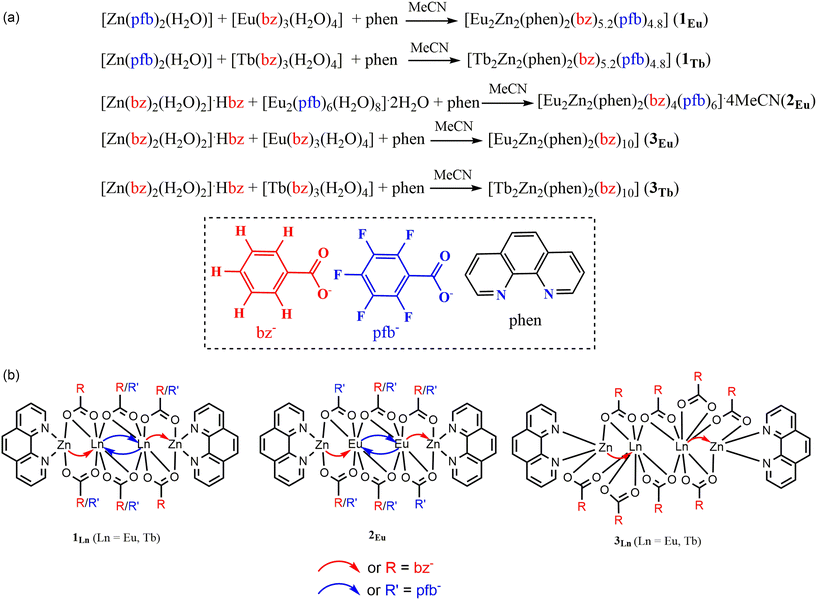
Luminescence enhancement by mixing carboxylate benzoate–pentafluorobenzoate ligands in polynuclear {Eu2Zn2} and {Tb2Zn2} complexes†
Alena E.
Bolot'ko
 *ab,
Maxim A.
Shmelev
*ab,
Maxim A.
Shmelev
 a,
Alexandr S.
Chistyakov
a,
Julia K.
Voronina
a,
Alexandr S.
Chistyakov
a,
Julia K.
Voronina
 a,
Evgeniya A.
Varaksina
c,
Natalia V.
Gogoleva
a,
Ilya V.
Taydakov
a,
Evgeniya A.
Varaksina
c,
Natalia V.
Gogoleva
a,
Ilya V.
Taydakov
 c,
Alexey A.
Sidorov
a and
Igor L.
Eremenko
c,
Alexey A.
Sidorov
a and
Igor L.
Eremenko
 a
a
aN. S. Kurnakov Institute of General and Inorganic Chemistry, Russian Academy of Sciences, Leninsky Prospect 31, 119991 Moscow, Russian Federation. E-mail: al.bolotko@gmail.com
bFaculty of Chemistry of the Higher School of Economics, Vavilova st, 7, 117312 Moscow, Russian Federation
cP.N. Lebedev Physical Institute of the Russian Academy of Sciences, Leninsky Prospect 53, 119991, Moscow, Russian Federation
First published on 4th March 2025
Abstract
This study investigates mixed-carboxylate benzoate (bz)–pentafluorobenzoate (pfb) {Eu2Zn2} and {Tb2Zn2} compounds with 1,10-phenanthroline (phen) molecules. It is demonstrated that variation of the synthesis conditions yields mixed-carboxylate compounds with different compositions: [Ln2Zn2(phen)2(bz)5.2(pfb)4.8] (Ln = Eu (1Eu), Tb (1Tb)) and [Eu2Zn2(phen)2(bz)4(pfb)6]·4MeCN (2Eu). In these structures, bz− and pfb− anions occupy specific positions in various ratios. Benzoate compounds [Ln2Zn2(phen)2(bz)10] (Ln = Eu (3Eu), Tb (3Tb)) were synthesized in order to compare the structures and photoluminescence properties of complexes 1 and 2 with their homoanionic analogues, and comparison was also made with previously reported pentafluorobenzoate complexes [Ln2Zn2(pfb)10(phen)2] (Ln = Eu (4Eu), Tb (4Tb)). It was found that the introduction of a second type of anion into the studied compounds improves the photoluminescence properties and alters the geometry of the metal core, the polyhedra of rare-earth elements (REEs), and the system of non-covalent interactions compared to benzoate and pentafluorobenzoate complexes.
Introduction
Luminescent lanthanide complexes have attracted significant attention due to their potential applications in producing luminescent sensors, lasers, diodes, catalysis, and biomedical imaging.1–6 They are also considered as single-molecule magnets for a new generation of high-density information storage and ultra-fast processing devices.7–11 Development of effective methods to improve the luminescence properties of coordination compounds remains a critical practical challenge. The rational selection of the ligand environment surrounding the lanthanide ion determines the structure of the coordination compound, the efficiency of luminescence sensitization of the lanthanide ion, and the probability of luminescence quenching.12–15The incorporation of a d-block ion, such as Zn2+ or Cd2+, with organic antenna molecules into the coordination environment of a lanthanide ion can also influence the luminescence efficiency of the compounds by inducing structural modification, altering the geometry of the coordination polyhedron of the metal ions, and minimizing the interionic interactions.16–20
The simultaneous incorporation of multiple different ionic co-ligands into a lanthanide compound can result in structural modifications and a significant increase in photoluminescence properties.21–24 For example, in europium compounds, it has been demonstrated that the combination of three ligands within a single complex can lead to a fivefold increase in luminescence efficiency.25 Similarly, the use of four ionic ligands in the synthesis of a samarium complex resulted in a compound with a record quantum yield of luminescence for this metal ion.26 While these studies have established a promising foundation, no investigations have yet explored how the ratio of ligands in the composition of such compounds affects their structure and photoluminescence properties, leaving the factors influencing these properties only partially understood.
This work aimed at studying mixed-carboxylate {Eu2Zn2} and {Tb2Zn2} compounds containing anions of benzoic acid (H(bz)) and pentafluorobenzoic acid (H(pfb)), as well as 1,10-phenanthroline molecules, and comparing their structures and photoluminescence properties with those of homoanionic pentafluorobenzoate and benzoate analogues. The combination of perfluorinated and non-fluorinated aromatic anions in the structure of a coordination compound can result in the formation of multiple non-covalent interactions.27 Such systems are characterized by the formation of a dense stacked arrangement of aromatic rings, with their distances approaching 3.4–3.6 Å due to “arene–perfluoroarene” interactions, which involve contributions from various non-covalent forces (π⋯π, C–F⋯π, C–H⋯F, etc.). These interactions can significantly influence the molecular and crystalline structures of the resulting compounds.28–32
Results and discussion
Synthesis of complexes
To obtain mixed-carboxylate zinc and lanthanide complexes, benzoate and pentafluorobenzoate salts of the corresponding metals were used: [Zn(pfb)2(H2O)],33 [Zn(bz)2(H2O)2]·Hbz,34 [Ln2(pfb)6(H2O)8]·2H2O (Ln = Eu, Tb),35 and [Ln(bz)3(H2O)4] (Ln = Eu, Tb),36 which were synthesized using previously described methods.It was found that the composition of the mixed-carboxylate complexes in this case changes depending on the metal salt used in the synthesis. In the reaction of zinc pentafluorobenzoate, rare-earth benzoate, and 1,10-phenanthroline (in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, with the anion ratio bz
1 ratio, with the anion ratio bz![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) pfb = 3
pfb = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) in acetonitrile, crystals of the compound [Ln2Zn2(phen)2(bz)5.2(pfb)4.8] (Scheme 1, Ln = Eu (1Eu), Tb (1Tb)) were obtained. Replacing the initial salts with zinc benzoate and europium pentafluorobenzoate, in a reaction with 1,10-phenanthroline (1
2) in acetonitrile, crystals of the compound [Ln2Zn2(phen)2(bz)5.2(pfb)4.8] (Scheme 1, Ln = Eu (1Eu), Tb (1Tb)) were obtained. Replacing the initial salts with zinc benzoate and europium pentafluorobenzoate, in a reaction with 1,10-phenanthroline (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, with the anion ratio bz
1 ratio, with the anion ratio bz![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) pfb = 2
pfb = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) in acetonitrile, led to the formation of crystals of [Eu2Zn2(phen)2(bz)4(pfb)6]·4MeCN composition (Scheme 1, 2Eu).
