolyte for Dendrite-free Lithium-Metal Batteries, ACS Appl. Mater. Interfaces, 2022, 14(5), 6859–6868 CrossRef CAS PubMed.
- Z. Wang, H. Zhou, C. Meng, W. Xiong, Y. Cai, P. Hu, H. Pang and A. Yuan, Enhancing Ion Transport: Function of Ionic Liquid Decorated MOFs in Polymer Electrolytes for All-Solid-State Lithium Batteries, ACS Appl. Energy Mater., 2020, 3(5), 4265–4274 CrossRef CAS.
- Z. Wu, Y. Yi, F. Hai, X. Tian, S. Zheng, J. Guo, W. Tang, W. Hua and M. Li, A Metal–Organic Framework Based Quasi-Solid-State Electrolyte Enabling Continuous Ion Transport for High-Safety and High-Energy-Density Lithium Metal Batteries, ACS Appl. Mater. Interfaces, 2023, 15(18), 22065–22074 CrossRef CAS PubMed.
- B. M. Mahimai, G. Sivasubramanian, S. Moorthy and P. Deivanayagam, Copper Metal Organic Framework-Encapsulated Ionic Liquid-Decorated Sulfonated Polystyrene-block-poly(ethylene-ranbutylene)-block-polystyrene Membranes for Fuel Cells, Ind. Eng. Chem. Res., 2022, 61(23), 8081–8090 CrossRef CAS.
- J. F. D. Montero, H. A. Tajiri, G. M. O. Barra, M. C. Fredel, C. A. M. Benfatti, R. S. Magini, A. L. Pimenta and J. C. M. Souza, Biofilm behavior on sulfonated poly(ether-ether-ketone) (sPEEK), Mater. Sci. Eng., C, 2017, 70, 456–460 CrossRef CAS PubMed.
- L. Sun, S. Qu, X. Lv, L. Ding, J. Duan and W. Wang, Sulfonated Poly Ether Ether Ketone Membranes Reinforced by Metal–Organic Frameworks/Ionic Liquids, ACS Appl. Polym. Mater., 2023, 5(12), 10081–10090 CrossRef CAS.
- C. Ru, Y. Gu, H. Na, H. Li and C. Zhao, Preparation of a Cross-Linked Sulfonated Poly(arylene ether ketone) Proton Exchange Membrane with Enhanced Proton Conductivity and Methanol Resistance by Introducing an Ionic Liquid-Impregnated Metal Organic Framework, ACS Appl. Mater. Interfaces, 2019, 11(35), 31899–31908 CrossRef CAS PubMed.
- R. Wang, Y. Feng, Y. Zhong, Y. Zou, M. Yang, Y. Liu and Y. Zhou, Enhancing Demulsification Performance for Oil–Water Separation through Encapsulating Ionic Liquids in the Pore of MIL-100(Fe), Langmuir, 2021, 37(27), 8232–8239 CrossRef CAS PubMed.
- S. Ranjbari, A. Ayati, M. N. Shahrak, B. Tanhaei and S. H. Tabrizi, Design of [BmIm]3PW12O40 Ionic Liquid Encapsulated-ZIF-8 Nanocomposite for Cationic Dye Adsorptive Removal: Modeling by Response Surface Methodology, Ind. Eng. Chem. Res., 2023, 62(11), 4636–4645 CrossRef CAS.
- N. A. Khan, Z. Hasan and S. H. Jhung, Ionic Liquids Supported on Metal-Organic Frameworks: Remarkable Adsorbents for Adsorptive Desulfurization, Chem. – Eur. J., 2014, 20, 376–380 CrossRef CAS PubMed.
- P. Li, Z. Li, S. Liu, C. Li, L. Ma, C. Yang, D. Han, C. Niu, X. Xin and F. Li, Imidazole/pyridine-based ionic liquids modified metal-organic frameworks for efficient adsorption of Congo red in water, J. Mol. Struct., 2024, 1303, 137599 CrossRef CAS.
