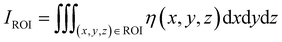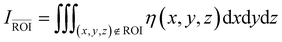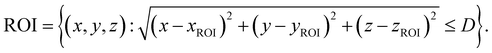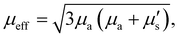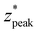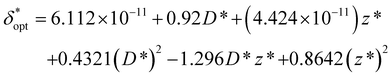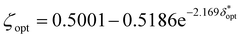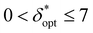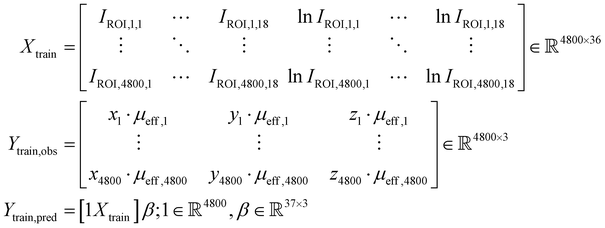Material-agnostic characterization of spatially offset Raman spectroscopy in turbid media via Monte Carlo simulations†
Zuriel Erikson
Joven
 a,
Piyush
Raj
a and
Ishan
Barman
a,
Piyush
Raj
a and
Ishan
Barman
 *abc
*abc
aDepartment of Mechanical Engineering, Johns Hopkins University, Baltimore, Maryland 21218, USA. E-mail: ibarman@jhu.edu
bDepartment of Oncology, Johns Hopkins University, Baltimore, Maryland 21287, USA
cThe Russell H. Morgan Department of Radiology and Radiological Science, The Johns Hopkins University, School of Medicine, Baltimore, Maryland 21205, USA
First published on 8th October 2024
Abstract
Spatially offset Raman spectroscopy (SORS) is a transformative method for probing subsurface chemical compositions in turbid media. This systematic study of Monte Carlo simulations provides closed-form characterizations of key SORS parameters, such as the distribution of spatial origins of collected Raman photons and optimal SORS geometry to selectively interrogate a subsurface region of interest. These results are unified across an extensive range of material properties by multiplying spatial dimensions by the medium's effective attenuation coefficient, which can be calculated when the absorption and reduced scattering coefficients are known from the literature or experimentation. This method of spatial nondimensionalization is validated via goodness-of-fit analysis on the aggregate models and by training a subsurface sample localization model on a heterogeneous population of materials. The findings reported here advance the understanding of SORS phenomena while providing a quantitative and widely applicable foundation for designing and interpreting SORS experiments, facilitating its application in disciplines such as biomedical, materials science, and cultural heritage fields.
Introduction
Spatially offset Raman spectroscopy (SORS) is a powerful technique for interrogating the subsurface chemical makeup of turbid media by measuring the shifts in frequency of scattered light.1,2 Unlike conventional Raman spectroscopy, which primarily captures surface information, SORS utilizes spatial offsets between the excitation laser and collection optics to probe deeper layers in a manner similar to diffuse reflectance spectroscopy.3–5 Such noninvasive and nondestructive sensing capabilities find applications in diverse fields, including medical diagnostics, materials science, and cultural heritage.6–16As the use of SORS grows, there is an unmet need for widely applicable protocols to optimize experimental parameters and quantify results. The probability of spontaneous Raman scattering is on the order of 10−8, and the Raman signal in SORS decays exponentially with increasing spatial offset.17–19 The scarcity of collected Raman photons necessitates the deliberate placement of the source and detector probes to maximize the Raman signal recorded from specific subsurface regions, as indefinitely increasing laser power risks irradiation damage to the sample and feasible signal acquisition time is constrained based on the specific application.20–24 Furthermore, while it is well established that the likelihood of a detected Raman photon originating from a specific layer generally decreases with depth (although not always monotonically), no widely applicable quantitative models describe the depth distribution of the spatial origins of collected Raman photons.25–27 Such models are necessary for interpreting collected Raman spectra for the localization, not just detection, of subsurface Raman scatterers. Localization abilities are crucial in various SORS applications, such as tumor margin estimation, cartilage degradation assessment, and painted layer analysis.28–31
Prior works demonstrate progress towards the aforementioned goals but are often tailored towards a singular SORS application or are otherwise too contrived to be universally applicable. For example, Keller et al. presented histograms of Raman photon generation depth for various spatial offsets obtained from Monte Carlo simulations of SORS in a two-layered breast cancer tissue model.32 Similarly, Zhang et al. presented Monte Carlo reconstructions of SORS spectra in human skin tissue and the relative contributions of each tissue layer.33 Studies of this type provide results that can guide other researchers in highly similar SORS applications but cannot be generalized to other applications with different material properties or non-planar geometry.33–35 Mosca et al. reported that the transport length of the material defines the spatial scale of SORS phenomena and that multiplying the spatial offset by the reduced scattering coefficient, μ′s, provides a nondimensional parameter that uniquely defines the collected Raman signal strength and the nondimensionalized 90th percentile probed depth via Monte Carlo simulations on non-absorbing samples with varying degrees of scattering.25 Spatial nondimensionalization is an effective method for generalizing reported results to a wide range of materials; these results, however, assume zero absorption and stratified layers, limiting their applicability. Kotturi et al. analyzes surface-enhanced SORS detection capabilities for disc-shaped implants, a step towards applicability in vivo where detection targets are not infinitely wide planes.36
In this work, the concept of spatial nondimensionalization is extended to absorbing materials by multiplying spatial variables by the material's effective attenuation coefficient, μeff. This paper uses this new variation of spatial nondimensionalization to present, to the best of the authors’ knowledge, the first closed-form expressions for key SORS experimental parameters as functions of the nondimensionalized spatial offset. Firstly, this study provides quantitative measures of location and dispersion for the depth distribution of the spatial origins of collected Raman photons for a given nondimensionalized spatial offset. One can calculate μeff from a material's absorption and reduced scattering coefficients, which can be determined from literature or via experimentation, multiply by the chosen spatial offset, and use the corresponding results presented here to quantitatively interpret the collected Raman spectra and estimate the depth of the Raman scatterers.36–39 One could also use these results to choose an optimal spatial offset to maximize the relative Raman signal collected from a specific layer. This study then extends this optimization concept beyond layered geometry to subsurface spherical samples, providing optimal placement of the excitation laser and detection probe as a function of the sample's nondimensionalized diameter and nondimensionalized depth. Such optimization enables the chemical interrogation of clinically relevant spherical inclusions in subsurface layers, such as tumors and microcalcifications.29,36–39 Finally, the power of spatial nondimensionalization is demonstrated by training a model to estimate the location of a subsurface spherical sample in a variety of turbid media from SORS measurements and evaluating its performance in materials outside of the training dataset.
