Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
High adsorption of ammonia in a titanium-based metal–organic framework†
Xiangdi
Zeng
a,
Jiangnan
Li
ab,
Meng
He
 a,
Wanpeng
Lu
a,
Danielle
Crawshaw
a,
Wanpeng
Lu
a,
Danielle
Crawshaw
 a,
Lixia
Guo
ab,
Yujie
Ma
a,
Lixia
Guo
ab,
Yujie
Ma
 a,
Meredydd
Kippax-Jones
ac,
Yongqiang
Cheng
d,
Pascal
Manuel
e,
Svemir
Rudić
a,
Meredydd
Kippax-Jones
ac,
Yongqiang
Cheng
d,
Pascal
Manuel
e,
Svemir
Rudić
 e,
Mark D.
Frogley
e,
Mark D.
Frogley
 c,
Martin
Schröder
c,
Martin
Schröder
 *a and
Sihai
Yang
*a and
Sihai
Yang
 *ab
*ab
aDepartment of Chemistry, University of Manchester, Manchester, M13 9PL, UK. E-mail: M.Schroder@manchester.ac.uk; Sihai.Yang@manchester.ac.uk
bCollege of Chemistry and Molecular Engineering, Beijing National Laboratory for Molecular Sciences, Peking University, Beijing, 100871, China. E-mail: Sihai.Yang@pku.edu.cn
cDiamond Light Source, Harwell Science Campus, Oxfordshire, OX11 0DE, UK
dNeutron Scattering Division, Neutron Sciences Directorate, Oak Ridge National Laboratory, Oak Ridge, TN 37831, USA
eISIS Neutron and Muon Facility, Rutherford Appleton Laboratory, Didcot, OX11 0QX, UK
First published on 26th April 2024
Abstract
We report the high adsorption of NH3 in a titanium-based metal–organic framework, MFM-300(Ti), comprising extended [TiO6]∞ chains linked by biphenyl-3,3′,5,5′-tetracarboxylate ligands. At 273 K and 1 bar, MFM-300(Ti) shows an exceptional NH3 uptake of 23.4 mmol g−1 with a record-high packing density of 0.84 g cm−3. Dynamic breakthrough experiments confirm the excellent uptake and separation of NH3 at low concentration (1000 ppm). The combination of in situ neutron powder diffraction and spectroscopic studies reveal strong, yet reversible binding interactions of NH3 to the framework oxygen sites.
Ammonia (NH3) is an important feedstock that is produced at a scale of ca. 150 million tonnes per year.1 In addition, the high volumetric (∼0.105 kg L−1) and gravimetric (17.7 wt%) hydrogen densities of NH3 make it a promising renewable fuel and a potential hydrogen carrier for on-board storage. However, the highly toxic and corrosive nature of NH3 poses challenges in its safe storage and transportation. At present, NH3 is transported as a compressed liquid either at 10 bar at 25 °C or at ambient pressures at low temperature (liquefication point of −33 °C).2 Thus, the development of robust sorbents for reversible NH3 adsorption under mild conditions is of great importance.
Porous materials, such as zeolites,3 active carbons,4 mesoporous silica,5 and organic polymers6 have been tested for NH3 adsorption. However, these materials demonstrate limited capacities; for example, 9.3 mmol g−1 in 13X zeolite,3 8.8 mmol g−1 in MCM-41,5 and 11.4 mmol g−1 in Amberlyst 15.7 Metal–organic frameworks (MOFs) have demonstrated potential for NH3 adsorption8 owing to their high accessible surface area, and their tailored porosity and pore environment. The MOFs displaying top performance for NH3 adsorption are primarily divalent/trivalent-based MOFs or MOF composites, such as LiCl@MIL-53-(OH)29 (33.9 mmol g−1 at 298 K and 1 bar) and Ni_acryl_TMA10 (23.5 mmol g−1 at 298 K and 1 bar). However, the stability of MOFs toward NH3 adsorption needs to be improved, and typically tetravalent metal-based MOFs (e.g., Zr4+, V4+, Hf4+) exhibit enhanced thermal and chemical stability. However, the reported Zr-based MOFs show limited NH3 adsorption capacities (8.40 mmol g−1 in UiO-6711). On the other hand, the study of V-based MFM-300(V) for NH3 adsorption has revealed that the incorporation of V4+ can effectively enhance the NH3 adsorption,12 which is attributed to the charge transfer within the framework and the formation of N2H4.
