Fine-tuning of optical band gap in mixed halide aziridinium lead perovskites†
Olesia I.
Kucheriv‡
 a,
Dmytro A.
Haleliuk‡
a,
Sergiu
Shova
b and
Il'ya A.
Gural'skiy
a,
Dmytro A.
Haleliuk‡
a,
Sergiu
Shova
b and
Il'ya A.
Gural'skiy
 *a
*a
aDepartment of Chemistry, Taras Shevchenko National University of Kyiv, Volodymyrska St. 64, 01601 Kyiv, Ukraine. E-mail: illia.guralskyi@univ.kiev.ua
bDepartment of Inorganic Polymers, “Petru Poni” Institute of Macromolecular Chemistry, 41A Aleea Gr. Ghica Voda, 700487 Iasi, Romania
First published on 29th November 2024
Abstract
Hybrid halide perovskites form a promising class of light-absorbing materials. Among the numerous 3D semiconducting perovskites, there is a group of emerging aziridinium-based hybrids that are considered to be prospective materials for optoelectronic applications. In this work, we report the mixed halide aziridinium perovskites of (AzrH)PbBrxI3−x series (AzrH = aziridinium). Small changes in the composition of perovskites are shown to have a defining impact on the optoelectronic properties of the reported materials. Halogen substitution allowed a variation in band gap values of these compounds, ranging from 1.57 to 2.23 eV, as established using electronic spectroscopy. Crystal structures of (AzrH)PbBrxI3−x perovskites were studied using single crystal and powder X-ray diffraction analysis. The lattice constant had a linear dependence on the Br content in the structure, thus strictly following Vegards's law. Importantly, the reported compounds displayed a preferential inclusion of iodine upon synthesis, revealing that the mixed halide perovskite composition cannot be estimated based on the precursors’ ratio only, and it should be post-synthetically checked. The reported results expand the range of hybrid perovskites with tuneable band gaps beyond the conventional methylammonium and formamidinium-based perovskites and offer a new series of metal-halide hybrids suitable for photovoltaic and other optoelectronic applications.
Introduction
Hybrid halide perovskites attract significant attention as photoabsorbers in various optoelectronic applications.1–3 The materials themselves were first reported in 1978 by Weber et al.4,5 However, the rapid developments in perovskite research started only in 2009 when Kojima et al. demonstrated the potential applications of hybrid perovskite in photovoltaic devices.6 Even though the efficiency of the first prototypes was quite low and they had some stability issues, the significant effort that has been put into the development of this research field allowed obtaining perovskite solar cells with efficiency exceeding 25%.7 Such increasing values of efficiency and inexpensive precursors, along with simple solution-based deposition approaches, make hybrid perovskites highly attractive materials for the rapidly growing industry of solar cell technology.8 In addition, hybrid perovskites have also been successfully implemented as active layers in light emitting diodes,9 lasers,10 thermoelectric couplers,11 used for water splitting applications,12 photodetection13 and more.Hybrid halide perovskites have a general formula ABX3, where A is an organic cation, B is a cation of divalent metal (usually Pb2+ or Sn2+), and X is a halogen anion (Cl−, Br− or I−). In its crystal structure, the hybrid perovskite is composed of [BX6] octahedra that are assembled in a corner-sharing manner into an infinite 3D framework. Among these octahedra there are cuboctahedral voids that are occupied by organic cations. Limited space in the framework voids sets very strict geometrical limitations on the size of the organic cation. A cation that is too big for the void will induce the formation of layered low-dimensional perovskites, while a cation that is too small will cause an excessive strain, preventing the formation of the framework. The most commonly studied and used hybrid perovskites are based on methylammonium (MA). MAPbI3 is considered to be a background standard material that is used for all the subsequent modifications.14 This cation is quite often replaced with formamidinium (FA), which also has high efficiency for photovoltaic applications.15–19 Simultaneously, in recent years, several new organic cations, such as aziridinium,20–23 methylhydrazinium24–27 and a few others,28–30 have been found to be suitable for the formation of 3D hybrid perovskite structures.
