Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Donor-free 9,10-dihydro-9,10-dialuminaanthracenes†
Paula L.
Lückert
,
Jannik
Gilmer
,
Alexander
Virovets
 ,
Hans-Wolfram
Lerner
,
Hans-Wolfram
Lerner
 and
Matthias
Wagner
and
Matthias
Wagner
 *
*
Institut für Anorganische und Analytische Chemie, Goethe-Universität Frankfurt, Max-von-Laue-Straße 7, D-60438 Frankfurt (Main), Germany. E-mail: matthias.wagner@chemie.uni-frankfurt.de
First published on 18th November 2024
Abstract
Despite their promising potential, e.g., as ditopic, cooperatively binding Lewis acids, 9,10-dihydro-9,10-dialuminaanthracenes (DAA-R2; R: terminal Al-bonded substituent) have remained unexplored for long due to the challenges in synthesizing the ligand-free species. We demonstrate that DAA-Me2 is accessible via the reaction of 1,2-(Me3Sn)2C6H4 with AlMe3, producing volatile SnMe4 as the sole byproduct. In non-coordinating solvents and in the solid state, DAA-Me2 exists as a dimer (DAA-Me2)2. Treatment of (DAA-Me2)2 with 4 equiv. AlBr3 cleaves the dimer, leads to quantitative Me/Br exchange, and forms the double AlBr3 adduct DAA-Br2·(AlBr3)2. Removal of AlBr3 with 2,2′-bipyridine gives free DAA-Br2, which also dimerizes in the absence of bases to form (DAA-Br2)2. (DAA-Me2)2 and (DAA-Br2)2 readily react with mono- (e.g., pyridine) or ditopic Lewis bases (e.g., potassium pyrazolide) to afford trans-diadducts or triptycene-type frameworks. Upon addition of [nBu4N]Br, DAA-Br2·(AlBr3)2 undergoes selective cleavage of Al–C bonds to produce the Br− chelate complex of 1,2-(Br2Al)2C6H4, a valuable synthon for 1,2-dideprotonated benzenes.
Introduction
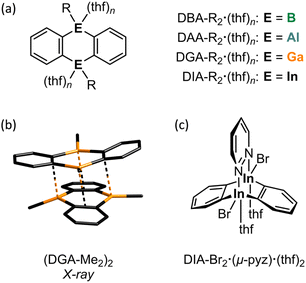
The incorporation of p-block elements other than carbon into polycyclic aromatic hydrocarbons (‘heteroatom doping’) has emerged as a powerful tool for imparting new and valuable chemical and physical properties to these compounds.1 Notable examples are 9,10-dihydro-9,10-diboraanthracenes (DBAs; Fig. 1a, E = B), which have found wide-ranging applications,2 including their use as fluorophores3 or homogeneous catalysts.4 To further enhance the utility of DBAs, various substituted derivatives have been developed,5,6 the B-doped acene scaffold has been expanded by benzannulation,7 and additional heteroatoms (such as N, O, and S) have been introduced into the delocalized π system.8Significantly less attention has been given to what is arguably the most impactful modification: the exchange of the B atoms for their higher homologues.9,10 While a few anthracenes containing Al (DAAs),11,12 Ga (DGAs),11,13 or In (DIAs)11,14–18 at the 9,10-positions are known, these compounds are typically isolated as their Lewis-base adducts, which inherently diminishes the desired reactivity. As an example, the synthesis of DAA-Me2·(thf)n according to Bickelhaupt et al.11 uses MeAlCl2 (ref. 19) and [Mg(thf)(o-C6H4)]4, prepared from [Hg(o-C6H4)]3,20,21 in THF;11,22 DGA-Me2·(thf)n and DIA-Me2·(thf)n were synthesized in a similar manner (Fig. 1a, E = Al, Ga, or In; n ≥ 2).11 The solubility requirements of the Mg2+ reagent necessitate the use of THF, which inevitably precludes the formation of ligand-free heteroanthracenes. Also in the synthesis of the octafluorinated congener of DAA-Me2·(thf)n, where 1,2-(Me3Sn)2C6F4 and Me2AlCl are employed as starting materials, the cyclocondensation of the initially formed intermediate into the target product must be initiated by adding THF.12 Donor-free DGA-R2 is accessible from 1,2-(ClHg)2C6H4 and GaR3 in p-xylene (R = Me, Et; 140 °C, 3 h).13 This protocol, however, poses considerable risks due to the toxic or pyrophoric precursors and particularly the extremely harmful byproduct HgR2, which is released in 4 equivalents. DGA-R2 dimerizes via Ga⋯π interactions or Ga–Cb–Ga two-electron three-center bonds (2e3c; Cb: bridging C atom),23 with (DGA-Me2)2 (Fig. 1b) and (DGA-Et2)2 having distinctly different molecular structures in the solid state (see below).
Moving on to In offers novel perspectives for several reasons: (i) due to the ‘inert-pair effect’, In(I) halides are more stable and easier to handle than their Al(I) or Ga(I) counterparts. Consequently, [Hg(o-C6H4)]3 in THF can conveniently be reacted with InBr in a combined transmetallation/redox reaction to furnish DIA-Br2·(thf)4 and elemental Hg, which is a significantly less concerning byproduct compared to HgMe2 mentioned earlier.14 (ii) Due to its larger atomic radius, each In site in DIA-Br2 can accommodate two Lewis bases within a trigonal-bipyramidal ligand sphere, instead of just one. Given the increasing significance of coordination networks and microporous solids,24,25 it is noteworthy that DIA-Br2·(thf)4, when combined with rigid, ditopic Lewis bases such as 1,4-diazine, has been used to self-assemble molecular stairs and ladders.16 When 1,2-diazine (pyridazine, pyz) is offered to DIA-Br2·(thf)4 instead of 1,4-diazine, the system switches to a chelating mode, leading to the formation of a triptycene-type structure DIA-Br2·(μ-pyz)·(thf)2 (Fig. 1c).17 This outcome points to the potential application of DIA-Br2·(thf)4 as a homogeneous Lewis acid catalyst with cooperating heteroatoms.18
Herein, we present efficient access routes to the first donor-free 9,10-dihydro-9,10-dialuminaanthracenes (DAA-Me2)2, (1)2, and (DAA-Br2)2, (2)2, which exist as dimers in non-coordinating solvents and in the solid state. We further describe selective reactions of (1)2 and (2)2 with (i) mono- and bidentate N- or O-Lewis bases and (ii) the Lewis acid AlBr3.19 Beyond their intriguing electronic structures, these compounds hold promise as preorganized, ditopic Lewis acids26 and rare ortho-dimetallated benzene building blocks for organic synthesis.