3) in acetonitrile, led to the formation of crystals of [Eu2Zn2(phen)2(bz)4(pfb)6]·4MeCN composition (Scheme 1, 2Eu).
The structure of the obtained tetranuclear complex 1 contains four benzoate anions, two pentafluorobenzoate anions, and four positions in which bz and pfb anions are simultaneously refined, with a ratio of 0.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.7, according to single-crystal X-ray diffraction (SCXRD) data. The occupancies of the disordered anions were determined during the refinement of SCXRD data using free variables, subsequently fixed to an accuracy of one decimal place, and found to be in good agreement with the results of CHN analysis. The phase purity of complex 1 was confirmed by powder X-ray diffraction (PXRD) (Fig. S1 and S2†). The reagent ratio in the first reaction (Scheme 1a) could theoretically support the quantitative formation of the heterometallic mixed-carboxylate complex [Ln2Zn2(phen)2(bz)6(pfb)4]. However, the significant increase in pentafluorobenzoate content in the obtained complex 1 suggests that the expected composition does not correspond to a thermodynamically stable compound. When the reagent ratios were altered and the initial synthesis was repeated, the occupancies of the anion positions in complex 1 [Ln2Zn2(phen)2(bz)5.2(pfb)4.8] were well reproduced, indicating that, despite its nonstoichiometric composition, the product is likely a co-crystal rather than a phase of variable composition or a solid solution.
0.7, according to single-crystal X-ray diffraction (SCXRD) data. The occupancies of the disordered anions were determined during the refinement of SCXRD data using free variables, subsequently fixed to an accuracy of one decimal place, and found to be in good agreement with the results of CHN analysis. The phase purity of complex 1 was confirmed by powder X-ray diffraction (PXRD) (Fig. S1 and S2†). The reagent ratio in the first reaction (Scheme 1a) could theoretically support the quantitative formation of the heterometallic mixed-carboxylate complex [Ln2Zn2(phen)2(bz)6(pfb)4]. However, the significant increase in pentafluorobenzoate content in the obtained complex 1 suggests that the expected composition does not correspond to a thermodynamically stable compound. When the reagent ratios were altered and the initial synthesis was repeated, the occupancies of the anion positions in complex 1 [Ln2Zn2(phen)2(bz)5.2(pfb)4.8] were well reproduced, indicating that, despite its nonstoichiometric composition, the product is likely a co-crystal rather than a phase of variable composition or a solid solution.
In the structure of compound 2Eu, simultaneous localization of benzoate and pentafluorobenzoate anions is also observed at four anion positions, with ratios of 0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8 and 0.8
0.8 and 0.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2, respectively. However, according to powder X-ray diffraction (PXRD) data, compound 2Eu is not phase-pure and contains impurities that could not be identified (Fig. S3†).
0.2, respectively. However, according to powder X-ray diffraction (PXRD) data, compound 2Eu is not phase-pure and contains impurities that could not be identified (Fig. S3†).
When the ratio of the initial reagents was altered (zinc salt, europium salt, and 1,10-phenanthroline in ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 or 2
1 or 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, respectively), it was not possible to isolate mixed-carboxylate compounds with different compositions. Only crystals of benzoate or pentafluorobenzoate complexes, [Ln2Zn2(phen)2(bz)10] (Ln = Eu(3Eu), Tb(3Tb)) and [Ln2Zn2(phen)2(pfb)10] (Ln = Eu(4Eu), Tb(4Tb)), were formed.37
2, respectively), it was not possible to isolate mixed-carboxylate compounds with different compositions. Only crystals of benzoate or pentafluorobenzoate complexes, [Ln2Zn2(phen)2(bz)10] (Ln = Eu(3Eu), Tb(3Tb)) and [Ln2Zn2(phen)2(pfb)10] (Ln = Eu(4Eu), Tb(4Tb)), were formed.37
To compare the photoluminescence properties of complex 1 with its homoanionic analogue, the previously described complex [Tb2Zn2(phen)2(bz)10] (Scheme 1, 3Tb)38 was synthesized, for which data on quantum yields and luminescence lifetimes were previously unavailable. Additionally, the complex [Eu2Zn2(phen)2(bz)10] (Scheme 1, 3Eu) was synthesized for the first time. The phase purity of complexes 3, as well as the isostructurality of complex 3Tb to the previously described complex 3Eu, was confirmed by powder X-ray diffraction (PXRD) (Fig. S4 and S5†). A comparison of the structures of compounds 1 and 2Eu with the previously obtained pentafluorobenzoate complexes [Ln2Zn2(phen)2(pfb)10] (Ln = Eu(4Eu), Tb(4Tb))37 was also made.
Using IR spectroscopy, the primary composition of the resulting compounds was confirmed (S6–S10†). Complexes 1Eu, 1Tb, and 2Eu are heteroanion compounds containing pfb- and bz-anions, whereas complexes 3Eu and 3Tb are homoanion compounds with only bz–anions. All the complexes contain 1,10-phenanthroline ligand molecules. Characteristic peaks corresponding to the out-of-plane skeletal bending of the aromatic system were observed in the range of 714–720 cm−1. Peaks associated with the out-of-plane C–H vibrations of the condensed aromatic system appear in the range of 837–847 cm−1, while skeletal stretching vibrations are located in the range of 1516–1520 cm−1.
For complexes 1Eu, 1Tb, and 2Eu, which contain pfb acid anions, intense vibrations of the aromatic system of this acid are observed at 1490–1491 cm−1. However, similar vibration peaks from the bz acid anions were found in all the obtained complexes, located in the range of 1558–1561 cm−1. Additionally, in complexes 1Eu, 1Tb, and 2Eu, skeletal C–F vibration bands were observed at 991 cm−1. The most intense peaks correspond to the vibrations of the carboxylate groups of the acid anions, located in the regions of 1600–1603 cm−1 for asymmetric vibrations and 1394–1398 cm−1 for symmetric vibrations.
