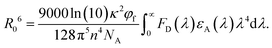Tunable color-stable hybrid white OLEDs by combining fluorescent and TADF emitters in a single emissive layer†
Upasana
Deori
 ,
Thamodharan
Viswanathan
,
Thamodharan
Viswanathan
 ,
Nisha
Yadav
,
Nisha
Yadav
 and
Pachaiyappan
Rajamalli
and
Pachaiyappan
Rajamalli
 *
*
Materials Research Centre, Indian Institute of Science, Bangalore–560012, Karnataka, India. E-mail: rajamalli@iisc.ac.in
First published on 14th November 2024
Abstract
Hybrid metal-free white organic light-emitting diodes (WOLEDs) based on the complementary color of a fluorescent dopant and thermally activated delayed fluorescence (TADF) emitters in a single emissive layer structure have drawn considerable attention and achieved enormous progress for their potential in complete exciton utilisation and a simple design structure. However, WOLEDs made of a single emissive layer suffer from poor color stability and insufficient blue emission for a well-balanced white light. Here, we designed and synthesised an orange fluorescent emitter with a peak maximum at 589 nm, which was utilised in fabricating WOLEDs by combining with a robust sky-blue TADF material. The resulting hybrid WOLEDs showed remarkable electroluminescence performances with a maximum external quantum efficiency (EQE) of 23.8%, and a balanced white emission could be attained with Commission Internationale de l’Eclairage (CIE) coordinates of (0.33, 0.43). The correlated color temperature (CCT) is measured to be 5863 K, resembling the sunlight temperature at noon. Furthermore, the white light can be tuned to cool and warm white emissions by controlling the concentration of the fluorescent dopant. All the WOLEDs exhibit excellent color stability, maintaining minimal shifts in the CIE coordinates even at high brightness/voltages.
1. Introduction
White organic light-emitting diodes (WOLEDs) have been considered a promising next-generation technology for flat-panel full-color displays and solid-state lighting, which opened a new market in the illumination sector due to their comprehensive merits of high efficiency, low energy consumption, low-cost, feasible flexibility, and well-balanced white color.1–5 Extensive research on materials and device structures has been devoted to exploring WOLEDs with high emission efficiency in the past years.6 The WOLEDs based on traditional all-fluorescent emitters can hardly exceed 25% of exciton utilisation due to theoretical limitations and end up with inadequate performance for lighting applications.7 Alternatively, phosphorescence and thermally activated delayed fluorescence (TADF) emitters have been exploited in developing highly efficient WOLEDs due to their near unity internal quantum efficiency that can harvest all the electrically generated excitons, including the dark triplet excitons.8–11 However, the phosphors contain expensive rare noble metals and suffer from color impurity and poor operational stability, particularly for the blue phosphorescent emitters, which remain a big obstacle and restrict their reliability in practical applications.12–14 Conversely, all TADF WOLEDs usually suffer from serious exciton-annihilation processes, inadequate energy transfer, and dissatisfactory Commission Internationale de I’Eclairage (CIE) coordinates due to an imbalance in the radiation decay rates of the emitters, thus leading to unbalanced white emission.15–17 To address these issues, fully organic and hybrid WOLEDs, constructed with fluorescent emitters and TADF materials, have gained noticeable attention and emerged as potential candidates to meet the aforementioned requirements.18,19White devices can be achieved by combining three primary color (red, green, and blue) or two complementary color (sky blue and orange) materials in the emissive layer, thus covering the visible spectrum.20,21 A sandwich-type emissive layer with two separate green/red layers was reported to manage excitons within their respective emissive zones, achieving a maximum external quantum efficiency (EQE) of 18.2%.22 However, such an approach required multiple emissive layers, introducing more intricate energy transfer paths and complications in the overall fabrication process.23–25 In principle, the WOLEDs fabricated with a single emissive layer are technically simple and potentially reduce the complexity of the device structure.26,27 These WOLEDs can be developed by combining a blue TADF material, which also behaves as a sensitiser, with an orange fluorescent emitter. The blue TADF emitter should emit its own color as well as transfer the generated excitons from its lowest singlet level (S1) through the forward Förster energy transfer (FRET) process to the orange fluorophore to achieve white