Production of natural zeolite-filled recycled PVDF filters and their application for gray water treatment†
Ayşenur
Katırcı
 a,
Seniyecan
Kahraman
a,
Seniyecan
Kahraman
 a and
Filiz
Uğur Nigiz
a and
Filiz
Uğur Nigiz
 *b
*b
aÇanakkale Onsekiz Mart University, School of Graduate Studies, Çanakkale, 17100, Türkiye
bÇanakkale Onsekiz Mart University, Chemical Engineering Department, Çanakkale, 17100, Türkiye. E-mail: filiz.ugur@comu.edu.tr
First published on 29th January 2025
Abstract
In this study, clinoptilolite (Clp)-doped poly(vinylidene fluoride) (PVDF) membranes (1–4 wt%) were prepared using an electrospinning method. Further, filtration tests on the simulated gray water components were investigated. Methylene blue (MB), linear alkyl benzene sulfonate (LAS), oil (soybean oil), and microplastic (MP) filtration were performed. MB filtration with the PVDF membrane resulted in over 99% of MB rejection. Oil rejection with the PVDF membrane without Clp was observed to be 95%, while the addition of Clp increased the oil rejection to over 99%. It was observed that LAS rejection increased as the Clp content increased. MP rejection using PVDF-based membranes was 100%. Considering all the test results, the membrane containing 3 wt% Clp showed the best performance, and the process parameters and rejection efficiencies were determined through experimental optimization. Synthetic gray water analyses included the chemical oxygen demand (COD), pH, conductivity, and total dissolved solids (TDS). COD rejection was 63.1%, while turbidity rejection was 97.2%.
Water impactWater is a critical resource for life. It directly affects ecosystems and human health. Our study titled “Production of natural zeolite-filled recycled PVDF filters and their application for gray water treatment” offers important insights for developing water-treatment technology. Gray water, which is a part of domestic wastewater, can be treated, preventing its damage to the environment. Gray water contains contaminants such as oils, dyes, and residues from soaps and detergents. Our research offers a sustainable and effective method to reduce this threat by removing these dangerous pollutants from wastewater sources. The impact of our research on water resources is multifaceted. Our methodology offers a reliable and sustainable approach for water treatment by removing impurities from gray water. This makes a direct contribution to eliminating environmental pollution, protecting ecosystems and safeguarding human health. Our work is exemplary in addressing the challenges faced during gray water treatment. It sets a precedent for future research and encourages the development of environmentally friendly and efficient water-treatment technologies. |
1 Introduction
Urbanization started with the industrial revolution, and since then, the population has been increasing rapidly. With the increasing population, water resources have become insufficient. Thus, water scarcity is a major problem that needs to be solved urgently. Among the techniques developed to prevent water scarcity, reduction in water usage, rainwater harvesting, and gray water treatment systems have shown great promises. In particular, gray water treatment systems are attracting increasing attention as they promote water reuse along with reducing environmental pollution caused by wastewater.1Gray water is produced through domestic activities in bathrooms and kitchens and through the use of washing machines and dishwashers. Gray water accounts for 75% of household wastewater.2 Therefore, its treatment is very important. In order to minimize the energy required for gray water treatment and improve its efficiency, it is recommended to treat gray water at source. Gray water can contain various contaminants, such as oils, dyes, and residues from soaps and detergents. In addition, gray water pollutants are formed and are present as dissolved, colloidal, and suspended forms. The anionic and cationic surfactants in gray water degrade slowly, and they are not biodegradable under anaerobic conditions.1,3
Gray water treatment processes consist of physical (sedimentation and filtration), chemical (coagulation and flocculation), and biological methods, as well as a combination of these methods. Biological treatment takes place in the presence of microorganisms, which decompose organic matter. In physical separation, large particles and suspended matter are retained, whereas in chemical separation, pollutants in the water are retained by changing the chemical properties of the water. Among these processes, membrane technologies are of great interest owing to their low energy requirement and high efficiency.4
Membranes used in wastewater treatment can be ceramic, inorganic, and polymeric. Polymeric membranes are made of a variety of materials, such as polyvinyl alcohol (PVA), polyether sulfone (PES) and polyvinylidene fluoride (PVDF).5,6 They are attractive owing to their high porosity, flexibility, high permeability, and easy functionality.7 PVDF, which has a semi-crystalline structure, is mostly used in commercial wastewater treatment owing to its high mechanical strength, thermal stability, and chemical resistance.8,9 However, PVDF is hydrophobic and susceptible to fouling when treating water containing organics that tend to foul.10 Therefore, researchers are aiming to develop better PVDF membranes by improving the preparation processes or/and the surface modification of plain PVDF membranes. Incorporation of the polymer with inorganic materials during membrane preparation is a widespread technique used to enhance both the hydrophilicity and separation performance.11 For this purpose, zeolites may be used as cost-effective natural materials.12
Natural zeolites may be preferred in wastewater treatment due to their low cost and abundant availability in nature. Natural zeolites are crystals with a three-dimensional structure based on repeated silicon–oxygen (SiO4) and aluminum–oxygen (AlO4) tetrahedral units.13 Zeolites are unique because of their structures, with many channels and cavities. In addition, their ability to absorb small molecules and their non-toxicity make them attractive for many applications.14 The most important natural zeolite is clinoptilolite (Clp).15
In this study, the wastewater treatment properties of electrospun PVDF were investigated with the incorporation of Clp in different ratios. In the literature, electrospun membranes seem to have been preferred by many researchers due to their excellent membrane formation, and good thermal, mechanical, and chemical resistance.16 However, it is important to rearrange the filler content in electrospun membranes. Bahi et al. (2017) prepared lignin-zeolite composite nanofiber membranes by electrospinning. The addition of zeolite improved the tensile strength, hydrophilicity, permeability, and separation factors of the membranes. However, the addition of zeolite at a high weight percentage resulted in decreased mechanical properties, permeability, and separation factors of the membranes.17 Therefore, in the present study, only 1–4 wt% Clp was added to the PVDF membranes. Also, in preliminary studies, agglomeration was observed in PVDF fibers containing 5 wt% Clp, hence we kept the percentage Clp below this. The membranes were characterized by scanning electron microscopy (SEM), Fourier transform infrared spectroscopy (FTIR), thermogravimetric analysis (TGA), differential scanning calorimetry (DSC), contact angle (CA) measurements, mechanical analysis, and antimicrobial activity tests. Methylene blue (MB), linear alkyl benzene sulfonate (LAS), oil (soybean oil), and microplastic (MP) filtration were performed with the PVDF membranes. The best-performing filter was selected, and the optimum process parameters and separation efficiencies were then determined by experimental optimization (response surface methodology). Then, synthetic gray water was prepared, and filtration was performed. The chemical oxygen demand (COD), pH, conductivity, and total dissolved solids (TDS) in the gray water were also determined. To the best of our knowledge, this is the first time in the literature that a membrane filter has been produced from recycled PVDF and its effect on multiple separation investigated.
2 Experimental section
2.1 Materials
The PVDF polymer used in this study was regenerated from hollow-fiber ultrafiltration membrane production waste from a local factory. The membrane was manufactured from Kynar720 (average molecular weight (Mw) of 263![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1) (Arkema). Dimethyl formamide (DMF) (with 99.0% purity) and acetone (with 99.0% purity) were purchased from Merck Chemicals, Turkey. Polyvinylpyrrolidone (PVP) (average mole weight of 360
000 g mol−1) (Arkema). Dimethyl formamide (DMF) (with 99.0% purity) and acetone (with 99.0% purity) were purchased from Merck Chemicals, Turkey. Polyvinylpyrrolidone (PVP) (average mole weight of 360![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1) as a pore-forming agent was obtained from Sigma Aldrich, Turkey. Clinoptilolite was kindly supplied from Rota Maden Inc., Turkey. The properties of Clp are given in the ESI† data (Table S1).