The structure of complexes
The obtained compounds 1–3 are molecular complexes based on a tetrahedral Zn–Ln–Ln–Zn metal core, where bz – and/or pfb anions function as bridging ligands, and phenanthroline molecules are bidentately coordinated to the terminal zinc atoms. The metal ions and phenanthroline molecules form a plane, relative to which the molecular geometry can be conveniently analyzed.The zinc and rare-earth ions in the structures of complexes 1–3 are linked by two bridging anions and one chelating–bridging carboxylate anion (Fig. 1). One of the bridging anions adopts an almost planar conformation and forms in the main plane of the molecule (Zn1–O3–O4–Eu1, the maximum deviation from the plane is observed in complex 3Eu, where the oxygen atom O4 is displaced from the plane by 0.111(6) Å). The maximum deviations from the plane in the molecules of the remaining compounds are within the margin of error (Fig. S11†). The carboxyl groups of the second bridging and chelating–bridging carboxylate anions are positioned above and below the main plane at angles close to 90°, with the aromatic fragments of the anions rotated relative to their respective carboxyl groups. The zinc ions in all the complexes are in a distorted octahedral environment (Fig. 2 and S12†), formed by four oxygen atoms from the carboxylate anions and two nitrogen atoms from the phenanthroline moiety (Table S4,† ZnO4N2 CShM(1Eu) = 2.792; CShM(1Tb) = 2.774, CShM(2Eu) = 2.577, CShM(3Eu) = 2.990).
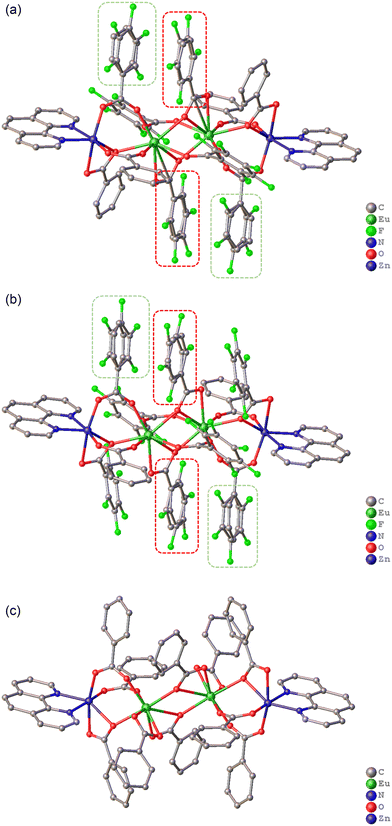 | ||
| Fig. 1 Molecular structure of Eu2Zn2 complexes 1Eu (a), 2Eu (b) and 3Eu. Dashed lines indicate pfb/bz anions with disordered positions. Solvent molecules and H-atoms omitted for clarity. | ||
 | ||
| Fig. 2 Coordination polyhedra of Zn and Eu metal ions and metal–metal distances in complexes 1Eu (a), 2Eu (b), 3Eu (c), and 4Eu (d). | ||
Four carboxylate anions are localized between the rare-earth ions in all complexes, two of which act as chelating–bridging ligands in all studied complexes and are positioned almost perpendicular to the main molecular plane. The other two anions, found in complexes 1Eu, 1Tb, and 2Eu, and the previously described 4Eu and 4Tb, which contain pfb anions, coordinate in the main plane and serve as bridging ligands. In the benzoate complex 3Eu, these two anions are coordinated as chelating to each rare-earth ion. As a result, the coordination polyhedra of the eight-coordinated rare-earth ions in the studied complexes differ, forming a square anti-prism in the pfb complexes 1Eu, 1Tb, and 2Eu (LnO8, CShM(1Eu) = 1.131); CShM(1Tb) = 1.108, CShM(2Eu) = 1.223, and a biaugmented trigonal prism (LnO8, CShM(3Eu) = 3.515) in the bz complex 3Eu (Fig. 2 and S12†).
Such a difference in the coordination pattern and polyhedron type leads to a change in the metal core. Thus, the distance between the rare-earth ions is expected to be the greatest in complex 3Eu, slightly smaller in 1Eu, and minimal in 2Eu (Table 1). This change correlates with the number of benzoate anions in the molecule; the fewer are the benzoate anions, the shorter is the distance between the lanthanides. It is interesting to note that the corresponding distance in the pfb complex 4Eu is about 4 Å, which would not allow making a conclusion about a direct dependence of the distance between the rare-earth ions on the number of bz anions. However, a detailed analysis of the geometry of complex 4Eu reveals significant differences compared to complexes 1–3. In particular, the main molecular plane cannot be identified, as the angle between the plane formed by the metal ions and the phenanthroline planes is 24.1(1)°. Additionally, the complex contains no anions with a planar structure.
| Bond | Distance, Å | |||
|---|---|---|---|---|
| 1Eu | 1Tb | 2Eu | 3Eu | |
| Zn–O | 2.029(4)–2.306(4) | 2.021(3)–2.293(3) | 1.991(6)–2.339(6) | 2.025(5)–2.313(6) |
| Ln–O | 2.298(4)–2.589(4) | 2.297(3)–2.581(3) | 2.284(6)–2.535(3) | 2.314(5)–2.637(5) |
| Zn–N | 2.099(5), 2.178(5) | 2.101(3), 2.178(4) | 2.086(6), 2.146(7) | 2.107(6), 2.177(6) |
| Zn⋯Ln | 3.875(1) | 3.860(1) | 3.883(1) | 3.801(1) |
| Ln⋯Ln | 3.963(1) | 3.945(1) | 3.923(1) | 4.145(1) |
| Angle, ° | ||||
| Zn–Ln–Ln | 162.28(2) | 162.42(2) | 160.25(2) | 146.86(2) |
In the final refinement of SCXRD data, it was found that the thermal parameters of the fluorine atoms in the pentafluorobenzoate anions, which occupy two positions in compounds 1 and 2Eu, were elongated. The corresponding ellipsoids were stretched along the C–F bond direction, indicating disorder in the pentafluorobenzoate fragment, which is distributed between two positions. Several attempts to refine the structures with this disorder were made but proved to be unsuccessful. Since this phenomenon was observed during the refinement of experimental data obtained for several crystals from the same sample, it became evident that the issue was unrelated to crystal quality or inaccuracies in data acquisition or refinement. Significant improvement in the experimental results was achieved by refining the occupancies of the corresponding fluorine atoms as free variables. As a result, the occupancies of all fluorine atoms in each disordered pfb ring were found to be consistent. This led us to hypothesize that the second component might not be fluorine atoms but rather hydrogen atoms, suggesting that the corresponding positions were occupied by a mixture of pentafluorobenzoic acid and benzoic acid anions in a specific ratio. Refinement of the corresponding models equalized the thermal parameters of all atoms and significantly reduced the R-factor values, confirming the validity of the structure solution.
Thus, the structures of compounds 1Eu, 1Tb, and 2Eu represent mixed-carboxylate complexes in which bz− and pfb− anions are refined in four positions (two in the independent part of the unit cell) with varying ratios, indicating the simultaneous presence of molecules with different compositions within the crystal. In other words, the crystals of 1Eu, 1Tb, and 2Eu are co-crystals of complexes where four anions, acting as bridging ligands between zinc and lanthanide ions and as chelating–bridging ligands between lanthanide ions, are either bz− or pfb−. Since SCXRD data represent an averaged superposition of the crystal as a whole, only the ratio of anions at each position can be discussed. Thus, in the crystals of compound 1, the ratio between fluorinated and non-fluorinated anions is 0.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3. In compound 2Eu, this ratio varies: in the terminal region, the pfb/bz ratio is 0.8
0.3. In compound 2Eu, this ratio varies: in the terminal region, the pfb/bz ratio is 0.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2, while in the central region, it is reversed to 0.2
0.2, while in the central region, it is reversed to 0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8.
0.8.