emission. Such an energy transfer process is known as hyperfluorescence.28–30 However, WOLEDs made of a single emissive layer suffer from low exciton utilisation, poor color stability, and lack of blue emission, resulting in unbalanced white light.31,32 The inevitable energy loss processes, such as direct charge trapping in the fluorescent emitter and the Dexter energy transfer (DET) from the triplet state (T1) of the TADF emitter to that of the fluorescent dopant, may contribute to the exciton loss in the device. Moreover, the sensitising FRET process often works more efficiently than the radiative decay of the blue TADF emitter, which results in inadequate blue emission.18 This is why an extremely low doping concentration of the fluorescent dopant is adopted to minimise the energy loss mechanisms. The energy transfer process is susceptible to the concentration of the fluorescent dopant, which allows the modulation of the white light emission by varying the doping percentage in the emissive layer. For instance, Song et al. developed a single emissive layer WOLED with a blue TADF emitter and extremely low concentration (0.03–0.05 wt%) of the yellow fluorescent dopant and realised a maximum EQE of 15.5% and CIE of (0.28, 0.35).33 It is challenging to control such a low concentration during fabrication. Another report by Xie et al. achieved an EQE of 21.8% with balanced white emission by employing a dual-host system, strategically modulating the energy transfer pathways within the emissive layer.34 Wei et al. reported WOLEDs with an EQEmax of 19.6% and CIE coordinates of (0.33, 0.45) using sterically shielded emitters to increase the intermolecular distance and prevent exciton loss processes.35 Numerous endeavours have been devoted to improving the performance of single emissive layer WOLEDs by choosing efficient new blue TADF emitters.36,37 Despite these efforts, white devices still required additional enhancements in their performances, primarily due to the persisting issues of unbalanced white emission and color shifts experienced at high brightness. Moreover, tuning the emission from cool white to warm white light from the same system by adjusting the concentrations would be beneficial, and it would enable simultaneous achievement of high-efficiency and balanced white color OLEDs.
In this work, we designed and synthesised a fluorescent emitter p-ditolylamino anthracene carbonitrile (p-DTAACN), showing orange emission with a peak maximum at 589 nm and an EQE of 5.2%. Due to its broad and long-wavelength emission, the emitter is ideal for fabricating white OLEDs to cover the maximum visible spectrum. In this regard, a previously reported sky-blue TADF emitter, (3,5-bis(3,6-di-tert-butyl-9H-carbazol-9-yl)phenyl)(pyridin-3-yl)methanone, 3BPy-mDTC, which radiates at 479 nm with an EQE of 24.6%,38,39 was used in constructing white light devices. By utilising these two complementary emitters, single emissive layer WOLEDs were developed. In addition, the white emission of the devices allowed the tuning of color from a cooler to a warmer tone by precisely varying the doping concentration of p-DTAACN from 0.5 wt% to 1.0 wt% in the emissive layer. Notably, 0.7 wt% of p-DTAACN exhibited a maximum EQE of 23.8% and a purer white emission with CIE coordinates of (0.33, 0.43) and a CCT of 5863 K. Furthermore, the devices also realised remarkable spectral stability in white electroluminescence (EL) spectra across varying operating voltages. These findings suggest a facile pathway for developing highly efficient and color-stable WOLEDs, offering practical applications through thoughtful design and optimisation.
2. Results and discussion
2.1. Synthesis and characterisation of the anthracene emitter
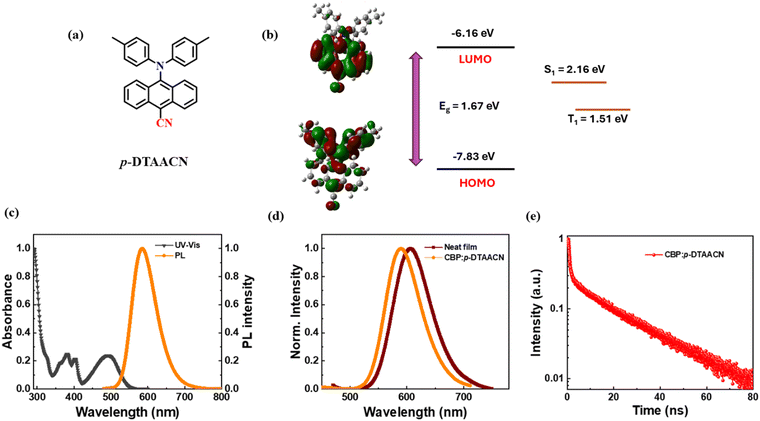
Using the Gaussian 16 programme, density functional theory (DFT) calculations were carried out to obtain insight into the electronic states of the p-DTAACN molecule. The result indicates that the highest occupied molecular orbital (HOMO) is primarily distributed on the electron-donating tolylamine units and extends to the anthracene unit. The lowest unoccupied molecular orbital (LUMO) is covered by the electron-accepting cyano-anthracene group (Fig. 1b). According to these findings, p-DTAACN is a donor–acceptor (D–A) dipole molecule with substantial charge transfer capabilities from the donor to acceptor (HOMO to LUMO), which is consistent with the emission spectra. Furthermore, the HOMO–LUMO energy gap was found to be Eg = 1.67 eV, and the calculated singlet and triplet energy levels were S1 = 2.163 eV and T1 = 1.510 eV. Though the emitter is a D–A compound, it is not expected to show TADF properties due to the large single-triplet gap.
