DOI:
10.1039/D4RE00597J
(Paper)
React. Chem. Eng., 2025,
10, 1038-1047
Residence time distribution effects on continuous-flow reaction in a polymer gel-based porous monolith: investigation of an asymmetric reaction with supported Hayashi–Jørgensen catalysts†
Received
6th December 2024
, Accepted 30th December 2024
First published on 30th December 2024
Abstract
Immobilized catalysts are easy to reuse and applicable to continuous-flow reactions, but their catalytic activity decreases due to poor diffusivity of reactants. To mitigate the molecular diffusion resistance, it is important to design support materials that boost the diffusion of reactants toward the catalyst. Gels are polymers consisting of cross-linked networks that swell in aqueous and/or organic solvents. The gels are insoluble, like solids, but create a unique reaction environment within the network, where the molecular diffusivity is quite fast, as in a homogeneous system. In this respect, monolithic porous gels (MPGs) composed of organic polymer have been developed for continuous-flow reactors to mitigate the diffusion resistance of the reactants. However, the effects of the gel properties (e.g. cross-linking structure) on the molecular diffusivity through the MPG under continuous-flow conditions have not been quantitatively evaluated so far. Here, we prepared MPGs supporting Hayashi–Jørgensen catalysts and assessed the diffusivity within the gel on the basis of the residence time distribution, as well as on the catalytic performance in Michael addition reactions. The MPG with low cross-linking density exhibited a high swelling porosity (εswollen), which corresponded to fast molecular diffusion of the reactants. The MPG with the lowest cross-linking density demonstrated the highest diffusivity and superior turnover frequency (εswollen = 100%, TOF = 2.4 h−1) in the continuous-flow reactions.
Introduction
In recent years, continuous-flow synthesis of fine chemicals has garnered significant interest.1–3 In continuous-flow systems, efficient heat and mass transfer, precise control of production amounts, automation, and safe operation can be achieved, offering many advantages over batch systems.4,5 Asymmetric reactions are important chemical transformations for fine chemicals, such as pharmaceuticals, agrochemicals, and fragrances.6,7 For efficient asymmetric reactions under continuous-flow conditions, immobilized catalysts supporting chiral molecules have attracted much interest. Since the catalysts are retained in the continuous-flow reactor, catalytic reaction and separation of products from the catalyst are achieved simultaneously, resulting in high process efficiency.
For continuous-flow reactions using immobilized catalysts, porous materials have emerged as promising supports. Due to the large surface area and high porosity of these porous materials, fast mass transport and efficient catalytic reactions have been realized.8,9 Among these, porous monoliths provide a single support with a flow-through pore network.10,11 The monoliths have flow-through macropores that allow fast permeation of fluid. Owing to the narrow pore-size distribution, mass transport of solutes through the macropores is relatively uniform and close to an ideal plug flow. These characteristics of monolith reactors are advantageous compared with packed-bed reactors.12 Moreover, it has been reported that the formulation of micro/mesopores on the monolith surface provides a hierarchical porous structure, which increases the number of active sites and hence the catalytic efficiency.13–15 Since the micro/mesopores are primarily accessible by diffusion, the mass transport of reactants in these pores is severely limited by diffusion resistance. As a result, some catalysts located on these pores are virtually inaccessible under continuous-flow conditions. In this case, introduction of micro/mesoporosity into the monoliths will not directly improve the reactor performance as expected, due to the low catalytic efficiency in the unavailable pores. To enhance catalytic efficiency and reactor performance, porous monolith catalysts with fast molecular diffusion into the micro/mesopores are needed.
Gels are polymer materials consisting of cross-linked networks that swell in good solvents. The gels are insoluble in the solvents and exhibit similarly to solid materials. Furthermore, the swollen network of the gel provides microporosity toward small solutes, which facilitates molecular diffusion in the matrix.16,17 It has been reported that solute diffusion through the gel can be considered to be as high as that in a solution.18 These unique features depend on the physical properties of the gel, which vary with the cross-linking structure. To date, polymer gel catalysts have been developed as immobilized catalysts for asymmetric reactions, which show easy recoverability and excellent catalytic efficiency.19–21 However, applications of gel catalysts to continuous-flow asymmetric reactions are scarce.22
In this respect, our research group has developed monolithic porous gels (MPGs), which are applicable to continuous-flow catalytic reactions. The MPGs are porous monoliths consisting of polymer gels that swell in the reaction media (Fig. 1). The MPGs have not only macropores, but also micropores, derived from the swollen gel network. This hierarchical porous structure of the MPG gives two different pathways for reactants. In the macropores, the reactants flow by convection of the reaction media. At the same time, the reactants rapidly transfer into the gel matrix by diffusion during the flow. As catalysts are immobilized in the gel network, the reactants can easily contact with the catalysts to form the desired products. Finally, the desired products diffuse out from the gel network and flow out of the reactor. Using the MPGs, excellent catalytic efficiencies have been realized in Suzuki–Miyaura cross-couplings23,24 and asymmetric aldol additions25 featuring fast molecular diffusion in the gel network. It is expected that the physical properties of the gel network in the MPGs contribute greatly to the accessibility toward the catalysts, and should vary with the cross-linking structure of the gel. Investigation of flow behavior through MPGs with different cross-linking structures would give insight into the mass transport in the continuous-flow reaction. However, quantitative studies on the effect of the cross-linking structure of MPGs on their catalytic performance have not been investigated so far.
