Open Access Article
Open Access ArticleScientific information about sugar-based emulsifiers: a comprehensive review
Aniruddha Pal
 a,
Monohar Hossain Mondal
a,
Monohar Hossain Mondal
 ab,
Achyut Adhikari
ab,
Achyut Adhikari
 c,
Ajaya Bhattarai
c,
Ajaya Bhattarai
 *d and
Bidyut Saha
*d and
Bidyut Saha
 *a
*a
aHomogeneous Catalysis Laboratory, Department of Chemistry, The University of Burdwan, Burdwan-713104, WB, India. E-mail: bsaha@chem.buruniv.ac.in
bChemical Sciences Laboratory, Government General Degree College, Singur, Hooghly 712409, WB, India
cCentral Department of Chemistry, Tribhuvan University, Kirtipur, Nepal
dDepartment of Chemistry, M.M.A.M.C., Tribhuvan University, Biratnagar, Nepal. E-mail: ajaya.bhattarai@mmamc.tu.edu.np
First published on 6th October 2021
Abstract
The instantaneous demand for foods, detergents, cosmetics, and personal care products that can be commercialized with value-added benefits including natural origin, environmental friendliness, and sustainability is increasing day by day. Accordingly, the associated industries are trying to identify bioactive ingredients that may be natural alternatives to synthetic ones. This review article is mainly aimed at the classification of natural saccharide-based emulsifiers (which are mainly bio-surfactants), their methods of preparation and their various types of applications in daily life activities. Different routes of production of mono and polysaccharide-based emulsifiers and their industrial advantages are exclusively highlighted. The readers can get an approach on how sugar-based emulsifiers are synthesized and used in the pharmaceutical, food, and personal care industries to contribute excellent physicochemical properties and feature excellent functional characteristics. Many of the synthetic procedures are associated with the use of natural ingredients to prepare emulsions concerning “eco-friendly” selective materials. In this report, an endeavour has been made towards contextual examples for the production methods of some saccharide-based emulsifiers and their advantages in various fields.
1. Introduction
The development of science and technology is mainly focused on the security and safety of the environment, people, and eco-system.1 The diverse and conventional chemistry requires convenient alterations that promote the applied aspect of ‘green chemistry’ in the daily life activities of our community. Natural resources have been proved to be the most effective and dependable alternatives to accelerate the ‘green chemistry’ worldwide. In this context, species having properties such as lesser toxicity, chemical stability, biodegradability, environmental safety, efficient solubilisation, and of course, recycling facility are of key interest.Emulsions are normally thermodynamically very unstable colloidal systems.2 They consist of small oil droplets suspended in an aqueous phase. An emulsion is a colloidal substance whose dispersion medium and dispersed phase both are liquid. By adsorbing onto droplet surfaces and lowering the interfacial tension, emulsifiers tend to produce an emulsion.
Once it gets produced, it turns into a facile breakup of the droplet. Simple emulsions may exist as either oil-in-water (o/w) or water-in-oil (w/o) systems.3 Emulsions are illustrated as fine colloidal systems containing emulsion droplets with a size larger than the dimension referred for a colloidal system, i.e. diameter > 1 μm. The investigations over several studies enable us to explore that oil and water emulsions are prepared as well as stabilized utilizing nano- or micro-solid particle adsorption at the interfacial juncture. It also supports that protein could stabilize solid- or semi-solid-like lipid materials such as structured emulsions and oil-in-water (o/w) emulsions. Some naturally available and readily found emulsifiers are polysaccharides, phospholipids, and proteins.4 Almost regularly consumed milk, which is an important constituent of our diet, is a good example of the emulsion of fat in water. The electrostatic repulsions caused by a charged oil–water interface can suppress the aggregation between oil droplets, thereby developing emulsion stability. An emulsion is an unstable system from a thermodynamic viewpoint since a liquid/liquid system has a natural tendency to separate and diminish its interfacial area, as well as its interfacial energy.5 To stabilize the emulsion, addition of an emulsifying agent or emulsifier is usually essential. The emulsifier plays an important role, as it exacerbates the stability of an emulsion by enhancing the kinetic stability.6 Inspired by the momentous applications of environmentally benign emulsifiers, the present report has focussed on the production and utilization of such important ‘green chemical’, the sugar-based emulsifiers collectively.
Before discussing polysaccharide-based emulsifiers,7 it is rational to interpret and discuss the reasons behind emulsion instability or, more specifically, why we need emulsifiers to stabilize emulsions. An emulsion should be stable until it achieved its purpose. A higher energy gradient is needed to stabilize the emulsion by preventing the fusion of droplets.8 Based on the droplet size and different densities of the droplets and the medium, emulsions may break down in many ways (Scheme 1).
