Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Divergent synthesis of δ-valerolactones and furanones via palladium or copper-catalyzed α-hydroxycyclopropanol ring opening cyclizations†
Pedro
de Andrade Horn‡
a,
Michael J. E.
Collins‡
b,
Cyrus C.
Gudeman
b,
Alexandra A.
Fresh
 a and
Mingji
Dai
a and
Mingji
Dai
 *bc
*bc
aDepartment of Chemistry, Purdue University, West Lafayette, IN 47907, USA
bDepartment of Chemistry, Emory University, Atlanta, GA 30322, USA. E-mail: mingji.dai@emory.edu; Tel: 001-404-727-4299
cDepartment of Pharmacology and Chemical Biology, Emory University, Atlanta, GA 30022, USA
First published on 6th August 2024
Abstract
Cyclopropanols are versatile starting materials which can undergo various ring opening reactions due to their intrisic ring strain. Herein, we report two transition metal-catalyzed α-hydroxycyclopropanol ring opening cyclizations to divergently transform the same α-hydroxycyclopropanol substrate into two different products of enhanced value. One is a palladium-catalyzed α-hydroxycyclopropanol ring opening carbonylative lactonization to synthesize δ-valerolactones. The other one is a copper-catalyzed α-hydroxycyclopropanol ring opening cyclization to access furanones.
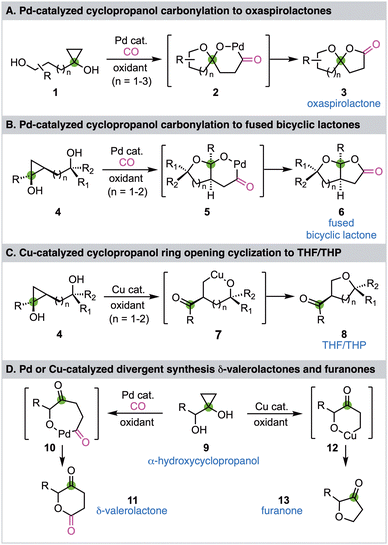
Cyclopropanols have been widely utilized in organic synthesis because of their unique and versatile reactivity profiles.1–3 Due to the intrinsic ring strain of the cyclopropane and the reactivity of the hydroxyl functional group, cyclopropanols can undergo various ring opening reactions. In general, a one-electron cyclopropanol ring opening pathway would generate a β-keto radical and a two-electron cyclopropanol ring opening pathway would give a homoenolate. If a transition metal is involved in the ring opening process, a metal homoenolate would be produced.4 The resulting β-keto radical and (metal) homoenolate can then engage in different downstream reactivity modes to give various products.5–7 We have been developing transition metal-catalyzed cyclopropanol ring opening reactions to facilitate chemical synthesis of biologically active natural products and pharmaceutically important compounds.8–11 In 2016 and 2020, we reported palladium-catalyzed hydroxycyclopropanol ring opening carbonylative lactonizations to synthesize either oxaspirolactones (Fig. 1A, 1 → 2 → 3)12 or fused bicyclic lactones (Fig. 1B, 4 → 5 → 6)13 and have applied these enabling carbonylation chemistry to synthesize a collection of natural products.14–16 While we were developing the fused bicyclic lactone synthesis, we discovered that the same cyclopropanol starting material 4 can undergo a copper-catalyzed cyclopropanol ring opening cyclization to give THF/THP-containing product 8 presumably via intermediate 7 (Fig. 1C).17 We then wondered if α-hydroxycyclopropanol 9 can undergo similar divergent pathways to synthesize δ-valerolactone 11via the palladium-catalyzed ring opening carbonylative lactonization process and furanone 13via the copper-catalyzed ring opening cyclization process (Fig. 1D). To realize these transformations, a few challenges need to be addressed. First, the 1,2-diol moiety of 9 can chelate on the transition metal and may deactivate the catalyst. Second, such α-hydroxycyclopropanol is prone to Pinnacol rearrangement to partially release the ring strain and produce a cyclobutanone.18 Third, the lactonization process would generate a six-membered lactone, which has not been reported from a cyclopropanol ring opening carbonylation process. Fourth, once the Pd or Cu homoenolate is generated, β-hydride elimination, dimerization, and over oxidation are always competing pathways which need to be suppressed. Despite these challenges, the proposed divergent synthesis of δ-valerolactone 11 and furanone 13 from α-hydroxycyclopropanol 9 would further expand cyclopropanol chemistry and offer alternative methods to prepare the target structures, which are frequently found in biologically active molecules, especially natural products.19–23 Herein, we report the details of our exploration which led to divergent approaches to either δ-valerolactone 11 or furanone 13 from the same α-hydroxycyclopropanol 9via a Pd-catalyzed ring opening carbonylative lactonization or a Cu-catalyzed ring opening cyclization, respectively.
 | ||
| Fig. 1 Our previous development in Pd and Cu-catalyzed cyclopropanol ring opening (carbonylative) cyclization and this work. | ||
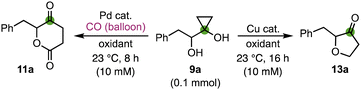
We started our investigation with cyclopropanol 9a, which was prepared via a modified Kulinkovich reaction (see the ESI†)24 and used as a model compound for optimization of the reaction conditions (Table 1). When it was treated with Pd(OAc)2 (10 mol%) and DDQ (2.0 eq.) in benzene at room temperature (the conditions we developed for the bicyclic lactone synthesis),13 desired product 11a was only produced in 13% yield (entry 1). Changing the palladium catalyst from Pd(OAc)2 to Pd(PPh3)4 (entry 2), Pd(TFA)2 (entry 3), and Pd(PPh3)2Cl2 (entry 4) only slightly affected the reaction yield (11–19%). Switching the solvent from benzene to THF increased the yield more significantly (entries 5 and 6). When the combination of Pd(OAc)2 and THF was used, the yield of the desired product 11a increased to 55% yield (entry 6). When the amount of DDQ was reduced from 2.0 equivalents to 1.2 equivalents, 11a was isolated in 59% yield (entry 7). THF was also found to be superior to MTBE (methyl tert-butyl ether), 1,4-dioxane, and DMSO (entries 8–10). Interestingly, when Cu(OTf)2 (2.0 eq.) was used as oxidant, instead of 11a, furanone 13a was obtained in 70% yield (entry 11), which encouraged us to further develop a general cyclopropanol ring opening cyclization to synthesize furanones.
| Entry | Conditions for 9a to 11a | Yielda (%) (11a) |
|---|---|---|
| a NMR yield. b Isolated yield. | ||
| 1 | Pd(OAc)2 (10 mol%), DDQ (2.0 eq.), PhH | 13b |
| 2 | Pd(PPh3)4 (10 mol%), DDQ (2.0 eq.), PhH | 18 |
| 3 | Pd(TFA)2 (10 mol%), DDQ (2.0 eq.), PhH | 11 |
| 4 | Pd(PPh3)2Cl2 (10 mol%), DDQ (2.0 eq.), PhH | 19 |
| 5 | Pd(PPh3)2Cl2 (10 mol%), DDQ (2.0 eq.), THF | 28 |
| 6 | Pd(OAc)2 (10 mol%), DDQ (2.0 eq.), THF | 55 |
| 7 | Pd(OAc)2 (10 mol%), DDQ (1.2 eq.), THF | 59b |
| 8 | Pd(OAc)2 (10 mol%), DDQ (1.2 eq.), MTBE | 25 |
| 9 | Pd(OAc)2 (10 mol%), DDQ (1.2 eq.), 1,4-dioxane | 35 |
| 10 | Pd(OAc)2 (10 mol%), DDQ (2.0 eq.), DMSO | 0 |
| 11 | Pd(OAc)2 (10 mol%), Cu(OTf)2 (2.0 eq.), PhH | 70 (13a) |
| Entry | Conditions for 9a to 13a | Yielda (%) (13a) |
|---|---|---|
| 12 | Cu(OTf)2 (10 mol%), DDQ (1.0 eq.), THF | 74 |
| 13 | CuCl2 (10 mol%), DDQ (1.0 eq.), THF | 66 |
| 14 | CuBr2 (10 mol%), DDQ (1.0 eq.), THF | 49 |
| 15 | CuSO4 (10 mol%), DDQ (1.0 eq.), THF | 0 |
| 16 | CuTC (10 mol%), DDQ (1.0 eq.), THF | 82 |
| 17 | Cu(OAc)2 (10 mol%), DDQ (1.0 eq.), THF | 93b |
| 18 | Cu(OAc)2 (5.0 mol%), DDQ (1.0 eq.), THF | 84 |
| 19 | Cu(OAc)2 (1.0 mol%), DDQ (1.0 eq.), THF | 58 |
| 20 | Cu(OAc)2 (10 mol%), TFBQ (1.0 eq.), THF | 74 |
| 21 | Cu(OAc)2 (10 mol%), Chloranil (1.0 eq.), THF | 69 |
| 22 | Cu(OAc)2 (10 mol%), 2,5-DCBQ (1.0 eq.), THF | 37 |
| 23 | Cu(OAc)2 (10 mol%), 2,6-DCBQ (1.0 eq.), THF | 19 |
| 24 | Cu(OAc)2 (1.0 eq.), THF | 79 |
| 25 | DDQ (1.0 eq.), THF | 0 |
We then started to further optimize the furanone synthesis conditions and learned that palladium catalyst is not necessary. With 10 mol% of Cu(OTf)2 as catalyst and DDQ (1.0 eq.) as oxidant, 13a was obtained in 74% yield (entry 12). While CuCl2 (entry 13), CuBr2 (entry 14), CuSO4 (entry 15), and CuTC (copper thiophene-2-carboxylate, entry 16) are less effective than Cu(OTf)2, the more economical Cu(OAc)2 (entry 17) is superior to Cu(OTf)2 and 13a was produced in 93% isolated yield. Further reducing the catalyst loading to 5 mol% (entry 18) and 1.0 mol% (entry 19) resulted in lower yields (84% and 58%, respectively). Other oxidants including TFBQ (tetrafluoro-1,4-benzoquinone, entry 20), Chloranil (entry 21), 2,5-DCBQ (2,5-dichloro-1,4-benzoquinone, entry 22) and 2,6-DCBQ (2,6-dichloro-1,4-benzoquinone, entry 23) gave reduced yield. In addition, if DDQ or its byproduct complicates the reaction or purification, the reaction can be conducted with a stoichiometric amount of Cu(OAc)2 to produce 13a in 79% yield (entry 24). Given the similar price of DDQ and Cu(OAc)2, the stoichiometric condition doesn’t significantly affect the overall cost. In addition, no product 13a was obtained without the copper catalyst (entry 25).25
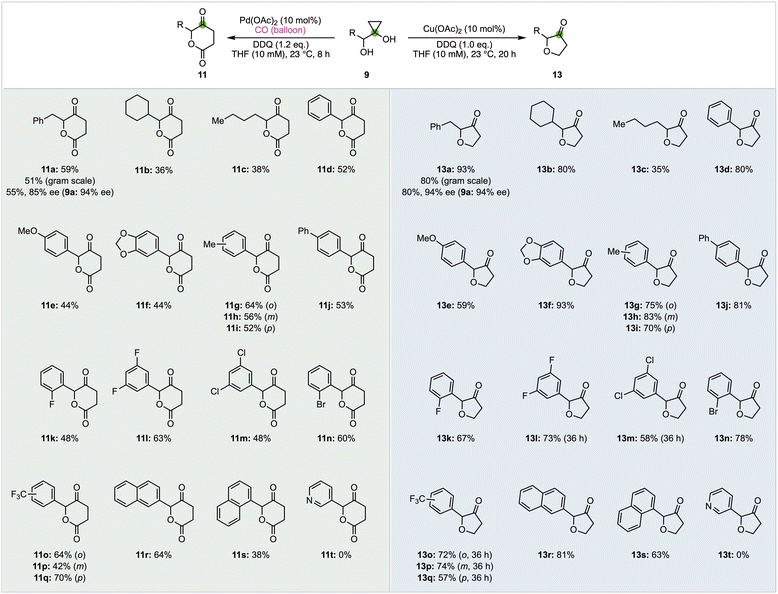
With both the δ-valerolactone and furanone synthesis conditions established, we started to probe the substrate scope of both transformations (Fig. 2). Twenty different α-hydroxycyclopropanols were prepared and subjected to the optimized reaction conditions. In general, the Cu-catalyzed furanone synthesis tends to give higher yield than the corresponding Pd-catalyzed δ-valerolactone synthesis. Both transformations can be conducted on gram scale with only a slight drop of the reaction yields. Alkyl (11/13a–c) and aryl groups (11/13d–s) are tolerated. The electronic properties of the aryl groups don't influence the reaction yield significantly. Halides such as fluoride, chloride, and bromide are not affected under both reaction conditions. The tolerance of chloride and bromide offers opportunity for cross coupling reactions to further functionalize the corresponding products.
While both 1- or 2-naphthyl group containing substrates are effective, no desired products (11t and 13t) were obtained for substrate 9t with a 3-pyridyl under both reaction conditions. Finally, we prepared enantio-enriched starting material 9a (94% ee) from L-phenylalanine and evaluated if both reaction conditions would erode the stereochemistry at the α-position of the newly formed ketone. When 9a (94% ee) was subjected to the carbonylation conditions, product 11a was obtained in 55% yield and 85% ee. When 9a (94% ee) was subjected to the furanone synthesis conditions, product 13a was obtained in 80% yield and 94% ee. These results indicate the mildness of the reaction conditions and the potential application of these two methods to prepare enantio-enriched δ-valerolactones and furanones.
In summary, two novel cyclopropanol ring opening cyclization reactions were developed to divergently transform the same α-hydroxycyclopropanol substrate to either a δ-valerolactone or a furanone. The δ-valerolactone synthesis was catalyzed by Pd(OAc)2 under carbon monoxide atmosphere and the furanone synthesis was catalyzed by Cu(OAc)2. DDQ was used as external oxidant in both reactions. An array of δ-valerolactones and furanones were prepared. The reactions can be scaled up to gram scale. Overall, these two cyclopropanol ring opening cyclization reactions provide mild alternatives to synthesize δ-valerolactones and furanones, which are frequently found in biologically active molecules.
This work was financially supported by NSF 2349014.
Data availability
The ESI† includes the experimental procedures, compound characterization data, and NMR spectra. ESI† is included in the submission and will be available to the readers via the Chemical Communication online publication system.Conflicts of interest
There are no conflicts to declare.Notes and references
- X. Cai, W. Liang and M. Dai, Tetrahedron, 2019, 75, 193–208 CrossRef CAS.
- O. G. Kulinkovich, Chem. Rev., 2003, 103, 2597–2632 CrossRef CAS PubMed.
- D. H. Gibson and C. H. DePuy, Chem. Rev., 1974, 74, 605–623 CrossRef CAS.
- A. Nikolaev and A. Orellana, Synthesis, 2016, 1741–1768 CAS.
- T. R. McDonald, L. R. Mills, M. S. West and S. A. L. Rousseaux, Chem. Rev., 2021, 121, 3–79 CrossRef CAS PubMed.
- L. R. Mills and S. A. L. Rousseaux, Eur. J. Org. Chem., 2019, 8–26 CrossRef CAS.
- H. Yan, G. S. Smith and F.-E. Chen, Green Syn. Catal., 2022, 3, 219–226 CrossRef CAS.
- Y. Li, Z. Ye, T. M. Bellman, T. Chi and M. Dai, Org. Lett., 2015, 17, 2186–2189 CrossRef CAS PubMed.
- Z. Ye and M. Dai, Org. Lett., 2015, 17, 2190–2193 CrossRef CAS PubMed.
- Z. Ye, K. E. Gettys, X. Shen and M. Dai, Org. Lett., 2015, 17, 6074–6077 CrossRef CAS PubMed.
- Z. Ye, X. Cai, J. Li and M. Dai, ACS Catal., 2018, 8, 5907–5914 CrossRef CAS PubMed.
- D. C. Davis, K. L. Walker, C. Hu, R. N. Zare, R. M. Waymouth and M. Dai, J. Am. Chem. Soc., 2016, 138, 10693–10699 CrossRef CAS PubMed.
- X. Cai, W. Liang, M. Liu, X. Li and M. Dai, J. Am. Chem. Soc., 2020, 142, 13677–13682 CrossRef CAS PubMed.
- K. Ma, X. Yin and M. Dai, Angew. Chem., Int. Ed., 2018, 57, 15209–15212 CrossRef CAS PubMed.
- X. Yin, K. Ma, Y. Dong and M. Dai, Org. Lett., 2020, 22, 5001–5004 CrossRef CAS PubMed.
- H. S. Sims, P. de Andrade Horn, R. Isshiki, M. Lim, Y. Xu, R. H. Grubbs and M. Dai, Angew. Chem., Int. Ed., 2022, 61, e202115633 CrossRef CAS PubMed.
- W. Liang, X. Cai and M. Dai, Chem. Sci., 2021, 12, 1311–1316 RSC.
- M. M. Hussain, H. Li, N. Hussain, M. Ureña, P. J. Carroll and P. J. Walsh, J. Am. Chem. Soc., 2009, 131, 6516–6524 CrossRef CAS PubMed.
- V. K. Omanakuttan, J. John and H. Hopf, Eur. J. Org. Chem., 2021, 163–201 CrossRef CAS.
- A. Husain, S. A. Khan, F. Iram, M. A. Iqbal and M. Asif, Eur. J. Med. Chem., 2019, 171, 66–92 CrossRef CAS PubMed.
- R. Liu, S. Yang, Z. Chen, X. Kong, H. Ding and X. Fang, Org. Lett., 2020, 22, 6948–6953 CrossRef CAS PubMed.
- Y. Matsuda, T. Iwabuchi, T. Wakimoto, T. Awakawa and I. Abe, J. Am. Chem. Soc., 2015, 137, 3393–3401 CrossRef CAS PubMed.
- M. Dangalov, A. Fernández-Figueiras, M. A. Ravutsov, E. Vakarelska, M. K. Marinova, N. R. Candeias and S. P. Simeonov, ACS Catal., 2023, 13, 1916–1925 CrossRef CAS PubMed.
- J. S. Kingsbury and E. J. Corey, J. Am. Chem. Soc., 2005, 127, 13813–13815 CrossRef CAS PubMed.
- Note: with Cu(OTf)2 (2.0 eq.) in toluene at room temperature, 9a underwent a Pinnacol rearrangement to give the corresponding cyclobutanone in 80% yield. The cyclobutanone is a known compound: T. Nordvik and U. H. Brinker, J. Org. Chem., 2003, 68, 9394–9399 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4cc03255a |
| ‡ Contributed equally. |
| This journal is © The Royal Society of Chemistry 2024 |