000 g mol−1) as a pore-forming agent was obtained from Sigma Aldrich, Turkey. Clinoptilolite was kindly supplied from Rota Maden Inc., Turkey. The properties of Clp are given in the ESI† data (Table S1).
2.2 Membrane production
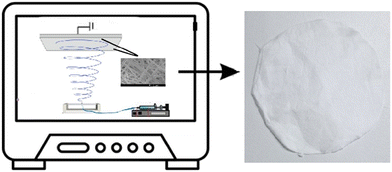
The waste ultrafiltration membranes were cut into 1 mm pieces, washed with deionized water, soaked in alcohol, and dried at 50 °C. In this study, regenerative membrane filters were produced by an electrospinning technique. For this purpose, PVDF-DMF-acetone solution containing 25 wt% PVDF (10 wt% PVP according to the weight of the PVDF) was prepared and stirred at 50 °C for 2 h. The homogeneous polymer solution was taken in a 10 ml syringe, placed in the electrospinning system, and finally collected on the plate at 17 kV, 0.05 mL min−1, 50% humidity conditions. The distance of the plate from the syringe tip was kept constant at 15 cm. The thickness of each film produced was 150 μm. For the preparation of Clp-loaded membranes, 1–4 wt% Clp was dissolved in DMF by means of an ultrasonic homogenizer (Bandelin HD4050) and added to the homogenous polymer solution using a priming method that was described in a previous study.18 Thus, it was ensured that the particles did not collapse and Clp was homogeneously distributed in the electrospinning process. The membranes were coded according to the Clp ratio as PVDF-(1,2,3,4)wt% Clp. The experimental film production system is shown in Fig. 1.2.3 Characterization
Scanning electron microscopy (SEM) analysis was carried out under low and high vacuum with a SEM device (JEOL JSM-7100-F). Attenuated total reflectance-Fourier transform infrared spectroscopy (FTIR-ATR) analysis was performed with a Cary 630 ATR-FTIR instrument. The wavelength range for the tests was 4000–650 cm−1. Contact angle tests (Dataphysics TBU OCA 11) were applied for all the membranes. Measurements were taken from 5 different points and the average values were recorded. The tensile strength and elongation at break values were determined using an ANKARIN universal testing machine according the ASTM D882 standard.Thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) tests were performed for thermal analysis. TGA (Mettler Toledo) was carried out up to 650 °C. DSC analyses were carried out in a temperature range of 10–250 °C in a nitrogen environment.
For the swelling tests, the weights of the samples were measured dry and after being kept in water for 24 h and the swelling percentage calculation was based on the weight difference.
For the porosity (empirical) analysis, each porosity value was determined by averaging three different measurements. The following equation was used:19
 | (1) |
The accumulation of Gram-negative bacteria Escherichia coli and Gram-positive bacteria Staphylococcus aureus on the filter was determined by the agar diffusion method.20 Selected Gram-negative and Gram-positive bacteria were cultured separately on tryptic soy broth medium, incubated for 24 h and diluted to 0.5 McFarland turbidity to 105 CFU ml−1. The bacterial suspension was spread over the entire surface of the agar plate using a sterile swab from the liquid medium with the adjusted bacterial density. Filter samples with a diameter of 6 mm were placed in Petri dishes and incubated at 37 °C for 24 h and the zone of inhibition diameter was measured. The transparent zone formed was evidence of the antimicrobial activity.
2.4 Filtration tests
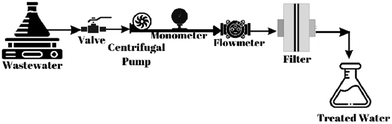
A schematic representation of the filtration test is shown in Fig. 2. To determine the filtration performance of all the filters, emulsified oil (soybean oil, 2%), cationic dye (MB, 5 mg L−1), anionic surfactant (LAS, 100 mg L−1), and MP (polyester, 200 mg L−1) rejection tests were performed separately. The test was performed at a flow rate of 0.6 mL min−1 at room temperature with an effective membrane diameter of 3 cm.For the determination of MB, the concentrations were read directly according to calibration curves using the UV-Probe program at 665 nm wavelength with an ultraviolet-visible (UV-vis) spectrophotometer (Shimadzu 1280).21 The calibration curve is given in the ESI† data (Fig. S1). For the determination of LAS, the concentrations were read directly according to the calibration curves using the MB method with a UV-vis spectrophotometer at a wavelength of 650 nm using the UV-Probe program.22 Details of the method and calibration curve are given in the ESI† data (Fig. S2). For the determination of oil, the oil solution was prepared and homogenized using an ultrasonic bath (Bandelin HD4050) for 1 h. The solution was kept in the ultrasonic bath until a stable milky color was obtained. Using a UV-vis spectrophotometer at a wavelength of 272 nm, the pre- and post-test concentrations were recorded.23,24 For the determination of MP, a product containing more than 90% polyester was taken and the polyester was removed with a fluff collector. A solution of 200 mg L−1 was then prepared. After filtration, the polyester in the water was collected on the membrane paper and weighed.25 The percentages (%) MB, LAS, MP, and oil rejection were calculated by the following equation:
 | (2) |
2.5 Determination of the process parameters and rejection efficiencies with the best-performing filter
Experimental optimization was performed with the filter with the highest rejection performance and the rejection percentages from the filtration tests were determined. Design expert 12, an experimental design program, was used to implement the response surface method. Within the scope of the study, the central composite design technique, which is a subheading of the response surface method, was preferred. The factors affecting the filtration, and the levels of the factors were created by considering the literature data. As a result, a design consisting of three factors and three levels was created. The factors and their levels are given in the ESI† data (Table S2).2.6 Synthetic gray water tests with the best-performing filter
Synthetic gray water was created by mixing the synthetic gray water contents given in the ESI† data (Table S3), and then the COD, suspended solid matter (SSM), turbidity, pH, conductivity, and TDS rejection performances of the filter were analyzed by performing filtration tests.The COD is the amount of oxygen required for the chemical oxidation of oxidizable substances in water. It is an important parameter in determining wastewater pollution. It was determined here by the potassium dichromate method using a spectrophotometer (Shimadzu UV-1280).22 The concentrations were read directly from the calibration curve with the help of the UV-Probe program at 450 nm wavelength using the spectrophotometer. Details of the methods and the calibration graph are given in the ESI† data (Fig. S3).
Turbidity can be observed in waters with suspended solids. It prevents the light transmission of water. Turbidity can be caused by organic or inorganic substances. Here, turbidity was determined directly from the calibration graph using a spectrophotometer at a wavelength of 450 nm with the UV-Probe program.22 Details of the methods and calibration graph are given in the ESI† data (Fig. S4). The percentage rejection based on the pre-, and post-filter values was calculated according to eqn (2).
The SSM is the sum of substances that settle or do not settle in water. It usually consists of rock fragments, mud, clay minerals, and/or colloidal organic matter fragments. The analysis was performed according to the standard methods 1989 S.2-75, GEMS S.22 (APHA, 1989). The gravimetric method was preferred here for the analysis. Whatman no. 42 filter papers were used, and the SSM was determined using the following formula:
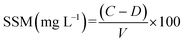 | (3) |
Conductivity, pH, and TDS are other routine tests were also performed. These tests were performed using a Mettler Toledo Seven Compact instrument.
