 Open Access Article
Open Access ArticleNanomaterials: innovative approaches for addressing key objectives in periodontitis treatment
Ruijianghan Shi†
 a,
Yujie Zhu†
a,
Yujie Zhu† a,
Weitong Lu
a,
Weitong Lu a,
Ruohan Zhai
a,
Ruohan Zhai a,
Mi Zhoua,
Sirong Shi*a and
Yang Chen*b
a,
Mi Zhoua,
Sirong Shi*a and
Yang Chen*b
aState Key Laboratory of Oral Diseases, National Center for Stomatology, National Clinical Research Center for Oral Diseases, West China Hospital of Stomatology, Sichuan University, Chengdu 610041, Sichuan, China. E-mail: sirongshi@scu.edu.cn; srjh15088429156@126.com; zyj13668024814@163.com
bDepartment of Pediatric Surgery, Department of Liver Surgery & Liver Transplantation Center, West China Hospital of Sichuan University, Chengdu 610041, Sichuan, China. E-mail: chenyangwz1116@163.com
First published on 2nd September 2024
Abstract
Periodontitis is a chronic inflammatory disease primarily caused by dental plaque, which is a significant global public health concern due to its high prevalence and severe impact on oral, and even systemic diseases. The current therapeutic plan focuses on three objectives: pathogenic bacteria inhibition, inflammation control, and osteogenic differentiation induction. Existing treatments still have plenty of drawbacks, thus, there is a pressing need for novel methods to achieve more effective treatment effects. Nanomaterials, as emerging materials, have been proven to exert their inherent biological properties or serve as stable drug delivery platforms, which may offer innovative solutions in periodontitis treatment. Nanomaterials utilized in periodontitis treatment fall into two categories, organic and inorganic nanomaterials. Organic nanomaterials are known for their biocompatibility and their potential to promote tissue regeneration and cell functions, including natural and synthetic polymers. Inorganic nanomaterials, such as metal, oxides, and mesoporous silica nanoparticles, exhibit unique physicochemical properties that make them suitable as antibacterial agents and drug delivery platforms. The inorganic nanosurface provides terrain induction for cell migration and osteogenic regeneration at defect sites by introducing different surface morphologies. Inorganic nanomaterials also play a role in antibacterial photodynamic therapy (aPDT) for eliminating pathogenic bacteria in the oral cavity. In this review, we will introduce multiple forms and applications of nanomaterials in periodontitis treatment and focus on their roles in addressing the key therapeutic objectives, to emphasize their promising future in achieving more effective and patient-friendly approaches toward periodontal tissue regeneration and overall health.
1 Introduction
Periodontitis is a chronic multifactorial inflammatory disease primarily induced by dental plaque, and it is characterized by progressive destruction of the dental tissue.1 It ranks as the most prevalent oral disease and is a significant public health concern.2,3 Periodontitis can lead to gingival inflammation, clinical attachment loss, and alveolar bone loss,4 making it the primary trigger of tooth loss and severely decreasing patients' quality of life. Nonetheless, epidemiological studies reveal a strong association between periodontitis and many systemic diseases including cardiovascular diseases,5 type II diabetes,6 rheumatoid arthritis,7 Alzheimer's disease,8 and cancer.9,10 Furthermore, the release of inflammatory agents can create a conducive environment for bacteria to enter the bloodstream, thus leading to odontogenic bacteremia, triggering severe complications.11 Consequently, the treatment of periodontitis holds promise for improving the management of systemic diseases, highlighting the therapeutic potential of addressing periodontitis.Nevertheless, treating periodontitis presents substantial medical and socio-economic challenges. Common treatment methods include non-surgical approaches such as scaling and root planning, surgical interventions like periodontal flap surgery, and adjunctive therapies involving local medications and systemic antibiotic. It's important to note that neither non-surgical nor extensive surgical techniques can completely eradicate periodontal microorganisms.12 Achieving satisfactory therapeutic results often requires combining multiple approaches. However, challenges such as significant patient discomfort, extended treatment durations, and uncertain efficacy cannot be disregarded. In summary, there remains a pressing need for ideal methods to treat periodontitis and achieve predictable periodontal tissue regeneration.
Nanomaterials are undergoing significant advancements in the field of medicine. Nanomaterials fall into two categories: organic nanomaterials and inorganic nanomaterials, all with considerable biological application potential. Possessing promising properties, nanomaterials offer potential applications across various dental therapeutic modalities, including local drug delivery, restorative materials, bone grafts, and implant surface modifications.13 The clinical utilization of nanomaterials in periodontitis treatment involves harnessing their inherent biological properties for therapy, employing them as drug delivery platforms, and combined with novel therapeutic technologies.
Organic nanomaterials encompass nature and synthetic polymers. They represent a prominent topic in biomedical research due to their excellent biocompatibility and their potential to promote cell adhesion, proliferation, cell-to-cell interactions, cell migration, and tissue regeneration.14 Inorganic nanomaterials encompass metal, oxides, and structured compounds like mesoporous silica nanoparticles. Inorganic nanomaterials exhibit exceptional physicochemical properties that offer valuable functionalities and hold significant promise in biomedicine.15 Currently, the application of inorganic nanomaterials in dental therapy centers on their roles as antibacterial agents16 and reliable drug delivery platforms.17 Meanwhile, by introducing different surface morphologies, inorganic nanosurface provides terrain induction for cell migration and osteogenic regeneration at defect sites. Additionally, they can serve as sensitizers or targeting moieties in antibacterial photodynamic therapy (aPDT), which is an effective approach for eliminating pathogenic bacteria in the oral cavity.18 In this review, we will provide insights into the diverse applications of classic nanomaterial structures in the context of periodontitis treatment and update the primary treatment scenarios they engage in (Fig. 1 and Table 1).
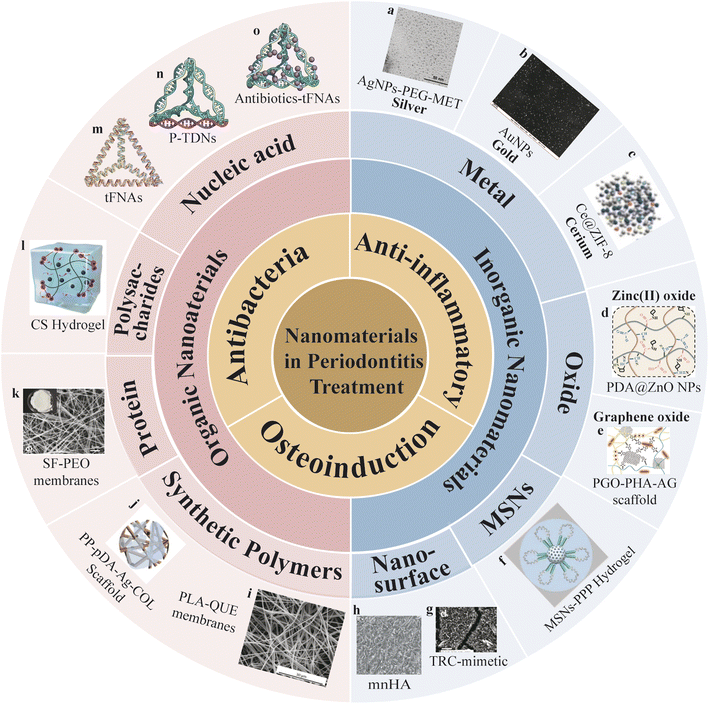 | ||
| Fig. 1 Nanomaterials in periodontitis treatment (a) figure of silver surface attached with metronidazole molecules via a polyethylene glycol (PEG) linker (AgNPs-PEG-MET). Adapted under the terms of the CC-BY Creative Commons Attribution 3.0 Unported license from ref. 19. Copyright 2023, Dove Medical Press Limited. (b) Figure of gold nanoparticles (AuNPs). Adapted with permission from ref. 20. Copyright 2023, Elsevier. (c) Figure of Ce-doped ZIF-8 nanoparticles (ZIF-8: Ce NPs). Adapted with permission from ref. 21. Copyright 2023, ACS. (d) Figure of PDA coated ZnO NPs (PDA@ZnO NPs). Adapted with permission from ref. 22. Copyright 2023, Wiley. (e) Figure of a polydopamine-mediated graphene oxide (PGO) and hydroxyapatite nanoparticle (PHA)-incorporated conductive alginate/gelatin (AG) scaffold (PGO-PHA-AG scaffold). Adapted under the terms of the CC BY-NC-ND license from ref. 23. Copyright 2022, KeAi Publishing. (f) Figure of MSN-incorporated PPP hydrogel. Adapted under the terms of the CC BY-NC-ND license 4.0 from ref. 24. Copyright 2022, KeAi Publishing. (g) Figure of tooth root cementum (TRC)-mimetic. Adapted under the terms of the CC BY-NC-ND license 4.0 from ref. 25. Copyright 2022, American Chemical Society. (h) Figure of hydroxyapatite (HA) bioceramics with micro-nano-hybrid surface (mmHA). Adapted under the terms of the CC-BY Creative Commons Attribution 3.0 Unported license from ref. 26. Copyright 2015, Dove Medical Press Limited. (i) Figure of electrospun polylactic acid nanofibers loaded with Quercetin (PLA-QUE) membranes. Adapted under the terms of the CC BY-NC-ND license from ref. 27. Copyright 2022, MDPI. (j) Figure of silver-modified/collagen-coated electrospun PLGA/PCL scaffold (PP-pDA-Ag-COL scaffolds). Adapted with permission from ref. 28. Copyright 2019, ACS. (k) Figure of silk fibroin/poly(ethylene oxide) (SF/PEO) membranes. Adapted with permission from ref. 29. Copyright 2019, Elsevier. (l) Figure of Chitosan (CS) hydrogel. Adapted under the terms of the CC BY-NC-ND license 4.0 from ref. 30. Copyright 2020, KeAi Publishing. (m) Figure of tFNAs. (n) Figure of tFNAs loaded with PNAs (P-TDNs). (o) Figure of antibotics-tFNAs. | ||
| Nanomaterials | Structure | Research mode | Therapeutic effect in periodontitis treatment | Mechanism | References | |
|---|---|---|---|---|---|---|
| a t-GL13K: tFNAs loaded with GL13K; P-TDNs: tFNAs loaded with asPNA; stFNA-miR: tFNAs loaded with miRNA; ASOs-tFNAs: tFNAs loaded with ASOs; tFNAs-Ery: tFNAs loaded with erythromycin; tFNAs-ampicillin: tFNAs loaded with ampicillin.b Methacrylic anhydride modified HA with minocycline hydrochloride-loaded mesoporous bioactive glass nanoparticles.c HA-based nano-loading system designed to encapsulate curcumin into nanoparticles.d Tannic acid modified electrospun silk fibroin nanofibrous membranes.e Silk fibroin nanofibrous membranes containing gelatin nanospheres.f Sodium alginate hydrogel composites doped with Cu2O nanoparticles and PDA-coated titanium dioxide nanoparticles.g AgNPs loaded with chlorhexidine and metronidazole.h Peptide-coated gold cluster.i Ce-doped ZIF-8 nanoparticles.j Amoxicillin linked to GO using a peptide linker.k Graphene quantum dots coupled with curcumin.l Zinc oxide nanoparticles incorporated into an albumin-based carrier.m Silver-modified/collagen-coated electrospun PLGA/PCL scaffold.n AgNPs incorporated into a composite film synthesized from poly(vinyl alcohol) cross-linked with oxidized chitosan.o PCL scaffolds coated with GO.p Chitosan/silk fibroin membranes incorporating reduced graphene oxide.q Copper-loaded MSNs integrated into a PLGA/gelatin fiber matrix.r Quaternary ammonium salts-modified core–shell MSNs containing Ag nanoparticles.s CeO2 coupled with chlorin e6. | ||||||
| Tetrahedral framework nucleic acid | Monomer | In vitro/vivo | Anti-inflammatory, osteogenic differentiation induction | MAPK/ERK pathway inhibited | 31 | |
| Conjugatea | t-GL13K | In vitro | Antibacterial | Bacterial uptake of AMPs increased | 31 | |
| Susceptibility to the protease-rich extracellular environment | ||||||
| P-TDNs | In vitro | Antibacterial | Bacterial uptake of asPNA increased → asPNA target ftsZ gene → bacterial growth inhibited | 32 | ||
| stFNA–miR | In vitro/vivo | Osteogenic differentiation induction | Bacterial uptake of miR increased → expression of HDAC5 downgraded, osteogenic differentiation upgraded | |||
| ASOs-tFNAs | In vitro | Antibacterial | Bacterial uptake of ASOs increased → EPS synthesis and biofilm thickness decreased, expression of gtfBCD, gbpB, ftf downgraded | 33 | ||
| tFNAs-Ery | In vitro | Antibacterial | Bacterial uptake of Ery increased → drug resistance inhibited | 34 | ||
| tFNAs-ampicillin | In vitro | Antibacterial | Bacterial uptake of ampicillin increased → bacterial cell wall synthesis-related genes downgraded, antibiotic sensibility-related genes upgraded | 35 | ||