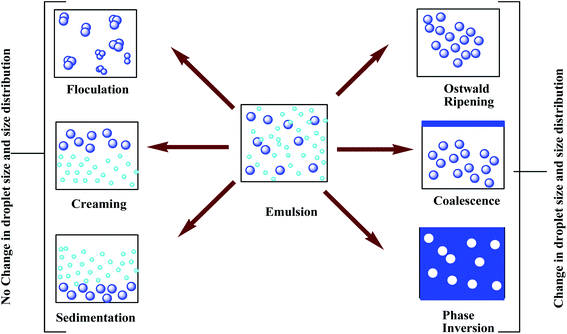 | ||
| Scheme 1 Different mechanisms of emulsion breakdown. This scheme is reproduced from ref. 13, with permission from Springer, Berlin, Heidelberg, copyright 2015. | ||
Sedimentation occurs when particles in suspension are settling out of the medium in which they entrain and come to rest against a barrier.9 This is due to external forces such as gravity, centrifugal acceleration, or electromagnetism. An emulsion could be stabilized by preventing such aggregation since the aggregate of droplets could sediment faster than individual tiny droplets. Flocculation occurs when floc-like colloids appear from the suspension.10 This process may be spontaneous or it may also be observed by adding a clarifying agent. In the case of flocculation, because of weak van der Waals attraction, the repulsive force is not adequately strong to keep the droplets apart. To prevent flocculation, it needs to overcome the attractive force acting between droplets. This can be done by introducing a repulsive force via the formation of the electrical double layer using an ionic surfactant. Ostwald ripening is best described by the alteration of the size of droplets over time in solid particles or liquid mediums.11 In Ostwald ripening, larger droplets of poly-dispersed emulsion have lower solubility than smaller ones. Smaller droplets recombine with larger droplets and become invisible over time. Ostwald ripening may be prevented by using a stabilizer which can reduce the interfacial tension between solid solutions and liquid sols. There's a process called coalescence that occurs when two or more droplets join together.12 This is because of the surface or film fluctuation which makes the droplets come close to each other. Coalescence can be reduced by using polysaccharides and polypeptides as weighting agents, which also coat the individual droplets. In phase inversion, dispersed phase and dispersion medium are interchanged, i.e. water-in-oil emulsion changes into oil-in-water emulsion and vice versa.13
It is legitimate to indicate here that a natural polymer like polysaccharides is composed of similar or different kinds of monosaccharide units bound together by two or more glycosidic linkages.14 Physicochemical properties of polysaccharides especially depend on their molar masses, electric charge, hydrophobicity, degree of branching, and polarity. The emulsification performance of polysaccharides enhances when glycoproteins or glycolipids are covalently bonded with them.15 However, several polysaccharides are amphiphilic and thus they can stabilize emulsions, being adsorbed onto the surface of oil droplets. In this review, we will discuss some natural emulsifiers which are polysaccharide-based bio-surfactants16 such as alkyl poly-glycosides, sorbitan esters, and sucrose esters.17–19 Bio-surfactants are categorized into two classes based on their molecular masses by Rosenberg and Ron.20 Surface-active agents fall into two categories: low-molecular-weight (such as glycolipids, lipopeptides, and phospholipids) and high-molecular-weight (such as polymeric and particulate surfactants).21 Now, considering a surfactant having amphiphilic properties, it is a very difficult task to substitute polyethylene glycol with a carbohydrate molecule for fatty alcohol or fatty acid.22–25 Though there are various ways of preparing a large number of carbohydrates, only very few of them satisfy the criteria for being raw material in the industrial aspect because of their cost, quality, and availability. For instance, glucose from starches, sucrose from sugarcane, and sorbitol from hydrogenation glucose derivatives are possible to prepare (Table 1).26 Nowadays, remainders of straw and hemicelluloses processing are used for making the derivatives of lactose, xylose, and other carbohydrates.
| Volume of production [t a−1] | Prices on averagea [€ kg−1] | |
|---|---|---|
| a Several factors cause agricultural commodities to be highly volatile on the market. Hence, the figures shown can only be indications. Table is reprinted with permission from ref. 27 copyright 1999 WILEY-VCH Verlag GmbH, Weinheim, Fed. Rep. of Germany. | ||
| Sorbitol | 650![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.80 |
| Glucose | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.30 |
| Sucrose | 150![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.25 |
2. Classification of polysaccharide-based emulsifiers
Surfactants are mostly derivatized using fats, oils, amino acids, etc.28 Production of surfactants especially bio-surfactants (BSs) is still not on a bigger industrial scale though researchers around the globe are emphasising these alternatives. The most important topic in the category of BSs nowadays is sugar-based BSs.29 or particularly polysaccharide-based BSs.30 There are a various types of polysaccharide-based BSs synthesized, such as alkyl polyglycosides, sorbitan esters, and sucrose esters.31 Here, a chart has been represented for different polysaccharide-based surfactants (Scheme 2). This information would help the readers to understand the huge variety of such emulsifiers. The classification is solely based on the types of substrates used for the production of such surfactants.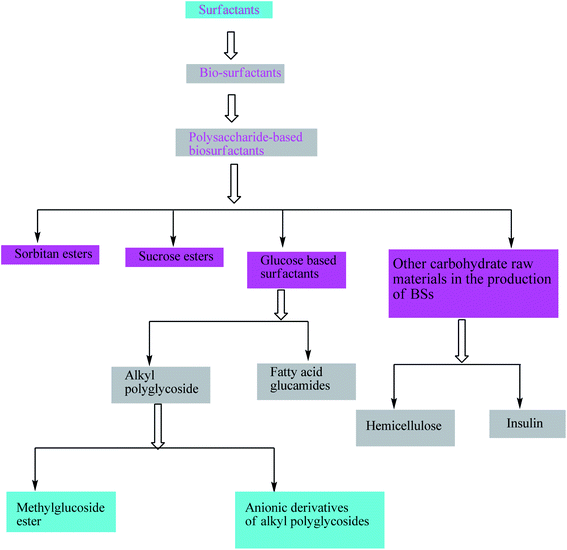 | ||
| Scheme 2 Different types of polysaccharide-based surfactants. The scheme is reprinted with permission from ref. 27 copyright 1999 WILEY-VCH Verlag GmbH, Weinheim, Germany. | ||
Surfactant is itself a class of chemical especially known for its wide range of applications and variety of production. BSs are a type of surfactant which has been introduced a few decades ago. As this review article is solely focused on only polysaccharide-based BSs, we have given the details of different types of polysaccharide-based BSs in the above diagram.
