 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
MXenes from MAX phases: synthesis, hybridization, and advances in supercapacitor applications
Tamal K. Paula,
Md. Abdul Khalequeab,
Md. Romzan Aliab,
Mohamed Aly Saad Aly *acd,
Md. Sadek Bacchuab,
Saidur Rahmanef and
Md. Zaved H. Khan*ab
*acd,
Md. Sadek Bacchuab,
Saidur Rahmanef and
Md. Zaved H. Khan*ab
aLaboratory of Nano-Bio and Advanced Materials Engineering (NAME), Jashore University of Science and Technology, Jashore 7408, Bangladesh. E-mail: mohamed.alysaadaly@ece.gatech.edu; zaved.khan@just.edu.bd
bDepartment of Chemical Engineering, Jashore University of Science and Technology, Jashore 7408, Bangladesh
cSchool of Electrical and Computer Engineering, Georgia Institute of Technology, Atlanta, GA 30332, USA
dDepartment of Electrical and Computer Engineering at Georgia Tech Shenzhen Institute (GTSI), Shenzhen, Guangdong 518052, China
eResearch Centre for Nano-Materials and Energy Technology, School of Engineering and Technology, Sunway University, Bandar Sunway, Malaysia
fDepartment of Engineering, Lancaster University, Lancaster, UK
First published on 24th March 2025
Abstract
MXenes, which are essentially 2D layered structures composed of transition metal carbides and nitrides obtained from MAX phases, have gained substantial interest in the field of energy storage, especially for their potential as electrodes in supercapacitors due to their unique properties such as high electrical conductivity, large surface area, and tunable surface chemistry that enable efficient charge storage. However, their practical implementation is hindered by challenges like self-restacking, oxidation, and restricted ion transport within the layered structure. This review focuses on the synthesis process of MXenes from MAX phases, highlighting the different etching techniques employed and how they significantly influence the resulting MXene structure and subsequent electrochemical performance. It further highlights the hybridization of MXenes with carbon-based materials, conducting polymers, and metal oxides to enhance charge storage capacity, cyclic stability, and ion diffusion. The influence of dimensional structuring (1D, 2D, and 3D architectures) on electrochemical performance is critically analyzed, showcasing their role in optimizing electrolyte accessibility and energy density. Additionally, the review highlights that while MXene-based supercapacitors have seen significant advancements in terms of energy storage efficiency through various material combinations and fabrication techniques, key challenges like large-scale production, long-term stability, and compatibility with electrolytes still need to be addressed. Future research should prioritize developing scalable synthesis methods, optimizing hybrid material interactions, and investigating new electrolyte systems to fully realize the potential of MXene-based supercapacitors for commercial applications. This comprehensive review provides a roadmap for researchers aiming to bridge the gap between laboratory research and commercial supercapacitor applications.
1 Introduction
Currently, environmental problems are considered the most concerning issues in the growing usage of fossil fuel-based products. To mitigate the adverse effect of fossil fuels on the environment, alternative environmentally friendly energy sources such as geothermal energy, wind energy, solar energy, and hydropower are extensively investigated. Although these sources are environmentally friendly, it largely depends on nature to consistently supply the energy demands. Therefore, in order to reduce the use of fossil fuels and lower the dependence on these natural sources, a new energy storage device needs to be explored.1 Therefore, scientists are trying to develop supercapacitors, parallel plate capacitors that can store energy as batteries,2,3 as a new alternative type of energy storage device. Supercapacitors have fast charge and discharge rates with excellent cyclic capability and high power density.4,5 Electrode materials play a crucial role for supercapacitor applications. It has been reported that electrode materials prepared from carbon based materials (CNT, rGO, etc.),6 transition metal based materials7 and conductive polymers8 have been used in supercapacitor-related applications. Among these active materials, transition metal nitrides and carbides known as MXene, discovered by Naguib et. al.,9 are extensively used for supercapacitor applications due to their high electrical conductivity, fast ion diffusion and excellent hydrophilic characteristics.10MXene can be synthesized from MAX phases, where M refers to transition metal such as V, Sc, Zr, Cr, Ti and Mo; a represents metal element such as Sn, Ga, Ti, Ge, In, Al, Si, Cd, P, As, S; and X indicates carbon (C) or nitrogen (N) atoms.11 MXene can be produced by etching the element “A” from the MAX phase using different etching agents such as HF, H3PO4, NaOH or LiF.11 The general formula of MXene is denoted by Mn+1XnTx, where M symbolizes a transition metal, X represents C or N atoms, T denotes the surface terminating groups such as –OH, –O, –F, introduced during etching process.12 This negative surface groups of MXene makes it an excellent substrate for hybridization with other materials.13 Furthermore, due to the inherent conductivity and the potentiality of charge transfer provided by the transition metal M changeable oxidation number, MXene exhibits exceptional electrochemical properties, and therefore, it is well suited for use in supercapacitor applications.12,14 Although MXene has the potential characteristics to fabricate excellent electrode materials for supercapacitor applications, it has some major issues that may reduce electrochemical performance. During the fabrication of MXene from MAX phases, a wide variety of negative functional groups are induced on the MXene surface, which is why, aggregation occurs in MXene suspension due to the van der walls interaction between these polar groups.15 Furthermore, the structural stability of pure MXene-based electrodes during the cyclic performance may be hampered due to the restacking nature of MXene.16 Additionally, Ti3C2Tx may be partially oxidized by oxygen or water molecules into the nonconductive titanium dioxide (TiO2), decreasing the redox reaction active sites and raising the charge transfer impedance.17 Scientists are trying to solve these flaws by preparing MXene-based hybrid materials to enhance their capacitive characteristics. To overcome these drawbacks, scientists took the advantages of wide surface terminating groups of MXenes, that allows MXene materials to interact with other active material. This interaction increases the interlayer spacing of MXene by avoiding the aggregation problem for which ion transport between the MXene based hybrids is enhanced. Therefore, the capacitive behavior of MXene based hybrid structure is improved. The most promising hybridization strategies include: (i) MXene/carbon-based hybrids: carbon nanotubes (CNTs), graphene, and activated carbon can be incorporated with MXenes to enhance conductivity, prevent restacking, and increase surface area for improved ion diffusion. (ii) MXene/conducting polymer hybrids: polyaniline (PANI), polypyrrole (PPy), and PEDOT:PSS provide pseudocapacitance, boosting energy storage capacity while maintaining flexibility and mechanical stability. (iii) MXene/metal compound hybrids: transition metal oxides (TMOs) and transition metal dichalcogenides (TMDs) improve charge storage due to their redox activity, increasing the overall capacitance and energy density. For example, Wang et al.18 prepared MXene/PDA film where PDA acted as an interlayer spacer, reducing self-stacking during cycling. In this hybrid structure, Ti made strong bonds with oxygen atoms in polydopamine whereas dopamine formed hydrogen bonds with surface functional groups, ensuring the stability of the structure.18 Liu et al. fabricated MXene/cellulose hybrid where cellulose increased the interlayer space of MXene and also ensured the good mechanical (124.6 MPa) and electromagnetic properties (36 dB).19 For using additive materials with MXene nanosheets, enhanced electrochemical performance has been achieved which is extensively discussed by many reports to enlighten the authors about the recent research of MXene in supercapacitors. For example, Luo et al. reported the application of MXene/conducting polymers (PPy, PANI, PEDOT:PSS) composites in the research of supercapacitors by discussing the preparation MXene/conducting polymers electrodes and their uses in supercapacitors.20 Thomas et al. highlighted the supercapacitor applications of MXene hybrids with carbonaceous materials, conducting polymers, transition metal dichalcogenides (TMDs), transition metal oxides (TMOs), etc., thorough their fundamental properties, synthesis tactics and etching procedures comprising various kind of MXenes.21 Besides the interaction of MXene and other active materials for excellent supercapacitor applications, some other factors, like electrolytes, dimensional structure of hybrid materials, fabrication technique of hybrid materials, are very important to enhance the electrochemical performance. These factors greatly influence capacitive performance. However, there are some reports whereas these factors are highlighted. For example, while the research conducted by Zang et al. primarily investigated ways to improve the capacitance of a material by manipulating its surface, creating films, and combining it with other materials (creating a composite), they did not delve deeply into other factors that could also significantly impact capacitance, such as the type of electrolyte used, the shape and size of the material (dimensional structures), and the specific methods used to create the material (fabrication techniques).4 Orangi et al. elaborately discussed the fabrication process of MXenes based electrode materials for energy storage applications, however, they did not extensively analyze the influence of multidimensional structural design, interlayer spacing, and ion diffusion on capacitive performance.22 Hu et al. shed light on the MXene-based supercapacitor performance focusing on structure, design, surface chemistry, electrode architecture and composites of MXenes, however, future challenges (aggregation, oxidation, scalability) and the possible solution for these hurdles weren't discussed.12 While the review reported by Panda et al. thoroughly examined how factors like MXene sheet size, shape, electrode architecture, and electrolyte type impact the performance of MXene-based supercapacitors, it notably lacked a comprehensive analysis of how the multidimensional structural design (1D, 2D, and 3D) of MXene materials specifically influences ion transport and the overall capacitive performance within the device.23 Among all these factors, interlayer multidimensional structure of MXene hybrid materials (1D, 2D, 3D) has also a great influence in capacitive performance, because electrolyte ion transportation path largely depends on it which can affect the electrochemical performance. Hu et al. discussed the progress on MXene symmetric supercapacitor focusing on 1D, 2D, 3D structures.12
Herein, the influence of multidimensional structural design of MXene hybridized materials for capacitive performance is elaborately discussed. To the best of the authors' knowledge this is the first report on the influence of the dimensional structure of MXene hybrid materials in supercapacitor applications. Moreover, this review systematically discusses the procedure of MXene synthesis from MAX phases, the preparation strategy of MXene-based hybrid materials and their multidimensional structural analysis in supercapacitors. The effect of interlayer spacing ion diffusion and the electrochemical performance of multidimensional MXene hybrids are also analyzed extensively. Finally, an overall guideline is provided to tackle the challenges of preparing MXene-based hybrid materials for next-generation supercapacitor applications.
2 Synthesis of MXenes
2.1 MAX phases to MXene
The protocol of MAX phase etching attracted a great deal of attention among scientific communities because of the great demand of using MXene in materials development research. In “Top-down” selective etching strategies, MAX phase is converted to MXene by breaking the bonds between ‘M’ and ‘A’. In this procedure, the etching reaction is significantly sensitive to air and moisture (<1 ppm H2O; <5 ppm O2).24 The etching agents are categorized as acids (HF, H3PO4), alkali (NaOH), fluoride salt + HCl (LiF, KF, NH4F), molten salt (LiF + KF, CdBr2, ZnCl2), NH4HF2 and others. Among these, fluoride salt + HCl (LiF, KF, NH4F) affects the multi-layered MXenes synthesis when intercalation fabrication method is used. That is, the etching agent mixture causes the interlayer space to expand by increasing lattice parameter and weaken interflake interactions.25 A summary on carbide, nitride, and carbonitride precursors etching events is represented in Table 1. In contrast, the delamination process is only suitable for a few layer-flakes exfoliation of MXenes. It is a mechanical exfoliation of MXenes, and it is comparatively challenging than intercalation.| MAX phase | MXene | Etching agent | Temperature (°C) | Time (hour) | Yield (%) | Ref. |
|---|---|---|---|---|---|---|
| Ti2AlC | Ti2CTx | 10% HF | Room temp. | 10 | 80 | 26 |
| V2AlC | V2CTx | 50% HF | Room temp. | 92 | 60 | 27 |
| Nb2CTx | Nb2CTx | 50% HF | Room temp. | 90 | 100 | 28 |
| Ti2AlN | Ti2NTx | KF + HCl | Room temp. | 24 | N/A | 29 |
| Ti3AlC2 | Ti3C2Tx | 50% HF | Room temp. | 2 | 100 | 9 |
| (Ti0.5Nb0.5)2AlC | (Ti0.5Nb0.5)2CTx | 51% HF | Room temp. | 28 | 80 | 30 |
| (V0.5Cr0.5)3AlC2 | (V0.5Cr0.5)3C2Tx | 50% HF | 69 | N/A | ||