The electrochemical behaviour of p-DTAACN was examined using the cyclic voltammetry (CV) technique to know HOMO and LUMO energy levels, and the voltammogram is shown in Fig. S6 (ESI†). The HOMO energy level for p-DTAACN was calculated to be −5.41 eV, obtained from the onset of oxidation potential. The corresponding LUMO energy level was estimated to be −3.03 eV from HOMO-Eg. These HOMO and LUMO values are desirable for smooth carrier injection in the device.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 770 cd m−2 and a maximum EQE of 5.2%. The corresponding maximum current efficiency (CE) and power efficiency (PE) are 18.8 cd A−1 and 14.7 lm W−1, respectively. The device radiated orange emission with an EL peak maximum at 589 nm, which is consistent with its PL maximum. Furthermore, the EL spectra at various operating voltages were measured (Fig. S8, ESI†), and no wavelength shift was observed, indicating the stability of EL spectra at high voltage. The CIE coordinate of the device was located at (0.57, 0.47).
770 cd m−2 and a maximum EQE of 5.2%. The corresponding maximum current efficiency (CE) and power efficiency (PE) are 18.8 cd A−1 and 14.7 lm W−1, respectively. The device radiated orange emission with an EL peak maximum at 589 nm, which is consistent with its PL maximum. Furthermore, the EL spectra at various operating voltages were measured (Fig. S8, ESI†), and no wavelength shift was observed, indicating the stability of EL spectra at high voltage. The CIE coordinate of the device was located at (0.57, 0.47).
| Device | V on [V] | L max [cd m−2] | EQEc [%] | CEc [cd A−1] | PEc [lm W−1] | λ max [nm] | CIEe (x, y) |
|---|---|---|---|---|---|---|---|
| a Turn-on voltage (Von) at 1 cd m−2. b Maximum luminance (Lmax). c Maximum external quantum efficiency (EQE), current efficiency (CE) and power efficiency (PE) measured at the maxima. d Electroluminescence at peak maxima. e CIE coordinates recorded at 8 V. | |||||||
| p-DTAACN | 3.2 | 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 770 770 |
5.2 | 18.8 | 14.7 | 589 | (0.57, 0.47) |
2.2. Hybrid white OLEDs
Encouraged by the EL performances of p-DTAACN, hybrid white OLEDs were fabricated by combining this orange fluorescent emitter with a complementary robust sky-blue TADF emitter within the emissive layer. Herein, a known highly efficient sterically shielded TADF emitter, 3BPy-mDTC, with tert-butyl units, has been utilised. The peripheral inert groups present in 3BPy-mDTC increased the intermolecular distance between the TADF emitter and fluorescent dopant. They can strictly suppress undesired energy loss processes, such as the DET from T1 of 3BPy-mDTC to T1 of p-DTAACN, leading to the accumulation of triplet excitons on p-DTAACN and their non-radiatively decay.38,39 In addition, 3BPy-mDTC has a small singlet–triplet splitting (ΔEST) of 0.05 eV, a high PLQY of 92% and an emission peak at 478 nm, and is thus preferred for effective exciton harvesting and complementary blue color. The p-DTAACN emitter, when precisely doped with 3BPy-mDTC, can span the entire visible spectrum, creating a favourable scenario for generating white light. As illustrated in Fig. 3, the TADF emitter not only emits blue light but is also employed as a sensitiser for energy transfer to the final dopant to produce orange emission, such that a well-balanced emission from both 3BPy-mDTC and p-DTAACN can be harvested for white light. As evident from Fig. S9 (ESI†), a clear overlap between the PL spectrum of 3BPy-mDTC and the absorption spectrum of p-DTAACN can be seen revealing that an efficient FRET process from S1 of 3BPy-mDTC to S1 of p-DTAACN is feasible. The radius of FRET (R0) can be expressed using the equation:Here,
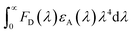 is the spectral overlap integral between the emission spectra of the donor (3BPy-mDTC) and the absorption spectra of the acceptor (p-DTAACN).40κ2 is the relative dipole orientation factor and taken as 2/3 for a random distribution system,41n is the refractive index, which is assumed to be 1.8,42NA is Avogadro's number, and φf is the PL quantum yield of the TADF emitter in the absence of a fluorescent dopant. The R0 value based on the spectral overlap for 3BPy-mDTC:p-DTAACN was estimated to be 5.78 nm, thus validating the effectiveness of the FRET process.
