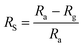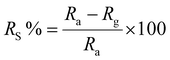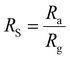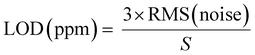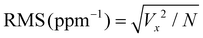Advancements in porous framework materials for chemiresistive hydrogen sensing: exploring MOFs and COFs
Nany
Thokala†
a,
Marilyn Esclance
DMello†
b,
Krishnaveni
Valle
a,
Kiran
Vankayala
 c and
Suresh Babu
Kalidindi
c and
Suresh Babu
Kalidindi
 *d
*d
aDepartment of Chemistry, Andhra University, Visakhapatnam, 530003, India
bCentre for Nano and Soft Matter Sciences, Bengaluru, 562162, India
cDepartment of Chemistry, Birla Institute of Technology and Science (BITS), Pilani, K. K. Birla Goa campus, Goa 403726, India
dDepartment of Chemistry, Central Tribal University of Andhra Pradesh (CTUAP), Andhra Pradesh 535003, India. E-mail: ksureshu@gmail.com; suresh.babu@ctuap.ac.in
First published on 8th January 2025
Abstract
Hydrogen is a zero-emissive fuel and has immense potential to replace carbon-emitting fuels in the future. The development of efficient H2 sensors is essential for preventing hazardous situations and facilitating the widespread usage of hydrogen. Chemiresistors are popular gas sensors owing to their attractive properties such as fast response, miniaturization, simple integration with electronics and low cost. Traditionally, semiconducting metal oxides (SMOs) and Pd-based materials have been widely investigated for chemiresistive H2 sensing applications. However, issues such as limited selectivity and poor reliability still hinder their use in real-time applications. Recent advancements have explored metal–organic frameworks (MOFs) and covalent organic frameworks (COFs), offering new perspectives and potential applications in this field. MOFs and COFs belong to the crystalline framework (CF) family of materials and are highly porous, designable materials with tunable pore surfaces featuring sites for H2 interactions. They exhibit good selectivity towards H2 with quick response/recovery times at relatively low temperatures compared to SMOs. Furthermore, they provide an additional advantage of sensing H2 in the absence of oxygen, even at high concentrations of H2. In this perspective article, we summarize recent advancements and challenges in the development of H2 sensors employing MOFs, COFs, and their hybrid composites as sensing elements. Additionally, we discuss our perspective on hybridizing MOFs/COFs with SMOs and other nanomaterials for the future development of advanced H2 sensors.
1. Introduction
In recent times, there have been significant advancements in the use of hydrogen as an efficient alternative source of energy. Often regarded as a fuel of the future, hydrogen (H2) is considered to be a key player in significantly overhauling the present energy challenges and global economy.1–4 The H2 economy envisions a clean and eco-friendly energy system with net zero emission and subsequent reduction in carbon footprints.5–7 H2 has a high energy content per unit mass (142 kJ g−1, about three times that of gasoline) and very low density (0.0899 kg m−3), which makes it 1/14 times lighter than air.8,9 Upon combustion, H2 yields only water and heat energy, and thus, it is free from polluting emissions. This aspect of net zero CO2 emission has attracted attention from researchers around the world to harness its potential for generating electricity and as a transportation fuel.10 However, commercializing H2 entails inherent risks associated with its explosive and flammable nature, given its wide flammable range of 4.1 to 75% v/v H2 and rapid diffusivity (0.756 cm2 s−1), which readily forms a flammable mixture with air.11,12 Any unexpected leakage of H2 into the air could be catastrophic.13–15 H2 gas is devoid of colour, odour, and taste. It is totally imperceptible to humans. Inhaling high concentrations of H2 may lead to asphyxiation and could be fatal.16 Therefore, it is imperative to deploy efficient H2 sensors as safety measures to check for leakages in H2-related energy systems. A meaningful impact of H2 energy can be achieved by implementing safety codes including sensors.In recent years, chemiresistors have become popular gas sensors and are in demand for detecting gases at trace levels. The chemiresistive technique is an interesting approach due to its attractive features such as straightforward design, ease of processing, cost-effectiveness, and suitability for making small, portable sensing devices.17,18 Indeed, advancements in the chemiresistive technique have gone beyond those anticipated since T. Seiyama introduced a resistance-based gas sensor using zinc oxide (ZnO) thin films in 1962.19 The work was inspired by the findings of B. W. H. Brattain (1952) and G. Heiland (1954), which revealed the distinctive behaviour of semiconducting materials (Ge) and semiconducting metal oxides (SMOs) such as ZnO in exhibiting variations in conduction properties upon exposure to different gases present in the surrounding atmosphere.20,21 The first commercial chemiresistive gas sensor based on tin oxide (SnO2) was developed by Taguchi in 1972 for detecting reducing gases.22,23 Essentially, chemiresistivity is a characteristic of certain materials wherein the intrinsic electrical resistance changes in response to the presence of specific chemical species (gases, vapors) in the surrounding environment. This phenomenon is the basis for chemiresistive sensors that exploit this property to detect and quantify specific gases.24 The construction of a typical conventional chemiresistive gas sensor includes systematically arranged components such as a heater (for thermal activation), a substrate that integrates electrodes, and the sensing material, as illustrated in Fig. 1. A sensing element/material in a chemiresistive sensor plays a crucial role in dictating sensitivity and selectivity. In this regard, SMOs have been explored widely in chemiresistive gas sensors for sensing various gases including H2.25 The detection of H2 using SMOs largely depends on the change in electrical resistance when electrons (e−) from the generated surface oxygen species (O2−, O− and O2−) interact with the H2 gas.26 The nature of these oxygen species is usually governed by two major factors, operating/sensing temperature (200 °C to 500 °C) and background gas, i.e. synthetic air. In the absence of either of these two factors, the detection of gases by SMOs is negligible. The limitations of SMOs can be listed as (i) high operating temperatures, (ii) the lack of selectivity of a target analyte among multiple gases, (iii) saturation of the signal at higher concentrations of the analyte, and (iv) a high consumption of power.27 Furthermore, limitations of low surface reactivity and sensing kinetics, especially at room temperature, must be addressed.28 On the other hand, carbon-based materials like carbon nanotubes (CNTs), graphene, its derivatives [graphene oxide (GO), reduced graphene oxide (rGO)] and conducting polymers have been explored for H2 sensing but with less success.29–31
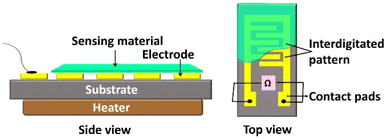 | ||
| Fig. 1 Schematic representation of a chemiresistive device. Chemiresistive sensing device (side and top views) with coated sensing material; the device also shows the presence of an interdigitated pattern of electrodes with contact pads to connect the probes to establish suitable contacts to measure the change in resistance of the sensing material. This figure has been reproduced from ref. 17 with permission from IOP, copyright@2021. | ||
Palladium (Pd) is renowned for its high affinity towards H2, which explains its frequent use in H2-based chemiresistive sensors. The formation of PdHx (palladium hydride) during H2 sensing is one of the most widely accepted mechanisms for Pd-based sensors.32,33 Pd exhibits high selectivity for chemisorbed H2 and is capable of dissociating H2 and hosting the H species in its interstitial lattice sites through the formation of PdHx. Initially, a solid solution known as the α-phase (α-PdHx) is formed. As the H2 concentration increases, a transition from the α-phase to the more stable β-phase (β-PdHx) occurs. Eventually, a two-phase equilibrium is achieved, where both α and β phases coexist. The phase transition from α to β is accompanied by volume expansion and lattice strain, which causes changes in electrical resistivity and corresponding changes in output signals.34 These changes can be effectively achieved under ambient conditions, which is why Pd is a highly preferred sensor element for H2 sensors. However, the formation of an irreversible β-phase may introduce hysteresis, resulting in a poor response, slow recovery, and poor stability of the sensor with inaccurate electrical read-outs.35 These issues can be addressed by modifying the Pd surface (the first contact with H2) or by selecting a substrate material with a large surface area and robust structure, capable of withstanding mechanical stress.36
With the pressing need for reliable and cost-effective sensors, alternative materials characterized by a large surface area and tuneable porosity are sought for improving the sensing performance.37–39 Amidst the debate, metal–organic frameworks (MOFs) and covalent–organic frameworks (COFs) are thriving materials from the family of crystalline frameworks (CFs) with inherent features of a high degree of porosity and large surface area, and thus, have immense potential to be utilized as sensor elements for chemiresistive H2 sensors.40 In this review, CFs specifically refer to MOFs and COFs. The unique characteristics of CFs include (a) ultrahigh porosity, (b) large surface area, (c) tailorable pore sizes/volume, (d) flexibility in modifying the internal surface area, and (e) good thermal/chemical stability.41 It is worth noting that the linkers can be rationally selected in the design of MOFs and COFs as they influence the MOF/COF–H2 interactions. MOFs possess topological diversity and are synthesized using different metal ions/nodes coordinated to many different organic linkers. Often transition metal ions are employed and can generally dictate the stability of MOFs based on the coordinative bond strength.42 Carboxylate based linkers [e.g. benzene-1,4-dicarboxylate (C8H6O4/H2BDC)] and nitrogen containing linkers (e.g. imidazoles) have been widely employed for MOF construction. Common examples of MOFs constructed using various metal ions/secondary building units (SBUs) and organic linkers are given in Fig. 2. Further functionalization of these linkers with –OH, –NH2, etc. have aided in regulating the pore chemistry at the atomic level.44 COFs are regarded as the organic counterparts to MOFs. However, they differ from MOFs by being rich in functionalities. They are exclusively constructed with dynamic covalent bonds and possess a low density.45–47 Notably, COFs are characterized by uninterrupted planar π-electron delocalisation due to extended π–π conjugations across the two-dimensional (2D) or three-dimensional (3D) structures.54–56 Some common examples of COFs constructed using organic linkers are represented in Fig. 3.48
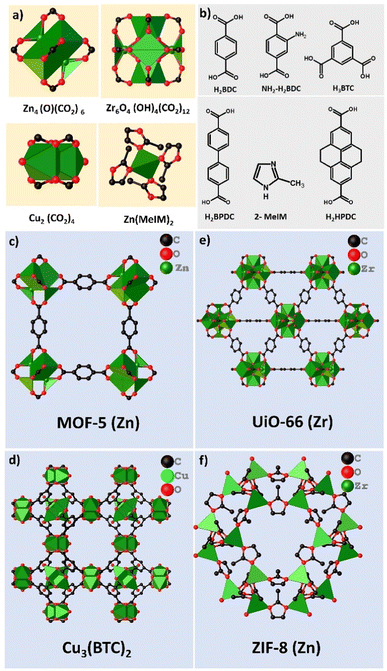 | ||
| Fig. 2 Representative examples of (a) secondary building units (SBUs), (b) organic linkers and structures of (c) MOF-5, (d) Cu3(BTC)2, (e) UiO-66 and (f) ZIF-8 MOFs. This figure has been adapted from ref. 43 with permission from Shodhganga, copyright@2022. | ||
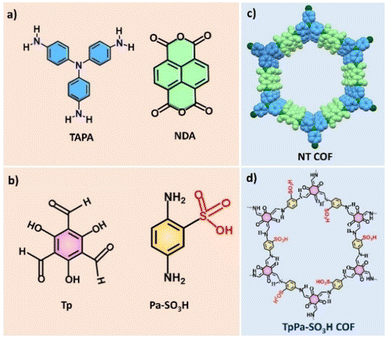 | ||
| Fig. 3 Representative examples of organic linkers and structures of COFs; (a), (b) common organic linkers used for COF synthesis; and structures of (c) NT COF and (d) TpPa-SO3H COF. | ||
In general, CFs have been majorly studied for gas storage and gas separation applications since their discovery due to their high surface area and tuneable porosity/pore size.75 At the same time, they have been overlooked for gas sensing applications, mostly due to their poor electrical conductivity or high electrical resistance. With an increasing understanding of the nature of electrical transport in these classes of materials and advancements in preparing conducting CFs, significant interest has emerged in the domain of chemiresistive sensors fashioned out of CFs. Typically, there are two pathways known to administer charge conduction in CFs: (a) through-bond and (b) through-space.49,50 “Through-bond” charge transport occurs via direct covalent or coordination bonds between metal ions and organic ligands. “Through-space” conduction occurs via non-covalent interactions, such as π–π stacking or other close spatial arrangements of electroactive components, allowing charges to move between sites that are not directly bonded but are in proximity. Charge propagation may occur through continuous energy bands or may hop/“jump” from one localized site to another via linkers or guests, as illustrated in Fig. 4.
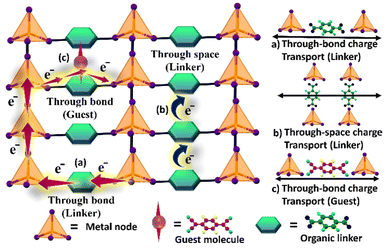 | ||
| Fig. 4 Two main charge transport regimes in MOFs: (a) through bond charge transfer via linker (band transport); (b) through space charge transfer via linker (hopping transport) and (c) through bond charge transfer via guest. Reproduced with modifications from ref. 82 from Wiley, copyright@2016. | ||
Hopping transport is more prevalent in MOFs, wherein the charges move between localized sites through a hopping electron transfer route across the metal nodes. The generated charges are activated via thermal/photoexcitation or chemical doping routes.51–54 Predominantly, through-space charge transfer is observed in 2D electrically conductive MOFs. COFs tend to favour both through-bond and through-space charge transduction.55–58 The in-plane conjugation within an individual 2D COF layer offers a bond channel with an overall in-plane delocalization of electrons (bond transfer). Whereas, the space charge transfer is constructed through the inter-planar π–π stacking layers in the structure. Electroactive CFs have been in the limelight in recent times, wherein redox-active conjugated functional building blocks can serve as mobile charge carriers. Notably, together, through-bond and hopping charge transfer cannot contribute to the conductivity in CFs as they generate different channels for conductivity.55 Overall, since the motion of generated charge within the framework critically determines the charge transport performance, a judicious choice of building units, redox-active guests/building blocks, mixed-valence systems, strong π–π stacking, or π-conjugation, and long-range crystallinity are essential in the CF's structure.59
M. G. Campbell et al. synthesized an electrically conductive 2D MOF, Cu3(HITP)2 (HITP = 2,3,6,7,10,11-hexaiminotriphenylene), with a hexagonal structure and a slipped-parallel stacking of the 2D sheets.60 At room temperature, Cu3(HITP)2 displayed a bulk electrical conductivity of 0.2 S cm−1, as measured using the two-probe method. The authors measured the electrical conductivity of Cu3(HITP)2 by pressing the MOF powder into a pellet. This work represented the first study of using a conductive 2D MOF for a chemiresistive sensing application. Cu3(HITP)2 was active for sensing ammonia (≤5 ppm, concentration) at room temperature. Moreover, significant studies carried out by E. X. Chen et al. using a zeolitic imidazolate framework (ZIF), especially ZIF-67 for sensing formaldehyde and trimethylamine, were among the first studies reported for MOF-based chemiresistive sensor applications.61 Simultaneously, L. M. Tao et al. reported covalent triazine frameworks (CTFs) for the detection of ammonia at room temperature.62 In another study, using the aromatic annulation of 2,3,9,10,16,17,23,24-octa-aminophthalocyanine nickel(II) and pyrene-4,5,9,10-tetraone, K. A. Mirica's group synthesized COF-DC-8 for the chemiresistive sensing of NH3, H2S, NO, and NO2 at ppb levels.63 Since then, many reports on chemiresistive sensing using CFs have been published by various groups, giving insights highlighting the importance of these materials.64–74,76–78 However, the existing literature lacks a thorough exploration of CFs in the context of developing chemiresistive H2 sensors. Therefore, this review focuses on the progress made in H2 sensing utilizing CFs and examines their potential for future applications.