- D. Shen, Q. Du, P. Wang, X. Tian, Y. Wang, R. Feng and J. Pan, Spatially confined conjugation of enzyme immobilized magnetic nanochains with poly(vinylphosphonic acid) polymers using droplet reactors for uranium extraction, Chem. Eng. J., 2024, 494, 152970 CrossRef CAS.
- S. Wu, J. Huang, C. Zhuo, F. Zhang, W. Sheng and M. Zhu, One-step fabrication of magnetic carbon nanocomposite as adsorbent for removal of methylene blue, J. Inorg. Organomet. Polym., 2016, 26, 632–639 CrossRef CAS.
- M. Zhu, Q. Chen, W. Tong, J. Kan and W. Sheng, Preparation and application of Fe3O4 nanomaterials, Prog. Chem., 2017, 29, 1366–1394 CAS.
- D. Lu, M. Qin, C. Liu, J. Deng, G. Shi and T. Zhou, Ionic Liquid-Functionalized Magnetic Metal–Organic Framework Nanocomposites for Efficient Extraction and Sensitive Detection of Fluoroquinolone Antibiotics in Environmental Water, ACS Appl. Mater. Interfaces, 2021, 13(4), 5357–5367 CrossRef CAS PubMed.
- A. Yohannesa and S. Yao, Preconcentration of tropane alkaloids by a metal organic framework (MOF)-immobilized ionic liquid with the same nucleus for their quantitation in Huashanshen tablets, Analyst, 2019, 144, 6989–7000 RSC.
- W. Rong, M. Ding, P. Ma, C. Ling, X. Liu and J. Yao, Poly(ionic liquids)-functionalized metal-organic frameworks for sustainable water purification, Colloids Surf., A, 2023, 674, 131901 CrossRef CAS.
- L. Yang, L. Bao, Y. Zhong, C. Hao, J. Chen, J. Wu and X. Wang, Fabrication of in situ metal-organic framework grown on sodium lignosulphonate hydrogel for removal of Pb2+, methylene blue and crystal violet from aqueous solution, J. Cleaner Prod., 2024, 434, 139831 CrossRef CAS.
- R. Hameed, A. Abbas, J. Lou, W. A. Khattak, B. Roha, B. Iqbal, G. Li, Q. Zhang and X. Zhao, Synthesis of biochar-ZnAl-layered double hydroxide composite for effective heavy metal adsorption: Exploring mechanisms and structural transformations, J. Environ. Chem. Eng., 2024, 12, 112687 CrossRef CAS.
- S. Wu, W. Shi, L. Cui and C. Xu, Enhancing contaminant rejection efficiency with ZIF-8 molecular sieving in sustainable mixed matrix membranes, Chem. Eng. J., 2024, 482, 148954 CrossRef CAS.
- S. Wu, W. Shi, K. Li, J. Cai and L. Chen, Recent advances on sustainable bio-based materials for water treatment: Fabrication, modification and application, J. Environ. Chem. Eng., 2022, 10, 108921 CrossRef CAS.
- K. Luo, Q. Wang, Q. Xin, Z. Lei, E. Hu, H. Wang, F. Liang and H. Wang, Uranium adsorption properties and mechanism of ZIF-8/microalgae composite adsorbent with crosslinked chitosan/tannic acid curing supported by quaternary phosphate ionic liquid, Desalination, 2024, 592, 118079 CrossRef CAS.
- I. Ahmed, T. Panja, N. A. Khan, M. Sarker, J.-S. Yu and S. H. Jhung, Nitrogen-Doped Porous Carbons from Ionic Liquids@MOF: Remarkable Adsorbents for Both Aqueous and Nonaqueous Media, ACS Appl. Mater. Interfaces, 2017, 9(11), 10276–10285 CrossRef CAS PubMed.
| This journal is © the Partner Organisations 2025 |