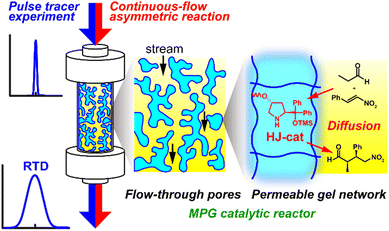 |
| | Fig. 1 Schematic of continuous-flow reactors using MPGs with different degrees of cross-linking. Mass transport of the reactants was estimated based on the RTD through the MPG reactors. | |
In this study, a series of the MPG reactors with different cross-linked structures was prepared, and the correlation between mass transport phenomena and catalytic performance was investigated (Fig. 1). As a model catalyst, the Hayashi–Jørgensen catalyst (HJ-cat) was chosen because of its broad applicability and high durability in asymmetric synthesis of important fine chemicals, such as γ-amino acid derivatives.26–28 Through copolymerization of the styryl monomer carrying HJ-cat (HJ-cat monomer), styrene, and divinylbenzene (DVB), an MPG supporting the HJ-cat was prepared. Owing to the flexibility of the polystyrene chains, this MPG swelled in organic solvents, such as toluene. In addition, varying the feed ratio of the DVB as a cross-linker provided MPGs with different degrees of cross-linking, which would show different swelling abilities in the organic solvent. For the flow behavior in the MPGs, the residence time distribution (RTD) was evaluated by pulse tracer experiments, which were correlated with the catalytic performance in the asymmetric Michael addition of propionaldehyde to trans-β-nitrostyrene under continuous-flow conditions.
Experimental
General
The samples for field emission scanning electron microscopy (FE-SEM) analysis were coated with platinum (approx. 4 nm thickness) using a JEOL JFC-1600 auto fine coater (JEOL Ltd., Tokyo, Japan). FE-SEM analysis was performed on a Hitachi SU8000 microscope (Hitachi High-Technologies Corporation, Tokyo, Japan). Mercury intrusion porosimetry (MIP) analysis was performed on a Micromeritics AutoPoreIV9520 (Micromeritics Instrument Co., Norcross, GA, USA). Elemental analysis (EA) was performed on a Yanako MT-6 CHN recorder (Yanaco Technical Science Co., Ltd., Tokyo, Japan). Solution nuclear magnetic resonance (NMR) spectra were recorded on a JEOL ECZ400S spectroscopy, operating at 400 MHz for 1H NMR. Chemical shift values for the 1H NMR spectra were referenced to Me4Si. High-performance liquid chromatography (HPLC) analyses were performed on a JASCO LC-2000Plus system equipped with a JASCO DG-980-50 degasser, a JASCO PU-980 pump, a Kanto Chemical Mightysil RP-18 GP 250-4.6 column (Kanto Chemical Co., Tokyo, Japan), a JASCO UV-2077Plus UV detector, and a JASCO CO-2065Plus column oven (JASCO Co., Tokyo, Japan). Acetonitrile/water (60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v) containing trifluoroacetic acid (0.1 vol%) was employed as a mobile phase in the HPLC measurements (1.0 mL min−1). HPLC analyses for enantiomeric excess were performed on a JASCO LC-2000Plus system equipped with a JASCO DG-980-50 degasser, a JASCO PU-986 pump, a CHIRALPAK OD-H 250-4.6 column (Daicel Co., Osaka, Japan), a JASCO MD-4010 photo diode array detector, and a JASCO CO-2060Plus column oven (JASCO Co., Tokyo, Japan). 2-Propanol/hexane (6.2
40 v/v) containing trifluoroacetic acid (0.1 vol%) was employed as a mobile phase in the HPLC measurements (1.0 mL min−1). HPLC analyses for enantiomeric excess were performed on a JASCO LC-2000Plus system equipped with a JASCO DG-980-50 degasser, a JASCO PU-986 pump, a CHIRALPAK OD-H 250-4.6 column (Daicel Co., Osaka, Japan), a JASCO MD-4010 photo diode array detector, and a JASCO CO-2060Plus column oven (JASCO Co., Tokyo, Japan). 2-Propanol/hexane (6.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 93.8 v/v) was employed as a mobile phase in the HPLC measurements (0.75 mL min−1).
93.8 v/v) was employed as a mobile phase in the HPLC measurements (0.75 mL min−1).
In the continuous-flow setup, an SGE glass gas-tight syringe (SGE Analytical Science Pty. Ltd., Melbourne, Australia), which was mounted on a YMC YSP-101 syringe pump (YMC Co. Ltd., Kyoto, Japan), and a Krone KDM-30 pressure gauge (Krone Co., Tokyo, Japan) were connected to the column with PTFE or PFA tubing (0.75 mm inner diameter) in a thermo-controlled incubator. For RTD studies, a FLOM VI-11 injection valve (FLOM Co., Tokyo, Japan) with a 10 μL loop, an Ocean Optics USB2000+ ultraviolet-visible (UV-vis) spectrometer (Ocean Optics Inc., Dunedin, FL, USA), an Ocean Optics tungsten halogen light source (Ocean Optics Inc.), and an FIA-Z-SMA-TEF Z-type flow cell (Ocean Optics Inc.) were included in the continuous-flow setup.
Materials were obtained from commercial suppliers unless otherwise noted. Styrene (>99.0% GC purity, stabilized with 4-tert-butylcatechol, Tokyo Chemical Industry Co., Ltd., Tokyo, Japan) and divinylbenzene (DVB) (3- and 4- mixture, contains ethylvinylbenzene and ethylbenzene, >50.0% GC purity, stabilized with 4-tert-butylcatechol, Tokyo Chemical Industry Co., Ltd.) were purified using a basic alumina column. 2,2′-Azo-bis(isobutyronitrile) (AIBN) (98.0% absorptiometry purity, FUJIFILM Wako Pure Chemical Co., Osaka, Japan) was purified by recrystallization from MeOH. The HJ-cat monomer was synthesized according to previous reports (Fig. S1, see ESI† for details).29
Preparation and characterization of MPGs
HJ-cat monomer (10 wt%), DVB (X = 20, 40, or 60 wt%), and styrene (90–X wt%) were used as the catalyst moiety, cross-linker, and gel matrix monomers, respectively (Scheme 1a and b). The total weight of these monomers was 300 mg. The monomers were admixed with a mixture solvent of toluene/1-decanol (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 w/w, 700 mg) and completely dissolved by shaking. AIBN (3 mg, 1 wt% with respect to the monomers) was added to the mixture and completely dissolved. The resulting monomer solution was degassed by N2 bubbling for 30 min and poured into a home-made column (stainless steel, 4.4 mm inner diameter (d) × 5.0 cm length (L)). After sealing with stainless steel plugs at both column ends, the column was heated in an incubator at 70 °C for 72 h. After the polymerization, the MPG column was washed by permeation of tetrahydrofuran (THF) for more than 1 h (2.972 mL h−1). For characterization of the MPG, the MPG was dried under vacuum and extruded from the column to give white solids.