Unlike gases such as NH3, SO2, etc., H2 is a non-polar molecule and as a result, its interactions with the surface of CFs are largely governed by weak van der Waals interactions.79,80 In a few MOFs, sites like metal nodes and functional groups of linkers do adsorb H2 reasonably strongly enough to change the resistance of the material. The hybridization of MOFs with SMOs and with Pd NPs is found to be an effective strategy for realizing synergetic H2 sensing properties.36,64 The well-defined porosity of MOFs is exploited for the sieving of gas molecules by preparing metal oxide@MOF core–shell materials, thereby enhancing the selectivity of metal oxides.64 Furthermore, nanomaterials derived from MOFs are also actively pursued in order to realize effective H2 sensors. COFs are generally enriched with a variety of functional groups and contain extended π–π conjugations within the plane. The functional groups are potential sites for interactions with H2via dipole–induced-dipole interactions and the extended π–π conjugation facilitates the conversion of interactions across the material into a readable signal.63,81 The versatility of COFs can be further enhanced by hybridizing COFs with H2-active metal nanoparticles (MNPs) such as Pd, Pt etc. This aspect enables one to utilize the sensing ability of COFs rather than utilizing them as mere supports to stabilize the nanoparticles.
This perspective article initially includes a short discussion on chemiresistive sensing measurements, and the parameters commonly measured/calculated for gas sensing studies (section 2). The article mainly encompasses the latest advancements in the application of porous CFs for chemiresistive H2 sensing (Scheme 1), section 3, wherein, the H2 gas sensors are classified as two types: MOFs and COFs. We begin with an overview of MOFs in section 3.1, covering discussions on pristine MOFs, followed by MOF hybrids (MOF-coated SMOs and metal NP-loaded MOFs), and MOF derivatives that are used for chemiresistive H2 sensing. The latest advancements in COFs for H2 sensing are discussed in section 3.2. Section 4 offers a comparative discussion on the H2 sensing mechanisms in SMOs and porous CFs. Finally, in section 5, we present our perspectives on opportunities for developing advanced sensing elements based on CF hybrids, with a focus on integrating MOFs/COFs with SMOs. It is anticipated that this unique structure combines the strengths offered by both of these materials (MOF/COFs and SMOs) while mitigating their inherent drawbacks.
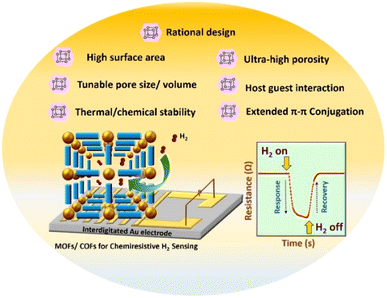 | ||
| Scheme 1 Schematic representation of the advantages of MOFs/COFs (CFs) for chemiresistive H2 sensing. Inset displays the resistance vs. time plot for chemiresistive H2 sensing measurements. | ||
2. Chemiresistive sensing measurements
2.1 Resistance measurements
The resistance (R) of a material can be measured by applying a current (I) and measuring the resulting potential (V), following Ohm's Law, V = R × I, where V is voltage and I is current. Common approaches to measure resistance include (i) the four-probe method, suitable for a wide range of materials possessing low resistivity including bulk materials, (ii) the two-probe method, generally used for materials with high resistivity, (iii) the four-point method (distinct from the four-probe method), and (iv) the van der Pauw method, particularly suitable for measuring very thin films and narrow samples where a set of contacts arranged in a particular geometric pattern are used to calculate the resistivity of the material more accurately.82,83 The two-probe method is particularly convenient for samples with high resistivity that cannot be measured using the four-probe method.84,85 This method involves two wires or contact needles (tips) typically made of tempered metal wires (Cu, Pt, W, or Au). These wires make contacts with the samples either using conductive adhesives made with Au, Ag, or graphitic pastes, or by attaching the wires to metal pads through soldering or wire bonding. Interdigitated electrodes (IDEs), patterned on aluminum (Al), silica, glass, or other materials, are suitable devices for two-probe measurements and are the most widely used architectures in chemiresistive sensing devices. Deposition of the sample is typically done either by drop casting the sample dispersed in a suitable solvent or by spraying or applying a slurry of the material on the IDEs. In the case of powdered samples, resistance is usually measured by making the powders into pressed pellets. The contacts on pressed pellets are established by applying adhesive conductive paste (Au, Ag) as equidistant dots.2.2 Gas sensing measurements
To obtain an active functional layer, the sample-loaded IDE or pressed pellet is heated using a heater to a designated temperature. The activation temperature varies from 100 to 200 °C, depending on the type of material. This process is typically conducted in a closed environment under an inert gas atmosphere (N2 or Ar) by applying a voltage bias (1–2 V for the IDE or up to 3–5 V for a pressed pellet). Under these conditions, the material attains a stable resistance value, and is considered as the baseline resistance, which is detected by a sourcemeter. Once stabilized, the material is exposed to the target analyte gas at different temperatures and varying concentrations to investigate the sensing behaviour of the sensing element, the MOF/COF in the present case. The gas switching and concentration adjustments are managed using mass flow controllers that precisely control the flow rates of the gases. Fig. 5 illustrates a schematic representation of the gas sensing setup, which includes a closed probe chamber with two probes, the sample material (in the form of a pellet or coated on the IDE), a heater, and inlet for gases to be introduced into the probe chamber. The heater is connected to a temperature control unit to maintain the required temperature, while the probes are interfaced with a sourcemeter and a computer for data acquisition. The performance of chemiresistive sensors is evaluated based on some key parameters, which collectively determine the accuracy and effectiveness of the sensor.86–88 These parameters are described below.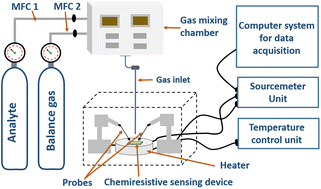 | ||
| Fig. 5 Schematic representation of the chemiresistive gas sensing setup commonly used in the literature. | ||
| S = Δy/Δx |
where Ra is the resistance of the material stabilised under an inert gas, and Rg is the resistance in the presence of the target gas. Ra and Rg are interchangeable depending on the reducing/oxidising nature of the target gas.
| LOD = 3Sa/b |
where RMS(noise) = root mean square (RMS) noise of the baseline, which is calculated using the variation method,
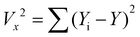 values are the experimental data points of the baseline from the ΔR/Ravs. time (s) plot, Yi and Y are the measured data points and fitted curve values, respectively. S is the slope of linear fitted data from ΔR/Ra of the sensor with the H2 concentration (ppm), N = number of data points.93–95
values are the experimental data points of the baseline from the ΔR/Ravs. time (s) plot, Yi and Y are the measured data points and fitted curve values, respectively. S is the slope of linear fitted data from ΔR/Ra of the sensor with the H2 concentration (ppm), N = number of data points.93–95
3. Porous CFs for chemiresistive H2 sensing
The versatility of MOFs and COFs is being actively explored to develop effective H2 sensors. Although MOFs and COFs both belong to the class of porous CFs and share similarities in their features, a clearer understanding of advancements in chemiresistive H2 sensing requires a distinct classification. Thus, CFs have been divided into two sections, section 3.1 and 3.2, based on their structural diversity, the nature of studies reported, and the type of sensing mechanisms operating in these materials. Section 3.1 is dedicated to MOF-based H2 sensors reported so far in the literature to the best of our knowledge. Section 3.2 highlights studies on COFs, demonstrating their potential as effective H2 sensors. A schematic illustrating the classification of CF materials for H2 sensing in the following discussion is given in Scheme 2.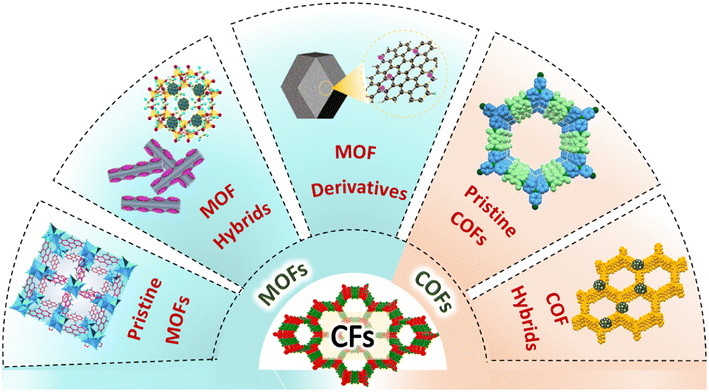 | ||
| Scheme 2 Schematic illustration depicting the classification of CF materials into MOFs and COFs, along with their respective subdivisions for H2 sensing applications. | ||
3.1 Applications of MOFs for chemiresistive H2 sensing
Leveraging the unique properties of MOFs to achieve optimum sensing performance for H2 detection, some key approaches have been adopted in the literature; these include utilizing pristine/functionalized MOFs, applying a MOF passive layer to cover metal oxides for molecular sieving, integrating metal nanoparticles (MNPs) with MOFs to form hybrids, and employing materials that are derived from MOFs. Table 1 summarizes recent studies reported in the literature in this direction.| S. no. | Material | Sensing temp. (°C) | H2 conc. | Sensing properties | Response time (s) | Recovery time (s) | Limit of detection (LOD) | Selectivity | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Pristine MOFs | |||||||||
| 1 | ZIF-8/ZIF-67 | RT | 5 ppm | ΔR/R0 = 81.6% | 856 s | 905 s | 1 ppm | C7H8, CO, NO2 and C2H5OH | 100 |
| 2 | Zn-BDC-NH2 | 50 | 10 ppm | R a/Rg = 2.93 | ∼40 s | ∼50 s | 1 ppm | CO, C6H6, C7H8 and CH4 | 101 |
| 3 | Co-MOF-I and Co-MOF-II | 200 | 50 ppm | ΔR/R0 × 100 = 53.8%; 101.4% | — | — | — | NO2, CO, O2, H2S | 102 |
| MOF@SMO hybrids | |||||||||
| 1 | ZnO@ZIF-8 NWs | 300 | 50 ppm | R gas/Rair = 1.44 | — | — | 10 ppm | C7H8 and C6H6 | 96 |
| 2 | ZnO@ZIF-8 nanorods | 250 | 50 ppm | ΔI/I0 = 80% | — | — | 10 ppm | C6H6, C7H8, C2H5OH, and CH3COCH3 | 113 |
| 3 | ZnO@ZIF-8 | 275 | 50 ppm | — | 50 s | 130 s | — | C6H6 | 114 |
| 4 | 16-ZnO@ZIF-8 | 290 | 1000 ppm | R a/Rg = 6 | — | — | — | C2H6O, C3H6O | 115 |
| MNS@MOF hybrids | |||||||||
| 1 | Pd nanowire@ZIF-8 | RT | 1% | ΔR/R0 = 3.5 | 10 s | 7 s | 600 ppm | O2 | 117 |
| 2 | Pt-Co-MOF@GO | 15 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ppm 000 ppm |
ΔR/R0 = 9% | 9 s | 12 s | 700 ppm | CH4, CO, CH3OH and CH3COCH3 | 118 |
| 3 | ZnO@Pd@ZIF-8 nanowires | 200 | 50 ppm | R gas/Rair = 6.7 | — | — | 10 ppm | C6H6, C7H8, C2H5OH, and CH3COCH3 | 119 |
| 4 | Pd/ZIF-67/PMMA | – | 1% | ΔI/I0 = 24.1% | 9.5 s (0.4% H2) | 8.8 s (0.4% H2) | 2 kPa | C6H6, C7H8, C2H5OH, CO and CH3COCH3 | 120 |
| 5 | PdII@CrPy | 60 | 1% | ΔR/R0 = 2% | 13 s | 10 s | 0.25% | SO2, NO2, CO, CH4 and CO2 | 126 |
| MOF-derived nanostructures | |||||||||
| 1 | ZnO@GC750 | RT | 5 ppm | ΔR/R0 = 97.8% | 34 s | 46 s | 0.1 ppm | HCHO, NO, NH3, CO, NO2 | 132 |
| 2 | ZIF-8rGO | 400 | 200 ppm | ΔR/R0 = 18 | 50 s | 7 s | 5 ppm | CO, CH4, NO2 and C2H5OH | 133 |
| 3 | Co–ZnO–N/C | RT | 1% | ΔR/R0 = 3.7% | 26 s | 17 s | 0.25% | SO2, NO2, CO, CH4 and CO2 | 134 |
| 4 | MOF-derived TiO2 | 30 | 1000 ppm | R a/Rg = 9.2 | 17 s | 150–200 s | 25 ppm | CO, C3H6 | 135 |
| 5 | Sn-SIM-3 | RT | 5% | — | 400 s | 5–10 s | — | — | 136 |
Enhancing the sensing capabilities of one MOF over another by developing composites of two MOFs (MOF@MOF) was reported by D. Matatagui et al. They combined ZIF-8 and ZIF-67 [Co(mlm)2, mlm = 2-methyl imidazole] into one composite with a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The ZIF-8/ZIF-67 composite was active for the sensing of H2 gas at 180 °C.100 The selectivity between H2, C7H8, C2H5OH, CO, and NO2 were compared. However, the responses towards H2 and C7H8 using the ZIF-8 + ZIF-67 composite were comparable and there was scope for improvement. In addition, insights into the H2 interactions with the MOF surface were not provided by this study. In principle, ZIF-8 and ZIF-67 are almost insulators and do not possess active sites for H2. They may show n-type behaviour similar to that observed for ZnO. The effect of H2 detection may be attributed to the presence of trace amounts of ZnO impurities that are formed during the synthesis of ZIF-8.