3. Production and utilization of saccharide-based BSs
This review article is a dedicated endeavour to collectively present different polysaccharide-based emulsifiers with the aim of gathering scientific knowledge on polysaccharide-based BSs, their production, and their application in different chemical and industrial fields. We have decided to provide acquired scientific knowledge of different classes of polysaccharide-based BSs, their production, and scientific information regarding them.3.1. Sorbitan esters
In the process of preparation of polysaccharide-based bio-surfactants, the first synthesized emulsifying agent was sorbitan ester. It is prepared by direct acid32 and base-catalyzed reactions of sorbitol containing a fatty acid at high temperatures. Stockburger33 explains how to produce sorbitan ester via acid-catalyzed anhydrization of anhydro sorbitol (a combination of sorbitans, isosorbide, and unreacted sorbitol). At a temperature less than 215 °C, the anhydro sorbitol is reacted with a fatty acid in the presence of a base. Sorbitan emulsifiers typically have a low HLB (hydrophilic lipophilic balance) value of about 1–8. More dedicated researchers are needed in association with modified technologies to improve the HLB value. It has been established that if these esters are reacted with ethylene oxide, a compound like sorbitan ester ethoxylate is produced with a higher value of HLB about 10–17.34 A schematic diagram of sorbitan emulsifier production is given in Scheme 3.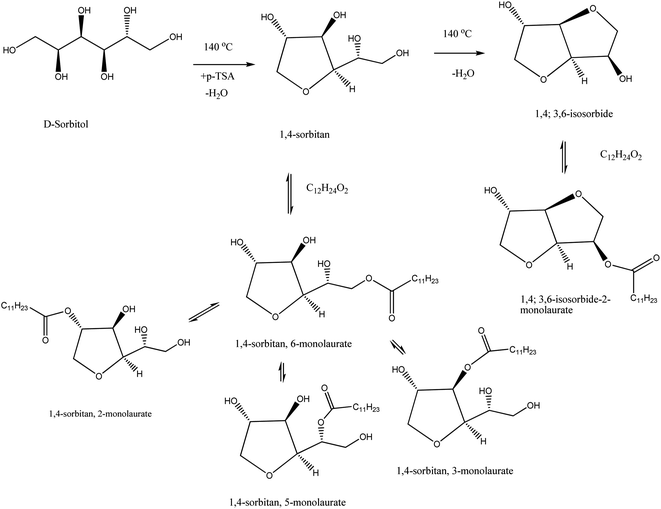 | ||
| Scheme 3 Esterification reactions hypothetically leading to sorbitan monolaurate. The scheme is reprinted with permission from ref. 35 copyright 2019 Carl Hanser Verlag GmbH & Co. KG. | ||
The interesting information about sorbitan esters is that approximately 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons of these surfactants are produced and utilized in the market every year. The main use of sorbitan ester is an emulsifier in food processing, cosmetics, polymerization, and pharmaceutical industries.
000 tons of these surfactants are produced and utilized in the market every year. The main use of sorbitan ester is an emulsifier in food processing, cosmetics, polymerization, and pharmaceutical industries.
Table 2 is reprinted with permission from ref. 27 copyright 1999 WILEY-VCH Verlag GmbH, Weinheim, Fed. Rep. of Germany.
| Specified suppliers | Fields of utilization | Production ability, worlda [t a−1] | |
|---|---|---|---|
| a Estimated figures are based on private communications and literature data, references given in the text. | |||
| Sorbitan esters | Akzo Nobel, Cognis, Kau, Dai-Ichi Kogyo Seiyaku, SEPPIC, Riken Vitamin | Pharmaceuticals, personal hygiene, foodstuffs, fiber, agrochemicals, platings, bombs | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| Sucrose esters | Jiangsu Weixi, Cognis, Evonik/Goldschmidt, Croda, Mitsubishi-Kagaku, Dai-Ichi Kogyo Seiyaku | Pharmaceuticals, personal care, food | <10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| Alkyl polyglycosides | Cognis, Akzo Nobel, China BASF, Dai-Ichi Kogyo Seiyaku, Research Institute for Daily Chemical Industry, LG, and SEPPIC | Personal care, agrochemicals, detergents | 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| Anionic alkyl polyglycoside derivatives | Cognis, Cesalpina | Personal care, pharmaceuticals | <10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
3.2. Sucrose esters
Unlike sorbitan esters, sucrose esters are very mild and their sole application is based on dermatological products. In many countries, it is an approved edible food processing emulsifier.36,37 Sucrose-fatty acid esters are widely accepted non-ionic surfactants usually applied as emulsifiers in food products and beverage industries. Sucrose is a very temperature-sensitive molecule and thus its esterification needs an optimum method and perfect control. For such reasons, sucrose emulsifier production is very difficult compared to sorbitan esters. Here also mixtures of mono-, di-, tri-, tetra- or penta-esters are observed. These emulsifiers were first introduced in the market by the Dai-Nippon manufacturing company in the late 1960s. The application of DMSO and DMF as solvents is of great concern for such emulsifier production as both DMSO and DMF have established health hazards. Industrial-scale productions of sucrose emulsifiers are still in the queue for two reasons – first, the difficulties that arrive in the product purification (i.e., removing DMSO and DMF from the product) and second, huge soap content is produced as a by-product.38–40 Researchers are needed in the purification process. Liquid–liquid extraction and crystallization in association with lyophilisation may be useful in this context.41–45 One of the most useful sucrose esters is sucrose octasulfate prepared by using pyridine/sulphur trioxide complex (Pyr·SO3) with sucrose (Scheme 4).46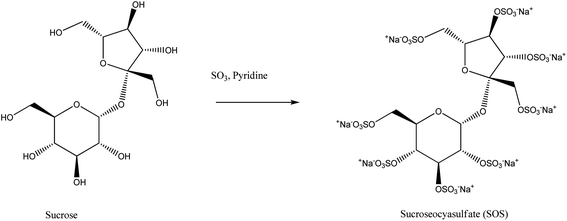 | ||
| Scheme 4 Synthesis of sucrose octasulfate using pyridine/sulphur trioxide complex. This scheme is reproduced from ref. 46 with permission from Springer Nature, copyright 2001. | ||
3.3. Glucose-based emulsifiers
Emil Fischer was the first one to discover a type of emulsifier named alkyl glycoside with the reaction of glucose and alcohol. It is established that such glycosidic reactions are highly chemo and regioselective because of the hemiacetal functionality in the glucose molecule at the C-1 position.47 Similar reactions are possible if fatty acids react with glucose. The fatty acid-derived glucose-based emulsifiers are termed glucamides. Initially, glucose reacts with methylamine, which undergoes hydrogenation and produces selective glucamine. We may get the concerned product by derivatizing glucamine with fatty acid via esterification.48,49Scheme 5 for glucamine synthesis has been taken from the literature.50 The reactions that produce glucamine-based trisiloxane surfactant (HAG) are cost-effective and generate no waste.