Herein, we report a novel Ti4+-based MOF, MFM-300(Ti) [Ti2(O)2(C16H6O8)], which displays a reversible NH3 uptake of 23.4 mmol g−1 at 273 K and 1 bar, among the best NH3 sorbent materials showing reversible adsorption to date. Significantly, the packing density of NH3 in MFM-300(Ti) (0.84 g cm−3, calculated using the pore volume derived from crystallographic data) is comparable to that of solid NH3 at –80 °C (0.82 g cm−3), due to the strong guest–guest interaction within the pores. In addition, breakthrough experiments confirm that MFM-300(Ti) can effectively capture and separate NH3 at low concentrations (1000 ppm). The binding sites of NH3 in MFM-300(Ti) have been determined by in situ neutron powder diffraction (NPD), and its binding dynamics investigated by a combination of in situ inelastic neutron scattering (INS) and synchrotron IR microspectroscopy.
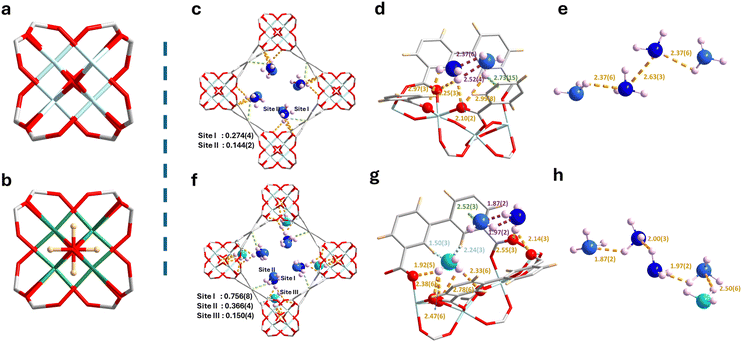
Solvated MFM-300(Ti), [Ti2(O)2(C16H6O8)(H2O)2(C3H6O)0.7], was prepared via a solvothermal reaction of {Ti8AF} clusters,13 H4L (biphenyl-3,3′,5,5′-tetracarboxylic acid, H4bptc), acetic acid, acetic anhydride and methanol at 180 °C for 12 h. Structure solution by NPD (Fig. 1a and b) confirms that MFM-300(Ti) crystallises in the tetragonal space group I4122 and is isostructural to other MFM-300 materials.14 The presence of cis-μ2-O moieties rather than cis-μ2-OH groups as observed in M3+-based analogues is confirmed by NPD and FTIR spectra (Fig. 1a and b, Fig. S7, ESI†). MFM-300(Ti) exhibits an open framework structure comprising of chains of [TiO4(O)2] moieties bridged by tetracarboxylate ligands L4− to afford 1D channels along the c axis with a diameter of 7.4 Å. Desolvated MFM-300(Ti) displays a Brunauer–Emmett–Teller (BET) surface area of 890 m2 g−1 and pore volume of 0.44 cm3 g−1, as determined from the CO2 isotherm at 195 K (Fig. S1, ESI†), consistent with the porosity derived from the crystal structure (pore volume of 0.45 cm3 g−1). Crystallites of MFM-300(Ti) show rod-like morphology (Fig. S2, ESI†). The excellent thermal stability up to 450 °C and the chemical stability of MFM-300(Ti) has been confirmed by thermogravimetric analysis (TGA; Fig. S3, ESI†), variable temperature powder X-ray diffraction (VT-PXRD; Fig. S4, ESI†), PXRD analysis of samples soaked in various solutions (Fig. 1c and Fig. S5, ESI†), and the CO2 adsorption measurements for samples after various treatments to confirm the porosity (Fig. S6, ESI†).