An important feature of the hybrid halide perovskites that makes them beneficial, in comparison with commercially used semiconductors, is the simplicity of their synthesis through widespread solution-based approaches. These approaches allow very simple, yet elegant, engineering of the chemical composition of the hybrid perovskites, which has a significant impact on their photophysical properties. In particular, the composition allows modification of the band gap of these materials. Modification of the A-site cation (for example, partial substitution of MA with FA) usually enables quite minor changes in the band gap values in the order of 0.1 eV.14 Simultaneously, the substitution of I with Br has been shown to allow band gap modification from 1.5 eV (for pure I perovskites) to ca. 2.3 eV (for pure Br perovskites). Such a substitution has been studied for both MA31–33 and FA34 perovskites. Supposedly, the same substitution is possible for the I and Cl combination, it could allow a band gap tuning in an even larger range. However, in practice, there are certain obstacles that emerge upon the introduction of chloride,35 making the Br/I system the most promising for further investigations.
In this paper, we report a series of new semiconducting materials for optoelectronic applications: mixed (AzrH)PbBrxI3−x perovskites in both single crystal and polycrystalline form, as well as their structural and spectroscopic characterization.
Results and discussion
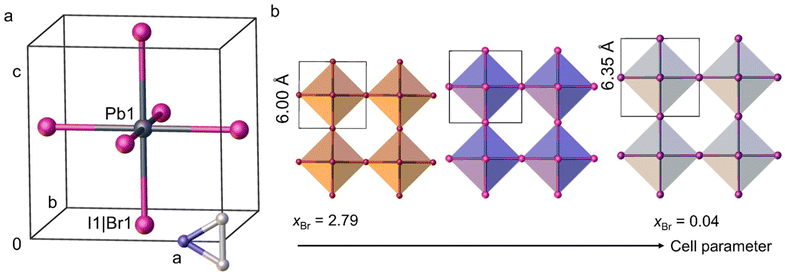
The crystal structures of aziridinium lead mixed halide perovskites were studied by performing seven SXRD measurements on crystals with different Br/I ratios (Tables 1 and S2.1–S8.3†). All obtained materials crystalize in cubic Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m space group, same as (AzrH)PbBr3 and (AzrH)PbI3 perovskites. In their crystal structure, the [PbX6] octahedra are connected with each other in an infinite 3D framework in a corner-sharing manner (Fig. 1). Voids between the inorganic octahedra are filled with organic aziridinium cations that are disordered among multiple positions at the temperatures of the experiments. It is worth noting that the offered solutions for aziridinium disorder are only a couple of multiple possible ways to model this cation. Aziridinium cation interacts with the inorganic framework via N–H⋯Hal hydrogen bonds.
m space group, same as (AzrH)PbBr3 and (AzrH)PbI3 perovskites. In their crystal structure, the [PbX6] octahedra are connected with each other in an infinite 3D framework in a corner-sharing manner (Fig. 1). Voids between the inorganic octahedra are filled with organic aziridinium cations that are disordered among multiple positions at the temperatures of the experiments. It is worth noting that the offered solutions for aziridinium disorder are only a couple of multiple possible ways to model this cation. Aziridinium cation interacts with the inorganic framework via N–H⋯Hal hydrogen bonds.