70 w/w, 700 mg) and completely dissolved by shaking. AIBN (3 mg, 1 wt% with respect to the monomers) was added to the mixture and completely dissolved. The resulting monomer solution was degassed by N2 bubbling for 30 min and poured into a home-made column (stainless steel, 4.4 mm inner diameter (d) × 5.0 cm length (L)). After sealing with stainless steel plugs at both column ends, the column was heated in an incubator at 70 °C for 72 h. After the polymerization, the MPG column was washed by permeation of tetrahydrofuran (THF) for more than 1 h (2.972 mL h−1). For characterization of the MPG, the MPG was dried under vacuum and extruded from the column to give white solids.
 |
| | Scheme 1 (a) Synthesis of MPG supporting HJ-cat. (b) Preparation of MPGs with different cross-linking degree. (c) Continuous-flow setup for asymmetric Michael addition of propionaldehyde to trans-β-nitrostyrene. | |
Permeability test for continuous-flow reactors
After polymerization and washing with THF, the MPG column was connected to the syringe pump and pressure gauge. Superficial velocity (u) was defined as follows:| | 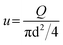 | (1) |
where Q is the flow rate of the feed. As a mobile phase, toluene was permeated through the MPG at various u, and the pressure loss (ΔP) was monitored. At steady state, the Darcy's permeability (kD) of the MPG was estimated as follows:| | 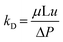 | (2) |
where μ is the viscosity of the feed solution.
Pulse tracer experiment for continuous-flow reactors
RTD in the continuous-flow system was evaluated using a pulse tracer. The MPG column was provided with fittings and attached to a syringe pump, injection valve of tracer, UV-vis spectrometer, deuterium halogen light source, and Z-type flow cell.30 As a mobile phase, toluene was continuously permeated through the MPG column (Q = 0.743 mL h−1). Methyl red (in EtOH, 1 g L−1) as a pulse tracer (10 μL) was injected into the stream from the injection valve. At the outlet of the column, UV-vis absorbance at 427-nm wavelength at each time (t) was measured to monitor the concentration of the tracer in the eluent (C(t)). RTD function (E(t)) and residence time (τ) were calculated as follows:| | 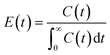 | (3) |
| | 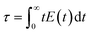 | (4) |
To evaluate deviation from ideal plug flow, the elution curves were treated with dimensionless numbers. Dimensionless time (θ) and dimensionless RTD function (E(θ)) were calculated as follows:| |  | (5) |
For parametric RTD analysis, the dimensionless variance (σ2) was further introduced, as follows:| |  | (7) |
Typical procedures for continuous-flow Michael addition
Michael addition of propionaldehyde to trans-β-nitrostyrene was performed under continuous-flow conditions using the MPG reactor (Scheme 1c). trans-β-Nitrostyrene (0.2 mol L−1, 1.0 eq.), propionaldehyde (0.3 mol L−1, 1.5 eq.), p-nitrophenol (0.02 mol L−1, 10 mol%), and nitrobenzene (0.024 mol L−1, 12 mol%, internal standard) were dissolved in toluene. The substrate solution was loaded in a glass syringe, and the syringe was attached to a syringe pump. The reactor was provided with fittings and attached to the syringe pump. At the control u, the substrate solution was continuously permeated through the MPG at 50 °C in a thermo-controlled incubator. The total elution volume of the substrate solution was normalized to be column volume (CV) as follows:| | 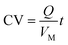 | (8) |
The elution from the reactor was continuously collected and analyzed using HPLC (Fig. S3 and S4†) to determine the yield of product, turnover number (TON), turnover frequency (TOF), space–time yield (STY), enantiomeric excess (ee), and diastereomeric ratio (d.r.), as follows:| | 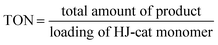 | (9) |
| |  | (10) |
| |  | (11) |
| |  | (12) |
| |  | (13) |
where tpeak corresponds to the elution time at a peak top in the RTD curves. The reaction mixture was purified by normal-phase silica gel column chromatography (ethyl acetate/hexane), and the chemical structure of the product was identified using 1H NMR (Fig. S2†).
Results and discussion
Characterization of MPG
The MPGs supporting HJ-cat were prepared through polymerization-induced phase separation in the presence of porogenic solvent (toluene/1-decanol).31 To clarify the effect of swelling ability of the MPGs on the molecular diffusivity within the gel network, the different cross-linked structures in the MPGs were fabricated by varying the degree of cross-linking. The feed ratio of the DVB (X = 20, 40, or 60 wt%) was tuned to prepare a series of MPGs with different degrees of cross-linking (MPG20, MPG40, and MPG60). Further decreasing the degree of cross-linking of the monolith would not be suitable due to significant swelling and low permeability (described in a later section). All the MPGs were obtained as white solids with high yields (90–93% yield, Table S1, see ESI†). From EA for nitrogen, loading of HJ-cat in the MPGs was estimated to be 0.23–0.28 mmol g−1 (Table S1†). FE-SEM confirmed that the dried MPG consisted of aggregates of primary particles with diameters of 0.5–2.0 μm and interconnected pores between the particles (Fig. 2a–c). MIP revealed that all the MPGs had narrow and monomodal pore size (Fig. 2e). The median pore diameters (Dmed) of the MPG20, MPG40, and MPG60 were 2190, 800, and 340 nm, respectively (Table S2†). The porosity of the dried MPGs (εdry) was high enough to be 81–82%. Higher cross-linking degree caused decreasing sizes of the primary particles and pores, suggesting phase separation at an early stage due to suppressed swelling by cross-linking. Since all the MPGs possessed large through pores and high porosity, high permeability and excellent mass transfer could be obtained in continuous-flow systems.