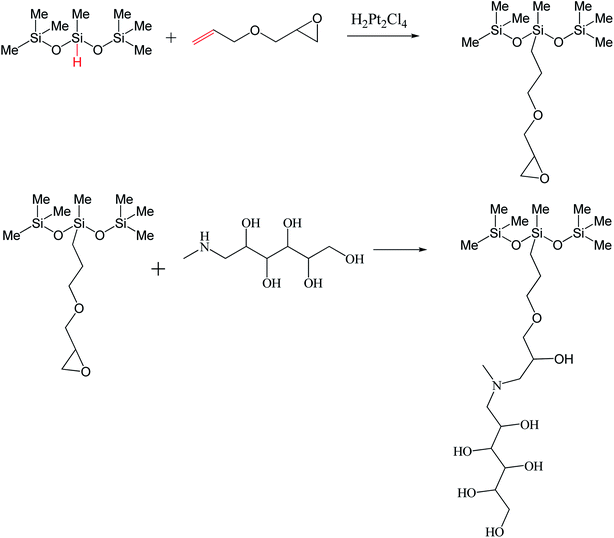 | ||
| Scheme 5 Synthesis of glucamine-based trisiloxane surfactant (HAG). The scheme is adopted from ref. 50 copyright 2019 American Chemical Society. | ||
According to Scheme 5, HAG was synthesized in two steps.
Hydrosilylation was used to graft allyl glycidyl ether onto 1,1,1,3,5,5,5-heptamethyltrisiloxane (HMTS). The prior adduct and N-methyl-D-glucamine were then linked by a ring-opening process.
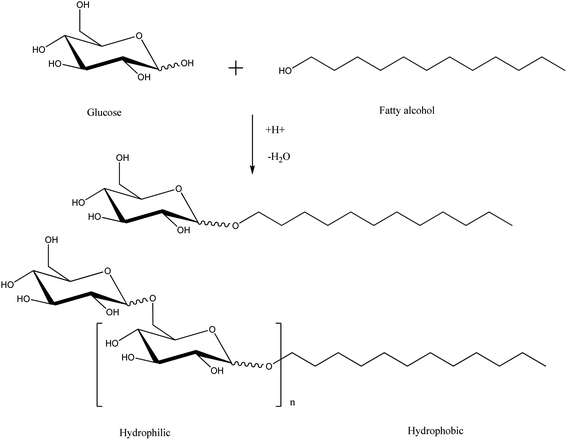 | ||
| Scheme 6 Acetalization of glucose with excess fat alcohol catalyzes the synthesis of alkyl polyglycosides. This scheme is reprinted with permission from ref. 27 copyright 1999 WILEY-VCH Verlag GmbH, Weinheim, Fed. Rep. of Germany. | ||
Thus, the industrial name of these products is alkyl polyglycosides. The alkyl chain length and the average number of glucose units linked to polyglycosides regulate the product quality.
The lower interfacial tension of a surfactant makes it highly applicable for industrial use. Alkyl polyglycosides are produced against hydrocarbon phases for lowering its interfacial tension.53 Unlike fatty alcohol alkyl ethoxylate whose interfacial tension is temperature dependent, alkyl polyglycosides are not, rather they depend on several molecular details such as sugar head group, length of the alkyl chain, functional groups, stereochemistry, and salt addition.53,54 The foam which has greater stability and high resistance to hard water is suitable for industrial purposes. The foam height increases with the increase in the length of the alkyl chain and reaches maximum for C9 alkyl polyglycosides and then decreases.55 The foam stability and foam height of alkyl polyglycosides are shown in Table 3.
| Sl. no. | Surfactants | Foam height (mm) | Foam half-life (min) | Wetting time (s) | Emulsion stability (s) |
|---|---|---|---|---|---|
| 1 | C8 alkylpolyglycoside | 20 | 31 | 67 | 230 |
| 2 | C9 alkylpolyglycoside | 60 | 19 | 61 | 210 |
| 3 | C10 alkylpolyglycoside | 36 | 15 | 51 | 288 |
| 4 | C12 alkylpolyglycoside | 30 | 210 | 53 | 310 |
| 5 | C14 alkylpolyglycoside | 18 | 90 | 57 | 426 |
The wetting power of a surfactant is a very important property because it simplifies the infiltration of alkali dyes into the threads in laundry cleaning and, thus, promotes the detergency or dyeing effect.56 It is clear from Table 3 that the wetting time of alkyl polyglycosides reduced with the increase in the length of the alkyl chain. Because of the higher penetration and wetting powers, alkyl polyglycosides are broadly used in crop protection formulation.57
The information in Table 3 is reprinted with permission from ref. 57 copyright 2001 American Oil Chemists' Society (AOCS).
For the manufacturing of high-quality products, we have to develop the industrial process. The crucial point for this is to establish reaction conditions which must be secure and economically adaptable. This may be achieved by adjusting the reaction parameters such as pressure, temperature, reaction time, and the glucose-to-fatty-alcohol ratio.58,59 At present, alkyl polyglycosides are directly manufactured by a special method that includes a smooth distillation technology for removing the excess fatty alcohol via bleaching and stabilization (Scheme 7).60–63
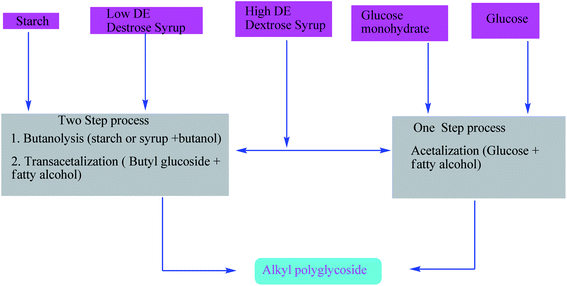 | ||
| Scheme 7 Manufacturing process for alkyl polyglycosides. The scheme is reprinted with permission from ref. 57 copyright 2001 American Oil Chemists' Society (AOCS). | ||
Mid-chain (C12/14) alkyl polyglycoside was first industrially introduced by Cognis. Currently, Cognis has the largest capacity worldwide in case of supply. Other manufacturers are listed in Table 2.