| Ta4AlC3 | Ta4C3Tx | 50% HF | 72 | 90 | ||
| Nb4AlC3 | Nb4C3Tx | 50% HF | Room temp. | 96 | 77 | 31 |
| Mo2TiAlC2 | Mo2TiC2Tx | 50% HF | Room temp. | 48 | 100 | 32 |
| Mo2TiAlC3 | Mo2TiC3Tx | 50% HF | 55 | 90 | ||
| (Mo2/3Y1/3)2AlC | Mo4/3CTx | 48% HF | Room temp. | 60 | N/A | 33 |
| 10% HF | Room temp. | 72 | N/A | 33 | ||
| Mo2TiAlC2 | Mo2TiC2Tx | 48–51% HF | Room temp. | 48 | N/A | 32 |
| Mo2Ti2AlC3 | Mo2Ti2C3Tx | 48–51% HF | 55 | 90 | N/A | 32 |
| (W2/3Sc1/3)2AlC | W4/3CTx | 48% HF | Room temp. | 30 | N/A | 34 |
| Zr3Al3C5 | Zr3C2Tx | 50% HF | Room temp. | 60 | N/A | 35 |
| Hf3[Al(Si)]4C6 | Hf3C2Tx | 35% HF | Room temp. | 60 | N/A | 36 |
| Ti2AlC | Ti2CTx | 0.9 M LiF + 6 M HCl | 40 | 15 | N/A | 37 |
| Mo2Ga2C | Mo2CTx | 3 M LiF + 12 M HCl | 35 | 384 | N/A | 38 |
| Mo2Ga2C | Mo2CTx | NH4Cl + HCl | 140–180 | 24 | N/A | 39 |
| Nb2AlC | Nb2CTx | 0.75 g NaBF4 + 37% HCl | 180 | 15–35 | N/A | 40 |
| V2AlC | V2CTx | 2 g LiF + 40 M HCl | 90 | 48 | N/A | 41 |
| V2AlC | V2CTx | 1.5 g NaF + HCl | 90 | 120 | 42 | |
| V2AlC | V2CTx | LiF + HCl | 90 | 120 | N/A | 43 |
| V2AlC | V2CTx | 2 g NaF + 1.24 g LiF + 4.48 g KF + 40 ml HCl | 90 | 72 | N/A | 44 |
| Ti3AlC2 | Ti3C2Tx | 0.75 g NaBF4 + 37% HCl | 180 | 8–32 | N/A | 40 |
| Ti3AlC2 | Ti3C2Tx | 1 g LiF + 6 M HCl | 35 | 24 | N/A | 45 |
| Ti3AlC2 | Ti3C2Tx | 3 M LiF + 6 M HCl | 40 | 45 | 100 | 46 |
| Ti3AlCN | Ti3CNTx | 0.66 g LiF + 6 M HCl | 35 | 12 | N/A | 33 |
| (Nb0.8Zr0.2)4AlC3 | (Nb0.8Zr0.2)4C3Tx | LiF +12 M HCl | 50 | 168 | N/A | 47 |
| (W2/3Sc1/3)2AlC | W4/3CTx | 4 g LiF + 12 M HCl | 35 | 48 | N/A | 34 |
| Ti3AlC2 | Ti3C2Tx | 1 M NH4HF2 | 80 | 12 | N/A | 48 |
| Ti3AlC2 | Ti3C2Tx | NH4F | 150 | 24 | N/A | 49 |
| Ti4AlN3 | Ti4N3Tx | 59% KF + 29% LiF + 12% NaF | 550 | 0.5 | N/A | 50 |
| Ti3AlC2 | Ti3C2Tx | 2.07 g LiF + 3.35 g NaF + 7.52 g KF | 30, 40, 50, 60 | 12, 24, 48 | N/A | 51 |
| Ti3AlC2 | Ti3C2Tx | SnF2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) 6) |
550 | 6 | N/A | 52 |
| Ti4AlN3 | Ti4N3Tx | KF + LiF + NaF | 550 | 0.5 | N/A | 29 |
 | ||
| Fig. 1 (a) Schematic representation of V2CTx synthesis from V2AlC; SEM images of (b) V2AlC phase and (c) V2CTx. (d) XRD patterns of V2AlC and V2CTx, (*) denotes residual V2AlC phase.27 | ||
 | ||
| Fig. 2 (a) Schematic representation of etching and delamination of Mo2Ga2C MAX phase; (b) digital images of delamination and filtration; (c) XRD patterns of (i) Mo2Ga2C (black), (ii) Mo2CTx–Li (green), (iii) Mo2CTx (red), (iv) Mo2CTx intercalated by TBAOH (blue) and (v) paper (purple), (d) same XRD patterns of (c) with the focus on the 2θ range of 2–10.5°.38 | ||
Furthermore, fluoride salt and HCl etching protocol was comparatively suitable to synthesize multilayer flakes than other processes. There were different kinds of MXene synthesized and reported such as Ti2CTx,37 Nb2CTx,56 V2CTx,41 V2CTx,42 V2CTx,43 V2CTx,44 Ti3C2Tx,40 Ti3C2Tx,45 Ti3C2Tx,46 Ti3CNTx,33 (Nb0.8Zr0.2)4C3Tx,47 W4/3CTx.34
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6),52 KF + LiF + NaF.29
6),52 KF + LiF + NaF.29
3 Possible hybridization of MXenes for supercapacitor application
MXene can be considered one of the best electrode materials for supercapacitor application. Lukatskaya et al. found that macroporous multilayered MXene (Ti3C2Tx) film handled up to 210 F g−1 at 10 V s−1 of scan rate.59 However, it has been reported that freestanding individual MXene electrodes suffers from restacking and oxidation (in contact with oxygen and water) problems,4 for which reason intercalation of MXene with other materials is very necessary. In the following section, the possible hybridization of MXene materials with their preparation process and capacitive behavior are highlighted.3.1 MXene/CNT hybrid
Carbon nanotube (CNT) possesses excellent electrical, thermal and mechanical properties. This increases the potential of utilizing CNT in developing promising materials in various applications such as wearable electronic devices, sensors, supercapacitors.60 Laser ablation, chemical vapor deposition (CVD) and arc discharge are the most commonly used methods to synthesis CNT. It is worth mentioning that as-prepared CNT may possess metallic impurities.61 Furthermore, CNT may be aggregated in colloidal suspension because of the van der Waals interaction between the sidewalls of CNT.62 These issues restrict the practical application of individual CNT. Thus, incorporating MXene material with CNT can be a possible solution. A hybrid material composed of MXene and Carbon Nanotubes (CNTs) overcomes the individual limitations of each component, exhibiting superior electrical and mechanical properties, a larger surface area, and high pore volume, making it a highly promising candidate for supercapacitor applications; researchers like Yu et al. have extensively explored the diverse applications of MXene/CNT hybrids, including various fabrication methods and structural architectures to optimize their performance across different applications.63 Here, the preparation process is summarized first, and then an elaborate discussion is made regarding the supercapacitor applications of multidimensional MXene/CNT hybrid material.To synthesize MXene/CNT hybrid material, mainly two approaches are involved: the integration of CNT and MXene with chemically reactive (chemical) and without chemically reactive (physical) process. Preparing MXene and CNT hybrid material without chemically reactive process is an easy technique that involves different techniques like mechanical mixing,64 co-dispersion and self-assembly.65 Mechanical Mixing is the frequently used technique involving ultrasonication of a certain amount of MXene and CNT dispersion followed by vacuum filtration to prepare a thin film. Yan et al. prepared MXene/CNT hybrid material by ultrasonic stirring of MXene and CNT colloidal suspension followed by filtering the mixed dispersion.64 Regarding the self-assembly method, Guo et al. developed MXene/CNT composite material by taking the advantage of electrostatic interaction between MXene and CNT.66 The terminating group (–OH, –F, –O etc.) of MXene makes it a highly negative charged particle, ensuring strong electrochemical interaction with positively charged CNT-polyethyleneimine.66 There are many chemically reactive process involved in preparing MXene/CNT hybrid material such as in situ technique,67 thermal treatment,68 microwave process69 and hydrothermal process.70 Regarding the chemical process, it may need high energy consumption, like 800 °C for thermal treatment,68 which makes this process unsuitable for scalable production. On the other hand, mechanical mixing, self-assembly, co-dispersion methods are the easiest and most widely used techniques for the fabrication of MXene/CNT hybrids and also, in this regard the hybridized materials provide superior mechanical strength due to the hydrogen bonding between the materials.
The as-prepared MXene/CNT hybrid material by the above mentioned techniques can be formed into one-dimension (1D), two-dimension 2D or three-dimension (3D) structures to meet the required demands for supercapacitor application. 1D MXene/CNT materials are found in the form of fiber or yarn.71 Yu et al. dropped MXene solution on CNT scaffold and after drying the MXene/CNT ink, it is peeled off and scrolled into a helical fiber by Archimedean spirals.72 2D MXene/CNT hybrid material can be prepared in the form of thin film, paper, nanosheets or coating on textile substrate.73 Weng et al. utilized layer by layer method to fabricate MXene/CNT composite film. For this, they sprayed MXene/PVA suspension (positively charged particle) on CNT/PSS (negatively charged particle) to prepare the composite layer.74 In case of 3D structure of MXene/CNT hybrid material, it forms in foams or aerogel.75,76 The as-prepared MXene/CNT hybrid material with different architectures exhibits unique mechanical, electrical, low density, making this hybrid structure a potential material for supercapacitor applications. The application of MXene/CNT hybrid material's structure in supercapacitor is extensively discussed in the next section.
 | ||
| Fig. 4 (a) Schematic illustration wet spun MXene/CNT hybrid fibers;77 (b) the CV curves and (c) GCD curves of symmetric supercapacitors at various scan rate and current density, respectively;77 (d) schematic illustration of biscrolling technique to fabricate MXene/CNT yarn;78 (e) (I) vacuum filtration technique to fabricate MXene/CNT technique with (II) top view of the 5 wt% mass ratio of MXene/CNT film, (III) loading test, (IV) rolling test and (V and VI) folding test of the MXene/CNT films;79 (f) SEM image of MXene/CNT hybrids with thickness (I) 7.65 μm, (II) 9.69 μm and (III) 10.05 μm for pure MXene, 5 wt% and 10 wt% mass ratio of MXne/CNT respectively;79 (g) the volumetric capacitance as a function of the CNT content;64 (h) cyclic performance of Ti3C2Tx/CNT hybrid supercapacitor;80 (i) schematic illustration of electrolyte ion transportation pathways of (I) vacuum-dried dense MXene film, (II) freeze-dried porous MXene film, and (III) freeze-dried MXene/CNT film.81 | ||
In addition, Wang et al. used “Biscrolling” technique to prepare Ti3C2Tx MXene/CNT yarn, as shown in Fig. 4(d).78 For this, they decorated five layers of CNT sheets on a glass substrate with fixing an electric motor at the end. Then Ti3C2Tx MXene dispersion were then dropped on the CNT sheets followed by pulling by motor to obtain MXene/CNT yarn. The biscrolled yarn showed volumetric and gravimetric capacitances of 1083 F cm−3, 532 F g−1 in 3 M H2SO4 electrolyte solution. Besides, they also fabricated symmetric supercapacitor with PVA/H2SO4 gel electrolyte that demonstrated the highest energy and power density of 8.54 mW h cm−3 and 530 mW cm−3 respectively. Regarding aqueous electrolyte solution, the highest gravimetric capacitance of biscrolled yarn (532 F g−1 (ref. 78)) than Wet Spun Yarn (295 F g−1 (ref. 77)) may be attributed to the molar concentration of H2SO4 electrolyte, because enhanced concentration increases the ion conductivity of electrolyte that causes the increase of specific capacitance of supercapacitor.4 In a similar studies, MXene suspension was drop-casted on CNT scaffold and then MXene/CNT film was peeled off and scrolled into fiber formation, and in this helical structured fiber, MXene was wrapped into CNT corridor.72 CNTs maintain highly orientation in this hybrid fiber, providing high mechanical strength electrical conductivity without sacrificing plenty of spaces, contributing to the more ion transportation for enhanced capacitance.72
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles) and coulombic efficiency (99.7%) for their high surface area and pseudocapacitance.83
000 cycles) and coulombic efficiency (99.7%) for their high surface area and pseudocapacitance.83Besides the widely used Ti3C2Tx-MXene, Nb2CTx MXene hybrid with MWCNTs was also used by Xiao et al.84 Here, the lower conductivity of Nb2CTx than Ti3C2Tx was improved by introducing MWCNT with the Nb2CTx-MXene. In addition, it has been found that the specific capacitance of Nb2CTx/MWCNT and pure Nb2CTx was 202 F g−1 and 186 F g−1 at 2 mV s−1 in three electrode system where 1 M H2SO4 as used as an electrolyte. This significant capacitive performance of Nb2CTx/MWCNT were mainly derived by introducing MWCNT as a conductive bridge.84
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles of the charge–discharge process 10 mA cm−2, as depicted in Fig. 4(h).80 The highest capacitance of 3D like MXene/CNT honeycomb sponges may be attributed to the formation of more porous structure in spongy film80 that results in providing more opening path for electrolyte ion exchange. The effect of this porous structure of MXene/CNT hybrids on supercapacitor applications are investigated by Zhang et al.81 They fabricated three different MXene-based films namely, a densely packed Ti3C2Tx film (D-MF) by vacuum filtration, a porous Ti3C2Tx film by freeze-drying (3D-PMF), and a porous Ti3C2Tx/CNT film by freeze-drying (3D-PMCF). Furthermore, they used these films to create symmetric supercapacitors (SSCs).81 They observed that 3D-PMCF had the highest area of the cyclic voltammetry (CV) curve compared with D-MF and 3D-PMF, indicating the highest specific capacitance (about 375 F g−1). This capacitance is attributed to the bigger pore volume (0.103 cm3 g−1) of 3D-PMCF than those of the other two samples (D-MF: 0.01 cm3 g; 3D-PMF: 0.065 cm3 g−1), resulting in improved ion accessibility. Here, CNT acts as the spacer to increase the pore volume of PMCF than PMF and D-MF samples and for increasing porous structure, the ion transportation is fast in case of 3D-PMCF than the other samples, as demonstrated in Fig. 4(i).81 Moreover, a new 3D structure of Ti3C2Tx-MXene/CNT hybrid was developed by Gao et al.85 Regarding this, at first, they prepared a new knotted CNT which was then dispersed in CTAB solution. After that MXene-knotted CNT composite electrodes were prepared by a self-assembly process which was further used for investigating the electrochemical performance in an organic electrolyte for maximizing ion accessibility. They found that MXene-knotted CNT hybrids showed high capacitance, up to 130 F g−1 (276 F cm−3) in organic electrolytes with a capacitance retention of ∼56% at scan rates from 10 mV s−1 to 10 V s−1. For comparison, they also prepared Ti3C2Tx-MXene/non-knotted MWCNT 2D structure, however, this 2D structure only displayed a capacitance retention of 39% from 10 to 500 mV s−1.85 This proves that the 3D structure design allows more electrolyte ion accessibility than 2D structure which means 3D like MXene/CNT possess more capacitive performance. An overall comparison of preparation, structure and electrochemical performance of MXene/CNT is shown in Table 2.