| Chitosan | CSnp (chitosan nanoparticle) | In vitro | Anti-inflammatory | Regulate paracrine signaling → macrophages polarization and PdLF migration increased | 36 | |
| Chitosan hydrogel | In vitro/vivo | Anti-inflammatory | NF-κB and p38 MAPK signaling inhibited | 30 | ||
| Exosome-mediated transfer of miR-1246 increased | ||||||
| Hyaluronic acid | MBGN-MNCl/MHA gelsb | In vitro | Anti-inflammatory, antibacterial, osteogenic differentiation induction | Irregular periodontal defects fitting | 37 | |
| Expression of bone-related genes increased | ||||||
| High load and sustained release of antibacterial MNCl | ||||||
| ROS-responsive HA@CUR NPsc | In vitro/vivo | ROS-responsive | Release and maintain CUR in response to ROS | 38 | ||
| Anti-inflammatory, antibacterial, osteogenic differentiation induction | Enhance cellular uptake at inflammation sites | |||||
| Silk fibroin | TA/SF nanofibrous membranesd | In vitro | Osteogenic differentiation induction | In situ formation of HAp → osteogenic differentiation upgraded | 39 | |
| Induce a conformational change in SF molecules from random coils to β-sheets | ||||||
| Cell proliferation promoted | ||||||
| SF/GN membranese | In vitro | Antibacterial, tissue regeneration | Prolonged release of vancomycin | 40 | ||
| High cell compatibility | ||||||
| Promote attachment, spreading, and proliferation of periodontal ligament cells | ||||||
| Synthetic polymers | A 3D aligned nanofibrous scaffold | In vitro/vivo | Osteogenic differentiation induction | Guide-oriented arrangement and elongation of cells | 41 | |
| Tri-Layered nanocomposite hydrogel scaffold with growth factors | In vitro/vivo | Osteogenic differentiation induction | Cementogenic, fibrogenic, and osteogenic differentiation of hDFCs increased | 42 | ||
| A three-layered graded membrane nanostructure | In vitro | Osteogenic differentiation induction | Adjust the bioresorption behavior for optimal osteogenesis in different sites for defect repairs | 43 | ||
| PDA coating of nanofibrous scaffolds | In vitro/vivo | Osteogenic differentiation induction | Hydroxyapatite mineralization increased | 44 | ||
| PDLSCs osteogenic differentiation increased | ||||||
| CTP-SA doped with Cu2O and TiO2@PDA nanoparticlesf | In vitro/vivo | Antibacterial, osteogenic differentiation induction | TiO2@PDA: generate ROS under blue light irradiation | 45 | ||
| Cu2O nanoparticles: antibacterial effect | ||||||
| Cu2: proliferation and osteogenic differentiation of BMSCs increased | ||||||
| Silver nanoparticles | Monomer | In vitro | Antibacterial | Disrupt ATP molecules → direct damage to the cell membrane | 16 | |
| Chitosan-fucoidan complex-coated AgNPs | In vitro | Antibacterial | Biocompatibility, drug encapsulation, and cellular uptake increased | 46 | ||
| Conjugate | AgNPs-CHL, AgNPs-PEG-METg | In vitro | Antibacterial, anti-inflammatory | Proinflammatory cytokines downgraded | 19 | |
| Levels of metalloproteinases MMP3 and MMP8 downgraded | ||||||
| Gold | Au25Sv9h | In vitro/vivo | Anti-inflammatory | NF-κB pathway inhibited | 47 | |
| AuNPs | In vitro | Anti-inflammatory | Macrophage: M1 → M2 | 48 | ||
| In vitro/vivo | Osteogenic differentiation induction | Wnt/β-catenin pathway, ERK/AMPK pathway and autophagy pathway upgraded | 49–51 | |||
| Cerium | ZIF-8: Ce NPsi | In vitro | Anti-inflammatory | SOD and CAT enzyme mimic activity → ROS scavenging | 52 | |
| Pro-inflammatory mediators downgraded | ||||||
| NF-κB signaling pathway inhibited | ||||||
| Macrophage: M1 → M2 | ||||||
| Quercetin-loaded ZIF-8: Ce NPs | In vitro | Anti-inflammatory | Anti-inflammation efficiency inceased | 53 | ||
| CeO2 NPs | In vitro/vivo | Anti-inflammatory and antibacterial | MAPK-NF-κB pathway inhibited | 54 | ||
| Nrf2-HO-1 pathway upgraded | ||||||
| Graphene oxide | Monomer | In vitro | Antibacterial | P. gingivalis inhibited | 55 | |
| Bacterial cell membranes' structural integrity inhibited | ||||||
| GO-AMOXj | In vitro | Antibacterial | Enzyme hydrolysis → controlled AMOX release | 56 | ||
| GQDs-Curk | In vitro | Antibacterial | Periodontal pathogens inhibited | 57 | ||
| Biofilm-related genes downgraded | ||||||
| Zinc(II) oxide | Mino-ZnO@Albl | In vitro | Antibacterial | Increase the encapsulation efficiency and loading capacity of minocycline | 58 | |
| Targeted drug release in acidic environments typical of infection sites | ||||||
| Demonstrates a broad antimicrobial spectrum | ||||||
| ZnO gel | In vivo | Antibacterial | Significant improvement in periodontal health outcomes after 1 month with PDT compared to SRP alone | 59 | ||
| Mesoporous silica nanoparticles | MSNs-antibiotics | In vitro | Antibacterial | Stable release of carried antibiotics | 60–62 | |
| MSN-Baicalin (BA) | In vitro | Anti-inflammatory | Cellular internalization increased by endocytosis and thiol-mediated mechanisms → immuno-inflammatory responses downgraded | 63 | ||
| MSN-resveratrol (RSV) | In vitro/vivo | Anti-inflammatory | SIRT1/AMPK pathway upgraded | 64 | ||
| NF-κB pathway inhibited | ||||||
| Macrophage: M1 → M2 | ||||||
| Composite of multiple materials | PP-pDA-Ag-COLm | In vitro/vivo | Antibacterial, osteogenic differentiation induction | Nanofibrous scaffold: MC3T3 cell adhesion and osteogenic differentiation increased | 28 | |
| AgNPs: antibacterial effect | ||||||
| Composite PVA/CS films incorporating AgNPsn | In vitro | Antibacterial | S. aureus inhibited | 65 | ||
| PEKK coated with/in an AgNP-in-epoxy lining | In vitro | Antibacterial | P. gingivalis inhibited | 66 | ||
| SF-GO nano-configurations | In vitro | Osteogenic differentiation induction | Cell proliferation and osteo/cementoblast differentiation, cementum physiological synthesis increased | 67 | ||
| P-GO scaffoldso | In vitro | Osteogenic differentiation induction | Osteogenic differentiation, cell proliferation, calcium deposition increased | 68 | ||
| SF/CS/rGO blended membranesp | In vitro | Osteogenic differentiation | Bone regeneration increased | 69 | ||
| PG-Cu@MSNs scaffoldq | In vitro/vivo | Antibacterial, osteogenic differentiation induction | Osteogenesis and antibacterial effects increased | 70 | ||
| Ag@QHMSr | In vitro | Antibacterial, osteogenic differentiation induction | Sustained Ag+ release → stable antibacterial effects | 71 | ||
| Osteogenic differentiation of BMSCs increased | ||||||
| BPNSs (Black phosphorus nanosheets) | In vivo | Antibacterial, anti-inflammatory | Rapid electron transport → ROS consumption capabilities | 72 | ||
| Antibacterial and anti-inflammatory capabilities → protect cells from oxidative stress and accompanying inflammatory reactions induced by aPDT | ||||||
| CeO2@Ce6s | In vitro/vivo | Antibacterial, anti-inflammatory | SOD and CAT enzyme mimic activity → ROS scavenging | 18 | ||
| Inorganic nanosurface | Titanium surfaces with microgrooves and nanopores | In vitro | Osteogenic differentiation induction | Actin cytoskeleton polymerization increased | 73 | |
| ERK and p38 MAPK pathways upgraded | ||||||
| Nuclear translocation and transcriptional activity increased → TAZ activation increased | ||||||
| Titanium surfaces mimicking TRC | In vivo | Osteogenic differentiation induction | Regulates periodontal ligament cells and coordinates phosphate metabolism | 25 | ||
| Hybrid nanorod and microrod (mnHA) surfaces | In vitro | Osteogenic differentiation induction | Wnt pathway upgraded | 26 | ||
2 Pathogenesis and nanomaterials' therapeutic strategies for periodontitis
To develop an accurate treatment plan, a comprehensive understanding of periodontitis pathogenesis is essential. Periodontitis, an inflammatory disease primarily induced by bacteria, is initiated by predominant pathogens like Porphyromonas gingivalis. These pathogens activate the host's defense mechanisms, leading to tissue destruction through the release of mediators that stimulate connective tissue breakdown, particularly affecting alveolar bone and periodontal connective tissue.74,75 As a result, the three key treatment objectives for periodontitis encompass inhibiting pathogenic bacteria, controlling inflammation, and promoting osteogenic differentiation, ultimately facilitating periodontal tissue regeneration and functional recovery (Fig. 2).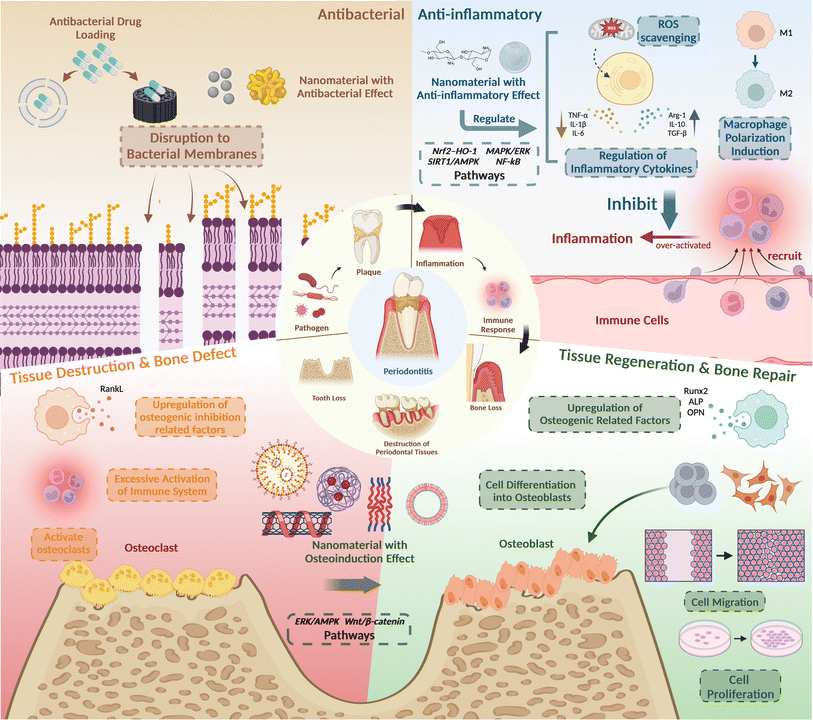 | ||
| Fig. 2 The pathological mechanism and treatment strategies of periodontitis. Created with https://BioRender.com/. | ||
The first objective is to utilize antimicrobial properties. The primary cause of periodontitis is plaque biofilm, a complex community of various bacteria, such as P. gingivalis. While antibiotics have traditionally been used to combat pathogens, their overuse can lead to antibiotic resistance, making these drugs less effective and potentially causing various side effects. In contrast, nanomaterials offer a promising alternative. Due to their small size, high surface energy, and charge density, nanomaterials can easily penetrate pathogen cells, effectively eradicating them. To be specific, the high surface energy of nanomaterials increased reactivity and interaction with biological membranes, enhancing their antimicrobial efficacy, and the positively charged nanoparticles interact electrostatically with the negatively charged bacterial cell wall, disrupting the cell membrane's permeability.76
The second objective aims to regulate inflammatory mediators and immune responses in the context of periodontitis. The interplay between pathogenic microorganisms and the immune system initiates a host defense response, which significantly contributes to periodontal tissue damage. This defense response exhibits a dual nature: on one hand, immune cells, particularly macrophages, serve as crucial guardians of tissue homeostasis and effective defenders against microbial invasion. Conversely, uncontrolled escalation and hyperactivation of these host defense mechanisms can trigger an inflammatory cascade. This inflammatory environment, characterized by the excessive production of proinflammatory mediators, ultimately leads to tissue damage. Therefore, mitigating an exaggerated immune response and suppressing inflammation represent fundamental objectives in periodontitis therapeutics. Recent scientific efforts to alleviate inflammation associated with periodontitis have predominantly focused on modulating macrophage and neutrophil polarization.77 Additionally, targeted interventions have been aimed at inhibiting inflammation by manipulating key signal transduction pathways, notably, p38 MAPK,30 NF-κB,47 and reactive oxygen species (ROS) scavenging.78
The third objective is tissue regeneration. The bacterial toxins produced by pathogenic microorganisms within the biofilm, along with the exacerbated host immune response, ultimately lead to defects in periodontal tissues and bone loss. This cascade of events can result in various periodontal dysfunctions, such as tooth movement, displacement, and even tooth loss.79 Therefore, the primary objective of periodontal treatment, and the ultimate goal, is to restore the structures and functions of periodontal tissues. Nanomaterials, owing to their exceptional biocompatibility and diverse biological capabilities, can serve as agents to induce osteogenic differentiation through various pathways and mechanisms. For instance, they can modulate pathways like Wnt/β-catenin signaling and the ERK/AMPK pathway to regulate cell migration, adhesion, and osteogenic differentiation of periodontal cells and stem cells. Ultimately, this facilitates periodontal tissue regeneration and bone repair, aligning with the therapeutic objectives of periodontal treatment.