3 Results and discussion
3.1 Characterization tests
Fig. 3 shows the contact angle (CA) values of PVDF and the PVDF-Clp doped filters with water on the surface. PVDF without Clp was superhydrophobic and the contact angle was measured as 155°. This value decreased gradually with the addition of Clp. According to these results, the surface hydrophobicity of the Clp-added PVDF materials decreased, just as expected. This has two advantages. One of them was the decrease in the fouling capacity of the surface due to the decrease in hydrophobicity and the increase in the antimicrobial effect. The other was that the water flux increased significantly due to the increase in the hydrophilicity of the fibers. The reason for the increase in hydrophilicity was the presence of the hydroxyl group in Clp. In similar studies in the literature, it has been reported that Clp films added at a very low rate significantly reduced the contact angle.26,27SEM analysis was performed to observe the morphological structures of the membranes. Fig. 4 shows the structures of the plain PVDF (Fig. 4a and d), PVDF-2 wt% Clp (Fig. 3b and e), and PVDF-4 wt% Clp (Fig. 4c, f, and g) membranes. The images confirmed that the electrospinning conditions used for the membrane preparations were suitable. As can be seen in the figures, there was a slight increase in the fiber diameters when the Clp ratio was increased, which was also reported by Nassrullah et al., 2020 and Hassan et al., 2022.28,29 The difference is very clear between Fig. 4a and c. It was also observed that the fiber surface porosity increased with the addition of Clp (Fig. 4g). Another important finding was that no pilling or agglomeration was observed in the fibers. However, in preliminary studies, agglomeration was observed in PVDF fibers containing 5% Clp by weight. For this reason, it was decided the top Clp addition would be up to 4% in this study, since the homogeneity would deteriorate with a higher Clp ratio.
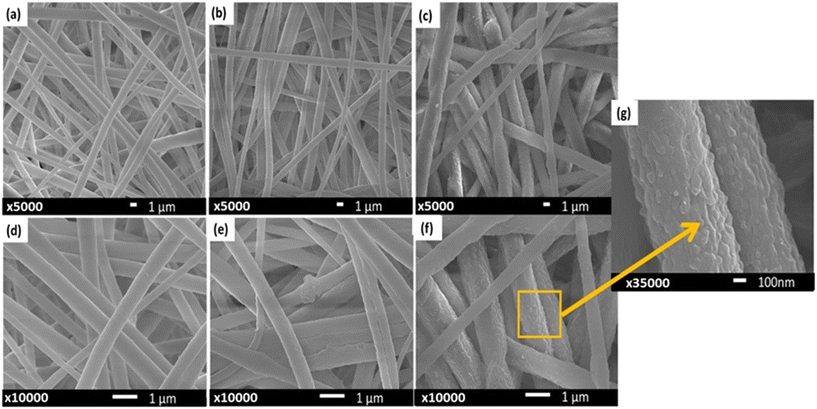 | ||
| Fig. 4 SEM analysis of the PVDF (a and d), and 2 wt% (b and e) and 4 wt% (c, f, and g) Clp-doped PVDF. | ||
Fig. 5 shows the ATR-FTIR analysis spectra of the PVDF and Clp-doped PVDF filters. Before FTIR analysis, the filters were first soaked in water and then dried. The PVP used as a pore former in the filter was expected to dissolve completely. However, the peaks observed in the 1670 cm−1 region showed that the PVP was completely insoluble in water, with the peaks related to the carboxyl C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds in it. The peaks observed in the 870 and 1170 cm−1 region in Fig. 5 were due to C–C and C–C–C bonds in the organic structure of PVDF. The peak at 1400 cm−1 was due to the C–F vibration of the CF2 bond in the PVDF structure.29,30
O bonds in it. The peaks observed in the 870 and 1170 cm−1 region in Fig. 5 were due to C–C and C–C–C bonds in the organic structure of PVDF. The peak at 1400 cm−1 was due to the C–F vibration of the CF2 bond in the PVDF structure.29,30
In the study by Lopes et al. (2014), while silicon-based bonds were clearly seen in the membranes prepared as a film with 4% NaY zeolite doping into PVDF, when the same membrane was prepared by electrospinning, it was observed that the appearances of the doped and undoped membranes were exactly the same. The reason for this is that due to the nature of the alpha, beta, and gamma phases formed due to the electrospinning process, there was not much difference seen with varying the additive ratio.31 Similarly, no significant difference was observed in this study with varying the Clp addition.
The porosity values of all the filters produced by electrospinning were calculated empirically using eqn (1). In the porosity tests, the filters were weighed in the same mass with a certain geometry, and the thickness of the films used was taken as 150 μm. The porosity mentioned here does not refer to the structural porosity of the fibers but rather the porosity in-between the fibers, which is important in the separation process. The water retention (water uptake) and porosity values of the PVDF membranes are shown in Fig. 6a. As can be seen, the porosity and water retention values increased as the Clp content increased. The main reason for this is the high water retention capacity of Clp and the hydrogen bonds it forms with water. The increase in porosity increased depending on the water retention value. Here, the effective value for the increase in porosity was taken as the water retention because the water retention value increased at a greater rate than the porosity. This actually shows that the empirical values increased because more water was retained. As seen from the SEM analyses, the increase in Clp increased the fiber diameters, which relatively reduced the distance between the fibers. This shows that while the porosity decreased, the hydrophilicity of the fibers increased. Similar results have been reported in the literature.28,29
 | ||
| Fig. 6 Porosity and water retention results (a) and mechanical analysis (b) of the PVDF and Clp-doped PVDF filters. | ||
Mechanical analysis of the filters was carried out using an ANKARIN Universal Test device. Mechanical analysis is essential, especially for a material that works under hydrodynamic pressure, such as a filter. Fig. 6b shows the mechanical analysis of all the filters depending on the Clp ratio. The PVDF membrane's mechanical characteristics can be altered in a number of ways by adding Clp. Zeolites are known for having remarkable rigidity and mechanical strength. Zeolite particles can serve as a reinforcing agent when incorporated into polymeric membranes, which enhances the membrane's overall mechanical qualities. They can improve the membrane's resistance to deformation, tensile strength, and flexural strength. Furthermore, compared to a large proportion of polymers, Clp has a higher modulus of elasticity.6 As shown in Fig. 7, the addition of Clp to PVDF increased both the stress and strain tolerance with up to 3 wt% doping. The reason for this increase is that the strength of the zeolite was higher than that of the polymer according to the rule of mixing. However, both the strength and elongation decreased after 3 wt%. This was attributed to the deterioration of the homogeneity and the inhibition of in-filter load transfer due to the excess amount of additives. Similar results were also reported in the literature.32,33
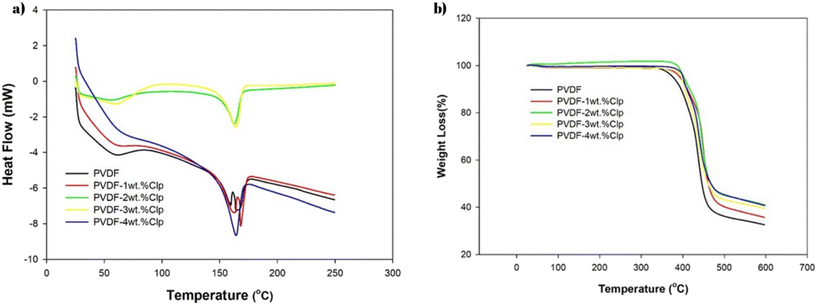
The DSC analysis results for the filters are given in Fig. 7a. The TGA analysis results for the PVDF and Clp-doped PVDF filters are given in Fig. 7b. DSC and TGA analyses provide information about the thermal behavior of polymeric filters and their glass transition temperatures, melting points, and melting enthalpies. Especially in the case of these filters that need to operate above a certain thermal value, the effect of temperature on the filter is very important. As seen in Fig. 7a and Table 3, the glass transition temperature of the PVDF was observed at 59.9 °C. Then, it showed two melting peaks, which have also been reported in the literature.34 The melting of the α-form crystalline structure was attributed to the endothermic transition at 160 °C, whereas the β-form of the PVDF crystals could be linked to the second endothermic peak at 167 °C. The literature reports confirm that because of PVDF's polymorphic nature, double melting transitions are commonly seen.35,36 This behavior can be explained by the fact that piezoelectric β-phases can occur in electrospun PVDF fibers due to the strong electric field and mechanical stress that are typical characteristics of the electrospinning process.