A large amount of non-ionic surfactant is produced by using vegetable oils and sugar. This process is completely based on renewable resources. C12/14 alkyl polyglycoside is used as a dishwasher agent, detergent, and cosmetic. Hard surface cleaners, agrochemicals, and products for institutional and industrial cleaning are the principal applications for C8/10 (or branched C8) alkyl polyglycosides (Table 2). According to Anastas and Warner, basic features of method and product and the environmental consequence for Glucopon, Plantaren, and Plantacare types by applying ‘12 principles of green chemistry’ are represented in Table 4.64
| (1) Prevention | Optimistic reaction procedure with re-usage of excess fatty alcohols |
| (2) Atom economy | Highest utilization: reaction 100% – water >90% |
| (3) Less hazardous chemical synthesis | The method is secure; proven suitable tox and ECOTOX information |
| (4) Designing safer chemical | Replacement of ethylene oxide by glucose |
| (5) Safer solvent and auxiliary | No solvent rather than water |
| (6) Design for energy efficiency | Distillation of excess fatty alcohols under atmospheric condition; minimize the requirement of energy |
| (7) Usage of renewable feedstock | Starting compounds (glucose and vegetable fatty alcohol) are completely reusable |
| (8) Minimize derivative avoid blocking | No uses of the protection group |
| (9) Catalysis | Acids are applied in a catalytic amount |
| (10) Design for degradations | Aerobic and anaerobic degradations proven |
| (11) Pollution prevention through real-time analysis | Process management via process information system PI |
| (12) Accident-prevention based on inherently safer chemistry | The use of less fugitive raw material makes the process safe |
Alkyl polyglycoside is present in the market in an adequate amount. Therefore, it is used as a starting component for the production of the main surfactant. Currently, by using these raw materials the properties of surfactants are also modified with great commitment.
Table 4 is reprinted with permission from ref. 27 copyright 1999 WILEY-VCH Verlag GmbH, Weinheim, Fed. Rep. of Germany.
Alkyl polyglycoside derivatives are prepared by an easier mechanism such as nucleophilic substitution. Along with esterification or ethoxylation of alkyl polyglycosides, ionic products of sulphate, as well as phosphate, may also be prepared (Scheme 8). But till now, only a few products, such as methyl glucoside ester and some selective ionic derivatives of alkyl polyglycoside are established in the market.65–68
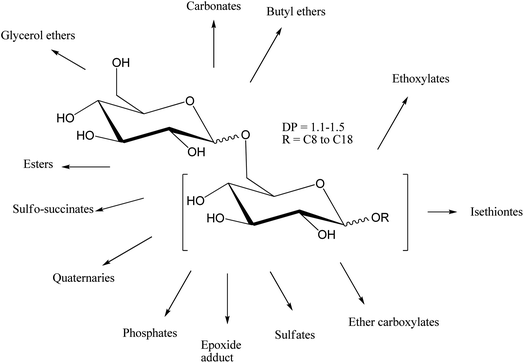 | ||
| Scheme 8 Overview of alkyl polyglycoside derivatives. The scheme is reprinted with permission from ref. 27 copyright 1999 WILEY-VCH Verlag GmbH, Weinheim, Fed. Rep. of Germany. | ||
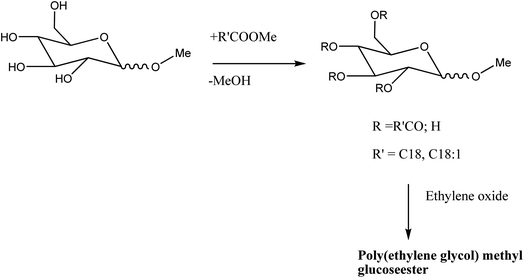 | ||
| Scheme 9 Synthesis of methyl glucoside esters. The scheme is reprinted with permission from ref. 27 copyright 1999 WILEY-VCH Verlag GmbH, Weinheim, Fed. Rep. of Germany. | ||
Unlike alkyl polyglycoside, methyl glucoside ester with the identical hydrophobic chain length is barely dissolved due to its lipophilic nature. However, they show excellent emulsification properties.36,69 It is used as an emollient, emulsifying agent, thickener for cosmetic applicants, and moisturizer also. A useful method for manufacturing methyl glucoside ester and its ethoxylated derivatives is treating Lubrizols with their Noveon lines. There are heavy market sizes of methyl glucoside including the ethoxylated products, i.e. around 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons per year (Table 2).
000 tons per year (Table 2).
3.4. Alkyl polyglycosides with anionic derivatives
Chemicals, a division of Lamberti Spa in Italy, released three non-ionic polyglycoside esters – citrates, sulfosuccinates, and tartrates – for use in personal care products a few years ago.70For their synthesis, alkyl polyglycosides are first prepared and then treated with citric acid, maleic anhydride, and tartaric acid, respectively.71
After the production of non-ionic polyglycoside esters, alkyl polyglycoside carboxylate, a new anionic surfactant, which is used in personal care cleansing applications, has been introduced to the market by Cogins. It is seen that this anionic derivative of alkyl polyglycosides shows better performance in personal care products than those non-ionic derivatives. The anionic surfactants have a relatively high tendency to form foam than non-ionic sugar-surfactants in shampoo and shower bath formulations. The sensorial effects are also better in body wash applications in the case of the anionic derivative. These advantages make the anionic derivative appropriate for many cosmetic products, for sensitive skin and hair. A new method is introduced by which these anionic derivatives can be made through an environment-friendly and cheaper procedure. This process is being carried out by treating sodium monochloroacetate with aqueous alkyl polyglycoside, and the speciality of the reaction is that it does not require any solvent (Scheme 10).72
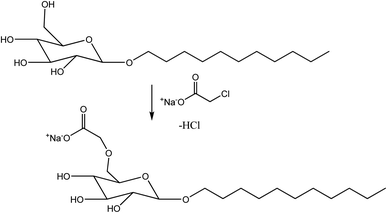 | ||
| Scheme 10 Synthesis of alkyl polyglycoside carboxylate. The scheme is reprinted with permission from ref. 27 copyright 1999 WILEY-VCH Verlag GmbH, Weinheim, Fed. Rep. of Germany. | ||
3.5. Fatty acid glucamides
Fatty acid glucamides are produced via a two-step reaction. In the first step, glucose reacts with methylamine via reductive alkylation to produce N-methyl glucamine. A reaction with a fatty acid methyl ester converts N-methyl glucamine to the corresponding fatty acid amide in the second step.Now, we have seen from the product that glucamides of fatty acids are made of one carbohydrate molecule linked with it.