3 Nanomaterials in periodontitis treatment application
In periodontitis treatment, different forms of nanomaterials have been explored and applied, showing promising therapeutic outcomes. Their therapy approach usually has multiple therapeutic effects addressing three major aspects: antibacterial activity, anti-inflammatory capability, and tissue regeneration facilitation. This multifaceted approach is crucial for periodontitis treatment and holds promise for offering innovative therapeutic strategies. Hence, as the commonly utilized treatment methods still hold their defects, several emerging therapeutic technologies employ nanomaterials aiming to achieve better healing efficiency, such as aPDT, tissue engineering, and drug delivery. In this section, we will delve into the latest advancements in the design strategies of nanomaterial in periodontitis treatment including organic nanomaterials, inorganic nanomaterials and nanosurface, summarize their applications across in vitro and in vivo studies, and introduce their combination with emerging therapeutic technologies.3.1 Organic nanomaterials
Remarkably, tFNAs have been found to be biologically active despite DNA's polyanionic nature. Their spatial configuration enables easy traversal of cellular plasma membranes, enhancing the effective delivery of nucleic acids with negative or neutral charge.81 tFNAs also show remarkable structural stability within living cells, lasting up to 48 hours,82 while also demonstrating exceptional biocompatibility.83 Moreover, research indicates that relatively high concentrations of tFNAs can stimulate cell proliferation, differentiation, and migration.84 Additionally, tFNAs offer programmability and unique modification sites, facilitating the incorporation of small molecules and nucleic acids, thereby serving as a versatile drug delivery platform.85
Zhou et al. present compelling evidence on tFNAs' anti-inflammatory and osteogenic differentiation-inducing properties in periodontitis treatment (Fig. 3). Their research demonstrates that tFNAs protect periodontal ligament stem cells (PDLSCs) from inflammation by suppressing the MAPK/ERK signaling pathway phosphorylation. In vivo experiments on mice's periodontium show reduced inflammatory cell infiltration and pro-inflammatory factor release in the tFNAs-treated group, highlighting their anti-inflammatory efficacy. Moreover, assessments of osteogenic markers in PDLSCs treated with tFNAs under inflammatory conditions show promising results. Mice with periodontitis receiving tFNAs injections in the gingival sulcus exhibit denser periodontal ligaments, increased collagen fiber density, intact cementum and Sharpey fibers, decreased osteoclast populations, and reduced bone resorption compared to control groups. Collectively, these findings underscore the therapeutic potential of tFNAs in bone regeneration.80
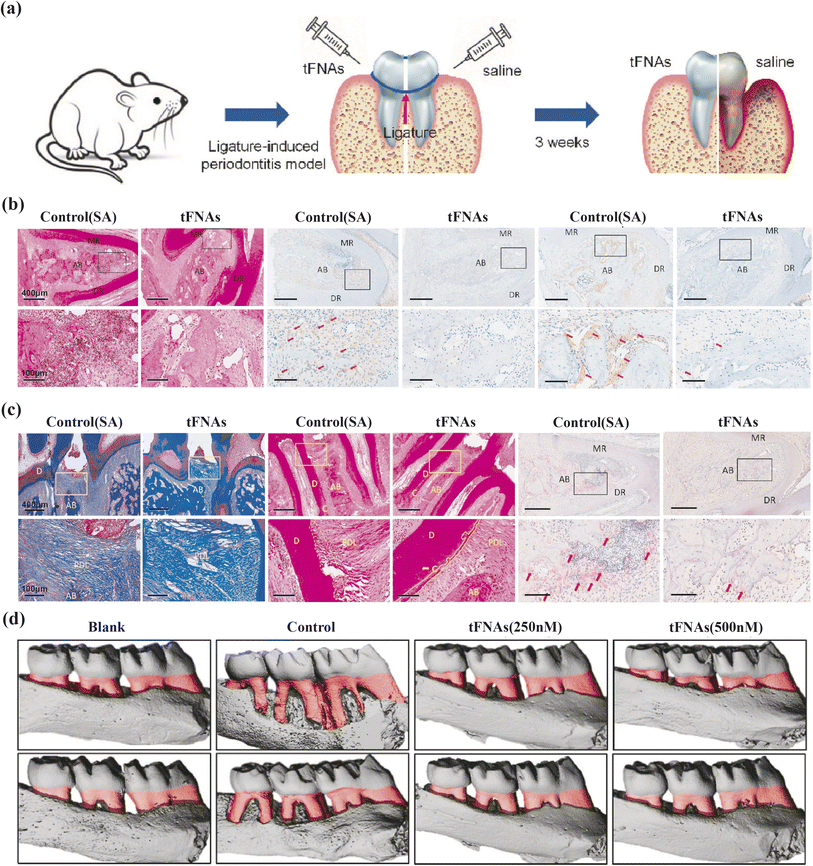 | ||
| Fig. 3 The protective effect of tFNAs on periodontium under inflammatory conditions. (a) Schematic diagram of the rat periodontitis experiment. (b) H&E staining (yellow arrow: inflammatory cells), the immunohistochemical results of IL-1β/IL-6 (red arrow) at 5× and 20× magnifcation. (c) Masson staining, H&E staining (yellow solid line: cementum, yellow dotted line: cementum absorbed by inflammation), TRAP staining (red arrow: osteoclasts) at 5× and 20× magnification. (d) The micro-CT 3D reconstruction images of left maxillary alveolar bone (red part: the exposure of root). MR/DR = mesial root/distal root; AB = alveolar ridge; D = dentin; C = cementum; PDL = periodontal ligament. Adapted under the terms of the CC BY-NC-ND license 4.0 from ref. 80. Copyright 2020, Elsevier. | ||
Additionally, findings from other researchers support tFNAs' ability to utilize their unique biological properties, including modulating periodontitis-related pathways and acting as drug delivery vectors, to provide therapeutic benefits. These studies offer additional validation of tFNAs' potential in periodontitis treatment, providing a complementary perspective on their therapeutic utility.
Effective antibacterial activity is crucial in combating periodontitis, which is primarily triggered by bacterial biofilm formation. In antimicrobial therapy, tFNAs serve as versatile drug delivery vehicles. Liu discussed the structure of t-GL13K, where tFNAs are used to deliver GL13K peptides.31 These peptides (antimicrobial peptide, AMPs) are antimicrobial agents that interact with microbial membranes through electrostatic and hydrophobic forces, causing irreversible membrane damage and cytoplasmic leakage.86 By increasing bacterial uptake and membrane interactions, t-GL13K showed improved effectiveness against Escherichia coli.87 Additionally, t-GL13K remained effective against P. gingivalis due to reduced susceptibility to extracellular protease-rich environments. Beyond traditional peptides, nucleic acids can be easily integrated with tFNAs using base pairing principles. Zhang and colleagues created the P-TDNs vector system, which incorporates antisense peptide nucleic acids (asPNA) into tFNAs. These synthetic DNA analogs target the ftsZ gene in bacteria, leading to a dose-dependent decrease in ftsZ expression and bacterial growth inhibition, demonstrating potential for asPNA delivery.32 MicroRNAs have also been linked with tFNAs, with Zhang and team designing antisense oligonucleotide sequences (ASOs) targeting conserved regions of the VicK protein-binding site, using tFNAs as the delivery method (ASOs-tFNAs) (Fig. 4). This strategy significantly reduced EPS production and biofilm thickness, facilitating the treatment of chronic biofilm-related infections that are resistant to standard treatments.33 Furthermore, tFNAs have been crucial in delivering small-molecules like erythromycin and methicillin, with research showing enhanced bacterial uptake and improved antibacterial activity, thereby addressing bacterial resistance challenges.34,35
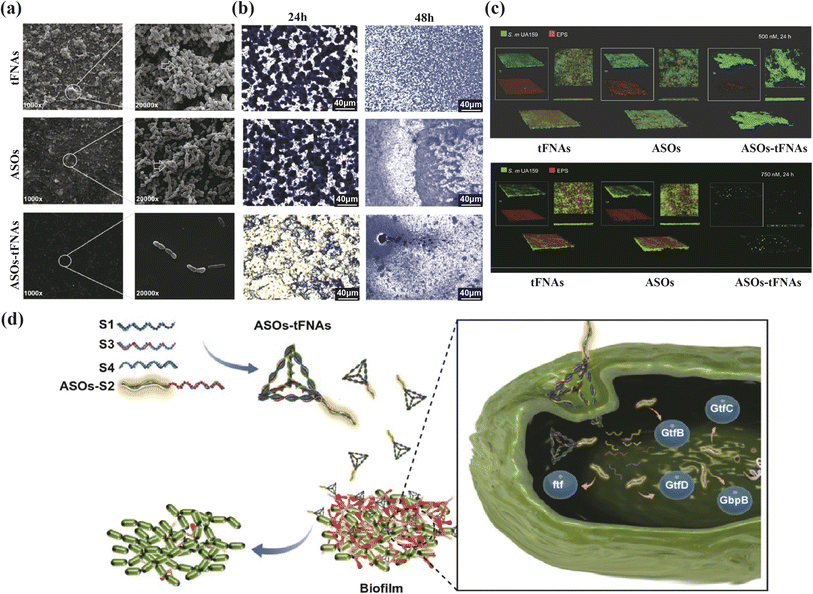 | ||
| Fig. 4 Multi-targeted ASOs-tFNAs for inhibiting biofilm formation and virulence. (a) SEM images of the structure of biofilms (24 h), (b) crystal violet staining of S. mutans cells (24 h, 48 h), (c) dual-label imaging and 3D visualization of EPS (red) and bacteria (green) in S. mutans biofilms, after treated with tFNAs, ASOs, or ASOs-tFNA. (d) Schematic diagram of the mechanism of ASOtFNAs delivery system. Adapted under the terms of the CC BY-NC-ND license 4.0 from ref. 33. Copyright 2020, Springer. | ||
Furthermore, tFNAs have exhibited the capacity to induce osteogenic differentiation in stem cells via the Wnt/β-catenin and Notch pathways, offering novel methods for tissue regeneration in periodontitis treatment. Shao et al. observed upregulated expression of key proteins in the Wnt pathway in adipose-derived stem cells (ADSCs) treated with tFNAs, promoting ADSC osteogenic differentiation.88 Zhou et al. demonstrated tFNAs' stimulation of the Notch pathway in dental pulp stem cells (DPSCs), enhancing osteogenic differentiation through increased expression of crucial Notch pathway factors.89 Alkaline phosphatase staining and calcium deposition assays further corroborated these findings. In addition to the osteogenic effect of the tFNAs itself, it can also carry microRNAs targeting osteogenic genes to transport them into cells, stabilizing the microRNA structure90 and increasing its endocytosis rate, while also exerting better therapeutic effects. Li's study involved the loading of miR-2861 onto tFNAs (stFNA-miR), which targets histone deacetylase 5 (HDAC5) to upgrade runt-related transcription factor 2 (Runx2) protein expression.91 This research investigated the impact of stFNA-miR on bone regeneration and drug toxicity in both in vitro and in vivo models. Statistical analysis confirmed the successful transportation of stFNA-miR into bone marrow-derived mesenchymal stem cells (BMSCs) and its release upon RNase H cleavage. Furthermore, the anticipated regulation of proteins was observed. In vivo experiments demonstrated promising outcomes for bone regeneration within the defect area.92
In conclusion, tFNAs represent a versatile and promising platform for drug delivery and therapeutic applications, showcasing exceptional biocompatibility, stability, cellular uptake capabilities, and diverse biological function. Their potential to transport a range of bioactive molecules into target cells positions them as valuable tools in advancing biomedical research and innovative therapies, such as in periodontitis treatment.
3.1.2.1 Polysaccharides—chitosan and hyaluronic acid.
3.1.2.1.1 Chitosan (CS). Chitosan, primarily sourced from crustacean exoskeletons, is a naturally derived biomaterial renowned for its attributes including biodegradability, biocompatibility, non-toxicity, hydrophilicity, and intrinsic antibacterial and antifungal properties.93
As a naturally derived biomaterial, CS possesses distinctive anti-inflammatory characteristics, capable of modulating various cytokines based on factors such as structure, deacetylation degree, relative molecular mass, and dosage.94 In the study conducted by Hebatullah, engineered bioactive chitosan-based nanoparticles (CSnp) were investigated for their potential to upregulate proteins with antioxidant and immunoregulatory properties, promoting the polarization of macrophages into the M2 phenotype and PdLF migration via paracrine signaling.36 However, further research is warranted to comprehensively assess the capacity of CSnp to enhance periapical tissue healing in vivo.
In most scenarios, CS is usually used as a drug-loading platform. Through chemical modifications, CS can acquire elasticity, flexibility, and induce minimal inflammatory responses owing to its β-(1,4) glycosidic bonds, rendering it an exceptional platform for drug delivery.95 Chitosan-based materials aiming periodontitis treatment comes in various terms including scaffolds,96 films,97 etc., however, the chitosan hydrogel has become a current research hotspot for its easily-modified mechanical properties98 and its ability to fit in different shapes and sizes of tissue defects by simply inject the pre-gel solution.99 For instance, Shen et al. fabricated DPSC-Exo-incorporated CS (DPSC-Exo/CS) and then proved its abilities to facilitate macrophages to convert to an anti-inflammatory phenotype, cut down epithelial lesion and speed up the alveolar bone coalescence in periodontitis mice.30 More research is underway to leverage the unique drug-carrying biological characteristics of CS.
3.1.2.1.2 Hyaluronic acid (HA). HA is a glycosaminoglycan and a natural component of the extracellular matrix,100 known for its high water retention, absorbency, and excellent biocompatibility. Biologically, HA exhibits significant anti-inflammatory properties by inhibiting the production of pro-inflammatory mediators,101 and it also possesses antimicrobial properties that inhibit the growth and adhesion of various microorganisms.102 Clinical randomized studies have shown that using HA as an adjunct to standard non-surgical periodontal therapy improves the antioxidant status in the oral cavity of periodontitis patients, reduces gingival inflammation, and increases periodontal attachment.103
However, HA's clinical efficacy is limited by its sensitivity to free radicals and susceptibility to degradation by hyaluronidase.104,105 To enhance its resistance to degradation and mechanical strength, a series of chemical modifications are necessary. Hu et al. addressed these limitations by modifying HA with methacrylic anhydride (MHA), achieving cross-linking under UV irradiation.37 This modification not only compensates for HA's instability and weak mechanical strength but also provides an injectable drug delivery approach, facilitating localized drug delivery to periodontal lesions. Despite laboratory evidence of HA's antimicrobial capability, systematic reviews of clinical trials indicate that HA as an adjunct to non-surgical mechanical treatment of periodontitis does not provide additional benefits in reducing the prevalence of P. gingivalis in subgingival biofilms.106 To further enhance the antibacterial and tissue regeneration capabilities of MHA gels, Hu et al. introduced minocycline hydrochloride (MNCl) loaded spherical mesoporous bioactive glass nanoparticles (MBGNs) to prepare MBGN-MNCl/MHA gels for treating irregular periodontal defects caused by periodontitis. Research data showed that MHA gels have good morphological adaptability, healing within the defect area after 30 seconds of UV irradiation, and exhibit excellent anti-inflammatory effects with low cytotoxicity. MBGNs (120 nm) have outstanding osteogenic properties, significantly promoting the expression of ALP, Runx2, OPN, and bone-related genes in MC3T3-E1 cells. Additionally, they can achieve a high load of 120 mg g−1 of antibacterial MNCl with sustained release, effectively inhibiting the proliferation of Streptococcus mutans. Hu et al.'s experimental results provide valuable insights for further chemical modifications of HA, enhancing its clinical utility in periodontal therapy.