Results and discussion
Syntheses
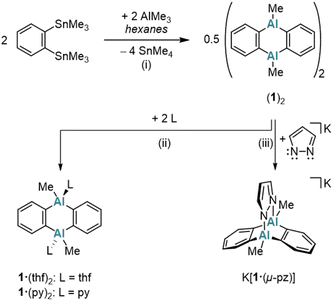
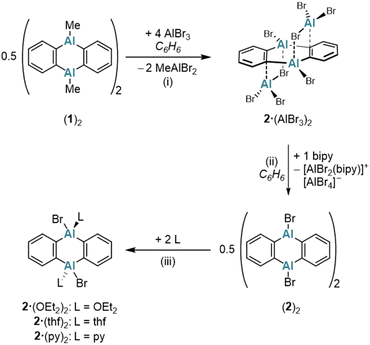
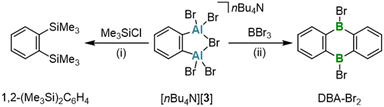
A classical protocol for the synthesis of DBA-X2 is based on reactions between 1,2-(Me3E)2C6H4 and BX3 in toluene, n-hexane, or under solvent-free conditions (E = Si, Sn; X = Cl, Br).3a,d,5 To extend this approach to the synthesis of DAAs with the aim to avoid the use of [Hg(o-C6H4)]3 and coordinating solvents, we explored whether 1,2-(Me3E)2C6H4 could also serve as a suitable o-phenylene source in the present case. Eisch et al. reported that the reaction of the corresponding stannane with AlCl3 in toluene gives 1,2-(Cl2Al)2C6H4.27 However, a serious drawback is that the Me3SnCl byproduct remains firmly complexed with the aryl alane, resulting in an inseparable polymeric ion pair. Although using AlMe2Cl somewhat mitigated this issue – yielding a weaker electron-pair acceptor in 1,2-(Me2Al)2C6H4 – the Me3SnCl could still not be completely removed.27 Considering modified approaches, we noted that the tetrafluoro species 1,2-(Me3Sn)2C6F4 reacts with Me2AlCl to form dimeric [1,2-(Cl(Me)Al)2C6F4]2 and SnMe4 (rather than Me3SnCl).28 This suggested that starting with AlMe3 (ref. 19) could prevent the formation of difficult-to-remove chlorostannanes altogether. Indeed, when 1,2-(Me3Sn)2C6H4 (ref. 29) is treated with an equimolar amount of AlMe3 in hexanes at elevated temperatures (150 °C, 3 d, sealed glass ampoule), SnMe4 is released and cyclocondensation to the heteroanthracene occurs (Scheme 1). The dimeric product (1)2 precipitates in pure form from the reaction mixture upon cooling to room temperature (yield: 76%); (1)2 is highly soluble in C6H6, toluene, CHCl3, or CH2Cl2. The volatile byproduct SnMe4 can be easily removed and, in principle, subjected to a redistribution reaction with SnCl4 to regenerate30 the Me3SnCl required for the synthesis of the starting material 1,2-(Me3Sn)2C6H4. In the presence of Lewis-basic ligands such as tetrahydrofuran (THF) or pyridine (py), (1)2 is cleaved into the monomers, which are obtained as the diadducts 1·(thf)2 (ref. 11) and 1·(py)2 (Scheme 1). Of particular interest is the coordination behavior of 1 toward bidentate ligands, as this reveals the potential of 1 as a preorganized, ditopic Lewis acid. Initial exploratory investigations with pyridazine (pyz) led to the following observations: (i) the room-temperature 1H NMR spectrum of an equimolar mixture of (1)2 and pyz in THF-d8 showed only minor changes of ± 0.03 ppm compared to the chemical shift values of the signals of 1·(thf)2 and free pyz. (ii) Upon gas-phase diffusion of n-hexane into such mixtures, however, the heteroadduct 1·(pyz)(thf) crystallized, which features a pyz ligand that coordinates to one Al site through one of its N atoms, while a thf ligand coordinates to the other Al site (Fig. S41†).16,31 In contrast, the boron and indium congeners DBA-H2 and DIA-Br2 show triptycene-type structures with E–(μ-pyz*)2–E′ moieties under comparable conditions in the solid state (E = B, In; pyz* = benzo[d]pyridazine).17,32 A THF-stable heterotriptycene motif can also be imposed on 1 by using the negatively charged, five-membered pyrazolato ([pz]−) ligand instead of the neutral, six-membered pyz ligand (cf. K[1·(μ-pz)]; Scheme 1).To expand the variety of donor-free DAAs, it would be desirable to obtain also an Al-halogenated derivative DAA-X2. One possibility is to start from (1)2 and to achieve the necessary Me/X exchange by the reaction with AlX3. AlBr3 was selected for this purpose because, unlike AlCl3, it is soluble in the non-coordinating solvent C6H6. Treatment of (1)2 in C6H6 with 8 equiv. AlBr3 leads to the instantaneous precipitation of 2·(AlBr3)2, which is generally poorly soluble in non-coordinating solvents (Scheme 2). If only 4 equiv. AlBr3 per (1)2 are used instead of 8 equiv., a structure similar to 2·(AlBr3)2 is obtained, but with the non-bridging Br positions partially occupied by Me groups [DAA-R2·(AlBrR2)2; R = Me or Br; according to X-ray crystallography, Fig. S42†]. Having achieved the aimed-for Me/Br exchange on 1, the next task is to remove the two coordinating AlBr3 molecules from 2·(AlBr3)2 to obtain the free heteroanthracene. The chelating ligand 2,2′-bipyridine (bipy) proved to be ideally suited for this purpose: In C6H6, the addition of 1 equiv. bipy to 2·(AlBr3)2 resulted in the formation of (2)2 after heating and sonication. NMR spectroscopy on the supernatant revealed exclusively signals of the free, dimeric (2)2, with no detectable bipy resonances (Scheme 2). We assume that the solid consists of species such as [AlBr2(bipy)][AlBr4], which, due to their salt-like nature, quantitatively separate from the target product.33 Similar to (1)2, (2)2 is converted to 2·(OEt2)2, 2·(thf)2, or 2·(py)2 upon addition of Et2O, THF, or py, respectively (Scheme 2).