Table 1 shows the glass transition temperature (Tg), melting temperature (Tm), and enthalpy of melting (ΔHm) of the electrospun PVDF and Clp-doped PVDF filters. The glass transition temperature decreased slightly with the addition of Clp and then increased. However, this increase did not affect the processability or glass transition temperature of the material. On the other hand, while the melting temperature increased gradually and slowly, there was a very high and continuous increase observed for the melting enthalpy. This shows that zeolite doping significantly increases the fiber crystallinity. Similar results have been reported in the literature.37
| Membrane | T g (°C) | T m (°C) | ΔHm (J g−1) |
|---|---|---|---|
| PVDF | 59.9 | 160–167 | 15.87 |
| PVDF-1 wt% Clp | 58.9 | 161.5–168.3 | 14.85 |
| PVDF-2 wt% Clp | 56.64 | 162.9 | 36.2 |
| PVDF-3 wt% Clp | 60.46 | 163.8 | 32.99 |
| PVDF-4 wt% Clp | 60.9 | 164 | 30.13 |
The TGA analysis results of the PVDF and Clp-doped PVDF filters are shown in Fig. 7b. Two-stage degradation was generally observed in the PVDF filters with Clp loading, and degradation due to water loss occurred below 100 °C. Mass losses due to PVDF degradation were observed between 400 °C and 450 °C. During degradation, PVDF releases hydrogen and fluorine, which combine to form hydrogen fluoride (CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CF2). Thus, carbon atoms become free to bond with each other.38
CF2). Thus, carbon atoms become free to bond with each other.38
The thermal resistance data of the PVDF and Clp-doped PVDF filters are listed in Table 2. As can be seen in the aforementioned figure and in the table, the first decomposition temperature increased from 377 °C to over 400 °C with the addition of Clp. This shows that the thermal resistance of the Clp-doped PVDFs was higher than that of the plain PVDF material. This increase decreased slightly after 3% loading. As can be seen in the table, Clp added at a maximum of 3% of the polymer increased the residual mass from 32.5% to 41%. This shows that the thermal heat transfer and thermal resistance to degradation increased with the addition of the zeolite.
| Membrane | T 5 (°C) | T 50 (°C) | Remaining (%) | Decomposition temperature (°C) |
|---|---|---|---|---|
| PVDF | 377 | 446 | 32.5 | 438 |
| PVDF-1 wt% Clp | 394 | 461 | 35.6 | 416–450 |
| PVDF-2 wt% Clp | 403 | 467 | 40.96 | 407–445 |
| PVDF-3 wt% Clp | 401 | 468 | 40 | 404–440 |
| PVDF-4 wt% Clp | 397 | 460 | 40.7 | 402–438 |
All the prepared films were tested for antimicrobial activity against both Gram-negative (E. coli) and Gram-positive (S. aureus) bacteria. During the antimicrobial test, both the disk diffusion experiment samples and the films were added directly to the medium and tested for culture growth. The disk diffusion results are shown in Fig. 8. In all the tests, it was observed that there was no microbial growth on the films, not even in the pure form of the polymer matrix.
 | ||
| Fig. 8 Disk diffusion results for Gram-positive (S. aureus) (a), and Gram-negative (E. coli) (b) bacteria. | ||
The zone diameters of all materials are given in the ESI† data (Table S4). According to this, Clp added to PVDF polymer showed more successful antimicrobial activity against the Gram-positive bacteria. This was probably due to the ability of Clp to be positively charged. As a result, the films were found to have both surface and environmental antimicrobial effects. In the literature, there are also studies indicating that Clp-added films show an antimicrobial effect.39
3.2 Filtration test results
The rejection results of the membranes are given in Fig. 9. It can be seen that the rejection of MB was over 99% (Fig. 9a). As can also be seen in the graph, the increase in rejection percentage was not very high when the Clp content was increased, and MB rejection only increased from 99% to 99.4%. Therefore, Clp was not very effective for improving MB rejection. However, it should be noted that MB is a cationic molecule and zeolites carry a negative charge in their alumina silicate frameworks. Therefore, they can attract MB molecules and increase the resulting rejection. In the literature, Kadja et al. (2023) fabricated a mixed matrix membrane (Mercapto functionalized-natural zeolites/PVDF) for achieving a better rejection of MB. They reported achieving an increase in the rejection of MB with the inclusion of Clp in the PVDF matrix.40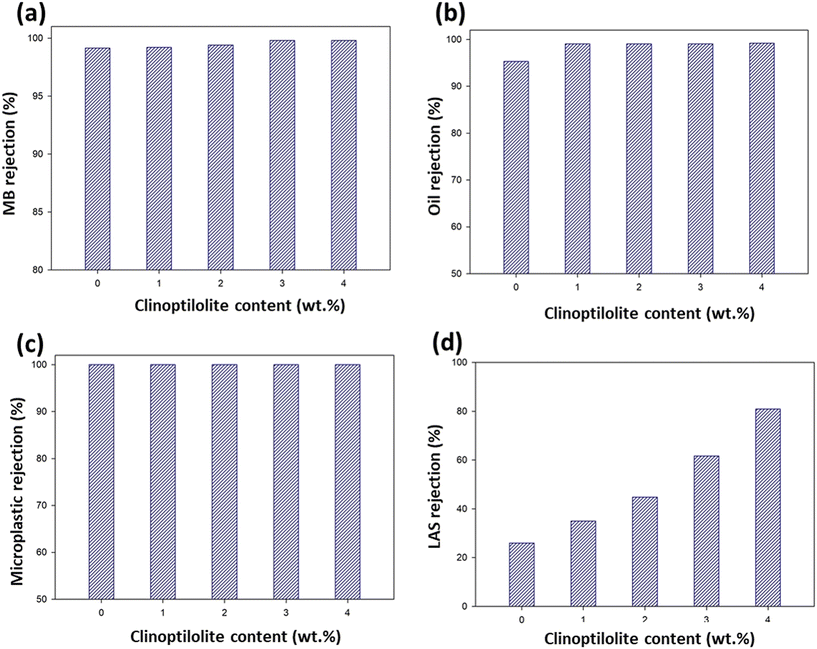 | ||
| Fig. 9 MB (a), oil (b), MP (c), and LAS (d) rejection graphs of the PVDF and Clp-doped PVDF filters. | ||
The oil rejection results are shown in Fig. 9b. It is clear from the figure that the oil rejection with all the membranes was greater than 95%. It was also observed that the percentage oil rejection increased with the increase in the amount of Clp, notably from 95% to 99%. In the literature, Nayak et al. (2022) used a PVDF membrane as an oil trap due to its high oleophilicity and reported that it removed more than 98% of an oil–water emulsion of soybean and silicone oils.41 Clp, a natural zeolite, has an oleophilic structure, and its water, oil, and gas absorption capacity is quite high. In addition, due to its crystal lattice, there is a lack of negative charge both on the crystal surface and in the channels. It can hold and especially provide positive ions by displacing them.42Fig. 9c also shows that 100% microplastic removal was achieved with all membranes due to the small pore size of the PVDF, which was also revealed in the porosity test and SEM analysis.
The LAS removal results are shown in Fig. 9d. As can be seen in the figure, Clp incorporation significantly increased the LAS rejection from 23% to 80.87%. Here, the presence of aluminum in the zeolite results in the formation of an unstable framework with an intrinsic cationic-exchange capacity.43 The positively charged groups are balanced by anionic counter ions, which increases the rejection of anionic pollutants, such as LAS. The reason for the increase in rejection as the amount of Clp increased is that the ion-exchange capacity increased with the amount of Clp.