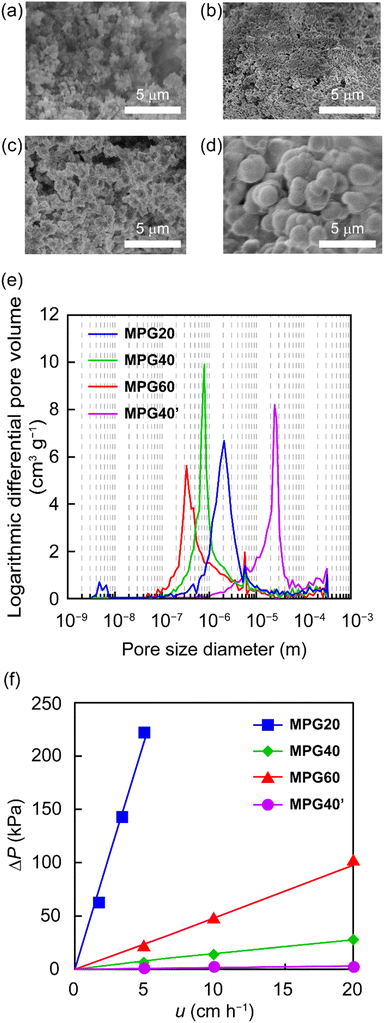 |
| | Fig. 2 FE-SEM images of (a) MPG20, (b) MPG40, (c) MPG60, and (d) MPG40′. (e) Pore-size distribution determined by MIP. (f) Continuous-flow permeability of the MPG reactors. Conditions: mobile phase = toluene, room temperature. | |
Permeability of the MPG column
To evaluate the permeability of the MPGs in the continuous-flow system, toluene was fed through the column, and the ΔP at steady state was measured. Within the measured range, ΔP was proportional to u (Fig. 2f). Using Darcy's law, the values of kD for MPG20, MPG40, and MPG60 were calculated to be 1.8 × 10−15, 5.6 × 10−14, and 1.8 × 10−14 m2, respectively. It has been reported that the kD value of packed-bed silica particles ranges from 10−15 to 10−16 m2.32 The MPG40 and MPG60 exhibit excellent permeability, although the kD value for MPG20 was one order of magnitude lower. This might be due to significant swelling of MPG20 in toluene due to less cross-linking, resulting in a reduced pore size in the swollen state. On the other hand, ΔP was proportional to u within the measured range, suggesting the absence of deformation of the MPG by hydrodynamic pressure. Using the MPG reactors, catalytic reactions in the continuous-flow systems could be successfully realized.
RTD and molecular diffusivity of pulse tracer through the MPG column
Mass transfer phenomena in the MPG reactors were evaluated using pulse tracer experiments with an in-line UV-vis spectrometer. Toluene was continuously fed to the MPG column (Q = 0.743 mL h−1) and a pulse tracer (methyl red) was injected at the inlet of the MPG column. The absorbance of the tracer was continuously monitored with the in-line UV-vis spectrometer at a wavelength of 427 nm. In the elution curve of the tracer, a single peak was obtained for all the MPGs (Fig. 3a). The σ2 values of the RTDs for the MPGs were calculated for deviation from an ideal plug flow (Fig. 3b). For MPG20, MPG40, and MPG60, the σ2 values were 0.015, 0.018, and 0.124, respectively (Table 1). MPG20 and MPG40 exhibited narrow RTDs, which were close to that of ideal plug flow.33 On the other hand, MPG60 showed a relatively broad RTD (Fig. 3b).
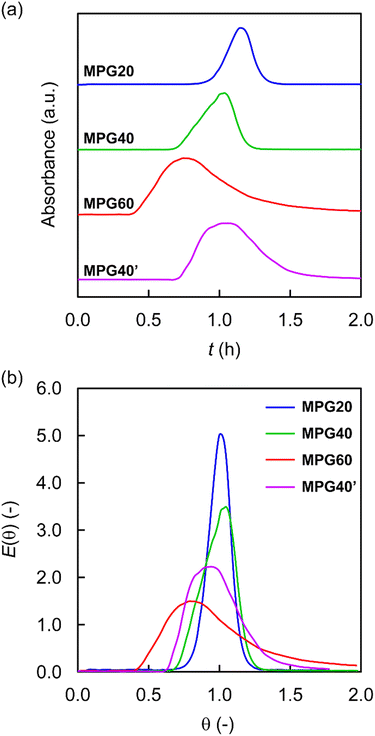 |
| | Fig. 3 (a) Elution curves and (b) dimensionless RTD curves in pulse tracer experiments for the MPG columns. Conditions: tracer = methyl red (in EtOH, 1 g L−1, 10 μL), mobile phase = toluene. | |
Table 1 RTD variance (σ2), porosity in swollen state (εswollen), diastereomeric ratio (d.r.), turnover frequency (TOF), and space–time yield (STY) for the MPGs
| Catalyst |
DVB [wt%] |
σ
2
[−] |
ε
swollen
[%] |
d.r.b [%] |
TOFb [h−1] |
|
Determined from the pulse tracer experiments.
Determined at 6 CV during the continuous-flow Michael additions.
|
|
MPG20
|
20 |
0.015 |
100 |
82 |
2.7 |
|
MPG40
|
40 |
0.018 |
89 |
81 |
2.3 |
|
MPG60
|
60 |
0.124 |
60 |
84 |
1.9 |
|
MPG40′
|
40 |
0.036 |
89 |
81 |
2.3 |
The molecular diffusion through the gel network of the MPGs was further evaluated from the RTD curves. It has been reported that the molecular diffusion of small molecules through the swollen gel is as fast as that in bulk liquid.18 Hence, under continuous-flow conditions, tracer molecules could flow through the volume fraction of the swollen gel network in the MPG column. Using the elution time of the tracer, the porosity of the swollen MPG (εswollen) was calculated as follows:34
| |  | (14) |
where
VM and
Vd are the swollen
MPG volume in the column and the dead volume from the injection valve to the UV-vis detector (0.113 mL), respectively. The
εswollen values for
MPG20,
MPG40, and
MPG60 were calculated as 100, 89, and 60%, respectively (
Table 1). Notably, the
MPG with a lower cross-linking degree exhibited a higher
εswollen.