1. The ZIF-8/ZIF-67 composite was active for the sensing of H2 gas at 180 °C.100 The selectivity between H2, C7H8, C2H5OH, CO, and NO2 were compared. However, the responses towards H2 and C7H8 using the ZIF-8 + ZIF-67 composite were comparable and there was scope for improvement. In addition, insights into the H2 interactions with the MOF surface were not provided by this study. In principle, ZIF-8 and ZIF-67 are almost insulators and do not possess active sites for H2. They may show n-type behaviour similar to that observed for ZnO. The effect of H2 detection may be attributed to the presence of trace amounts of ZnO impurities that are formed during the synthesis of ZIF-8.
The functionalization of MOFs is a very straightforward approach, as shown in Scheme 3 and this was explored by H. W. Kim et al. for sensing H2 at 50 °C by developing an amine-functionalized Zn-based MOF (Zn-BDC-NH2).101 A maximum response of Rair/Rgas = 2.93 and response/recovery times of 44/160 s, respectively, towards 10 ppm H2 were observed. The H2 sensing characteristics were attributed to the redox reactions occurring on the surface of the MOF between H2 and adsorbed oxygen. D. K. Nguyen et al. demonstrated two Co-MOF-74 materials (Co-MOF-I = C99H54N9O45Co9 and Co-MOF-II = C72H36N9O45Co9) for H2 sensing applications.102 The pore apertures of Co-MOF-I and Co-MOF-II are 27.6 Å and 23.2 Å and they possess a high density of +2 and +3 states of Co ions. The MOFs were synthesized via a solvothermal route at 100 °C and activated using anhydrous methanol in supercritical CO2 to achieve guest-free MOF structures. The MOF samples were dispersed in 2-propanol and dropcast on Pt-interdigitated electrodes for further sensing experiments. The H2 sensing studies were carried out at 200 °C in the presence of 50 ppm H2. Co-MOF-I and Co-MOF-II showed responses (S%) of 53.8 and 101.4%, respectively. The selectivity was tested and the sequence of the response was as follows: H2 > NO2 > CO > O2 > H2S, which depended on the molecular diameter of the gas, with the exception of CO gas. Furthermore, the high density of unsaturated Co ions also aided in increasing the response/sensitivity towards H2. This was also compared with MOF-74, containing different metal clusters (Ni and Mg), which displayed a much lower response to H2 (1.3 to 3.9%) at 200 °C. However, the actual role played by the Co ions in the MOFs that leads to the higher response to H2 is unclear.
Although the examples on pristine MOFs discussed in section 3.1.1 have shown potential for the chemiresistive sensing of H2, they are plagued by inherent challenges of limited charge migration and poor electrical conductivity. Moreover, this results in inferior signal transduction at low temperatures and it is essential to heat the samples for the hopping transport of electrons to prevail throughout the surface of the MOF. Also, these studies have not highlighted the role of H2 selectivity. In order to address inherent challenges of pristine MOFs, the hybridisation of MOFs with semiconductor metal oxides and metal nanoparticles (Pd/Pt) has been developed. Section 3.1.2 discusses the studies reported on MOF hybrids for H2 sensing and highlights their advantages over pristine MOFs.
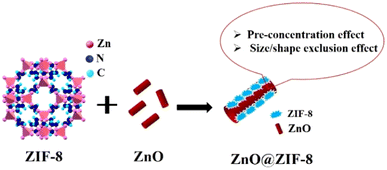
3.1.2.1 Hybrids of MOFs with semiconductor metal oxides (MOF@SMO). Most of the MOF hybrids have been demonstrated to act as molecular sieves owing to their well-defined porous structures with the exception of some studies discussed in section 3.1.2.2. The molecular (gas) sieving ability of MOFs provides unique advantages to realize better sensitivity in comparison with non-MOF based materials in gas sensing applications. Two effects, namely, the pre-concentration effect and the shape/size exclusion effect, can govern the sensitivity and selectivity of MOF-based sensors, respectively.103 Molecular sieving is traditionally a non-equilibrium kinetic separation that depends mainly on the following factors: (a) gas diffusivity, (b) temperature, (c) pressure and (d) framework structure (pore size). Molecular sieving typically does not involve any strong binding sites unlike those in the case of equilibrium separations.104 However, due to extremely low diffusion kinetics of certain gases, energy provided in the form of heat (temperature dictated by the environment) and pressure (dictated by the gradient concentration) plays a crucial role in determining the selectivity of the MOF for a given gas.103
One of the well explored MOF-based micromembranes is ZIF-8 (Zn(MeIM)2, MeIM = 2-methylimidazole) with a pore aperture of 3.4 Å (as determined by single crystal XRD). The pore aperture is flexible as a result of rotation of the MeIM linker and can be tunable in the range of 4 to 4.2 Å.105,106 ZIF-8 has offered a kinetic separation-based selectivity not only for gas molecules with a molecular diameter comparable to the pore-aperture size but also for larger molecules like para-xylene at high temperatures.107 Studies demonstrating the permeability of ZIFs for H2 gas, and thus permitting effective selectivity over other gas molecules, are well reported.108,109 The surface of traditional SMOs, like ZnO, SnO2, etc. have been modified by growing a suitable ZIF micromembrane (Scheme 4). Nanorods/nanowires of these SMOs have advantages over spherical nanoparticles in terms of their larger surface area for the adsorption of gases and to achieve good sensitivity, in addition to possessing facile percolation paths for conduction.110 It should be noted that the H2 sensitivity using bulk/pristine SMOs like ZnO is not adequate. In general, ZnO is a direct band gap semiconductor material possessing numerous oxygen vacancy sites with no specific interactions for H2. ZnO is an active sensor only when these oxygen vacancies are made available at high operating temperatures.111 For example, a study on ZnO nanorod arrays showed a response (ΔR/R) of 0.1% in the presence of 0.1% H2 at 350 °C.103 In another study, ZnO nanoassemblies showed a response of 11% for 0.5% H2 at 400 °C.112 Selectivity to a target gas is one of the major concerns with ZnO-based sensors that often results in mixed signals. The main objective of improving the selectivity of ZnO for H2 has led to researchers modifying the ZnO surface by coating it with an appropriate material like MOFs. The design of MOF-based membranes to enhance the selectivity of ZnO towards H2 was first explored by M. Drobek et al. using 10 wt% of ZIF-8 as a molecular sieving element that was grown on n-type ZnO nanowire (with a length of 25 μm) with ZnO thin films and 2-methyl imidazolate linker solution (Fig. 6).96 The nanowires (NWs) of the ZnO core were encapsulated with a ZIF-8 shell/layer of 50–250 nm and exhibited a surface area of 1760 ± 260 m2 g−1. The devices fabricated using ZnO@ZIF-8 NWs demonstrated the selective sensing of H2 at 300 °C and showed a maximum response of Rair/Rgas = 1.44 (where Rair/Rgas are the resistance of the chemiresistor in the presence and absence of H2) towards 50 ppm of H2. In this case, it is reported that ZIF-8 acts as a selective membrane barrier and permits more diffusion of small H2 molecules (kinetic diameter = 2.89 Å) compared to larger C7H8 and C6H6 molecules (kinetic diameter = 5.92 Å and 5.27 Å, respectively). Analogous to this study, T. Zhou et al. demonstrated the applicability of two different MOF coatings, namely, ZIF-8 and ZIF-71 [Zn(dcIm)2, dcIm = 4,5 dichloroimidazole] grown on a ZnO nanorod array (NRA) for selective H2 sensing.113 ZnO@ZIF NRAs were synthesized by utilizing self-assembling templates of ZnO NRAs to provide the Zn2+ metal ions, followed by solvothermal treatment. In the case of ZnO@ZIF-8 NRAs, the ZIF-8 shell thickness was reported to be 70–100 nm whereas, for ZnO@ZIF-71 NRAs, the ZIF-71 shell thickness was optimised to be 50 nm. The H2 sensing characteristics were compared between ZnO NRAs, ZnO@ZIF-8 NRAs and ZnO@ZIF-71 NRAs at 250 °C. ZnO@ZIF-8 NRAs were able to selectively detect H2 over benzene (5.85 Å), ethanol (4.53 Å), and acetone (4.60 Å), owing to the presence of the smaller pore aperture of ZIF-8 (3.4 Å). Conversely, ZnO@ZIF-71 NRAs show a selective response towards ethanol and acetone as the pore aperture of ZIF-71 is between 4.8 and 5.4 Å, and hence, enable gas permeation with ease.
 | ||
| Fig. 6 (a) Schematic of the synthesis steps yielding the ZIF-membrane encapsulated ZnO nanowires (ZnO@ZIF-8 NWs), (b) FESEM images of networked ZnO nanowires and ZnO@ZIF-8 composite nanowires, (c) and (d) responses of the pristine ZnO nanowires and ZnO@ZIF-8 composite nanowires in contact with 10, 30, and 50 ppm H2 gas. Reproduced with permission from ref. 96, American Chemical Society, copyright@2016. | ||
Another report by A. I. Khudiar et al. reported the encapsulation of ZnO nanorods (NRs) using ZIF-8 to form a hybrid and was termed ZnO@ZIF-8.114 Thin films of ZIF-8 were synthesized on Al2O3 substrate via a solvothermal and sacrificial template route similar to the self-assembling template route used by T. Zhou et al. described above.113 Platelet-like structures of ZIF-8 were coated on ZnO NRs (∼7 μm length); this was evident from electron microscopy studies. The optimal sensing temperature was reported to be 275 °C and the selectivity studies compared H2 and benzene. The transport limitation offered by the ZIF-8 coating over ZnO NRs enabled H2 to be selectively detected with response and recovery times of 50 s and 130 s. Although, the pristine ZnO NRs lacked the selective detection of H2, the response and recovery times were shorter than those of the ZnO@ZIF-8-based sensor. This was ascribed to the uninterrupted interaction between H2 and the uncoated ZnO surface. Notably, the thickness of the MOF coating on ZnO nanostructures plays a significant role in sieving and sensing H2. Studies carried out by S. Zhang et al. demonstrated the effect of the ZIF-8 film thickness coated on ZnO nanostructures; this was achieved via altering the processing time of ZIF-8 film formation.115 Sensor studies demonstrated that 1000 ppm H2 was optimum when the measurement was carried out at 290 °C. The molecular sieving effect of the ZIF-8 film on ZnO particles was more effective at sensing H2 only with a growth time of >8 h with >100 nm thickness. Among the synthesized hybrids, 16-ZnO@ZIF-8 showed a response of Rair/Rgas = 6 towards 1000 ppm H2. The selectivity studies were carried out for C2H6O and C3H6O. The pore aperture size of ZIF-8@ZnO is ∼4 Å, which is larger than H2 = 2.89 Å, and smaller than C2H6O = 4.53 Å and C3H6O = 4.60 Å. A smaller gas molecule like H2 could interact with the ZnO core more easily than C2H6O and C3H6O.
The above discussed literature explores using ZIF-based MOF encapsulated ZnO nanostructures (ZnO@MOF composites) to improve H2 sensor selectivity. However, thicker ZIF films over ZnO can occlude the adsorption of even H2 gas, thus decreasing the sensitivity of the sensor. Optimization of the uniform ZIF shell coating is a crucial step to improve the sensitivity of ZnO@MOF composites for H2 sensing. Furthermore, the studies discussed so far have only demonstrated the selectivity of ZnO@MOF in the presence of molecules that are much bigger (in terms of kinetic diameter) than H2, for example, C7H8, C6H6, C2H6O and C3H6O. Despite this fact, investigation of the selectivity of ZnO@MOF composites towards small gas molecules, like CO, CO2, SO2 and NOx, which have kinetic diameters close to that of H2, is essential. Moreover, redox-active SMOs like SnO2, CeO2, Fe2O3, Co3O4etc. as the core coated with a suitable MOF layer have not been explored for H2 sensing.
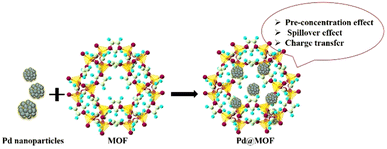
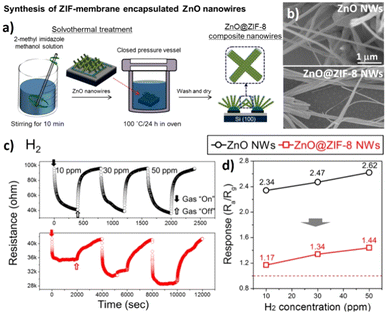
3.1.2.2 Hybrids of MOFs decorated with metal ions/nanostructures (MNS@MOF). Functionalization of MOFs with metal ions/nanostructures is another approach to achieve enhanced performance. The incredible features of MOFs, especially ZIF-8, which exhibit good permeability for H2 and ease of synthesis, as detailed in section 3.1.1, have led researchers to explore functionalizing MOFs with nanostructures. Among all the noble metals, Pd is a good chemisorption component that can interact with H2 under ambient conditions via interstitial metal hydride (PdHx) formation, as discussed in Section 1.116 Pd nanoparticles (NPs) are either confined to the pores of MOFs or they are highly dispersed on the MOF matrix (Scheme 5). Although these Pd-based H2 sensors display very fast response times, they are limited to detecting H2 only at lower concentrations (1–2% max). On the other hand, the sensitivity of Pd-based sensors is known to degrade in the presence of gases like O2, CO, SO2 and H2S due to the formation of strong Pd–O intermediates, giving water as a by-product.116 As a result, the kinetics/rate of H2 adsorption will not only decrease but also block the adsorption sites on the Pd surface for further adsorption of gases. W.T. Koo et al. optimised the self-assembled growth of ZIF-8 on Pd NWs to address the interference in sensing H2 caused by the presence of O2 gas.117 The primary step in preparing Pd NWs@ZIF-8 is to synthesize Pd NWs (diameter = 200 ± 50 nm) followed by growing a layer of ZIF-8 using a nanofiltration method. Pd NWs@ZIF-8 were self-assembled over 2–6 h to obtain a maximum thickness of 330 nm of ZIF-8 layer grown on the surface of Pd NWs. The gas sensing characteristics were tested as a function of the thickness of the ZIF-8 coating on Pd NWs obtained at different time intervals (2 h to 6 h). Pd NWs@ZIF-8_4 h exhibited the highest response of 3.5% towards 1% H2 at room temperature. The response and recovery times were 10-fold faster than those of pristine Pd NWs. The sensing mechanism was explained by the molecular sieving effect of ZIF-8, which further enhanced the kinetics of response and recovery in the presence of H2. In another study, nanocomposites of Pt NPs, Co-BDC MOF (BDC = 1,4 benzene dicarboxylic acid) and graphene oxide (GO) based p-type sensors were demonstrated by S. Fardindoost et al.118 Pt was sputtered on Co-MOF@GO that was synthesized by a solvothermal route using as-synthesized GO powder and Co(NO3)2·6H2O and a BDC linker. A flower-like morphology with an interlayer distance of 0.43 nm in GO–Co was observed. The optimum sensing temperature was reported to be 85 °C. Pt-sputtered Co-MOF@GO showed a response of ΔR/R0 = 10% towards 1.5% H2. The response and recovery times are reported in the range of 216–230 s. The interaction between H2 and Pt-sputtered Co-MOF@GO is known to facilitate the formation of Pt–Hx intermediates, which undergo redox reactions that occur on the surface of GO. In another study, M. J. Weber et al. prepared Pd NPs (prepared by an atomic layer deposition method) decorated on ZnO with ZIF-8 membrane (ZIF-8/Pd/ZnO) to enhance the selectivity of ZnO NWs for H2 sensing and achieved a maximum signal response.119 The optimal sensing temperature was reported to be 200 °C and the highest response of Rair/Rgas = 6.7 for 50 ppm H2 was observed (Fig. 7). Although the response of Pd/ZnO NWs was ∼20% greater than that of ZIF-8/Pd/ZnO, it failed to outdo ZIF-8/Pd/ZnO in terms of selectivity towards C6H6/C2H5OH/C7H8/CH3COCH3. Three factors are reported to influence the sensor performance; (a) concentration effect of the ZIF-8 membrane, (b) spill-over effect of Pd NWs and (c) redox reactions on the surface of ZnO NWs. It should be noted that often H2 gas produced from the steam reforming of methane is contaminated with CO. It is essential to decipher the H2 sensor characteristics in the presence of CO for real-time applications. In order to address the detrimental effect of the presence of CO on the sensing characteristics of Pd-based nanostructures for H2 sensing, the Pd/ZIF-67/PMMA [PMMA = poly(methyl methacrylate)] based sensing element was reported by B. Xie et al.120 The composite material consisted of a MOF as an intermediate layer between the Pd nanocluster and PMMA polymer. The synergistic effects of ZIF-67 and PMMA membrane impacted the electronic structure of Pd NCs (NCs = nanocubes). Pd/ZIF-67/PMMA showed the highest response of ΔI/I0 = 24.1% towards 1% H2. The response and recovery times were in the range of 8–10 s in the presence of 0.4% H2. The authors proposed that PMMA acted as a permeable layer for H2 whereas ZIF-67 assisted in enhancing the sensitivity and improving the response/recovery times. It was also reported that the Pd/ZIF-67/PMMA sensor showed a high tolerance towards CO poisoning because of the PMMA layer, as compared to that observed in the case of pristine Pd and Pd/ZIF-67. Selectivity studies with volatile organic compounds – VOCs (C6H6, CH3COCH3, C7H8, C2H5OH) – using Pd/ZIF-67/PMMA revealed that the sensor did not show any response to VOCs, thus exhibiting high selectivity.