is the spectral overlap integral between the emission spectra of the donor (3BPy-mDTC) and the absorption spectra of the acceptor (p-DTAACN).40κ2 is the relative dipole orientation factor and taken as 2/3 for a random distribution system,41n is the refractive index, which is assumed to be 1.8,42NA is Avogadro's number, and φf is the PL quantum yield of the TADF emitter in the absence of a fluorescent dopant. The R0 value based on the spectral overlap for 3BPy-mDTC:p-DTAACN was estimated to be 5.78 nm, thus validating the effectiveness of the FRET process.
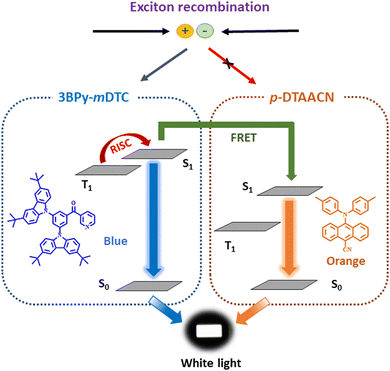 | ||
| Fig. 3 A schematic representation of the energy transfer mechanism to generate white light through simultaneous emissions from both the TADF sensitiser and fluorescent emitter. | ||
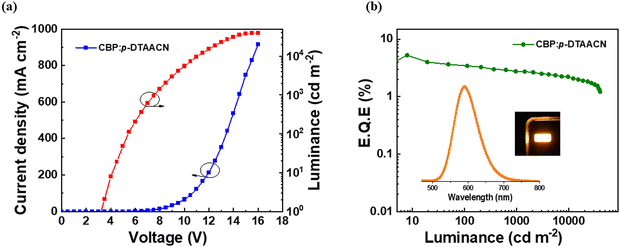
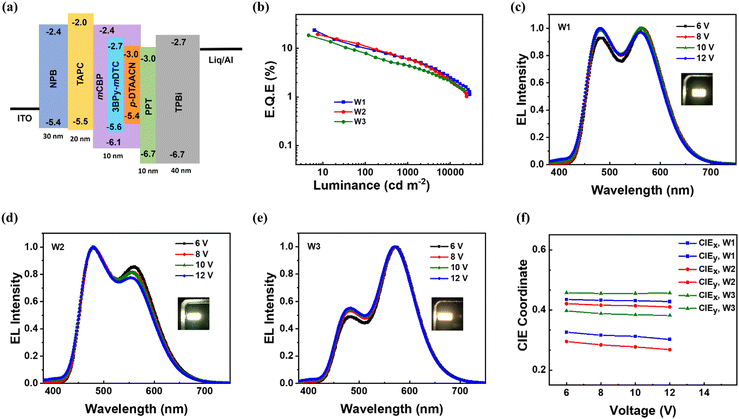
The J–V–L characteristics of W1 plotted in Fig. S10a (ESI†) suggest good electrical conductivity with a turn-on voltage of 3.6 V. The WOLED showcased high efficiency as described by the EQE-Luminance characteristics in Fig. 4b, with a maximum EQE, PE and CE of 23.8%, 57.9 lm W−1 and 73.7 cd A−1, respectively. The luminance value of W1 reached a maximum of 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 003 cd m−2. The steric protection in 3BPy-mDTC inhibited the DET and blocked exciton loss in the device, thus being responsible for higher efficiency.43,44 The EL spectra of W1 are shown in Fig. 4c, where two main emission bands are clearly visible with peak maxima at 476 and 560 nm, corresponding to the contribution of 3BPy-mDTC and p-DTAACN, respectively. The emission intensities of both blue and orange peaks are identical, thus displaying a pure white color. The CIE coordinates of W1 are (0.33, 0.43), and the correlated color temperature (CCT) is measured to be 5863 K, closely resembling the temperature of daylight at noon.45,46
003 cd m−2. The steric protection in 3BPy-mDTC inhibited the DET and blocked exciton loss in the device, thus being responsible for higher efficiency.43,44 The EL spectra of W1 are shown in Fig. 4c, where two main emission bands are clearly visible with peak maxima at 476 and 560 nm, corresponding to the contribution of 3BPy-mDTC and p-DTAACN, respectively. The emission intensities of both blue and orange peaks are identical, thus displaying a pure white color. The CIE coordinates of W1 are (0.33, 0.43), and the correlated color temperature (CCT) is measured to be 5863 K, closely resembling the temperature of daylight at noon.45,46