Beyond leveraging HA's inherent biological functions, HA can also serve as a tool for targeted drug delivery in periodontitis treatment by specifically binding to various receptors, such as CD44, and facilitating endocytosis.107 Chen et al. developed an HA-based nano-loading system designed to encapsulate curcumin (CUR) into nanoparticles (NPs), targeting macrophages, fibroblasts, and epithelial cells with high CD44 expression, thereby achieving targeted delivery of CUR.38 Since excessive ROS produced during periodontitis can cause DNA damage, protein denaturation, and periodontal tissue damage, the researchers further modified HA using the pinacol ester of 4-hydroxybenzeneboronic acid (PBAP) to create an HA-PBAP ROS-responsive smart nano-loading system. This modification enabled HA@CUR NPs to release the drug in response to ROS, enhancing cellular uptake at inflammation sites and maintaining drug release at the lesion. The research demonstrated that HA@CUR NPs exhibit high antibacterial, anti-inflammatory, ROS scavenging, and immunomodulatory capabilities. Moreover, HA's ability to achieve long-term retention in the oral cavity ensures prolonged treatment for periodontitis, thereby benefiting patient compliance. As a novel multifunctional nanocarrier, HA@CUR NPs offer a promising reference for clinical applications in treating periodontitis.
3.1.2.2 Protein-silk fibroin (SF). Silk fibroin, a high-purity protein extracted from silk, boasts a rich amino acid composition with over 20 constituents, including glycine, alanine, and serine.108 This natural polymer offers exceptional mechanical attributes, encompassing biocompatibility, permeability to oxygen and water, degradability, and inducing minimal inflammatory responses.29 In terms of fabrication versatility, SF lends itself to flexible processing, enabling the production of nanoparticles,109 nanofibers,29 and scaffold14 in periodontitis therapy.
Silk fibroin has long been recognized as a strong candidate for guided tissue regeneration (GTR) membranes due to its excellent mechanical properties. However, the regeneration process of SF solution can disrupt the protein conformation and the parallel fiber structure of natural SF fibers, which limits their application in GTR treatments.110 Furthermore, SF-based membranes traditionally exhibit limited bone repair capabilities.111 To address these limitations, Zheng et al. developed a straightforward method for the direct modification of electrospun SF nanofibrous membranes, enhancing both their mechanical properties and osteogenic functions.39 Tannic acid (TA), with its rich phenolic hydroxyl moieties, provides abundant chelate sites for Ca2+ ions, thereby accelerating the in situ formation of hydroxyapatite (HAp) on the SF nanofibrous membranes. Additionally, TA induces a conformational change in SF molecules from random coils to β-sheets. The results indicated that the TA modification improved the hydrophilicity and mechanical performance of the SF membranes. Further experiment evaluated SF-based nanofibrous membranes for cell growth, proliferation, and osteogenic differentiation. The ST film-MI7d group showed higher OD values on day 5, indicating better osteoblast proliferation due to its porous structure and HAp content. Flow cytometry revealed low necrosis and apoptosis, confirming no adverse effects on cell viability. The living rate of MC3T3 cells was high across all groups, demonstrating good cytocompatibility. The ST film-MI7d group had lower ALP activity on day 7 but the highest on day 14, indicating positive effects on osteogenic differentiation. This group also showed the highest ECM mineralization and calcium deposition, suggesting that HAp formation on the film enhances osteogenesis by regulating protein synthesis and promoting bone mineral generation. Due to the simplicity and efficiency of the method, TA-mediated SF fiber modification holds significant potential for future applications.
In addition to their role in tissue regeneration and repair, enhancing the antibacterial activity of SF fiber-based biomaterials is crucial for achieving comprehensive treatment effects for periodontitis. Song et al. addressed this by developing novel silk fibroin nanofibrous membranes containing gelatin nanospheres (SF/GN membranes).40 Utilizing core–shell electrospinning technology, they incorporated substantial amounts of positively charged gelatin type A nanospheres (GANs) and negatively charged gelatin type B nanospheres (GBNs) loaded with positively charged vancomycin into the silk fibroin nanofibers. By adjusting the weight ratio between the nanospheres at the nanoscale using single-nozzle electrospinning, they achieved a prolonged release of vancomycin (up to 14 days). The experiment demonstrated that SF nanofibrous membranes with GBNs sustained vancomycin release for over 14 days, while those without GBNs stopped after 2 days. The presence of GBNs prolonged the release due to attractive interactions with vancomycin. Antibacterial tests showed that vancomycin-loaded membranes inhibited S. aureus, with larger inhibition zones for higher vancomycin loading. Membranes with vancomycin-loaded GBNs maintained antibacterial effects after the initial burst release, indicating enhanced sustained release and efficacy. Furthermore, they also found that SF/GN membranes exhibited excellent cytocompatibility and supported cell viability and proliferation. Live/dead assays and DNA content tests confirmed high cell viability and proliferation on membranes containing GBNs, compared to those without. SEM images showed enhanced cell attachment and spreading on GBN-containing membranes. These results underscored the beneficial role of SF/GN membranes (loaded with GBNs) in promoting cell adhesion and growth by exposing cell recognition sites upon membrane dissolution.
In practice, biomimetic scaffolds and stem cell engineering therapy often share common design principles and are frequently combined to enhance therapeutic outcomes. For instance, Zhang et al. integrated PCL-PEG (PCE) nanofibers into porous chitosan (CS) to create a 3D aligned layer-by-layer nanofibrous scaffold. They assessed its potential for topographic guidance and periodontium regeneration by introducing the 3D scaffold into an in vitro rBMSCs model and a rat periodontal defect model. In this scaffold, PEG improved water wettability and degradation of PCL, while CS enhanced material adhesion and provided anti-inflammatory properties. Their findings indicated that the nanoscale arrangement of scaffolds offered cues for cell alignment, promoting the alignment of adhered cells in the same direction. Furthermore, the scaffold directed the osteoblastic differentiation of MSCs, consistent with results in the rat model.41 To further enhance hard tissue regeneration, Sowmya et al. introduced rhCEMP1, rhFGF2, and PRP-derived growth factors into a three-layer nanocomposite hydrogel scaffold containing nBGC and chitin-PLGA polymeric blend. They evaluated cementogenic, fibrogenic, and osteogenic differentiation of hDFCs on the scaffold and in rabbit periodontal defect models. Positive results were observed in groups with growth factors.42 As stem cells, including BMSCs, DFCs, and PDLSCs, possess robust osteogenic differentiation abilities, numerous studies are exploring the use of stem cells as a crucial component in periodontal regeneration.48,120 Consequently, the potential combination of stem cells and scaffolds holds promise for advancing tissue engineering in periodontitis treatment.
In the realm of guided bone regeneration therapy, membrane forms are widely accepted. Polymer membranes, in particular, have emerged as promising materials that overcome limitations associated with non-resorbable and weak membranes. These materials offer absorbability, high strength, and ease of use. Liao et al. employed nano HA/collagen/PLA (nHAC/PLA) as a barrier membrane to create osteoblast/complex constructs and nano carbonated hydroxyapatite/collagen (nCHAC) complexes as bioactive components. These materials effectively induced the differentiation of undifferentiated cells at the recipient site of the graft into osteoblasts and promotion of new bone formation.43 Hasani-Sadrabadi et al. developed a PDA-coated PCL (PDA-PCL) membrane modified with functional proteins, including cytokines, growth factors, and extracellular proteins, to precisely control the chemical characteristics of the matrix based on physical patterns, thereby facilitating periodontal tissue regeneration.44 Beyond traditional membrane forms, novel structures such as injectable sodium alginate hydrogel composites (CTP-SA) have also been investigated. These composites avoid the need for precise membrane tailoring before implantation. Xu et al. reported a CTP-SA doped with Cu2O nanoparticles and PDA-coated titanium dioxide (TiO2@PDA) nanoparticles. Under blue light irradiation, TiO2@PDA generated ROS, exhibiting antibacterial properties and oxidizing Cu+ in Cu2O nanoparticles to Cu2+, promoting osteogenesis. This technique allows CTP-SA to transform between antibacterial and osteogenic modes, offering a novel GTR strategy in terms of form and treatment cycle.45
3.2 Inorganic nanomaterials—metal, oxide, silica and inorganic nanosurface
Inorganic nanomaterials, encompassing metals, oxides, and silicates, play a pivotal role in the development of advanced periodontitis treatment strategies. Their synthesis and processing are relatively straightforward, allowing these materials to be reduced to nanoscale dimensions through physical and chemical methods. In this nanosized form, they can be effectively incorporated into various periodontitis treatment modalities. Moreover, introducing diverse surface morphologies through inorganic nanosurface modification provides terrain induction for cell migration and osteogenic regeneration at defect sites. These tailored surface features can guide cellular behavior, promoting more effective tissue integration and enhancing the regenerative processes essential for healing and repair. Notably, due to their distinctive physical properties, inorganic materials such as metals are particularly useful in aPDT, where they synergistically enhance the antibacterial effects of aPDT. This section explores the different subcategories of inorganic nanomaterials employed in periodontitis treatment, and the application of aPDT will be discussed in Section 3.2.5.3.2.1.1 Silver nanoparticles (AgNPs). AgNPs, characterized by their unique physicochemical properties,121 enhanced antibacterial activities,122 and excellent drug loading capabilities,123 demonstrated promise in dental therapy. In much of the research, AgNPs are typically synthesized using the chemical deoxidization method, where Ag+ ions in AgNO3 are chemically reduced to Ag0 atoms using NaBH4.124 However, concerns about the cost and toxicity associated with this chemical deoxidization method have been raised. To adopt a greener synthesis approach, researchers have turned to various natural products, leading to the production of diverse biosynthesized AgNPs, including those synthesized from plant extracts121 and by endophytic fungi Fusarium semitectum.125
AgNPs exhibit a broad-spectrum antibacterial effect, including drug-resistant strains. This effect is attributed to the release of Ag+ ions, which disrupt ATP molecules and cause direct damage to the cell membrane.16 Importantly, AgNPs have a low potential to induce antibacterial resistance.126 Due to their potent antibacterial activity, AgNPs are employed either independently or integrated into other nanostructures for early-stage antibacterial treatment of periodontitis.
In scenarios where AgNPs are applied separately for antibacterial therapy to enhance their biocompatibility, biodegradability, and cellular uptake, Venkatesan et al. developed chitosan-fucoidan complex-coated AgNPs. This approach aimed to leverage better drug encapsulation and cellular absorption abilities of chitosan-fucoidan polyelectrolytes/nanoparticles.46 However, the primary application of AgNPs is to serve as novel antibacterial agents within various nanomaterials, such as guided tissue regeneration and guided bone regeneration (GBR) scaffolds. Qian et al., for instance, introduced a novel silver-modified/collagen-coated electrospun PLGA/PCL scaffold (PP-pDA-Ag-COL) impregnated with AgNPs to impart antibacterial properties. In their study, the scaffolds can release silver ions out of AgNPs in a controlled speed, thus significantly inhibited the growth of S. mutans and Staphylococcus aureus, promoting both bacterial inhibition and the proliferation and differentiation of MC3T3 cells.28 Similarly, Constantin et al. incorporated AgNPs into a composite film synthesized from poly(vinyl alcohol) (PVA) cross-linked with oxidized chitosan (OxCS), demonstrating significant antimicrobial activity against S. aureus.65 Additionally, Lee et al. employed an epoxy lining to incorporate AgNPs onto the Polyetheretherketone (PEKK) surface and evaluated their antibacterial activity against P. gingivalis using the disc diffusion method. Their results indicated that 0.5% Ag-PEKK and Ag-PEEK exhibited diffusive properties that suppressed the growth of P. gingivalis.66 In summary, AgNPs' exceptional antibacterial properties position them as promising nanomaterials for the antibacterial treatment of periodontitis.
In addition to their remarkable antibacterial properties mentioned earlier, AgNPs can also serve as an effective drug delivery platform. By co-administering them with an antibacterial agent, it becomes possible to synergistically harness the antibacterial effects of both AgNPs and the medication, thereby significantly enhancing antibacterial treatment efficacy. Steckiewicz et al. conducted experiments in which they combined AgNPs with chlorhexidine (AgNPs-CHL) and metronidazole (AgNPs-PEG-MET) and assessed their antibacterial and anti-inflammatory properties in vitro. The results demonstrated their successful downgrading in the production of proinflammatory cytokines like IL-1β, and the inhibition of tissue degradation by cutting down the production of metalloproteinases MMP3 and MMP8.19
3.2.1.2 Gold. Gold nanostructures have gained prominence in biomedicine for their potential in treating diverse diseases.48 Unlike some metal nanoparticles that can induce oxidative stress and toxicity, gold nanostructures have exhibited favorable biocompatibility and cellular internalization, characterized by vesicular accumulation reminiscent of autophagosomes,127 possibly through a ROS-associated mechanism.128
In the context of inflammation control, gold nanostructures have demonstrated significant regulatory effects on relevant signaling pathways. Yuan et al. investigated a peptide-coated gold cluster (Au25Sv9) in an inflammatory osteoclast model and inflammation-induced bone destruction mice model.47 Their findings indicate that Au25Sv9 dose-dependently suppressed the secretion and transcriptional regulation of pro-inflammatory cytokines including TNF-α, IL-1β, and IL-6, while suppressing NF-κB activation. These results underscore the anti-inflammatory potential of the gold cluster in vitro and in vivo. Ni et al. synthesized gold nanoparticles (AuNPs) to examine their effects on macrophages of varying sizes (Fig. 5).48 Notably, AuNPs, particularly those with a diameter of 45 nm, promoted M2-related factors expression, such as Arg-1, IL-10, and TGF-β, while downregulating CD86, a surface marker associated with the M1 phenotype. This effect suggests the anti-inflammatory capability of AuNPs by promoting the transition of macrophages from M1 phenotype to M2 phenotype.