000 cycles of the charge–discharge process 10 mA cm−2, as depicted in Fig. 4(h).80 The highest capacitance of 3D like MXene/CNT honeycomb sponges may be attributed to the formation of more porous structure in spongy film80 that results in providing more opening path for electrolyte ion exchange. The effect of this porous structure of MXene/CNT hybrids on supercapacitor applications are investigated by Zhang et al.81 They fabricated three different MXene-based films namely, a densely packed Ti3C2Tx film (D-MF) by vacuum filtration, a porous Ti3C2Tx film by freeze-drying (3D-PMF), and a porous Ti3C2Tx/CNT film by freeze-drying (3D-PMCF). Furthermore, they used these films to create symmetric supercapacitors (SSCs).81 They observed that 3D-PMCF had the highest area of the cyclic voltammetry (CV) curve compared with D-MF and 3D-PMF, indicating the highest specific capacitance (about 375 F g−1). This capacitance is attributed to the bigger pore volume (0.103 cm3 g−1) of 3D-PMCF than those of the other two samples (D-MF: 0.01 cm3 g; 3D-PMF: 0.065 cm3 g−1), resulting in improved ion accessibility. Here, CNT acts as the spacer to increase the pore volume of PMCF than PMF and D-MF samples and for increasing porous structure, the ion transportation is fast in case of 3D-PMCF than the other samples, as demonstrated in Fig. 4(i).81 Moreover, a new 3D structure of Ti3C2Tx-MXene/CNT hybrid was developed by Gao et al.85 Regarding this, at first, they prepared a new knotted CNT which was then dispersed in CTAB solution. After that MXene-knotted CNT composite electrodes were prepared by a self-assembly process which was further used for investigating the electrochemical performance in an organic electrolyte for maximizing ion accessibility. They found that MXene-knotted CNT hybrids showed high capacitance, up to 130 F g−1 (276 F cm−3) in organic electrolytes with a capacitance retention of ∼56% at scan rates from 10 mV s−1 to 10 V s−1. For comparison, they also prepared Ti3C2Tx-MXene/non-knotted MWCNT 2D structure, however, this 2D structure only displayed a capacitance retention of 39% from 10 to 500 mV s−1.85 This proves that the 3D structure design allows more electrolyte ion accessibility than 2D structure which means 3D like MXene/CNT possess more capacitive performance. An overall comparison of preparation, structure and electrochemical performance of MXene/CNT is shown in Table 2.
| Hybrid materialsa | Preparation method & structure | Electrolyte | Optimum A. C.a (F cm−2) | Optimum G. C.a (F g−1) | Optimum V. C.a (F cm−3) | C. R.a | Ref. |
|---|---|---|---|---|---|---|---|
| a A. C. = Areal Capacitance, G. C. = Gravimetric Capacitance, V. C. = Volumetric Capacitance, C. R. = Capacitance Retention, CNT = Carbon Nanotube, MWCNT = Multiwall Carbon Nanotube, SWCNT = Single Wall Carbon Nanotube. | |||||||
| Ti3C2Tx/CNT | Wet spun, fiber (1D) | 1 M H2SO4 | — | ∼295 | — | — | 77 |
| PVA/H2SO4 | 83% after 5000 cycles | ||||||
| 33 | |||||||
| MXene/CNT | Helical yarn (1D) | 3 M H2SO4 | 3.188 | 532 | 1083 | — | 78 |
| Ti3C2Tx/CNT/MnO2 | Hydrothermal and coating, composite fiber (1D) | 1 M Na2SO4 | — | 181.8 | — | 91% after 5000 cycles | 71 |
| Ti3C2Tx/CNT | Drop casting and scrolling, helical fiber (1D) | PVA/LiCl | — | — | 22.7 | 84% at current density of 1A cm−3 | 72 |
| 6 M LiCl | Approximately 90 | 95% at 1 A cm−3 | |||||
| Ti3C2Tx/CNT | Vacuum filtration, composite film (2D) | 1 M H2SO4 | — | 300 | — | 92% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
79 |
| Ti3C2Tx/SWCNT | Vacuum filtration of sandwiched hybrids & composite paper (2D) | 1 M MgSO4 | — | — | 390 | No degradation after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
86 |
| Vacuum filtration of random mixed hybrids & composite paper (2D) | 300 | ||||||
| Ti3C2Tx/MWCNT | Vacuum filtration of sandwiched hybrids, composite paper (2D) | — | — | 321 | — | ||
| Vacuum filtration of random mixed hybrids, composite paper (2D) | 366 | ||||||
| Ti3C2Tx/CNT | In situ growth, composite material (2D) | 3 M H2SO4 | — | 299.52 | — | 84.2% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
87 |
| Alkali induced Ti3C2Tx/CNT | Vacuum filtration, composite film (2D) | 3 M H2SO4 | — | 401.4 | — | 99.0% after 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
82 |
| Ti3C2Tx/CNT | Focused ion beam, hybrid composite film (2D) | PVA/H2SO4 | 0.317 | — | — | — | 88 |
| Ti3C2Tx/SCNT | Self-assembly, composite film (2D) | 1 M KOH | 0.22 | — | 314 | 95% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
89 |
| Nb2CTx/CNT | MXene/CNT slurry coating on carbon paper, (2D) | 1 M H2SO4 | — | 202 | — | 80.3% after 5000 cycles | 84 |
| Ti3C2Tx/CNT | Layer-by-layer assembly, composite film (2D) | 0.1 ml H2SO4/PVA gel | 61.38 | — | 87.68 | 67.2% at current density of 5 mA cm−2 | 90 |
| MXene/CNT/MnO2 | Vacuum filtration assisted layer by layer strategy, composite film (2D) | 1 M Na2SO4 | — | 221 | — | Good retention during 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
91 |
| Ti3C2Tx/CNT | Dip coating, composite film (2D) | 0.5 M Na2SO4 | 2.26 | 56.6 | — | 94.3% after 1000 cycles | 92 |
| Ti3C2Tx/CNT | Freeze drying, porous composite film (3D) | 3 M H2SO4 | — | 375.0 | — | 95.9% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
81 |
| Freeze drying, porous MXene film (3D) | 323.3 | — | |||||
| Vacuum filtration, MXene dense film (2D) | 286.8 | — | |||||
| Ti3C2Tx/knotted CNT | Self-assembly, MXene-knotted CNT structure (3D) | 1 M EMIM-TFSI/ACN | — | 130 | 276 | Almost no decay after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
85 |
| Ti3C2Tx/CNT | Electrophoretic deposition, sponge (3D) | 6 M KOH | 0.661 | 468 | — | 92.8% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
80 |
3.2 MXene/PPy
The use of conductive polymer like polypyrrole with the notable 2D MXene structure has opened a new era for the fabrication of wearable, flexible, lightweight, and portable devices. Due to the surface termination group of Mn+1XnTx, where Tx represents –O, –OH, and/or –F terminating groups, MXene exhibits superior reinforcing properties towards the conducting polymers.93,94 As MXene and conducting polymer exhibit excellent interfacial bonding, the hybrid material of MXene/conductive polymer offers significant advantages ranging from versatility, compatibility and high performance products. For instance, polypyrrole is a conductive polymer that is widely used for preparing energy storage devices.95 Intercalation of polypyrrole with MXene solves the degradation problem of MXene in the presence of water and oxygen,96 attracting scientists to produce novel MXene/PPy material for next generation wearable and flexible supercapacitor-based devices. In addition, the intercalation of PPy can expand the interlayer spaces of MXene with porous structure which may offer an excellent transmission of electrolyte ion during charge/discharge cycles.97,98 Due to the increasing of interlayer spacing and strong interfacial bonding between polypyrrole and MXene materials, an ion transfer path is created,99 resulting in the highest capacitance. In this section, the preparation process and application of MXene/PPy hybrids are described elaborately. | ||
| Fig. 5 (a) Cross section of MXene/PPy wrapped around the cotton fiber;102 (b) specific capacitance of PPy@cotton fiber and (PPy/MXene)@cotton fiber with different mass loading of electrochemically active substance;102 (c and d) cross-section of porous PPy/l-Ti3C2 film and dense PPy film respectively;103 (e) schematic diagram of MXene/PPy–PVA hydrogel fabrication process;104 (f) SEM image MXene/PPy–PVA hydrogel with porous structure;104 (g) comparison of specific capacitance between MXene/PPy–PVA and MXene/PVA hydrogel at different current density.104 | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles which was due to the hydrogen bonding, increased interlayer space of composite film and surface redox processes of the PPy and MXene.105 Although the MXene/PPy can exhibit interesting result, some drawbacks, such as time consuming in electrolyte ion transportation or filling out electrolyte gel in cell assembly still exist that can be improved by using liquid electrolytes as spacer.106 Fan et al. used an innovative strategy by addressing this challenges, where they introduced both polymerized polypyrrole (PPy) particles and ionic liquid (ILs)-based microemulsion particles as “dual spacers”, to fabricate functionalized Ti3C2-MXene composite films for high-performance and wide-temperature application in supercapacitors. Their prepared composite electrode displayed excellent rate capability between 4 °C and 50 °C as well as high gravimetric energy density of 31.2 W h kg−1.106
000 cycles which was due to the hydrogen bonding, increased interlayer space of composite film and surface redox processes of the PPy and MXene.105 Although the MXene/PPy can exhibit interesting result, some drawbacks, such as time consuming in electrolyte ion transportation or filling out electrolyte gel in cell assembly still exist that can be improved by using liquid electrolytes as spacer.106 Fan et al. used an innovative strategy by addressing this challenges, where they introduced both polymerized polypyrrole (PPy) particles and ionic liquid (ILs)-based microemulsion particles as “dual spacers”, to fabricate functionalized Ti3C2-MXene composite films for high-performance and wide-temperature application in supercapacitors. Their prepared composite electrode displayed excellent rate capability between 4 °C and 50 °C as well as high gravimetric energy density of 31.2 W h kg−1.106![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles) were fond which was attributed to the PPy nanowires matrix which connected separated MXene blocks through porous structure, enabling highly ions and charges transport for high supercapacitor performance.107
000 cycles) were fond which was attributed to the PPy nanowires matrix which connected separated MXene blocks through porous structure, enabling highly ions and charges transport for high supercapacitor performance.107Besides the in situ polymerization process, the electrochemical deposition technique was also used to fabricate 3D carambola-like structures.108 First, 2D Ti3C2Tx-MXene was added with pyrrole monomer and then an electric field was applied.108 In this case, the pyrrole monomer was polymerized in the layered space of Ti3C2Tx-MXene where the wide functional groups of MXene nanosheets acted as a core polymer, forming carambola like structure. When the current density was increased from 0.5 A g−1 to 8 A g−1, the as decorated carambola like MXene/PPy displayed 50% capacitance retention which was about 2.4% for pure PPy film. This excellent capacity retention was attributed to the formation 3D structure due to providing more pathways to promote the electrolyte ions. In addition, the symmetric supercapacitor decorated by 3D carambola-like MXene/PPy structure which showed a high specific capacitance of 184 F g−1 at a scan rate of 10 mV s−1 and superior capacity retention of about 86.4% after 5000 cycles.108 An overall comparison of preparation, structure and electrochemical performance of MXene/PPy is shown in Table 3.
| Hybrid materialsa | Preparation method and structure | Electrolyte | Optimum (A. C.)a (F cm−2) | Optimum (G. C.)a (F g−1) | Optimum (V. C.)a (F cm−3) | C. R.a | Ref. |
|---|---|---|---|---|---|---|---|
| a A. C. = Areal Capacitance, G. C. = Gravimetric Capacitance, V. C. = Volumetric Capacitance, C. R. = Capacitance Retention, PPy = Polypyrrole, PVA = Poly Vinyl Alcohol. | |||||||
| PPy/MXene@cotton | In situ polymerization, porous core–shell structure (1D) | 1 M H2SO4 | — | 506.6 | 0.456 | 83.3% after 2000 cycles | 102 |
| Ti3C2Tx/PPy | In situ polymerization of pyrrole, composite film (2D) | 1 M H2SO4 | — | 437 | — | 78% after 1000 cycles | 109 |
| Ti3C2Tx/PPy | In situ polymerization, composite film (2D) | 0.5 M Na2SO4 | 2.11 | 52.75 | — | — | 110 |
| Ti3C2Tx/PPy | In situ polymerization of pyrrole, organ like composite (2D) | 1 M Na2SO4 | — | 184.36 | — | 83.33% after 4000 cycles | 111 |
| Ti3C2/PPy | In situ polymerization, composite film (2D) | 1 M H2SO4 | — | 416 | 1000 | 92% after 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
105 |
| Ti3C2/PPy | Electrochemical polymerization, freestanding composite film (2D) | 0.5 M H2SO4 | 0.203 | — | 406 | 100% after 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
103 |
| PVA/H2SO4 | 0.035 | 2.39 | No decay after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
||||
| Ti3C2Tx/PPy | Electrophoretic deposition and electrochemical polymerization, composite film (2D) | 2 M H2SO4 | 0.109 | — | — | 96% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
101 |
| PVA/H2SO4 | 0.0867 | — | |||||
| PPy/Ti3C2 | In situ polymerization & heterostructure nanocomposite (2D) | 1 M H2SO4 (3 electrode) | — | 458 | — | 83.64% after 1000 cycles | 112 |
| 1 M H2SO4 (2 electrode) | 155.61 | 73.68% after 4000 cycles | |||||
| Ti3C2Tx/PPy | Electrostatic self-assembly and in situ polymerization, textile electrode (2D) | 1 M Na2SO4 | 1.295 | 439 | — | 94.8% after 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
113 |
| Ti3C2Tx/PPy | Dip-dry and electrochemical deposition, textile electrode (2D) | 1 M H2SO4 | — | 343.20 | — | — | 114 |
| Ti3C2/PPy/PVA | Freeze drying and in situ polymerization, hydrogel (3D) | 1 M H2SO4 | — | 614 | 100% over 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
104 | |
| H2SO4/PVA gel | — | 184 | — | 83% over 100 cycles | |||
| MXene/PPy | Electrochemical polymerization & carambola-like composite (3D) | 1 M H2SO4 | — | 416 | — | 86.4% after 5000 cycles | 108 |
| Ti3C2Tx@PPy NW | In situ polymerization & porous composite structure (3D) | 3 M KOH | — | 610 | — | 100% after 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
107 |
3.3 MXene/PANI hybrid
At present, as a conductive polymer, polyaniline (PANI) is widely used for various purposes such as super capacitors, electrodes, electromagnetic shielding, wearable gas sensor and human motion monitoring sensor. The intrinsic conductivity, low cost, ease of processibility, thermal and environmental stability and faradaic pseudo capacitance makes PANI an excellent substrate for supercapacitor applications.115,116 The choice of PANI for the supercapacitor devices has been facing flaws, for example, PANI gets stacked during the preparation of thin film. This is because in aniline monomer, lone electron pair of nitrogen atom is attracted by the benzene due to the resonance, causing electron cloud in benzene structure. Thus, it aggregates in aqueous solution and shows improper film forming properties.117 To improve the low dispersibility of polyaniline and get better electrochemical performance, it is necessary to disperse the polyaniline uniformly, thus the interaction of MXene with PANI can solve the low dispersibility problem. Furthermore, it also solves the restacking problem as well as increase the interlayer space of MXene,118 improving the surface wettability of Ti3C2 for more active sites and provide faradaic reactions, thus improving the electrochemical performance.119 In the coming subsection, the preparation and application of MXene/PANI in supercapacitor is discussed.During the preparation process of MXene/PANI hybrids, polyaniline acts as a conductive bridge for linking adjacent layers of MXene together, accelerating the charge transfer among different MXene layers.127 The anchored PANI on the MXene surface can provide many active sites for rapidly transferring electrolyte ions.128 In case of MXene/PANI hybrids, positively charged aniline and negatively charged functional groups (e.g., Ti–OH– and Ti–F–) on the surface of MXene would attract each other. The electrostatic interactions promote nanostructured PANI anchored on the surface of MXene, and then the formed PANI nanostructures prevent MXene layers from stacking and collapsing.120 Cai et al.129 proposed possible mechanism of polyaniline and MXene. They mentioned that cationic radicals of aniline monomer are produced during the polymerization of aniline. Negatively charged Ti3C2Tx nanosheets are able to attract positively charged radicals by electrostatic adsorption, as demonstrated in Fig. 6(a).129 Thus, aniline monomers can be anchored on the surface of Ti3C2Tx nanosheets, and the oxygen and hydroxyl functional groups act as anchored sites.129
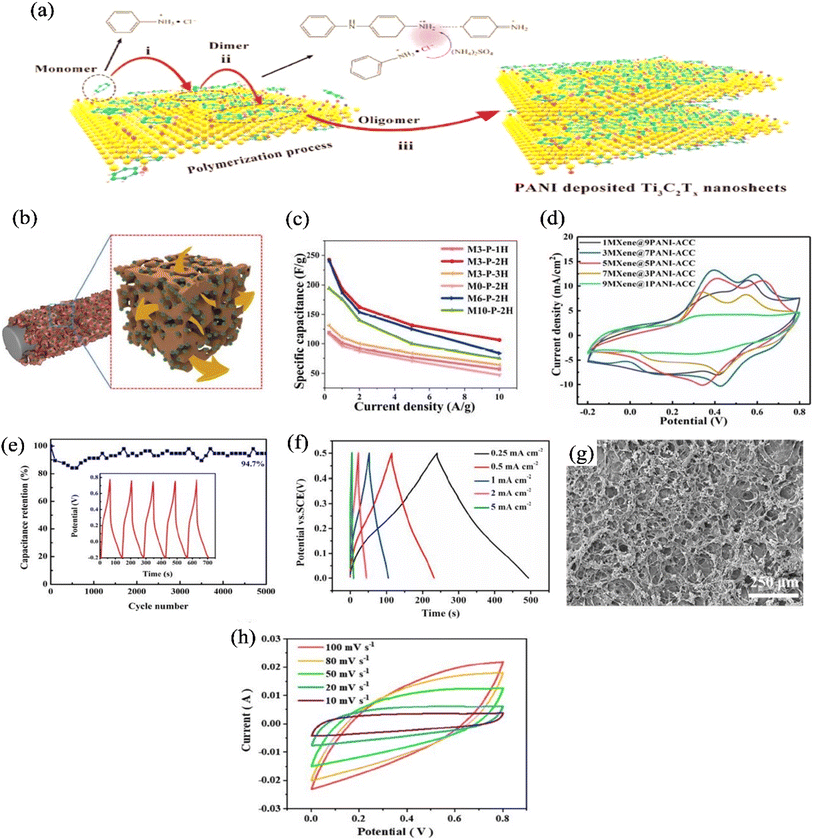 | ||
| Fig. 6 (a) Schematic illustration of MXene/PANI polymerization mechanism,129 (b) ion migration of MXene/PANI coated activated carbon cloth;130 (c) capacitive performance of CF@MXene/PANI composite fiber with different mass loading of MXene and polymerization time;131 (d) CV curves of MXene/PANI coated activated carbon cloth electrode with different mass ratio of MXene and PANI;130 (e) cyclic performance of Ti3C2/PANI-NT electrodes (5000 cycles at 1 A g−1), the inset exhibits the GCD curves of the last five charge–discharge cycles;132 (f) GCD curve of organ-like Ti3C2 MXenes/polyaniline hybrids at different current density with almost triangular characteristics;133 (g) SEM image of PANI@Ti3C2Tx/PVA hydrogel with sponge structure;134 (h) cyclic voltammetry (CV) curve of PANI@Ti3C2Tx/PVA with rectangular shape and broad redox peak.134 | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 in lieu of using 1
7 in lieu of using 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, 5
9, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 7
5, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 9
3, 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mass ratio, because 3MXene@7PANI carbon cloth exhibited the largest peak current and integration area than other composition, as shown in Fig. 6(d), confirming the highest capacity.130 From the above discussion, it can be conferred that excess amount of MXene or PANI can impede the individual capacitive performance which may not allow us the purpose of using MXene/PANI composites; therefore, before using MXene/PANI based composite fiber in supercapacitor applications, the content of MXene/PANI must be optimized.