To further understand the impact of varying p-DTAACN doping concentration on the emission pattern and color of white light, two devices (W2 and W3) were constructed with increasing and decreasing percentage of p-DTAACN doping compared to W1. The device structure mirrored that of W1, except for the emissive layer, where W2 contained 0.5 wt% p-DTAACN while W3 contained 1.0 wt%. Fig. 4 compares the electroluminescence performances of the WOLEDs, and the detailed parameters are illustrated in Table 2. W2 and W3 both displayed commendable performances, and their maximum EQE values were 19.6% and 18.7%, respectively. The maximum PE and CE values for W2 and W3 are 42.1 lm W−1, 53.7 lm W−1 and 60.2 cd A−1, 68.4 cd A−1, respectively (Fig. S10b, ESI†). The performances of W2 and W3 are slightly inferior to W1; however, the color tunability of the WOLEDs could be achieved. The EL spectra of W2 and W3 are shown in Fig. 4(d and e), along with their photographs. W2 depicted a cooler tone of white light attributed to an intensified blue region with a CIE of (0.29, 0.42) and a CCT of 6863 K measured at 8 V, implying that the majority of excitons in the emissive layer are consumed for blue emission rather than being transferred to p-DTAACN. As the concentration of p-DTAACN increased to 1.0 wt%, the CIE coordinates and CCT of W3 gradually moved from a cooler to a warmer region of (0.39, 0.46) and 4350 K, respectively. The standard reference considered for illumination applications is mostly warm white light known for its human-eye-friendly characteristics and has a CIE of (0.44, 0.40), representing a dominant orange region.47,48 Device W3 displayed such eye-protecting warm color, close to the standard warm light, with reasonably low blue intensity and enhanced orange emission. It is noteworthy that with varying concentrations of p-DTAACN from 0.5 wt% to 1.0 wt%, the WOLEDs could tune the light with cool, pure and warm white emissions. The color rendering index (CRI) was also measured for the WOLEDs,49,50 with values of 62 for W1 and 60 for both W2 and W3.
| Device | V on [V] | L max [cd m−2] | EQEc [%] | CEc [cd A−1] | PEc [lm W−1] | CIEd (x, y) | CCTd [K] |
|---|---|---|---|---|---|---|---|
| a Turn-on voltage (Von) at 1 cd m−2. b Maximum luminance (Lmax). c Maximum external quantum efficiency (EQE), current efficiency (CE) and power efficiency (PE) measured at the maxima. d CIE coordinates and CCT recorded at 8 V. | |||||||
| W1 | 3.6 | 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 003 003 |
23.8 | 73.7 | 57.9 | (0.33, 0.43) | 5863 |
| W2 | 3.6 | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 239 239 |
19.6 | 60.2 | 42.1 | (0.29, 0.42) | 6863 |
| W3 | 3.5 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 763 763 |
18.7 | 57.4 | 39.7 | (0.39, 0.46) | 4350 |
Additionally, the devices exhibited color-stable white EL spectra at various driving voltages. The CIE–voltage curve (Fig. 4f) vividly demonstrated higher spectral stability of devices W1–W3. As the voltage increases from 6 V to 12 V, a minimal spectral change in the EL spectra and a slight deviation in the CIE coordinates were observed. The luminance at 6 and 12 V ranges from ∼300 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cd m−2, revealing the uniform exciton recombination zones for devices W1–W3 across a broad luminance range.
000 cd m−2, revealing the uniform exciton recombination zones for devices W1–W3 across a broad luminance range.
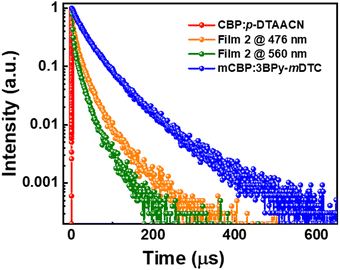
Next, the transient PL decay curves of the doped white films, mCBP:7 wt% 3BPy-mDTC:x wt% p-DTAACN where x = 0.5, 0.7 and 1.0 for film 1, film 2 and film 3, respectively, were recorded at emission peak maxima at 476 nm and 560 nm. The decay curves are shown in Fig. 5 and Fig. S12 (ESI†). It is noted that film 1 exhibited a reduced delayed lifetime of 32.1 μs at 476 nm, compared to the 3BPy-mDTC film without a dopant (48.4 μs), suggesting that the energy transfer process occurred effectively from the 3BPy-mDTC to the p-DTAACN dopant. The delayed lifetime at 560 nm was 22.7 μs, longer than that for the p-DTAACN film, indicating the contribution of triplet excitons in the emission. For film 2, the delayed lifetime is 22.5 μs, shorter than that for film 1, which is due to the increased concentration of p-DTAACN, thus enhancing the FRET process. As previously stated, ksr of 3BPy-mDTC is faster than that of p-DTAACN; hence, excitons of 3BPy-mDTC can radiate quickly and with efficient FRET, the transferred excitons can also decay rapidly. The W1 device, in particular, observed a purer white colour attributed to the balanced ksr between the blue emission of 3BPy-mDTC and the orange emission of p-DTAACN, which could be the reason for achieving a well-balanced white light within the system and showcasing high electroluminescence performance. On further increasing p-DTAACN doping percentage, film 3 exhibited even shorter delayed lifetimes of 19.9 μs in comparison to film 1 and film 2. The increased concentration of p-DTAACN in the film induced a strong energy transfer process from 3BPy-mDTC to p-DTAACN, leading to a dramatic decrease in the delayed lifetime. Consequently, the performances demonstrated that both blue and orange components are highly sensitive over p-DTAACN doping concentration in the emitting layer, and with precise control, the devices hold potential in tuning the color from cool white to warm white emission. Also, by selecting appropriate sky-blue and orange emitters with balanced radiative decay rates, simultaneous realisation of high efficiency and color-stable white light is possible in single emissive layer WOLEDs.
3. Conclusions
In summary, an orange emitter, p-DTAACN, based on the anthracene core was synthesised which showed a maximum EQE of 5.2% and an EL peak at 589 nm. By combining this material with the sky-blue TADF emitter 3BPy-mDTC, efficient, spectral stable and color-tunable single emissive layer WOLEDs were successfully fabricated. The steric protection in the blue emitter helped in blocking the energy loss processes and provided maximum exciton utilisation. The white devices can be tuned from cool, pure and warm white emission with control over the p-DTAACN doping concentration in the range of 0.5–1.0 wt%, thereby modulating the energy transfer process. The well-balanced white device achieved an EQEmax of 23.8%, a PEmax of 57.9 lm W−1, CIE coordinates of (0.33, 0.43), and a CCT of 5863 K, respectively. The radiative decay rates of both blue and orange emitters complement each other, and with the optimised concentration of p-DTAACN, sufficient blue light can be produced, which benefits in balancing the white emission. The results presented can bring new insights and testify the potential of WOLEDs in designing highly efficient, color-stable and human-eye-friendly light sources that may broaden our scope of mind for future lighting applications.Data availability
Data of this paper is available from authors based on request.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
The authors are extremely grateful to the Department of Science and Technology (DSTFIST: SR/FST/PSII009/2010) for the instrumental facility at MRC, IISc, and SAMat Research Facilities, JNCASR, Bengaluru, for lifetime measurement facilities. The authors thank the Indian Science Technology and Engineering Facilities Map (I-STEM) for enabling access to the SC-XRD, Bruker LT D8 Quest at Indian Institute of Science, Bangalore, to carry out this work. P. R. thanks IISc and the Science & Engineering Research Board (SERB), India, for the SERB-Power Grant (SPG) (Grant no. SPG/2020/000107). T. V. thanks Science and Engineering Research Board (SERB), India, for a National Postdoctoral fellowship (NPDF-File no. PDF/2023/001648).References
- J. Kido, K. Hongawa, K. Okuyama and K. Nagai, Appl. Phys. Lett., 1994, 64, 815–817 CrossRef CAS.
- J. Kido, M. Kimura and K. Nagai, Science, 1995, 267, 1332–1334 CrossRef CAS PubMed.
- S. Reineke, F. Lindner, G. Schwartz, N. Seidler, K. Walzer, B. Lüssem and K. Leo, Nature, 2009, 459, 234–238 CrossRef CAS PubMed.
- M. Du, Y. Feng, D. Zhu, T. Peng, Y. Liu, Y. Wang and M. R. Bryce, Adv. Mater., 2016, 28, 5963–5968 CrossRef CAS.
- Z. Wu, Y. Liu, L. Yu, C. Zhao, D. Yang, X. Qiao, J. Chen, C. Yang, H. Kleemann, K. Leo and D. Ma, Nat. Commun., 2019, 10, 2380 CrossRef.
- X. Tang, X.-Y. Liu, Z.-Q. Jiang and L.-S. Liao, Adv. Funct. Mater., 2019, 29, 1807541 CrossRef.