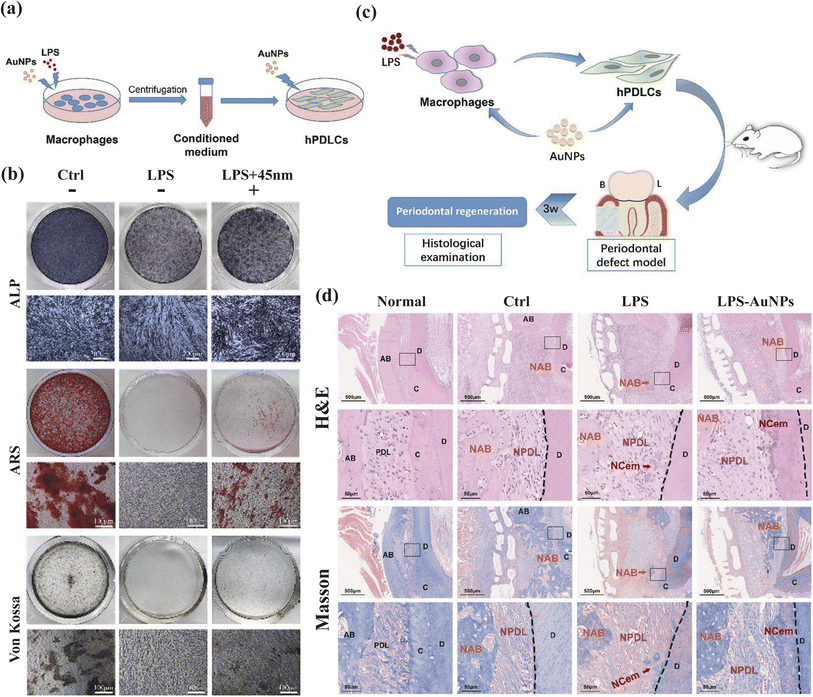 | ||
| Fig. 5 AuNPs modulate the crosstalk between macrophages and periodontal ligament cells for periodontitis treatment. (a and b) AuNPs promote osteogenic and cementogenic differentiation of hPDLCs in LPS-induced inflammatory conditions. (A) Schematic diagram. (b) ALP staining (day 7); ARS and Von Kossa staining (day 21). (c and d) The macrophage-hPDLCs coculture system modulated by AuNPs showed better periodontal regeneration potential in a rat periodontal defect model. (c) Schematic diagram. (B = buccal side, L = lingual side). (d) H&E and Masson staining (3 weeks). Adapted under the terms of the CC BY-NC-ND license 4.0 from ref. 48. Copyright 2019, Elsevier. | ||
Beyond their anti-inflammatory properties, gold nanostructures have gained recognition for their remarkable bone regeneration capabilities. Zhang's study revealed that the osteogenic differentiation function of AuNPs is size-dependent, inhibitory at 5 nm, and promotive at 45 nm.127 This differentiation was confirmed by the expression of osteogenic genes, that Alkaline Phosphatase (ALP) and Collagen Type I (COL1) were produced earlier than Osteopontin (OPN), Osteocalcin (OCN), conforming to the biological behavior of osteogenic differentiation.49 Zhang's team delved into the underlying mechanisms, demonstrating that 45 nm AuNPs activate the Wnt/β-catenin pathway, effectively enhancing hPDLSC proliferation,50 and promoting hPDLSC differentiation into osteoblasts through the ERK/AMPK pathway.51 Moreover, TEM images of AuNPs-treated PDLPs revealed an increase in autophagic vesicles, prompting an investigation into the autophagy pathway. In the 13 and 45 nm AuNPs treatment groups, heightened mRNA expression of LC3 and Beclin1, along with an increase in LC3-II, suggested autophagosome formation. Inhibition experiments at different autophagy stages reversed the AuNPs-induced osteogenesis, highlighting the link between autophagy and osteogenesis.127 Lastly, Zhang's team combined AuNPs with PDLSC sheets, harnessing the osteogenic induction ability of AuNPs alongside the accessibility and multipotency of PDLSC sheets, offering a novel strategy for treating bone defects.129
3.2.1.3 Cerium. In the field of biomedical research, nanomaterials incorporating cerium (Ce) have garnered significant attention due to their distinctive properties, notably the presence of abundant oxygen vacancies and the capability for transformation between Ce(III) and Ce(IV) ions. These unique characteristics position Ce-based nanomaterials as promising candidates for addressing oxidative stress-related ailments.130 Li et al. demonstrated the anti-oxidative potential of Ce-doped ZIF-8 nanoparticles (ZIF-8: Ce NPs) in periodontitis treatment, confirming their safety. These Ce-endowed ZIF-8 NPs exhibited superoxide dismutase (SOD) and catalase (CAT) enzyme mimic capability, facilitating ROS elimination, and thereby exerting multiple biological effects. These effects encompass protection against oxidative stress-induced cell damage through Zn2+ activity, modulation of pro-inflammatory mediators, and suppression of the NF-κB pathway, along with the induction of macrophage transition from the M1 to M2 phenotype.52 To further enhance the coordinated regulation of host immunity, researchers introduced the antioxidant drug quercetin onto nano-octahedral ceria, resulting in improved inflammation mitigation efficiency.53 Moreover, cerium oxide nanoparticles (CeO2 NPs) have demonstrated SOD and CAT mimetic activities, along with oxidase-like properties.131 Leveraging these distinctive attributes, CeO2 NPs have been applied in the treatment of periodontitis. Research has revealed that CeO2 NPs possess anti-inflammatory and antioxidant capabilities by suppressing the MAPK-NF-κB signaling pathway and upgrading the Nrf2-HO-1 pathway. These actions were accompanied by the regulation of associated protein expression in RAW 264.7 cell inflammation model induced by LPS, as well as a cut down in inflammation observed in a periodontitis rat model.54
3.2.2.1 Graphene oxide (GO). Graphene is a two-dimensional sheet of sp2-hybridized carbon, recognized as the thinnest and strongest element known.132 It is celebrated for its exceptional biocompatibility in the field of biomedicine. Furthermore, graphene exhibits potential antibacterial and tissue regenerative properties, rendering it a promising candidate for dental treatments, particularly in the context of periodontal disease.55,133 Due to its capacity for facile functionalization through various functional groups, multiple derivatives of graphene have been developed to attain specific properties suited to diverse applications. In dentistry, the primary focus has been on graphene, graphene oxide, graphene quantum dots (GQDs), and reduced graphene oxide (rGO) due to their remarkable antibacterial and tissue regenerative capabilities.
GO, in particular, stands out for its superior chemical stability and water solubility.134 Additionally, it maintains an atomic structure even thinner than graphene itself.135 He et al. produced GO nanosheets from natural graphite using a modified Hummers' method and assessed their antibacterial activity against P. gingivalis at varying concentrations. The results demonstrated a reduction in bacterial cell activity with increasing GO concentration, along with structural integrity loss in the cell membrane and cell wall, as confirmed by TEM images, thus highlighting its potent antimicrobial activity.55 To combine the antimicrobial properties of GO with its potential as a drug carrier, Trusek et al. attached amoxicillin (AMOX) to GO using a peptide linker (Leu-Leu-Gly). They dispersed this composite within a hydrogel containing the enzyme responsible for AMOX release from GO, resulting in the formation of GO-AMOX Alginate Capsules. This capsule exhibited excellent physical stability and showed no cytotoxicity. With the use of bromelain (BROM) as the enzyme, the capsule effectively released AMOX, holding significant medical potential.56
Furthermore, numerous studies have reported optimal cell adhesion and proliferation on plastic or glass plates coated with GO, garnering substantial interest in the field of tissue engineering.136 Vera-Sánchez et al. developed SF-GO nano-configurations as scaffold supports for human periodontal ligament stem cells (hPDLSCs). SF exhibited excellent compatibility with film-format GO, promoting cell proliferation and favoring osteo/cementoblast differentiation, particularly by stimulating cementum physiological synthesis.67 Park et al. fabricated P-GO scaffolds and assessed their impact on the adhesion, proliferation, as well as osteogenic differentiation of PDLSCs. GO was used as an osteogenic-inducing coating material to enhance the capacity of PCL scaffolds, the experimental group exhibited increased cell proliferation, greater presence of cytoskeletal elements and nuclei on the GO-coated scaffold surface, and a 60% increase in calcium deposition, underscoring its remarkable bioactivity and osteogenic differentiation potential.68
In addition to GO, other common nanomaterials used in the treatment of periodontitis include rGO and GQDs. Jabbari et al. fabricated chitosan/silk fibroin (CS/SF) membranes incorporating rGO as promising candidates for guided bone regeneration in bone tissue engineering.69 Moreover, GQDs coupled with curcumin (Cur) were found to enhance aPDT by suppressing periodontal pathogens in both planktonic and biofilm forms and downregulating the expression pattern of biofilm-related genes, as demonstrated by Pourhajibagher et al.57
3.2.2.2 Zinc(II) oxide (ZnO NPs). Metal oxide nanoparticles, especially when in nano-size, exhibit inherent properties including antibacterial efficacy and compatibility with human cells.137 These nanoparticles offer versatile and tunable shapes, achievable through various synthesis methods by modulating reaction conditions.138,139
ZnO nanoparticles are particularly renowned for their shape controllability, biocompatibility, and antimicrobial prowess. Recent investigations have predominantly concentrated on the integration of ZnO NPs into nanofibers,140 membrane,141 and scaffolds to confer antibacterial attributes. For instance, Mou et al. addressed the limitations of conventional minocycline treatments by developing a novel nanohydrogel, Mino-ZnO@Alb, which incorporates ZnO NPs into an albumin-based carrier.58 This formulation aims to optimize drug delivery through several innovative features. Firstly, ZnO NPs enhance the stability of the albumin nanoparticles (Alb NPs) and increase the encapsulation efficiency (EE) and loading capacity (LD) of minocycline. This is achieved through coordination bonding between ZnO and both albumin and the drug molecules, thereby improving drug retention and controlled release characteristics under physiological conditions. According to their experimental results, the Mino-ZnO@Alb nanohydrogel exhibits pH-responsiveness, enabling targeted drug release in acidic environments typical of infection sites. This feature enhances therapeutic efficacy while minimizing systemic side effects. The nanohydrogel also demonstrates a broad antimicrobial spectrum due to the combined effects of minocycline and ZnO NPs, making it suitable for combating infections caused by various pathogens. Furthermore, the formulation's biocompatibility and biodegradability ensure minimal cytotoxicity and potential for tissue repair, which are critical for applications in periodontal treatments and other localized therapies. By reducing the dosage of minocycline required for effective treatment, the nanohydrogel aims to enhance patient compliance and reduce the risk of systemic toxicity associated with conventional minocycline formulations.
Moreover, the application of ZnO NPs in aPDT has advanced to clinical research, as detailed in Section 3.2.5.
In the context of antibacterial therapy, MSNs have been harnessed for the transport of antibiotics such as meropenem,61 tetracycline,60 and vancomycin,62 all exhibit antibacterial effects against the corresponding pathogenic bacteria by carrying antibiotics. Furthermore, MSNs have been explored for the management of host inflammatory responses triggered by subgingival tooth biofilms. Baicalin (BA), a natural flavonoid with potent anti-inflammatory properties but limited solubility and bioavailability, has been effectively harnessed.143 Li et al. engineered a red-emissive MSNs-based nanosystem with CPD capping to precisely control the release of baicalin in response to glutathione. This system utilizes endocytosis and thiol-mediated mechanisms for cellular internalization, offering precise modulation of immuno-inflammatory responses.63 Also, resveratrol (RSV) has great potential in the therapy of diabetes periodontitis due to its effective antioxidant and anti-inflammatory properties, so it has become a heated topic to overcome its poor water solubility, fast decomposition, and short serum half-life.146 By grafting RSV onto MSNs (MSN-RSV), the bioavailability of RSV was enhanced, regulating macrophage polarization through the activation of the SIRT1/AMPK pathway and suppression of the NF-κB pathway. Additionally, MSN-RSV displayed the ability to modulate glucose metabolism, ameliorate insulin resistance (IR), and control glucose homeostasis.64
Furthermore, the synergy of MSNs with other drug delivery systems can yield combined effects. For instance, Lian et al. integrated copper-loaded MSNs (Cu@MSNs) into a PLGA/gelatin fiber matrix, creating a composite PG-Cu@MSNs fibrous scaffold for guided bone regeneration. This scaffold exhibits dual functionality, promoting both osteogenesis and antibacterial effects.70 MSNs can also serve as carriers for other nanomaterials, such as AgNPs, to construct composite nanostructures. Quaternary ammonium salts (QAS)-modified core–shell MSNs containing Ag nanoparticles (Ag@QHMS) demonstrate sustained Ag+ release, leading to effective and stable concentration-dependent antibacterial effects and enhancing osteogenic differentiation of BMSCs.71
Hu et al. successfully engineered titanium surfaces featuring hierarchical topographies comprising microgrooves and nanopores using a combination of selective laser melting (SLM) and alkali heat treatment (AHT). This innovative approach induced differentiation of PDLSCs by promoting actin cytoskeleton polymerization and activating the ERK and p38 MAPK signaling pathways. This, in turn, facilitated TAZ activation through nuclear translocation and increased transcriptional activity.73 Conversely, Yamada et al. adopted a biomimetic approach, seeking to replicate the morphology and physicochemical properties of living tissues.25,150 They crafted titanium surfaces mimicking tooth root cementum (TRC), creating a specialized topographical and mechanical microenvironment that regulates periodontal ligament cells and coordinates phosphate metabolism, ultimately inducing endogenous periodontium regeneration. In the realm of bioceramics, Mao's team prepared hybrid nanorod and microrod (mnHA) surfaces on hydroxyapatite bioceramics. These surfaces were found to possess the capacity to induce osteogenic and cementogenic differentiation of hPDLSCs through the Wnt pathway.26
The mechanism underlying aPDT consists of two pivotal components: a non-toxic light-absorbing dye (photosensitizer or PS) and visible light of an appropriate wavelength. In this process, the PS is activated by light, instigating a phototoxic reaction that generates ROS. These ROS, in turn, inflict damage on biomolecules and cellular structures through oxidation, culminating in the demise of microorganisms.152 Nevertheless, the accumulation of ROS post-aPDT can elevate oxidative stress in periodontal tissues, initiating adverse endogenous immune responses, and ultimately leading to cell death and tissue degradation.153 Consequently, managing the dual effects of ROS in aPDT has emerged as a critical challenge.