In the filtration tests, high and very close values were obtained for MB, MP, and oil filtration. Therefore, the indicative process was LAS filtration. The highest rejection was obtained with PVDF-4 wt% Clp, namely 80.87%, whereas the rejection by PVDF-3 wt% Clp was 61.66%. However, in the mechanical analysis after 3 wt% Clp was added, both the strength and elongation decreased. Therefore, PVDF with 3 wt% Clp showed the best performance and was selected as the best filter membrane. Therefore, the oil, MB, and MP rejection percentages of the best-performing PVDF-3 wt% Clp membranes were compared with the results from other studies in the literature. The comparable values are listed in Table 3. As can be seen in the table, PVDF membranes are remarkable for their high separation properties. Improvements in the preparation process of PVDF membranes and surface modification of pure membranes can increase their separation performances.
3.3 Determination of the optimum process parameters by experimental optimization
There are many parameters that can affect the membrane performance, such as temperature, pH, flow rate, and membrane structure. In this study, the effects of varying the concentration of the impurities, the feed temperature, and the feed flow rate on the rejection were investigated using a central composite design of response surface methodology. In the three-factor experimental design, each parameter is represented by letters. Here, the parameter represented by the letter A is the feed flow rate (L min−1), while the letters B and C are the feed temperature (°C) and the concentration of impurities (mg L−1), respectively.Fig. 10 shows 3D plots of the model and the model and fit statistics. Accordingly, the second-order equation model was determined as the most appropriate model for MB separation. According to this model, the R2 value was obtained above 98%. As can be seen in Fig. 10, a high separation efficiency was observed at low temperature and low flow rates, and these results were largely consistent with the model.
 | ||
| Fig. 10 3D plots of MB rejection by the PVDF-3 wt% Clp filter (5 mg L−1 (a), 10 mg L−1 (b), 15 mg L−1 (c)), verification plot (d), model summary statistics (e) and fit statistics (f). | ||
Details of the analysis of variance (ANOVA) of the model are provided in the ESI† data (Table S6). According to this analysis, a high negative effect of the flow rate and temperature could be seen from the low p values and high F values, showing that the concentration also has a significant negative effect. According to the formula obtained (eqn (4)), it could be concluded that the highest effect was seen with varying the temperature.
| R = 98.41 − 0.3600A − 0.8600B − 0.1500C − 0.1500AB − 0.0250AC − 0.1000BC − 0.0423A2 +0.4577B2 + 0.0077C2 | (4) |
 | ||
| Fig. 11 3D plots of oil rejection by the PVDF-3 wt% Clp filter (1% (a), 2% (b), 3% (c)), verification plot (d), model summary statistics (e), and fit statistics (f). | ||
The equation obtained according to the modeling is given below (eqn (5)). The equation shows that the most influential factor for oil separation was the oil concentration in the feed.
| R = 98.37 − 0.2050A − 0.3950B − 0.4750C + 0.0187AB + 0.0937AC − 0.1313BC + 0.0194A2 + 0.1694B2 + 0.3694C2 | (5) |
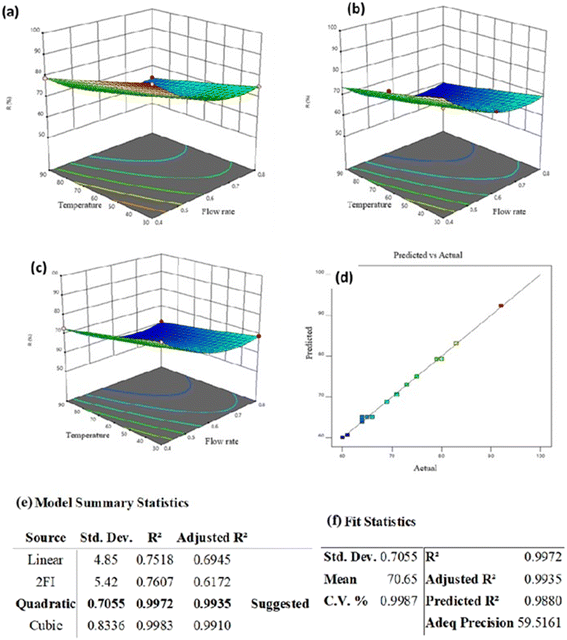 | ||
| Fig. 12 3D plots of LAS rejection by the PVDF-3 wt% Clp filter (50 mg L−1 (a), 100 mg L−1 (b), 150 mg L−1 (c)), verification plot (d), model summary statistics (e), and fit statistics (f). | ||
Eqn (6) shows that the most effective factor for rejection was the feed flow rate.
| R = +65.11 − 7.40A − 5.30B − 3.10C − 0.5AB − 0.75AC + 0.75BC + 6.8A2 + 0.3028B2 + 2.30C2 | (6) |
3.4 Results of the synthetic gray water tests
Synthetic gray components were mixed to form synthetic wastewater. Then, filtration tests were performed with the PVDF-3 wt% Clp filter. The COD, SSM, turbidity, pH, conductivity, and TDS separation performances of the filter were analyzed. The synthetic gray water analysis data are shown in Table 4. Fig. S5 in the ESI† data shows images of the synthetic gray water before filtration (a), after filtration, and the membrane after filtration (c).| Parameters | Before filtration | After filtration | Rejection (%) |
|---|---|---|---|
| pH | 6.25 | 7.20 | — |
| Conductivity (μs) | 112.5 | 88.5 | — |
| Turbidity (NTU) | 598.9 | 16.7 | 97.2 |
| TDS (mg L−1) | 71.9 | 59.4 | — |
| COD (mg O2 per L) | 1207.8 | 445.6 | 63.1 |
As can be seen in Table 4, the COD was decreased from 1207.8 mg O2 per L before filtration to 445.6 mg O2 per L after filtration, and hence 63.1% rejection was achieved. Affandi and Razak (2017) reported a 64% COD rejection in their study with an electrospun Nylon 6 nanofiber membrane. This shows that the electrospun nanofiber membrane has the ability to reduce the COD.62 Hosseini et al. (2024) reported 11% COD rejection in wastewater treatment using Nylon 6/zeolite nanofiber membranes produced by an electrospinning method.63 Zendehdel and Nouri (2021) reported 49%, 61%, and 66% COD rejection rates in filtration with concentrations of 500, 480, and 420 mg O2 per L at pH 8.45, 8.40, and 8.42, respectively, using electrospun PES nanofibers.64
As a result of the SSM analysis, it was found that 99.8% of the SSM were successfully retained by the filter. Accordingly, the rejection of the turbidity elements also increased. Particles and colloids formed by the SSM cause turbidity in water and can cause clogging in the filters and pipes used in such treatment.65 Therefore, the SSM affects the turbidity. Turbidity also prevents the light transmission of water. Turbidity can be caused by organic or inorganic substances. Here, the rejection percentages were calculated from the difference in the turbidity values before and after filtration. Here, 97.2% of the turbidity-causing elements could be removed, leading to a major improvement in the water quality; whereby the treated water had an excellent and clear physical appearance, free of SSM.
According to the American Public Health Association (APHA 1995), the conductivity, pH, and TDS are important parameters in gray water analysis.66 In this study, the pH value was 6.25 before filtration and 7.20 after filtration. In the study conducted by Chaabane et al. (2017), the pH values of gray water samples after filtration ranged between 7.35–8.82.66 According to the study conducted by Zipf et al. (2016), the pH values of gray water consisting of domestic waste were 5.7 and 6.4. After various filtrations, the pH was found to be in the range of 7.5–7.6.67 In another study by Yovo et al. (2016), the pH value before filtration was 6.4, while the pH value after filtration was 7.04.68 Therefore, the pH value above 7 after filtration and the increase in pH value after filtration were similar to the previous literature reports.