1 mass ratio, because 3MXene@7PANI carbon cloth exhibited the largest peak current and integration area than other composition, as shown in Fig. 6(d), confirming the highest capacity.130 From the above discussion, it can be conferred that excess amount of MXene or PANI can impede the individual capacitive performance which may not allow us the purpose of using MXene/PANI composites; therefore, before using MXene/PANI based composite fiber in supercapacitor applications, the content of MXene/PANI must be optimized.| Hybrid materialsa | Preparation method & structure | Electrolyte | Optimum A. C.a (F cm−2) | Optimum G. C.a (F g−1) | Optimum V. C.a (F cm−3) | C. R.a | Ref. |
|---|---|---|---|---|---|---|---|
| a A. C. = Areal Capacitance, G. C. = Gravimetric Capacitance, V. C. = Volumetric Capacitance and C. R. = Capacitance Retention, PANI = Polyaniline. | |||||||
| Ti3C2Tx/PANI/carbon fiber | Drop coating & hierarchical structures (1D) | 1 M H2SO4 | 1.347 | — | — | 81% after 5000 cycles | 130 |
| CF@Ti3C2Tx/PANI | In situ “co-growth” & 1D fiber with 3D coating layer. | 1 M H2SO4 | — | 193.75 | — | 89% after 2000 cycles | 131 |
| Ti3C2Tx/PANI | Oxidant free in situ polymerization, freestanding hybrid film (2D) | 3 M H2SO4 | — | 503 | 1682 | 98.3% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
118 |
| Ti3C2/PANI-nanotube | In situ polymerization, composite film (2D) | 1 M H2SO4 | — | 596.6 | — | 94.7% after 5000 cycles | 132 |
| Ti3C2Tx/PANI | Chemical oxidative polymerization, composite film (2D) | 1 M H2SO4 | — | 556.2 | — | 91.6% after 5000 cycles | 142 |
| PANI/Ti3C2Tx | Self-assembly strategy, nanohybrid film (2D) | 1 M H2SO4 | — | 462 | — | 84.5% after 5000 cycles | 143 |
| Graphene decorated Ti3C2Tx/PANI | In situ oxidative polymerization, composite film (2D) | 1 M H2SO4 | — | 452 | 606 | 80.4% after 5000 cycles | 144 |
| Ti3C2/PANI | Electrochemical polymerization, organ like composite (2D) | 0.5 M H2SO4 | 0.228 | — | — | 85% after 1000 cycles | 133 |
| PANI/Ti3C2 | In situ polymerization, composite film (2D) | 1 M Na2SO4 | — | 164 | — | 96% after 3000 cycles | 119 |
| Ti3C2Tx/PANI | Electrostatic self-assembly, porous sandwich structured (3D) | — | 0.959 | 645.7 | — | 98% after 5000 cycles | 145 |
| PANI@Ti3C2Tx/PVA | Sol gel and in situ polymerization porous sponge structure (3D) | PVA/H2SO4 | 0.103 | — | — | 99% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
134 |
| Ti3C2Tx/PANI | Hydrothermal reaction, hierarchical architecture (3D) | 6 M KOH | — | 563 | — | 95.15% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
127 |
| Ti3C2Tx/PANI | Solvent-assisted self-assembly and blade coating, composite film (2D) | 1 M H2SO4 | — | 560 | 1167 | 97.5% after 5000 cycles | 135 |
| PANI/Ti3C2Tx | Self-assembly, porous structure (3D) | 3 M H2SO4 | — | 510 | 1632 | 85.7% from 1 to 100 A g−1 | 146 |
3.4 MXene/graphene hybrid
2D graphene material possesses excellent electrical, thermal and mechanical properties for which this notable material has attracted scientists' attention to fabricate supercapacitor-based devices. Furthermore, graphene has a broad operating area (2630 m2 g−1) and light weight structure, making graphene a great material to prepare supercapacitor-based devices.147 Although the use of graphene materials (GO and rGO) enhances the electrochemical performance, it has some shortcomings like π–π bond attraction that enhances the aggregation of individual graphene suspension for which it may surpass the use of individual graphene based material for supercapacitor applications.148Fabricating MXene/graphene hybrid material can solve the aforementioned problems. During the fabrication of MXene/graphene hybrids, MXene material intercalate into the graphene sheets, thus solving the aggregation problems of graphene and the hydrophilicity of MXene can improve electrochemical performance of MXene/graphene hybrid materials. For the supercapacitor applications, graphene oxide (GO) and reduced graphene oxide (rGO), derivatives of graphene, are currently extensively used. In this section, the preparation and supercapacitors application of MXene-based graphene hybrid material are highlighted.
In addition to the 2D composite film structure shown in Fig. 7(a),149 3D like hydrogel of MXene/rGO hybrids was also fabricated for supercapacitor application. Through a graphene oxide (GO)– aided self-assembly technique, Chen et al.151 developed 3D macroscopic hydrogel with enhanced porous structure. Regarding this, they kept Ti3C2Tx and GO mixture solution at 70 °C under N2 atmosphere for 30 hours in the presence of NaHSO3. The as-prepared hydrogel, depicted in Fig. 7(b) was washed with DI water. Furthermore, in order to prevent MXene from oxidizing, Zhao et al.154 added ascorbic acid to the Ti3C2Tx and GO suspension that was then undergone hydrothermal treatment at 65 °C for 3 hours. After cooling down, the resultant hydrogel was dialyzed in ethanol solvent for 6 hours followed by freeze drying. The as-prepared hydrogel exhibited high-conductive 3D Ti3C2Tx/rGO porous structure. Shao et al.155 and Saha et al.156 also prepared MXene/rGO gel like electrodes for supercapacitor application.
 | ||
| Fig. 7 (a) Flexible (i) and freestanding (ii) MXene/rGO hybrid film,149 (b) (i) synthesis process of Ti3C2Tx/rGO hydrogel (ii), digital and SEM image of Ti3C2Tx/rGO,151 (c) rectangular shape of MXene/rGO (i) under relaxed state indicating double layer capacitive behavior, identical CV curves of MXene/rGO (ii) under different stretch condition indicating excellent electrochemical properties,152 (d) triangle like symmetrical GCD curve of lignosulphonate modified MXene/rGO hybrid,153 indicating the pseudocapacitive behavior. | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. Zhou et al.152 fabricated Ti3C2Tx/rGO hybrid materials for stretchable supercapacitor applications. Their prepared electrode showed almost rectangular CV curve at relaxed state under different scan rates resulting double layer capacitive behavior. Moreover, the identical CV curve under different strains proved excellent electrochemical and structural integrity under larger strains. Fig. 7(c) shows the CV curve at relaxed state and deformation state.152 Ma et al.153 followed a novel strategy where they modified MXene surface with lignosulfonate, by products of the sulfite process in the wood pulping process, and they took the advantage π–π interaction between lignosulfonate and graphene to form a 3D ultrathick aerogel structure. The as-prepared aerogel structure showed highly symmetrical GCD curve, as shown in Fig. 7(d), with 386 F g−1 specific capacitance which indicated a high coulombic efficiency and higher capacitive behavior. The developed 3D structure of graphene incorporated MXene hybrid material can enhance the potential window due to the enhanced interlayer space and the faster ion diffusion of MXene hybrids.163 This may result in enhanced electrochemical performance of electrodes for supercapacitor application. In addition to the 3D structure, Xu et al.164 prepared micro-supercapacitors by MXene/rGO (EGMX) hybrid film, showing an excellent volumetric and gravimetric capacitance of 370 F cm−3 and 405 F g−1, respectively. An overall outlook of MXene/graphene hybrids in supercapacitor application is presented in Table 5.
000 cycles. Zhou et al.152 fabricated Ti3C2Tx/rGO hybrid materials for stretchable supercapacitor applications. Their prepared electrode showed almost rectangular CV curve at relaxed state under different scan rates resulting double layer capacitive behavior. Moreover, the identical CV curve under different strains proved excellent electrochemical and structural integrity under larger strains. Fig. 7(c) shows the CV curve at relaxed state and deformation state.152 Ma et al.153 followed a novel strategy where they modified MXene surface with lignosulfonate, by products of the sulfite process in the wood pulping process, and they took the advantage π–π interaction between lignosulfonate and graphene to form a 3D ultrathick aerogel structure. The as-prepared aerogel structure showed highly symmetrical GCD curve, as shown in Fig. 7(d), with 386 F g−1 specific capacitance which indicated a high coulombic efficiency and higher capacitive behavior. The developed 3D structure of graphene incorporated MXene hybrid material can enhance the potential window due to the enhanced interlayer space and the faster ion diffusion of MXene hybrids.163 This may result in enhanced electrochemical performance of electrodes for supercapacitor application. In addition to the 3D structure, Xu et al.164 prepared micro-supercapacitors by MXene/rGO (EGMX) hybrid film, showing an excellent volumetric and gravimetric capacitance of 370 F cm−3 and 405 F g−1, respectively. An overall outlook of MXene/graphene hybrids in supercapacitor application is presented in Table 5.