Li et al. addressed this challenge by combining Black phosphorus nanosheets (BPNSs) with ICG/aPDT, harnessing the potent ROS elimination capability and low cytotoxicity of BPNSs to safeguard periodontal structures.72 BPNSs, characterized by a two-dimensional folded bilayer structure along the Z-axis, facilitate rapid electron transport. Their elemental state promotes swift oxidation reactions, forming P–O bonds, which confer these nanomaterials with robust ROS consumption capabilities.154 Consequently, their research revealed that despite exerting antibacterial and anti-inflammatory capabilities, BPNSs also protect healthy cells from oxidative stress and accompanying inflammatory reactions induced by aPDT.
As previously discussed, CeO2 NPs possess the ability to combat chronic inflammation and oxidative stress.131 Hence, Sun et al. coupled CeO2 with the highly efficient red-light-excited photosensitizer Chlorin e6 (Ce6) through silane encapsulation, harnessing the SOD and CAT functions of CeO2 nanoparticles within the aPDT system. This innovative approach mitigated the issue of local ROS accumulation.18 Results demonstrated that CeO2@Ce6 NPs exhibited formidable sterilization and anti-inflammatory capabilities, validated through both in vitro and in vivo experiments.
Mathew et al. synthesized a nano ZnO gel containing ZnO NPs via a bio-hydrothermal method to minimize potential risks or hazards.59 Previous studies indicated its biocompatibility with no toxicity against red blood cells and mouse fibroblast cell lines, contrasting with moderate toxicity of conventional ZnO powder in vitro.155 This bio-synthesis approach suggests reduced ecological toxicity when compared to chemically synthesized ZnO. This study evaluated the efficacy of photodynamic therapy (PDT) using bio-hydrothermally synthesized nano ZnO gel combined with visible light as an adjunct to scaling and root planing (SRP) for treating periodontitis. Results showed significant improvement in periodontal health outcomes after 1 month with PDT compared to SRP alone. By 3 months, all adjunctive therapies, including PDT, demonstrated similar clinical parameter improvements and reduction in P. gingivalis levels, indicating sustained benefits in periodontal treatment. These findings underscore the potential of using nano ZnO gel and visible light PDT as adjunctive therapies for periodontitis, suggesting further validation through longitudinal studies with larger samples and extended follow-up to confirm efficacy and safety in clinical practice.
In summary, nanomaterials can play an important role in aPDT therapy with their unique structural, physical, or chemical properties.
4 Conclusions and perspectives
Periodontitis is a disease with complex pathogenesis, along with the bacterial and biological factor diversity of the human oral cavity. Therefore, the difficulty of the current treatment is to accurately target various biological mechanisms in the pathogenesis process and maximize the biological effects. This review briefly describes several typical nanostructures for periodontitis treatment and introduces their application scenarios, including direct therapeutic effects, drug loading, and combination with emerging treatment technologies. In summary, as a novel technology, nanomaterials are a valid therapeutic modality that is feasible in periodontitis treatment. However, it should be noted that the current technology still has limitations, such as manufacturing difficulties, high costs, limited efficacy, and especially the scarcity of clinical studies, which reveals that in vivo investigations are urgently needed. Hence, we hope that more in-depth research can be carried out in the academic community in the future to realize the market application of nanomaterials in periodontitis treatment.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was supported by the National Natural Science Foundation of China (82101077), Sichuan Science and Technology Program (2023NSFSC1516), Postdoctoral Science Foundation of China (2021M692271, 2023T160455), West China School/Hospital of Stomatology Sichuan University, no. RCDWJS2023-5, Fundamental Research Funds for the Central Universities, and Research and Develop Program, West China Hospital of Stomatology Sichuan University.References
- P. de Pablo, et al., Periodontitis in systemic rheumatic diseases, Nat. Rev. Rheumatol., 2009, 5(4), 218–224 CrossRef PubMed.
- M. X. Chen, et al., Global, regional, and national burden of severe periodontitis, 1990–2019: An analysis of the Global Burden of Disease Study 2019, J. Clin. Periodontol., 2021, 48(9), 1165–1188 CrossRef PubMed.
- L. Wu, et al., Global, regional, and national burden of periodontitis from 1990 to 2019: Results from the Global Burden of Disease study 2019, J. Periodontol., 2022, 93(10), 1445–1454 CrossRef PubMed.
- M. Di Stefano, et al., Impact of Oral Microbiome in Periodontal Health and Periodontitis: A Critical Review on Prevention and Treatment, Int. J. Mol. Sci., 2022, 23(9), 5142 CrossRef CAS PubMed.
- M. Sanz, et al., Periodontitis and cardiovascular diseases: Consensus report, J. Clin. Periodontol., 2020, 47(3), 268–288 CrossRef PubMed.
- P. M. Preshaw and S. M. Bissett, Periodontitis and diabetes, Br. Dent. J., 2019, 227(7), 577–584 CrossRef PubMed.
- F. Ceccarelli, et al., Periodontitis and Rheumatoid Arthritis: The Same Inflammatory Mediators?, Mediators Inflammation, 2019, 2019, 6034546 Search PubMed.
- M. A. Beydoun, et al., Helicobacter pylori, periodontal pathogens, and their interactive association with incident all-cause and Alzheimer's disease dementia in a large national survey, Mol. Psychiatry, 2021, 26(10), 6038–6053 CrossRef CAS PubMed.
- J. Deberin, et al., Antibodies to Oral Pathobionts and Colon Cancer Risk in the CLUE I Cohort Study, Int. J. Cancer, 2023, 153(2), 302–311 CrossRef PubMed.
- Y. T. Shi, et al., Ligature-Induced Periodontitis Drives Colorectal Cancer: An Experimental Model in Mice, J. Dent. Res., 2023, 102(6), 689–698 CrossRef CAS PubMed.
- E. J. Boother, et al., Cerebral Abscess Associated With Odontogenic Bacteremias, Hypoxemia, and Iron Loading in Immunocompetent Patients With Right-to-Left Shunting Through Pulmonary Arteriovenous Malformations, Clin. Infect. Dis., 2017, 65(4), 595–603 CrossRef CAS PubMed.
- G. D. Tribble and R. J. Lamont, Bacterial invasion of epithelial cells and spreading in periodontal tissue, Periodontol., 2010, 52(1), 68–83 CrossRef PubMed.
- S. K. Bhavikatti, S. Bhardwaj and M. L. Prabhuji, Current applications of nanotechnology in dentistry: a review, Gen Dent, 2014, 62(4), 72–77 Search PubMed.
- S. Gokila, et al., Development of 3D scaffolds using nanochitosan/silk-fibroin/hyaluronic acid biomaterials for tissue engineering applications, Int. J. Biol. Macromol., 2018, 120(Pt A), 876–885 Search PubMed.
- X. Wang, et al., Inorganic nanomaterials with rapid clearance for biomedical applications, Chem. Soc. Rev., 2021, 50(15), 8669–8742 RSC.
- S. Prasath and K. Palaniappan, Is using nanosilver mattresses/pillows safe? A review of potential health implications of silver nanoparticles on human health, Environ. Geochem. Health, 2019, 41(5), 2295–2313 CrossRef CAS PubMed.
- Z. Liu, et al., Nanofibrous Spongy Microspheres To Distinctly Release miRNA and Growth Factors To Enrich Regulatory T Cells and Rescue Periodontal Bone Loss, ACS Nano, 2018, 12(10), 9785–9799 CrossRef CAS PubMed.
- Y. Sun, et al., A versatile nanocomposite based on nanoceria for antibacterial enhancement and protection from aPDT-aggravated inflammation via modulation of macrophage polarization, Biomaterials, 2021, 268, 120614 CrossRef CAS PubMed.
- K. P. Steckiewicz, et al., Silver Nanoparticles as Chlorhexidine and Metronidazole Drug Delivery Platforms: Their Potential Use in Treating Periodontitis, Int. J. Nanomed., 2022, 17, 495–517 CrossRef CAS PubMed.
- Q. Wang, et al., Gold nanoparticles enhance proliferation and osteogenic differentiation of periodontal ligament stem cells by PINK1-mediated mitophagy, Arch. Oral Biol., 2023, 150, 105692 CrossRef CAS PubMed.
- Q. Yan, et al., Nanoparticles of Cerium-Doped Zeolitic Imidazolate Framework-8 Promote Soft Tissue Integration by Reprogramming the Metabolic Pathways of Macrophages, ACS Biomater. Sci. Eng., 2023, 9(7), 4241–4254 CrossRef CAS PubMed.
- W. Liao, et al., Antibacterial Collagen-Based Nanocomposite Dressings for Promoting Infected Wound Healing, Adv. Healthcare Mater., 2023, 12(15), e2203054 CrossRef PubMed.
- Y. Li, et al., Polydopamine-mediated graphene oxide and nanohydroxyapatite-incorporated conductive scaffold with an immunomodulatory ability accelerates periodontal bone regeneration in diabetes, Bioact. Mater., 2022, 18, 213–227 CAS.
- H. Wang, et al., Bioinspired drug-delivery system emulating the natural bone healing cascade for diabetic periodontal bone regeneration, Bioact. Mater., 2023, 21, 324–339 CAS.
- M. Yamada, et al., Titanium Nanosurface with a Biomimetic Physical Microenvironment to Induce Endogenous Regeneration of the Periodontium, ACS Appl. Mater. Interfaces, 2022, 14(24), 27703–27719 CrossRef CAS PubMed.
- L. Mao, et al., Effect of micro-nano-hybrid structured hydroxyapatite bioceramics on osteogenic and cementogenic differentiation of human periodontal ligament stem cell via Wnt signaling pathway, Int. J. Nanomed., 2015, 10, 7031–7044 CrossRef CAS PubMed.
- F. Di Cristo, et al., PLA Nanofibers for Microenvironmental-Responsive Quercetin Release in Local Periodontal Treatment, Molecules, 2022, 27(7), 2205 CrossRef CAS PubMed.
- Y. Qian, et al., Triple PLGA/PCL Scaffold Modification Including Silver Impregnation, Collagen Coating, and Electrospinning Significantly Improve Biocompatibility, Antimicrobial, and Osteogenic Properties for Orofacial Tissue Regeneration, ACS Appl. Mater. Interfaces, 2019, 11(41), 37381–37396 CrossRef CAS PubMed.
- R. Serôdio, et al., Ultrasound sonication prior to electrospinning tailors silk fibroin/PEO membranes for periodontal regeneration, Mater. Sci. Eng., C, 2019, 98, 969–981 CrossRef PubMed.
- Z. Shen, et al., Chitosan hydrogel incorporated with dental pulp stem cell-derived exosomes alleviates periodontitis in mice via a macrophage-dependent mechanism, Bioact. Mater., 2020, 5(4), 1113–1126 Search PubMed.
- Y. Liu, et al., Tetrahedral Framework Nucleic Acids Deliver Antimicrobial Peptides with Improved Effects and Less Susceptibility to Bacterial Degradation, Nano Lett., 2020, 20(5), 3602–3610 CrossRef CAS PubMed.
- Y. Zhang, et al., Inhibiting Methicillin-Resistant Staphylococcus aureus by Tetrahedral DNA Nanostructure-Enabled Antisense Peptide Nucleic Acid Delivery, Nano Lett., 2018, 18(9), 5652–5659 CrossRef CAS PubMed.
- Y. Zhang, et al., Multi-targeted Antisense Oligonucleotide Delivery by a Framework Nucleic Acid for Inhibiting Biofilm Formation and Virulence, Nanomicro Lett., 2020, 12(1), 74 CAS.
- Y. Sun, et al., Erythromycin loaded by tetrahedral framework nucleic acids are more antimicrobial sensitive against Escherichia coli (E. coli), Bioact. Mater., 2021, 6(8), 2281–2290 CAS.
- Y. Sun, et al., Tetrahedral Framework Nucleic Acids Loading Ampicillin Improve the Drug Susceptibility against Methicillin-Resistant Staphylococcus aureus, ACS Appl. Mater. Interfaces, 2020, 12(33), 36957–36966 CrossRef CAS PubMed.