The conductivity is another important factor to consider. Here, the conductivity was 112.5 μs before filtration and 88.5 μs after filtration. The conductivity is the ability of a substance to conduct electric current. Water gains conductivity due to the presence of dissolved ions in it. Since dissolved ions will decrease after filtration, it is natural that the conductivity will decrease.69 There is a strong relationship between the TDS and conductivity. The TDS parameter indicates the total solids dissolved in water while the electrical conductivity indicates the conductivity of the current coming from them.70 Therefore, it is reasonable to expect both to decrease after filtration. Here, TDS was 71.9 mg L−1 before filtration and 59.4 mg L−1 after filtration.
After the synthetic gray water test, the PVDF-3 wt% Clp membrane was used in 6 cycles of experiments to test its reusability. It was observed that the flux loss was 12% after the 6th experiment. At this stage, a cleaning procedure was applied to the membrane. Membrane cleaning was performed for 30 min with alkaline (0.1 M NaOH) and acidic (0.6 M HCl) cleaning solutions to remove organic and inorganic contaminants. It was found that the same results were obtained in the filtration tests performed after the cleaning test; thus demonstrating the reusability of the membrane after cleaning.
4 Conclusions
In this study, Clp-doped recycled PVDF electrospun nanofiber membrane filters were synthesized and used for gray water purification. Clp increased the hydrophilicity, porosity, and antimicrobial activity of the membranes. The addition of Clp to PVDF increased both the stress and strain tolerance for up to 3 wt% doping. However, after 3 wt%, both the strength and elongation decreased. Therefore, the PVDF-3 wt% Clp filter showed the best performance and was selected as the best filter. According to the filtration result, MB rejection above 99% was observed. The oil rejection performance of the PVDF membranes without Clp was 95%, while the addition of Clp increased the oil rejection rate to over 99%. It was also observed that LAS rejection increased as the Clp content was increased, and the highest rejection percentage was 80.87% with PVDF containing 4 wt% Clp. Next, experimental optimization was performed to determine the rejection efficiencies based on varying the process parameters. It was observed that the rejection decreased with increasing the temperature, flow rate, and concentration. The most effective factor for MB rejection was the temperature, while for oil it was the feed concentration, and for LAS it was the flow rate. According to the synthetic gray water tests, COD rejection was found to be 63.1% and the turbidity rejection was 97.2%. According to these results, it could be seen that the filtration process based on recycled PVDF could achieve very successful results.Abbreviations
| ANOVA: | Analysis of variance |
| APHA: | American public health association |
| CFU: | Colony-forming unit |
| CA: | Contact angle |
| Clp: | Clinoptilolite |
| COD: | Chemical oxygen demand |
| DMF: | Dimethylformamide |
| DSC: | Differential scanning calorimetry |
| FTIR: | Fourier transform infrared spectroscopy |
| LAS: | Linear alkylbenzene sulfonate |
| MB: | Methylene blue |
| MP: | Microplastic |
| NTU: | Nephelometric turbidity unit |
| PES: | Polyether sulfone |
| PVA: | Polyvinyl alcohol |
| PVDF: | Polyvinylidene fluoride |
| PVP: | Polyvinylpyrrolidone |
| SEM: | Scanning electron microscopy |
| SSM: | Suspended solid matter |
| TDS: | Total dissolved solids |
| TGA: | Thermogravimetric analysis |
| UV-vis: | Ultraviolet-visible |
Data availability
The data that support the findings of this study are available from the corresponding author, Filiz Uğur Nigiz, upon reasonable request.Author contributions
Ayşenur Katırcı: investigation, methodology, writing – original draft, carried out the experiment. Seniyecan Kahraman: investigation, methodology, writing – original draft, carried out the experiment. Filiz Uğur Nigiz: investigation, visualization, conceptualization, methodology, writing – original draft, methodology, data curation, validation, writing – review & editing.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This study was financially supported by the Scientific and Technological Research Council of Türkiye (Grant Number:123Y119) and by the Scientific Research Project Unit of Çanakkale Onsekiz Mart University (Grant number: FHD-2024-4693).References
- G. A. Edwin, P. Gopalsamy and N. Muthu, Characterization of domestic gray water from point source to determine the potential for urban residential reuse: a short review, Appl. Water Sci., 2014, 4, 39–49 CrossRef CAS.
- L. Hernández Leal, N. Vieno, H. Temmink, G. Zeeman and C. J. Buisman, Occurrence of xenobiotics in gray water and removal in three biological treatment systems, Environ. Sci. Technol., 2010, 44(17), 6835–6842 CrossRef.
- L. A. Ghunmi, G. Zeeman, M. Fayyad and J. B. van Lier, Grey water treatment systems: A review, Crit. Rev. Environ. Sci. Technol., 2011, 41(7), 657–698 CrossRef.
- M. Kabiri, A. Akbarpour and M. Akbari, Evaluation of the efficiency of a gray water treatment system based on aeration and filtration, Water Reuse, 2021, 11(3), 361–372 CAS.
- Y. Zhu, D. Yang, J. Li, Z. Yue, J. Zhou and X. Wang, The preparation of ultrathin and porous electrospinning membranes of HKUST-1/PLA with good antibacterial and filtration performances, J. Porous Mater., 2023, 30, 1011–1019 CrossRef CAS.
- R. F. Darmayanti, M. Muharja, A. Widjaja, N. Widiastuti, R. A. Rachman, A. R. Widyanto and B. Piluharto, Performance of modified hollow fiber membrane silver nanoparticles-zeolites Na–Y/PVDF composite used in membrane bioreactor for industrial wastewater treatment, Heliyon, 2023, 9(11), e21350 CrossRef CAS PubMed.
- P. S. Goh and A. F. Ismail, A review on inorganic membranes for desalination and wastewater treatment, Desalination, 2018, 434, 60–80 CrossRef CAS.
- Q. Zhang, J. Wang, K. Huo, M. Dou, C. Han, W. Wang and B. Gao, Constructing high-porosity composite membranes by loading novel CD@ DCN in PVDF: Achieving superior permeability and selectivity simultaneously, J. Water Process Eng., 2024, 65, 105777 CrossRef.
- X. Wu, B. Zheng, Y. Tian and S. Jia, Developing PVDF hollow fiber membrane enhanced by braided tube and modified by bionic coating for treating aldehyde-containing wastewater, J. Water Process Eng., 2024, 67, 106239 CrossRef.
- X. Huang, W. Wang, Y. Liu, H. Wang, Z. Zhang, W. Fan and L. Li, Treatment of oily wastewater by PVP grafted PVDF ultrafiltration membranes, Chem. Eng. J., 2015, 273, 421–429 CrossRef CAS.
- V. R. Pereira, A. M. Isloor, U. K. Bhat and A. F. Ismail, Preparation and antifouling properties of PVDF ultrafiltration membranes with polyaniline (PANI) nanofibers and hydrolysed PSMA (H-PSMA) as additives, Desalination, 2014, 351, 220–227 CrossRef CAS.
- J. Kim and B. V. Bruggen, The use of nanoparticles in polymeric and ceramic membrane structures: review of manufacturing procedures and performance improvement for water treatment, Environ. Pollut., 2010, 158, 2335–2349 CrossRef CAS PubMed.
- F. Morante-Carballo, N. Montalván-Burbano, P. Carrión-Mero and K. Jácome-Francis, Worldwide Research Analysis on Natural Zeolites as Environmental Remediation Materials, Sustainability, 2021, 13(11), 6378 CrossRef CAS.
- H. Baykara, M. C. Martinez and D. V. Rey, Preparation and Determination of Antimicrobial Property of Cation-Exchanged Ecuadorian Natural Zeolite to be Used as Filler for Polyethylene and Polypropylene Matrices, J. Polym. Environ., 2018, 26, 2566–2578 CrossRef CAS.