The amazingly high porosity in the swollen MPGs (up to 100% εswollen) indicated that the large volume fraction of the MPG column was accessible under continuous-flow conditions. The polystyrene-based MPGs functioned as organogels, which swelled in toluene. The swelling behavior of the MPG column allowed the small molecules to be transported to the interior of the gel network by diffusion under continuous-flow conditions. Furthermore, decreasing the cross-linking degree of the MPGs increased the εswollen owing to low cross-linking of the gel network and good swelling in toluene. The swollen nature of the low-cross-linked MPGs accelerated the molecular diffusion of solutes through the gel network.23–25 Additionally, the low-cross-linked gel might contribute to the small σ2, which is close to ideal plug flow, owing to faster molecular diffusion. The narrow RTDs and fast molecular diffusion in the MPG columns could dramatically reduce the mass transfer resistance to catalytic sites, and hence lead to excellent catalytic efficiency in continuous-flow reactions.
Catalytic performance of MPG reactors in Michael addition
The catalytic performance of the MPG columns was evaluated in the asymmetric Michael addition of propionaldehyde (0.2 mol L−1, 1.0 eq.) to trans-β-nitrostyrene (0.3 mol L−1, 1.5 eq.) in toluene under continuous-flow conditions (Scheme 1c). Since the elution curves of the tracer showed different tpeak through each MPG column, the flow rate was adjusted to set the tpeak to 1.0 h, in order to fix a mean residence time. Along with permeation of the substrate solution, the yield of γ-nitroaldehyde gradually increased and reached an almost constant value within 6 CV (Fig. 4). The yields at steady state were 74–79%, 62–67%, and 41–49% for MPG20, MPG40, and MPG60, respectively. In addition, all the MPGs processed asymmetric Michael additions with high selectivity, to give a d.r. of 81–84% (Fig. 4 and Table 1). The ee was 91% for MPG20 in 1–6 CV. At 6 CV, the TOFs for MPG20, MPG40, and MPG60 were estimated to be 2.7, 2.3, and 1.9 h−1, respectively (Table 1). To evaluate the productivity of the MPG reactors, STYs were calculated. At 6 CV, the STYs for MPG20, MPG40, and MPG60 were 167, 123, and 55 mmol h−1 L−1, respectively (Table 1). Interestingly, decreasing the DVB content resulted in dramatic improvements, with 1.4- and 3.0-fold increases in the TOF and STY, respectively. Clearly, the higher STYs of the MPGs strongly relate to the higher εswollen of the gel.
 |
| | Fig. 4 Yield and d.r. in the continuous-flow Michael addition with the MPG reactors. | |
In the continuous-flow Michael addition, a decreased degree of cross-linking of the MPG significantly increased the catalytic activity (Fig. 4). From the RTD tests, a lower degree of cross-linking of the MPG exhibited a higher εswollen, meaning that a larger volume fraction of the gel network was available for molecular diffusion of the reactants. As a result, efficient contact between catalysts and reactants was realized within the gel network of the MPG, leading to an excellent TOF. Furthermore, a higher εswollen of the MPG corresponded to larger accessibility in the reactor volume available for catalytic reactions. This feature of the MPGs minimized the inaccessible volume of the reactor, which significantly improved the STY with increase in the εswollen (Fig. 5a and b). Design of immobilized catalysts based on a swollen support with higher εswollen is a powerful method for enhancing the catalytic efficiency and productivity of the continuous-flow reactors.
 |
| | Fig. 5 (a) STY vs. εswollen diagram. (b) Schematic of the MPGs with different cross-linking degree for high TOF and STY in continuous-flow reactions. | |
Effect of porous structure of the MPG on catalytic performance
The effects of the surface morphologies of the MPGs on the catalytic performance were confirmed. Compared with the original MPG, a reference MPG with different macroporous structures but the same degree of cross-linking in the gel was prepared. For preparation of the reference MPG, the composition of the porogenic solvent was varied (toluene/1-decanol = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 w/w), while the feed ratio of the DVB was fixed at 40 wt%. Compared with MPG40, the resulting MPG40′ was expected to have a different macroporous structure but the same degree of cross-linking (X = 40 wt%). The MPG40′ showed a smaller specific surface area than MPG40 (1 vs. 17 m2 g−1, Table S2†). The pulse experiment was performed, giving an εswollen of 89% for MPG40′ (Fig. 3 and Table 1), which was the same as for the original MPG40 (89%). The σ2 of MPG40′ (0.036, Table 1) was slightly larger than that of the MPG40 (0.018). In the continuous-flow Michael addition, MPG40′ exhibited comparable TOF, STY, and d.r. (2.3 h−1, 106 mmol h−1 L−1, and 81%, Fig. 4 and Table 1) values to those of MPG40 (2.3 h−1, 123 mmol h−1 L−1, and 81%). These results suggested little effect of surface morphology on the molecular diffusivity through the MPG and on catalytic performance in continuous-flow reactions.
80 w/w), while the feed ratio of the DVB was fixed at 40 wt%. Compared with MPG40, the resulting MPG40′ was expected to have a different macroporous structure but the same degree of cross-linking (X = 40 wt%). The MPG40′ showed a smaller specific surface area than MPG40 (1 vs. 17 m2 g−1, Table S2†). The pulse experiment was performed, giving an εswollen of 89% for MPG40′ (Fig. 3 and Table 1), which was the same as for the original MPG40 (89%). The σ2 of MPG40′ (0.036, Table 1) was slightly larger than that of the MPG40 (0.018). In the continuous-flow Michael addition, MPG40′ exhibited comparable TOF, STY, and d.r. (2.3 h−1, 106 mmol h−1 L−1, and 81%, Fig. 4 and Table 1) values to those of MPG40 (2.3 h−1, 123 mmol h−1 L−1, and 81%). These results suggested little effect of surface morphology on the molecular diffusivity through the MPG and on catalytic performance in continuous-flow reactions.