This may be one reason for the less solubility of fatty acid glucamides and the tendency to crystallize smoothly from the aqueous solution. Scheme 11 depicts the manufacturing method for fatty acid glucamides. However, a problem arises during the process of manufacturing fatty acid glucamides. A large amount of N-methyl glucamine remains unreacted, which acts as precursors for nitrosamines.73 To avoid this problem, Procter and Gamble have introduced an alternative method that deals with the reaction with acetic anhydride. By using acetic anhydride, secondary amines form corresponding acetates as the desired product.71,72,74,75
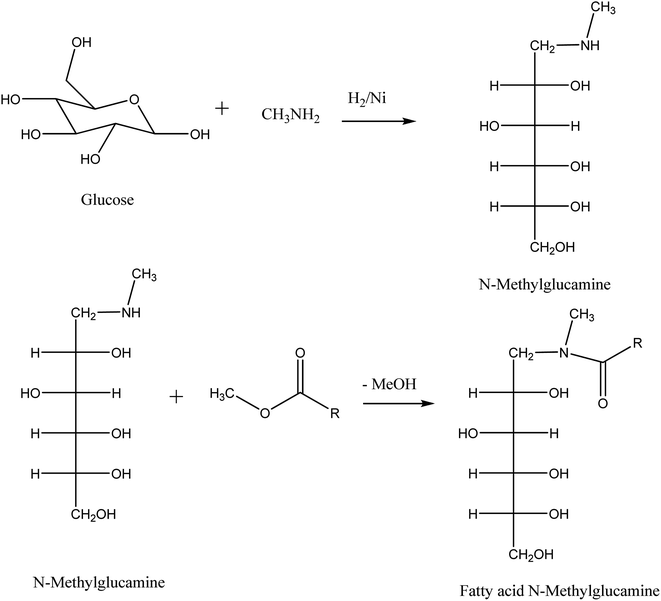 | ||
| Scheme 11 Synthesis of fatty acid glucamides in two steps. The scheme is reprinted with permission from ref. 27 copyright 1999 WILEY-VCH Verlag GmbH, Weinheim, Fed. Rep. of Germany. | ||
3.6. Carbohydrate raw materials used in the production of polysaccharide-based BSs
Chicory root is the main source of extraction for the commercial production of inulin. Researchers are also developing surfactant-type derivatives of it. Inulin derivatives are traded in the market as Inutec.77,78
3.7. Preparative/synthetic methods of sugar-based emulsifiers
To isolate sugar beet pectin, three-volume portions of pure isopropyl alcohol were prepared and the mixture constant agitation was carried out for one hour. The mixture was cleaned by separating sugar beet pectin from it using cotton canvas and then suspending it in 20% aqueous isopropyl alcohol. Overnight drying at 50 °C in a well-ventilated oven followed a second separation of the material. Using a 0.5 mm sieve, 335 g of sugar beet pectin was isolated after milling the dried pectin. Details about the various methods of preparation of sugar beet pectin can be found in the literature.84
4. Conclusion and future direction
Nowadays, it has been found that there is a tendency of consumers for claiming more plant-based natural cosmetics, food, and beverages. For this reason, scientists have focused on the classification, production, and application of natural emulsifiers such as bio-surfactants, mainly polysaccharides rather than synthetic ingredients. Some of those emulsifiers form tiny droplets of oil-in-water emulsions, which are stable in various circumstances and thus become appropriate for producing food and cosmetic products.However, we still need to investigate and examine far more significant outcomes to successfully unveil several natural emulsifiers. The natural emulsifiers which have greater functionalities such as stability to freezing/thawing, protection of encapsulated components against the degradation of chemicals, or controlled release properties are very much effective in the food industry. From the above-mentioned points, we can conclude that a good natural emulsifier should be amphiphilic, so that it can stabilize emulsions, being adsorbed onto the surface of oil droplets. Therefore, each newly revealed natural emulsifier should be cautiously classified according to the above criteria.
It is noteworthy for this review that surface-active agents or surfactants are one type of emulsifier. Beyond this discussion, future scientific research is additionally needed to spot, isolate, purify, and characterize new forms of natural emulsifiers, and to check their efficacy in cosmetics, detergent, food, and other products.
Author contributions
The project was conceived by Aniruddha Pal, Monohar Hossain Mondal, and Bidyut Saha. The manuscript was written by Aniruddha Pal, Monohar Hossain Mondal, Ajaya Bhattarai, and Bidyut Saha and edited by Achyut Adhikari. The manuscript was revised several times by Bidyut Saha, Achyut Adhikari and Ajaya Bhattarai.Conflicts of interest
The authors declare that there is no conflict of interest.References
- D. J. McClements, Curr. Opin. Colloid Interface Sci., 2012, 17, 235–245 CrossRef CAS.
- Y. Maphosa and V. A. Jideani, in Science and Technology Behind Nanoemulsions, InTech, 2018 Search PubMed.
- Q. Chang, Colloid and Interface Chemistry for Water Quality Control, Elsevier Academic Press, 2016, ISBN 9780128093191 Search PubMed.
- Specialty Oils and Fats in Food and Nutrition, Properties, Processing and Applications, ed. G. Talbot, Elsevier (Woodhead Publishing), 1st edn, 2015, ISBN 9781782423768 Search PubMed.
- F. Goodarzi and S. Zendehboudi, Can. J. Chem. Eng., 2018, 97, 281–309 CrossRef.
- F. Teng, M. He, J. Xu, F. Chen, C. Wu, Z. Wang and Y. Li, Sci. Rep., 2020, 10, 14010 CrossRef CAS PubMed.
- K. Łupina, D. Kowalczyk, E. Zięba, W. Kazimierczak, M. Mężyńska, M. Basiura-Cembala and A. E. Wiącek, Food Hydrocolloids, 2019, 96, 555–567 CrossRef.
- T. F. Tadros, Emulsion formation and stability, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, Belitz, 2013 Search PubMed.
- R. J. Wakeman, A-to-Z Guide to Thermodynamics, Heat and Mass Transfer, and Fluids Engineering, 2011 Search PubMed.
- J. Gregory, in Encyclopedia of Colloid and Interface Science, Springer Berlin Heidelberg, 2013, pp. 459–491 Search PubMed.
- Remington, The Science and Practice of Pharmacy, ed. A. Adejare, Elsevier, 23rd edn, 2021, ISBN: 9780128223895 Search PubMed.
- H. Xu, C. Chang, N. Yi, P. Tao, C. Song, J. Wu, T. Deng and W. Shang, ACS Omega, 2019, 4, 17615–17622 CrossRef CAS PubMed.
- E. Piacentini, Encyclopedia of Membranes, Springer-Verlag Berlin Heidelberg, 2014, p. 1 Search PubMed.