 | ||
| Fig. 7 (a and b) SEM images of ZIF-8/Pd/ZnO NWs, (c) GI-XRD diffraction pattern (the ZIF-8, ZnO and Pd peaks are indicated), (d) dynamic normalized resistance curves of Pd/ZnO and ZIF-8/Pd/ZnO NW gas sensors when the devices were exposed to 10, 30 and 50 ppm H2, C6H6, C2H5OH, C7H8 and CH3COCH3 gases, (e) calibration curves obtained using bare ZnO, Pd/ZnO and ZIF-8/Pd/ZnO NW-based gas sensors, (f) calibration curves of bare ZnO, Pd/ZnO and ZIF-8/Pd/ZnO NW gas sensors to 10, 30 and 50 ppm H2, C6H6, C2H5OH, C7H8 and CH3COCH3 interfering gases. Reproduced with permission from ref. 119, American Chemical Society, copyright@2018. | ||
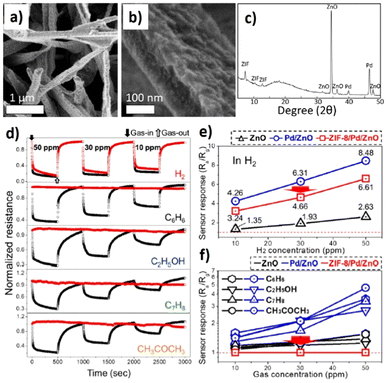
The rapid development of electrically conductive 2D MOFs is another driving force that has led to increased research in developing MOFs as sensing elements in chemiresistive sensing.67,121 The research groups of Mircea Dincă and Martí-Gastaldo have extensively investigated electrically conductive MOFs for the detection of VOCs and small molecules.53,122 Additionally, the phase of developing redox-active MOFs has been highly sought after in recent times.123 In fact, the MOFs synthesized traditionally using metal centres like Zn(II) and carboxylates (e.g. BDC) or imidazolates (2-MeIM) are often redox inactive.124 The construction of MOFs using 3d transition metal centres (first row transition series) that show redox-state changes may often result in changes to the topology/geometry of the product MOF. Besides, the use of redox-active ligands can lead to the availability of radical states (e.g. anion radicals of pyrazine ligands) but require an inert atmosphere during synthesis.123 In an attempt to design 2D conductive MOFs using transition metal ions with strongly reducing features in conjugation with organic linkers, K. S. Pedersen et al. synthesized CrCl2(pyz)2 [CrIIICl2(pyz)2, pyz = pyrazine] for the first time. CrCl2(pyz)2 showed a strong degree of π–d conjugation and strong redox activity was displayed by the pyrazine ligand.125 Instead of an ionic conduction pathway through the pores, the movement of electrons/holes (charge transfer phenomenon) through the framework was reported to influence the redox activity. Inspired by fascinating structure dependent properties displayed by the redox-active CrCl2(pyz)2 MOF, our group investigated the use of CrCl2(pyz)2 as a sensor element in a chemiresistor configuration for H2 sensing (Fig. 8).126 The sites responsible for the interactions with H2 in the target gas were systematically studied by anchoring Pd(II) ions on CrCl2(pyz)2. It was observed that the CrCl2(pyz)2 MOF enabled a high dispersion of Pd(II) through charge transfer interactions, occurring in the vicinity of the negatively charged pyrazine linker. PdII@CrPy showed a response of ΔR/R0 = 2% towards 1% H2 concentration at 60 °C. The response and recovery times were in the range of 5–8 s. The limit of detection for H2 was found to be 0.25%. PdII@CrPy displayed reversible and long-term stability towards H2via a partial charge transfer mechanism demonstrated through density functional theory (DFT) calculations. In this study, an inert atmosphere of N2 was used as a background gas unlike in the previous studies described wherein air was majorly used. This particular study gives an insight into sensing H2 above ppm levels using 2D conductive MOFs.
 | ||
| Fig. 8 Structures of CrCl2(pyrazine)2 MOF and PdII@CrCl2(pyrazine)2: (a) staggered stacking layers of CrCl2(pyrazine)2, (b) secondary building unit in CrCl2(pyrazine)2, (c) pyrazine anion radical in ligand reaction with Pd2+ precursor, (d) chemiresistive sensing of H2 using PdII@CrCl2(pyrazine)2, (e) comparison of the gas sensing performance of CrPy and PdII@CrPy to 1% H2 gas; response–recovery curves of PdII@CrPy for 1% H2 and (f) selectivity studies of gases tested near their TLV limits include 10 ppm SO2, 10 ppm CO, 10 ppm NO2, 0.05% CH4, 0.5% H2 and 0.5% CO2. The figure is reproduced with permission from ref. 126, Elsevier, copyright@2022. | ||
It should be noted that the majority of studies described in this section have demonstrated H2 sensing at ultra-low ppm levels. The detection of H2 in the range of 1–4% is also a great challenge as the sensing surface begins to get saturated at these concentrations. Composites of Pd/Pt@MOFs are very attractive for room temperature sensing; however, the stability and sluggish recovery speeds pose challenges for their H2 sensing proficiencies. Downsizing Pd/Pt NPs to atomic levels and high dispersion could overcome some of the glitches associated with metal surface saturation attributed to the formation of β-PdHx systems in the future.
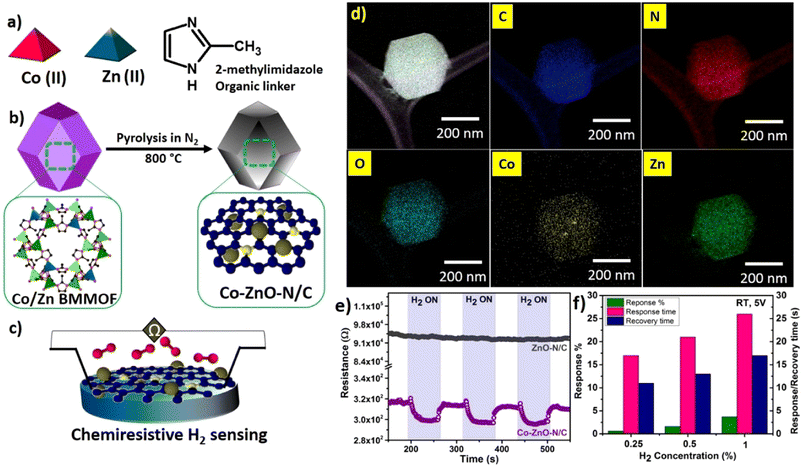 | ||
| Fig. 9 (a) Precursors used for the synthesis of Co/Zn bimetallic MOF (BMMOF), (b) pyrolysis of Co/Zn BMMOF to form Co–ZnO–N/C, and (c) chemiresistive sensing of H2 using Co–ZnO–N/C, (d) high annular dark-field imaging by using a scanning transmission electron microscope with energy-dispersive X-ray spectroscopy (EDS) mapping of C, N, O, Co, and Zn; (e) comparison of the gas sensing performance of Co–ZnO–N/C and ZnO–N/C for 1% H2 gas and (f) effect of H2 concentration (0.25, 0.5, and 1%) on the overall gas sensing characteristics at room temperature. Reproduced with permission from ref. 132, American Chemical Society, copyright@2023. | ||
The examples discussed above are some of the rare studies in the direction of using non-noble metals for chemiresistive sensing of H2. A MOF-derived route for developing room temperature H2 sensors may be quite attractive research in terms of academic curiosity. However, arguably MOFs may offer certain practical limitations like longer synthetic methodologies and the use of very high calcination temperatures (600–800 °C).
3.2. Application of COFs for chemiresistive H2 sensing
The significant structure–property relationship has rendered COFs intriguing materials for multiple functionalities including gas adsorption, energy storage devices, optoelectronic devices, catalysis, chemical sensing, and bio-related applications.137–142 Initially, COFs were perceived as less optimal materials for chemiresistive gas sensing applications compared to other uses, primarily due to their inherent bulky nature and low conductivity. Through efforts over the past decade, COFs have gradually emerged in chemiresistive sensing applications, effectively detecting gases such as NO2, CO2, NH3, VOCs and humidity.143–145 However, the potential of COFs for sensing H2 gas has been overlooked. In this section we discuss the emerging prospects of COFs as chemiresistive H2 gas sensors. A summary of the literature on COF-based chemiresistive H2 sensors is provided in Table 2.| S. No. | Material | Sensing temp. | H2 conc. | Sensing properties | Response time (s) | Recovery time (s) | Limit of detection (LOD) | Selectivity | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 1. | eNT COF | 200 °C | 1% | ΔR/R0 = 30.7 | 4.5 | 3.9 | 0.14% | — | 152 |
| 2. | TpPa-SO3H CONs | 120 °C | 1% | ΔR/R0 = 12 ± 1 | 5.5 ± 1 | 2.6 ± 0.5 | 0.2% | — | 154 |
| 3. | ePd@TpPa-SO3H | 120 °C | 1% | ΔR/R0 = 29 ± 1 | 5.3 | 3.1 | 0.2% | SO2, NO2, and CH4 | 156 |
| 4. | Pd@amicPT | RT | 1% | ΔR/R0 = 67.3 | 5.3 | 3.5 | 0.18% | NH3, NO2, SO2, and CO | 157 |
In the context of H2 sensing, there has been limited exploration of using COFs. The bulkiness of COFs results from the dense stacking of layers driven by π–π interactions, leading to hindered access to the active sites.146 It is rather a challenge to fabricate sensors using bulk materials, as it often results in inhomogeneous films due to poor dispersibility in solvents. The unstable suspensions pose difficulties in device fabrication and impact sensor performance.147,148 Addressing these issues, COFs have been delaminated into nanosheets that exhibit improved functionalities including high efficiency for energy storage, enhanced gas adsorption, ion diffusion and better processability, compared to their bulk counterparts.149–151 The significant improvement and unique features observed for exfoliated COFs have propelled the development of various strategies for the delamination of COFs. The commonly employed routes include mechanical exfoliation, solvent/liquid-assisted exfoliation, and chemical exfoliation. Solvent-assisted exfoliation is a mild method for exfoliation, espoused for its non-damaging approach. Exploiting the advantages of exfoliation, our group was the first to report COF-based H2 chemiresistive sensors and has conducted notable work in H2 sensing with COFs. The following provides a discussion on these works.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio) at room temperature. The exfoliated NT COF (eNT COF) retained its basic structure, as supported by powder X-ray diffraction (PXRD) patterns and Fourier-transform infrared (FT-IR) studies. Exfoliation to a-few-layers-thick 2D sheets with a thickness of 17–20 nm was confirmed by atomic force microscopy (AFM) analysis. The eNT COF dispersion, when integrated into the chemiresistor, demonstrated an appreciable sensing response of 30.7 ± 0.26% at 200 °C for 1% H2 with 4.5 ± 0.6/3.9 ± 0.3 s response/recovery times, respectively (Fig. 10b). The sensitivity of eNT COF towards H2 was ascribed to exfoliation, which permitted the effective interaction of H2 with the functional active sites that were previously buried within the interiors of the COF. The colloidal dispersion of eNT COF allowed straightforward device fabrication by simple drop casting, which facilitated the creation of a more uniform and homogeneous sensing film. The eNT COF sensor was used to quantify varying concentrations of H2 gas, ranging from 1 to 0.2%, at 200 °C. The sensing mechanism, elucidated by DFT calculations, suggested an electrostatic interaction between H2 molecules and the carbonyl oxygen (C
2 ratio) at room temperature. The exfoliated NT COF (eNT COF) retained its basic structure, as supported by powder X-ray diffraction (PXRD) patterns and Fourier-transform infrared (FT-IR) studies. Exfoliation to a-few-layers-thick 2D sheets with a thickness of 17–20 nm was confirmed by atomic force microscopy (AFM) analysis. The eNT COF dispersion, when integrated into the chemiresistor, demonstrated an appreciable sensing response of 30.7 ± 0.26% at 200 °C for 1% H2 with 4.5 ± 0.6/3.9 ± 0.3 s response/recovery times, respectively (Fig. 10b). The sensitivity of eNT COF towards H2 was ascribed to exfoliation, which permitted the effective interaction of H2 with the functional active sites that were previously buried within the interiors of the COF. The colloidal dispersion of eNT COF allowed straightforward device fabrication by simple drop casting, which facilitated the creation of a more uniform and homogeneous sensing film. The eNT COF sensor was used to quantify varying concentrations of H2 gas, ranging from 1 to 0.2%, at 200 °C. The sensing mechanism, elucidated by DFT calculations, suggested an electrostatic interaction between H2 molecules and the carbonyl oxygen (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) atoms of the COF, resulting in increased negative charge density on the carbonyl oxygen (Fig. 10c). Unlike SMOs, where the interaction of H2 is with surface-adsorbed oxygen species, here the electrostatic interaction of H2 occurs with functionalities of the COF. In a recent study by our group, a simple and green in situ gas exfoliation method to exfoliate an imine-linked TpPa–SO3H COF, synthesized from triformylphloroglucinol (Tp) and 2,5-diaminobenzene sulfonic acid (Pa–SO3H) was demonstrated. The approach utilised the catalytic activity of the –SO3H groups to induce the hydrolysis of ammonia borane (AB), producing H2 gas within the COF layers, which facilitated exfoliation of the COF into covalent organic nanosheets (CONs). CONs realized through this route not only retained the basic structure but exhibited improved photoelectrochemical and photoluminescence properties and good H2 sensing characteristics with a response of 12.5 ± 1%. The response/recovery times were noted as 5.5 ± 1–2.6 ± 0.5s, respectively, at an operating temperature of 120 °C, towards 1% H2 concentration.154