Methods
Monte Carlo simulations
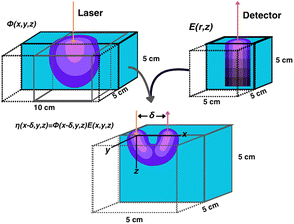
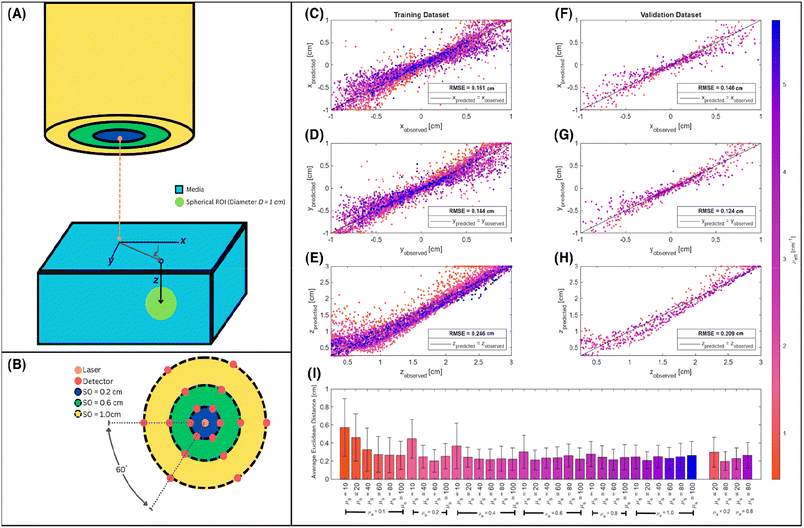
Numerical simulations of photon propagation in turbid media were performed using MCmatlab 4.4.5, a library in MATLAB (Mathworks) for Monte Carlo modeling of light transport in tissue.40,58 The MATLAB code used in this study is freely accessible on GitHub (https://github.com/zurieljoven/SORS-Simulations/). These simulations were performed in homogeneous media assuming constant material properties. This assumption is justified by the relatively unchanging properties within the higher wavelength (700 nm onwards) regions commonly used in Raman spectroscopy in biological tissue.18,20,41 Thirty-six materials were modeled by pairing a scattering coefficient (μs = 10, 20, 40, 60, 80, 100 cm−1) with an absorption coefficient (μa = 0.1, 0.2, 0.4, 0.6, 0.8, 1.0 cm−1); all materials had a scattering anisotropy factor of g = 0.9.As illustrated in Fig. 1, the distribution of spatial origins of collected Raman photons in a typical two-point SORS setup was obtained for each material and spatial offset in a three-step process modified from Wang et al.42 First, the normalized fluence rate (NFR) distribution ϕ(x, y, z) was calculated for a Gaussian beam centered on the top of a 10 × 5 × 5 cm cuboid of uniform material, where (x, y, z) represent local position relative to the incident spot, with z denoting depth. The NFR reflects the likelihood of Raman scattering, assuming the probability of Raman photon generation is proportional to NFR. This assumption of linearity is used in previously published simulation studies to improve computational efficiency.19,25,26,33,34,34,42 The 36 NFR distributions were calculated using MCmatlab simulations, with 108 photons launched from the incident laser and a voxel size of 0.2 × 0.2 × 0.2 mm.
Second, the escape functions E(rdetector, θdetector, zdetector) for each material, i.e., the probability of a Raman photon being detected after generation at a given location relative to the detector fiber, was calculated. Because the simulated media are homogeneous, the distribution of escape function values is axisymmetric about the detector fiber. The escape function values E(rdetector, zdetector) were obtained by placing isotropic Raman annular sources of radii r = 0.1, 0.3, …, 2.1 cm, radial thickness Δr = 0.2 cm, depths z = 0.05, 0.15, …, 4.95 cm, and vertical thickness Δz = 0.1 cm, and dividing the number of collected photons by the number of launched photons. MCmatlab simulations were performed for each of the 36 materials by isotropically launching 106 photons from each annulus in a 5 × 5 × 5 cm cuboid of homogeneous media and a voxel size of 1 × 1 × 1 mm. If no photons reached the detector, the simulation was repeated with 107 photons; if still no photons reached the detector, the corresponding escape function value was deemed negligible. The assumption of directional isotropy of Raman scattering is standard and validated by Keller et al.26,34,43–45 The escape function values are then mapped to a Cartesian representation E(xdetector, ydetector, zdetector) with a voxel size of 0.2 × 0.2 × 0.2 mm via bilinear interpolation in the (rdetector, zdetector) coordinate space.
Third, the distributions of the spatial origins of collected Raman photons η(x, y, z) at a given spatial offset δ were calculated for each material via term-by-term multiplication of the NFR and escape function value distributions, offset by δ in the +x direction:
| η(x − δ, y, z) = ϕ(x − δ, y, z)E(x, y, z). |
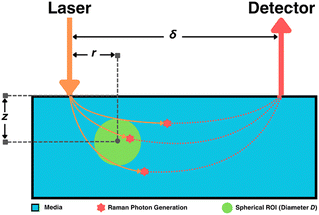
Subsurface sample signal acquisition
For a spherical region of interest (ROI) of diameter D, centered at (xROI, yROI, zROI) within the media, the Raman photon signal-to-noise ratio SNRphoton was defined aswhere
represents the signal (Raman signal from inside the ROI),
represents the noise (Raman signal from outside the ROI), and
The SNRphoton gives the ratio of collected Raman photons originating from within the ROI to those originating outside the ROI, assuming uniform turbidity and Raman cross-section across the ROI boundary. By the linearity of Raman scattering, the SNRphoton for unequal Raman cross-section can be obtained via multiplication by the ratio of the Raman cross-sections. The contrast-to-noise ratio may also be orders of magnitude larger than SNRphoton depending on the spectral shape of the Raman scatterers, e.g., if the Raman spectrum of the subsurface sample exhibits a tall and sharp peak where that of the surrounding media does not. This contrast-to-noise ratio can be deduced via linear combination of the constituent Raman spectra, weighted by the corresponding I values multiplied by their respective Raman cross-sections. However, to maintain the broadest applicability of the reported results, this study was performed without consideration of specific Raman cross-section values or Raman spectra. For the same reason, as well as for computational efficiency, the deviations in turbidity effects caused by the subsurface sample were neglected, modeling the sample as the same material as the surrounding bulk media. Refractions and reflections due to refractive index mismatch across the ROI boundary were also neglected.
Spatial nondimensionalization
To generalize SORS phenomena across varying degrees of turbidity within a material, the following definition for the effective attenuation coefficient was adopted from prior literature:46–49where
| μ′s = μs(1 − g) |
| μ eff [cm−1] | μ a [cm−1] | ||||||
|---|---|---|---|---|---|---|---|
| 0.1 | 0.2 | 0.4 | 0.6 | 0.8 | 1.0 | ||
| μ s [cm−1] | 10 | 0.58 | 0.85 | 1.30 | 1.70 | 2.08 | 2.45 |
| 20 | 0.79 | 1.15 | 1.70 | 2.16 | 2.59 | 3.00 | |
| 40 | 1.11 | 1.59 | 2.30 | 2.88 | 3.39 | 3.87 | |
| 60 | 1.35 | 1.93 | 2.77 | 3.45 | 4.04 | 4.58 | |
| 80 | 1.56 | 2.22 | 3.17 | 3.93 | 4.60 | 5.20 | |
| 100 | 1.74 | 2.47 | 3.53 | 4.37 | 5.09 | 5.74 | |
Spatial nondimensionalization was performed by multiplying spatial dimensions by the relevant μeff value. In this paper, asterisks (*) denote nondimensionalized variables, and [–] denotes unitless space.