In another attempt to generate the AlBr3-free (2)2, Br− ions were used as alternative ligands instead of bipy. However, the reaction between [nBu4N]Br (2 equiv.) and 2·(AlBr3)2 in C6H6 furnished the 1,2-dialumino-substituted benzene derivative [nBu4N][3], rather than the initially expected products (2)2 and [nBu4N][AlBr4] (Scheme 3). Formally, 2·(AlBr3)2 is a dimer of 1,2-(Br2Al)2C6H4, and [3]− is the Br− adduct of this ditopic, chelating Lewis acid (a comparable F− adduct of the boron-based ditopic Lewis acid 1,2-[(C6F5)2B]2C6F4 has been characterized by NMR spectroscopy).34
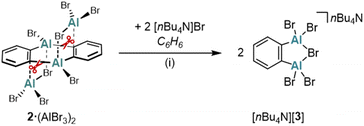 | ||
| Scheme 3 The reaction of 2·(AlBr3)2 with [nBu4N]Br yields the 1,2-dialumino-substituted benzene derivative [nBu4N][3]. (i) 2 equiv. [nBu4N]Br, C6H6, 70 °C, 1.5 h, sonication. | ||
Solid-state structures
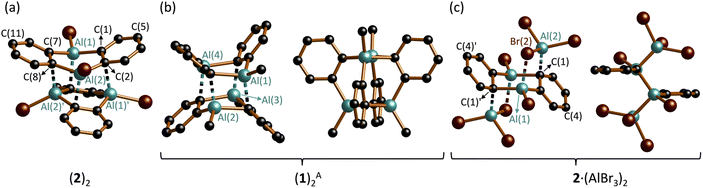
Note: Whenever we want to indicate individual DAA units in a molecular structure, we will hereafter use dashed lines for any interactions between a respective unit and the rest of the molecule. This is not intended to imply any judgements about the nature or strength of the interaction.The crystal of (2)2 is a true racemate of discrete chiral C2-symmetric units, best described as dimers of DAA-Br2 molecules (Fig. 2a; note that the corresponding B-doped DBA-Br2 is monomeric in the solid state3a).35 The Al2C4 cores of the individual monomers adopt distorted, shallow boat conformations [dihedral angles Al(1)C(1)C(7)//C(1)C(2)C(7)C(8) = 35.3(2)°, Al(2)C(2)C(8)//C(1)C(2)C(7)C(8) = 6.0(2)°]. The Al atoms of monomer M (or M′) interact with two C(ipso) atoms, both bonded to the same Al atom of monomer M′ (or M), as schematically depicted in Fig. 3a. The corresponding bond lengths Al(1)′–C(2) and Al(2)′–C(8) measure 2.261(3) and 2.191(3) Å, respectively; the angles including these Al–C bonds and the C(2)⋯C(5) or C(8)⋯C(11) vectors across the corresponding phenylene rings are Al(1)′–C(2)⋯C(5) = 101.9(1) and Al(2)′–C(8)⋯(C11) = 111.6(2)°. In summary, (2)2 forms a cage structure with six-membered rings serving as the base and top, and one four-membered, two five-membered, and one six-membered ring(s) constituting the belt (Fig. S44 and S45†). To facilitate the analysis of M⋯M′ interactions in (2)2, we assume that each bridging Cb is sp2-hybridized, neglecting contributions from Wheland-type36 electronic structures with sp3-hybridized Cb atoms. Within this model, an Al′ atom from monomer M′ can engage with monomer M either through the unhybridized pz orbital of Cb (Al⋯π(Ar) interaction) or via the Al–Cb σ bond (to generate a 2e3c bond). Of the four M⋯M′ interactions present in (2)2, two are pairwise identical. Intrinsic bond orbitals (IBOs) of the remaining two distinct interaction types are illustrated in Fig. S52.† Both types have contributions from Al⋯π(Ar) and 2e3c interactions, but to varying degrees: Based on the interpretation of IBOs, Wiberg bond indices (WBIs), and Mayer bond orders (MBOs), the two M⋯M′ interactions within the four-membered ring of the belt appear to be dominated by 2e3c bonding, whereas the other two intermonomer bonds are predominantly of the Al⋯π(Ar) type (Fig. S52 and S53†). (2)2 can be compared with the dimeric 1,4-dichloro-2,3,5,6-tetramethyl-1,4-dialumina-2,5-cyclohexadiene [(DAChd)2], where the two non-planar monomers are rotated by 90° relative to each other and are linked via four Al⋯π(olefin) bonds (Fig. 3b). The distance between two Al atoms of different monomers within the same dimer is about 3.00 Å, which was regarded as ‘relatively short’; according to ab initio calculations, Al⋯Al′ interactions contribute to the stability of the system.37 Indeed, the dimers remained intact under mass spectrometry conditions up to temperatures of 140 °C.38 In (2)2, the Al⋯Al′ distances range from 2.717(2) Å (across the four-membered ring) to 3.635(2) Å (across the six-membered ring). A third dimeric structural motif comparable to (2)2 and (DAChd)2 is observed in (DGA-Me2)2 (Fig. 1b): the primary distinction among these three cases lies in the degree of rotation of the monomer units relative to each other.13
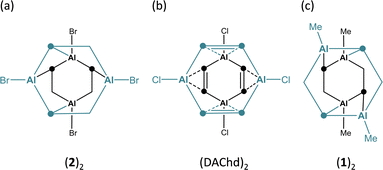 | ||
| Fig. 3 Schematic representations to illustrate the intermonomer contacts in (a) (2)2, (b) (DAChd)2, and (c) (1)2. | ||
(1)2 crystallizes with three crystallographically independent molecules in the unit cell, each displaying approximate D2d symmetry [(1)2A–(1)2C].39 Since their key structural parameters are very similar (Fig. S35†), only (1)2A will be discussed in detail. Although its molecular formula corresponds to a dimer of DAA-Me2, the assignment of two distinct DAA units within the total ensemble is less unambiguous than in the case of (2)2, owing to the higher symmetry of (1)2A. For the purposes of the discussion to follow, the six-membered rings containing Al(1)/Al(4) and Al(2)/Al(3) are defined as belonging to monomers M and M′ (Fig. 2b, left; an alternative definition is possible but leads to the same conclusions). Both monomeric units exhibit twist-boat conformations. Unlike (2)2, the Al atoms of monomer M (or M′) in (1)2A interact with two diagonally opposite C(ipso) atoms attached to different Al atoms of monomer M′ (or M) (cf.Fig. 3c). The respective ‘intermonomeric’ Al–C bond lengths range from 2.100(2) to 2.132(2) Å, which are shorter than those of (2)2. While these findings are informative for the comparison of the solid-state structures of (1)2 and (2)2, an alternative analysis of the (1)2 scaffold is more appropriate to account for its high symmetry: the cluster comprises four equivalent C6H4–Al(Me) fragments, each featuring a C(Ar)–Al σ bond; the second deprotonated o-phenylene C atom bridges two additional Al atoms, forming an Al′–Cb–Al′′ 2e3c bond (Fig. 2b, right). Consequently, each Al vertex is tetracoordinated by C atoms. The Al4 core of (1)2 adopts a strongly distorted tetrahedral geometry with Al⋯Al distances of av. 2.666 and av. 3.611 Å. Overall, the framework of (1)2 resembles that of (DGA-Et2)2 (ref. 13) and of the o-phenylene magnesium tetramer [Mg(thf)(o-C6H4)]4, with the Al atoms replaced by Mg atoms and the Me substituents by thf ligands (Fig. S36 and S37†).21 To conclude the discussion of structures (1)2 and (2)2, we find it remarkable that both species prefer to form discrete dimers in a cluster-like arrangement rather than coordination polymers with Al–(μ-Me/Br)2–Al′ bridges, as seen in Al2Me6 (ref. 40) and Al2Br6;41 the diboraanthracene (DBA-H2)∞ is indeed polymeric via B–(μ-H)2–B′ linkages in the solid state.42 Furthermore, it is worth noting the following result from quantum chemical calculations (SMD(C6H6)/ωB97XD/def2-TZVPP//SMD(C6H6)/ωB97XD/def2-TZVPP): after Me/Br or Br/Me exchange, the resulting (1Br)2 or (2Me)2 remain minima on the potential-energy surface. For R = Me, the crystallographically observed structure (1)2 is more stable than (2Me)2 by 4.9 kcal mol−1. Yet, for R = Br, structure (2)2 is less stable than (1Br)2 by 3.5 kcal mol−1, in the absence of crystal-packing effects.43
In the Ci-symmetric compound 2·(AlBr3)2, two AlBr3 moieties coordinate to opposite sides of the DAA-Br2 core, which consequently adopts a chair conformation (Fig. 2c).44 Similar to (1)2, the binding sites are two diagonally opposite C(ipso) atoms (cf.Fig. 3c). The respective bonds are relatively short (Al(2)–C(1) = 2.032(4) Å), and the C(1) atoms are strongly pyramidalized (Al(2)–C(1)⋯C(4) = 134.0(2)°, Al(1)–C(1)′⋯C(4)′ = 124.0(2)°). Each Al(2)–C(1) bond is reinforced by a Br atom that bridges the Al atoms of DAA-Br2 and AlBr3 (Al(1)–Br(2) = 2.449(1) Å, Al(2)–Br(2) = 2.411(1) Å). In summary, the largely symmetric Al(1)⋯Al(1)′-bridging mode of the phenylene ring in 2·(AlBr3)2 more closely resembles the situation in (1)2A than in (2)2. Compound 2·(AlBr3)2 is formally the dimer of 1,2-(Br2Al)2C6H4. The related [1,2-(Cl(Me)Al)2C6F4]2, which was characterized by Gabbaï et al. with X-ray diffraction, has a markedly different molecular structure: It features two stacked 1,2-phenylene rings, two distinct types of Al⋯Al′-bridging Cl− ions, and lacks any Al–Cb–Al 2e3c bonds.28