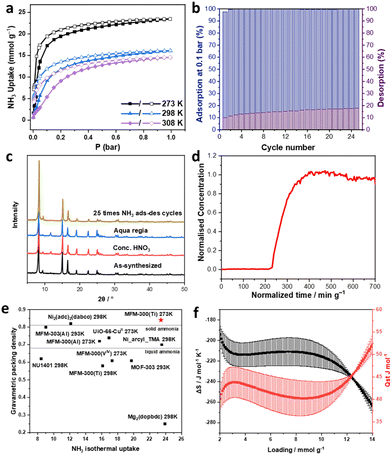
The adsorption isotherms for NH3 in MFM-300(Ti) were measured between 273 and 308 K at 1 bar (Fig. 2a). MFM-300(Ti) exhibits an exceptional and fully reversible NH3 uptake of 23.4 mmol g−1 at 273 K and 1.0 bar, comparable to state-of-the-art sorbents (Table S1, ESI† and Fig. 2e). Hysteresis in the NH3 isotherms was observed at all temperatures, indicating the presence of strong host–guest interactions. The pressure-swing experiment was conducted at 298 K from 0 to 0.1 bar. Little change in sorption capacity or structure was observed for MFM-300(Ti) after 25 cycles of adsorption and desorption of NH3 (Fig. 2b and c), confirming the excellent stability of MFM-300(Ti) towards NH3. The residual NH3 observed on desorption during cyclic pressure-swing experiments suggests the presence of strong binding sites in the pore, consistent with the observed hysteresis. The dynamic NH3 uptake of MFM-300(Ti) at low concentration (1000 ppm) was recorded as 0.6 mmol g−1 at 298 K, demonstrating an excellent capture capability (Fig. 2d). The heat of adsorption (Qst) of NH3 in MFM-300(Ti) was calculated to be 39–52 kJ mol−1 (Fig. 2f and Table S2, ESI†), comparable with other MOFs incorporating strong binding sites, such as UiO-66Cu(II)15 (25–55 kJ mol−1).
In situ NPD data for ND3-loaded MFM-300(Ti) were collected at 10 K (ND3/Ti = 0.42, 1.27), and Rietveld refinements afforded distinct binding sites for ND3. At low-loading (ND3/Ti = 0.42; MFM-300(Ti)·0.84 ND3) (Fig. 1c and d), two binding sites (I and II) are observed. Site I is anchored by hydrogen bonding between the ND3 molecules and the carboxylate oxygen atoms [ND3⋯Oligand = 2.10(2)–2.97(3) Å]. Site II is adjacent to site I, stabilised by hydrogen bonding interactions [ND3⋯Oligand = 2.99(8) Å], electrostatic interactions [ND3⋯aromatic rings = 2.52(3)–2.73(15) Å], and guest–guest interactions [ND3⋯ND3 = 1.87(2)–2.52(4) Å]. Interestingly, when the loading was increased to 1.27 ND3/Ti (MFM-300(Ti)·2.54 ND3) (Fig. 2e and f), an additional binding site was observed even closer to the [TiO6]∞ chain and stabilised by multiple supramolecular interactions [C–Haromatic⋯N = 1.50(3)–2.24(3) Å, ND3⋯Oligand = 1.92(5)–2.38(6) Å, and ND3⋯Obridge = 2.33(6)–2.78(6) Å]. In addition, the adsorbed ND3 molecules propagated to form a 1D network within the channel of MFM-300(Ti) (Fig. 1e and h). Moreover, the hydrogen bonding distance [N–D⋯N = 2.37(6)–2.52(3) Å] between adsorbed ND3 molecules in MFM-300(Ti)·0.84 ND3 decreases to [N–D⋯N = 1.87(3)–2.00(3) Å] in MFM-300(Ti)·2.54 ND3. This tighter host–guest and guest–guest interaction with increasing NH3 loading is consistent with the trend of Qst (Fig. 2f). Notably, the shortest distance between adsorbed ND3 molecules in MFM-300(Ti) is 1.87 Å, which is notably shorter than those previously observed [2.33 Å in MFM-300(Fe); 3.05 Å in MFM-300(VIV)]15 and reflects highly efficient packing of NH3 molecules in MFM-300(Ti), consistent with the high packing density derived from the adsorption isotherms. Compared with MFM-300(MIII),15 the replacement of μ2-OH by μ2-O in MFM-300(Ti) reduces the steric hindrance between the bridging hydroxyl groups and guest molecules, providing additional binding sites and boosting the storage of NH3. In MFM-300(VIV), the presence of the vanadium centre promotes charge transfer between NH3 molecules, resulting in the observation of both N2D4 and ND4+ molecules in the pore,15 which limits the reusability of the MOF. The utilisation of Ti4+ ions not only improves the structural stability of the material, but also hinders charge transfer to enhance the adsorption reversibility of NH3.