- H. Hussein and A. Kishen, Engineered Chitosan-based Nanoparticles Modulate Macrophage-Periodontal Ligament Fibroblast Interactions in Biofilm-mediated Inflammation, J. Endod., 2021, 47(9), 1435–1444 CrossRef PubMed.
- Z. Hu, et al., An injectable gel based on photo-cross-linkable hyaluronic acid and mesoporous bioactive glass nanoparticles for periodontitis treatment, Int. J. Biol. Macromol., 2024, 257(Pt 1), 128596 CrossRef CAS PubMed.
- J. Chen, et al., The application of phenylboronic acid pinacol ester functionalized ROS-responsive multifunctional nanoparticles in the treatment of Periodontitis, J. Nanobiotechnol., 2024, 22(1), 181 CrossRef CAS PubMed.
- X. Zheng, et al., A facile strategy to construct silk fibroin based GTR membranes with appropriate mechanical performance and enhanced osteogenic capacity, J. Mater. Chem. B, 2020, 8(45), 10407–10415 RSC.
- J. Song, et al., Electrospun Nanofibrous Silk Fibroin Membranes Containing Gelatin Nanospheres for Controlled Delivery of Biomolecules, Adv. Healthcare Mater., 2017, 6(14), 1700014 CrossRef PubMed.
- W. Jiang, et al., Incorporation of aligned PCL-PEG nanofibers into porous chitosan scaffolds improved the orientation of collagen fibers in regenerated periodontium, Acta Biomater., 2015, 25, 240–252 CrossRef CAS PubMed.
- S. Sowmya, et al., Tri-Layered Nanocomposite Hydrogel Scaffold for the Concurrent Regeneration of Cementum, Periodontal Ligament, and Alveolar Bone, Adv. Healthcare Mater., 2017, 6(7), 1601251 CrossRef PubMed.
- S. Liao, et al., A three-layered nano-carbonated hydroxyapatite/collagen/PLGA composite membrane for guided tissue regeneration, Biomaterials, 2005, 26(36), 7564–7571 CrossRef CAS PubMed.
- M. M. Hasani-Sadrabadi, et al., Hierarchically Patterned Polydopamine-Containing Membranes for Periodontal Tissue Engineering, ACS Nano, 2019, 13(4), 3830–3838 CrossRef CAS PubMed.
- Y. Xu, et al., Jelly-Inspired Injectable Guided Tissue Regeneration Strategy with Shape Auto-Matched and Dual-Light-Defined Antibacterial/Osteogenic Pattern Switch Properties, ACS Appl. Mater. Interfaces, 2020, 12(49), 54497–54506 CrossRef CAS PubMed.
- J. Venkatesan, et al., Preparation, Characterization and Biological Applications of Biosynthesized Silver Nanoparticles with Chitosan-Fucoidan Coating, Molecules, 2018, 23(6), 1429 CrossRef PubMed.
- Q. Yuan, et al., Gold Clusters Prevent Inflammation-Induced Bone Erosion through Inhibiting the Activation of NF-κB Pathway, Theranostics, 2019, 9(7), 1825–1836 CrossRef CAS PubMed.
- C. Ni, et al., Gold nanoparticles modulate the crosstalk between macrophages and periodontal ligament cells for periodontitis treatment, Biomaterials, 2019, 206, 115–132 CrossRef CAS PubMed.
- S. Patil and S. Paul, A comprehensive review on the role of various materials in the osteogenic differentiation of mesenchymal stem cells with a special focus on the association of heat shock proteins and nanoparticles, Cells Tissues Organs, 2014, 199(2–3), 81–102 CrossRef CAS PubMed.
- C. Li, et al., The role of the Wnt/β-catenin signaling pathway in the proliferation of gold nanoparticle-treated human periodontal ligament stem cells, Stem Cell Res. Ther., 2018, 9(1), 214 CrossRef CAS PubMed.
- C. Niu, et al., Gold nanoparticles promote osteogenic differentiation of human periodontal ligament stem cells via the p38 MAPK signaling pathway, Mol. Med. Rep., 2017, 16(4), 4879–4886 CrossRef CAS PubMed.
- X. Li, et al., Novel nanoparticles of cerium-doped zeolitic imidazolate frameworks with dual benefits of antibacterial and anti-inflammatory functions against periodontitis, J. Mater. Chem. B, 2019, 7(44), 6955–6971 RSC.
- Y. Wang, et al., Quercetin-Loaded Ceria Nanocomposite Potentiate Dual-Directional Immunoregulation via Macrophage Polarization against Periodontal Inflammation, Small, 2021, 17(41), e2101505 CrossRef PubMed.
- Y. Yu, et al., Cerium oxide nanozyme attenuates periodontal bone destruction by inhibiting the ROS-NFκB pathway, Nanoscale, 2022, 14(7), 2628–2637 RSC.
- J. He, et al., Killing dental pathogens using antibacterial graphene oxide, ACS Appl. Mater. Interfaces, 2015, 7(9), 5605–5611 CrossRef CAS PubMed.
- A. Trusek and E. Kijak, Drug Carriers Based on Graphene Oxide and Hydrogel: Opportunities and Challenges in Infection Control Tested by Amoxicillin Release, Materials, 2021, 14(12), 3182 CrossRef CAS PubMed.
- M. Pourhajibagher, et al., Photoexcitation triggering via semiconductor Graphene Quantum Dots by photochemical doping with Curcumin versus perio-pathogens mixed biofilms, Photodiagn. Photodyn. Ther., 2019, 28, 125–131 CrossRef CAS PubMed.
- J. Mou, et al., Hydrogel containing minocycline and zinc oxide-loaded serum albumin nanopartical for periodontitis application: preparation, characterization and evaluation, Drug Delivery, 2019, 26(1), 179–187 CrossRef CAS PubMed.
- C. A. Mathew, et al., Antimicrobial photocatalysis using bio-hydrothermally synthesized Zinc oxide nanoparticles in the management of periodontitis: a prospective split-mouth, double-blind, randomized, controlled clinical trial, J. Appl. Oral Sci., 2023, 31, e20230271 CrossRef CAS PubMed.
- B. Koneru, et al., Tetracycline-Containing MCM-41 Mesoporous Silica Nanoparticles for the Treatment of Escherichia coli, Molecules, 2015, 20(11), 19690–19698 CrossRef CAS PubMed.
- M. Y. Memar, et al., Biocompatibility, cytotoxicity and antibacterial effects of meropenem-loaded mesoporous silica nanoparticles against carbapenem-resistant Enterobacteriaceae, Artif. Cells, Nanomed., Biotechnol., 2020, 48(1), 1354–1361 CrossRef CAS PubMed.
- M. Y. Memar, et al., Antibacterial and biofilm-inhibitory effects of vancomycin-loaded mesoporous silica nanoparticles on methicillin-resistant staphylococcus aureus and gram-negative bacteria, Arch. Microbiol., 2023, 205(4), 109 CrossRef CAS PubMed.
- X. Li, et al., A glutathione-responsive silica-based nanosystem capped with in-situ polymerized cell-penetrating poly(disulfide)s for precisely modulating immuno-inflammatory responses, J. Colloid Interface Sci., 2022, 614, 322–336 CrossRef CAS PubMed.
- Y. Tan, et al., Grafting resveratrol onto mesoporous silica nanoparticles towards efficient sustainable immunoregulation and insulin resistance alleviation for diabetic periodontitis therapy, J. Mater. Chem. B, 2022, 10(25), 4840–4855 RSC.
- M. Constantin, et al., PVA/Chitosan Thin Films Containing Silver Nanoparticles and Ibuprofen for the Treatment of Periodontal Disease, Polymers, 2022, 15(1), 4 CrossRef PubMed.
- W. F. Lee, et al., Characterization and Antibacterial Properties of Polyetherketoneketone Coated with a Silver Nanoparticle-in-Epoxy Lining, Polymers, 2022, 14(14), 2906 CrossRef CAS PubMed.
- M. Vera-Sánchez, et al., Silk-Fibroin and Graphene Oxide Composites Promote Human Periodontal Ligament Stem Cell Spontaneous Differentiation into Osteo/Cementoblast-Like Cells, Stem Cells Dev., 2016, 25(22), 1742–1754 CrossRef PubMed.
- J. Park, et al., Enhanced Osteogenic Differentiation of Periodontal Ligament Stem Cells Using a Graphene Oxide-Coated Poly(ε-caprolactone) Scaffold, Polymers, 2021, 13(5), 797 CrossRef CAS PubMed.
- F. Jabbari, S. Hesaraki and B. Houshmand, The physical, mechanical, and biological properties of silk fibroin/chitosan/reduced graphene oxide composite membranes for guided bone regeneration, J. Biomater. Sci., Polym. Ed., 2019, 30(18), 1779–1802 CrossRef CAS PubMed.
- M. Lian, et al., A multifunctional electrowritten bi-layered scaffold for guided bone regeneration, Acta Biomater., 2020, 118, 83–99 CrossRef CAS PubMed.
- D. Li, et al., A Multifunctional Antibacterial and Osteogenic Nanomedicine: QAS-Modified Core-Shell Mesoporous Silica Containing Ag Nanoparticles, BioMed Res. Int., 2020, 2020, 4567049 Search PubMed.
- X. Li, et al., Combined Black Phosphorus Nanosheets with ICG/aPDT is an Effective Anti-Inflammatory Treatment for Periodontal Disorders, Int. J. Nanomed., 2023, 18, 813–827 CrossRef CAS PubMed.
- P. Hu, et al., The Role and Activation Mechanism of TAZ in Hierarchical Microgroove/Nanopore Topography-Mediated Regulation of Stem Cell Differentiation, Int. J. Nanomed., 2021, 16, 1021–1036 CrossRef PubMed.
- S. Filoche, L. Wong and C. H. Sissons, Oral biofilms: emerging concepts in microbial ecology, J. Dent. Res., 2010, 89(1), 8–18 CrossRef CAS PubMed.
- R. C. Page, The role of inflammatory mediators in the pathogenesis of periodontal disease, J. Periodontal Res., 1991, 26(3 Pt 2), 230–242 CrossRef CAS PubMed.
- P. Yudaev, et al., Polymeric Dental Nanomaterials: Antimicrobial Action, Polymers, 2022, 14(5), 864 CrossRef CAS PubMed.
- X. Xu, et al., Nanosilicate-functionalized nanofibrous membrane facilitated periodontal regeneration potential by harnessing periodontal ligament cell-mediated osteogenesis and immunomodulation, J. Nanobiotechnol., 2023, 21(1), 223 CrossRef CAS PubMed.
- Q. Ou, et al., More natural more better: triple natural anti-oxidant puerarin/ferulic acid/polydopamine incorporated hydrogel for wound healing, J. Nanobiotechnol., 2021, 19(1), 237 CrossRef CAS PubMed.
- B. L. Pihlstrom, B. S. Michalowicz and N. W. Johnson, Periodontal diseases, Lancet, 2005, 366(9499), 1809–1820 CrossRef PubMed.
- M. Zhou, et al., The protective effect of tetrahedral framework nucleic acids on periodontium under inflammatory conditions, Bioact. Mater., 2021, 6(6), 1676–1688 CAS.
- Q. Li, et al., Aptamer-Modified Tetrahedral DNA Nanostructure for Tumor-Targeted Drug Delivery, ACS Appl. Mater. Interfaces, 2017, 9(42), 36695–36701 CrossRef CAS PubMed.
- A. S. Walsh, et al., DNA cage delivery to mammalian cells, ACS Nano, 2011, 5(7), 5427–5432 CrossRef CAS PubMed.
- Q. Zhang, et al., Anti-inflammatory and Antioxidative Effects of Tetrahedral DNA Nanostructures via the Modulation of Macrophage Responses, ACS Appl. Mater. Interfaces, 2018, 10(4), 3421–3430 CrossRef CAS PubMed.
- W. Ma, et al., Self-Assembled Tetrahedral DNA Nanostructures Promote Neural Stem Cell Proliferation and Neuronal Differentiation, ACS Appl. Mater. Interfaces, 2018, 10(9), 7892–7900 CrossRef CAS PubMed.
- T. Zhang, et al., Design, fabrication and applications of tetrahedral DNA nanostructure-based multifunctional complexes in drug delivery and biomedical treatment, Nat. Protoc., 2020, 15(8), 2728–2757 CrossRef CAS PubMed.
- H. Lee, et al., Scolopendin 2, a cationic antimicrobial peptide from centipede, and its membrane-active mechanism, Biochim. Biophys. Acta, 2015, 1848(2), 634–642 CrossRef CAS PubMed.
- D. I. Andersson, D. Hughes and J. Z. Kubicek-Sutherland, Mechanisms and consequences of bacterial resistance to antimicrobial peptides, Drug Resistance Updates, 2016, 26, 43–57 CrossRef CAS PubMed.
- X. R. Shao, et al., Effect of tetrahedral DNA nanostructures on osteogenic differentiation of mesenchymal stem cells via activation of the Wnt/β-catenin signaling pathway, Nanomedicine, 2017, 13(5), 1809–1819 CrossRef CAS PubMed.
- M. Zhou, et al., Effect of tetrahedral DNA nanostructures on proliferation and osteo/odontogenic differentiation of dental pulp stem cells via activation of the notch signaling pathway, Nanomedicine, 2018, 14(4), 1227–1236 CrossRef CAS PubMed.
- F. Wang, T. Zuroske and J. K. Watts, RNA therapeutics on the rise, Nat. Rev. Drug Discovery, 2020, 19(7), 441–442 CrossRef CAS PubMed.
- L. Hong, H. Sun and B. A. Amendt, MicroRNA function in craniofacial bone formation, regeneration and repair, Bone, 2021, 144, 115789 CrossRef CAS PubMed.
- S. Li, et al., Bioswitchable Delivery of microRNA by Framework Nucleic Acids: Application to Bone Regeneration, Small, 2021, 17(47), e2104359 CrossRef PubMed.