| Hybrid materialsa | Preparation method and structure | Electrolyte | Optimum A. C.a (F cm−2) | Optimum G. C.a (F g−1) | Optimum V. C.a (F cm−3) | C. R.a | Ref. |
|---|---|---|---|---|---|---|---|
| a A. C. = Areal Capacitance, G. C. = Gravimetric Capacitance, V. C. = Volumetric Capacitance, C. R. = Capacitance Retention, EG = Exfoliated Graphene, MSC = Micro Supercapacitor, ASSSs = All-Solid-State Supercapacitors, rGO = reduced Graphene Oxide. | |||||||
| Ti3C2Tx/EG | Self-assembly and vacuum filtration, thin film (2D) | PVA/H3PO4 gel | — | — | 216 for ASSSs | 85.2% after 2500 cycles for ASSSs | 160 |
| 0.00326 for MSCs | 33 | 82% after 2500 cycles | |||||
| Ti3C2Tx/rGO | Self-assembly and filtration, composite film (2D) | 2 M KOH | — | 154.3 | — | 85% after 6000 cycles | 159 |
| rGO/Ti3C2Tx | GO/Ti3C2Tx solution was thermally reduced to obtain rGO/Ti3C2Tx, porous composite film (2D) | 6 M KOH | — | 405 | 370 | No change after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
164 |
| MXene/rGO | Wet spinning strategy, fiber structure (1D) | 1 M H2SO4 | 0.372 | — | 586.40 | Excellent cycling after 3000 cycles | 157 |
| Ti3C2Tx/rGO | Electrostatic self-assembly, composite film (2D) | 3 M H2SO4 | — | 335.4 | 1040 | No degradation after 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
149 |
| Ti3C2Tx/rGO | Self-assembly and freeze drying, aerogel architecture (3D) | 1 M H2SO4 | 0.346 | — | — | 91% after 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
165 |
| Ti3C2Tx/rGO | Self-assembly of MXene and holey graphene oxide, followed by an annealing, composite film (2D) | 3 M H2SO4 | — | 438 | 1445 | 93% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
161 |
| Ti3C2Tx/rGO | In situ reduction and thermal annealing process, aerogel structure (3D) | 6 M KOH | — | 345 | — | 85% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
162 |
3.5 MXene/nanocellulose hybrid
With the increasing demand for energy storage devices and the growing concern of environmental problems, natural resources have been explored extensively to fabricate supercapacitor devices. Due to the attractive properties such as large surface area, exceptional chemical structure and high porosity, nanocellulose drew a great deal of the attention of large number of scientists to develop supercapacitor devices. Furthermore, due to light the weight characteristics of nanocellulose, it can be used as a suitable substrate for the fabrication of next generation wearable supercapacitor-based devices. Although nanocellulose is an insulating material, surface modification is possible due to the abundant hydroxyl group of nanocellulose that allows it to act as a binder of active material for supercapacitor related application.166 In addition, the porous structure of nanocellulose allows the ions transportation through nanocellulose based electrodes, thus enhancing the electrochemical performance of supercapacitor.167 In spite of having great potentiality to use nanocellulose in fabricating supercapacitor, the composition of nanocellulose base electrode materials (ratio of nanocellulose and active material such as CNT, rGO and MXene) need to be optimized to get the best performance from supercapacitor devices. Among the different active materials, MXene, a newly discovered transitional materials, were used extensively for electrode materials for its high metallic conductivity that can reach up to 8000 S cm−1.168,169 However, for supercapacitor application, MXene have been suffering from restacking problem resulting in poor ion transportation. This flaw, can be solved by incorporating nanocellulose with MXene, resulting in an increase of the interlayer spacing of MXene. This facilitates the ion transportation path and further enhances the electrochemical performance of supercapacitor. In this section, the preparation process and the application of MXene/nanocellulose hybrids in supercapacitor are highlighted. | ||
| Fig. 8 (a) SEM image of the layer by layer composition of MXene/cellulose,173 (b) zeta potential distribution of the MXene nanosheets dispersed in water,174 (c) hydrogen bonding between MXene sheets and CNC,175 (d) schematic illustration of wet spinning of LC-MXene/CNC fibers176 (e) enhanced MXene layered space when incorporating with cellulose,177 (f) CV curves for MXene paper, MXene aerogel, and functionalized MXene aerogel composite,178 (g) schematic diagram of ionic transport pathway of MXene film and 3D like MXene/bacterial cellulose composite.179 | ||
During the exfoliation process of MXene from MAX phase by using different etching agents such as HF, NaOH, H3PO4 and LiF, abundant terminating groups (–OH, –O and –F) are usually induced. This negative terminating group of MXene can be confirmed by negative zeta potential, as it can be seen in Fig. 8b,174 which can form hydrogen bond with the hydroxyl group (–OH) of cellulose, providing strong bonding with the interface.181 Moreover, the polar groups of both MXene and cellulose possess strong interaction via hydrogen bonding, facilitating the solution mixture of MXene and nanocellulose to get the hybrid film. Fig. 8(c) shows the hydrogen bonding of MXene and nanocellulose.175
Although nanocellulose can facilitate ion transportation between the MXene nanosheets, it may sometimes slightly decrease the electrochemical performance because of being an electrochemically inactive material. Tian et al. showed that 5%, 10% and 20% loading of CNF with Ti3C2Tx exhibited tensile strength of 139 MPa, 181 MPa and 340 MPa with decreasing capacitance of 369 F g−1, 324 F g−1 and 298 F g−1, respectively.190 This slight reduction of capacitance with enhanced mechanical strength does not limit the ion transportation for supercapacitor application, thus proving the practical application of CNF/Ti3C2Tx hybrid film.190
However, introducing additional active material with 3D MXene/cellulose hybrids can improve the mechanical and electrochemical performance with multifunctional applications which may prove the promising wearable electronics. It has been found that, Cai et al.194 introduced in situ grown SnS2 onto MXene nanosheets followed by adding CNF. By adding SnS2, extra H+ storage is achieved during the charge–discharge process which contributed specific capacitance of 171.6 F g−1 with high mechanical strength (78.3 MPa).194 Moreover, more H+ transport were activated by SnS2 under solar intensity that contributed 60% increase in capacitance under solar intensity of 1 kW m−2.194 Besides, 3D like MXene/Ag nanowires (NWs)/cellulose composite displayed a high capacitance of 505 F g−1 with excellent conductivity, 58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 843 S m−1, and mechanical properties, tensile strength of 34 MPa and Young's modulus of 6 GPa.195
843 S m−1, and mechanical properties, tensile strength of 34 MPa and Young's modulus of 6 GPa.195
3.6 Challenges and future perspective
MXene is a newly discovered material with high electrical conductivity, excellent hydrophilicity characteristic due to the surface terminating groups and also it has an intrinsic capability for the fabrication of electrode materials in supercapacitor related applications. Due to the tunable surface groups of MXene and synthesis process of multiple MXene compositions, there may exist some problems for the fabrication of MXene based hybrids electrodes which should have been introduced properly. For example,• There exists almost 20 different MXene composition and during etching of “A” element from MAX phases, tunable functional groups appeared on MXene surface. For this etching of MAX phase, different etching elements and synthesis conditions are applied, as summarized in Table 1. This synthesis procedure led to producing multilayered MXene of different compositions and wide variety of surface groups. Therefore, it is needed to further study to address the cause and solution of restacking problems of different MXene compositions. Moreover, the fabrication process of MXene hybrids should also be investigated to get the best output of MXene based electrodes. Furthermore, the most popular etching methods used to synthesis MXene, HF and LiF/HCL, are considered hazardous procedures. Therefore, due to the over growing concern of the environment, it is urgent to explore new environmentally friendly process to synthesis MXene.
• During the process of individual MXene material, aggregation problem appears due to its strong hydrophilicity that may reduce the electrochemical performance of MXene materials. For this reason, preparation of MXene based hybrid materials is the best solution in this regard. Introducing active materials with the MXene can increase the interlayer spacing and further solve the stacking problem of MXne, thus allowing the use as electrode material in supercapacitor related applications. However, the ratio of MXene and hybridized material during the preparation of electrodes should be properly investigated to guarantee high performance for supercapacitor-based application.
• Furthermore, the investigation of electrolyte performance for supercapacitor applications is needed. The capacitance property of MXene based hybrid materials largely depends on the electrolyte. There are several electrolytes used such as aqueous electrolyte, ionic electrolyte and organic electrolyte. There is different composition of MXene, and thus there are many possible MXene based hybridization compositions, so the influence of electrolyte on the performance of MXene hybridized materials should be studied elaborately for supercapacitor applications.
4 Conclusion
MXenes, derived from MAX phases, show great promise for supercapacitor applications due to their high conductivity, hydrophilic nature, and customizable surface chemistry; however, issues like self-restacking, oxidation, and limited ion transport restrict their full potential. By combining MXenes with carbon materials, conducting polymers, and metal oxides, researchers can significantly improve their electrochemical performance through enhanced charge storage, cyclic stability, and ion diffusion. Designing MXene-based structures in 1D, 2D, and 3D formats further optimizes electrolyte access and charge transport. Despite these advancements, challenges remain in scaling up production, achieving long-term stability, and ensuring electrolyte compatibility. Future research should prioritize developing scalable synthesis methods, innovative hybridization strategies, and environmentally friendly processing techniques to enable MXene-based supercapacitors to bridge the gap between high energy and power density, paving the way for next-generation energy storage technologies.Data availability
No primary research results, software or code have been included, and no new data were generated or analysed as part of this review.Author contributions
Tamal K. Paul: conceptualized the main idea, structured, and wrote the original manuscript; Md. Abdul Khaleque: wrote and revised of the manuscript; Md. Romzan Ali: wrote and revised the manuscript; Mohamed Aly Saad Aly: updated the main concepts and ideas, wrote, reviewed, and edited the manuscript, supervised and evaluated the overall work; Md. Sadek Bacchu: wrote sections of the manuscript; Saidur Rahman: designed, wrote, reviewed and edited sections of the manuscript; Md. Zaved Hossain Khan: contributed to the main concept, supervised and evaluated the overall concepts.Conflicts of interest
The authors declare that there is no conflict of interest.Acknowledgements
This research received specific funding facilities from Georgia Tech Shenzhen Institute (GTSI).References
- Y. Tian, et al., N-doped graphitic carbon encapsulating cobalt nanoparticles derived from novel metal–organic frameworks for electrocatalytic oxygen evolution reaction, Chin. Chem. Lett., 2023, 34(8), 108056, DOI:10.1016/j.cclet.2022.108056.
- J. Huang, B. G. Sumpter and V. Meunier, Theoretical Model for Nanoporous Carbon Supercapacitors, Angew. Chem., 2008, 120(3), 530–534, DOI:10.1002/ANGE.200703864.
- P. Liu, Z. Song, L. Miao, Y. Lv, L. Gan and M. Liu, Boosting Spatial Charge Storage in Ion-Compatible Pores of Carbon Superstructures for Advanced Zinc-Ion Capacitors, Small, 2024, 20(32), 2400774, DOI:10.1002/smll.202400774.
- X. Zang, et al., Enhancing Capacitance Performance of Ti3C2Tx MXene as Electrode Materials of Supercapacitor: From Controlled Preparation to Composite Structure Construction, Nano-Micro Lett., 2020, 12(1), 77, DOI:10.1007/s40820-020-0415-5.
- J. Guo, et al., Modular assembly of superstructures from polyphenol-functionalized building blocks, Nat. Nanotechnol., 2016, 11(12), 1105–1111, DOI:10.1038/nnano.2016.172.
- Y. Wang, et al., Recent progress in carbon-based materials for supercapacitor electrodes: a review, J. Mater. Sci., 2021, 56(1), 173–200, DOI:10.1007/s10853-020-05157-6.
- M. Cui and X. Meng, Overview of transition metal-based composite materials for supercapacitor electrodes, Nanoscale Adv., 2020, 2(12), 5516–5528, 10.1039/D0NA00573H.
- I. Shown, A. Ganguly, L. Chen and K. Chen, Conducting polymer-based flexible supercapacitor, Energy Sci. Eng., 2015, 3(1), 2–26, DOI:10.1002/ese3.50.
- M. Naguib, et al., Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2, Adv. Mater., 2011, 23(37), 4248–4253, DOI:10.1002/adma.201102306.
- B. Anasori, M. R. Lukatskaya and Y. Gogotsi, 2D metal carbides and nitrides (MXenes) for energy storage, Nat. Rev. Mater., 2017, 2(2), 16098, DOI:10.1038/natrevmats.2016.98.
- A. Sohan, P. Banoth, M. Aleksandrova, A. Nirmala Grace and P. Kollu, Review on MXene synthesis, properties, and recent research exploring electrode architecture for supercapacitor applications, Int. J. Energy Res., 2021, 45(14), 19746–19771, DOI:10.1002/er.7068.
- M. Hu, H. Zhang, T. Hu, B. Fan, X. Wang and Z. Li, Emerging 2D MXenes for supercapacitors: status, challenges and prospects, Chem. Soc. Rev., 2020, 49(18), 6666–6693, 10.1039/D0CS00175A.
- L. Huang, L. Ding and H. Wang, MXene-Based Membranes for Separation Applications, Small Sci., 2021, 1(7), 2100013, DOI:10.1002/smsc.202100013.
- L. Luo, et al., Flexible and free-standing MXene decorated biomass-derived carbon cloth membrane anodes for superior lithium-ion capacitors, J. Energy Storage, 2024, 103(PB), 114430, DOI:10.1016/j.est.2024.114430.
- R. B. Rakhi, B. Ahmed, D. Anjum and H. N. Alshareef, Direct Chemical Synthesis of MnO 2 Nanowhiskers on Transition-Metal Carbide Surfaces for Supercapacitor Applications, ACS Appl. Mater. Interfaces, 2016, 8(29), 18806–18814, DOI:10.1021/acsami.6b04481.
- T. Shang, et al., 3D Macroscopic Architectures from Self-Assembled MXene Hydrogels, Adv. Funct. Mater., 2019, 29(33), 1903960, DOI:10.1002/adfm.201903960.
- M. Guo, C. Liu, Z. Zhang, J. Zhou, Y. Tang and S. Luo, Flexible Ti 3 C 2 T x @Al electrodes with Ultrahigh Areal Capacitance: In Situ Regulation of Interlayer Conductivity and Spacing, Adv. Funct. Mater., 2018, 28(37), 1803196, DOI:10.1002/adfm.201803196.
- H. Wang, et al., In situ polymerized Ti3C2Tx/PDA electrode with superior areal capacitance for supercapacitors, J. Alloys Compd., 2019, 778, 858–865, DOI:10.1016/j.jallcom.2018.11.172.
- D. Liu, et al., Highly Sensitive Multifunctional Electronic Skin Based on Nanocellulose/MXene Composite Films with Good Electromagnetic Shielding Biocompatible Antibacterial Properties, Biomacromolecules, 2022, 23(1), 182–195, DOI:10.1021/acs.biomac.1c01203.
- W. Luo, et al., Overview of MXene/conducting polymer composites for supercapacitors, J. Energy Storage, 2022, 52(PB), 105008, DOI:10.1016/j.est.2022.105008.
- S. A. Thomas, A. Patra, B. M. Al-Shehri, M. Selvaraj, A. Aravind and C. S. Rout, MXene based hybrid materials for supercapacitors: Recent developments and future perspectives, J. Energy Storage, 2022, 55(PD), 105765, DOI:10.1016/j.est.2022.105765.
- J. Orangi and M. Beidaghi, A Review of the Effects of Electrode Fabrication and Assembly Processes on the Structure and Electrochemical Performance of 2D MXenes, Adv. Funct. Mater., 2020, 30(47), 2005305, DOI:10.1002/adfm.202005305.
- S. Panda, K. Deshmukh, S. K. Khadheer Pasha, J. Theerthagiri, S. Manickam and M. Y. Choi, MXene based emerging materials for supercapacitor applications: Recent advances, challenges, and future perspectives, Coord. Chem. Rev., 2022, 462, 214518, DOI:10.1016/j.ccr.2022.214518.
- A. Jawaid, et al., Halogen Etch of Ti 3 AlC 2 MAX Phase for MXene Fabrication, ACS Nano, 2021, 15(2), 2771–2777, DOI:10.1021/acsnano.0c08630.
- K. R. G. Lim, M. Shekhirev, B. C. Wyatt, B. Anasori, Y. Gogotsi and Z. W. Seh, Fundamentals of MXene synthesis, Nat. Synth., 2022, 1(8), 601–614, DOI:10.1038/s44160-022-00104-6.
- Y. Meng, et al., Fast Li + diffusion in interlayer-expanded vanadium disulfide nanosheets for Li +/Mg 2+ hybrid-ion batteries, J. Mater. Chem. A, 2018, 6(14), 5782–5788, 10.1039/C8TA00418H.
- A. VahidMohammadi, A. Hadjikhani, S. Shahbazmohamadi and M. Beidaghi, Two-Dimensional Vanadium Carbide (MXene) as a High-Capacity Cathode Material for Rechargeable Aluminum Batteries, ACS Nano, 2017, 11(11), 11135–11144, DOI:10.1021/acsnano.7b05350.
- M. Naguib, et al., New Two-Dimensional Niobium and Vanadium Carbides as Promising Materials for Li-Ion Batteries, J. Am. Chem. Soc., 2013, 135(43), 15966–15969, DOI:10.1021/ja405735d.