The compound [nBu4N][3] is asymmetric in the solid state, although the anionic component approximates the C2v point group (Fig. 4a). [3]− can be described as a ditopic Lewis acid (i.e., 1,2-(Br2Al)2C6H4), where the two vicinally positioned Al sites cooperate in bonding to the same Br− anion, with an average bond length of Al–(μ-Br) = 2.443 Å. As expected, these bonds are longer than the Al–Br bonds to the terminal Br atoms, which range from 2.282(2) to 2.294(2) Å. When the bridging Br− ion is excluded from consideration, the sum of angles within the remaining CAlBr2 fragments averages 343.7°, which lies between the typical values of a planar (360°) and a tetrahedral geometry (328.5°). The endocyclic angles (μ-Br)–Al–C and Al–(μ-Br)–Al are av. 101.8° and 90.7(1)°, respectively.
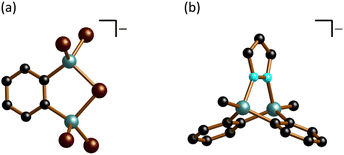 | ||
| Fig. 4 Molecular structures in the solid state: (a) [nBu4N][3]; (b) [K(thf)1.5][1·(μ-pz)]. Counter cations and H atoms omitted for clarity. C: black, Br: brown, Al: turquoise, N: cyan. | ||
In the crystal lattice, [K(thf)1.5][1·(μ-pz)] forms a one-dimensional coordination polymer, with [K(thf)]+ and [K(thf)2]+ cations bonding simultaneously to phenylene rings of two different anions (Fig. S40†). The anion [1·(μ-pz)]− represents a rare heterotriptycene with Al atoms at the bridgehead positions (Fig. 4b).45–47 The Al–N bonds to the bridging pyrazolato ([pz]−) ring (av. 1.973 Å) are longer than the Al–N(pz) bonds in [R2Al–(μ-pz)2–AlR2] (R = Me: av. 1.921 Å, tBu: av. 1.929 Å)48 or in bicyclic [HAl(μ-3,5-tBu2pz)2(μ-CH2NtBu)AlH] (av. 1.914 Å).45
The monomeric complexes 1·(py)2 × C6H6 and 2·(py)2 × C6H6 are isostructural in the crystalline state and show planar, Ci-symmetric DAA-R2 units coordinated by two py ligands from opposite sides (trans configuration; R = Br, Me). Also, the thf diadducts 1·(thf)2 and 2·(thf)2 adopt trans configurations. Remarkably, the OEt2 ligands in the corresponding diadduct 2·(OEt2)2 are positioned in a cis arrangement in the solid state (full details are given in the ESI†).
NMR analysis
For all molecules presented in this work, the 27Al NMR resonances were broadened beyond detection. NMR spectroscopic analysis was not possible for 2·(AlBr3)2 and [nBu4N][3], due to their low solubility in all suitable solvents. In the cases of the Et2O/thf/py diadducts of 1 and 2 as well as K[1·(μ-pz)] and (1)2, the number of 1H and 13C NMR signals, their chemical shift values, and the integral ratios of the proton resonances were consistent with the (symmetry-averaged) molecular structures determined by X-ray analysis (full details are given in the ESI†). (2)2 is the only example that requires a more thorough consideration: Its solid-state structure has insufficient symmetry to align with the 1H and 13C NMR spectra obtained in solution (C6D6), which show only two and three signals, respectively. There are two possible explanations for the observed NMR features: (i) (2)2 may dissociate in solution into the monomeric units 2. (ii) The dimeric structure may persist but experience significant fluctuations due to librational motion of the monomers relative to each other, resulting in an average D2d symmetry. A quantum-chemical analysis renders the conversion of (2)2 → 2 × 2 unlikely, as it would be endergonic with ΔG = 28.0 kcal mol−1 (which is in line with the shortened Al⋯Al′ distances discussed in the crystallographic section). In contrast, any activation barrier to be overcome during the librational motion does not exceed ΔG‡ = 3.0 kcal mol−1 (Scheme S1†), which makes option (ii) more probable.Potential of [nBu4N][3] as synthesis equivalent of the 1,2-dideprotonated benzene nucleophile
The arylaluminum species described here, while interesting in their own right, also hold potential as ortho-dimetallated starting materials for organic synthesis. Such nucleophilic building blocks, which complement their ubiquitous, polarity inverted o-dihalogenated analogues, are just as valuable as they are difficult to access:49 A major challenge is to avoid the unwanted formation of benzyne on the way to 1,2-M2C6H4 (M = Li, MgBr). The key starting material for most o-dimetallated benzenes therefore still remains o-phenylene mercury, [Hg(o-C6H4)]3, which is obtained from 1,2-Br2C6H4 and sodium amalgam in a process that takes several days.50,51 [Hg(o-C6H4)]3 can subsequently be converted into the organolithium, -magnesium, or -zinc species 1,2-Li2C6H4, [Mg(thf)(o-C6H4)]4, or [Zn(thf)2(o-C6H4)]n [n = 2 (crystalline state) or 3 (solution)] by reaction with metallic Li,50,52 Mg,21 or Zn,53 respectively; the required reaction times range from half a day to weeks. Taken together, the widespread use of o-dimetallated benzenes is hindered not only by the well-recognized environmental and health concerns associated with organomercury compounds but also by the apparently poor reproducibility in the synthesis of [Hg(o-C6H4)]3: while Wittig claimed to have obtained yields of 50%, Massey explicitly stated that, despite ‘10 years of experience in the field’, they consistently observed yields as low as 1–2%.50,51 Even though, in favorable cases, the in situ generation of nucleophilic intermediates from 1,2-Br2C6H4 and Mg can be achieved in the presence of the electrophile, resulting in satisfactory yields (as demonstrated in the synthesis of 1,2-(Me3Sn)2C6H4),29 there is a persistent demand for efficient access to additional o-dimetallated benzenes.Recognizing the potential of our aryl aluminium compound [nBu4N][3] as an ideal candidate to address this need, we conducted several proof-of-concept experiments. For this purpose, we selected target compounds with published synthesis protocols that require prolonged reaction times and/or high temperatures (Scheme 4). This allows us to identify potential advantages of our new starting material through direct comparison: For instance, the reaction between [nBu4N][3] and 2 equiv. Me3SiCl in C6D6 furnished the disubstituted benzene 1,2-(Me3Si)2C6H4 at room temperature after 1 d (quantitative conversion according to NMR spectroscopy; Fig. S25†). In contrast, the established synthesis of the same product from 1,2-Br2C6H4, Mg, and Me3SiCl via a Grignard-type reaction in THF requires stirring for 2 d, with the temperature gradually increasing from 0 °C to room temperature.54 Particularly noteworthy is the conversion of [nBu4N][3] with neat BBr3, which instantaneously affords the corresponding 9,10-dihydro-9,10-diboraanthracene already at room temperature (Scheme 4). The traditional route to DBA-Br2via 1,2-(Me3Si)2C6H4 and BBr3 requires heating to 120 °C for 6 d.3a,55
Already this selection of straightforward conversions highlights the potential of [3]− as a synthetic equivalent of 1,2-dideprotonated benzene. Despite its poor solubility in non-polar solvents like C6H6, it still undergoes smooth heterogeneous reactions. The products, however, are nicely soluble in C6H6, allowing for easy separation from any unreacted starting material and Al-containing byproduct salts, which greatly simplifies the purification process.