| (AzrH)PbBr0.04I2.96 | (AzrH)PbBr0.09I2.91 | (AzrH)PbBr0.85I2.15 | (AzrH)PbBr1.96I1.04 | (AzrH)PbBr2.01I0.99 | (AzrH)PbBr2.08I0.92 | (AzrH)PbBr2.79I0.21 | |
|---|---|---|---|---|---|---|---|
| R 1 = ∑||Fo| − |Fc||/∑|Fo| and wR2 = [∑w(Fo2 − Fc2)2/∑w(Fo2)2]1/2 for Fo2 > 2σ(Fo2). | |||||||
| Temperature (K) | 250 | 250 | 293 | 230 | 293 | 250 | 293 |
| Crystal system | Cubic | Cubic | Cubic | Cubic | Cubic | Cubic | Cubic |
| Space group |
Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m m |
Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m m |
Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m m |
Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m m |
Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m m |
Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m m |
Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m m |
| a (Å) | 6.35060(10) | 6.33861(18) | 6.2570(2) | 6.10960(10) | 6.0891(2) | 6.0775(3) | 5.9968(3) |
| Volume (Å3) | 256.120(12) | 254.67(2) | 244.96(2) | 228.054(11) | 225.77(2) | 224.48(3) | 215.65(3) |
| Z | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| ρ calc. (g cm−3) | 4.046 | 4.054 | 3.972 | 3.887 | 3.909 | 3.907 | 3.806 |
| Goodness-of-fit on F2 | 1.132 | 1.250 | 1.185 | 1.227 | 1.001 | 1.116 | 1.174 |
| R 1 [I> = 2σ(I)] | 0.0300 | 0.0140 | 0.0663 | 0.0171 | 0.0353 | 0.0176 | 0.0437 |
| wR2 [all data] | 0.0861 | 0.0428 | 0.1852 | 0.0578 | 0.0847 | 0.0423 | 0.1073 |
Single crystal XRD experiments are a precise tool for determining unit cell parameters for all perovskites in the obtained series and to refine the ratio of Br and I as these halogens have considerably different contributions to the electronic density in the structure. The plot of cell parameter a as a function of Br content x is given in Fig. 2. The obtained plot has a linear dependence that can be linearized by the following function:
| a = 6.3577 − 1.2996xBr. | (1) |
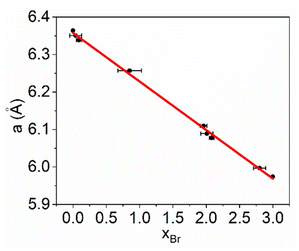 | ||
| Fig. 2 Dependance of the cell parameter a on the xBr content in mixed halide (AzrH)PbBrxI3−x perovskites. | ||
A linear descent of the given dependance with xBr growth is observed in (AzrH)PbBrxI3-x mixed halide perovskites, following the empirical Vegard's law. This suggests that these materials form random solid solutions. A similar linear trend of cell parameter dependance has been observed for MAPbBrxI3−x solid solutions for the xBr = 0.24–2.73 range, in which the formation of the cubic form is supported.36 Meanwhile, iodine-rich samples of MAPbBrxI3−x tend to form a tetragonal phase.
According to Goldschmidt's rule, the formation of a perovskite 3D structure is possible, when the tolerance factor t lies in the range of 0.8–1:
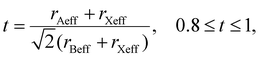 | (2) |
The value of the tolerance factor is 0.956 for (AzrH)PbBr3 and 0.939 for (AzrH)PbI3. As the Pb–X bond lengths follow Vegard's rule in the (AzrH)PbBrxI3−x series, the tolerance factor in these mixed halide perovskites also drops linearly with the decrease in the Br content (Table S1†). It is worth noting that the Pb–Hal bond length obtained from the SXRD experiments is always lower than the sum of calculated effective ionic radii (by 0.163 Å for (AzrH)PbBr3 and by 0.208 Å for (AzrH)PbI3), indicating the partially covalent nature of the Pb–Hal bonds in the hybrid perovskites.
In addition, a series of polycrystalline (AzrH)PbBrxI3−x mixed halide perovskite samples were obtained in bulk form. The phase purity of the polycrystalline samples was established using PXRD measurements (Fig. 3). Experimental PXRD patterns are in good agreement with patterns calculated from the SXRD data, confirming the formation of a pure cubic Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phase for all the obtained materials. Upon increase in the Br content (i.e., x) in the obtained materials, 2θ values of some characteristic peaks shift from 27.97° (002), 31.36° (012), 40.11° (022), and 42.42° (003) for xBr = 0.00 to 29.78° (002), 33.41° (012), 42.60° (022) and 45.24° (003) for xBr = 2.73. In addition, the peak at 24.13°, corresponding to the (111) plane for xBr = 0.00, almost disappears for xBr = 2.73.
m phase for all the obtained materials. Upon increase in the Br content (i.e., x) in the obtained materials, 2θ values of some characteristic peaks shift from 27.97° (002), 31.36° (012), 40.11° (022), and 42.42° (003) for xBr = 0.00 to 29.78° (002), 33.41° (012), 42.60° (022) and 45.24° (003) for xBr = 2.73. In addition, the peak at 24.13°, corresponding to the (111) plane for xBr = 0.00, almost disappears for xBr = 2.73.