The dynamics of host–guest binding has been analysed using in situ synchrotron IR microspectroscopy, INS, and density functional theory (DFT) calculations. The absence of the characteristic IR band of hydroxyl group at ∼3600 cm−1 in activated MFM-300(Ti) (Fig. S7, ESI†) confirmed the presence of cis-μ2-O as a result of the tetravalent Ti4+ sites, consistent with the NPD analysis. On loading NH3, a new band at 3381 cm−1 assigned to a ν(N–H) stretching vibration is observed, confirming the adsorption in the framework (Fig. 3). The band at 1614 cm−1 can be assigned to vas(COO−), which broadens on loading of NH3. The bands at 1470 cm−1, 1414 cm−1,1360 cm−1, and 1320 cm−1 can be assigned to different vibrations vs(COO−) of the carboxylate group in the framework, and all show redshifts on increasing loading of NH3. This suggests the presence of strong interactions between NH3 and the carboxylate groups of the bridging ligand. Simultaneously, as the concentration of NH3 increases from 2% to 5%, two bands at 1548 and 1500 cm−1 corresponding to aromatic C–C stretching vibrations merge into one band (1525 cm−1), which is not observed in other MFM-300 materials. This indicates a notable change in the conjugated structure of the aromatic rings, which is further evidenced by the elongation of the C–C bonds in the biphenyl linker from 1.458(2) Å in bare MFM-300(Ti) to ∼1.497(4) Å in MFM-300(Ti)·0.84 ND3 and further to ∼1.500(7) Å in MFM-300(Ti)·2.54 ND3, as determined by the NPD analysis (Table S9, ESI†).
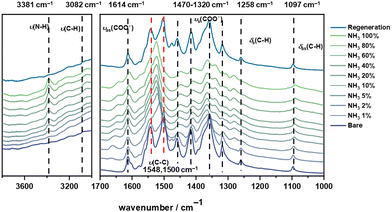 | ||
| Fig. 3 In situ synchrotron FT-IR spectra of MFM-300(Ti) as a function of NH3 adsorption and of regenerated MFM-300(Ti) under a flow of dry N2 at 100 mL min−1 at 298 K for 2 h. | ||
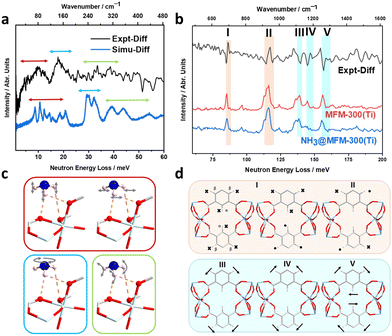
A combination of INS and DFT calculations has been employed to elucidate the dynamic behaviour of adsorbed NH3 within MFM-300(Ti). The congruence between experimental and simulated INS spectra for MFM-300(Ti) (Fig. S12, ESI†) and NH3-loaded MFM-300(Ti) (Fig. S13, ESI†) is remarkable, allowing the assignments of INS peaks. Distinct peaks were observed in the INS difference spectra, which were obtained by subtracting the features of the bare MFM-300(Ti) and the sample cell from the spectra of NH3-loaded MFM-300(Ti) (Fig. 4b). Translational and rotational modes of adsorbed NH3 molecules around its C3 axis are observed between 4.4–14.4 meV and 16.6–23.5 meV, respectively. The bands at 26.5–41.7 meV correspond to the rocking motions of NH3 around the N centre (Fig. 4c and d). In comparison to the spectrum of NH3 in the solid state, where each NH3 molecule forms a 3D hydrogen bonding network with six adjacent NH3 molecules, the bands in all regions for adsorbed NH3 exhibit shifts to lower energy and display broader features (for solid NH3, 6.3–23.1 meV translational modes; 27.6–35.5 meV rotational modes; 36.7–56.9 meV rocking modes) (Fig. 4a). This phenomenon suggests a more dynamic environment for the adsorbed NH3 molecules within MFM-300(Ti). Specifically, the rotational modes show more significant red shifts, attributed to the higher rotational flexibility of NH3 in its adsorbed environment in MFM-300(Ti).