The presence of disordered anion positions in complexes 1 and 2Eu was unexpected. A review of literature data on crystals of similar organic systems (comprising benzoic acid and its fluorinated derivatives) revealed that disordered anion positions are typically observed only in systems containing mono-fluorinated or specific isomers of di- or trifluorobenzoic acids.39 For systems containing benzoic/3,4,5-trifluorobenzoic (tetra- or pentafluorobenzoic) acids, only organic co-crystals with fixed compositions are reported to form.39,40
Analysis of molecular packing in the studied crystal structures revealed, as anticipated, that the primary structure-forming interactions are π⋯π interactions between aromatic fragments (Fig. 3 and Table 2). In the crystals of complexes 1Eu and 1Tb, infinite layers are created through π⋯π stacking interactions between phenanthroline rings, which are aligned parallel to the 0a-axis (Fig. 3(a)). The three-dimensional crystal packing arises due to stacking interactions between the aromatic rings of disordered bz and pfb fragments. In the crystal of complex 2Eu, paired π⋯π interactions occur between the phenanthroline and benzoate anion fragments of the molecules, resulting in infinite chains parallel to the b0c-plane (Fig. 3(b)). These chains are further interconnected by weak C–H⋯F and C–F⋯π interactions (Tables S2 and S3†). Additionally, in 2Eu, the nitrogen atoms of acetonitrile solvate molecules form short contacts with the disordered aromatic bz/pfb fragments, with centroid distances of 3.50(3)/3.93(2) Å and angles (γ) of 23.43°/17.02°, suggesting π-system interactions between the aromatic ring and acetonitrile. In the crystal of 3Eu, stacking interactions between four aromatic fragments (two central phenanthroline rings and two benzene rings on either side of the phenanthrolines) form an infinite layer parallel to the b0c-plane (Fig. 3(c) and Table 2). These layers are connected through C–H⋯O interactions (Table S3†). Previously reported pentafluorobenzoate complex 4 forms chains due to π⋯π interactions between the phenanthroline fragments of neighboring molecules. In addition, acetonitrile solvate molecules are integrated into the π⋯π interaction system, interacting with the phenanthroline of one molecule and the pfb− anion of another, with the shortest interatomic distances measured at 3.21(2) and 3.289 Å, respectively.37
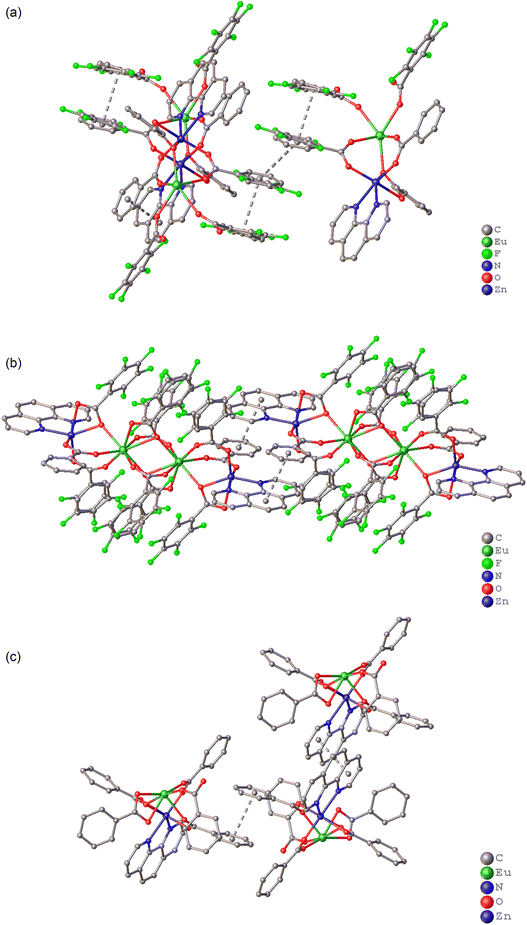 | ||
| Fig. 3 Fragment of the crystal packing for complexes 1Eu (a), 2Eu (b), and 3Eu (c). Dashed lines indicate π⋯π interactions. Solvent molecules and H-atoms omitted for clarity. | ||
| Interactions | Cg⋯Cg, Å | Symmetry code | Cg⋯Perp, Å | α, ° |
|---|---|---|---|---|
| Cg is the centroid of aromatic rings, Perp is a perpendicular to the plane of the ring, and α is the angle between the planes of aromatic fragments. | ||||
| Complex 1Eu | ||||
| phen⋯bz | 3.652(4) | 1 − x,−y,2 − z | 3.326(3) | 6.0(4) |
| phen⋯bz | 3.684(5) | 1 − x,−y,2 − z | 3.442(3) | 5.8(4) |
| bz/pfb⋯bz/pfb | 3.612(5) | 3.301(3) | 8.7(4) | |
| Complex 1Tb | ||||
| phen⋯bz | 3.657(3) | 1 − x,−y,2 − z | 3.335(2) | 6.3(3) |
| phen⋯bz | 3.697(3) | 1 − x,−y,2 − z | 3.445(3) | 5.6(3) |
| bz/pfb⋯bz/pfb | 3.605(4) | 3.458(3) | 8.9(3) | |
| Complex 2Eu | ||||
| bz/pfb⋯bz/pfb | 3.650(9) | 1 − x,−y,1 − z | 3.547(7) | 6.8(8) |
| phen⋯bz | 3.687(7) | 1 − x,1 − y,−z | 3.687(7) | 4.3(5) |
| Complex 3Eu | ||||
| phen⋯phen | 3.412(5) | 1 − x,2 − y,1 − z | 3.280(4) | 0.0(4) |
| bz⋯bz | 3.638(6) | −x,1 − y,−z | 3.546(4) | 0.0(5) |
Luminescence properties of complexes
To evaluate the influence of mixed carboxylate ligands on the efficiency of energy transfer processes, the photophysical properties of mixed carboxylate benzoate-pentafluororbenzoate of the compounds 1 benzoate compound 3 were investigated in detail and compared with previously studied compounds 4Eu4andTb with pentafluorobenzoic acid anions.37 The luminescence spectra of the europium complexes 1Eu, 3Eu and 4Eu obtained at 300 K and 77 K display line-like luminescence associated with the f–f transition of Eu3+ (Fig. S14† and Fig. 4). The singlet transition 5D0 → 7F0 at about 580 nm is unique and symmetrical and indicates one type of emission center for each of the compounds studied. The 5D0 → 7F1 transition splits into two components which is related to the axial D4d symmetry of the coordination polyhedron obtained by X-ray diffraction data (Table S4†) The transition 5D0 → 7F2 is very sensitive to the ligand environment and according to Judd–Ofelt theory is strictly forbidden at the site with the inversion center. In contrast, the transition 5D0 → 7F1 is allowed by the Laporte selection rules and its integrated intensity does not depend on the environment in the first approximation. This property is often used to compare the emission spectra of different europium complexes. The ratio of the 5D0 → 7F2 transition to the magnetic dipole transition 5D0 → 7F1 is 4.48 for 1Eu, 4.08 for 3Eu and 5.97 for 4Eu, which indicate that the Eu3+ ion site is non-centrosymmetric. A similar pattern of Stark splitting of the f–f transition indicates roughly the same charge distribution in the inner coordination sphere of the europium ion for the complexes 1Eu and 3Eu while for complex 4Eu it has some differences.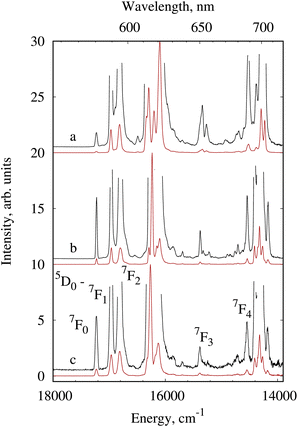 | ||
| Fig. 4 Luminescence spectra of 4Eu (a), 1Eu (b) and 3Eu (c) at 77 K, λex = 280 nm. The black line indicates the spectra at an enlarged scale. | ||
The terbium complexes 4Tb, 1Tb and 3Tb under ligand excitation and at 77 K exhibit the characteristic luminescence from the 5D4 level of the terbium ion (Fig. 5). The highest intensity was detected for the transition 5D4 → 7F5 located in the spectral range of 535–555 nm. Three transitions 5D4 → 7F2–0 have an extremely low intensity typical of terbium complexes. In contrast to europium complexes, the splitting pattern of the 5D4 → 7F5 transition of Tb3+ seems quite similar for three terbium compounds. However, due to the large value of the quantum number J of the initial and final state of Tb3+, a huge number of overlapping spectral bands are observed when degeneracy is removed by a crystal field. For the terbium compounds studied, detailed comparison of the spectral band splitting is complicated even at low temperature.