Conclusions
Anthracenes incorporating Group 13 elements at the 9,10-positions have significant potential as redox-active systems and versatile ditopic Lewis acids, offering a broad range of applications. Their planar structures expose the reactive heteroelement sites, while the rigid 1,2-phenylene bridges bring these sites into close proximity, promoting acid–base binding cooperativity. Additionally, the delocalized π systems enable electronic communication between the dopant atoms. While 9,10-dihydro-9,10-diboraanthracenes have been extensively studied and higher homologues have received some attention, 9,10-dihydro-9,10-dialuminaanthracenes (DAA-R2; R: terminal Al-bonded substituent) have remained almost entirely unexplored, despite Al being by far the most earth-abundant Group 13 element.In this work, we synthesized DAA-Me2 (1) in the absence of stabilizing ligands, resulting in its dimerization through Al⋯π(Ar) interactions to form (1)2. Despite dimerization, (1)2 serves as an effective synthesis equivalent for ‘free’ DAA-Me2, as it readily reacts with mono- or bidentate Lewis bases to afford trans-diadducts such as 1·(py)2, or triptycene-type structures like K[1·(μ-pz)] (py: pyridine; Hpz: pyrazole). Notably, (1)2 can be cleaved into its monomers not only by Lewis bases but also by the strong Lewis acid AlBr3, which displaces the original DAA-Me2 partner by establishing new Br3Al⋯π(Ar) bonds and Al–Br–Al′ bridges in its place. This reaction also exchanges all Al-bonded Me substituents of DAA-Me2 for Br atoms, leading to 2·(AlBr3)2, the double AlBr3 adduct of DAA-Br2 (2), which can also be viewed as a dimer of 1,2-(Br2Al)2C6H4. The donor-free (2)2 can be liberated from 2·(AlBr3)2 using 2,2′-bipyridine. In contrast, treatment of 2·(AlBr3)2 with Br− ions splits two Al–C bonds to give the adduct [nBu4N][3], in which one molecule of 1,2-(Br2Al)2C6H4 chelates one Br− ion. The anion [3]− has proven to be an excellent synthon for a 1,2-dideprotonated benzene. Such compounds are rare but of exceptional synthetic value; the broader utility of [3]− in this regard is currently under investigation in our laboratories.
Data availability
The datasets supporting this article have been uploaded as part of the ESI.†Author contributions
P. L. L. performed the experimental studies and characterized all new compounds. P. L. L. and J. G. performed the quantum-chemical calculations. A. V. performed the X-ray crystal structure analyses of all compounds. H.-W. L. and M. W. supervised the project. The manuscript was written by P. L. L. and M. W. and edited by all co-authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are grateful to Felipe Fantuzzi (University of Kent) and Eugenia Peresypkina (University of Frankfurt) for helpful discussions. We thank the center for scientific computing (CSC) Frankfurt and the University of Kent for providing HPC resources that contributed to the computational investigation of this work. We acknowledge Liselotte Michels (University of Würzburg) and the microanalytical laboratory Pascher for the elemental analyses.Notes and references
- (a) A. Borissov, Y. K. Maurya, L. Moshniaha, W.-S. Wong, M. Żyła-Karwowska and M. Stępień, Chem. Rev., 2022, 122, 565–788 CrossRef CAS PubMed; (b) M. Stępień, E. Gońka, M. Żyła and N. Sprutta, Chem. Rev., 2017, 117, 3479–3716 CrossRef PubMed; (c) L. Ji, S. Griesbeck and T. B. Marder, Chem. Sci., 2017, 8, 846–863 RSC.
- (a) S. E. Prey and M. Wagner, Adv. Synth. Catal., 2021, 363, 2290–2309 CrossRef CAS; (b) E. von Grotthuss, A. John, T. Kaese and M. Wagner, Asian J. Org. Chem., 2018, 7, 37–53 CrossRef CAS; (c) L. Schweighauser and H. A. Wegner, Chem.–Eur. J., 2016, 22, 14094–14103 CrossRef CAS.
- (a) E. Januszewski, A. Lorbach, R. Grewal, M. Bolte, J. W. Bats, H.-W. Lerner and M. Wagner, Chem.–Eur. J., 2011, 17, 12696–12705 CrossRef CAS; (b) E. Januszewski, M. Bolte, H.-W. Lerner and M. Wagner, Organometallics, 2012, 31, 8420–8425 CrossRef CAS; (c) C. Hoffend, F. Schödel, M. Bolte, H.-W. Lerner and M. Wagner, Chem.–Eur. J., 2012, 18, 15394–15405 CrossRef CAS; (d) C. Reus, S. Weidlich, M. Bolte, H.-W. Lerner and M. Wagner, J. Am. Chem. Soc., 2013, 135, 12892–12907 CrossRef CAS PubMed; (e) C. Reus, F. Guo, A. John, M. Winhold, H.-W. Lerner, F. Jäkle and M. Wagner, Macromolecules, 2014, 47, 3727–3735 CrossRef CAS.