O) atoms of the COF, resulting in increased negative charge density on the carbonyl oxygen (Fig. 10c). Unlike SMOs, where the interaction of H2 is with surface-adsorbed oxygen species, here the electrostatic interaction of H2 occurs with functionalities of the COF. In a recent study by our group, a simple and green in situ gas exfoliation method to exfoliate an imine-linked TpPa–SO3H COF, synthesized from triformylphloroglucinol (Tp) and 2,5-diaminobenzene sulfonic acid (Pa–SO3H) was demonstrated. The approach utilised the catalytic activity of the –SO3H groups to induce the hydrolysis of ammonia borane (AB), producing H2 gas within the COF layers, which facilitated exfoliation of the COF into covalent organic nanosheets (CONs). CONs realized through this route not only retained the basic structure but exhibited improved photoelectrochemical and photoluminescence properties and good H2 sensing characteristics with a response of 12.5 ± 1%. The response/recovery times were noted as 5.5 ± 1–2.6 ± 0.5s, respectively, at an operating temperature of 120 °C, towards 1% H2 concentration.154
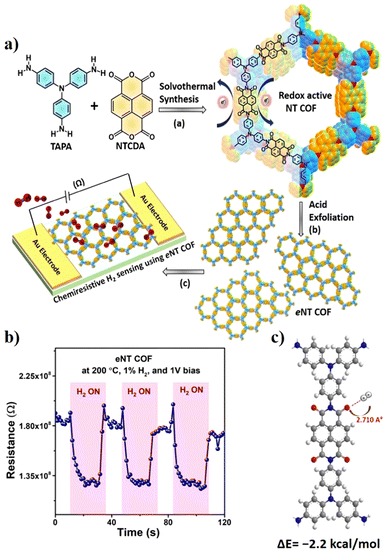 | ||
| Fig. 10 (a) Schematic illustration of the structure of the NT COF showing the solvothermal synthesis of the NT COF, acid exfoliation of the NT COF (eNT COF) and a chemiresistive H2 gas sensing device using eNT COF. (b) Response and recovery curve of eNT COF at optimum temperature with 1% H2 concentration. (c) Molecular electrostatic potential plot of the optimized binding site. Reproduced with permission from ref. 152, Royal Society of Chemistry, Copyright@2023. | ||
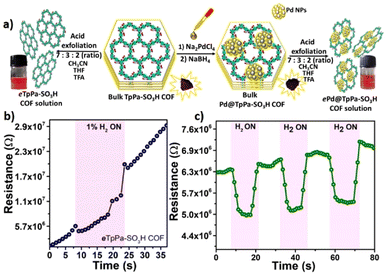 | ||
| Fig. 11 (a) Schematic illustration depicting the experimental protocol followed to prepare exfoliated TpPa-SO3H COF and Pd loaded COF; the Tyndal light scattering effect of dispersions of ePd@TpPa-SO3H COF is also shown. (b) H2 gas sensing performance of eTpPa-SO3H COF at 80 °C. (c) Response and recovery curve of ePd@TpPa-SO3H COF at optimum temperature with 1% H2 concentration. Reproduced with permission from ref. 156, American Chemical Society, copyright@2023. | ||
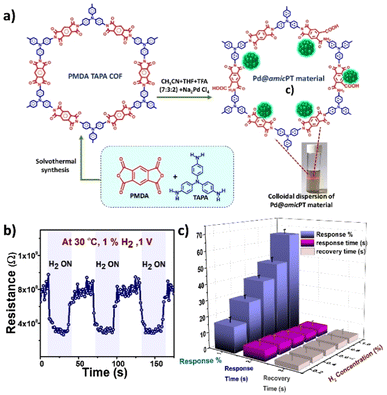 | ||
| Fig. 12 (a) Schematic illustration of the synthesis of PT COF and Pd@amicPT material; the Tyndal light scattering effect of the dispersion is also shown. (b) H2 gas sensing performance of the Pd@amicPT sensor at optimum temperature, 30 °C, for 1% H2. (c) Effect of concentrations ranging from 0.2 to 1% of H2. Reproduced with permission from ref. 157, Elsevier, copyright@2024. | ||
These studies illustrate the significant potential of COFs as emerging materials that can effectively serve as chemiresistive sensing elements for H2. The hybridization of COFs with Pd also offers benefits such as high selectivity and a noticeable improvement in response% and reaction kinetics of the sensor. COFs have yielded promising findings, pushing the boundaries for the effective detection of H2 under ambient conditions, below its flammability limit.
4. H2 sensing mechanism: SMO vs. porous CFs
Commonly adapted mechanisms for H2 sensing in SMOs and porous CFs are discussed in this section.4.1 H2 sensing mechanism in SMOs
In general, H2 detection by SMO-based sensors is primarily influenced by oxygen (O2) ions, oxygen-deficient sites, and ions present in the interstitial sites of SMOs, which play a vital role in electrical conductance.26 Typically, the charge carriers are free electrons (e−) in n-type SMOs and holes (h+) in p-type SMOs. When oxygen, an oxidising molecule from air, adsorbs on the surface of the SMO, it acquires a negative charge by capturing e− from the conduction band, forming adsorbed oxygen species such as O2−(ads), O−(ads) and O2−(ads) (ads: denotes an adsorption site on the surface); this results in the formation of an electron-depleted region or hole-accumulation region in n- or p-type SMOs, respectively.159 Variations in electrical conductivity occur when these chemisorbed oxygen species interact with the H2 gas. In most of the n-type SMOs, such as ZnO, In2O3, and WO3, a redox reaction occurs between H2 and adsorbed oxygen species, producing water and free e−. The produced e− thins the depletion layer, resulting in a significant decrease in the device resistance, as illustrated in Scheme 7a.160,161 For a p-type SMO, exposure to H2 removes the holes and increases the resistance (Scheme 7a).162–164 Under certain circumstances, a direct interaction of H2 with the SMO, resulting in the formation of a metallized region and causing a reduction in resistance, is also reported.165,166 In either case, the SMO reverts to its original state, and the resistance returns to its initial value when re-exposed to air, implying the reversibility of the interaction and re-adsorption of oxygen onto the SMO (Scheme 7a). This widely accepted mechanism infers that the effective interaction between H2 and chemisorbed oxygen species critically influences the sensing capability of the SMO-based sensor.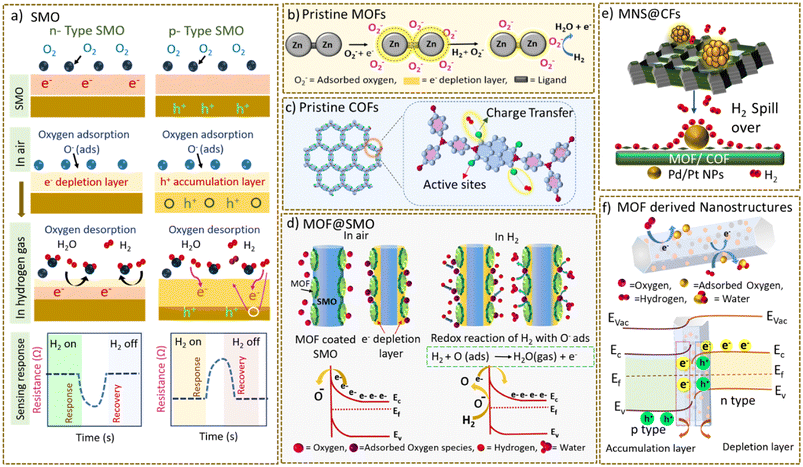 | ||
| Scheme 7 Schematic illustration of the sensing mechanism for the detection of H2. (a) In n-type and p-type SMO. The scheme illustrates the corresponding resistance vs. time plot displaying a decrease in n-type SMO and an increase in the resistance in p-type SMO, upon exposure to H2 gas. This figure has been reproduced from ref. 167 with permission from Shodhganga, copyright@2023. (b) H2 sensing mechanism in pristine MOFs. This figure has been reproduced from ref. 101 with permission with modification, Elsevier, copyright@2022. (c) H2 sensing mechanism in pristine COFs. (d) H2 sensing mechanism in SMO@MOF hybrid materials, adapted with permission with modification from ref. 171, Elsevier, copyright@2019. (e) Spill-over effect in metal NP loaded MOF/COF composites. (f) H2 sensing mechanism in MOF-derived materials, adapted with permission with modification from ref. 171, Elsevier, copyright@2019. | ||
4.2 Sensing mechanism in pristine CFs
The plausible H2 sensing mechanism proposed for pristine MOFs in the existing literature is an extrapolation of a similar concept of electron depletion or hole accumulation that occurs in SMOs (Scheme 7b). The changes in resistance are dictated by the type of MOF (n-type or p-type) in addition to interactions between H2 gas and the framework, more specifically with ligands. For instance, in the combined ZIF-8 and ZIF-67 sensor studied by D. Matataguia et al., the chemiresistive response was represented by an increase in the resistance of the material.100 The response is attributed to the p-type electrical behaviour of the materials and reductive nature of H2. In Co-MOF-I and Co-MOF-II (Co-MOF-74 based sensors), demonstrated by D. K. Nguyen et al., the sensing behaviour of the MOFs is explained using the concept of an electron-depletion mechanism, which involves redox changes in the chemisorbed oxygen species and an interaction with the reducing H2 environment, resulting in a decrease in resistance.102 An analogous pathway is reported by H. W. Kim et al. for a Zn-BDC-NH2 based sensor, which is known to exhibit n-type behaviour.101 However, these studies lack substantial experimental evidence and are rather an adaptation of the mechanism followed by SMO MOF-based materials. Therefore, deriving a clear sensing mechanism is difficult and a thorough understanding of the interaction of H2 with pristine MOFs requires further comprehensive studies in this area.Our understanding of the H2 sensing mechanism in pristine COFs is in the relatively nascent stage, as the field is in the early stages. In pristine COFs, charge transfer interactions between H2 and functional groups present in the structure of the COFs play a determining role in chemiresistive sensing that distinguish COFs from the mechanism that operates in SMOs.64,103,143–145 For instance, as reported in our study, an electrostatic interaction between H2 and the carbonyl oxygen in eNT COF may be responsible for changes in the resistance of eNT COF, as revealed from DFT calculations. Mulliken charge analysis also indicates charge transfer from the H2 molecule to the oxygen atom of the NDI linker, which results in a chemiresistive response (described in section 3.2.1).153 Calculations also revealed that the negative charge density on the oxygen atom increased under an electric field, which further strengthened the interaction with H2. It is noteworthy that there is very little focus on the direct use of pristine COFs as H2 sensors; however, there are reports in the literature on other analyte gases such as NO2, NH3, H2S, and VOCs.63,168–170 All these studies revealed the critical role of functional groups such as the triazine core, imine linkages etc. in the sensing mechanism. A schematic illustrating the interaction of H2 with the functional groups in COFs is shown in Scheme 7c.
4.3 Sensing mechanism in hybrid CFs
In the hybrids of SMOs encapsulated by MOFs, the well-defined and highly porous structure of a MOF functions as a molecular sieving layer, which increases the permeability and concentration of the H2 interaction. The pre-concentrated H2 molecules undergo typical redox reactions with the chemisorbed oxygen species, on the SMO surface (described in section 4.1). This process brings about a change in the resistance of the overall sensing material, which is measured as a H2 sensing signal (Scheme 7d).In composites of Pd@MOF, the permeability and interaction of H2 are primarily governed by three effects: (a) pre-concentration, (b) spill-over, and (c) charge transfer. Pd exhibits a reversible interaction with H2 that makes it particularly beneficial for real-time H2 detection. The pre-concentrated H2 molecules are easily dissociated to hydrogen atoms by the Pd NPs, owing to their high catalytic activity. These dissociated hydrogen atoms then easily migrate (spill over) to the surface of the MOF (Scheme 7e). Notably, Pd@MOF composites exhibit a decrease in resistance when exposed to H2, behaviour that can be attributed to the formation of hydrides, and an associated phase transition from α-PdHx to β-PdHx, as discussed in section 1. The phase change causes a volume expansion, while reducing discontinuities within the Pd nanostructures, thus resulting in effective charge transfer from Pd to the underlying material and, consequently, lowering the resistance.116 A similar role of Pd is anticipated in the case of Pd@COF hybrid materials,157,158 wherein the formation of PdHx species increases the charge carrier concentration in the material owing to its lower work function compared to Pd. The sensing response is generally produced as a negative sigmoidal curve.
4.4 Sensing mechanism in MOF-derived materials
Due to the challenges associated with poor electrical conductivity and charge migration of pristine MOFs, these MOFs have been pyrolyzed at high temperature to obtain nanostructures different from the parent MOF. The MOF-derived nanostructures possess graphitic carbon/a nitrogen-doped carbon network and metal oxide dispersed throughout the matrix.133 The graphitic carbon provides facile charge transport pathways while the derived metal oxide nanostructures possess abundant oxygen adsorption sites/vacancies in addition to multi-component n–n/p–n heterojunctions (Scheme 7d).134 These materials have shown rapid charge migration and improved H2 interactions. This is followed by the redox reactions occurring at the surface of the MOF-derived metal oxide analogous to the phenomenon occurring in the presence of SMOs. In summary, improved charge distribution due to synergistic interactions and the subsequent surface redox reaction with H2 lead to the sensing of H2 in the case of MOF-derived materials.134Taken together with the discussion on H2 sensing, MOFs and COFs exhibit different sensing mechanisms towards H2 as compared to that of SMOs. It should be noted that the highly porous nature, large surface area, and diverse functionalities resulting from a variety of linkers in MOFs/COFs could be additional gears that might provide a new mechanism of H2 sensing. Although limited studies exist on MOFs that follow the electron depletion/accumulation concept, we presume that in pristine MOFs and COFs, sensing is primarily governed by functional groups and linkers, as well as metal clusters in the case of MOFs. When examining the sensing mechanisms of MOFs for analytes other than H2, there is a clear indication that the metal nodes present in MOFs can coordinate with the analytes, enabling selective and sensitive detection of gases.53,60,64 In metal NP-loaded MOF and COF hybrids, similar behaviour is expected from both materials, with the NPs playing a crucial role in facilitating charge transfer.