Results and discussion
Unified analysis of depth distribution of the spatial origins collected Raman photons
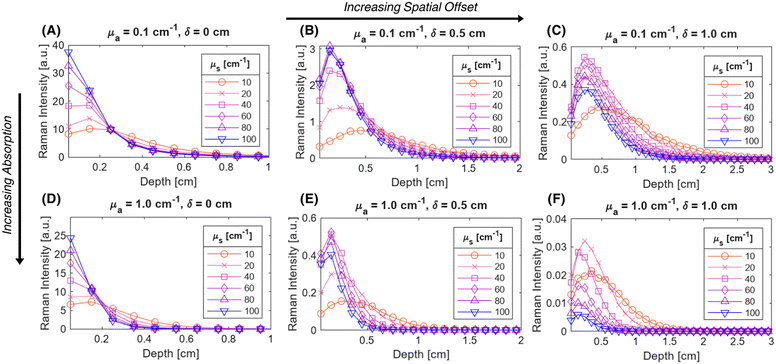
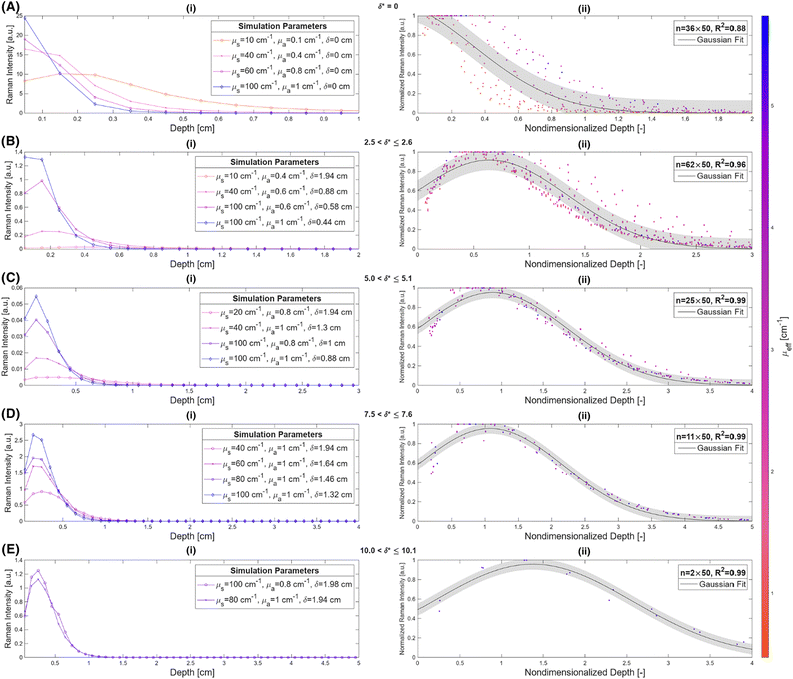
Fig. 2 illustrates the depth dependence of collected Raman intensity, η(z), which resembles previously published trends.25,26,33,34,42 Raman intensity generally decreases with depth, but this relationship is not necessarily monotonic nor uniform across varying values of μs, μa, or δ. In particular, at zero spatial offset, Raman intensity is greatest at z = 0 cm and appears to exponentially decay in deeper layers (Fig. 2A and B). However, at nonzero spatial offsets, intensity increases and peaks at nonzero depth, followed by the same exponential decay, akin to a left-truncated Gaussian curve (Fig. 2C–F). The location of the peak of the distribution zpeak, as well as the degree of spread (which can be characterized by its full-width half maximum, or FWHM), vary with μs, μa, and δ. As scattering increases, the peak moves to shallower layers. When intensity is maximum at the surface, which occurs when δ ≈ 0, the negative slope is steeper for higher values of μs, implying that an extrapolated peak would occur at a more negative value; this supports the observation that μs and zpeak are inversely correlated. It is difficult to directly observe the effect of μs on the FWHM of the distribution for δ = 0 cm, but the spread appears to decrease with increased scattering for nonzero spatial offset. Interestingly, while increasing μs (keeping μa, δ constant) appears to uniformly push the location of peak intensity to the left in Fig. 2, the value of peak intensity monotonically increases with μs for δ = 0 cm (Fig. 2A and D) but increases then decreases for nonzero spatial offset (Fig. 2B–C, E and F). One explanation is that for low scattering media, the chance of sufficient scattering events occurring such that the photons, which were launched directly downwards, “turn around” towards the surface, will be lower than in highly scattering media. However, at much higher levels of scattering, there is a chance for photons to “get lost” and end up absorbed by the media or escape the surface at a different location than the detector. This phenomenon is especially prominent at higher values of μa and δ, since higher μa increases the chance of losing the photons to absorption, and higher δ requires the photons to travel a longer distance to reach the detector, which also increases the chance that absorption or irreversible escape at the surface will occur before detection (Fig. 2C, E and F). With increasing absorption, the entire distribution appears to shrink in both intensity and depth; for example, the maximum Raman intensity observed for μa = 1.0 cm−1, δ = 0.5 cm was less than 20% of the maximum Raman intensity observed for μa = 0.1 cm−1 at the same spatial offset (Fig. 2B and E). Additionally, for this spatial offset, the distributions appear to all settle around zero intensity between 1.5 and 2 cm for the lower absorption but settle between 0.5 and 1 cm for all high absorption cases except μs = 10 cm−1 (Fig. 2B and E). This phenomenon, which is also observed for δ = 0 and 1.0 cm, results in a decrease in the zpeak and FWHM with increasing absorption. Increased μa directly increases light attenuation, therefore intensity decays more rapidly at deeper layers, leading to a shallower zpeak, lower overall intensity values and a smaller spread in the distribution. Conversely, for fixed μs and μa, increasing spatial offset appears to stretch the distribution to deeper layers, increasing zpeak and FWHM. At nonzero spatial offsets, photons must also travel horizontally from source to detector to be collected; photons that travel close to the surface are likely to escape at the media–air interface before reaching the detector, but photons that travel at much deeper layers may never reach the detector before being absorbed. This results in a nonzero peak depth for Raman intensity that balances these counteracting phenomena (Fig. 2B, C, E and F). Additionally, at larger spatial offsets, each phenomenon has more chances to occur, but as photons escaping the surface is irreversible (whereas photons traveling deeper can scatter back to shallower layers), a greater weight of the collected Raman photon distribution shifts to the right tail of the depth distribution, resulting in a larger distribution spread.In order to unify the interdependent effects of μs, μa and δ on the distribution parameters, the associated nondimensionalized spatial offset δ* = δ·μeff was calculated for each distribution. The distributions were also nondimensionalized in depth by calculating z* = z·μeff. Fig. 3 shows that distributions with similar δ* values, even when the values of μs, μa and δ themselves differ, follow the same Raman intensity versus z* distribution when normalized to unit peak; i.e., δ* uniquely determines the peak location and spread of the distribution. Distributions were grouped into bins of 0.1 [–] spatial offset, and (left-truncated) Gaussian curves were fit to the aggregate data from the normalized and nondimensionalized Raman intensity distributions (Fig. 3A–E.ii); good correspondence in the fitting is evidenced by all R2 values being higher than 0.88 (Fig. 4A). Plotting the fit parameters for nondimensionalized peak location, 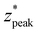 , and nondimensionalized full-width half maximum, FWHM*, it is observable that both generally increase with δ*, but at a decreasing rate (Fig. 4B–D). The same can be said for the nondimensionalized “probed depth” quantity
, and nondimensionalized full-width half maximum, FWHM*, it is observable that both generally increase with δ*, but at a decreasing rate (Fig. 4B–D). The same can be said for the nondimensionalized “probed depth” quantity 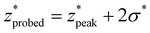 (where σ* is the nondimensionalized standard deviation of the fit distribution), a proxy for the upper limit of the distribution commonly used for Gaussian curves; only a minuscule (small single-digit) percentage of collected Raman photons originate from this depth or deeper, though the exact number is variable due to the left-truncation of the distributions at z* = 0.50–52 These relationships can be characterized by closed-form equations of the form y = c − ae−bx (where x represents the nondimensionalized spatial offset and y represents a Gaussian curve parameter) via a second least-squares regression, with R2 ≥ 0.96 (Table 2). These unified characterizations at different δ* demonstrate that spatial offset determines the general shape of the intensity versus depth distribution, while turbidity determines the scaling, resulting in multiple SORS setups behaving identically (with respect to the depth dependence on collected Raman intensity) as long as δ·μeff are equal. The utility of such equations is their ability to guide SORS setups for a given μeff, as the nondimensionalized parameters can be divided by μeff and a specific spatial offset can be chosen to obtain a specific peak layer (or other distribution parameter).