- S. Wang, T. Terdkiatburana and M. O. Tadé, Adsorption of Cu(II), Pb(II) and humic acid on natural zeolite tuff in single and binary systems, Sep. Purif. Technol., 2008, 62(1), 64–70 CrossRef CAS.
- S. Agarwal, J. H. Wendorff and A. Greiner, Use of electrospinning technique for biomedical applications, Polymer, 2008, 49(26), 5603–5621 CrossRef CAS.
- A. Bahi, J. Shao, M. Mohseni and F. K. Ko, Membranes based on electrospun lignin-zeolite composite nanofibers, Sep. Purif. Technol., 2017, 187, 207–213 CrossRef CAS.
- F. U. Nigiz, H. Dogan and N. D. Hilmioglu, Pervaporation of ethanol/water mixtures using clinoptilolite and 4A filled sodium alginate membranes, Desalination, 2012, 300, 24–31 CrossRef CAS.
- M. Badini Pourazar, T. Mohammadi, M. R. Jafari Nasr, O. Bakhtiari and M. Javanbakht, Preparation and characterization of poly (vinylidene fluoride)-13X zeolite mixed matrix membranes for lithium ion batteries' separator with enhanced performance, J. Appl. Polym. Sci., 2020, 137(44), 49367 CrossRef CAS.
- E. Eliuz, Antimicrobial activity of citric acid against Escherichia coli, Staphylococcus aureus and Candida albicans as a sanitizer agent, Eurasian Journal of Forest Science, 2020, 8(3), 295–301 CrossRef.
- R. Karthik, R. Muthezhilan, A. Jaffar Hussain, K. Ramalingam and V. Rekha, Effective removal of Methylene Blue dye from water using three different low-cost adsorbents, Desalin. Water Treat., 2016, 57(23), 10626–10631 CrossRef CAS.
- A. D. Eaton, L. S. Clesceri, E. W. Rice and A. E. Greenberg, Standard methods for the examination of water and wastewater, Washington DC, 21st edn, 2005 Search PubMed.
- G. R. Alther, Removing oils from water with organoclays, J. - Am. Water Works Assoc., 2002, 94(7), 115–121 CrossRef CAS.
- U. C. Paul, D. Fragouli, I. S. Bayer and A. Athanassiou, Functionalized cellulose networks for efficient oil removal from oil–water emulsions, Polymer, 2016, 8(2), 52 Search PubMed.
- W. J. Shim, S. H. Hong and S. E. Eo, Identification methods in microplastic analysis: a review, Anal. Methods, 2017, 9(9), 1384–1391 RSC.
- H. Etemadi, R. Kazemi, N. Ghasemian and E. Shokri, Effect of Transmembrane pressure on antifouling properties of PVC/Clinoptilolite ultrafiltration nanocomposite membranes, Chem. Eng. Technol., 2022, 45(6), 1192–1200 CrossRef CAS.
- S. M. Hosseinifard, M. A. Aroon and B. Dahrazma, Application of PVDF/HDTMA-modified clinoptilolite nanocomposite membranes in removal of reactive dye from aqueous solution, Sep. Purif. Technol., 2020, 251, 117294 CrossRef CAS.
- H. Nassrullah, O. Makanjuola, I. Janajreh, F. A. AlMarzooqi and R. Hashaikeh, Incorporation of nanosized LTL zeolites in dual-layered PVDF-HFP/cellulose membrane for enhanced membrane distillation performance, J. Membr. Sci., 2020, 611, 118298 CrossRef CAS.
- F. Hassan, R. Mushtaq, S. Saghar, U. Younas, M. Pervaiz, A. muteb Aljuwayid and M. Sillanpaa, Fabrication of graphene-oxide and zeolite loaded polyvinylidene fluoride reverse osmosis membrane for saltwater remediation, Chemosphere, 2022, 307, 136012 CrossRef CAS.
- M. K. Chan, S. J. Tan, A. T. Yeow, S. C. Ng and W. J. Lau, Zeolite-Based Poly (vinylidene fluoride) Ultrafiltration Membrane: Characterization and Molecular Weight Cut-Off Estimation with Support Vector Regression Modelling, Membranes, 2024, 14(4), 91 CrossRef CAS.
- A. C. Lopes, C. Ribeiro, V. Sencadas, G. Botelho and S. Lanceros-Méndez, Effect of filler content on morphology and physical–chemical characteristics of poly (vinylidene fluoride)/NaY zeolite-filled membranes, J. Mater. Sci., 2014, 49, 3361–3370 CrossRef CAS.
- Y. Wang, R. Guo, D. Guan, Z. Luo, Z. Zhang and R. Lin, Development of a Low-Shrinkage-Lightweight Engineered Cementitious Composite Based on Heavily Doped Zeolites, Polymers, 2023, 15(16), 3474 CrossRef CAS.
- Q. Fang, X. Liu, N. Wang, C. Ma and F. Yang, The effect of zeolite particle modified by PEG on rubber composite properties, Sci. Eng. Compos. Mater., 2015, 22(6), 607–612 CrossRef CAS.
- C. Merlini, G. M. O. Barra, T. M. Araujo and A. Pegoretti, Electrically pressure sensitive poly (vinylidene fluoride)/polypyrrole electrospun mats, RSC Adv., 2014, 4(30), 15749–15758 RSC.
- K. P. Pramoda, A. Mohamed, I. Yee Phang and T. Liu, Crystal transformation and thermomechanical properties of poly (vinylidene fluoride)/clay nanocomposites, Polym. Int., 2005, 54(1), 226–232 CrossRef CAS.
- S. Lanceros-Mendez, J. F. Mano, A. M. Costa and V. H. Schmidt, FTIR and DSC studies of mechanically deformed β-PVDF films, J. Macromol. Sci., Part B:Phys., 2001, 40(3–4), 517–527 CrossRef.
- Y. Shen and A. C. Lua, Preparation and characterization of mixed matrix membranes based on PVDF and three inorganic fillers (fumed nonporous silica, zeolite 4A and mesoporous MCM-41) for gas separation, Chem. Eng. J., 2012, 192, 201–210 CrossRef CAS.
- G. C. Dias, M. F. Cardoso, A. O. Sanches, M. C. Santos and L. F. Malmonge, PVDF/Clay Spheres Obtained through Phase Inversion for Cu Ion Removal, Polymer, 2023, 15(12), 2643 CAS.
- A. Nikolov, L. Dobreva, S. Danova, J. Miteva-Staleva, E. Krumova, V. Rashev and N. Vilhelmova-Ilieva, Natural and modified zeolite clinoptilolite with antimicrobial properties: A review, Acta Microbiol. Bulg., 2023, 39(2), 147–161 CrossRef.
- G. T. Kadja, E. Dwihermiati, F. Sagita, K. Mukhoibibah, K. Umam, M. Ledyastuti and C. L. Radiman, Mercapto functionalized–natural zeolites/PVDF mixed matrix membrane for enhanced removal of methylene blue, Inorg. Chem. Commun., 2023, 157, 111263 CrossRef CAS.
- K. Nayak, A. Kumar and B. P. Tripathi, Molecular grafting and zwitterionization based antifouling and underwater superoleophobic PVDF membranes for oil/water separation, J. Membr. Sci., 2022, 643, 120038 CrossRef CAS.
- Ö. Bilgin, Investigation of the raw material properties of gordes zeolite ores and searching their useability in different sectors, Doctoral Dissertation, Institute of Science, Dokuz Eylul University, Turkey, 2009 Search PubMed.
- G. L. D. Rivera, A. M. Hernández, A. F. P. Cabello, E. L. R. Barragán, A. L. Montes, G. A. F. Escamilla and D. A. D. H. Del Río, Removal of chromate anions and immobilization using surfactant-modified zeolites, J. Water Process Eng., 2021, 39, 101717 CrossRef.