5. Conclusion and future perspectives
MOFs/COFs belonging to the CF family are demonstrated to be promising materials for next-generation chemiresistive H2 gas sensors through recent examples. Owing to their beneficial structural features, such as rich pore chemistry, high surface area, and well-defined active sites, these materials provide wide opportunities for exploration. While MOFs and their hybrids have been relatively well-explored for H2 sensing, the exploration of COFs in this area represents a promising venture that still needs to be fully exploited. In our opinion, COFs carry greater promise as materials for H2 sensing due to their rich functional diversity. COFs are constructed with linkages, such as boronic esters, imines, imides, hydrazones, azines, triazines, and polar groups (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, NH, S
O, NH, S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), that can act as active sites for reversible interactions with the analyte. Unlike MOFs, where charge transport relies on hopping transport or “guest-promoted” mechanisms, COFs offer more efficient charge transport, arising from the framework itself, due to enhanced in-plane/interplanar charge transport routes. This makes COFs particularly promising for advanced H2 sensing applications. However, CFs exhibit certain limitations that cannot be overlooked and must be addressed to enhance their performance as sensors:
O), that can act as active sites for reversible interactions with the analyte. Unlike MOFs, where charge transport relies on hopping transport or “guest-promoted” mechanisms, COFs offer more efficient charge transport, arising from the framework itself, due to enhanced in-plane/interplanar charge transport routes. This makes COFs particularly promising for advanced H2 sensing applications. However, CFs exhibit certain limitations that cannot be overlooked and must be addressed to enhance their performance as sensors:
(i) Limited detection range: CFs are generally effective at detecting higher analyte concentrations, typically above 0.1 ppm, but struggle to achieve sensitivity at lower trace levels.
(ii) Long-term drift: some CF-based sensors experience long-term drift in baseline resistance, affecting their performance and reliability over time.
(iii) Difficulty in processability: the large-scale green synthesis of CFs is not straightforward given control of their long-range-order growth. Moreover, device fabrication is a cumbersome process, as most CF samples exist as bulk materials or powders, making solution processability difficult.
Chemiresistors based on SMOs and CFs complement each other with respect to their H2 sensing characteristics (Fig. 13). SMOs have the ability to detect H2 at low concentrations (in ppm levels) with good stability (less drift in baseline over time), making them ideal for the trace level detection of H2. However, they require high temperatures (≥300 °C), saturation of signals at higher concentrations and the presence of oxygen. Additionally, SMOs suffer from low selectivity, particularly with gases such as CO and SO2. On the other hand, CF sensors work very well for detecting gases at higher concentrations (0.2–5%), with excellent selectivity. They can operate efficiently under inert conditions as the sensing mechanism is different from that of SMOs. Importantly, the CF-based sensors have the ability to work at moderate temperatures (low temperature range from room temperature to 250 °C), making them suitable candidates for the development of energy-saving chemiresistive gas sensors. Furthermore, most of the reported CF-based sensors exhibited good stability towards humidity. However, the gas sensing data discussed in this review clearly suggest that baseline drift over time is a common phenomenon in these classes of materials. Though CFs do not appear suitable for trace level H2 detection, their ability to handle higher concentrations, where semi-conductor oxides may fail, makes them a valuable complement in comprehensive gas sensing systems.
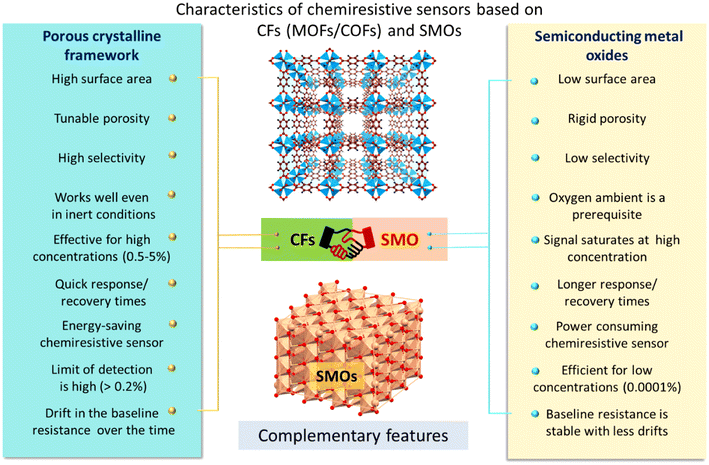 | ||
| Fig. 13 Schematic representation of the characteristic features of CFs and SMOs to complement each other in developing efficient hybrid materials for chemiresistive sensing devices. | ||
In order to design efficient H2 sensors based on pristine CFs, the synthetic design strategies should aim to create notable active sites for H2 interactions. These include functional groups in the organic linker and open metal centers in the secondary building units. Additionally, it is crucial that the interactions with H2 are efficiently converted into measurable signals.172 To achieve this, the framework must possess facile electron-conducting pathways. The incorporation of redox-active linkers (tetrathiafulvalene (TTF) based linkers, metal-bis(dithiolene), naphthalene diimide (NDI) based linkers and quinone-based linkers) can significantly enhance charge transfer properties in CFs.173,174 Additionally, in MOFs, the use of transition metal nodes with partially filled d-orbitals (e.g., Fe or Co) has shown promise for creating MOFs with tunable band gaps, contributing to their semiconducting nature.175 Furthermore, effective signal transduction between particles is critical for the overall performance of the sensing material. Therefore, the utilization of methods such as layer-by-layer assembly, electrophoretic deposition, and spin coating should be an integral part of the device fabrication process.176
Considering the versatility and adaptability of MOFs and COFs, we foresee that the challenges posed by individual materials in chemiresistive sensing can be addressed through hybridization with other materials to form composites, such as CFs hybridized with SMOs (CF@SMO), MOFs with other MOFs (MOF@MOF), MOFs with COFs (MOF@COF), COFs with MOFs (COF@MOF) or employing conducting MOFs/COFs. The prospects for some of these systems in realizing high-performance sensing are discussed below.
5.1 SMO–CF hybrids
Upon reviewing the strengths and limitations of CFs and SMOs, we recognize their potential for synergistic complementarity. Modifying CFs with SMOs could enhance various sensor parameters. The approach involves hybridizing SMOs with CFs to form composites like SMO@MOFs or SMO@COFs, leveraging the strengths of each material. This strategy anticipates that the hybrid composite material could offer improved properties such as broader detection ranges with better sensitivity. For example, in a study, our group demonstrated the assembly of cobalt-imidazole based ZIF-67 MOF onto SnO2 nanoparticles to create SnO2@ZIF-67 material.177 The ZIF-67 MOF is effective at capturing CO2. Coating SnO2 with ZIF-67 significantly boosted the sensitivity of SnO2 towards CO2 at relatively low temperature. The response of SnO2@ZIF-67 is reported to be 16.5% at 205 °C for 5% of CO2, which is one of the highest values recorded for a SnO2-based sensor. The response represents a 3-fold increase compared to pristine SnO2 under the same conditions. The study is a demonstration of the superior performance of SMO@MOF hybrid materials and provides ways to design novel sensors for H2 by utilizing the same strategy.Recent research has highlighted the effective use of 2D materials having p–n heterojunctions in gas sensing applications.178 By carefully selecting p–n junction configurations composed of MOFs/COFs and SMOs, researchers can tailor their properties to achieve selectivity and robust sensing characteristics. For example, Andre et al. developed a p–n heterojunction using n-type In2O3 and p-type reduced graphene oxide (rGO), which exhibited an excellent sensing performance for NH3.179 We expect that creating such heterojunctions with COFs/MOFs might substantially enhance the H2 sensing abilities.
5.2 MOF@COF and COF@MOF composites
Current research has focused on the integration of MOFs with MOFs and MOFs with COFs, which have exciting electrochemical, biosensing, and chemiresistive sensor applications.66,180,181 For example, Cho et al. integrated a 2D MOF (Ni-HHTP) with a 3D MOF (UiO-66-NH2), which showed excellent porosity, conductivity, and new interfacial properties.182 This hybrid MOF was successfully employed for the chemiresistive sensing of toxic H2S gas, achieving enhanced sensitivity with a remarkably low detection limit of 1.4 ppb. While such approaches have not yet been explored for H2 sensing, further research in this direction holds promise for enhanced sensing capabilities.5.3 Conducting MOFs/COFs
A notable number of conducting MOFs and COFs have been documented in the literature, offering their versatility across various applications,183 including chemiresistive sensing.67 These conductive CF sensors operate effectively at room temperature and exhibit impressive selectivity and sensitivity towards low-concentration gases. A study by Yao et al. demonstrated the effectiveness of a conducting MOF chemiresistor, Cu3(HHTP)(THQ), utilizing conductive organic linkages, HHTP (2,3,6,7,10,11-hexahydrotriphenylene) and THQ (tetrahydroxy-1,4-quinone), which showed excellent NH3 detection capabilities under ambient conditions.184 In contrast, research on conducting COF sensors remains relatively limited. Meng et al. reported a prominent example, COF-DC-8, of a 2D-COF employing 2,3,9,10,16,17,23,24-octa-amino phthalocyanine nickel(II) and pyrene-4,5,9,10-tetraone linkages, signifying superior detection of various gases such as NH3, H2S, NO, and NO2 at low concentrations (ppb level) at room temperature.63 However, reports based on H2 sensors are very limited in this direction. Considering initial studies in this area and promising prospects for future research, we believe that MOF/COF-based chemiresistors have great potential to surpass state-of-the-art H2 sensing characteristics. The area also provides enough opportunities for theoretical and experimental researchers to understand the interactions between H2 and MOF/COF linkers, which are responsible for H2 sensing. This may enable the rational design of MOFs/COFs with suitable linkers to realize enhanced sensing performance. Furthermore, the molecular level design of CFs with redox-active linkers with functional groups such as imine, phenazine, carbonyl etc. that have the ability to interact with H2 are particularly beneficial for H2 sensing materials. Furthermore, interactive guest molecules and the incorporation of mixed-valence systems are crucial for optimizing the structure and enhancing the sensing capabilities of CF materials. Thus, the proper amalgamation of synthetic chemists, materials chemists, device physicists and theoreticians may further enhance this area and lead to the development of effective porous CF-based H2 sensors.Author contributions
M.E.D. and N.T. contributed equally. The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
S. B. K. acknowledges the Science and Engineering Research Board (SERB), Department of Science and Technology (DST), Government of India, for funding through a core research grant (CRG/2019/003925).References
- A. Mehtab, S. A. Ali, I. Sadiq, S. Shaheen, H. Khan, M. Fazil, N. A. Pandit, F. Naaz and T. Ahmad, ACS Sustainable Resour. Manage., 2024, 1, 604–620 CrossRef CAS
.
- D. Guan, B. Wang, J. Zhang, R. Shi, K. Jiao, L. Li, Y. Wang, B. Xie, Q. Zhang, J. Yu, Y. Zhu, Z. Shao and M. Ni, Energy Environ. Sci., 2023, 16, 4926–4943 RSC
.
- T. T. Le, P. Sharma, B. J. Bora, V. D. Tran, T. H. Truong, H. C. Le and P. Q. P. Nguyen, Int. J. Hydrogen Energy, 2024, 54, 791–816 CrossRef CAS
.
- A. Midilli and I. Dincer, Int. J. Hydrogen Energy, 2008, 33, 4209–4222 CrossRef CAS
.
- P. A. Le, V. D. Trung, P. L. Nguyen, T. V. Bac Phung, J. Natsuki and T. Natsuki, RSC Adv., 2023, 13, 28262–28287 RSC
.
- V. R. Bakuru, M. E. Dmello and S. B. Kalidindi, ChemPhysChem, 2019, 20, 1177–1215 CrossRef CAS
.
- M. Yue, H. Lambert, E. Pahon, R. Roche, S. Jemei and D. Hissel, Renewable Sustainable Energy Rev., 2021, 146, 111180 CrossRef
.
- S. Evro, B. A. Oni and O. S. Tomomewo, Int. J. Hydrogen Energy, 2024, 78, 1449–1467 CrossRef CAS
.
- G. Nicoletti, N. Arcuri, G. Nicoletti and R. Bruno, Energy Convers. Manage., 2015, 89, 205–213 CrossRef
.
- A. Roy and S. Pramanik, Int. J. Hydrogen Energy, 2024, 49, 792–821 CrossRef CAS
.
- D. Hu, P. Gao, Z. Cheng, Y. Shen, R. He, F. Yi, M. Lu, J. Wang and S. Liu, Energy Fuels, 2024, 38, 4803–4835 CrossRef CAS
.
- Q. Hassan, I. Azzawi, A. Zuhair and H. Salman, Sustainability, 2023, 15, 1–26 Search PubMed
.
- L. Guo, J. Su, Z. Wang, J. Shi, X. Guan, W. Cao and Z. Ou, Int. J. Hydrogen Energy, 2024, 51, 1055–1078 CrossRef CAS
.
- S. Foorginezhad, M. Mohseni-Dargah, Z. Falahati, R. Abbassi, A. Razmjou and M. Asadnia, J. Power Sources, 2021, 489, 229450 CrossRef CAS
.
- T. M. Swager, T. N. Pioch, H. Feng, H. M. Bergman, S.-X. L. Luo and J. J. I. I. Valenza, ACS Sens., 2024, 9, 2205–2227 CrossRef CAS
.
- S. K. Arya, S. Krishnan, H. Silva, S. Jean and S. Bhansali, Analyst, 2012, 137, 2743–2756 RSC
.
- B. K. S. Reddy and P. H. Borse, J. Electrochem. Soc., 2021, 168, 057521 CrossRef CAS
.
- W. T. Koo, H. J. Cho, D. H. Kim, Y. H. Kim, H. Shin, R. M. Penner and I. D. Kim, ACS Nano, 2020, 14, 14284–14322 CrossRef CAS
.
- T. Seiyama, K. Fujiishi, M. Nagatani and A. Kato, J. Soc. Chem. Ind., Jpn., 1963, 66, 652–655 CAS
.
- B. W. H. Brattain and J. Bardeent, Am. Teleph. Telegr. Co., 1952, 32, 1–41 Search PubMed
.
- G. Heiland, Z. Phys., 1954, 138, 459–464 CrossRef CAS
.
-
N. Taguchi, US Pat, 3631436, 1971 Search PubMed
.