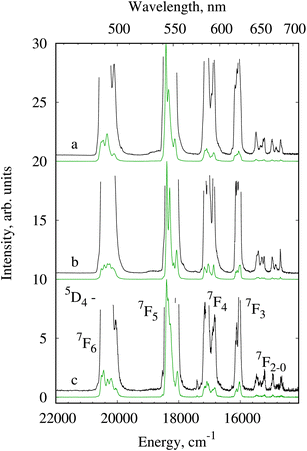 | ||
| Fig. 5 Luminescence spectra of 4Tb (a), 1Tb (b) and 3Tb (c) at 77 K, λex = 280 nm. The black line indicates the spectra in an enlarged scale. | ||
The broadband emission in the 340–450 nm spectral range is clearly observed in the emission spectra of the investigated compounds (Fig. S15†). The observed bands are typical of heteronuclear compounds and early were associated with the luminescence of d-block.41 The presence of the ligand emission indicates incomplete energy transfer to the lanthanide ion. To estimate the energy losses, the ratio of the d-block luminescence intensity to the intensity of the lanthanide luminescence band (5D4 → 7F5 and 5D0 → 7F1 for terbium and europium complexes, respectively) was calculated. The obtained ratios are 0.12, 0.05 and 0.22 for 3Tb, 1Tb and 4Tb and 1.07, 0.41 and 0.64 for 3Eu, 1Eu and 4Eu, respectively. It can be noted that the relative intensity of broadband luminescence of heteroanionic compounds is noticeably lower than that of homoanionic compounds, which indicates lower energy losses during energy transfer to the lanthanide ion in mixed carboxylate compounds.
The excitation spectra of compounds 1 and 3 obtained at 300 K and 77 K are presented in Fig. S14 and S16.† The long-wavelength maximum of the broadband excitation line located at 345 nm can be associated with absorption of the 1,10-phenantroline ligand which is an effective and widely used sensitizer for excitation of europium and terbium ions.42 The excitation spectrum of the complex 4Eu contains a weak shoulder in the wavelength range 340–390 nm, which is absent in the isostructural terbium complex and can be assigned to a ligand-to-metal charge transfer (LMCT) state.
The absolute quantum yield of lanthanide-centered emission for 3Eu is the lowest in the series of investigated complexes which may partially be due to weak quenching of the excited state of Eu3+ by the CH high-energy oscillator of the bz anion (Table 3). A gradual decrease in the nonradiative rate constant Arad occurs when bz is replaced by the pfb anion. In addition, compounds 4Eu and 4Tb show a slight increase in the observed luminescence lifetime compared to compounds 1 and 3. However, the best quantum yield is observed for compounds 1 containing both bz and pfb anions. According to the sensitization efficiency values for europium compounds, mixed carboxylate compounds provide better energy transfer to the lanthanide ion than homoanionic compounds. This effect may be associated with a significant restructuring of the system of non-covalent interactions during the formation of a heteroanionic complex. As indicated above, the lowest energy losses for the luminescence of the d-block are observed for the heteroanion complex, which contributes to a more efficient transfer of excitation to the lanthanide ion. Moreover, a significant decrease in the sensitization efficiency for the complex 4Eu is due to energy quenching by the low-lying LMCT state observed in the excitation spectrum of this compound. According to the literature, an enhancement of the luminescence properties of mixed-carboxylate complexes can be observed due to a reduction in the polyhedral symmetry of the molecule. In our case, the lowest polyhedral symmetry is observed for the benzoate compound 3Eu (C2áμ¥), whereas the polyhedra of our other europium(III) complexes show only minor differences (D4d). Thus, the effect of polyhedral symmetry on quantum yields in our case cannot be confirmed. The main difference in the crystal structure of type 1 complexes lies in a more developed system of stacking interactions compared to complexes 2 and 3. This sophisticated system enhances charge delocalization and may lead to an improvement in photoluminescence properties.
According to the literature,21,22,25 an enhancement of the luminescence properties of mixed-carboxylate complexes can be observed due to a reduction in the polyhedral symmetry of the molecule. In our case, the lowest polyhedral symmetry is observed for the benzoate compound 3Eu (C2áμ¥), whereas the polyhedra of our other europium(III) complexes show only minor differences (D4d) (Table S4†). Thus, the effect of polyhedral symmetry on quantum yields in our case cannot be confirmed. The main difference in the crystal structure of type 1 complexes lies in a more developed system of stacking interactions compared to complexes 2 and 3. This sophisticated system enhances charge delocalization and may lead to an improvement in photoluminescence properties.
Experimental section
General information
All operations related to the synthesis of complexes were performed in air using commercially available solvents and reagents: MeCN (>99%, KHIMMED), EtOH (96%, FEREIN), 1,10-phenanthroline monohydrate (phen, 99%, “Aldrich-Chemie”, Germany), H(pfb) (99%, “P&M Invest”), H(bz) (99%, “Aldrich-Chemie”). Compounds [Zn(pfb)2(H2O)],33 [Zn(bz)2(H2O)2]·Hbz,34 [Eu2(pfb)6(H2O)8]·2H2O35 and [Ln(bz)3(H2O)4] Ln = Eu, Tb36 were obtained using known methods.IR spectra of compounds 1–2 were recorded using a Jasco FT/IR-4700 spectrophotometer (China) in the range 550–4000 cm−1. The IR spectra of compounds 3 were recorded using a PerkinElmer Spectrum 65 spectrophotometer (PerkinElmer, Waltham, MA, USA) equipped with a Quest ATR accessory (Specac, Orpington BR5 3FQ, UK) by the attenuated total reflectance (ATR) method in the range 400–4000 cm−1. (abbreviations: w = weak, m = medium, s = strong, sy = symmetric, as = asymmetric, ar = aromatic).