- R. Zhang, Y. Liu, Y. Li, Q. Han, T. Zhang, K. Zeng and C. Zhao, Polyvinylidene fluoride membrane modified by tea polyphenol for dye removal, J. Mater. Sci., 2020, 55(1), 389–403 CrossRef CAS.
- R. Mohamat, S. A. Bakar, A. Mohamed, M. Muqoyyanah, S. N. E. A. M. Kamal, M. H. D. Othman, R. Rohani and S. Ramakrishna, Methylene blue rejection and antifouling properties of different carbonaceous additives-based polyvinylidene fluoride membrane, Mater. Today Commun., 2023, 35, 105862 CrossRef CAS.
- A. B. Suriani, M. Muqoyyanah, A. Mohamed, M. H. D. Othman, R. Rohani, I. I. Yusoff and H. A. Khalil, Incorporation of electrochemically exfoliated graphene oxide and TiO2 into polyvinylidene fluoride-based nanofiltration membrane for Dye Rejection, Water, Air, Soil Pollut., 2019, 230, 1–13 CrossRef CAS.
- K. Valizadeh, A. Heydarinasab, S. S. Hosseini and S. Bazgir, Fabrication of modified PVDF membrane in the presence of PVI polymer and evaluation of its performance in the filtration process, J. Ind. Eng. Chem., 2022, 106, 411–428 CrossRef CAS.
- S. Pourziad, M. R. Omidkhah and M. Abdollahi, Preparation of fouling-resistant and self-cleaning PVDF membrane via surface-initiated atom transfer radical polymerization for emulsified oil/water separation, Can. J. Chem. Eng., 2019, 97, 1581–1588 CrossRef CAS.
- S. Pourziad, M. R. Omidkhah and M. Abdollahi, Improved antifouling and self-cleaning ability of PVDF ultrafiltration membrane grafted with polymer brushes for oily water treatment, J. Ind. Eng. Chem., 2020, 83, 401–408 CrossRef CAS.
- Y. Chen, H. Liu, C. Lin, C. Cheng, F. Li, C. Fang and L. Zhu, Designing poly (vinylidene fluoride) membranes with narrow pore size distribution for microplastics removal from water, J. Appl. Polym. Sci., 2023, 140(34), e54305 CrossRef CAS.
- X. Lv, Q. Dong, Z. Zuo, Y. Liu, X. Huang and W. M. Wu, Microplastics in a municipal wastewater treatment plant: Fate, dynamic distribution, removal efficiencies, and control strategies, J. Cleaner Prod., 2019, 225, 579–586 CrossRef CAS.
- X. Zhang, H. N. Li, C. Y. Zhu, X. J. Huang, A. Greiner and Z. K. Xu, Biomimetic gill-inspired membranes with direct-through micropores for water remediation by efficiently removing microplastic particles, Chem. Eng. J., 2022, 434, 134758 CrossRef CAS.
- B. Xu, W. Gao, B. Liao, H. Bai, Y. Qiao and W. Turek, A Review of Temperature Effects on Membrane Filtration, Membranes, 2023, 14(1), 5 Search PubMed.
- R. R. Sharma, R. Agrawal and S. Chellam, Temperature effects on sieving characteristics of thin-film composite nanofiltration membranes: pore size distributions and transport parameters, J. Membr. Sci., 2003, 223(1–2), 69–87 CrossRef CAS.
- H. Q. Dang, W. E. Price and L. D. Nghiem, The effects of feed solution temperature on pore size and trace organic contaminant rejection by the nanofiltration membrane NF270, Sep. Purif. Technol., 2014, 125, 43–51 CrossRef CAS.
- H. P. Ngang, B. S. Ooi, A. L. Ahmad and S. O. Lai, Preparation of PVDF–TiO2 mixed-matrix membrane and its evaluation on dye adsorption and UV-cleaning properties, Chem. Eng. J., 2012, 197, 359–367 CrossRef CAS.
- T. W. Cheng, C. J. Han, K. J. Hwang, C. D. Ho and W. J. Cooper, Influence of feed composition on distillate flux and membrane fouling in direct contact membrane distillation, Sep. Purif. Technol., 2010, 45(7), 967–974 CAS.
- J. H. Huang, C. F. Zhou, G. M. Zeng, X. Li, J. Niu, H. J. Huang and S. B. He, Micellar-enhanced ultrafiltration of methylene blue from dye wastewater via a polysulfone hollow fiber membrane, J. Membr. Sci., 2010, 365(1–2), 138–144 CrossRef CAS.
- P. Ni, J. Zeng, H. Chen, F. Yang and X. Yi, Effect of different factors on treatment of oily wastewater by TiO2/Al2O3-PVDF ultrafiltration membrane, Environ. Technol., 2022, 43(19), 2981–2989 CrossRef CAS.
- K. Masoudnia, A. Raisi, A. Aroujalian and M. Fathizadeh, Treatment of oily wastewaters using the microfiltration process: Effect of operating parameters and membrane fouling study, Sep. Purif. Technol., 2013, 48(10), 1544–1555 CAS.
- M. A. Ahmad, B. S. Zainal, N. H. Jamadon, T. C. S. Yaw and L. C. Abdullah, Filtration analysis and fouling mechanisms of PVDF membrane for POME treatment, J. Water Reuse Desalin., 2020, 10(3), 187–199 CrossRef CAS.
- N. D. N. Affandi and N. N. A. Razak, Removal of pigment from textile wastewater by electrospun nanofibre membrane, J. Mech. Eng., 2017, 4, 190–201 Search PubMed.
- M. Hosseini, M. Soleimani, N. Mirghaffari and S. Borhani, Improving performance of electrospun nylon 6 nanofiber membrane by zeolite nanoparticles for wastewater treatment of herbal essence industries, Int. J. Environ. Sci. Technol., 2024, 21(2), 1493–1508 CrossRef CAS.
- M. Zendehdel and F. H. Nouri, Electrospun polyethersulfone/Ag-Clinoptilolite to remove chemical oxygen demand from real wastewater, Polym. Polym. Compos., 2021, 29(9_suppl), S1498–S1509 CAS.
- Y. Boyjoo, V. K. Pareek and M. Ang, A review of greywater characteristics and treatment processes, Water Sci. Technol., 2013, 67(7), 1403–1424 CrossRef CAS PubMed.
- S. Chaabane, K. Riahi, H. Hamrouni and B. B. Thayer, Suitability assessment of grey water quality treated with an upflow-downflow siliceous sand/marble waste filtration system for agricultural and industrial purposes, Environ. Sci. Pollut. Res., 2017, 24, 9870–9885 CrossRef CAS.
- M. S. Zipf, I. G. Pinheiro and M. G. Conegero, Simplified greywater treatment systems: Slow filters of sand and slate waste followed by granular activated carbon, J. Environ. Manage., 2016, 176, 119–127 CrossRef CAS PubMed.
- F. Yovo, B. Dimon, F. Suanon, M. Aina, I. C. Agani, V. D. Wotto and A. F. C. Togbe, Treatment performance of an autonomous gray water treatment system (SAUTEG) with the macrophytes Thalia geniculata, Am. J. Environ. Prot., 2016, 5(6), 187–198 Search PubMed.
- S. Singh, N. Pradhan, N. Ojha, B. Roy and S. Bose, Grey water treatment and its application in cultivation of plants, Asian J. Microbiol., Biotechnol. Environ. Sci., 2016, 18(4), 1043–1053 Search PubMed.
- S. M. Laghari, Z. M. Ali, M. M. Tunio and A. J. Laghari, Investigation on Indigenous Material as a Filter Medium for Decontamination of Greywater, Pak. J. Anal. Environ. Chem., 2016, 17(1), 6 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ew01070a |
| This journal is © The Royal Society of Chemistry 2025 |