- Selected examples of neutral DBA catalysts: (a) S. N. Kessler and H. A. Wegner, Org. Lett., 2010, 12, 4062–4065 CrossRef CAS PubMed; (b) S. N. Kessler and H. A. Wegner, Org. Lett., 2012, 14, 3268–3271 CrossRef CAS PubMed; (c) S. N. Kessler, M. Neuburger and H. A. Wegner, J. Am. Chem. Soc., 2012, 134, 17885–17888 CrossRef CAS PubMed; (d) L. Schweighauser, I. Bodoky, S. N. Kessler, D. Häussinger, C. Donsbach and H. A. Wegner, Org. Lett., 2016, 18, 1330–1333 CrossRef CAS PubMed; (e) L. Hong, S. Ahles, M. A. Strauss, C. Logemann and H. A. Wegner, Org. Chem. Front., 2017, 4, 871–875 RSC; (f) S. Ahles, J. Ruhl, M. A. Strauss and H. A. Wegner, Org. Lett., 2019, 21, 3927–3930 CrossRef CAS PubMed; (g) S. Beeck, S. Ahles and H. A. Wegner, Chem.–Eur. J., 2022, 28, e202104085 CrossRef CAS PubMed . Selected examples of dianionic [DBA]2− catalysts/element–element bond activators:; (h) A. Lorbach, M. Bolte, H.-W. Lerner and M. Wagner, Organometallics, 2010, 29, 5762–5765 CrossRef CAS; (i) E. von Grotthuss, M. Diefenbach, M. Bolte, H.-W. Lerner, M. C. Holthausen and M. Wagner, Angew. Chem., Int. Ed., 2016, 55, 14067–14071 CrossRef CAS PubMed; (j) E. von Grotthuss, S. E. Prey, M. Bolte, H.-W. Lerner and M. Wagner, Angew. Chem., Int. Ed., 2018, 57, 16491–16495 CrossRef CAS; (k) E. von Grotthuss, S. E. Prey, M. Bolte, H.-W. Lerner and M. Wagner, J. Am. Chem. Soc., 2019, 141, 6082–6091 CrossRef CAS PubMed; (l) H. Budy, S. E. Prey, C. D. Buch, M. Bolte, H.-W. Lerner and M. Wagner, Chem. Commun., 2022, 58, 254–257 RSC; (m) S. E. Prey, C. Herok, F. Fantuzzi, M. Bolte, H.-W. Lerner, B. Engels and M. Wagner, Chem. Sci., 2023, 14, 849–860 RSC.
- S. Brend'amour, J. Gilmer, M. Bolte, H.-W. Lerner and M. Wagner, Chem.–Eur. J., 2018, 24, 16910–16918 CrossRef.
- (a) T. Jin, M. Bolte, H.-W. Lerner and M. Wagner, Org. Chem. Front., 2022, 9, 5611–5616 RSC; (b) T. Jin, M. Bolte, H.-W. Lerner, J.-M. Mewes and M. Wagner, Chem.–Eur. J., 2022, 28, e202202234 CrossRef CAS; (c) M. Metzler, M. Bolte, A. Virovets, H.-W. Lerner and M. Wagner, Org. Lett., 2023, 25, 5827–5832 CrossRef CAS PubMed.
- (a) S. Kirschner, J.-M. Mewes, M. Bolte, H.-W. Lerner, A. Dreuw and M. Wagner, Chem.–Eur. J., 2017, 23, 5104–5116 CrossRef CAS; (b) A. John, M. Bolte, H.-W. Lerner and M. Wagner, Angew. Chem., Int. Ed., 2017, 56, 5588–5592 CrossRef CAS; (c) A. John, M. Bolte, H.-W. Lerner, G. Meng, S. Wang, T. Peng and M. Wagner, J. Mater. Chem. C, 2018, 6, 10881–10887 RSC; (d) A. John, S. Kirschner, M. K. Fengel, M. Bolte, H.-W. Lerner and M. Wagner, Dalton Trans., 2019, 48, 1871–1877 RSC; (e) S. Kirschner, I. Uecker, M. Bolte, H.-W. Lerner and M. Wagner, Organometallics, 2019, 38, 2818–2823 CrossRef CAS; (f) J. Jovaišaitė, S. Kirschner, S. Raišys, G. Kreiza, P. Baronas, S. Juršėnas and M. Wagner, Angew. Chem., Int. Ed., 2023, 62, e202215071 CrossRef.
- (a) T. Kaehler, M. Bolte, H.-W. Lerner and M. Wagner, Angew. Chem., Int. Ed., 2019, 58, 11379–11384 CrossRef CAS PubMed; (b) T. Jin, L. Kunze, S. Breimaier, M. Bolte, H.-W. Lerner, F. Jäkle, R. F. Winter, M. Braun, J.-M. Mewes and M. Wagner, J. Am. Chem. Soc., 2022, 144, 13704–13716 CrossRef CAS PubMed; (c) M. Metzler, A. Virovets, H.-W. Lerner and M. Wagner, J. Am. Chem. Soc., 2023, 145, 23824–23831 CrossRef CAS.
- M. Melaimi and F. P. Gabbaï, Adv. Organomet. Chem., 2005, 53, 61–99 CrossRef CAS.
- M. Oishi, Product Subclass 9: Triorganoaluminum Compounds, in Science of Synthesis, ed. H. Yamamoto, Georg Thieme Verlag KG, Stuttgart, 2004, pp. 261–385 Search PubMed.
- M. A. Dam, T. Nijbacker, F. J. J. de Kanter, O. S. Akkerman, F. Bickelhaupt and A. L. Spek, Organometallics, 1999, 18, 1706–1709 CrossRef CAS.
- M. Tschinkl, T. M. Cocker, R. E. Bachman, R. E. Taylor and F. P. Gabbaï, J. Organomet. Chem., 2000, 604, 132–136 CrossRef CAS.
- P. Jutzi, H. Sielemann, B. Neumann and H.-G. Stammler, Inorg. Chim. Acta, 2005, 358, 4208–4216 CrossRef CAS.
- F. P. Gabbaï, A. Schier, J. Riede and D. Schichl, Organometallics, 1996, 15, 4119–4121 CrossRef.
- M. A. Dam, T. Nijbacker, B. C. de Pater, F. J. J. de Kanter, O. S. Akkerman, F. Bickelhaupt, W. J. J. Smeets and A. L. Spek, Organometallics, 1997, 16, 511–512 CrossRef CAS.
- F. P. Gabbaï, A. Schier and J. Riede, Angew. Chem., Int. Ed., 1998, 37, 622–624 CrossRef.
- F. P. Gabbaï, A. Schier, J. Riede and M. J. Hynes, Chem. Commun., 1998, 897–898 RSC.
- M. Tschinkl, A. Schier, J. Riede and F. P. Gabbaï, Inorg. Chem., 1998, 37, 5097–5101 CrossRef CAS.
- We are aware that the compounds AlMe3, AlBr3, and MeAlCl2 are monomeric neither in solution nor in the solid state. However, for simplicity, the monomeric forms were used in calculating the quantities employed..