- M. Strukelj, R. H. Jordan and A. Dodabalapur, J. Am. Chem. Soc., 1996, 118, 1213–1214 CrossRef CAS.
- S. Lee, H. Shin and J.-J. Kim, Adv. Mater., 2014, 26, 5864–5868 CrossRef CAS PubMed.
- D. Zhang, M. Cai, Y. Zhang, D. Zhang and L. Duan, ACS Appl. Mater. Interfaces, 2015, 7, 28693–28700 CrossRef CAS PubMed.
- J. Liang, C. Li, X. Zhuang, K. Ye, Y. Liu and Y. Wang, Adv. Funct. Mater., 2018, 28, 1707002 CrossRef.
- D. de, S. Pereira, P. L. dos Santos, J. S. Ward, P. Data, M. Okazaki, Y. Takeda, S. Minakata, M. R. Bryce and A. P. Monkman, Sci. Rep., 2017, 7, 6234 CrossRef.
- Q. Wang and D. Ma, Chem. Soc. Rev., 2010, 39, 2387–2398 RSC.
- Y. Zhang, J. Lee and S. R. Forrest, Nat. Commun., 2014, 5, 5008 CrossRef CAS.
- P. Heimel, A. Mondal, F. May, W. Kowalsky, C. Lennartz, D. Andrienko and R. Lovrincic, Nat. Commun., 2018, 9, 4990 CrossRef.
- D. Luo, Q. Chen, Y. Gao, M. Zhang and B. Liu, ACS Energy Lett., 2018, 3, 1531–1538 CrossRef CAS.
- J. Zhao, X. Chen, Z. Yang, Z. Chi, Z. Yang, Y. Zhang, J. Xu, Z. Chi and M. P. Aldred, J. Mater. Chem. C, 2018, 6, 3226–3232 RSC.
- D. Zhang, X. Cao, Q. Wu, M. Zhang, N. Sun, X. Zhang and Y. Tao, J. Mater. Chem. C, 2018, 6, 3675–3682 RSC.
- T. Higuchi, H. Nakanotani and C. Adachi, Adv. Mater., 2015, 27, 2019–2023 CrossRef CAS.
- Z. Wang, X.-L. Li, Z. Ma, X. Cai, C. Cai and S.-J. Su, Adv. Funct. Mater., 2018, 28, 1706922 CrossRef.
- Z. Wu, L. Yu, X. Zhou, Q. Guo, J. Luo, X. Qiao, D. Yang, J. Chen, C. Yang and D. Ma, Adv. Opt. Mater., 2016, 4, 1067–1074 CrossRef CAS.
- T. Higuchi, H. Nakanotani and C. Adachi, Adv. Mater., 2015, 27, 2019–2023 CrossRef CAS.
- Z. Wu, Q. Wang, L. Yu, J. Chen, X. Qiao, T. Ahamad, S. M. Alshehri, C. Yang and D. Ma, ACS Appl. Mater. Interfaces, 2016, 8, 28780–28788 CrossRef CAS.
- H. Liu, J. Chen, Y. Fu, Z. Zhao and B. Z. Tang, Adv. Funct. Mater., 2021, 31, 2103273 CrossRef CAS.
- Y. Chen, Q. Sun, Y. Dai, D. Yang, X. Qiao and D. Ma, J. Mater. Chem. C, 2020, 8, 13777–13785 RSC.
- X. Tang, Y. Li, Y.-K. Qu, C.-C. Peng, A. Khan, Z.-Q. Jiang and L.-S. Liao, Adv. Funct. Mater., 2020, 30, 1910633 CrossRef CAS.
- W. Song, I. Lee and J. Y. Lee, Adv. Mater., 2015, 27, 4358–4363 CrossRef CAS PubMed.
- J. Zhang, C. Han, F. Du, C. Duan, Y. Wei and H. Xu, Adv. Funct. Mater., 2020, 30, 2005165 CrossRef CAS.
- H. Nakanotani, T. Higuchi, T. Furukawa, K. Masui, K. Morimoto, M. Numata, H. Tanaka, Y. Sagara, T. Yasuda and C. Adachi, Nat. Commun., 2014, 5, 4016 CrossRef CAS.
- U. Deori, N. Yadav, G. P. Nanda, K. L. Kumawat and P. Rajamalli, ACS Appl. Electron. Mater., 2023, 5, 4959–4967 CrossRef CAS.
- U. Deori, G. P. Nanda, C. Murawski and P. Rajamalli, Chem. Sci., 2024, 15, 17739–17759 RSC.