- M. M. Islam, et al., Chitosan based bioactive materials in tissue engineering applications-A review, Bioact. Mater., 2020, 5(1), 164–183 Search PubMed.
- D. Fong and C. D. Hoemann, Chitosan immunomodulatory properties: perspectives on the impact of structural properties and dosage, Future Sci. OA, 2018, 4(1), Fso225 CrossRef CAS PubMed.
- Z. Gu, et al., Preparation of chitosan/silk fibroin blending membrane fixed with alginate dialdehyde for wound dressing, Int. J. Biol. Macromol., 2013, 58, 121–126 CrossRef CAS PubMed.
- G. Atia, et al., Drug-Loaded Chitosan Scaffolds for Periodontal Tissue Regeneration, Polymers, 2022, 14(15), 3192 CrossRef CAS PubMed.
- S. Senel, et al., Chitosan films and hydrogels of chlorhexidine gluconate for oral mucosal delivery, Int. J. Pharm., 2000, 193(2), 197–203 CrossRef CAS PubMed.
- M. C. G. Pellá, et al., Chitosan-based hydrogels: From preparation to biomedical applications, Carbohydr. Polym., 2018, 196, 233–245 CrossRef PubMed.
- A. Aguilar, et al., Application of Chitosan in Bone and Dental Engineering, Molecules, 2019, 24(16), 3009 CrossRef CAS PubMed.
- R. C. Gupta, et al., Hyaluronic Acid: Molecular Mechanisms and Therapeutic Trajectory, Front. Vet. Sci., 2019, 6, 192 CrossRef PubMed.
- M. Chen, et al., High molecular weight hyaluronic acid regulates P. gingivalis-induced inflammation and migration in human gingival fibroblasts via MAPK and NF-κB signaling pathway, Arch. Oral Biol., 2019, 98, 75–80 CrossRef CAS PubMed.
- A. Ardizzoni, et al., Influence of hyaluronic acid on bacterial and fungal species, including clinically relevant opportunistic pathogens, J. Mater. Sci.: Mater. Med., 2011, 22(10), 2329–2338 CrossRef CAS PubMed.
- I. Olszewska-Czyz, et al., The Influence of Hyaluronic Acid Adjunctive Therapy of Periodontitis on Salivary Markers of Oxidative Stress: Randomized, Controlled Clinical Trial, Antioxidants, 2022, 11(1), 135 CrossRef CAS PubMed.
- S. A. Unterman, et al., Hyaluronic acid-binding scaffold for articular cartilage repair, Tissue Eng., Part A, 2012, 18(23–24), 2497–2506 CrossRef CAS PubMed.
- K. S. Masters, et al., Crosslinked hyaluronan scaffolds as a biologically active carrier for valvular interstitial cells, Biomaterials, 2005, 26(15), 2517–2525 CrossRef CAS PubMed.
- F. A. Alshehri and M. S. Alharbi, The Effect of Adjunctive Use of Hyaluronic Acid on Prevalence of Porphyromonas gingivalis in Subgingival Biofilm in Patients with Chronic Periodontitis: A Systematic Review, Pharmaceutics, 2023, 15(7), 1883 CrossRef CAS PubMed.
- W. L. Hynes and S. L. Walton, Hyaluronidases of Gram-positive bacteria, FEMS Microbiol. Lett., 2000, 183(2), 201–207 CrossRef CAS PubMed.
- L. Safonova, et al., Silk Fibroin/Spidroin Electrospun Scaffolds for Full-Thickness Skin Wound Healing in Rats, Pharmaceutics, 2021, 13(10), 1704 CrossRef CAS PubMed.
- A. Giménez-Siurana, et al., Chemoprevention of Experimental Periodontitis in Diabetic Rats with Silk Fibroin Nanoparticles Loaded with Resveratrol, Antioxidants, 2020, 9(1), 85 CrossRef PubMed.
- C. Holland, et al., Natural and unnatural silks, Polymer, 2007, 48(12), 3388–3392 CrossRef CAS.
- S. Bai, et al., Bioinspired Mineral–Organic Bone Adhesives for Stable Fracture Fixation and Accelerated Bone Regeneration, Adv. Funct. Mater., 2020, 30(5), 1908381 CrossRef CAS.
- P. Zhao, et al., Electrospun Nanofibers for Periodontal Treatment: A Recent Progress, Int. J. Nanomed., 2022, 17, 4137–4162 CrossRef CAS PubMed.
- M. Y. Mahmoud, J. M. Steinbach-Rankins and D. R. Demuth, Functional assessment of peptide-modified PLGA nanoparticles against oral biofilms in a murine model of periodontitis, J. Controlled Release, 2019, 297, 3–13 CrossRef CAS PubMed.
- E. Malikmammadov, et al., PCL and PCL-based materials in biomedical applications, J. Biomater. Sci., Polym. Ed., 2018, 29(7–9), 863–893 CrossRef CAS PubMed.
- J. P. Binotto, et al., Poly (Lactic Acid) membrane and Sedum dendroideum extract favors the repair of burns in rats, Acta Cir. Bras., 2020, 35(3), e202000302 CrossRef PubMed.
- J. M. Lü, et al., Current advances in research and clinical applications of PLGA-based nanotechnology, Expert Rev. Mol. Diagn., 2009, 9(4), 325–341 CrossRef PubMed.
- H. D. Kim, et al., Biomimetic Materials and Fabrication Approaches for Bone Tissue Engineering, Adv. Healthcare Mater., 2017, 6(23), 1700612 CrossRef PubMed.
- J. Han, et al., Stem cells, tissue engineering and periodontal regeneration, Aust. Dent. J., 2014, 59(Suppl 1), 117–130 CrossRef PubMed.
- Y. Ren, et al., Barrier Membranes for Guided Bone Regeneration (GBR): A Focus on Recent Advances in Collagen Membranes, Int. J. Mol. Sci., 2022, 23(23), 14987 CrossRef CAS PubMed.
- L. Wang, et al., Bone marrow mesenchymal stem cell sheets with high expression of hBD3 and CTGF promote periodontal regeneration, Biomater. Adv., 2022, 133, 112657 CrossRef CAS PubMed.
- O. Ahmed, et al., Plant Extract-Synthesized Silver Nanoparticles for Application in Dental Therapy, Pharmaceutics, 2022, 14(2), 380 CrossRef CAS PubMed.
- R. A. Bapat, et al., An overview of application of silver nanoparticles for biomaterials in dentistry, Mater. Sci. Eng., C, 2018, 91, 881–898 CrossRef CAS PubMed.
- S. J. Lee, et al., Electrospun chitosan nanofibers with controlled levels of silver nanoparticles. Preparation, characterization and antibacterial activity, Carbohydr. Polym., 2014, 111, 530–537 CrossRef CAS PubMed.
- Y. Xu, et al., Silver nanoparticles promote osteogenic differentiation of human periodontal ligament fibroblasts by regulating the RhoA-TAZ axis, Cell Biol. Int., 2019, 43(8), 910–920 CrossRef CAS PubMed.
- K. R. Halkai, et al., Antibiofilm efficacy of biosynthesized silver nanoparticles against endodontic-periodontal pathogens: An in vitro study, J. Conservative Dent., 2018, 21(6), 662–666 CrossRef CAS PubMed.
- J. J. Yan, et al., The Sequence Characteristics and Expression Models Reveal Superoxide Dismutase Involved in Cold Response and Fruiting Body Development in Volvariella volvacea, Int. J. Mol. Sci., 2016, 17(1), 34 CrossRef PubMed.
- Y. Zhang, et al., Size-dependent Effects of Gold Nanoparticles on Osteogenic Differentiation of Human Periodontal Ligament Progenitor Cells, Theranostics, 2017, 7(5), 1214–1224 CrossRef CAS PubMed.
- J. J. Li, et al., Autophagy and oxidative stress associated with gold nanoparticles, Biomaterials, 2010, 31(23), 5996–6003 CrossRef CAS PubMed.
- Y. Zhang, et al., Gold Nanoparticles Promote the Bone Regeneration of Periodontal Ligament Stem Cell Sheets Through Activation of Autophagy, Int. J. Nanomed., 2021, 16, 61–73 CrossRef PubMed.
- C. Mahapatra, et al., Nano-shape varied cerium oxide nanomaterials rescue human dental stem cells from oxidative insult through intracellular or extracellular actions, Acta Biomater., 2017, 50, 142–153 CrossRef CAS PubMed.
- Z. Chen, et al., Enzyme Mimicry for Combating Bacteria and Biofilms, Acc. Chem. Res., 2018, 51(3), 789–799 CrossRef CAS PubMed.
- M. J. Allen, V. C. Tung and R. B. Kaner, Honeycomb carbon: a review of graphene, Chem. Rev., 2010, 110(1), 132–145 CrossRef CAS PubMed.
- R. Guazzo, et al., Graphene-Based Nanomaterials for Tissue Engineering in the Dental Field, Nanomaterials, 2018, 8(5), 349 CrossRef PubMed.
- Y. Zhu, et al., Graphene and graphene oxide: synthesis, properties, and applications, Adv. Mater., 2010, 22(35), 3906–3924 CrossRef CAS PubMed.
- H. Y. Jeong, et al., Graphene oxide thin films for flexible nonvolatile memory applications, Nano Lett., 2010, 10(11), 4381–4386 CrossRef CAS PubMed.
- S. Goenka, V. Sant and S. Sant, Graphene-based nanomaterials for drug delivery and tissue engineering, J. Controlled Release, 2014, 173, 75–88 CrossRef CAS PubMed.
- N. Padmavathy and R. Vijayaraghavan, Enhanced bioactivity of ZnO nanoparticles-an antimicrobial study, Sci. Technol. Adv. Mater., 2008, 9(3), 035004 CrossRef PubMed.
- J. Wojnarowicz, et al., Effect of Microwave Radiation Power on the Size of Aggregates of ZnO NPs Prepared Using Microwave Solvothermal Synthesis, Nanomaterials, 2018, 8(5), 343 CrossRef PubMed.
- X. Bai, et al., Solvothermal synthesis of ZnO nanoparticles and anti-infection application in vivo, ACS Appl. Mater. Interfaces, 2015, 7(2), 1308–1317 CrossRef CAS PubMed.
- A. M. Dias, et al., Polycaprolactone nanofibers loaded oxytetracycline hydrochloride and zinc oxide for treatment of periodontal disease, Mater. Sci. Eng., C, 2019, 103, 109798 CrossRef CAS PubMed.
- E. A. Münchow, et al., Development and characterization of novel ZnO-loaded electrospun membranes for periodontal regeneration, Dent. Mater., 2015, 31(9), 1038–1051 CrossRef PubMed.
- M. Gisbert-Garzarán, D. Lozano and M. Vallet-Regí, Mesoporous Silica Nanoparticles for Targeting Subcellular Organelles, Int. J. Mol. Sci., 2020, 21(24), 9696 CrossRef PubMed.
- X. Li, et al., Nanoparticle-encapsulated baicalein markedly modulates pro-inflammatory response in gingival epithelial cells, Nanoscale, 2017, 9(35), 12897–12907 RSC.
- D. Gkiliopoulos, et al., SBA-15 Mesoporous Silica as Delivery Vehicle for rhBMP-2 Bone Morphogenic Protein for Dental Applications, Nanomaterials, 2022, 12(5), 822 CrossRef CAS PubMed.
- S. Sharifi, et al., Effect of Curcumin-Loaded Mesoporous Silica Nanoparticles on the Head and Neck Cancer Cell Line, HN5, Curr. Issues Mol. Biol., 2022, 44(11), 5247–5259 CrossRef CAS PubMed.
- G. Bhattarai, et al., Resveratrol prevents alveolar bone loss in an experimental rat model of periodontitis, Acta Biomater., 2016, 29, 398–408 CrossRef CAS PubMed.
- P. Capellato, S. E. A. Camargo and D. Sachs, Biological Response to Nanosurface Modification on Metallic Biomaterials, Curr. Osteoporos. Rep., 2020, 18(6), 790–795 CrossRef PubMed.
- H. Fan and Z. Guo, Bioinspired surfaces with wettability: biomolecule adhesion behaviors, Biomater. Sci., 2020, 8(6), 1502–1535 RSC.
- C. Zhao, et al., The role of the micro-pattern and nano-topography of hydroxyapatite bioceramics on stimulating osteogenic differentiation of mesenchymal stem cells, Acta Biomater., 2018, 73, 509–521 CrossRef CAS PubMed.
- J. Hwang, et al., Biomimetics: forecasting the future of science, engineering, and medicine, Int. J. Nanomed., 2015, 10, 5701–5713 CAS.
- F. Cieplik, et al., Antimicrobial photodynamic therapy for inactivation of biofilms formed by oral key pathogens, Front. Microbiol., 2014, 5, 405 Search PubMed.
- E. T. Carrera, et al., The application of antimicrobial photodynamic therapy (aPDT) in dentistry: a critical review, Laser Phys., 2016, 26(12), 123001 CrossRef PubMed.
- J. Zhou, et al., Fusobacterium nucleatum Accelerates Atherosclerosis via Macrophage-Driven Aberrant Proinflammatory Response and Lipid Metabolism, Front. Microbiol., 2022, 13, 798685 CrossRef PubMed.
- L. Qin, et al., Black phosphorus nanosheets and gemcitabine encapsulated thermo-sensitive hydrogel for synergistic photothermal-chemotherapy, J. Colloid Interface Sci., 2019, 556, 232–238 CrossRef CAS PubMed.
- P. Shubha, et al., In vitro and In vivo evaluation of green-hydrothermal synthesized ZnO nanoparticles, J. Drug Delivery Sci. Technol., 2019, 49, 692–699 CrossRef CAS.
Footnote |
| † Ruijianghan Shi and Yujie Zhu contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |