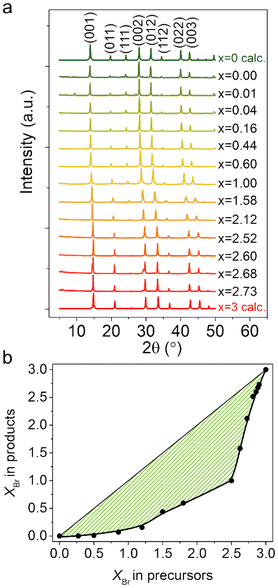 | ||
| Fig. 3 (a) Experimental PXRD patterns of polycrystalline (AzrH)PbBrxI3−x mixed halide perovskite samples, in comparison with the calculated patterns for (AzrH)PbI3 and (AzrH)PbBr3.23 The mentioned Br content x is determined from cell lattice parameters. (b) The dependance of xBr in the obtained products on the xBr in the precursor solutions shows a significant preferential inclusion of iodine. A line is drawn to guide the eye. | ||
The direct refinement of the halogen ratio from PXRD peak intensity is not very precise, as the intensity of peaks is usually strongly affected by preferential orientation and other factors. Meanwhile, indexing of the PXRD patterns provides precise values of unit cell parameters, especially in the case of the title perovskite materials with cubic structures. This is the reason why we used unit cell parameters to extract the halogen ratio by means of dependance as shown in Fig. 2.
Indexing of patterns and comparison of obtained cell parameters revealed a notable difference in the Br/I ratio in the precursor solutions and in the resulting mixed halide perovskites (Fig. 3b). In the case of (AzrH)PbBrxI3−x series, a significant preferential inclusion of iodine is observed. From the chemical point of view, this effect can be explained in line with hard and soft acids and bases theory, according to which the iodine anion is a soft Lewis base, while the bromine anion is a relatively harder base. Considering that the lead cation is a soft Lewis acid,37 creating a bond with iodine is more energetically beneficial for it. In addition, this observation makes an important demonstration of the fact that estimation of mixed halide composition from the precursors’ ratio is not always possible.
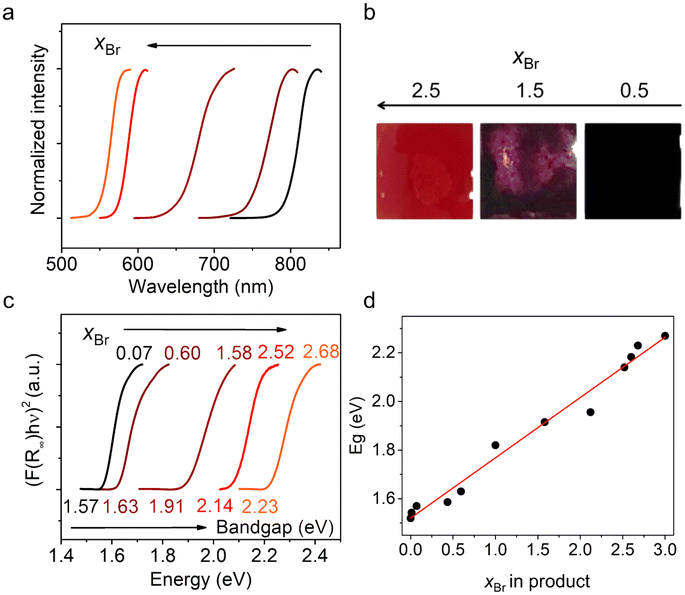
The optical properties of polycrystalline (AzrH)PbBrxI3−x mixed halide perovskite samples were studied by measuring their optical reflectance spectra (Fig. 4). All materials displayed a characteristic cut-off of reflectance, which is typical for semiconducting materials. The cut-off wavelength shifted gradually from 794 nm for x = 0.07 to 550 nm for x = 2.68, thus covering a yellow-to-red range of visible spectrum and even partially covering the near-IR range. Optical photographs of selected powder samples are given in Fig. 4b that show a change in the samples’ color upon halogen substitution from orange to black.
Optical band gap values can be extracted from the experimentally determined absorption coefficient using a Tauc plot based on the equation:
| (αhυ)1/n = A(hυ − Eg), | (3) |
When reflectance spectra are obtained experimentally, instead of the absorbance spectra, the Kubelka–Munk function has to be applied. According to this:
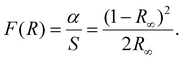 | (4) |
The function F(R) is directly proportional to the absorption coefficient α and inversely proportional to the scattering S, while R∞ is the diffuse reflectance of the sample. Thus, the optical band gap of the studied materials value can be extracted by plotting the following function:
| (hυF(R∞))2 = A(hυ − Eg). | (5) |
The Tauc plots for polycrystalline (AzrH)PbBrxI3−x mixed halide perovskite samples are shown in Fig. 4c. The band gap of the studied materials varies in the range of 1.57 eV (for xBr = 0.07) − 2.23 eV (for xBr = 2.68). The conservation of a cubic structure for the whole series of (AzrH)PbBrxI3−x solid solutions is important and a linear dependance of the cell parameter on the Br content causes a linear dependance of band gap on the Br content (Fig. 4d). This dependance can be linearized as follows:
| Eg = 1.520 + 0.248xBr. | (6) |
This linear dependance provides additional proof that the materials from the studied series are semiconductors that follow Vegard's law.
For comparison, the Eg dependance on the cell parameter in MAPbBrxI3−x series (xBr = 0.24–2.73) has been shown to obey the dependency described by a quadratic equation. Simultaneously, the iodine-rich samples tend to display significant deviation from this dependence, which can be explained by the formation of a tetragonal phase for this composition.36
Notably, these samples do not have any considerable fluorescence at room temperature. However, the title Br perovskite is known to have fluorescence at low temperatures or when processed to NPs (sometimes referred to as perovskite quantum dots). Therefore, the linear dependance of band gap on xBr content in (AzrH)PbBrxI3−x series has a significant advantage for further application in optoelectronic devices, such as light emitting diodes, due to the possibility of conducting more straightforward prediction of photoluminescence wavelength upon fabrication of devices.
Experimental
Chemicals and reagents
Aziridine, lead bromide, lead iodide, hydrobromic (48%), and hydroiodic acids (57%) were purchased from UkrOrgSyntez Ltd.Synthetic procedures
Br![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) I ratio I ratio |
x Br in precursors | x Br in productsa | PbI2 | PbBr2 | HI | HBr | H2O | Azr | MeCN | H2O | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mmol | mg | mmol | mg | mL | mL | mL | μL | mL | mL | |||
| a x Br in products established by indexing their PXRD patterns and comparing the cell parameters obtained with results derived from the SXRD measurements. | ||||||||||||
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2.90 | 2.73 | 0.0065 | 3.00 | 0.1951 | 71.60 | 0.02 | 0.60 | 1.00 | 63.00 | 0.10 | 0.20 |
25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) 1 1
|
2.88 | 2.68 | 0.0065 | 3.00 | 0.1627 | 59.70 | 0.03 | 0.60 | 1.00 | 53.00 | 0.10 | 0.20 |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) 1 1
|
2.86 | 2.60 | 0.0108 | 5.00 | 0.2169 | 79.60 | 0.04 | 0.60 | 1.00 | 71.00 | 0.10 | 0.20 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) 1 1
|
2.81 | 2.52 | 0.0108 | 5.00 | 0.1627 | 59.70 | 0.05 | 0.60 | 1.00 | 54.00 | 0.20 | 0.20 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) 1 1
|
2.73 | 2.12 | 0.0108 | 5.00 | 0.1084 | 39.80 | 0.05 | 0.40 | 0.80 | 37.00 | 0.20 | 0.20 |
7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) 1 1
|
2.63 | 1.58 | 0.0325 | 15.00 | 0.2278 | 83.60 | 0.10 | 0.60 | 0.80 | 82.00 | 0.50 | 0.20 |
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) 1 1
|
2.50 | 1.00 | 0.0217 | 10.00 | 0.1084 | 39.80 | 0.10 | 0.40 | 0.80 | 41.00 | 0.50 | 0.20 |
1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) 1 1
|
1.80 | 0.60 | 0.0868 | 40.00 | 0.1302 | 47.80 | 0.40 | 0.50 | 0.80 | 68.00 | 0.50 | 0.20 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) 1 1
|
1.50 | 0.44 | 0.0868 | 40.00 | 0.0872 | 32.00 | 0.24 | 0.20 | 0.80 | 54.00 | 0.80 | 0.20 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) 1.5 1.5
|
1.20 | 0.16 | 0.1226 | 56.50 | 0.0817 | 30.00 | 0.30 | 0.17 | 0.50 | 64.00 | 0.80 | 0.20 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) 2.5 2.5
|
0.86 | 0.07 | 0.1022 | 47.10 | 0.0409 | 15.00 | 0.40 | 0.13 | 0.50 | 45.00 | 1.00 | 0.20 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) 5 5
|
0.50 | 0.01 | 0.1362 | 62.80 | 0.0272 | 10.00 | 0.40 | 0.07 | 0.50 | 31.00 | 1.00 | 0.20 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) 10 10
|
0.27 | 0.00 | 0.1356 | 62.50 | 0.0136 | 5.00 | 0.30 | 0.03 | 0.50 | 47.00 | 1.00 | 0.20 |
6 eq. of aziridine were dissolved in a water/acetonitrile mixture. Afterward, the latter solution was added portion-wise to the lead halide solution with constant stirring.
Measurements and characterization
Optical reflectance measurements were performed on a Shimadzu spectrometer RF6000.
The PXRD patterns were acquired on a Shimadzu XRD-6000 diffractometer using Cu-Kα radiation (5–50°, 0.05° step) and Benchtop Rigaku Miniflex 600 diffractometer using Cu-Kα radiation (2–50°, 0.025° step).
Conclusions
In this work, we describe a new series of mixed halide lead perovskites with an aziridinium cation. Each of the reported materials contains a single crystalline phase which has a three-dimensional cubic structure. The cell parameters display a linear decrease with a decrease in the bromide content, proving that the reported materials obey the empirical Vegard's law and form solid solutions. It was established that iodine has significant preferential inclusion into the framework upon synthesis. Importantly, the variation in halogen content allows for fine-tuning of the absorption edge of aziridinium lead perovskite: a decrease in the bromide content leads to a linear red shift of the absorption cut-off. The optical band gap can be tuned in the 1.57–2.23 eV range. This effect makes the mixed halide aziridinium perovskites appealing candidates for further optoelectronic applications.Author contributions
Olesia I. Kucheriv – writing – original draft, formal analysis; Dmytro A. Haleliuk – investigation, formal analysis; Sergiu Shova – investigation, formal analysis; Il'ya A. Gural'skiy – supervision, conceptualization, funding acquisition, writing – review & editing.Data availability
Crystallographic data for (AzrH)PbBr0.04I2.96, (AzrH)PbBr0.09I2.91, (AzrH)PbBr0.85I2.15, (AzrH)PbBr1.96I1.04, (AzrH)PbBr2.01I0.99, (AzrH)PbBr2.08I0.92, and (AzrH)PbBr2.79I0.21 were deposited at Cambridge Crystallographic Data Center under CCDC numbers 2390779–2390785† and can be obtained from https://www.ccdc.cam.ac.uk/.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the financial support from the Ministry of Education and Science of Ukraine (grant no 24BF037-02 and grant no 24DF037-03H financed by the external aid instrument of the European Union to fulfill the obligations of Ukraine in the European Union Framework Program for Scientific Research and Innovation “Horizon 2020”). The authors also acknowledge the courage of the Armed Forces of Ukraine that made the submission of this manuscript possible.References
- Y. Zhao and K. Zhu, Chem. Soc. Rev., 2016, 45, 655–689 RSC.
- W. Li, Z. Wang, F. Deschler, S. Gao, R. H. Friend and A. K. Cheetham, Nat. Rev. Mater., 2017, 2, 16099 CrossRef.
- A. Younis, C. Lin, X. Guan, S. Shahrokhi, C. Huang, Y. Wang, T. He, S. Singh, L. Hu, J. R. D. Retamal, J. He and T. Wu, Adv. Mater., 2021, 33, 2005000 CrossRef CAS.
- D. Weber, Z. Naturforsch., B, 1978, 33, 862–865 CrossRef.
- D. Weber, Z. Naturforsch., B, 1978, 33, 1443–1445 CrossRef.
- A. Kojima, K. Teshima, Y. Shirai and T. Miyasaka, J. Am. Chem. Soc., 2009, 131, 6050–6051 CrossRef CAS PubMed.
- M. Kim, J. Jeong, H. Lu, T. K. Lee, F. T. Eickemeyer, Y. Liu, I. W. Choi, S. J. Choi, Y. Jo, H.-B. Kim, S. Mo, Y.-K. Kim, H. Lee, N. G. An, S. Cho, W. R. Tress, S. M. Zakeeruddin, A. Hagfeldt, J. Y. Kim, M. Grätzel and D. S. Kim, Science, 2022, 375, 302–306 CrossRef CAS PubMed.
- B. Niu, H. Liu, Y. Huang, E. Gu, M. Yan, Z. Shen, K. Yan, B. Yan, J. Yao, Y. Fang, H. Chen and C. Li, Adv. Mater., 2023, 35, 2212258 CrossRef CAS.
- S. Ju, Y. Zhu, H. Hu, Y. Liu, Z. Xu, J. Zheng, C. Mao, Y. Yu, K. Yang, L. Lin, T. Guo and F. Li, Light: Sci. Appl., 2022, 11, 331 CrossRef CAS PubMed.
- Y.-J. Lu, T. L. Shen, K. Peng, P. Cheng, S. Chang, M. Lu, C. W. Chu, T. Guo and H. A. Atwater, ACS Photonics, 2021, 8, 335–342 CrossRef CAS.
- W. Tang, J. Zhang, S. Ratnasingham, F. Liscio, K. Chen, T. Liu, K. Wan, E. S. Galindez, E. Bilotti, M. Reece, M. Baxendale, S. Milita, M. A. McLachlan, L. Su and O. Fenwick, J. Mater. Chem. A, 2020, 8, 13594–13599 RSC.
- H. Park, I. J. Park, M. G. Lee, K. C. Kwon, S. Hong, D. H. Kim, S. A. Lee, T. H. Lee, C. Kim, C. W. Moon, D. Son, G. H. Jung, H. S. Yang, J. R. Lee, J. Lee, N. Park, S. Y. Kim, J. Y. Kim and H. W. Jang, ACS Appl. Mater. Interfaces, 2019, 11, 33835–33843 CrossRef CAS.
- N. R. Al Amin, C.-C. Lee, Y.-C. Huang, C.-J. Shih, R. Estrada, S. Biring, M.-H. Kuo, C.-F. Li, Y.-C. Huang and S.-W. Liu, ACS Appl. Mater. Interfaces, 2023, 15, 21284–21295 CrossRef CAS.
- T. J. Jacobsson, J.-P. Correa-Baena, M. Pazoki, M. Saliba, K. Schenk, M. Grätzel and A. Hagfeldt, Energy Environ. Sci., 2016, 9, 1706–1724 RSC.
- Y. Zhang, Y. Li, L. Zhang, H. Hu, Z. Tang, B. Xu and N. Park, Adv. Energy Mater., 2021, 11, 2102538 CrossRef CAS.
- J. Jeong, M. Kim, J. Seo, H. Lu, P. Ahlawat, A. Mishra, Y. Yang, M. A. Hope, F. T. Eickemeyer, M. Kim, Y. J. Yoon, I. W. Choi, B. P. Darwich, S. J. Choi, Y. Jo, J. H. Lee, B. Walker, S. M. Zakeeruddin, L. Emsley, U. Rothlisberger, A. Hagfeldt, D. S. Kim, M. Grätzel and J. Y. Kim, Nature, 2021, 592, 381–385 CrossRef CAS PubMed.
- Z. Xiong, L. Lan, Y. Wang, C. Lu, S. Qin, S. Chen, L. Zhou, C. Zhu, S. Li, L. Meng, K. Sun and Y. Li, ACS Energy Lett., 2021, 6, 3824–3830 CrossRef CAS.
- Z. Qin, Y. Chen, X. Wang, N. Wei, X. Liu, H. Chen, Y. Miao and Y. Zhao, Adv. Mater., 2022, 34, 2203143 CrossRef CAS.
- Z. Huang, Y. Bai, X. Huang, J. Li, Y. Wu, Y. Chen, K. Li, X. Niu, N. Li, G. Liu, Y. Zhang, H. Zai, Q. Chen, T. Lei, L. Wang and H. Zhou, Nature, 2023, 623, 531–537 CrossRef CAS.