Since the surface area of the MPG had little influence on its catalytic performance, it could be anticipated that most of the catalytic sites were preferentially located in the interior rather than on the exterior of the gel phase in the MPGs.35,36 In this case, efficient access of reactants toward the interior of the gel network in the MPGs improved the catalytic activity due to the increased number of the catalysts. Thus, the degree of cross-linking of the MPGs had a critical effect on their catalytic efficiency and reactor performance in the continuous-flow asymmetric reaction.
Comparison of MPG reactors with GP reactors
Finally, to emphasize the importance of the porous nature and monolithic structure of the MPG, a reference catalyst was prepared from gel particles (GPs). The polystyrene-based GP supporting HJ-cat was synthesized through a suspension copolymerization process (DVB 40 wt%, Fig. S5, see ESI†). The resulting GP was sieved into particles ranging in size from 420 to 640 μm in diameter, and packed into a stainless steel column. In the RTD curve for the GP column, a broader peak was obtained than for the MPG40 column (Fig. 6a). The σ2 value for the GP column was calculated as 0.086, which was larger than that for the MPG40 column (0.018, Fig. 6b). Additionally, the εswollen for the GP column was estimated to be 65%, which is fairly low compared to that of MPG40 (89%). In the continuous-flow Michael addition, the d.r. and TOF for the GP column were 77–81% and 1.9 h−1, respectively. The higher TOF of the MPG40 (2.3 h−1, Table 1) than for GP (1.9 h−1) was noted. Moreover, the STY for the GP column (99 mmol h−1 L−1, Fig. 6c) was lower than that for MPG40 (123 mmol h−1 L−1). The narrow pore-size distribution (Fig. 2e) and the small primary particles (0.5–2.0 μm diameters, Fig. 2a–d) of the MPG were beneficial for a narrow RTD and fast diffusivity through the gel network. These characteristic of the MPG contribute to its amazingly high εswollen, resulting in the high catalytic efficiency and productivity of the reactor.
 |
| | Fig. 6 (a) Elution curves and (b) dimensionless RTD curves of pulse tracers. (c) STY of MPG40 and the GP in the continuous-flow Michael addition. The MPG40 and GP were prepared with the same feed ratio of DVB content (40 wt%) as that for MPG40. | |
Conclusions
In conclusion, monolithic porous gels, MPGs, were developed as support materials for immobilization of HJ-cat in continuous-flow asymmetric catalysis. Pulse tracer experiments were performed to determine RTD and molecular diffusivity of the reactants through the MPG reactor. The variance, σ2, and porosity in swollen state, εswollen, were calculated, and greatly influenced the catalytic performance in the continuous-flow Michael addition. The MPG with the lowest degree of cross-linking achieved both a small σ2 and large εswollen, demonstrating ideal plug flow and remarkably high molecular diffusivity. This led to dramatic improvement in the catalytic performance of the MPG column, where the TOF and STY were up to 1.4 and 3.0 times higher, respectively. This was attributed to the increased number of effective catalytic sites owing to enhanced molecular diffusivity through the gel network in the MPG catalysts. These findings provide a design criterion relating to swelling of immobilized catalysts for improving the catalytic performance of continuous-flow reactors.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its ESI† materials.
Author contributions
H. Shigeeda, H. Matsumoto, and Y. Miura designed the experiments. H. Shigeeda performed the experiments. H. Shigeeda, H. Matsumoto, and Y. Miura wrote the manuscript. M. Nagao made contributions to the discussions during this work. All authors have given approval to the final version of the manuscript.
Conflicts of interest
There are no conflicts to declare.
Acknowledgements
The authors greatly appreciate Prof. Yujiro Hayashi for his significant contribution to the synthesis of the HJ-cat monomer and fruitful discussions during this work. This work was supported by JSPS KAKENHI grant numbers JP24K17560, JP23K26708, JP23H02015, JP22H04553, JP22H05372, and JP22K19068, the Sumitomo Foundation, the Yashima Environment Technology Foundation, and the Tobemaki Foundation.
References
- S. V. Ley, Y. Chen, A. Robinson, B. Otter, E. Godineau and C. Battilocchio, A comment on continuous flow technologies within the agrochemical industry, Org. Process Res. Dev., 2021, 25, 713–720 CrossRef CAS.
- A. Gioiello, A. Piccinno, A. M. Lozza and B. Cerra, The medicinal chemistry in the era of machines and automation: recent advances in continuous flow technology, J. Med. Chem., 2020, 63, 6624–6647 CrossRef CAS PubMed.
- G. Gambacorta, J. S. Sharley and I. R. Baxendale, A comprehensive review of flow chemistry techniques tailored to the flavours and fragrances industries, Beilstein J. Org. Chem., 2021, 17, 1181–1312 CrossRef CAS PubMed.
- J. Britton and C. L. Raston, Multi-step continuous-flow synthesis, Chem. Soc. Rev., 2017, 46, 1250–1271 RSC.
- R. L. Hartman, J. P. McMullen and K. F. Jensen, Deciding Whether To Go with the Flow: Evaluating the Merits of Flow Reactors for Synthesis, Angew. Chem., Int. Ed., 2011, 50, 7502–7519 CrossRef CAS PubMed.
- C. Wiles and P. Watts, Continuous flow reactors: a perspective, Green Chem., 2012, 14, 38–54 RSC.
- R. Porta, M. Benaglia and A. Puglisi, Flow chemistry: recent develop- ments in the synthesis of pharmaceutical products, Org. Process Res. Dev., 2016, 20, 2–25 CrossRef CAS.
- P. Kumar, A. Das and B. Maji, Phosphorus containing porous organic polymers: synthetic techniques and applications in organic synthesis and catalysis, Org. Biomol. Chem., 2021, 19, 4174–4192 RSC.
- T. Drake, P. Ji and W. Lin, Site Isolation in Metal–Organic Frameworks Enables Novel Transition Metal Catalysis, Acc. Chem. Res., 2018, 51, 2129–2138 CrossRef CAS PubMed.