- G. Neri, Chemosensors, 2015, 3, 1–20 CrossRef CAS
.
- H. Wohltjen, W. R. Barger, A. W. Snow and N. L. Jarvis, IEEE Trans. Electron Devices, 1985, 32, 1170–1174 Search PubMed
.
- H. Gu, Z. Wang and Y. Hu, Sensors, 2012, 12, 5517–5550 CrossRef CAS
.
- M. B. Rahmani, M. H. Yaacob and Y. M. Sabri, Sens. Actuators, B, 2017, 251, 57–64 CrossRef CAS
.
- C. Kuru, C. Choi, A. Kargar, D. Choi, Y. J. Kim, C. H. Liu, S. Yavuz and S. Jin, Adv. Sci., 2015, 2, 1–5 Search PubMed
.
- L. Gao, Y. Tian, A. Hussain, Y. Guan and G. Xu, Anal. Bioanal. Chem., 2024, 416, 3697–3715 CrossRef CAS PubMed
.
- P. Dariyal, S. Sharma, S. Chauhan, P. Singh and S. R. Dhakate, Nanoscale Adv., 2021, 3, 6514–6544 RSC
.
- E. Llobet, Sens. Actuators, B, 2013, 179, 32–45 CrossRef CAS
.
- A. Ilnicka and J. P. Lukaszewicz, Processes, 2020, 8, 633 CrossRef CAS
.
- E. Kowalska, E. Czerwosz, R. Diduszko, A. Kaminska and M. Danila, Sens. Actuators, A, 2013, 203, 434–440 CrossRef CAS
.
- Q. Li, L. Wang, A. Xiao, L. Zhu and Z. Yang, Int. J. Hydrogen Energy DOI:10.1016/j.ijhydene.2024.01.001
.
- I. Darmadi, F. A. A. Nugroho and C. Langhammer, ACS Sens., 2020, 5, 3306–3327 CrossRef CAS
.
- C. Wadell, F. A. A. Nugroho, E. Lidström, B. Iandolo, J. B. Wagner and C. Langhammer, Nano Lett., 2015, 15, 3563–3570 CrossRef CAS PubMed
.
- A. Mirzaei, H. R. Yousefi, F. Falsafi, M. Bonyani, J. H. Lee, J. H. Kim, H. W. Kim and S. S. Kim, Int. J. Hydrogen Energy, 2019, 44, 20552–20571 CrossRef CAS
.
- M. Tiemann, Chem. – Eur. J., 2007, 13, 8376–8388 CrossRef CAS PubMed
.
- P. M. Bulemo and J. Y. Cheong, ACS Appl. Nano Mater., 2023, 6, 1027–1049 CrossRef CAS
.
- J. Zhang, Z. Qin, D. Zeng and C. Xie, Phys. Chem. Chem. Phys., 2017, 19, 6313–6329 RSC
.
- A. Sharma, S. B. Eadi, H. Noothalapati, M. Otyepka, H. D. Lee and K. Jayaramulu, Chem. Soc. Rev., 2024, 53, 2530–2577 RSC
.
- J. Ha, J. H. Lee and H. R. Moon, Chem. Front., 2020, 7, 12–27 CAS
.
- D. J. Tranchemontagne, J. L. Mendoza-Cortés, M. O'Keeffe and O. M. Yaghi, Chem. Soc. Rev., 2009, 38, 1257–1283 RSC
.
- https://hdl.handle.net/10603/426879 .
- H. Ghasempour, K. Y. Wang, J. A. Powell, F. Zarekarizi, X. L. Lv, A. Morsali and H. Zhou, Coord. Chem. Rev., 2021, 426, 213542 CrossRef CAS
.
- A. P. Côté, A. I. Benin, N. W. Ockwig, M. O'Keeffe, A. J. Matzger and O. M. Yaghi, Science, 2005, 310, 1166–1170 CrossRef PubMed
.
- C. S. Diercks and O. M. Yaghi, Science, 2017, 355, eaal1585 CrossRef PubMed
.
- N. Huang, P. Wang and D. Jiang, Nat. Rev. Mater., 2016, 1, 16068 CrossRef CAS
.
- H. R. Abuzeid, A. F. M. El-Mahdy and S. W. Kuo, Giant, 2021, 6, 100054 CrossRef CAS
.
- R. K. Parashar, P. Jash, M. Zharnikov and P. C. Mondal, Angew. Chem., Int. Ed., 2024, 63, e202317413 CrossRef CAS
.
- L. S. Xie, G. Skorupskii and M. Dincă, Chem. Rev., 2020, 120, 8536–8580 CrossRef CAS PubMed
.
- M. Ko, L. Mendecki and K. A. Mirica, Chem. Commun., 2018, 54, 7873–7891 RSC
.
- R. Saha and C. J. Gomez Garcia, Chem. Soc. Rev., 2024, 9490–9559 RSC
.
- V. Rubio-Giménez, N. Almora-Barrios, G. Escorcia-Ariza, M. Galbiati, M. Sessolo, S. Tatay and C. Martí-Gastaldo, Angew. Chemie, 2018, 130, 15306–15310 CrossRef
.
- M. Souto and D. F. Perepichka, J. Mater. Chem. C, 2021, 9, 10668–10676 RSC
.
- R. Gutzler and D. F. Perepichka, J. Am. Chem. Soc., 2013, 135, 16585–16594 CrossRef CAS PubMed
.
- M. R. Rao, Y. Fang, S. De Feyter and D. F. Perepichka, J. Am. Chem. Soc., 2017, 139, 2421–2427 CrossRef CAS
.
- M. Yu, R. Dong and X. Feng, J. Am. Chem. Soc., 2020, 142, 12903–12915 CrossRef CAS
.
- D. M. D'Alessandro, Chem. Commun., 2016, 52, 8957–8971 RSC
.
- S. Lin, P. M. Usov and A. J. Morris, Chem. Commun., 2018, 54, 6965–6974 RSC
.
- M. G. Campbell, D. Sheberla, S. F. Liu, T. M. Swager and M. DincƏ, Angew. Chem., Int. Ed., 2015, 54, 4349–4352 CrossRef CAS PubMed
.
- E. X. Chen, H. R. Fu, R. Lin, Y. X. Tan and J. Zhang, ACS Appl. Mater. Interfaces, 2014, 6, 22871–22875 CrossRef CAS
.
- L. M. Tao, F. Niu, D. Zhang, T. M. Wang and Q. H. Wang, New J. Chem., 2014, 38, 2774–2777 RSC
.
- Z. Meng, R. M. Stolz and K. A. Mirica, J. Am. Chem. Soc., 2019, 141, 11929–11937 CrossRef CAS PubMed
.
- W.-T. Koo, J.-S. Jang and I.-D. Kim, Chem, 2019, 5, 1938–1963 CAS
.
- J. D. Bhaliya, V. R. Shah, G. Patel and K. Deshmukh, J. Inorg. Organomet. Polym., 2023, 33, 1453–1494 CrossRef CAS
.
- C. Park, J. W. Baek, E. Shin and I. Kim, ACS Nanosci., 2023, 3, 353–374 CrossRef CAS
.
- A. Sharma, S. B. Eadi, H. Noothalapati, M. Otyepka, H. D. Lee and K. Jayaramulu, Chem. Soc. Rev., 2024, 53, 2530–2577 RSC
.
- A. Mei, Z. Yang, M. Zhou, W. Jin, Y. Liu and W. Chen, Anal. Sens., 2024, 4, e202300078 CAS
.
- M. Zhou, Y. Li and G. Xu, TrAC, Trends Anal. Chem., 2024, 174, 117679 CrossRef CAS
.
- M. Kim, M. Pander and H. R. Moon, ACS Appl. Electron. Mater., 2024, 6, 3024–3038 CrossRef CAS
.
- G. H. Pham and C. Z. Dinu, RSC Sustainability, 2023, 1, 1125–1149 RSC
.
- Y.-M. Jo, Y. K. Jo, J.-H. Lee, H. W. Jang, I.-S. Hwang and D. J. Yoo, Adv. Mater., 2023, 35, 2206842 CrossRef CAS
.
- H. Wei, H. Zhang, B. Song, K. Yuan, H. Xiao, Y. Cao and Q. Cao, Int. J. Environ. Res. Public Health, 2023, 20, 4388–4401 CrossRef CAS
.
- H. Yuan, N. Li, W. Fan, H. Cai and D. Zhao, Adv. Sci., 2022, 9, 1–27 Search PubMed
.
- Ankit, N. Saini, H. Pandey and K. Pandey, Macromol. Symp., 2024, 413, 2300058 CrossRef CAS
.
- N. Garg, A. Deep and A. L. Sharma, Coord. Chem. Rev., 2021, 445, 214073 CrossRef CAS
.
- A. Chidambaram and K. C. Stylianou, Inorg. Chem. Front., 2018, 5, 979–998 RSC
.
- H. Y. Li, S. N. Zhao, S. Q. Zang and J. Li, Chem. Soc. Rev., 2020, 49, 6364–6401 RSC
.
- A. Melillo, A. Franconetti, M. Alvaro, B. Ferrer and H. Garcia, Chem. – Eur. J., 2023, 29, e202202625 CrossRef CAS PubMed
.
- V. Zeleňák and I. Saldan, Nanomaterials, 2021, 11, 1638 CrossRef PubMed
.
- Z. Meng and K. A. Mirica, Chem. Soc. Rev., 2021, 50, 13498–13558 RSC
.
- L. Sun, M. G. Campbell and M. DincƏ, Angew. Chem., Int. Ed., 2016, 55, 3566–3579 CrossRef CAS PubMed
.
- R. Saha, K. Gupta and C. J. Gómez García, Cryst. Growth Des., 2024, 24, 2235–2265 CrossRef CAS
.
- S. G. Ibrahim and A. U. Ubale, J. Mol. Struct., 2014, 1076, 291–298 CrossRef CAS
.
- G. Givaja, P. Amo-Ochoa, C. J. Gómez-García and F. Zamora, Chem. Soc. Rev., 2012, 41, 115–147 RSC
.
- J. F. Fennell, S. F. Liu, J. M. Azzarelli, J. G. Weis, S. Rochat, K. A. Mirica, J. B. Ravnsbæk and T. M. Swager, Angew. Chem., Int. Ed., 2016, 55, 1266–1281 CrossRef CAS PubMed
.
- M. A. Franco, P. P. Conti, R. S. Andre and D. S. Correa, Sens. Actuators Rep., 2022, 4, 100100 CrossRef
.
- R. Tang, Y. Shi, Z. Hou and L. Wei, Sensors, 2017, 17, 882–897 CrossRef PubMed
.
- N. Le Hung, E. Ahn, S. Park, H. Jung, H. Kim, S.-K. Hong, D. Kim and C. Hwang, J. Vac. Sci. Technol., A, 2009, 27, 1347–1351 CrossRef
.
- F. Rumiche, H. H. Wang and J. E. Indacochea, Sens. Actuators, B, 2012, 163, 97–106 CrossRef CAS
.
- K. Chikkadi, M. Muoth, W. Liu, V. Maiwald and C. Hierold, Sens. Actuators, B, 2014, 196, 682–690 CrossRef CAS
.
- A. Shrivastava and V. Gupta, Chron. Young Sci., 2011, 2, 21 Search PubMed
.
- R. Kumar, R. N. Jenjeti and S. Sampath, ACS Sens., 2020, 5, 404–411 Search PubMed
.
- F. Gu, M. Di, D. Han, S. Hong and Z. Wang, ACS Sens., 2020, 5, 2611–2619 CrossRef CAS
.
- J. Burgués, J. M. Jiménez-Soto and S. Marco, Anal. Chim. Acta, 2018, 1013, 13–25 CrossRef
.
- M. Drobek, J. H. Kim, M. Bechelany, C. Vallicari, A. Julbe and S. S. Kim, ACS Appl. Mater. Interfaces, 2016, 8, 8323–8328 CrossRef CAS
.
- N. Stock and S. Biswas, Chem. Rev., 2012, 112(2), 933–969 CrossRef CAS
.
- W.-J. Li, M. Tu, R. Cao and R. A. Fischer, J. Mater. Chem. A, 2016, 4, 12356–12369 RSC
.
- M. Rivera-Torrente, L. D. B. Mandemaker, M. Filez, G. Delen, B. Seoane, F. Meirer and B. M. Weckhuysen, Chem. Soc. Rev., 2020, 49, 6694–6732 RSC
.
- D. Matatagui, A. Sainz-Vidal, I. Gràcia, E. Figueras, C. Cané and J. M. Saniger, Sens. Actuators, B, 2018, 274, 601–608 CrossRef CAS
.
- D. H. Yang, T. T. T. Nguyen, S. T. Navale, L. H. T. Nguyen, Y. T. Dang, N. X. D. Mai, T. B. Phan, J.-Y. Kim, T. L. H. Doan, S. S. Kim and H. W. Kim, Sens. Actuators, B, 2022, 368, 132120 CrossRef CAS
.
- D. K. Nguyen, J. H. Lee, T. L. H. Doan, T. B. Nguyen, S. Park, S. S. Kim and B. T. Phan, Appl. Surf. Sci., 2020, 523, 146487 CrossRef CAS
.
- R. Zhang, L. Lu, Y. Chang and M. Liu, J. Hazard. Mater., 2022, 429, 128321 CrossRef CAS PubMed
.
- Y. Wang and D. Zhao, Cryst. Growth Des., 2017, 17, 2291–2308 CrossRef CAS
.
- C. Zhang, R. P. Lively, K. Zhang, J. R. Johnson, O. Karvan and W. J. Koros, J. Phys. Chem. Lett., 2012, 3, 2130–2134 CrossRef CAS PubMed
.
- Q. Ke, Y. Duan, Y. Ji, D. Zhao, H. Zhang, C. Duan, L. Li and Y. Wei, J. Chem. Eng. Data, 2021, 66, 3483–3492 CrossRef CAS
.
- D. Peralta, G. Chaplais, A. Simon-Masseron, K. Barthelet, C. Chizallet, A. A. Quoineaud and G. D. Pirngruber, J. Am. Chem. Soc., 2012, 134, 8115–8126 CrossRef CAS
.
- A. Huang, Q. Liu, N. Wang and Y. Zhu, J. Am. Chem. Soc., 2014, 136, 14686–14689 CrossRef CAS PubMed
.
- Y. Pan, B. Wang and Z. Lai, J. Membr. Sci., 2012, 421–422, 292–298 CrossRef CAS
.
- C. W. Kung, A. E. Platero-Prats, R. J. Drout, J. Kang, T. C. Wang, C. O. Audu, M. C. Hersam, K. W. Chapman, O. K. Farha and J. T. Hupp, ACS Appl. Mater. Interfaces, 2018, 10, 30532–30540 CrossRef CAS
.
