Open Access Article
Open Access ArticleGreen dipolar aprotic solvents for the dynamic polycondensation of high-performance polyimide membranes†
E.
San José
 ab,
M. R.
de la Viuda
ab,
M. R.
de la Viuda
 *ab,
F. J.
Carmona
ab,
C.
Soto
ab,
L.
Palacio
*ab,
F. J.
Carmona
ab,
C.
Soto
ab,
L.
Palacio
 ab,
P.
Prádanos
ab,
P.
Prádanos
 ab,
A.
Hernández
ab and
A.
Tena
ab,
A.
Hernández
ab and
A.
Tena
 *ab
*ab
aSurfaces and Porous Materials (SMAP), CSIC-Associated Research Unit, Universidad de Valladolid, 47011, Valladolid, Spain. E-mail: monicarosa.viuda@uva.es; a.tena@uva.es
bInstitute of Sustainable Processes (ISP), 47011, Valladolid, Spain
First published on 18th October 2024
Abstract
The legislation is limiting the use of harmful organic solvents in industrial processes. The establishment of clear guidelines for minimizing solvent residues and the development and implementation of circular methodologies, together with growing environmental and health awareness, will promote the replacement of traditional solvents by more sustainable alternatives. In general, high-performance polymers, such as polyimides, are synthesized under specific reaction conditions. This work defines and develops clear guidelines, integrating them into a decision map to evaluate the potential of an alternative solvent for application in the synthesis of polyimides. Since every industrial application demands explicit criteria, our study focused on the development of polyimides for the membrane industry. More than 130 solvents were evaluated, and 10 solvents were found to have the potential to be employed in the synthesis of polyimides. The outcome was verified with 7 of those solvents, namely, γ-valerolactone (GVL), cyrene (Cy), dimethyl carbonate (DMC), dimethyl isosorbide (DMI), dimethyl sulfoxide (DMSO), 3-methoxy-N,N-dimethylpropanamide (commercially known as KJCMPA®-100), and the reference N-methyl-2-pyrrolidone (NMP), and tested for the synthesis of 3 polyimides (6FDA-HAB, 6FDA-6FpDA and 6FDA-DAM), obtaining extremely consistent outcomes. This work found four solvents, GVL, DMI, DMSO, and KJCMPA, that could substitute NMP, and other harmful solvents, in the synthesis of high-performance polymers. GVL provided even better results in terms of the molecular weight of the polyimides than the reference NMP, showing a realistic potential for its direct substitution. This work also reports more than 40 alternative solvents derived from the identified solvents. Finally, the key action points that should be taken into account for imminent advances in the subject were recognized.
1. Introduction
There is a continuously growing awareness of the need to replace conventional toxic chemicals with more environmentally friendly chemicals in conventional processes. The consequences of using certain types of chemicals raise concerns worldwide, necessitating their regulation. The EU's Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) legislation offers protection to chemical manufacturers, distributors and users. Main manufacturing and producer countries in Asia-Pacific regions, such as China, Korea and Japan, have increased their restrictions in terms of safety during the use and manipulation of chemicals.1 Future steps for the regulation of chemical substances such as solvents will probably evolve towards more rigorous control in terms of risk assessments, as well as the promotion of actions in recycling and circularity. Therefore, academia and industry face challenges in transforming conventional industrial chemistry while maintaining product cost, material performance, and process competitiveness.In this sense, dipolar aprotic solvents are ubiquitous in chemistry owing to their ability to dissolve a wide variety of materials (often including salts), their versatility as reaction media, and often their low cost.2 Dipolar solvents present unique features such as their ability to separate regions of high and low electronegativity and the absence of any ability to donate hydrogen bonds. Their extensive use is also favoured by the abundance of existing literature supporting their use in countless transformations and processes.2 Dipolar aprotic solvents such as N-methyl pyrrolidone (NMP), dimethylformamide (DMF), and N,N-dimethylacetamide (DMAC) are heavily used in the polymer industry, for instance, in the synthesis of common and high-performance polymers such as polyamides or polyimides. Nevertheless, these solvents pose serious hazards to the human health or environmental safety, and their use is restricted or prioritized for future restriction as Substances of Very High Concern (SVHC) under the REACH (CE No. 1907/2006) regulation.3 The limited number of green dipolar aprotic solvents provides very few opportunities to replace traditional dipolar solvents, resulting in a substantial absence of appropriate substitutes. Additionally, the alternative solvent must present specific and appropriate characteristics to be employed in the specific process. For instance, NMP is generally used as the preferred solvent for extraction processes in the oil and gas industry, for the synthesis of cathodes in the battery production, for the coatings of semiconductor materials in electronics, for the production of fibers in the safety clothing, or for the production and processing of materials in the coating and membrane industry.4
This work is focused on the development of an effective methodology to find greener solvents, which can be employed in the polymer-based membrane industry. The membrane industry is recognized as an environmentally friendly and modern technology that requires low energy consumption and has simple operational conditions compared to other traditional separation technologies. An ideal strategy for the membrane processing would be based on minimizing or even eliminating the use of solvents for the membrane production.5 However, the materials are generally synthesized and processed from solvents such as NMP or DMF. The necessity to find alternative solvents has boosted the number of publications related to the search for greener ones in the membrane field. The polymeric membrane production workflow process generally implies two key steps: the synthesis of the polymeric material and the processing of the material as the membrane, which in an ideal situation would directly occur from the synthesis avoiding precipitation of the polymer, together with cleaning and drying. The main part of the research activities has been mainly focussed in the processing of the materials based on phase inversion processes, while very little attention has been paid to the synthetic protocol of the more or less dense polymer materials. Cyrene has been one of the most studied bio-derived solvents for the membrane fabrication processes. Polymers such as cellulose acetate (CA), poly(vinylidene fluoride) (PVDF), polyethersulfone (PES), polysulfone (PSf) and polyimide (PI) membranes have been processed as porous membranes6 using solvents such as dihydrolevoglucosenone (Cyrene™) and derivatives from this solvent. Other greener solvents such as dimethyl isosorbide (DMI),7 methyl lactate,8N,N-dimethyl lactamide and succindiamide,9 γ-valerolactone,10 γ-butyrolactone,11 methyl-5-(dimethylamino)-2-methyl-5-oxopentanoate (Rhodiasolv®PolarClean),12 and derivatives of dialkyl carbonates (DACs) have been used. Deep eutectic solvents (DESs)13–15 will have an important impact in the coming years in the chemical industry.16 By the moment, the use of DESs in the polymer and membrane industry is still limited to their use as an element to change the membrane formation behaviour as well as the hydrophobicity of the materials.
Hong et al. had quantified the total environmental impact of membrane production, which could be subdivided into polymer synthesis (32–34%), polymer washing (16–17%), membrane casting (23–26%), and membrane drying (21–23%).17 On a life cycle basis, nearly half of the environmental burden was attributed to membrane casting and drying, while another one-third of the impacts came from the synthesis of polymers. By the approach of processing the membranes directly from the polymer synthesis, it will be possible to save unnecessary steps reducing up to 50% of the possible environmental impact of the process.17 Therefore, more efforts should be devoted to the search for greener solvents which can be used at any step of the process. This is especially critical for the polymer synthesis and membrane formation. Therefore, the use of greener solvents in the polymer synthesis and membrane processing will boost the transition toward a more sustainable membrane production process.
In this work, we developed a tool which can be easily implemented by any researcher or industry for the selection and substitution of the regulated toxic solvents by more sustainable solvents which will come to the market in the coming years. For that purpose, we analysed the key-factors involved in the polymer synthesis and membrane formation process. We selected three well-reported polyimides which are generally tested as gas separation membranes. These, or variations in them, are often synthesized by a very large number of research groups in the topic. In this way, a decision tree for the selection of the currently suitable solvents was developed. As a result of the selection procedure, three selected polyimides were synthesized in an ensemble of 6 of the more suitable solvents. The polymers resulting from these experiments were compared with the three reference polymers synthesized in NMP.
2. Definition of the necessary criteria to be met by the alternative solvents
As general methodology, the selection of alternative solvents was developed taking into account the generally accepted criteria for the harmonized system of classification and labelling of chemicals in terms of safety, health and environmental criteria.18 In addition, different solvent guidelines,19–24 the Globally Harmonized System (GHS)18 and European regulations attending to the classification used in REACH25,26 were considered. In this sense, this type of general preliminary classification of any chemical or solvent allows a simple and quick evaluation of its sustainability and capacity to be used in a process. However, the polymer synthesis and membrane manufacture contain specific characteristics which must be taken into account. In this selection tool, up to 138 solvents will be analyzed.The health criterion considers the risk of exposure. In this case, relating this criterion to the exposure limits imposed by authorities would be the most appropriate. However, these limits are only established for common solvents and their values are not unified,29–31 which would drastically reduce the applicability and scope of this, and any other proposed study. To simplify the analysis, this criterion was related to the hazard statements in the GHS/CLP system and a scoring system developed within the project CHEM21,20 where the health score of any solvent is equal to the figure corresponding to the highest hazard according to Fig. 1. In this regard, any substance with a score above 5 or that fits with the pictogram of mortals or highly toxic, shown in Fig. 1, would be directly discarded. According to the Safety Data Sheet (SDS), the solvent NMP holds multiple health hazards such as acute toxicity (category 4 – harmful if swallowed, in contact with skin, inhaled), skin corrosion/irritation (category 2 – Causes skin irritation), eye damage/irritation (category 2A – causes eye irritation), aspiration hazard (category 2 – may be harmful if swallowed and enters airways), carcinogen (group 2B – the agent (mixture) may damage fertility or the unborn child), toxicity single exposure (category 3: respiratory tract irritation (sore throat and cough)), and toxicity repeated exposure (category 2: may cause damage to organs through prolonged or repeated exposure in organs such as the kidney, liver, spleen, and blood). These health hazards, attending to the GHS classification determines that the codes associated with them are: H315, H319, H335 and H360. Attending to Fig. 1, we will select the hazard with the highest score. In this case, it is H360, which scores 9 points in the health criteria, and that will be the qualification for the solvent, NMP in this case. In the elaboration of the decision tree, it has been considered that any solvent presenting acute toxicity (fatal or toxic) or a score higher than 5 will be discarded from the list of sustainable chemicals.
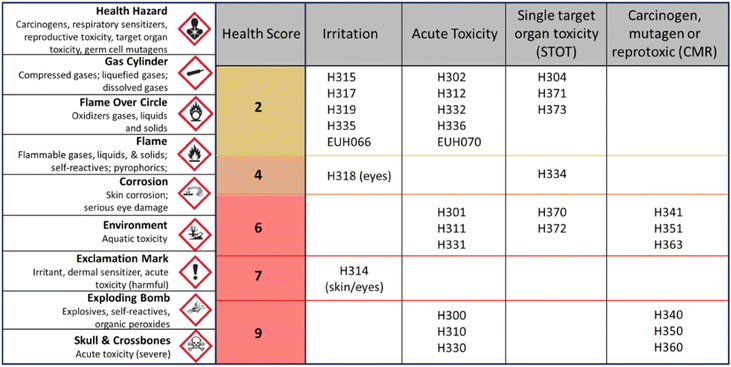 | ||
| Fig. 1 Score for the chemicals according to the health criteria. One extra point is added if BP < 85 °C. Adapted from ref. 20. | ||
The environmental impact of solvents was judged here on the basis of a life cycle analysis that pays attention to both the production and disposal methods. Actually, the source of the solvents needs an in-depth analysis because sometimes even the biodegradable solvents might come totally or partially from the petrochemical industry (e.g. methanol and n-butanol). Moreover, the boiling point also plays an important role in the environmental impact. A solvent with a low BP leads to the generation of volatile organic compounds (VOCs), while a solvent with high BP is not easily recyclable. Therefore, 85 °C–139 °C (ref. 20) has been accepted as the ideal temperature range when a solvent has to be disposed. However, the tendency in the industry is to design processes which tend to have partial or complete circularity. For instance, solvents such as DMF, DMAc, and NMP can be biologically degraded by using bacteria.32 Therefore, although the solvents such as NMP are categorized as H402 – harmful to aquatic life, the existence of effective methodologies to degrade it will eventually allow the minimization or elimination of the possible environmental impact of this solvent. Then, even if the boiling point of the solvent exceeds the established temperature range, the possibility of using separation processes or reutilization of the solvent must be considered. This consideration leads to an urgent need of developing efficient technologies to recover the solvents. In general, the solvent recovery by distillation techniques is complex and requires high energy consumption to be carried out. Water extraction can be used instead; however, the resulting solvent/water mixture is difficult and costly to separate, leading to a very poor quality of the fuel for incineration. Membrane technology is facing this challenge by the development of processes such as organic solvent nanofiltration (OSN),33 which is going to be a key technology in all the chemical sectors in the coming years. Biologically driven processes, by bacteria or algae, leading to the production of valuable compounds which, finally, can be integrated in the process loop,34 can also be suggested. The development of an easily adaptable, energy-efficient and environmentally friendly technology that could recover or create value from the solvents is critical for the incorporation of green solvents into the chemical industry.
Additionally, the degradation of the solvents must be considered.35 For instance, although36 the boiling point of a identified green solvent such as the Cyrene™, 227 °C, surpasses the proposed temperature range, it satisfies the environmental criterion because of its safe handling and since it is environmentally friendly and decompose in water and carbon dioxide. In addition, this solvent is produced from lignocellulosic biomass in a two-step solvent-free process, with more than 90% efficiency.37
Until this part of the development of the solvent selection diagram, the concept could be applied to any chemical process where a new solvent is aimed to be introduced. In fact, several research papers have tackled similar questions24 organizing the answer in different tools to support a consistent decision. However, the targeted chemical industry has precise features which depend on the limiting conditions of the explicit processes. In this case, the targeted procedure is the synthesis and processing of polymeric membranes applied in gas separation processes.
With respect to the polyimide production, there are several methodologies to obtain the corresponding material including the two-step reaction, anhydride-isocyanate reaction, transimidization reaction, aromatic nucleophilic substitution, and even synthesis in aqueous media.40 However, a vast majority of commercial polyimides are synthesized by the conventional two-step polycondensation reaction.41 Generally, this reaction occurs between a diamine monomer and dianhydride monomer in the presence of an aprotic solvent such as N,N-dimethylformamide (DMF), N,N-dimethylacetamide (DMAC) or, N-methyl pyrrolidone (NMP) as a main solvent. Dipolar aprotic solvent is used in order to promote the nucleophilic substitution reactions (SN2 and SNAr). This type of aprotic solvents favor the SN2 reactions as well as increase the reaction rate as they cannot experience a hydrogen bond with the nucleophiles as a protic solvent would do.42 This great rate enhancement effect for SNAr reactions43 by using dipolar aprotic solvents has been widely studied.44 Therefore, a greener solvent that aims to substitute undesired solvents must be adequate to carry out a typical polycondensation reaction. Apart from the solubility and reactivity, the solvent characteristics such as viscosity, polarity or boiling point can also affect the final polymer characteristics such as molecular weight, glass transition temperature, or degradation stability. At this point, in order to process the material as membrane, two paths can be taken into account for the polymer: precipitation of the material; or, processing of the polymer directly as a membrane. Processing the polymer directly from the synthesis will eliminate all these steps, and it will be much more efficient from the point of view of cost, energy consumption, and environmental issues.
Phase inversion is the most common method employed for the preparation of polymeric membranes. The membrane is obtained from a solution which is cast into a desired shape, i.e., a flat sheet or hollow fiber membrane, and turned into a porous solid via non-solvent-induced phase separation (NIPS), thermally induced phase separation (TIPS), vapor-induced phase separation (VIPS) or their combinations.45 In order to carry out the process, the membranes are generally processed from high-viscous solutions formed by the polymer, the solvent and, eventually, certain additives, which can go from a non-solvent, in order to increase the viscosity of the solution, to a pore former such as polypyrrolidone (PVP),46–48 which is added in order to promote the formation of a porous structure. In any case, the polymer solution usually contains polymers at a concentration greater than 15 wt% (up to 25 wt% or even higher polymer contents). The great majority of the membranes are processed by the NIPS method. When the polymer cannot be solubilized under this concentration condition, the material can be processed as a thin-film composite membrane (TFCM).49
For simplicity, environmental and economic reasons, water is usually selected as the non-solvent phase in the phase inversion process. Therefore, a suitable alternative solvent must present certain miscibility with water. The first stage of the process occurs at the interlayer formed between the polymer solution and the water, where the concentration of polymer is much higher.50 A low solvent miscibility would hinder the solvent exchange and slow down the kinetics of the process, leading to an ineffective formation of the selective layer. Attending to it, alternative solvents should show a miscibility of, or greater than, 100 milligrams of solvent per 1 milliliter of water. Although on a commercial scale, a very large excess of water vs. solvent in the polymer solution (<0.001 wt%) is guaranteed.
3. Elaboration of the decision tree for a gas separation membrane process
3.1. Discussion on the elaboration of the tree
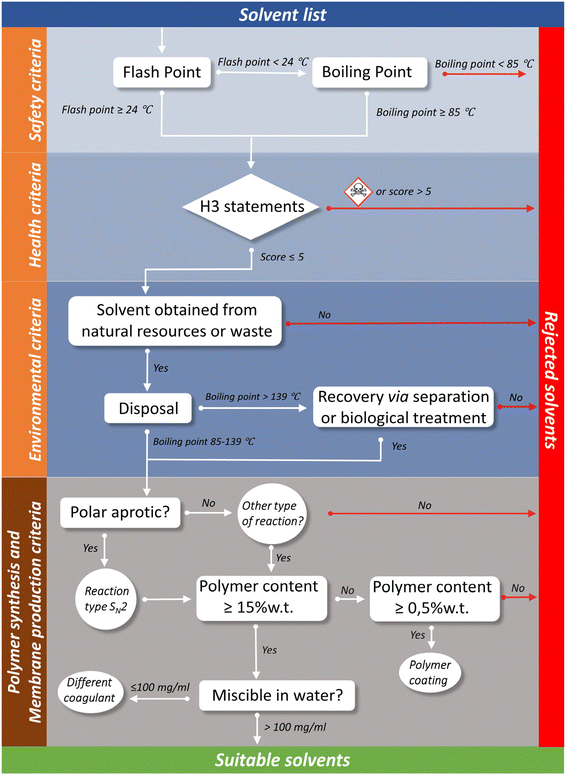
Including all general and specific criteria discussed previously allow us to define the conditions of the new solvents to be considered as possible candidates to substitute the traditional toxic solvents employed in the synthesis of polyimide membranes for gas separation applications. In this sense, the decision tree has been organized in four different sections: (a) safety criteria; (b) health criteria; (c) environmental criteria; and (d) Polymer synthesis and membrane production criteria.(a) Safety criteria: as described before, this section will be based on the flash point and boiling point of the solvents. Solvents with a flash point ≥24 °C or/and boiling point ≥85 °C will be considered safe and analysed in the next section. The rest of the solvents will be directly dismissed.
(b) Health criteria: this section is critical with any solvent labelled as acute toxicity, which will be directly rejected. Additionally, any solvent ranked with more than 5 points according to Table 1 will not continue to the next section.
(c) Environmental criteria: this criterion focuses on the solvent production protocol. Only solvents produced from natural resources or waste will be considered. The disposal methodology will be analyzed as a function of the boiling point of the solvent. Solvents presenting a boiling point in the range between 85 and 139 °C will continue to the next analysis. Solvents with a boiling point >139 °C will be eliminated from the selection unless a specific recovery or treatment methodology exists. However, the continuous advances in the recovery of solvents via separation technologies as well as the progress in biological treatments of the waste allow us to guess that future development of green solvents should be conducted together with the analysis of their circularity cycle.
(d) Polymer synthesis and membrane production criteria: this section is very specific to the polymeric membrane topic despite the possibility of application to any process where polyimides, or other polymers, are synthesized. Polycondensation reactions, type SN2, as well as other types of reactions must be possible in order to obtain the required polymer. Additionally, the solvent should be able to solubilize the polymer at elevated amounts of solid contents (>15 wt%). Under these conditions, the membrane will be formed by coagulation of the mixture solvent and polymer in water. Therefore, there must be certain miscibility between the solvent and the water (>100 mg mL−1). For lower concentrations in the solutions (>0.5 wt%) still the creation of TFCM would be possible.
Fig. 2 shows the outcome of the defined criteria in the shape of a diagram for the search of more sustainable solvents applied in a membrane formation process, as well as in the synthesis of polymers via polycondensation reactions, i.e., polyimides. Skipping the synthetic part of the process, the diagram will directly go from the environmental impact section to the minimum polymer concentration needed in a solution to carry out the phase inversion process. Adopting the established criteria will lead to the selection of the correct solvent for a specific membrane formation process.
3.2. Selection of solvents
The criteria for the classification of solvents are very broad. For instance, M. Durand et al., proposed up to 10 different types of solvents attending to the solvent molecular structure.51 Similar approach has been carried out in order to classify green solvents by different sustainability criteria.52 In this work, the authors define up to 7 types of green solvents, according to their nature and production methodology.An extensive screening process following the criteria established in the decision tree shown in Fig. 2 was conducted encompassing 132 solvents. The solvents analyzed are presented in Table 1, according to their nature (apolar, polar protic, and polar aprotic). Therefore, the evaluation of the suitability of green solvents was carried out for 36 apolar solvents, 31 polar protic solvents and 65 polar aprotic solvents.
Table 1 shows that 73 (36 from safety and 37 from health criteria → first two light blue columns) out of 132 solvents analyzed have already been excluded after evaluation of the first two stages of the diagram. A thoughtful analysis of the production methodologies for these solvents, which is included in the third criteria of the diagram, was performed. However, this point is very controversial for the consideration of a solvent as green. Synthetic methodologies are continuously explored for the production of greener and more efficient solvents. However, the existence of a more sustainable synthetic route does not warrant that the solvent will be produced in this way. Chemical processes with relatively low and disrupted solvent necessity will benefit from green synthetic methodologies, while processes with very high solvent consumption will, most likely, still be mainly fed by solvents produced from traditional methodologies or a mixture of both, green and traditional. Referring to the study, only 16 out of the 59 solvents analyzed at the third stage were excluded at this point (darker blue column). Advances in the utilization of waste to generate value as well as the creation of new sustainable synthetic routes for chemicals, for instance, from carbon dioxide, will most likely evolve to the discovery of novel procedures to synthesize these and many other chemicals.
At this point, as it can be seen in Table 1, only 43 solvents out of 132 evaluated (around 32%) have met the 3 first criteria stages of the diagram. The specific criteria of the process are now introduced into the evaluation. Despite showing all the general criteria, 33 out of the 43 have been rejected due to the specific synthetic criteria (grey column). Hence, every specific feature of a particular chemical process should be considered. In the polyimide synthesis, the synthetic protocol involves a polycondensation reaction where the reaction mechanism is SN2. For this type of reaction, the solvent must be polar aprotic. As an example, the considered greener solvent N,N′-dimethyl lactamide (DML), commercialized by BASF under the trade name Agnique® AMD 3L, is a bio-based, biodegradable, label-free polar solvent. This solvent has been tested for the membrane formation process with various polymers.53–55 However, DML is a protic solvent, and this type of solvent cannot be used in the synthetic process, which offers a possible solution for membrane formation but not for the entire process. Then, only 23 of those 43 solvents (grey and green columns), due to their polar aprotic nature, can actually be applied in the synthetic process. In a more detailed way, the solvent must be able to dissolve the polymer. From those solvents, only 10 are able to dissolve the polymers (green column). Therefore, 10 out of the 132 solvents analyzed, less than 8%, are susceptible to be applied in membrane applications.
The list of the aprotic solvents as well as the criteria to be excluded is included in Table S1 in the ESI.† These types of solvents, as just mentioned, are the preferred type of media to carry out the polycondensation reaction. The list of analyzed non-polar and protic solvents, which, in principle, cannot be used for these reactions, can be found in the ESI.† As mentioned before in Table 1, 10 out the 65 polar aprotic solvents analyzed have initially met the required conditions to transform the membrane process into a more sustainable one. These solvents are: γ-valerolactone, cyrene™, dimethyl carbonate, N-octyl pyrrolidone, diethyl carbonate, dimethyl isosorbide, γ-butyrolactone, N-butyl pyrrolidone, methyl-5-(dimethylamino)-2-methyl-5-oxopentanoate, and dimethyl sulfoxide. Some of the physico-chemical properties of the selected (and non-selected) polar aprotic solvents analyzed in the decision tree are included in Table S2.†
Some of these solvents have been already tested in the membrane field. The focus of these studies searching greener solvents have been carried out in the processing of the membranes from polymers such as cellulose acetate (CA), poly(vinylidene fluoride) (PVDF), polyethersulfone (PES), polysulfone (PSf) and polyimide (PI).36 However, as it has been previously discussed, the option of processing the material directly from the synthesis will potentially reduce up to 50% of the environmental impact derived from the solvent utilization. Therefore, the goal is to find possible solvents which can be used for the entire membrane process, which includes the polymer synthesis and membrane formation. In this sense, the selected polymeric membrane material will define which type of solvent will be needed for the process.
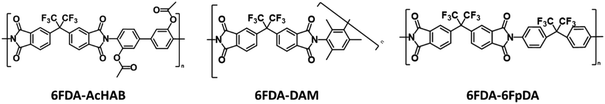
3.3. Selection of the polymers
As mentioned, polyimides are considered high-performance polymers with superior properties for multiple applications including their application as membranes for gas separation processes. Therefore, finding more sustainable solvents for this type of polymers will have a significant impact in multiple industrial sectors such as electronics or aerospace. The reactivity of the monomers will determine some of the properties of the final materials such as molecular weight or glass transition temperature. Therefore, in this work, three different polyimides, which have been often tested for gas separation applications, have been synthesized. The selected polyimides are: 6FDA-HAB-Ac, 6FDA-6FpDA and 6FDA-DAM (Fig. 3). These polymers present a common dianhydride structure, 6FDA and various diamine monomers with different reactivities (HAB, 6FDA and DAM). The selection is based on testing the solvents for the synthesis of polyimides presenting different reactivities. The synthetic methodology selected has been the classical two-step polycondensation reaction (as shown in the general reaction schedule in Fig. S1†). This method is well-extended, studied and applied in the main part of the industrial polymer production. In this specific case, the polycondensation reaction occurs between a dianhydride, 6FDA, and the corresponding diamine. More details about the synthetic protocol can be found in the ESI.† The dianhydride 6FDA is often selected for the design of polymers applied in the gas separation field due to the presence of –CF3 groups which considerably increase the chain stiffness,56 which usually leads to polymeric materials presenting a good balance between selectivity and permeability for several separations.57Fig. 3 shows the structure of the monomers and polymers synthesized in this work and the separation performances associated with the corresponding polyimide.In order to avoid any disparity on the results due to the synthetic process conditions, the synthetic protocol will be exactly the same for the materials and solvents employed in this work. The synthetic process will involve the formation of poly(amic acid) (PAA) in the first step, where the molecular weight of the polymers is built. The formation of this PAA involves the nucleophilic attack of the amino group on one of the carboxyl groups of the anhydride, causing the opening of the anhydride ring, by means of an acid-based reaction. Several factors might affect the PAA formation such as the chemical solubility, polymer concentration, temperature, and time, among the monomer's reactivity and purity. At this point, the presence of impurities, reactive gases or even water might drastically affect the outcome of the reaction.
The second step of the process is the transformation of PAA into the final polyimide (PI). This process can be carried out by three different methodologies: azeotropic imidization, thermal imidization, or chemical imidization. The first two processes depend on the chemical structure of the polymers as well as the solubility of the polymer, for instance, in the mixture between the solvent and the azeotropic solvent employed. The thermal imidization often leads to crosslinked structures due to the presence of secondary reactions between different polymer chains, which finally affects the solubility of the polymers and adds complexity to the structural characterization of the materials. The last one, the chemical imidization, has been selected for this study. This methodology is more standardized and is more independent of the structure and is more reproducible. This selected procedure for the second step of the reaction, the chemical imidization of PAA, involves closing the ring, while water is released, in order to obtain the corresponding polyimides (PIs).
3.4. Solubility parameter evaluation
The polymer synthesis in solution as well as the membrane formation process involves, minimum, a binary polymer–solvent system. In polymer chemistry, predicting and understanding the polymer–solvent phase equilibrium in binary systems is quite important. The thermodynamic properties of polymeric solutions are generally expressed in terms of a polymer–solvent interaction parameter called the Flory–Huggins χ parameter.58 This χ parameter depends on several factors such as polymer molecular weight, reaction temperature, or volume fractions. The experimental characterization of such parameters still present certain technical complexity. Computational modelling is simplifying the process by applying empirical models, which are basically based on the distance between the polymer and the solvent solubility parameters.59,60 One of the most often employed is the Hansen solubility parameter (HSP) δT.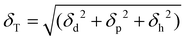 | (1) |
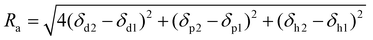 | (2) |
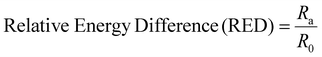 | (3) |
Empirical methods such as the group contribution methods have been extensively studied and widely used for the calculations of the molecular interactions.62 Nevertheless, different methodologies including models such as UNIFAC-FV and entropic-FV, and advanced statistical-associating fluid theory (SAFT) have been developed in the last years.63 The predictive capacity has evolved, especially by the contribution of Klamt and collaborators, by developing the COSMO-RS method.64,65 This method is based on rapid calculations by quantum chemistry, which can be applied to a diverse variety of polymer–solvent systems.66 However, these atomistic simulations are still facing some challenges in the topic,67 leading to a very large variability in reported results (as it is shown in Table S3† for the solvent DMSO). In recent years, high speed predictions can be achieved by employing the machine learning approach, which is fed by the growing free accessible data and using continuously improving computational resources. In any case, experimental polymer–solvent interaction parameters are always required in order to train the model. In general, the studied techniques proposed to obtain experimental χ parameter values, such as vapor pressure, osmotic pressure, or inverse chromatography, are still presenting some limitations besides being technically difficult and costly.63 Additionally, the determination of the solubility parameter of polymers is complex because most polymers, like most polyimides or other high-performance polymers, cannot be vaporized without decomposition. The HSP for polymers are generally calculated simply and conveniently by using typical contribution methods. This makes it difficult to create highly generalizable predictive models of χ parameters that can be applied to a wide variety of polymeric systems. In any case, it can be guessed that the continuous evolution in the topic as well as the interactions between international researchers all over the globe, such as the massive collaborative effort allowing the continuous development of the Q-Chem quantum chemistry program,68 will contribute to the refining of the predictive tools.
It is clear that, although concepts and criteria are clear, still some variability can be found. Then, more uniform criteria, in particular development of really reliable methodology to determine experimentally and computationally the solubility parameters associated with a solvent or substance, are to be developed.
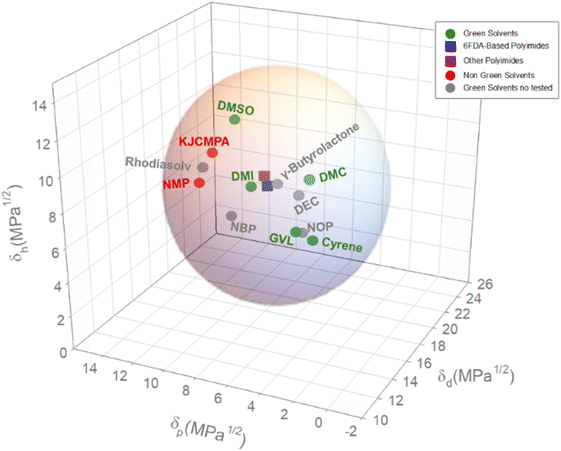
Table 2 shows all the solvents that have met all the criteria as well as their solubility parameters. The list of solvents was ordered according to their total solubility parameter δT calculated according to eqn (1). Two additional solvents have been added to the list: NMP, which will be used here as reference; and 3-methoxy-N,N-dimethylpropanamide (commercially known as KJCMPA®-100), which, at the beginning if this study, was considered as a sustainable solvent particularly based on the synthetic path. KJCMPA®-100 is a biodegradable linear amphiphilic amide solvent employed at electronic component degreasing and inkjet printing.69 However, the solvent has been recently qualified, among other hazard statements, as suspected of damaging fertility or the unborn child. In any case, and having this in mind, we will include the obtained analysis of the use of this solvent in the synthesis of the different polymers. Besides, 5 solvents from the list have been selected to carry out this study based on their availability, type of solvent, and δT values. Based on this, the solvents selected are as follows: γ-Valerolactone (GVL), Cyrene™ (Cy), Dimethyl Carbonate (DMC), Dimethyl Isosorbide (DMI), and Dimethyl Sulfoxide (DMSO). This selection involves somehow the rest of the structures, as it will be discussed in section 4. Table 2 also includes the current cost of these solvents. The most usual solvents such as NMP or DMSO will generally present the lower cost while less traditional or novel solvents such as Cyrene™ or N-butyl pyrrolidone show more elevated costs. Solvents such as carbonate-based solvents generally present very moderate prices favored by the production simplicity and cost. It is important to highlight that the cost shown in Table 2 is for low amount of solvents. Ordering higher solvent amounts will rapidly drop the cost. For instance, NMP shows a cost of around 15 € per kg on a kilogram scale, however ordering the solvent on a ton scale will drop to cost to 1–2 € per kg. In any case, the cost will be determined by the supply and demand law. Great industrial demand of a chemical will favor the search for more efficient and cost-effective synthetic routes. Therefore, the initial cost of a novel solvent will always be much higher than the cost of traditional solvents. Nevertheless, this fact should not be an initial stopper for its implementation in a process since a radical reduction in the cost can be expected.
| Solvent | δ d (Mpa1/2) | δ p (Mpa1/2) | δ h (Mpa1/2) | δ T (Mpa1/2) | Cost € per kg |
|---|---|---|---|---|---|
| a Rhodiasolv® PolarClean. b KJCMPA®-100. c Purity between 95–98%. d Price not provided by the producer. | |||||
| γ-Valerolactone | 15.5 | 4.7 | 6.6 | 17.5 | 40–50 |
| Cyrene™ | 17.2 | 4.5 | 5.4 | 18.6 | 320 |
| Dimethyl carbonate | 15.5 | 3.9 | 9.7 | 18.7 | 24–28 |
| N-Octyl pyrrolidone | 17.3 | 5.2 | 5.7 | 18.9 | 70–85 |
| Diethyl carbonate | 17.6 | 5.6 | 7.7 | 20 | 30–40 |
| Dimethyl isosorbide | 16.8 | 8.2 | 8.1 | 20.4 | 48–80 |
| γ-Butyrolactone | 17.7 | 7 | 8.1 | 20.7 | 58 |
| N-Butyl pyrrolidone | 17.5 | 9.9 | 5.8 | 20.9 | 420–540c |
| Methyl-5-(dimethylamino)-2-methyl-5-oxopentanoatea | 15.8 | 10.7 | 9.2 | 21.2 | —c,d |
| Dimethyl sulfoxide | 18.4 | 10.1 | 11.1 | 23.7 | 24–30 |
| 3-Methoxy-N,N-dimethylpropanamide | 17.2 | 10.9 | 9.5 | 22.5 | 120–140c |
| N-Methyl pyrrolidone | 18 | 12.3 | 7.2 | 23 | 15–25 |
Similarly, as it was discussed for solvents, in polymers, the Hansen Solubility Parameters can also be estimated. As mentioned above, Ra, defined in eqn (2), determines the distance between the HSP vectors of the solvent and the molecule. Then, the polymer will be soluble when similar energy of vaporization is shown. In other words, when the value of Ra is equal to or less than 1. For Ra > 1, the materials will be considered as non-soluble. Therefore, the appropriate HSP should be calculated for solvents and polymers. However, the precision of the method depends on the number of different solvents tested.70 In general, a limited number of HSPs are accessible for polymers such as PVDF,71 polycarbonate,72 poly(ether sulfone),73 or PMMA.74 However, the HSP of the polymers will differ depending on the chemical nature of the structure. The list of HSP for polyimides, reported in literature, can be found in Table 3. The polymers are divided into 2 groups: (a) 6FDA-based polyimides and (b) other polyimides.
| (a) 6FDA-based polyimides | δ d (Mpa1/2) | δ p (Mpa1/2) | δ h (Mpa1/2) | δ T (Mpa1/2) | Ref. |
|---|---|---|---|---|---|
| 6FDA-durene | 18.9 | 9.5 | 8.8 | 22.9 | 75 |
6FDA-DAM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DAP 2 DAP 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
17.9 | 9.8 | 7.7 | 21.9 | 76 |
| 6FDA-PFDAB | 16.2 | 3.5 | 6.7 | 17.9 | 77 |
| 6FDA-mPDA | 17.7 | 5.6 | 7.2 | 19.9 | 77 |
| 6FDA-DABA | 17.1 | 9.7 | 8.8 | 21.5 | 78 |
| 6FDA-2.6 DAT | 19.2 | 9.3 | 6.9 | 22.4 | 79 |
| 6FDA-DAPI | 18.78 | 7.12 | 8.11 | 21.7 | 80 |
| Average of 6FDA-based polymers | 18.0 ± 1.1 | 7.8 ± 2.5 | 7.7 ± 0.9 | 21.2 ± 1.7 |
| (b) Other polyimides | δ d (Mpa1/2) | δ p (Mpa1/2) | δ h (Mpa1/2) | δ T (Mpa1/2) | Ref. |
|---|---|---|---|---|---|
| Poly(ether imide) | 17.3 | 5.4 | 6.3 | 19.2 | 73 |
| P84® | 19 | 17.5 | 9.2 | 27.4 | 81 |
| P84® | 20.4 | 12.3 | 10.3 | 25.9 | 82 |
| Matrimid® 5218 | 18.7 | 9.5 | 6.7 | 22.0 | 83 |
| Matrimid® 5218 | 20.9 | 11.3 | 9.7 | 25.7 | 11 |
| Ultem® 1000 | 19.6 | 7.6 | 9 | 22.9 | 84 |
| Ultem® 1000 | 18.3 | 7.8 | 7.2 | 21.2 | 85 |
| ODPA-PFDAB | 16.5 | 4.1 | 7.5 | 18.6 | 77 |
| ODPA-m-PDA | 18.8 | 7.2 | 8.8 | 22.0 | 77 |
| BTDA-PFDAB | 16.8 | 4.3 | 7.4 | 18.8 | 77 |
| BTDA-m-PDA | 18.8 | 7.3 | 8.4 | 21.8 | 77 |
| PMDA-PFDAB | 17.2 | 4.9 | 7.9 | 19.5 | 77 |
| PMDA-m-PDA | 18.4 | 9.4 | 9.4 | 22.7 | 77 |
| BPADA-DAPI | 19.3 | 5.5 | 8.2 | 21.6 | 80 |
| PMDA-DAPI | 19.4 | 9.4 | 9.5 | 23.6 | 80 |
| Average of other polyimides | 18.6 ± 1.2 | 8.3 ± 3.5 | 8.3 ± 1.2 | 22.2 ± 2.6 |
It can be seen in Table 3, despite including polyimides with very different chemical structures, that the average HSP values for the two groups, 6FDA-based and other polyimides, present very similar values. Once we understand the HSP of the polymers, it is possible to calculate the Ra and RED values. Since we have two different mean values reported for polyimides, depending on their structure, two different values for Ra and RED are provided in Table 4.
| Solvent | 6FDA-based polyimides | Other polyimides | ||
|---|---|---|---|---|
| R a | RED | R a | RED | |
| a Rhodiasolv® PolarClean. b Commercially known as KJCMPA®-100. | ||||
| γ-Valerolactone | 5.9 | 0.83 | 7.3 | 1.02 |
| Cyrene™ | 4.3 | 0.60 | 5.5 | 0.77 |
| Dimethyl carbonate | 6.6 | 0.92 | 7.7 | 1.06 |
| N-Octyl pyrrolidone | 3.6 | 0.50 | 4.8 | 0.67 |
| Diethyl carbonate | 2.3 | 0.32 | 3.4 | 0.47 |
| Dimethyl isosorbide | 2.4 | 0.33 | 3.6 | 0.50 |
| γ-Butyrolactone | 1.0 | 0.14 | 2.2 | 0.30 |
| N-Butyl pyrrolidone | 3.0 | 0.42 | 3.8 | 0.53 |
| Methyl-5-(dimethylamino)-2-methyl-5-oxopentanoatea | 5.4 | 0.75 | 6.2 | 0.86 |
| Dimethyl sulfoxide | 4.2 | 0.58 | 3.3 | 0.46 |
| 3-Methoxy-N,N-dimethylpropanamide | 4.5 | 0.63 | 4.4 | 0.62 |
| N-Methyl pyrrolidone | 3.9 | 0.54 | 4.0 | 0.56 |
As it can be seen in Table 4, all the RED values, irrespective of whether 6FDA is included or not in the polyimide structure, are ≤1, which, as mentioned above in the explanation of eqn (3), would mean that all the selected solvents are likely to dissolve polyimides. According to the RED values, from the selected alternative solvents, DMSO, Cyrene™ and KJCMPDA®-100 will show similar solubility performances to those of NMP; DMI will be the best solvent since it shows the lower RED value from the selected solvents; and GVL and DMC will show the biggest limitation in terms of solubility. From the rest of the solvents present in Table 4, it seems that γ-butyrolactone has the potential to be the most likely solvent to dissolve the polyimides, while N-octyl- and N-butyl-pyrrolidone show, as expected, similar potentials to that of NMP. The solvent Rhodiasolv® PolarClean showed a little higher RED value than NMP while showing the potential to dissolve any type of polyimide. This ability of the solvents to dissolve the polyimides is also represented in Fig. 4, where visually it is clear how all the solvents presented in Table 4 have the ability to dissolve the polymer.
3.5. Evaluation of the selected solvents as media for the synthesis of the polyimides
As mentioned before, in order to avoid any disparity in the results, the synthetic protocol is exactly the same for the materials and solvents employed in this work. We have a broad experience in the synthesis of polyimides.86–88 In this sense, some critical reaction aspects are related to the polymer concentration, reaction time, and imidization protocol, among others. The reaction conditions have been tested initially for the control reaction in NMP. The three selected polymers, namely 6FDA-HAB-Ac, 6FDA-DAM, and 6FDA-6FpDA, were synthesized under the same reaction conditions. In a 100 mL three-necked flask, fitted with a mechanical stirrer, the corresponding diamine (5 mmol) and anhydrous solvent (5 mL) were added. The solution was stirred at room temperature in a nitrogen atmosphere until the diamine was completely dissolved. Then, the solution was cooled to 0 °C in an ice bath. The required amount of dianhydride 6FDA (5 mmol) and additional solvent (3 mL) were slowly added to the reaction vessel. The polymer concentration in the reaction was around 27.5 wt%. After 1 h, the ice bath was removed, and the poly(amic) acid solution was allowed under agitation at room temperature for 11 hours while the viscosity of the solution increased considerably. The reaction is followed by 5 hours of acetylation in the presence of an excess of acetic anhydride (20 mmol) and a base (15 mmol). The mixture was left to stir at room temperature for 5 h followed by heating to 60 °C for one hour. Afterwards, the solution was cooled down to room temperature, before polymer precipitation as thin fibers in tap water. Finally, the fibers were washed several times in water. The resulting polymers were filtered and dried at 200 °C under vacuum for 24 h to remove the remaining residual solvent.During this time, PAA is formed due to the nucleophilic attack of the carbonyls in the dianhydride to the diamine compound. Since we have here a common dianhydride, the electrophilicity of the carbonyl, which is generally correlated with the electron affinity (Ea), is the same for all the reactions. However, the nucleophilic character of the different amines will determine the reaction rate. The pKa value is used to evaluate the nucleophilicity of the amine,89 and higher values will indicate a stronger nucleophilicity. The pKa values and other properties of the selected diamines are compared in Table 5. All the diamines show small differences in the pKa values associated with the diamines with DAM (5.0) > HAB (4.9) > 6FpDA (4.0). However, the presence of hydroxyl groups in the HAB monomer allows the presence of two different pKa values leading to a, in principle, more favoured reactivity of the HAB monomer due to the conservation of the nucleophilicity once the reaction has started.
| Dianhydride | Diamines | |||
|---|---|---|---|---|
| 6FDA | HAB | 6FpDA | DAM | |
| a All the data have been reported from the Advanced Chemistry Development (ACD/Labs) Software: calculated at 20 °C and 760 Torr. b Calculated at 760 Torr. c Calculated at 25 °C. d The first value corresponds to the amino group and the second to the hydroxyl. | ||||
| Molecular structure |
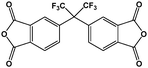
|
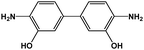
|
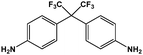
|
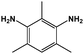
|
| Molecular weight | 444.24 | 216.24 | 334.26 | 150.22 |
| Density/g cm−3 | 1.70 ± 0.06 | 1.38 ± 0.06 | 1.39 ± 0.06 | 1.05 ± 0.06 |
| pKa | 4.9/9.2d ± 0.3 | 4.0 ± 0.3 | 5.0 ± 0.1 | |
| Molar volume/cm3 mol−1 | 262 ± 3 | 156 ± 3 | 240 ± 3 | 143 ± 3 |
| Polar surface area/Å | 86.7 | 92.5 | 52.0 | 52.0 |
| Boiling point/°C | 490 ± 50 | 440 ± 50 | 350 ± 40 | 300 ± 40 |
| Enthalpy of vaporization/kJ mol−1 | 76 ± 3 | 73 ± 3 | 60 ± 3 | 54.0 ± 3 |
| Flash point/°C | 240 ± 20 | 220 ± 30 | 159 ± 19 | 160 ± 30 |
| Vapor pressure/Torr | 6.40 × 10−10 | 2.24 × 10−8 | 4.18 × 10−5 | 1.32 × 10−3 |
After the reaction is finished, the polymers are precipitated in water and washed 4 times in order to eliminate unreactive chemicals and most of the employed solvents. Afterwards, and in order to eliminate the residual solvent, all the polymers are thermally treated at 200 °C under vacuum for 12 hours. The exact conditions for the synthesis and drying protocol are reported in the ESI.† The visual aspect of the small samples of the 21 materials (3 polymers × 7 solvents) synthesized is shown in Fig. 5.
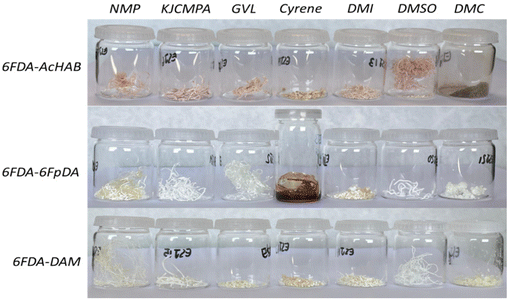 | ||
| Fig. 5 Polymers 6FDA-AcHAB, 6FDA-6FpDA and 6FDA-DAM synthesized in that order in NMP, KJCMPA, GVL, Cyrene™, DMI, DMSO, and DMC. | ||
From Fig. 5, it is simple to determine visual differences between the polymers obtained. For instance, 6FDA-6FpDA synthesized from Cyrene™ is oil-like while for the rest of the solvents the colour was mainly white fibers. Similarly, 6FDA-AcHAB, where Ac stands for the functionalization of the –OH to –OAc, synthesized in DMC is a powder while the same polymer synthesized in other solvents precipitated again as a fiber-like structure. This is, generally, a simple indication of the molecular weight of the polymers. However, other factors such as water miscibility and coagulation rate might have an influence on the formation of such structures. At this point, some aspects of the reaction were annotated, and are reported in Table 6.
| Solvent/polymer | Observations during the synthetic procedure | ||
|---|---|---|---|
| 6FDA-DAM | 6FDA-AcHAB | 6FDA-6FpDA | |
| Cyrene™ | Viscosity drops after imidization | Viscosity drops after imidization | Viscosity drops after imidization |
| DMC | Heated to 70 °C to dissolve the diamine | Heated to 70 °C to dissolve the diamine | Heated to 70 °C to dissolve the diamine |
| DMSO | Frozen after cooling | Frozen after cooling heated to 70 °C to dissolve the diamine | Frozen after cooling |
As Table 6 reports, during the reactions in Cyrene™, the viscosity visibly dropped after the chemical imidization was carried out for all the polyimides. Cyrene™ presents an elevated viscosity of 1.2473 g cm−3 compared to, for instance, 1.028 g cm−3 for the NMP, which might limit the reaction kinetics at elevated solid concentrations. For the polycondensation reactions carried out in Cyrene™ used as the solvent, after the chemical imidization, the drop in the viscosity of the solution is remarkable. This drop might be explained by the reaction between Cyrene™ and the dianhydride present in the reaction in the presence of a base,90 such as the pyridine, added during the chemical imidization protocol. This would displace the equilibrium of the reaction leading to a low or lower molecular weight polymer and most likely to an incomplete transformation from PAA to PI. Far from qualify, the solvent is inefficient for the reaction, which would only mean that the standard polycondensation reaction employed should be adapted in the case of using Cyrene™ as the solvent. In the same way, when the employed solvent was DMC, the diamines needed to be heated at 70 °C to be dissolved. Under these circumstances, and, in the presence of nucleophiles, the DMC can react as it has been described before.91,92 Again, due to this phenomenon, that might be expected to have an influence in the final molecular weight of the polymer due to its effect on the imbalance of the stoichiometry of the reaction, preventing high molecular weights to be reached. In the case of the DMSO, it is known that a high pure solvent is frozen when it is stored under refrigerated conditions due to its low freezing point (18.5 °C). However, apart from practical limitations to carry on the reaction and an evident limitation on the initial reaction kinetics, no major impact on the polycondensation reaction is expected.
1H-NMR was carried out for all the samples to have a more specific information about the structure formed. The 1H-NMR spectra provided crucial structural details, where all the hydrogen atoms present in the structure can be perfectly identified, which confirms the successful synthesis of polymers. For instance, the peaks corresponding to specific functional groups validate the formation of the desired chemical linkages between monomers, verifying the expected polymer backbone structure regardless of the solvent used. Fig. S2† shows the 1H-NMR spectra with the signals assigned to the respective protons present in the twenty-one synthesized polymers together with their general structures and their peak assignations.
Only a selected area is shown in Fig. S2,† while the entire information on the spectra is included in the ESI.† In the case of 6FDA-AcHAB, due to chemical imidization in the absence of a protective group, the hydroxyl group (–OH) is acetylated, being found as an –OCOCH3 group in the polymer, as shown in Fig. S2.† The typical –OH group peak disappeared and a peak around 2.17 ppm indicated the functionalization of this hydroxyl group. In the 1H-NMR corresponding to the polymers synthesized in Cyrene™ and DMC, extra peaks are detected. As mentioned above, these peaks are related to the compounds generated from the reaction between the monomers and the solvents. As a result, instead of clear and defined peaks for the corresponding polyimides, the signals appeared duplicated, as a clear indication of the presence of different configurations of similar structures, as for instance, the existence of a small percentage of PAA which has not been converted completely to PI.93 For the rest of the solvents, the reference NMP, and the alternative solvents KJMPA, GVL, DMI and DMSO, the 1H-NMR spectra confirm the structure of the polyimides by replicating exactly the same signals. The detailed peaks for every polymer can be found in the ESI.†
The chemical structures and completion of imidization processes are confirmed by FT-IR spectroscopy. In order to simplify the process and to get a uniform and continuous contact between the sample and the device, small films were formed. Given the structural similarity of homopolymers regarding their characteristic vibrational bands, variations between solvents used were not discernible. Across all polymers, complete cyclization was evident attending to the absence of amide peaks. For all the polyimides, distinctive peaks94 were noted such as symmetric C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (1778 cm−1), asymmetric C
O (1778 cm−1), asymmetric C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (1721 cm−1), C–N stretching (1366 cm−1), and imide ring deformation (714 cm−1). The FTIR-ATR spectrum of the synthesized polymer is included in Fig. S3.†
O (1721 cm−1), C–N stretching (1366 cm−1), and imide ring deformation (714 cm−1). The FTIR-ATR spectrum of the synthesized polymer is included in Fig. S3.†
Once the polymeric structures have been confirmed, the real effectiveness of the successful polycondensation reaction by using alternative solvents should be evaluated as a function of the molecular weight. The molecular weight and polydispersity of 6FDA-based polymers were evaluated by GPC. The corresponding results are summarized in Table 7. A narrow molecular weight distribution, corresponding to low polydispersity (PDI) values, typically leads to a uniform synthetic procedure where the process minimized the branching side reactions. This uniformity minimizes weak points or inconsistencies within the polymer synthesis, enhancing the reproducibility of the synthetic process.
| Solvent | Parameter | Polymers | ||
|---|---|---|---|---|
| 6FDA-DAM | 6FDA-AcHAB | 6FDA-6FpDA | ||
| a Oligomers were formed. | ||||
| NMP | M n/kDa | 65 | 79 | 46 |
| M w/kDa | 100 | 122 | 78 | |
| PDI | 1.5 | 1.5 | 1.7 | |
| KJCMPA | M n/kDa | 42 | 72 | 54 |
| M w/kDa | 64 | 109 | 80 | |
| PDI | 1.5 | 1.5 | 1.5 | |
| GVL | M n/kDa | 34 | 14 | 62 |
| M w/kDa | 50 | 201 | 90 | |
| PDI | 1.4 | 1.4 | 1.4 | |
| Cyrene™ | M n/kDa | 50 | 16 | 9 |
| M w/kDa | 85 | 25 | 12 | |
| PDI | 1.7 | 1.6 | 1.4 | |
| DMI | M n/kDa | 48 | 28 | 38 |
| M w/kDa | 77 | 85 | 51 | |
| PDI | 1.5 | 1.5 | 1.3 | |
| DMSO | M n/kDa | 26 | 19 | 25 |
| M w/kDa | 41 | 41 | 42 | |
| PDI | 1.6 | 2.1 | 1.7 | |
| DMCa | M n/kDa | — | 9 | 7 |
| M w/kDa | — | 13 | 10 | |
| PDI | — | 1.5 | 1.4 | |
In any case, the molecular weight values obtained for the polyimides synthesized in Cyrene™ and DMC are the lowest obtained, where DMC is clearly finding decisive limitations for all the polyimides. This observation fits very well with the possibility of presenting secondary reactions between the reactants and the solvents, which would unbalance the stoichiometry of the reaction, leading to low molecular weights. In the case of the DMSO, the slower kinetics and limitation in the miscibility of the monomers at the initial stage due to the low freezing point could have an important effect on the final molecular weight. The cooling down step of this reaction might not be the right strategy when using DMSO as the solvent. In general, for the three polymers, the highest molecular weight was obtained when the synthesis was performed in GVL, with the molecular weight values in the following order: GVL > NMP > KJCMPA > DMI > DMSO > Cyrene™ > DMC.
Since the influence of solubility of both monomers and polymers on the solvent is crucial, this trend in molecular weights depending on the solvent used in the synthesis is almost the same regardless of the polymer synthesized with the exceptions of 6FDA-DAM synthesized in DMI, and 6FDA-6FpDA synthesized in NMP. In all cases, the highest molecular weights were obtained for polymers formed with the diamine monomer HAB, followed by 6FpDA, and DAM, with the exceptions mentioned. Since the tendency is robust, this would, in principle, indicate the order of reactivity of the monomers under the specific reaction conditions. An adjustment of the reaction's conditions in terms of temperature, time, concentration, or others most probably would allow reaching molecular weight values comparable to that obtained in NMP. It is remarkable that the molecular weight of 6FDA-diamine polymers synthesized using GVL is directly comparable to that prepared using NMP under the same reaction conditions. Possibly, minor adjustment of the polycondensation reaction conditions using KJCMPA, DMI, and DMSO as solvents would lead to comparable results.
4. Identification of other alternative solvents
The solvents selected to carry out the experimental proof of concept can also present possible derivatives, which, at the same time, have the potential of being employed as greener solvents. In this section, their obtention protocols as well as some of their reported derivatives are analysed. These derivatives are formed by various strategies such as variation in member rings (in cyclic compounds), differences in the chain length and/or functionality, or disparity in the symmetry of the compounds. The solvents are organized by similarity to the solvents selected for the Experimental section.γ-Valerolactone (GVL) can be synthesized from multiple bio reactants such as levulinic acid (LA), formic acid, furfural, HMF, or one catalytic dehydration of hexose and pentose sugars.95 Novel approaches to synthesize GVL have been taken into account in order to improve the sustainability of the solvent96,97 production. Other derivatives related to the GVL included in the list of more sustainable solvents to be applied are the commonly named δ-valerolactone, γ-butyrolactone, and ε-caprolactone.98Fig. 6 shows the GVL chemical structure as well as other lactone-type derivatives, which can also be employed as more sustainable solvents. There is a systematic way of naming the lactone derivatives as a function of the ring size (as shown in Fig. 6a). In this way, α-, β-, γ-, δ-, and ω-lactones contain correspondingly 3-, 4-, 5-, 6-, and 7-membered rings.99 It is important to highlight that the list of derivatives can be as high as different functionalities can be added to the compounds. In Fig. 6, some derivatives for the γ-butyrolactone are shown as examples.
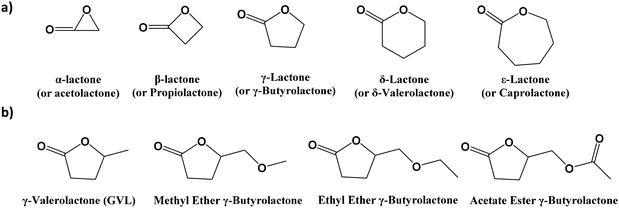 | ||
| Fig. 6 (a) Types of lactones as a function of the ring size; (b) γ-valerolactone (GVL) and other examples of aprotic lactone-type solvents. | ||
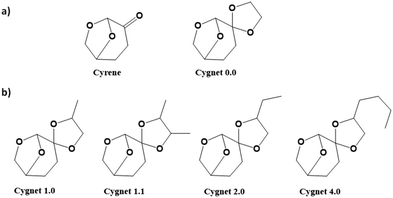
Cyrene (Cy) : is synthesized from levoglucosenone, which is a derivative of the cellulose6,100 and commercialized by the Norwegian Circa Group under the designation Cyrene™. Then, using the Cyrene as a platform, other derivatives can be obtained. In this way, the formation of different 7-membered ring ketal-based derivatives leads to the denominated Cygnet (Fig. 7a). This new category of solvents is named as a function of the substituents of the ketal (as shown in Fig. 7b). Then, it is possible to form as many solvents as different combinations of substituents (R1 and R2) can be placed at the ketal group. Cygnet 0.0, which according to Fig. 7a corresponds with the ketal's groups where R1 and R2 are equal to hydrogen, shows similar Hansen parameters to those of, for instance, the chlorinated solvent dichloromethane. The price of these solvents directly depends on the cost of levoglucosenone.
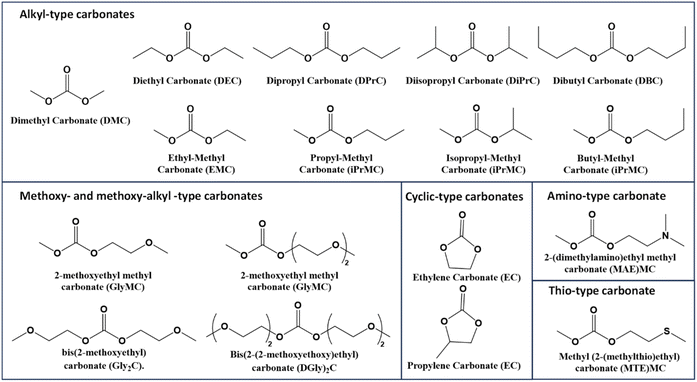
Dimethyl carbonate (DMC) is widely used in paints, inks, and coatings, as well as in the chemical industry as alternatives to chlorine (halogen) reagents such as methyl and acyl halides in alkylation and carbonylation reactions;101 phosgene in the synthesis of urethanes, carbamates and isocyanates; or as direct substitutes of solvents such as dichloromethane and chloroform. Although DMC was originally synthesized from phosgene, the synthetic protocol has evolved towards more sustainable sources. Nowadays, the DMC is mainly produced from the reaction between CO2 and oxyrane which evolves under basic conditions to the formation of the solvent. Other synthetic routes such as the catalytic oxidative carbonylation of methanol using O2 as the oxidant102,103 are gaining relevance. Other types of green synthetic protocols for obtaining carbonate-based derivatives are the transesterification of urea104 or alkylation of salt carbonates.105 This type of compound behaves in a very special way since nucleophiles can discriminate between the electrophilic sites of DMC and undergo a methoxycarbonylation reaction via a BAc2 mechanism or a methylation reaction via a BAl2 mechanism106 where the methyl groups behave as soft electrophilic sites of the molecule. Carbonate-based derivatives107 can be easily formed by varying the reactants during the formation process. Some of these derivatives have been tested as solvents for the membrane formation process.108 At the same time, the development of unsymmetrical carbonate derivatives by, for instance, the transcarbonylation of DMC, leads to alkyl methyl carbonates (AMC)109 showing different polarities, solubilities, and performances. Another approach for the synthesis of carbonates is the carbonation of epoxides or diol derivatives, which can lead to symmetrical and unsymmetrical carbonates as a function of the chemical structure of the initial epoxy compounds.110 A strategy to enhance the polarity and, thus, the solubility in water oxygen can be incorporated to the structure. In that sense, the synthesis of (methoxy ethyl)carbonates meets the criteria, which can be very useful, for instance, in coatings and paints. Using the same approach, it is possible to synthesize symmetrical and unsymmetrical amino- and thio-carbonates.109 Therefore, several methodologies and initial compounds can be considered for the formation of carbonate-based compounds leading to a very large number of molecules with the potential of becoming more sustainable media for synthetic reactions. Fig. 8 shows some examples of the mentioned carbonate derivatives.
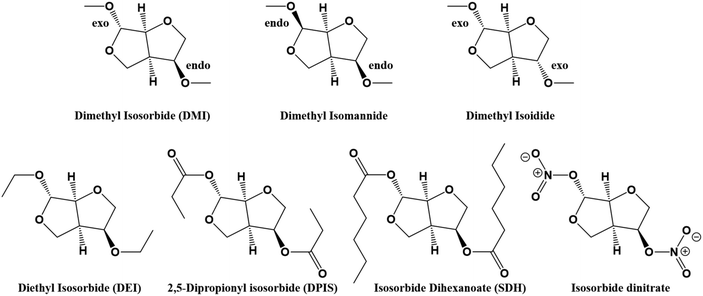
Dimethyl isosorbide (DMI) is a cheap, sugar-based solvent synthesized via methylation of the anhydro sugar isosorbide, known as 1,4:3,6-dianhydro-D-glucitol, which is obtained from D-glucitol.111 Isosorbide is also a bio-based platform chemical, produced, for instance, from biomass,112 which can be employed for obtaining a great variability of compounds such as pharmaceuticals, cosmetics, green solvents, plasticizers, surfactants, and even biopolymers.113,114 The functionalization of the hydroxyl groups present in the isosorbide leads to different chemicals by, for instance, Williamson etherification.115 The functionalization of the isosorbide and its epimers (isomannide and isoidide) leads to compounds with different properties.116,117 The epimer containing two exo hydroxyl groups is the isoidide, which is produced by the isomerization of isomannide and isosorbide (see Fig. 9).118 Green synthetic protocol for obtaining DMI has been developed employing precisely DMC. In this way, the isosorbide reacts with dimethyl carbonate (DMC), which acts as the reagent and solvent, in the presence of a base, yielding DMI.119 The large number of possibilities in the composition of carbonates shown above allows obtaining different functionalities in isosorbides.120 Multiple isosorbide-derivatives such as isosorbide suberate, isosorbide dioctoate, or isosorbide dihexanoate can be synthesized via the esterification121 process.122 The functionalization of the hydroxyl groups will directly affect the performances of the compounds. For instance, 2,5-dipropionyl isosorbide (DPIS) is a liquid while diacetyl isosorbide,123 which is only one methyl group shorter, is a solid. The main use of these derivatives is as green plasticizers,122,124 showing great potential to substitute the frequently used phthalate-based plasticizers. Fig. 9 shows some examples of derivatives from isosorbide. Similar structures could be formed from other stereoisomers.
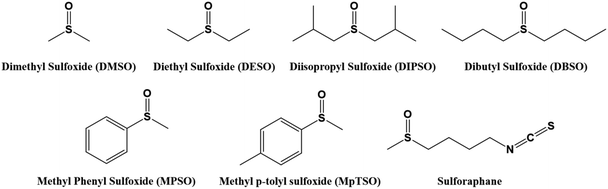
Dimethyl sulfoxide (DMSO) is produced in the industry by the oxidation of dimethyl sulfide (DMS), which is a by-product of the wood pulp generated in the paper production, with nitrogen dioxide or oxygen.125 The applications of DMSO involve its use as a solvent and a synthon in organic chemistry, especially as a source of –Me, –SMe, or –SO2Me.126–129 DMSO is normally recognised for the smell which makes the work with it in big quantities unkind. Arkema and other companies developed a new grade of DMSO renamed as DMSO EVOL™ which basically present the same characteristics and performances with a more pleasant fruit-like odor.130 One isotopologue of dimethyl sulfoxide, concretely DMSO-d6, produced by heating DMSO in heavy water (D2O) in the presence of a base, is one of the most common solvents used in NMR spectroscopy. Following the same criteria as those for the previous solvents, and as it is shown in Fig. 10, the substitution of the methyl groups by other alkyl functionalities will lead to symmetrical and unsymmetrical dialkylsulfoxides.131 Then, the structure design might lead to symmetrical derivatives such as diethyl sulfoxide (DESO), used as preservatory of cells, tissues and organs;132 or unsymmetrical derivatives such as the methyl phenyl sulfoxide (PMSO) which shows special ability to differentiate oxidizing species like, for instance, the characterization of specific iron oxides [FeIVO]2+.133 Derivatives such as the sulforaphane can be found naturally in vegetables such as broccoli, cabbage or Brussels sprouts.
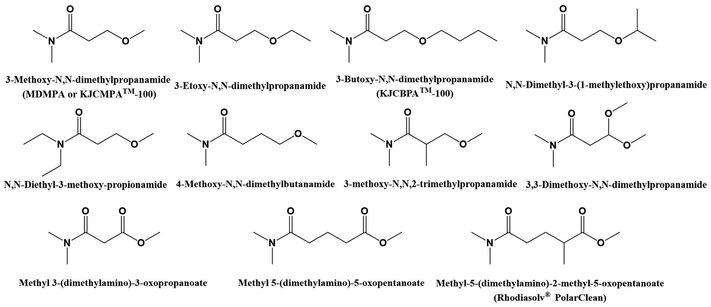
3-Methoxy-N,N-dimethylpropanamide, commercialized under the trade name KJCMPA™-100®, can be synthesized from the combination of levulinic acid (LA) and dimethylamine. The LA can be extracted from a very large variety of agricultural waste (lignocellulosic biomass) such as cotton, sugar cane bagasse, Cicer arietinum, rice straw, corn stover, sweet sorghum bagasse and miscanthus; marine and fresh water algae; and bacterial sources like cyanobacteria.134 Until few months ago, this solvent was considered as a very promising green alternative to conventional polar aprotic solvents. In fact, at the beginning of this work, even at the beginning of this year, the solvent was identified as a safe solvent,69,135 compared to traditional ones. In the last months, the solvent has been classified with the hazard statements H319, H361, and H373. The statement H361, according to the health criteria, obtains a score of 6 (higher than 5), which discards it from the list of sustainable chemicals. Similarly to previous examples, the modification of the structure of LA in, for instance, length, type of substituents or configuration would lead to different derivatives. The extension of the chain to 3-butoxy-N,N-dimethylpropanamide, also known under the trade name KJCBPA™-100, leads to a lower toxicity where the GHS Hazard Statements for this compound are H302 and H319. Attending to similar statement, the structure of the molecule has been functionalized and some examples are shown in Fig. 11. One of these derivatives is the commercially available Solvay known as Rhodiasolv® PolarClean, for which the chemical structure is methyl-5-(dimethylamino)-2-methyl-5-oxopentanoate136 (as shown in Fig. 11). PolarClean is a solvent produced from 2-methyleglutaronitrile.137 This solvent is also placed in the list of solvents meeting all the criteria to be applied in the membrane industry. Then, it is clear that there is a need to understand which type of chemical structure will lead to undesirable features trying to avoid their use.
N-Methyl-2-pyrrolidone (NMP)
is probably one of the most employed polar aprotic solvents in the polymer and membrane field. The solvent structure entails a 5-membered ring lactam. The solvent can be obtained from multiple sources including the reaction between the γ-lactone, which, as mentioned above, can be produced from various bio-based chemicals, and the methylamine. It can be considered that sustainable methods to produce it have been industrially developed. In this way, more than 150![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons of NMP are produced every year. This solvent has a list of hazard statements such as H315 – causes skin irritation, H319 – serious eye irritation, H335 – specific target organ toxicity (single exposure) may cause respiratory irritation, and H360D – reproductive toxicity may damage the unborn child, which greatly limit its use. One consequence is that the NMP shall not be placed on the market as a material on its own or in mixtures in a concentration equal to or greater than 0.3 wt%.138 Furthermore, the specific properties of the NMP, such as excellent solubility and coalescence capacity, low volatility, and outstanding miscibility with numerous solvents, including water, make use of this solvent critical in multiple industries including paints, batteries, coatings, wiring, polymers or membranes.139 Of course, multiple efforts have been made to minimize the hazardous character of this chemical. The strategy is similar to the previous solvents, by using structurally homologues substances with different lengths of carbon side chains. For instance, one of the first industrially offered replacements was N-butyl-2-pyrrolidone (NBP), commercially known as TamiSolve™ NxG, which contains very similar physical properties140,141 to those of the NMP, showing good results in the initial toxicity analysis. As expected, the solvent has been tested for the critical applications mentioned before including membrane formation142–144 or polyimide synthesis.75 Paradoxically, derivatives with shorter, the N-ethyl-2-pyrrolidone (NEP), and longer side chains, the N-octyl-2-pyrrolidone (NOP), have been classified with the statements H360D – reproductive toxicity may damage the unborn child and H314 – causes severe skin burns and eye damage, respectively, which, according to Fig. 1, is enough to eliminate them from the selection list. The same situation occurs for bicycle-type compounds such as 1-cyclohexyl-2-pyrrolidone, which is labelled as H314, while 1-benzyl-2-pyrrolidinone is labelled as a safer chemical. Similar to the lactone-based derivatives, cycles with different ring members can be formed with the same functionality (as shown in Fig. 12). Interestingly enough, changing the number of ring members results in very diverse hazard statements. For more than 5-membered ring structures, the compounds show hazards which would pass the analysis of the decision tree.
000 tons of NMP are produced every year. This solvent has a list of hazard statements such as H315 – causes skin irritation, H319 – serious eye irritation, H335 – specific target organ toxicity (single exposure) may cause respiratory irritation, and H360D – reproductive toxicity may damage the unborn child, which greatly limit its use. One consequence is that the NMP shall not be placed on the market as a material on its own or in mixtures in a concentration equal to or greater than 0.3 wt%.138 Furthermore, the specific properties of the NMP, such as excellent solubility and coalescence capacity, low volatility, and outstanding miscibility with numerous solvents, including water, make use of this solvent critical in multiple industries including paints, batteries, coatings, wiring, polymers or membranes.139 Of course, multiple efforts have been made to minimize the hazardous character of this chemical. The strategy is similar to the previous solvents, by using structurally homologues substances with different lengths of carbon side chains. For instance, one of the first industrially offered replacements was N-butyl-2-pyrrolidone (NBP), commercially known as TamiSolve™ NxG, which contains very similar physical properties140,141 to those of the NMP, showing good results in the initial toxicity analysis. As expected, the solvent has been tested for the critical applications mentioned before including membrane formation142–144 or polyimide synthesis.75 Paradoxically, derivatives with shorter, the N-ethyl-2-pyrrolidone (NEP), and longer side chains, the N-octyl-2-pyrrolidone (NOP), have been classified with the statements H360D – reproductive toxicity may damage the unborn child and H314 – causes severe skin burns and eye damage, respectively, which, according to Fig. 1, is enough to eliminate them from the selection list. The same situation occurs for bicycle-type compounds such as 1-cyclohexyl-2-pyrrolidone, which is labelled as H314, while 1-benzyl-2-pyrrolidinone is labelled as a safer chemical. Similar to the lactone-based derivatives, cycles with different ring members can be formed with the same functionality (as shown in Fig. 12). Interestingly enough, changing the number of ring members results in very diverse hazard statements. For more than 5-membered ring structures, the compounds show hazards which would pass the analysis of the decision tree.
Other emerging polar aprotic solvents derived or extracted from natural sources will slowly start gaining interest in the field as soon as their production methodologies gain reliability. Some examples to mention might be p-cymene or limonene. Both compounds are derivatives from natural sources. However, they both present immiscibility with water, which limits their application at the last step of the membrane formation process. Triethyl phosphate (TEP) might be an efficient alternative for traditional solvents. TEP was obtained by the combination between ethanol and phosphoric acid. This solvent has been already tested in the membrane formation process, especially in the case of the PVDF polymer, with positive results.145,146Sulfolane has been identified as a sustainable reaction medium replacing other solvents, particularly in reactions occurring at very high temperatures due to its excellent high boiling point (285 °C).147 However, the solvent has been recently labelled as H360 (which scores the maximum in Fig. 1). Identifying other families of solvents, probably ionic liquids (ILs) and deep eutectic solvents (DESs)148–150 are presenting sufficient versatility and tuned capabilities to be, at least, tested for the entire membrane process. Both types of compounds have been heavily tested for a large variety of chemical reactions as well as common characteristics such as non-volatility, non-flammability, or high viscosity. ILs present an almost unlimited number of combinations increasing their synthetic flexibility and tunability for a specific reaction.151 Aspects such as biodegradability or aquatic toxicity remain open in the IL topic.152 The use of nontoxic and naturally derived precursors would minimize those risks. In the case of DESs, the preparation is simpler than ILs, presenting lower toxicity and cost. However, the testing on a polymer synthesis process, especially high-performance polymers such as polyimides, is still limited153 for both types of compounds.
Alternative solutions such as the use of supercritical or subcritical fluids are often employed in extraction processes especially involving food products. The chemical characteristics of a solvent might radically change under super- or subcritical conditions. The supercritical CO2 has been employed for the synthesis as well as modification of aromatic polyimides.154,155 Supercritical water has been widely used for the synthesis of polyimides and other polymers.156,157 An innovative use of supercritical CO2 has been used for the formation of very thin membranes.5 The formation of porous substrate and selective layer at once will be, no doubt, in the coming years very employed since it avoids the use of solvent in the last part of the membrane formation process. Redefinition of the technical and engineering parts of the entire process has to be carried out in order to be able to covert the synthesis and processing of the materials from the same supercritical process.
As it can be seen from the discussion in this section, here we describe more than 60 alternative polar aprotic solvents, selected solvents and derivatives from those selected solvents, apart from other emerging solvents and alternative solutions, which have the potential of substitute the traditional solvents. This list can be extended by following the strategies describes for the formation of derivatives such as changing the length of carbon side chain, inclusion of functionalities or variation in the member rings in cycles. Research evolution has proven that the processes can be tuned until a sustainable synthetic protocol is found. In the same way, the cost of the solvents is not discussed here since the offer and demand law will finally push the price of any chemical significantly down if the substance is needed on an industrial scale. However, the possibility and design for the scaling up of the processes should be taken into account immediately after the concept has been proven.
5. Conclusions
While the research criteria on the selection of greener solvent media are generally focused on the safety, health and environmental impacts of the solvents, the specific criteria for the process must be introduced into the discussion. In this work, the specific case of the synthesis of polyimides for their processing as a gas separation membrane has been developed. A simple decision diagram has been developed attending to the general as well as specific criteria of the polymer synthesis and membrane formation processes. As a result of the analysis of more than 130 solvents, ten commercially available greener solvents have been identified: γ-valerolactone, cyrene, dimethyl carbonate, N-octyl pyrrolidone, diethyl carbonate, dimethyl isosorbide, γ-butyrolactone, N-butyl pyrrolidone, methyl-5-(dimethylamino)-2-methyl-5-oxopentanoate, and dimethyl sulfoxide.Five of them selected and tested for the synthesis of polyimides present differences in their reactivity. Other two solvents were tested as reference: N-methyl pyrrolidone (NMP) and 3-methoxy-N,N-dimethylpropanamide (known by the trade name KJCMPA®-100). The synthetic protocol for the three polyimides was optimized by using NMP. The solvent was just replaced by the alternative solvents. In all the cases, the polycondensation reaction was possible, independent of the solvent and polymer, and the final polymer structure was identified by 1H-NMR spectroscopy. By using the defined synthetic protocol, the solvents cyrene and dimethyl carbonate presented some encounters, indicating that the reaction protocol should be adapted to these solvents. In general, for the three polymers, the highest molecular weight was consistently obtained when the synthesis was performed in GVL, with the molecular weight values in the following order: GVL > NMP > KJCMPA > DMI > DMSO > Cyrene > DMC. Therefore, for the selected polyimides, the GVL could directly substitute NMP in a synthetic process.
This work has also identified more than 60 possible alternative solvents following the chemical structure of the selected solvents obtained through the decision diagram as well as other emerging solvents. This list can be extended by simple strategies such as changing the length of carbon side chain, inclusion of functionalities or variation in the member rings in cycles.
6. Future directions and key action points
1. Development of solvents derived from green platforms. Ensuring a continuous and stable source of starting biobased molecules158 will lead to a large number of possible novel solvents. An existing list158,159 of the 10 key future compounds obtained from biorefineries includes: ethanol, furans, glycerol and derivatives, bio-hydrocarbons, lactic acid, succinic acid, hydroxy propionic acid/aldehyde, levulinic acid, D-sorbitol and xylitol. In our opinion, many other such as compounds obtained from the exploitation of the urban and industrial waste treatments will join this and other lists.160,1612. Development of simple, well-documented, reproducible and scalable synthetic protocols for the novel solvents. Rather than the solvent cost, which, at the end, will be dominated by the supply and demand law, ensuring a fast and continuous accessibility to the solvents will determine their implementation at the industrial level.
3. Development of simple methodologies for the recycling and reutilization of solvents. In this point, the membrane technology, as well as other separation technologies, will be fundamental to reach a high recovery degree of the solvents. Several membranes are already commercialized for the organic solvent nanofiltration.82 However, significantly increasing the effort to improve the performance and knowledge of separations involving solvents and water will be very important for the reutilization of solvents in the membrane production process. In this way, the solvent can be integrated again into the synthetic process for a number of cycles, which will depend on the degree of recovery and purity (separation performances and process design) obtained from the separation process.
4. Development of simple tools for the determination of a preliminary idea of the environmental impact of the solvents such as carbon foot print. Several solvents have already been presented as alternative solvents and many more will appear in the coming years. However, sometimes researchers and chemical companies should work to avoid the temptation to use the absence of environmental and health information to keep the solvents ahead of legislation.
5. Biodegradability studies of solvents and solvent mixtures and their optimization to obtain valuable products (as the one expressed in the first point). When reutilization is not possible, the effective methodology for obtaining valuable compounds that can be integrated in a cycle will minimize the generation of wastes.
6. Study of the biodegradability of the polymeric materials will promote an entire circularity. The use of easily degradable materials will simply not be logic in certain applications where the materials must stand very aggressive media162 in terms of contaminants, pH, or temperatures. However, in certain applications, the separation can occur under relatively mild conditions where a very wide range of materials can be employed and the biodegradability of the membranes, after use, will be highly beneficial.
7. More experiences should be carried out covering the entire process in order to show to the industry the real outcomes and benefits of the approach. In the membrane technology, this would mean to explore the connection of synthesis with the processing of the material as well as testing the performances.
8. Machine learning and artificial intelligence for the learning of production, reutilisation and biodegradability of the novel green-based solvents. The anticipation of the actions will allow us to have a better perspective of the opportunities offered by the use of certain chemicals and compounds.
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was funded by the Project TED2021-131170A-I00 financed by MCIN/AEI/10.13039/501100011033 and the European Union Next Generation EU/PRTR. We are also grateful to the ECLOSION project MIG-202111071 (Mission2, Spanish “Plan Estatal I + D + I”), funded by Next Generation EU. Authors are thankful to the Spanish Research Agency for the funding of the project PID2023-149594OB. We are grateful as well to the Regional Government of Castilla y León and the EU-FEDER initiative for their programs CL-EI-2021-07 and UIC082.References
- P. R. C. Ministry of Ecology and Environment, Measures for the Environmental Management Registration of New Chemical Substances, Protection Order No. 7 of Ministry of Ecology and Environment, 2021, MEE Order No. 12.
- A. Jordan, C. G. J. Hall, L. R. Thorp and H. F. Sneddon, Chem. Rev., 2022, 122, 6749–6794 CrossRef CAS PubMed.
- J. H. Clark, T. J. Farmer, A. J. Hunt and J. Sherwood, Int. J. Mol. Sci., 2015, 16, 17101–17159 CrossRef CAS PubMed.
- A. European Chemicals, How to comply with REACH Restriction 71, guideline for users of NMP (1-methyl-2-pyrrolidone), European Chemicals Agency, 2019 Search PubMed.
- D. Cuadra-Rodríguez, C. Soto, F. J. Carmona, A. Tena, L. Palacio, M. A. Rodríguez-Pérez and J. Pinto, J. CO2 Util., 2023, 74, 102536 CrossRef.
- R. A. Milescu, A. Zhenova, M. Vastano, R. Gammons, S. Lin, C. H. Lau, J. H. Clark, C. R. McElroy and A. Pellis, ChemSusChem, 2021, 14, 3367–3381 CrossRef CAS PubMed.
- F. Russo, F. Galiano, F. Pedace, F. Aricò and A. Figoli, ACS Sustainable Chem. Eng., 2020, 8, 659–668 CrossRef CAS.
- M. A. Rasool, C. Van Goethem and I. F. J. Vankelecom, Sep. Purif. Technol., 2020, 232, 115903 CrossRef.
- F. P. Byrne, C. M. Nussbaumer, E. J. Savin, R. A. Milescu, C. R. McElroy, J. H. Clark, B. M. A. van Vugt-Lussenburg, B. van der Burg, M. Y. Meima, H. E. Buist, E. D. Kroese, A. J. Hunt and T. J. Farmer, ChemSusChem, 2020, 13, 3212–3221 CrossRef CAS PubMed.
- M. A. Rasool and I. F. J. Vankelecom, Green Chem., 2019, 21, 1054–1064 RSC.
- M. A. Rasool and I. F. J. Vankelecom, Membranes, 2021, 11, 418 CrossRef CAS PubMed.
- P. Ortiz-Albo, V. D. Alves, I. Kumakiri, J. Crespo and L. A. Neves, J. Membr. Sci., 2024, 693, 122346 CrossRef CAS.
- R. Cabezas, E. Zurob, B. Gomez, G. Merlet, A. Plaza, C. Araya-Lopez, J. Romero, F. Olea, E. Quijada-Maldonado, L. Pino-Soto, T. Gonzalez and R. Castro-Muñoz, Ind. Eng. Chem. Res., 2022, 61, 17397–17422 CrossRef CAS.
- R. Castro-Muñoz, F. Galiano, A. Figoli and G. Boczkaj, J. Environ. Chem. Eng., 2022, 10, 106414 CrossRef.
- M. Taghizadeh, A. Taghizadeh, V. Vatanpour, M. R. Ganjali and M. R. Saeb, Sep. Purif. Technol., 2021, 258, 118015 CrossRef CAS.
- A. Paiva, R. Craveiro, I. Aroso, M. Martins, R. L. Reis and A. R. C. Duarte, ACS Sustainable Chem. Eng., 2014, 2, 1063–1071 CrossRef CAS.
- S. U. Hong, Y. Wang, L. S. Soh and W. F. Yong, Green Chem., 2023, 25, 4501–4512 RSC.
- United-Nations, Globally Harmonized System of Classification and Labelling of Chemicals (GHS), New York, 2019 Search PubMed.
- P. Anastas and N. Eghbali, Chem. Soc. Rev., 2010, 39, 301–312 RSC.
- D. Prat, A. Wells, J. Hayler, H. Sneddon, C. R. McElroy, S. Abou-Shehada and P. J. Dunn, Green Chem., 2016, 18, 288–296 RSC.
- R. K. Henderson, C. Jiménez-González, D. J. C. Constable, S. R. Alston, G. G. A. Inglis, G. Fisher, J. Sherwood, S. P. Binks and A. D. Curzons, Green Chem., 2011, 13, 854–862 RSC.
- K. Alfonsi, J. Colberg, P. J. Dunn, T. Fevig, S. Jennings, T. A. Johnson, H. P. Kleine, C. Knight, M. A. Nagy, D. A. Perry and M. Stefaniak, Green Chem., 2008, 10, 31–36 RSC.
- T. V. T. Phan, C. Gallardo and J. Mane, Green Chem., 2015, 17, 2846–2852 RSC.
- F. P. Byrne, S. Jin, G. Paggiola, T. H. M. Petchey, J. H. Clark, T. J. Farmer, A. J. Hunt, C. R. McElroy and J. Sherwood, Sustainable Chem. Processes, 2016, 4, 7 CrossRef.
- European-Parliament, European-Parliament, Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) 2006 Search PubMed.
- R. Recio, L. Palacio, P. Pradanos, A. Hernandez, A. E. Lozano, A. Marcos, J. G. de la Campa and J. de Abajo, J. Membr. Sci., 2007, 293, 22–28 CrossRef CAS.
- European-Parliament, Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008Classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006 2008 Search PubMed.
- D. Prat, J. Hayler and A. Wells, Green Chem., 2014, 16, 4546–4551 RSC.
- Republique-Française, Décrete n° 2012-746 du 9 mai 2012fixant des valeurs limites d’exposition professionnelle contraignantes pour certains agents chimiques 2012 Search PubMed.
- BAUA, Federal Institute for Occupational Safety and HealthTRGS 900 Arbeitsplatzgrenzwerte 2006 Search PubMed.
- ACGIH, American Conference of Governmental Industrial Hygienists, https://www.acgih.org/, (accessed 2024/06/27).
- G. Bhojani, S. Jani and N. K. Saha, J. Cleaner Prod., 2022, 334, 130098 CrossRef CAS.
- X. Q. Cheng, K. Konstas, C. M. Doherty, C. D. Wood, X. Mulet, Z. Xie, D. Ng, M. R. Hill, L. Shao and C. H. Lau, ACS Appl. Mater. Interfaces, 2017, 9, 14401–14408 CrossRef CAS PubMed.
- R. Muñoz and B. Guieysse, Water Res., 2006, 40, 2799–2815 CrossRef PubMed.
- P. G. Jessop, Green Chem., 2011, 13, 1391–1398 RSC.
- W. Xie, T. Li, A. Tiraferri, E. Drioli, A. Figoli, J. C. Crittenden and B. Liu, ACS Sustainable Chem. Eng., 2021, 9, 50–75 CrossRef CAS.
- C. J. Clarke, W.-C. Tu, O. Levers, A. Bröhl and J. P. Hallett, Chem. Rev., 2018, 118, 747–800 CrossRef CAS PubMed.
- Mordor-Intelligence, Polyimides (PI) Market SIZE & SHARE ANALYSIS – GROWTH TRENDS & FORECASTS UP TO 2029, https://www.mordorintelligence.com/industry-reports/polyimides-market, (accessed 2024/06/28).
- Business-Research, Polyimide Plastic Market Size, Share, Growth, And Industry Analysis By Type (PI Profile, PI Film, PI Resin, PI Coating, and Others) By Application (Electrical Industry, Aerospace Industry, Automotive Industry, Medical Industry, and Others), Regional Insights, and Forecast To 2031, https://www.businessresearchinsights.com/market-reports/polyimide-plastic-market-106907, (accessed 2024/06/28).
- Z. Xu, Z. L. Croft, D. Guo, K. Cao and G. Liu, J. Polym. Sci., 2021, 59, 943–962 CrossRef CAS.
- J. K. Fink, High Performance Polymers, William Andrew, Elsevier, Norwich, NY, USA, 2008 Search PubMed.
- A. J. Parker, Chem. Rev., 1969, 69, 1–32 CrossRef CAS.
- J. Miller and A. J. Parker, J. Am. Chem. Soc., 1961, 83, 117–123 CrossRef CAS.
- N. Teo and S. C. Jana, Langmuir, 2018, 34, 8581–8590 CrossRef CAS PubMed.
- D. Zou, S. P. Nunes, I. F. J. Vankelecom, A. Figoli and Y. M. Lee, Green Chem., 2021, 23, 9815–9843 RSC.
- H. Strathmann, K. Kock, P. Amar and R. W. Baker, Desalination, 1975, 16, 179–203 CrossRef CAS.
- G. R. Guillen, Y. Pan, M. Li and E. M. V. Hoek, Ind. Eng. Chem. Res., 2011, 50, 3798–3817 CrossRef CAS.
- P. Vandezande, X. Li, L. E. M. Gevers and I. F. J. Vankelecom, J. Membr. Sci., 2009, 330, 307–318 CrossRef CAS.
- M. d. l. A. Ramirez-Kantun, F. Weigelt, S. Neumann, S. Shishatskiy and T. Brinkmann, J. Membr. Sci., 2024, 695, 122519 CrossRef CAS.
- M. Mulder, in Encyclopedia of Separation Science, ed. I. D. Wilson, Academic Press, Oxford, 2000, pp. 3331–3346 Search PubMed.
- M. Durand, V. Molinier, W. Kunz and J.-M. Aubry, Chem. – Eur. J., 2011, 17, 5155–5164 CrossRef CAS PubMed.
- L. Moity, M. Durand, A. Benazzouz, C. Pierlot, V. Molinier and J.-M. Aubry, Green Chem., 2012, 14, 1132–1145 RSC.
- C. Kahrs and J. Schwellenbach, Polymer, 2020, 186, 122071 CrossRef CAS.
- O. Gronwald and M. Weber, J. Appl. Polym. Sci., 2020, 137, 48419 CrossRef CAS.
- S. Uebele, K. S. Johann, T. Goetz, O. Gronwald, M. Ulbricht and T. Schiestel, J. Appl. Polym. Sci., 2021, 138, 50935 CrossRef CAS.
- H. Kita, K. Tanaka and T. Koga, Kobunshi, 2008, 57, 894–897 CrossRef CAS.
- A. Tena, S. Shishatskiy, D. Meis, J. Wind, V. Filiz and V. Abetz, Macromolecules, 2017, 50, 5839–5849 CrossRef CAS.
- P. J. Flory, J. Chem. Phys., 1942, 10, 51–61 CrossRef CAS.
- J. H. Hildebrand and R. L. Scott, The solubility of nonelectrolytes, Dover Publications Inc., New York, NY, USA, 1964 Search PubMed.
- C. M. Hansen, The three dimensional solubility parameter and solvent diffusion coefficient, Danish Technical Press, Copenhagen, Denmark, 1967 Search PubMed.
- C. M. Hansen, Hansen solubility parameters: a user's handbook, CRC Press, BocaRaton, end edition edn., 2007 Search PubMed.
- T. Oishi and J. M. Prausnitz, Ind. Eng. Chem. Process Des. Dev., 1978, 17, 333–339 CrossRef CAS.
- Y. Aoki, S. Wu, T. Tsurimoto, Y. Hayashi, S. Minami, O. Tadamichi, K. Shiratori and R. Yoshida, Macromolecules, 2023, 56, 5446–5456 CrossRef CAS.
- A. Klamt, V. Jonas, T. Bürger and J. C. W. Lohrenz, J. Phys. Chem. A, 1998, 102, 5074–5085 CrossRef CAS.
- A. Klamt, J. Phys. Chem., 1995, 99, 2224–2235 CrossRef CAS.
- P. B. Staudt, R. L. Simões, L. Jacques, N. S. M. Cardozo and R. d. P. Soares, Fluid Phase Equilib., 2018, 472, 75–84 CrossRef CAS.
- G. M. C. Silva, D. A. Pantano, S. Loehlé and J. A. P. Coutinho, Ind. Eng. Chem. Res., 2023, 62, 20936–20944 CrossRef CAS.
- E. Epifanovsky, A. T. B. Gilbert, X. Feng, J. Lee, Y. Mao, N. Mardirossian, P. Pokhilko, A. F. White, M. P. Coons, A. L. Dempwolff, Z. Gan, D. Hait, P. R. Horn, L. D. Jacobson, I. Kaliman, J. Kussmann, A. W. Lange, K. U. Lao, D. S. Levine, J. Liu, S. C. McKenzie, A. F. Morrison, K. D. Nanda, F. Plasser, D. R. Rehn, M. L. Vidal, Z.-Q. You, Y. Zhu, B. Alam, B. J. Albrecht, A. Aldossary, E. Alguire, J. H. Andersen, V. Athavale, D. Barton, K. Begam, A. Behn, N. Bellonzi, Y. A. Bernard, E. J. Berquist, H. G. A. Burton, A. Carreras, K. Carter-Fenk, R. Chakraborty, A. D. Chien, K. D. Closser, V. Cofer-Shabica, S. Dasgupta, M. de Wergifosse, J. Deng, M. Diedenhofen, H. Do, S. Ehlert, P.-T. Fang, S. Fatehi, Q. Feng, T. Friedhoff, J. Gayvert, Q. Ge, G. Gidofalvi, M. Goldey, J. Gomes, C. E. González-Espinoza, S. Gulania, A. O. Gunina, M. W. D. Hanson-Heine, P. H. P. Harbach, A. Hauser, M. F. Herbst, M. Hernández Vera, M. Hodecker, Z. C. Holden, S. Houck, X. Huang, K. Hui, B. C. Huynh, M. Ivanov, Á. Jász, H. Ji, H. Jiang, B. Kaduk, S. Kähler, K. Khistyaev, J. Kim, G. Kis, P. Klunzinger, Z. Koczor-Benda, J. H. Koh, D. Kosenkov, L. Koulias, T. Kowalczyk, C. M. Krauter, K. Kue, A. Kunitsa, T. Kus, I. Ladjánszki, A. Landau, K. V. Lawler, D. Lefrancois, S. Lehtola, R. R. Li, Y.-P. Li, J. Liang, M. Liebenthal, H.-H. Lin, Y.-S. Lin, F. Liu, K.-Y. Liu, M. Loipersberger, A. Luenser, A. Manjanath, P. Manohar, E. Mansoor, S. F. Manzer, S.-P. Mao, A. V. Marenich, T. Markovich, S. Mason, S. A. Maurer, P. F. McLaughlin, M. F. S. J. Menger, J.-M. Mewes, S. A. Mewes, P. Morgante, J. W. Mullinax, K. J. Oosterbaan, G. Paran, A. C. Paul, S. K. Paul, F. Pavošević, Z. Pei, S. Prager, E. I. Proynov, Á. Rák, E. Ramos-Cordoba, B. Rana, A. E. Rask, A. Rettig, R. M. Richard, F. Rob, E. Rossomme, T. Scheele, M. Scheurer, M. Schneider, N. Sergueev, S. M. Sharada, W. Skomorowski, D. W. Small, C. J. Stein, Y.-C. Su, E. J. Sundstrom, Z. Tao, J. Thirman, G. J. Tornai, T. Tsuchimochi, N. M. Tubman, S. P. Veccham, O. Vydrov, J. Wenzel, J. Witte, A. Yamada, K. Yao, S. Yeganeh, S. R. Yost, A. Zech, I. Y. Zhang, X. Zhang, Y. Zhang, D. Zuev, A. Aspuru-Guzik, A. T. Bell, N. A. Besley, K. B. Bravaya, B. R. Brooks, D. Casanova, J.-D. Chai, S. Coriani, C. J. Cramer, G. Cserey, A. E. DePrince III, R. A. DiStasio Jr., A. Dreuw, B. D. Dunietz, T. R. Furlani, W. A. Goddard III, S. Hammes-Schiffer, T. Head-Gordon, W. J. Hehre, C.-P. Hsu, T.-C. Jagau, Y. Jung, A. Klamt, J. Kong, D. S. Lambrecht, W. Liang, N. J. Mayhall, C. W. McCurdy, J. B. Neaton, C. Ochsenfeld, J. A. Parkhill, R. Peverati, V. A. Rassolov, Y. Shao, L. V. Slipchenko, T. Stauch, R. P. Steele, J. E. Subotnik, A. J. W. Thom, A. Tkatchenko, D. G. Truhlar, T. Van Voorhis, T. A. Wesolowski, K. B. Whaley, H. L. Woodcock III, P. M. Zimmerman, S. Faraji, P. M. W. Gill, M. Head-Gordon, J. M. Herbert and A. I. Krylov, J. Chem. Phys., 2021, 155, 084801 CrossRef CAS PubMed.
- G. Yang, M. Zhang, I. Majeed, W. Fan, J. Zhao and Z. Zeng, ACS Sustainable Chem. Eng., 2023, 11, 14582–14590 CrossRef CAS.
- S. Venkatram, C. Kim, A. Chandrasekaran and R. Ramprasad, J. Chem. Inf. Model., 2019, 59, 4188–4194 CrossRef CAS PubMed.
- S.-i. Sawada, C. Ursino, F. Galiano, S. Simone, E. Drioli and A. Figoli, J. Membr. Sci., 2015, 493, 232–242 CrossRef CAS.
- H. W. Milliman, D. Boris and D. A. Schiraldi, Macromolecules, 2012, 45, 1931–1936 CrossRef CAS.
- Y. Liu and B. Shi, Polym. Bull., 2008, 61, 501–509 CrossRef CAS.
- M. Vayer, A. Vital and C. Sinturel, Eur. Polym. J., 2017, 93, 132–139 CrossRef CAS.
- L. Sing Soh, S. Uyin Hong, C. Zeng Liang and W. Fen Yong, Chem. Eng. J., 2023, 478, 147451 CrossRef CAS.
- B. Chen, G. Zhao, C. H. Lau, F. Wang, S. Fan, C. Niu, Z. Ren, G. Tang, P. Qin, Y. Liu and P. Li, Sep. Purif. Technol., 2024, 331, 125724 CrossRef CAS.
- J.-H. Kim, S.-B. Lee and S. Y. Kim, J. Appl. Polym. Sci., 2000, 77, 2756–2767 CrossRef CAS.
- F. Huang, T. D. Largier, W. Khan, W. Zheng and C. J. Cornelius, J. Membr. Sci., 2023, 683, 121864 CrossRef CAS.
- J. Ren, T.-S. Chung, D. Li, R. Wang and Y. Liu, J. Membr. Sci., 2002, 207, 227–240 CrossRef CAS.
- J. Li, J. Lu, H. Sang, Y. Zhang, B. Qi, J. Luo, Y. Wan and Y. Zhuang, ACS Appl. Polym. Mater., 2023, 5, 5641–5649 CrossRef CAS.
- M. Etxeberria-Benavides, O. Karvan, F. Kapteijn, J. Gascon and O. David, Membranes, 2020, 10, 4 CrossRef CAS PubMed.
- Y. Thiermeyer, S. Blumenschein and M. Skiborowski, Sep. Purif. Technol., 2021, 265, 118492 CrossRef CAS.
- G. Dong, H. Li and V. Chen, J. Membr. Sci., 2010, 353, 17–27 CrossRef CAS.
- C. M. Hansen and L. Just, Ind. Eng. Chem. Res., 2001, 40, 21–25 CrossRef CAS.
- F. Cakar, D. Sakar, O. Cankurtaran and F. Karaman, Eur. Polym. J., 2007, 43, 507–513 CrossRef CAS.
- L. Escorial, M. de la Viuda, S. Rodriguez, A. Tena, A. Marcos, L. Palacio, P. Pradanos, A. E. Lozano and A. Hernandez, Eur. Polym. J., 2018, 103, 390–399 CrossRef CAS.
- A. Tena, R. Vazquez-Guilló, A. Marcos-Fernández, A. Hernández and R. Mallavia, RSC Adv., 2015, 5, 41497–41505 RSC.
- A. Torres, C. Soto, J. Carmona, B. Comesaña-Gandara, M. de la Viuda, L. Palacio, P. Prádanos, M. T. Simorte, I. Sanz, R. Muñoz, A. Tena and A. Hernández, Polymers, 2024, 16, 13 CrossRef CAS PubMed.
- C. E. Sroog, J. Polym. Sci., Part D: Macromol. Rev., 1976, 11, 161–208 CAS.
- P. Ray, T. Hughes, C. Smith, M. Hibbert, K. Saito and G. P. Simon, Polym. Chem., 2019, 10, 3334–3341 RSC.
- P. Worsawat, P. Noppawan, C. Croise, N. Supanchaiyamat, C. R. McElroy and A. J. Hunt, Org. Biomol. Chem., 2023, 21, 1070–1081 RSC.
- P. Tundo, S. Memoli, D. Hérault and K. Hill, Green Chem., 2004, 6, 609–612 RSC.
- İ. Yazgan, Polym. Bull., 2020, 77, 1191–1203 CrossRef.
- A. Tena, Á. Marcos-Fernández, M. de la Viuda, L. Palacio, P. Prádanos, Á. E. Lozano, J. de Abajo and A. Hernández, Eur. Polym. J., 2015, 62, 130–138 CrossRef CAS.
- X.-L. Du, Q.-Y. Bi, Y.-M. Liu, Y. Cao and K.-N. Fan, ChemSusChem, 2011, 4, 1838–1843 CrossRef CAS PubMed.
- T. Raj, K. Chandrasekhar, R. Banu, J.-J. Yoon, G. Kumar and S.-H. Kim, Fuel, 2021, 303, 121333 CrossRef CAS.
- F. Valentini, G. Brufani, B. Di Erasmo and L. Vaccaro, Curr. Opin. Green Sustain. Chem., 2022, 36, 100634 CrossRef CAS.
- U. M. Fornefeld-Schwarz and P. Svejda, J. Chem. Eng. Data, 1999, 44, 597–604 CrossRef CAS.
- S. K. Sartori, M. A. N. Diaz and G. Diaz-Muñoz, Tetrahedron, 2021, 84, 132001 CrossRef CAS.
- A. Alves Costa Pacheco, J. Sherwood, A. Zhenova, C. R. McElroy, A. J. Hunt, H. L. Parker, T. J. Farmer, A. Constantinou, M. De bruyn, A. C. Whitwood, W. Raverty and J. H. Clark, ChemSusChem, 2016, 9, 3503–3512 CrossRef PubMed.
- P. Tundo and F. Aricò, ChemSusChem, 2023, 16, e202300748 CrossRef CAS PubMed.
- M.-j. Chen, J.-h. Yang, N. Zhao and F.-k. Xiao, J. Fuel Chem. Technol., 2023, 51, 804–811 CrossRef CAS.
- S.-H. Pyo, J. H. Park, T.-S. Chang and R. Hatti-Kaul, Curr. Opin. Green Sustain. Chem., 2017, 5, 61–66 CrossRef.
- B. M. Bhanage, S.-i. Fujita, Y. Ikushima and M. Arai, Green Chem., 2003, 5, 429–432 RSC.
- P. Tundo, M. Musolino and F. Aricò, Green Chem., 2018, 20, 28–85 RSC.
- P. Tundo, F. Aricò, A. E. Rosamilia, M. Rigo, A. Maranzana and G. Tonachini, Pure Appl. Chem., 2009, 81, 1971–1979 CAS.
- C. C. Truong, D. K. Mishra and V. Mishra, in Green Sustainable Process for Chemical and Environmental Engineering and Science, ed. Inamuddin, R. Boddula, M. I. Ahamed and A. M. Asiri, Elsevier, 2021, pp. 253–275 Search PubMed.
- M. A. Rasool, P. P. Pescarmona and I. F. J. Vankelecom, ACS Sustainable Chem. Eng., 2019, 7, 13774–13785 CrossRef CAS.
- G. Trapasso, C. Salaris, M. Reich, E. Logunova, C. Salata, K. Kümmerer, A. Figoli and F. Aricò, Sustainable Chem. Pharm., 2022, 26, 100639 CrossRef CAS.
- Ł. Kotyrba, A. Chrobok and A. Siewniak, Catalysts, 2022, 12, 309 CrossRef.
- D. Dalla Torre, M. Annatelli and F. Aricò, Catal. Today, 2023, 423, 113892 CrossRef CAS.
- S. Van de Vyver, J. Geboers, P. A. Jacobs and B. F. Sels, ChemCatChem, 2011, 3, 82–94 CrossRef CAS.
- M. Rose and R. Palkovits, ChemSusChem, 2012, 5, 167–176 CrossRef CAS PubMed.
- F. Aricò, Curr. Opin. Green Sustain. Chem., 2020, 21, 82–88 CrossRef.
- A. Caouthar, P. Roger, M. Tessier, S. Chatti, J. C. Blais and M. Bortolussi, Eur. Polym. J., 2007, 43, 220–230 CrossRef CAS.
- F. Aricò, S. Evaristo and P. Tundo, Sci. Open Res., 2014, 1–11 Search PubMed.
- P. Che, H. Ma, X. Nie, W. Yu and J. Xu, Green Chem., 2022, 24, 7545–7555 RSC.
- A. C. Cope and T. Y. Shen, J. Am. Chem. Soc., 1956, 78, 3177–3182 CrossRef CAS.
- P. Tundo, F. Aricò, G. Gauthier, L. Rossi, A. E. Rosamilia, H. S. Bevinakatti, R. L. Sievert and C. P. Newman, ChemSusChem, 2010, 3, 566–570 CrossRef CAS PubMed.
- O. Gómez-de-Miranda-Jiménez-de-Aberasturi, A. Centeno-Pedrazo, S. Prieto Fernández, R. Rodriguez Alonso, S. Medel, J. María Cuevas, L. G. Monsegue, S. De Wildeman, E. Benedetti, D. Klein, H. Henneken and J. R. Ochoa-Gómez, Green Chem. Lett. Rev., 2021, 14, 534–544 CrossRef.
- A. Alhanish and M. Abu Ghalia, Biotechnol. Prog., 2021, 37, e3210 CrossRef CAS PubMed.
- B. Yin and M. Hakkarainen, J. Appl. Polym. Sci., 2011, 119, 2400–2407 CrossRef CAS.
- K. Städtke, A. W. Göpfert and A. Inayat, Green Chem., 2023, 25, 7292–7308 RSC.
- Y. Yang, Z. Xiong, L. Zhang, Z. Tang, R. Zhang and J. Zhu, Mater. Des., 2016, 91, 262–268 CrossRef CAS.
- K. Capriotti and C. Joseph, J. Clin. Aesthet. Dermatol., 2012, 5, 24–26 Search PubMed.
- X.-F. Wu and K. Natte, Adv. Synth. Catal., 2016, 358, 336–352 CrossRef CAS.
- Z. Tashrifi, M. M. Khanaposhtani, B. Larijani and M. Mahdavi, Adv. Synth. Catal., 2020, 362, 65–86 CrossRef CAS.
- J.-C. Xiang, Q.-H. Gao and A.-X. Wu, in Solvents as Reagents in Organic Synthesis, 2017, pp. 315–353 Search PubMed.
- H. Lu, Z. Tong, L. Peng, Z. Wang, S.-F. Yin, N. Kambe and R. Qiu, Top. Curr. Chem., 2022, 380, 55 CrossRef CAS PubMed.
- T. Marino, F. Galiano, S. Simone and A. Figoli, Environ. Sci. Pollut. Res., 2019, 26, 14774–14785 CrossRef CAS PubMed.
- W. J. McDowell and H. D. Harmon, J. Inorg. Nucl. Chem., 1971, 33, 3107–3117 CrossRef CAS.
- S. Bonora, S. A. Markarian, A. Trinchero and K. R. Grigorian, Thermochim. Acta, 2005, 433, 19–26 CrossRef CAS.
- Y. Chen, C. J. Miller, J. Xie and T. D. Waite, Environ. Sci. Technol., 2023, 57, 18617–18625 CrossRef CAS PubMed.
- A. Victor, P. Sharma, I. N. Pulidindi and A. Gedanken, Catalysts, 2022, 12, 909 CrossRef CAS.
- R. Sliz, I. S. Roy, P. Molaiyan, J. Välikangas, T. Jakkila, M. Christophliemk, T. Hu, H. H. Nguyen, E. Hannila, S. Illikainen, U. Lassi and T. Fabritius, Batteries Supercaps, 2024, 7, e202300527 CrossRef CAS.
- A. Kumar, A. Sharma, B. G. de la Torre and F. Albericio, Green Chem. Lett. Rev., 2021, 14, 545–550 CrossRef CAS.
- X. Dong, A. Al-Jumaily and I. C. Escobar, Membranes, 2018, 8, 23 CrossRef PubMed.
- A. Hunt and N. Dale, Economic valuation in 1-Methyl-2-pyrrolidone (NMP) regulation, Organisation for Economic Co-operation and Development, 2018 Search PubMed.
- J. Sherwood, T. J. Farmer and J. H. Clark, Chem, 2018, 4, 2010–2012 CAS.
- J. Sherwood, H. L. Parker, K. Moonen, T. J. Farmer and A. J. Hunt, Green Chem., 2016, 18, 3990–3996 RSC.
- B. G. de la Torre, A. Kumar, M. Alhassan, C. Bucher, F. Albericio and J. Lopez, Green Chem., 2020, 22, 3162–3169 RSC.
- X. Jiang, W. F. Yong, J. Gao, D.-D. Shao and S.-P. Sun, J. Membr. Sci., 2021, 635, 119530 CrossRef CAS.
- N. Alqadhi, M. H. Abdellah, S. Nematulloev, O. F. Mohammed, M. A. Abdulhamid and G. Szekely, Sep. Purif. Technol., 2024, 328, 125072 CrossRef CAS.
- H. Yaacoubi and L. F. Dumée, in Green Membrane Technologies towards Environmental Sustainability, ed. L. F. Dumée, M. Sadrzadeh and M. M. A. Shirazi, Elsevier, 2023, pp. 515–554 Search PubMed.
- J. Chang, J. Zuo, L. Zhang, G. S. O'Brien and T.-S. Chung, J. Membr. Sci., 2017, 539, 295–304 CrossRef CAS.
- S. Fadhil, T. Marino, H. F. Makki, Q. F. Alsalhy, S. Blefari, F. Macedonio, E. D. Nicolò, L. Giorno, E. Drioli and A. Figoli, Chem. Eng. Process., 2016, 102, 16–26 CrossRef CAS.
- U. Tilstam, Org. Process Res. Dev., 2012, 16, 1449–1454 CrossRef CAS.
- A. Prabhune and R. Dey, J. Mol. Liq., 2023, 379, 121676 CrossRef CAS.
- D. O. Abranches, M. A. R. Martins, L. P. Silva, N. Schaeffer, S. P. Pinho and J. A. P. Coutinho, Chem. Commun., 2019, 55, 10253–10256 RSC.
- D. S. Freitas, D. Rocha, J. Noro, T. G. Castro, A. Cavaco-Paulo and C. Silva, ACS Sustainable Chem. Eng., 2023, 11, 16594–16607 CrossRef CAS.
- H. Niedermeyer, J. P. Hallett, I. J. Villar-Garcia, P. A. Hunt and T. Welton, Chem. Soc. Rev., 2012, 41, 7780–7802 RSC.
- M. Cvjetko Bubalo, S. Vidović, I. Radojčić Redovniković and S. Jokić, J. Chem. Technol. Biotechnol., 2015, 90, 1631–1639 CrossRef CAS.
- F. del Monte, D. Carriazo, M. C. Serrano, M. C. Gutiérrez and M. L. Ferrer, ChemSusChem, 2014, 7, 999–1009 CrossRef CAS PubMed.
- E. E. Said-Galiyev, Y. S. Vygodskii, L. N. Nikitin, R. A. Vinokur, M. O. Gallyamov, I. V. Pototskaya, V. V. Kireev, A. R. Khokhlov and K. Schaumburg, J. Supercrit. Fluids, 2003, 26, 147–156 CrossRef CAS.
- I. A. Ronova, M. Bruma, O. V. Sinitsyna, I. Sava, A. Y. Nikolaev, S. Chisca and N. G. Ryvkina, Struct. Chem., 2014, 25, 1687–1694 CrossRef CAS.
- M. J. Taublaender, M. Reiter and M. M. Unterlass, Macromolecules, 2019, 52, 6318–6329 CrossRef CAS.
- B. Baumgartner, M. Puchberger and M. M. Unterlass, Polym. Chem., 2015, 6, 5773–5781 RSC.
- J. J. Bozell and G. R. Petersen, Green Chem., 2010, 12, 539–554 RSC.
- F. Aricò and P. Tundo, Beilstein J. Org. Chem., 2016, 12, 2256–2266 CrossRef PubMed.
- F. Rivera, L. Villareal, P. Prádanos, A. Hernández, L. Palacio and R. Muñoz, Bioresour. Technol., 2022, 362, 127829 CrossRef CAS PubMed.
- M. Salamanca, L. Palacio, A. Hernandez, M. Peña and P. Prádanos, Membranes, 2023, 13, 266 CrossRef CAS PubMed.
- F. Radmanesh, A. Tena, E. J. R. Sudhölter and N. E. Benes, Mater. Today Nano, 2023, 24, 100379 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4gc04279d |
| This journal is © The Royal Society of Chemistry 2024 |