- O. I. Kucheriv, V. Y. Sirenko, H. R. Petrosova, V. A. Pavlenko, S. Shova and I. A. Gural'skiy, Inorg. Chem. Front., 2023, 10, 6953–6963 RSC.
- O. A. Semenikhin, O. I. Kucheriv, L. Sacarescu, S. Shova and I. A. Gural'skiy, Chem. Commun., 2023, 59, 3566–3569 RSC.
- M. Mączka, M. Ptak, A. Gągor, J. K. Zaręba, X. Liang, S. Balčiūnas, O. A. Semenikhin, O. I. Kucheriv, I. A. Gural'skiy, S. Shova, A. Walsh, J. Banys and M. Šimėnas, Chem. Mater., 2023, 35, 9725–9738 CrossRef.
- H. R. Petrosova, O. I. Kucheriv, S. Shova and I. A. Gural'skiy, Chem. Commun., 2022, 58, 5745–5748 RSC.
- D. Drozdowski, A. Gągor, D. Stefańska, J. K. Zareba, K. Fedoruk, M. Mączka and A. Sieradzki, J. Phys. Chem. C, 2022, 126, 1600–1610 CrossRef CAS.
- M. Mączka, M. Ptak, A. Gagor, D. Stefańska, J. K. Zareba and A. Sieradzki, Chem. Mater., 2020, 32, 1667–1673 CrossRef.
- M. Mączka, M. Ptak, D. L. M. Vasconcelos, L. Giriunas, P. T. C. Freire, M. Bertmer, J. Banys and M. Simenas, J. Phys. Chem. C, 2020, 124, 26999–27008 CrossRef.
- M. Mączka, A. Gagor, J. K. Zareba, D. Stefanska, M. Drozd, S. Balciunas, M. Šimėnas, J. Banys and A. Sieradzki, Chem. Mater., 2020, 32, 4072–4082 CrossRef.
- H. Y. Zhang and R. G. Xiong, Chem. Commun., 2022, 59, 920–923 RSC.
- H. Y. Zhang, X. G. Chen, Z. X. Zhang, X. J. Song, T. Zhang, Q. Pan, Y. Zhang and R. G. Xiong, Adv. Mater., 2020, 32, 1–8 Search PubMed.
- S. Huang, P. Huang, L. Wang, J. Han, Y. Chen and H. Zhong, Adv. Mater., 2019, 31, 1903830 CrossRef CAS.
- A. Sadhanala, F. Deschler, T. H. Thomas, S. E. Dutton, K. C. Goedel, F. C. Hanusch, M. L. Lai, U. Steiner, T. Bein, P. Docampo, D. Cahen and R. H. Friend, J. Phys. Chem. Lett., 2014, 5, 2501–2505 CrossRef CAS PubMed.
- S. Aharon, B. El Cohen and L. Etgar, J. Phys. Chem. C, 2014, 118, 17160–17165 CrossRef CAS.
- E. T. Hoke, D. J. Slotcavage, E. R. Dohner, A. R. Bowring, H. I. Karunadasa and M. D. McGehee, Chem. Sci., 2015, 6, 613–617 RSC.
- G. E. Eperon, S. D. Stranks, C. Menelaou, M. B. Johnston, L. M. Herz and H. J. Snaith, Energy Environ. Sci., 2014, 7, 982 RSC.
- H. Yu, F. Wang, F. Xie, W. Li, J. Chen and N. Zhao, Adv. Funct. Mater., 2014, 24, 7102–7108 CrossRef CAS.
- Y. Nakamura, N. Shibayama, A. Hori, T. Matsushita, H. Segawa and T. Kondo, Inorg. Chem., 2020, 59, 6709–6716 CrossRef CAS.
- R. A. Kerner, T. H. Schloemer, P. Schulz, J. J. Berry, J. Schwartz, A. Sellinger and B. P. Rand, J. Mater. Chem. C, 2019, 7, 5251–5259 RSC.
- G. M. Sheldrick, Acta Crystallogr., Sect. C:Struct. Chem., 2015, 71, 3–8 Search PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. A:Found. Crystallogr., 2008, 64, 112–122 CrossRef CAS.
- O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard and H. Puschmann, J. Appl. Crystallogr., 2009, 42, 339–341 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Crystallographic tables, Goldschmidt's tolerance factors. CCDC 2390779–2390785. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4dt02879a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |