Open Access Article
Open Access ArticleMicrowave-assisted synthesis of highly photoluminescent core/shell CuInZnSe/ZnS quantum dots as photovoltaic absorbers†
Shubham
Shishodia
ab,
Hervé
Rinnert
 b,
Lavinia
Balan
b,
Lavinia
Balan
 c,
Jordane
Jasniewski
c,
Jordane
Jasniewski
 d,
Stéphanie
Bruyère
b,
Ghouti
Medjahdi
b,
Thomas
Gries
b and
Raphaël
Schneider
d,
Stéphanie
Bruyère
b,
Ghouti
Medjahdi
b,
Thomas
Gries
b and
Raphaël
Schneider
 *a
*a
aUniversité de Lorraine, CNRS, LRGP, F-54000 Nancy, France. E-mail: raphael.schneider@univ-lorraine.fr
bUniversité de Lorraine, CNRS, IJL, F-54000 Nancy, France
cCEMHTI-UPR 3079 CNRS, Site Haute Température, 1D avenue de la Recherche Scientifique, 45071 Orléans, France
dUniversité de Lorraine, LIBio, F-54000 Nancy, France
First published on 9th January 2025
Abstract
Water-dispersible core/shell CuInZnSe/ZnS (CIZSe/ZnS) quantum dots (QDs) were efficiently synthesized under microwave irradiation using N-acetylcysteine (NAC) and sodium citrate as capping agents. The photoluminescence (PL) emission of CIZSe/ZnS QDs can be tuned from 593 to 733 nm with varying the Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu molar ratio in the CIZSe core. CIZSe/ZnS QDs prepared with a Zn
Cu molar ratio in the CIZSe core. CIZSe/ZnS QDs prepared with a Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu ratio of 0.5 exhibit the highest PL quantum yield (54%) and the longest PL lifetime (515 ns) originating from the recombination of donor–acceptor pairs. The potential of CIZSe/ZnS QDs as photoabsorbers in QD-sensitized solar cells was also evaluated. An adequate type-II band alignment is observed between TiO2 and CIZSe/ZnS QDs, indicating that photogenerated electrons in CIZSe/ZnS QDs could efficiently be injected into TiO2.
Cu ratio of 0.5 exhibit the highest PL quantum yield (54%) and the longest PL lifetime (515 ns) originating from the recombination of donor–acceptor pairs. The potential of CIZSe/ZnS QDs as photoabsorbers in QD-sensitized solar cells was also evaluated. An adequate type-II band alignment is observed between TiO2 and CIZSe/ZnS QDs, indicating that photogenerated electrons in CIZSe/ZnS QDs could efficiently be injected into TiO2.
Introduction
Over the past three decades, binary group II–VI semiconductor nanocrystals (quantum dots, QDs) with a relatively narrow bandgap, such as CdSe or CdTe, have been extensively studied due to their tunable and narrow photoluminescence (PL) emission, high PL quantum yield (PL QY) and high photostability.1,2 However, their practical applications are limited due to the toxicity concern of Cd and the associated environmental issues.3 Recently, many efforts have been made to prepare eco-friendly QDs with PL characteristics comparable to Cd-based ones.I–III–VI2 QDs, like AgInS2 or CuInS2, and their quaternary derivatives AgInZnS or CuInZnS obtained after shelling and alloying with ZnS, have been demonstrated to be excellent alternatives to II–VI QDs for numerous applications including QD-sensitized solar cells (QDSSCs), fluorescence bio-imaging and light-emitting diodes.4–7 I–III–VI2 QDs exhibit a narrow bandgap, high absorption coefficients, a broad light absorption ranging from the UV to the NIR and a high thermal stability, conditions required for any use of these nanocrystals in technologies related to the conversion of solar energy.7–9 Moreover, I–III–VI2 QDs exhibit a high tolerance to stoichiometric deviation and defects, allowing a composition-dependent band structure which can be used in particular when coupling with other semiconductors to promote the transport and the separation of charge carriers.10–13
Recently, CuInSe2 (CISe) QDs have attracted high interest for photovoltaic applications due to their small bandgap (1.04 eV), high light absorption coefficient (ca. 105 cm−1) and large Bohr exciton radius (10.6 nm).8,14–16 Hydrophobic CISe core QDs and core/shell CISe/ZnS QDs capped with oleylamine, dodecanethiol or oleic acid can be prepared by thermal decomposition of metal precursors and Se in a high boiling point solvent like 1-octadecene followed by the deposition of the ZnS shell.16–21 These nanocrystals can also be synthesized by the hot injection of a Se precursor into a mixture of metal precursors.22–33 The optical properties of the QDs are excellent with optical absorption covering the entire visible and near IR range and PL QYs up to almost 100%.33 However, the use of these oil-dispersible nanocrystals in QDSSCs requires an additional ligand exchange step, for example with 3-mercaptopropionic acid (3-MPA), in order to make them hydrophilic and allow their anchoring onto TiO2 films.
An alternative is the preparation of CIZSe core QDs in an aqueous medium. The synthesis processes in an aqueous medium use fewer toxic precursors and ligands than those necessary for synthesis in an organic medium and the reaction conditions are generally milder. However, the preparation of CIZSe and CIZSe/ZnS QDs in an aqueous medium has been significantly less studied than in organic media. Syntheses are most commonly performed at reflux or under microwave activation using a mixture of glutathione (GSH) and sodium citrate as ligands.34–37 The use of gelatin and thioglycolic acid (TGA) or of trithiocyanuric acid when the synthesis is carried out in an autoclave has also been reported.38,39 The nanocrystals produced using these methods are of very small sizes (between 2 and 5.5 nm). Except the Mn2+-doped CIZSe/ZnS QDs whose PL QY is 40%,37 PL QYs are between 4 and 23.3%.
Herein, we report a microwave-assisted aqueous phase synthesis of core/shell CIZSe/ZnS QDs using N-acetylcysteine (NAC) and sodium citrate (SC) as capping ligands. By varying the Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu molar ratio from 5 to 0 in the CIZSe core, the PL emission can be tuned from the visible (593 nm) to the NIR (733 nm). Nanocrystals prepared with the Zn
Cu molar ratio from 5 to 0 in the CIZSe core, the PL emission can be tuned from the visible (593 nm) to the NIR (733 nm). Nanocrystals prepared with the Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu ratio of 0.5 exhibit the highest PL QY (54%) and the longest PL decay lifetime (515 ns). These nanocrystals exhibit a small diameter (2.2 ± 0.1 nm), indicating that the synthesis method developed allows a good size control. The potential of CIZSe/ZnS QDs as absorbers for photovoltaic cells was also investigated.
Cu ratio of 0.5 exhibit the highest PL QY (54%) and the longest PL decay lifetime (515 ns). These nanocrystals exhibit a small diameter (2.2 ± 0.1 nm), indicating that the synthesis method developed allows a good size control. The potential of CIZSe/ZnS QDs as absorbers for photovoltaic cells was also investigated.
Results and discussion
Synthesis and optical properties of CIZSe/ZnS QDs
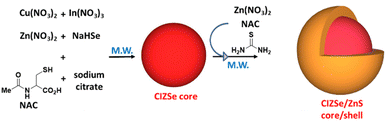
CIZSe core nanocrystals were prepared via a colloidal synthetic method under microwave (MW) irradiation using Cu(NO3)2, In(NO3)3 and Zn(NO3)2 as metal precursors (Scheme 1). Note that Cu2+ cations will be reduced in situ to Cu+ by Se2− anions. To regulate the reactivities of Cu+, In3+ and Zn2+ cations, a soft organic base (an organic thiol) was associated with the soft Lewis acid Cu+ while the hard Lewis base sodium citrate was used to bind with the hard Lewis acid In3+. With an intermediate hardness between those of In3+ and Cu+, Zn2+ ions can bind to both sodium citrate and thiol. To tune the optical absorption and the PL emission relative to conventional CISe QDs, Zn2+ ions were doped in the CISe core and the Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu elemental ratio was varied while maintaining the amount of In.36,40 In preliminary experiments not described in detail here, we varied the time and power of microwave irradiation, the sulfur ligand associated to sodium citrate and the pH of the reaction medium to optimize the PL intensity of CIZSe/ZnS QDs. These trials showed that two pulses of 180 W for 2.5 min for the CIZSe core and one of 180 W for 2 min for the shell deposition allow to obtain CIZSe/ZnS QDs with the strongest PL intensity. Among the studied thiol ligands (N-acetylcysteine, glutathione, 3-mercaptopropionic acid, 2-mercaptopropionic acid, mercaptosuccinic acid, dimercaptosuccinic acid, and thioglycolic acid), the best results were obtained using NAC (Fig. S1†). Finally, an adjustment of the pH of the reaction mixture to 6 before microwave heating allows to obtain the highest PL intensity (Fig. S2†).
Cu elemental ratio was varied while maintaining the amount of In.36,40 In preliminary experiments not described in detail here, we varied the time and power of microwave irradiation, the sulfur ligand associated to sodium citrate and the pH of the reaction medium to optimize the PL intensity of CIZSe/ZnS QDs. These trials showed that two pulses of 180 W for 2.5 min for the CIZSe core and one of 180 W for 2 min for the shell deposition allow to obtain CIZSe/ZnS QDs with the strongest PL intensity. Among the studied thiol ligands (N-acetylcysteine, glutathione, 3-mercaptopropionic acid, 2-mercaptopropionic acid, mercaptosuccinic acid, dimercaptosuccinic acid, and thioglycolic acid), the best results were obtained using NAC (Fig. S1†). Finally, an adjustment of the pH of the reaction mixture to 6 before microwave heating allows to obtain the highest PL intensity (Fig. S2†).
In a typical CIZSe/ZnS QDs synthesis, the metal precursors (Cu(NO3)2, In(NO3)3 and Zn(NO3)2) and the ligands (NAC and sodium citrate) were dissolved in water and the pH adjusted to 6. Next, freshly prepared NaHSe was quickly injected under stirring and the mixture transferred into a MW oven for 5 min. After the core growth, a ZnS shell was deposited on the surface of CIZSe QDs by adding Zn(NO3)2, thiourea and NAC and further heating under MW irradiation for 2 min (Scheme 1).
The presence of Cu in the core is essential to observe a PL signal. Core CIZSe QDs exhibit a low PL intensity due to high density of surface and intrinsic defects like In substituted Cu (InCu), which cause non-radiative recombination of the photo-excited carrier (see Fig. S3† for QDs prepared with a Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu ratio of 0.5). The presence of the InCu-related absorption can be observed at ca. 1200 nm on UV-visible-near infrared diffuse reflectance spectra (Fig. S3†).39 The PL QYs of CIZSe QDs are lower than 2%. However, after ZnS shelling, the PL QYs markedly increase, reaching values up to 54% for QDs prepared with a Zn
Cu ratio of 0.5). The presence of the InCu-related absorption can be observed at ca. 1200 nm on UV-visible-near infrared diffuse reflectance spectra (Fig. S3†).39 The PL QYs of CIZSe QDs are lower than 2%. However, after ZnS shelling, the PL QYs markedly increase, reaching values up to 54% for QDs prepared with a Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu ratio of 0.5 (vide infra), through the effective passivation of CIZSe QDs surface which allows to remove some surface-related non-radiative centers and thus improve the optical stability. A significant blue-shift from 722 to 685 nm of the PL peak position is observed after the ZnS shelling, indicating the further incorporation of Zn2+ cations into the CIZSe core, resulting in an increase in the bandgap energy (Fig. S4†).
Cu ratio of 0.5 (vide infra), through the effective passivation of CIZSe QDs surface which allows to remove some surface-related non-radiative centers and thus improve the optical stability. A significant blue-shift from 722 to 685 nm of the PL peak position is observed after the ZnS shelling, indicating the further incorporation of Zn2+ cations into the CIZSe core, resulting in an increase in the bandgap energy (Fig. S4†).
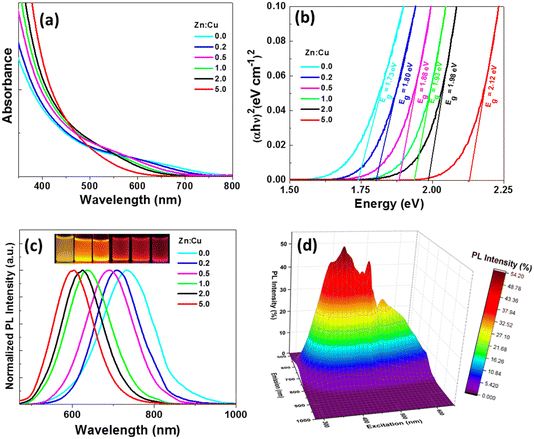
CIZSe/ZnS QDs absorb over the whole visible region and even in the near infrared for QDs containing little or no Zn (Fig. 1a). No distinctive excitonic peak can be observed for CIZSe/ZnS QDs due to their heterogeneous composition distributions that generate a wide distribution of vibrational states (intrinsic and surface trap states), which is a typical feature of I–III–VI2 QDs.41–43 A gradual red-shift of the UV-visible band edge is observed when decreasing the Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu ratio, indicating a composition-dependent variation of the energy bandgap. Fig. 1b shows the plots of (αhγ)2vs. hγ (where α is the absorption coefficient and hν is the photon energy) used to determine the energy bandgap of CIZSe/ZnS QDs. Eg values were obtained by extrapolating the linear portion of the curve to the intercept with the x axis and were found to be 1.73, 1.80, 1.88, 1.93, 1.98 and 2.12 eV for Zn
Cu ratio, indicating a composition-dependent variation of the energy bandgap. Fig. 1b shows the plots of (αhγ)2vs. hγ (where α is the absorption coefficient and hν is the photon energy) used to determine the energy bandgap of CIZSe/ZnS QDs. Eg values were obtained by extrapolating the linear portion of the curve to the intercept with the x axis and were found to be 1.73, 1.80, 1.88, 1.93, 1.98 and 2.12 eV for Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu ratios of 0, 0.2, 0.5, 1.0, 2.0 and 5.0, respectively.
Cu ratios of 0, 0.2, 0.5, 1.0, 2.0 and 5.0, respectively.
As seen in Fig. 1c, the PL emission can be tuned from 593 to 733 nm when increasing the Cu content, is broad (full-width at half-maximum, fwhm, of ca. 125 nm) and shows a large Stokes shift. The broad PL emissions are associated to intragap surface states and intrinsic defect states involving donor–acceptor pairs (DAPs).41–43 The sample prepared with a Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu ratio of 0.5 has the highest PL QY (54%) (Table 1). The PL QYs of other CIZSe/ZnS are between 21 and 33%. The excitation wavelength was tuned from 275 to 650 nm and the PL emission was found to vary as shown in the excitation-emission 3D map (Fig. 1d). The excitation-dependent PL likely originates from the different relaxations pathways previously mentioned. The highest PL emission intensity is observed at 644 nm when CIZSe/ZnS QDs are excited at 396 nm.
Cu ratio of 0.5 has the highest PL QY (54%) (Table 1). The PL QYs of other CIZSe/ZnS are between 21 and 33%. The excitation wavelength was tuned from 275 to 650 nm and the PL emission was found to vary as shown in the excitation-emission 3D map (Fig. 1d). The excitation-dependent PL likely originates from the different relaxations pathways previously mentioned. The highest PL emission intensity is observed at 644 nm when CIZSe/ZnS QDs are excited at 396 nm.
Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu ratio Cu ratio |
PL QY (%) | A 1 | τ 1 (ns) | A 2 | τ 2 (ns) | A 3 | τ 3 (ns) | τ av (ns) |
|---|---|---|---|---|---|---|---|---|
| 0 | 21 | 0.128 | 210 | 0.0059 | 674 | 0.0004 | 7080 | 252 |
| 0.2 | 31 | 0.169 | 229 | 0.0086 | 801 | 0.0013 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
332 |
| 0.5 | 54 | 0.116 | 268 | 0.0007 | 1120 | 0.0027 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
515 |
| 1 | 33 | 0.072 | 277 | 0.0024 | 1210 | 0.0010 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
455 |
| 2 | 24 | 0.201 | 225 | 0.0079 | 899 | 0.0012 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
310 |
| 5 | 24 | 0.185 | 271 | 0.0217 | 948 | 0.0022 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
442 |
The PL emission wavelength can also be regulated through changing the precursor molar ratio of Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) In or In
In or In![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Se in the reaction. When increasing the Cu
Se in the reaction. When increasing the Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) In molar ratio, the PL emission of CIZSe/ZnS QDs was found to shift to long-wavelength region due to the bandgap narrowing (Fig. S5†).44 With the increase of the Se content in CIZSe/ZnS nanocrystals, the PL emission shifts to shorter wavelengths, indicating an increase of the bandgap (Fig. S6†). Besides, all CIZSe and CIZSe/ZnS QDs show a broad absorption from 250 to 650 nm.
In molar ratio, the PL emission of CIZSe/ZnS QDs was found to shift to long-wavelength region due to the bandgap narrowing (Fig. S5†).44 With the increase of the Se content in CIZSe/ZnS nanocrystals, the PL emission shifts to shorter wavelengths, indicating an increase of the bandgap (Fig. S6†). Besides, all CIZSe and CIZSe/ZnS QDs show a broad absorption from 250 to 650 nm.
The photostability of a colloidal dispersion of CIZSe/ZnS QDs (Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu = 0.5) was also compared to that of Rhodamine B (RhB) under continuous irradiation of a Hg/Xe lamp (light irradiance of 50 mW cm−2), at 20 °C and under ambient conditions (Fig. S7†). A decrease in PL intensity of CIZSe/ZnS QDs (Zn
Cu = 0.5) was also compared to that of Rhodamine B (RhB) under continuous irradiation of a Hg/Xe lamp (light irradiance of 50 mW cm−2), at 20 °C and under ambient conditions (Fig. S7†). A decrease in PL intensity of CIZSe/ZnS QDs (Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu = 0.5) of about 17% is observed after 90 min of irradiation before reaching a quasi-plateau after 240 min irradiation. This decrease likely originates from a photo-oxidation of QDs during the irradiation. Noteworthy is also that the fwhm of the PL signal is almost constant and that no additional PL signals related to defects could be observed. For RhB, the decrease in PL intensity is faster and regular throughout the irradiation and only 40% of the initial intensity is maintained after 240 min irradiation, indicating that CIZSe/ZnS QDs are more photostable than a conventional organic dye like RhB.
Cu = 0.5) of about 17% is observed after 90 min of irradiation before reaching a quasi-plateau after 240 min irradiation. This decrease likely originates from a photo-oxidation of QDs during the irradiation. Noteworthy is also that the fwhm of the PL signal is almost constant and that no additional PL signals related to defects could be observed. For RhB, the decrease in PL intensity is faster and regular throughout the irradiation and only 40% of the initial intensity is maintained after 240 min irradiation, indicating that CIZSe/ZnS QDs are more photostable than a conventional organic dye like RhB.
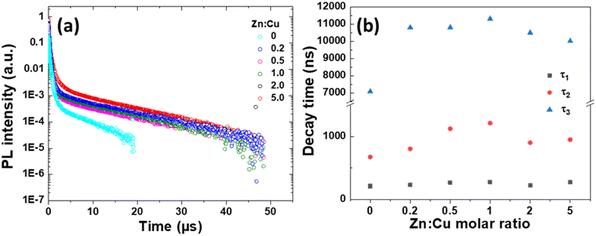
The PL emission mechanism of CIZSe/ZnS QDs was investigated by measuring their PL lifetime (Fig. 2a). The normalized PL decay curves were fitted by a tri-exponential function:
I(t) = A1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/τ1) + A2 exp(−t/τ1) + A2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/τ2) + A3 exp(−t/τ2) + A3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/τ3) exp(−t/τ3) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu molar ratio is shown in Fig. 2b. As can be seen, the short PL lifetime τ1 is only weakly influenced by the Zn content of the nanocrystals and is likely related to non-radiative processes as previously described.32,41–43 The highest PL QYs are measured for samples prepared with Zn
Cu molar ratio is shown in Fig. 2b. As can be seen, the short PL lifetime τ1 is only weakly influenced by the Zn content of the nanocrystals and is likely related to non-radiative processes as previously described.32,41–43 The highest PL QYs are measured for samples prepared with Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu ratios of 0.2, 0.5 and 1, which correspond to CIZSe/ZnS QDs exhibiting the highest medium (τ2) and longest (τ3) decays. These decays correspond to radiative processes dependent on surface defect states and DAPs. Regarding the Zn
Cu ratios of 0.2, 0.5 and 1, which correspond to CIZSe/ZnS QDs exhibiting the highest medium (τ2) and longest (τ3) decays. These decays correspond to radiative processes dependent on surface defect states and DAPs. Regarding the Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu ratio, τ2, and to a lesser extent τ3, increase with the Zn
Cu ratio, τ2, and to a lesser extent τ3, increase with the Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu ratio from 0 to 1, indicating a promotion of energy transfer processes. As a result, the average PL lifetimes τav increases up to 515 ns for CIZSe/ZnS QDs prepared with a Zn
Cu ratio from 0 to 1, indicating a promotion of energy transfer processes. As a result, the average PL lifetimes τav increases up to 515 ns for CIZSe/ZnS QDs prepared with a Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu ratio of 0.5. By further increasing the Zn
Cu ratio of 0.5. By further increasing the Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu ratio (2 or 5), a weak decrease of τ2 and τ3 values is observed. The high PL QY determined for the sample prepared with a Zn
Cu ratio (2 or 5), a weak decrease of τ2 and τ3 values is observed. The high PL QY determined for the sample prepared with a Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu ratio of 0.5 likely results from the low contribution of non-radiative processes combined to the large weight of radiative processes involved in the PL emission. The average PL lifetimes determined for CIZSe/ZnS QDs are significantly higher than those of CISe/ZnS QDs prepared in aqueous phase (ca. 150–250 ns)35,36,39 and are similar to those of CISe/ZnS QDs prepared in organic phase,18 suggesting that the synthesis method developed in this work (incorporation of Zn2+ ions in the CISe core before depositing the ZnS shell and microwave heating) allows to restrain nonradiative processes.
Cu ratio of 0.5 likely results from the low contribution of non-radiative processes combined to the large weight of radiative processes involved in the PL emission. The average PL lifetimes determined for CIZSe/ZnS QDs are significantly higher than those of CISe/ZnS QDs prepared in aqueous phase (ca. 150–250 ns)35,36,39 and are similar to those of CISe/ZnS QDs prepared in organic phase,18 suggesting that the synthesis method developed in this work (incorporation of Zn2+ ions in the CISe core before depositing the ZnS shell and microwave heating) allows to restrain nonradiative processes.
Structural characterizations
The crystalline structure of core/shell CIZSe/ZnS QDs was first assessed by XRD. The XRD patterns show that CIZSe/ZnS QDs exhibit the chalcopyrite tetragonal structure as indicated by (112), (204)/(220) and (312)/(116) reflections (JCPDS No. 01-087-2265), which agrees well with literature.45 The broadness of XRD signals also confirms the nanocrystalline nature of the samples. With the increasing Zn2+ content, the XRD signals slightly shift to higher angles due to the lattice contraction originating from the incorporation of higher amounts of Zn2+ ions with a smaller ion radius (0.6 nm) than that of In3+ (0.62 nm). CIZSe/ZnS QDs prepared with Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu molar ratios of 0, 0.2, 0.5 and 1.0 show a pure tetragonal phase. However, cubic In(OH)3 (JCPDS No. 04-008-9898) was detected for QDs prepared with the highest Zn
Cu molar ratios of 0, 0.2, 0.5 and 1.0 show a pure tetragonal phase. However, cubic In(OH)3 (JCPDS No. 04-008-9898) was detected for QDs prepared with the highest Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu ratios (2.0 and 5.0). The formation of In(OH)3 likely originates from the In3+ → Zn2+ cation exchange either during the core growth and/or the ZnS shelling followed by the reaction of In3+ cations with hydroxide anions present in the reaction medium. The presence of In(OH)3 was confirmed by TEM (Fig. S8a†) and the associated SAED pattern (Fig. S8b†) which shows the co-existence of CIZSe/ZnS and In(OH)3 nanocrystals in samples prepared with Zn
Cu ratios (2.0 and 5.0). The formation of In(OH)3 likely originates from the In3+ → Zn2+ cation exchange either during the core growth and/or the ZnS shelling followed by the reaction of In3+ cations with hydroxide anions present in the reaction medium. The presence of In(OH)3 was confirmed by TEM (Fig. S8a†) and the associated SAED pattern (Fig. S8b†) which shows the co-existence of CIZSe/ZnS and In(OH)3 nanocrystals in samples prepared with Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu ratios of 2.0 and 5.0.
Cu ratios of 2.0 and 5.0.
Dynamic light scattering (DLS) measurements conducted on NAC-capped CIZSe/ZnS QDs prepared with a Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu ratio of 0.5 and selected as representative are shown in Fig. 3b. The volume-weighted size distribution shows that the sample is composed of very small QDs with an average size of ca. 2 nm and that the colloidal dispersion is almost monodisperse (PDI = 0.2). The intensity-weighted distribution seems to indicate that the sample is more heterogeneous but this analysis gives a much greater weight to nanoparticles of larger size which only represent a very small fraction of the sample.
Cu ratio of 0.5 and selected as representative are shown in Fig. 3b. The volume-weighted size distribution shows that the sample is composed of very small QDs with an average size of ca. 2 nm and that the colloidal dispersion is almost monodisperse (PDI = 0.2). The intensity-weighted distribution seems to indicate that the sample is more heterogeneous but this analysis gives a much greater weight to nanoparticles of larger size which only represent a very small fraction of the sample.
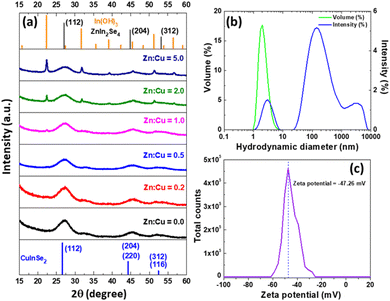 | ||
Fig. 3 (a) XRD patterns of CIZSe/ZnS QDs. (b) Hydrodynamic diameter and (c) ζ potential of CIZSe/ZnS QDs prepared with a Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu ratio of 0.5. Cu ratio of 0.5. | ||
The carboxylate group of NAC provides to CIZSe/ZnS QDs a zeta (ζ) potential of −47 mV at pH = 7 (Fig. 3c). This highly negative ζ potential will not only influence the colloidal stability of the dots in aqueous media but also their optical properties such as recombination dynamics.46
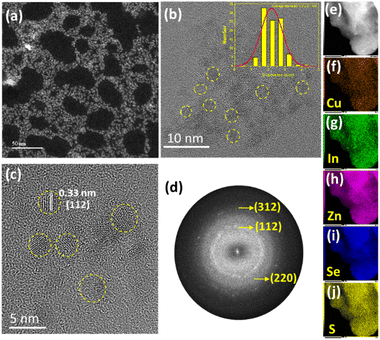
The diameter of CIZSe/ZnS QDs was determined using HAADF-STEM and TEM (Fig. 4a and b). Quasi-spherical nanoparticles with an average diameter of 2.2 ± 0.1 nm can be observed (inset of Fig. 4b). The lattice spacing of 0.33 nm measured on the HR-TEM image can be indexed to the (112) plane of CuInSe2. The HR-TEM image and the associated selected area electron diffraction (SAED) pattern confirm the high crystallinity of CIZSe/ZnS QDs (Fig. 4c and d). No interface can be seen between the CIZSe core and the ZnS shell likely due to the thin layer of ZnS covering the CIZSe core and/or the alloying of ZnS with the core. The EDS analysis and the associated elemental confirm the presence of Cu, In, Zn, S and Se elements as well as their homogenous distribution in CIZSe/ZnS QDs (Fig. 4e–j and S9†).
The presence of NAC at the surface of QDs was confirmed by FT-IR (Fig. S10†). The signals observed at 3374, 2574 and 1718 cm−1 for NAC correspond to N–H, S–H and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretchings, respectively. Compared to NAC, all QDs exhibit a broad signal at ca. 3300 cm−1 corresponding to the O–H stretching of the carboxylic acid group and/or to chemisorbed water molecules. The peaks with the highest intensity at ca. 1585 and 1380 cm−1 can be assigned to the asymmetric and symmetric stretching modes of the carboxylate function, respectively. This high density of carboxylate functions at the surface of QDs allows QDs to be dispersible in aqueous media and of high colloidal stability, which agrees with ζ potential measurements. Fig. S11† plots the thermogravimetric analyses from room temperature to 800 °C of CIZSe/ZnS QDs. A first weight loss of ca. 7% is observed between 30 and 150 °C and likely corresponds to the removal of chemisorbed water molecules and of neutral ligands (L-ligands) from the QDs surface. A second weight loss of ca. 20% originating from the decomposition of organic ligands ionically bound (X-ligands) to the QDs surface occurs between 250 and 450 °C.47 These results show that ligands participate in ca. 25% of the mass of QDs.
O stretchings, respectively. Compared to NAC, all QDs exhibit a broad signal at ca. 3300 cm−1 corresponding to the O–H stretching of the carboxylic acid group and/or to chemisorbed water molecules. The peaks with the highest intensity at ca. 1585 and 1380 cm−1 can be assigned to the asymmetric and symmetric stretching modes of the carboxylate function, respectively. This high density of carboxylate functions at the surface of QDs allows QDs to be dispersible in aqueous media and of high colloidal stability, which agrees with ζ potential measurements. Fig. S11† plots the thermogravimetric analyses from room temperature to 800 °C of CIZSe/ZnS QDs. A first weight loss of ca. 7% is observed between 30 and 150 °C and likely corresponds to the removal of chemisorbed water molecules and of neutral ligands (L-ligands) from the QDs surface. A second weight loss of ca. 20% originating from the decomposition of organic ligands ionically bound (X-ligands) to the QDs surface occurs between 250 and 450 °C.47 These results show that ligands participate in ca. 25% of the mass of QDs.
XPS was further used to determine the chemical composition as well as the bonding state of elements composing CIZSe/ZnS QDs. The XPS survey spectrum of CIZSe/ZnS QDs prepared with a Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu ratio of 0.5 indicates that Cu, In, Zn, Se, S, C, N, O and Na are present and that no other elements are detected, indicating the purity of the nanocrystals (Fig. S12†). C, N, O and Na elements correspond to the capping ligands (NAC and sodium citrate) of CIZSe/ZnS QDs. The HR XPS spectrum of Cu 2p3/2 shows a single signal at 932.08 eV corresponding to Cu in the +1 oxidation state in CuInSe2,30,32 confirming that the Cu2+ precursor is reduced into Cu+ during the synthesis (Fig. S13a†). The In 3d5/2 peaks at 444.34 and 451.86 eV are indicative of In(+3) (Fig. S13b†) while the Zn 2p3/2 signal at 1021.40 eV corresponds to Zn(+2) (Fig. S13c†).30,32 The signals of Se 3d5/2 appear at 53.61 and 54.46 eV and show the presence of Se(−2) (Fig. S13d and e†). The presence of two signals suggest that Se(−2) is involved in different bonds with In3+, Cu+ and Zn2+. The S 2p core level spectrum shows two signals at 161.70 and 162.90 eV, corresponding to Cu–S and In–S bonds, respectively (Fig. S13d†).48 The quantification of XPS signals indicates that the Cu/In/Zn ratio is of ca. 1/7.9/24.2 while the theoretical ratio is of 1/10/85. ICP-OES analyses carried out on CIZSe/ZnS QDs after purification show that the percentages in In and especially in Zn are lower than theoretical values (Table S1†). This deviation suggests that the Cu precursor reacts faster than In and Zn ones with Se2− in the reaction medium.
Cu ratio of 0.5 indicates that Cu, In, Zn, Se, S, C, N, O and Na are present and that no other elements are detected, indicating the purity of the nanocrystals (Fig. S12†). C, N, O and Na elements correspond to the capping ligands (NAC and sodium citrate) of CIZSe/ZnS QDs. The HR XPS spectrum of Cu 2p3/2 shows a single signal at 932.08 eV corresponding to Cu in the +1 oxidation state in CuInSe2,30,32 confirming that the Cu2+ precursor is reduced into Cu+ during the synthesis (Fig. S13a†). The In 3d5/2 peaks at 444.34 and 451.86 eV are indicative of In(+3) (Fig. S13b†) while the Zn 2p3/2 signal at 1021.40 eV corresponds to Zn(+2) (Fig. S13c†).30,32 The signals of Se 3d5/2 appear at 53.61 and 54.46 eV and show the presence of Se(−2) (Fig. S13d and e†). The presence of two signals suggest that Se(−2) is involved in different bonds with In3+, Cu+ and Zn2+. The S 2p core level spectrum shows two signals at 161.70 and 162.90 eV, corresponding to Cu–S and In–S bonds, respectively (Fig. S13d†).48 The quantification of XPS signals indicates that the Cu/In/Zn ratio is of ca. 1/7.9/24.2 while the theoretical ratio is of 1/10/85. ICP-OES analyses carried out on CIZSe/ZnS QDs after purification show that the percentages in In and especially in Zn are lower than theoretical values (Table S1†). This deviation suggests that the Cu precursor reacts faster than In and Zn ones with Se2− in the reaction medium.
Finally, XRD, TEM, FT-IR and XPS analyses further confirm that CIZSe/ZnS QDs were successfully prepared by the microwave-assisted hydrothermal method developed in this work.
Photoelectrochemical properties
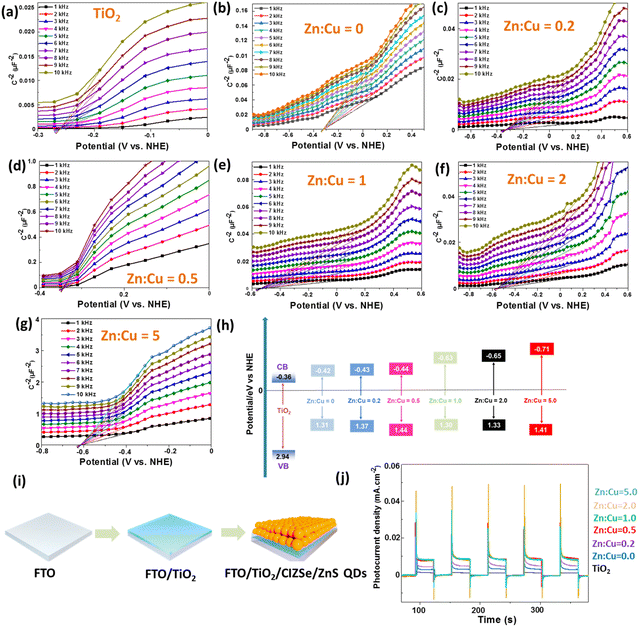
In a first step, the flat band potentials of TiO2 and CIZSe/ZnS QDs were determined using Mott–Schottky plots recorded after QDs deposition on FTO by dip-coating (Fig. 5a–g). The flat band potential VFB was obtained by extrapolation of the linear part of the Mott–Schottky plots. For CIZSe/ZnS QDs prepared with Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu molar ratios of 0, 0.2, 0.5, 1, 2 and 5, VFB values were determined to be of −0.32, −0.33, −0.34, −0.53, −0.55 and −0.61 V vs. NHE, respectively, while the VFB value for TiO2 is −0.26 V. The positive slopes of Mott–Schottky plots indicate that all CIZSe/ZnS QDs are n-type semiconductors. For this type of semiconductor, the conduction band (CB) edge is located at a slightly lower potential (ca. 0.1 V) with respect to its VFB.49 The position of the valence band (VB) of these QDs was determined using the energy bandgap estimated using the Tauc plots (Fig. 1b) and the results are described in Fig. 5h. The position of the valence band is only slightly impacted by the Zn
Cu molar ratios of 0, 0.2, 0.5, 1, 2 and 5, VFB values were determined to be of −0.32, −0.33, −0.34, −0.53, −0.55 and −0.61 V vs. NHE, respectively, while the VFB value for TiO2 is −0.26 V. The positive slopes of Mott–Schottky plots indicate that all CIZSe/ZnS QDs are n-type semiconductors. For this type of semiconductor, the conduction band (CB) edge is located at a slightly lower potential (ca. 0.1 V) with respect to its VFB.49 The position of the valence band (VB) of these QDs was determined using the energy bandgap estimated using the Tauc plots (Fig. 1b) and the results are described in Fig. 5h. The position of the valence band is only slightly impacted by the Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu ratio and varies between +1.30 to +1.44 V. On the other hand, when the Zn
Cu ratio and varies between +1.30 to +1.44 V. On the other hand, when the Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu ratio increases from 0 to 5, the position of the conduction band is moved towards increasingly negative values (from −0.42 to −0.71 eV). This phenomenon is directly related to the increase in bandgap energy with the increase in Zn content in the CIZSe cores.
Cu ratio increases from 0 to 5, the position of the conduction band is moved towards increasingly negative values (from −0.42 to −0.71 eV). This phenomenon is directly related to the increase in bandgap energy with the increase in Zn content in the CIZSe cores.
In QDSSCs, QDs anchored on the TiO2 photoanode act as light absorbers. Upon photoexcitation, electrons are injected from the QD excited state into the conduction band of the TiO2 layer that provides a fast channel for these electrons to transfer to the external circuit. Thus, engineering photoanodes of high stability and with high electrical conductivity is of high importance for enhancing the photoelectric conversion efficiency.50 As the electron injection rate from QDs into the conduction band of TiO2 plays a key role on photovoltaic performance, the interaction of CIZSe/ZnS QDs (Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu = 5.0) with a TiO2 colloid was investigated by PL.51 As can be seen on Fig. S14a,† the addition of 50 μL of a TiO2 colloid to 3 mL of an aqueous dispersion of CIZSe/ZnS QDs leads to an immediate decrease of a factor two of the PL intensity, indicating that QDs strongly bind to TiO2 nanoparticles and that photo-excited electrons in QDs efficiently transfer to TiO2. The decrease in PL intensity is not linear and is less pronounced after adding a larger amount of the TiO2 colloid (up to 550 μL) (Fig. S14b†).
Cu = 5.0) with a TiO2 colloid was investigated by PL.51 As can be seen on Fig. S14a,† the addition of 50 μL of a TiO2 colloid to 3 mL of an aqueous dispersion of CIZSe/ZnS QDs leads to an immediate decrease of a factor two of the PL intensity, indicating that QDs strongly bind to TiO2 nanoparticles and that photo-excited electrons in QDs efficiently transfer to TiO2. The decrease in PL intensity is not linear and is less pronounced after adding a larger amount of the TiO2 colloid (up to 550 μL) (Fig. S14b†).
As previously shown, a suitable type II band alignment is observed for all CIZSe/ZnS QDs and TiO2, which should favor the charge transfer from QDs to TiO2.52 This has been demonstrated by deposition of a TiO2 layer with a thickness of ca. 125 nm onto FTO by magnetron sputtering followed by CIZSe/ZnS QDs via dip coating (Fig. 5i). The transient photocurrent responses of the TiO2/CIZSe/ZnS samples prepared with Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu ratios of 5.0, 2.0, 1.0 and 0.5 are about six times higher than that of TiO2, which confirms the improved carriers separation and transfer (Fig. 5j).
Cu ratios of 5.0, 2.0, 1.0 and 0.5 are about six times higher than that of TiO2, which confirms the improved carriers separation and transfer (Fig. 5j).
Conclusions
In summary, a fast and green synthetic route to prepare high quality water dispersible CIZSe/ZnS QDs was developed. CIZSe/ZnS QDs of small size (ca. 2.2 nm) and with excellent optical properties (PL QY up to 54% and PL lifetime up to 515 ns) were produced. The use of CIZSe/ZnS QDs as light absorbers for QDSSCs was explored. An adequate band alignment of QDs relative to TiO2 was demonstrated for all QDs prepared indicating that photogenerated electrons in QDs can effectively transfer to TiO2 and increase the photocurrent of the electrode. Future work will include the improvement of the device performance.Experimental section
Chemicals
In(NO3)3 hydrate (99.99%, Thermo Fisher Scientific), Cu(NO3)2·3H2O (99%, Merck), Zn(NO3)2·6H2O (99%, Thermo Fisher Scientific), trisodium citrate dihydrate (99%, Thermo Fisher Scientific), N-acetyl-L-cysteine (>98%, Thermo Fisher Scientific), SeO2 (99.999%, Thermo Fisher Scientific), NaBH4 (99%, Merck), thiourea CH4N2S (99+%, Thermo Fisher Scientific) and NaOH (VWR Chemicals) were used as received. Deionized Milli-Q water was used as solvent.Synthesis of Cu–In–Zn–Se (CIZSe) core QDs
Typically, for the synthesis of CIZSe QDs with Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu molar ratio of 0.5 as an example, In(NO3)3·5H2O (0.20 mmol), Cu(NO3)2·3H2O (0.04 mmol), Zn(NO3)2·6H2O (0.02 mmol), trisodium citrate dihydrate (0.34 mmol), and N-acetyl-L-cysteine (0.243 mmol) were dissolved into 30 mL of Milli-Q water in a 50 mL three-necked flask. The pH of the resultant mixture was adjusted to 6 by adding dropwise a 2 M NaOH solution under stirring. Separately, the selenium precursor was prepared at room temperature by adding SeO2 (1 mmol) and NaBH4 (2 mmol) into 5 mL of Milli-Q water, which was then reacted for 10 min under strong magnetic stirring and nitrogen protection to form a transparent NaHSe precursor solution. Then, 1.6 mL of the NaHSe solution was added into the three necked flask containing optically clear solution of nitrate salts and ligands under intermediate magnetic stirring and nitrogen purging, which turned reddish-brown instantaneously. Next, the reddish-brown mixture was kept under constant stirring and nitrogen protection for 15 minutes before being transferred to a Teflon-sealed microwave reactor. The microwave reactor was heated in a microwave oven at 180 W for two brief pulses of 2.5 min each with an interval of 1 min in between to form Cu–In–Zn–Se core QDs. Aliquots were taken for the core Zn–Cu–In–Se core QDs.
Cu molar ratio of 0.5 as an example, In(NO3)3·5H2O (0.20 mmol), Cu(NO3)2·3H2O (0.04 mmol), Zn(NO3)2·6H2O (0.02 mmol), trisodium citrate dihydrate (0.34 mmol), and N-acetyl-L-cysteine (0.243 mmol) were dissolved into 30 mL of Milli-Q water in a 50 mL three-necked flask. The pH of the resultant mixture was adjusted to 6 by adding dropwise a 2 M NaOH solution under stirring. Separately, the selenium precursor was prepared at room temperature by adding SeO2 (1 mmol) and NaBH4 (2 mmol) into 5 mL of Milli-Q water, which was then reacted for 10 min under strong magnetic stirring and nitrogen protection to form a transparent NaHSe precursor solution. Then, 1.6 mL of the NaHSe solution was added into the three necked flask containing optically clear solution of nitrate salts and ligands under intermediate magnetic stirring and nitrogen purging, which turned reddish-brown instantaneously. Next, the reddish-brown mixture was kept under constant stirring and nitrogen protection for 15 minutes before being transferred to a Teflon-sealed microwave reactor. The microwave reactor was heated in a microwave oven at 180 W for two brief pulses of 2.5 min each with an interval of 1 min in between to form Cu–In–Zn–Se core QDs. Aliquots were taken for the core Zn–Cu–In–Se core QDs.
Synthesis of core/shell CIZSe/ZnS QDs
After the core synthesis, the shell solution consisting of precursors, Zn(NO3)2·6H2O (1.68 mmol), N-acetyl-L-cysteine (2.58 mmol) and thiourea CH4N2S (4.38 mmol) in 10 mL of Milli-Q water at pH 6 was simultaneously added dropwise to the microwave reactor which was further subjected to microwave heating at 180 W for 2 min to form core/shell Zn–Cu–In–Se/ZnS QDs. Aliquots were taken and the resulting QDs were subjected to dialysis to purify and remove unwanted impurities. Followed by precipitation of synthesized QDs with propan-2-ol, centrifugation for 10 min at 8000 rpm, washing for three cycles and keeping the resultant QDs to dry under normal conditions. For the synthesis of Zn–Cu–In–Se core QDs with varying Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu molar ratios (from 0.0 to 5.0), the feed molar ratios of Zn and Cu precursors in core were varied with the total amount of Zn and Cu unaltered while keeping all the other reaction conditions the same.
Cu molar ratios (from 0.0 to 5.0), the feed molar ratios of Zn and Cu precursors in core were varied with the total amount of Zn and Cu unaltered while keeping all the other reaction conditions the same.
Characterization
The morphology and structure of CIZSe/ZnS QDs were determined by transmission electron microscopy (TEM) on a JEOL ARM 200F cold FEG TEM/STEM equipped with a GIF quantum ER model 965, operating at 200 kV with a pixel resolution of 0.12 nm. High-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) and two-dimensional elemental mapping using energy-dispersive X-ray spectroscopy (EDX) were employed to determine the chemical composition of QDs.Powder X-ray diffraction (XRD) patterns were obtained using Cu Kα radiation (λ = 0.15418 nm) on a Panalytical X'Pert Pro MPD diffractometer instrument.
X-ray photoelectron spectra (XPS) were recorded on a Gammadata Scienta SES 200-2 spectrometer. Inductively Coupled Plasma-Optical Emission Spectrometer (ICP-OES) measurements were conducted on a Varian 720-ES equipment.
Thermogravimetric analysis (TGA) was conducted under air from 20 to 800 °C at a heating rate of 10 °C min−1 using a TGA/DSC1 STAR equipment (Mettler-Toledo).
Dynamic light scattering (DLS) experiments and zeta potential measurements were carried out on a Zetasizer Nano ZS at 20 °C in water (green laser beam 532 nm) with a disposable capillary cell (DTS1070) (Malvern-Panalytical, UK). All measurements were performed in triplicate.
The Fourier transform infrared (FT-IR) spectra were recorded on a Bruker ALPHA spectrometer. The UV-visible absorption spectra were recorded using a Thermo Scientific Evolution 220 spectrometer. UV-visible-near infrared diffuse reflectance spectra were recorded a Shimadzu 2600–2700 spectrometer. The PL measurements were conducted using a SAFAS Xenius spectrophotometer equipped with a Xenon lamp as the excitation source. PL spectra were spectrally corrected and PL QYs were determined relative to Rhodamine 6G in ethanol (PL QY = 94%).
For the time resolved photoluminescence (TR-PL) experiments, the QDs were pumped by the 355 nm line of a frequency-tripled YAG (yttrium aluminium garnet):Nd laser. The laser pulse frequency, energy and duration were typically equal to 10 Hz, 50 μJ and 10 ns, respectively. The PL signal was analysed by a monochromator equipped with a 600 grooves per mm grating and by a photomultiplier tube cooled at 190 K. The rise time of the detector is equal to around 3 ns.
Mott–Schottky plots at 1–10 kHz frequencies were determined from EIS measurements in the dark, using 0.5 M Na2SO4 (pH = 7). The Mott–Schottky measurements are based on eqn (1):
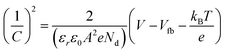 | (1) |
| VNHE = VAg/AgCl + 0.197 | (2) |
For Mott–Schottky measurements, the samples were prepared as follows. The 125 nm thick TiO2 layer was deposited onto FTO substrates (fluorine doped tin oxide coated glass, TEC 7 Ω sq−1) by DC magnetron sputtering of a pure Ti target (80 W for 15 min 48 s) and next annealed for 2 h at 500 °C. CIZSe/ZnS QDs were deposited onto FTO by dip-coating (20 cycles) from the purified stock solution. The deposited sample dried for 12 h under ambient conditions and then heated for 5 h at 300 °C under nitrogen.
Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
Thomas Gries and Raphaël Schneider supervised this study. Shubham Shishodia performed the synthesis and some characterization of QDs and photoelectro-measurements. Hervé Rinnert, Lavinia Balan, Jordane Jasniewski, Stéphanie Bruyère, and Ghouti Medjahdi performed the characterization of QDs. Shubham Shishodia, Thomas Gries, and Raphaël Schneider wrote the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the European Union's Horizon 2020 Research and Innovation Program under Grant Agreement 963530 and from the Agence Nationale de la Recherche ANR (project QDSOC).Notes and references
- D. Bera, L. Qian, T. K. Tseng and P. H. Holloway, Materials, 2010, 3, 2260–2345 CrossRef CAS
.
- M. Liu, N. Yazdani, M. Yarema, M. Jansen, V. Wood and E. H. Sargent, Nat. Electron., 2021, 4, 548–558 CrossRef
.
- X. Huang and M. Tang, J. Appl. Toxicol., 2021, 41, 342–361 CrossRef CAS PubMed
.
- O. Yarema, M. Yarema and V. Wood, Chem. Mater., 2018, 30, 1446–1461 CrossRef CAS
.
- T. Torimoto, T. Kameyama, T. Uematsu and S. Kuwabata, J. Photochem. Photobiol., C, 2023, 54, 100569 CrossRef CAS
.
- L. Yang, S. Zhang, B. Xu, J. Jiang, B. Cai, X. Lv, Y. Zou, Z. Fan, H. Yang and H. Zeng, Nano Lett., 2023, 23, 2443–2453 CrossRef CAS PubMed
.
- S. Shishodia, B. Chouchene, T. Gries and R. Schneider, Nanomaterials, 2023, 13, 2889 CrossRef CAS PubMed
.
- L. Jin, G. S. Selopal, X. Tong, D. F. Perepichka, Z. M. Wang and F. Rosei, Adv. Mater., 2024, 2402912 CrossRef CAS
.
- L. Jin, J. Liu, X. Liu, D. Benetti, G. S. Selopal, X. Tong, E. Hamzehpoor, F. Li, D. F. Perepichka, Z. M. Wang and F. Rosei, Small Methods, 2024, 8, 2300133 CrossRef CAS
.
- M. Mrad, B. Chouchene, T. Ben Chaabane, T. Gries, G. Medjahdi, L. Balan and R. Schneider, Catalysts, 2022, 12, 1585 CrossRef CAS
.
- J. Du, R. Singh, I. Fedin, A. S. Fuhr and V. I. Klimov, Nat. Energy, 2020, 5, 409–417 CrossRef CAS
.
- L. J. Lim, X. Zhao and Z. K. Tan, Adv. Mater., 2023, 35, 2301887 CrossRef CAS
.
- Z. Guan, J. Pan, Q. Li, G. Li and J. Yang, ACS Sustainable Chem. Eng., 2019, 7, 7736–7742 CrossRef CAS
.
- C. F. Du, T. You, L. Jiang, S. Q. Yang, K. Zou, K. L. Han and W. Q. Deng, RSC Adv., 2014, 4, 33855–33860 RSC
.
- H. J. Yun, J. Lim, J. Roh, D. C. Jin Neo, M. Law and V. I. Klimov, Nat. Commun., 2020, 11, 5280 CrossRef CAS PubMed
.
- J. Yang, J. Y. Kim, J. H. Yu, T. Y. Ahn, H. Lee, T. S. Choi, Y. W. Kim, J. Joo, M. J. Ko and T. Yyeon, Phys. Chem. Chem. Phys., 2013, 15, 20517–20525 RSC
.
- F. Liu, J. Zhu, Y. Xu, L. Zhou and S. Dai, Nanoscale, 2016, 8, 10021–10025 RSC
.
- X. Tong, Y. Zhou, L. Jin, K. Basu, R. Adhikari, G. S. Selopal, X. Tong, H. Zhao, S. Sun, A. Vomiero, Z. M. Wang and F. Rosei, Nano Energy, 2017, 31, 441–449 CrossRef CAS
.
- F. E. S. Gorris, M. Deffner, S. Priyadarshi, C. Klinke, H. Weller and H. Lange, Adv. Opt. Mater., 2020, 8, 1901058 CrossRef CAS
.
- I. Mehmood, Y. Liu, K. Chen, A. H. Shah and W. Chen, RSC Adv., 2017, 7, 33106–33112 RSC
.
- S. M. Harvey, D. W. Houck, W. Liu, Y. Liu, D. J. Gosztola, B. A. Korgel, M. R. Wasielewski and R. D. Schaller, ACS Nano, 2021, 15, 19588–19599 CrossRef CAS PubMed
.
- H. Zhong, Z. Wang, E. Bovero, Z. Lu, F. C. J. M. van Veggel and G. D. Scholes, J. Phys. Chem. C, 2011, 115, 12396–12402 CrossRef CAS
.
- H. McDaniel, N. Fuke, J. M. Pietryga and V. I. Klimov, J. Phys. Chem. Lett., 2013, 4, 355–361 CrossRef CAS
.
- M. G. Panthani, C. J. Stolle, D. K. Reid, D. J. Rhee, T. B. Harvey, V. A. Akhavan, Y. Yu and B. A. Korgel, J. Phys. Chem. Lett., 2013, 4, 2030–2034 CrossRef CAS PubMed
.
- B. K. Graeser, C. J. Hages, W. C. Yang, N. J. Carter, C. K. Miskin, E. A. Stach and R. Agrawal, Chem. Mater., 2014, 26, 4060–4063 CrossRef CAS
.
- W. Li, Z. Pan and X. Zhong, J. Mater. Chem. A, 2015, 3, 1649–1655 RSC
.
- J. Du, Z. Du, J. S. Hu, Z. Pan, Q. Shen, J. Sun, D. Long, H. Dong, L. Sun, X. Zhong and L. J. Wan, J. Am. Chem. Soc., 2016, 138, 4201–4216 CrossRef CAS
.
- L. Zhang, Z. Pan, W. Wang, J. Du, Z. Ren, Q. Shen and X. Zhong, J. Mater. Chem. A, 2017, 5, 21442–21451 RSC
.
- H. Song, Y. Lin, M. Zhou, H. Rao, Z. Pan and X. Zhong, Angew. Chem., Int. Ed., 2021, 60, 6137–6144 CrossRef CAS PubMed
.
- R. Guo, J. Meng, W. Lin, A. Liu, T. Pullerits, K. Zheng and J. Tian, J. Chem. Eng., 2021, 403, 126452 CrossRef CAS
.
- S. Yamashita, M. Tanabe, T. Araki, M. Shiomi, T. Nishi and Y. Kudo, J. Phys. Chem. C, 2022, 126, 14558–14565 Search PubMed
.
- B. Luo, J. Liu, H. Guo, X. Liu, R. Song, K. Shen, Z. M. Wang, D. Jing, G. S. Selopal and F. Rosei, Nano Energy, 2021, 88, 106220 CrossRef CAS
.
- W. Lian, D. Tu, X. Weng, K. Yang, F. Li, D. Huang, H. Zhu, Z. Xie and X. Chen, Adv. Mater., 2024, 36, 2311011 CrossRef CAS
.
- M. A. Abate, K. Dehvari, J. Y. Chang and K. Waki, Dalton Trans., 2019, 48, 16115–16122 RSC
.
- Y. Jia, H. Liu, P. Cai, X. Liu, L. Wang, L. Ding, G. Xu, W. Wang, M. Jiao and X. Luo, Chem. Commun., 2021, 57, 4178–4181 RSC
.
- H. Liu, P. Cai, K. J. McHugh, C. F. Perkinson, L. Li, S. Wang, W. Wang, M. Jiao, X. Luo and L. Jing, Nano Res., 2022, 15, 8351–8359 CrossRef CAS
.
- N. Irmania, K. Dehvari and J. Y. Chang, J. Biomater. Appl., 2022, 36, 1617–1628 CrossRef CAS PubMed
.
- X. Kang, Y. Yang, L. Huang, Y. Tao, L. Wang and D. Pan, Green Chem., 2015, 17, 4482–4488 RSC
.
- S. Qu, X. Yuan, Y. Li, X. Li, X. Zhou, X. Xue, K. Zhang, J. Xu and C. Yuan, Nanoscale Adv., 2021, 3, 2334–2342 RSC
.
- P. Priyadarshini, S. Senapati, S. Bisoyi, S. Samal and R. Naik, J. Alloys Compd., 2023, 945, 169222 CrossRef CAS
.
- P. Galiyeva, H. Rinnert, L. Balan, H. Alem, G. Medjahdi, B. Uralbekov and R. Schneider, Appl. Surf. Sci., 2021, 562, 150143 CrossRef CAS
.
- P. Galiyeva, H. Alem, H. Rinnert, L. Balan, S. Blanchard, G. Medjahdi, B. Uralbekov and R. Schneider, Inorg. Chem. Front., 2019, 6, 1422–1431 RSC
.
- M. Mrad, T. Ben Chaabane, H. Rinnert, L. Balan, J. Jasniewski, G. Medjahdi and R. Schneider, Inorg. Chem., 2020, 59, 6220–6231 CrossRef CAS
.
- H. McDaniel, N. Fuke, J. M. Pietryga and V. I. Klimov, J. Phys. Chem. Lett., 2013, 4, 355–361 CrossRef CAS PubMed
.
- J. Ning, Y. Xiong, F. Huang, Z. Duan, S. V. Kershaw and A. L. Rogach, Chem. Mater., 2020, 32, 7842–7849 CrossRef CAS
.
- A. Radchanka, V. Hrybouskaya, A. Iodchik, A. W. Achtstein and M. Artemyev, J. Phys. Chem. Lett., 2022, 13, 4912–4917 CrossRef CAS PubMed
.
- B. Shakeri and R. W. Meulenberg, Langmuir, 2015, 31, 13433–13440 CrossRef CAS
.
- W. Yue, S. Han, R. Peng, W. Shen, H. Geng, F. Wu, S. Taoe and M. Wang, J. Mater. Chem., 2010, 20, 7570–7578 RSC
.
- Y. Matsumoto, M. Omae, I. Watanabe and E. Sato, J. Electrochem. Soc., 1986, 133, 711–716 CrossRef CAS
.
- Q. Li, T. Zhang, D. Cui and F. Li, Dalton Trans., 2024, 53, 7742–7750 RSC
.
- S. M. Kobosko, D. H. Jara and P. V. Kamat, ACS Appl. Mater. Interfaces, 2017, 9, 33379–33388 CrossRef CAS
.
- S. U. Rahayu, Y. R. Wang, J. B. Shi and M. W. Le, Sustain. Energy Fuels, 2024, 8, 113–124 RSC
.
Footnote |
| † Electronic supplementary information (ESI) available: UV-visible and PL emission spectra, FT-IR spectra, EDX analysis, TGA analysis, XPS spectra, ICP-OES analysis, TEM image and SAED pattern, PL quenching by TiO2. See DOI: https://doi.org/10.1039/d4na00893f |
| This journal is © The Royal Society of Chemistry 2025 |