- B. Soundiraraju and B. K. George, Two-Dimensional Titanium Nitride (Ti 2 N) MXene: Synthesis, Characterization, and Potential Application as Surface-Enhanced Raman Scattering Substrate, ACS Nano, 2017, 11(9), 8892–8900, DOI:10.1021/acsnano.7b03129.
- X. Yuan, M. Zhang and Y. Wu, MXene-based sensors for detecting human physiological information, Sens. Mater., 2020, 32(12), 4047–4065, DOI:10.18494/SAM.2020.2990.
- P. K. Kalambate, et al., Recent advances in MXene–based electrochemical sensors and biosensors, TrAC, Trends Anal. Chem., 2019, 120, 15643, DOI:10.1016/j.trac.2019.115643.
- B. Anasori, et al., Two-Dimensional, Ordered, Double Transition Metals Carbides (MXenes), ACS Nano, 2015, 9(10), 9507–9516, DOI:10.1021/acsnano.5b03591.
- J. Pang, et al., Applications of 2D MXenes in energy conversion and storage systems, Chem. Soc. Rev., 2019, 48(1), 72–133, 10.1039/C8CS00324F.
- B. Anasori, et al., A Tungsten-Based Nanolaminated Ternary Carbide: (W,Ti) 4 C 4– x, Inorg. Chem., 2019, 58(2), 1100–1106, DOI:10.1021/acs.inorgchem.8b02226.
- J. Zhou, et al., A Two-Dimensional Zirconium Carbide by Selective Etching of Al 3 C 3 from Nanolaminated Zr 3 Al 3 C 5, Angew. Chem., Int. Ed., 2016, 55(16), 5008–5013, DOI:10.1002/anie.201510432.
- J. Zhou, et al., Synthesis and Electrochemical Properties of Two-Dimensional Hafnium Carbide, ACS Nano, 2017, 11(4), 3841–3850, DOI:10.1021/acsnano.7b00030.
- S. Kajiyama, et al., Sodium-Ion Intercalation Mechanism in MXene Nanosheets, ACS Nano, 2016, 10(3), 3334–3341, DOI:10.1021/acsnano.5b06958.
- J. Halim, et al., Synthesis and Characterization of 2D Molybdenum Carbide (MXene), Adv. Funct. Mater., 2016, 26(18), 3118–3127, DOI:10.1002/adfm.201505328.
- Y. Guo, et al., Synthesis of two-dimensional carbide Mo2CTx MXene by hydrothermal etching with fluorides and its thermal stability, Ceram. Int., 2020, 46(11), 19550–19556, DOI:10.1016/j.ceramint.2020.05.008.
- K. Kor and K. Zarei, Development and characterization of an electrochemical sensor for furosemide detection based on electropolymerized molecularly imprinted polymer, Talanta, 2016, 146, 181–187, DOI:10.1016/j.talanta.2015.08.042.
- F. Liu, et al., Preparation of High-Purity V 2 C MXene and Electrochemical Properties as Li-Ion Batteries, J. Electrochem. Soc., 2017, 164(4), A709–A713, DOI:10.1149/2.0641704jes.
- M. Wu, Y. He, L. Wang, Q. Xia and A. Zhou, Synthesis and electrochemical properties of V2C MXene by etching in opened/closed environments, J. Adv. Ceram., 2020, 9(6), 749–758, DOI:10.1007/s40145-020-0411-8.
- L. Wang, D. Liu, W. Lian, Q. Hu, X. Liu and A. Zhou, The preparation of V2CTx by facile hydrothermal-assisted etching processing and its performance in lithium-ion battery, J. Mater. Res. Technol., 2020, 9(1), 984–993, DOI:10.1016/j.jmrt.2019.11.038.
- M. Wu, B. Wang, Q. Hu, L. Wang and A. Zhou, The Synthesis Process and Thermal Stability of V2C MXene, Materials, 2018, 11(11), 2112, DOI:10.3390/ma11112112.
- X. Sang, et al., Atomic Defects in Monolayer Titanium Carbide (Ti3C2Tx) MXene, ACS Nano, 2016, 10(10), 9193–9200, DOI:10.1021/acsnano.6b05240.
- M. Ghidiu, M. R. Lukatskaya, M.-Q. Zhao, Y. Gogotsi and M. W. Barsoum, Conductive two-dimensional titanium carbide ‘clay’ with high volumetric capacitance, Nature, 2014, 516(7529), 78–81, DOI:10.1038/nature13970.
- J. Yang, et al., Two-Dimensional Nb-Based M 4 C 3 Solid Solutions (MXenes), J. Am. Ceram. Soc., 2016, 99(2), 660–666, DOI:10.1111/jace.13922.
- J. Halim, et al., Transparent conductive two-dimensional titanium carbide epitaxial thin films, Chem. Mater., 2014, 26(7), 2374–2381, DOI:10.1021/cm500641a.
- C. Shen, et al., Synthesis and Electrochemical Properties of Two-Dimensional RGO/Ti3C2Tx Nanocomposites, Nanomaterials, 2018, 8(2), 80, DOI:10.3390/nano8020080.
- P. Urbankowski, et al., Synthesis of two-dimensional titanium nitride Ti 4 N 3 (MXene), Nanoscale, 2016, 8(22), 11385–11391, 10.1039/C6NR02253G.
- F. Liu, et al., Preparation of Ti 3 C 2 and Ti 2 C MXenes by fluoride salts etching and methane adsorptive properties, Appl. Surf. Sci., 2017, 416, 781–789, DOI:10.1016/j.apsusc.2017.04.239.
- K. Arole, et al., Water-dispersible Ti3C2Tz MXene nanosheets by molten salt etching, iScience, 2021, 24(12), 103403, DOI:10.1016/j.isci.2021.103403.
- P. K. Kannan, D. J. Late, H. Morgan and C. S. Rout, Recent developments in 2D layered inorganic nanomaterials for sensing, Nanoscale, 2015, 7(32), 13293–13312, 10.1039/C5NR03633J.
- X. Wu, P. Ma, Y. Sun, F. Du, D. Song and G. Xu, Application of MXene in Electrochemical Sensors: A Review, Electroanalysis, 2021, 33(8), 1827–1851, DOI:10.1002/elan.202100192.
- S. Sun, C. Liao, A. M. Hafez, H. Zhu and S. Wu, Two-dimensional MXenes for energy storage, Chem. Eng. J., 2018, 338, 27–45, DOI:10.1016/j.cej.2017.12.155.
- C. Peng, et al., A hydrothermal etching route to synthesis of 2D MXene (Ti3C2, Nb2C): Enhanced exfoliation and improved adsorption performance, Ceram. Int., 2018, 44(15), 18886–18893, DOI:10.1016/j.ceramint.2018.07.124.
- T. Li, et al., Fluorine-Free Synthesis of High-Purity Ti 3 C 2 T x (T=OH, O) via Alkali Treatment, Angew. Chem., Int. Ed., 2018, 57(21), 6115–6119, DOI:10.1002/anie.201800887.
- S. Yang, et al., Fluoride-Free Synthesis of Two-Dimensional Titanium Carbide (MXene) Using A Binary Aqueous System, Angew. Chem., Int. Ed., 2018, 57(47), 15491–15495, DOI:10.1002/anie.201809662.
- M. R. Lukatskaya, et al., Ultra-high-rate pseudocapacitive energy storage in two-dimensional transition metal carbides, Nat. Energy, 2017, 2(8), 17105, DOI:10.1038/nenergy.2017.105.
- J. C. Stallard, W. Tan, F. R. Smail, T. S. Gspann, A. M. Boies and N. A. Fleck, The mechanical and electrical properties of direct-spun carbon nanotube mats, Extreme Mech. Lett., 2018, 21, 65–75, DOI:10.1016/j.eml.2018.03.003.
- S. A. Romanov, A. A. Alekseeva, E. M. Khabushev, D. V. Krasnikov and A. G. Nasibulin, Rapid, efficient, and non-destructive purification of single-walled carbon nanotube films from metallic impurities by Joule heating, Carbon, 2020, 168, 193–200, DOI:10.1016/j.carbon.2020.06.068.
- S. Zhang, et al., Multiwall-carbon-nanotube/cellulose composite fibers with enhanced mechanical and electrical properties by cellulose grafting, RSC Adv., 2018, 8(11), 5678–5684, 10.1039/C7RA11304H.
- L. P. Yu, X. H. Zhou, L. Lu, L. Xu and F. J. Wang, MXene/Carbon Nanotube Hybrids: Synthesis, Structures, Properties, and Applications, ChemSusChem, 2021, 14(23), 5079–5111, DOI:10.1002/cssc.202101614.
- P. Yan, et al., Enhanced supercapacitive performance of delaminated two-dimensional titanium carbide/carbon nanotube composites in alkaline electrolyte, J. Power Sources, 2015, 284, 38–43, DOI:10.1016/j.jpowsour.2015.03.017.
- J. Yang, Z. Pan, J. Zhong, S. Li, J. Wang and P.-Y. Chen, Electrostatic self-assembly of heterostructured black phosphorus–MXene nanocomposites for flexible microsupercapacitors with high rate performance, Energy Storage Mater., 2021, 36, 257–264, DOI:10.1016/j.ensm.2020.12.025.
- D. Guo, et al., MXene based self-assembled cathode and antifouling separator for high-rate and dendrite-inhibited Li–S battery, Nano Energy, 2019, 61, 478–485, DOI:10.1016/j.nanoen.2019.05.011.
- H. Li, R. Chen, M. Ali, H. Lee and M. J. Ko, In Situ Grown MWCNTs/MXenes Nanocomposites on Carbon Cloth for High-Performance Flexible Supercapacitors, Adv. Funct. Mater., 2020, 30(47), 2002739, DOI:10.1002/adfm.202002739.
- C. Xiong, G. Y. Zhu, H. R. Jiang, Q. Chen and T. S. Zhao, Achieving multiplexed functionality in a hierarchical MXene-based sulfur host for high-rate, high-loading lithium-sulfur batteries, Energy Storage Mater., 2020, 33, 147–157, DOI:10.1016/j.ensm.2020.08.006.
- W. Zheng, P. Zhang, J. Chen, W. B. Tian, Y. M. Zhang and Z. M. Sun, In situ synthesis of CNTs@Ti 3 C 2 hybrid structures by microwave irradiation for high-performance anodes in lithium ion batteries, J. Mater. Chem. A, 2018, 6(8), 3543–3551, 10.1039/C7TA10394H.
- L. Lv, C. Guo, W. Sun and Y. Wang, Strong Surface-Bound Sulfur in Carbon Nanotube Bridged Hierarchical Mo 2 C-Based MXene Nanosheets for Lithium–Sulfur Batteries, Small, 2018, 15(3), 1804338, DOI:10.1002/smll.201804338.
- Q. Liu, et al., Fabrication of a fibrous MnO2@MXene/CNT electrode for high-performance flexible supercapacitor, Ceram. Int., 2020, 46(8), 11874–11881, DOI:10.1016/j.ceramint.2020.01.222.
- C. Yu, et al., A Solid-State Fibriform Supercapacitor Boosted by Host-Guest Hybridization between the Carbon Nanotube Scaffold and MXene Nanosheets, Small, 2018, 14(29), 1801203, DOI:10.1002/smll.201801203.
- M. Hu, et al., MXene-coated silk-derived carbon cloth toward flexible electrode for supercapacitor application, J. Energy Chem., 2018, 27(1), 161–166, DOI:10.1016/j.jechem.2017.10.030.
- G. Weng, et al., Layer-by-Layer Assembly of Cross-Functional Semi-transparent MXene-Carbon Nanotubes Composite Films for Next-Generation Electromagnetic Interference Shielding, Adv. Funct. Mater., 2018, 28(44), 1803360, DOI:10.1002/adfm.201803360.
- H. Wang, et al., High-Performance Foam-Shaped Strain Sensor Based on Carbon Nanotubes and Ti 3 C 2 T x MXene for the Monitoring of Human Activities, ACS Nano, 2021, 15(6), 9690–9700, DOI:10.1021/acsnano.1c00259.
- P. Sambyal, et al., Ultralight and Mechanically Robust Ti 3 C 2 T x Hybrid Aerogel Reinforced by Carbon Nanotubes for Electromagnetic Interference Shielding, ACS Appl. Mater. Interfaces, 2019, 11(41), 38046–38054, DOI:10.1021/acsami.9b12550.
- X. Zhao, J. Zhang, K. Lv, N. Kong, Y. Shao and J. Tao, Carbon nanotubes boosts the toughness and conductivity of wet-spun MXene fibers for fiber-shaped super capacitors, Carbon, 2022, 200(August), 38–46, DOI:10.1016/j.carbon.2022.08.045.
- Z. Wang, et al., High-Performance Biscrolled MXene/Carbon Nanotube Yarn Supercapacitors, Small, 2018, 14(37), 1802225, DOI:10.1002/smll.201802225.
- H. Chen, et al., Carbon nanotubes enhance flexible MXene films for high-rate supercapacitors, J. Mater. Sci., 2020, 55(3), 1148–1156, DOI:10.1007/s10853-019-04003-8.
- R. Yang, et al., Anchoring Oxidized MXene Nanosheets on Porous Carbon Nanotube Sponge for Enhancing Ion Transport and Pseudocapacitive Performance, ACS Appl. Mater. Interfaces, 2022, 14(37), 41997–42006, DOI:10.1021/acsami.2c10659.
- P. Zhang, et al., In Situ Ice Template Approach to Fabricate 3D Flexible MXene Film-Based Electrode for High Performance Supercapacitors, Adv. Funct. Mater., 2020, 30(47), 2000922, DOI:10.1002/adfm.202000922.
- K. Li, P. Zhang, R. A. Soomro and B. Xu, Alkali-Induced Porous MXene/Carbon Nanotube-Based Film Electrodes for Supercapacitors, ACS Appl. Nano Mater., 2022, 5(3), 4180–4186, DOI:10.1021/acsanm.2c00109.
- Q. Wang, J. Liu, G. Tian and D. Zhang, Co@N-CNT/MXenes in situ grown on carbon nanotube film for multifunctional sensors and flexible supercapacitors, Nanoscale, 2021, 13(34), 14460–14468, 10.1039/D1NR03641F.
- J. Xiao, J. Wen, J. Zhao, X. Ma, H. Gao and X. Zhang, A safe etching route to synthesize highly crystalline Nb2CTx MXene for high performance asymmetric supercapacitor applications, Electrochim. Acta, 2020, 337, 135803, DOI:10.1016/j.electacta.2020.135803.
- X. Gao, et al., Maximizing ion accessibility in MXene-knotted carbon nanotube composite electrodes for high-rate electrochemical energy storage, Nat. Commun., 2020, 11(1), 6160, DOI:10.1038/s41467-020-19992-3.