- S. Damodaran, K. L. Parkin and O. R. Fennema, Fennema's Food Chemistry, CRC Press, Boca Raton, FL, 4th edn, 2007 Search PubMed.
- E. Dickinson, Food Hydrocolloids, 2003, 17, 25–39 CrossRef CAS.
- R. A. Ghashoghchi, M. R. Hosseini and A. Ahmadi, Appl. Clay Sci., 2017, 138, 81–88 CrossRef CAS.
- S. Patel and A. Goyal, Int. J. Food Prop., 2015, 18, 986–998 CrossRef CAS.
- P. A. Williams and G. O. Phillips, Gum arabic, Handbook of Hydrocolloids, 2nd edn, 2009, p. 252 Search PubMed.
- S.-P. Nie, C. Wang, S. W. Cui, Q. Wang, M.-Y. Xie and G. O. Phillips, Food Hydrocolloids, 2013, 31, 42–48 CrossRef CAS.
- E. Z. Ron and E. Rosenberg, Curr. Opin. Biotechnol., 2002, 13, 249–252 CrossRef CAS PubMed.
- L. Cheung, J. Wanasundara and M. T. Nickerson, Food Biophys., 2014, 10, 30–38 CrossRef.
- C. Schulz, in AutoCAD Praktikum, Vieweg+Teubner Verlag, 1992, pp. 33–61 Search PubMed.
- M. Biermann, K. Schmid and P. Schulz, Starch/Staerke, 1993, 45, 281–288 CrossRef CAS.
- W. Ruback and S. Schmidt, Alkyl polyglucoside, a carbohydrate-based surfactant, in Carbohydrates as Organic Raw Materials III, ed. H. v. Bekkum, H. Röper and F. Voragen, VCH, Weinheim, 1996 Search PubMed.
- A. G. L. Borthwick and S. A. Joynes, J. Environ. Eng., 1992, 118, 905–922 CrossRef.
- F. W. Lichtentaler, Carbohydrates as Renewable Raw Materials: A Major Challenge of Green Chemistry, in Methods and Reagents for Green Chemistry: An Introduction, ed. P. Tundo, A. Perosa and F. Zecchini, Wiley & Sons, Inc., Hoboken, New Jersey, 2007, pp. 23–63 Search PubMed.
- K. Hill and O. Rhode, Fett/Lipid, 1999, 101, 25–33 CrossRef CAS.
- J. Falbe, Surfactants in Consumer Products – Theory, Technology and Application, Springer Verlag, Berlin, Heidelberg, 1987, p. 547, ISBN 3-540-17019-7 Search PubMed.
- M. Enayati, Y. Gong, J. M. Goddard and A. Abbaspourrad, Food Chem., 2018, 266, 508–513 CrossRef CAS PubMed.
- Y. Liu, J. Wu, L. Huang, J. Qiao, N. Wang, D. Yu, G. Zhang, S. Yu and Q. Guan, Int. J. Biol. Macromol., 2020, 154, 499–510 CrossRef CAS PubMed.
- H. Baumann and M. Biermann, Neue Tenside ausnativen Rohstoffen, in Nachwachsende Rohstoffe, Perspektivenfür die Chemie, ed. M. Eggersdorfer, S. Warwel, and G. Wulff, VCH, Weinheim, 1993, pp. 33–35 Search PubMed.
- K. R. Brown, US Pat., 2, 322, 820, 1943 Search PubMed.
- G. J. Stockburger, US Pat., 4, 297, 290, 1981 Search PubMed.
- M. Biermann, F. Lange, R. Piorr, U. Ploog, H. Rutzen, J. Schindler and R. Schmid, Surfactants in Consumer Products, ed. J. Falbe, Springer-Verlag, Berlin, 1987 Search PubMed.
- J. Giacometti, Č. Milin, N. Wolf and F. Giacometti, J. Agric. Food Chem., 1996, 44, 3950, DOI:10.1021/jf950314j.
- N. B. Desai, Cosmet. Toilet., 1990, 105, 99 CAS.
- S. Nakamura, Inform., 1997, 8, 866 Search PubMed.
- M. Cecchim, DESS Ingenierie Documentaire, ENSSIB de Lyon., 2001, p. 87 Search PubMed.
- L. I. Osipow, F. D. Snell and W. C. Finchler, Ind. Eng. Chem., 1956, 48, 1459, DOI:10.1021/ie51400a026.
- H. B. Hass, Early History of Sucrose Esters, in Sugar Esters, ed. H. B. Hass and The Sugar Research Foundation, Noyes Development Corp., Park Ridge, NJ, 1968, p. 1 Search PubMed.
- L. I. Osipow and W. Rosenblatt, J. Am. Oil Chem. Soc., 1967, 44, 307, DOI:10.1007/BF02635621.
- M. K. Matsson, B. Kronberg and P. M. Claesson, Langmuir, 2004, 20(10), 4051–4058 CrossRef CAS PubMed.
- V. Pian, C. Le Hen-Ferrenbach, M. Beuché and M. Roussel, New Sucrose Polystearate Effective Emulsifier Combining Skin Touch with Moisturizing Properties, ISFCC, Florence, 2005 Search PubMed.
- C. Le Hen-Ferrenbach, M. Beuché and M. Roussel, DE 054432, Cognis, 2005 Search PubMed.
- X. Zhang, B. Li, J. Wang, H. Li and B. Zhao, J. Agric. Food Chem., 2017, 65(49), 10767–10774 CrossRef CAS PubMed.
- T. Plat and R. J. Linhardt, J. Surfactants Deterg., 2001, 4, 415, DOI:10.1007/s11743-001-0196-y.
- E. Fischer, Ber. Dtsch. Chem. Ges., 1893, 26, 2400, DOI:10.1002/cber.18930260327.
- C. J. Drummond, C. Fong, I. Krodkiewska, B. J. Boyd, and I. J. Baker, in Novel Surfactants/114, Marcel Dekker, New York, 2003, Ch. 3 Search PubMed.
- P. Jürges and A. Turowaki, in Perspektiven Nachwachsender Rohstoffe in der Chemie, ed. H. Eierdanz, VCH, Weinheim, 1996, p. 61 Search PubMed.
- J. Li, Y. Bai, W. Wang, X. Tai and G. Wang, ACS Sustainable Chem. Eng., 2019, 7, 4390–4398 CrossRef CAS.
- H. Kelkenberg, Tenside, Surfactants, Deterg., 1988, 25, 8, DOI:10.1515/tsd-1988-250107.
- K. Hill, W. von Rybinski and G. Stoll, Alkyl Polyglycosides: Technology, Properties and Applications, VCH, Weinheim, 1997 Search PubMed.
- T. Tharwat, B. Levecke and K. Booten, Chim. Oggi, 2006, 55–58 CAS.
- S. Glaur, Y. Wu, P. Shuler, T. Yongchun and W. A. Goddard, Tenside, Surfactants, Deterg., 2010, 47, 87 CrossRef.
- Novel Surfactant, Preparation, application, biodegradability, ed. H. Krishter, Marcel Dekkar, Inc, New York Basel, 2nd edn, 2003 Search PubMed.
- M. M. A. El-Sukkary, N. A. Syed, I. Aiad and W. I. M. El-Azab, J. Am. Chem. Soc., 2008, 11, 129 CAS.
- D. Geetha and R. Tyagi, Tenside, Surfactants, Deterg., 2012, 49, 417, DOI:10.3139/113.110212.
- W. Von Rybinski and K. Hill, Angew. Chem., Int. Ed., 1998, 37, 1228 CrossRef.
- W. Von Rybinski and K. Hill, Alkyl Polyglycosides—Properties and Applications of a new Class of Surfactants, Marcel Dekker, New York, 1998, Ch. 2 Search PubMed.
- D. Balzer and H. Lüders, Nonionic surfactants: alkyl polyglucosides, Marcel Dekker, New York, 2000 Search PubMed.
- D. E. Koeltzow and A. D. Urfer, J. Am. Oil Chem. Soc., 1984, 61, 1651, DOI:10.1007/BF02541651.
- A. J. J. Straathof, H. van Bekkum and A. P. G. Kieboom, Starch/Staerke, 1988, 40, 438, DOI:10.1002/star.19880401109.
- T. Böcker and J. Thiem, Tenside, Surfactants, Deterg., 1989, 26, 318, DOI:10.1515/tsd-1989-260508.
- P. T. Anastas and J. C. Warner, Principles of Green Chemistry, Oxford University Press, New York, 1998 Search PubMed.
- W. Aulmann and W. Sterzel, Toxicology of alkyl polyglycosides, in Alkyl Polyglycosides, ed. K. Hill, W. Rybinski, and G. Stoll, VCH, Weinheim, New York, Basel, Cambridge, Tokyo 1997, pp. 151–167 Search PubMed.
- W. Matthies, B. Jackwerth and H. U. Kraechter, Dermatological properties of alkyl polyglucosides, in Alkyl Polyglycosides, ed. K. Hill, W. Rybinski, and G. Stoll, VCH, Weinheim, New York, Basel, Cambridge, Tokyo, 1997 Search PubMed.
- J. Steber, W. Guhl, N. Stelter and F. R. Schröder, Ecological evaluation of alkyl polyglycosides, in Alkyl Polyglycosides, ed. K. Hill, W. Rybinski, and G. Stoll, VCH, Weinheim, New York, Basel, Cambridge, Tokyo, 1997 Search PubMed.
- M. Stalmans, E. Matthijs, E. Weeg and S. Morris, SOFW J., 1993, 119, 794 CAS.
- O. Rhode, M. Weuthen and D. Nickel, New Nonionic Derivatives of Alkyl Polyglycosides Synthesis and Properties, in Alkyl Polyglycosides, ed. K. Hill, W. Rybinski, and G. Stoll, VCH, Weinheim, New York, Basel, Cambridge, Tokyo, 1997 Search PubMed.
- Anonymous, Parfums, Cosmet., Actual., 1998, 139, 44 Search PubMed.
- P. Bernardi, D. Fornara, L. Paglino and T. Verzotti, The 1st Concise Surfactants Directory, 1996, p. 15 Search PubMed.
- W. Santos, Chem. Mark. Rep., 1996, 249, 10 Search PubMed.
- R. G. Laughlin, Y. C. Fu, F. C. Wireko, J. J. Scheibel and R. L. Munyon, N-Alkanoyl-N-Alkyl-1-Glycamines, in Novel Surfactants: Preparation, Applications, and Biodegradability, Marcel Dekker, New York, 2004, pp. 1–34 Search PubMed.
- J. J. Scheibel, D. S. Connor, R. E. Shumate and J. C. T. R. B. S. Laurent, US Pat., 598462, Patent EP-B 0558515, 1990 Search PubMed.
- Procter & Gamble, Chem. Abstr., 1992, 117, 114045 Search PubMed.
- A. Behler, H. Hensen and W. Seipel, Alkyl Polyglycoside Carboxylate - A New Anionic Surfactant, Proceedings 6th World Surfactant Congress, World Surfactant Congress, CESIO, Berlin, 2004 Search PubMed.
- M. Watts and J. Lim, ICIS Chem. Bus., 8, 2007 Search PubMed.
- K. Booten and B. Levecke, Specialty Chemicals Magazine, 2004, p. 14 Search PubMed.
- M. Akhtar, E. Dickinson, J. Mazoyer and V. Langendorff, Food Hydrocolloids, 2002, 16, 249–256 CrossRef CAS.
- J. Leroux, V. Langendorff, G. Schick, V. Vaishnav and J. Mazoyer, Food Hydrocolloids, 2003, 17, 455–462 CrossRef CAS.
- P. A. Williams, C. Sayers, C. Viebke, C. Senan, J. Mazoyer and P. Boulenguer, J. Agric. Food Chem., 2005, 53, 3592–3597 CrossRef CAS PubMed.
- T. Funami, G. Zhang, M. Hiroe, S. Noda, M. Nakauma, I. Asai, M. K. Cowman, S. Al-Assaf and G. O. Phillips, Food Hydrocolloids, 2007, 21, 1319–1329 CrossRef CAS.
- C. K. Siew and P. A. Williams, J. Agric. Food Chem., 2008, 56, 4164–4171 CrossRef CAS PubMed.
- H. C. Buchholt, T. M. I. E. Christensen, B. Fallesen, M.-C. Ralet and J.-F. Thibault, Carbohydr. Polym., 2004, 58, 149–161 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2021 |