(where σ* is the nondimensionalized standard deviation of the fit distribution), a proxy for the upper limit of the distribution commonly used for Gaussian curves; only a minuscule (small single-digit) percentage of collected Raman photons originate from this depth or deeper, though the exact number is variable due to the left-truncation of the distributions at z* = 0.50–52 These relationships can be characterized by closed-form equations of the form y = c − ae−bx (where x represents the nondimensionalized spatial offset and y represents a Gaussian curve parameter) via a second least-squares regression, with R2 ≥ 0.96 (Table 2). These unified characterizations at different δ* demonstrate that spatial offset determines the general shape of the intensity versus depth distribution, while turbidity determines the scaling, resulting in multiple SORS setups behaving identically (with respect to the depth dependence on collected Raman intensity) as long as δ·μeff are equal. The utility of such equations is their ability to guide SORS setups for a given μeff, as the nondimensionalized parameters can be divided by μeff and a specific spatial offset can be chosen to obtain a specific peak layer (or other distribution parameter).
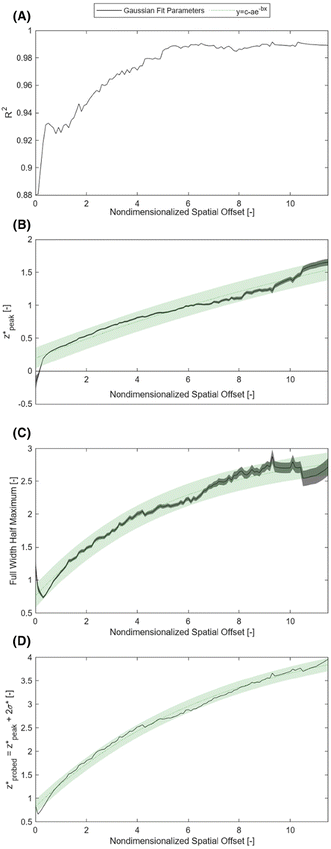 | ||
| Fig. 4 Gaussian fit parameters (black) and overlaid y = c − ae−bx regression curves (green); values for a, b, and c presented in Table 2. All shaded areas represent 95% confidence prediction intervals. | ||
These formulas show good correspondence with the plotted values, except for small δ* (Fig. 4B–D). At smaller δ*, R2 is lower (Fig. 4A), and larger divergence between the individual nondimensionalized and normalized data points and the aggregate fit is observed (Fig. 3A.ii). Also at small δ*, 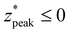 , resulting in distributions with peak intensity at the surface and monotonic decay with depth; more negative
, resulting in distributions with peak intensity at the surface and monotonic decay with depth; more negative 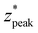 values result in steeper negative slopes at z* = 0 (Fig. 4B). FWHM* (which is a scalar multiple of σ*) initially decreases before increasing in the form of y = c − ae−bx (Fig. 4C); the relative minimum appears to occur at the same δ* where the
values result in steeper negative slopes at z* = 0 (Fig. 4B). FWHM* (which is a scalar multiple of σ*) initially decreases before increasing in the form of y = c − ae−bx (Fig. 4C); the relative minimum appears to occur at the same δ* where the 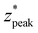 versus δ* slope starts to decrease (Fig. 4B). A similar trend is observed in the nondimensionalized probed depth, though the dip results from counterplay between the increasing
versus δ* slope starts to decrease (Fig. 4B). A similar trend is observed in the nondimensionalized probed depth, though the dip results from counterplay between the increasing 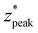 and decreasing FWHM* at small δ* (Fig. 4D). The growth equations for all three parameters imply the existence of asymptotic limits (Table 2). The significance of such upper bounds is particularly profound for
and decreasing FWHM* at small δ* (Fig. 4D). The growth equations for all three parameters imply the existence of asymptotic limits (Table 2). The significance of such upper bounds is particularly profound for 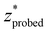 , as this implies that, for a fixed μeff, there is a hard limit on the deepest probed depth attainable by arbitrarily increasing spatial offset. Plus, reaching such a high value for probed depth requires such a large spatial offset that the overall collected Raman signal may be extraordinarily low, further supporting this conclusion that SORS cannot probe infinitely deep layers due to both theoretical and practical limitations.
, as this implies that, for a fixed μeff, there is a hard limit on the deepest probed depth attainable by arbitrarily increasing spatial offset. Plus, reaching such a high value for probed depth requires such a large spatial offset that the overall collected Raman signal may be extraordinarily low, further supporting this conclusion that SORS cannot probe infinitely deep layers due to both theoretical and practical limitations.
Optimal source-detector placement assuming a priori knowledge of subsurface sample size and depth
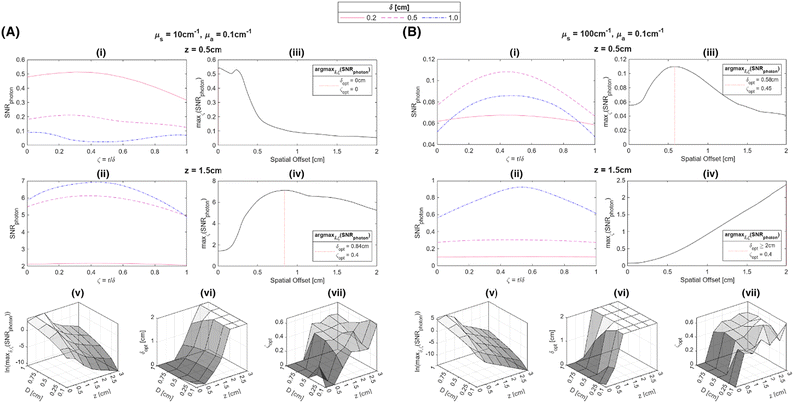
The use of spatial nondimensionalization to enable a unified analysis of the spatial distribution of collected Raman photons was extended from stratified media to spherical samples. Spherical samples of diameter D = 0.1, 0.25, 0.5, 0.75, 1.0 cm were placed at depths z = 0, 0.5, 1.0, 1.5, 2.0, 2.5, 3.0 cm. The source and detector probes were placed at spatial offsets δ = 0, 0.02,…, 2.0 cm such that the plane connecting the two probes and the center of the sphere contained the basis vector in the z direction, with various displacements r = 0, 0.02, ⋯, δ cm between the laser and the center of the sphere (Fig. 5). SNRphoton was plotted against ζ = r/δ for four different materials at z = 0.5, 1.5 cm, at δ = 0.02, 0.5, 1.0 cm (Fig. 6A–B.i-ii and Fig. S1A–B.i-ii in the ESI†). These plots show that for each material and fixed depth, different spatial offsets yield a different optimal ζ that maximizes SNRphoton. For example, in the majority of plotted cases, SNRphoton appears to be maximum when ζ ≈ 0.5, i.e., the source and detector probes are placed such that the spherical sample is somewhere in the middle between them.The natural collected photon pathways from the laser to detector extend to deeper layers in the middle, as represented in Fig. 5 and corroborated by results in the previous subsection. However, in the cases of μs = 10 cm−1, z = 0.5 cm, δ = 1.0 cm, SNRphoton is actually minimum around ζ ≈ 0.5, i.e., it is more optimal to place either the source or the detector nearly on top of the sample (Fig. 6A.i and Fig. S1A.i†). In these cases, the natural photon pathways extend to layers deeper than the depth of the spherical sample. The peak around ζ ≈ 0 is due to higher ϕ closer to the laser, though this is counteracted by a lower E further from the detector; conversely, the secondary peak around ζ ≈ 1 is due to higher E closer to the detector, though ϕ is lower further from the laser. This phenomenon is illustrated in the ESI (Fig. S2†).