- M.-Q. Zhao, et al., Flexible MXene/Carbon Nanotube Composite Paper with High Volumetric Capacitance, Adv. Mater., 2015, 27(2), 339–345, DOI:10.1002/adma.201404140.
- Y. Sun, et al., Improved pseudocapacitances of supercapacitors based on electrodes of nitrogen-doped Ti3C2Tx nanosheets with in-situ growth of carbon nanotubes, J. Alloys Compd., 2021, 859, 158347, DOI:10.1016/j.jallcom.2020.158347.
- E. Kim, et al., Microsupercapacitor with a 500 nm gap between MXene/CNT electrodes, Nano Energy, 2021, 81, 105616, DOI:10.1016/j.nanoen.2020.105616.
- Q. Fu, et al., Self-assembled Ti3C2Tx/SCNT composite electrode with improved electrochemical performance for supercapacitor, J. Colloid Interface Sci., 2018, 511, 128–134, DOI:10.1016/j.jcis.2017.09.104.
- R. Wang, et al., MXene-carbon nanotubes layer-by-layer assembly based on-chip micro-supercapacitor with improved capacitive performance, Electrochim. Acta, 2021, 386, 138420, DOI:10.1016/j.electacta.2021.138420.
- Y.-L. Huang and S.-W. Bian, Vacuum-filtration assisted layer-by-layer strategy to design MXene/carbon nanotube@MnO 2 all-in-one supercapacitors, J. Mater. Chem. A, 2021, 9(37), 21347–21356, 10.1039/D1TA06089A.
- X. Li, J. Zhu, W. Liang and I. Zhitomirsky, MXene (Ti3C2Tx) anodes for asymmetric supercapacitors with high active mass loading, Mater. Chem. Phys., 2021, 268, 124748, DOI:10.1016/j.matchemphys.2021.124748.
- L. Gao, et al., MXene/Polymer Membranes: Synthesis, Properties, and Emerging Applications, Chem. Mater., 2020, 32(5), 1703–1747, DOI:10.1021/acs.chemmater.9b04408.
- X. Chen, et al., MXene/Polymer Nanocomposites: Preparation, Properties, and Applications, Polym. Rev., 2021, 61(1), 80–115, DOI:10.1080/15583724.2020.1729179.
- A. Brzózka, et al., Polypyrrole–Nickel Hydroxide Hybrid Nanowires as Future Materials for Energy Storage, Nanomaterials, 2019, 9(2), 307, DOI:10.3390/nano9020307.
- S. Seyedin, et al., Facile Solution Processing of Stable MXene Dispersions towards Conductive Composite Fibers, Glob. Chall., 2019, 3(10), 1900037, DOI:10.1002/gch2.201900037.
- Y. Tong, et al., Hybridizing polypyrrole chains with laminated and two-dimensional Ti3C2Tx toward high-performance electromagnetic wave absorption, Appl. Surf. Sci., 2018, 434, 283–293, DOI:10.1016/j.apsusc.2017.10.140.
- P. Chen, et al., Highly selective NH3 gas sensor based on polypyrrole/Ti3C2Tx nanocomposites operating at room temperature, J. Mater. Sci.:Mater. Electron., 2022, 33(9), 6168–6177, DOI:10.1007/s10854-022-07792-y.
- J. Shao, J.-W. Wang, D.-N. Liu, L. Wei, S.-Q. Wu and H. Ren, A novel high permittivity percolative composite with modified MXene, Polymer, 2019, 174, 86–95, DOI:10.1016/j.polymer.2019.04.057.
- E. Yildirim, et al., A Dual-Surface Mechanism of Oxidant-Free Pyrrole Polymerization in the Two-Dimensional Titanium Carbide (MXene) Interlayer Nanospace, J. Phys. Chem. C, 2022, 126(3), 1316–1325, DOI:10.1021/acs.jpcc.1c08231.
- C. Zhang, S. Xu, D. Cai, J. Cao, L. Wang and W. Han, Planar supercapacitor with high areal capacitance based on Ti3C2/Polypyrrole composite film, Electrochim. Acta, 2020, 330, 135277, DOI:10.1016/j.electacta.2019.135277.
- L. Yang, F. Lin, F. Zabihi, S. Yang and M. Zhu, High specific capacitance cotton fiber electrode enhanced with PPy and MXene by in situ hybrid polymerization, Int. J. Biol. Macromol., 2021, 181, 1063–1071, DOI:10.1016/j.ijbiomac.2021.04.112.
- M. Zhu, et al., Highly Flexible, Freestanding Supercapacitor Electrode with Enhanced Performance Obtained by Hybridizing Polypyrrole Chains with MXene, Adv. Energy Mater., 2016, 6(21), 1600969, DOI:10.1002/aenm.201600969.
- W. Zhang, et al., A multidimensional nanostructural design towards electrochemically stable and mechanically strong hydrogel electrodes, Nanoscale, 2020, 12(12), 6637–6643, 10.1039/D0NR01414A.
- M. Boota, B. Anasori, C. Voigt, M. Q. Zhao, M. W. Barsoum and Y. Gogotsi, Pseudocapacitive Electrodes Produced by Oxidant-Free Polymerization of Pyrrole between the Layers of 2D Titanium Carbide (MXene), Adv. Mater., 2016, 28(7), 1517–1522, DOI:10.1002/adma.201504705.
- Q. Fan, et al., Ti3C2-MXene composite films functionalized with polypyrrole and ionic liquid-based microemulsion particles for supercapacitor applications, Chem. Eng. J., 2021, 428, 131107, DOI:10.1016/j.cej.2021.131107.
- T. A. Le, N. Q. Tran, Y. Hong and H. Lee, Intertwined Titanium Carbide MXene within a 3 D Tangled Polypyrrole Nanowires Matrix for Enhanced Supercapacitor Performances, Chem.–Eur. J., 2018, 25(4), 201804291, DOI:10.1002/chem.201804291.
- X. Jian, et al., Three-dimensional carambola-like MXene/polypyrrole composite produced by one-step co-electrodeposition method for electrochemical energy storage, Electrochim. Acta, 2019, 318, 820–827, DOI:10.1016/j.electacta.2019.06.045.
- J. Cao, Y. Han, X. Zheng and Q. Wang, Preparation and electrochemical performance of modified Ti 3 C 2 T x/polypyrrole composites, J. Appl. Polym. Sci., 2019, 136(4), 47003, DOI:10.1002/app.47003.
- W. Liang and I. Zhitomirsky, MXene-polypyrrole electrodes for asymmetric supercapacitors, Electrochim. Acta, 2022, 406, 139843, DOI:10.1016/j.electacta.2022.139843.
- W. Wu, et al., Enhanced electrochemical performances of organ-like Ti3C2 MXenes/polypyrrole composites as supercapacitors electrode materials, Ceram. Int., 2019, 45(6), 7328–7337, DOI:10.1016/j.ceramint.2019.01.016.
- D. Wei, W. Wu, J. Zhu, C. Wang, C. Zhao and L. Wang, A facile strategy of polypyrrole nanospheres grown on Ti3C2-MXene nanosheets as advanced supercapacitor electrodes, J. Electroanal. Chem., 2020, 877, 114538, DOI:10.1016/j.jelechem.2020.114538.
- X. Li, et al., Interfacing MXene flakes on fiber fabric as an ultrafast electron transport layer for high performance textile electrodes, Energy Storage Mater., 2020, 33, 62–70, DOI:10.1016/j.ensm.2020.05.004.
- J. Yan, et al., Polypyrrole–MXene coated textile-based flexible energy storage device, RSC Adv., 2018, 8(69), 39742–39748, 10.1039/C8RA08403C.
- A. Khosrozadeh, M. A. Darabi, M. Xing and Q. Wang, Flexible Electrode Design: Fabrication of Freestanding Polyaniline-Based Composite Films for High-Performance Supercapacitors, ACS Appl. Mater. Interfaces, 2016, 8(18), 11379–11389, DOI:10.1021/acsami.5b11256.
- S. Zhang, G. Sun, Y. He, R. Fu, Y. Gu and S. Chen, Preparation, Characterization, and Electrochromic Properties of Nanocellulose-Based Polyaniline Nanocomposite Films, ACS Appl. Mater. Interfaces, 2017, 9(19), 16426–16434, DOI:10.1021/acsami.7b02794.
- H. Yu, P. Chen, W. Chen and Y. Liu, Effect of cellulose nanofibers on induced polymerization of aniline and formation of nanostructured conducting composite, Cellulose, 2014, 21(3), 1757–1767, DOI:10.1007/s10570-014-0189-3.
- A. VahidMohammadi, et al., Thick and freestanding MXene/PANI pseudocapacitive electrodes with ultrahigh specific capacitance, J. Mater. Chem. A, 2018, 6(44), 22123–22133, 10.1039/C8TA05807E.
- Y. Ren, et al., Synthesis of polyaniline nanoparticles deposited on two-dimensional titanium carbide for high-performance supercapacitors, Mater. Lett., 2018, 214, 84–87, DOI:10.1016/j.matlet.2017.11.060.
- Z. He, et al., Recent Advances in MXene/Polyaniline-Based Composites for Electrochemical Devices and Electromagnetic Interference Shielding Applications, ACS Omega, 2021, 6(35), 22468–22477, DOI:10.1021/acsomega.1c02996.
- X. Li, et al., Toward agricultural ammonia volatilization monitoring: A flexible polyaniline/Ti3C2T hybrid sensitive films based gas sensor, Sens. Actuators, B, 2020, 316, 128144, DOI:10.1016/j.snb.2020.128144.
- H. Wei, et al., Ti3C2Tx MXene/polyaniline (PANI) sandwich intercalation structure composites constructed for microwave absorption, Compos. Sci. Technol., 2019, 169, 52–59, DOI:10.1016/j.compscitech.2018.10.016.
- L. Zhao, K. Wang, W. Wei, L. Wang and W. Han, High-performance flexible sensing devices based on polyaniline/MXene nanocomposites, InfoMat, 2019, 1(3), 407–416, DOI:10.1002/inf2.12032.
- J. Yun, et al., Layer-by-Layer Assembly of Polyaniline Nanofibers and MXene Thin-Film Electrodes for Electrochemical Energy Storage, ACS Appl. Mater. Interfaces, 2019, 11(51), 47929–47938, DOI:10.1021/acsami.9b16692.
- G. Yin, Y. Wang, W. Wang, Z. Qu and D. Yu, A Flexible Electromagnetic Interference Shielding Fabric Prepared by Construction of PANI/MXene Conductive Network via Layer-by-Layer Assembly, Adv. Mater. Interfaces, 2021, 8(6), 2001893, DOI:10.1002/admi.202001893.
- X. Jia, B. Shen, L. Zhang and W. Zheng, Construction of compressible Polymer/MXene composite foams for high-performance absorption-dominated electromagnetic shielding with ultra-low reflectivity, Carbon, 2021, 173, 932–940, DOI:10.1016/j.carbon.2020.11.036.
- Y. Li, P. Kamdem and X.-J. Jin, Hierarchical architecture of MXene/PANI hybrid electrode for advanced asymmetric supercapacitors, J. Alloys Compd., 2021, 850, 156608, DOI:10.1016/j.jallcom.2020.156608.
- X. Lu, J. Zhu, W. Wu and B. Zhang, Hierarchical architecture of PANI@TiO2/Ti3C2Tx ternary composite electrode for enhanced electrochemical performance, Electrochim. Acta, 2017, 228, 282–289, DOI:10.1016/j.electacta.2017.01.025.
- M. Cai, et al., Ti3C2Tx/PANI composites with tunable conductivity towards anticorrosion application, Chem. Eng. J., 2021, 410, 128310, DOI:10.1016/j.cej.2020.128310.
- W. L. Liu, et al., High-performance supercapacitor electrodes of MXene/PANI/carbon fiber hybrid composites with 2D/0D/1D hierarchical nanostructures, J. Alloys Compd., 2022, 926, 166855, DOI:10.1016/j.jallcom.2022.166855.
- L. Cheng, Y. Qu and J. Sun, Preparation of CF@MXene/PANI fiber electrodes for high-performance flexible supercapacitors, J. Mater. Sci.:Mater. Electron., 2023, 34(2), 1–12, DOI:10.1007/s10854-022-09562-2.
- W. Wu, C. Wang, C. Zhao, D. Wei, J. Zhu and Y. Xu, Facile strategy of hollow polyaniline nanotubes supported on Ti3C2-MXene nanosheets for High-performance symmetric supercapacitors, J. Colloid Interface Sci., 2020, 580, 601–613, DOI:10.1016/j.jcis.2020.07.052.
- W. Wu, et al., Organ-like Ti3C2 Mxenes/polyaniline composites by chemical grafting as high-performance supercapacitors, J. Electroanal. Chem., 2019, 847, 113203, DOI:10.1016/j.jelechem.2019.113203.
- S. Cao, et al., Fabrication of PANI@Ti3C2Tx/PVA hydrogel composite as flexible supercapacitor electrode with good electrochemical performance, Ceram. Int., 2022, 48(11), 15721–15728, DOI:10.1016/j.ceramint.2022.02.108.
- Y. Wang, et al., Scalable fabrication of polyaniline nanodots decorated MXene film electrodes enabled by viscous functional inks for high-energy-density asymmetric supercapacitors, Chem. Eng. J., 2021, 405, 126664, DOI:10.1016/j.cej.2020.126664.
- F. Ye, B. Xu, R. Chen, R. Li and G. Chang, A high performance flexible cotton-based supercapacitor prepared by in-situ polyaniline and MXene coating, J. Energy Storage, 2023, 62, 106803, DOI:10.1016/j.est.2023.106803.
- M. Boota and Y. Gogotsi, MXene—Conducting Polymer Asymmetric Pseudocapacitors, Adv. Energy Mater., 2019, 9(7), 1802917, DOI:10.1002/aenm.201802917.
- K. Li, et al., 3D MXene Architectures for Efficient Energy Storage and Conversion, Adv. Funct. Mater., 2020, 30(47), 2000842, DOI:10.1002/adfm.202000842.
- P. Das, S. Mondal and S. Malik, Fully organic polyaniline nanotubes as electrode material for durable supercapacitor, J. Energy Storage, 2021, 39, 102662, DOI:10.1016/j.est.2021.102662.
- D. Ge, et al., Foldable supercapacitors from triple networks of macroporous cellulose fibers, single-walled carbon nanotubes and polyaniline nanoribbons, Nano Energy, 2015, 11, 568–578, DOI:10.1016/j.nanoen.2014.11.023.
- M. R. Waikar, A. A. Shaikh and R. G. Sonkawade, PANINFs synthesized electrochemically as an electrode material for energy storage application, Polym. Bull., 2019, 76(9), 4703–4718, DOI:10.1007/s00289-018-2634-1.