- The literature presents contradicting information regarding the degree of association of [Hg(o-C6H4)]n: Wittig and Bickelhaupt have proposed values of n = 6 (or 4) based on calotte model inspections and molecular weight determinations in solution (ref. 50). Massey et al. reported that n equals 3 (ref. 51). We will adopt a value of n = 3 throughout, as it is supported by the results of single-crystal X-ray structure analyses and mass spectrometric investigations: (a) C. M. Woodard, G. Hughes and A. G. Massey, J. Organomet. Chem., 1976, 112, 9–19 CrossRef CAS; (b) D. S. Brown, A. G. Massey and D. A. Wickens, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1978, 34, 1695–1697 CrossRef; (c) D. S. Brown, A. G. Massey and D. A. Wickens, Inorg. Chim. Acta, 1980, 44, 193–194 CrossRef.
- (a) M. A. G. M. Tinga, O. S. Akkerman, F. Bickelhaupt, E. Horn and A. L. Spek, J. Am. Chem. Soc., 1991, 113, 3604–3605 CrossRef CAS; (b) M. A. G. M. Tinga, G. Schat, O. S. Akkerman, F. Bickelhaupt, E. Horn, H. Kooijman, W. J. J. Smeets and A. L. Spek, J. Am. Chem. Soc., 1993, 115, 2808–2817 CrossRef CAS.
- This study focuses exclusively on heteroanthracenes that lack substituents on the phenylene rings. Substituents attached to Al/Ga/In are denoted after the hyphen; for example, DAA-Me2 refers to a 9,10-dihydro-9,10-dialuminaanthracene with methyl substituents at the Al sites. The type and number of coordinated Lewis-basic ligands (if present) are added in parentheses..
- One example of a corresponding B–Cb–B 2e3c bond has been crystallographically characterized: A. Hübner, M. Diefenbach, M. Bolte, H.-W. Lerner, M. C. Holthausen and M. Wagner, Angew. Chem., Int. Ed., 2012, 51, 12514–12518 CrossRef.
- C. Janiak, Angew. Chem., Int. Ed., 1997, 36, 1431–1434 CrossRef CAS.
- X. Zheng, M. Kato, Y. Uemura, D. Matsumura, I. Yagi, K. Takahashi, S. Noro and T. Nakamura, Inorg. Chem., 2023, 62, 1257–1263 CrossRef CAS.
- A. Y. Timoshkin, Chem.–Eur. J., 2024, 30, e202302457 CrossRef CAS PubMed.
- J. J. Eisch, K. Mackenzie, H. Windisch and C. Krüger, Eur. J. Inorg. Chem., 1999, 153–162 CrossRef CAS.
- (a) M. Tschinkl, R. E. Bachman and F. P. Gabbaï, Chem. Commun., 1999, 1367–1368 RSC; (b) For a more recent example of a similar reaction, see: P. Federmann, R. Müller, F. Beckmann, C. Lau, B. Cula, M. Kaupp and C. Limberg, Chem.–Eur. J., 2022, 28, e202200404 CrossRef CAS PubMed.
- J. J. Eisch and B. W. Kotowicz, Eur. J. Inorg. Chem., 1998, 761–769 CrossRef CAS.
- W. J. Scott, G. T. Crisp and J. K. Stille, Org. Synth., 1990, 68, 116 CrossRef CAS.
- An analogous phenomenon has already been observed for mixtures of certain N- or P-ligands and B- or In-Lewis acids: (a) M. Fontani, F. Peters, W. Scherer, W. Wachter, M. Wagner and P. Zanello, Eur. J. Inorg. Chem., 1998, 1453–1465 CrossRef CAS; (b) ref. 16; (c) M. Grosche, E. Herdtweck, F. Peters and M. Wagner, Organometallics, 1999, 18, 4669–4672 CrossRef CAS; (d) R. E. Dinnebier, M. Wagner, F. Peters, K. Shankland and W. I. F. David, Z. Anorg. Allg. Chem., 2000, 626, 1400–1405 CrossRef CAS; (e) M. Scheibitz, J. W. Bats, M. Bolte and M. Wagner, Eur. J. Inorg. Chem., 2003, 2049–2053 CrossRef CAS; (f) M. Scheibitz, M. Bolte, H.-W. Lerner and M. Wagner, Organometallics, 2004, 23, 3556–3559 CrossRef CAS.
- A. Lorbach, M. Bolte, H.-W. Lerner and M. Wagner, Chem. Commun., 2010, 46, 3592–3594 RSC.
- (i) The addition of monodentate pyridine (2 equiv.) also releases (2)2. However, the AlBr3/py adduct(s) that are formed as byproduct(s) remain soluble and are therefore difficult to remove. (ii) Adding 2 equiv. bipy to 2·(AlBr3)2 results in the precipitation of all Al-containing compounds (in C6D6); the 1H NMR spectrum of the supernatant shows no resonances, except for the solvent signal. (iii) As control experiments, we have also prepared 2:1 and 1:1 mixtures of AlBr3 and bipy in C6D6. In both cases, a precipitate formed; the supernatants showed either no bipy signals (2:1 mixture) or significant bipy signals (1:1 mixture). NMR analysis of each precipitate in CD3CN displayed a strong resonance at δ(27Al) = 81.0 ppm, assignable to the [AlBr4]− anion (literature value: 80.0 ppm as taken from: J. W. Akitt, Aluminum, Gallium, Indium, and Thallium, in Multinuclear NMR, ed. J. Mason, Plenum Press, New York, 1987, ch. 9, p. 269. The 1H resonance patterns of both precipitates were essentially identical, though rather complex, indicating the presence of various bipy-containing cations. Overall, the NMR characteristics of the precipitate that had been isolated (in quantitative yield) during the synthesis of (2)2 are comparable to those observed in the control experiments. For an earlier study of the AlBr3/bipy system, see: J. Y. Corey and R. Lamberg, Inorg. Nucl. Chem. Lett., 1972, 8, 275–280.
- V. C. Williams, W. E. Piers, W. Clegg, M. R. J. Elsegood, S. Collins and T. B. Marder, J. Am. Chem. Soc., 1999, 121, 3244–3245 CrossRef CAS.
- Deposition Numbers 2385628 (for (1)2), 2385629 (for 1·(py)2), 2385630 (for K[1·(μ-pz)]), 2385631 (for 1·(pyz)(thf)), 2385632 (for DAA-R2·(AlBrR2)2 (R = Me or Br)), 2385633 (for α-2·(AlBr3)2), 2385634 (for β-2·(AlBr3)2), 2385635 (for (2)2), 2385636 (for 2·(thf)2), 2385637 (for 2·(py)2), 2385638 (for [nBu4N][3]), 2385639 (for 1·(thf)2), and 2385640 (for 2·(OEt2)2) contain the ESI† crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service..
- C. A. Reed, K.-C. Kim, E. S. Stoyanov, D. Stasko, F. S. Tham, L. J. Mueller and P. D. W. Boyd, J. Am. Chem. Soc., 2003, 125, 1796–1804 CrossRef CAS PubMed.