Ultimately, different choices for δ result in a different maximum attainable SNRphoton value and choice of ζ that maximizes SNRphoton. This dependence of maxζ(SNRphoton) on δ for the different materials and sample depths are plotted in Fig. 5A–D.iii–iv, along with the value of δ and corresponding ζ that obtains the absolute maximum attainable SNRphoton for a given material and sample depth:
| δopt, ζopt = argmaxδ,ζ(SNRphoton) |
For each material, δopt is smaller for z = 0.5 cm than for z = 1.5 cm. The dependencies of the absolute maximum attainable SNRphoton and values of δopt and ζopt on the diameter and depth of the spherical samples are plotted in Fig. 6A, B.v–vii and Fig. S1A, B.v–vii.† Note that the “ridge” in the maxδ,ζ (SNRphoton) plots at high D and low z is due to the fact that when z < D, part of the spherical ROI is above the surface, effectively decreasing the volume of the subsurface sample (Fig. 6A, B.v and Fig. S1A, B.v†). Otherwise, these plots show a relatively log-linear relationship between maxδ,ζ(SNRphoton) and sample size and depth for all materials. Additionally, δopt primarily exhibits exponential or parabolic growth in z for all materials with minimal variation in D, though these plots are truncated at δopt = 2 cm, the maximum spatial offset for which η was calculated (Fig. 6A, B.vi and Fig. S1A, B.vi†). ζopt appears to grow primarily in z as well, settling around ζopt ≈ 0.5 (Fig. 6A, B.vii and Fig. S1A, B.vii†). Interestingly, these plots do not exhibit any values of ζopt ≈ 1, indicating that it is seemingly never optimal to place the detector probe above the subsurface sample instead of the laser. This implies that the Raman photon detection efficiency near the detector is overshadowed by the laser beam depletion during photon migration.
In order to obtain generalized characterizations of optimal source-detector placement across varying degrees of turbidity, maximum attainable SNRphoton values and corresponding δopt and ζopt values were obtained for μs = 10, 20, 40, 60, 80, 100 cm−1, μa = 0.1, 0.2, 0.4, 0.6, 0.8, 1.0 cm−1, D = 0.1, 1.0 cm, and z = 0.5, 1.0, 2.0 cm (n = 216 data points). The data were nondimensionalized in the same manner as the previous subsection. The dependencies of maxδ*,ζ(SNRphoton) and 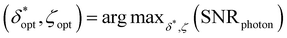 on nondimensionalized sample diameter and nondimensionalized sample depth are plotted in Fig. 7; note that data points where δopt = 2 cm were excluded because the true value of δopt may be higher than 2 cm (n = 124 data points remain). Explicit formulas for ln(maxδ*,ζ(SNRphoton)) and
on nondimensionalized sample diameter and nondimensionalized sample depth are plotted in Fig. 7; note that data points where δopt = 2 cm were excluded because the true value of δopt may be higher than 2 cm (n = 124 data points remain). Explicit formulas for ln(maxδ*,ζ(SNRphoton)) and 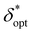 as functions of D* and z*were obtained via least-absolute-residuals regression and are plotted in Fig. 7A and B and summarized in Table 3. The R2 values and residual plots showcase good correspondence with the aggregate data, save for some outliers at extremely high μeff values (Fig. 7D and E); the domain for which these functions are assumed to be valid was obtained as a convex hull of the data projected onto the (D*, z*) plane (shaded region in Fig. 7F). To the left of this region, the sample may protrude from the surface, and to the right of this region, δopt may exceed 2 cm. The ζopt values were unable to be fit as an explicit function of D* and z*, but was fit to an explicit function of
as functions of D* and z*were obtained via least-absolute-residuals regression and are plotted in Fig. 7A and B and summarized in Table 3. The R2 values and residual plots showcase good correspondence with the aggregate data, save for some outliers at extremely high μeff values (Fig. 7D and E); the domain for which these functions are assumed to be valid was obtained as a convex hull of the data projected onto the (D*, z*) plane (shaded region in Fig. 7F). To the left of this region, the sample may protrude from the surface, and to the right of this region, δopt may exceed 2 cm. The ζopt values were unable to be fit as an explicit function of D* and z*, but was fit to an explicit function of 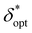 via least-absolute-residuals regression and summarized in Table 3 (Fig. 7C and G). These correlations can be used to inform optimal SORS geometry if the sample diameter and depth are known a priori by multiplying these parameters by μeff, obtaining
via least-absolute-residuals regression and summarized in Table 3 (Fig. 7C and G). These correlations can be used to inform optimal SORS geometry if the sample diameter and depth are known a priori by multiplying these parameters by μeff, obtaining 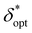 and the corresponding ζopt from Table 3, and then dividing
and the corresponding ζopt from Table 3, and then dividing  by μeff. For example, computerized tomography (CT) or magnetic resonance imaging (MRI) scans can identify suspicious subsurface masses such as potential tumors, and the proposed SORS methodology can be subsequently applied for optimal chemical interrogation.14,29,36–39,53,54
by μeff. For example, computerized tomography (CT) or magnetic resonance imaging (MRI) scans can identify suspicious subsurface masses such as potential tumors, and the proposed SORS methodology can be subsequently applied for optimal chemical interrogation.14,29,36–39,53,54
 | ||
Fig. 7 Aggregate optimization data closely follow unified regression models. Raw data (scatter points) of maxδ*,ζ(SNRphoton) (A),  (B), and ζopt (C) versus (D*, z*) overlaid with regression models (A and B) or bilinear interpolation (C) in gray. Residuals plots (D and E) and valid domain (F) for the regression models. (G) Raw data of ζoptversus (B), and ζopt (C) versus (D*, z*) overlaid with regression models (A and B) or bilinear interpolation (C) in gray. Residuals plots (D and E) and valid domain (F) for the regression models. (G) Raw data of ζoptversus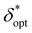 overlaid with regression of the form y = c − ae−bx and 95% confidence prediction interval. Details on all regression models presented in Table 3. overlaid with regression of the form y = c − ae−bx and 95% confidence prediction interval. Details on all regression models presented in Table 3. | ||
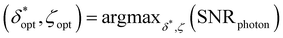 obtained via least-absolute-residuals regression (n = 124)
obtained via least-absolute-residuals regression (n = 124)
Estimation of subsurface sample location from multi-detector probe measurements
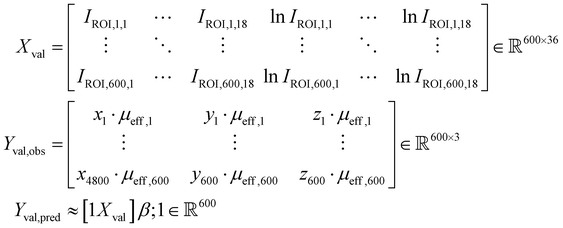
The potential utility for these generalized characterizations for SORS applications was demonstrated via attempts to estimate the location of spherical subsurface samples using an array of detector fibers and a single source laser. Training was performed on an aggregate dataset spanning multiple materials, and testing was performed on materials not used for training; nondimensionalization enables the training of a widely applicable model on a heterogeneous dataset. One example use case is calibrating SORS measurements for in vivo clinical applications, as optical properties are variable between different patients.41,55–57 Eighteen detector probes were placed surrounding a laser as illustrated in Fig. 8A and B. Spherical samples of diameter D = 1.0 cm were placed at random locations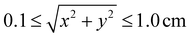 and 0.25 ≤ z ≤ 3.0 cm, and the 18 signals IROI from each detector were recorded for each sample; 150 samples were recorded for each of the 36 materials. The 600 data points from the (μs = 20 cm−1, μa = 0.2 cm−1), (μs = 20 cm−1, μa = 0.8 cm−1), (μs = 80 cm−1, μa = 0.2 cm−1), and (μs = 80 cm−1, μa = 0.8 cm−1) materials were reserved as a hold-out dataset for validation. The other 4800 data points were used for the following partial-least-squares (PLS) regression:
and 0.25 ≤ z ≤ 3.0 cm, and the 18 signals IROI from each detector were recorded for each sample; 150 samples were recorded for each of the 36 materials. The 600 data points from the (μs = 20 cm−1, μa = 0.2 cm−1), (μs = 20 cm−1, μa = 0.8 cm−1), (μs = 80 cm−1, μa = 0.2 cm−1), and (μs = 80 cm−1, μa = 0.8 cm−1) materials were reserved as a hold-out dataset for validation. The other 4800 data points were used for the following partial-least-squares (PLS) regression:The matrices X and Y are designed to capture potential log-linear relationships similar to those observed in Fig. 6A. PLS was performed with 37 components to estimate β. The resultant model was used to predict subsurface sample locations from the Raman signals in the 600 validation data points:
Fig. 8C–I summarizes the predictive accuracy of this regression model. The predicted versus observed values closely follow the identity line for both the training (Fig. 8C–E) and validation (Fig. 8F–H) datasets, though the root-mean-squared-error (RMSE) is consistently lower for the validation dataset. This is likely simply due to the smaller number of materials in the validation dataset compared to the number used for training, as a single unified model will struggle to account for all edge cases. In particular, a number of low μeff value data points appear far above the identity line in shallow depths in the training dataset, and follows a shape reminiscent of a curved checkmark (Fig. 8E). A similar (but less prominent) trend is observed for the validation dataset (Fig. 8H). Most localization attempts in both datasets have a mean error (Euclidean distance) of 0.2–0.4 cm between the predicted and observed sample locations. The error is highest for low values of scattering and absorption coefficients, which corroborates the observations in Fig. 8E and H. One potential explanation is that the linear model captures monotonic relationships, but not reversals of this trends. Such reversals have been observed, for example, in Fig. 5A–D.v, where SNRphoton first increases before decreasing with depth, and in Fig. 2B, C, E and F, where Raman intensity first increases before decreasing with scattering. As a result, the model, trained on aggregate data from a heterogeneous population of materials, successfully captures the dominant inverse relationship between Raman intensity and nondimensionalized depth, but erroneously predicts that lower Raman intensity for lower scattering values must be due to deeper buried samples. Nevertheless, while the predicted versus observed plots correlate nicely with the identity line, the distance error in the validation dataset being around 20% of the sample diameter demonstrates significant room for improvement. Optimization of regression parameters or the use of nonlinear models such as neural networks may prove more suitable for these and more complex localization tasks, and further exploration is needed to evaluate the potential translation to in vivo analysis.
Limitations
While a number of simplifying assumptions were made to enable generalization across a wide range of materials, these same assumptions may limit the applicability of the reported results. Firstly, the assumption that the bulk media can be modeled with a single set of optical properties, even when subsurface samples are present, may not accurately reflect the heterogeneity of samples such as human skin.33,41,42 Additionally, the presented results only apply for the tested range of optical properties, and are further limited in their valid domains (Tables 2 and 3). The assumption that Raman scattering is directly proportional to fluence rate allows for the optimization methods in this paper to result in the same optimal experimental parameters to maximize true Raman signal and contrast-to-noise ratios, but can be problematic if the Raman cross-section is exceedingly low. Neglecting specific Raman cross-sections and spectral shapes also breaks the applicability of spatial nondimensionalization for training models on SORS measurements on a heterogeneous population if the Raman signatures vary between samples. This irrespectivity also limited the characterizations of the depth distributions to descriptors of peak depth and distribution spread, as descriptors of the Raman intensity values in arbitrary units lack physical utility without accounting for Raman cross-section and spectra. Also, these calculations for η assume a semi-infinite medium; η was only calculated for a finite domain, leading to potential inaccuracies arising from an inability to capture nonzero values at large values of x, y, or z. Investigation of such edge effects revealed that voxels touching the border of the 5 × 5 × 5 cm (excluding the top surface) accounted for less than 0.25% of collected Raman photons in each calculated distribution (and accounted for 0% in nearly half of all cases). Hence, these effects were safely neglected. Finally, while the use of Monte Carlo simulations enables the acquisition of a large and standardized dataset for systematic analysis, experimental validation and empirical modeling should be performed in future works, noting whether significant discrepancies arise between the characterizations derived from simulated versus physical data.Conclusions
This thorough and systematic Monte Carlo simulation study advances progress towards a quantitative and universal protocol for optimizing and interpreting SORS experiments. In particular, closed-form expressions for the measures of location, dispersion, and upper limits for the depth distribution of collected Raman intensity, as well as for optimal source and detector probe placement, are reported as functions of spatial parameters multiplied by the bulk medium's effective attenuation coefficient. Such results facilitate the selective interrogation of subsurface regions of interest, as well as estimation of the location of subsurface Raman scatterers. The power of spatial nondimensionalization to enable unified analyses was demonstrated by the high R2 values of regression models used to derive the closed-form expressions, as well as successful localization of subsurface samples in materials outside the training dataset. These advancements contribute to a deeper understanding of SORS phenomena and facilitate its development for use in biomedical research, materials characterization, cultural heritage fields, and more.Author contributions
Z.E.J., P.R., and I.B. designed the research. Z.E.J and P.R. performed the Monte Carlo simulations. Z.E.J. analyzed the data. Z.E.J. wrote the initial draft of the manuscript. All authors contributed to editing the manuscript. I.B. supervised the study and secured funding for the project.Data availability
The MATLAB code and raw data used to generate the simulation results for this paper are freely available on GitHub (https://github.com/zurieljoven/SORS-Simulations/) and Figshare (SORS Excitation and Escape Function Distributions, 10.6084/m9.figshare.26354497).Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported, in whole or in part, by the Bill & Melinda Gates Foundation INV-051765. I. B. is supported by the National Institute of General Medical Sciences (1R35GM149272).References
- P. Matousek, I. P. Clark, E. R. C. Draper, M. D. Morris, A. E. Goodship, N. Everall, M. Towrie, W. F. Finney and A. W. Parker, Subsurface Probing in Diffusely Scattering Media Using Spatially Offset Raman Spectroscopy, Appl. Spectrosc., 2005, 59(4), 393–400 CrossRef CAS PubMed.
- N. Stone, K. Faulds, D. Graham and P. Matousek, Prospects of Deep Raman Spectroscopy for Noninvasive Detection of Conjugated Surface Enhanced Resonance Raman Scattering Nanoparticles Buried within 25 Mm of Mammalian Tissue, Anal. Chem., 2010, 82(10), 3969–3973 CrossRef CAS.
- S. Mosca, C. Conti, N. Stone and P. Matousek, Spatially Offset Raman Spectroscopy, Nat. Rev. Methods Primers, 2021, 1(1), 1–16 CrossRef.
- Principles and Techniques of Diffuse–Reflectance Spectroscopy - Kortüm - 1963 - Angewandte Chemie International Edition in English - Wiley Online Library.
- C. Conti, M. Realini, C. Colombo and P. Matousek, Comparison of Key Modalities of Micro-Scale Spatially Offset Raman Spectroscopy, Analyst, 2015, 140(24), 8127–8133 RSC.
- M. Park, A. Somborn, D. Schlehuber, V. Keuter and G. Deerberg, Raman Spectroscopy in Crop Quality Assessment: Focusing on Sensing Secondary Metabolites: A Review, Hortic. Res., 2023, 10(5), uhad074 CrossRef CAS PubMed.
- U. J. Kim, S. Lee, H. Kim, Y. Roh, S. Han, H. Kim, Y. Park, S. Kim, M. J. Chung, H. Son and H. Choo, Drug Classification with a Spectral Barcode Obtained with a Smartphone Raman Spectrometer, Nat. Commun., 2023, 14(1), 5262 CrossRef CAS.
- C. Eliasson, N. A. Macleod and P. Matousek, Noninvasive Detection of Concealed Liquid Explosives Using Raman Spectroscopy, Anal. Chem., 2007, 79(21), 8185–8189 CrossRef CAS.
- R. J. Hopkins, S. H. Pelfrey and N. C. Shand, Short-Wave Infrared Excited Spatially Offset Raman Spectroscopy (SORS) for through-Barrier Detection, Analyst, 2012, 137(19), 4408–4410 RSC.
- H. Xie, R. Stevenson, N. Stone, A. Hernandez-Santana, K. Faulds and D. Graham, Tracking Bisphosphonates through a 20 Mm Thick Porcine Tissue by Using Surface-Enhanced Spatially Offset Raman Spectroscopy, Angew. Chem., Int. Ed., 2012, 51(34), 8509–8511 CrossRef CAS PubMed.
- B. Sharma, K. Ma, M. R. Glucksberg and R. P. Van Duyne, Seeing through Bone with Surface-Enhanced Spatially Offset Raman Spectroscopy, J. Am. Chem. Soc., 2013, 135(46), 17290–17293 CrossRef CAS.
- F. Nicolson, L. E. Jamieson, S. Mabbott, K. Plakas, N. C. Shand, M. R. Detty, D. Graham and K. Faulds, Through Tissue Imaging of a Live Breast Cancer Tumour Model Using Handheld Surface Enhanced Spatially Offset Resonance Raman Spectroscopy (SESORRS), Chem. Sci., 2018, 9(15), 3788–3792 RSC.
- Z. Liu, M. Huang, Q. Zhu, J. Qin and M. S. Kim, A Packaged Food Internal Raman Signal Separation Method Based on Spatially Offset Raman Spectroscopy Combined with FastICA, Spectrochim. Acta, Part A, 2022, 275, 121154 CrossRef CAS.
- F. Nicolson, M. F. Kircher, N. Stone and P. Matousek, Spatially Offset Raman Spectroscopy for Biomedical Applications, Chem. Soc. Rev., 2021, 50(1), 556–568 RSC.
- P. Matousek, Inverse Spatially Offset Raman Spectroscopy for Deep Noninvasive Probing of Turbid Media, Appl. Spectrosc., 2006, 60(11), 1341–1347 CrossRef CAS.
- S. M. Asiala, N. C. Shand, K. Faulds and D. Graham, Surface-Enhanced, Spatially Offset Raman Spectroscopy (SESORS) in Tissue Analogues, ACS Appl. Mater. Interfaces, 2017, 9(30), 25488–25494 CrossRef CAS PubMed.
- R. R. Jones, D. C. Hooper, L. Zhang, D. Wolverson and V. K. Valev, Raman Techniques: Fundamentals and Frontiers, Nanoscale Res. Lett., 2019, 14, 231 CrossRef PubMed.
- J. R. Durig, Practical Raman Spectroscopy, in TrAC Trends in Analytical Chemistry, 1990, vol. 9, p. IX Search PubMed.
- S. Mosca, P. Dey, M. Salimi, B. Gardner, F. Palombo, N. Stone and P. Matousek, Estimating the Reduced Scattering Coefficient of Turbid Media Using Spatially Offset Raman Spectroscopy, Anal. Chem., 2021, 93(7), 3386–3392 CrossRef CAS.
- F. Angelini and F. Colao, Optimization of Laser Wavelength, Power and Pulse Duration for Eye-Safe Raman Spectroscopy, JEOS-RP., 2019, 15(1), 2 CrossRef.
- D. Pestov, R. K. Murawski, G. O. Ariunbold, X. Wang, M. Zhi, A. V. Sokolov, V. A. Sautenkov, Y. V. Rostovtsev, A. Dogariu, Y. Huang and M. O. Scully, Optimizing the Laser-Pulse Configuration for Coherent Raman Spectroscopy, Science, 2007, 316(5822), 265–268 CrossRef CAS.
- Y. Wang and R. L. McCreery, Evaluation of a Diode Laser/Charge Coupled Device Spectrometer for near-Infrared Raman Spectroscopy, Anal. Chem., 1989, 61(23), 2647–2651 CrossRef CAS.
- G. Insero, F. Fusi and G. Romano, The Safe Use of Lasers in Biomedicine: Principles of Laser-Matter Interaction, J. Public Health Res., 2023, 12(3), 22799036231187077 CrossRef.
- Y. Zhang, R. Chen, F. Liu, P. Miao, L. Lin and J. Ye, In Vivo Surface-Enhanced Transmission Raman Spectroscopy under Maximum Permissible Exposure: Toward Photosafe Detection of Deep-Seated Tumors, Small Methods, 2023, 7(2), 2201334 CrossRef CAS PubMed.
- S. Mosca, P. Dey, M. Salimi, B. Gardner, F. Palombo, N. Stone and P. Matousek, Spatially Offset Raman Spectroscopy—How Deep?, Anal. Chem., 2021, 93(17), 6755–6762 CrossRef CAS PubMed.
- M. D. Keller, R. H. Wilson, M.-A. Mycek and A. Mahadevan-Jansen, Monte Carlo Model of Spatially Offset Raman Spectroscopy for Breast Tumor Margin Analysis, Appl. Spectrosc., 2010, 64(6), 607–614 CrossRef CAS PubMed.
- M. E. Berry, S. M. McCabe, N. C. Shand, D. Graham and K. Faulds, Depth Prediction of Nanotags in Tissue Using Surface Enhanced Spatially Offset Raman Scattering (SESORS), Chem. Commun., 2022, 58(11), 1756–1759 RSC.
- P. Raj, L. Wu, C. Almeida, L. Conway, S. Tanwar, J. Middendorf and I. Barman, Shining Light on Osteoarthritis: Spatially Offset Raman Spectroscopy as a Window into Cartilage Health, ACS Sens., 2024, 9(7), 3794–3804 CrossRef CAS PubMed.
- M. D. Keller, E. Vargis, N. de Matos Granja, R. H. Wilson, M.-A. Mycek, M. C. Kelley and A. Mahadevan-Jansen, Development of a Spatially Offset Raman Spectroscopy Probe for Breast Tumor Surgical Margin Evaluation, J. Biomed. Opt., 2011, 16(7), 077006 CrossRef PubMed.
- G. Thomas, T.-Q. Nguyen, I. J. Pence, B. Caldwell, M. E. O'Connor, J. Giltnane, M. E. Sanders, A. Grau, I. Meszoely, M. Hooks, M. C. Kelley and A. Mahadevan-Jansen, Evaluating Feasibility of an Automated 3-Dimensional Scanner Using Raman Spectroscopy for Intraoperative Breast Margin Assessment, Sci. Rep., 2017, 7(1), 13548 CrossRef CAS.
- C. Conti, C. Colombo, M. Realini and P. Matousek, Subsurface Analysis of Painted Sculptures and Plasters Using Micrometre-Scale Spatially Offset Raman Spectroscopy (Micro-SORS), J. Raman Spectrosc., 2015, 46(5), 476–482 CrossRef CAS.
- M. D. Keller, S. K. Majumder and A. Mahadevan-Jansen, Spatially Offset Raman Spectroscopy of Layered Soft Tissues, Opt. Lett., 2009, 34(7), 926–928 CrossRef.
- Y.-H. Zhang, H.-Z. Zhu, Y.-J. Dong, J. Zeng, X.-P. Han, I. A. Bratchenko, F.-R. Zhang, S.-Y. Xu and S. Wang, Reconstructing in Vivo Spatially Offset Raman Spectroscopy of Human Skin Tissue Using a GPU-Accelerated Monte Carlo Platform, Chin. Phys. B, 2023, 32(11), 118702 CrossRef CAS.
- P. Matousek, M. D. Morris, N. Everall, I. P. Clark, M. Towrie, E. Draper, A. Goodship and A. W. Parker, Numerical Simulations of Subsurface Probing in Diffusely Scattering Media Using Spatially Offset Raman Spectroscopy, Appl. Spectrosc., 2005, 59(12), 1485–1492 CrossRef CAS PubMed.
- K. Buckley, J. G. Kerns, A. W. Parker, A. E. Goodship and P. Matousek, Decomposition of in Vivo Spatially Offset Raman Spectroscopy Data Using Multivariate Analysis Techniques, J. Raman Spectrosc., 2014, 45(2), 188–192 CrossRef CAS.
- D. Kotturi, S. Paterson and M. McShane, Surface-Enhanced Spatially Offset Raman Spectroscopy in Tissue, Biosensors, 2024, 14(2), 81 CrossRef CAS.
- P. Matousek and N. Stone, Development of Deep Subsurface Raman Spectroscopy for Medical Diagnosis and Disease Monitoring, Chem. Soc. Rev., 2016, 45(7), 1794–1802 RSC.
- R. Vanna, C. Morasso, B. Marcinnò, F. Piccotti, E. Torti, D. Altamura, S. Albasini, M. Agozzino, L. Villani, L. Sorrentino, O. Bunk, F. Leporati, C. Giannini and F. Corsi, Raman Spectroscopy Reveals That Biochemical Composition of Breast Microcalcifications Correlates with Histopathologic Features, Cancer Res., 2020, 80(8), 1762–1772 CrossRef CAS.
- I. Barman, N. C. Dingari, A. Saha, S. McGee, L. H. Galindo, W. Liu, D. Plecha, N. Klein, R. R. Dasari and M. Fitzmaurice, Application of Raman Spectroscopy to Identify Microcalcifications and Underlying Breast Lesions at Stereotactic Core Needle Biopsy, Cancer Res., 2013, 73(11), 3206–3215 CrossRef CAS.
- D. Marti, R. N. N. Aasbjerg, P. E. E. Andersen and A. K. K. Hansen, MCmatlab: An Open-Source, User-Friendly, MATLAB-Integrated Three-Dimensional Monte Carlo Light Transport Solver with Heat Diffusion and Tissue Damage, J. Biomed. Opt., 2018, 23(12), 121622 CAS.
- J. L. Sandell and T. C. Zhu, A Review of In-Vivo Optical Properties of Human Tissues and Its Impact on PDT, J. Biophotonics, 2011, 4(11–12), 773–787 CrossRef PubMed.
- S. Wang, J. Zhao, H. Lui, Q. He, J. Bai and H. Zeng, Monte Carlo Simulation of in Vivo Raman Spectral Measurements of Human Skin with a Multi-Layered Tissue Optical Model, J. Biophotonics, 2014, 7(9), 703–712 CrossRef CAS PubMed.
- S. Sathyendranath and T. Platt, Analytic Model of Ocean Color, Appl. Opt., 1997, 36(12), 2620–2629 CrossRef CAS PubMed.
- W.-C. Shih, K. L. Bechtel and M. S. Feld, Intrinsic Raman Spectroscopy for Quantitative Biological Spectroscopy Part I: Theory and Simulations, Opt. Express, 2008, 16(17), 12726 CrossRef CAS PubMed.
- A. M. K. Enejder, T.-W. Koo, J. Oh, M. Hunter, S. Sasic, M. S. Feld and G. L. Horowitz, Blood Analysis by Raman Spectroscopy, Opt. Lett., 2002, 27(22), 2004–2006 CrossRef CAS PubMed.
- A. M. Chiarelli, K. A. Low, E. L. Maclin, M. A. Fletcher, T. S. Kong, B. Zimmerman, C. H. Tan, B. P. Sutton, M. Fabiani and G. Gratton, The Optical Effective Attenuation Coefficient as an Informative Measure of Brain Health in Aging, Photonics, 2019, 6(3), 79 CrossRef PubMed.
- A. M. Chiarelli, E. L. Maclin, K. A. Low, S. Fantini, M. Fabiani and G. Gratton, Low-Resolution Mapping of the Effective Attenuation Coefficient of the Human Head: A Multidistance Approach Applied to High-Density Optical Recordings, Neurophotonics, 2017, 4(2), 021103 CrossRef PubMed.
- S.-Å. Stark and S. Carlsson, A Phantom for the Measurement of Transfer Functions in Nuclear Medicine Image Processing, Radiat. Prot. Dosim., 1993, 49(1–3), 303–305 CrossRef.
- S.-Å. Starck and S. Carlsson, The Determination of the Effective Attenuation Coefficient from Effective Organ Depth and Modulation Transfer Function in Gamma Camera Imaging, Phys. Med. Biol., 1997, 42(10), 1957–1964 CrossRef CAS PubMed.
- E. K. Harris and D. L. DeMets, Estimation of Normal Ranges and Cumulative Proportions by Transforming Observed Distributions to Gaussian Form, Clin. Chem., 1972, 18(7), 605–612 CrossRef CAS.
- Business Statistics: Based on Schaum's Outline of Theory and Problems of Business Statistics, Third Edition, ed. L. J. Kazmier, McGraw-Hill, New York, 2003 Search PubMed.
- D. Dowson and A. Wragg, Maximum-Entropy Distributions Having Prescribed First and Second Moments (Corresp.), IEEE Trans. Inf. Theory, 1973, 19(5), 689–693 CrossRef.
- M. F. Kircher and J. K. Willmann, Molecular Body Imaging: MR Imaging, CT, and US. Part I. Principles, Radiology, 2012, 263(3), 633–643 CrossRef PubMed.
- M. A. Pysz, S. S. Gambhir and J. K. Willmann, Molecular Imaging: Current Status and Emerging Strategies, Clin. Radiol., 2010, 65(7), 500–516 CrossRef CAS PubMed.
- M. N. Hannan, A. K. Sharma and T. M. Baran, Preliminary Measurements of Optical Properties in Human Abscess Cavities Prior to Methylene Blue Photodynamic Therapy, Proc. SPIE-Int. Soc. Opt. Eng., 2023, 12359, 123590A Search PubMed.
- M. Späth, M. Rohde, D. Ni, F. Knieling, F. Stelzle, M. Schmidt, F. Klämpfl and M. Hohmann, The Influence of the Optical Properties on the Determination of Capillary Diameters, Sci. Rep., 2022, 12(1), 270 CrossRef.
- R. Al-Halawani, M. Qassem and P. A. Kyriacou, Monte Carlo Simulation of the Effect of Melanin Concentration on Light-Tissue Interactions in Transmittance and Reflectance Finger Photoplethysmography, Sci. Rep., 2024, 14(1), 8145 CrossRef CAS.
- S. L. Jacques and L. Wang, Monte Carlo Modeling of Light Transport in Tissue, in Optical-Thermal Response of Laser-Irradiated Tissue. Lasers, Photonics, and Electro-Optics, Springer, Boston, MA, 1995, pp 73–100 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Raw data, Monte Carlo code, additional Raman photon signal-to-noise ratio plots, and visualizations of collected Raman intensity distributions. See DOI: https://doi.org/10.1039/d4an01044b |
| This journal is © The Royal Society of Chemistry 2024 |