- H. Xu, D. Zheng, F. Liu, W. Li and J. Lin, Synthesis of an MXene/polyaniline composite with excellent electrochemical properties, J. Mater. Chem. A, 2020, 8(12), 5853–5858, 10.1039/D0TA00572J.
- J. Zhou, et al., Ultrahigh rate capability of 1D/2D polyaniline/titanium carbide (MXene) nanohybrid for advanced asymmetric supercapacitors, Nano Res., 2022, 15(1), 285–295, DOI:10.1007/s12274-021-3472-2.
- S. Wang, Z. Ma, Q. Lü and H. Yang, Two-Dimensional Ti 3 C 2 T X/Polyaniline Nanocomposite from the Decoration of Small-Sized Graphene Nanosheets: Promoted Pseudocapacitive Electrode Performance for Supercapacitors, ChemElectroChem, 2019, 6(10), 2748–2754, DOI:10.1002/celc.201900433.
- B. Chen, Q. Song, Z. Zhou and C. Lu, A Novel Sandwiched Porous MXene/Polyaniline Nanofibers Composite Film for High Capacitance Supercapacitor Electrode, Adv. Mater. Interfaces, 2021, 8(12), 2002168, DOI:10.1002/admi.202002168.
- K. Li, et al., An Ultrafast Conducting Polymer@MXene Positive Electrode with High Volumetric Capacitance for Advanced Asymmetric Supercapacitors, Small, 2020, 16(4), 1906851, DOI:10.1002/smll.201906851.
- M. T. U. Malik, A. Sarker, S. M. S. Mahmud Rahat and S. B. Shuchi, Performance enhancement of graphene/GO/rGO based supercapacitors: A comparative review, Mater. Today Commun., 2021, 28, 102685, DOI:10.1016/j.mtcomm.2021.102685.
- Y. Shao, et al., Graphene-based materials for flexible supercapacitors, Chem. Soc. Rev., 2015, 44(11), 3639–3665, 10.1039/C4CS00316K.
- J. Yan, et al., Flexible MXene/Graphene Films for Ultrafast Supercapacitors with Outstanding Volumetric Capacitance, Adv. Funct. Mater., 2017, 27(30), 1–10, DOI:10.1002/adfm.201701264.
- L. Liao, D. Jiang, K. Zheng, M. Zhang and J. Liu, Industry-Scale and Environmentally Stable Ti 3 C 2 T x MXene Based Film for Flexible Energy Storage Devices, Adv. Funct. Mater., 2021, 31(35), 2103960, DOI:10.1002/adfm.202103960.
- Y. Chen, X. Xie, X. Xin, Z.-R. Tang and Y.-J. Xu, Ti 3 C 2 T x -Based Three-Dimensional Hydrogel by a Graphene Oxide-Assisted Self-Convergence Process for Enhanced Photoredox Catalysis, ACS Nano, 2019, 13(1), 295–304, DOI:10.1021/acsnano.8b06136.
- Y. Zhou, et al., Ti 3 C 2 T x MXene-Reduced Graphene Oxide Composite Electrodes for Stretchable Supercapacitors, ACS Nano, 2020, 14(3), 3576–3586, DOI:10.1021/acsnano.9b10066.
- L. Ma, T. Zhao, F. Xu, T. You and X. Zhang, A dual utilization strategy of lignosulfonate for MXene asymmetric supercapacitor with high area energy density, Chem. Eng. J., 2021, 405(35), 126694, DOI:10.1016/j.cej.2020.126694.
- S. Zhao, et al., Highly Electrically Conductive Three-Dimensional Ti 3 C 2 T x MXene/Reduced Graphene Oxide Hybrid Aerogels with Excellent Electromagnetic Interference Shielding Performances, ACS Nano, 2018, 12(11), 11193–11202, DOI:10.1021/acsnano.8b05739.
- L. Shao, et al., MXene/RGO composite aerogels with light and high-strength for supercapacitor electrode materials, Compos. Commun., 2020, 19(November 2019), 108–113, DOI:10.1016/j.coco.2020.03.006.
- S. Saha, K. Arole, M. Radovic, J. L. Lutkenhaus and M. J. Green, One-step hydrothermal synthesis of porous Ti 3 C 2 T z MXene/rGO gels for supercapacitor applications, Nanoscale, 2021, 13(39), 16543–16553, 10.1039/D1NR02114A.
- Q. Yang, et al., MXene/graphene hybrid fibers for high performance flexible supercapacitors, J. Mater. Chem. A, 2017, 5(42), 22113–22119, 10.1039/C7TA07999K.
- S. Seyedin, E. R. S. Yanza and J. M. Razal, Knittable energy storing fiber with high volumetric performance made from predominantly MXene nanosheets, J. Mater. Chem. A, 2017, 5(46), 24076–24082, 10.1039/C7TA08355F.
- C. Zhao, Q. Wang, H. Zhang, S. Passerini and X. Qian, Two-Dimensional Titanium Carbide/RGO Composite for High-Performance Supercapacitors, ACS Appl. Mater. Interfaces, 2016, 8(24), 15661–15667, DOI:10.1021/acsami.6b04767.
- H. Li, et al., Flexible All-Solid-State Supercapacitors with High Volumetric Capacitances Boosted by Solution Processable MXene and Electrochemically Exfoliated Graphene, Adv. Energy Mater., 2017, 7(4), 2–7, DOI:10.1002/aenm.201601847.
- Z. Fan, et al., Modified MXene/Holey Graphene Films for Advanced Supercapacitor Electrodes with Superior Energy Storage, Adv. Sci., 2018, 5(10), 1800750, DOI:10.1002/advs.201800750.
- R. Liu, et al., Fabrication of Cobaltosic Oxide Nanoparticle-Doped 3 D MXene/Graphene Hybrid Porous Aerogels for All-Solid-State Supercapacitors, Chem.–Eur. J., 2019, 25(21), 5547–5554, DOI:10.1002/chem.201806342.
- A. M. Navarro-Suárez, K. Maleski, T. Makaryan, J. Yan, B. Anasori and Y. Gogotsi, 2D Titanium Carbide/Reduced Graphene Oxide Heterostructures for Supercapacitor Applications, Batteries Supercaps, 2018, 1(1), 33–38, DOI:10.1002/batt.201800014.
- S. Xu, G. Wei, J. Li, W. Han and Y. Gogotsi, Flexible MXene–graphene electrodes with high volumetric capacitance for integrated co-cathode energy conversion/storage devices, J. Mater. Chem. A, 2017, 5(33), 17442–17451, 10.1039/C7TA05721K.
- Y. Yue, et al., Highly Self-Healable 3D Microsupercapacitor with MXene–Graphene Composite Aerogel, ACS Nano, 2018, 12(5), 4224–4232, DOI:10.1021/acsnano.7b07528.
- J. Kim, D. Lee, Y. Lee, W. Chen and S. Lee, Nanocellulose for Energy Storage Systems: Beyond the Limits of Synthetic Materials, Adv. Mater., 2019, 31(20), 1804826, DOI:10.1002/adma.201804826.
- N. A. A. Sezali, H. L. Ong, N. Jullok, A. R. Villagracia and R. Doong, A Review on Nanocellulose and Its Application in Supercapacitors, Macromol. Mater. Eng., 2021, 306(12), 2100556, DOI:10.1002/mame.202100556.
- M. Zhang, et al., Mixed analogous heterostructure based on MXene and prussian blue analog derivative for high-performance flexible energy storage, Chem. Eng. J., 2020, 387, 123170, DOI:10.1016/j.cej.2019.123170.
- M. Alhabeb, et al., Guidelines for Synthesis and Processing of Two-Dimensional Titanium Carbide (Ti 3 C 2 T x MXene), Chem. Mater., 2017, 29(18), 7633–7644, DOI:10.1021/acs.chemmater.7b02847.
- S. Feng, Y. Yi, B. Chen, P. Deng, Z. Zhou and C. Lu, Rheology-Guided Assembly of a Highly Aligned MXene/Cellulose Nanofiber Composite Film for High-Performance Electromagnetic Interference Shielding and Infrared Stealth, ACS Appl. Mater. Interfaces, 2022, 14(31), 36060–36070, DOI:10.1021/acsami.2c11292.
- Z. Zhou, Q. Song, B. Huang, S. Feng and C. Lu, Facile Fabrication of Densely Packed Ti 3 C 2 MXene/Nanocellulose Composite Films for Enhancing Electromagnetic Interference Shielding and Electro-/Photothermal Performance, ACS Nano, 2021, 15(7), 12405–12417, DOI:10.1021/acsnano.1c04526.
- S. Feng, Z. Zhan, Y. Yi, Z. Zhou and C. Lu, Facile fabrication of MXene/cellulose fiber composite film with homogeneous and aligned structure via wet co-milling for enhancing electromagnetic interference shielding performance, Composites, Part A, 2022, 157, 106907, DOI:10.1016/j.compositesa.2022.106907.
- B. Zhou, et al., Flexible, Robust, and Multifunctional Electromagnetic Interference Shielding Film with Alternating Cellulose Nanofiber and MXene Layers, ACS Appl. Mater. Interfaces, 2020, 12(4), 4895–4905, DOI:10.1021/acsami.9b19768.
- C. Sun, C. Wu, X. Gu, C. Wang and Q. Wang, Interface Engineering via Ti3C2Tx MXene Electrolyte Additive toward Dendrite-Free Zinc Deposition, Nano-Micro Lett., 2021, 13(1), 89, DOI:10.1007/s40820-021-00612-8.
- M. Pi, L. Jiang, Z. Wang, W. Cui, L. Shi and R. Ran, Robust and ultrasensitive hydrogel sensors enhanced by MXene/cellulose nanocrystals, J. Mater. Sci., 2021, 56(14), 8871–8886, DOI:10.1007/s10853-020-05644-w.
- K. A. S. Usman, et al., Inducing liquid crystallinity in dilute MXene dispersions for facile processing of multifunctional fibers, J. Mater. Chem. A, 2022, 10(9), 4770–4781, 10.1039/D1TA09547A.
- Z. Zhang, Z. Yao and Z. Jiang, Fast self-assembled microfibrillated cellulose@MXene film with high-performance energy storage and superior mechanical strength, Chin. Chem. Lett., 2021, 32(11), 3575–3578, DOI:10.1016/j.cclet.2021.03.025.
- C. Cai, Z. Wei, L. Deng and Y. Fu, Temperature-Invariant Superelastic Multifunctional MXene Aerogels for High-Performance Photoresponsive Supercapacitors and Wearable Strain Sensors, ACS Appl. Mater. Interfaces, 2021, 13(45), 54170–54184, DOI:10.1021/acsami.1c16318.
- Y. Wang, et al., Engineering 3D Ion Transport Channels for Flexible MXene Films with Superior Capacitive Performance, Adv. Funct. Mater., 2019, 29(14), 1900326, DOI:10.1002/adfm.201900326.
- G. Song, et al., Highly flexible few-layer Ti 3 C 2 MXene/cellulose nanofiber heat-spreader films with enhanced thermal conductivity, New J. Chem., 2020, 44(17), 7186–7193, 10.1039/D0NJ00672F.
- K. A. S. Usman, et al., Tough and Fatigue Resistant Cellulose Nanocrystal Stitched Ti3C2Tx MXene Films, Macromol. Rapid Commun., 2022, 43(11), 1–10, DOI:10.1002/marc.202200114.
- W. Zhang, X.-X. Ji and M.-G. Ma, Emerging MXene/cellulose composites: Design strategies and diverse applications, Chem. Eng. J., 2023, 458, 141402, DOI:10.1016/j.cej.2023.141402.
- L.-X. Liu, et al., Tough and electrically conductive Ti3C2T MXene–based core–shell fibers for high–performance electromagnetic interference shielding and heating application, Chem. Eng. J., 2022, 430, 133074, DOI:10.1016/j.cej.2021.133074.
- J. Zhang, et al., Additive-Free MXene Liquid Crystals and Fibers, ACS Cent. Sci., 2020, 6(2), 254–265, DOI:10.1021/acscentsci.9b01217.
- J. Zhang, et al., Scalable Manufacturing of Free-Standing, Strong Ti 3 C 2 T x MXene Films with Outstanding Conductivity, Adv. Mater., 2020, 32(23), 2001093, DOI:10.1002/adma.202001093.
- W. Eom, et al., Large-scale wet-spinning of highly electroconductive MXene fibers, Nat. Commun., 2020, 11(1), 2825, DOI:10.1038/s41467-020-16671-1.
- G. Zhou, et al., Electrostatic Self-assembly of Ti 3 C 2 T x MXene/Cellulose Nanofiber Composite Films for Wearable Supercapacitor and Joule Heater, Energy Environ. Mater., 2022, 0–3, DOI:10.1002/eem2.12454.
- J. Chen, H. Chen, M. Chen, W. Zhou, Q. Tian and C.-P. Wong, Nacre-inspired surface-engineered MXene/nanocellulose composite film for high-performance supercapacitors and zinc-ion capacitors, Chem. Eng. J., 2022, 428, 131380, DOI:10.1016/j.cej.2021.131380.
- A. S. Etman, J. Halim and J. Rosen, Fabrication of Mo 1.33 CT z (MXene)–cellulose freestanding electrodes for supercapacitor applications, Mater. Adv., 2021, 2(2), 743–753, 10.1039/D0MA00922A.
- W. Tian, et al., Multifunctional Nanocomposites with High Strength and Capacitance Using 2D MXene and 1D Nanocellulose, Adv. Mater., 2019, 31(41), 1902977, DOI:10.1002/adma.201902977.
- X. Zhao, et al., All-weather-available, continuous steam generation based on the synergistic photo-thermal and electro-thermal conversion by MXene-based aerogels, Mater. Horiz., 2020, 7(3), 855–865, 10.1039/C9MH01443H.
- M. Zhao, et al., Hollow MXene Spheres and 3D Macroporous MXene Frameworks for Na-Ion Storage, Adv. Mater., 2017, 29(37), 1702410, DOI:10.1002/adma.201702410.
- J. Liu, et al., Hydrophobic, Flexible, and Lightweight MXene Foams for High-Performance Electromagnetic-Interference Shielding, Adv. Mater., 2017, 29(38), 1702367, DOI:10.1002/adma.201702367.
- C. Cai, W. Zhou and Y. Fu, Bioinspired MXene nacre with mechanical robustness for highly flexible all-solid-state photothermo-supercapacitor, Chem. Eng. J., 2021, 418(January), 129275, DOI:10.1016/j.cej.2021.129275.
- H. Tang, et al., Scalable manufacturing of leaf-like MXene/Ag NWs/cellulose composite paper electrode for all-solid-state supercapacitor, EcoMat, 2022, 4(6), 1–13, DOI:10.1002/eom2.12247.
| This journal is © The Royal Society of Chemistry 2025 |