- (a) H. Schnöckel, M. Leimkühler, R. Lotz and R. Mattes, Angew. Chem., Int. Ed., 1986, 25, 921–922 CrossRef; (b) R. Ahlrichs, M. Häser, H. Schnöckel and M. Tacke, Chem. Phys. Lett., 1989, 154, 104–110 CrossRef CAS; (c) C. Üffing, A. Ecker, R. Köppe, K. Merzweiler and H. Schnöckel, Chem.–Eur. J., 1998, 4, 2142–2147 CrossRef.
- Donor adducts of 1,4-dialuminacyclohexadienes have been prepared by Hoberg et al. through the cyclocondensation of Na2[cis-(Et3Al)(Ph)C=C(Ph)(AlEt3)] or via photochemical reactions: (a) H. Hoberg, V. Gotor, A. Milchereit, C. Krüger and J. C. Sekutowski, Angew. Chem., Int. Ed., 1977, 16, 539 CrossRef; (b) H. Hoberg and F. Aznar, J. Organomet. Chem., 1979, 164, C13–C15 CrossRef CAS; (c) H. Hoberg and F. Aznar, J. Organomet. Chem., 1980, 193, 161–163 CrossRef CAS.
- To facilitate the structure discussion, we will from now on treat (1)2A as if it had an exact D2d symmetry in the solid state..
- R. G. Vranka and E. L. Amma, J. Am. Chem. Soc., 1967, 89, 3121–3126 CrossRef CAS.
- D. D. Eley, J. H. Taylor and S. C. Wallwork, J. Chem. Soc., 1961, 3867–3873 RSC.
- A. Lorbach, M. Bolte, H. Li, H.-W. Lerner, M. C. Holthausen, F. Jäkle and M. Wagner, Angew. Chem., Int. Ed., 2009, 48, 4584–4588 CrossRef CAS.
- A. Nangia, Molecular Conformation and Crystal Lattice Energy Factors in Conformational Polymorphs, in Models, Mysteries and Magic of Molecules, ed. J. C. A. Boeyens and J. F. Ogilvie, Springer, Dordrecht, 2008, ch. 3, pp. 63–86 Search PubMed.
- The compound 2·(AlBr3)2 crystallizes in two polymorphous modifications that differ by the number of crystallographically unique molecules (two molecules in the denser α-form and one in the less dense β-form). Since the geometrical characteristics of the molecules are similar, we are focusing here on the β-form. More details are provided in the ESI.†.
- W. Zheng, A. Stasch, J. Prust, H.-W. Roesky, F. Cimpoesu, M. Noltemeyer and H.-G. Schmidt, Angew. Chem., Int. Ed., 2001, 40, 3461–3464 CrossRef CAS.
- Selected examples of bicyclic compounds [RAl(μ-3,5-tBu2pz)2(μ-E)AlR]: (a) R = H, E = O–Te: W. Zheng, N. C. Mösch-Zanetti, H.-W. Roesky, M. Noltemeyer, M. Hewitt, H.-G. Schmidt and T. R. Schneider, Angew. Chem., Int. Ed., 2000, 39, 4276–4279 CrossRef CAS; (b) R = PhCC, E = S–Te: W. Zheng, H. Hohmeister, N. C. Mösch-Zanetti, H.-W. Roesky, M. Noltemeyer and H.-G. Schmidt, Inorg. Chem., 2001, 40, 2363–2367 CrossRef CAS.
- Boron-containing heterotriptycenes are known: (a) ref. 32; (b) Ö. Seven, S. Popp, M. Bolte, H.-W. Lerner and M. Wagner, Dalton Trans., 2014, 43, 8241–8253 RSC; (c) A. Ben Saida, A. Chardon, A. Osi, N. Tumanov, J. Wouters, A. I. Adjieufack, B. Champagne and G. Berionni, Angew. Chem., Int. Ed., 2019, 58, 16889–16893 CrossRef CAS; (d) A. Chardon, A. Osi, D. Mahaut, T.-H. Doan, N. Tumanov, J. Wouters, L. Fusaro, B. Champagne and G. Berionni, Angew. Chem., Int. Ed., 2020, 59, 12402–12406 CrossRef CAS; (e) A. Osi, D. Mahaut, N. Tumanov, L. Fusaro, J. Wouters, B. Champagne, A. Chardon and G. Berionni, Angew. Chem., Int. Ed., 2022, 61, e202112342 CrossRef CAS; (f) M. Henkelmann, A. Omlor, M. Bolte, V. Schünemann, H.-W. Lerner, J. Noga, P. Hrobárik and M. Wagner, Chem. Sci., 2022, 13, 1608–1617 RSC; (g) A. Osi, N. Tumanov, J. Wouters, A. Chardon and G. Berionni, Synthesis, 2023, 55, 347–353 CrossRef CAS; (h) S. E. Prey, J. Gilmer, S. V. Teichmann, L. Čaić, M. Wenisch, M. Bolte, A. Virovets, H.-W. Lerner, F. Fantuzzi and M. Wagner, Chem. Sci., 2023, 14, 5316–5322 RSC.
- (a) C.-C. Chang, T.-Y. Her, F.-Y. Hsieh, C.-Y. Yang, M. Y. Chiang, G.-H. Lee, Y. Wang and S.-M. Peng, J. Chin. Chem. Soc., 1994, 41, 783–789 CrossRef CAS; (b) J. Lewiński, J. Zachara, T. Kopeć, I. Madura and I. Prowotorow, Inorg. Chem. Commun., 1999, 2, 131–134 CrossRef.
- (a) F. Bickelhaupt, Angew. Chem., Int. Ed., 1987, 26, 990–1005 CrossRef; (b) F. Bickelhaupt, Pure Appl. Chem., 1990, 62, 699–706 CrossRef CAS.
- G. Wittig and F. Bickelhaupt, Chem. Ber., 1958, 91, 883–894 CrossRef CAS.
- N. A. A. Al-Jabar and A. G. Massey, J. Organomet. Chem., 1984, 275, 9–18 CrossRef CAS.
- H. J. S. Winkler and G. Wittig, J. Org. Chem., 1963, 28, 1733–1740 CrossRef CAS.
- M. S. Goedheijt, T. Nijbacker, O. S. Akkerman, F. Bickelhaupt, N. Veldman and A. L. Spek, Angew. Chem., Int. Ed., 1996, 35, 1550–1552 CrossRef CAS.
- A. Lorbach, C. Reus, M. Bolte, H.-W. Lerner and M. Wagner, Adv. Synth. Catal., 2010, 352, 3443–3449 CrossRef CAS . A simplified version of this published protocol, which we refer to here, can be found in the ESI.†.
- The reaction of [nBu4N][3] with Me2SiCl2 or Me3SnCl gives 9,9,10,10-tetramethyl-9,10-dihydro-9,10-disilaanthracene or 1,2-(Me2BrSn)2C6H4, respectively. These reactions are not yet optimized and represent the onset of an investigation into the full scope of the reaction, which will be reported at a later stage..
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic procedures, NMR spectra, X-ray crystallographic data and computational details. CCDC 2385628–2385640. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4sc06940d |
| This journal is © The Royal Society of Chemistry 2025 |