- F. Svec and J. M. J. Frechet, New Designs of Macroporous Polymers and Supports: From Separation to Biocatalysis, Science, 1996, 273, 205–211 CrossRef CAS PubMed.
- F. Svec, Porous polymer monoliths: amazingly wide variety of techniques enabling their preparation, J. Chromatogr. A, 2010, 1217, 902–924 CrossRef CAS PubMed.
- V. Chiroli, M. Benaglia, A. Puglisi, R. Porta, R. P. Jumde and A. Mandoli, A chiral organocatalytic polymer-based monolithic reactor, Green Chem., 2014, 16, 2798–2806 RSC.
- A. Sachse, A. Galarneau, F. Fajula, F. Di Renzo, P. Creux and B. Coq, Functional silica monoliths with hierarchical uniform porosity as continuous flow catalytic reactors, Microporous Mesoporous Mater., 2011, 140(1–3), 58–68 CrossRef CAS.
- J. Lee and J. Y. Chang, A hierarchically porous catalytic monolith prepared from a Pickering high internal phase emulsion stabilized by microporous organic polymer particles, Chem. Eng. J., 2020, 381, 122767 CrossRef CAS.
- L. Tan and B. Tan, Functionalized hierarchical porous polymeric monoliths as versatile platforms to support uniform and ultrafine metal nanoparticles for heterogeneous catalysis, Chem. Eng. J., 2020, 390, 124485 CrossRef CAS.
- Y. Osada and J. P. Gong, Soft and Wet Materials: Polymer Gels, Adv. Mater., 1998, 10, 827–837 CrossRef CAS.
- M. A. Kuzina, D. D. Kartsev, A. V. Stratonovich and P. A. Levkin, Organogels versus Hydrogels: Advantages, Challenges, and Applications, Adv. Funct. Mater., 2023, 33, 2301421 CrossRef CAS.
- P. C. Carman and R. A. W. Haul, Measurement of diffusion coefficients, Proc. R. Soc. A., 1954, 222, 109–118 CAS.
- K. Hawkins, A. K. Patterson, P. A. Clarke and D. K. Smith, Catalytic Gels for a Prebiotically Relevant Asymmetric Aldol Reaction in Water: From Organocatalyst Design to Hydrogel Discovery and Back Again, J. Am. Chem. Soc., 2020, 142, 4379–4389 CrossRef CAS PubMed.
- R. Porcar, M. I. Burguete, P. Lozano, E. Garcia-Verdugo and S. V. Luis, Supramolecular Interactions Based on Ionic Liquids for Tuning of the Catalytic Efficiency of (L)-Proline, ACS Sustainable Chem. Eng., 2016, 4, 6062–6071 CrossRef CAS.
- A. Lu, D. Moatsou, D. A. Longbottom and R. K. O'Reilly, Tuning the catalytic activity of L-proline functionalized hydrophobic nanogel particles in water, Chem. Sci., 2013, 4, 965–969 RSC.
- C. J. Schmiegel, R. Baier and D. Kuckling, Direct Asymmetric Aldol Reaction in Continuous Flow Using Gel-Bound Organocatalysts, Eur. J. Org. Chem., 2021, 17, 2578–2586 CrossRef.
- H. Matsumoto, H. Seto, T. Akiyoshi, M. Shibuya, Y. Hoshino and Y. Miura, Macroporous gel with a permeable reaction platform for catalytic flow synthesis, ACS Omega, 2017, 2, 8796–8802 CrossRef CAS PubMed.
- H. Matsumoto, H. Seto, T. Akiyoshi, M. Shibuya, Y. Hoshino and Y. Miura, Macroporous monolith with polymer gel matrix as continuous-flow catalytic reactor, Chem. Lett., 2017, 46, 1065–1067 CrossRef.
- H. Matsumoto, H. Hattori, M. Nagao, Y. Hoshino and Y. Miura, Continuous-Flow Asymmetric Aldol Addition Using Immobilized Chiral Catalyst on Organogel-Based Porous Monolith, J. Chem. Eng. Jpn., 2024, 57, 2384402 CrossRef.
- Y. Hayashi, H. Gotoh, T. Hayashi and M. Shoji, Diphenylprolinol silyl ethers as efficient organocatalysts for the asymmetric Michael reaction of aldehydes and nitroalkenes, Angew. Chem., Int. Ed., 2005, 44, 4212–4215 CrossRef CAS PubMed.
- K. A. Jørgensen, M. Johannsen, S. Yao, H. Audrain and J. Thorhauge, Catalytic asymmetric addition reactions of carbonyls. A common catalytic approach, Acc. Chem. Res., 1999, 32, 605–613 CrossRef.
- Y. Hayashi, S. Hattori and S. Koshino, Asymmetric Flow Reactions Catalyzed by Immobilized Diphenylprolinol Alkyl Ether: Michael Reaction and Domino Reactions, Chem. – Asian J., 2022, 17, e202200314 CrossRef CAS PubMed.
- H. Ochiai, A. Nishiyama, N. Haraguchi and S. Itsuno, Polymer-Supported Chiral Cis-Disubstituted Pyrrolidine Catalysts and Their Application to Batch and Continuous-Flow Systems, Org. Process Res. Dev., 2020, 24, 2228–2233 CrossRef CAS.
- V. Sans, N. Karbass, M. I. Burguete, E. García-Verdugo and S. V. Luis, Residence time distribution, a simple tool to understand the behaviour of polymeric mini-flow reactors, RSC Adv., 2012, 2, 8721–8728 RSC.
- C. Viklund, F. Svec, J. M. J. Fréchet and K. Irgum, Preparation of porous poly(styrene-co-divinylbenzene) monoliths with controlled pore size distributions initiated by stable free radicals and their pore surface functionalization by grafting, Chem. Mater., 1996, 8, 744–750 CrossRef CAS.
- S. Jung, S. Ehlert, J. A. Mora, K. Kraiczek, M. Dittmann, G. P. Rozing and U. Tallarek, Packing density, permeability, and separation efficiency of packed microchips at different particle-aspect ratios, J. Chromatogr. A, 2009, 1216, 264–273 CrossRef CAS PubMed.