- Z. Wen, L. Zhu, Z. Zhang and Z. Ye, Sens. Actuators, B, 2015, 208, 112–121 CrossRef CAS
.
- H. Yue, Z. Shi, Q. Wang, Z. Cao, H. Dong, Y. Qiao, Y. Yin and S. Yang, ACS Appl. Mater. Interfaces, 2014, 6, 17067–17074 CrossRef CAS PubMed
.
- T. Zhou, Y. Sang, X. Wang, C. Wu, D. Zeng and C. Xie, Sens. Actuators, B, 2018, 258, 1099–1106 CrossRef CAS
.
- A. I. Khudiar, A. K. Elttayef, M. K. Khalaf and A. M. Oufi, Mater. Res. Express, 2020, 6, 126450 Search PubMed
.
- R. Lv, Q. Zhang, W. Wang, Y. Lin and S. Zhang, Sensors, 2021, 21, 4069 CrossRef CAS PubMed
.
- T. B. Flanagan and W. A. Oates, Annu. Rev. Mater. Sci., 1991, 21, 269–304 CrossRef CAS
.
- W. T. Koo, S. Qiao, A. F. Ogata, G. Jha, J. S. Jang, V. T. Chen, I. D. Kim and R. M. Penner, ACS Nano, 2017, 11, 9276–9285 Search PubMed
.
- S. Fardindoost, S. Hatamie, A. I. Zad and F. R. Astaraei, Nanotechnology, 2018, 29, 015501 CrossRef PubMed
.
- M. Weber, J. H. Kim, J. H. Lee, J. Y. Kim, I. Iatsunskyi, E. Coy, M. Drobek, A. Julbe, M. Bechelany and S. S. Kim, ACS Appl. Mater. Interfaces, 2018, 10, 34765–34773 CrossRef CAS PubMed
.
- B. Xie, B. Ding, P. Mao, Y. Wang, Y. Liu, M. Chen, C. Zhou, H. min Wen, S. Xia, M. Han, R. E. Palmer, G. Wang and J. Hu, Small, 2022, 18, 1–13 Search PubMed
.
- M. K. Smith, K. E. Jensen, P. A. Pivak and K. A. Mirica, Chem. Mater., 2016, 28, 5264–5268 CrossRef CAS
.
- M. G. Campbell, S. F. Liu, T. M. Swager and M. Dincă, J. Am. Chem. Soc., 2015, 137, 13780–13783 CrossRef CAS
.
- D. M. D'Alessandro, Chem. Commun., 2016, 52, 8957–8971 RSC
.
- H. Feng, J. Wang, Z. Tong and H.-Y. Qu, Chem. Eng. J., 2022, 442, 136158 CrossRef
.
- K. S. Pedersen, P. Perlepe, M. L. Aubrey, D. N. Woodruff, S. E. Reyes-Lillo, A. Reinholdt, L. Voigt, Z. Li, K. Borup, M. Rouzières, D. Samohvalov, F. Wilhelm, A. Rogalev, J. B. Neaton, J. R. Long and R. Clérac, Nat. Chem., 2018, 10, 1056–1061 CrossRef CAS PubMed
.
- M. E. Dmello, R. C. Sahoo, R. Raghunathan, H. S. S. R. Matte, P. Yadav, G. V. Shanbhag and S. B. Kalidindi, Int. J. Hydrogen Energy, 2022, 47, 9477–9483 CrossRef CAS
.
- X. Liu, G. Verma, Z. Chen, B. Hu, Q. Huang, H. Yang, S. Ma and X. Wang, The Innovation, 2022, 3, 100281 CrossRef CAS PubMed
.
- N. Cui, K. Bi, W. Sun, Q. Wu, Y. Li, T. Xu, B. Lv and S. Zhang, Catalysts, 2021, 11, 1–12 CrossRef
.
- J. Ren, Y. Huang, H. Zhu, B. Zhang, H. Zhu, S. Shen, G. Tan, F. Wu, H. He, S. Lan, X. Xia and Q. Liu, Carbon Energy, 2020, 2, 176–202 CrossRef CAS
.
- C. Montoro, J.-Y. Kim, A. Mirzaei, J.-H. Lee, S. Sayegh, E. Makhoul, I. Iatsunskyi, E. Coy, M. Bechelany, H. W. Kim and S. S. Kim, Composites, Part B, 2024, 283, 111637 CrossRef CAS
.
- J. Lin, W. Ho, X. Qin, C.-F. Leung, V. K.-M. Au and S.-c. Lee, Small, 2022, 18, 2105484 CrossRef CAS
.
- A. Sharma, K. Karuppasamy, D. Vikraman, Y. Cho, K. Adaikalam, J. G. Korvink, H. S. Kim and B. Sharma, ACS Appl. Mater. Interfaces, 2022, 14, 44516–44526 CrossRef CAS
.
- S. Zhou, J. Ji, T. Qiu, L. Wang, W. Ni, S. Li, W. Yan, M. Ling and C. Liang, Inorg. Chem. Front., 2022, 9, 599–606 RSC
.
- M. E. Dmello, S. Vishwanathan, V. R. Bakuru, G. V. Shanbhag and S. B. Kalidindi, ACS Appl. Nano Mater., 2023, 6, 238–247 CrossRef CAS
.
- M. Guo, N. Luo, Y. Bai, Z. Xue, Q. Hu and J. Xu, Sens. Actuators, B, 2024, 398, 134151 CrossRef CAS
.
- L. Zhang, J. Xiong, B. Ding, C. Fan, G. Liu and H. Li, Sens. Actuators, B Chem, 2024, 407, 135471 CrossRef CAS
.
- Y. Kumar, I. Ahmad, A. Rawat, R. K. Pandey, P. Mohanty and R. Pandey, ACS Appl. Mater. Interfaces, 2024, 16, 11605–11616 CrossRef CAS PubMed
.
- S. Ruidas, L. Pradhan, B. Mohanty, S. Dalapati, S. Kumar, B. K. Jena and A. Bhaumik, ACS Appl. Energy Mater., 2024, 7, 2872–2880 CrossRef CAS
.
- W. Huang, W. Zhang, S. Yang, L. Wang and G. Yu, Small, 2024, 20, 2308019 CrossRef CAS
.
- Y. Yusran, H. Li, X. Guan, Q. Fang and S. Qiu, EnergyChem, 2020, 2, 100035 CrossRef
.
- Z. Alsudairy, N. Brown, A. Campbell, A. Ambus, B. Brown, K. Smith-Petty and X. Li, Mater. Chem. Front., 2023, 7, 3298–3331 RSC
.
- S. Zhang, D. Liu and G. Wang, Molecules, 2022, 27, 2586–2630 CrossRef CAS PubMed
.
- A. Mei, Z. Yang, M. Zhou, W. Jin, Y. Liu and W. Chen, Anal. Sens., 2024, 4, e202300078 CAS
.
- M. Zhou, Y. Li and G. Xu, TrAC Trends Anal. Chem., 2024, 174, 117679 CrossRef CAS
.
- Y. Shi, L. Ni, Z. Wang, M. Chen and L. Feng, Coord. Chem. Rev., 2024, 505, 215691 CrossRef CAS
.
- Z. Wang, Y. Li, P. Liu, Q. Qi, F. Zhang, G. Lu, X. Zhao and X. Huang, Nanoscale, 2019, 11, 5330–5335 RSC
.
- Y. Tao, W. Ji, X. Ding and B. H. Han, J. Mater. Chem. A, 2021, 9, 7336–7365 RSC
.
- B. J. Smith, L. R. Parent, A. C. Overholts, P. A. Beaucage, R. P. Bisbey, A. D. Chavez, N. Hwang, C. Park, A. M. Evans, N. C. Gianneschi and W. R. Dichtel, ACS Cent. Sci., 2017, 3, 58–65 CrossRef CAS PubMed
.
- S. Wang, Q. Wang, P. Shao, Y. Han, X. Gao, L. Ma, S. Yuan, X. Ma, J. Zhou, X. Feng and B. Wang, J. Am. Chem. Soc., 2017, 139, 4258–4261 CrossRef CAS PubMed
.
- H. Duan, K. Li, M. Xie, J. Chen, H. Zhou, X. Wu, G. Ning, A. I. Cooper and D. Li, J. Am. Chem. Soc., 2021, 143, 19446–19453 CrossRef CAS
.
- Y. Yusran, H. Li, X. Guan, D. Li, L. Tang, M. Xue, Z. Zhuang, Y. Yan, V. Valtchev, S. Qiu and Q. Fang, Adv. Mater., 2020, 32, 1–9 Search PubMed
.
- N. Thokala, K. Vankayala, A. D. Gaonkar, G. Periyasamy, K. Fazl-Ur-Rahman, K. Valle, M. E. Dmello, K. Basavaiah and S. B. Kalidindi, Sens. Diagn., 2023, 2, 1176–1180 RSC
.
- D. W. Burke, C. Sun, I. Castano, N. C. Flanders, A. M. Evans, E. Vitaku, D. C. McLeod, R. H. Lambeth, L. X. Chen, N. C. Gianneschi and W. R. Dichtel, Angew. Chem., Int. Ed., 2020, 59, 5165–5171 CrossRef CAS
.
- V. Krishnaveni, M. E. Dmello, N. Thokala, A. Bonda, V. K. Sriramadasu, S. Akkenapally, S. B. Bubanale, K. Basavaiah, S. Bhattacharyya and S. B. Kalidindi, ACS Appl. Nano Mater., 2024, 7, 23416–23422 CrossRef CAS
.
- J. Chen, Y. Wang, Y. Yu, J. Wang, J. Liu, H. Ihara and H. Qiu, Exploration, 2023, 3, 20220144 CrossRef PubMed
.
- V. Krishnaveni, M. Esclance Dmello, P. Sahoo, N. Thokala, V. R. Bakuru, K. Vankayala, K. Basavaiah and S. B. Kalidindi, ACS Appl. Nano Mater., 2023, 6, 10960–10966 CrossRef CAS
.
- N. Thokala, K. Vankayala, K. Basavaiah and S. B. Kalidindi, Int. J. Hydrogen Energy, 2024, 81, 270–279 CrossRef CAS
.
- G. Y. Lee, J. Lee, H. T. Vo, S. Kim, H. Lee and T. Park, Sci. Rep., 2017, 7, 1–10 CrossRef
.
- I. D. Kim, A. Rothschild and H. L. Tuller, Acta Mater., 2013, 61, 974–1000 CrossRef CAS
.
- Z. Li, S. Yan, Z. Wu, H. Li, J. Wang, W. Shen, Z. Wang and Y. Fu, Int. J. Hydrogen Energy, 2018, 43, 22746–22755 CrossRef CAS
.
- E. Amani, K. Khojier and S. Zoriasatain, Int. J. Hydrogen Energy, 2017, 42, 29620–29628 CrossRef CAS
.
- H. Steinebach, S. Kannan, L. Rieth and F. Solzbacher, Sens. Actuators, B, 2010, 151, 162–168 CrossRef CAS
.
- M. Govindhan, B. Sidhureddy and A. Chen, ACS Appl. Nano Mater., 2018, 1, 6005–6014 CrossRef CAS
.
- D. P. Volanti, A. A. Felix, M. O. Orlandi, G. Whitfield, D. J. Yang, E. Longo, H. L. Tuller and J. A. Varela, Adv. Funct. Mater., 2013, 23, 1759–1766 CrossRef CAS
.
- C. Wang, G. Zhou, J. Li, B. Yan and W. Duan, Phys. Rev. B:Condens. Matter Mater. Phys., 2008, 77, 1–7 Search PubMed
.
- O. K. Varghese, D. Gong, M. Paulose, K. G. Ong and C. A. Grimes, Sens. Actuators, B, 2003, 93, 338–344 CrossRef CAS
.
- https://hdl.handle.net/10603/537933 .
- F. Niu, Z. W. Shao, L. M. Tao and Y. Ding, Sens. Actuators, B, 2020, 321, 128513 CrossRef CAS
.
- W. C. Ko, M. S. Kim, Y. J. Kwon, J. Jeong, W. R. Kim, H. Choi, J. K. Park and Y. K. Jeong, J. Mater. Chem. A, 2020, 8, 19246–19253 RSC
.
- F. Niu, Z. W. Shao, J. L. Zhu, L. M. Tao and Y. Ding, J. Mater. Chem. C, 2021, 9, 8562–8569 RSC
.
- X. Wu, S. Xiong, Y. Gong, Y. Gong, W. Wu, Z. Mao, Q. Liu, S. Hu and X. Long, Sens. Actuators, B, 2019, 292, 32–39 CrossRef CAS
.
- H. Yuan, N. Li, W. Fan, H. Cai and D. Zhao, Adv. Sci., 2022, 9, 1–27 Search PubMed
.
- M. Souto, K. Strutyński, M. Melle-Franco and J. Rocha, Chem. – Eur. J., 2020, 26, 10912–10935 CrossRef CAS PubMed
.
- G. Benedetto and K. A. Mirica, Acc. Chem. Res., 2024, 57, 2775–2789 CrossRef CAS
.
- M. Ko, L. Mendecki and K. A. Mirica, Chem. Commun., 2018, 54, 7873–7891 RSC
.
- W. Li, Z. Zhu, Q. Chen, J. Li and M. Tu, Cell Rep. Phys. Sci., 2023, 4, 101679 CrossRef CAS
.
- M. E. Dmello, N. G. Sundaram and S. B. Kalidindi, Chem. – Eur. J., 2018, 24, 9220–9223 CrossRef CAS PubMed
.
- M. Mathew, P. V. Shinde, R. Samal and C. S. Rout, J. Mater. Sci., 2021, 56, 9575–9604 CrossRef CAS
.
- R. S. Andre, L. A. Mercante, M. H. M. Facure, L. H. C. Mattoso and D. S. Correa, Appl. Surf. Sci., 2019, 473, 133–140 CrossRef CAS
.
- B. Mohan, R. Kumari, G. Singh, K. Singh, A. J. L. Pombeiro, X. Yang and P. Ren, Environ. Int., 2023, 175, 107928 Search PubMed
.
- C. Liu, J. Wang, J. Wan and C. Yu, Coord. Chem. Rev., 2021, 432, 213743 CrossRef CAS
.
- S. Cho, C. Park, M. Jeon, J. H. Lee, O. Kwon, S. Seong, J. Kim, I.-D. Kim and H. R. Moon, Chem. Eng. J., 2022, 449, 137780 Search PubMed
.
- K. K. Liu, Z. Meng, Y. Fang and H.-L. Jiang, eScience., 2023, 3, 100133 Search PubMed
.
- M.-S. Yao, P. Wang, Y.-F. Gu, T. Koganezawa, H. Ashitani, Y. Kubota, Z.-M. Wang, Z.-Y. Fan, K.-i. Otake and S. Kitagawa, Dalton Trans., 2021, 50, 13236–13245 RSC
.
Footnote |
| † These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2025 |