The vibrational features of the framework have been observed in the high energy region of the difference-INS spectra. Features at (I) 84.9, (II) 117.6, (III) 126.5, (IV) 145.1, and (V) 155.9 meV can be assigned to C–H out-of-C6-plane wagging (in-phase along the ring, meaning H atoms move in the same direction), C–H out-of-C6-plane twisting (anti-phase along the ring, meaning neighbouring H atoms move in opposite directions), C–H in-C6-plane scissoring mode, in phase, C–H in-C6-phane scissoring mode, anti-phase, and C–H in-C6-plane rocking mode, respectively (Fig. 4b and d). Compared with other MFM-300(M) materials, MFM-300(Ti) shows more versatile C–H bending modes, and the changes in the C–H bending peaks upon adsorption of NH3 indicate the strong host–guest interactions between the benzyl ring of the framework and NH3 molecules. These results are in excellent agreement with the NPD and FTIR study.
In summary, we report the high capacity and reversible adsorption of NH3 within a novel titanium-based MOF, MFM-300(Ti). This framework features a unique structure composed of extended [TiO6]∞ chains linked by tetra-topic ligands [(C16H6O8)4−]. Notably, at 273 K and 1 bar, MFM-300(Ti) shows an exceptional NH3 uptake of 23.4 mmol g−1 and a record-high packing density of 0.84 g cm−3. The volumetric and gravimetric adsorption capacity is 0.36 g cm−3 and 0.28 g g−1, respectively. Dynamic breakthrough experiments confirm excellent adsorption of NH3 at low concentration (1000 ppm). The combination of in situ NPD, INS, FTIR and DFT studies reveal the molecular details on the host–guest binding interactions. This study has established MFM-300(Ti) as a highly efficient sorbent for NH3, demonstrating its potential for practical applications.
We thank EPSRC (EP/I011870, EP/V056409), University of Manchester, National Science Foundation of China and BNLMS for funding. This project has received funding from the European Research Council (ERC) under the European Union Horizon 2020 research and innovation programme (grant agreement no. 742401, NANOCHEM and PoC665632). We are grateful to the STFC/ISIS Facility and Diamond Light Source for access to beamlines WISH/TOSCA and B22(SM30398), respectively. The computing resources were made available through the VirtuES and the ICE-MAN projects, funded by Laboratory Directed Research and Development program and Compute and Data Environment for Science (CADES) at ORNL.
Conflicts of interest
There are no conflicts to declare.Notes and references
- NITROGEN (FIXED)—AMMONIA U.S. Geological Survey, Mineral Commodity Summaries, 2022, https://pubs.usgs.gov/periodicals/mcs2022/mcs2022-nitrogen.pdf.
- D. R. MacFarlane, P. V. Cherepanov, J. Choi, B. H. Suryanto, R. Y. Hodgetts, J. M. Bakker, F. M. F. Vallana and A. N. Simonov, Joule, 2020, 4, 1186–1205 CrossRef CAS.
- J. Helminen, J. Helenius, E. Paatero and I. Turunen, AIChE J., 2000, 46, 1541–1555 CrossRef CAS.
- T. Zeng, H. Huang, N. Kobayashi and J. Li, Nat. Resour., 2017, 8, 611–631 CAS.
- A. M. B. Furtado, Y. Wang, T. G. Glover and M. D. LeVan, Microporous Mesoporous Mater., 2011, 142, 730–739 CrossRef CAS.
- C. J. Doonan, D. J. Tranchemontagne, T. G. Glover, J. R. Hunt and O. M. Yaghi, Nat. Chem., 2010, 2, 235–238 CrossRef CAS PubMed.
- J. Helminen, J. Helenius, E. Paatero and I. Turunen, J. Chem. Eng. Data, 2001, 46, 391–399 CrossRef CAS.
- X. Han, S. Yang and M. Schröder, J. Am. Chem. Soc., 2023, 145, 1998–2012 CrossRef CAS PubMed.
- Y. Shi, Z. Wang, Z. Li, H. Wang, D. Xiong, J. Qiu, X. Tian, G. Feng and J. Wang, Angew. Chem., Int. Ed., 2022, 61, e202212032 CrossRef CAS PubMed.
- D. W. Kim, D. W. Kang, M. Kang, D. S. Choi, H. Yun, S. Y. Kim, S. M. Lee, J.-H. Lee and C. S. Hong, J. Am. Chem. Soc., 2022, 144, 9672–9683 CrossRef CAS PubMed.
- T. Yoskamtorn, P. Zhao, X.-P. Wu, K. Purchase, F. Orlandi, P. Manuel, J. Taylor, Y. Li, S. Day, L. Ye, C. C. Tang, Y. Zhao and S. C. E. Tsang, J. Am. Chem. Soc., 2021, 143, 3205–3218 CrossRef CAS PubMed.
- X. Han, W. Lu, Y. Chen, I. Da Silva, J. Li, L. Lin, W. Li, A. M. Sheveleva, H. G. Godfrey, Z. Lu, F. Tuna, E. J. L. McInnes, Y. Cheng, L. L. Daemen, L. J. M. McPherson, S. J. Teat, M. D. Frogley, S. Rudić, P. Manuel, A. J. Ramirez-Cuesta, S. Yang and M. Schröder, J. Am. Chem. Soc., 2021, 143, 3153–3161 CrossRef CAS PubMed.
- S. Wang, M. Cabrero-Antonino, S. Navalón, C.-C. Cao, A. Tissot, I. Dovgaliuk, J. Marrot, C. Martineau-Corcos, L. Yu, H. Wang, W. Shepard, H. García and C. Serre, Chem, 2020, 6, 3409–3427 CAS.
- S. Yang, J. Sun, A. J. Ramirez-Cuesta, S. K. Callear, W. I. David, D. P. Anderson, R. Newby, A. J. Blake, J. E. Parker, C. C. Tang and M. Schröder, Nat. Chem., 2012, 4, 887–894 CrossRef CAS PubMed.
- Y. Ma, W. Lu, X. Han, Y. Chen, I. Da Silva, D. Lee, A. M. Sheveleva, Z. Wang, J. Li, W. Li, M. Fan, S. Xu, F. Tuna, E. J. L. McInnes, Y. Cheng, S. Rudić, P. Manuel, M. D. Frogley, A. J. Ramirez-Cuesta, M. Schröder and S. Yang, J. Am. Chem. Soc., 2022, 144, 8624–8632 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2296493, 2296494 and 2296495. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4cc01449a |
| This journal is © The Royal Society of Chemistry 2024 |