The powder diffraction patterns were obtained using the Bruker D8 Advance diffractometer with a LynxEye detector in Bragg–Brentano geometry. The sample was finely dispersed on a silicon holder with a zero background, λ(CuKα) = 1.54060 Å. The acquired data were refined using the Topas 4 software.43
Photoluminescence excitation, emission spectra, and luminescence decays were recorded at room temperature with a Horiba-Jobin–Yvon Fluorolog-QM spectrofluorimeter equipped with a 75 W ArcTune xenon lamp and a Hamamatsu R-FL-QM-R13456 photomultiplier sensitive in the 200–980 nm emission range. The luminescence quantum yield (QLnL) values were measured by the absolute method, employing the same setup equipped with a G8 Spectralon®-covered sphere (GMP SA, Switzerland) and Hamamatsu R13456 photomultiplier. A diffusing screen was mounted inside the sphere to avoid direct irradiation of the detector. The measurements were carried out at ambient temperature. The samples in quartz cells were placed near the center of the sphere. A NIST-traceable 45 W quartz tungsten-halogen bulb emission standard (Oriel) was employed to measure the instrument response function. All QY measurements were repeated at least three times to achieve an experimental error below 15%. The photophysical parameters were calculated via Werts’ formula.44
Synthesis of complexes
The yield of 1Eu was 0.059 g (44%) based on [Eu(bz)3(H2O)4]. Found, %: C, 46.2; H, 1.8; N, 2.3. For C94H42Eu2Zn2F24N4O20 calculated, %: C, 46.3; H, 1.7; N, 2.3. IR (ATR), ν/cm−1: IR-spectrum (ATR; ν, cm−1): 3065 w, 1725 w, 1648 m, 1600 s [as(COO−)], 1561 m [ar(C–C)], 1519 m [C–C], 1490 s [ar(C–C)], 1394 s [sy(COO−)], 1289 w, 1227 w, 1175 w, 1144 w, 1108 m, 991 s [C–F], 932 w, 844 m [ar(C–C)], 768 m, 719 s [ar(C–C)], 643 w, 579 w, 505 w, 441 m, 425 m.
The yield of 1Tb was 0.075 g (62%) based on [Tb(bz)3(H2O)4]·H2O. Found, %: C, 46.1; H, 1.8; N, 2.3. For C94H42Tb2Zn2F24N4O20 calculated, %: C, 46.0; H, 1.7; N, 2.2. IR (ATR), ν/cm−1: 1735 w, 1688 w, 1642 m, 1602 m [as(COO−)], 1558 m [ar(C–C)], 1520 m [C–C], 1491 s [ar(C–C)], 1394 s [sy(COO−)], 1290 w, 1176 w, 1149 w, 1107 m, 1069 w, 991 s [C–F], 934 w, 837 w [ar(C–C)], 762 m, 720 s [ar(C–C)], 680 m, 638 w, 576 w, 504 w, 433 m.
The yield of 2Eu was 0.042 g (22%) based on [Eu2(pfb)6(H2O)8]·2H2O. Found, %: C, 44.9; H, 1.3; N, 3.5. For C100H45Eu2Zn2F30N7O20 calculated, %: C, 45.0; H, 1.7; N, 3.7. IR (ATR), ν/cm−1: 3076 w, 1725 w, 1648 m, 1600 m [as(COO–)], 1561 m [ar(C–C)], 1519 m [C–C], 1490 s [ar(C–C)], 1394 s [sy(COO–)], 1289 w, 1226 w, 1175 w, 1144 w, 1108 m, 991 s [C–F], 932 w, 844 w [ar(C–C)], 768 m, 719 s [ar(C–C)], 677 m, 643 w, 579 w, 506 w, 441 m, 425 m.
The yield of 3Eu was 0.034 g (41.2%) based on [Zn(bz)2(H2O)2]·Hbz. Found, %: C, 56.1; H, 3.2; N, 3.0. For C94H66O20N4Eu2Zn2 calculated, %: C 56.3; H 3.3; N 2.8. IR-spectrum (ATR; ν, cm−1): 3054 w, 2360 w, 2341 w, 1603 s [as(COO−)], 1561 s [ar(C–C)], 1516 w [C–C], 1492 w, 1446 w, 1426 m, 1398 s [sy(COO−)], 1347 w, 1318 w, 1304 w, 1173 w, 1141 w, 1103 w, 1069 w, 1024 w, 847 m [ar(C–C)], 819 w, 807 w, 768 w, 714 s [ar(C–C)], 660 m, 642 w, 618 w, 606 w, 589 w, 578 w, 560 w.
The yield of 3Tb was 0.029 g (34.6%) based on [Zn(bz)2(H2O)2]·Hbz. Found, %: C, 55.8; H, 3.1; N, 2.9. For C94H66O20N4Tb2Zn2 calculated, %: C 55.9; H 3.3; N 2.7; IR-spectrum (ATR; ν, cm−1): 3734 w, 2360 m, 2341 m, 1603 s [as(COO−)], 1561 s [ar(C–C)], 1542 m, 1516 w [C–C], 1492 w, 1446 w, 1426 m, 1398 s [sy(COO−)], 1363 w, 1346 w, 1318 w, 1304 w, 1221 w, 1174 w, 1141 w, 1121 w, 1103 w, 1070 w, 1024 w, 983 w, 942 w, 848 m [ar(C–C)], 819 w, 807 w, 768 w, 714 s [ar(C–C)], 679 m, 643 w, 589 w, 574 w, 565 w.
Conclusions
In our studies, benzoate–pentafluorobenzoate [Ln2Zn2(phen)2(bz)5.2(pfb)4.8] (Ln = Eu (1Eu), Tb (1Tb)) and [Eu2Zn2(phen)2(bz)4(pfb)6]·4MeCN (2Eu) were obtained, in which the simultaneous localization of benzoate and pentafluorobenzoate anions occurs in nearby positions in different ratios. It was found that the use of different metal salts results in complexes with varying compositions, while varying the ratio of the starting reagents only led to the formation of either benzoate or pentafluorobenzoate complexes. In the case of complexes 1 and 2, it was discovered that the introduction of a heteroanion significantly alters the geometry of the metal framework, the coordination polyhedron of the lanthanide ion, as well as the reorganization of the non-covalent interaction system. Photoluminescence studies revealed that the mixed carboxylate complexes provide better energy transfer to the lanthanide ion than the homoanionic complexes, which leads to a higher quantum yield for complex 1 compared to the analogous benzoate and pentafluorobenzoate complexes 3 and 4. Thus, the developed approach to the direct enhancement of luminescence by mixing fluorinated and non-fluorinated ligands in the structures of polynuclear {Zn2Ln2} complexes can be used in the design of bright luminescent compounds. For instance, such compounds may be potentially applied as components of emissive layers in organic light-emitting diodes. In this case, future studies on the efficiency and brightness of the electroluminescence and stability of the compounds in the presence of an electric field should be carried out as a logical continuation of this research.Author contributions
Conceptualization and validation: M. A. S. and J. K. V.; methodology: A. E. B., M. A. S., A. S. C., N. V. G., and E. A. V.; formal analysis: A. E. B., M. A. S., A. S. C., E. A. V., and I. V. T.; investigation: A. E. B., M. A. S., A. S. C., and E. A. V.; writing – original draft preparation: A. E. B., M. A. S., and A. S. C.; writing – review and editing: A. A. S., J. K. V., and I. L. E.; supervision: I. L. E. All authors have read and agreed to the published version of the manuscript.Data availability
The data supporting this article have been included as part of the ESI.†CCDC 2343086 (1Eu), 2343087 (1Tb), 2343088 (2Eu), and 2390847 (3Eu) contain the supplementary crystallographic data for this paper.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Russian Science Foundation research grant no. 22-73-10192.IR spectroscopy, X-ray diffraction, powder X-ray diffraction and CHN analyses of the complexes were performed using the equipment of the JRC PMR IGIC RAS as part of the state assignment of the IGIC RAS in the field of fundamental scientific research. Photophysical measurements were carried out with financial support from the Ministry of Science and Higher Education of the Russian Federation, using the equipment of the Research Center for Molecular Structure Studies, INEOS RAS.
References
- Q. Liu, S. Z. Ge, J. C. Zhong, Y. Q. Sun and Y. P. Chen, Two novel 2D lanthanide–cadmium heterometal–organic frameworks based on nanosized heart-like Ln6Cd6O12 wheel-clusters exhibiting luminescence sensing to the polarization and concentration of cations, Dalton Trans., 2013, 42(18), 6314 RSC.
- A. A. Sidorov, N. V. Gogoleva, E. S. Bazhina, S. A. Nikolaevskii, M. A. Shmelev and E. N. Zorina-Tikhonova, et al., Some aspects of the formation and structural features of low nuclearity heterometallic carboxylates, Pure Appl. Chem., 2020, 92(7), 1093–1110 CrossRef CAS.
- Q. Liu, F. Wan, L. X. Qiu, Y. Q. Sun and Y. P. Chen, Four 2D Ln–Cd heterometal–organic coordination polymers based on tetranuclear Ln–Cd oxo-cluster with highly selective luminescent sensing of organic molecules and metal cations, RSC Adv., 2014, 4(51), 27013–27021 RSC.
- H. X. Feng, M. J. Xin and L. W. Sheng, Lanthanide Metalloligand Strategy toward d–f Heterometallic Metal–Organic Frameworks: Magnetism and Symmetric-Dependent Luminescent Properties, Inorg. Chem., 2014, 53(12), 5922–5930 Search PubMed.
- R. A. Jones, A. J. Gnanam, J. F. Arambula, J. N. Jones, J. Swaminathan and X. Yang, et al., Lanthanide nano-drums: a new class of molecular nanoparticles for potential biomedical applications, Faraday Discuss., 2014, 175, 241–255 RSC.
- J. Tang, L. Wang, D. Mao, W. Wang, L. Zhang and S. Wu, et al., Ytterbium pentafluorobenzoate as a novel fluorous Lewis acid catalyst in the synthesis of 2,4-disubstituted quinolines, Tetrahedron, 2011, 67(44), 8465–8469 CrossRef CAS.
- S. M. Yakout, Spintronics: Future Technology for New Data Storage and Communication Devices, J. Supercond. Novel Magn., 2020, 33(9), 2557–2580 CrossRef CAS.
- H. Tokoro, A. Namai and O. S. Ichi, Advances in magnetic films of epsilon-iron oxide toward next-generation high-density recording media, Dalton Trans., 2021, 50(2), 452–459 RSC.
- H. Lian, X. Cheng, H. Hao, J. Han, M. T. Lau and Z. Li, et al., Metal-containing organic compounds for memory and data storage applications, Chem. Soc. Rev., 2022, 51(6), 1926–1982 RSC.
- J. T. Chen, T. D. Zhou and W. B. Sun, Multifunctional lanthanide-based single-molecule magnets exhibiting luminescence thermometry and photochromic and ferroelectric properties, Dalton Trans., 2023, 52(15), 4643–4657 Search PubMed.
- F. Manna, M. Oggianu, N. Avarvari and M. L. Mercuri, Lanthanide-Based Metal–Organic Frameworks with Single-Molecule Magnet Properties, Magnetochemistry, 2023, 9(7), 190 CrossRef CAS.
- T. J. Summers, M. G. Taylor, L. J. Augustine, J. Janssen, D. Perez and E. R. Batista, et al., On the Importance of Configuration Search to the Predictivity of Lanthanide Selectivity, JACS Au, 2024, 5(2), 631–641 CrossRef PubMed.
- J. T. Chen, T. D. Zhou and W. B. Sun, Multifunctional lanthanide-based single-molecule magnets exhibiting luminescence thermometry and photochromic and ferroelectric properties, Dalton Trans., 2023, 52(15), 4643–4657 RSC.
- D. Parker, J. D. Fradgley and K. L. Wong, The design of responsive luminescent lanthanide probes and sensors, Chem. Soc. Rev., 2021, 50(14), 8193–8213 RSC.
- Y. J. Chen, C. X. Dou, P. P. Yin, J. T. Chen, X. G. Yang and B. Li, et al., U-type π-conjugated phosphorescent ligand sensitized lanthanide metal–organic frameworks for efficient white-light-emitting diodes, Dalton Trans., 2023, 52(39), 13872–13877 RSC.
- M. A. Shmelev, R. A. Polunin, N. V. Gogoleva, I. S. Evstifeev, P. N. Vasilyev and A. A. Dmitriev, et al., Cadmium-Inspired Self-Polymerization of {LnIIICd2} Units: Structure, Magnetic and Photoluminescent Properties of Novel Trimethylacetate 1D-Polymers (Ln = Sm, Eu, Tb, Dy, Ho, Er, Yb), Molecules, 2021, 26(14), 4296 CrossRef CAS PubMed.
- Y. Meng, Y. Cheng, X. Yang, C. Wang, K. Yang and D. Schipper, Construction of a Zn(II)–Eu(III) Nanoring with Temperature-Dependent Luminescence for the Qualitative and Quantitative Detection of Neopterin as an Inflammatory Marker, Inorg. Chem., 2024, 63(16), 7199–7205 CrossRef CAS PubMed.
- M. A. Shmelev, Yu. K. Voronina, N. V. Gogoleva, A. A. Sidorov, M. A. Kiskin and F. M. Dolgushin, et al., Influence of the steric properties of pyridine ligands on the structure of complexes containing the {LnCd2(bzo)7} fragment, Russ. Chem. Bull., 2020, 69(8), 1544–1560 CrossRef CAS.
- Y. Zhang, W. Feng, H. Liu, Z. Zhang, X. Lü and J. Song, et al., Photo-luminescent hetero-trinuclear Zn2Ln (Ln = Nd, Yb, Er or Gd) complexes based on the binuclear Zn2L precursor, Inorg. Chem. Commun., 2012, 24, 148–152 CrossRef CAS.
- F. Q. Song, H. Cheng, N. N. Zhao, X. Q. Song and L. Wang, Anion-Dependent Structure and Luminescence Diversity in Zn II -Ln III Heterometallic Architectures Supported by a Salicylamide-Imine Ligand, Inorg. Chem., 2021, 60(22), 17051–17062 CrossRef CAS PubMed.
- N. B. D. Lima, A. I. S. Silva, S. M. C. Gonçalves and A. M. Simas, Synthesis of mixed ligand europium complexes: Verification of predicted luminescence intensification, J. Lumin., 2016, 170, 505–512 CrossRef CAS.
- N. B. D. Lima, A. I. S. Silva, V. F. C. Santos, S. M. C. Gonçalves and A. M. Simas, Europium complexes: choice of efficient synthetic routes from RM1 thermodynamic quantities as figures of merit, RSC Adv., 2017, 7(34), 20811–20823 RSC.
- M. A. Shmelev, G. N. Kuznetsova, N. V. Gogoleva, F. M. Dolgushin, Yu. V. Nelyubina and M. A. Kiskin, et al., Heteroleptic cadmium(II) and terbium(III) pentafluorobenzoate–benzoate and pentafluorobenzoate-2-furancarboxylate compounds, Russ. Chem. Bull., 2021, 70(5), 830–838 CrossRef CAS.
- H. L. Wang, X. F. Ma, H. H. Zou, K. Wang, B. Li and Z. L. Chen, et al., Mixed chelating ligands used to regulate the luminescence of Ln(III) complexes and single-ion magnet behavior in Dy-based analogues, Dalton Trans., 2018, 47(44), 15929–15940 RSC.
- N. B. D. Lima, S. M. C. Gonçalves, S. A. Júnior and A. M. Simas, A Comprehensive Strategy to Boost the Quantum Yield of Luminescence of Europium Complexes, Sci. Rep., 2013, 3(1), 2395 CrossRef PubMed.
- L. L. L. S. Melo, G. P. Castro and S. M. C. Gonçalves, Substantial Intensification of the Quantum Yield of Samarium(III) Complexes by Mixing Ligands: Microwave-Assisted Synthesis and Luminescence Properties, Inorg. Chem., 2019, 58(5), 3265–3270 CrossRef CAS PubMed.
- J. K. Voronina, D. S. Yambulatov, A. S. Chistyakov, A. E. Bolot'ko, L. M. Efromeev and M. A. Shmelev, et al., Influence of the Arene/Perfluoroarene Ratio on the Structure and Non-Covalent Interactions in Crystals of Cd(II), Cd(II)-Tb(III) and Cu(II) Compounds, Crystals, 2023, 13(4), 678 CrossRef CAS.
- C. E. Smith, P. S. Smith, R. L. l. Thomas, E. G. Robins, J. C. Collings and C. Dai, et al., Arene-perfluoroarene interactions in crystal engineering: structural preferences in polyfluorinated tolans, J. Mater. Chem., 2004, 14(3), 413–420 RSC.
- L. Wang, J. Deng, M. Jiang, C. Zhen, F. Li and S. Li, et al., Arene–perfluoroarene interactions in molecular cocrystals for enhanced photocatalytic activity, J. Mater. Chem. A, 2023, 11(21), 11235–11244 RSC.
-
J. C. Collings, K. P. Roscoe, E. G. Robins, A. S. Batsanov, L. M. Stimson and J. A. K. Howard, et al., Arene-perfluoroarene interactions in crystal engineering 8: Structures of 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes of hexafluorobenzene with fused-ring polyaromatic hydrocarbonsyz, in New Journal of Chemistry, Royal Society of Chemistry, 2002, pp. 1740–1746 Search PubMed.
1 complexes of hexafluorobenzene with fused-ring polyaromatic hydrocarbonsyz, in New Journal of Chemistry, Royal Society of Chemistry, 2002, pp. 1740–1746 Search PubMed. - X. H. Zhou, J. Luo, S. Huang, T. D. Kim, Z. Shi and Y. J. Cheng, et al., Supramolecular self-assembled dendritic nonlinear optical chromophores: Fine-tuning of arene-perfluoroarene interactions for ultralarge electro-optic activity and enhanced thermal stability, Adv. Mater., 2009, 21(19), 1976–1981 CrossRef CAS.
- T. Itoh, M. Kondo, M. Kanaike and S. Masaoka, Arene-perfluoroarene interactions for crystal engineering of metal complexes: Controlled self-assembly of paddle-wheel dimers, CrystEngComm, 2013, 15(31), 6122–6126 RSC.
- M. A. Shmelev, Yu. K. Voronina, N. V. Gogoleva, M. A. Kiskin, A. A. Sidorov and I. L. Eremenko, Synthesis and Crystal Structure of {Eu2IIICd2}, {Tb2IIICd2} and {Eu2IIIZn2} Complexes with Pentafluorobenzoic Acid Anions and Acetonitrile, Russ. J. Coord. Chem., 2022, 48(4), 224–232 CrossRef CAS.
- B. T. Usubaliev, P. S. Abdurakhmanova, M. K. Munshieva and D. M. Ganbarov, Clathrate [Zn(C6H5COO)2(H2O)2] · 2CH3COOH: Synthesis and crystal structure, Russ. J. Coord. Chem., 2010, 36(11), 865–869 CrossRef CAS.
- S. V. Larionov, L. A. Glinskaya, T. G. Leonova, R. F. Klevtsova, E. M. Uskov and V. E. Platonov, et al., Luminescence properties of complexes Ln(Phen)(C6F5COO)3 (Ln = Tb, Eu) and Ln(C6F5COO)3 · nH2O (Ln = Tb, n = 2; Ln = Eu, n = 1). Structures of the [Tb2(H2O)8(C6F5COO)6] complex and its isomer in the supramolecular compound [Tb2(H2O)8(C6F5COO)6] · 2C6F5COOH, Russ. J. Coord. Chem., 2009, 35(11), 798–806 CrossRef CAS.
- M. S. Khiyalov, I. R. Amiraslanov, F. N. Musaev and K. S. Mamedov, Structural and thermographic investigation of dysprosium (3) pentaaquobenzoate, Koord. Khim., 1982, 8(4), 548 Search PubMed.
- M. A. Shmelev, M. A. Kiskin, J. K. Voronina, K. A. Babeshkin, N. N. Efimov and E. A. Varaksina, et al., Molecular and Polymer Ln2M2 (Ln = Eu, Gd, Tb, Dy; M = Zn, Cd) Complexes with Pentafluorobenzoate Anions: The Role of Temperature and Stacking Effects in the Structure; Magnetic and Luminescent Properties, Materials, 2020, 13(24), 5689 Search PubMed.
- M. Yin and J. Sun, Synthesis and characterization of two tetranuclear heterometallic Tb(III)–Zn(II) complexes, J. Coord. Chem., 2005, 58(4), 335–342 CrossRef CAS.
- R. K. R. Jetti, R. Boese, P. K. Thallapally and G. R. Desiraju, Five New Pseudopolymorphs of s ym -Trinitrobenzene, Cryst. Growth Des., 2003, 3(6), 1033–1040 CrossRef CAS.
- D. Sharada, A. Saha and B. K. Saha, Charge transfer complexes as colour changing and disappearing–reappearing colour materials, New J. Chem., 2019, 43(20), 7562–7566 RSC.
- M. A. Shmelev, J. K. Voronina, M. A. Evtyukhin, F. M. Dolgushin, E. A. Varaksina and I. V. Taydakov, et al., Synthesis, Structure and Photoluminescence Properties of Cd and Cd-Ln Pentafluorobenzoates with 2,2′:6′,2′-Terpyridine Derivatives, Inorganics, 2022, 10(11), 194 Search PubMed.
- L. N. Puntus, K. A. Lyssenko, M. Yu. Antipin and J. C. G. Bünzli, Role of Inner- and Outer-Sphere Bonding in the Sensitization of Eu III -Luminescence Deciphered by Combined Analysis of Experimental Electron Density Distribution Function and Photophysical Data, Inorg. Chem., 2008, 47(23), 11095–11107 CrossRef CAS PubMed.
- N. V. Y. Scarlett and I. C. Madsen, Powder Diffr., 2006, 21(4), 278 CrossRef CAS.
- M. H. V. Werts, R. T. F. Jukes and J. W. Verhoeven, The emission spectrum and the radiative lifetime of Eu3+ in luminescent lanthanide complexes, Phys. Chem. Chem. Phys., 2002, 4, 1542–1548 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: PXRD patterns for 1Eu, 1Tb, 2Eu, and 3Eu; continuous shape measures (CShM) for Zn and Ln coordination polyhedra; tables of hydrogen bonds and C–F⋯π and C–N⋯π interaction parameters; main crystallography data and refinement details; emission spectra of 1–4 at 300 K and 77 K and excitation spectra of 1–4 at 300 K and 77 K. CCDC 2343086 (1Eu), 2343087 (1Tb), 2343088 (2Eu), 2390847 (3Eu). For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4dt03414g |
| This journal is © The Royal Society of Chemistry 2025 |