- S. R. L. Gobert, S. Kuhn, L. Braeken and L. C. J. Thomassen, Characterization of milli-and microflow reactors: mixing efficiency and residence time distribution, Org. Process Res. Dev., 2017, 21, 531–542 CrossRef CAS.
- J. Urban, S. Eeltink, P. Jandera and P. J. Schoenmakers, Characterization of polymer-based monolithic capillary columns by inverse size-exclusion chromatography and mercury-intrusion porosimetry, J. Chromatogr. A, 2008, 1182, 161–168 CrossRef CAS PubMed.
- B. Altava, M. I. Burguete, E. García-Verdugo and S. V. Luis, Chiral catalysts immobilized on achiral polymers: effect of the polymer support on the performance of the catalyst, Chem. Soc. Rev., 2018, 47, 2722–2771 RSC.
- S. Itsuno and M. M. Hassan, Polymer-immobilized chiral catalysts, RSC Adv., 2014, 4, 52023–52043 RSC.
|
| This journal is © The Royal Society of Chemistry 2025 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article ,
Masanori
Nagao
,
Masanori
Nagao
 and
Yoshiko
Miura
and
Yoshiko
Miura
 *
*

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v) containing trifluoroacetic acid (0.1 vol%) was employed as a mobile phase in the HPLC measurements (1.0 mL min−1). HPLC analyses for enantiomeric excess were performed on a JASCO LC-2000Plus system equipped with a JASCO DG-980-50 degasser, a JASCO PU-986 pump, a CHIRALPAK OD-H 250-4.6 column (Daicel Co., Osaka, Japan), a JASCO MD-4010 photo diode array detector, and a JASCO CO-2060Plus column oven (JASCO Co., Tokyo, Japan). 2-Propanol/hexane (6.2
40 v/v) containing trifluoroacetic acid (0.1 vol%) was employed as a mobile phase in the HPLC measurements (1.0 mL min−1). HPLC analyses for enantiomeric excess were performed on a JASCO LC-2000Plus system equipped with a JASCO DG-980-50 degasser, a JASCO PU-986 pump, a CHIRALPAK OD-H 250-4.6 column (Daicel Co., Osaka, Japan), a JASCO MD-4010 photo diode array detector, and a JASCO CO-2060Plus column oven (JASCO Co., Tokyo, Japan). 2-Propanol/hexane (6.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 93.8 v/v) was employed as a mobile phase in the HPLC measurements (0.75 mL min−1).
93.8 v/v) was employed as a mobile phase in the HPLC measurements (0.75 mL min−1).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 w/w, 700 mg) and completely dissolved by shaking. AIBN (3 mg, 1 wt% with respect to the monomers) was added to the mixture and completely dissolved. The resulting monomer solution was degassed by N2 bubbling for 30 min and poured into a home-made column (stainless steel, 4.4 mm inner diameter (d) × 5.0 cm length (L)). After sealing with stainless steel plugs at both column ends, the column was heated in an incubator at 70 °C for 72 h. After the polymerization, the MPG column was washed by permeation of tetrahydrofuran (THF) for more than 1 h (2.972 mL h−1). For characterization of the MPG, the MPG was dried under vacuum and extruded from the column to give white solids.
70 w/w, 700 mg) and completely dissolved by shaking. AIBN (3 mg, 1 wt% with respect to the monomers) was added to the mixture and completely dissolved. The resulting monomer solution was degassed by N2 bubbling for 30 min and poured into a home-made column (stainless steel, 4.4 mm inner diameter (d) × 5.0 cm length (L)). After sealing with stainless steel plugs at both column ends, the column was heated in an incubator at 70 °C for 72 h. After the polymerization, the MPG column was washed by permeation of tetrahydrofuran (THF) for more than 1 h (2.972 mL h−1). For characterization of the MPG, the MPG was dried under vacuum and extruded from the column to give white solids.
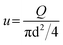
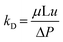
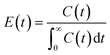
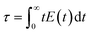


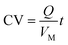
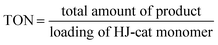







![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 w/w), while the feed ratio of the DVB was fixed at 40 wt%. Compared with MPG40, the resulting MPG40′ was expected to have a different macroporous structure but the same degree of cross-linking (X = 40 wt%). The MPG40′ showed a smaller specific surface area than MPG40 (1 vs. 17 m2 g−1, Table S2†). The pulse experiment was performed, giving an εswollen of 89% for MPG40′ (Fig. 3 and Table 1), which was the same as for the original MPG40 (89%). The σ2 of MPG40′ (0.036, Table 1) was slightly larger than that of the MPG40 (0.018). In the continuous-flow Michael addition, MPG40′ exhibited comparable TOF, STY, and d.r. (2.3 h−1, 106 mmol h−1 L−1, and 81%, Fig. 4 and Table 1) values to those of MPG40 (2.3 h−1, 123 mmol h−1 L−1, and 81%). These results suggested little effect of surface morphology on the molecular diffusivity through the MPG and on catalytic performance in continuous-flow reactions.
80 w/w), while the feed ratio of the DVB was fixed at 40 wt%. Compared with MPG40, the resulting MPG40′ was expected to have a different macroporous structure but the same degree of cross-linking (X = 40 wt%). The MPG40′ showed a smaller specific surface area than MPG40 (1 vs. 17 m2 g−1, Table S2†). The pulse experiment was performed, giving an εswollen of 89% for MPG40′ (Fig. 3 and Table 1), which was the same as for the original MPG40 (89%). The σ2 of MPG40′ (0.036, Table 1) was slightly larger than that of the MPG40 (0.018). In the continuous-flow Michael addition, MPG40′ exhibited comparable TOF, STY, and d.r. (2.3 h−1, 106 mmol h−1 L−1, and 81%, Fig. 4 and Table 1) values to those of MPG40 (2.3 h−1, 123 mmol h−1 L−1, and 81%). These results suggested little effect of surface morphology on the molecular diffusivity through the MPG and on catalytic performance in continuous-flow reactions.