- B. Zhao, T. Zhang, W. Li, Z. Su, B. Chu, X. Yan, F. Jin, Y. Gao and H. Wu, Org. Electron., 2015, 23, 208–212 CrossRef CAS.
- Y.-N. Hu, X.-C. Fan, F. Huang, Y.-Z. Shi, H. Wang, Y.-C. Cheng, M.-Y. Chen, K. Wang, J. Yu and X.-H. Zhang, Adv. Opt. Mater., 2023, 11, 2202267 CrossRef CAS.
- W. Song, I. H. Lee, S.-H. Hwang and J. Y. Lee, Org. Electron., 2015, 23, 138–143 CrossRef CAS.
- F. M. Xie, S. J. Zou, Y. Li, L. Y. Lu, R. Yang, X. Y. Zeng, G. H. Zhang, J. Chen and J. X. Tang, ACS Appl. Mater. Interfaces, 2020, 12, 16736–16742 CrossRef CAS.
- P. Wei, D. Zhang and L. Duan, Adv. Funct. Mater., 2020, 30, 1–10 Search PubMed.
- S.-J. Zou, F.-M. Xie, Y.-Q. Li, Y.-Z. Shi, Y. Shen, Z.-G. Ma, J.-D. Chen, H.-X. Wei, X.-H. Zhang and J.-X. Tang, Mater. Today Energy, 2021, 21, 100745 CrossRef CAS.
- Z. Liu, B. Zhao, Y. Gao, H. Chen, B. Dong, Y. Xu, J. Li, H. Wang and W. Li, Opt. Mater., 2020, 110, 110510 CrossRef CAS.
- P. Rajamalli, V. Thangaraji, N. Senthilkumar, C. C. Ren-Wu, H. W. Lin and C. H. Cheng, J. Mater. Chem. C, 2017, 5, 2919–2926 RSC.
- B. Sk, E. Ravindran, U. Deori, N. Yadav, G. P. Nanda and P. Rajamalli, J. Mater. Chem. C, 2022, 10, 4886–4893 RSC.
- K. Gao, K. Liu, X. L. Li, X. Cai, D. Chen, Z. Xu, Z. He, B. Li, Z. Qiao, D. Chen, Y. Cao and S. J. Su, J. Mater. Chem. C, 2017, 5, 10406–10416 RSC.
- H. Wang, B. Yue, Z. Xie, B. Gao, Y. Xu, L. Liu, H. Sun and Y. Ma, Phys. Chem. Chem. Phys., 2013, 15, 3527 RSC.
- N. Aizawa, S. Shikita and T. Yasuda, Chem. Mater., 2017, 29, 7014–7022 CrossRef CAS.
- D. Zhang, X. Song, M. Cai and L. Duan, Adv. Mater., 2018, 30, 1705250 CrossRef.
- W. Xie, X. Peng, M. Li, W. Qiu, W. Li, Q. Gu, Y. Jiao, Z. Chen, Y. Gan, K. K. Liu and S.-J. Su, Adv. Opt. Mater., 2022, 10, 2200665 CrossRef CAS.
- J.-H. Jou, M.-H. Wu, S.-M. Shen, H.-C. Wang, S.-Z. Chen, S.-H. Chen, C.-R. Lin and Y.-L. Hsieh, Appl. Phys. Lett., 2009, 95, 13307 CrossRef.
- J. Zhou, Z. Kou, L. Wang, B. Wang, X. Chen, X. Sun and Z. Zheng, J. Phys. D: Appl. Phys., 2021, 54, 265107 CrossRef CAS.
- G. Schwartz, S. Reineke, T. C. Rosenow, K. Walzer and K. Leo, Adv. Funct. Mater., 2009, 19, 1319–1333 CrossRef CAS.
- J. Jou, C. Hsieh, J. Tseng, S. Peng, Y. Jou, J. H. Hong, S. Shen, M. Tang, P. Chen and C. Lin, Adv. Funct. Mater., 2013, 23, 2750–2757 CrossRef CAS.
- Y. Miao, G. Wang, M. Yin, Y. Guo, B. Zhao and H. Wang, Chem. Eng. J., 2023, 461, 141921 CrossRef CAS.
- L. Lu, Y. Guo, B. Zhao, H. Wang and Y. Miao, Mater. Today, 2024, 74, 109–120 CrossRef.
- N. Yadav, U. Deori, E. Ravindran, B. Sk and P. Rajamalli, J. Mater. Chem. C, 2023, 11, 16368–16376 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2352021. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4tc03934c |
| This journal is © The Royal Society of Chemistry 2025 |